Highlights
Rhodium ion incorporation helps the nucleation of perovskite grain, passivates the defects in the grain boundaries and enhances the film quality, charge carrier lifetime and mobility.
After optimizing 1% rhodium into perovskite film, the solar cells achieve an efficiency of 20.71% without obvious hysteresis.
Electronic supplementary material
The online version of this article (10.1007/s40820-020-00457-7) contains supplementary material, which is available to authorized users.
Keywords: Perovskite solar cells, Grain boundary passivation, Rhodium incorporation
Abstract
Organic cation and halide anion defects are omnipresent in the perovskite films, which will destroy perovskite electronic structure and downgrade the properties of devices. Defect passivation in halide perovskites is crucial to the application of solar cells. Herein, tiny amounts of trivalent rhodium ion incorporation can help the nucleation of perovskite grain and passivate the defects in the grain boundaries, which can improve efficiency and stability of perovskite solar cells. Through first-principle calculations, rhodium ion incorporation into the perovskite structure can induce ordered arrangement and tune bandgap. In experiment, rhodium ion incorporation with perovskite can contribute to preparing larger crystalline and uniform film, reducing trap-state density and enlarging charge carrier lifetime. After optimizing the content of 1% rhodium, the devices achieved an efficiency up to 20.71% without obvious hysteresis, from 19.09% of that pristine perovskite. In addition, the unencapsulated solar cells maintain 92% of its initial efficiency after 500 h in dry air. This work highlights the advantages of trivalent rhodium ion incorporation in the characteristics of perovskite solar cells, which will promote the future industrial application.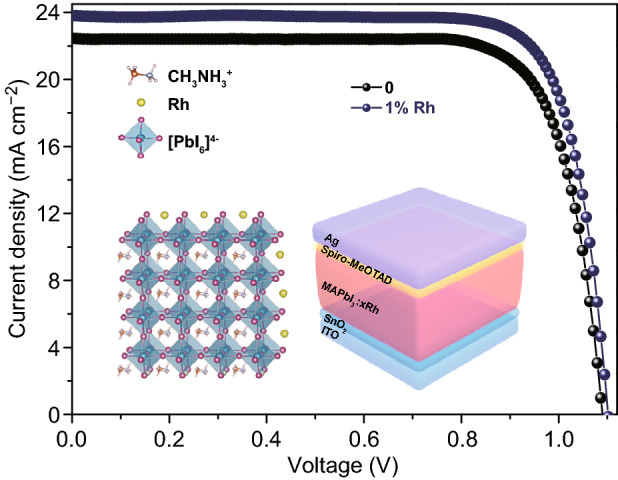
Electronic supplementary material
The online version of this article (10.1007/s40820-020-00457-7) contains supplementary material, which is available to authorized users.
Introduction
Halide perovskites have attracted great attention owing to the eminent optoelectronic properties, such as suitable energy bandgap [1–6], high absorption coefficient [7–9], long charge diffusion length [10–13] and high carrier mobility [14, 15]. Those advantages boost the improvement in perovskite solar cells (PSCs), being with certified power conversion efficiency (PCE) up to 25.2% [16].
However, pristine perovskite has inevitable internal defects, such as Pb and I vacancy defects, especially in gain boundary, which reduce the performance and stability of PSCs. To date, doping metal ion is an effective strategy to reduce defects, which can improve efficiency and stability of PSCs [17]. For example, doping with monovalent metal cations (Cu+, Ag+ or Li+) was applied to reduce trap-state density [18, 19], improved perovskite crystallinity and film quality, thus enhanced performance of PSCs. By doping bivalent cations (Mn2+, Co2+ or Zn2+), tuned electronic band structures and crystalline morphology were received and improved the stability of PSCs [20–27]. Trivalent metal cations (In3+, Eu3+ and Al3+) were often used to decrease deep defects, optimize film morphology and increase efficiency and stability of PSCs [28–31]. Especially for Eu3+ doping, device achieves long-term durability with 92% of the original PCE under continuous illumination for 1500 h [31]. Hence, finding more trivalent metal ions to obtain excellent properties of PSCs is important. The radius of Rh3+ is 67 pm, which is less than Pb2+ (119 pm). A smaller radius allows both interstitial doping and the possibility of partial replacement of Pb2+. Moreover, 4d orbital of Rh3+ probably tunes electrical properties of perovskite films by heterovalent incorporation [32, 33]. Clearly, compared to elements doped without d orbitals (such as, Al3+), such method definitely advances hybrid perovskite materials [30]. Therefore, it is anticipated that Rh3+ can be used in hybrid perovskite to enhance the properties of devices.
Here, we utilized Rh3+ to be incorporated with MAPbI3 for developing MAPbI3:xRh3+ (where x is excessive rhodium mol ratio, and x = 0, 0.5, 1.0 and 5.0 mol%). Rh3+ incorporation with tiny amounts can help the nucleation of perovskite grain and passivate the defects in perovskite film boundaries. In addition, 1% Rh3+ incorporation with perovskite films possesses larger crystalline and less pinhole, which leads to reduce trap-state density and enlarge charge carrier lifetime. Planar heterojunction PSCs with 1% Rh3+ incorporation exhibit high PCE of 20.71% and significantly suppressed photocurrent hysteresis. Compared to other studies of PSCs, the PSCs based on Rh3+ incorporation MAPbI3 achieved higher PCE (Table S11). Meanwhile, the devices of 1% Rh3+ incorporation have high stability within 500 h without encapsulation in dry air. This work highlights the advantages of Rh3+ incorporation in PSCs, which can promote the future industrial application.
Experimental Section
First-Principle Calculation
All of the density functional theory (DFT) calculations were employed by VASP code [34]. Generalized gradient approximation (GGA) of the projector augmented wave (PAW) was employed [35]. The plane-wave energy cutoff is 500 eV. The energy cutoff convergence is 1 × 10−4 eV, and the force cutoff convergence is − 0.09 eV Å−1 [36, 37]. For perovskite structure, 3 × 3×3 Monkhorst–Pack grid is taken [38]. The results of calculated lattice constants are well agreed with the experimental from Rietveld refinement (Table S2).
Preparation of Perovskite Single Crystals
For MAPbI3 single crystal, PbI2 (2.835 g) and MAI (0.978 g) were mixed in 5 mL γ-butyrolactone under stirring at 70 °C for 2 h. After heating the solution at 150 °C for 24 h, some obvious single crystals were obtained, which were washed with γ-butyrolactone and dried at 50 °C. Select a relatively large single crystal as the seed crystal, the above reaction was repeated once [11]. For MAPbI3:xRh (where x = 0.5, 1 and 5%) single crystal, x is excessive RhI3 incorporation, and the mixed PbI2 and MAI solution was added 0.5, 1 and 5 mol% (0.015, 0.030 and 0.149 g) RhI3, respectively.
Preparation of Devices
SnO2 dense layer (2.67%, diluted by deionized water) was prepared for the cleaned ITO substrate by spin-coated method. For MAPbI3:xRh (where x = 0, 0.5, 1, 5%) solution, 159 mg MAI, 470 mg PbI2 and 0, 0.5, 1 and 5 mol % (0, 2.42, 4.84 and 24.2 mg) RhI3 were separately dissolved in 0.8 mL DMSO:DMF (1:4) solution under room temperature. In total, 35 μL perovskite solution dipped on the SnO2 layers and then spun at 4000 rpm for 20 s. In total, 300 μL chlorobenzene was dipped on the substrate to enhance the film quality when the spinning at 10 s. Then, the substrate was annealed at hot plate. The hole transporting layer Spiro-OMeTAD was spin coated as our previous work [21]. Finally, the PSCs were evaporated 150 nm Ag (0.8 Å s−1) with area of 0.09 cm2.
Results and Discussion
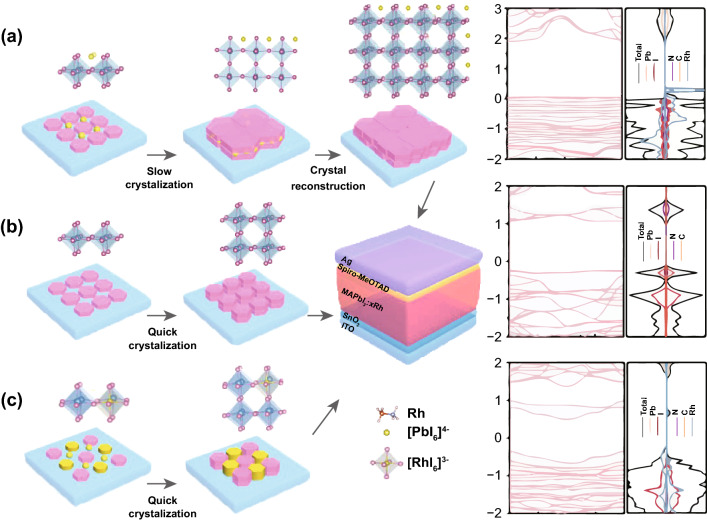
The characteristics of device are closely related to the morphology of the absorption film. Figure 1 shows top-view SEM pictures of MAPbI3:xRh (x = 0, 0.5%, 1% and 5%) (x represents Rh excessive incorporation). At low Rh3+-incorporated concentration, such as x = 0.5% and 1%, the grain size becomes larger than pristine MAPbI3 film without Rh3+-incorporated (Fig. 1f, g). However, at high Rh3+-incorporated concentration (where x = 5%), the quality of film becomes worse (Fig. 1h). The phenomenon was explained both theoretically and experimentally. From the experimental perspective, when the Rh3+-incorporated concentration is 0.5% and 1%, a small quantity of Rh3+ aggregated near the octahedral [PbI6]4−, decreasing the process of perovskite crystallization, organizing large crystalline and uniform perovskite films (Fig. 2a). However, when the Rh3+-incorporated concentration is over 5%, on the one hand, a large number of excessive Rh3+ aggregate at octahedral [PbI6]4− and prevent the crystallization of perovskite, resulting in the poor-quality film; on the other hand, both Rh3+ and Pb2+ may be acted as the nuclei to quick crystallization and form discontinuous perovskite films (Fig. 2c) [24]. Thus, when perovskite thin films are based on MAPbI3:xRh (where x = 5%), the film appears pinhole (Fig. 1h). The reason for this change in perovskite films is the different speed of film formation (Fig. 2). From a theoretical point of view, when the Rh3+-incorporated concentration of Rh3+ is 1%, Rh3+ through chemical bonds with the surrounding [PbI6]4− is to induce ordered arrangement and reduce defects [18]. However, when the Rh3+-incorporated concentration of Rh3+ is up to 5%, partial Rh3+ may replace Pb2+ to form additional perovskite structures. From intersecting surface SEM pictures of PSCs fabricated by MAPbI3 and MAPbI3:xRh (where x = 1%), there is no pinhole in the cross section after Rh3+ incorporation. This shows that Rh3+ incorporation makes both plane and cross section of perovskite continuous [39]. The film thicknesses with its deviations from (where x = 0, 1%) layers are 380 and 400 nm. Electron energy loss spectroscopy (EELS) mapping analysis of MAPbI3:xRh (where x = 1%) shows that the atomic % is also similar to the ratio of experimental preparation (Table S1). From EELS mapping, most of rhodium atoms are distributed at the grain boundary. By grain boundary passivation of rhodium ions, larger perovskite grains without pinholes film were formed (Fig. 1g). In order to explore physical mechanism of MAPbI3:xRh materials, we prepared the MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%) single crystal (Fig. S3). From Fig. 1k, l, XRD was measured to research the crystallinity of MAPbI3:xRh single crystalline. The peaks of 14° and 28° that correspond to the (101) and (202) lattice planes can be clearly seen from XRD, which is indicated that the MAPbI3:xRh possesses excellent crystallinity. No other peaks are observed for the MAPbI3:xRh perovskites, which are indicated that the Rh3+ and Pb2+ cations have not formed different kinds of phases. Moreover, the diffraction angles are slight lessening when it is 0.5% Rh3+ incorporation MAPbI3 (Fig. 1k). The reason of that is the unit cell of MAPbI3:xRh (where x = 0.5%) was enlarging from Rietveld refinement result (Table S2). However, when there is excessive 5% Rh3+ incorporation, the peak shift to a higher diffraction angle indicates the lattice parameters decrease. Maybe smaller Rh3+ cations partially displace the larger Pb2+ cations and the cell volume decreases (Table S2).
Fig. 1.
SEM pictures of MAPbI3:xRh [where x = 0 (a, e), 0.5% (b, f), 1% (c, g) and 5% (d, h)] films. i, j Cross-sectional pictures of PSCs fabricated by MAPbI3:xRh (where x = 0, 1%). k, l XRD patterns of the MAPbI3:xRh single crystal (where x = 0, 0.5%, 1% and 5%)
Fig. 2.
Schematic plot of mechanism for Rh3+-induced perovskite crystallization. a 1% Rh3+-incorporated in MAPbI3 through Rh-N band and Rh-I band to form large crystalline structure. b Pristine MAPbI3 film formed by quick crystallization. c 5% Rh3+-incorporated in MAPbI3 film. The diagram on the right is the corresponding band structures and density of states from the first principles (a interstitial 6% Rh incorporation, b MAPbI3, c MAPb0.875Rh0.125I3)
We set up the corresponding model and study electronic and optical properties of MAPbI3:xRh (where x = 0, 6%, 8% and 12.5%) and MAPb0.875Rh0.125I3 (Figs. 2 and S1, S5) based on the result of XRD Rietveld refinement. In perovskite system, iodide ion and methylamine ion are easy to dissociate, which is destroyed perovskite structure and resulted in iodine vacancy defect and methylamine ion vacancy defect. From first-principle calculation, the binding energy (formula as E = Edoped − Ebulk − Edoping atom) is − 3.45 eV [40], indicating that Rh3+ can easily insert into the interstices of perovskite through the chemical bonds with iodine ion to enhance the stability of perovskite structure and prevent the ions from escaping and reduce iodine vacancy defects. Therefore, the crystallization of perovskite, tiny amount of Rh3+ is near the octahedral [PbI6]4− to induce ordered arrangement of organic cations to form high-quality film with few defects (Fig. 2a). From first-principle calculation, the bandgap values of MAPbI3:xRh (where x = 0, 6%, 8% and 12.5%) and MAPb0.875Rh0.125I3 are 1.31, 1.84, 1.14, 0.80 and 1.15 eV, respectively. The calculated bandgap of 1.31 is similar to experimental bandgap of 1.57 eV. There is a small error in the results of experiment and calculation. For the density of states (DOS) of MAPbI3, valence band maximum (VBM) and conduction band minimum (CBM) are mainly affected by orbitals hybridization of I and Pb. When rhodium ions are interatrial in perovskite structure, hybridization between rhodium and other atomic orbitals affects the distribution of DOS. From Fig. 2a, the rhodium atom makes the conduction band minimum move toward a higher energy level, thus increasing the bandgap. In Fig. 2b, DOS and band structure shows that the MAPbI3 are nonmagnetic. From Fig. 2a, the spin-up and spin-down band structure is not imperfect symmetry, suggesting MAPbI3:xRh possesses magnetism. The calculated total magnetic moment of MAPbI3:xRh (where x = 6%) atom is 2.254 μB mainly attributed to Rh atom. This indicates the potential application of this material in the field of magnetism.
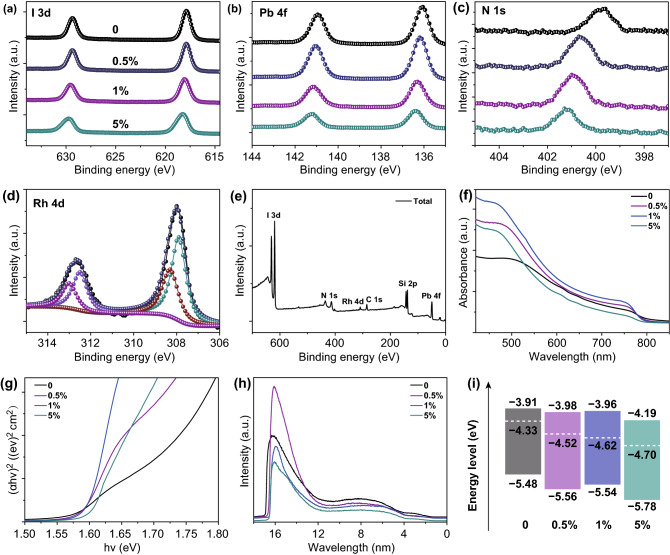
XPS was also further to verify Rh3+ incorporation and study chemical bonding states of the MAPbI3:xRh structure. Figure 3a shows a separation of approximately 11.6 eV from I 3d3/2and 3d5/2 spectra. As the Rh concentration increased, the binding energy (BE) of I 3d is slightly toward higher. From Fig. 3b, the BE of the Pb 4f7/2 was about 136.5 eV. As the Rh concentration is increased, the BE of Pb 4f is also slightly toward higher. This small transition to high binding energy may be the shorter Rh–I distance than Pb–I distance, which is lead to higher energy of Pb(Rh)–I bond. We can also observe from the Rietveld refinement above (Table S2). In addition, as the Rh3+-incorporated concentration increases, the 1s orbital of N also moves to a higher binding energy. Stronger interactions of Rh–I bond can reduce iodine vacancy defects. The peak at 315 and 308 eV of MAPbI3:xRh (where x = 1%) film indicates the presence of Rh elements (Fig. 3d).
Fig. 3.
XPS of a I 3d, b Pb 4f, c N 1s from MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%). d Rh 4d from MAPbI3:xRh (where x = 1%). e XPS of total elements from the MAPbI3:xRh (where x = 1%). f Absorbance coefficient of MAPbI3:xRh. g Estimated bandgap potential of perovskite films. h UPS of MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%). i Energy level diagram of MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%)
In order to study the distribution of energy level, absorbance coefficient and ultraviolet photoelectron spectroscopy (UPS) spectra were tested. When the Rh3+-incorporated concentration increased from 0 to 1%, the intensity of the absorption spectrum also increased. However, absorption intensity of 5% Rh3+ incorporation decreases, which may be related to the quality deterioration of perovskite films. We also calculated the absorption spectra, which were generally consistent with the experiment (Fig. S5a). The bandgap of MAPbI3:xRh is calculated by converting the UV/Vis absorption spectrum into Tauc plots (Fig. 3g). On the basis of the Kubelka–Munk theory [21], the bandgap of MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%) is determined to be 1.570, 1.58, 1.58 and 1.59 eV, respectively. From Figs. 3f and S5a, with the increase of Rh3+-incorporated concentration, the absorption range was slightly blue shift and basically consistent with the increase of bandgap. Figure 3h shows UPS image of MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%). The specific energy levels were calculated from UPS and UV/Vis absorption (Fig. 3i). The formula for calculating the Fermi energy level is EF = 21.22 − EB [21] (where EF is the Fermi level, and EB is high binding energy cutoff). The high binding energy cutoff of MAPbI3 is 16.89 eV. EF of MAPbI3 is − 4.33 eV (21.22–16.89). The VBM of MAPbI3 is the EF minus low binding energy as − 5.48 eV (− 4.33–1.15). The CBM of MAPbI3 is the sum of VBM and bandgap as − 3.91 eV. Other detail energy levels of MAPbI3:xRh can be calculated in this way. Energy level diagram of MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%) is shown in Fig. 3i. The detailed analysis of energy levels is shown in Table S3.
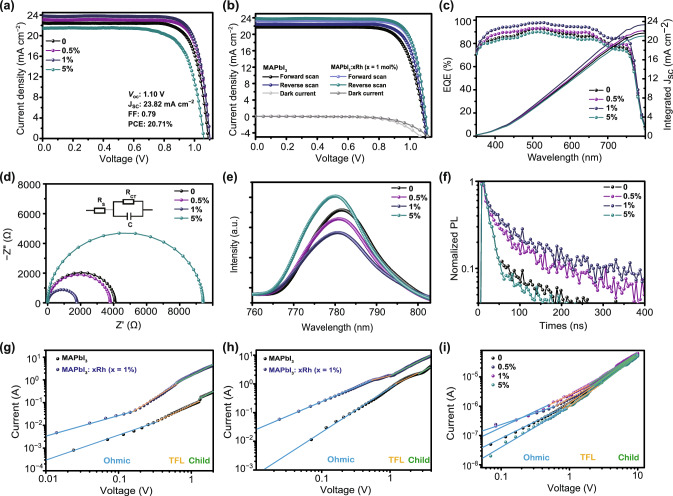
The current density versus voltage (J–V) curve is important to study photovoltaic properties. J–V curves of PSCs are fabricated by structure of ITO/SnO2/MAPbI3:xRh/Spiro-MeOTAD/Ag (Fig. 4a). Device photovoltaic parameters with MAPbI3:xRh are shown in detail (Table 1). PSCs fabricated by pristine perovskite possess short-circuit current density (Jsc) of 22.46 mA cm−2, open-circuit voltage (Voc) of 1.09 V, fill factor (FF) of 0.78, PCE of 19.09%. The PSCs prepared by MAPbI3:xRh (where x = 1%) possess high PCE of 20.71% (Table 1), which is increased approximately 10% compared to that of based on MAPbI3. However, the properties of PSCs based on MAPbI3:xRh (where x = 5%) have decreased. The MAPbI3:xRh (where x = 5%) films possess poor-quality films, which leads to large leakage current, low Jsc. To ensure repeatability of device performance, over 40 PSCs are fabricated and characterized (Tables S5–S8). PSCs based on MAPbI3 show a wide photoresponse in the range of 350-800 nm, and external quantum efficiency (EQE) values were close to 85%. EQE values of devices fabricated by 1% Rh incorporation are risen to more than 90%. The photocurrent of 21.62, 22.13, 23.37 and 21.00 mA cm−2 is obtained by integration of EQE spectrum in the range of 350–800 nm, which is similar to the Jsc from the results of J–V measurement.
Fig. 4.
a J–V, c EQE data and d Nyquist plots of PSCs fabricated by MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%). b Forward and reverse direction J–V of PSC fabricated by MAPbI3:xRh (where x = 0, 1%). e PL spectra of the MAPbI3:xRh/SnO2 films. f Time-resolved PL spectrum of perovskite films based on MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%). I–V characteristic of g hole-only device, h electron-only device prepared by MAPbI3 and MAPbI3:xRh (x = 1%). i Perovskite-only device fabricated by MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%)
Table 1.
Device photovoltaic parameters of devices based on MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%)
| Devices | Voc (V) | Jsc (mA cm−2) | FF | PCE (%) | |
|---|---|---|---|---|---|
| MAPbI3 | Best | 1.09 | 22.46 | 0.78 | 19.09 |
| Average | 1.07 ± 0.02 | 22.60 ± 0.8 | 0.75 ± 0.04 | 18.19 ± 0.96 | |
| 0.5% Rh | Best | 1.09 | 23.13 | 0.79 | 20.01 |
| Average | 1.08 ± 0.02 | 22.72 ± 0.43 | 0.78 ± 0.03 | 19.37 ± 1.02 | |
| 1% Rh | Best | 1.10 | 23.82 | 0.79 | 20.71 |
| Average | 1.10 ± 0.03 | 23.47 ± 0.41 | 0.76 ± 0.05 | 19.56 ± 1.01 | |
| 5% Rh | Best | 1.06 | 21.44 | 0.73 | 16.76 |
| Average | 1.03 ± 0.03 | 21.30 ± 1.06 | 0.67 ± 0.06 | 14.98 ± 1.78 |
Charge transfer characteristics of PSCs were studied by electrochemical impedance spectroscopy (EIS). The EIS was tested in the dark under applied bias of VOC. Under such a condition, the charge carrier recombination resistance (RREC) attained the lowest value (RREC ≪ RCT), and RCT is the charge transfer resistance. Semicircle from EIS measurement is formed by series resistor (Rs), charge transport resistor (Rct) and capacitor (CPE1). The inset of Fig. 4d is equivalent circuit diagram [41]. Because all devices have the same structure, RS values are basically the same (Fig. S6). The radius of the arc represents the value level of Rct. The lowest Rct of MAPbI3:xRh (x = 1%) reveals that a tiny amount of Rh-incorporated can enhance charge transport capacity. Therefore, supreme Jsc is obtained from PSCs based on MAPbI3:xRh (x = 1%). Figure 4e presents steady-state photoluminescence (PL) spectrum of MAPbI3:xRh/SnO2 films. Excitation wavelength is 465 nm. For MAPbI3 films, a peak was around 780 nm. With the increase of Rh-incorporated content, the PL peak to appear slight blue shift. From Fig. 4e, the lower intensity of MAPbI3:xRh (where x = 0.5%, 1%) film indicated extract electron carriers more effectively to SnO2 electron transport layer, which is consistent with the larger FF.
The device measured in the forward and reverse scanning directions. Hysteresis characteristics of photocurrent are analyzed by the results of J–V curves. Photocurrent hysteresis can be expressed by photocurrent hysteresis index (HI). The formula is (Eq. 1) [28]:
| 1 |
The HI of PSCs fabricated by MAPbI3:xRh (x = 0, 1%) is 0.042 and 0.020. Previous reports have shown that photocurrent hysteresis of devices mainly came from trap-induced carrier prevention. Therefore, lower photocurrent hysteresis indexes of devices fabricated by MAPbI3:xRh (x = 1%) suggest that higher trap-induced carrier prevention is taken.
The time-resolved PL spectrums of MAPbI3:xRh films are shown in Fig. 4f. Usually, τ1 is attributed to bimolecular recombination of photogenerated carriers, while τ2 is due to trap-assisted recombination. Table S4 shows the details of parameters of carrier lifetimes. The decay time of pristine perovskite is τ1 = 67 ns and τ2 = 693 ns. The decay time of MAPbI3:xRh (x = 1%) is τ1 = 63 ns and τ2 = 818 ns. The passivation of rhodium ion mainly reduces grain boundaries and defects and increases carrier lifetime. To find the difference in average carrier lifetime (τavg), the formula was defined as follows (Eq. 2) [42]:
| 2 |
The τavg of pristine MAPbI3 film is only 671 ns. MAPbI3:xRh (where x = 0.5%, 1%) films possess longer τavg, which is 726 and 796 ns. Because there is no charge transport layer, nonradiative recombination is the main reason for decay lifetime. Rh3+ incorporation has long lifetime, which is reduced recombination and improve photocurrent of PSCs.
Space charge limited current (SCLC) was measured to study the mobility and defects density of perovskite films. Figure S7 shows device structures of the hole-only diode and the electron-only diode are shown. For electron-only device, SnO2 and PCBM layer are used as the electron transport (blocking holes) layer being coated on both sides of the perovskite. For the hole-only device, the NiO and Spiro-MeOTAD layer is utilized as hole transport (blocking electrons) layer being coated on both sides of the perovskite. The current versus voltage (I–V) was tested under dark conditions by a Keithley model 2400. Three regions were evident in the experimental data. I–V characteristics show three different regions: a linear ohmic region at low voltage (represented by the blue line); a trap-filling region from mediate voltage to the trap-filled limit voltage (VTFL) (represented by the orange line); a Child’s region (represented by the green line). The formula for trap density is [28]:
| 3 |
(where V is the relative dielectric constant of perovskite hybrid materials, ε0 is vacuum permittivity, L is the thickness of perovskite layer). The charge carrier mobility (μ) is estimated at the quadratic dependence region. The Mott–Gurney’s law is (Eq. 4) [28]:
| 4 |
(where Id is dark current density, and V is the applied voltage). The trap-filling process for the hole-only device set is at VTFL = 0.33 V for MAPbI3 and VTFL = 0.14 V for MAPbI3:xRh (where x = 1%); the trap-filling process for the electron-only device set is at VTFL = 1.12 V for MAPbI3 and VTFL = 0.57 V for MAPbI3:xRh (where x = 1%). As shown in Table S10, the hole trap density of 1% Rh3+-incorporated is 3.10 × 1015 cm−3 lower than MAPbI3 (8.09 × 1015 cm−3). The hole mobility of MAPbI3:xRh (where x = 0, 1%) is 1.29 × 10−3 and 2.51 × 10−2 cm2 V−1 s−1. Similarly, the electron trap density of 1% Rh3+-incorporated is 1.26 × 1015 cm−3 smaller than MAPbI3 (2.74 × 1016 cm−3). The electron mobility of MAPbI3:xRh (where x = 0, 1%) is 5.38 × 10−3 and 1.26 × 10−2 cm2 V−1 s−1. The decrease of trap densities of MAPbI3:xRh (where x = 1%) leads to both electron and hole mobility increase. We also measured I–V curves of perovskite-only device by MAPbI3:xRh (where x = 0, 0.5%, 1% and 5%). Because the trap density is proportional to VTFL, the trap density of MAPbI3:xRh (where x = 1%) is minimum (Fig. 4i). In polycrystalline perovskite films, there are many defects such as vacancies, dislocations and bond deformation because of the confusion of atomic arrangement on grain boundaries. Defects are mainly distributed on the gain boundaries [43]. The results of defect density show that rhodium ion incorporation mainly plays the role of passivation grain boundary.
XRD patterns of MAPbI3 and 1% Rh-incorporated perovskite films are exposed in the humid air before and after two months (Fig. 5). The aging rate of perovskite films is related to PbI2 separated. Peak value of PbI2 is higher, and perovskite film aging is faster. From Fig. 5b, the perovskite films based on MAPbI3:xRh (where x = 0.5% and 1%) aged slower than that based on MAPbI3 films. The addition of Rh3+ made the perovskite structure more stable once again. The perovskite film stability directly affects the PSCs stability. The PSCs were stored in dry atmosphere without encapsulation for 500 h (Fig. 5c). Figure 5c shows that PSCs based on MAPbI3:xRh (where x = 1%) possess higher stability than that of MAPbI3, maintained 92% of initial PCE after 500 h. Detail performance of PSCs is fabricated by MAPbI3 and MAPbI3:xRh with different aged time at dry air (Fig. S11 and Table S12).
Fig. 5.
a, b XRD of perovskite films based on MAPbI3 and 1% Rh3+-incorporated is exposed in the air before and after two months. c PCE changes of perovskite solar cells located in dry air without encapsulation for 500 h
Conclusion
Herein, Rh3+ incorporation with tiny amount can help nucleation of perovskite grain, passivate grain boundary defects and improve properties of PSCs. In addition, Rh3+ incorporation with perovskite can contribute to preparing larger crystalline and uniform film, reducing trap-state density and enlarging charge carrier lifetime. Therefore, planar heterojunction PSCs by MAPbI3:xRh3+ perovskite materials possess PCE of 20.71% without obvious photocurrent hysteresis. Meanwhile, the devices of Rh3+-incorporated have high stability within 500 h without encapsulation in dry air. This work highlights the advantages of Rh3+ incorporation in the capabilities of PSCs, which will promote the future industrial application.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Ministry of Education of China (IRT1148), the National Natural Science Foundation of China (U1732126, 11804166, 51602161, 51372119), China Postdoctoral Science Foundation (2018M630587), the Priority Academic Program Development of Jiangsu Higher Education Institutions (YX03001), Guangdong Science and Technology Program (2017B030314002), Graduate Research Innovation Fund of Jiangsu Province (KYCX18_0863, KYCX18_0847, KYCX18_0869).
Contributor Information
Liang Chu, Email: chuliang@njupt.edu.cn.
Xing’ao Li, Email: iamxali@njupt.edu.cn.
Wei Huang, Email: iamwhuang@njupt.edu.cn.
References
- 1.Shi B, Duan L, Zhao Y, Luo J, Zhang X. Semitransparent perovskite solar cells: from materials and devices to applications. Adv. Mater. 2019;32(3):1806474. doi: 10.1002/adma.201806474. [DOI] [PubMed] [Google Scholar]
- 2.Cho KT, Grancini G, Lee Y, Oveisi E, Ryu J, et al. Selective growth of layered perovskites for stable and efficient photovoltaics. Energy Environ. Sci. 2018;11(4):952–959. doi: 10.1039/C7EE03513F. [DOI] [Google Scholar]
- 3.Liu Y, Yang Z, Cui D, Ren X, Sun J, et al. Two-inch-sized perovskite CH3NH3PbX3 (X = Cl, Br, I) crystals: growth and characterization. Adv. Mater. 2015;27(35):5176–5183. doi: 10.1002/adma.201502597. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Q, Zhao Y, Zhang X, Yang X, Chen Y, et al. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics. 2019;13(7):460–466. doi: 10.1038/s41566-019-0398-2. [DOI] [Google Scholar]
- 5.Liu W, Chu L, Hu R, Zhang R, Ma Y, et al. Diameter engineering on TiO2 nanorod arrays for improved hole-conductor-free perovskite solar cells. Sol. Energy. 2018;166:42–49. doi: 10.1016/j.solener.2018.03.037. [DOI] [Google Scholar]
- 6.Ghosh S, Singh T. Role of ionic liquids in organic–inorganic metal halide perovskite solar cells efficiency and stability. Nano Energy. 2019;63:103828. doi: 10.1016/j.nanoen.2019.06.024. [DOI] [Google Scholar]
- 7.Chen C, Song Z, Xiao C, Zhao D, Shrestha N, et al. Achieving a high open-circuit voltage in inverted wide-bandgap perovskite solar cells with a graded perovskite homojunction. Nano Energy. 2019;61:141–147. doi: 10.1016/j.nanoen.2019.04.069. [DOI] [Google Scholar]
- 8.Chu L, Zhang J, Liu W, Zhang R, Yang J, et al. A facile and green approach to synthesize mesoporous anatase TiO2 nanomaterials for efficient dye-sensitized and hole-conductor-free perovskite solar cells. ACS Sustain. Chem. Eng. 2018;6(4):5588–5597. doi: 10.1021/acssuschemeng.8b00607. [DOI] [Google Scholar]
- 9.Chu L, Ahmad W, Liu W, Yang J, Zhang R, et al. Lead-free halide double perovskite materials: a new superstar toward green and stable optoelectronic applications. Nano-Micro Lett. 2019;11(1):16. doi: 10.1007/s40820-019-0244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosconi E, Merabet B, Meggiolaro D, Zaoui A, De Angelis F. First-principles modeling of bismuth doping in the MAPbI3 perovskite. J. Phys. Chem. C. 2018;122(25):14107–14112. doi: 10.1021/acs.jpcc.8b01307. [DOI] [Google Scholar]
- 11.Xie LQ, Chen L, Nan ZA, Lin HX, Wang T, et al. Understanding the cubic phase stabilization and crystallization kinetics in mixed cations and halides perovskite single crystals. J. Am. Chem. Soc. 2017;139(9):3320–3323. doi: 10.1021/jacs.6b12432. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Chu L, Liu N, Cheng Y, Wu F, et al. Simultaneously enhanced efficiency and stability of perovskite solar cells with TiO2@CdS core–shell nanorods electron transport layer. Adv. Mater. Interfaces. 2019;6(5):1801976. doi: 10.1002/admi.201801976. [DOI] [Google Scholar]
- 13.Huang Y, Li L, Liu Z, Jiao H, He Y, et al. The intrinsic properties of FA(1−x)MAxPbI3 perovskite single crystals. J. Mater. Chem. A. 2017;5(18):8537–8544. doi: 10.1039/C7TA01441D. [DOI] [Google Scholar]
- 14.Manser JS, Kamat PV. Band filling with free charge carriers in organometal halide perovskites. Nat. Photonics. 2014;8(9):737–743. doi: 10.1038/nphoton.2014.171. [DOI] [Google Scholar]
- 15.National renewable energy laboratory, Best research-cell efficiencies. www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20190802.pdf
- 16.Zhang J, Wang Q, Wang L, Li XA, Huang W. Layer-controllable WS2-reduced graphene oxide hybrid nanosheets with high electrocatalytic activity for hydrogen evolution. Nanoscale. 2015;7(23):10391–10397. doi: 10.1039/C5NR01896J. [DOI] [PubMed] [Google Scholar]
- 17.Kim YC, Jeon NJ, Noh JH, Yang WS, Seo J, et al. Beneficial effects of PbI2 incorporated in organo-lead halide perovskite solar cells. Adv. Energy Mater. 2016;6(4):1502104. doi: 10.1002/aenm.201502104. [DOI] [Google Scholar]
- 18.Zhao W, Yao Z, Yu F, Yang D, Liu SF. Alkali metal doping for improved CH3NH3PbI3 perovskite solar cells. Adv. Sci. 2018;5(2):1700131. doi: 10.1002/advs.201700131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang CC, Gao CH, Li M, Ma XJ, et al. N-type doping of organic–inorganic hybrid perovskites toward high-performance photovoltaic devices. Sol. RRL. 2019;3(2):1800269. doi: 10.1002/solr.201800269. [DOI] [Google Scholar]
- 20.Zhang J, Chen R, Wu Y, Shang M, Zeng Z, Zhang Y, Zhu Y, Han L. Extrinsic movable ions in MAPbI3 modulate energy band alignment in perovskite solar cells. Adv. Energy Mater. 2018;8(5):1701981. doi: 10.1002/aenm.201701981. [DOI] [Google Scholar]
- 21.Liu W, Chu L, Liu N, Ma Y, Hu R, et al. Efficient perovskite solar cells fabricated by manganese cations incorporated in hybrid perovskites. J. Mater. Chem. C. 2019;7(38):11943–11952. doi: 10.1039/C9TC03375K. [DOI] [Google Scholar]
- 22.Shi Z, Guo J, Chen Y, Li Q, Pan Y, Zhang H, Xia Y, Huang W. Lead-free organic–inorganic hybrid perovskites for photovoltaic applications: recent advances and perspectives. Adv. Mater. 2017;29(16):1605005. doi: 10.1002/adma.201605005. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Zheng L, Zhang X, Cao Y, Meng T, et al. Efficient perovskite solar cells fabricated by Co partially substituted hybrid perovskite. Adv. Energy Mater. 2018;8(14):1703178. doi: 10.1002/aenm.201703178. [DOI] [Google Scholar]
- 24.Gong X, Guan L, Pan H, Sun Q, Zhao X, et al. Highly efficient perovskite solar cells via nickel passivation. Adv. Funct. Mater. 2018;28(50):1804286. doi: 10.1002/adfm.201804286. [DOI] [Google Scholar]
- 25.Klug MT, Osherov A, Haghighirad AA, Stranks SD, Brown PR, et al. Tailoring metal halide perovskites through metal substitution: influence on photovoltaic and material properties. Energy Environ. Sci. 2017;10(1):236–246. doi: 10.1039/C6EE03201J. [DOI] [Google Scholar]
- 26.Xiao Z, Song Z, Yan Y. From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 2019;31(47):e1803792. doi: 10.1002/adma.201803792. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Chen S, Zheng Q. Defect passivation of CsPbI2Br perovskites through Zn(II) doping: toward efficient and stable solar cells. Sci. China Chem. 2019;62:1044–1050. doi: 10.1007/s11426-019-9486-0. [DOI] [Google Scholar]
- 28.Wang K, Zheng L, Zhu T, Yao X, Yi C, et al. Efficient perovskite solar cells by hybrid perovskites incorporated with heterovalent neodymium cations. Nano Energy. 2019;61:352–360. doi: 10.1016/j.nanoen.2019.04.073. [DOI] [Google Scholar]
- 29.Wang ZK, Li M, Yang YG, Hu Y, Ma H, et al. High efficiency Pb–In binary metal perovskite solar cells. Adv. Mater. 2016;28(31):6695–6703. doi: 10.1002/adma.201600626. [DOI] [PubMed] [Google Scholar]
- 30.Wang JW, Wang Z, Pathak S, Zhang W, de Quilettes DW, et al. Efficient perovskite solar cells by metal ion doping. Energy Environ. Sci. 2016;9(9):2892–2901. doi: 10.1039/C6EE01969B. [DOI] [Google Scholar]
- 31.Wang L, Zhou H, Hu J, Huang B, Sun M, et al. A Eu3+–Eu2+ ion redox shuttle imparts operational durability to Pb–I perovskite solar cells. Science. 2019;363(6424):265–270. doi: 10.1126/science.aau5701. [DOI] [PubMed] [Google Scholar]
- 32.Maeda K. Rhodium-doped barium titanate perovskite as a stable p-type semiconductor photocatalyst for hydrogen evolution under visible light. ACS Appl. Mater. Interfaces. 2014;6(3):2167–2173. doi: 10.1021/am405293e. [DOI] [PubMed] [Google Scholar]
- 33.Iwashina K, Kudo A. Rh-doped SrTiO3 photocatalyst electrode showing cathodic photocurrent for water splitting under visible-light irradiation. J. Am. Chem. Soc. 2011;133(34):13272–13275. doi: 10.1021/ja2050315. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Tao S, Chen Y, Niu X, Onwudinanti CK, et al. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat. Energy. 2019;4(5):408–415. doi: 10.1038/s41560-019-0382-6. [DOI] [Google Scholar]
- 35.Wei L, Ma W, Lian C, Meng S. Benign interfacial iodine vacancies in perovskite solar cells. J. Phys. Chem. C. 2017;121(11):5905–5913. doi: 10.1021/acs.jpcc.6b12583. [DOI] [Google Scholar]
- 36.Niu X, Li Y, Zhang Y, Zhou Z, Wang J. Greatly enhanced photoabsorption and photothermal conversion of antimonene quantum dots through spontaneously partial oxidation. ACS Appl. Mater. Interfaces. 2019;11(19):17987–17993. doi: 10.1021/acsami.9b02771. [DOI] [PubMed] [Google Scholar]
- 37.Niu X, Bai X, Zhou Z, Wang J. Rational design and characterization of direct Z-scheme photocatalyst for overall water splitting from excited state dynamics simulations. ACS Catal. 2020;10:1976–1983. doi: 10.1021/acscatal.9b04753. [DOI] [Google Scholar]
- 38.Zhou L, Su J, Lin Z, Chen D, Zhu W, et al. Theoretical and experimental investigation of mixed Pb–In halide perovskites. J. Phys. Chem. C. 2018;122(28):15945–15953. doi: 10.1021/acs.jpcc.8b05267. [DOI] [Google Scholar]
- 39.Bai D, Zhang J, Jin Z, Bian H, Wang K, et al. Interstitial Mn2+-driven high-aspect-ratio grain growth for low-trap-density microcrystalline films for record efficiency CsPbI2Br solar cells. ACS Energy Lett. 2018;3(4):970–978. doi: 10.1021/acsenergylett.8b00270. [DOI] [Google Scholar]
- 40.Zhao Y, Zhu P, Wang M, Huang S, Zhao Z, et al. A polymerization-assisted grain growth strategy for efficient and stable perovskite solar cells. Adv. Mater. 2020 doi: 10.1002/adma.201907769. [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Zhang J, Gan X, Yu L, Yuan H, et al. Pb-reduced CsPb0.9Zn0.1I2Br thin films for efficient perovskite solar cells. Adv. Energy Mater. 2019;9(25):1900896. doi: 10.1002/aenm.201900896. [DOI] [Google Scholar]
- 42.Ma Y, Zhang H, Zhang Y, Hu R, Jiang M, et al. Enhancing the performance of inverted perovskite solar cells via grain boundary passivation with carbon quantum dots. ACS Appl. Mater. Interfaces. 2018;11(3):3044–3052. doi: 10.1021/acsami.8b18867. [DOI] [PubMed] [Google Scholar]
- 43.Ni Z, Bao C, Liu Y, Wu W, Chen S, Jiang Q, et al. Resolving spatial and energetic distributions of trap states in metal halide perovskite solar cells. Science. 2020;367(6484):1352–1358. doi: 10.1126/science.aba0893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.