Highlights
An in situ molecular foaming and activation strategy is designed and investigated for the synthesis of hierarchically porous N-doped carbon foams (HPNCFs).
The prepared HPNCFs possess 3D macropores, uniform micropores and mesopores, ultrahigh surface areas and high N contents and show high performances in supercapacitors and CO2 capture.
Electronic supplementary material
The online version of this article (10.1007/s40820-020-0389-3) contains supplementary material, which is available to authorized users.
Keywords: Porous carbon foams, Hierarchical pore structure, Nitrogen doping, Supercapacitors, CO2 capture
Abstract
Hierarchically porous carbon materials are promising for energy storage, separation and catalysis. It is desirable but fairly challenging to simultaneously create ultrahigh surface areas, large pore volumes and high N contents in these materials. Herein, we demonstrate a facile acid–base enabled in situ molecular foaming and activation strategy for the synthesis of hierarchically macro-/meso-/microporous N-doped carbon foams (HPNCFs). The key design for the synthesis is the selection of histidine (His) and potassium bicarbonate (PBC) to allow the formation of 3D foam structures by in situ foaming, the PBC/His acid–base reaction to enable a molecular mixing and subsequent a uniform chemical activation, and the stable imidazole moiety in His to sustain high N contents after carbonization. The formation mechanism of the HPNCFs is studied in detail. The prepared HPNCFs possess 3D macroporous frameworks with thin well-graphitized carbon walls, ultrahigh surface areas (up to 3200 m2 g−1), large pore volumes (up to 2.0 cm3 g−1), high micropore volumes (up to 0.67 cm3 g−1), narrowly distributed micropores and mesopores and high N contents (up to 14.6 wt%) with pyrrolic N as the predominant N site. The HPNCFs are promising for supercapacitors with high specific capacitances (185–240 F g−1), good rate capability and excellent stability. They are also excellent for CO2 capture with a high adsorption capacity (~ 4.13 mmol g−1), a large isosteric heat of adsorption (26.5 kJ mol−1) and an excellent CO2/N2 selectivity (~ 24).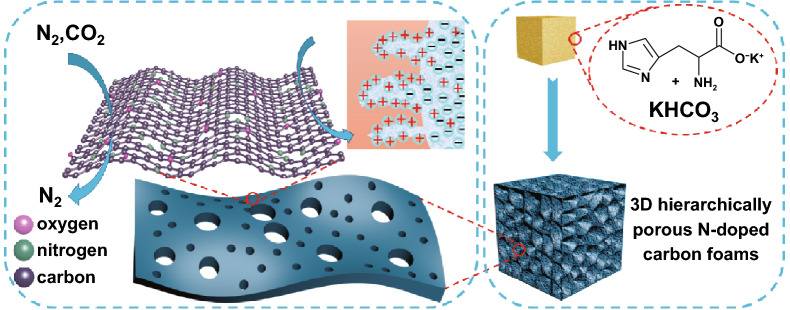
Electronic supplementary material
The online version of this article (10.1007/s40820-020-0389-3) contains supplementary material, which is available to authorized users.
Introduction
Porous carbon materials have received wide interest because of their attractive physicochemical properties, easy compatibility with other elements, and low cost and toxicity, as well as wide applications in energy storage and conversion, adsorption and catalysis [1–7]. A well-controlled pore structure is significantly important for these materials in various applications. Taking supercapacitors and CO2 capture as examples [4, 8–13], which are important for upgrading fossil fuel utilization in a greener and more sustainable way, uniform micropores and high surface areas are desirable to provide abundant active sites for enhanced storage of small-sized ions and gas molecules. However, the accessibility of micropores within thick carbon walls is low, even inaccessible for bulk molecular diffusion [4, 14, 15]. To overcome this limitation, creating mesopores in microporous carbon walls to establish short diffusion paths and large pore volumes is effective, especially so for improving charge storage at a high current density [16, 17]. In addition, a macroporous network can act as a molecule- or ion-buffering reservoir for shortening diffusion time [14]. Therefore, it is attractive to construct hierarchically macro-/meso-/microporous carbon materials (HPCs) with well-defined structures and ultrahigh surface areas to further enhance their potential.
There are a series of methods reported in the literature for the synthesis of HPCs [18]. Based on the type of precursors adopted, three general methods can be categorized. The first method is the use of preformed carbon nanomaterials, such as graphene, for the construction of low-density HPCs in the form of aerogels [19, 20]. These materials possess moderate surface areas and lack of control in the micro-/mesopore structure. The second method is carbonization of natural biomass or synthetic polymers [21, 22]. For example, carbonization of natural biomass can result in various interesting HPCs [23–34]. Control of the pore size and distribution in these HPCs is relatively difficult. In addition, their carbon walls are often relatively thick such that the accessibility of the micropores may be restricted. The third method is the templating synthesis starting from molecular precursors [35–38]. Various templates can be adopted to confine the carbonization of different precursors. In particular, the dual templating approach, in which colloid nanospheres and surfactants act as the hard and soft templates, is capable of synthesizing ordered hierarchical structures with uniform and controllable pore sizes [35, 39–42]. Nevertheless, this templating method is complicated, costly and time-consuming. Comparatively, the salt templating approach starting from molecularly mixed precursors and salts, sometimes combining with ice templating, chemical blowing and leavening, is general and cost-effective for generating various HPCs with interesting structures [43–55]. During carbonization of the precursors, the diffusion and growth of the salts or their thermal decomposition products play the templating role generating hierarchical pores. The resulted HPCs often possess broad pore size distributions with variable surface areas. In order to improve the microporosity and surface area of HPCs, post-activation with various chemical activators, especially KOH, is often adopted. A disadvantage for post-activation is the high dosage of activator and the non-uniform mixing between carbon and activator, which can subsequently result in uneven activation and excessive etching. In spite of the above development, it is still fairly challenging to simultaneously create ultrahigh surface areas, uniform micro-/mesopores and large pore volumes in HPCs.
On the other hand, doping of nitrogen (N) in porous carbon materials can increase the electronic conductivity, wettability and basicity, which is highly desirable for supercapacitor and selective CO2 capture [16, 56–60]. There are two major methods for N doping in carbon materials. The first method is post-treatment under high temperatures by exposing preformed carbon materials in ammonia or other N-containing substances [61, 62]. This method may cause uneven distribution of N and structure change. The more common method is in situ doping by directly pyrolysis of a single N-containing organic precursor or precursor mixture [35, 60, 63–67]. Although a series of porous N-doped carbon materials have been reported, the construction of three-dimensional HPCs with ultrahigh surface areas, large pore volumes, and high N contents sustained at high carbonization temperatures is still highly demanded.
In this work, we demonstrate an acid–base enabled in situ foaming and activation strategy for the synthesis of hierarchically macro-/meso-/microporous N-doped carbon foams (HPNCFs). Our concept for the synthesis design lies in the following aspects. First, the selected amino acid (His) as the carbon precursor shows a self-foaming behavior under heat treatment, and the selected salt PBC undergoes decomposition releasing CO2 to facilitate the foaming process, allowing the formation of 3D macroporous frameworks with thin walls. Second, the PBC/His acid–base reaction allows a molecular mixing for subsequent in situ uniform activation to generate narrowly distributed micropores and mesopores. Third, the imidazole moiety of His allows the sustain of a high N content after carbonization. The formation mechanism of the HPNCFs is illustrated by a detailed study. The resultant HPNCFs possess attractive properties, including 3D foam structures constructed by thin carbon walls, ultrahigh surface areas (~ 3200 m2 g−1), large pore volumes (~ 2.0 cm3 g−1), narrowly distributed micropores and mesopores and high N contents (~ 14.6 wt%). They are promising for supercapacitors showing high specific capacitances, a good rate capability and an excellent stability, as well as attractive for CO2 capture with a large adsorption capacity and an excellent CO2/N2 selectivity.
Experimental Section
Preparation of HPNCFs
The HPNCFs were prepared by a simple acid–base enabled in situ foaming and activation method. The details of the chemicals adopted for the synthesis can be found in Supporting Information. Briefly, a certain amount (0.9687–3.8748 g) of PBC was dissolved in deionized water (100 mL), and then, 2.0 g of His was added into the above solution. After completely dissolved, the obtained clear solution was transferred to an eggplant-shaped steaming bottle. The solvent was removed at 50 °C under a reduced pressure of 20–50 mbar in a rotary evaporation unit. The resulted mixture was collected in a ceramic boat and was subject to a thermal treatment in a tube furnace. The sample was heated from room temperature to various target temperatures (400–900 °C) with a heating speed of 2 °C min−1 and kept isothermal at the target temperature for 3 h under flowing N2 atmosphere (~ 60 mL min−1). Then, after natural cooling, the obtained composites were soaked in water at 60 °C overnight, followed by filtration, washing with deionized water and ethanol several times and drying in a vacuum oven at 60 °C for 10 h, leading to the final samples, which were denoted as HPNCF-X-Y, where X stands for the molar ratio of PBC/His, and Y for the carbonization temperature (in °C), respectively. A control sample was also prepared by carbonizing pure His without the addition of PBC following the same procedure. The characterization details of the obtained samples are provided in Supporting Information.
Electrochemical Tests
The electrochemical tests were measured on a CHI 760e electrochemical workstation to evaluate the supercapacitor charge storage performance of the HPNCFs. In the measurement system, a Pt plate was used as the counter electrode, and a Hg/HgO electrode was served as the reference electrode. The working electrode was fabricated from the mixture of a specific HPNCF sample (80 wt%), a conductive agent (acetylene black, 10 wt%) and a binding agent PTFE (10 wt%). To prepare the working electrode, the mixture was dispersed in 2.5 mL of ethanol in an ultrasonic cleaner to form a uniform slurry and then was dried at 80 °C for 2 h to evaporate the liquid. The dried black powder was pressed onto a neat nickel foam (~ 1 × 1 cm2) to obtain a thin electrode membrane. The composite foam was then dried in a vacuum oven at 60 °C for 12 h. Cyclic voltammetry (CV), galvanostatic charge and discharge (GCD), electrochemical impedance spectroscopy (EIS) and cyclic tests were carried out through a three-electrode system from 0.0 to − 1.0 V with a 6.0 M KOH solution as the electrolyte. CV experiments were conducted under different sweep rates of 5–100 mV s−1. EIS was carried out over a frequency range of 100–0.01 Hz with a 5.0 mV AC potential amplitude. The specific capacitance (Cs, F g−1) of the work electrode is calculated by using Eq. 1:
| 1 |
where I stands for the current density (A g−1), Δt is the discharging time (s), ΔV is the potential range in volt and m for the mass (g) of the active HPNCF material, respectively.
CO2 Adsorption Tests
The gas adsorption performance for CO2 and N2 was evaluated by measuring the adsorption/desorption isotherms of single components and 273 and 298 K on the Micromeritics ASAP 2020 analyzer. The capacity was retrieved from the adsorption isotherms. The adsorption selectivity was calculated by using Henry’s law. The isosteric heat of adsorption (ΔHads) was calculated by using the Clausius–Clapeyron equation (Eq. 2):
| 2 |
where T1 and T2 (K) are the two temperatures for adsorption isotherms measurement, P1 and P2 are the pressure points on the two isotherms wherein the adsorption capacities are the same.
Results and Discussion
HPNCFs Formation Mechanism
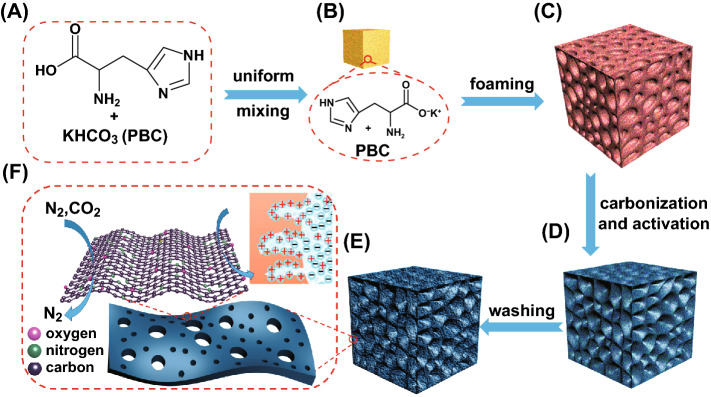
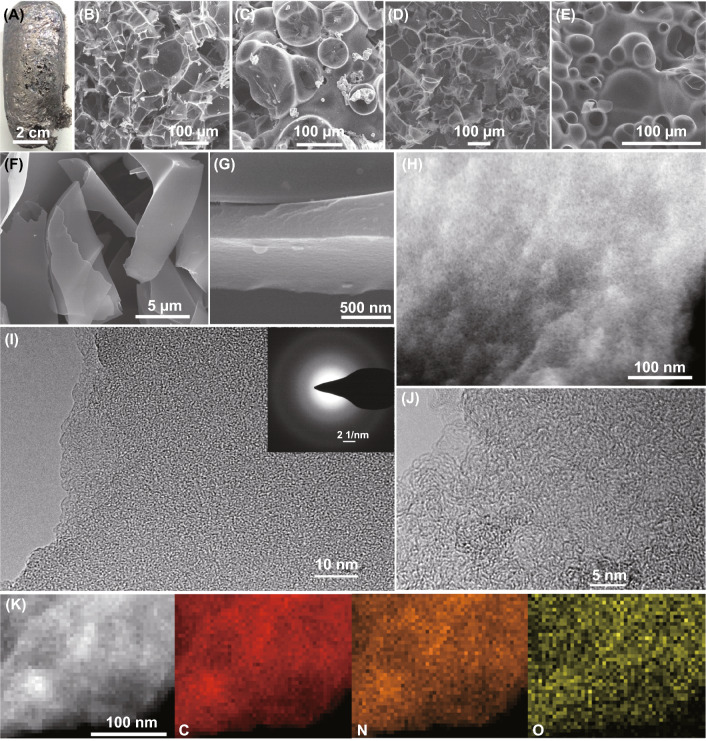
To synthesize the HPNCFs, an acid–base enabled in situ foaming and activation strategy has been proposed (Scheme 1). To demonstrate the formation mechanism and the unique features of resulted HPNCFs, the synthesis process with its intermediates has been analyzed. First, PBC and His can partially neutralize each other by the reaction between the bicarbonate ions of PBC and the carboxylic group of His (Eq. 3, and Scheme 1A, B). This can be validated by the decreased pH values of the mixed PBC/His solutions compared with the pure PBC solutions (Fig. S1) and the observed CO2 gas bubbling during mixing PBC and His in water. Such a neutralization reaction renders the molecular mixing of His and PBC (Scheme 1B), further revealed by the formation of a uniform PBC/His composite with evenly distributed C, O, N and K elements (Fig. S2). Such a molecular mixing is beneficial for in situ foaming and chemical activation. The wide-angle XRD pattern of the dried PBC/His mixture shows a group of diffraction peaks different from those of the pure PBC and His (Fig. S3), indicating the formation of a new phase probably assigned to the potassium salt of His. Next, the solid is subject to heating for in situ foaming, carbonization and chemical activation. During the heating process, sophisticated physicochemical changes are involved. Pure His is stable up to ~ 270 °C, followed by melting, polymerization and gradual carbonization at increasing temperatures (Fig. 1A, curve a). The PBC/His mixture shows a gradual mass loss at 100–270 °C (Fig. 1A, curve b), attributed to the decomposition of PBC releasing CO2 and H2O as shown in Eq. 4, indicating that the foaming probably starts at about 270 °C. The TG curve of PBC with a weight loss of ~ 30 wt% at 100–210 °C further validates the gas releasing process (Fig. 1A, curve c). This gas formation process may partially overlap with the melting of His, facilitating in situ foaming of the molten/dissolved His liquid (Scheme 1C), leading to the development of a macroporous spongy structure, similar to the leavening process [52]. The optical image after the heating treatment verifies the occurrence of foaming and the formation of a 3D macroporous foam (Fig. 2A). At 270–600 °C, the thermal behaviors of pure His and the PBC/His mixture are similar (Fig. 1A, curves a and b), indicating that chemical activation does not obviously occur at this stage. In this stage, condensation of His occurs with continuous weight loss due to the removal of water, CO2 and volatile N-containing molecular fragments. These released gases can lead to increased porosity in the 3D foams. The wide-angle XRD pattern of the composite obtained at 400 °C reveals the formation of a K2CO3·1.5H2O phase (JCPDS No. 11-0655) (Fig. 1B, curve a), indicating the decomposition of PBC. At a temperature of 600 °C, two crystalline phases assigned to K2CO3·1.5H2O and K2CO3 (JCPDS No. 49-1093) (Fig. 1B, curve b) can be observed. The C, N, O, and K elements are still uniformly distributed in the composite (Fig. S4a-c). The FTIR spectra of the composites obtained at 400 and 600 °C show several bands ~ 2950, 2450, 1430, 840, and 710 cm−1 (Fig. S5a, b), indicating the presence of K2CO3. In the temperature range of 700–900 °C, the TG curves of pure His and the PBC/His derived mixture are significantly different (Fig. 1A, curves a and b), because dramatic chemical activation occurs at this stage with the carbonaceous walls becoming increasingly thinner (Scheme 1D). At a temperature of 700–900 °C, His can be carbonized. Meanwhile, potassium oxide (K2O) can be formed because of the decomposition of K2CO3 and the reaction of K2CO3 with carbon as shown in Eqs. 5 and 6. The produced K2O and CO2 can in situ react with carbon as shown in Eqs. 7 and 8, generating plenty of small pores and potassium (K). The K vapor can diffuse into the carbon matrix and intercalated into graphitic layers, leading to continuous reaction with oxygen-containing carbon walls. The wide-angle XRD patterns of the composites obtained at 700–900 °C show the formation of various crystalline phases including K2O (JCPDS Nos. 26-1327 and 27-0431), K (JCPDS Nos. 89-3993 and 89-4080) and K2CO3 (minor) (Fig. 1B, curves c-e), confirming the occurrence of dramatic chemical activation. It should be pointed out that further decomposition of K2CO3 decomposed from pristine PBC cannot be observed at 700–900 °C for pure PBC (69 wt% remained, Fig. 1A, curve c), indicating that the presence of carbon can promote the decomposition of K2CO3 into K2O and K. From the PBC/His mixture with a molar ratio of 2.0, a low residual of only ~ 10.4 wt% at 900 °C confirms the formation and escape of K vapor. The elements are still uniformly distributed in the composites obtained at 700–900 °C (Fig. S4d-f), indicating that uniform chemical activation can be achieved. The FTIR spectra of the composites obtained at 700–900 °C show that the bands attributed to K2CO3 become weakened (Fig. S5c-e), in accordance with the result that K2CO3 can be converted to K2O and K in this temperature range. Interestingly, N2 sorption results show that the composites obtained at 400–900 °C possess no detectable porosity (Scheme 1D, Fig. S6a, b). This is mostly because the generated pores in the carbon walls by activation are occupied by K, K2O and other salts, verifying the occurrence of in situ molecular level activation. SEM images confirm the formation of a foam structure in the composite obtained at 900 °C, with the presence of a 3D macroporous structure inside and hollow carbon capsules on the surface originating from gas foaming (Fig. 2B, C). Finally, washing with water results in the HPNCFs (Scheme 1E). The foam structures are well maintained (Fig. 2D, E). Moreover, due to the presence of an imidazole moiety in His, a high N content (6.31 wt%) can be sustained even at 900 °C. The HPNCFs are suitable for supercapacitors and CO2 adsorption (Scheme 1F). The 3D macropores act as buffer reservoirs for guest molecules, the mesopores enhance mass transfer, and the micropores and N sites offer high specific surface areas and abundant active sites.
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
Scheme 1.
Schematic illustration of the synthesis process of the HPNCFs: A precursor selection, B the composite obtained after molecular mixing by acid–base reaction, C the composite obtained after in situ foaming at low temperatures, D the composite obtained after in situ carbonization and chemical activation at high temperatures, E the HPNCFs obtained after washing by water, and F structure model of the HPNCFs and their applications in CO2/N2 separation and charge storage
Fig. 1.
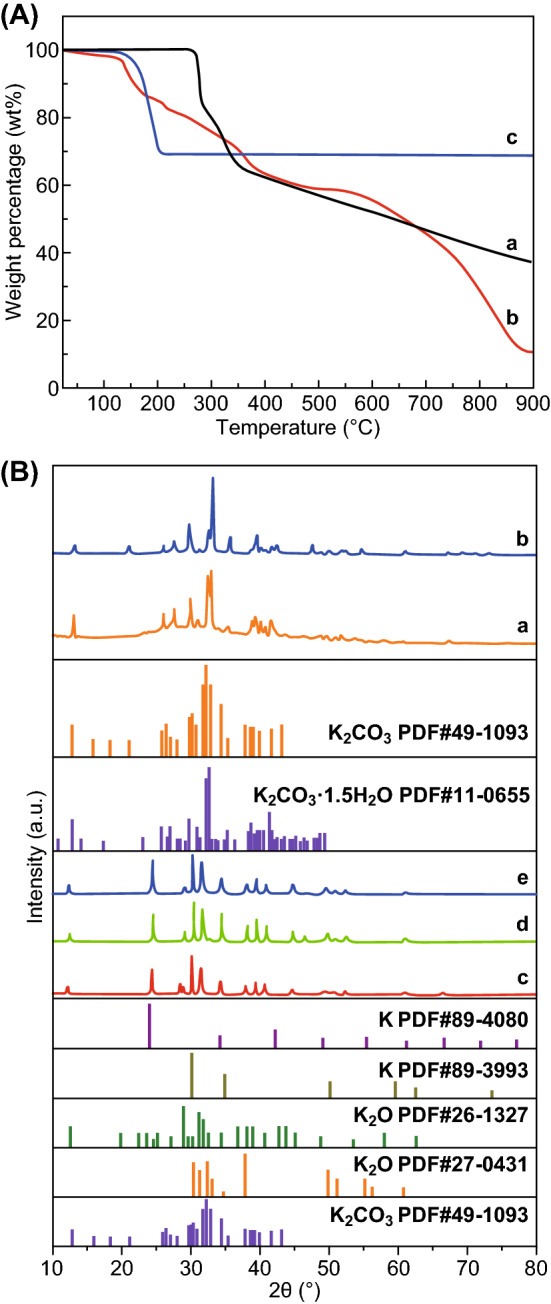
A TG curve under N2 of the pure His (a), PBC/His mixture with a molar ratio of 2.0 (b) and pure PBC (c). B Wide-angle XRD patterns of the composites obtained by heating the PBC/His mixture with a molar ratio of 2.0 at 400 (a), 600 (b), 700 (c), 800 (d) and 900 °C (e)
Fig. 2.
A Optical, B–G SEM, H DF-STEM, I TEM, inset I SAED pattern, J HRTEM, and K elemental maps of the HPNCF-2.0-900 sample before (A–C) and after (D–K) water washing
Morphology and Structure of HPNCFs
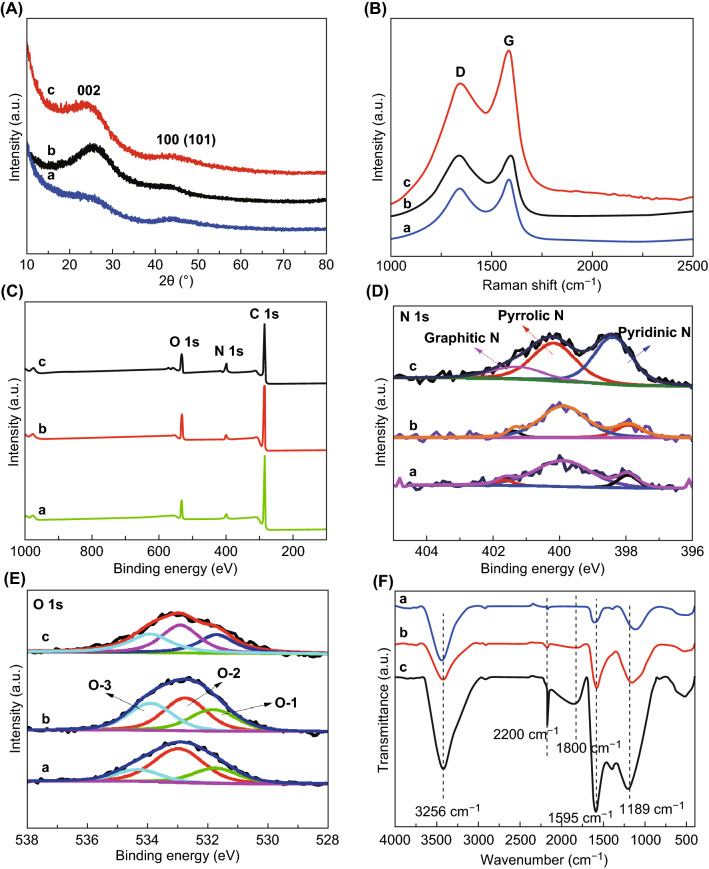
All the HPNCFs possess a 3D hierarchically macro-/meso-/microporous structure. By using the sample HPNCF-2.0-900 obtained with a PBC/His molar ratio of 2.0 at 900 °C as a typical example, the wide-angle XRD pattern shows two weak and broad diffraction peaks at ~ 25 and 44º corresponding to (002) and (100)/(101) planes of graphitized carbon (Fig. 3A, curve a). No other peaks can be detected, implying that the K-containing substances can be washed off by water. The Raman spectrum of the sample displays two distinct bands at ~ 1341 cm−1 (D band) and ~ 1586 cm−1 (G band) (Fig. 3B, curve a). The first one can be assigned to carbon of amorphous or defective nature, and the second one to sp2 hybridized carbon of graphitic nature. The intensity ratio of the D and G bands (ID/IG) is 0.97, indicating a good graphitization degree. The sample shows a 3D foam morphology with interconnected macropores inside and some hollow carbon capsules on the surface (Fig. 2D, E). The carbon skeleton is made up of thin and smooth carbon plates (Fig. 2F). The thickness of the carbon plates is down to ~ 300 nm (Fig. 2G). Such an open 3D thin foam structure accounts for the ultralow density (about 0.03 g cm−3) of the sample. TEM and dark-field scanning TEM (DF-STEM) images show the presence of uniform micropores and small mesopores within the carbon plates (Fig. 2H, I), which are originating from the etching of carbon by chemical activation. The micropores are in a highly disordered orientation, and they are distributed uniformly throughout the carbon plates because of the in situ chemical activation. HRTEM image shows that very short and randomly orientated (002) graphitic layers with a d-spacing of ~ 0.37 nm can be observed (Fig. 2J). Two very weak diffraction rings indexed to the (002) and (101) crystal plate of graphitic carbon can be observed in the selected area electron diffraction (SAED) pattern (inset in Fig. 2I), further indicating that the carbon walls are moderately graphitized.
Fig. 3.
A Wide-angle XRD patterns, B Raman spectra, C XPS survey spectra, D N 1s XPS spectra, E O 1s XPS spectra, and F FTIR spectra of HPNCF-2.0-900 (a), HPNCF-2.0-800 (b), and HPNCF-2.0-700 (c)
Heat treatment at 700–900 °C with a fixed PBC/His molar ratio of 2.0 does not obviously influence the overall morphology and structure of the resultant HPNCFs. An obvious difference is that the thickness of carbon plates becomes thinner with the increase in temperature (Fig. 4A, B, and 2F). This can be explained by two facts. The first one is that the carbon yield of His decreases sharply with the increase in temperature. The second one is that the chemical activation becomes increasingly violent at higher temperatures. The wide-angle XRD patterns show that the sample obtained at 800 °C possesses the best resolved (002) diffraction peak (Fig. 3A). This is because a higher temperature induces better carbonization, but also triggers more intensive chemical activation increasing structure disorder. The medium temperature of 800 °C balances the two factors leading to the most carbonized walls. The Raman spectra of the samples obtained at 700–900 °C are similar, with the lowest ID/IG ratio observed for the sample obtained at 700 °C (Fig. 3B), which is due to the more intensive activation at higher temperatures causing loss of graphitic order.
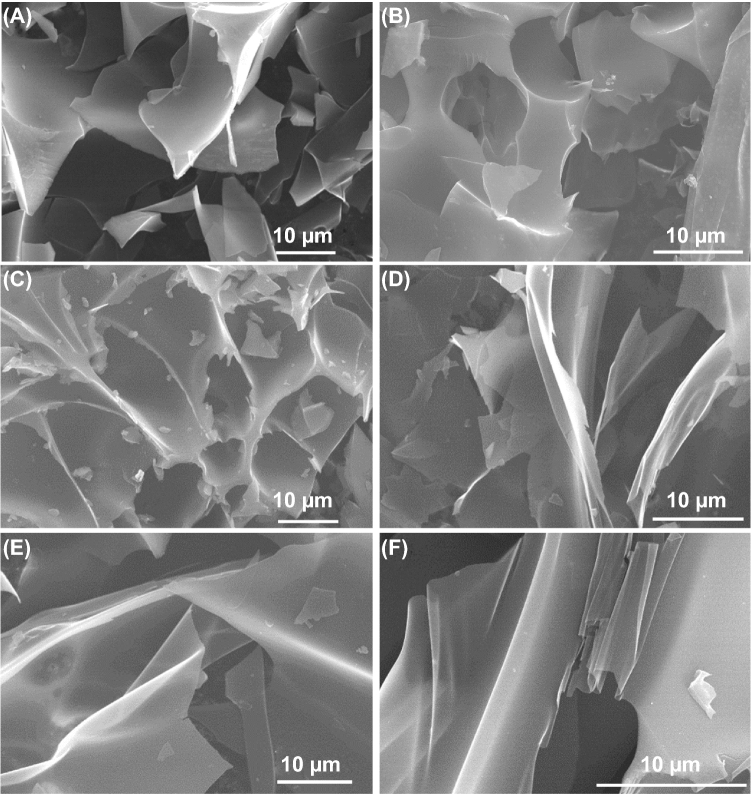
Fig. 4.
SEM images of A HPNCF-2.0-700, B HPNCF-2.0-800, C HPNCF-0.75-900, D HPNCF-1.0-900, E HPNCF-1.5-900, and F HPNCF-2.5-900
PBC/His molar ratios of 0.75–2.5 with the fixed temperature of 900 °C also have no obvious influence on the overall morphology and structure of the resultant HPNCFs. There is also a general trend that the carbon plates become increasingly thinner with the increase in PBC dosage (Fig. 4C–F). This is because of the decreased carbon yield and the more violent chemical activation with higher PBC dosages. The wide-angle XRD patterns show that the intensity of the (002) diffraction peak decreases with the increase in the PBC/His ratio (Fig. S7A), because the enhanced chemical activation can result in increased carbon etching. The Raman spectra show a general increasing trend for the ID/IG ratio with the increase in the PBC/His ratio (Fig. S7B), further revealing that the enhanced chemical activation reduces graphitic order.
Textural Properties
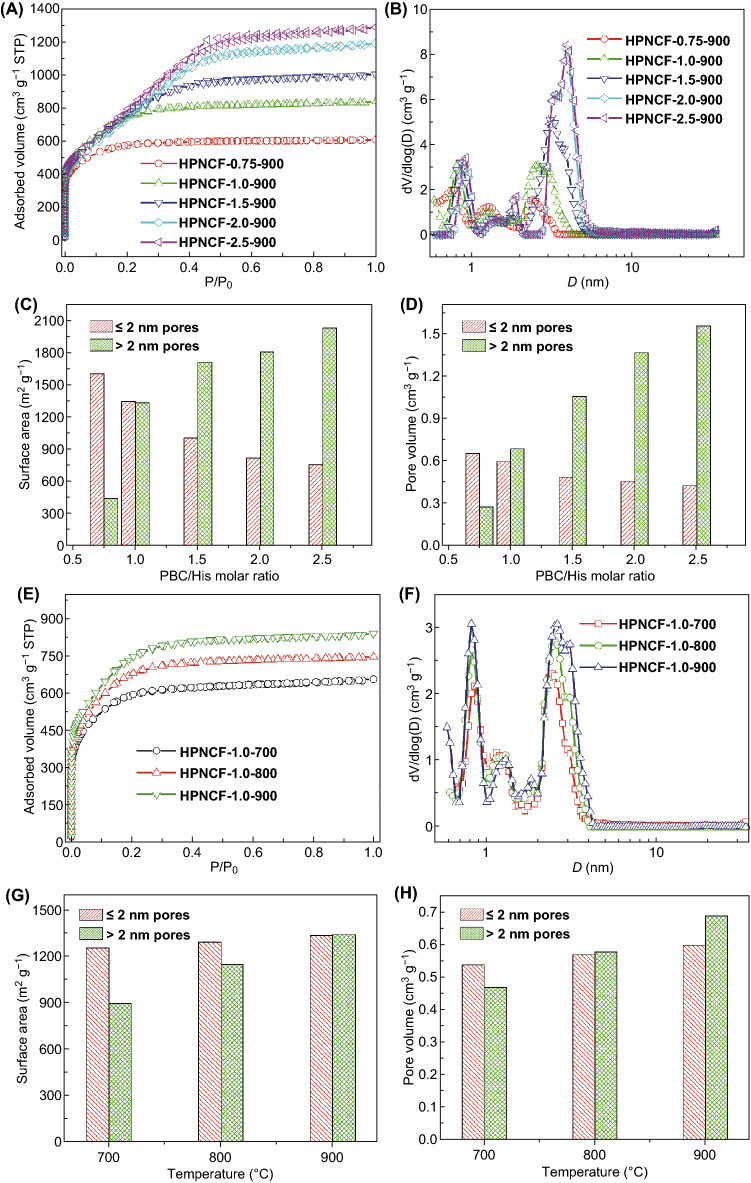
All the HPNCFs possess ultrahigh surface areas, high micropore surface areas, large pore volumes and narrowly distributed micropores and small mesopores (Fig. 5 and Table 1). The representative sample HPNCF-2.0-900 displays N2 adsorption/desorption isotherms of combinative types I and IV (Fig. 5A), indicative of a hierarchical meso-/microporous material. Notably, at P/P0 < 0.1, there is a sharp and large N2 uptake because of the N2 filling in micropores. At a P/P0 range of 0.2–0.5, there is a N2 condensation step with a slight H2-type hysteresis in the desorption branch, indicating the presence of uniform and small mesopores. The sample possesses an ultrahigh specific surface area of ~ 2634 m2 g−1 and a large total pore volume of ~ 1.83 cm3 g−1. Moreover, the micropore surface area and micropore volume are up to 823 m2 g−1 and 0.46 cm3 g−1. These parameters are highly competitive compared with many HPCs in the literature (Table S1). The corresponding pore size distribution (PSD) curve of the sample reveals two narrow peaks centered at ~ 0.91 and 1.8 nm and a third peak centered at ~ 4.0 nm (Fig. 5B). In a sharp contrast, the control sample prepared from His without the addition of PBC is predominantly microporous (pore size < 0.8 nm) with a much lower surface area of 400 m2 g−1 and a significantly smaller pore volume of 0.25 cm3 g−1 (Fig. S6d). This result confirms the high efficiency of the acid–base enabled in situ chemical activation method.
Fig. 5.
N2 sorption isotherms (A, E) and the PSD curves (B, F) of the HPNCFs obtained at various PBC/His molar ratios and temperatures, and the corresponding pore-size-dependent surface area (C, G) and pore volume (D, H) variations with the increase in the PBC/His molar ratio at a fixed temperature of 900 °C (C, D) and with the increase in temperature at a fixed PBC/His molar ratio of 1.0 (G, H)
Table 1.
Summary of the textural properties and the N and O contents of the HPNCFs obtained at various PBC/His molar ratios and temperatures
| Sample name | SBET (m2 g−1) | Smicropore (m2 g−1) | Vtotal (cm3 g−1) | Vmicropore (cm3 g−1) | Micropore (nm) | Mesopore (nm) | N (wt%) | O (wt%) |
|---|---|---|---|---|---|---|---|---|
| HPNCF-0.75-900 | 2056 | 1611 | 0.94 | 0.66 | 0.77, 1.3 | 2.3 | 3.91 | 7.71 |
| HPNCF-1.0-900 | 2686 | 1348 | 1.29 | 0.60 | 0.82, 1.3, 1.8 | 2.6 | 4.62 | 11.87 |
| HPNCF-1.5-900 | 2730 | 1011 | 1.55 | 0.49 | 0.86, 1.4, 1.8 | 3.3 | 5.29 | 9.60 |
| HPNCF-2.0-900 | 2634 | 823 | 1.83 | 0.46 | 0.91, 1.4, 1.8 | 4.0 | 6.31 | 8.03 |
| HPNCF-2.5-900 | 2793 | 760 | 1.99 | 0.43 | 0.92, 1.5, 1.9 | 4.0 | 5.90 | 6.12 |
| HPNCF-1.0-700 | 2162 | 1261 | 1.01 | 0.54 | 0.85, 1.1 | 2.4 | 14.62 | 16.90 |
| HPNCF-1.0-800 | 2452 | 1298 | 1.15 | 0.57 | 0.82, 1.2 | 2.5 | 9.10 | 14.15 |
| HPNCF-2.0-700 | 3209 | 1381 | 1.66 | 0.67 | 0.88, 1.8 | 3.1 | 14.52 | 16.48 |
| HPNCF-2.0-800 | 2305 | 730 | 1.52 | 0.39 | 0.91, 1.8 | 3.9 | 9.96 | 13.44 |
All the HPNCFs obtained at 900 °C with various PBC/His molar ratios of 0.75–2.5 possess hierarchical meso-/micropores (Fig. 5A–D and Table 1). The sample HPNCF-0.75-900 is mainly microporous showing predominant type I N2 adsorption/desorption isotherms (Fig. 5A). With the increase in the PBC/His molar ratio, the resultant samples are more obviously mesoporous, indicating the intensified chemical activation and carbon wall etching with the increase in PBC (Fig. 5A-D). With the PBC/His molar ratio increased from 0.75 to 2.5, the total surface area increases from 2056 to 2793 m2 g−1, and the total pore volume increases sharply from 0.94 to 1.99 cm3 g−1 (Table 1). However, the micropore surface area decreases from 1611 to 760 m2 g−1 (Fig. 5C and Table 1), and the micropore volume decreases from 0.66 to 0.43 cm3 g−1 (Fig. 5D and Table 1). On the other hand, with the increase in the PBC/His molar ratio, the PSD curves show that the mesopore size increases from 2.3 to 4.0 nm, and the micropore size also increases to some extent (Fig. 5B and Table 1). The above trends clearly confirm that the increase in PBC can enhance the chemical activation to etch more carbon walls and generate pores with increased sizes.
All the HPNCFs obtained with a fixed PBC/His molar ratio at 700–900 °C possess similar hierarchical meso-/micropores (Fig. 5E–H, S8 and Table 1). At a low PBC/His molar ratio of 1.0, there are clear increasing trends for the total surface area (2162–2686 m2 g−1), micropore surface area (1261–1348 m2 g−1), total pore volume (1.01–1.29 cm3 g−1) and micropore volume (0.54–0.60 cm3 g−1) with the temperature increased from 700 to 900 °C (Fig. 5E–H, and Table 1), while the pore size increases slightly (Fig. 5F, Table 1). Differently, at a high PBC/His molar ratio of 2.0, with the temperature increased from 700 to 900 °C, while the total pore volume (1.66–1.83 cm3 g−1) and pore size (3.1–4.0 nm) increases obviously, the total surface area (3209–2634 m2 g−1), micropore surface area (1381–823 m2 g−1) and micropore volume (0.67–0.46 cm3 g−1) decrease obviously (Fig. S8 and Table 1). Therefore, enhanced chemical activation generating more micropores can be achieved with the increase in temperature at a relatively low PBC dosage. Significant etching of carbon walls generating mesopores can be promoted with the increase in temperature at a relatively high PBC dosage.
Chemical Composition and Surface Property
Elemental analyses reveal that all the HPNCFs are composed of C as the main component and O, N, and H as the minor ones. The DF-STEM image and the corresponding elemental maps show that the elements are evenly distributed in the sample (Fig. 2K). All the samples have high N contents (3.9–14.6 wt%) (Table 1), because the His precursor carries a theoretical N content of 22 wt% with a stable imidazole ring [66]. At a fixed PBC/His molar ratio of 2.0, with the temperate increased from 700 to 900 °C, the N content of the HPNCFs decreases from 14.52 to 6.31 wt% (Table 1), because the intensive carbonization process can break down the less stable N-containing moieties. At 900 °C, the N content of the HPNCFs generally increases (from about 4–6 wt%) with the increase in the PBC/His molar ratio (Table 1). This is probably because the etching of carbon becomes increasingly intensive with the increase in PBC, rendering relatively increased N contents. On the other hand, all the HPNCFs possess high O contents of 6.12–16.90 wt% (Table 1). With the increase in temperature, the O content decreases obviously. At the fixed temperature, there is a decreasing trend for the O content with the increase in PBC dosage. This is in agreement with the intensified carbon etching releasing CO and CO2 with the increase in PBC.
XPS survey spectra of the HPNCFs show three obvious bands assigned to C, N and O (Fig. 3C). The N contents estimated from the XPS analyses are in agreement with the results from element analyses. The high-resolution N 1s XPS spectra of the HPNCFs obtained at 700–900 °C can be well fitted into three component peaks centered at 401.4, 400.1 and 398 eV (Fig. 3D), respectively, which can be assigned to graphitic, pyrrolic and pyridinic N, respectively. Notably, with the increase in the temperature, the content of graphitic N is minimized and the content pyridinic N decreases significantly (Fig. 3D). Normally, with the increase in heating temperature, graphitic and pyridinic N are the most stable N sites in N-doped carbon materials. In the present case, the observed opposite trend is mostly because the enhanced chemical activation at higher temperatures breaks down the aromatic rings of the graphitic and pyridinic N sites. This allows a selective N-type doping in carbon materials, which will be studied in detail in our future work. On the other hand, the high-resolution O 1s XPS spectra (Fig. 3E) of all the HPNCFs can also be fitted into three components centered at 531.7, 532.6 and 533.7 eV, corresponding to the quinone-type oxygen (C=O, O-1), phenol-type oxygen (C–OH or C–O–C, O-2) and carboxyl-type oxygen (COO–, O-3), respectively. Among them, the O-2 band shows the highest intensity for all the samples. With the increase in the temperature, the O-2 and O-3 oxygen bands become weakened because of their relatively lower thermal stability.
The FTIR spectra of all the HPNCFs exhibit a broad absorption band at 3400 cm−1 with a small shoulder at ~ 3256 cm−1 (Fig. 3F), indicating the presence of hydroxyl groups probably from the sample surface and the adsorbed water. This peak becomes weaker with the increase in temperature. A strong sharp peak at ~ 2200 cm−1 and an obvious band at ~ 1800 cm−1 can be observed for the sample obtained at 700 °C (Fig. 3F, curve c), mostly assigned to the N=C=O and C=O moieties in the sample, and there might be some CO2 molecules adsorbed on the N sites contributing to the band at ~ 2200 cm−1. These two bands become significantly weakened in the samples obtained at higher temperatures (Fig. 3F, curves a and b), in agreement with the elemental analyses and XPS results showing dramatically decreased N and O contents. The observed broad bands at ~ 1189 and at 1595 cm−1 reveal the presence of benzene rings, C–O (O-2) and C–N bonding configurations. These two bands become gradually weakened with the increase in temperature because of the decreased N and O contents.
Performance in Supercapacitors
The HPNCFs are desirable for supercapacitors. They possess ultrahigh surface areas and uniform micropores for charge storage, uniform mesopores for fast mass transfer, and 3D macropores for electrolyte storage (Scheme 1F). Moreover, their O- and N-containing groups can facilitate the infiltration and diffusion of aqueous electrolytes. These groups can also provide oxidation–reduction pathways, which can supply faradaic pseudocapacitance.
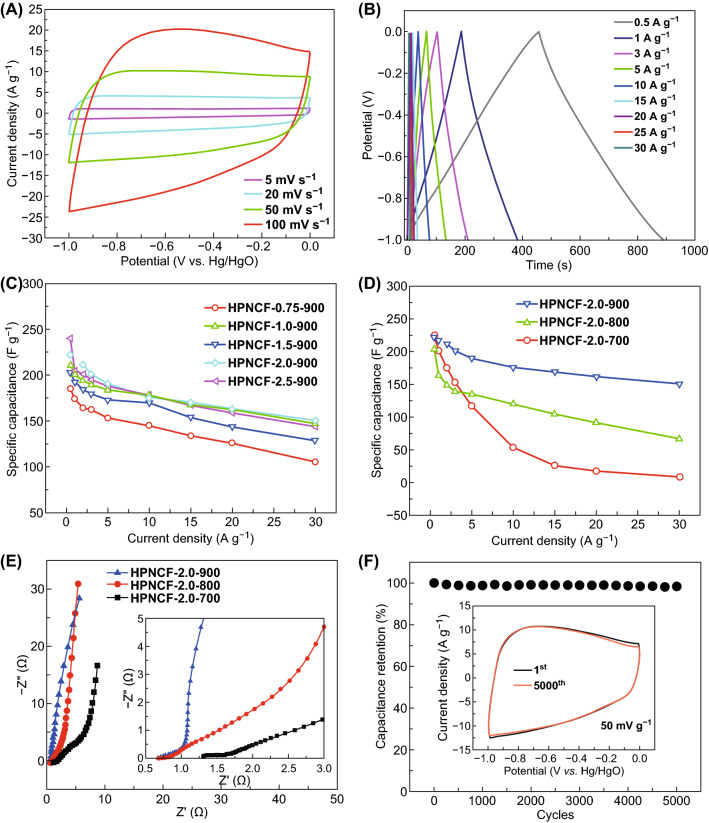
The CV curves of the electrode made of the typical sample HPNCF-2.0-900 shows a quasi-rectangular shape over a scan rate of 5–100 mV s−1 in a potential window of − 1.0 to 0 V by using a 6.0-M KOH aqueous electrolyte (Fig. 6A), indicating a typical characteristic of electrical double-layer capacitance (EDLC). At the scan rate of 5 mV/s, there are small and weak redox peaks in the CV curve at ~ − 0.4 V (Fig. S9), mostly because of the redox reactions induced by the N- and O-containing groups. The GCD curves of the HPNCF-2.0-900 electrode at the current densities of 0.5–30 A g−1 show similar isosceles triangle shapes with no obvious IR drop (Fig. 6B), indicating a high rate capability. The specific capacitance of the HPNCF-2.0-900 sample at a current density of 0.5 A g−1 is ~ 222 F g−1. At the high current density of 30 A g−1, a high specific capacitance of ~ 150 F g−1 (~ 68% of the capacitance at 0.5 A g−1) can be maintained (Fig. 6C). Such performance is better than or comparable to those of many reported N-doped carbon materials (Table S1). In the Nyquist plot of the HPNCF-2.0-900 electrode, there is no visible semicircle in the high-frequency range, and a steep line nearly parallel to the vertical axis can be observed in the low-frequency region (Fig. 6E), indicative of a fast charge transfer and ionic diffusion process and a typical double-layer capacitance behavior [68, 69]. From the intercept on the X-axis, the equivalent series resistance (ESR), which reflects the electrolyte ionic resistance, the working electrode electronic resistance and contact resistance at the interface of electrode/electrolyte [70], is only ~ 0.67 Ω. To further evaluate the impedance of electrochemical system, in the equivalent circuit, Rs (the cell resistance of electrolyte and electrode) is only ~ 0.68 Ω, and Rct (the charge transfer resistance) is only ~ 2.36 Ω (Fig. S10a), verifying that excellent charge transfer with a low resistance can be proceeded on the HPNCF-2.0-900 electrode. The rapid charge transfer is closely related to the structural features of HPNCF-2-900; that is, the 3D macroporous carbon network with hierarchal meso-/micropores can host large amount of electrolytes with a high wettability, provide abundant and accessible sites for charge storage and shorten the transfer paths of electrons and ions (Scheme 1F) [6, 28]. These features also make the HPNCF-2-900 electrode very stable. After 5000 cycles, the CV curves keep the same (inset in Fig. 6F), and only a slight capacitance loss of 1.84% can be observed (Fig. 6F).
Fig. 6.
A CV and B GCD curves, and F cycling test of the electrode made of the sample HPNCF-2.0-900. C Rate capability of the HPNCFs obtained at various PBC/His molar ratios with a fixed temperature of 900 °C. D, E Rate capability and Nyquist plots of the HPNCFs obtained at various temperatures with a fixed PBC/His molar ratio of 2.0
The temperature influences the supercapacitor performance significantly of the resultant HPNCFs. The sample obtained at 800 °C shows quasi-rectangular CV curves at low scan rates, but the CV curves are much distorted at high scan rates (Fig. S11b). Distorted CV curves can be observed for the sample obtained at 700 °C at all scan rates (Fig. S11a). The specific capacitances of the samples obtained at 700–900 °C at a low current density of 0.5 A g−1 are close (204–225 F g−1) (Fig. 6D). However, the capacitance maintains only 33 and 4% for the samples obtained at 800 and 700 °C (Fig. 6D), respectively, indicative of their poor rate capabilities because of their low electronic conductivity and large electric resistance. The Nyquist plot for the sample obtained at 700 °C shows a semicircle at the high-frequency region (Fig. 6E). In the low-frequency region, the samples obtained at 800 and 700 °C show small slopes for the straight lines (Fig. 6E). The ESR is estimated to be 0.79 and 1.32 Ω for the sample obtained at 800 and 700 °C. Besides, from the equivalent circuits (Fig. S10b, c), the Rs values for the samples obtained at 800 and 700 °C are 0.80 and 1.42 Ω, and the Rct values are up to ~ 70 and 1750 Ω, respectively. The above results reveal that the electrical resistance increases significantly for samples obtained at low temperatures, thus leading to the low rate capability.
For the HPNCFs samples obtained at various PBC/His molar ratios (0.75–2.5) at 900 °C, their specific capacitances are close (185–240 F g−1) at a current density of 0.5 A g−1 (Fig. 6C). Generally, the samples obtained at high PBC/His molar ratios possess relatively higher specific capacitances, probably due to the enhanced surface areas. On the other hand, their rate capability is similar, with 60–70% capacitance retained at 30 A g−1 (Fig. 6C), because these samples possess similar hierarchical macro-/meso-/micropores, as well as similar chemical compositions. These results indicate that the HPNCFs obtained at 900 °C are attractive for charge storage because of their hierarchical porosity, high surface areas and low electronic resistance.
CO2 Capture Performance
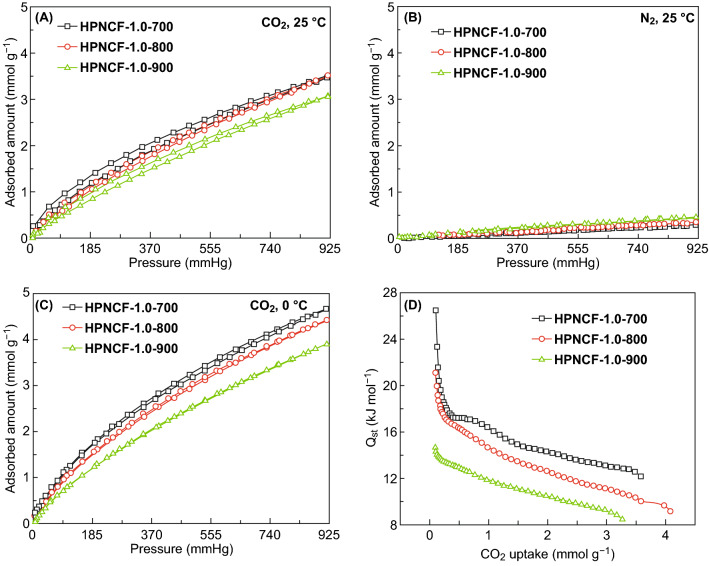
The HPNCFs possess high surface areas, large micropore volumes and high N contents. These features make them promising for CO2 capture [71–74]. The HPNCFs obtained with a PBC/His molar ratio of 1.0 at 700–900 °C are typical for CO2 capture because they are mainly microporous (Table 1). The CO2 adsorption isotherms at 25 °C of the sample HPNCF-1.0-700 show gradual uptake of CO2 with the increase in pressure, and a high adsorption capacity of ~ 3.1 mmol g−1 can be achieved at a CO2 pressure of 760 torr (Fig. 7A, and Table 2). The adsorption capacity at a CO2 pressure of 114 torr, the normal CO2 partial pressure of industrial flue gas, is up to ~ 0.81 mmol g−1. The adsorption capacities at these two pressures can be increased to ~ 4.13 and 1.27 mmol g−1 at an adsorption temperature of 0 °C (Fig. 7C, and Table 2). The CO2 adsorption capacity of the HPNCFs decreases with the increase in the temperature from 700 to 900 °C (Fig. 7A, C), in spite of the increase in the total surface area and pore volume (Table 1). This is because CO2 is a small acidic molecule and its adsorption capacity is more related to the micropore surface area and the N content. The sample obtained at 700 °C possesses the highest micropore surface area and N content, thus showing the best CO2 adsorption capacity. Because of the strong interactions of CO2 molecules with the N sites and micropore walls, the sample obtained at 700 °C also shows the highest CO2 isosteric heat of adsorption, up to ~ 26.5 kJ mol−1, about two times of that for the sample obtained at 900 °C (Fig. 7D). On the other hand, the adsorption capacities of N2 on the above HPNCFs samples are quite low (Fig. 7B), because of the weak interactions between N2 and the adsorbents. As a result, an excellent CO2/N2 adsorption selectivity of ~ 24 at 25 °C on the sample obtained at 700 °C can be achieved (Table 2). The adsorption selectivity for the HPNCFs decreases with the increase in the temperature (Table 2), which is because of the much decreased N content and micropore surface area. The CO2 capture performance (CO2 adsorption capacity and CO2/N2 adsorption selectivity) of the typical HPNCFs is competitive among typical N-doped HPCs (Table S1). In addition, during cyclic tests in CO2 capture on the typical sample HPNCF-1.0-700 (Fig. S12), both rapid adsorption and desorption of CO2 processes can be observed, indicative of a predominant physisorption. After six adsorption–desorption cycles, 92% of the initial adsorption capacity can be retained, indicating a high cyclic stability for CO2 capture.
Fig. 7.
CO2 (A, C) and N2 (B) sorption isotherms at 25 (A, B) and 0 °C (C), and the corresponding isosteric heat of adsorption curves (D) of HPNCF-1.0-700, HPNCF-1.0-800, and HPNCF-1.0-900
Table 2.
Summary of the CO2 capture performance of the typical HPNCFs
| Sample name | CO2 capacity at 25 °C | CO2 capacity at 0 °C | CO2/N2 selectivity at 25 °C | ||
|---|---|---|---|---|---|
| 114 torr | 760 torr | 114 torr | 760 torr | ||
| HPNCF-1.0-700 | 0.81 | 3.06 | 1.27 | 4.13 | 24.0 |
| HPNCF-1.0-800 | 0.69 | 3.04 | 1.07 | 3.93 | 17.3 |
| HPNCF-1.0-900 | 0.56 | 2.63 | 0.83 | 3.41 | 9.0 |
Conclusions
In summary, novel HPNCFs have been synthesized by the acid–base enabled in situ foaming and activation strategy. The key of the strategy is the self-foaming nature of His and the CO2 releasing behavior of PBC allowing the formation of 3D foam structure and the acid–base reaction enabling a molecular mixing for in situ chemical activation. With the increase in temperature, His undergoes gradual carbonization with high N contents sustained. Simultaneously, phase evolutions from PBC to K2CO3·1.5H2O and K2CO3 and then to K2O and K are elucidated. The K-containing substances can in situ react with carbon walls to achieve uniform chemical activation. The HPNCFs possess 3D macroporous frameworks with thin well-graphitized carbon walls, ultrahigh surface areas (2056–3200 m2 g−1), high micropore surface areas (760–1611 m2 g−1), large pore volumes (0.94–2.0 cm3 g−1), high micropore volumes (0.39–0.67 cm3 g−1), narrowly distributed micropores (0.8–1.9 nm) and mesopores (2.3–4.0 nm), and high N contents (3.9–14.6 wt%) with pyrrolic N as the predominant N site. The increase in the PBC/His ratio results in increases in total surface area, total pore volume, pore size and N content, but decreases in micropore surface area, micropore volume and carbon wall thickness. The increase in temperature results in sharp decreases in carbon wall thickness and N content. The temperature increase induces enhancement for all the textural parameters at low PBC/His ratios, while it leads to increase in pore volume and pore size but decrease in surface area at high PBC/His ratios. The HPNCFs are promising for supercapacitors and CO2 capture. The HPNCFs obtained at 900 °C show high specific capacitance (185–240 F g−1), good rate capability and excellent stability due to the high surface area for charge storage, low electric resistance and short paths for fast electrolyte and electron transfer. The HPNCFs obtained at 700 °C show a high CO2 adsorption capacity (4.13 and 1.27 mmol g−1 at 114 and 760 torr), a large isosteric heat of adsorption (26.5 kJ mol−1) and an excellent CO2/N2 adsorption selectivity (~ 24). Finally, the in situ foaming and activation strategy may be extended for the synthesis of other carbon-based hierarchical structures for various applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Financial support from the National Natural Science Foundation of China (Nos. 21875153, 21501125), the Natural Science Foundation of Jiangsu Province (BK20150312), and the Jiangsu Shuangchuang Team Program is appreciated. We also thank the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions and the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201708) for support.
References
- 1.Liu J, Wickramaratne NP, Qiao SZ, Jaroniec M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015;14:763–774. doi: 10.1038/nmat4317. [DOI] [PubMed] [Google Scholar]
- 2.Zhang LL, Zhao XS. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009;38:2520–2531. doi: 10.1039/B813846J. [DOI] [PubMed] [Google Scholar]
- 3.Arico AS, Bruce P, Scrosati B, Tarascon JM, Van Schalkwijk W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005;4:366–377. doi: 10.1142/9789814317665_0022. [DOI] [PubMed] [Google Scholar]
- 4.Wang DW, Li F, Liu M, Lu GQ, Cheng H-M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed. 2008;47:373–376. doi: 10.1002/anie.200702721. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Zhao D. Ordered mesoporous materials as adsorbents. Chem. Commun. 2011;47:3332–3338. doi: 10.1039/c0cc04909c. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Kim J, Chung DY, Yoo JM, Lee HS, et al. Design principle of Fe–N–C electrocatalysts: how to optimize multimodal porous structures? J. Am. Chem. Soc. 2019;141:2035–2045. doi: 10.1021/jacs.8b11129. [DOI] [PubMed] [Google Scholar]
- 7.Tian H, Liang J, Liu J. Nanoengineering carbon spheres as nanoreactors for sustainable energy applications. Adv. Mater. 2019;31:e1903886. doi: 10.1002/adma.201903886. [DOI] [PubMed] [Google Scholar]
- 8.Simon P, Gogotsi Y. Materials for electrochemical capacitors. Nat. Mater. 2008;7:845–854. doi: 10.1142/9789814287005_0033. [DOI] [PubMed] [Google Scholar]
- 9.Kondrat S, Pérez CR, Presser V, Gogotsi Y, Kornyshev AA. Effect of pore size and its dispersity on the energy storage in nanoporous supercapacitors. Energy Environ. Sci. 2012;5:6474. doi: 10.1039/c2ee03092f. [DOI] [Google Scholar]
- 10.Sevilla M, Ferrero GA, Diez N, Fuertes AB. One-step synthesis of ultra-high surface area nanoporous carbons and their application for electrochemical energy storage. Carbon. 2018;131:193–200. doi: 10.1016/j.carbon.2018.02.021. [DOI] [Google Scholar]
- 11.Creamer AE, Gao B. Carbon-based adsorbents for postcombustion CO2 capture: a critical review. Environ. Sci. Technol. 2016;50:7276–7289. doi: 10.1021/acs.est.6b00627. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Huang L, Yang R, Zhang Z, Wu J, Gao Y, Wang Q, O’Hare D, Zhong Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014;7:3478–3518. doi: 10.1039/C4EE01647E. [DOI] [Google Scholar]
- 13.Sevilla M, Al-Jumialy ASM, Fuertes AB, Mokaya R. Optimization of the pore structure of biomass-based carbons in relation to their use for CO2 capture under low- and high-pressure regimes. ACS Appl. Mater. Interfaces. 2018;10:1623–1633. doi: 10.1021/acsami.7b10433. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Yang X, Zhang F, Long G, Zhang T, et al. Controlling the effective surface area and pore size distribution of sp2 carbon materials and their impact on the capacitance performance of these materials. J. Am. Chem. Soc. 2013;135:5921–5929. doi: 10.1021/ja402552h. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharjya D, Kim M-S, Bae T-S, Yu J-S. High performance supercapacitor prepared from hollow mesoporous carbon capsules with hierarchical nanoarchitecture. J. Power Sources. 2013;244:799–805. doi: 10.1016/j.jpowsour.2013.01.112. [DOI] [Google Scholar]
- 16.Lin TQ, Chen IW, Liu FX, Yang CY, Bi H, Xu FF, Huang FQ. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science. 2015;350:1508–1513. doi: 10.1126/science.aab3798. [DOI] [PubMed] [Google Scholar]
- 17.Yao L, Wu Q, Zhang P, Zhang J, Wang D, et al. Scalable 2D hierarchical porous carbon nanosheets for flexible supercapacitors with ultrahigh energy density. Adv. Mater. 2018;30:1706054. doi: 10.1002/adma.201706054. [DOI] [PubMed] [Google Scholar]
- 18.Yang XY, Chen LH, Li Y, Rooke JC, Sanchez C, Su BL. Hierarchically porous materials: synthesis strategies and structure design. Chem. Soc. Rev. 2017;46:481–558. doi: 10.1039/C6CS00829A. [DOI] [PubMed] [Google Scholar]
- 19.Xu YX, Lin ZY, Zhong X, Huang XQ, Weiss NO, Huang Y, Duan XF. Holey graphene frameworks for highly efficient capacitive energy storage. Nat. Commun. 2014;5:4554. doi: 10.1038/ncomms5554. [DOI] [PubMed] [Google Scholar]
- 20.Xu YX, Chen CY, Zhao ZP, Lin ZY, Lee C, et al. Solution processable holey graphene oxide and its derived macrostructures for high-performance supercapacitors. Nano Lett. 2015;15:4605–4610. doi: 10.1021/acs.nanolett.5b01212. [DOI] [PubMed] [Google Scholar]
- 21.Guan T, Zhao J, Zhang G, Wang J, Zhang D, Li K. Template-free synthesis of honeycomblike porous carbon rich in specific 2–5 nm mesopores from a pitch-based polymer for a high-performance supercapacitor. ACS Sustain. Chem. Eng. 2019;7:2116–2126. doi: 10.1021/acssuschemeng.8b04736. [DOI] [Google Scholar]
- 22.Miao L, Qian X, Zhu D, Chen T, Ping G, et al. From interpenetrating polymer networks to hierarchical porous carbons for advanced supercapacitor electrodes. Chin. Chem. Lett. 2019;30:1445–1449. doi: 10.1016/j.cclet.2019.03.010. [DOI] [Google Scholar]
- 23.Qu G, Jia S, Wang H, Cao F, Li L, et al. Asymmetric supercapacitor based on porous N-doped carbon derived from pomelo peel and NiO arrays. ACS Appl. Mater. Interfaces. 2016;8:20822–20830. doi: 10.1021/acsami.6b06630. [DOI] [PubMed] [Google Scholar]
- 24.Karnan M, Subramani K, Sudhan N, Ilayaraja N, Sathish M. Aloe vera derived activated high-surface-area carbon for flexible and high-energy supercapacitors. ACS Appl. Mater. Interfaces. 2016;8:35191–35202. doi: 10.1021/acsami.6b10704. [DOI] [PubMed] [Google Scholar]
- 25.Zhang CY, Zhu XH, Cao M, Li ML, Li N, Lai LQ, Zhu JL, Wei DC. Hierarchical porous carbon materials derived from sheep manure for high-capacity supercapacitors. ChemSusChem. 2016;9:932–937. doi: 10.1002/cssc.201501624. [DOI] [PubMed] [Google Scholar]
- 26.Chen CJ, Zhang Y, Li YJ, Dai JQ, Song JW, et al. All-wood, low tortuosity, aqueous, biodegradable supercapacitors with ultra-high capacitance. Energy Environ. Sci. 2017;10:538–545. doi: 10.1039/C6EE03716J. [DOI] [Google Scholar]
- 27.Dong X, Jin H, Wang R, Zhang J, Feng X, et al. High volumetric capacitance, ultralong life supercapacitors enabled by waxberry-derived hierarchical porous carbon materials. Adv. Energy Mater. 2018;8:1702695. doi: 10.1002/aenm.201702695. [DOI] [Google Scholar]
- 28.Zhang Q, Han K, Li S, Li M, Li J, Ren K. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors. Nanoscale. 2018;10:2427–2437. doi: 10.1039/C7NR07158B. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Xiong L, Xu D, Cai J, Huang L, Zhou J, Zhang L. Distinctive construction of chitin-derived hierarchically porous carbon microspheres/polyaniline for high-rate supercapacitors. ACS Appl. Mater. Interfaces. 2018;10:28918–28927. doi: 10.1021/acsami.8b05891. [DOI] [PubMed] [Google Scholar]
- 30.Kesavan T, Sasidharan M. Palm spathe derived N-doped carbon nanosheets as a high performance electrode for Li-ion batteries and supercapacitors. ACS Sustain. Chem. Eng. 2019;7:12160–12169. doi: 10.1021/acssuschemeng.9b01261. [DOI] [Google Scholar]
- 31.Peng L, Liang Y, Huang J, Xing L, Hu H, Xiao Y, Dong H, Liu Y, Zheng M. Mixed-biomass wastes derived hierarchically porous carbons for high-performance electrochemical energy storage. ACS Sustain. Chem. Eng. 2019;7:10393–10402. doi: 10.1021/acssuschemeng.9b00477. [DOI] [Google Scholar]
- 32.Peng X, Zhang L, Chen Z, Zhong L, Zhao D, et al. Hierarchically porous carbon plates derived from wood as bifunctional ORR/OER electrodes. Adv. Mater. 2019;31:1900341. doi: 10.1002/adma.201900341. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Zhang M, Wang L, Hou Y, Guo C, Xin H, Xu S. A biomass carbon material with microtubule bundling and natural O-doping derived from goldenberry calyx and its electrochemical performance in supercapacitor. Chin. Chem. Lett. 2019 doi: 10.1016/j.cclet.2019.05.045. [DOI] [Google Scholar]
- 34.Xuan C, Peng Z, Wang J, Lei W, Xia K, Wu Z, Xiao W, Wang D. Biomass derived nitrogen doped carbon with porous architecture as efficient electrode materials for supercapacitors. Chin. Chem. Lett. 2017;28:2227–2230. doi: 10.1016/j.cclet.2017.09.009. [DOI] [Google Scholar]
- 35.Han P, Chung S-H, Manthiram A. Pyrrolic-type nitrogen-doped hierarchical macro/mesoporous carbon as a bifunctional host for high-performance thick cathodes for lithium–sulfur batteries. Small. 2019;15:1900690. doi: 10.1002/smll.201900690. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Sun Y, Zhang L, Li K, Yi Y, et al. Space-confined polymerization: controlled fabrication of nitrogen-doped polymer and carbon microspheres with refined hierarchical architectures. Adv. Mater. 2019;31:e1807876. doi: 10.1002/adma.201807876. [DOI] [PubMed] [Google Scholar]
- 37.Qiu D, Guan J, Li M, Kang C, Wei J, Li Y, Xie Z, Wang F, Yang R. Kinetics enhanced nitrogen-doped hierarchical porous hollow carbon spheres boosting advanced potassium-ion hybrid capacitors. Adv. Funct. Mater. 2019;29:1903496. doi: 10.1002/adfm.201903496. [DOI] [Google Scholar]
- 38.Sun S, Han F, Wu X, Fan Z. One-step synthesis of biomass derived O, N-codoped hierarchical porous carbon with high surface area for supercapacitors. Chin. Chem. Lett. 2019 doi: 10.1016/j.cclet.2019.11.023. [DOI] [Google Scholar]
- 39.Petkovich ND, Stein A. Controlling macro- and mesostructures with hierarchical porosity through combined hard and soft templating. Chem. Soc. Rev. 2013;42:3721–3739. doi: 10.1039/C2CS35308C. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Jiang R, Dou Y, Wu Z, Huang T, et al. Synthesis of mesoporous carbon spheres with a hierarchical pore structure for the electrochemical double-layer capacitor. Carbon. 2011;49:1248–1257. doi: 10.1016/j.carbon.2010.11.043. [DOI] [Google Scholar]
- 41.Chou T-C, Huang C-H, Doong R-A, Hu C-C. Architectural design of hierarchically ordered porous carbons for high-rate electrochemical capacitors. J. Mater. Chem. A. 2013;1:2886–2895. doi: 10.1039/C2TA01190E. [DOI] [Google Scholar]
- 42.Hasegawa G, Kanamori K, Kiyomura T, Kurata H, Abe T, Nakanishi K. Hierarchically porous carbon monoliths comprising ordered mesoporous nanorod assemblies for high-voltage aqueous supercapacitors. Chem. Mater. 2016;28:3944–3950. doi: 10.1021/acs.chemmater.6b01261. [DOI] [Google Scholar]
- 43.You B, Kang F, Yin P, Zhang Q. Hydrogel-derived heteroatom-doped porous carbon network for supercapacitor and electrocatalytic oxygen reduction. Carbon. 2016;103:9–15. doi: 10.1016/j.carbon.2016.03.009. [DOI] [Google Scholar]
- 44.Zhang F, Liu T, Li M, Yu M, Luo Y, Tong Y, Li Y. Multiscale pore network boosts capacitance of carbon electrodes for ultrafast charging. Nano Lett. 2017;17:3097–3104. doi: 10.1021/acs.nanolett.7b00533. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Tian L, Liang F, Wang J, Han L, et al. Molten salt synthesis of hierarchical porous N-doped carbon submicrospheres for multifunctional applications: high performance supercapacitor, dye removal and CO2 capture. Carbon. 2019;141:739–747. doi: 10.1016/j.carbon.2018.09.061. [DOI] [Google Scholar]
- 46.Zhu S, Li L, He C, Zhao N, Liu E, Shi C, Zhang M. Soluble salt self-assembly-assisted synthesis of three-dimensional hierarchical porous carbon networks for supercapacitors. J. Mater. Chem. A. 2015;3:22266–22273. doi: 10.1039/C5TA04646G. [DOI] [Google Scholar]
- 47.Zhang F, Liu T, Hou G, Kou T, Yue L, Guan R, Li Y. Hierarchically porous carbon foams for electric double layer capacitors. Nano Res. 2016;9:2875–2888. doi: 10.1007/s12274-016-1173-z. [DOI] [Google Scholar]
- 48.Li W, Zhang F, Dou Y, Wu Z, Liu H, et al. A self-template strategy for the synthesis of mesoporous carbon nanofibers as advanced supercapacitor electrodes. Adv. Energy Mater. 2011;1:382–386. doi: 10.1002/aenm.201000096. [DOI] [Google Scholar]
- 49.Zhao S, Yan T, Wang H, Zhang J, Shi L, Zhang D. Creating 3D hierarchical carbon architectures with micro-, meso-, and macropores via a simple self-blowing strategy for a flow-through deionization capacitor. ACS Appl. Mater. Interfaces. 2016;8:18027–18035. doi: 10.1021/acsami.6b03704. [DOI] [PubMed] [Google Scholar]
- 50.Hao J, Liao Y, Zhong Y, Shu D, He C, et al. Three-dimensional graphene layers prepared by a gas-foaming method for supercapacitor applications. Carbon. 2015;94:879–887. doi: 10.1016/j.carbon.2015.07.069. [DOI] [Google Scholar]
- 51.Hao J, Shu D, Guo S, Gao A, He C, et al. Preparation of three-dimensional nitrogen-doped graphene layers by gas foaming method and its electrochemical capactive behavior. Electrochim. Acta. 2016;193:293–301. doi: 10.1016/j.electacta.2016.02.048. [DOI] [Google Scholar]
- 52.Deng J, Xiong T, Xu F, Li M, Han C, Gong Y, Wang H, Wang Y. Inspired by bread leavening: one-pot synthesis of hierarchically porous carbon for supercapacitors. Green Chem. 2015;17:4053–4060. doi: 10.1039/C5GC00523J. [DOI] [Google Scholar]
- 53.Guan L, Pan L, Peng T, Qian T, Huang Y, et al. Green and scalable synthesis of porous carbon nanosheet-assembled hierarchical architectures for robust capacitive energy harvesting. Carbon. 2019;152:537–544. doi: 10.1016/j.carbon.2019.06.05. [DOI] [Google Scholar]
- 54.Diez N, Ferrero GA, Sevilla M, Fuertes AB. A sustainable approach to hierarchically porous carbons from tannic acid and their utilization in supercapacitive energy storage systems. J. Mater. Chem. A. 2019;7:14280–14290. doi: 10.1039/C9TA01712G. [DOI] [Google Scholar]
- 55.Shi R, Han C, Li H, Xu L, Zhang T, et al. NaCl-templated synthesis of hierarchical porous carbon with extremely large specific surface area and improved graphitization degree for high energy density lithium ion capacitors. J. Mater. Chem. A. 2018;6:17057–17066. doi: 10.1039/C8TA05853A. [DOI] [Google Scholar]
- 56.Wang JG, Liu HZ, Sun HH, Hua W, Wang HW, Liu XR, Wei BQ. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon. 2018;127:85–92. doi: 10.1016/j.carbon.2017.10.084. [DOI] [Google Scholar]
- 57.Li B, Dai F, Xiao Q, Yang L, Shen J, Zhang C, Cai M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016;9:102–106. doi: 10.1039/C5EE03149D. [DOI] [Google Scholar]
- 58.Zhao J, Lai H, Lyu Z, Jiang Y, Xie K, et al. Hydrophilic hierarchical nitrogen-doped carbon nanocages for ultrahigh supercapacitive performance. Adv. Mater. 2015;27:3541–3545. doi: 10.1002/adma.201500945. [DOI] [PubMed] [Google Scholar]
- 59.Zou K, Cai P, Liu C, Li J, Gao X, et al. A kinetically well-matched full-carbon sodium-ion capacitor. J. Mater. Chem. A. 2019;7:13540–13549. doi: 10.1039/C9TA03797G. [DOI] [Google Scholar]
- 60.Wei J, Zhou D, Sun Z, Deng Y, Xia Y, Zhao D. A controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for CO2 capture and supercapacitors. Adv. Funct. Mater. 2013;23:2322–2328. doi: 10.1002/adfm.201202764. [DOI] [Google Scholar]
- 61.Wang X, Lee JS, Zhu Q, Liu J, Wang Y, Dai S. Ammonia-treated ordered mesoporous carbons as catalytic materials for oxygen reduction reaction. Chem. Mater. 2010;22:2178–2180. doi: 10.1021/cm100139d. [DOI] [Google Scholar]
- 62.Wu Z, Webley PA, Zhao D. Post-enrichment of nitrogen in soft-templated ordered mesoporous carbon materials for highly efficient phenol removal and CO2 capture. J. Mater. Chem. 2012;22:11379–11389. doi: 10.1039/C2JM16183D. [DOI] [Google Scholar]
- 63.Song Z, Li L, Zhu D, Miao L, Duan H, et al. Synergistic design of a N, O co-doped honeycomb carbon electrode and an ionogel electrolyte enabling all-solid-state supercapacitors with an ultrahigh energy density. J. Mater. Chem. A. 2019;7:816–826. doi: 10.1039/C8TA10406A. [DOI] [Google Scholar]
- 64.Hao L, Ning J, Luo B, Wang B, Zhang Y, et al. Structural evolution of 2D microporous covalent triazine-based framework toward the study of high-performance supercapacitors. J. Am. Chem. Soc. 2015;137:219–225. doi: 10.1021/ja508693y. [DOI] [PubMed] [Google Scholar]
- 65.Yao Y, Chen Z, Zhang A, Zhu J, Wei X, et al. Surface-coating synthesis of nitrogen-doped inverse opal carbon materials with ultrathin micro/mesoporous graphene-like walls for oxygen reduction and supercapacitors. J. Mater. Chem. A. 2017;5:25237–25248. doi: 10.1039/C7TA08354H. [DOI] [Google Scholar]
- 66.Gao X, Chen Z, Yao Y, Zhou M, Liu Y, et al. Direct heating amino acids with silica: a universal solvent-free assembly approach to highly nitrogen-doped mesoporous carbon materials. Adv. Funct. Mater. 2016;26:6649–6661. doi: 10.1002/adfm.201601640. [DOI] [Google Scholar]
- 67.Benzigar MR, Talapaneni SN, Joseph S, Ramadass K, Singh G, et al. Recent advances in functionalized micro and mesoporous carbon materials: synthesis and applications. Chem. Soc. Rev. 2018;47:2680–2721. doi: 10.1039/C7CS00787F. [DOI] [PubMed] [Google Scholar]
- 68.Song Z, Zhu D, Li L, Chen T, Duan H, et al. Ultrahigh energy density of a N, O codoped carbon nanosphere based all-solid-state symmetric supercapacitor. J. Mater. Chem. A. 2019;7:1177–1186. doi: 10.1039/C8TA10158B. [DOI] [Google Scholar]
- 69.Portet C, Yushin G, Gogotsi Y. Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors. Carbon. 2007;45:2511–2518. doi: 10.1016/j.carbon.2007.08.024. [DOI] [Google Scholar]
- 70.Celzard A, Collas F, Mareche JF, Furdin G, Rey I. Porous electrodes-based double-layer supercapacitors: pore structure versus series resistance. J. Power Sources. 2002;108:153–162. doi: 10.1016/S0378-7753(02)00030-7. [DOI] [Google Scholar]
- 71.To JW, He J, Mei J, Haghpanah R, Chen Z, et al. Hierarchical N-doped carbon as CO2 adsorbent with high CO2 selectivity from rationally designed polypyrrole precursor. J. Am. Chem. Soc. 2016;138:1001–1009. doi: 10.1021/jacs.5b11955. [DOI] [PubMed] [Google Scholar]
- 72.Lee JH, Lee HJ, Lim SY, Kim BG, Choi JW. Combined CO2-philicity and ordered mesoporosity for highly selective CO2 capture at high temperatures. J. Am. Chem. Soc. 2015;137:7210–7216. doi: 10.1021/jacs.5b03579. [DOI] [PubMed] [Google Scholar]
- 73.Oschatz M, Antonietti MA. Search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018;11:57–70. doi: 10.1039/C7EE02110K. [DOI] [Google Scholar]
- 74.Hwang J, Walczak R, Oschatz M, Tarakina NV, Schmidt B. Micro-blooming: hierarchically porous nitrogen-doped carbon flowers derived from metal-organic mesocrystals. Small. 2019;15:e1901986. doi: 10.1002/smll.201901986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.