Abstract
Aneurysmal subarachnoid hemorrhage (aSAH) is a subtype of hemorrhagic stroke with significant morbidity and mortality. Aneurysmal bleeding causes elevated intracranial pressure, decreased cerebral blood flow, global cerebral ischemia, brain edema, blood component extravasation, and accumulation of breakdown products. These post-SAH injuries can disrupt the integrity and function of the blood-brain barrier (BBB), and brain tissues are directly exposed to the neurotoxic blood contents and immune cells, which leads to secondary brain injuries including inflammation and oxidative stress, and other cascades. Though the exact mechanisms are not fully clarified, multiple interconnected and/or independent signaling pathways have been reported to be involved in BBB disruption after SAH. In addition, alleviation of BBB disruption through various pathways or chemicals has a neuroprotective effect on SAH. Hence, BBB permeability plays an important role in the pathological course and outcomes of SAH. This review discusses the recent understandings of the underlying mechanisms and potential therapeutic targets in BBB disruption after SAH, emphasizing the dysfunction of tight junctions and endothelial cells in the development of BBB disruption. The emerging molecular targets, including toll-like receptor 4, netrin-1, lipocalin-2, tropomyosin-related kinase receptor B, and receptor tyrosine kinase ErbB4, are also summarized in detail. Finally, we discussed the emerging treatments for BBB disruption after SAH and put forward our perspectives on future research.
Keywords: Subarachnoid hemorrhage, early brain injury, blood-brain barrier, endothelial cell, tight junction, toll-like receptor 4
1. Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a subtype of hemorrhagic stroke that accounts for about 5% of strokes, and is one of the most life-threatening diseases [1, 2]. Despite the improvement of therapies and clinical management, morbidity remains at 9 to 15 cases per 100,000 person-years, and mortality remains at 40–50% [2-4]. Of those who survive, more than 40% suffer long-term neurological sequelae, and only a fifth may have no residual symptoms [5]. The recent research focus of SAH has shifted from vasospasm to early brain injury (EBI), which is defined as the pathophysiological event that occurs within 72 hours after SAH. EBI has been considered as the main determinant of poor outcome in patients with SAH [6].
The rapidly increased intracranial pressure in EBI after SAH causes significantly decreased cerebral blood flow (CBF) and global cerebral ischemia, along with the extravasation of the blood and degradation products, collectively leading to brain injury [7, 8]. These pathological changes can also result in endothelial cell (EC) contraction and disassembly of tight junctions, and then further induce an increase in vascular permeability, which is related to blood-brain barrier (BBB) damage and brain edema after SAH [9]. Researchers found that aSAH patients with unfavorable outcomes tend to have increased BBB permeability and suggested that the early evaluation of BBB permeability may predict unfavorable outcomes in patients with SAH and help guide clinical management [10, 11]. The increase in microvascular permeability after SAH is also related to delayed cerebral ischemia (DCI) and poor prognosis [12]. Ivanidze et al. indicated that BBB permeability has the potential to become a biomarker in predicting delayed infarction related to DCI before the onset of clinical symptoms and alterations of CBF [13]. Thus, BBB disruption serves as an important component of both EBI and DCI.
According to recent reports (January 2014 - October 2019) regarding BBB disruption after SAH, this review mainly focuses on the underlying mechanisms, including EC apoptosis and disruption of tight junctions, and gives an update on the emerging therapeutic targets in BBB disruption after SAH.
2. Characteristics of the Blood-Brain Barrier
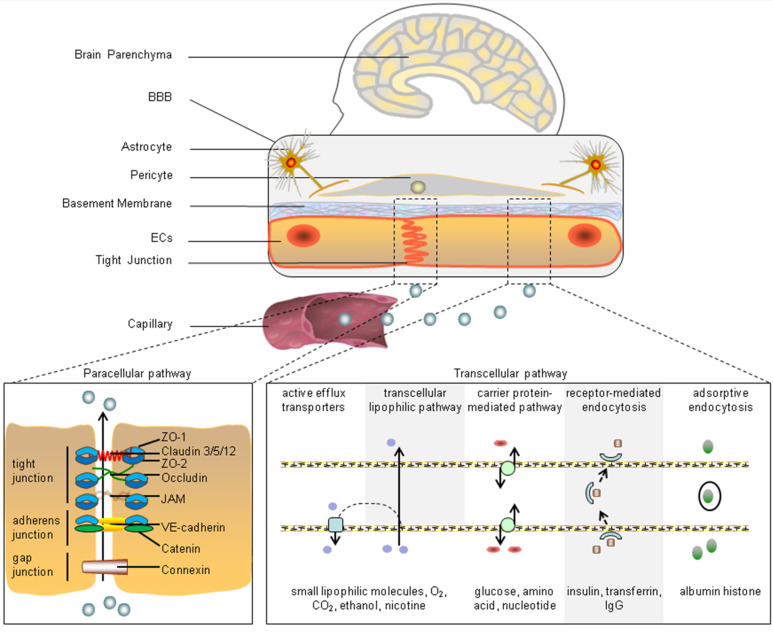
The BBB is a component of the neurovascular unit. It serves as the blood-brain interface and mediates substance communication between the peripheral blood and central nervous system (CNS) [14]. As shown in Fig. (1), microvascular endothelial cells, tight junctions, astrocytes, and pericytes are the main components of the BBB and can affect BBB functions [15]. Importantly, according to the requirements of different brain regions and the types of blood vessels, the compositions of the BBB can change and take in other components, including the extracellular matrix, vascular smooth muscle cells, and glial cells [16].
Fig. (1).
Components of the BBB and the transport pathways of substances across the BBB. The typical BBB is mainly composed of ECs, tight junctions, a basement membrane, pericytes, and astrocytes. The substances in capillaries are transported into the brain parenchyma through the paracellular pathway (tight junction, adherens junction, and gap junction complexes) and transcellular pathway (transcellular lipophilic pathway, carrier protein-mediated pathway, receptor-mediated endocytosis, adsorptive endocytosis, and active efflux transporters). ZO-1: (zonula occludens-1); JAM: (junctional adhesion molecule); VE-cadherin: (vascular endothelial-cadherin); lgG: (immunoglobulin G). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Endothelial Cells
A monolayer of cerebral ECs that are connected by tight junctions and adherens junctions is the first line of defense of the brain against circulating factors [17]. The differences of cerebral ECs from the peripheral ECs are that they form a stronger barrier through the expression of tight junction proteins and restrict vesicle-mediated transcellular transport and transporters by decreasing pinocytosis [18]. The ECs can readily and efficiently transport proteins and molecules into and out of the brain tissues since they make up the largest surface area at the blood-brain interface [16]. Simultaneously, transcellular transport is limited by vesicle transport activity in ECs and plasma membrane components, such as caveolin-1 [19, 20]. ECs are polarized cells with a luminal and abluminal side, so the expression of transporters and cellular machinery is different at each side. The ECs are the most often used cell type in BBB models in vitro [16]. For instance, the ECs were used in the studies of Alzheimer's disease as well as the regulation of BBB function [21, 22].
2.2. Tight Junctions
The junctional complexes that are located between ECs comprise tight junctions, adherens junctions, and gap junctions;their function is to reduce paracellular permeability [20]. The tight junctions comprise various proteins including occludin, zonula occludens -1 (ZO-1), and claudin-5 [7]. The tight junction proteins are structurally identical phosphoproteins, and the phosphorylation can change their interaction, redistribution, and transmembrane protein localization [7]. Other proteins participating in the tight junction system are the junctional adhesion molecules (JAMs). As integral membrane proteins, the JAMs belong to the immunoglobulin superfamily, of which several isoforms have been discovered. The homotypic and heterotypic interactions between JAMs and JAM family members can oppose ECs and are involved in leukocyte migration across EC layers [23].
2.3. Astrocytes
Astrocytes are the most abundant cells in the CNS and participate in the regulation of nearly all aspects of neuronal function, e.g., development, survival, metabolism, and neurotransmission [24]. The main function of astrocytes in the BBB is to provide maintenance and repair support through the release of several effector molecules. Astrocytes also contribute to maintaining BBB function by inducing barrier properties and polarization of transporters [25-27]. Astrocytes also provide a cellular connection between neuronal activity and blood vessels, termed neurovascular coupling, and this connection allows astrocytes to regulate the CBF and brain water content in response to neuronal activity by relaying signals [16, 28]. The astrocytic end-feet ensheath most portions of the microvessels and capillaries, and help to regulate ion and water transportation [14]. Moreover, the astrocytic end-feet are polarized and guided to cerebral vascular walls by pericytes [29].
2.4. Pericytes
Pericytes are mural cells that cover the abluminal surface of microvessels and are embedded in the vascular basement membrane. The pericytes are connected to ECs by way of gap junctions, adherens junctions, and peg-and-socket connections [30, 31]. Cerebral pericytes have distinct properties to their peripheral counterparts, and the proportion of pericytes in the CNS is much greater than that of peripheral tissues [30]. Pericytes can maintain and stabilize the monolayer of ECs by regulating angiogenesis and the deposition of the extracellular matrix. They are also essential for the development of tight junctions and BBB function in both developing and adult brains [16, 31, 32]. In addition, the contractility of pericytes is conducive to the regulation of CBF by changing capillary diameter in response to neural activity [28, 31, 33].
All of the above cell types can communicate with each other to establish the BBB and regulate its structure and function [16]. Neurons and basement membranes also significantly contribute to BBB integrity and function. Neurons remain close (less than 8-20 µm) to brain capillaries and connect with the astrocytic end-feet of the neurovascular unit 34. Neurons can interact with the extracellular matrix and release factors to stimulate angiogenesis, and they also contribute to the regulation of blood flow and microvascular permeability [35]. The cerebrovascular basement membranes are composed of collagen IV, laminin, perlecan, nidogen, and fibronectin [36, 37]. The basement membranes act as physical barriers surrounding the abluminal surface of ECs and anchor the cells in the BBB. The components of the basement membrane can mediate various signaling mechanisms in ECs and pericytes, thereby affecting BBB stability and function [38, 39].
3. Blood-Brain Barrier Disruption After Subarachnoid Hemorrhage
BBB dysfunction has been reported to occur within hours after SAH and has been linked with pathologic changes, including brain edema, microthrombosis, inflammation, and abnormal cerebral metabolism [13, 40-42]. BBB dysfunction is also associated with the development of delayed ischemia in patients with SAH [43]. In addition, the transfer of fluid and molecules into the brain parenchyma plays a key role in brain injury after SAH. Therefore, exploring the exact molecular mechanisms of BBB damage is crucial for studies on SAH.
3.1. Animal Models Used in Subarachnoid Hemorrhage Studies
The brain injuries that occur after SAH are very complicated, and more than 72 animal models using multifarious species have been proposed with more or less modifications according to the research objectives [44]. However, no successful translation of preclinical findings into clinical trials has been reported, and this may be attributed to the fact that standard guidance in the performance and modeling of experimental SAH, such as the differences in outcome evaluation and methodological disadvantages, has not been established [45]. Many biological factors, including age, sex, genetic background, strain-related differences, comorbidities, and anesthetics, have been shown to strongly affect outcome evaluations [44, 45]. Currently, the endovascular perforation method is widely used in mice or rats to study EBI after SAH [45]. The endovascular perforation models can well imitate the natural course of aSAH, and acute physiological and pathological changes in the models, such as the elevation of intracranial pressure, blood distribution in the subarachnoid region, and even the high mortality rates, are similar to clinical findings in aSAH [46]. In addition, a novel grading system evaluating the SAH bleeding scale in endovascular perforation rat models can categorize animals according to the severity of bleeding, and the system substantially enhances the practicability and applicability of endovascular perforation models [47]. Hence, the endovascular perforation method is becoming increasingly popular in studies of SAH and can also imitate the natural course of BBB disruption in SAH. Current findings of BBB disruption described in this review are mostly obtained from this model.
3.2. Characteristics of Blood-Brain Barrier Disruption after Subarachnoid Hemorrhage
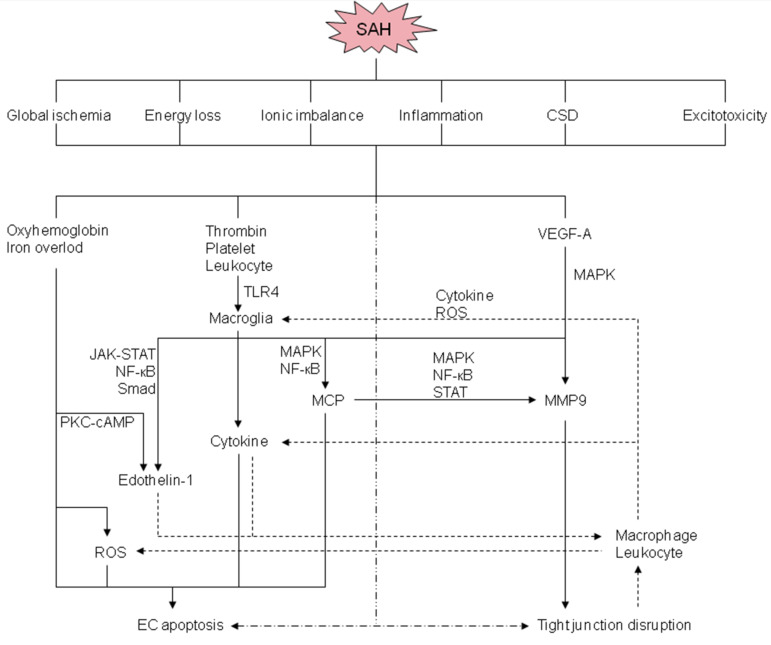
In both ischemic and hemorrhagic stroke, BBB disruption is a prominent pathological characteristic and is usually associated with poor neurological outcomes [8, 48, 49]. BBB disruption occurs very soon after SAH onset, even before MRI changes, and researchers have provided evidence that BBB injury starts as early as 30 minutes, peaks at 3 hours, and a later injury peaks at 72 hours post-SAH, and these changes were evaluated using tight junction proteins, such as occludin and ZO-1 [50]. As shown in Fig. (2), the extravasated blood components, such as oxyhemoglobin and immune cells, are the main cause of BBB disruption after SAH, while in ischemic stroke, the focal ischemia itself plays a significant role in BBB disruption [48, 49]. Interestingly, BBB disruption after aSAH is distinct from that which occurs after intracerebral hemorrhage or cerebral ischemia, and this may be attributed to the combination of the immediate development of transient global ischemia after aneurysmal rupture, delayed cerebral ischemia, and extravasated blood components at days 4 to 14 after onset [8]. The clinical data have shown that global brain edema is observed in 8-67% of patients with aSAH at admission, and about 12% of patients develop delayed cerebral edema within two weeks after bleeding onset [8]. The global brain edema or increased BBB permeability that occurs at the acute stage was reported to be correlated with unfavorable outcomes in aSAH, and researchers have also indicated that BBB injury was an important manifestation of pathological mechanisms of EBI in experimental SAH models [8, 10]. Furthermore, aSAH is experienced by relatively young people (mean age: 55 years) as compared with other types of strokes [51]. The comorbidities after all types of stroke also influence BBB disruption and the outcomes, and the comorbidities that occur in patients with aSAH and other types of strokes are different [44, 48]. Therefore, the BBB injury that occurs after aSAH is unique, and thus, the research and therapeutic approaches should also differ from other types of stroke.
Fig. (2).
Potential mechanisms and signaling pathways of BBB disruption after SAH. CSD: Cortical spreading depolarization; JAK: Janus kinase; MAPK: mitogen-activated protein kinase; MCP: matricellular protein; MMP-9: matrix metalloproteinase-9; NF-κΒ: nuclear factor kappa-light-chain-enhancer of activated B cells; PKC-cAMP: protein kinase C-cyclic adenosine monophosphate; ROS: reactive oxygen species; STAT: signal transducer and activator of transcription; TLR4: Toll-like receptor 4; VEGF-A: vascular endothelial growth factor-A. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
As global brain edema is a prognostic factor in patients with aSAH, timely and effective therapy, as well as clinical management, may contribute to an improvement of outcomes [44]. In patients with poor-grade SAH, drug treatments including hypertonic saline, hyperosmolar agents, therapeutic hypothermia, barbiturates, and decompressive craniectomy have been used in clinical work to reduce brain edema and/or to control intracranial pressure, while the exact efficacy has not been retrospectively analyzed or well illuminated [52, 53]. Many chemicals have been reported to protect against EBI and/or cerebral vasospasm, but the drugs that can effectively prevent vasospasm have failed to improve outcomes in patients with SAH [44, 54]. The poor therapeutic effect may be attributed to the limited therapeutic window of EBI or acute BBB disruption, because EBI, as well as BBB injury, have occurred within minutes after the initial bleeding; thus, the early administration of drugs even within 24-72 hours of the bleeding might prevent cerebral vasospasm, but not EBI [44]. The complexity and changeability of EBI drive researchers to clarify the exact mechanisms of BBB disruption after SAH and develop effective therapies against it.
3.3. Mechanisms of Blood-brain Barrier Disruption After Subarachnoid Hemorrhage
The neurovascular unit that is also known as the BBB mainly consists of a continuous EC layer, astrocytic end-feet, and pericytes. The function of the BBB is maintained by the adjacent ECs strictly bound by the tight junctions, and this structure can prevent the extravasation of intravascular contents into the brain and the invasion of immune cells. Under physiological conditions, the BBB can regulate brain homeostasis by selectively allowing specific substances, such as water, ions, and small molecular weight particles, to permeate [55]. While under pathological conditions, after the disruption of the BBB, detrimental blood contents such as thrombin and fibrinogen will enter the brain parenchyma, leaving the brain tissues directly exposed to these toxic substances [49]. Increased BBB permeability can also lead to the abnormal infiltration and transmigration of immune cells like leukocytes into the brain parenchyma, and these cells then release various cytokines, chemokines, reactive oxygen species (ROS), and proteases [56]. The adverse effects of these released substances induce further deterioration of brain tissue, brain edema, intracranial pressure, neuronal apoptosis, and epilepsy [57]. Thus, BBB damage is regarded as an important therapeutic target to treat EBI and improves the outcomes of aSAH. Researchers also found that early identification of BBB disruption might serve as a pivotal predictive biomarker for neurological outcome in patients with aSAH [11].
Multiple mechanisms are involved in the development of BBB disruption after aSAH, with endothelial cell apoptosis and disruption of tight junctions being the main mechanisms that have been widely studied. The well-studied mechanisms involved in BBB disruption after SAH are shown in Fig. (2). In the acute stage, all the exudative blood components, cerebral ischemia, and brain tissue damage will contribute to BBB disruption, as well as many other cascades, such as inflammation, oxidative stress, and the disorder of ions. After the rupture of cerebral aneurysms, the blood contents instantaneously extravasate out from the intravascular lumen under arterial pressure and then the surrounding brain tissues will suffer from mechanical damage, and the subsequent elevated intracranial pressure, global cerebral ischemia, and brain edema [58]. Brain edema further increases the intracranial pressure and decreases cerebral perfusion pressure and CBF [59]. A previous report showed that the diffuse brain edema is an independent risk factor for poor outcomes and appears in about 8% of patients with SAH during the early stage [60]. In addition, the blood components and degradation products rapidly spread in the subarachnoid space, which further extends the following injuries.
4. Potential Therapeutic Approaches to Blood-brain Barrier Disruption
Several therapeutic approaches have been reported to reduce BBB disruption after SAH (Table 1) and intracranial hematoma in preclinical models. Importantly, the exact mechanisms of these approaches to alleviate the damage of the brain parenchyma are undefined, and the specific agents that could target the BBB have not been examined [61]. Though astrocytes and pericytes are important to the integrity of BBB, the relevant studies are few in SAH. Thus, this paper mainly focuses on EC apoptosis and disruption of tight junctions to review the mechanisms and potential therapeutic targets of BBB disruption after SAH.
Table 1.
Tight junction and adherens junction protein changes after SAH.
| Models | Changes | Interventions | Refs. |
|---|---|---|---|
| Rat/EP | Occludin redistribution (IHC) | Glibenclamide | Simard et al. [62] |
| Rat/BI | Occludin/ZO-1 ↓ (WB) 48h | HIF-1α, MMPs, aquaporin-4 | Wang et al. [63] |
| Rat/EP | Occludin/ZO-1 ↓ (WB/IHC) 24h | c-Jun N-terminal kinase inhibition | Chen et al. [64] |
| Mice/EP | Claudin-5/ZO-1/JAM-A/VE-cadherin ↓ (WB) 24h | Isoflurane | Altay et al. [65] |
| Rat/EP | Occludin/ZO-1/claudin-5 ↓ (WB) 24h | Melatonin | Chen et al. [66] |
| Rat/EP | Occludin/ZO-1 ↓, claudin-1/5 ↔ (WB, IHC) 0.5 – 168h | None | Li et al. [7] |
| Rat/EP | Occludin/ZO-1/claudin-5/VE-cadherin ↓ (WB, IHC) 24h | Norrin/frizzled-4 | Chen et al. [67] |
| Mice/EP | ZO-1/VE-cadherin ↓ (WB, IHC) 24 h | Thrombomodulin | Xu et al. [68] |
| Rat/EP | ZO-1 ↓ (WB) 24h | Progranulin | Zhou et al. [69] |
| Rat/BI | ZO-1 ↓ (WB) 24h | Fisetin (flavonoid) | Zhou et al. [70] |
| Rat/BI | Claudin-5/occludin ↓ (WB) 24h | PARP inhibition | Chen et al. [71] |
| Mice/EP | ZO-1 ↓ (WB) 24 h | Tenascin-C | Fujimoto et al. [72] |
| Rat/EP | Claudin-5/occludin ↓ (WB) 24h | Ethyl pyruvate | Fang et al. [73] |
| Rat/EP | Claudin-5/occludin ↓ (WB) 24h | Valproic acid | Ying et al. [74] |
| Rat/BI | Occludin/ZO-1 ↓ | Matrine (alkaloid) | Liu et al. [75] |
| Mice/EP | Occludin/ZO-1/claudin-5 ↓ (WB) 48h | Apolipoprotein E | Pang et al. [76] |
| Mice/EP | Occludin/ZO-1 ↓ (WB) 24h | Curcumin | Yuan et al. [77] |
| Rat/EP | Claudin-5/ZO-1 ↓ (WB) 24h | Eph receptor-A4/Fasudil (ROCK inhibition) | Fan et al. [78] |
| Rat/EP | ZO-1 ↓ (IHC) 24 h | Tropomyosin-related kinase receptor B activation | Qi et al. [59] |
| Rat/EP | Occludin/Claudin-5 ↓(WB) 24h | Nrg1 isoform β1 | Qian et al. [79] |
↓: decrease; ↔: no change; EP: endovascular perforation; BI: blood injection; WB: western blot; IHC: immunohistochemistry; ROCK: RhoA kinase; HIF-1a: hypoxia-inducible factor-1a; MMPs: matrix metalloproteinases; PARP: Poly (ADP-ribose) polymerase.
4.1. Endothelial Cell Dysfunction
Under physiological conditions, the ECs of the cerebral capillaries can maintain the function of the BBB. However, the aneurysmal rupture could lead to EC injury and apoptosis, and then cause BBB disruption. Researchers found that multiple factors, such as oxidative stress, oxyhemoglobin, and iron overload could induce apoptosis of ECs within 10 minutes to 24 hours after SAH [58, 80]. Oxyhemoglobin, a representative blood component in the subarachnoid space after aSAH, has cytotoxic effects on ECs through the activation of caspase-3, caspase-8, caspase-9, matrix metalloproteinase (MMP)-9, and elevation of intracellular Ca2+ and the production of free radicals [58]. The ECs are very susceptible to oxidative stress injury, and the excessive production of free radicals from disrupted mitochondrial respiration; the extracellular hemoglobin following erythrolysis and the subsequent iron overload after SAH would also cause serious damage to the ECs [81]. In addition, the overproduction of ROS can activate pro-apoptotic signals, including p53 and caspases-3 and -9, to promote cell apoptosis and further aggravate BBB disruption [57].
Many other factors involved in EC injuries after SAH contain heme, thrombin, platelets, fibrinogen, and leukocytes, and these factors can activate microglia and toll-like receptor 4 (TLR4). TLR4 belongs to the toll-like receptor family, and it can recognize damage-associated molecular patterns and induce inflammatory cascades, likely through nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) and/or activator protein-1, which is mainly mediated by mitogen-activated protein kinases (MAPKs) [8, 82]. In addition, the activation of TLR4 facilitates the production of pro-inflammatory cytokines and mediators, including tumor necrosis factor-α, interleukins-1β, -6, -8, and -12, and MMP-9 [82]. These pro-inflammatory mediators can induce caspase-dependent EC apoptosis and even BBB dysfunction, and upregulate specific cell adhesion molecules on ECs. This can consequently promote the migration of macrophages and neutrophils into the subarachnoid space and brain tissues, and the immune cells release many inflammatory factors, including endothelins and oxidative radicals through the activation of Janus kinase-2, signal transducers and activators of transcription-3, NF-κB, and Smad signaling pathways [58, 83]. Oxyhemoglobin can also induce the production of endothelin-1 in endothelial cells and vascular smooth muscle cells through protein kinase C-cyclic adenosine monophosphate signaling, and the binding of endothelin-1 to endothelin-A receptors can activate macrophages, increase neutrophil-vessel wall interactions, and induce the production of free radicals. All the above pathological and molecular mechanisms were reported to cause EC dysfunction in myocardial ischemia [58, 84]. While clazosentan, an endothelin receptor antagonist, was reported to decrease large-artery vasospasm, it cannot prevent oxidative stress, microthromboembolism, or endothelial nitric oxide synthase dysfunction after SAH in mice [85].
ErbB4, which is a member of the epidermal growth factor receptor tyrosine kinase family, plays an important role in the regulation of axonal guidance, neurite outgrowth, and synaptic signaling [86]. ErbB4 was reported to facilitate the survival of ECs and preserve the BBB integrity in oxidative stress injury [87]. In a study of SAH, ErbB4 was found to be expressed in ECs and neurons, and the activation of ErbB4 improved BBB disruption after SAH and increased the expression of tight junction proteins, including occludin and claudin-5. The potential protective mechanism might be via the ErbB4/YAP/PIK3CB signaling pathway [79].
Focal adhesion kinase (FAK), which is a cytoplasmic protein tyrosine kinase, is involved in the regulation of EC function and endothelial barrier integrity [88]. In a study of embryonic development, a lack of FAK in ECs resulted in the disruption of barrier integrity and the abnormal distribution of vascular endothelial cadherin [88]. A recent study showed FAK phosphorylation was increased after SAH, and the administration of exogenous netrin-1 (NTN-1) can further increase the p-FAK level, providing effective improvement of BBB disruption, such as restoring the tight junction proteins (ZO-1 and occludin.) [89]. As a multifunctional protein, NTN-1 was recently found to maintain BBB integrity in models of traumatic brain injury and experimental autoimmune encephalomyelitis [90, 91]. While in SAH, both endogenic NTN-1 and its receptor Deleted in Colorectal Cancer (DCC), which are mainly expressed in ECs, were found to increase at 24 hours after SAH onset. Exogenous NTN-1 can preserve BBB integrity and attenuate EBI, and the protective effects of NTN-1 on the BBB are mediated through the DCC/FAK/RhoA-related signaling pathway [89].
The cortical spreading depolarization, excitotoxicity, and loss of energy stores caused by the derangement in neurotransmitter release and inhibition of reuptake can induce ionic imbalance and contribute to the apoptosis of ECs after SAH [57]. Moreover, activated astrocytes and microglia can synthesize glutamate, which serves as a major excitatory transmitter, and glutamate can induce toxic effects such as massive Ca2+ influx by over-activation of N-methyl D-aspartate receptors, finally leading to apoptosis and necrosis of multifarious cells after SAH [57].
4.2. Tight Junction Disruption
The permeability of the BBB can be disrupted by the contraction of ECs and disassembly of tight junctions, leading to brain edema formation after SAH [58]. There are two major routes for the transport of substances across the BBB: the paracellular (tight junctions between cells) and the transcellular (caveolin- and clathrin-mediated endocytosis and macropinocytosis) pathways. Tight junction disruption causes the diffusion of exudate into the extracellular space of the brain and elevates the osmotic pressure, which further leads to extracellular fluid accumulation [59]. The phosphorylation of tight junction proteins can regulate their interaction, redistribution, and transmembrane protein localization [7]. ZO-1 anchors the junction to the actin cytoskeleton and links to the cytoplasmic tail of occludin [92]. Downregulation of ZO-1 and occludin in tight junctions would cause capillary leakage, which is responsible for the increase in BBB permeability [93, 94]. Furthermore, there is no significant change after SAH in the expression of caveolin-1 and claudin-5, which are specific microstructures on the plasma membrane surface and can mediate transcellular transport pathways, while occludin and ZO-1 were downregulated during the acute stage. Therefore, the downregulation of ZO-1 and occludin contributes to the damage of tight junctions in the BBB disruption after SAH [7, 19]. This may also indicate that the disruption or increased permeability of the BBB was largely caused by the dysfunction of paracellular pathways.
MMP-9, which is induced by inflammatory cytokines and ROS, can degrade the extracellular matrix of the cerebral microvessel basal lamina, such as collagen IV, fibronectin, laminin, and inter-endothelial tight junction proteins (ZO-1), and thus, cause early BBB disruption after SAH [58, 95]. The activation of NF-κB can not only orchestrate the inflammatory cascade, but also directly mediates the transcription of MMP-9 and tissue inhibitor of MMP-1. In addition, the balanced interaction between MMP-9 and tissue inhibitor of MMP-1 can determine the severity of BBB disruption after SAH [96]. Previous reports have shown that the BBB disruption that occurs after SAH is induced by MAPK-mediated MMP-9 activation [97]. The MAPK-mediated activation of MMP-9 and subsequent degradation of ZO-1 in brain capillary ECs of mice can upregulate tenascin-C and periostin matricellular proteins [72, 98-100]. A positive feedback mechanism formed by tenascin-C and periostin will further upregulate them to aggravate BBB disruption that occurs after SAH [96]. MMP-9 in ECs can also be activated by galectin-3, which is another matricellular protein, and then cause BBB disruption through MAPK, as well as signal transducer and activator of transcription pathways [101, 102]. The reactive astrocytes and capillary ECs can induce another matricellular protein osteopontin in a delayed fashion, whereas the prevention of MMP-9 activation and BBB disruption occurs through the inactivation of MAPK and NF-κB [103, 104].
Heat shock protein 70 (HSP 70), a member of the HSP family of evolutionarily conserved molecular chaperones, can decrease the activity of MMP-9, and then mediate BBB disruption and cell death through aberrant proteolysis. This helps to reduce the inflammation and brain edema that occurs during EBI [105]. The activation of TLR4 after SAH can activate both NF-κB and MAPKs, and it can also upregulate pro-inflammatory cytokines and mediators, as well as matricellular proteins [82]. Importantly, selective inhibition of TLR4 has been reported to maintain BBB function after SAH through inhibiting the upregulation of the molecules mentioned above and activation of MAPKs and MMP-9 [97].
Increasing evidence suggests that many molecules, including vascular endothelial growth factor (VEGF)-A, VEGF-B, angiopoietin (Ang)-1, Ang-2, MAPKs, and MAPK phosphatase-1 are activated simultaneously or at different stages during BBB disruption [103]. VEGF-A serves as a potent inducer of BBB disruption, while VEGF-B and Ang-1 can inhibit the effects of VEGF-A and are potently anti-permeable. The potential mechanism may be through the regulation of MAPK activation. Besides, MAPK phosphatase-1 is an endogenous MAPK inhibitor, and Ang-2 is a natural inhibitor of Ang-1 in vivo [106]. Taken together, the molecular changes of BBB disruption after SAH include the downregulation of Ang-1 and MAPK phosphatase-1, and the upregulation of MAPKs and VEGF-A, whereas the improvement of the damaged BBB was associated with MAPK phosphatase-1 upregulation, MAPK inactivation, and VEGF-A downregulation [103]. Recombinant osteopontin was reported to regulate these molecules and then preserves and restores BBB function, interestingly, the expressions of Ang-2 and VEGF-B were unchanged after SAH [103]. Another recent study found that lipocalin-2 (LCN2) contributes to the BBB disruption after SAH, especially during the early stages; and animals deficient in LCN2 did not show the early peak (4 hours) of BBB disruption; thus, the authors considered the hypoxia-HIF-1α-LCN2-VEGF-A pathway might serve as a potential mechanism [107].
In addition to the molecules above, activating transcription factor 6 (ATF6), which is a single-pass endoplasmic reticulum-transmembrane protein, was also reported to be related to BBB disruption and is upregulated during EBI. A study showed that the administration of apelin-13 can improve the BBB damage through the inhibition of the ATF6/CHOP pathway [108]. Furthermore, the activation of β-catenin transcription could be a promising therapeutic target for maintaining BBB integrity via a type of endogenous protective mechanism. β-catenin is an adherens junction protein and a Wnt pathway signal transducer. In ECs, the adherens junction proteins link to the actin cytoskeleton, which is part of the BBB. Therefore, disruption of β-catenin would cause downregulation of tight junction proteins, BBB disruption, and neuroinflammation, while the activation of Wnt/β-catenin signaling attenuates these pathological changes [109]. Actually, in experimental SAH animal models, some interventions including stabilizing β-catenin or promoting β-catenin nuclear translocation have been reported to protect BBB integrity via an increase in the expression of tight junction proteins, such as claudin-3, claudin-5, occludin, and ZO-1, and an adherens junction protein vascular endothelial-cadherin [110]. Nevertheless, over-activation of the Wnt/β-catenin pathway may cause accumulation of β-catenin in the nucleus and promote the transcription of oncogenes, leading to subsequent tumorigenesis and tumor progression of several cancers [111]. Another study on SAH in rats found the activation of tropomyosin-related kinase receptor B (TrkB), which is a member of the receptor tyrosine kinase family and has been verified to ameliorate the EBI, can restore the tight junction protein ZO-1, and preserve the BBB integrity, and this might be related to the activation of the Wnt/β-catenin pathway [59]. Further studies are needed to verify these possibilities and their potential roles in the treatment of BBB disruption. The therapeutic roles of astrocytes and pericytes in BBB dysfunction after SAH should also be illuminated by basic and clinical trials.
Conclusion
Many pathological and molecular pathways have been reported to be involved in the disruption of the BBB after SAH, but the exact mechanisms remain unclear. Although a lot of therapeutic agents and methods were proposed to improve EC apoptosis and tight junction disruption in experimental SAH studies, these achievements have not been replicated in a clinical setting. Possibly, multiple signaling pathways work independently and/or reciprocally to cause disruption of the BBB after SAH, but the complex pathogeneses make it difficult to inhibit or alleviate BBB disruption after SAH in vivo through the inhibition of only one specific pathway or molecule. The apoptosis of ECs and disruption of tight junctions are two major pathological changes and represent key therapeutic targets for BBB injuries. Hence, the novel therapeutic targets against apoptosis of ECs and disruption of tight junctions, as well as other pathological injuries need to be further explored, and this could contribute to the improvement of outcomes in patients with SAH. To verify if treatments against BBB disruption can benefit patients with SAH and the exact mechanisms involved, further scientific experiments, especially clinical studies, are required.
Acknowledgements
Declared none.
list of Abbreviations
- Ang-1
Angiopoietin 1
- ATF6
Activating transcription factor 6
- BBB
Blood-brain barrier
- CBF
Cerebral blood flow
- CNS
Central nervous system
- DCC
Deleted in Colorectal Cancer
- DCI
Delayed cerebral ischemia
- EBI
Early brain injury
- ECs
Endothelial cells
- FAK
Focal adhesion kinase
- JAMs
Junctional adhesion molecules
- LCN2
Lipocalin-2
- MAPKs
Mitogen-activated protein kinases
- MMP9
Metalloproteinase 9
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B
- NTN-1
Netrin-1
- SAH
Subarachnoid hemorrhage
- TLR4
Toll-like receptor 4
- TrkB
Tropomyosin-related kinase receptor B
- VEGF-A
Vascular endothelial growth factor A
- ZO-1
Zonula occludens-1
Consent for Publication
Not applicable.
Funding
This work was supported by the Heilongjiang Natural Science Foundation, China (ZD2018018 and YQ2019H015), Innovation Fund of Harbin Medical University, China (YTSKYCX2018-38HYD), and National Natural Science Foundation of China (81901190).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Connolly E.S., Jr, Rabinstein A.A., Carhuapoma J.R., Derdeyn C.P., Dion J., Higashida R.T., Hoh B.L., Kirkness C.J., Naidech A.M., Ogilvy C.S., Patel A.B., Thompson B.G., Vespa P. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn J., Kerr R.S., Rinkel G.J.E. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 3.Feigin V.L., Rinkel G.J., Lawes C.M., Algra A., Bennett D.A., van Gijn J., Anderson C.S. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36(12):2773–2780. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 4.Daou B.J., Koduri S., Thompson B.G., Chaudhary N., Pandey A.S. Clinical and experimental aspects of aneurysmal subarachnoid hemorrhage. CNS Neurosci. Ther. 2019;25(10):1096–1112. doi: 10.1111/cns.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong C.M., Tosun C., Kurland D.B., Gerzanich V., Schreibman D., Simard J.M. Biomarkers as outcome predictors in subarachnoid hemorrhage--a systematic review. Biomarkers. 2014;19(2):95–108. doi: 10.3109/1354750X.2014.881418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehba F.A., Hou J., Pluta R.M., Zhang J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012;97(1):14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z., Liang G., Ma T., Li J., Wang P., Liu L., Yu B., Liu Y., Xue Y. Blood-brain barrier permeability change and regulation mechanism after subarachnoid hemorrhage. Metab. Brain Dis. 2015;30(2):597–603. doi: 10.1007/s11011-014-9609-1. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H., Fujimoto M., Kawakita F., Liu L., Nakatsuka Y., Nakano F. Tenascin-C in brain injuries and edema after subarachnoid hemorrhage: Findings from basic and clinical studies. J. Neurosci. Res. 2018;98(1):42–56. doi: 10.1002/jnr.24330. [DOI] [PubMed] [Google Scholar]
- 9.Kahles T., Luedike P., Endres M., Galla H.J., Steinmetz H., Busse R., Neumann-Haefelin T., Brandes R.P. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38(11):3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 10.Ivanidze J., Ferraro R.A., Giambrone A.E., Segal A.Z., Gupta A., Sanelli P.C. Blood-brain barrier permeability in aneurysmal subarachnoid hemorrhage: Correlation with clinical outcomes. AJR Am. J. Roentgenol. 2018;211(4):891–895. doi: 10.2214/AJR.17.18237. [DOI] [PubMed] [Google Scholar]
- 11.Lublinsky S., Major S., Kola V., Horst V., Santos E., Platz J., Sakowitz O., Scheel M., Dohmen C., Graf R., Vatter H., Wolf S., Vajkoczy P., Shelef I., Woitzik J., Martus P., Dreier J.P., Friedman A. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. EBioMedicine. 2019;43:460–472. doi: 10.1016/j.ebiom.2019.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germanò A., d’Avella D., Imperatore C., Caruso G., Tomasello F. Time-course of blood-brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir. (Wien) 2000;142(5):575–580. doi: 10.1007/s007010050472. [DOI] [PubMed] [Google Scholar]
- 13.Ivanidze J., Kesavabhotla K., Kallas O.N., Mir D., Baradaran H., Gupta A., Segal A.Z., Claassen J., Sanelli P.C. Evaluating blood-brain barrier permeability in delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 2015;36(5):850–854. doi: 10.3174/ajnr.A4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 15.Ueno M. Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr. Med. Chem. 2007;14(11):1199–1206. doi: 10.2174/092986707780597943. [DOI] [PubMed] [Google Scholar]
- 16.Rhea E.M., Banks W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019;13:521. doi: 10.3389/fnins.2019.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tietz S., Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015;209(4):493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reese T.S., Karnovsky M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng J., Huang Q., Wang F., Liu Y., Wang Z., Wang Z. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles J. Mol. Neurosci. MN. 2012;46(3):677–687. doi: 10.1007/s12031-011-9629-9. [DOI] [PubMed] [Google Scholar]
- 20.Stamatovic S.M., Keep R.F., Andjelkovic A.V. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr. Neuropharmacol. 2008;6(3):179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin M., Zhu L., Wang J., Xue Y., Shang X. miR-424-5p maybe regulate blood-brain barrier permeability in a model in vitro with Abeta incubated endothelial cells. Biochem. Biophys. Res. Commun. 2019;517(3):525–531. doi: 10.1016/j.bbrc.2019.07.075. [DOI] [PubMed] [Google Scholar]
- 22.Kimura I., Dohgu S., Takata F., Matsumoto J., Kawahara Y., Nishihira M., Sakada S., Saisho T., Yamauchi A., Kataoka Y. Activation of the α7 nicotinic acetylcholine receptor upregulates blood-brain barrier function through increased claudin-5 and occludin expression in rat brain endothelial cells. Neurosci. Lett. 2019;694:9–13. doi: 10.1016/j.neulet.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Kealy J., Greene C., Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 2018;133664 doi: 10.1016/j.neulet.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 24.García-Cáceres C., Quarta C., Varela L., Gao Y., Gruber T., Legutko B., Jastroch M., Johansson P., Ninkovic J., Yi C.X., Le Thuc O., Szigeti-Buck K., Cai W., Meyer C.W., Pfluger P.T., Fernandez A.M., Luquet S., Woods S.C., Torres-Alemán I., Kahn C.R., Götz M., Horvath T.L., Tschöp M.H. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell. 2016;166(4):867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez J.I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P.J., Terouz S., Sabbagh M., Wosik K., Bourbonnière L., Bernard M., van Horssen J., de Vries H.E., Charron F., Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 26.Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., Berk B.C., Zlokovic B.V. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamazaki Y., Kanekiyo T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017;18(9):E1965. doi: 10.3390/ijms18091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armulik A., Genové G., Mäe M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B.R., Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 30.Bonkowski D., Katyshev V., Balabanov R.D., Borisov A., Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS. 2011;8(1):8. doi: 10.1186/2045-8118-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler E.A., Bell R.D., Zlokovic B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton N.B., Attwell D., Hall C.N. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front. Neuroenergetics. 2010;2:2. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlageter K.E., Molnar P., Lapin G.D., Groothuis D.R. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc. Res. 1999;58(3):312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 35.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Morris A.W., Carare R.O., Schreiber S., Hawkes C.A. The cerebrovascular basement membrane: Role in the clearance of β-amyloid and cerebral amyloid angiopathy. Front. Aging Neurosci. 2014;6:251. doi: 10.3389/fnagi.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yousif L.F., Di Russo J., Sorokin L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adhes. Migr. 2013;7(1):101–110. doi: 10.4161/cam.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baeten K.M., Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 2011;71(11):1018–1039. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilling T., Korte D., Hoheisel D., Galla H.J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998;71(3):1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 40.Gu H., Fei Z.H., Wang Y.Q., Yang J.G., Zhao C.H., Cai Y., Zhong X.M. Angiopoietin-1 and Angiopoietin-2 Expression Imbalance Influence in Early Period After Subarachnoid Hemorrhage. Int. Neurourol. J. 2016;20(4):288–295. doi: 10.5213/inj.1632692.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrowski R.P., Colohan A.R., Zhang J.H. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol. Res. 2006;28(4):399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 42.Song J.N., Chen H., Zhang M., Zhao Y.L., Ma X.D. Dynamic change in cerebral microcirculation and focal cerebral metabolism in experimental subarachnoid hemorrhage in rabbits. Metab. Brain Dis. 2013;28(1):33–43. doi: 10.1007/s11011-012-9369-8. [DOI] [PubMed] [Google Scholar]
- 43.Russin J.J., Montagne A., D’Amore F., He S., Shiroishi M.S., Rennert R.C., Depetris J., Zlokovic B.V., Mack W.J. Permeability imaging as a predictor of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2018;38(6):973–979. doi: 10.1177/0271678X18768670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki H., Nakano F. To Improve Translational Research in Subarachnoid Hemorrhage. Transl. Stroke Res. 2018;9(1):1–3. doi: 10.1007/s12975-017-0546-2. [DOI] [PubMed] [Google Scholar]
- 45.Marbacher S., Grüter B., Schöpf S., Croci D., Nevzati E., D’Alonzo D., Lattmann J., Roth T., Bircher B., Wolfert C., Muroi C., Dutilh G., Widmer H.R., Fandino J. Systematic Review of In Vivo Animal Models of Subarachnoid Hemorrhage: Species, Standard Parameters, and Outcomes. Transl. Stroke Res. 2018;••• doi: 10.1007/s12975-018-0657-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz A.Y., Masago A., Sehba F.A., Bederson J.B. Experimental models of subarachnoid hemorrhage in the rat: a refinement of the endovascular filament model. J. Neurosci. Methods. 2000;96(2):161–167. doi: 10.1016/S0165-0270(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 47.Sugawara T., Ayer R., Jadhav V., Zhang J.H. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J. Neurosci. Methods. 2008;167(2):327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang X., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., Keep R.F., Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keep R.F., Zhou N., Xiang J., Andjelkovic A.V., Hua Y., Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang K.C., Tang S.C., Lee J.E., Li Y.I., Huang Y.S., Yang W.S., Jeng J.S., Arumugam T.V., Tu Y.K. Cerebrospinal fluid high mobility group box 1 is associated with neuronal death in subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2017;37(2):435–443. doi: 10.1177/0271678X16629484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki H. What is early brain injury? Transl. Stroke Res. 2015;6(1):1–3. doi: 10.1007/s12975-014-0380-8. [DOI] [PubMed] [Google Scholar]
- 52.de Oliveira Manoel A.L., Goffi A., Zampieri F.G., Turkel-Parrella D., Duggal A., Marotta T.R., Macdonald R.L., Abrahamson S. The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Crit. Care. 2016;20:272. doi: 10.1186/s13054-016-1432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komotar R.J., Schmidt J.M., Starke R.M., Claassen J., Wartenberg K.E., Lee K., Badjatia N., Connolly E.S., Jr, Mayer S.A. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64(3):397–410. doi: 10.1227/01.NEU.0000338946.42939.C7. [DOI] [PubMed] [Google Scholar]
- 54.Sun X.G., Duan H., Jing G., Wang G., Hou Y., Zhang M. Inhibition of TREM-1 attenuates early brain injury after subarachnoid hemorrhage via downregulation of p38MAPK/MMP-9 and preservation of ZO-1. Neuroscience. 2019;406:369–375. doi: 10.1016/j.neuroscience.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Chow B.W., Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38(10):598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimoto M., Shiba M., Kawakita F., Liu L., Shimojo N., Imanaka-Yoshida K., Yoshida T., Suzuki H. Effects of Tenascin-C Knockout on Cerebral Vasospasm After Experimental Subarachnoid Hemorrhage in Mice. Mol. Neurobiol. 2018;55(3):1951–1958. doi: 10.1007/s12035-017-0466-x. [DOI] [PubMed] [Google Scholar]
- 57.Chen S., Feng H., Sherchan P., Klebe D., Zhao G., Sun X., Zhang J., Tang J., Zhang J.H. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog. Neurobiol. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peeyush Kumar T., McBride D.W., Dash P.K., Matsumura K., Rubi A., Blackburn S.L. Endothelial Cell Dysfunction and Injury in Subarachnoid Hemorrhage. Mol. Neurobiol. 2019;56(3):1992–2006. doi: 10.1007/s12035-018-1213-7. [DOI] [PubMed] [Google Scholar]
- 59.Qi X., Liu J., Wu J., Bi Y., Han C., Zhang G., Lou M., Lu J., Tang J. Initiating TrkB/Akt Signaling Cascade Preserves Blood-Brain Barrier after Subarachnoid Hemorrhage in Rats. Cell Transplant. 2019;28(8):1002–1008. doi: 10.1177/0963689719857649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caner B., Hou J., Altay O., Fujii M., Zhang J.H. Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J. Neurochem. 2012;123(Suppl. 2):12–21. doi: 10.1111/j.1471-4159.2012.07939.x. [DOI] [PubMed] [Google Scholar]
- 61.Keep R.F., Andjelkovic A.V., Xiang J., Stamatovic S.M., Antonetti D.A., Hua Y., Xi G. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J. Cereb. Blood Flow Metab. 2018;38(8):1255–1275. doi: 10.1177/0271678X18774666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simard J.M., Geng Z., Woo S.K., Ivanova S., Tosun C., Melnichenko L., Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009;29(2):317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Meng CJ, Shen XM, Shu Z, Ma C, Zhu GQ. 2012.
- 64.Chen D., Wei X.T., Guan J.H., Yuan J.W., Peng Y.T., Song L., Liu Y.H. Inhibition of c-Jun N-terminal kinase prevents blood-brain barrier disruption and normalizes the expression of tight junction proteins clautin-5 and ZO-1 in a rat model of subarachnoid hemorrhage. Acta Neurochir. (Wien) 2012;154(8):1469–1476. doi: 10.1007/s00701-012-1328-y. [DOI] [PubMed] [Google Scholar]
- 65.Altay O., Suzuki H., Hasegawa Y., Caner B., Krafft P.R., Fujii M., Tang J., Zhang J.H. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke. 2012;43(9):2513–2516. doi: 10.1161/STROKEAHA.112.661728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J., Chen G., Li J., Qian C., Mo H., Gu C., Yan F., Yan W., Wang L. Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: a possible role for the regulation of pro-inflammatory cytokines. J. Pineal Res. 2014;57(3):340–347. doi: 10.1111/jpi.12173. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y., Zhang Y., Tang J., Liu F., Hu Q., Luo C., Tang J., Feng H., Zhang J.H. Norrin protected blood-brain barrier via frizzled-4/β-catenin pathway after subarachnoid hemorrhage in rats. Stroke. 2015;46(2):529–536. doi: 10.1161/STROKEAHA.114.007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu T., Zhang W.G., Sun J., Zhang Y., Lu J.F., Han H.B., Zhou C.M., Yan J.H. Protective effects of thrombomodulin on microvascular permeability after subarachnoid hemorrhage in mouse model. Neuroscience. 2015;299:18–27. doi: 10.1016/j.neuroscience.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 69.Zhou C., Xie G., Wang C., Zhang Z., Chen Q., Zhang L., Wu L., Wei Y., Ding H., Hang C., Zhou M., Shi J. Decreased progranulin levels in patients and rats with subarachnoid hemorrhage: a potential role in inhibiting inflammation by suppressing neutrophil recruitment. J. Neuroinflammation. 2015;12:200. doi: 10.1186/s12974-015-0415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou C.H., Wang C.X., Xie G.B., Wu L.Y., Wei Y.X., Wang Q., Zhang H.S., Hang C.H., Zhou M.L., Shi J.X. Fisetin alleviates early brain injury following experimental subarachnoid hemorrhage in rats possibly by suppressing TLR 4/NF-κB signaling pathway. Brain Res. 2015;1629:250–259. doi: 10.1016/j.brainres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Chen T., Wang W., Li J.R., Xu H.Z., Peng Y.C., Fan L.F., Yan F., Gu C., Wang L., Chen G. PARP inhibition attenuates early brain injury through NF-κB/MMP-9 pathway in a rat model of subarachnoid hemorrhage. Brain Res. 2016;1644:32–38. doi: 10.1016/j.brainres.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Fujimoto M., Shiba M., Kawakita F., Liu L., Shimojo N., Imanaka-Yoshida K., Yoshida T., Suzuki H. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J. Neurosurg. 2016;124(6):1693–1702. doi: 10.3171/2015.4.JNS15484. [DOI] [PubMed] [Google Scholar]
- 73.Fang R., Zheng X., Zhang M. Ethyl pyruvate alleviates early brain injury following subarachnoid hemorrhage in rats. Acta Neurochir. (Wien) 2016;158(6):1069–1076. doi: 10.1007/s00701-016-2795-3. [DOI] [PubMed] [Google Scholar]
- 74.Ying G.Y., Jing C.H., Li J.R., Wu C., Yan F., Chen J.Y., Wang L., Dixon B.J., Chen G. Neuroprotective Effects of Valproic Acid on Blood-Brain Barrier Disruption and Apoptosis-Related Early Brain Injury in Rats Subjected to Subarachnoid Hemorrhage Are Modulated by Heat Shock Protein 70/Matrix Metalloproteinases and Heat Shock Protein 70/AKT Pathways. Neurosurgery. 2016;79(2):286–295. doi: 10.1227/NEU.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 75.Liu X., Zhang X., Ma K., Zhang R., Hou P., Sun B., Yuan S., Wang Z., Liu Z. Matrine alleviates early brain injury after experimental subarachnoid hemorrhage in rats: possible involvement of PI3K/Akt-mediated NF-κB inhibition and Keap1/Nrf2-dependent HO-1 inductionn. Cell. Mol. Biol. 2016;62(11):38–44. [PubMed] [Google Scholar]
- 76.Pang J., Wu Y., Peng J., Yang P., Kuai L., Qin X., Cao F., Sun X., Chen L., Vitek M.P., Jiang Y. Potential implications of Apolipoprotein E in early brain injury after experimental subarachnoid hemorrhage: Involvement in the modulation of blood-brain barrier integrity. Oncotarget. 2016;7(35):56030–56044. doi: 10.18632/oncotarget.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan J., Liu W., Zhu H., Zhang X., Feng Y., Chen Y., Feng H., Lin J. Curcumin attenuates blood-brain barrier disruption after subarachnoid hemorrhage in mice. J. Surg. Res. 2017;207:85–91. doi: 10.1016/j.jss.2016.08.090. [DOI] [PubMed] [Google Scholar]
- 78.Fan R., Enkhjargal B., Camara R., Yan F., Gong L. ShengtaoYao; Tang, J.; Chen, Y.; Zhang, J.H. Critical role of EphA4 in early brain injury after subarachnoid hemorrhage in rat. Exp. Neurol. 2017;296:41–48. doi: 10.1016/j.expneurol.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian H., Dou Z., Ruan W., He P., Zhang J.H., Yan F. ErbB4 Preserves Blood-Brain Barrier Integrity via the YAP/PIK3CB Pathway After Subarachnoid Hemorrhage in Rats. Front. Neurosci. 2018;12:492. doi: 10.3389/fnins.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Friedrich V., Flores R., Sehba F.A. Cell death starts early after subarachnoid hemorrhage. Neurosci. Lett. 2012;512(1):6–11. doi: 10.1016/j.neulet.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ayer RE, Zhang JH. 2008.
- 82.Okada T., Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen. Res. 2017;12(2):193–196. doi: 10.4103/1673-5374.200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen G., Zhang S., Shi J., Ai J., Hang C. Effects of recombinant human erythropoietin (rhEPO) on JAK2/STAT3 pathway and endothelial apoptosis in the rabbit basilar artery after subarachnoid hemorrhage. Cytokine. 2009;45(3):162–168. doi: 10.1016/j.cyto.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 84.Singhal A.K., Symons J.D., Boudina S., Jaishy B., Shiu Y.T. Role of Endothelial Cells in Myocardial Ischemia-Reperfusion Injury. Vasc. Dis. Prev. 2010;7:1–14. doi: 10.2174/1874120701007010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sabri M., Ai J., Macdonald R.L. Dissociation of vasospasm and secondary effects of experimental subarachnoid hemorrhage by clazosentan. Stroke. 2011;42(5):1454–1460. doi: 10.1161/STROKEAHA.110.604728. [DOI] [PubMed] [Google Scholar]
- 86.Shamir A., Kwon O.B., Karavanova I., Vullhorst D., Leiva-Salcedo E., Janssen M.J., Buonanno A. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J. Neurosci. 2012;32(9):2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lok J., Sardi S.P., Guo S., Besancon E., Ha D.M., Rosell A., Kim W.J., Corfas G., Lo E.H. Neuregulin-1 signaling in brain endothelial cells. J. Cereb. Blood Flow Metab. 2009;29(1):39–43. doi: 10.1038/jcbfm.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao X., Peng X., Sun S., Park A.Y., Guan J.L. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J. Cell Biol. 2010;189(6):955–965. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie Z., Enkhjargal B., Reis C., Huang L., Wan W., Tang J., Cheng Y., Zhang J.H. Netrin-1 Preserves Blood-Brain Barrier Integrity Through Deleted in Colorectal Cancer/Focal Adhesion Kinase/RhoA Signaling Pathway Following Subarachnoid Hemorrhage in Rats. J. Am. Heart Assoc. 2017;6(5):e005198. doi: 10.1161/JAHA.116.005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Podjaski C., Alvarez J.I., Bourbonniere L., Larouche S., Terouz S., Bin J.M., Lécuyer M.A., Saint-Laurent O., Larochelle C., Darlington P.J., Arbour N., Antel J.P., Kennedy T.E., Prat A. Netrin 1 regulates blood-brain barrier function and neuroinflammation. Brain. 2015;138(Pt 6):1598–1612. doi: 10.1093/brain/awv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen J., Qian S., Yang Q., Deng L., Mo Y., Yu Y. Overexpression of netrin-1 increases the expression of tight junction-associated proteins, claudin-5, occludin, and ZO-1, following traumatic brain injury in rats. Exp. Ther. Med. 2014;8(3):881–886. doi: 10.3892/etm.2014.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aijaz S., Balda M.S., Matter K. Tight junctions: molecular architecture and function. Int. Rev. Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- 93.Fujii M., Duris K., Altay O., Soejima Y., Sherchan P., Zhang J.H. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem. Int. 2012;60(3):327–333. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kondo T., Hafezi-Moghadam A., Thomas K., Wagner D.D., Kahn C.R. Mice lacking insulin or insulin-like growth factor 1 receptors in vascular endothelial cells maintain normal blood-brain barrier. Biochem. Biophys. Res. Commun. 2004;317(2):315–320. doi: 10.1016/j.bbrc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 95.Guo Z., Sun X., He Z., Jiang Y., Zhang X., Zhang J.H. Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol. Res. 2010;32(7):715–720. doi: 10.1179/016164109X12478302362491. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki H., Ayer R., Sugawara T., Chen W., Sozen T., Hasegawa Y., Kanamaru K., Zhang J.H. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit. Care Med. 2010;38(2):612–618. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okada T., Kawakita F., Nishikawa H., Nakano F., Liu L., Suzuki H. Selective Toll-Like Receptor 4 Antagonists Prevent Acute Blood-Brain Barrier Disruption After Subarachnoid Hemorrhage in Mice. Mol. Neurobiol. 2019;56(2):976–985. doi: 10.1007/s12035-018-1145-2. [DOI] [PubMed] [Google Scholar]
- 98.Liu L., Kawakita F., Fujimoto M., Nakano F., Imanaka-Yoshida K., Yoshida T., Suzuki H. Role of Periostin in Early Brain Injury After Subarachnoid Hemorrhage in Mice. Stroke. 2017;48(4):1108–1111. doi: 10.1161/STROKEAHA.117.016629. [DOI] [PubMed] [Google Scholar]
- 99.Nishikawa H., Suzuki H. Implications of periostin in the development of subarachnoid hemorrhage-induced brain injuries. Neural Regen. Res. 2017;12(12):1982–1984. doi: 10.4103/1673-5374.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiba M., Suzuki H. Lessons from tenascin-C knockout mice and potential clinical application to subarachnoid hemorrhage. Neural Regen. Res. 2019;14(2):262–264. doi: 10.4103/1673-5374.244789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakatsuka Y., Shiba M., Nishikawa H., Terashima M., Kawakita F., Fujimoto M., Suzuki H. pSEED group. Acute-Phase Plasma Osteopontin as an Independent Predictor for Poor Outcome After Aneurysmal Subarachnoid Hemorrhage. Mol. Neurobiol. 2018;55(8):6841–6849. doi: 10.1007/s12035-018-0893-3. [DOI] [PubMed] [Google Scholar]
- 102.Nishikawa H., Liu L., Nakano F., Kawakita F., Kanamaru H., Nakatsuka Y., Okada T., Suzuki H. Modified Citrus Pectin Prevents Blood-Brain Barrier Disruption in Mouse Subarachnoid Hemorrhage by Inhibiting Galectin-3. Stroke. 2018;49(11):2743–2751. doi: 10.1161/STROKEAHA.118.021757. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki H., Hasegawa Y., Kanamaru K., Zhang J.H. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41(8):1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki H., Nishikawa H., Kawakita F. Matricellular proteins as possible biomarkers for early brain injury after aneurysmal subarachnoid hemorrhage. Neural Regen. Res. 2018;13(7):1175–1178. doi: 10.4103/1673-5374.235022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shao A., Zhou Y., Yao Y., Zhang W., Zhang J., Deng Y. The role and therapeutic potential of heat shock proteins in haemorrhagic stroke. J. Cell. Mol. Med. 2019;23(9):5846–5858. doi: 10.1111/jcmm.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nag S., Manias J.L., Stewart D.J. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009;118(2):197–217. doi: 10.1007/s00401-009-0541-0. [DOI] [PubMed] [Google Scholar]
- 107.Toyota Y., Wei J., Xi G., Keep R.F., Hua Y. White matter T2 hyperintensities and blood-brain barrier disruption in the hyperacute stage of subarachnoid hemorrhage in male mice: The role of lipocalin-2. CNS Neurosci. Ther. 2019;25(10):1207–1214. doi: 10.1111/cns.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu W., Gao L., Li T., Zheng J., Shao A., Zhang J. Apelin-13 Alleviates Early Brain Injury after Subarachnoid Hemorrhage via Suppression of Endoplasmic Reticulum Stress-mediated Apoptosis and Blood-Brain Barrier Disruption: Possible Involvement of ATF6/CHOP Pathway. Neuroscience. 2018;388:284–296. doi: 10.1016/j.neuroscience.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 109.Tran K.A., Zhang X., Predescu D., Huang X., Machado R.F., Göthert J.R., Malik A.B., Valyi-Nagy T., Zhao Y.Y. Endothelial β-Catenin Signaling Is Required for Maintaining Adult Blood-Brain Barrier Integrity and Central Nervous System Homeostasis. Circulation. 2016;133(2):177–186. doi: 10.1161/CIRCULATIONAHA.115.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zuo S., Ge H., Li Q., Zhang X., Hu R., Hu S., Liu X., Zhang J.H., Chen Y., Feng H. Artesunate Protected Blood-Brain Barrier via Sphingosine 1 Phosphate Receptor 1/Phosphatidylinositol 3 Kinase Pathway After Subarachnoid Hemorrhage in Rats. Mol. Neurobiol. 2017;54(2):1213–1228. doi: 10.1007/s12035-016-9732-6. [DOI] [PubMed] [Google Scholar]
- 111.Shang S., Hua F., Hu Z.W. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8(20):33972–33989. doi: 10.18632/oncotarget.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]