Abstract
Subarachnoid hemorrhage (SAH) is a type of hemorrhagic stroke associated with high mortality and morbidity. The blood-brain-barrier (BBB) is a structure consisting primarily of cerebral microvascular endothelial cells, end feet of astrocytes, extracellular matrix, and pericytes. Post-SAH pathophysiology included early brain injury and delayed cerebral ischemia. BBB disruption was a critical mechanism of early brain injury and was associated with other pathophysiological events. These pathophysiological events may propel the development of secondary brain injury, known as delayed cerebral ischemia. Imaging advancements to measure BBB after SAH primarily focused on exploring innovative methods to predict clinical outcome, delayed cerebral ischemia, and delayed infarction related to delayed cerebral ischemia in acute periods. These predictions are based on detecting abnormal changes in BBB permeability. The parameters of BBB permeability are described by changes in computed tomography (CT) perfusion and magnetic resonance imaging (MRI). Kep seems to be a stable and sensitive indicator in CT perfusion, whereas Ktrans is a reliable parameter for dynamic contrast-enhanced MRI. Future prediction models that utilize both the volume of BBB disruption and stable parameters of BBB may be a promising direction to develop practical clinical tools. These tools could provide greater accuracy in predicting clinical outcome and risk of deterioration. Therapeutic interventional exploration targeting BBB disruption is also promising, considering the extended duration of post-SAH BBB disruption.
Keywords: Subarachnoid hemorrhage, blood brain barrier, imaging, clinical trial, early brain injury, delayed cerebral ischemia
1. INTRODUCTION
Subarachnoid hemorrhage (SAH) is a type of hemorrhagic stroke with high mortality and morbidity [1]. Although mortality has reportedly decreased in recent decades [2], survivors of SAH are commonly left with severe disabilities, cognitive deficits, and mental problems [3]. SAH is an important public health concern that warrants further exploration of diagnostic methods, biopathological mechanisms, and therapeutic targets to achieve better outcomes for patients. The pathophysiological events after SAH were divided into early brain injury and delayed cerebral ischemia. Although consensus has not been reached regarding a uniform definition of early brain injury, early brain injury was defined roughly as any type of pathophysiological event that occurs within 72h, other than iatrogenic brain injury, which induces injury to the brain immediately [4]. The main components of early brain injury include intracranial hypertension, cell apoptosis, hydrocephalus, blood-brain barrier (BBB) dysfunction, loss of autoregulation, and cerebral edema.
Delayed cerebral ischemia is defined as angiographic vasospasm that lasts for at least 2 hours, and is associated with the decline of neurological function. Moreover, delayed cerebral ischemia reportedly affects 20-30% of SAH patients [5, 6]. It was reportedly the most important independent predictive factor of poor outcome and cognitive deficits after SAH [7, 8]. The BBB is a structure primarily consisting of cerebral microvascular endothelial cells, pericytes, extracellular matrix, and the end feet of astrocytes Fig. (1). The BBB primarily functions to maintain brain homeostasis by preventing the entry of neurotoxic plasma components, blood cells, and pathogens from the peripheral blood [9]. An experimental SAH model has shown that a significant increase in BBB permeability can be observed at 24-36 hours, peaking at 48 hours, and normalizing on day 3. Several preclinical SAH studies have demonstrated functional disruption in the BBB [10], and it was associated with the impairment of the basal lamina and microvasculature [11]. Recent studies aim to prevent delayed cerebral ischemia by using vasospasm-reducing drugs, such as clazosentan. However, they failed to improve the clinical outcomes [12, 13]. Moreover, there are fewer studies investigating post-SAH BBB breakdown compared with vasospasm [14]. This review aimed to summarize the latest advances in clinical trials regarding SAH, as well as the progress made in detecting post-SAH changes in BBB permeability on imaging. Lastly, the important role of BBB disruption is emphasized and the recommended direction for future studies is discussed.
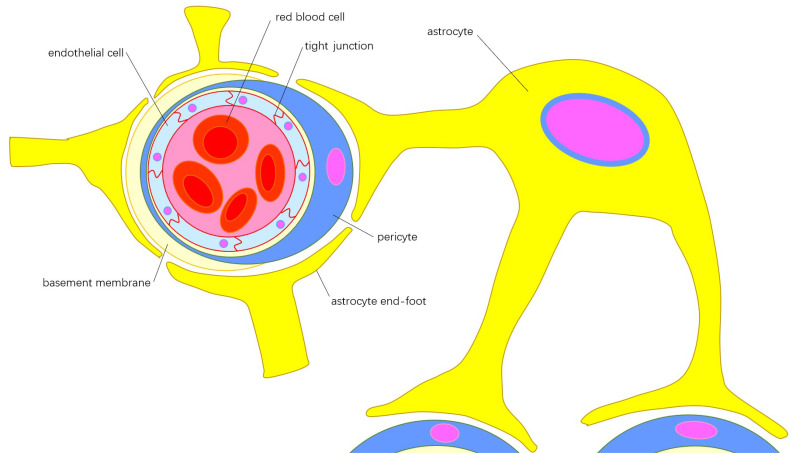
Fig. (1).
Components of blood brain barrier: Cerebral microvascular endothelial cells connected by tight junctions form the internal layer of the microvessel wall, which is embedded in the extracellular matrix, known as the basement membrane. Pericytes insert into the basement membrane and encircle the internal layer of microvessel wall and part of the basement membrane. The end-feet from different astrocytes cover the surface of pericytes, forming the outer layer of the microvessel wall. The functions of endothelial cells and astrocytes can be integrated by the pericyte though polarization of the astrocytic end-feet. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2. SUBARACHNOID HEMORRHAGE (SAH)
SAH refers to bleeding within the subarachnoid space, which exists between the arachnoid and pia, and is filled with cerebrospinal fluid [15]. Survivors of SAH often endure numerous ongoing complications, including disabilities, cognitive disorders, and psychological problems [16]. SAH accounts for 3% of all strokes, and is most commonly caused by a ruptured aneurysm [17]. Early brain injury was first described in 2004, and is increasingly considered a critical factor in the development of delayed cerebral ischemia and prediction of unfavorable outcomes [18, 19]. Early brain injury include acute cerebral ischemia, energy dysfunction following cortical spreading depolarizations, mitochondrial dysfunction, etc [19, 20]. In recent decades, vasospasm has been considered the primary culprit of delayed cerebral ischemia [21]. However, delayed cerebral ischemia is now attributed to cerebral vasospasm, micro thombosis, and cortical spreading depolarization [22]. Moreover, delayed cerebral ischemia was the result of these independent courses acting in concert [23]. Delayed cerebral ischemia can occur in 30% of SAH patients. The current standard prophylactic intervention for delayed cerebral ischemia is limited to oral nimodipine and maintaining euvolemia and normal circulating volume [24] Fig. (2).
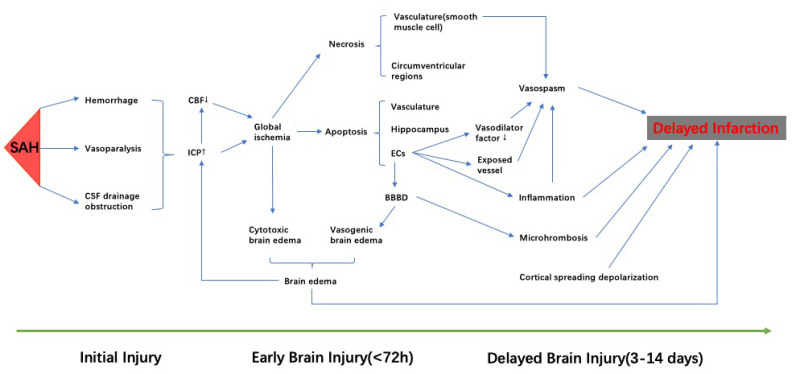
Fig. (2).
The pathophysiological events occurred after SAH. Initial injury damages brain. Increased ICP and decreased CBF leads to global ischemia, which contributes to the cytotoxic brain edema. Cell apoptosis, resulting from ischemic damage, can be observed in the vasculature, hippocampus, and blood brain barrier, and was the major cell death after SAH. Apoptosis of endothelial cells results in disruption of blood-brain barrier, which contributes directly to vasogenic brain edema. Brain edema, including cytotoxic and vasogenic, aggressively increases ICP. Apoptosis of endothelial cells reduces the secretion of vasodilation factors and exposes the vessel to vasoactive and toxic metabolites, which aggravates vasospasm. Necrosis is not the primary route of cell death in SAH, but the necrosis of smooth muscle cells partially contributes to vasospasm. Cortical spreading depolarization, microthrombosis, inflammation, brain edema, and vasospasm are thought to be a part of delayed brain injury, and may develop to delayed infarction. SAH: subarachnoid hemorrhage; CBF: cerebral blood flow; ICP: intracranial pressure. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. BLOOD BRAIN BARRIER IN THE PATHOPHYSIOLOGY OF SAH
BBB maintains brain homeostasis by preventing the entry of potentially toxic molecules from the peripheral blood. BBB dysfunction reportedly occurs in numerous neurological disorders, including stroke, traumatic brain injury, intracerebral hemorrhage, tumors, and neurological disorders [9, 25]. It consists of endothelial cells, basement membrane, pericytes, and astrocytic end-feet. Endothelial cells, which comprise the BBB, differ from peripheral vessels in several ways, such as tight junction proteins, adherens junction proteins, fewer fenestrations, increased mitochondrial content, and pinocytosis [10, 14]. Tight junction proteins, such as zona occludens-1 and zona occludens-2, connect endothelial cells, forming a barrier that restricts the communication of water-soluble substances between the blood and the brain [26]. The functions of endothelial cells and astrocytes can be integrated by pericytes through the polarization of astrocytic end-feet. Pericyte-derived laminin also plays an essential role in maintaining BBB integrity [27, 28]. Pericytes may be associated with ischemic events through their effects on BBB integrity [29]. Regarding endothelial cells, the pathophysiological events after SAH include the contraction, apoptosis, and the inflammatory response. These pathophysiological events reportedly contribute to BBB disruption, indicating the importance of endothelial impairment in BBB breakdown [14, 30]. While microglia are not a component of the BBB, these cells can modulate their influence on the BBB dynamically in central nervous system disorders [31]. According to recent reports, the microglial effects exerted on BBB integrity are protective at the very beginning. However, the persistent existence of infection and inflammation alters the predominant microglial phenotype, causing damage to BBB integrity [32]. Moreover, there has not been a confirmation as to when the interaction between these components change after SAH, nor has there been confirmation regarding the exact time course of BBB disruption. Some studies report that the significant increase in BBB permeability can be observed at 24-36 hours, peaking at 48 hours, and returning to baseline on 72 hours [33]. Recent studies report that white matter T2-hyperintensities can be detected on MRI in mice at 4 hours post-SAH. These white matter T2-hyperintensities correlated with the area of albumin leakage caused by BBB disruption, suggesting that BBB disruption occurred in the acute phase after SAH [34, 35]. There is also evidence that shows that BBB disruption can be observed as early as 30 minutes with two peaks: occurring 3 hours and again at 72 hours after onset [36]. The time course of BBB disruption still cannot be confirmed by these studies, but it is obvious that BBB disruption is a pathophysiological change that occurs very early and lasts for an extended period. It is associated with brain edema [37], thrombosis [38], inflammation [39], and other pathophysiological events that may propel the development of delayed cerebral ischemia [40, 41]. The causality between early brain injury and delayed cerebral ischemia has been widely accepted [42]. BBB disruption plays a critical role as a bridge connecting early brain injury and delayed cerebral ischemia.
4. LATEST ADVANCEMENTS IN CLINICAL TRIALS
The latest clinical trials focus on the treatment and prevention of delayed cerebral ischemia. Pharmacological agents, including cilostazol, dantrolene, epoprostenol, and simvastatin, have been studied. The feasibility of epoprostenol was preliminarily demonstrated, whereas the efficacy still requires further confirmation. Research on intraventricular nimodipine reveals a potential way to achieve better clinical efficacy, as well as fewer side effects [43]. Application of induced hypertension failed and may even lead to serious adverse events [44, 45].
Cortical spreading depolarization could be induced and may invade normal brain tissue in many detrimental conditions, such as ischemia, hypoxia, and status epilepticus. In addition, electrophysiological evidence proved that cortical spreading depolarization could also be recorded in patients with aneurysmal subarachnoid hemorrhage [46]. Resistance vessels induced by cortical spreading depolarization can cause abnormal perfusion. In regions with low perfusion levels, such as the ischemic penumbra, this abnormal perfusion contributes to lesion progression [46]. Cilostazol, an antiplatelet drug and inhibitor of phosphodiesterase-3, reduced delayed cerebral ischemia in a recent double-blind, randomized controlled trial, but did not decrease cerebral vasospasm [47]. Previous studies have shown that cilostazol can reduce cerebral vasospasm and delayed cerebral ischemia, but can also improve poor outcomes after SAH [48]. Recently, a randomized controlled trial was carried out in Japan. This trial aimed to study this medicine from a new perspective, through which cilostazol could alleviate delayed cerebral ischemia by decreasing cortical spreading depolarization [49]. The clinical trial showed that cilostazol treatment did not significantly decrease the occurrence of delayed cerebral ischemia in SAH patients with clip ligation, which does not coincide with previous studies. This result may be due to the small sample size, which did not meet the due volume in the initial design. However, shorter durations of depression and lower occurrence of isoelectric cortical spreading depolarization were observed in the cilostazol group. Since cilostazol’s abilities to reduce delayed cerebral ischemia and improve poor outcomes have been proven by many previous convincing studies, this trial is a beneficial exploration of the mechanism behind this therapeutic method. Further studies with larger samples that target this mechanism will assist us in better understanding the effects of cilostazol and the pathophysiological changes after SAH.
A sustained increase in intracellular Ca2+ levels in vascular smooth muscle cells is characteristic of vasospasm following SAH. This ionic disturbance is mediated by the ryanodine receptor [50]. Dantrolene is a ryanodine receptor blocker with a neuroprotective effect [51]. In a previous SAH animal experiment, dantrolene showed not only a synergistic inhibition on vasospasm with nimodipine, but also suppressed cerebral vasoconstriction alone [52]. Therefore, there are high expectations regarding dantrolene and its efficacy in improving clinical outcomes after SAH. A previous pilot study reported that dantrolene might reduce cerebral vasospasm after SAH with a single dose of intravenous injection. Moreover, the feasibility of this treatment was preliminarily confirmed in this pilot study [53]. Lately, a single-center, randomized, double-blind, placebo-controlled preliminary trial was conducted to confirm the feasibility, tolerability, and safety of dantrolene administered intravenously [54]. The results showed that intravenous injection of dantrolene is tolerable, feasible, and safe. However, dantrolene did not attenuate cerebral vasospasm outcomes and delayed cerebral ischemia, compared with the placebo group. While this study has drawn convincing conclusions, the report of this work suggests that the timing of dantrolene administration is ambiguous. An accurate and practical administration time would be necessary, in the future, to reduce heterogeneity among studies. This will ease clinical transformation. Since feasibility and tolerance were confirmed, a large sample trial aiming to explore the efficacy of dantrolene would be the next step. It would also be an interesting direction to further explore the synergistic effect of dantrolene and nimodipine.
Delayed cerebral ischemia was considered a major cause of unfavorable clinical outcomes after SAH [6]. Patients often have prolonged management for SAH in the intensive care unit because of the need to monitor for delayed cerebral ischemia [16]. Triple-H therapy is a widespread method utilized. This treatment was used to alleviate delayed cerebral ischemia after SAH by increasing cerebral blood flow [55]. However, there was a lack of strong evidence supporting the efficacy of this treatment. Previous studies have reported an additional risk of hypervolemia and an absence of benefit. Hemodilution actually reduced cerebral oxygen transportation, though cerebral blood flow was increased [56-58]. Compared with hypervolemia and hemodilution, induced hypertension is a more promising way to increase cerebral blood flow [59]. This trial evaluated the effect of induced hypertension on delayed cerebral ischemia after SAH [44]. The results did not support the hypothesis that cerebral blood flow can be increased by induced hypertension. However, there might be a small effect that cannot be detected due to a small sample volume, as discussed in the report of this work. This is because a trend toward improved cerebral blood flow after intervention can only be observed in the induced hypertension group. Most regions with this trend have the lowest cerebral perfusion before intervention. According to the results, 225 to 250 patients per group would be needed to differentiate overall changes in cerebral blood flow. Therefore, a study targeting the region with the lowest perfusion might be more practical because the effects of intervention would be more obvious, and a smaller sample volume would be enough to differentiate them.
No significant improvements in clinical outcomes and occurrence of serious adverse events were observed in the induced hypertension groups [45]. The trial was prematurely terminated primarily due to slow recruitment and a lack of effect on overall cerebral blood flow, as reported previously. The slow recruitment resulted from difficulty in obtaining consent and the low occurrence rate of delayed cerebral ischemia. The occurrence of delayed cerebral ischemia in patients included was 16.53%, which is much lower than the 30% that was previously reported, and also lower than the 25-30% reported in the protocol of this trial [60, 61]. Regarding the lack of effect on overall cerebral blood flow, many possible explanations have been discussed in the article. The most likely explanations include: (1)the small sample volume is unable to discover the efficacy, (2)induced hypertension is actually not effective. The former explanation could be resolved using a large sample trial in the future, but this trial has proven that this would be difficult to attain. However, it could be plausible with participation from more centers, or if the trial was conducted in regions with higher SAH morbidity. The latter was indirectly supported by the failure of clinical trials targeting cerebral vasospasm in recent years. Mechanisms, such as BBB disruption, spreading depolarization, and micro thrombosis, may play a more important role than cerebral vasospasm alone. A recent retrospective analysis reported a significant decrease in mean transit time after induced hypertension [45]. An increased mean transit time is a parameter sensitive to cerebral hemodynamic, which takes into consideration the flow and volume of cerebral blood [62]. It was associated with the development of delayed cerebral ischemia in a previous report [63]. Future studies may consider using the mean transit time as a parameter to better evaluate the effect of induced hypertension. So far, there is still a lack of sound evidence supporting the clinical application of induced hypertension as a treatment for post-SAH delayed cerebral ischemia.
Nimodipine is an L-type Ca2+ channel antagonist recommended as a standard treatment for SAH. It is the only drug proven to improve the clinical outcome of patients with SAH [64, 65]. The acute effects of nimodipine could decrease mean arterial blood pressure and reduce cerebral blood flow, depending on the plasma concentration. However, the cerebral spinal fluid concentration of nimodipine is still lower than the optimal therapeutic demand [66]. The reduced nimodipine dosage because of hypotension is detrimental for SAH patients, which was associated with an unfavorable clinical outcome in previous studies [59, 67]. Ventricular drug administration might limit the effect of nimodipine within the brain, avoiding the fluctuation of peripheral blood pressure, while increasing the efficacy. A randomized controlled trial with 72 SAH patients was carried out in North America. This trial attempted to determine the safety, tolerability, pharmacokinetics, and clinical effects of nimodipine administered via intraventricular sustained-release [43]. The results showed that the maximum tolerated dose of intraventricular nimodipine is 800mg. The relative risk reduction of unfavorable outcome was also observed in intraventricular nimodipine groups compared with enteral nimodipine groups. The results of the trial are exciting, and once the feasibility and efficacy of this treatment are confirmed in future studies, the safety concerns regarding nimodipine-induced hypotension can be addressed. However, the results should not be over-interpreted without evaluating the limitations. All patients included in the trial had an external ventricular drain (EVD) inserted as a standard of care. They can only represent a selected population of patients with SAH. Additionally, the small sample size and non-blind design limited the strength of the results. However, large sample sizes and double-blind designs were applied in the latest ongoing clinical trial of ventricular nimodipine [68]. Future studies may also consider lumbar as the method of drug administration, which is easier to apply in clinical practice. This will also assist in expanding the population of subjects. The main additional risk introduced by this treatment is ventriculitis/meningitis. It occurred in 5 patients (9%) treated with intraventricular nimodipine, but there were no cases of this occurring in the enteral nimodipine group. Although 4 of the 5 patients eventually had a favorable outcome, ventriculitis/meningitis is still a serious complication that cannot be ignored. According to a previous report, the EVD-related infection rate ranges from 5-20%. However, this rate can be reduced to 1.4% if an EVD care bundle, including routine daily CSF sampling, is implemented and strictly observed [69, 70]. A strict and practical protocol for EVD is necessary to reduce the additional risk of ventricular nimodipine.
Cerebral vasospasm was implicated as the primary factor for delayed cerebral ischemia. It could influence 70% of SAH patients in the days after ictus and was thought to induce cerebral ischemia by reducing cerebral blood flow [71]. Though the mechanisms behind this pathophysiology event are poorly understood now, vascular endothelial cells and smooth muscle cells appear to play an important role in cerebral vasospasm [72]. Endothelial cells can endogenously secrete prostacyclin, which could alleviate vasospasm by vasodilating, and by improving delayed cerebral ischemia by inhibiting leukocytic adhesion and platelet aggregation, as well as enhancing membrane stabilization [73, 74]. The effects of prostacyclin on vasospasm and cerebral blood flow have been observed in an in vitro experiment [75]. The reduction of vasospasm by prostacyclin has been observed in 5 SAH patients in a previous pilot trial [76]. However, stronger evidence supporting prostacyclin as an effective treatment is needed before clinical application. A pilot trial was performed in Denmark with 90 SAH patients. The trial aimed to explore the possible effects of continuous prostacyclin infusion on relevant factors related to delayed ischemic neurological deficits [77]. The results revealed that no significant difference was observed among the three groups. The outcome of the trial included global and regional changes in cerebral blood flow, the incidence of delayed cerebral ischemia, and the clinical outcome 3 months after SAH. These disappointing results might be attributable to the fact that the intervention was initiated at day 5, which is too late compared with other effective pharmacological treatments, such as nimodipine. However, this time point might be appropriate, according to the previous study on prostacyclin. Considering the pathophysiological changes occurred almost immediately after SAH, initiating continuous infusion as early as possible might maximize the possible efficacy of prostacyclin. However, no sound evidence was found to support that there were any existing beneficial effects [78]. Currently, there is no sound evidence supporting the clinical application of prostacyclin after SAH. The necessity of further exploration on the efficacy of prostacyclin in SAH currently warrants reconsideration.
Statins are a type of 3-hydroxy-3-methylglutaryl co-enzyme A (HMG-CoA) reductase inhibitor. It reportedly increases cerebral blood flow by improving endothelial vasomotor function [79], blocks multiple aspects of inflammation by suppressing cytokine responses, and increases nitric oxide bioavailability by upregulating endothelial nitride oxide synthase [79, 80]. Experimental evidence suggests that simvastatin treatment might reduce neurological deficits after SAH [81]. Additionally, three pilot trials have shown that simvastatin can reduce the occurrence of cerebral vasospasm and delayed cerebral ischemia after SAH [82-84]. Recently, a multicenter randomized controlled double-blinded clinical trial was carried out to verify the superiority of high- dose simvastatin compared with low-dose simvastatin in SAH. However, no differences between the 80 mg simvastatin group and the 40mg simvastatin group were observed. The outcome of the trial included the incidence of delayed cerebral ischemia and found evidence of favorable outcomes at 3 months [85]. The trial was designed without a placebo control group, thus, the efficacy of simvastatin cannot be confirmed by this single trial alone. According to the report of this work, the reasoning behind this unusual design is that there was a previous randomized controlled trial and that has already compared efficacy between low-dose simvastatin and placebo. In that previous trial, it was confirmed that there was no difference in clinical outcomes at 6 months between the 40 mg simvastatin group and the placebo group [86]. The two trials were combined in systematic reviews aiming to summarize the clinical trials referring to simvastatin treatment after SAH [87, 88]. In addition to these two trials, other previously conducted trials were also included. Conclusions of the reviews showed that the application of simvastatin showed no benefit in clinical outcome and the occurrence of delayed cerebral ischemia. Another trial targeting the effects of simvastatin on cerebral blood flow and static autoregulation did not observe a significant difference between the simvastatin group and the placebo group [89]. A propensity analysis included 102 SAH patients. This analysis reports a significant decrease in the occurrence of cerebral vasospasm without improvement in clinical outcome after simvastatin treatment [90]. In this analysis, simvastatin administration was initiated within 48 hours after SAH, and the outcome was defined as the Montreal Cognitive Assessment at 3 months after SAH. This timepoint of drug administration differed from the latest clinical trials but was applied in a previous pilot trial. In a meta-regression analysis, there was weak evidence supporting a dose-dependent reduction in cerebral vasospasm, delayed cerebral ischemia, and mortality associated with the application of statin after SAH [91]. Simvastatin application of 40mg or 80mg per day within 96 hours after SAH was proven to be ineffective in improving delayed cerebral ischemia and clinical outcome, but the potential benefits of statin treatment cannot be dismissed. Future trials should consider initiating statin application within 48 hours, and using higher doses of the drug, if tolerable. Increasing the drug concentration in the cerebrospinal fluid by ventricular drug delivery is also an interesting way to more easily observe the statin’s effects. Until now, no sound evidence existed to support the benefits found from simvastatin application after SAH, and simvastatin should not be considered routine therapy.
5. IMAGING ADVANCEMENT TARGET ON BBB PERMEABILITY ALTERATION
After the ictus of SAH, several early pathophysiological events can be commonly observed in BBB components, such as the endothelium. Examples of these events include raised intracranial pressure, decreased cerebral blood flow, and global ischemia injury [92, 93]. Apoptosis can occur in the endothelium accompanied by increasing BBB permeability, which can be alleviated by apelin-13 [94, 95]. Cerebral vasospasm also partially contributed to the dysfunction of cerebrovascular endothelium [96]. Considering that BBB permeability increases before vasospasm, vasospasm could be predicted by measuring an increase in BBB permeability. In a retrospective analysis of 83 SAH patients who were suspicious of having vasospasms, CT perfusion and digital subtraction angiography (DSA) was completed within 24 hours [97]. BBB permeability values, cerebral blood volume, and cerebral blood flow derived from CT perfusion in the region of interest were obtained. Patients were classified as “no vasospasm”, “mild”, “moderate”, and “severe” according to severities of vasospasm in DSA. The results showed a trend of higher BBB permeability values in patients with higher severity of vasospasm, but this trend was not supported by multivariate analysis. Multivariate analysis suggests that mean transit time, cerebral blood volume, and severity of hydrocephalus are predictors of increasing BBB permeability. An increase of mean transit time was associated with both BBB permeability and vasospasm, which indicated that the mean transit time was a viable measurement for the pathophysiological changes of the endothelium. DSA data obtained early in the disease course and retrospective design may limit the value of this retrospective analysis. The conclusions of the analysis require more prospective studies with a broader group of patients to confirm.
Current methods to assess patients with a high risk of delayed infarction due to delayed cerebral ischemia are limited by low precision and stability [98]. After assessing delayed cerebral ischemia using parameters derived from imaging data, CT perfusion was reportedly superior to non-contrast CT and CT angiography [99, 100]. Additionally, CT perfusion is increasingly applied in patients with SAH because of its increased potency and the inconsequential, additional time needed for an examination compared with non-contrast CT and CT angiography [101]. However, there remains a lack of studies that describe BBB permeability alterations in patients with delayed cerebral ischemia. The permeability surface-area product (PS) is a parameter derived from CT perfusion. PS can indirectly measure changes in BBB permeability and has been quantified in acute ischemic stroke [102]. A retrospective analysis of 23 SAH patients with delayed cerebral ischemia revealed that PS significantly increased before the delayed infarction occurred [103]. CT perfusion was performed on days 0-3 after aneurysm rupture, and at that time, non-contrast CT revealed that no infarction was found in all of the patients. Regions of interest were selected by choosing the regions with a delayed infarction that could not be seen until 48h after ictus in follow-up non-contrast CT, and contralateral corresponding control regions were determined at the same time. CT perfusion parameters were generated from regions corresponding to the regions of interest and control. The results of the analysis showed that PS increased significantly in regions of interest compared to the control, but no differences were observed in mean transit time and cerebral blood flow. These results indicate that PS derived from CT perfusion may be a potential predictor for delayed cerebral ischemia-related delayed infarction and BBB permeability. Another retrospective analysis with 50 SAH patients of a prospective cohort was carried out in the year 2015 [104]. The CT perfusion was performed within 72 hours after the ictus of SAH. The results of this analysis indicate that significantly higher mean transit times can be observed in patients with delayed cerebral ischemia and in patients suffering cerebral infarction in the later course of the disease. However, an interesting fact is that the regions measured with the highest mean transit time were inconsistent with eventual infarction regions confirmed by discharge MRI. Besides, differences in mean transit time, early cerebral blood flow, and PS between patients with good outcome(mRS<2) and poor outcome (mRS>2) were not observed. Additionally, whether delayed cerebral ischemia and cerebral infarction occurred or not, there were no observed differences in cerebral blood flow and PS. These results did not support the association between BBB permeability and delayed cerebral ischemia or poor outcome. A possible explanation may be that the patients have a relatively good clinical condition (average WFNS score: 2). The chosen parameters may also contribute to the absence of this association. Parameters, such as mean transit time and cerebral blood flow, are highly dependent on hemodynamic conditions, which would influence the reliability of determining when there are abnormal changes in the blood flow. An early increase in BBB permeability was associated with global cerebral edema and delayed cerebral ischemia in recent studies. In these studies, BBB permeability was indicated by other parameters derived from CT perfusion, with the exception of mean transit time and cerebral blood flow [103, 105]. The perfusion deficits in CT perfusion were strongly associated with the development of delayed cerebral ischemia and poor clinical outcome in previous studies [63, 106]. Therefore, other BBB permeability parameters from CT perfusion may provide some predictive information for clinical outcomes. A retrospective cross-sectional analysis revealed the possibility of predicting the clinical outcome of SAH patients by extracting four parameters from CT perfusion. These parameters all indicate an increase in BBB permeability [107]. Twenty-two SAH patients were included and classified according to the clinical outcome assessed by permanent neurologic deficits and modified Rankin scores. All patients received perfusion CT on days 0-3 after ictus. Increased PS and Ve, and decreased Kep were observed in patients with unfavorable clinical outcomes. These changes in parameters all indicated an increasing BBB permeability. This recent work showed the potential of the BBB as a biomarker for the prognostication of SAH patients.
Global cerebral edema is the result of brief global ischemia and intracranial circulatory arrest caused by increasing ICP at the ictus of SAH [108, 109]. Global cerebral edema consists of cytotoxic and vasogenic edema [110]. Cytotoxic edema refers to intracellular water accumulation generated from dysfunction of cellular homeostasis caused by brief ischemia. Moreover, vasogenic edema was attributed to the disruption of BBB [94, 111]. Both BBB disruption and global cerebral edema led to excessive glutamate, which regulated tight junction degradation and cell contraction [112]. Global cerebral edema was reportedly an important risk factor for cognitive dysfunction after SAH in previous studies [113], but the method to diagnose global cerebral edema is difficult under the circumstances of diffuse SAH and increased ICP [114]. Measuring BBB permeability may be another method to detect and monitor global cerebral edema. BBB permeability parameters were extracted from the extended CT perfusion of 22 SAH patients and retrospectively analyzed. The observed results indicate that BBB permeability may be a central target in exploring the potential quantitative imaging biomarker for detecting global cerebral edema after SAH [105]. Patients with SAH were divided into two groups, global cerebral edema and non-global cerebral edema, according to the non-contrast CT. CT perfusion was performed in days 0-3 after ictus. Six parameters (Kep, Ktrans, Vp, F, Ve, PS) derived from CT perfusion were chosen to quantitatively evaluate BBB permeability differences between the two groups. The differences of parameters (Kep, Ktrans, Vp, F, Ve) indicate a significant increase in BBB permeability and a reduction in cerebral blood flow in patients with global cerebral edema. Of the six chosen parameters, Kep represents the flow that traverses the blood vessel wall from the extravascular extracellular space to the intravascular space. This parameter is independent of cerebral blood flow, which maintains stability in low blood flow conditions that are commonly seen in patients with global cerebral edema. This method might enhance SAH management and improve clinical outcomes.
In MRI, BBB disruption appears with abnormal brain tissue, but there was no quantitative assessment based on MRI for evaluating BBB disruption in patients with SAH [115]. The recent clinical tools for predicting delayed cerebral ischemia after SAH have unsatisfactory specificity and sensitivity [116]. A retrospective study semi-automatically analyzed data from MRI of 124 SAH patients at 4 time points (24–48 h, 6–8 days, 12–15 days, and 6–12months after ictus) in the Co-Operative Studies of Brain Injury Depolarizations (COSBID). This study revealed the potential of BBB disruption as a predictor for poor clinical outcomes [115]. The results showed that the volume of BBB disruption in the brain is significantly higher in patients with disease progression. BBB disruption can be detected within 24-48h after the ictus of SAH and can be observed in both normal and abnormal brain tissue. Except for those existing in normal brain tissue, BBB disruption was most likely found in a 1 cm ring around the abnormal lesion. The logistic regression model combining BBB disruption with MRI-based data has a greater predictive value compared to the model combining clinical data with WFNS, eGOS, or RMS. The detection of BBB disruption in MRI requires future prospective studies to investigate because it could be an early biomarker for predicting clinical outcome.
Since dynamic contrast-enhanced MRI was developed by Weinberg in the 1980s, it has made great contributions to in-vivo studies of the microvasculature [117]. Dynamic contrast-enhanced MRI is capable of evaluating the changes in BBB permeability by analyzing specific parameters and modeling options according to previous studies [118]. A small sample prospective study was published recently, and it enrolled 20 SAH patients who underwent a dynamic contrast-enhanced MRI on day 4 after SAH. The BBB permeability parameters Ve, Vp, and Ktrans derived from dynamic contrast-enhanced MRI were compared among different groups that were categorized according to their clinical outcomes (i.e., delayed cerebral ischemia, radiological vasospasm only, and no vasospasm or ischemia) [119]. Regions of interest of dynamic contrast-enhanced MRI were placed in standardized representative vascular territories, and the parameters were derived from these regions. The results showed that no significant differences in Ve or Vp were observed among the three groups. The results of the intergroup analysis showed significantly higher Ktrans in the delayed cerebral ischemia group compared to the radiological vasospasm-only group, the no vasospasm, or the ischemia group. The receiver operating characteristic curve illustrated that patients prone to developing delayed cerebral ischemia could be precisely distinct from patients with better outcomes by these parameters.
Imaging advancements on BBB disruption in patients after SAH generally aimed to explore a novel method to predict clinical outcomes, delayed cerebral ischemia, and delayed infarction related to delayed cerebral ischemia in the early disease course. These new methods were achieved by measuring the parameter changes in BBB permeability. CT perfusion and MRI appear to be the most popular detection method. These endeavors currently remain in the early phases of exploration. Therefore, the chosen parameters may vary. It is reasonable to anticipate that new parameters will be introduced within the next several years. Among the parameters in the current studies, Kep seems to be a stable and sensitive mark in CT perfusion. Ktrans is a reliable parameter for dynamic contrast-enhanced MRI. The prediction model combining the volume of BBB disruption derived from MRI and clinical data has shown better predictive value than most tools used. Applicating the volume of BBB disruption from CT perfusion, and predict models combining the volume of BBB disruption with stable parameters would be a promising way to develop practical clinical tools. These tools can be used to predict disease progression and clinical outcomes more precisely. Considering that the BBB plays an important role in the neurovascular unit and serves as a link between early brain injury and delayed cerebral ischemia, more efforts should be made to investigate the potential of therapeutic interventions targeting BBB disruption.
CONCLUSION
Clinical trials have made recent progress through ventricular administration of nimodipine, which was delivered through EVD, and may address the concerns for hypotension in the current strategy. BBB disruption is an early pathophysiological event between early brain injury and delayed cerebral ischemia, and it warrants more attention to the great potential depicted in the results of recent imaging studies, especially in regard to predicting delayed cerebral ischemia and clinical outcomes. Exploration of therapeutic interventions targeting BBB disruption is also promising when considering the extended duration of BBB disruption in the disease course of SAH.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by the National Natural Science Foundation of China, No. 81971107, and the Fundamental Research Funds for the Central Universities, China, No. 2019QNA7038, awarded to Dr. Sheng Chen.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Lovelock C.E., Rinkel G.J., Rothwell P.M. Time trends in outcome of subarachnoid hemorrhage: Population-based study and systematic review. Neurology. 2010;74(19):1494–1501. doi: 10.1212/WNL.0b013e3181dd42b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieuwkamp D.J., Setz L.E., Algra A., Linn F.H., de Rooij N.K., Rinkel G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khindi T., Macdonald R.L., Schweizer T.A. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H. What is early brain injury? Transl. Stroke Res. 2015;6(1):1–3. doi: 10.1007/s12975-014-0380-8. [DOI] [PubMed] [Google Scholar]
- 5.Eagles M.E., Jaja B.N.R., Macdonald R.L. Incorporating a modified graeb score to the modified fisher scale for improved risk prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurgery. 2018;82(3):299–305. doi: 10.1093/neuros/nyx165. [DOI] [PubMed] [Google Scholar]
- 6.Dorhout Mees S.M., Kerr R.S., Rinkel G.J., Algra A., Molyneux A.J. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the International Subarachnoid Aneurysm Trial (ISAT). J. Neurol. 2012;259(4):679–683. doi: 10.1007/s00415-011-6243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosengart A.J., Schultheiss K.E., Tolentino J., Macdonald R.L. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–2321. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 8.Eagles M.E., Tso M.K., Macdonald R.L. Cognitive impairment, functional outcome, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;S1878-8750(19):30020–8. doi: 10.1016/j.wneu.2018.12.152. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tso M.K., Macdonald R.L. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl. Stroke Res. 2014;5(2):174–189. doi: 10.1007/s12975-014-0323-4. [DOI] [PubMed] [Google Scholar]
- 11.Schöller K., Trinkl A., Klopotowski M., Thal S.C., Plesnila N., Trabold R., Hamann G.F., Schmid-Elsaesser R., Zausinger S. Characterization of microvascular basal lamina damage and blood- brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald R.L., Kassell N.F., Mayer S., Ruefenacht D., Schmiedek P., Weidauer S., Frey A., Roux S., Pasqualin A. CONSCIOUS-1 Investigators. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39(11):3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Bach D., Frey A., Marr A., Roux S., Kassell N. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011;10(7):618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- 14.Peeyush Kumar T., McBride D.W., Dash P.K., Matsumura K., Rubi A., Blackburn S.L. Endothelial cell dysfunction and injury in subarachnoid hemorrhage. Mol. Neurobiol. 2019;56(3):1992–2006. doi: 10.1007/s12035-018-1213-7. [DOI] [PubMed] [Google Scholar]
- 15.Smith M., Citerio G. What’s new in subarachnoid hemorrhage. Intensive Care Med. 2015;41(1):123–126. doi: 10.1007/s00134-014-3548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muehlschlegel S. Subarachnoid hemorrhage. Continuum (Minneap. Minn.) 2018;24(6):1623–1657. doi: 10.1212/CON.0000000000000679. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., de Ferranti S.D., Ferguson J.F., Fornage M., Gillespie C., Isasi C.R., Jiménez M.C., Jordan L.C., Judd S.E., Lackland D., Lichtman J.H., Lisabeth L., Liu S., Longenecker C.T., Lutsey P.L., Mackey J.S., Matchar D.B., Matsushita K., Mussolino M.E., Nasir K., O’Flaherty M., Palaniappan L.P., Pandey A., Pandey D.K., Reeves M.J., Ritchey M.D., Rodriguez C.J., Roth G.A., Rosamond W.D., Sampson U.K.A., Satou G.M., Shah S.H., Spartano N.L., Tirschwell D.L., Tsao C.W., Voeks J.H., Willey J.Z., Wilkins J.T., Wu J.H., Alger H.M., Wong S.S., Muntner P. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 18.Kusaka G., Ishikawa M., Nanda A., Granger D.N., Zhang J.H. Signaling pathways for early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2004;24(8):916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 19.Rass V., Helbok R. Early brain injury after poor-grade subarachnoid hemorrhage. Curr. Neurol. Neurosci. Rep. 2019;19(10):78. doi: 10.1007/s11910-019-0990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehba F.A., Hou J., Pluta R.M., Zhang J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012;97(1):14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhar R., Diringer M.N. Relationship between angiographic vasospasm, cerebral blood flow, and cerebral infarction after subarachnoid hemorrhage. Acta Neurochir. Suppl. (Wien) 2015;120:161–165. doi: 10.1007/978-3-319-04981-6_27. [DOI] [PubMed] [Google Scholar]
- 22.Macdonald R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014;10(1):44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 23.Hansen-Schwartz J., Vajkoczy P., Macdonald R.L., Pluta R.M., Zhang J.H. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol. Sci. 2007;28(6):252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Connolly E.S., Jr, Rabinstein A.A., Carhuapoma J.R., Derdeyn C.P., Dion J., Higashida R.T., Hoh B.L., Kirkness C.J., Naidech A.M., Ogilvy C.S., Patel A.B., Thompson B.G., Vespa P. American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z., Nelson A.R., Betsholtz C., Zlokovic B.V. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 27.Gautam J., Cao Y., Yao Y. Pericytic laminin maintains blood-brain barrier integrity in an age-dependent manner. Transl. Stroke Res. 2020;11(2):228–242. doi: 10.1007/s12975-019-00709-8. [DOI] [PubMed] [Google Scholar]
- 28.Armulik A., Genové G., Mäe M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B.R., Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Zhang X., Wei Q., Leng S., Li C., Han B., Bai Y., Zhang H., Yao H. Activation of sigma-1 receptor enhanced pericyte survival via the interplay between apoptosis and autophagy: Implications for blood-brain barrier integrity in stroke. Transl. Stroke Res. 11(2):267–287. doi: 10.1007/s12975-019-00711-0. [DOI] [PubMed] [Google Scholar]
- 30.Xu W., Mo J., Ocak U., Travis Z.D., Enkhjargal B., Zhang T., Wu P., Peng J., Li T., Zuo Y., Shao A., Tang J., Zhang J., Zhang J.H. Activation of melanocortin 1 receptor attenuates early brain injury in a rat model of subarachnoid hemorrhage via the suppression of neuroinflammation through ampk/tbk1/nf-kappab pathway in rats. Neurotherapeutics. 2020;17(1):294–308. doi: 10.1007/s13311-019-00772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurgur H., Pinteaux E. Microglia in the neurovascular unit: Blood-brain barrier-microglia interactions after central nervous system disorders. Neuroscience. 2019;405:55–67. doi: 10.1016/j.neuroscience.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 32.Haruwaka K., Ikegami A., Tachibana Y., Ohno N., Konishi H., Hashimoto A., Matsumoto M., Kato D., Ono R., Kiyama H., Moorhouse A.J., Nabekura J., Wake H. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019;10(1):5816. doi: 10.1038/s41467-019-13812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Germanò A., d’Avella D., Imperatore C., Caruso G., Tomasello F. Time-course of blood-brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir. (Wien) 2000;142(5):575–580. doi: 10.1007/s007010050472. [DOI] [PubMed] [Google Scholar]
- 34.Toyota Y., Wei J., Xi G., Keep R.F., Hua Y. White matter T2 hyperintensities and blood-brain barrier disruption in the hyperacute stage of subarachnoid hemorrhage in male mice: The role of lipocalin-2. CNS Neurosci. Ther. 2019;25(10):1207–1214. doi: 10.1111/cns.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egashira Y., Hua Y., Keep R.F., Iwama T., Xi G. Lipocalin 2 and blood-brain barrier disruption in white matter after experimental subarachnoid hemorrhage. Acta Neurochir. Suppl. (Wien) 2016;121:131–134. doi: 10.1007/978-3-319-18497-5_23. [DOI] [PubMed] [Google Scholar]
- 36.Wang K.C., Tang S.C., Lee J.E., Li Y.I., Huang Y.S., Yang W.S., Jeng J.S., Arumugam T.V., Tu Y.K. Cerebrospinal fluid high mobility group box 1 is associated with neuronal death in subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2017;37(2):435–443. doi: 10.1177/0271678X16629484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrowski R.P., Colohan A.R., Zhang J.H. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol. Res. 2006;28(4):399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 38.Pisapia J.M., Xu X., Kelly J., Yeung J., Carrion G., Tong H., Meghan S., El-Falaky O.M., Grady M.S., Smith D.H., Zaitsev S., Muzykantov V.R., Stiefel M.F., Stein S.C. Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp. Neurol. 2012;233(1):357–363. doi: 10.1016/j.expneurol.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao A., Zhu Z., Li L., Zhang S., Zhang J. Emerging therapeutic targets associated with the immune system in patients with intracerebral haemorrhage (ICH): From mechanisms to translation. EBioMedicine. 2019;45:615–623. doi: 10.1016/j.ebiom.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaichana K.L., Pradilla G., Huang J., Tamargo R.J. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg. 2010;73(1):22–41. doi: 10.1016/j.surneu.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Shao Z., Tu S., Shao A. Pathophysiological mechanisms and potential therapeutic targets in intracerebral hemorrhage. Front. Pharmacol. 2019;10:1079. doi: 10.3389/fphar.2019.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao A., Zhou Y., Yao Y., Zhang W., Zhang J., Deng Y. The role and therapeutic potential of heat shock proteins in haemorrhagic stroke. J. Cell. Mol. Med. 2019;23(9):5846–5858. doi: 10.1111/jcmm.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hänggi D., Etminan N., Aldrich F., Steiger H.J., Mayer S.A., Diringer M.N., Hoh B.L., Mocco J., Faleck H.J., Macdonald R.L., Investigators N. NEWTON Investigators. Randomized, open-label, phase 1/2a study to determine the maximum tolerated dose of intraventricular sustained release nimodipine for subarachnoid hemorrhage (newton nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage). Stroke. 2017;48(1):145–151. doi: 10.1161/STROKEAHA.116.014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gathier C.S., Dankbaar J.W., van der Jagt M., Verweij B.H., Oldenbeuving A.W., Rinkel G.J.E., van den Bergh W.M., Slooter A.J.C., Grp H.S. HIMALAIA Study Group. Effects of induced hypertension on cerebral perfusion in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage a randomized clinical trial. Stroke. 2015;46(11):3277–3281. doi: 10.1161/STROKEAHA.115.010537. [DOI] [PubMed] [Google Scholar]
- 45.Gathier C.S., van den Bergh W.M., van der Jagt M., Verweij B.H., Dankbaar J.W., Müller M.C., Oldenbeuving A.W., Rinkel G.J.E., Slooter A.J.C., Grp H.S. HIMALAIA Study Group. Induced hypertension for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage a randomized clinical trial. Stroke. 2018;49(1):76–83. doi: 10.1161/STROKEAHA.117.017956. [DOI] [PubMed] [Google Scholar]
- 46.Dreier J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011;17(4):439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 47.Matsuda N., Naraoka M., Ohkuma H., Shimamura N., Ito K., Asano K., Hasegawa S., Takemura A. Effect of cilostazol on cerebral vasospasm and outcome in patients with aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo- controlled trial. Cerebrovasc. Dis. 2016;42(1-2):97–105. doi: 10.1159/000445509. [DOI] [PubMed] [Google Scholar]
- 48.Niu P.P., Yang G., Xing Y.Q., Guo Z.N., Yang Y. Effect of cilostazol in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J. Neurol. Sci. 2014;336(1-2):146–151. doi: 10.1016/j.jns.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 49.Sugimoto K., Nomura S., Shirao S., Inoue T., Ishihara H., Kawano R., Kawano A., Oka F., Suehiro E., Sadahiro H., Shinoyama M., Oku T., Maruta Y., Hirayama Y., Hiyoshi K., Kiyohira M., Yoneda H., Okazaki K., Dreier J.P., Suzuki M. Cilostazol decreases duration of spreading depolarization and spreading ischemia after aneurysmal subarachnoid hemorrhage. Ann. Neurol. 2018;84(6):873–885. doi: 10.1002/ana.25361. [DOI] [PubMed] [Google Scholar]
- 50.Tani E., Matsumoto T. Continuous elevation of intracellular Ca2+ is essential for the development of cerebral vasospasm. Curr. Vasc. Pharmacol. 2004;2(1):13–21. doi: 10.2174/1570161043476492. [DOI] [PubMed] [Google Scholar]
- 51.Muehlschlegel S., Sims J.R. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit. Care. 2009;10(1):103–115. doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salomone S., Soydan G., Moskowitz M.A., Sims J.R. Inhibition of cerebral vasoconstriction by dantrolene and nimodipine. Neurocrit. Care. 2009;10(1):93–102. doi: 10.1007/s12028-008-9153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muehlschlegel S., Rordorf G., Sims J. Effects of a single dose of dantrolene in patients with cerebral vasospasm after subarachnoid hemorrhage: a prospective pilot study. Stroke. 2011;42(5):1301–1306. doi: 10.1161/STROKEAHA.110.603159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muehlschlegel S., Carandang R., Hall W., Kini N., Izzy S., Garland B., Ouillette C., van der Bom I.M.J., Flood T.F., Gounis M.J., Weaver J.P., Barton B., Wakhloo A.K. Dantrolene for cerebral vasospasm after subarachnoid haemorrhage: a randomised double blind placebo-controlled safety trial. J. Neurol. Neurosurg. Psychiatry. 2015;86(9):1029–1035. doi: 10.1136/jnnp-2014-308778. [DOI] [PubMed] [Google Scholar]
- 55.Sen J., Belli A., Albon H., Morgan L., Petzold A., Kitchen N. Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2003;2(10):614–621. doi: 10.1016/S1474-4422(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 56.Rinkel G.J., Feigin V.L., Algra A., van Gijn J. Circulatory volume expansion therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2004;(4):CD000483. doi: 10.1002/14651858.CD000483.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekelund A., Reinstrup P., Ryding E., Andersson A.M., Molund T., Kristiansson K.A., Romner B., Brandt L., Säveland H. Effects of iso- and hypervolemic hemodilution on regional cerebral blood flow and oxygen delivery for patients with vasospasm after aneurysmal subarachnoid hemorrhage. Acta Neurochir. (Wien) 2002;144(7):703–712. doi: 10.1007/s00701-002-0959-9. [DOI] [PubMed] [Google Scholar]
- 58.Raabe A., Beck J., Keller M., Vatter H., Zimmermann M., Seifert V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J. Neurosurg. 2005;103(6):974–981. doi: 10.3171/jns.2005.103.6.0974. [DOI] [PubMed] [Google Scholar]
- 59.Dankbaar J.W., Slooter A.J., Rinkel G.J., Schaaf I.C. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systematic review. Crit. Care. 2010;14(1):R23. doi: 10.1186/cc8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geraghty J.R., Testai F.D. Delayed cerebral ischemia after subarachnoid hemorrhage: Beyond vasospasm and towards a multifactorial pathophysiology. Curr. Atheroscler. Rep. 2017;19(12):50. doi: 10.1007/s11883-017-0690-x. [DOI] [PubMed] [Google Scholar]
- 61.Gathier C.S., van den Bergh W.M., Slooter A.J. Himalaia (hypertension induction in the management of aneurysmal subarachnoid haemorrhage with secondary ischaemia): A randomized single-blind controlled trial of induced hypertension vs. No induced hypertension in the treatment of delayed cerebral ischemia after subarachnoid hemorrhage. Int. J. Stroke. 2014;9(3):375–380. doi: 10.1111/ijs.12055. [DOI] [PubMed] [Google Scholar]
- 62.Murphy A., de Oliveira Manoel A.L., Macdonald R.L., Baker A., Lee T.Y., Marotta T., Montanera W., Aviv R., Bharatha A. Changes in cerebral perfusion with induced hypertension in aneurysmal subarachnoid hemorrhage: A pilot and feasibility study. Neurocrit. Care. 2017;27(1):3–10. doi: 10.1007/s12028-017-0379-6. [DOI] [PubMed] [Google Scholar]
- 63.Sanelli P.C., Anumula N., Johnson C.E., Comunale J.P., Tsiouris A.J., Riina H., Segal A.Z., Stieg P.E., Zimmerman R.D., Mushlin A.I. Evaluating CT perfusion using outcome measures of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 2013;34(2):292–298. doi: 10.3174/ajnr.A3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorhout Mees S.M., Rinkel G.J., Feigin V.L., Algra A., van den Bergh W.M., Vermeulen M., van Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007;(3):CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pickard J.D., Murray G.D., Illingworth R., Shaw M.D., Teasdale G.M., Foy P.M., Humphrey P.R., Lang D.A., Nelson R., Richards P. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989;298(6674):636–642. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi H.A., Ko S.B., Chen H., Gilmore E., Carpenter A.M., Lee D., Claassen J., Mayer S.A., Schmidt J.M., Lee K., Connelly E.S., Paik M., Badjatia N. Acute effects of nimodipine on cerebral vasculature and brain metabolism in high grade subarachnoid hemorrhage patients. Neurocrit. Care. 2012;16(3):363–367. doi: 10.1007/s12028-012-9670-8. [DOI] [PubMed] [Google Scholar]
- 67.Sandow N., Diesing D., Sarrafzadeh A., Vajkoczy P., Wolf S. Nimodipine dose reductions in the treatment of patients with aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2016;25(1):29–39. doi: 10.1007/s12028-015-0230-x. [DOI] [PubMed] [Google Scholar]
- 68.Hänggi D., Etminan N., Mayer S.A., Aldrich E.F., Diringer M.N., Schmutzhard E., Faleck H.J., Ng D., Saville B.R., Macdonald R.L. NEWTON Investigators. Clinical trial protocol: Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group, efficacy, and safety study comparing eg-1962 to standard of care oral nimodipine in adults with aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2019;30(1):88–97. doi: 10.1007/s12028-018-0575-z. [DOI] [PubMed] [Google Scholar]
- 69.Beer R., Lackner P., Pfausler B., Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J. Neurol. 2008;255(11):1617–1624. doi: 10.1007/s00415-008-0059-8. [DOI] [PubMed] [Google Scholar]
- 70.Champey J., Mourey C., Francony G., Pavese P., Gay E., Gergele L., Manet R., Velly L., Bruder N., Payen J.F. Strategies to reduce external ventricular drain-related infections: a multicenter retrospective study. J. Neurosurg. 2018:1–6. doi: 10.3171/2018.1.Jns172486. [DOI] [PubMed] [Google Scholar]
- 71.Ecker A., Riemenschneider P.A. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J. Neurosurg. 1951;8(6):660–667. doi: 10.3171/jns.1951.8.6.0660. [DOI] [PubMed] [Google Scholar]
- 72.Hansen-Schwartz J. Cerebral vasospasm: a consideration of the various cellular mechanisms involved in the pathophysiology. Neurocrit. Care. 2004;1(2):235–246. doi: 10.1385/NCC:1:2:235. [DOI] [PubMed] [Google Scholar]
- 73.Koskinen L.D., Sundström N., Hägglund L., Eklund A., Olivecrona M. Prostacyclin affects the relation between brain interstitial glycerol and cerebrovascular pressure reactivity in severe traumatic brain injury. Neurocrit. Care. 2019;31(3):494–500. doi: 10.1007/s12028-019-00741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moncada S., Higgs E.A., Vane J.R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1(8001):18–20. doi: 10.1016/S0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- 75.Brandt L., Ljunggren B., Andersson K.E., Hindfelt B., Uski T. Effects of indomethacin and prostacyclin on isolated human pial arteries contracted by CSF from patients with aneurysmal SAH. J. Neurosurg. 1981;55(6):877–883. doi: 10.3171/jns.1981.55.6.0877. [DOI] [PubMed] [Google Scholar]
- 76.Koskinen L.O., Olivecrona M., Rodling-Wahlström M., Naredi S. Prostacyclin treatment normalises the MCA flow velocity in nimodipine-resistant cerebral vasospasm after aneurysmal subarachnoid haemorrhage: a pilot study. Acta Neurochir. (Wien) 2009;151(6):595–599. doi: 10.1007/s00701-009-0295-4. [DOI] [PubMed] [Google Scholar]
- 77.Rasmussen R., Wetterslev J., Stavngaard T., Juhler M., Skjøth-Rasmussen J., Grände P.O., Olsen N.V. Effects of prostacyclin on cerebral blood flow and vasospasm after subarachnoid hemorrhage: randomized, pilot trial. Stroke. 2015;46(1):37–41. doi: 10.1161/STROKEAHA.114.007470. [DOI] [PubMed] [Google Scholar]
- 78.Gybel-Brask M., Rasmussen R., Stensballe J., Johansson P.I., Ostrowski S.R. Effect of delayed onset prostacyclin on markers of endothelial function and damage after subarachnoid hemorrhage. Acta Neurochir. (Wien) 2017;159(6):1073–1078. doi: 10.1007/s00701-017-3168-2. [DOI] [PubMed] [Google Scholar]
- 79.Endres M., Laufs U., Huang Z., Nakamura T., Huang P., Moskowitz M.A., Liao J.K. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1998;95(15):8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol. Sci. 2002;23(10):482–486. doi: 10.1016/S0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- 81.McGirt M.J., Lynch J.R., Parra A., Sheng H., Pearlstein R.D., Laskowitz D.T., Pelligrino D.A., Warner D.S. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33(12):2950–2956. doi: 10.1161/01.STR.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 82.Chou S.H., Smith E.E., Badjatia N., Nogueira R.G., Sims J.R., II, Ogilvy C.S., Rordorf G.A., Ayata C. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke. 2008;39(10):2891–2893. doi: 10.1161/STROKEAHA.107.505875. [DOI] [PubMed] [Google Scholar]
- 83.Lynch J.R., Wang H., McGirt M.J., Floyd J., Friedman A.H., Coon A.L., Blessing R., Alexander M.J., Graffagnino C., Warner D.S., Laskowitz D.T. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36(9):2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 84.Tseng M.Y., Czosnyka M., Richards H., Pickard J.D., Kirkpatrick P.J. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36(8):1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 85.Wong G.K.C., Chan D.Y.C., Siu D.Y.W., Zee B.C.Y., Poon W.S., Chan M.T.V., Gin T., Leung M. HDS-SAH Investigators. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: multicenter randomized controlled double-blinded clinical trial. Stroke. 2015;46(2):382–388. doi: 10.1161/STROKEAHA.114.007006. [DOI] [PubMed] [Google Scholar]
- 86.Kirkpatrick P.J., Turner C.L., Smith C., Hutchinson P.J., Murray G.D. STASH Collaborators. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. 2014;13(7):666–675. doi: 10.1016/S1474-4422(14)70084-5. [DOI] [PubMed] [Google Scholar]
- 87.Lin J., Liu H., Jiang J., Jia C., Zhang B., Gao X. Clinical evidence of efficacy of simvastatin for aneurysmal subarachnoid hemorrhage. J. Int. Med. Res. 2017;45(6):2128–2138. doi: 10.1177/0300060517713803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu H., Xu X. Effect of simvastatin in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Am. J. Emerg. Med. 2017;35(12):1940–1945. doi: 10.1016/j.ajem.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Diringer M.N., Dhar R., Scalfani M., Zazulia A.R., Chicoine M., Powers W.J., Derdeyn C.P. Effect of high-dose simvastatin on cerebral blood flow and static autoregulation in subarachnoid hemorrhage. Neurocrit. Care. 2016;25(1):56–63. doi: 10.1007/s12028-015-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong G.K., Wong A., Zee B.C., Poon W.S., Chan M.T., Gin T., Siu D.Y., Mok V.C. Cognitive outcome in acute simvastatin treatment for aneurysmal subarachnoid hemorrhage: A propensity matched analysis. J. Neurol. Sci. 2015;358(1-2):58–61. doi: 10.1016/j.jns.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 91.To M.S., Prakash S., Poonnoose S.I., Bihari S. Dose-dependent effects of statins for patients with aneurysmal subarachnoid hemorrhage: Meta-regression analysis. World Neurosurg. 2018;113:153–162. doi: 10.1016/j.wneu.2018.01.184. [DOI] [PubMed] [Google Scholar]
- 92.Bederson J.B., Germano I.M., Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26(6):1086–1091. doi: 10.1161/01.STR.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 93.Voldby B., Enevoldsen E.M. Intracranial pressure changes following aneurysm rupture. Part 1: clinical and angiographic correlations. J. Neurosurg. 1982;56(2):186–196. doi: 10.3171/jns.1982.56.2.0186. [DOI] [PubMed] [Google Scholar]
- 94.Cahill J., Calvert J.W., Zhang J.H. Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2006;26(11):1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 95.Xu W., Gao L., Li T., Zheng J., Shao A., Zhang J. Apelin-13 alleviates early brain injury after subarachnoid hemorrhage via suppression of endoplasmic reticulum stress-mediated apoptosis and blood-brain barrier disruption: Possible involvement of atf6/chop pathway. Neuroscience. 2018;388:284–296. doi: 10.1016/j.neuroscience.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 96.Zubkov A.Y., Ogihara K., Bernanke D.H., Parent A.D., Zhang J. Apoptosis of endothelial cells in vessels affected by cerebral vasospasm. Surg. Neurol. 2000;53(3):260–266. doi: 10.1016/S0090-3019(99)00187-1. [DOI] [PubMed] [Google Scholar]
- 97.Kishore S., Ko N., Soares B.P., Higashida R.T., Tong E., Bhogal S., Bredno J., Cheng S.C., Wintermark M. Perfusion-CT assessment of blood-brain barrier permeability in patients with aneurysmal subarachnoid hemorrhage. J. Neuroradiol. 2012;39(5):317–325. doi: 10.1016/j.neurad.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt J.M., Wartenberg K.E., Fernandez A., Claassen J., Rincon F., Ostapkovich N.D., Badjatia N., Parra A., Connolly E.S., Mayer S.A. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J. Neurosurg. 2008;109(6):1052–1059. doi: 10.3171/JNS.2008.109.12.1052. [DOI] [PubMed] [Google Scholar]
- 99.Dankbaar J.W., de Rooij N.K., Velthuis B.K., Frijns C.J., Rinkel G.J., van der Schaaf I.C. Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke. 2009;40(11):3493–3498. doi: 10.1161/STROKEAHA.109.559013. [DOI] [PubMed] [Google Scholar]
- 100.Sanelli P.C., Ugorec I., Johnson C.E., Tan J., Segal A.Z., Fink M., Heier L.A., Tsiouris A.J., Comunale J.P., John M., Stieg P.E., Zimmerman R.D., Mushlin A.I. Using quantitative CT perfusion for evaluation of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 2011;32(11):2047–2053. doi: 10.3174/ajnr.A2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wintermark M., Sincic R., Sridhar D., Chien J.D. Cerebral perfusion CT: technique and clinical applications. J. Neuroradiol. 2008;35(5):253–260. doi: 10.1016/j.neurad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 102.Aviv R.I., d’Esterre C.D., Murphy B.D., Hopyan J.J., Buck B., Mallia G., Li V., Zhang L., Symons S.P., Lee T.Y. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009;250(3):867–877. doi: 10.1148/radiol.2503080257. [DOI] [PubMed] [Google Scholar]
- 103.Ivanidze J., Kesavabhotla K., Kallas O.N., Mir D., Baradaran H., Gupta A., Segal A.Z., Claassen J., Sanelli P.C. Evaluating blood-brain barrier permeability in delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 2015;36(5):850–854. doi: 10.3174/ajnr.A4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murphy A., Manoel A.L., Burgers K., Kouzmina E., Lee T., Macdonald R.L., Bharatha A. Early CT perfusion changes and blood-brain barrier permeability after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2015;57(8):767–773. doi: 10.1007/s00234-015-1529-1. [DOI] [PubMed] [Google Scholar]
- 105.Ivanidze J., Kallas O.N., Gupta A., Weidman E., Baradaran H., Mir D., Giambrone A., Segal A.Z., Claassen J., Sanelli P.C. Application of blood-brain barrier permeability imaging in global cerebral edema. AJNR Am. J. Neuroradiol. 2016;37(9):1599–1603. doi: 10.3174/ajnr.A4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mir D.I., Gupta A., Dunning A., Puchi L., Robinson C.L., Epstein H.A., Sanelli P.C. CT perfusion for detection of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 2014;35(5):866–871. doi: 10.3174/ajnr.A3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ivanidze J., Ferraro R.A., Giambrone A.E., Segal A.Z., Gupta A., Sanelli P.C. AJR Am. J. Roentgenol. 2018;211(4):891–895. doi: 10.2214/AJR.17.18237. [DOI] [PubMed] [Google Scholar]
- 108.Mocco J., Prickett C.S., Komotar R.J., Connolly E.S., Mayer S.A. Potential mechanisms and clinical significance of global cerebral edema following aneurysmal subarachnoid hemorrhage. Neurosurg. Focus. 2007;22(5):E7. doi: 10.3171/foc.2007.22.5.8. [DOI] [PubMed] [Google Scholar]
- 109.Shigeno T., Fritschka E., Brock M., Schramm J., Shigeno S. Cervoś-Navarro, J. Cerebral edema following experimental subarachnoid hemorrhage. Stroke. 1982;13(3):368–379. doi: 10.1161/01.STR.13.3.368. [DOI] [PubMed] [Google Scholar]
- 110.Dreier J.P., Lemale C.L., Kola V., Friedman A., Schoknecht K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology. 2018;134(Pt B):189–207. doi: 10.1016/j.neuropharm.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 111.Hayman E.G., Wessell A., Gerzanich V., Sheth K.N., Simard J.M. Mechanisms of global cerebral edema formation in aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2017;26(2):301–310. doi: 10.1007/s12028-016-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang C., Jiang M., Wang W-Q., Zhao S-J., Yin Y-X., Mi Q-J., Yang M-F., Song Y-Q., Sun B-L., Zhang Z-Y. Selective mglur1 negative allosteric modulator reduces blood-brain barrier permeability and cerebral edema after experimental subarachnoid hemorrhage. Transl. Stroke Res. 2019;11(4):799–811. doi: 10.1007/s12975-019-00758-z. [DOI] [PubMed] [Google Scholar]
- 113.Kreiter K.T., Copeland D., Bernardini G.L., Bates J.E., Peery S., Claassen J., Du Y.E., Stern Y., Connolly E.S., Mayer S.A. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002;33(1):200–208. doi: 10.1161/hs0102.101080. [DOI] [PubMed] [Google Scholar]
- 114.Claassen J., Carhuapoma J.R., Kreiter K.T., Du E.Y., Connolly E.S., Mayer S.A. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33(5):1225–1232. doi: 10.1161/01.STR.0000015624.29071.1F. [DOI] [PubMed] [Google Scholar]
- 115.Lublinsky S., Major S., Kola V., Horst V., Santos E., Platz J., Sakowitz O., Scheel M., Dohmen C., Graf R., Vatter H., Wolf S., Vajkoczy P., Shelef I., Woitzik J., Martus P., Dreier J.P., Friedman A. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. EBioMedicine. 2019;43:460–472. doi: 10.1016/j.ebiom.2019.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Rooij N.K., Greving J.P., Rinkel G.J., Frijns C.J. Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage: development and validation of a practical risk chart. Stroke. 2013;44(5):1288–1294. doi: 10.1161/STROKEAHA.113.001125. [DOI] [PubMed] [Google Scholar]
- 117.Moroz J., Reinsberg S.A. Dynamic Contrast-Enhanced MRI. Methods Mol. Biol. 2018;1718:71–87. doi: 10.1007/978-1-4939-7531-0_5. [DOI] [PubMed] [Google Scholar]
- 118.Barnes S.R., Ng T.S., Montagne A., Law M., Zlokovic B.V., Jacobs R.E. Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn. Reson. Med. 2016;75(5):1967–1977. doi: 10.1002/mrm.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Russin J.J., Montagne A., D’Amore F., He S., Shiroishi M.S., Rennert R.C., Depetris J., Zlokovic B.V., Mack W.J. Permeability imaging as a predictor of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2018;38(6):973–979. doi: 10.1177/0271678X18768670. [DOI] [PMC free article] [PubMed] [Google Scholar]