Abstract
Stroke is one of the leading causes of mortality and morbidity worldwide. The blood-brain barrier (BBB) is a characteristic structure of microvessel within the brain. Under normal physiological conditions, the BBB plays a role in the prevention of harmful substances entering into the brain parenchyma within the central nervous system. However, stroke stimuli induce the breakdown of BBB leading to the influx of cytotoxic substances, vasogenic brain edema, and hemorrhagic transformation. Therefore, BBB disruption is a major complication, which needs to be addressed in order to improve clinical outcomes in stroke. In this review, we first discuss the structure and function of the BBB. Next, we discuss the progress of the techniques utilized to study BBB breakdown in in-vitro and in-vivo studies, along with biomarkers and imaging techniques in clinical settings. Lastly, we highlight the mechanisms of stroke-induced neuroinflammation and apoptotic process of endothelial cells causing BBB breakdown, and the potential therapeutic targets to protect BBB integrity after stroke. Secondary products arising from stroke-induced tissue damage provide transformation of myeloid cells such as microglia and macrophages to pro-inflammatory phenotype followed by further BBB disruption via neuroinflammation and apoptosis of endothelial cells. In contrast, these myeloid cells are also polarized to anti-inflammatory phenotype, repairing compromised BBB. Therefore, therapeutic strategies to induce anti-inflammatory phenotypes of the myeloid cells may protect BBB in order to improve clinical outcomes of stroke patients.
Keywords: Blood-brain barrier, macrophage, microglia, neuroinflammation, programmed cell death, stroke, tight junction
1. INTRODUCTION
Despite recent advances in clinical care and management, stroke is still one of the leading causes of mortality and morbidity worldwide [1, 2], and costs an estimated 33.9 billion dollars each year in the US [2]. Stroke is a huge public health concern in both human and financial resources. Therefore, further diagnostic techniques and novel treatments are required to improve outcomes of both ischemic and hemorrhagic strokes.
The blood-brain barrier (BBB) is a characteristic structure of microvessels within the brain and maintains a homeostatic environment in the central nervous system (CNS). The BBB also works as an essential physical and chemical barrier to protect the brain tissue from exposure to potentially toxic or harmful substances by restricting transduction [3, 4]. The BBB can be disrupted by ischemic and hemorrhagic stroke, which can lead to influx of water molecules and blood components into the brain extracellular space resulting in serious clinical consequences such as vasogenic brain edema and hemorrhagic transformation [5-8]. In clinical settings, brain edema was an independent risk factor for mortality and adverse outcomes in both ischemic and hemorrhagic stroke patients, particularly within the first week following onset [9, 10], and the increasing microvascular permeability predicted the development of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage (SAH) [11]. In contrast, hemorrhagic transformation is a major risk in post-ischemic stroke patients treated with thrombolytic therapy and thrombectomy [12-15]. A risk of hemorrhagic transformation reached 2.4-8.8% following the use of tissue plasminogen activator (t-PA) [16, 17], and 8-9.9% following mechanical angioplasty along with thrombolytic therapy [18, 19]. BBB disruption is a key factor to predict the risk of hemorrhagic transformation following thrombolytic therapy and thrombectomy. Therefore, the diagnostic technique to predict comprised BBB prior to these treatments would be useful to reduce the risk of hemorrhagic transformation in patients with acute ischemic stroke, and may change the time window for these therapies from the current fixed time to tailored time depending on a patient’s status [12-15, 20]. Furthermore, enhanced BBB permeability also allows macrophages and neutrophils in plasma to migrate into the brain parenchyma, and exacerbates neuroinflammation and brain injuries [21-24]. BBB disruption was also associated with abnormal brain function, specifically with hyper-synchronized activity and seizures [25-33]. Thus, BBB disruption is considered as a major factor to determine functional outcome and an important therapeutic target to prevent further brain injury in acute stroke.
In this review, we focus on BBB disruption after stroke. First, we discuss the structure and function of cells forming the BBB. Secondly, we identify techniques to measure the integrity of the BBB in in-vitro and in-vivo studies, and biomarkers as well as imaging techniques for clinical evaluation. Finally, we discuss mechanisms of post-stroke BBB breakdown via neuroinflammation as well as apoptosis and potential therapeutic target to preserve the BBB integrity after stroke.
2. THE STRUCTURE AND FUNCTION OF BBB
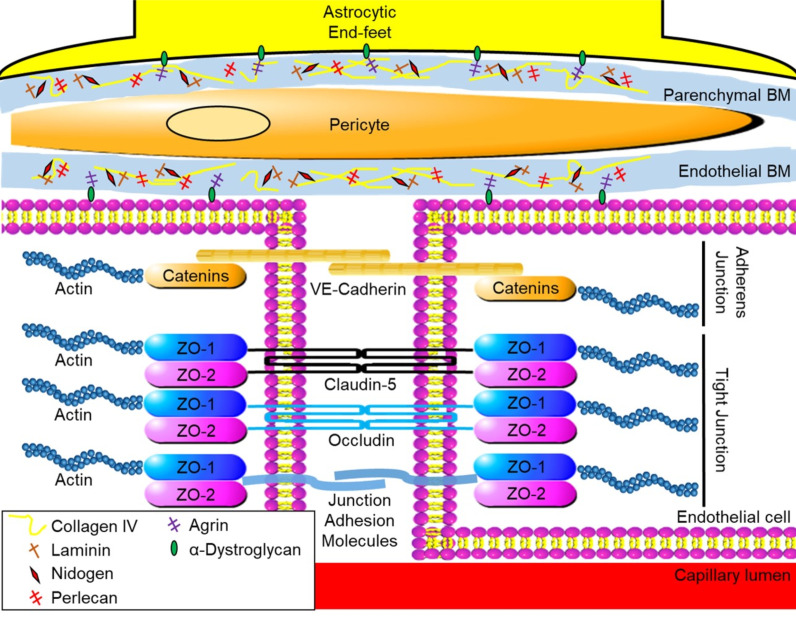
The innermost layer of the BBB is composed of endothelial cells forming a junction complex. The endothelial cells are surrounded by the basement membrane or basal lamina (BM) [34]. Structurally, the BM is classified into endothelial BM and parenchymal BM (which are separated by pericytes) [35]. Moreover, astrocytic end-feet embrace the BM, which further strengthens the BBB and helps maintain the environment (Fig. 1) [3, 36-42].
Fig. (1).
Physical structure of blood-brain barrier of brain capillary. BM: basement membrane, VE-cadherin: vascular endothelial- cadherin, ZO: zonula occludens. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The junction complex forms the tight and the adherens junctions between endothelial cells to restrict paracellular transport [43-47]. The main components of tight junction proteins are transmembrane adhesion proteins such as claudin-5, occludin, and junction adhesion molecules and cytoplasmic accessory proteins such as zonula occludens (ZO)-1 and -2 [45, 48-50]. The transmembrane adhesion proteins connect with the proteins on adjacent cells and occlude the intercellular space [45]. Cytoplasmic accessory proteins provide a foundation for the transmembrane adhesion proteins with actin cytoskeleton in intracellular space [43, 45]. Meanwhile, adherens junction proteins are located at the basolateral side of the intercellular space. The main components of adherens junction proteins consist of transmembrane vascular endothelial (VE)-cadherin and cytoplasmic accessory proteins such as α-, β-, and γ-catenin [45]. The cytoplasmic accessory proteins in adherens junctions also provide physical support via the actin cytoskeleton. The function of tight junctions are to serve both as physical and biochemical barriers and transporter systems [45, 48, 51-53]. In addition, brain endothelial cells have no fenestration within the cells [3]. All of these characteristics of brain capillary lead to a very low vascular permeability. Lipid-soluble small molecules up to 500 Da can pass the tight junction transcellularly, while water-soluble small molecules can overcome the tight junction paracellularly [48]. However, large molecules over 1000 Da cannot cross the BBB through the pathways or junctions [3, 54]. The endothelium also has a couple of transportation routes to cross the BBB such as carrier-mediated transcellular transport (transcytosis), receptor-mediated transcytosis, and adsorptive-mediated transcytosis [55]. Small endogenous molecules such as amino acids, glucose, and ketones are transported across the BBB via transport proteins through the carrier-mediated transcytosis [56, 57]. According to this carrier function, selective nutrients can enter the brain parenchyma and maintain the environment in the brain [48]. Endogenous larger molecules, including insulin and transferrin, are recognized by receptors located on the endothelial cell surface, and transported to get passed the BBB [56, 57]. Adsorptive-mediated transcytosis is a pathway for a positively charged large plasma protein such as albumin, which binds to a negatively charged area on the endothelial cell surface to be absorbed into the brain parenchyma by endocytosis [56, 57]. In experimental models of stroke, tight junction breakdown has been commonly examined by measuring the expression of ZO-1, claudin-5, occludin, and other junction proteins [45, 58]. The adherens junction protein, VE-cadherin, was also suppressed in post-stroke models [58-60]. However, VE-cadherin and β-catenin can work as a temporary barrier to restrict the extravasation of large molecules prior to the reappearance of transmembrane adhesion proteins of tight junction [61].
The BM is mainly composed of extracellular matrix (ECM) proteins including collagen IV, fibronectin, laminin, nidogen, and heparin sulfate proteoglycan such as perlecan and agrin [35, 47]. These proteins provide structural support as well as signaling transduction [45, 51-53]. Collagen IV is composed of trimeric protein containing six kinds of α-chains (COL4A1–6), and the most abundant in the BM [35, 62-65]. Collagen IV is secreted by endothelial cells, astrocytes, and pericytes, and plays roles in retaining laminin, nidogen, and perlecan [52, 66]. COL4A1 and 2 were highly expressed in BMs and ablation of COL4A1 and 2 resulted in embryonic death due to abnormal BM structure as well as fragile vessels [35, 66]. Missense mutations of COL4A1 and 2 resulted in brain malformation and occurrence of intracerebral hemorrhage (ICH) in mice [67-69]. Collagen IV expression may be decreased after stroke, but may be upregulated in later repairing phases of BBB [70, 71].
Fibronectin is a group of glycoproteins with disulfide-linked dimer. Two types of fibronectin exist: soluble plasma fibronectin and insoluble cellular fibronectin. The latter form is a major component of ECM and is produced by fibroblasts in the BM [51, 72-74]. Fibronectin provides cell adhesion, migration, and cytoskeletal organization [75]. In an in-vitro study, fibronectin promoted survival and proliferation of endothelial cells in the CNS, suggesting angiogenic effects [76]. Fibronectin-inactivated mice resulted in embryonic death due to notochord and somite absence as well as heart and embryonic vessel deformities [77]. Fibronectin is an indispensable factor for cerebral vascular development and integrity [78-80].
Laminin is a trimeric protein which consists of a combination of α, β and γ chains forming 15 different isoforms [81-85]. The major laminin isoforms in the BBB are laminin-111 (α1β1γ1), -211 (α2β1γ1), -411 (α4β1γ1), and -511 (α5β1γ1). Brain microvascular endothelial cells release laminin-411 and -511, which are mainly located in endothelial BM, while astrocytes solely produce laminin-111 and -211, which are predominantly found in the parenchymal BM [86-90]. Laminin organizes BM structure as well as barrier function of the BBB [85, 91]. Deletion of the laminin failed to organize collagen IV and perlecan during developing embryoid bodies [92]. Laminin α4 knockout mice resulted in the lack of vasculature followed by occurrence of ICH [41, 93]. Null mutation of LAMC1 gene coding for laminin γ1 in mice lacked BMs resulting in embryonic death leading to leaky vasculature causing ICH [92, 94]. Other mutations in laminin subunits such as α1 and β1 also led to BMs disorganization [95-98]. In adult mice, ablation of astrocytic laminin led to the disruption of BMs and vascular smooth muscle cells, resulting in ICH [93]. Laminin provides adult angiogenesis, while fibronectin contributes to embryonic angiogenesis [78, 99]. Laminin is also involved in tissue repair after stroke. Laminin expression including laminin-111, -211 -411, and -511 was upregulated in the area of ischemic penumbra after transient ischemia and promoted subsequent neurogenesis and angiogenesis via integrin signaling pathways [100-102], while endothelial laminin expression in the ischemic core region was downregulated due to loss of vascular component [103]. Laminin provided vascular scaffolds via β1 integrin, promoting neuronal migration toward damaged area on 18 days after ischemic stroke [104-106]. An in-vitro study demonstrated that cerebral endothelial cell-induced laminin promoted neurite outgrowth [107, 108].
Nidogen (enactin) is composed of two isoforms: nidogen-1 and -2, which are produced by endothelial cells and astrocytes, respectively [109]. The function of nidogen is to link collagen IV and laminin to provide BM stabilization [35, 52]. In particular, nidogen-1 synthesis may be a key regulator for adhesive properties of astrocyte to BM [109]. Knockout of either nidogen-1 or -2 in mice was still able to form functionally normal BMs, although its expression level was downregulated in cerebral vessels [110-112]. However, deletion of both nidogen-1 and -2 resulted in perinatal death due to severe BM defects [113-115]. Nidogen-1 null mice promote upregulation and redistribution of nidogen-2, while nitrogen-2 null mice do not change expressions of nidogen-1 [116, 117]. Therefore, nidogen-2 possibly compensates the expression of nidogen-1.
Perlecan and agrin are heparan sulfate proteoglycans found in the BM [118, 119]. Perlecan is also known as heparan sulfate proteoglycan 2 [119]. Perlecan has five different domains (domain I-V) and three glycosaminoglycan chains with NH2 terminal, which interact with a large number of molecules such as ECM proteins and heparin-binding growth factors to mediate a variety of cell signaling to control migration, proliferation, and differentiation [120-123]. Perlecan is an indispensable factor to organize BMs and promote cerebral angiogenesis during embryonic period similar to fibronectin [124, 125]. Deletion of perlecan during the embryonic stages in mice resulted in death [35, 124-126]. Lacking of perlecan only in the BM causes microvessels to bleed and dilate in mice [82]. In experimental ischemic stroke, a perlecan level was downregulated by 43–63% within 2 h from onset [127, 128]. In contrast, post-stroke perlecan domain V treatment promoted brain angiogenesis via the induction of vascular endothelial growth factor (VEGF) from brain endothelial cells [129]. Treatment with exogenous recombinant domain V of human perlecan after ischemic stroke exerted neuroprotective effect as well as infarction volume reduction in both wild-type mice and perlecan-deficient mice [127, 130]. Endogenous perlecan also seems to be actively processed into potentially beneficial protein fragments such as the C-terminal fragment of domain V after stroke [131]. The fragment promoted recovery of BBB injury after ischemic stroke [132]. Perlecan may be an important factor for BBB development as well as BBB reformation after the breakdown. Agrin has multiple isoforms by alternative splicing [133, 134]. Agrin in the BM consists of isoforms without a peptide insert at site B/z (B/z-negative form) [135]. During development, appearance of agrin in BM had the same time window as that of BBB formation [136]. Agrin binds to α-dystroglycan located on astrocytic end-feet or endothelial cells and acts as an anchor to connect endothelial cells and astrocytes to the BM [137]. Agrin also plays a role in the maintenance of BBB properties by polarizing astrocytes, clustering aquaporin-4 into orthogonal arrays of particles, expressing claudin-5 as well as occludin, and suppressing potential harmful matricellular protein tenascin-C [138, 139].
Perivascular cells (pericytes and astrocytes) also have a role in regulating BBB function, forming the neurovascular unit [56, 140-143]. Pericytes are involved in physical support and maintenance of endothelial cells as well as paracrine signaling to the endothelium for stabilization of the BBB [57, 72, 144-152]. Lack of pericytes showed an increase in endothelial transcytosis, failure of tight junction formation, and induction of genes related to increased vascular permeability in mice [153]. The detachment of pericytes was enhanced by VEGF, leading to production of matrix metalloproteinase (MMP)-9 as well as induction of leaky BBB especially in the brain cortex, striatum, and hippocampus in both in-vivo and in-vitro studies [148, 154-161]. VEGF is a glycoprotein regulating vascular permeability and angiogenesis during embryonic angiogenesis mainly via VEGF receptor (VEGFR) 2 located on endothelial cells [162]. VEGF enhanced BBB permeability in both adult mice with normal physiological conditions and inflammatory diseases [162]. Some studies demonstrated that VEGF signaling pathways included potentially detrimental endothelial nitric oxide synthase and matricellular protein tenascin-C [162, 163]. VEGF and VEGFR2 expressions were significantly increased at 24 h post-SAH [162], and a VEGF inhibitor exerted neuroprotective effects via protection of the BBB after SAH [154, 162]. However, when it comes to ICH and CI, elevation of serum VEGF was correlated with positive outcomes in clinical settings [164, 165]. The findings may be related to endothelial cell regeneration and angiogenesis by VEGF [166]. Astrocytes are the most abundant glial cells and are responsible for maintaining the environment in the brain [56, 167, 168]. Astrocytic end-feet are responsible for 90% of the BMs, which form the outermost layer of BBB, and control BBB integrity, cellular link to neurons, and ion and water transport [47, 57, 93, 152, 169-172]. An in-vivo study showed that lack of astrocytes by 3-chloropropanediol treatment caused microvascular damage, resulting in BBB breakdown without neuroinflammation [173]. Mice lacking glial fibrillary acidic protein, an astrocyte-specific filament protein, also showed an increase in infarction size in both permanent and transient focal ischemia compared to wild-type mice [174]. On the other hand, in post-ischemic stroke and inflammatory diseases in the CNS, astrocytes were reported to activate immune cell infiltration and release VEGF and MMPs to degrade tight junction proteins (claudin-5 and occludin) as well as the ECM in both in-vivo and in-vitro study, causing BBB disruption [163, 175-178].
3. ASSESSMENT OF BBB PERMEABILITY
3.1. Assessment of BBB Permeability in In-Vitro and In-Vivo Studies
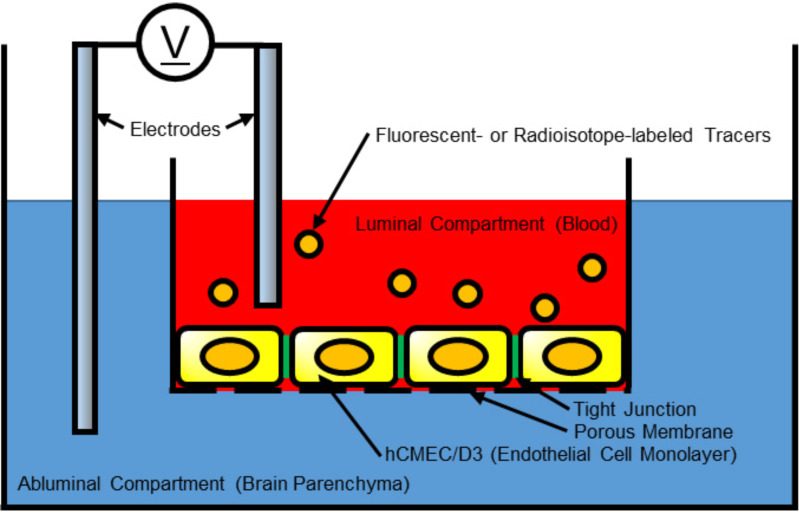
Transendothelial electrical resistance (TEER) would be the most common technique to evaluate the integrity of tight junction in cell culture models of endothelial monolayers [48, 179, 180]. BBB function in TEER is examined by measuring electrical resistance across a cellular monolayer. Representative setup for TEER measurement is equipped with endothelial cell lines, porous membrane, tracer compounds, and electrodes (Fig. 2). Some brain endothelial cell lines such as rat RBE4, rat GP8, and human cerebral microvascular endothelial cells (hCMEC/D3) express appropriate BBB protein characteristics [37]. Other cell types regulating BBB integrity such as pericytes, and astrocytes have also been tested in co-culture with brain endothelial cells [181]. The models using hCMEC/D3 in co-culture with astrocytes showed significant increases in TEER values as well as decreases in the passage of permeability tracers through the endothelial monolayer [182-184]. Brain endothelial cells produced from human pluripotent stem cells can provide cell lines that have highly accurate and repetitive structure of BBB in terms of permeability for small molecules, expression of nutrient transporters, and polarized efflux transporter activity [185]. Endothelial cells are grown on cell culture medium with porous membrane, which separates the compartments between luminal side and abluminal side. The tracer compounds generally consist of either fluorescent- or radioisotope- tagged molecules (Table 1). Tracer permeability via porous membrane is measured using different sized tracer compounds [45]. Electrodes are placed in both compartments and are used to monitor the electrical resistance to pass through the endothelial cells via transcellular and paracellular pathways [179]. The electrical resistance through transcellular pathway is primarily defined by individual cell and membrane, while the paracellular electrical resistance is based on formation of the tight junctions with adjacent cells [186]. Tracer compounds in the luminal side pass through endothelial cells and pericyte layers, and permeability is measured by the concentration of tracers in the abluminal side after a given period of time and is expressed by endothelial permeability coefficient Pe (cm/s) [187]. Overall, the TEER value reflects the integrity and permeability of endothelial cells in culture [188, 189]. The main advantage of the TEER is a non-invasive method and is able to monitor live cells continuously in various stages from BBB disruption to repair after stroke [48].
Fig. (2).
Transendothelial electrical resistance system to determine the blood-brain barrier integrity in in-vitro study. Monolayer endothelial cells, human cerebral microvascular endothelial cells (hCMEC)/D3 grown on porous membrane are separating the luminal compartment from the abluminal compartment. The resistance of tracers to pass through tight junction is measured by electrodes located in both compartments. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
The tracers used for in-vitro measurement of blood-brain barrier permeability.
| Tracer | Molecular Weight (Da) |
|---|---|
| Radioisotope-labeled marker | - |
| 14C-α-aminoisobutyric acid | 103 |
| 14C-mannitol | 180 |
| 14C-sucrose | 342 |
| 14C-methotrexate | 455 |
| 14C-inulin | 5k |
| 14C-labeled dextran | 70k |
| Fluorescence-labeled marker | - |
| Sodium fluorescein | 376 |
| Lucifer yellow | 444 |
| Horseradish peroxidase | 40k |
| Fluorescein isothiocynate-dextran | 62k |
| Fluorescein isothiocynate-albumin | 67k |
Alternatively, the recent advances of BBB organoids are useful for the permeability assay and drug pharmacokinetics researches [190]. An organoid is an in-vitro and 3-dimensional artificial miniature organ showing realistic micro-anatomy. BBB organoids consist of human primary brain endothelial cells, pericytes, and astrocytes, providing better characteristics of BBB integrity including tight junctions and adherens junctions, as well as more realistic function associated with molecular transporters and drug efflux pumps expression compared to conventional static culture systems [190].
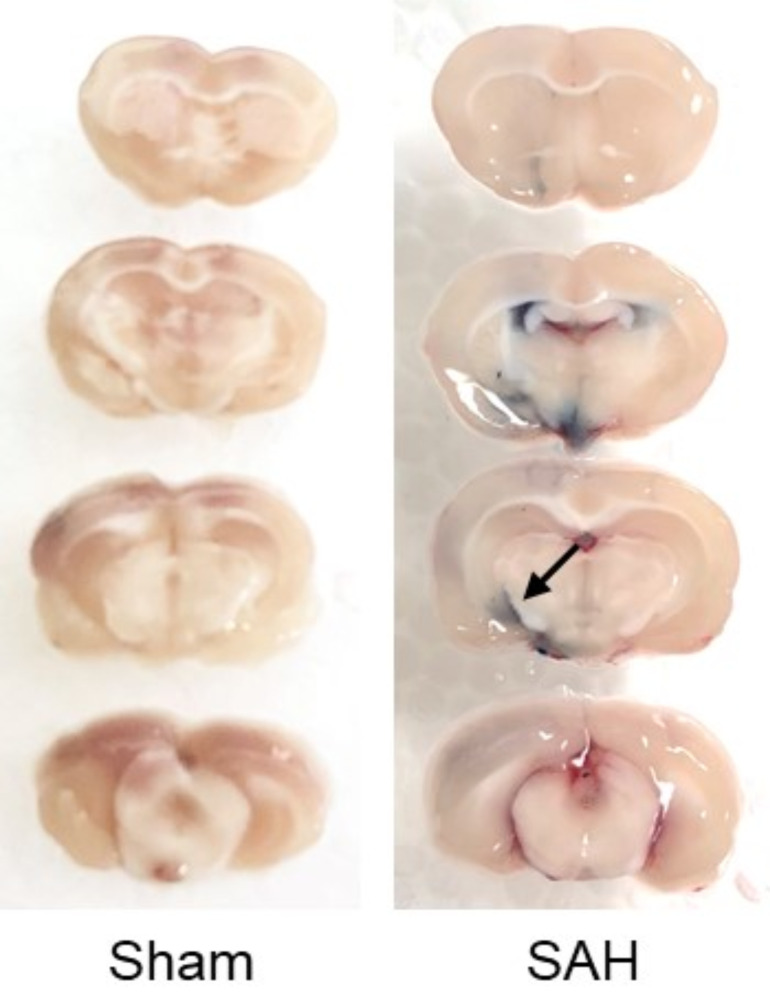
In in-vivo studies, the measurement of extravasated Evans blue dye is widely accepted to evaluate the BBB disruption. Evans blue is a water-soluble molecule in blue with molecular weight of 960.8 Da, and binds strongly to endogenous albumin following an intravenous injection [4]. This forms a large molecular complex with a molecular weight of 60,000 Da, and the BBB breakdown leads to the leakage of this large complex from the intravascular area to brain parenchyma [4]. Extravasated Evans blue-albumin complex is identified macroscopically and as red fluorescence microscopically (Fig. 3), and is measured by colorimetric and spectrophotometric methods [191]. The advantage of this method is cost-effective as well as to be able to assess the BBB disruption quantitatively. However, data acquisition is limited to only a single time point due to the methods requiring animal sacrifice. In addition, it may not reflect the active BBB disruption because the result shows total extravasation of Evans blue-albumin complex from stroke onset to the sacrificed time point. Moreover, the spatial information identifying compromised BBB is inferior to immunohistochemical or immunofluorescence staining techniques.
Fig. (3).
Evans blue dye test at 24 h after subarachnoid hemorrhage (SAH) induction in rats. Four % Evans blue was injected intraperitoneally at 2 h before brain harvesting. Extravasated Evans blue complex is shown in a SAH-operated rat (arrow). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
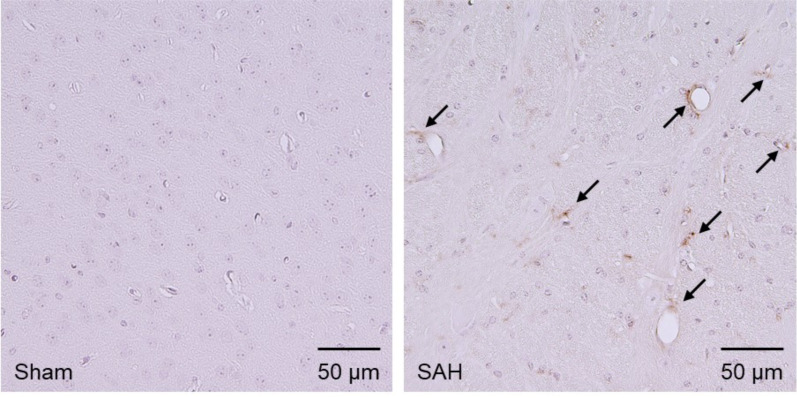
Another in-vivo study to assess BBB disruption is immunohistochemical or immunofluorescence staining of blood components. This method measures the leakage of blood components from the intravascular area into the brain parenchyma [192]. Currently, albumin, fibrinogen, and immunoglobulins (Igs; IgG and IgM) staining are used for this method [192]. Extravasated these blood components are identified microscopically (Fig. 4). The advantage is that this method is capable of evaluating the BBB disruption without administration of any exogenous tracers cost-effectively and semi-quantitatively [45, 193]. In addition, spatially visualized information is obtained. However, as is the case with Evans blue dye method, animal sacrifice is required for data acquisition and only the total extravasation can be obtained since stroke induction. In addition, slight BBB disruption may fail to be detected due to the generally huge size of blood components [192].
Fig. (4).
Immunohistochemical staining for immunoglobulin G (IgG) in the left temporal cortex at 1.0 mm posterior to the bregma at 24 hours after subarachnoid hemorrhage (SAH) induction in mice. Compared with sham-operated mice, extravasated IgG is stained in SAH-operated mice (arrows). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Transendothelial leukocyte migration is also a technique for evaluation of BBB disruption in both in-vivo and in-vitro studies [45, 194]. This method can show the severity of brain injury after stroke as well as BBB disruption because leukocyte infiltration causes neuroinflammation in the brain following stroke [195, 196]. However, leukocytes require several steps to migrate into parenchyma such as leukocytes endothelial adhesion, activation and crawling at the endothelial cells, and transmigration [197]. Therefore, this method may be influenced depending on the function of leukocyte to cross into the brain parenchyma [194, 198]. As another in-vivo method, the leakage of radioisotope-labeled sucrose and inulin has been measured to evaluate the BBB disruption because these carbohydrates also do not cross the BBB in normal physiological conditions and are stable metabolically [199, 200].
3.2. Identifying Clinical Biomarkers of BBB Breakdown
Currently, the most widely accepted clinical biomarkers to evaluate BBB integrity are the cerebrospinal fluid (CSF) albumin or IgG per serum albumin or IgG ratio measurement [201-205]. Serum albumin and IgG are hardly transported within CSF in normal conditions of the BBB. However, these proteins easily enter into the brain parenchyma and CSF under the compromised BBB, resulting in an increase in the CSF per serum ratio when the serum and CSF samples are collected simultaneously. However, this technique is not commonly performed in clinical settings because the acquisition of CSF data requires invasive technique such as lumber puncture.
Previous clinical studies to identify clinical biomarkers of the BBB breakdown are limited. Evaluation of BBB integrity would be useful to estimate the risk of hemorrhagic transformation after thrombolytic therapy and thrombectomy in ischemic stroke [170]. Therefore, some clinical studies focused on the relationships between serum biomarkers and hemorrhagic transformation following ischemic stroke. A study demonstrated that serum levels of tight junction proteins occludin and S100 on admission were significantly elevated in patients with hemorrhagic infarction compared to those without post-stroke hemorrhagic complications, suggesting that it can be potential clinical biomarkers to identify BBB disruption attributed to the hemorrhagic transformation following cerebral infarction (CI) [206]. In addition, patients with hemorrhagic infarction showed an increase of serum claudin-5 per ZO-1 ratio based on the data on admission, and a decrease of serum VEGF levels compared to patients without hemorrhagic transformation [206]. Serum S100B was also a good biomarker to predict BBB damage in traumatic brain injury [205]. S100B is an abundant protein in the brain expressed in astrocytes, though it is not specific [207]. Therefore, this protein is considered as one of the candidates to detect BBB damage caused by compromised astrocytes. Another study showed that plasma levels of cellular fibronectin and MMP-9 were correlated with hemorrhagic transformation after thrombolytic therapy in CI [208, 209]. The cellular fibronectin is predominantly expressed in BM and one of the essential components of BBB [51, 72-74]. MMP-9 is known as a proteolytic enzyme induced by inflammatory cytokines as well as reactive oxygen species (ROS), and degrades the ECM of cerebral microvessel BM [210, 211]. Therefore, elevation levels of cellular fibronectin and MMP-9 suggest the degradation of microvessel components leading to BBB disruption after ischemic injury via the inflammatory response [208, 209]. CSF MMP-9 expression could be increased following stroke as well. A clinical study showed that blood and CSF levels of MMP-9 correlated with each other, and that higher MMP-9 levels in the CSF within 14 days post-SAH were associated with worse 3-month clinical outcomes [212]. Neutrophils may be an important source of MMP-9 in the CSF [7, 212, 213]. On the other hand, post-traumatic BBB dysfunction as measured by albumin CSF per serum ratio was correlated with serum ubiquitin C-terminal hydrolase L1 (UCH-L1) elevation between 12 and 24 h after trauma [205]. UCH-L1 is used as a brain-specific biomarker because it is a neuron-specific protein and abundant in neuronal soma [205]. Thus, recent studies have expanded clinical biomarkers to assess BBB integrity. However, none of them is still less than the ideal biomarkers, which provide accurate results and is capable of simple and prompt measurement [170].
3.3. Imaging Techniques Measuring BBB Permeability in Clinical Settings
A number of imaging techniques for evaluating BBB permeability have been used in clinical settings. Dynamic contrast-enhanced computed tomography (DCE-CT) scan has been used to assess the post-stroke BBB disruption [14, 214-216]. Images are acquired by scanning during an intravenous injection of an iodinated contrast agent, which cannot pass the intact BBB. The major advantage of DCE-CT for the purpose of BBB permeability imaging is to be able to scan faster than other imaging modalities, and therefore it may be applicable to post-stroke patients who are unable to tolerate the longer scan due to disturbance of consciousness or requirement of immediate interventions. Disadvantages of DCE-CT scan include risks related to radiation, and adverse reactions due to an injection of iodinated contrast agent. The resolution of imaging is inferior to that acquired by dynamic contrast enhanced magnetic resonance (MR) imaging (DCE-MRI) scan [22]. Besides, the extent of BBB damage evaluated by DCE-CT scan may fluctuate depending on the cerebral blood flow, and therefore BBB disruption cannot be detected in severe ischemic region because of the insufficient blood supply [20].
DCE-MRI scan is the most widely adopted minimally invasive imaging technique for evaluating and measuring BBB breakdown [26, 33, 217-219]. This method commonly uses contrast agents containing gadolinium, which is a MR-visible tracer and does not cross the normal BBB. The MR images acquired by dynamic T1-weighted imaging (T1WI) following a contrast agent injection reflect the quantitative extravasation caused by the BBB breakdown [220, 221]. BBB permeability is calculated by evaluating the differences of intensity on MRI before and after an injection of a contrast agent [222]. A previous study showed that parenchymal enhancement on MRI T1WI after 2 h from thrombolytic therapy predicted the subsequent hemorrhagic transformation [13]. Another study demonstrated that delayed gadolinium enhancement of CSF space on fluid-attenuated inversion recovery images was also associated with hemorrhagic transformation and poor clinical outcome following focal CI [223]. The advantage of DCE-MRI is that non-iodine contrast agents can reduce risks for adverse reactions, and that high contrast as well as resolution images can be obtained [218]. Disadvantage of DCE-MRI includes longer scanning time [22]. It takes up to 1 h for processing full brain permeability by DCE-MRI scans [224]. The time-consuming nature may be a critical factor that leads to worse clinical outcomes in patients with acute ischemic or hemorrhagic stroke before intervention. Other limitations include potential artifacts as a major concern, and that this method is still difficult to detect slight BBB injury [20, 225-227]. In contrast, recent studies showed that arterial spin labeling (ASL) techniques of MRI could be used for evaluation of BBB integrity based on the different diffusion or transverse relaxations by separating the intravascular and extravascular signaling [228]. ASL technique does not require contrast agents containing gadolinium, but reliable areas to measure barrier function are currently limited to gray matter and white matter [228].
Single photon emitting computed tomography (SPECT) has also been used to measure BBB permeability [229, 230]. 99mTc-diethylenetriaminepentaacetic acid (99mTc-DTPA) brain scintigraphy is the common technique for the assessment of BBB integrity [229]. 99mTc-DTPA is a non-diffusible tracer and its uptake takes place at the site of severe tissue damage [229]. DTPA-SPECT has been used in the past to detect the BBB injury due to infection, trauma, brain metastasis, and stroke [229, 231-234]. BBB intensity as measured by 99mTc-DTPA uptake was also correlated with post-stroke seizures as well as neurologic poor outcomes [229, 230]. Advantage of 99mTc-DTPA SPECT is more cost-effective compared with DCE-MRI [229]. However, the low anatomical resolution and ionizing agent are disadvantages of DTPA-SPECT. Alternatively, 68Ga ethylenediaminetetraacetic acid (68Ga EDTA) tracer in positron emission tomography is also used to obtain the image of subtle leakage [228]. However, due to the disadvantage including use of ionizing radiation, high costs, lack of available infrastructure, and low spatial information, this technique has been limited to studies related to cerebral small vessel diseases [228].
4. INFLAMMATORY RESPONSE AND PROGRAMMED CELL DEATH INVOLVED IN BBB DISRUPTION AND THE POTENTIAL THERAPEUTIC TARGETS IN STROKE
4.1. Pathophysiological Changes in the Acute Phase after Stroke
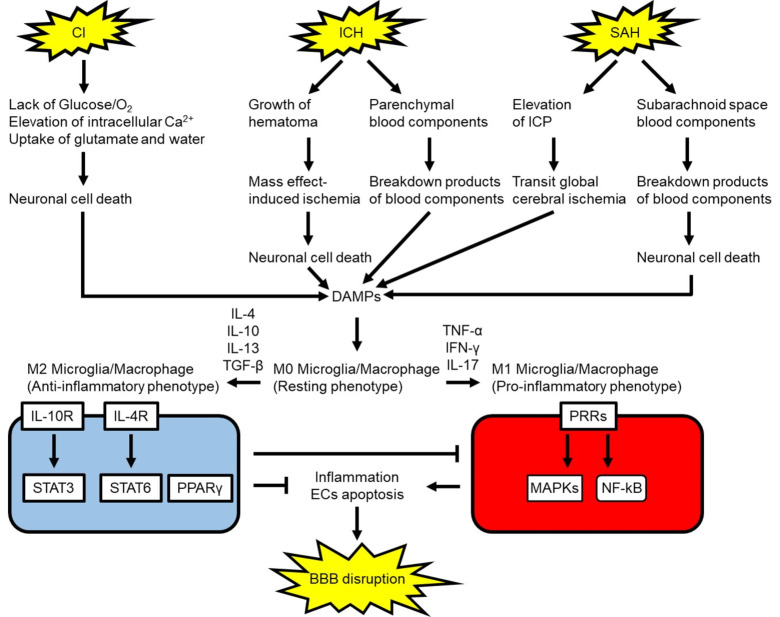
BBB disruption is initiated by various factors induced after stroke such as pro-inflammatory substances, ROS, and MMPs [235]. In ischemic stroke, insufficient blood flow leads to the lack of O2 and glucose supply as well as depletion of adenosine triphosphate (ATP) [55, 236]. Dysfunction of ion transport arising from a shortage of ATP results in excessive releases of glutamate and intracellular Ca2+ overload [236]. Uptake of glutamate facilitates astrocyte swelling, leading to compression of vessels in the ischemic regions and further reduction of vascular blood flow [237]. Intracellular Ca2+ overload activates a variety of cytotoxic factors which cause dysfunction of mitochondria and endoplasmic reticulum as well as generation of ROS, resulting in cerebral tissue damage and releasing damage-associated molecular patterns (DAMPs) [238, 239]. ROS signaling includes apoptotic factors such as p53, caspases-3 and -9, causing BBB disruption [23]. DAMPs are endogenous molecules released as a result of tissue damage or secondary products arising from blood components breakdown. These DAMPs include heme, fibrinogen, high-mobility group box 1 (HMGB1), S100P, heat shock proteins, and matricellular proteins such as tenascin-C and galectin-3 [240, 241]. ICH can cause secondary ischemia by the hematoma mass effect as well as increased intracranial pressure (ICP) [142]. ICH-induced ischemia destroys cerebral tissues and releases DAMPs [242]. In addition, the extravasated blood components within brain parenchyma are also processed and cause DAMPs production [55]. When it comes to SAH, a ruptured intracranial aneurysm leads to transient global cerebral ischemia due to immediate ICP elevation in a hyper-acute phase and releases a variety of cytotoxic factors including ROS, hemoglobin degradation products including iron, and other DAMPs into the subarachnoid space [10, 210, 243-246]. In the most severe cases, arterial bleeding causes persistent elevated ICP and global cerebral ischemia leading to brain death [247]. In surviving SAH cases, the subsequent processes cause delayed cerebral ischemia due to cerebral vasospasm, neuroinflammation, apoptosis of endothelial cells and so on (Fig. 5) [10, 210, 243-247].
Fig. (5).
Possible microglia/macrophages polarization signaling following stroke and response to blood-brain barrier (BBB). CI: cerebral infarction, DAMP: damage-associated molecular pattern, ECs: endothelial cells, ICH: intracerebral hemorrhage, ICP: intracranial pressure, IFN-γ: interferon-γ, IL: interleukin, IL-4R: IL-4 receptor, IL-10R: IL-10 receptor, MAPK: mitogen-activated protein kinase, NF-κB: nuclear factor-κB, PPARγ: peroxisome proliferator activated receptor γ, PRR: pattern recognition receptor, SAH: subarachnoid hemorrhage, STAT: signal transducer and activator of transcription, TGF-β: transforming growth factor-β, TNF-α: tumor necrosis factor-α. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.2. Time Window of BBB Opening after Stroke
In experimental models of ischemic stroke, the BBB was undamaged up to 2 h following onset [248]. BBB disruption was observed 6 h after reperfusion in experimental models of transient focal cerebral ischemia [248, 249]. Clinically, BBB breakdown occurs within 48-72 h after ischemic stroke [229, 248]. In contrast, blood injection models of ICH enhanced BBB permeability within 12 to 24 h, while BBB disruption was detected within 5 h in a collagenase injection model of ICH [45]. Expressions of the tight junction markers, ZO-1, claudin-5, and occludin were downregulated 1 to 3 days from onset in both collagenase and blood injection models of ICH [250-258]. Clinically, BBB permeability in patients with ICH increases after 24 h of onset [55]. Perihematomal vasogenic edema peaked at around 72 h in experimental animal models of ICH, while 10 days in ICH patients [55, 259]. On the other hand, BBB disruption associated with SAH occurred as early as 10 min and prolonged up to 7 days after onset in experimental rodent models [195, 260]. Another study demonstrated a biphasic opening of BBB with a peak at 3 and 72 h in rat endovascular perforation models of SAH [261]. This biphasic pattern possibly arises from BBB breakdown caused by ICP elevation in a super-acute phase and the subsequent cytotoxic factors because these peaks coincided with the reduction of cerebral blood flow [261]. Clinically, SAH-induced global brain edema is found in 8–67% of patients at admission, while late-onset brain edema took place in 12% of SAH patients within 2 weeks of onset [244].
4.3. Polarization of Myeloid Cells to Inflammatory Phenotype after Stroke
Inflammatory responses following stroke originate from myeloid cells: that is, microglia/macrophage activation and leukocyte infiltration [262-266]. Microglia/macrophages are polarized following occurrence of stroke, transforming into pro-inflammatory or anti-inflammatory phenotypes (Fig. 5) [267-271]. Resting microglia/macrophages (M0 phenotype) stimulated with DAMPs are polarized to pro-inflammatory microglia/macrophages (M1 phenotype) under the presence of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-17. Microglia/macrophage were activated by 4 h and prolonged for at least 4 weeks in an ICH model produced by an intracerebral blood injection in rats [272]. Activated microglia induce pro-inflammatory mediators and cytokines via activation of pattern recognition receptors (PRRs) which include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), and receptors for advanced glycation end products (RAGEs) [273-275]. Pro-inflammatory cytokines and mediators induce specific cell adhesion molecules on endothelial cells, causing neuroinflammation, degradation of BM and tight junction proteins in brain capillary leading to BBB disruption, and apoptosis of various cells including caspase-dependent endothelial cell apoptosis [210, 244, 245, 276]. Apoptosis of ECs was increased following stroke, leading to enhancement of the BBB permeability [245]. Caspase-3 positive human brain microvascular endothelial cells were also increased in oxygen-glucose deprivation administration models of ischemic stroke [277]. In-vivo studies in hemorrhagic stroke demonstrated that cell death in endothelial cells of microvessels was increased after SAH as well as in the perihematomal area in a collagenase model of ICH [277]. In addition, both apoptosis and autophagy of endothelial cells were induced in the hippocampus after SAH [277]. Moreover compromised BBB allows macrophages and leucocytes into brain parenchyma, leading to further inflammatory responses [275]. The transmigration and infiltration of leukocytes were enhanced with BBB permeability and caused further induction of cytokines as well as MMPs after ICH [278-281].
4.4. The Involvement of PPRs in an Acute Phase after Stroke
TLRs are cell surface receptors involved in innate immunity and inflammatory response. Among TLR family members, TLR4 plays the most important role after stroke and has been researched extensively [275]. DAMPs bind to TLR4, which in turn activates transcriptional factor nuclear factor-κΒ (NF-κB) as well as mitogen-activated protein kinases (MAPKs) via myeloid differentiation primary response protein 88 and toll receptor–associated activator of interferon dependent signaling pathway leading to the release of pro-inflammatory cytokines and mediators such as IL-1β, IL-6, IL-8, IL-12, TNF-α, and MMPs into the brain tissue [244, 275]. Therefore, TLR4 is possibly one of good therapeutic targets to prevent BBB disruption following stroke. Our recent study showed that selective TLR4 antagonists attenuated post-SAH neurobehavioral impairments as well as BBB disruption via inactivation of MAPK c-Jun N-terminal kinases and MMP-9 in mice [282]. Post-ischemic BBB disruption was also improved by Tanshinone II A via suppression of TLR4, RAGE, HMGB1, and NF-κB expression [283]. On the other hand, NLRs signaling is also known as one of the innate immune system [284]. Currently, four NLR family members have been described, and NLRP3 inflammasome has been studied most [285, 286]. NLRP3 inflammasome is a cytosolic receptor and no direct ligand has been known. However, some non-protein DAMPs such as ATP and uric acid activate NLRP3 inflammasome indirectly in response to stroke stimuli [287]. ATP binds to P2X7 receptor (P2X7R) located on cell surface of neurons and glial cells, and leads to activation of the downstream signaling NLRP3 inflammasome and MAPKs followed by production of pro-inflammatory substances resulting in BBB injury [288-295]. Uric acid also activates NLRP3 inflammasome although the exact mechanisms have not been revealed [241, 296]. A P2X7 antagonist BBG inhibited MAPK p38 activation as well as NLPR3 inflammasome, and alleviated inflammation-associated neurologic deficits as well as neuronal apoptosis following SAH [293, 297]. In contrast, a selective NLRP3 antagonist MCC950 improved post-SAH neurological impairments via the reduction of BBB disruption as measured by albumin levels in the brain tissues as well as suppression of the release of pro-inflammatory cytokines such as TNF-α, IL-1ß, and IL-6 and MMP-9 in SAH [298]. In addition, ruscogenin, a major bioactive steroid sapogeni, attenuated cerebral ischemia-induced BBB disruption via inactivation of NLRP3 inflammasome and MAPKs [299]. Post-ICH BBB disruption was also ameliorated by selective NLRP3 inflammasome antagonist MCC950 [300]. RAGE is a cell surface receptor expressed on various CNS cells including myeloid cells. RAGE is activated by DAMPs such as HMGB1 and S100, and induces activation of NF-κB as well as MAPKs via Ras activation [301-306]. The soluble form of RAGE (sRAGE) has been used for the treatment targeting RAGE because it shows competitive antagonistic blocking against full-length RAGE [307]. Post-SAH sRAGE treatment reduced neuronal cell death via suppression of inflammation in rats [307].
4.5. The Involvement of MMPs towards BBB Disruption after Stroke
MMPs are key mediators of BBB breakdown, endothelial upregulation of adhesion molecules, and infiltration of inflammatory cells in stroke [47, 196, 266, 308-310]. MMP-2 is found in CSF, astrocytes, microglia, macrophages, and is upregulated with hypoxia-inducible factor-1α (HIF-1α) [310, 311], while MMP-3 is produced by microglia, macrophage, and neurons [210, 211, 311-314]. HIF-1α is a dimeric protein complex, which is induced via responses to growth factors as well as hypoxic state, and enhances vascular permeability and the expression of VEGF [315, 316]. A HIF-1α inhibiter, YC-1, suppressed MMP-2 as well as VEGF and prevented BBB injury in ischemic stroke [310]. Knockout of HIF-1α only in endothelial cells reduced BBB damage and infarct size after transient occlusion of middle cerebral artery in mice with diabetes, although diabetes was associated with BBB disruption [315]. In contrast, MMP-9 is induced by neutrophils, microglia, and macrophages with ROS as well as inflammatory cytokines such as TNF-α and IL-1β [210, 211, 312-314].
MMPs are potential therapeutic targets to block BBB injury, oxidative stress, and inflammatory responses [317-324]. The pan-MMP inhibitors GM6001 and BB-1101 as well as genetic deletion of MMP-12 improved post-ICH brain injury [325-328]. Clinically, minocycline reduced MMPs induction and exerted anti-inflammatory effects [329]. Treatment with minocycline also showed better neurological outcomes in patients with multiple sclerosis mainly via BBB-protection effects [330]. However, other studies have found that MMP inhibition following stroke has detrimental effects. A pan-MMP inhibitor BB-1101 blocked an increase in brain MMP-2 levels, but it did not have any effect on infarct size at 48 h after focal ischemia and had significant adverse effects on neurologic function in rats after 3 to 4 weeks of onset [321]. Another pan-MMP inhibitor BB-94 exacerbated brain injury after ICH [331]. This may be because the function of MMPs differs depending on the subtype, and the angiogenetic effect shows both protective and detrimental responses at different phases, severity, and types of stroke. MMP-2 induced early degradation of tight junction proteins within several hours in animal models with reperfusion injury after transient ischemia, while MMP-3 as well as MMP-9 lead to delayed opening of BBB via neuroinflammation at 24 to 72 h after onset [311, 317, 321, 322]. However, the action of MMP-2 towards BBB injury and neuronal death after stroke is controversial [47]. MMP-2 is upregulated in peripheral blood and CSF as well as perihematomal tissues in ICH models [314, 332, 333], while some studies have shown no obvious increase in MMP-2 expression after ischemic stroke [334-336]. Effects of ischemia-induced MMP-2 seem to depend on the reperfusion time as well as the severity of stroke [47]. Some studies demonstrated that MMP-2 induced proteolytic effects on endothelial cells after CI [311, 317, 322, 337], and that MMP-2−deficient mice subjected to transient focal ischemia display smaller ischemic lesions, less edema, and hemorrhagic volumes than wild-type mice [338, 339]. However, another study showed that MMP-2 knockout mice provided upregulation of MMP-9 and no significant improvement of ischemic lesion size compared with wild-type mice in both transient focal ischemia and permanent ischemia models [340]. A clinical study reported that plasma MMP-2 was increased only in patients with lacunar stroke within 12 h and was correlated with stable or recovering symptoms. In contrast, plasma MMP-9 was increased in patients with severe stroke after 7 days of onset and was associated with worse clinical outcomes [321]. MMP-9 is a proteolytic enzyme which plays critical roles in regard to BBB disruption due to the effects degrading ECM proteins in cerebral microvessel BM such as collagen IV, laminin, and fibronectin as well as endothelial tight junction proteins such as ZO-1 [210, 211, 243, 317]. In patients with ischemic stroke, MMP-9 in infarcted brain tissue is increased at least by 48 h from onset [341]. In addition, serum levels of MMP-9 were associated with hemorrhagic transformation in patients with ischemic stroke [208, 209]. In contrast, knockout of MMP-9 significantly suppressed brain edema as well as BBB disruption in transient focal ischemic models in mice [340]. MMP-9 was also upregulated in blood, CSF and perihematomal tissues in animal ICH models [314, 332, 333]. In both clinical and experimental studies, MMP-9 concentrations correlated with neurological deterioration, hematoma expansion, and perihematomal edema [332, 342]. MMP-9 was suppressed by depletion of circulating blood neutrophils, which inhibited neuroinflammation as well as BBB disruption after ICH in rats [280]. In addition, MMP-9 inhibitor SB-3CT also reduced SAH-induced brain edema [211]. Knockout of MMP-9 in mice showed better neurological recovery, less brain swelling, and mortality compared with wild-type animals after SAH [321]. MMPs are potential targets to be addressed in order to protect BBB integrity after stroke. However, further experimental studies would be required before clinical studies because extended and broad inhibition of MMPs might be detrimental rather than protective [321].
4.6. Polarization of Myeloid Cells to Anti-Inflammatory Phenotype after Stroke
A number of studies have shown that acute inhibition of microglia reduces BBB disruption after stroke [265, 266, 281, 343]. However, microglia can have beneficial as well as detrimental effects [45, 265, 266]. IL-4, IL-10, and transforming growth factor (TGF)-β induce alternative anti-inflammatory microglia/macrophages (M2 phenotype), which provide inhibition of M1 phenotype functions as well as inflammation resolution by releasing anti-inflammatory cytokine such as IL-4, IL-10, and TGF-β via mainly STAT3, STAT6 and peroxisome proliferator activated receptor (PPAR)γ (Fig. 5) [237, 242, 344-346]. In addition, M2 microglia-induced Ym1/2, IL-10, and TGF-β improved stroke outcome via induction of angiogenesis, and suppression of BBB injury [347]. Furthermore, macrophages absorb cytotoxic substances including DAMPs and digest them by lysosomes via degradative endocytic pathways [348]. Therefore, these myeloid cells are considered to play an essential role in debris removal, hematoma phagocytosis in hemorrhagic stroke, and repair of tissues including endothelial cells [265, 266].
When M1 phenotype microglia predominate at damaged tissues, M2 phenotype microglia are decreased [349]. However, it is not well understood when and why the proinflammatory M1 phenotype is switched to the anti-inflammatory M2 phenotype, and microglia can express both M1 and M2 phenotypes simultaneously [349]. The expression levels of cytokines such as Ym1/2, IL-10, and TGF-β produced by M2 phenotypes were increased between 1 and 3 days with a peak at 3 to 5 days after CI, returning to normal levels within 14 days [347]. M1 phenotype markers such as TNF-α, IL-6 and IL-1β increased from days 3 to 14 after ischemia [347]. An AMPK activator, metformin, provided functional recovery and tissue repair by promoting the polarization of microglia toward a M2 phenotype via the suppression of NF-κB-mediated inflammatory signaling after ischemic stroke in mice [350]. Some experimental studies demonstrated that PPARγ agonists induced the polarization of M2 phenotype in microglia and showed neuroprotective effects after ICH [264, 351, 352]. The synthetic PPAR agonist HU-211 also suppressed the BBB disruption and production of cytokines in traumatic brain injury [353]. On the other hand, promoting BBB repair may play a role in detrimental effects because compromised BBB helps clearance of cytotoxic substances from brain parenchyma [45]. Thus, tailored treatments depending on severity of stroke and the time window of treatment should be considered in the future studies.
4.7. Roles of Pericytes and Astrocytes in BBB Disruption after Stroke
Pericytes are potentially involved in both BBB protection and damage after stroke depending on the situations [160]. Pericytes protected BBB function via maintaining endothelial cells and tight junctions under in-vitro hypoxic damage [354, 355]. In contrast, ischemia induces microvessels contraction by pericytes, followed by the degeneration [356]. Pericytes were detached from brain microvessels within 2 h after cerebral ischemia and weakened the intercellular contacts and signaling interactions between endothelial cells and pericytes [156, 158, 357, 358]. The detachment of pericytes promoted BBB disruption as well as leukocyte infiltration, resulting in further neuronal damage after stroke [160, 359]. On the other hand, viable pericytes may release pro-inflammatory substances by the stimulation of DAMPs and inflammatory cytokines, resulting in BBB dysfunction [160]. In addition, pericytes may transform into microglia to be involved in inflammation and inflammation-mediated tissue damages [160]. However, in a later phase, pericytes migrated into the peripheral region of ischemic core and acted as vessel coverage in middle cerebral artery occlusion in mice, suggesting a compensatory mechanism to limit BBB breakdown [360, 361].
Astrocyte also regulates BBB function, and astrocyte-induced factors may have both beneficial and detrimental effects on BBB after stroke. Selective knockout of the astrocytic Na+/H+ exchanger isoform 1 attenuated astrogliosis and BBB disruption after ischemic stroke in mice [362]. Pyr3, a specific transient receptor potential canonical channel 3 inhibitor, suppressed the pathological activation of astrocytes and prevented BBB breakdown after ICH in rats [363]. In contrast, astrocyte-derived angiopoietin-1 (ANG-1), sonic hedgehog, glial-derived neurotrophic factor, retinoic acid, insulin-like growth factor-1, and apolipoprotein E protected apoptosis of endothelial cells and induced tight junction repair. In addition, these factors also decreased endothelial cell adhesion molecules and reduced leukocyte infiltration [364]. Lower plasma ANG-1 levels were associated with poor outcomes after ischemic stroke [365], while higher serum ANG-1 resulted in good outcome in patients with intracerebral hemorrhage [165]. On the other hand, astrocyte-derived VEGFs, MMPs, nitric oxides, and endothelins are involved in endothelial cell apoptosis and downregulation of tight junction proteins, as well as upregulation of endothelial cell adhesion molecules, which induced leukocyte transmigration [364].
4.8. Alternative Potential Therapeutic Options against BBB Disruption after Stroke
Another potential target to prevent BBB disruption following stroke is matricellular proteins. Matricellular proteins are inducible and secretory non-structural proteins belonging to the ECM proteins [366]. Matricellular proteins are induced by various stimuli including stroke and are associated with aggravation or improvement of BBB disruption in stroke [244, 367]. Our recent studies showed that the expression of matricellular proteins including tenascin-C, periostin, and galectin-3 was enhanced after SAH and lead to BBB disruption via MAPKs and MMP-9 activation in mice [368-371]. A galectin-3 inhibitor, citrus pectin, attenuated BBB disruption via inactivation of TLR4 as well as MMP-9 [372, 373]. Tenascin-C and periostin enhanced the expression each other, resulting in BBB disruption via activation of MAPKs signaling pathway [374]. Treatment with neutralizing antibody against periostin suppressed tenascin-C induction as well as BBB disruption after SAH [369]. In contrast, another matricellular protein osteopontin was expressed in reactive astrocytes as well as capillary endothelial cells, and protected BBB integrity via inactivating MAPKs and NF-κB [244, 375]. Osteopontin induced by macrophages also seems to promote the repair of the compromised BBB associated with phagocytosis of fragmented cell debris, formation of connective tissue matrix, and resolution of damaged brain tissues after stroke [376]. Osteopontin knockout failed to repair the ischemia-induced damage of neurovascular unit in mice, resulting in incomplete coverage by perivascular astrocytic end-feet and persistently leaky compromised BBB [377]. On the other hand, ischemia-induced BBB disruption was ameliorated by aperin-13 and salidroside, which exerted anti-apoptotic effect via activation of phosphatidylinositol 3-kinase/Akt signaling pathways [324]. Inhibitors of cyclooxgyenases-2 reduced delayed BBB damage arising from neuroinflammation in experimental models of stroke [311]. Non-invasive vagus nerve stimulation also could suppressed BBB injury as well as infarct size by inhibiting MMP-2 and MMP-9 in ischemic stroke [378]. Alternatively, hypertonic saline prevented BBB dysfunction in ischemic stroke models via regulating VEGF and VEGFR2 as well as aquaporin-4 [379, 380]. Aquaporin-4 is a water channel protein widely expressed throughout the CNS including astrocytic end-feet [381]. Aquaporin-4 deficiency alleviated ischemia-induced brain edema, while it played a role in repairing compromised BBB in a delayed phase of stroke [237, 382]. Therefore, aquaporin-4-targeted therapy is possibly required to match the time window for treatment to obtain beneficial effects towards BBB protection.
CONCLUSION
BBB disruption is a pathological change causing brain edema, hemorrhagic transformation, and neuroinflammation after stroke. Stroke-induced neuroinflammation and apoptosis of endothelial cells are involved in BBB disruption. Therefore, a multitude of therapies to address these pathophysiological changes have been proposed in both experimental and clinical studies, but currently the clinical benefits for patients with stroke still remain insufficient. On the other hand, BBB disruption may also exert beneficial effects for clearance of cytotoxic substances from brain parenchyma. Thus, further studies to capture BBB disruption and to determine appropriate treatments depending on patients’ status would be needed to improve clinical outcomes of stroke patients.
Acknowledgements
We wish to express our gratitude to Yuanjian Fang for providing the photographs of Evans blue dye.
LIST OF ABBREVIATIONS
- 68Ga EDTA
68Ga ethylenediaminetetraacetic acid
- 99mTc-DTPA
99mTc-diethylenetriaminepentaacetic acid
- ASL
arterial spin labeling
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- BM
basement membrane
- CI
cerebral infarction
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DAMP
damage-associated molecular pattern
- DCE-CT
dynamic contrast-enhanced computed tomography
- DCE-MRI
dynamic contrast enhanced magnetic resonance imaging
- EC
endothelial cell
- ECM
extracellular matrix
- GFAP
glial fibrillary acidic protein
- hCMEC
human cerebral microvascular endothelial cell
- HIF-1α
hypoxia-inducible factor-1α
- HMGB1
high-mobility group box 1
- ICH
intracerebral hemorrhage
- ICP
intracranial pressure
- IFN-γ
interferon-γ
- Ig
immunoglobulin
- IL
interleukin
- IL-10R
IL-10 receptor
- IL-4R
IL-4 receptor
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MR
magnetic resonance
- NF-κB
nuclear factor-κB
- NLR
nucleotide-binding oligomerization domain-like receptor
- P2X7R
P2X7 receptor
- PPARγ
peroxisome proliferator activated receptor γ
- PRR
pattern recognition receptor
- RAGE
receptors for advanced glycation end product
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhage
- SPECT
single photo-emitting computed tomography
- sRAGE
soluble form of RAGE
- STAT
signal transducer and activator of transcription
- T1WI
T1-weighted imaging
- TEER
transendothelial electrical resistance
- TGF-β
transforming growth factor-β
- TIMPs
tissue inhibitor of metalloproteinases
- TLR
toll-like receptors
- TNF-α
tumor necrosis factor α
- t-PA
tissue plasminogen activator
- UCH-L1
ubiquitin C-terminal hydrolase L1
- VE-cadherin
vascular endothelial- cadherin
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- ZO
zonula occludens
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Feigin V.L., Norrving B., Mensah G.A. Global Burden of Stroke. Circ. Res. 2017;120(3):439–448. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., Isasi C.R., Jiménez M.C., Jordan L.C., Judd S.E., Lackland D., Lichtman J.H., Lisabeth L., Liu S., Longenecker C.T., Mackey R.H., Matsushita K., Mozaffarian D., Mussolino M.E., Nasir K., Neumar R.W., Palaniappan L., Pandey D.K., Thiagarajan R.R., Reeves M.J., Ritchey M., Rodriguez C.J., Roth G.A., Rosamond W.D., Sasson C., Towfighi A., Tsao C.W., Turner M.B., Virani S.S., Voeks J.H., Willey J.Z., Wilkins J.T., Wu J.H., Alger H.M., Wong S.S., Muntner P., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakarpandian B., Shen M-C., Nichols J.B., Mills I.R., Sidoryk-Wegrzynowicz M., Aschner M., Pant K. SyM-BBB: a microfluidic Blood Brain Barrier model. Lab Chip. 2013;13(6):1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdullahi W., Tripathi D., Ronaldson P.T. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018;315(3):C343–C356. doi: 10.1152/ajpcell.00095.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alluri H., Wiggins-Dohlvik K., Davis M.L., Huang J.H., Tharakan B. Blood-brain barrier dysfunction following traumatic brain injury. Metab. Brain Dis. 2015;30(5):1093–1104. doi: 10.1007/s11011-015-9651-7. [DOI] [PubMed] [Google Scholar]
- 6.Mracsko E., Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front. Cell. Neurosci. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner R.J., Sharp F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell. Neurosci. 2016;10:56. doi: 10.3389/fncel.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987;72(3):236–239. doi: 10.1007/BF00691095. [DOI] [PubMed] [Google Scholar]
- 9.Balami J.S., Chen R-L., Grunwald I.Q., Buchan A.M. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011;10(4):357–371. doi: 10.1016/S1474-4422(10)70313-6. [DOI] [PubMed] [Google Scholar]
- 10.Claassen J., Carhuapoma J.R., Kreiter K.T., Du E.Y., Connolly E.S., Mayer S.A. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33(5):1225–1232. doi: 10.1161/01.STR.0000015624.29071.1F. [DOI] [PubMed] [Google Scholar]
- 11.Russin J.J., Montagne A., D’Amore F., He S., Shiroishi M.S., Rennert R.C., Depetris J., Zlokovic B.V., Mack W.J. Permeability imaging as a predictor of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2018;38(6):973–979. doi: 10.1177/0271678X18768670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latour L.L., Kang D-W., Ezzeddine M.A., Chalela J.A., Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann. Neurol. 2004;56(4):468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- 13.Hjort N., Wu O., Ashkanian M., Sølling C., Mouridsen K., Christensen S., Gyldensted C., Andersen G., Østergaard L. MRI detection of early blood-brain barrier disruption: parenchymal enhancement predicts focal hemorrhagic transformation after thrombolysis. Stroke. 2008;39(3):1025–1028. doi: 10.1161/STROKEAHA.107.497719. [DOI] [PubMed] [Google Scholar]
- 14.Edgell R.C., Vora N.A. Neuroimaging markers of hemorrhagic risk with stroke reperfusion therapy. Neurology. 2012;79(13) Suppl. 1:S100–S104. doi: 10.1212/WNL.0b013e3182695848. [DOI] [PubMed] [Google Scholar]
- 15.Khatri R., McKinney A.M., Swenson B., Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13) Suppl. 1:S52–S57. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- 16.Shobha N., Buchan A.M., Hill M.D. Canadian Alteplase for Stroke Effectiveness Study (CASES). Thrombolysis at 3-4.5 hours after acute ischemic stroke onset--evidence from the Canadian Alteplase for Stroke Effectiveness Study (CASES) registry. Cerebrovasc. Dis. 2011;31(3):223–228. doi: 10.1159/000321893. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W., Furlan A.J., Al-Rawi Y., Davalos A., Fiebach J.B., Gruber F., Kaste M., Lipka L.J., Pedraza S., Ringleb P.A., Rowley H.A., Schneider D., Schwamm L.H., Leal J.S., Söhngen M., Teal P.A., Wilhelm-Ogunbiyi K., Wintermark M., Warach S. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith W.S., Sung G., Starkman S., Saver J.L., Kidwell C.S., Gobin Y.P., Lutsep H.L., Nesbit G.M., Grobelny T., Rymer M.M., Silverman I.E., Higashida R.T., Budzik R.F., Marks M.P., MERCI Trial Investigators Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36(7):1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 19.Smith W.S., Sung G., Saver J., Budzik R., Duckwiler G., Liebeskind D.S., Lutsep H.L., Rymer M.M., Higashida R.T., Starkman S., Gobin Y.P., Frei D., Grobelny T., Hellinger F., Huddle D., Kidwell C., Koroshetz W., Marks M., Nesbit G., Silverman I.E., Multi MERCI Investigators Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39(4):1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 20.Chen H., Zhu G., Liu N., Li Y., Xia Y. Applications and development of permeability imaging in ischemic stroke. Exp. Ther. Med. 2018;16(3):2203–2207. doi: 10.3892/etm.2018.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucke-Wold B.P., Logsdon A.F., Manoranjan B., Turner R.C., McConnell E., Vates G.E., Huber J.D., Rosen C.L., Simard J.M. Aneurysmal subarachnoid hemorrhage and neuroinflammation: A comprehensive review. Int. J. Mol. Sci. 2016;17(4):497. doi: 10.3390/ijms17040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veksler R., Shelef I., Friedman A. Blood-brain barrier imaging in human neuropathologies. Arch. Med. Res. 2014;45(8):646–652. doi: 10.1016/j.arcmed.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S., Feng H., Sherchan P., Klebe D., Zhao G., Sun X., Zhang J., Tang J., Zhang J.H. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog. Neurobiol. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattringer T., Valdes Hernandez M., Heye A., Armitage P.A., Makin S., Chappell F., Pinter D., Doubal F., Enzinger C., Fazekas F., Wardlaw J.M. Predictors of Lesion Cavitation After Recent Small Subcortical Stroke. Transl. Stroke Res. 2019;11(3):402–411. doi: 10.1007/s12975-019-00741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlovsky L., Seiffert E., Heinemann U., Korn A., Golan H., Friedman A. Persistent BBB disruption may underlie alpha interferon-induced seizures. J. Neurol. 2005;252(1):42–46. doi: 10.1007/s00415-005-0596-3. [DOI] [PubMed] [Google Scholar]
- 26.Tomkins O., Shelef I., Kaizerman I., Eliushin A., Afawi Z., Misk A., Gidon M., Cohen A., Zumsteg D., Friedman A. Blood-brain barrier disruption in post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry. 2008;79(7):774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- 27.Seiffert E., Dreier J.P., Ivens S., Bechmann I., Tomkins O., Heinemann U., Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004;24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomkins O., Friedman O., Ivens S., Reiffurth C., Major S., Dreier J.P., Heinemann U., Friedman A. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol. Dis. 2007;25(2):367–377. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Lapilover E.G., Lippmann K., Salar S., Maslarova A., Dreier J.P., Heinemann U., Friedman A. Peri-infarct blood-brain barrier dysfunction facilitates induction of spreading depolarization associated with epileptiform discharges. Neurobiol. Dis. 2012;48(3):495–506. doi: 10.1016/j.nbd.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David Y., Cacheaux L.P., Ivens S., Lapilover E., Heinemann U., Kaufer D., Friedman A. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J. Neurosci. 2009;29(34):10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman A., Kaufer D., Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85(2-3):142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivens S., Kaufer D., Flores L.P., Bechmann I., Zumsteg D., Tomkins O., Seiffert E., Heinemann U., Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130(Pt 2):535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 33.Tomkins O., Feintuch A., Benifla M., Cohen A., Friedman A., Shelef I. Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy. Cardiovasc. Psychiatry Neurol. 2011;2011:765923. doi: 10.1155/2011/765923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goulay R., Mena Romo L., Hol E.M., Dijkhuizen R.M. From Stroke to Dementia: a Comprehensive Review Exposing Tight Interactions Between Stroke and Amyloid-β Formation. Transl. Stroke Res. 2019;11(4):601–614. doi: 10.1007/s12975-019-00755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L., Nirwane A., Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018;4(2):78–82. doi: 10.1136/svn-2018-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raja R., Rosenberg G.A., Caprihan A. MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies. 2018. [DOI] [PMC free article] [PubMed]
- 37.Wilhelm I., Fazakas C., Krizbai I.A. In vitro models of the blood-brain barrier. Acta Neurobiol. Exp. (Warsz.) 2011;71(1):113–128. doi: 10.55782/ane-2011-1828. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins R.A., O’Kane R.L., Simpson I.A., Viña J.R. Structure of the blood-brain barrier and its role in the transport of amino acids. J. Nutr. 2006;136(1) Suppl.:218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 39.He Y., Yao Y., Tsirka S.E., Cao Y. Cell-culture models of the blood-brain barrier. Stroke. 2014;45(8):2514–2526. doi: 10.1161/STROKEAHA.114.005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Yao Y., Chen Z-L., Norris E.H., Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janzer R.C., Raff M.C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 43.Bazzoni G., Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 44.Fenstermacher J., Gross P., Sposito N., Acuff V., Pettersen S., Gruber K. Structural and functional variations in capillary systems within the brain. Ann. N. Y. Acad. Sci. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 45.Keep R.F., Andjelkovic A.V., Xiang J., Stamatovic S.M., Antonetti D.A., Hua Y., Xi G. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J. Cereb. Blood Flow Metab. 2018;38(8):1255–1275. doi: 10.1177/0271678X18774666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kniesel U., Wolburg H. Tight junctions of the blood-brain barrier. Cell. Mol. Neurobiol. 2000;20(1):57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang C., Hawkins K.E., Doré S., Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019;316(2):C135–C153. doi: 10.1152/ajpcell.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasan B., Kolli A.R., Esch M.B., Abaci H.E., Shuler M.L., Hickman J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015;20(2):107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamatovic S.M., Johnson A.M., Keep R.F., Andjelkovic A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers. 2016;4(1):e1154641. doi: 10.1080/21688370.2016.1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Kim S-H., Turnbull J., Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011;209(2):139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 52.Baeten K.M., Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 2011;71(11):1018–1039. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hynes R.O. 2009.
- 54.Talegaonkar S., Mishra P.R. Intranasal delivery : An approach to bypass the blood brain barrier. Indian J. Pharmacol. 2004;36:140–147. [Google Scholar]
- 55.Keep R.F., Zhou N., Xiang J., Andjelkovic A.V., Hua Y., Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 57.Deeken J.F., Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin. Cancer Res. 2007;13(6):1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 58.Altay O., Suzuki H., Hasegawa Y., Caner B., Krafft P.R., Fujii M., Tang J., Zhang J.H. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke. 2012;43(9):2513–2516. doi: 10.1161/STROKEAHA.112.661728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Zhang Y., Tang J., Liu F., Hu Q., Luo C., Tang J., Feng H., Zhang J.H. Norrin protected blood-brain barrier via frizzled-4/β-catenin pathway after subarachnoid hemorrhage in rats. Stroke. 2015;46(2):529–536. doi: 10.1161/STROKEAHA.114.007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pang J., Wu Y., Peng J., Yang P., Kuai L., Qin X., Cao F., Sun X., Chen L., Vitek M.P., Jiang Y. Potential implications of Apolipoprotein E in early brain injury after experimental subarachnoid hemorrhage: Involvement in the modulation of blood-brain barrier integrity. Oncotarget. 2016;7(35):56030–56044. doi: 10.18632/oncotarget.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willis C.L.L., Camire R.B.B., Brule S.A.A., Ray D.E.E. Partial recovery of the damaged rat blood-brain barrier is mediated by adherens junction complexes, extracellular matrix remodeling and macrophage infiltration following focal astrocyte loss. Neuroscience. 2013;250:773–785. doi: 10.1016/j.neuroscience.2013.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patino M.G., Neiders M.E., Andreana S., Noble B., Cohen R.E. Collagen: an overview. Implant Dent. 2002;11(3):280–285. doi: 10.1097/00008505-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 63.Hudson B.G., Reeders S.T., Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J. Biol. Chem. 1993;268(35):26033–26036. [PubMed] [Google Scholar]
- 64.Filie J.D., Burbelo P.D., Kozak C.A. Genetic mapping of the alpha 1 and alpha 2 (IV) collagen genes to mouse chromosome 8. Mamm. Genome. 1995;6(7):487. doi: 10.1007/BF00360662. [DOI] [PubMed] [Google Scholar]
- 65.Sado Y., Kagawa M., Naito I., Ueki Y., Seki T., Momota R., Oohashi T., Ninomiya Y. Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J. Biochem. 1998;123(5):767–776. doi: 10.1093/oxfordjournals.jbchem.a022003. [DOI] [PubMed] [Google Scholar]
- 66.Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 67.Favor J., Gloeckner C.J., Janik D., Klempt M., Neuhäuser-Klaus A., Pretsch W., Schmahl W., Quintanilla-Fend L. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics. 2007;175(2):725–736. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuo D.S., Labelle-Dumais C., Mao M., Jeanne M., Kauffman W.B., Allen J., Favor J., Gould D.B. Allelic heterogeneity contributes to variability in ocular dysgenesis, myopathy and brain malformations caused by Col4a1 and Col4a2 mutations. Hum. Mol. Genet. 2014;23(7):1709–1722. doi: 10.1093/hmg/ddt560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeanne M., Labelle-Dumais C., Jorgensen J., Kauffman W.B., Mancini G.M., Favor J., Valant V., Greenberg S.M., Rosand J., Gould D.B. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am. J. Hum. Genet. 2012;90(1):91–101. doi: 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamann G.F., Liebetrau M., Martens H., Burggraf D., Kloss C.U.A., Bültemeier G., Wunderlich N., Jäger G., Pfefferkorn T. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J. Cereb. Blood Flow Metab. 2002;22(5):526–533. doi: 10.1097/00004647-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Härtig W., Mages B., Aleithe S., Nitzsche B., Altmann S., Barthel H., Krueger M., Michalski D. Damaged Neocortical Perineuronal Nets Due to Experimental Focal Cerebral Ischemia in Mice, Rats and Sheep. Front. Integr. Nuerosci. 2017;11:15. doi: 10.3389/fnint.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milner R., Hung S., Wang X., Spatz M., del Zoppo G.J. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J. Cereb. Blood Flow Metab. 2008;28(4):812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pankov R., Yamada K.M. Fibronectin at a glance. J. Cell Sci. 2002;115(Pt 20):3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 74.To W.S., Midwood K.S. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsiao C-T., Cheng H-W., Huang C-M., Li H-R., Ou M-H., Huang J-R., Khoo K-H., Yu H.W., Chen Y-Q., Wang Y-K., Chiou A., Kuo J-C. Fibronectin in cell adhesion and migration via N-glycosylation. Oncotarget. 2017;8(41):70653–70668. doi: 10.18632/oncotarget.19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J., Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through alpha5beta1 and alphavbeta3 integrins via MAP kinase signalling. J. Neurochem. 2006;96(1):148–159. doi: 10.1111/j.1471-4159.2005.03521.x. [DOI] [PubMed] [Google Scholar]
- 77.George E.L., Georges-Labouesse E.N., Patel-King R.S., Rayburn H., Hynes R.O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 78.Milner R., Campbell I.L. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol. Cell. Neurosci. 2002;20(4):616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- 79.Sakai T., Johnson K.J., Murozono M., Sakai K., Magnuson M.A., Wieloch T., Cronberg T., Isshiki A., Erickson H.P., Fässler R. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat. Med. 2001;7(3):324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Reheman A., Spring C.M., Kalantari J., Marshall A.H., Wolberg A.S., Gross P.L., Weitz J.I., Rand M.L., Mosher D.F., Freedman J., Ni H. Plasma fibronectin supports hemostasis and regulates thrombosis. J. Clin. Invest. 2014;124(10):4281–4293. doi: 10.1172/JCI74630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colognato H., Yurchenco P.D. Form and function: the laminin family of heterotrimers. Dev. Dyn. 2000;218(2):213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213:AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 82.Hallmann R., Horn N., Selg M., Wendler O., Pausch F., Sorokin L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 83.Li S., Edgar D., Fässler R., Wadsworth W., Yurchenco P.D. The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell. 2003;4(5):613–624. doi: 10.1016/S1534-5807(03)00128-X. [DOI] [PubMed] [Google Scholar]
- 84.Miner J.H., Yurchenco P.D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 85.Miner J.H., Li C., Mudd J.L., Go G., Sutherland A.E. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131(10):2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- 86.Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O., Sorokin L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001;153(5):933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorokin L.M., Pausch F., Frieser M., Kröger S., Ohage E., Deutzmann R. Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev. Biol. 1997;189(2):285–300. doi: 10.1006/dbio.1997.8668. [DOI] [PubMed] [Google Scholar]
- 88.Jucker M., Tian M., Norton D.D., Sherman C., Kusiak J.W. Laminin alpha 2 is a component of brain capillary basement membrane: reduced expression in dystrophic dy mice. Neuroscience. 1996;71(4):1153–1161. doi: 10.1016/0306-4522(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 89.Sorokin L., Girg W., Göpfert T., Hallmann R., Deutzmann R. Expression of novel 400-kDa laminin chains by mouse and bovine endothelial cells. Eur. J. Biochem. 1994;223(2):603–610. doi: 10.1111/j.1432-1033.1994.tb19031.x. [DOI] [PubMed] [Google Scholar]
- 90.Tilling T., Engelbertz C., Decker S., Korte D., Hüwel S., Galla H-J.J. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res. 2002;310(1):19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 91.Tilling T., Korte D., Hoheisel D., Galla H.J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998;71(3):1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 92.Smyth N., Vatansever H.S., Murray P., Meyer M., Frie C., Paulsson M., Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 1999;144(1):151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Z-L.L., Yao Y., Norris E.H., Kruyer A., Jno-Charles O., Akhmerov A., Strickland S. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J. Cell Biol. 2013;202(2):381–395. doi: 10.1083/jcb.201212032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thyboll J., Kortesmaa J., Cao R., Soininen R., Wang L., Iivanainen A., Sorokin L., Risling M., Cao Y., Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol. Cell. Biol. 2002;22(4):1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halfter W., Dong S., Yip Y-P., Willem M., Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 2002;22(14):6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knöll R., Postel R., Wang J., Krätzner R., Hennecke G., Vacaru A.M., Vakeel P., Schubert C., Murthy K., Rana B.K., Kube D., Knöll G., Schäfer K., Hayashi T., Holm T., Kimura A., Schork N., Toliat M.R., Nürnberg P., Schultheiss H-P., Schaper W., Schaper J., Bos E., Den Hertog J., van Eeden F.J.M., Peters P.J., Hasenfuss G., Chien K.R., Bakkers J. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation. 2007;116(5):515–525. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- 97.Miyagoe Y., Hanaoka K., Nonaka I., Hayasaka M., Nabeshima Y., Arahata K., Nabeshima Y., Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415(1):33–39. doi: 10.1016/S0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 98.Miner J.H., Cunningham J., Sanes J.R. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J. Cell Biol. 1998;143(6):1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yousif L.F., Di Russo J., Sorokin L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adhes. Migr. 2013;7(1):101–110. doi: 10.4161/cam.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edwards D.N., Bix G.J. Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am. J. Physiol. Cell Physiol. 2019;316(2):C252–C263. doi: 10.1152/ajpcell.00151.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jucker M., Bialobok P., Kleinman H.K., Walker L.C., Hagg T., Ingram D.K. Laminin-like and laminin-binding protein-like immunoreactive astrocytes in rat hippocampus after transient ischemia. Antibody to laminin-binding protein is a sensitive marker of neural injury and degeneration. Ann. N. Y. Acad. Sci. 1993;679:245–252. doi: 10.1111/j.1749-6632.1993.tb18304.x. [DOI] [PubMed] [Google Scholar]
- 102.Szabó A., Kálmán M. Disappearance of the post-lesional laminin immunopositivity of brain vessels is parallel with the formation of gliovascular junctions and common basal lamina. A double-labelling immunohistochemical study. Neuropathol. Appl. Neurobiol. 2004;30(2):169–177. doi: 10.1046/j.0305-1846.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 103.Li L., Liu F., Welser-Alves J.V., McCullough L.D., Milner R. Upregulation of fibronectin and the α5β1 and αvβ3 integrins on blood vessels within the cerebral ischemic penumbra. Exp. Neurol. 2012;233(1):283–291. doi: 10.1016/j.expneurol.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujioka T., Kaneko N., Ajioka I., Nakaguchi K., Omata T., Ohba H., Fässler R., García-Verdugo J.M., Sekiguchi K., Matsukawa N., Sawamoto K. β1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine. 2017;16:195–203. doi: 10.1016/j.ebiom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gautam J., Cao Y., Yao Y. Pericytic Laminin Maintains Blood-Brain Barrier Integrity in an Age-Dependent Manner. Transl. Stroke Res. 2020;11(2):228–242. doi: 10.1007/s12975-019-00709-8. [DOI] [PubMed] [Google Scholar]
- 106.Gautam J., Miner J.H., Yao Y. Loss of Endothelial Laminin α5 Exacerbates Hemorrhagic Brain Injury. Transl. Stroke Res. 2019;10(6):705–718. doi: 10.1007/s12975-019-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pákáski M., Kása P., Joó F., Wolff J.R. Cerebral endothelial cell-derived laminin promotes the outgrowth of neurites in CNS neuronal cultures. Int. J. Dev. Neurosci. 1990;8(2):193–198. doi: 10.1016/0736-5748(90)90010-Y. [DOI] [PubMed] [Google Scholar]
- 108.Hatakeyama M., Ninomiya I., Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020;15(1):16–19. doi: 10.4103/1673-5374.264442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grimpe B., Probst J.C., Hager G. Suppression of nidogen-1 translation by antisense targeting affects the adhesive properties of cultured astrocytes. Glia. 1999;28(2):138–149. doi: 10.1002/(SICI)1098-1136(199911)28:2<138:AID-GLIA5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 110.Kang S.H., Kramer J.M. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol. Biol. Cell. 2000;11(11):3911–3923. doi: 10.1091/mbc.11.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong L., Chen Y., Lewis M., Hsieh J-C., Reing J., Chaillet J.R., Howell C.Y., Melhem M., Inoue S., Kuszak J.R., DeGeest K., Chung A.E. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab. Invest. 2002;82(12):1617–1630. doi: 10.1097/01.LAB.0000042240.52093.0F. [DOI] [PubMed] [Google Scholar]
- 112.Murshed M., Smyth N., Miosge N., Karolat J., Krieg T., Paulsson M., Nischt R. The absence of nidogen 1 does not affect murine basement membrane formation. Mol. Cell. Biol. 2000;20(18):7007–7012. doi: 10.1128/MCB.20.18.7007-7012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mokkapati S., Baranowsky A., Mirancea N., Smyth N., Breitkreutz D., Nischt R. Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J. Invest. Dermatol. 2008;128(9):2259–2267. doi: 10.1038/jid.2008.65. [DOI] [PubMed] [Google Scholar]
- 114.Böse K., Nischt R., Page A., Bader B.L., Paulsson M., Smyth N. Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J. Biol. Chem. 2006;281(51):39620–39629. doi: 10.1074/jbc.M607886200. [DOI] [PubMed] [Google Scholar]
- 115.Bader B.L., Smyth N., Nedbal S., Miosge N., Baranowsky A., Mokkapati S., Murshed M., Nischt R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell. Biol. 2005;25(15):6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schymeinsky J., Nedbal S., Miosge N., Pöschl E., Rao C., Beier D.R., Skarnes W.C., Timpl R., Bader B.L. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol. Cell. Biol. 2002;22(19):6820–6830. doi: 10.1128/MCB.22.19.6820-6830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miosge N., Sasaki T., Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002;21(7):611–621. doi: 10.1016/S0945-053X(02)00070-7. [DOI] [PubMed] [Google Scholar]
- 118.Agrawal S., Anderson P., Durbeej M., van Rooijen N., Ivars F., Opdenakker G., Sorokin L.M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006;203(4):1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knox S.M., Whitelock J.M. Perlecan: how does one molecule do so many things? Cell. Mol. Life Sci. 2006;63(21):2435–2445. doi: 10.1007/s00018-006-6162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whitelock J.M., Melrose J., Iozzo R.V. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47(43):11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Costell M., Sasaki T., Mann K., Yamada Y., Timpl R. Structural characterization of recombinant domain II of the basement membrane proteoglycan perlecan. FEBS Lett. 1996;396(2-3):127–131. doi: 10.1016/0014-5793(96)01082-4. [DOI] [PubMed] [Google Scholar]
- 122.Dolan M., Horchar T., Rigatti B., Hassell J.R. Identification of sites in domain I of perlecan that regulate heparan sulfate synthesis. J. Biol. Chem. 1997;272(7):4316–4322. doi: 10.1074/jbc.272.7.4316. [DOI] [PubMed] [Google Scholar]
- 123.Hopf M., Göhring W., Mann K., Timpl R. Mapping of binding sites for nidogens, fibulin-2, fibronectin and heparin to different IG modules of perlecan. J. Mol. Biol. 2001;311(3):529–541. doi: 10.1006/jmbi.2001.4878. [DOI] [PubMed] [Google Scholar]
- 124.Handler M., Yurchenco P.D., Iozzo R.V. Developmental expression of perlecan during murine embryogenesis. Dev. Dyn. 1997;210(2):130–145. doi: 10.1002/(SICI)1097-0177(199710)210:2<130:AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 125.Costell M., Gustafsson E., Aszódi A., Mörgelin M., Bloch W., Hunziker E., Addicks K., Timpl R., Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999;147(5):1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ford-Perriss M., Turner K., Guimond S., Apedaile A., Haubeck H-D., Turnbull J., Murphy M. Localisation of specific heparan sulfate proteoglycans during the proliferative phase of brain development. Dev. Dyn. 2003;227(2):170–184. doi: 10.1002/dvdy.10298. [DOI] [PubMed] [Google Scholar]
- 127.Clarke D.N., Al Ahmad A., Lee B., Parham C., Auckland L., Fertala A., Kahle M., Shaw C.S., Roberts J., Bix G.J. Perlecan Domain V induces VEGf secretion in brain endothelial cells through integrin α5β1 and ERK-dependent signaling pathways. PLoS One. 2012;7(9):e45257. doi: 10.1371/journal.pone.0045257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li L., Welser J.V., Milner R. Absence of the alpha v beta 3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in alpha 5 beta 1 integrin expression. J. Cereb. Blood Flow Metab. 2010;30(5):1031–1043. doi: 10.1038/jcbfm.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trout A.L., Kahle M.P., Roberts J.M., Marcelo A., de Hoog L., Boychuk J.A., Grupke S.L., Berretta A., Gowing E.K., Boychuk C.R., Gorman A.A., Edwards D.N., Rutkai I., Biose I.J., Ishibashi-Ueda H., Ihara M., Smith B.N., Clarkson A.N., Bix G.J. Perlecan Domain-V Enhances Neurogenic Brain Repair After Stroke in Mice. Transl. Stroke Res. 2020;••• doi: 10.1007/s12975-020-00800-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee B., Clarke D., Al Ahmad A., Kahle M., Parham C., Auckland L., Shaw C., Fidanboylu M., Orr A.W., Ogunshola O., Fertala A., Thomas S.A., Bix G.J. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J. Clin. Invest. 2011;121(8):3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roberts J., Kahle M.P., Bix G.J. Perlecan and the blood-brain barrier: beneficial proteolysis? Front. Pharmacol. 2012;3:155. doi: 10.3389/fphar.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Al-Ahmad A.J., Lee B., Saini M., Bix G.J. Perlecan domain V modulates astrogliosis in vitro and after focal cerebral ischemia through multiple receptors and increased nerve growth factor release. Glia. 2011;59(12):1822–1840. doi: 10.1002/glia.21227. [DOI] [PubMed] [Google Scholar]
- 133.Baumann E., Preston E., Slinn J., Stanimirovic D. Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and SPARC, and the blood-brain barrier disruption after global cerebral ischemia. Brain Res. 2009;1269:185–197. doi: 10.1016/j.brainres.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 134.Steiner E., Enzmann G.U., Lyck R., Lin S., Rüegg M.A., Kröger S., Engelhardt B. The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions. Cell Tissue Res. 2014;358(2):465–479. doi: 10.1007/s00441-014-1969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gautam M., Noakes P.G., Moscoso L., Rupp F., Scheller R.H., Merlie J.P., Sanes J.R. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85(4):525–535. doi: 10.1016/S0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 136.Barber A.J., Lieth E. Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev. Dyn. 1997;208(1):62–74. doi: 10.1002/(SICI)1097-0177(199701)208:1<62:AID-AJA6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 137.Gesemann M., Brancaccio A., Schumacher B., Ruegg M.A. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J. Biol. Chem. 1998;273(1):600–605. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- 138.Rascher G., Fischmann A., Kröger S., Duffner F., Grote E-H., Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104(1):85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- 139.Noell S., Fallier-Becker P., Deutsch U., Mack A.F., Wolburg H. Agrin defines polarized distribution of orthogonal arrays of particles in astrocytes. Cell Tissue Res. 2009;337(2):185–195. doi: 10.1007/s00441-009-0812-z. [DOI] [PubMed] [Google Scholar]
- 140.del Zoppo G.J. Aging and the neurovascular unit. Ann. N. Y. Acad. Sci. 2012;1268:127–133. doi: 10.1111/j.1749-6632.2012.06686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mäe M., Armulik A., Betsholtz C. Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr. Pharm. Des. 2011;17(26):2750–2754. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- 142.Ronaldson P.T., Davis T.P. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr. Pharm. Des. 2012;18(25):3624–3644. doi: 10.2174/138161212802002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stanimirovic D.B., Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J. Cereb. Blood Flow Metab. 2012;32(7):1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zonta M., Angulo M.C., Gobbo S., Rosengarten B., Hossmann K-A., Pozzan T., Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 145.Armulik A., Genové G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 146.Armulik A., Genové G., Mäe M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B.R., Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 147.Bergers G., Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol. 2005;7(4):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Winkler E.A., Bell R.D., Zlokovic B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alvarez J.I., Katayama T., Prat A. Glial influence on the blood brain barrier. Glia. 2013;61(12):1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hamby M.E., Sofroniew M.V. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7(4):494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maragakis N.J., Rothstein J.D. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006;2(12):679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 152.Wong A.D., Ye M., Levy A.F., Rothstein J.D., Bergles D.E., Searson P.C. The blood-brain barrier: an engineering perspective. Front. Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bai Y., Zhu X., Chao J., Zhang Y., Qian C., Li P., Liu D., Han B., Zhao L., Zhang J., Buch S., Teng G., Hu G., Yao H. Pericytes contribute to the disruption of the cerebral endothelial barrier via increasing VEGF expression: implications for stroke. PLoS One. 2015;10(4):e0124362. doi: 10.1371/journal.pone.0124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bell R.D., Winkler E.A., Sagare A.P., Singh I., LaRue B., Deane R., Zlokovic B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duz B., Oztas E., Erginay T., Erdogan E., Gonul E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology. 2007;55(3):279–284. doi: 10.1016/j.cryobiol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 157.Fukuda S., Fini C.A., Mabuchi T., Koziol J.A., Eggleston L.L., Jr, del Zoppo G.J. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35(4):998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gonul E., Duz B., Kahraman S., Kayali H., Kubar A., Timurkaynak E. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc. Res. 2002;64(1):116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 159.Yamagishi S., Yonekura H., Yamamoto Y., Fujimori H., Sakurai S., Tanaka N., Yamamoto H. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab. Invest. 1999;79(4):501–509. [PubMed] [Google Scholar]
- 160.Yang S., Jin H., Zhu Y., Wan Y., Opoku E.N., Zhu L., Hu B. Diverse Functions and Mechanisms of Pericytes in Ischemic Stroke. Curr. Neuropharmacol. 2017;15(6):892–905. doi: 10.2174/1570159X15666170112170226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Villaseñor R., Kuennecke B., Ozmen L., Ammann M., Kugler C., Grüninger F., Loetscher H., Freskgård P-O., Collin L. Region-specific permeability of the blood-brain barrier upon pericyte loss. J. Cereb. Blood Flow Metab. 2017;37(12):3683–3694. doi: 10.1177/0271678X17697340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu L., Fujimoto M., Kawakita F., Nakano F., Imanaka-Yoshida K., Yoshida T., Suzuki H. Anti-Vascular Endothelial Growth Factor Treatment Suppresses Early Brain Injury After Subarachnoid Hemorrhage in Mice. Mol. Neurobiol. 2016;53(7):4529–4538. doi: 10.1007/s12035-015-9386-9. [DOI] [PubMed] [Google Scholar]
- 163.Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N., Mahase S., Dutta D.J., Seto J., Kramer E.G., Ferrara N., Sofroniew M.V., John G.R. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122(7):2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee S-C., Lee K-Y., Kim Y-J., Kim S.H., Koh S-H., Lee Y.J. Serum VEGF levels in acute ischaemic strokes are correlated with long-term prognosis. Eur. J. Neurol. 2010;17(1):45–51. doi: 10.1111/j.1468-1331.2009.02731.x. [DOI] [PubMed] [Google Scholar]
- 165.Sobrino T., Arias S., Rodríguez-González R., Brea D., Silva Y., de la Ossa N.P., Agulla J., Blanco M., Pumar J.M., Serena J., Dávalos A., Castillo J. High serum levels of growth factors are associated with good outcome in intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2009;29(12):1968–1974. doi: 10.1038/jcbfm.2009.182. [DOI] [PubMed] [Google Scholar]
- 166.Yu S., Yao S., Wen Y., Wang Y., Wang H., Xu Q. Angiogenic microspheres promote neural regeneration and motor function recovery after spinal cord injury in rats. Sci. Rep. 2016;6:33428. doi: 10.1038/srep33428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hayashi Y., Nomura M., Yamagishi S., Harada S., Yamashita J., Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19(1):13–26. doi: 10.1002/(SICI)1098-1136(199701)19:1<13:AID-GLIA2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 168.Sobue K., Yamamoto N., Yoneda K., Hodgson M.E., Yamashiro K., Tsuruoka N., Tsuda T., Katsuya H., Miura Y., Asai K., Kato T. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci. Res. 1999;35(2):155–164. doi: 10.1016/S0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 169.Ahsan M.S., Yamazaki M., Maruyama S., Kobayashi T., Ida-Yonemochi H., Hasegawa M., Henry Ademola A., Cheng J., Saku T. Differential expression of perlecan receptors, α-dystroglycan and integrin β1, before and after invasion of oral squamous cell carcinoma. J. Oral Pathol. Med. 2011;40(7):552–559. doi: 10.1111/j.1600-0714.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 170.Li W., Pan R., Qi Z., Liu K.J. Current progress in searching for clinically useful biomarkers of blood-brain barrier damage following cerebral ischemia. Brain Circ. 2018;4(4):145–152. doi: 10.4103/bc.bc_11_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Montgomery D.L. Astrocytes: form, functions, and roles in disease. Vet. Pathol. 1994;31(2):145–167. doi: 10.1177/030098589403100201. [DOI] [PubMed] [Google Scholar]
- 173.Willis C.L., Nolan C.C., Reith S.N., Lister T., Prior M.J.W., Guerin C.J., Mavroudis G., Ray D.E. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45(4):325–337. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
- 174.Nawashiro H., Brenner M., Fukui S., Shima K., Hallenbeck J.M. High susceptibility to cerebral ischemia in GFAP-null mice. J. Cereb. Blood Flow Metab. 2000;20(7):1040–1044. doi: 10.1097/00004647-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 175.Argaw A.T., Gurfein B.T., Zhang Y., Zameer A., John G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA. 2009;106(6):1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Argaw A.T., Zhang Y., Snyder B.J., Zhao M-L., Kopp N., Lee S.C., Raine C.S., Brosnan C.F., John G.R. IL-1β regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006;177(8):5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 177.Dobrogowska D.H., Lossinsky A.S., Tarnawski M., Vorbrodt A.W. Increased blood-brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J. Neurocytol. 1998;27(3):163–173. doi: 10.1023/A:1006907608230. [DOI] [PubMed] [Google Scholar]
- 178.Proescholdt M.A., Jacobson S., Tresser N., Oldfield E.H., Merrill M.J. Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J. Neuropathol. Exp. Neurol. 2002;61(10):914–925. doi: 10.1093/jnen/61.10.914. [DOI] [PubMed] [Google Scholar]
- 179.Powell D.W. Barrier function of epithelia. Am. J. Physiol. 1981;241(4):G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 180.Crone C., Christensen O. Electrical resistance of a capillary endothelium. J. Gen. Physiol. 1981;77(4):349–371. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Deli M.A., Abrahám C.S., Kataoka Y., Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Daniels B.P., Cruz-Orengo L., Pasieka T.J., Couraud P-O., Romero I.A., Weksler B., Cooper J.A., Doering T.L., Klein R.S. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J. Neurosci. Methods. 2013;212(1):173–179. doi: 10.1016/j.jneumeth.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Neuhaus J., Risau W., Wolburg H. Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann. N. Y. Acad. Sci. 1991;633:578–580. doi: 10.1111/j.1749-6632.1991.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 184.Tao-Cheng J.H., Nagy Z., Brightman M.W. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J. Neurosci. 1987;7(10):3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lippmann E.S., Azarin S.M., Kay J.E., Nessler R.A., Wilson H.K., Al-Ahmad A., Palecek S.P., Shusta E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012;30(8):783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen S., Einspanier R., Schoen J. Transepithelial electrical resistance (TEER): a functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015;144(5):509–515. doi: 10.1007/s00418-015-1351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Watson P.M.D., Paterson J.C., Thom G., Ginman U., Lundquist S., Webster C.I. Modelling the endothelial blood-CNS barriers: a method for the production of robust in vitro models of the rat blood-brain barrier and blood-spinal cord barrier. BMC Neurosci. 2013;14:59. doi: 10.1186/1471-2202-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Anderson J.M. Molecular structure of tight junctions and their role in epithelial transport. News Physiol. Sci. 2001;16:126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- 189.Lo C.M., Keese C.R., Giaever I. Cell-substrate contact: another factor may influence transepithelial electrical resistance of cell layers cultured on permeable filters. Exp. Cell Res. 1999;250(2):576–580. doi: 10.1006/excr.1999.4538. [DOI] [PubMed] [Google Scholar]
- 190.Bergmann S., Lawler S.E., Qu Y., Fadzen C.M., Wolfe J.M., Regan M.S., Pentelute B.L., Agar N.Y.R., Cho C-F. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018;13(12):2827–2843. doi: 10.1038/s41596-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nagaraja T.N., Keenan K.A., Fenstermacher J.D., Knight R.A. Acute leakage patterns of fluorescent plasma flow markers after transient focal cerebral ischemia suggest large openings in blood-brain barrier. Microcirculation. 2008;15(1):1–14. doi: 10.1080/10739680701409811. [DOI] [PubMed] [Google Scholar]
- 192.Kassner A., Merali Z. Assessment of Blood-Brain Barrier Disruption in Stroke. Stroke. 2015;46(11):3310–3315. doi: 10.1161/STROKEAHA.115.008861. [DOI] [PubMed] [Google Scholar]
- 193.Jin A.Y., Tuor U.I., Rushforth D., Kaur J., Muller R.N., Petterson J.L., Boutry S., Barber P.A. Reduced blood brain barrier breakdown in P-selectin deficient mice following transient ischemic stroke: a future therapeutic target for treatment of stroke. BMC Neurosci. 2010;11:12. doi: 10.1186/1471-2202-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sladojevic N., Stamatovic S.M., Keep R.F., Grailer J.J., Sarma J.V., Ward P.A., Andjelkovic A.V. Inhibition of junctional adhesion molecule-A/LFA interaction attenuates leukocyte trafficking and inflammation in brain ischemia/reperfusion injury. Neurobiol. Dis. 2014;67:57–70. doi: 10.1016/j.nbd.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tso M.K., Macdonald R.L. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl. Stroke Res. 2014;5(2):174–189. doi: 10.1007/s12975-014-0323-4. [DOI] [PubMed] [Google Scholar]
- 196.Zhou Y., Wang Y., Wang J., Anne Stetler R., Yang Q-W. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog. Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 197.Takeshita Y., Ransohoff R.M. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol. Rev. 2012;248(1):228–239. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Coisne C., Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid. Redox Signal. 2011;15:1285–1303. doi: 10.1089/ars.2011.3929. [DOI] [PubMed] [Google Scholar]
- 199.Miah M.K., Chowdhury E.A., Bickel U., Mehvar R. Evaluation of [14C] and [13C]Sucrose as Blood-Brain Barrier Permeability Markers. J. Pharm. Sci. 2017;106(6):1659–1669. doi: 10.1016/j.xphs.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 200.Pfefferkorn T., Rosenberg G.A. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34(8):2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 201.Tibbling G., Link H., Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Invest. 1977;37(5):385–390. doi: 10.3109/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 202.Link H., Tibbling G. Principles of albumin and IgG analyses in neurological disorders. II. Relation of the concentration of the proteins in serum and cerebrospinal fluid. Scand. J. Clin. Lab. Invest. 1977;37(5):391–396. doi: 10.3109/00365517709091497. [DOI] [PubMed] [Google Scholar]
- 203.Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin. Chim. Acta. 2001;310(2):173–186. doi: 10.1016/S0009-8981(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 204.Reiber H. The discrimination between different blood-CSF barrier dysfunctions and inflammatory reactions of the CNS by a recent evaluation graph for the protein profile of cerebrospinal fluid. J. Neurol. 1980;224(2):89–99. doi: 10.1007/BF00313347. [DOI] [PubMed] [Google Scholar]
- 205.Blyth B.J., Farahvar A., He H., Nayak A., Yang C., Shaw G., Bazarian J.J. Elevated serum ubiquitin carboxy-terminal hydrolase L1 is associated with abnormal blood-brain barrier function after traumatic brain injury. J. Neurotrauma. 2011;28(12):2453–2462. doi: 10.1089/neu.2010.1653. [DOI] [PubMed] [Google Scholar]
- 206.Kazmierski R., Michalak S., Wencel-Warot A., Nowinski W.L. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology. 2012;79(16):1677–1685. doi: 10.1212/WNL.0b013e31826e9a83. [DOI] [PubMed] [Google Scholar]
- 207.Blyth B.J., Farhavar A., Gee C., Hawthorn B., He H., Nayak A., Stöcklein V., Bazarian J.J. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J. Neurotrauma. 2009;26(9):1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Castellanos M., Sobrino T., Millán M., García M., Arenillas J., Nombela F., Brea D., Perez de la Ossa N., Serena J., Vivancos J., Castillo J., Dávalos A. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke. 2007;38(6):1855–1859. doi: 10.1161/STROKEAHA.106.481556. [DOI] [PubMed] [Google Scholar]
- 209.Castellanos M., Leira R., Serena J., Blanco M., Pedraza S., Castillo J., Dávalos A. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35(7):1671–1676. doi: 10.1161/01.STR.0000131656.47979.39. [DOI] [PubMed] [Google Scholar]
- 210.Peeyush Kumar T., McBride D.W., Dash P.K., Matsumura K., Rubi A., Blackburn S.L. Endothelial Cell Dysfunction and Injury in Subarachnoid Hemorrhage. Mol. Neurobiol. 2019;56(3):1992–2006. doi: 10.1007/s12035-018-1213-7. [DOI] [PubMed] [Google Scholar]
- 211.Guo Z., Sun X., He Z., Jiang Y., Zhang X., Zhang J.H. Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol. Res. 2010;32(7):715–720. doi: 10.1179/016164109X12478302362491. [DOI] [PubMed] [Google Scholar]
- 212.Chou S.H-Y., Feske S.K., Simmons S.L., Konigsberg R.G.J., Orzell S.C., Marckmann A., Bourget G., Bauer D.J., De Jager P.L., Du R., Arai K., Lo E.H., Ning M.M. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl. Stroke Res. 2011;2(4):600–607. doi: 10.1007/s12975-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Steliga A., Kowiański P., Czuba E., Waśkow M., Moryś J., Lietzau G. Neurovascular Unit as a Source of Ischemic Stroke Biomarkers-Limitations of Experimental Studies and Perspectives for Clinical Application. Transl. Stroke Res. 2019;11(4):553–579. doi: 10.1007/s12975-019-00744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ozkul-Wermester O., Guegan-Massardier E., Triquenot A., Borden A., Perot G., Gérardin E. Increased blood-brain barrier permeability on perfusion computed tomography predicts hemorrhagic transformation in acute ischemic stroke. Eur. Neurol. 2014;72(1-2):45–53. doi: 10.1159/000358297. [DOI] [PubMed] [Google Scholar]
- 215.Hom J., Dankbaar J.W., Soares B.P., Schneider T., Cheng S-C., Bredno J., Lau B.C., Smith W., Dillon W.P., Wintermark M. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am. J. Neuroradiol. 2011;32(1):41–48. doi: 10.3174/ajnr.A2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lansberg M.G., Thijs V.N., Bammer R., Kemp S., Wijman C.A.C., Marks M.P., Albers G.W., DEFUSE Investigators Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38(8):2275–2278. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rebeles F., Fink J., Anzai Y., Maravilla K.R. Blood-brain barrier imaging and therapeutic potentials. Top. Magn. Reson. Imaging. 2006;17(2):107–116. doi: 10.1097/RMR.0b013e31802f5df9. [DOI] [PubMed] [Google Scholar]
- 218.Nagaraja T.N., Knight R.A., Ewing J.R., Karki K., Nagesh V., Fenstermacher J.D. Multiparametric magnetic resonance imaging and repeated measurements of blood-brain barrier permeability to contrast agents. Methods Mol. Biol. 2011;686:193–212. doi: 10.1007/978-1-60761-938-3_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Heye A.K., Culling R.D. Valdés Hernández, Mdel.C.; Thrippleton, M.J.; Wardlaw, J.M. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin. 2014;6:262–274. doi: 10.1016/j.nicl.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sourbron S.P., Buckley D.L. Tracer kinetic modelling in MRI: estimating perfusion and capillary permeability. Phys. Med. Biol. 2012;57(2):R1–R33. doi: 10.1088/0031-9155/57/2/R1. [DOI] [PubMed] [Google Scholar]
- 221.Knight R.A., Nagaraja T.N., Ewing J.R., Nagesh V., Whitton P.A., Bershad E., Fagan S.C., Fenstermacher J.D. Quantitation and localization of blood-to-brain influx by magnetic resonance imaging and quantitative autoradiography in a model of transient focal ischemia. Magn. Reson. Med. 2005;54(4):813–821. doi: 10.1002/mrm.20629. [DOI] [PubMed] [Google Scholar]
- 222.Schellenberg A.E., Buist R., Yong V.W., Del Bigio M.R., Peeling J. Magnetic resonance imaging of blood-spinal cord barrier disruption in mice with experimental autoimmune encephalomyelitis. Magn. Reson. Med. 2007;58(2):298–305. doi: 10.1002/mrm.21289. [DOI] [PubMed] [Google Scholar]
- 223.Warach S., Latour L.L. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35(11) Suppl. 1:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 224.Chassidim Y., Veksler R., Lublinsky S., Pell G.S., Friedman A., Shelef I. Quantitative imaging assessment of blood-brain barrier permeability in humans. Fluids Barriers CNS. 2013;10(1):9. doi: 10.1186/2045-8118-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Roberts C., Issa B., Stone A., Jackson A., Waterton J.C., Parker G.J.M. Comparative study into the robustness of compartmental modeling and model-free analysis in DCE-MRI studies. J. Magn. Reson. Imaging. 2006;23(4):554–563. doi: 10.1002/jmri.20529. [DOI] [PubMed] [Google Scholar]
- 226.Alonzi R., Taylor N.J., Stirling J.J., d’Arcy J.A., Collins D.J., Saunders M.I., Hoskin P.J., Padhani A.R. Reproducibility and correlation between quantitative and semiquantitative dynamic and intrinsic susceptibility-weighted MRI parameters in the benign and malignant human prostate. J. Magn. Reson. Imaging. 2010;32(1):155–164. doi: 10.1002/jmri.22215. [DOI] [PubMed] [Google Scholar]
- 227.Jackson A., Jayson G.C., Li K.L., Zhu X.P., Checkley D.R., Tessier J.J.L., Waterton J.C. Reproducibility of quantitative dynamic contrast-enhanced MRI in newly presenting glioma. Br. J. Radiol. 2003;76(903):153–162. doi: 10.1259/bjr/70653746. [DOI] [PubMed] [Google Scholar]
- 228.Thrippleton M.J., Backes W.H., Sourbron S., Ingrisch M., van Osch M.J.P., Dichgans M., Fazekas F., Ropele S., Frayne R., van Oostenbrugge R.J., Smith E.E., Wardlaw J.M. Quantifying blood-brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimers Dement. 2019;15(6):840–858. doi: 10.1016/j.jalz.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lorberboym M., Lampl Y., Sadeh M. Correlation of 99mTc-DTPA SPECT of the blood-brain barrier with neurologic outcome after acute stroke. J. Nucl. Med. 2003;44(12):1898–1904. [PubMed] [Google Scholar]
- 230.Gilad R., Lampl Y., Eilam A., Boaz M., Loyberboim M. SPECT-DTPA as a tool for evaluating the blood-brain barrier in post-stroke seizures. J. Neurol. 2012;259(10):2041–2044. doi: 10.1007/s00415-012-6445-2. [DOI] [PubMed] [Google Scholar]
- 231.Olsen T.S. Post-stroke epilepsy. Curr. Atheroscler. Rep. 2001;3(4):340–344. doi: 10.1007/s11883-001-0029-4. [DOI] [PubMed] [Google Scholar]
- 232.Barth A., Haldemann A.R., Reubi J.C., Godoy N., Rösler H., Kinser J.A., Seiler R.W. Noninvasive differentiation of meningiomas from other brain tumours using combined 111Indium-octreotide/99mtechnetium-DTPA brain scintigraphy. Acta Neurochir. (Wien) 1996;138(10):1179–1185. doi: 10.1007/BF01809748. [DOI] [PubMed] [Google Scholar]
- 233.Inoue Y., Momose T., Machida K., Honda N., Mamiya T., Takahashi T., Sasaki Y. Delayed imaging of Tc-99m-DTPA-HSA SPECT in subacute cerebral infarction. Radiat. Med. 1993;11(5):214–216. [PubMed] [Google Scholar]
- 234.Shih W.J., Domstad P.A., DeLand F.H. Opportunistic intracranial infection in AIDS detection by technetium-99m DTPA brain scintigraphy. J. Nucl. Med. 1986;27(4):498–501. [PubMed] [Google Scholar]
- 235.Keep R.F., Xiang J., Ennis S.R., Andjelkovic A., Hua Y., Xi G., Hoff J.T. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir. Suppl. (Wien) 2008;105:73–77. doi: 10.1007/978-3-211-09469-3_15. [DOI] [PubMed] [Google Scholar]
- 236.Sun Y., Feng X., Ding Y., Li M., Yao J., Wang L., Gao Z. Phased Treatment Strategies for Cerebral Ischemia Based on Glutamate Receptors. Front. Cell. Neurosci. 2019;13:168. doi: 10.3389/fncel.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jiang X., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., Keep R.F., Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lipton S.A., Rosenberg P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 239.Ankarcrona M., Dypbukt J.M., Bonfoco E., Zhivotovsky B., Orrenius S., Lipton S.A., Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 240.Geraghty J.R., Davis J.L., Testai F.D. Neuroinflammation and Microvascular Dysfunction After Experimental Subarachnoid Hemorrhage: Emerging Components of Early Brain Injury Related to Outcome. Neurocrit. Care. 2019;31(2):373–389. doi: 10.1007/s12028-019-00710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tang D., Kang R., Coyne C.B., Zeh H.J., Lotze M.T. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 2012;249(1):158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sifat A.E., Vaidya B., Abbruscato T.J. Blood-Brain Barrier Protection as a Therapeutic Strategy for Acute Ischemic Stroke. AAPS J. 2017;19(4):957–972. doi: 10.1208/s12248-017-0091-7. [DOI] [PubMed] [Google Scholar]
- 243.Lee C.Z., Xue Z., Zhu Y., Yang G-Y.Y.G-Y., Young W.L. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38(9):2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- 244.Suzuki H., Fujimoto M., Kawakita F., Liu L., Nakatsuka Y., Nakano F., Nishikawa H., Okada T., Kanamaru H., Imanaka-Yoshida K., Yoshida T., Shiba M. Tenascin-C in brain injuries and edema after subarachnoid hemorrhage: Findings from basic and clinical studies. J. Neurosci. Res. 2020;98(1):42–56. doi: 10.1002/jnr.24330. [DOI] [PubMed] [Google Scholar]
- 245.Kanamaru H., Suzuki H. Potential therapeutic molecular targets for blood-brain barrier disruption after subarachnoid hemorrhage. Neural Regen. Res. 2019;14(7):1138–1143. doi: 10.4103/1673-5374.251190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Friedrich V., Flores R., Sehba F.A. Cell death starts early after subarachnoid hemorrhage. Neurosci. Lett. 2012;512(1):6–11. doi: 10.1016/j.neulet.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Suzuki H. What is early brain injury? Transl. Stroke Res. 2015;6(1):1–3. doi: 10.1007/s12975-014-0380-8. [DOI] [PubMed] [Google Scholar]
- 248.Yang G.Y., Betz A.L. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25(8):1658–1664. doi: 10.1161/01.STR.25.8.1658. [DOI] [PubMed] [Google Scholar]
- 249.Li Y., Xu L., Zeng K., Xu Z., Suo D., Peng L., Ren T., Sun Z., Yang W., Jin X., Yang L. Propane-2-sulfonic acid octadec-9-enyl-amide, a novel PPARα/γ dual agonist, protects against ischemia-induced brain damage in mice by inhibiting inflammatory responses. Brain Behav. Immun. 2017;66:289–301. doi: 10.1016/j.bbi.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 250.Krafft P.R., Caner B., Klebe D., Rolland W.B., Tang J., Zhang J.H. PHA-543613 preserves blood-brain barrier integrity after intracerebral hemorrhage in mice. Stroke. 2013;44(6):1743–1747. doi: 10.1161/STROKEAHA.111.000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li Z., Chen X., Zhang X., Ren X., Chen X., Cao J., Zang W., Liu X., Guo F. Small Interfering RNA Targeting Dickkopf-1 Contributes to Neuroprotection After Intracerebral Hemorrhage in Rats. J. Mol. Neurosci. 2017;61(2):279–288. doi: 10.1007/s12031-017-0883-3. [DOI] [PubMed] [Google Scholar]
- 252.Sun H., Tang Y., Guan X., Li L., Wang D. Effects of selective hypothermia on blood-brain barrier integrity and tight junction protein expression levels after intracerebral hemorrhage in rats. Biol. Chem. 2013;394(10):1317–1324. doi: 10.1515/hsz-2013-0142. [DOI] [PubMed] [Google Scholar]
- 253.Wang T., Chen X., Wang Z., Zhang M., Meng H., Gao Y., Luo B., Tao L., Chen Y. Poloxamer-188 can attenuate blood-brain barrier damage to exert neuroprotective effect in mice intracerebral hemorrhage model. J. Mol. Neurosci. 2015;55(1):240–250. doi: 10.1007/s12031-014-0313-8. [DOI] [PubMed] [Google Scholar]
- 254.Wanyong Y., Zefeng T., Xiufeng X., Dawei D., Xiaoyan L., Ying Z., Yaogao F. Tempol alleviates intracerebral hemorrhage-induced brain injury possibly by attenuating nitrative stress. Neuroreport. 2015;26(14):842–849. doi: 10.1097/WNR.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 255.Xie R-X., Li D-W., Liu X-C., Yang M-F., Fang J., Sun B-L., Zhang Z-Y., Yang X-Y. Carnosine Attenuates Brain Oxidative Stress and Apoptosis After Intracerebral Hemorrhage in Rats. Neurochem. Res. 2017;42(2):541–551. doi: 10.1007/s11064-016-2104-9. [DOI] [PubMed] [Google Scholar]
- 256.Yang Y., Zhang Y., Wang Z., Wang S., Gao M., Xu R., Liang C., Zhang H. Attenuation of Acute Phase Injury in Rat Intracranial Hemorrhage by Cerebrolysin that Inhibits Brain Edema and Inflammatory Response. Neurochem. Res. 2016;41(4):748–757. doi: 10.1007/s11064-015-1745-4. [DOI] [PubMed] [Google Scholar]
- 257.Sun Y., Dai M., Wang Y., Wang W., Sun Q., Yang G-Y., Bian L. Neuroprotection and sensorimotor functional improvement by curcumin after intracerebral hemorrhage in mice. J. Neurotrauma. 2011;28(12):2513–2521. doi: 10.1089/neu.2011.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nadeau C.A., Dietrich K., Wilkinson C.M., Crawford A.M., George G.N., Nichol H.K., Colbourne F. Prolonged Blood-Brain Barrier Injury Occurs After Experimental Intracerebral Hemorrhage and Is Not Acutely Associated with Additional Bleeding. Transl. Stroke Res. 2019;10(3):287–297. doi: 10.1007/s12975-018-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Keep R.F., Hua Y., Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fumoto T., Naraoka M., Katagai T., Li Y., Shimamura N., Ohkuma H. The Role of Oxidative Stress in Microvascular Disturbances after Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 2019;10(6):684–694. doi: 10.1007/s12975-018-0685-0. [DOI] [PubMed] [Google Scholar]
- 261.Li Z., Liang G., Ma T., Li J., Wang P., Liu L., Yu B., Liu Y., Xue Y. Blood-brain barrier permeability change and regulation mechanism after subarachnoid hemorrhage. Metab. Brain Dis. 2015;30(2):597–603. doi: 10.1007/s11011-014-9609-1. [DOI] [PubMed] [Google Scholar]
- 262.Suzuki H. Inflammation: a Good Research Target to Improve Outcomes of Poor-Grade Subarachnoid Hemorrhage. Transl. Stroke Res. 2019;10(6):597–600. doi: 10.1007/s12975-019-00713-y. [DOI] [PubMed] [Google Scholar]
- 263.Xi G., Keep R.F., Hoff J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 264.Aronowski J., Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Taylor R.A., Sansing L.H. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin. Dev. Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang J., Doré S. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2007;27(5):894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 267.Venkatesan C., Chrzaszcz M., Choi N., Wainwright M.S. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J. Neuroinflammation. 2010;7:32. doi: 10.1186/1742-2094-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Loane D.J., Byrnes K.R. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wan S., Cheng Y., Jin H., Guo D., Hua Y., Keep R.F., Xi G. Microglia Activation and Polarization After Intracerebral Hemorrhage in Mice: the Role of Protease-Activated Receptor-1. Transl. Stroke Res. 2016;7(6):478–487. doi: 10.1007/s12975-016-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhao H., Garton T., Keep R.F., Hua Y., Xi G. Microglia/Macrophage Polarization After Experimental Intracerebral Hemorrhage. Transl. Stroke Res. 2015;6(6):407–409. doi: 10.1007/s12975-015-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zheng Z.V., Lyu H., Lam S.Y.E., Lam P.K., Poon W.S., Wong G.K.C. The dynamics of microglial polarization reveal the resident neuroinflammatory responses after subarachnoid hemorrhage. Transl. Stroke Res. 2019;11(3):433–449. doi: 10.1007/s12975-019-00728-5. [DOI] [PubMed] [Google Scholar]
- 272.Xue M., Del Bigio M.R. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci. Lett. 2000;283(3):230–232. doi: 10.1016/S0304-3940(00)00971-X. [DOI] [PubMed] [Google Scholar]
- 273.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014;289(51):35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chaudhry S.R., Hafez A., Rezai Jahromi B., Kinfe T.M., Lamprecht A., Niemelä M., Muhammad S. Role of damage associated molecular pattern molecules (DAMPs) in aneurysmal subarachnoid hemorrhage (aSAH). Int. J. Mol. Sci. 2018;19(7):19. doi: 10.3390/ijms19072035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Okada T., Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen. Res. 2017;12(2):193–196. doi: 10.4103/1673-5374.200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen G., Zhang S., Shi J., Ai J., Hang C. Effects of recombinant human erythropoietin (rhEPO) on JAK2/STAT3 pathway and endothelial apoptosis in the rabbit basilar artery after subarachnoid hemorrhage. Cytokine. 2009;45(3):162–168. doi: 10.1016/j.cyto.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 277.Zille M., Ikhsan M., Jiang Y., Lampe J., Wenzel J., Schwaninger M. The impact of endothelial cell death in the brain and its role after stroke: A systematic review. Cell Stress. 2019;3(11):330–347. doi: 10.15698/cst2019.11.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Greenwood J., Heasman S.J., Alvarez J.I., Prat A., Lyck R., Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 2011;37(1):24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 279.Engelhardt B. Immune cell entry into the central nervous system: involvement of adhesion molecules and chemokines. J. Neurol. Sci. 2008;274(1-2):23–26. doi: 10.1016/j.jns.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 280.Moxon-Emre I., Schlichter L.C. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J. Neuropathol. Exp. Neurol. 2011;70(3):218–235. doi: 10.1097/NEN.0b013e31820d94a5. [DOI] [PubMed] [Google Scholar]
- 281.Wasserman J.K., Schlichter L.C. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp. Neurol. 2007;207(2):227–237. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 282.Okada T., Kawakita F., Nishikawa H., Nakano F., Liu L., Suzuki H. Selective Toll-Like Receptor 4 Antagonists Prevent Acute Blood-Brain Barrier Disruption After Subarachnoid Hemorrhage in Mice. Mol. Neurobiol. 2019;56(2):976–985. doi: 10.1007/s12035-018-1145-2. [DOI] [PubMed] [Google Scholar]
- 283.Wang L., Zhang X., Liu L., Cui L., Yang R., Li M., Du W. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. 2010;1321:143–151. doi: 10.1016/j.brainres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 284.Chen G., Shaw M.H., Kim Y-G., Nuñez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 285.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 286.Abderrazak A., Syrovets T., Couchie D., El Hadri K., Friguet B., Simmet T., Rouis M. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Okada T., Suzuki H. Mechanisms of neuroinflammation and inflammatory mediators involved in brain injury following subarachnoid hemorrhage. Histol. Histopathol. 2020;•••:18208. doi: 10.14670/HH-18-208. [DOI] [PubMed] [Google Scholar]
- 288.Di Virgilio F., Dal Ben D., Sarti A.C., Giuliani A.L., Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47(1):15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 289.Monif M., Reid C.A., Powell K.L., Smart M.L., Williams D.A. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J. Neurosci. 2009;29(12):3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tang Y., Illes P. Regulation of adult neural progenitor cell functions by purinergic signaling. Glia. 2017;65(2):213–230. doi: 10.1002/glia.23056. [DOI] [PubMed] [Google Scholar]
- 291.Mariathasan S., Weiss D.S., Newton K., McBride J., O’Rourke K., Roose-Girma M., Lee W.P., Weinrauch Y., Monack D.M., Dixit V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 292.Yamasaki K., Muto J., Taylor K.R., Cogen A.L., Audish D., Bertin J., Grant E.P., Coyle A.J., Misaghi A., Hoffman H.M., Gallo R.L. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 2009;284(19):12762–12771. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chen S., Ma Q., Krafft P.R., Hu Q., Rolland W., II, Sherchan P., Zhang J., Tang J., Zhang J.H. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol. Dis. 2013;58:296–307. doi: 10.1016/j.nbd.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Khalafalla M.G., Woods L.T., Camden J.M., Khan A.A., Limesand K.H., Petris M.J., Erb L., Weisman G.A. P2X7 receptor antagonism prevents IL-1β release from salivary epithelial cells and reduces inflammation in a mouse model of autoimmune exocrinopathy. J. Biol. Chem. 2017;292(40):16626–16637. doi: 10.1074/jbc.M117.790741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lister M.F., Sharkey J., Sawatzky D.A., Hodgkiss J.P., Davidson D.J., Rossi A.G., Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J. Inflamm. (Lond.) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chen S., Ma Q., Krafft P.R., Chen Y., Tang J., Zhang J., Zhang J.H. P2X7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Crit. Care Med. 2013;41(12):e466–e474. doi: 10.1097/CCM.0b013e31829a8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Luo Y., Lu J., Ruan W., Guo X., Chen S. MCC950 attenuated early brain injury by suppressing NLRP3 inflammasome after experimental SAH in rats. Brain Res. Bull. 2019;146:320–326. doi: 10.1016/j.brainresbull.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 299.Cao G., Jiang N., Hu Y., Zhang Y., Wang G., Yin M., Ma X., Zhou K., Qi J., Yu B., Kou J. Ruscogenin Attenuates Cerebral Ischemia-Induced Blood-Brain Barrier Dysfunction by Suppressing TXNIP/NLRP3 Inflammasome Activation and the MAPK Pathway. Int. J. Mol. Sci. 2016;17(9):1418. doi: 10.3390/ijms17091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ren H., Kong Y., Liu Z., Zang D., Yang X., Wood K., Li M., Liu Q. Selective NLRP3 (Pyrin Domain-Containing Protein 3) Inflammasome Inhibitor Reduces Brain Injury After Intracerebral Hemorrhage. Stroke. 2018;49(1):184–192. doi: 10.1161/STROKEAHA.117.018904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bianchi R., Kastrisianaki E., Giambanco I., Donato R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J. Biol. Chem. 2011;286(9):7214–7226. doi: 10.1074/jbc.M110.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lee E.J., Park J.H. Receptor for Advanced Glycation Endproducts (RAGE), Its Ligands, and Soluble RAGE: Potential Biomarkers for Diagnosis and Therapeutic Targets for Human Renal Diseases. Genomics Inform. 2013;11(4):224–229. doi: 10.5808/GI.2013.11.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rani S.G., Sepuru K.M., Yu C. Interaction of S100A13 with C2 domain of receptor for advanced glycation end products (RAGE). Biochim. Biophys. Acta. 2014;1844(9):1718–1728. doi: 10.1016/j.bbapap.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 304.Rovere-Querini P., Capobianco A., Scaffidi P., Valentinis B., Catalanotti F., Giazzon M., Dumitriu I.E., Müller S., Iannacone M., Traversari C., Bianchi M.E., Manfredi A.A. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5(8):825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kim S-W., Lim C-M., Kim J-B., Shin J-H., Lee S., Lee M., Lee J-K. Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox. Res. 2011;20(2):159–169. doi: 10.1007/s12640-010-9231-x. [DOI] [PubMed] [Google Scholar]
- 306.Sparvero L.J., Asafu-Adjei D., Kang R., Tang D., Amin N. Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; Lotze, M.T. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wang K-C., Tang S-C., Lee J-E., Li Y-I., Huang Y-S., Yang W-S., Jeng J-S., Arumugam T.V., Tu Y-K. Cerebrospinal fluid high mobility group box 1 is associated with neuronal death in subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2017;37(2):435–443. doi: 10.1177/0271678X16629484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Taylor R.A., Chang C-F., Goods B.A., Hammond M.D., Mac Grory B., Ai Y., Steinschneider A.F., Renfroe S.C., Askenase M.H., McCullough L.D., Kasner S.E., Mullen M.T., Hafler D.A., Love J.C., Sansing L.H. TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Invest. 2017;127(1):280–292. doi: 10.1172/JCI88647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hua Y., Wu J., Keep R.F., Nakamura T., Hoff J.T., Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58(3):542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. [DOI] [PubMed] [Google Scholar]
- 310.Shen Y., Gu J., Liu Z., Xu C., Qian S., Zhang X., Zhou B., Guan Q., Sun Y., Wang Y., Jin X. Inhibition of HIF-1α Reduced Blood Brain Barrier Damage by Regulating MMP-2 and VEGF During Acute Cerebral Ischemia. Front. Cell. Neurosci. 2018;12:288. doi: 10.3389/fncel.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yang Y., Rosenberg G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Könnecke H., Bechmann I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin. Dev. Immunol. 2013;2013:914104. doi: 10.1155/2013/914104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhao L-R., Navalitloha Y., Singhal S., Mehta J., Piao C-S., Guo W-P., Kessler J.A., Groothuis D.R. Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Exp. Neurol. 2007;204(2):569–573. doi: 10.1016/j.expneurol.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Xue M., Yong V.W. Matrix metalloproteinases in intracerebral hemorrhage. Neurol. Res. 2008;30(8):775–782. doi: 10.1179/174313208X341102. [DOI] [PubMed] [Google Scholar]
- 315.Zhang Z., Yan J., Shi H. Role of Hypoxia Inducible Factor 1 in Hyperglycemia-Exacerbated Blood-Brain Barrier Disruption in Ischemic Stroke. Neurobiol. Dis. 2016;95:82–92. doi: 10.1016/j.nbd.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chen W., Jadhav V., Tang J., Zhang J.H. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiol. Dis. 2008;31(3):433–441. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yang Y., Estrada E.Y., Thompson J.F., Liu W., Rosenberg G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 318.Yang Y., Rosenberg G.A. MMP-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol. Biol. 2011;762:333–345. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhang S., An Q., Wang T., Gao S., Zhou G. Autophagy- and MMP-2/9-mediated Reduction and Redistribution of ZO-1 Contribute to Hyperglycemia-increased Blood-Brain Barrier Permeability During Early Reperfusion in Stroke. Neuroscience. 2018;377:126–137. doi: 10.1016/j.neuroscience.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 320.Kumari R., Willing L.B., Patel S.D., Baskerville K.A., Simpson I.A. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J. Neurochem. 2011;119(5):1029–1040. doi: 10.1111/j.1471-4159.2011.07487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lakhan S.E., Kirchgessner A., Tepper D., Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front. Neurol. 2013;4:32. doi: 10.3389/fneur.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Liu J., Jin X., Liu K.J., Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. 2012;32(9):3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lischper M., Beuck S., Thanabalasundaram G., Pieper C., Galla H-J. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010;1326:114–127. doi: 10.1016/j.brainres.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 324.Li Y., Zhong W., Jiang Z., Tang X. New progress in the approaches for blood-brain barrier protection in acute ischemic stroke. Brain Res. Bull. 2019;144:46–57. doi: 10.1016/j.brainresbull.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 325.Wang J., Tsirka S.E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128(Pt 7):1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 326.Xue M., Hollenberg M.D., Demchuk A., Yong V.W. Relative importance of proteinase-activated receptor-1 versus matrix metalloproteinases in intracerebral hemorrhage-mediated neurotoxicity in mice. Stroke. 2009;40(6):2199–2204. doi: 10.1161/STROKEAHA.108.540393. [DOI] [PubMed] [Google Scholar]
- 327.Rosenberg G.A., Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48(4):921–926. doi: 10.1212/WNL.48.4.921. [DOI] [PubMed] [Google Scholar]
- 328.Wells J.E.A., Biernaskie J., Szymanska A., Larsen P.H., Yong V.W., Corbett D. Matrix metalloproteinase (MMP)-12 expression has a negative impact on sensorimotor function following intracerebral haemorrhage in mice. Eur. J. Neurosci. 2005;21(1):187–196. doi: 10.1111/j.1460-9568.2004.03829.x. [DOI] [PubMed] [Google Scholar]
- 329.Matsukawa N., Yasuhara T., Hara K., Xu L., Maki M., Yu G., Kaneko Y., Ojika K., Hess D.C., Borlongan C.V. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zabad R.K., Metz L.M., Todoruk T.R., Zhang Y., Mitchell J.R., Yeung M., Patry D.G., Bell R.B., Yong V.W. The clinical response to minocycline in multiple sclerosis is accompanied by beneficial immune changes: a pilot study. Mult. Scler. 2007;13(4):517–526. doi: 10.1177/1352458506070319. [DOI] [PubMed] [Google Scholar]
- 331.Grossetete M., Rosenberg G.A. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J. Cereb. Blood Flow Metab. 2008;28(4):752–763. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- 332.Florczak-Rzepka M., Grond-Ginsbach C., Montaner J., Steiner T. Matrix metalloproteinases in human spontaneous intracerebral hemorrhage: an update. Cerebrovasc. Dis. 2012;34(4):249–262. doi: 10.1159/000341686. [DOI] [PubMed] [Google Scholar]
- 333.Brouns R., Wauters A., De Surgeloose D., Mariën P., De Deyn P.P. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur. Neurol. 2011;65(1):23–31. doi: 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- 334.Amantea D., Nappi G., Bernardi G., Bagetta G., Corasaniti M.T. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276(1):13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- 335.Lee J.E., Yoon Y.J., Moseley M.E., Yenari M.A. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J. Neurosurg. 2005;103(2):289–297. doi: 10.3171/jns.2005.103.2.0289. [DOI] [PubMed] [Google Scholar]
- 336.Park K-P., Rosell A., Foerch C., Xing C., Kim W.J., Lee S., Opdenakker G., Furie K.L., Lo E.H. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009;40(8):2836–2842. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Reuter B., Rodemer C., Grudzenski S., Meairs S., Bugert P., Hennerici M.G., Fatar M. Effect of simvastatin on MMPs and TIMPs in human brain endothelial cells and experimental stroke. Transl. Stroke Res. 2015;6(2):156–159. doi: 10.1007/s12975-014-0381-7. [DOI] [PubMed] [Google Scholar]
- 338.Lu A., Suofu Y., Guan F., Broderick J.P., Wagner K.R., Clark J.F. Matrix metalloproteinase-2 deletions protect against hemorrhagic transformation after 1 h of cerebral ischemia and 23 h of reperfusion. Neuroscience. 2013;253:361–367. doi: 10.1016/j.neuroscience.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Suofu Y., Clark J.F., Broderick J.P., Kurosawa Y., Wagner K.R., Lu A. Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience. 2012;212:180–189. doi: 10.1016/j.neuroscience.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Asahi M., Sumii T., Fini M.E., Itohara S., Lo E.H. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12(13):3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- 341.Clark A.W., Krekoski C.A., Bou S.S., Chapman K.R., Edwards D.R. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci. Lett. 1997;238(1-2):53–56. doi: 10.1016/S0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 342.Li N., Liu Y.F., Ma L., Worthmann H., Wang Y.L., Wang Y.J., Gao Y.P., Raab P., Dengler R., Weissenborn K., Zhao X.Q. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke. 2013;44(3):658–663. doi: 10.1161/STROKEAHA.112.673590. [DOI] [PubMed] [Google Scholar]
- 343.Shi W., Wang Z., Pu J., Wang R., Guo Z., Liu C., Sun J., Gao L., Zhou R. Changes of blood-brain barrier permeability following intracerebral hemorrhage and the therapeutic effect of minocycline in rats. Acta Neurochir. Suppl. (Wien) 2011;110(Pt 2):61–67. doi: 10.1007/978-3-7091-0356-2_12. [DOI] [PubMed] [Google Scholar]
- 344.Orihuela R., McPherson C.A., Harry G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016;173(4):649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lobo-Silva D., Carriche G.M., Castro A.G., Roque S., Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflammation. 2016;13(1):297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Holtman I.R., Skola D., Glass C.K. Transcriptional control of microglia phenotypes in health and disease. J. Clin. Invest. 2017;127(9):3220–3229. doi: 10.1172/JCI90604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Jiang C.T., Wu W.F., Deng Y.H., Ge J.W. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep., 2020, 21(5), 2006. RE:view. 2018 doi: 10.3892/mmr.2020.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Fumagalli S., Fiordaliso F., Perego C., Corbelli A., Mariani A., De Paola M., De Simoni M-G. The phagocytic state of brain myeloid cells after ischemia revealed by superresolution structured illumination microscopy. J. Neuroinflammation. 2019;16(1):9. doi: 10.1186/s12974-019-1401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kanazawa M., Ninomiya I., Hatakeyama M., Takahashi T., Shimohata T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017;18(10):2135. doi: 10.3390/ijms18102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zhao S.C., Ma L.S., Chu Z.H., Xu H., Wu W.Q., Liu F. Regulation of microglial activation in stroke. Acta Pharmacol. Sin. 2017;38(4):445–458. doi: 10.1038/aps.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Zhao X., Zhang Y., Strong R., Grotta J.C., Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-γ, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J. Cereb. Blood Flow Metab. 2006;26(6):811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- 352.Zhao X., Grotta J., Gonzales N., Aronowski J. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke. 2009;40(3) Suppl.:S92–S94. doi: 10.1161/STROKEAHA.108.533158. [DOI] [PubMed] [Google Scholar]
- 353.Neher M.D., Weckbach S., Huber-Lang M.S., Stahel P.F. New insights into the role of peroxisome proliferator-activated receptors in regulating the inflammatory response after tissue injury. PPAR Res. 2012;2012:728461. doi: 10.1155/2012/728461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Al Ahmad A., Gassmann M., Ogunshola O.O. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J. Cell. Physiol. 2009;218(3):612–622. doi: 10.1002/jcp.21638. [DOI] [PubMed] [Google Scholar]
- 355.Hayashi K., Nakao S., Nakaoke R., Nakagawa S., Kitagawa N., Niwa M. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul. Pept. 2004;123(1-3):77–83. doi: 10.1016/j.regpep.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 356.Yemisci M., Gursoy-Ozdemir Y., Vural A., Can A., Topalkara K., Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009;15(9):1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 357.Nishioku T., Dohgu S., Takata F., Eto T., Ishikawa N., Kodama K.B., Nakagawa S., Yamauchi A., Kataoka Y. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell. Mol. Neurobiol. 2009;29(3):309–316. doi: 10.1007/s10571-008-9322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Jung K-H., Chu K., Lee S-T., Bahn J-J., Jeon D., Kim J-H., Kim S., Won C-H., Kim M., Lee S.K., Roh J-K. Multipotent PDGFRβ-expressing cells in the circulation of stroke patients. Neurobiol. Dis. 2011;41(2):489–497. doi: 10.1016/j.nbd.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 359.Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., O’Farrell F.M., Buchan A.M., Lauritzen M., Attwell D., O’Farrell F.M., Buchan A.M., Lauritzen M., Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nahirney P.C., Reeson P., Brown C.E. Ultrastructural analysis of blood-brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J. Cereb. Blood Flow Metab. 2016;36(2):413–425. doi: 10.1177/0271678X15608396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Renner O., Tsimpas A., Kostin S., Valable S., Petit E., Schaper W., Marti H.H. Time- and cell type-specific induction of platelet-derived growth factor receptor-β during cerebral ischemia. Brain Res. Mol. Brain Res. 2003;113(1-2):44–51. doi: 10.1016/S0169-328X(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 362.Begum G., Song S., Wang S., Zhao H., Bhuiyan M.I.H., Li E., Nepomuceno R., Ye Q., Sun M., Calderon M.J., Stolz D.B., St Croix C., Watkins S.C., Chen Y., He P., Shull G.E., Sun D. Selective knockout of astrocytic Na+ /H+ exchanger isoform 1 reduces astrogliosis, BBB damage, infarction, and improves neurological function after ischemic stroke. Glia. 2018;66(1):126–144. doi: 10.1002/glia.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Chiu C-D., Yao N-W., Guo J-H., Shen C-C., Lee H-T., Chiu Y-P., Ji H-R., Chen X., Chen C-C., Chang C. Inhibition of astrocytic activity alleviates sequela in acute stages of intracerebral hemorrhage. Oncotarget. 2017;8(55):94850–94861. doi: 10.18632/oncotarget.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Michinaga S., Koyama Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int. J. Mol. Sci. 2019;20(3):571. doi: 10.3390/ijms20030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Golledge J., Clancy P., Maguire J., Lincz L., Koblar S., McEvoy M., Attia J., Levi C., Sturm J., Almeida O.P., Yeap B.B., Flicker L., Norman P.E., Hankey G.J. Plasma angiopoietin-1 is lower after ischemic stroke and associated with major disability but not stroke incidence. Stroke. 2014;45(4):1064–1068. doi: 10.1161/STROKEAHA.113.004339. [DOI] [PubMed] [Google Scholar]
- 366.Kawakita F., Kanamaru H., Asada R., Suzuki H. Potential roles of matricellular proteins in stroke. Exp. Neurol. 2019;322:113057. doi: 10.1016/j.expneurol.2019.113057. [DOI] [PubMed] [Google Scholar]
- 367.Murphy-Ullrich J.E., Sage E.H. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Fujimoto M., Shiba M., Kawakita F., Liu L., Shimojo N., Imanaka-Yoshida K., Yoshida T., Suzuki H. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J. Neurosurg. 2016;124(6):1693–1702. doi: 10.3171/2015.4.JNS15484. [DOI] [PubMed] [Google Scholar]
- 369.Liu L., Kawakita F., Fujimoto M., Nakano F., Imanaka-Yoshida K., Yoshida T., Suzuki H. Role of Periostin in Early Brain Injury After Subarachnoid Hemorrhage in Mice. Stroke. 2017;48(4):1108–1111. doi: 10.1161/STROKEAHA.117.016629. [DOI] [PubMed] [Google Scholar]
- 370.Nishikawa H., Suzuki H. Implications of periostin in the development of subarachnoid hemorrhage-induced brain injuries. Neural Regen. Res. 2017;12(12):1982–1984. doi: 10.4103/1673-5374.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Shiba M., Suzuki H. Lessons from tenascin-C knockout mice and potential clinical application to subarachnoid hemorrhage. Neural Regen. Res. 2019;14(2):262–264. doi: 10.4103/1673-5374.244789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Nishikawa H., Suzuki H. Possible Role of Inflammation and Galectin-3 in Brain Injury after Subarachnoid Hemorrhage. Brain Sci. 2018;8(2):8. doi: 10.3390/brainsci8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Nishikawa H., Liu L., Nakano F., Kawakita F., Kanamaru H., Nakatsuka Y., Okada T., Suzuki H. Modified citrus pectin prevents blood-brain barrier disruption in mouse Subarachnoid hemorrhage by inhibiting Galectin-3. Stroke. 2018;49(11):2743–2751. doi: 10.1161/STROKEAHA.118.021757. [DOI] [PubMed] [Google Scholar]
- 374.Kawakita F., Suzuki H. Periostin in cerebrovascular disease. Neural Regen. Res. 2020;15(1):63–64. doi: 10.4103/1673-5374.264456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Suzuki H., Ayer R., Sugawara T., Chen W., Sozen T., Hasegawa Y., Kanamaru K., Zhang J.H. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit. Care Med. 2010;38(2):612–618. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zhang W., Zhu L., An C., Wang R., Yang L., Yu W., Li P., Gao Y. The blood brain barrier in cerebral ischemic injury – Disruption and repair. Brain Hemorrhages. 2020;1:34–53. doi: 10.1016/j.hest.2019.12.004. [DOI] [Google Scholar]
- 377.Gliem M., Krammes K., Liaw L., van Rooijen N., Hartung H-P., Jander S. Macrophage-derived osteopontin induces reactive astrocyte polarization and promotes re-establishment of the blood brain barrier after ischemic stroke. Glia. 2015;63(12):2198–2207. doi: 10.1002/glia.22885. [DOI] [PubMed] [Google Scholar]
- 378.Yang Y., Yang L.Y., Orban L., Cuylear D., Thompson J., Simon B., Yang Y. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. 2018;11(4):689–698. doi: 10.1016/j.brs.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Huang L., Cao W., Deng Y., Zhu G., Han Y., Zeng H. Hypertonic saline alleviates experimentally induced cerebral oedema through suppression of vascular endothelial growth factor and its receptor VEGFR2 expression in astrocytes. BMC Neurosci. 2016;17(1):64. doi: 10.1186/s12868-016-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Cao C., Yu X., Liao Z., Zhu N., Huo H., Wang M., Ji G., She H., Luo Z., Yue S. Hypertonic saline reduces lipopolysaccharide-induced mouse brain edema through inhibiting aquaporin 4 expression. Crit. Care. 2012;16(5):R186. doi: 10.1186/cc11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Oklinski M.K., Skowronski M.T., Skowronska A., Rützler M., Nørgaard K., Nieland J.D., Kwon T-H., Nielsen S. Aquaporins in the Spinal Cord. Int. J. Mol. Sci. 2016;17(12):2050. doi: 10.3390/ijms17122050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Bonomini F., Rezzani R. Aquaporin and blood brain barrier. Curr. Neuropharmacol. 2010;8(2):92–96. doi: 10.2174/157015910791233132. [DOI] [PMC free article] [PubMed] [Google Scholar]