Abstract
Highlights
The formation of manganese oxide induces self-assembly of block copolymers to form polymeric vesicles.
The polymeric vesicles possessed strong stability and high drug loading capacity.
The drug-loaded polymeric vesicles have been demonstrated, especially in in vivo studies, to exhibit a higher efficacy of tumor suppression without known cardiotoxicity.
Abstract
Molecular self-assembly is crucially fundamental to nature. However, the aqueous self-assembly of polymers is still a challenge. To achieve self-assembly of block copolymers [(polyacrylic acid–block–polyethylene glycol–block–polyacrylic acid (PAA68–b–PEG86–b–PAA68)] in an aqueous phase, manganese oxide (MnO2) is first generated to drive phase separation of the PAA block to form the PAA68–b–PEG86–b–PAA68/MnO2 polymeric assembly that exhibits a stable structure in a physiological medium. The polymeric assembly exhibits vesicular morphology with a diameter of approximately 30 nm and high doxorubicin (DOX) loading capacity of approximately 94%. The transformation from MnO2 to Mn2+ caused by endogenous glutathione (GSH) facilitates the disassembly of PAA68–b–PEG86–b–PAA68/MnO2 to enable its drug delivery at the tumor sites. The toxicity of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 to tumor cells has been verified in vitro and in vivo. Notably, drug-loaded polymeric vesicles have been demonstrated, especially in in vivo studies, to overcome the cardiotoxicity of DOX. We expect this work to encourage the potential application of polymer self-assembly.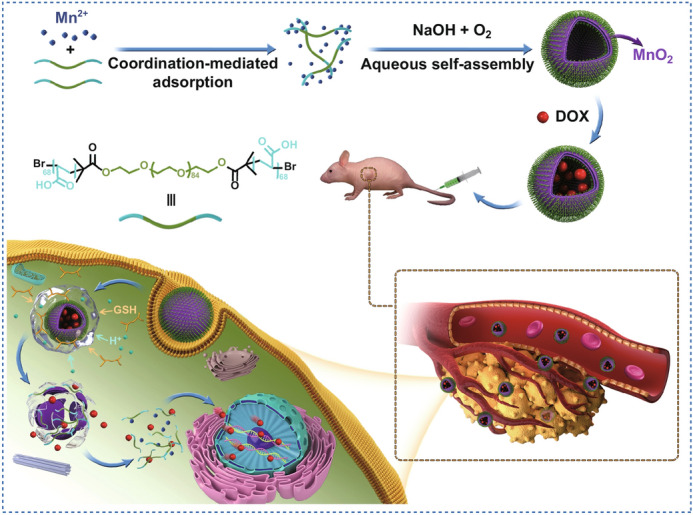
Electronic supplementary material
The online version of this article (10.1007/s40820-020-00447-9) contains supplementary material, which is available to authorized users.
Keywords: Polymer, Aqueous self-assembly, Vesicles, Tumor microenvironment, Drug delivery system
Introduction
In nature, self-assembly is one of the most crucial approaches that enables the building of micro- and nanostructures [1]. In biology, from structures of plant to those of multicellular organisms, it plays an important role in generating sophisticated superstructures through different interactions between a large number of building blocks [2–4]. Inspired by nature, polymer self-assembly has attracted extensive attention since the 1960s and serves as a “bottom-up” strategy for building complexes naturally [5, 6]. The self-assembly of block copolymers in solution developed by Armes, Kataoka, Discher, and Eisenberg has been utilized to design a series of polymeric architectures [7–10].
In principle, block copolymers can be self-assembled into a wide variety of polymer architectures, but in practice this is usually achieved in co-solvents, often with the aid of both water and an organic solvent [8–10]. As far as we know, self-assembly in nature occurs in aqueous phases, not organic phases, and aqueous self-assembly of block copolymer is an environmentally friendly method with the absence of organic solvents or surfactants. It is important to note that the use of organic solvents or surfactants in traditional self-assembly methods has restricted their application because of the side effects to humans. No matter the application, polymeric architectures formed by block copolymer self-assembly in drug delivery have already become an intriguing focus for a number of research areas, especially in cancer treatments.
The advancement of high-quality therapeutic methods based on polymeric architectures has attracted considerable attention for the treatment of cancer over the past 10 years [11, 12]. Moreover, we have gradually increased our understanding of tumor microenvironments and cells that contain various cooperating components (such as lysosomes and mitochondria) [13–15]. This understanding has facilitated the advancement of numerous nanoparticle-based drug delivery systems (nano-DDSs), both inorganic and organic, to fight against cancers [11, 16, 17]. Organic nanoparticle-based DDSs, especially the polymer micelle-based DDSs, are considered a major strategy to alleviate the side effects of antitumor drugs and improve their therapeutic efficacy [18, 19]. A wide range of self-assembly methods have been extensively utilized to fabricate polymeric nanoparticles such as polymerization- or precipitation-induced self-assembly [20, 21].
However, the inherently inferior stability of polymer micelle-based DDSs at low concentrations could be harmful to humans or impede high-quality therapy [22]. Both covalent cross-linking bonds (including disulfide, borate ester, Schiff base, and ketal bonds) and non-covalent interactions (including electrostatic, hydrophobic, and coordination interaction) have been widely used to develop nano-DDSs for overcoming this limitation [23–29]. However, only a few of these stable inorganic cross-linked block copolymer architectures with a redox potential-responsive property can be utilized to deliver anticancer agents by responding to tumor microenvironments. Therefore, researchers are enthusiastically developing stable block copolymer micelles that can fulfill their target delivery [30, 31].
Although numerous advancements have been achieved, there are still some major barriers that need to be overcome, such as the need for enhanced stability to prevent premature leakage of anticancer agents during the delivery process, selective delivery at tumor sites, and biodegradability of polymeric micelle-based DDSs after completion of drug delivery. However, no easy-to-use fabrication system has been developed. Transformation of MnO2 to Mn2+ ions has attracted extensive attention because of the stability of MnO2 in biological fluids and its disintegration by endogenous glutathione (GSH) inside lysosomes and endosomes at tumor sites [32, 33].
Moreover, Mn2+ ions released from the MnO2 component within MnO2-based systems can be easily metabolized by the kidneys [34]. Therefore, the MnO2 component was introduced in the fabrication of biomaterials because of its remarkable biocompatibility [35]. Few reported studies have described the in situ self-assembly of block copolymers using MnO2 formation in an aqueous phase. Based on the current knowledge of self-assembly of block copolymers, we hypothesized that block copolymer self-assembly is achievable by MnO2 formation in an aqueous phase without additional agents such as organic solvents or surfactants to form a stable polymeric architecture, which could extend the capability of self-assemblies beyond traditional methods.
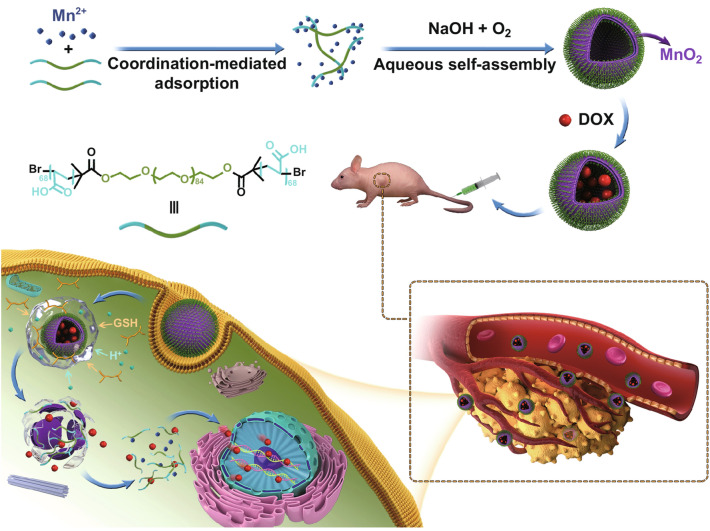
Herein, we describe the use of the self-assembly approach in an aqueous phase in the fabrication of polymeric vesicles with high stability for selective delivery of anticancer agents. The formation of MnO2 under mild conditions is used to induce the in situ self-assembly of block copolymers (PAA68–b–PEG86–b–PAA68) to form PAA68–b–PEG86–b–PAA68/MnO2 polymeric vesicles (Scheme 1), which exhibit the advantage of stability, which prevents the diffusion of encapsulated anticancer agents during the delivery process.
Scheme 1.
Schematic illustration of aqueous self-assembly of polymer and its tumor microenvironment-activated release
Thus, for the PAA68–b–PEG86–b–PAA68/MnO2 vesicles, MnO2 not only serves as a nucleating agent, but it also acts as an interlocking agent in adjusting the self-assembly process of block copolymer with the goal of optimizing their morphology and structural stability. In this design, the introduction of MnO2 enhances the structural stability of these PAA68–b–PEG86–b–PAA68/MnO2 vesicles. Compared to the silica/organosilica cross-linked block copolymer architectures [22], the PAA68–b–PEG86–b–PAA68/MnO2 vesicles can respond to GSH and weak acidic conditions to unload their cargos.
Moreover, these polymeric vesicles were further degraded to hydrophilic linear polymers and Mn2+ ions after effectively delivery (Scheme 1). On the other hand, the as-released Mn2+ ions from the decomposition of the PAA68–b–PEG86–b–PAA68/MnO2 vesicles as a T2 contrast agent could endow the DDSs with excellent characteristics for magnetic resonance imaging (MRI) [36]. Therefore, through the self-assembly of copolymers by the formation of MnO2 in an aqueous phase, polymeric micelles with controlled morphology and stability were fabricated for the selective delivery of anticancer agents, which may be extended to the development of polymeric architectures.
Experimental Section
Materials
Polyethylene glycol (HO–PEG86–OH) and doxorubicin (DOX) hydrochloride were obtained from Beijing Kaizheng Bioengineering Development Co. Ltd. Tert-butyl acrylate (tBA) was obtained from J & K Chem. Ltd. Copper (I) bromide [Cu(I)Br, 99.5%], N,N,N′,N′,N″-pentamethyl diethylenetriamine (PMDETA, 99% purity), dimethylformamide (DMF), 2-bromoisobutyryl bromide (BIBB, 98% purity), sodium hydroxide (NaOH), and manganese chloride tetrahydrate (MnCl2·4H2O) were obtained from Aladdin Ind. Co. Tetrahydrofuran (THF) and toluene were provided by Tianjin Chem. Co. Ltd. Fetal bovine serum (FBS) was purchased from ScienCell (Carlsbad, CA, USA).
Leibovitz’s L15 medium, 4′,6-diamidino-2-phenylindole (DAPI), paraformaldehyde, and cell counting kit-8 (CCK-8) were obtained from Boster Biotechnology (Wuhan, China). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Thermo Scientific. The human breast cancer MCF-7 (Catalog No.: SCSP-531) and human embryonic kidney HEK-293 (Catalog No.: GNHu43) cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The two cell lines were used within 20 passages in this study. Additionally, deionized water was adopted for all experiments.
Synthesis of PAA68–b–PEG86–b–PAA68
The synthetic steps of these copolymers are shown in the Supporting Information according to our previously reported studies [18, 19].
Aqueous Self-assembly of PAA68–b–PEG86–b–PAA68
The formation of MnO2 was used to induce self-assembly of block copolymers (PAA68–b–PEG86–b–PAA68) to form the MnO2–polymer hybrid vesicles (PAA68–b–PEG86–b–PAA68/MnO2). Briefly, 50 mg PAA68–b–PEG86–b–PAA68 was dispersed in 50 mL water under magnetic stirring with slight ultrasound sonication for 10 min using the Ultrasonic Cleaner (KQ-300DE). Then, 10 mL MnCl2 solution (360 mg) was slowly added with vigorous magnetic stirring. The mixture was transferred into dialysis tubes (molecular weight cutoff [MWCO], 1000) and then immersed in abundant deionized water to remove excess Mn2+ ions.
After dialysis against deionized water, the pH value of the mixture was adjusted to pH 11 using a 1.0 M NaOH aqueous solution. The PAA68–b–PEG86–b–PAA68/MnO2 vesicles were obtained under vigorous stirring for 24 h and stored at 4 °C. To optimize the effect of the Mn2+ concentrations on the morphology of the MnO2–polymer hybrids, 60 and 180 mg MnCl2·4H2O were successively added to induce the self-assembly of PAA68–b–PEG86–b–PAA68 according to the above procedures.
DOX Loading
DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 was prepared using 10 mg DOX and 10 mg PAA68–b–PEG86–b–PAA68/MnO2 in 10 mL phosphate-buffered saline (PBS) solution (pH 7.4) according to the reported procedures [18]. After centrifugation, the DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 was obtained as a precipitate and the DOX mass in the supernatant was determined using an ultraviolet (UV) spectrophotometer at 482 nm. The DOX encapsulation efficiency and DOX loading capacity for PAA68–b–PEG86–b–PAA68/MnO2 were evaluated using the following equations: DOX loading capacity (%) = (DOX mass in polymeric vesicle/polymeric vesicle mass) × 100 and DOX encapsulation efficiency (%) = (DOX mass in polymeric vesicle/feeding DOX mass) × 100.
Additionally, the above measurements were repeated with three samples from different batches to obtain the final loading capacity and encapsulation efficiency of DOX. The effect of two different DOX concentrations (2.5 mg DOX vs 10.0 mg PAA68–b–PEG86–b–PAA68/MnO2 and 5.0 mg DOX vs 10.0 mg PAA68–b–PEG86–b–PAA68/MnO2) on the loading capacity, drug release, and average hydrodynamic diameters (Dh) of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 were studied according to the above procedures and were, respectively, denoted as DOX1-loaded PAA68–b–PEG86–b–PAA68/MnO2 and DOX2-loaded PAA68–b–PEG86–b–PAA68/MnO2.
Cell Toxicity Assays
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the biocompatibility of PAA68–b–PEG86–b–PAA68/MnO2 vesicles with HEK-293 and MCF-7 cells. Furthermore, the growth inhibitory ability of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 was also evaluated on MCF-7 cells using the MTT assay. After the cells were seeded in 96-well plates at 5000 cells/well for 24 h and had reached a confluency of over 80%, 200 μL medium containing the PAA68–b–PEG86–b–PAA68/MnO2, DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2, or free DOX samples of different concentrations were added and incubated at 37 °C with 0.5% CO2 for 1, 2, or 3 days.
Typically, the concentrations of PAA68–b–PEG86–b–PAA68/MnO2, free DOX, or DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 (equivalent DOX concentrations) were 0.1, 0.5, 1.0, 2.5, and 5.0 μg mL−1. Then, the cell viability was detected using the MTT assay: 20 μL MTT (5 mg mL−1) was added to each well and incubated for 4 h. Then, the cell-bound dye was dissolved in 150 μL dimethyl sulfoxide (DMSO) and the absorbance was recorded using a microplate reader at 490 nm.
Controlled Release
For controlled release evaluation of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2, 50 mg was equally dispersed in samples of 10.0 mL PBS (pH 7.4, pH 7.4 in the presence of 10 μM GSH, pH 6.5, pH 5.0, and pH 5.0 in the presence of 10 mM GSH). After filtration using 0.2-μm ultrafiltration membranes, the PBS dispersions of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 were transferred into dialysis tubes (MWCO, 1000) and immersed in 140.0 mL of PBS at the corresponding pH at 37 °C. A 5.0 mL aliquot of the solution was collected to detect the drug concentration using a UV spectrophotometer at 482 nm at specific time intervals (0, 1, 3, 6, 12, 24, 48, and 60 h), and 5.0 mL fresh PBS of the corresponding pH was supplemented after each sampling.
Each controlled release analysis was repeated three times, and the final cumulative release ratio of DOX was the average of three measurements. DOX release from both DOX1-loaded PAA68–b–PEG86–b–PAA68/MnO2 and DOX2-loaded PAA68–b–PEG86–b–PAA68/MnO2 was analyzed at pH 7.4 in the presence of 10 μM GSH at pH 5.0 and 10 mM GSH according to the above procedures.
Confocal Laser Scanning Microscopy Analysis
The confocal laser scanning microscopy (CLSM) technique was used to investigate the cellular uptake of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 vesicles and free DOX by MCF-7 cells at excitation wavelengths of 482 nm for DOX and 406 nm for Hoechst, as reported previously [36].
Flow Cytometric Analysis
Flow cytometry was used to detect the intracellular release of the as-loaded DOX from DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 vesicles by measuring the cell-associated fluorescence using a flow cytometer (BD FACSCalibur) at specific time intervals (1, 3, 6, 9, and 12 h), after free DOX (2.5 μg mL−1) or DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 (equivalent DOX concentration: 2.5 μg mL−1) was added to the MCF-7 cells and incubated at 37 °C exposed to an atmosphere of 0.5% CO2. The experimental details are available from our previously reported studies [37].
Animal Experiments
Female nude BALB/c mice aged 5–6 weeks were obtained from Hunan Slack Scene of Laboratory Animal Co., Ltd. (Hunan, China) and Shanghai Laboratory Animal Center (Shanghai, China). Animals were treated according to protocols established by the ethics committee of Zhengzhou University, and the in vivo experiments were approved and conducted in accordance with the guidelines of the ethics committee of Zhengzhou University. MCF-7 cells (1 × 106) were subcutaneously injected into the right flank of the nude mice (n = 5 per group).
When the tumor volumes reached 100 mm3, the mice were randomized into saline, PAA68–b–PEG86–b–PAA68/MnO2, free DOX, and DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 groups. The groups were treated with corresponding samples intravenously (5 mg kg−1 day−1) once every 3 days. Tumor size was then measured every 3 days using Vernier calipers. Tumor volume was calculated using the following formula: volume = (length × width2)/2. Tumors were weighed after 31 days, and, subsequently, the hearts and livers were sectioned for histological evaluation using hematoxylin and eosin (H&E) staining.
Characterization
Proton (1H) nuclear magnetic resonance (NMR) spectra were recorded using a Bruker Avance (II) 400 MHz spectrometer at room temperature. Automatic tuning module (ATM) and double probe were utilized for the NMR spectrometer. 1H NMR spectra were measured in deuterated chloroform (CDCl3) or deuterium oxide (D2O, heavy water) using tetramethylsilane as the internal standard, and the concentration of the samples was 2 mg mL−1. Fourier transform infrared (FT-IR) spectra were recorded using a Bruker IFS 66 v/s IR spectrometer at 4000–400 cm−1 with a resolution of 4 cm−1. The number average molecular weight (Mn) and polydispersity (PDI) of the copolymers were measured using gel permeation chromatography (GPC) in THF at 35 °C. The vesicular morphology was analyzed using a JEM-1200 EX/S transmission electron microscope (TEM).
X-ray photoelectron spectroscopy (XPS) of PAA68–b–PEG86–b–PAA68/MnO2 was performed using an Elementar Vario EL instrument (Elementar Analysensysteme GmbH, Munich, Germany). An Agilent 7700× inductively coupled plasma mass spectrometer (ICP-MS) was used to detect the content of MnO2 in the PAA68–b–PEG86–b–PAA68/MnO2 vesicles. For ICP-MS analysis, the samples were treated with 10% hydrochloric acid for 8 h and then heated to remove the hydrochloric acid. The dynamic light scattering (DLS) measurements were performed using a light scattering system BI-200SM device. The release performance of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 was assessed using a PerkinElmer Lambda 35 UV–Vis spectrometer at room temperature.
Results and Discussion
Preparation of PAA68–b–PEG86–b–PAA68
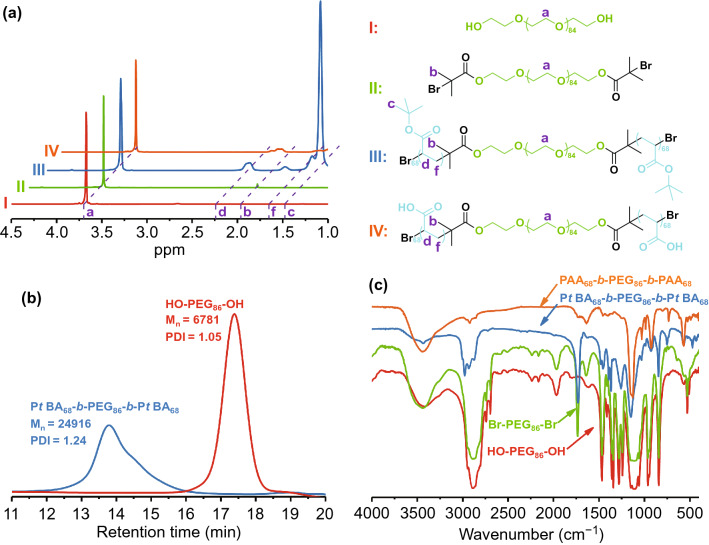
To achieve aqueous self-assembly, a water-soluble block copolymer PAA68–b–PEG86–b–PAA68 was prepared using rational design. Furthermore, we selected biocompatible PEG as a unique block to surmount the biological barriers. In this work, the PAA68–b–PEG86–b–PAA68 triblock copolymer was synthesized using a combination of acylation reaction, atomic transfer radical polymerization (ATRP), and hydrolysis reaction (Fig. S1). In the 1H NMR spectrum of HO–PEG86–OH, the characteristic peak at 3.68 ppm was assigned to the inner methylene protons (Fig. 1a). After the acylation reaction, the presence of the –CH3 proton peak at 1.94 ppm in the 1H NMR spectrum of Br–PEG86–Br (Fig. 1a) clearly confirmed the successful acylation reaction.
Fig. 1.
a 1H NMR spectra of HO–PEG86–OH (I), Br–PEG86–Br (II), PtBA68–b–PEG86–b–PtBA68 (III), and PAA68–b–PEG86–b–PAA68 (IV). b GPC spectra of HO–PEG86–OH and PtBA68–b–PEG86–b–PtBA68. c FT-IR spectra of HO–PEG86–OH, Br–PEG86–Br, PtBA68–b–PEG86–b–PtBA68, and PAA68–b–PEG86–b–PAA68
In addition to the characteristic proton peaks of the inner methylene and methyl protons, the characteristic proton peaks at 1.44 ppm clearly revealed the presence of t-butyl groups in PtBA–b–PEG86–b–PtBA (Fig. 1a). The peak area ratio of a to c was used to evaluate the polymerization degree to tBA, and the well-defined PtBA68–b–PEG86–b–PtBA68 block copolymer was successfully obtained. According to the gel permeation chromatography (GPC) results (Fig. 1b), the Mn of PtBA68–b–PEG86–b–PtBA68 was 24,916 Da, which was in good agreement with the 1H NMR spectral result of PtBA68–b–PEG86–b–PtBA68. Moreover, the PDI (Mw/Mn) of PtBA68–b–PEG86–b–PtBA68 was 1.24, indicating the existence of a narrow MW distribution. Figure 1c shows that the characteristic absorbance peaks at 1735 and 1394 cm−1 were associated with ester groups of Br–PEG86–Br and t-butyl groups, respectively, to PtBA–b–PEG86–b–PtBA. These findings were consistent with the results of the 1H NMR spectral analysis. The characteristic proton peaks of the t-butyl groups disappeared in the 1H NMR spectrum of PAA68–b–PEG86–b–PAA68, demonstrating the successful hydrolysis of t-butyl groups in PtBA68–b–PEG86–b–PtBA68. In addition, the characteristic absorbance of carboxyl stretching vibration in t-butyl groups at 1735 cm−1 disappeared in the FT-IR spectrum of the PAA68–b–PEG86–b–PAA68, demonstrating its generation from PtBA68–b–PEG86–b–PtBA68. Therefore, the resultant PAA68–b–PEG86–b–PAA68 copolymer possessed abundant carboxyl groups to chelate the Mn2+ ions [36, 38].
Preparation of PAA68–b–PEG86–b–PAA68/MnO2
The formation of MnO2 was incorporated to induce in situ self-assembly of the water-soluble block copolymer in an aqueous phase. Inspired by the diversities of the polymer topologies, the MnO2–polymer architectures with vesicular morphology were obtained through phase separation. After adequately mixing the PAA68–b–PEG86–b–PAA68 and excess MnCl2·4H2O in 60 mL aqueous solution under magnetic stirring, 1 M NaOH aqueous solution was introduced into the above mixtures. Subsequently, the MnO2 formed induced in situ self-assembly of PAA68–b–PEG86–b–PAA68 to obtain the PAA68–b–PEG86–b–PAA68/MnO2 architectures. The morphology of the MnO2–polymer hybrids changed from irregular spheres to uniform vesicles as the Mn2+ feed concentrations increased as shown in Figs. 2a and S2.
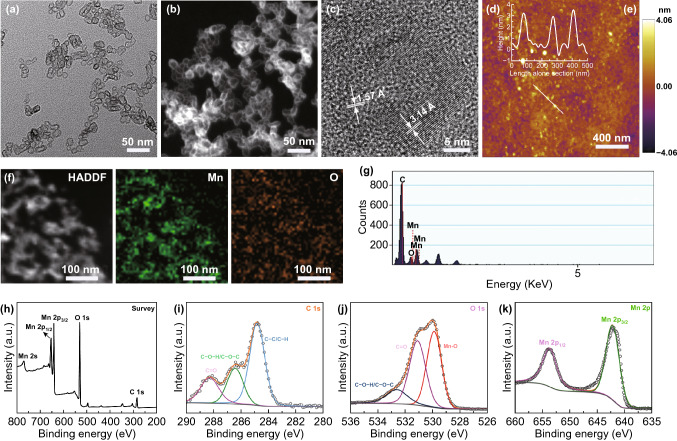
Fig. 2.
a TEM, b and f HADDF-STEM, c high-magnification TEM, d and e AFM height images of PAA68–b–PEG86–b–PAA68/MnO2. g EDS, h XPS, and high-resolution (HR) XPS spectra of i C 1s, j O 1s, and k Mn 2p of PAA68–b–PEG86–b–PAA68/MnO2 are presented
We made an important discovery that well-defined assemblies of PAA68–b–PEG86–b–PAA68 were not obtained with lower amounts of MnCl2·4H2O (1 and 3 mg mL−1), but amounts higher than 6 mg mL−1 produced well-defined vesicles. TEM images (Fig. 2a) showed that PAA68–b–PEG86–b–PAA68/MnO2 exhibited a vesicular morphology with a diameter of approximately 30 nm, which was consistent with the results of the determinations using the high-angle angular dark-field scanning transmission electron microscopy (HADDF-STEM, Fig. 2b) and atomic force microscopy (AFM, Fig. 2d, e). Moreover, the HADDF-STEM image of PAA68–b–PEG86–b–PAA68/MnO2 also revealed the vesicular morphology.
Notably, the AFM result showed that PAA68–b–PEG86–b–PAA68/MnO2 displayed a closed form, similar to vesicles with a hollow structure. Especially in the DLS (Fig. 3a), importantly, the hydrodynamic diameter of the PAA68–b–PEG86–b–PAA68/MnO2 architecture was much bigger than the size observed with TEM. This finding was attributed to the extreme stretch of hydrophilic PEG segments in the PAA68–b–PEG86–b–PAA68/MnO2 architecture in the aqueous phase, whereas these vesicles collapsed during the TEM analysis.
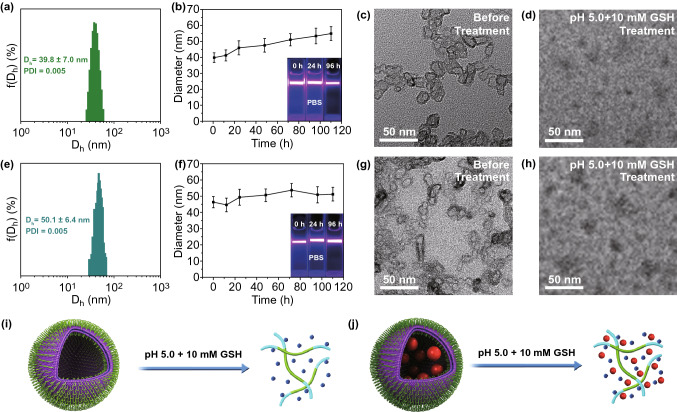
Fig. 3.
a Dh distributions, b hydrodynamic particle size and Tyndall effect of dispersed stability of PAA68–b–PEG86–b–PAA68/MnO2 in PBS at different times. TEM images c before and d after dissociation of PAA68–b–PEG86–b–PAA68/MnO2. e Dh distributions, f hydrodynamic particle size and Tyndall effect of dispersed stability of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 in PBS at different times. TEM images g before and h after dissociation of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2. Schematic illustrations of i disintegration of PAA68–b–PEG86–b–PAA68/MnO2 and j DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 at pH 5.0 with 10 mM GSH
As shown in Fig. 2f, the oxygen and Mn were uniformly distributed in the polymeric vesicles, indicating that Mn2+ ions were transformed into MnO2. Moreover, the result agreed with the energy-dispersive spectrometer (EDS) analysis (Fig. 2g) and XPS results (Fig. 2h), further indicating the existence of MnO2. To confirm the chemical valence of elemental Mn, the high-resolution (HR) XPS spectra of PAA68–b–PEG86–b–PAA68/MnO2 were analyzed and are presented in Fig. 2k. As shown in Fig. 2i-k, the C 1 s spectra of these samples were deconvoluted into three peaks that were assigned to a C–H/C–C band at 284.8 eV, C–O–C band at 286.7 eV, and C=O band at 288.2 eV, indicating the existence of a copolymer.
The deconvolutions of the O 1s spectra of PAA68–b–PEG86–b–PAA68/MnO2 indicated the obvious formation of MnO2 in these polymeric architectures because of the special peaks at 530.6 eV. In the Mn 2p spectrum (Fig. 2k), two specific peaks at binding energies of 653.1 and 641.1 eV were observed, indicating the existence of Mn4+ in MnO2 [39]. Additionally, the HRTEM image of the PAA68–b–PEG86–b–PAA68/MnO2 vesicle (Fig. 2c) exhibited characteristic lattice fringes with interplanar distance of 1.57 and 3.14 Å, also demonstrating the presence of MnO2 [40]. Moreover, the ICP-MS used to detect the content of MnO2 in the PAA68–b–PEG86–b–PAA68/MnO2 vesicles revealed that it was approximately 4 wt%.
Based on these results, polymeric vesicles with a controlled morphology and stability were successfully fabricated using the self-assembly approach in an aqueous phase. Compared to polymerization-induced self-assembly [41], MnO2 formation gradually made the soluble PAA block insoluble, which drove in situ self-assembly to form the MnO2–polymer polymeric vesicles. During the self-assembly, formation of MnO2 was used to drive the phase separation of one of the blocks and further induce in situ self-assembly of block copolymers (PAA68–b–PEG86–b–PAA68) to form the MnO2–polymer hybrids (PAA68–b–PEG86–b–PAA68/MnO2). Consequently, we determined that the self-assembly process in water should be performed via three steps: (1) complete dissolution of the copolymer segments in the aqueous phase at the initial stage, (2) reduction in the solvability of the PAA/MnO2 segments to drive the phase separation in the MnO2 formation process, and (3) nucleation of the PAA/MnO2 segments to induce the self-assembly of the copolymer chains at the final stage. Because of the vesicular morphology of PAA68–b–PEG86–b–PAA68/MnO2, we further explored its functions and properties for broader applications.
Structural Stability and Degradability of PAA68–b–PEG86–b–PAA68/MnO2
After successfully fabricating the polymer–inorganic architecture, we tested the structural stability of the PAA68–b–PEG86–b–PAA68/MnO2 vesicles in PBS. Figure 3b shows that PAA68–b–PEG86–b–PAA68/MnO2 exhibited a series of stable hydrodynamic diameters during 110 h. In addition, the Tyndall effects, which depend on the support of the corresponding dispersion system at different points in time, were observed as shown in Fig. 3b, demonstrating the excellent stability of the PAA68–b–PEG86–b–PAA68/MnO2 structure [42]. Subsequently, the dispersion time was extended to 20 days in PBS, and the corresponding Tyndall effect (Fig. S3) further indicated that the PAA68–b–PEG86–b–PAA68/MnO2 vesicles possessed excellent dispersity.
When the dispersion time was extended to 100 days in PBS, stable dispersion was maintained as shown in Fig. S5b. We also explored the structural stability of the PAA68–b–PEG86–b–PAA68/MnO2 vesicles in DMEM with FBS (10%, v/v) as shown in Fig. S4. When the PAA68–b–PEG86–b–PAA68/MnO2 hybrid vesicles were treated with DMEM with FBS (10%, v/v), an obvious Tyndall effect was observed even when the reaction time was extended to 6 days, indicating their favorable stability. We believed that the favorable stability of the PAA68–b–PEG86–b–PAA68/MnO2 vesicles was due to the introduction of MnO2.
Therefore, the introduction of MnO2 played a crucial role in the preparation of PAA68–b–PEG86–b–PAA68/MnO2 vesicles that exhibited excellent dispersity and good stability. As expected, the complete hydrolysis preserved abundant carboxyl groups and further supported the MnO2 cross-linking to introduce pH/GSH dual sensitivity in the final product. After treatment with an acidic and reductive dual-sensitive trigger (pH 5.0 and 10 mM GSH), the dissociation of PAA68–b–PEG86–b–PAA68/MnO2 was observed as presented in Figs. 3d and S5c compared to that of fresh PAA68–b–PEG86–b–PAA68/MnO2 (Figs. 3c and S5a), indicating the disintegration of PAA68–b–PEG86–b–PAA68/MnO2.
These degradable products, which have received US Food and Drug Administration (FDA) approval, included PEG and PAA [43, 44]. Other products include the as-released Mn2+ ions that can be metabolized easier by the kidneys [35, 36]. The transformation of MnO2 to Mn2+ ions could contribute to the disintegration of PAA68–b–PEG86–b–PAA68/MnO2 (Fig. 3i), which would facilitate the wider application of polymer–inorganic hybrids as DDS, especially in cancer treatments where the delivery of anticancer agents is the focus.
Dual-Responsive Drug Release from DOX-Loaded PAA68–b–PEG86–b–PAA68/MnO2 In Vitro
Taking advantage of the electrostatic interaction in an aqueous phase [45], the chemotherapeutic agent (DOX), a broad spectrum anticancer drug with known cardiotoxicity [46], was encapsulated in PAA68–b–PEG86–b–PAA68/MnO2. The PAA68–b–PEG86–b–PAA68/MnO2 polymeric vesicles showed a high drug encapsulation efficiency and drug loading capacity (both up to ~ 94%) derived from their abundant carboxyl groups. Moreover, the interpenetrating network between the copolymer and MnO2 in PAA68–b–PEG86–b–PAA68/MnO2 polymeric vesicles also probably contributed to the efficient loading of DOX.
After DOX loading, the vesicular morphology of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 vesicles was observed (Fig. 3g) and its Dh (Fig. 3e) increased more than that of the PAA68–b–PEG86–b–PAA68/MnO2 vesicles (Fig. 3a), suggesting the introduction of DOX molecules. The structural stability of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 was evaluated as shown in Fig. 3f, implying that DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 exhibited a favorable stability in PBS during 110 h. In addition, the Tyndall effect, which depends on the support of the corresponding dispersion system at different points in time, was observed as shown in Fig. 3f, demonstrating the excellent stability of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2.
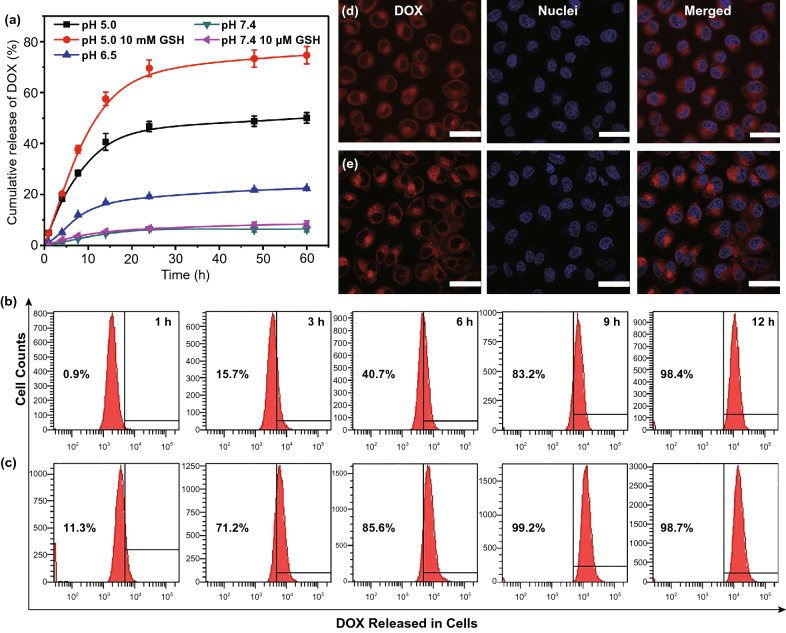
After treatment with an acidic and reductive dual-sensitive trigger (pH 5.0 and 10 mM GSH), the dissociation of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 was observed as shown in Fig. 3h, j, indicating the disintegration of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2. The DOX release curve showed that only 6% drug cumulative release occurred under normal conditions, whereas the drug cumulative release proportion reached 22% and 50% with decreasing pH from 6.5 to 5.0 within 60 h (Fig. 4a). In addition, the introduction of 10 μM GSH only slightly increased DOX release compared to that at pH 7.4 as presented in Fig. 4a.
Fig. 4.
a Cumulative release of DOX from DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 in simulated body fluids. b Intracellular DOX release from DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 in MCF-7 cells over a range of different times. c Free DOX used as control group. d CLSM visualization of cellular DOX release from DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 after incubation for 9 h. e Free DOX used as control group, scale bar: 50 μm. For CLSM and flow cytometric analyses, equivalent DOX dose was 2.5 μg mL−1
After treatment with GSH as high as 10 mM (a biologically relevant level [2-10 mM]) at pH 5.0 [18, 36], the DOX release was obviously facilitated, and a passive release plateau was observed over 24 h. Importantly, the final cumulative release ratio of DOX culminated at 75% at pH 5.0 in the presence of 10 mM GSH as presented in Fig. 4a. When the feed concentrations of DOX were decreased from 1.00 to 0.25 or 0.50 mg mL−1, a similar drug encapsulation efficiency of 95% or 93% was obtained. After DOX loading, the DOX1-loaded PAA68–b–PEG86–b–PAA68/MnO2 and DOX2-loaded PAA68–b–PEG86–b–PAA68/MnO2 vesicles displayed a similar Dh of approximately 50 nm as shown in Fig. S6.
Furthermore, the DOX1-loaded PAA68–b–PEG86–b–PAA68/MnO2 and DOX2-loaded PAA68–b–PEG86–b–PAA68/MnO2 vesicles also presented a similar trend of DOX release (Fig. S6) to that of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 (Fig. 4a) under the same conditions, indicating that the introduction of DOX molecules had a negligible influence on the structure of PAA68–b–PEG86–b–PAA68/MnO2. The intracellular DOX release from DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 was precisely evaluated at different time intervals as shown in Fig. 4b. At each incubation time, the DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 exhibited a slow cellular release in contrast to that of free DOX as shown in Fig. 4c, indicating that the DOX-loaded DDS was first degraded before and then the drugs were unloaded.
The DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 unloaded almost all its cargos when the incubation time was increased to 12 h, which almost agreed with the accumulative release of DOX from DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 at pH 5.0 in the presence of 10 mM GSH as presented in Fig. 4a. The CLSM technique was used to detect the intracellular distribution of the as-released DOX molecules from DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 as visualized in MCF-7 cells. After incubation for 9 h, the obvious red fluorescence of DOX molecules was observed at the cell nucleus as shown in Fig. 4d, suggesting that the DOX molecule was more efficiently delivered to the cell nucleus by the PAA68–b–PEG86–b–PAA68/MnO2 than the free DOX was (Fig. 4e). These results demonstrate that the polymeric vesicles responded to tumor acidity and reduction-triggered activation, achieving on-demand release of DOX.
Cytotoxicity of DOX-Loaded PAA68–b–PEG86–b–PAA68/MnO2 In Vitro
To further elucidate the toxicity of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2, its effects on the viability of MCF-7 cells were evaluated and free DOX and PAA68–b–PEG86–b–PAA68/MnO2 were selected as two controls as shown in Fig. S7. After treatment with PAA68–b–PEG86–b–PAA68/MnO2, both MCF-7 and HEK-293 cells maintained a viability of approximately 90% over the full tested concentration range over 3 days, indicating that PAA68–b–PEG86–b–PAA68/MnO2 exhibited a favorable biocompatibility. After incubation with DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2, the viability of MCF-7 cells decreased from 92.3 to 54.2% as the DOX equivalent dose to DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 increased from 0.1 to 5.0 μg within 1 day.
More intriguingly, the inhibitory effects of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 on MCF-7 cells were apparently enhanced when the incubation time was increased to 2 or 3 days. In addition, DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 displayed a similar trend with that of free DOX against MCF-7 cells (Fig. S7). These analyses validate the important fact that DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 induced cell death, whereas the PAA68–b–PEG86–b–PAA68/MnO2 vesicles exhibited favorable biocompatibility.
Therapeutic Effect of DOX-Loaded PAA68–b–PEG86–b–PAA68/MnO2 In Vivo
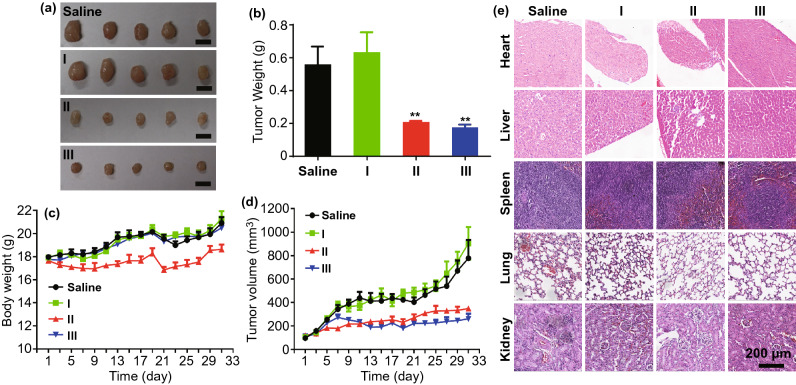
The antitumor efficacy of DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 in vivo was evaluated in mice bearing MCF-7 orthotopic xenografts. After 31 days, the tumor size apparently increased in the saline group (Fig. 5a). Moreover, the tumor size also showed similar trends in the PAA68–b–PEG86–b–PAA68/MnO2 group. After treatment with DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 for 31 days, there was an obvious decrease in the tumor size compared to that of both the saline and PAA68–b–PEG86–b–PAA68/MnO2 groups (Fig. 5a). This decrease was consistent with the results of the free DOX group, indicating that DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 exhibited an excellent antitumor efficacy.
Fig. 5.
Cancer chemotherapy in vivo using PAA68–b–PEG86–b–PAA68/MnO2 (I), free DOX (II), and DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 (III) in MCF-7 tumor-bearing mice. a Digital images of tumors from MCF-7 tumor-bearing mice after 31 days in vivo cancer chemotherapy, scale bar: 1 cm. b Weight of tumor excised from MCF-7 tumor-bearing mice after treatment. c Body weight changes of MCF-7 tumor-bearing mice during therapy. d Tumor size change of MCF-7 tumor-bearing mice during therapy. e Histological analyses of major tissues from MCF-7 tumor-bearing mice after 31 days in vivo cancer chemotherapy, scale bar: 200 μm
In addition, the tumor tissues of these mice were excised and weighed at the end of the experiment as shown in Fig. 5b, and the results indicate that DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 group exhibited similar tumor suppression to that in the DOX group. During treatment, the body weight of mice in the DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 group exhibited a similar trend with that of the PAA68–b–PEG86–b–PAA68/MnO2 group (Fig. 5c). In contrast, after 31 days, the body weight of mice in the free DOX group was obviously reduced by approximately 24%, revealing the severe toxicity of free DOX (Fig. 5c). As shown in Fig. 5d, the tumor size of the DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 and free DOX group slightly increase from ~ 100 to ~ 220 and 290 mm3, respectively.
In contrast, the tumor size of the PAA68–b–PEG86–b–PAA68 group obviously increases from approximately 100 to approximately 910 mm3. According to the histological analyses of the major organs, DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 did not induce obvious pathological changes in these tissues in contrast to the obvious pathological changes induced by the free DOX group as shown in Fig. 5e. Particularly, the liver and heart of the DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 group over came the cardiotoxicity of DOX. All the observations demonstrated that DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 exhibited higher tumor suppression without known cardiotoxicity, most probably because of the unique structure of the MnO2–polymer hybrid vesicles.
Conclusions
In conclusion, a simple and efficient aqueous self-assembly strategy was investigated for the construction of polymeric vesicles. In this self-assembly process, the formation of MnO2 not only induced in situ self-assembly of block copolymers, but also served as an interlocking agent to endow the polymeric vesicles with a fairly stable structure for the delivery process. Accordingly, the process of self-assembly is performed in three steps: (1) complete dissolution of the copolymer segments in an aqueous phase at the initial stage, (2) reduction in the solvability of the PAA block to drive the phase separation in the nucleation process, and (3) nucleation of PAA/MnO2 segments to induce self-assembly of the copolymer chains at the final stage.
Compared to other polymeric architectures formed using traditional self-assembly strategies, the polymeric vesicles respond to GSH and weak acidic conditions at tumor sites to unloading the cargo and are further degraded to hydrophilic linear polymers and Mn2+ ions after achieving drug delivery. DOX-loaded PAA68–b–PEG86–b–PAA68/MnO2 vesicles have been demonstrated, especially in in vivo studies, to overcome the cardiotoxicity of DOX. Moreover, other metallic oxides (including zinc oxide and ferroferric oxide) may also be utilized to induce in situ self-assembly of block copolymers using this strategy. Therefore, the as-proposed self-assembly strategy could facilitate the widening of the fabrication of a variety of polymeric architectures, and we expect that some versatile polymeric systems will be crafted using this environmentally friendly approach.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This project was granted financial support from the National Natural Science Foundation of China (21704093), Project funded by China Postdoctoral Science Foundation (2018M632795), Supports Plan for College Science and Technology Innovation Team of Henan Province (16IRTSTHN001), and Science & Technology Innovation Talent Plan of Henan Province (174200510018).
Contributor Information
Zhongyi Liu, Email: liuzhongyi@zzu.edu.cn.
Xiaojing Shi, Email: shixiaojing@zzu.edu.cn.
Xubo Zhao, Email: xbz2016@zzu.edu.cn.
References
- 1.Laurent J, Blin G, Chatelain F, Vanneaux V, Fuchs A, Larghero J, Thery M. Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nat. Biomed. Eng. 2017;1(12):939–956. doi: 10.1038/s41551-017-0166-x. [DOI] [PubMed] [Google Scholar]
- 2.Bell OA, Wu GL, Haataja JS, Brommel F, Fey N, et al. Self-assembly of a functional oligo(aniline)-based amphiphile into helical conductive nanowires. J. Am. Chem. Soc. 2015;137(45):14288–14294. doi: 10.1021/jacs.5b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dora Tang T-Y, Che Hak CR, Thompson AJ, Kuimova MK, Williams DS, Perriman AW, Mann S. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat. Chem. 2014;6(6):527–533. doi: 10.1038/nchem.1921. [DOI] [PubMed] [Google Scholar]
- 4.Karimi M, Zangabad PS, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR. Smart nanostructures for cargo delivery: uncaging and activating by light. J. Am. Chem. Soc. 2017;139(13):4584–4610. doi: 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman SJ. Note on colloidal dispersions from block copolymers. Appl. Polym. Sci. 1962;6(21):S15–S16. doi: 10.1002/app.1962.070062121. [DOI] [Google Scholar]
- 6.Krause S. Dilute solution properties of a styrene-methyl methacrylate block copolymer. J. Phys. Chem. 1964;68(7):1948–1955. doi: 10.1021/j100789a046. [DOI] [Google Scholar]
- 7.Warren NJ, Armes SP. Polymerization-induced self-assembly of block copolymer nano-objects via RAFT aqueous dispersion polymerization. J. Am. Chem. Soc. 2014;136(29):10174–10185. doi: 10.1021/ja502843f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018;118(14):6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 9.Discher BM, Won Y-Y, Ege DS, Lee JC-M, Bates FS, Discher DE, Hammer DA. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284(5417):1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 10.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 11.Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, Zboril R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016;116(9):5338–5431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar A, Traverso G, Farokhzad OC, Weissleder R, Langer R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017;1(8):0063. doi: 10.1038/s41570-017-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen DQ, Zhang GQ, Li RM, Guan MR, Wang XY, et al. Biodegradable, hydrogen peroxide, and glutathione dual responsive nanoparticles for potential programmable paclitaxel Release. J. Am. Chem. Soc. 2018;140(24):7373–7376. doi: 10.1021/jacs.7b12025. [DOI] [PubMed] [Google Scholar]
- 15.Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017;110:3–12. doi: 10.1016/j.addr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng HQ, Zhang YN, Liu LF, Wan W, Guo P, Nyström AM, Zou XD. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016;138(3):962–968. doi: 10.1021/jacs.5b11720. [DOI] [PubMed] [Google Scholar]
- 17.Grossen P, Witzigmann D, Sieber S, Huwyler J. PEG-PCL-based nanomedicines: a biodegradable drug delivery system and its application. J. Controlled Release. 2017;260:46–60. doi: 10.1016/j.jconrel.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Zhao XB, Liu P. Reduction-responsive core-shell-corona micelles based on triblock copolymers: novel synthetic strategy, characterization, and application as a tumor microenvironment-responsive drug delivery system. ACS Appl. Mater. Interfaces. 2015;7(1):166–174. doi: 10.1021/am505531e. [DOI] [PubMed] [Google Scholar]
- 19.Zhao XB, Qi MZ, Liang S, Tian K, Zhou TT, Jia X, Li JG, Liu P. Synthesis of photo- and pH dual-sensitive amphiphilic copolymer PEG43-b-P(AA76-co-NBA35-co-tBA9) and its micellization as leakage free drug delivery system for UV-triggered intracellular delivery of doxorubicin. ACS Appl. Mater. Interfaces. 2016;8(34):22127–22134. doi: 10.1021/acsami.6b08935. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W-J, Hong C-Y, Pan C-Y. Polymerization-induced self-assembly of functionalized block copolymer nanoparticles and their application in drug delivery. Macromol. Rapid Commun. 2018;40(2):1800279. doi: 10.1002/marc.201800279. [DOI] [PubMed] [Google Scholar]
- 21.Xiao YT, Liu J, Guo MY, Zhou HG, Jin J, et al. Synergistic combination chemotherapy using carrier-free celastrol and doxorubicin nanocrystals for overcoming drug resistance. Nanoscale. 2018;10(26):12639–12649. doi: 10.1039/c8nr02700e. [DOI] [PubMed] [Google Scholar]
- 22.Niu DC, Li YS, Shi JL. Silica/organosilica cross-linked block copolymer micelles: a versatile theranostic platform. Chem. Soc. Rev. 2017;46(3):569–585. doi: 10.1039/c6cs00495d. [DOI] [PubMed] [Google Scholar]
- 23.Miao YL, Qiu YD, Yang WJ, Guo YQ, Hou HW, Liu ZY, Zhao XB. Charge reversible and biodegradable nanocarriers showing dual pH-/reduction-sensitive disintegration for rapid site-specific drug delivery. Colloids Surf. B. 2018;169:313–320. doi: 10.1016/j.colsurfb.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Lee H, In I, Park SY. pH/redox/photo responsive polymeric micelle via boronate ester and disulfide bonds with spiropyran-based photochromic polymer for cell imaging and anticancer drug delivery. Eur. Polym. J. 2014;57:1–10. doi: 10.1016/j.eurpolymj.2014.04.020. [DOI] [Google Scholar]
- 25.Zhang H, Fei JB, Yan XH, Wang AH, Li JB. Enzyme-responsive release of doxorubicin from monodisperse dipeptide-based nanocarriers for highly efficient cancer treatment in vitro. Adv. Funct. Mater. 2015;25(8):1193–1204. doi: 10.1002/adfm.201403119. [DOI] [Google Scholar]
- 26.Louage B, Zhang QL, Vanparijs N, Voorhaar L, Casteele SV, et al. Degradable ketal-based block copolymer nanoparticles for anticancer drug delivery: a systematic evaluation. Biomacromolecules. 2015;16(1):336–350. doi: 10.1021/bm5015409. [DOI] [PubMed] [Google Scholar]
- 27.Chen WS, Ouyang J, Liu H, Chen M, Zeng K, et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv. Mater. 2017;29(5):1603864. doi: 10.1002/adma.201603864. [DOI] [PubMed] [Google Scholar]
- 28.Qiu M, Ouyang J, Sun HL, Meng FH, Cheng R, et al. Biodegradable micelles based on poly(ethylene glycol)-b-polylipopeptide copolymer: a robust and versatile nanoplatform for anticancer drug delivery. ACS Appl. Mater. Interfaces. 2017;9(33):27587–27595. doi: 10.1021/acsami.7b10533. [DOI] [PubMed] [Google Scholar]
- 29.Ding JX, Chen LH, Xiao CS, Chen L, Zhuang XL, Chen XS. Noncovalent interaction-assisted polymeric micelles for controlled drug delivery. Chem. Commun. 2014;50(77):11274–11290. doi: 10.1039/c4cc03153a. [DOI] [PubMed] [Google Scholar]
- 30.Bawa KK, Oh JK. Stimulus-responsive degradable polylactide-based block copolymer nanoassemblies for controlled/enhanced drug delivery. Mol. Pharm. 2017;14(8):2460–2474. doi: 10.1021/acs.molpharmaceut.7b00284. [DOI] [PubMed] [Google Scholar]
- 31.Saravanakumar G, Park H, Kim J, Park D, Pramanick S, Kim DH, Kim WJ. Miktoarm amphiphilic block copolymer with singlet oxygen-labile stereospecific β-aminoacrylate junction: synthesis, self-assembly, and photodynamically triggered drug release. Biomacromolecules. 2018;19(6):2202–2213. doi: 10.1021/acs.biomac.8b00290. [DOI] [PubMed] [Google Scholar]
- 32.Du B, Ding XY, Wang H, Du Q, Xu TG, Huang JS, Zhou J, Cheng GY. Development of an interactive tumor vascular suppression strategy to inhibit multidrug resistance and metastasis with pH/H2O2 responsive and oxygen-producing nanohybrids. J. Mater. Chem. B. 2019;7(31):4784–4793. doi: 10.1039/C9TB00546C. [DOI] [PubMed] [Google Scholar]
- 33.Lin XD, Fang Y, Tao ZH, Gao X, Wang TL, Zhao MY, Wang S, Liu YQ. Tumor-microenvironment-induced all-in-one nanoplatform for multimodal imaging-guided chemical and photothermal therapy of cancer. ACS Appl. Mater. Interfaces. 2019;11(28):25043–25053. doi: 10.1021/acsami.9b07643. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Ye DL, Wu MY, Chen HR, Zhang LL, Shi JL, Wang LZ. Break-up of two-dimensional MnO2 nanosheets promotes ultrasensitive pH-triggered theranostics of cancer. Adv. Mater. 2014;26(41):7019–7026. doi: 10.1002/adma.201402572. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Chen HR, Shi JL. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv. Mater. 2013;25(23):3144–3176. doi: 10.1002/adma.201205292. [DOI] [PubMed] [Google Scholar]
- 36.Zhao XB, Qiu YD, Miao YL, Liu ZY, Yang WJ, Hou HW. Unconventional preparation of polymer/amorphous manganese oxide-based biodegradable nanohybrids for low premature release and acid/glutathione-activated magnetic resonance imaging. ACS Appl. Nano Mater. 2018;1(6):2621–2631. doi: 10.1021/acsanm.8b00307. [DOI] [Google Scholar]
- 37.Zhao XB, Liu L, Li XR, Zeng J, Jia X, Liu P. Biocompatible graphene oxide nanoparticle-based drug delivery platform for tumor microenvironment-responsive triggered release of doxorubicin. Langmuir. 2014;30(34):10419–10429. doi: 10.1021/la502952f. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Feng LZ, Liu JJ, Zhu WW, Dong ZL, Wu YF, Liu Z. Intelligent albumin-MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016;28(33):7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- 39.Xia H, Feng JK, Wang HL, Lai MO, Lu L. MnO2 nanotube and nanowire arrays by electrochemical deposition for supercapacitors. J. Power Sources. 2010;195(13):4410–4413. doi: 10.1016/j.jpowsour.2010.01.075. [DOI] [Google Scholar]
- 40.Mastrikov YA, Heifets E, Kotomin EA, Maiera J. Atomic, electronic and thermodynamic properties of cubic and orthorhombic LaMnO3 surfaces. Surf. Sci. 2009;603(2):326–335. doi: 10.1016/j.susc.2008.11.034. [DOI] [Google Scholar]
- 41.Canning SL, Smith GN, Armes SP. A critical appraisal of RAFT-mediated polymerization-induced self-assembly. Macromolecules. 2016;49(6):1985–2001. doi: 10.1021/acs.macromol.5b02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LL, Zeng C, Xu H, Yin PC, Chen DC, et al. A highly soluble, crystalline covalent organic framework compatible with device implementation. Chem. Sci. 2019;10(4):1023–1028. doi: 10.1039/C8SC04255A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris JM, Chess RB. Effect of PEGylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 44.Liao Q, Shao QL, Wang HY, Qiu G, Lu XH. Hydroxypropylcellulose templated synthesis of surfactant-free poly(acrylic acid) nanogels in aqueous media. Carbohydr. Polym. 2012;87(4):2648–2654. doi: 10.1016/j.carbpol.2011.11.056. [DOI] [Google Scholar]
- 45.Yang WJ, Zhao XB. Glutathione-induced structural transform of double-cross-linked PEGylated nanogel for efficient intracellular anticancer drug delivery. Mol. Pharm. 2019;16(6):2826–2837. doi: 10.1021/acs.molpharmaceut.9b00467. [DOI] [PubMed] [Google Scholar]
- 46.Pugazhendhi A, Edison TNJI, Velmurugan BK, Jacob JA, Karuppusamy I. Toxicity of doxorubicin (dox) to different experimental organ systems. Life Sci. 2018;200:26–30. doi: 10.1016/j.lfs.2018.03.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.