Highlights
The 2.27-nm-thick hybridized quasi-2D structure of La2O3 crystalline nanoparticles embedded in La2O3 amorphous nanosheets (La2O3@NP-NS) exhibited a low overpotential of 310 mV at 10 mA cm−2.
The mass activity of La2O3@NP-NS reached as high as 6666.7 A g−1 at overpotential of 310 mV. Such a high mass activity was more than three orders of magnitude higher than that of benchmark IrO2 (4.4 A g−1) and RuO2 (2.05 A g−1) and five orders of magnitude higher than that of commercial La2O3 (0.048 A g−1).
Electronic supplementary material
The online version of this article (10.1007/s40820-020-0387-5) contains supplementary material, which is available to authorized users.
Keywords: Oxygen evolution reaction, Multiphase hybrid, Two-dimensional nanomaterials, Rare-earth oxides, Ionic layer epitaxy
Abstract
Electrochemical catalysts for oxygen evolution reaction are a critical component for many renewable energy applications. To improve their catalytic kinetics and mass activity are essential for sustainable industrial applications. Here, we report a rare-earth metal-based oxide electrocatalyst comprised of ultrathin amorphous La2O3 nanosheets hybridized with uniform La2O3 nanoparticles (La2O3@NP-NS). Significantly improved OER performance is observed from the nanosheets with a nanometer-scale thickness. The as-synthesized 2.27-nm La2O3@NP-NS exhibits excellent catalytic kinetics with an overpotential of 310 mV at 10 mA cm−2, a small Tafel slope of 43.1 mV dec−1, and electrochemical impedance of 38 Ω. More importantly, due to the ultrasmall thickness, its mass activity, and turnover frequency reach as high as 6666.7 A g−1 and 5.79 s−1, respectively, at an overpotential of 310 mV. Such a high mass activity is more than three orders of magnitude higher than benchmark OER electrocatalysts, such as IrO2 and RuO2. This work presents a sustainable approach toward the development of highly efficient electrocatalysts with largely reduced mass loading of precious elements.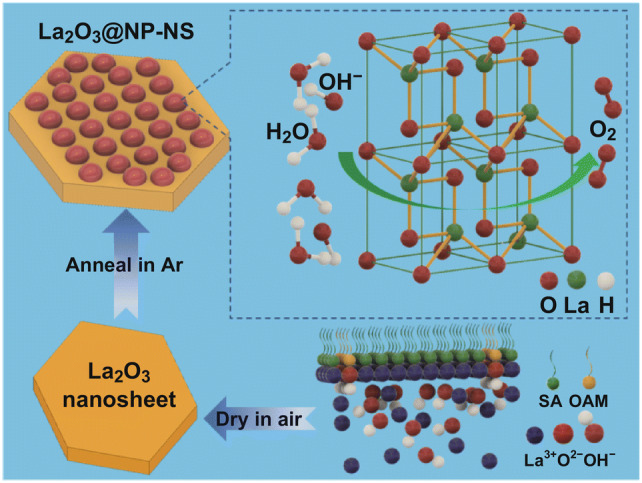
Electronic supplementary material
The online version of this article (10.1007/s40820-020-0387-5) contains supplementary material, which is available to authorized users.
Introduction
Today, oxygen evolution reaction (OER) is becoming an increasingly important process in many clean, renewable, and scalable electrochemical energy conversion and storage systems, such as fuel cells, electrochemical water splitting, solar fuel generation, and metal–air batteries [1–10]. To lead the exciting experimental developments of these energy systems to industrial applications, materials that catalyze OER with a high mass activity, a low overpotential, and a robust kinetic are highly desired [11–13]. Recent breakthroughs to lower the overpotential have revealed a large number of promising OER catalysts including carbon-based materials (e.g., graphene, CNT, and g-C3N4), and alternatives of transition metals (e.g., Mn, Co, Ni, and Fe) [13–25]. However, the low mass activity, high cost, and complicated fabrication procedure are still hindering scalable implementations of these materials in replacing the benchmark IrO2 and RuO2 that have high cost and limited supply [5, 13, 15, 26]. Therefore, development of high mass activity and high-efficient OER electrocatalysts based on earth-abundant elements is crucial and urgently needed to drive today’s advanced energy technologies forward [3, 13, 17].
Recently, rare-earth metal-based materials, especially rare-earth metal oxides, are found promising for a wide range of catalytic applications, including steam methane reforming, photocatalysis, water–gas shift reactions, thermochemical water splitting, and organocatalytic reactions [27]. Among them, La2O3 is showing a great potential as an alternative electrocatalytic candidate owing to its multiple oxidation states, excellent chemical stability, and low toxicity [27–29]. However, the electrochemical catalytic performance of commercial La2O3 powders is far from satisfaction mainly because of their low ratio of active catalytic sites and poor conductivity [5, 30]. Morphological alterations, particularly the two-dimensional (2D) geometry with just one or a few atomic layers, are a promising solution for improving the catalytic performance due to the abundant active sites, delocalized spin states, high electrical conductivity, and low mass loading [31, 32]. Furthermore, hybridization of 2D materials with nanoparticles (NPs) could offer an even greater boost to the electrochemical properties by combining the structural and electronic advantages of different morphologies [30, 33–35], such as lower the over potential of hydrogen evolution reaction (HER), and even raise the reversible capacity of lithium-ion batteries [36, 37]. Inspired by these previous advancements, reducing the dimension of La2O3 to a 2D structure and hybridizing with electrochemical active NPs may be a promising route leading La2O3 toward a high-performance electrochemical catalytic material in many energy conversion and storage systems. Based on this rationale, here, we report an ultrathin La2O3 nanosheets–NP hybrid structure (La2O3@NP-NS). The La2O3@NP-NS exhibited excellent OER performance with a low overpotential of 310 mV at 10 mA cm−2 and a very small Tafel slope of 43.1 mV dec−1 when the thickness of the La2O3 nanosheets was reduced to 2.27 nm. As a result of its uniform and ultrasmall thickness, they achieved a high mass activity, which was more than three orders of magnitude higher than benchmark IrO2 and RuO2, and five orders of magnitude higher than commercial La2O3 powder at the overpotential of 310 mV. This development presents an effective and scalable approach toward high-performance OER catalysts with a minimal use of precious rare-earth elements.
Experimental
Synthesis of 2.27-nm La2O3@NP-NS
Firstly, La2O3 nanosheets were synthesized by ionic layer epitaxy (ILE). In a typical process, 15 mL aqueous solution containing 0.2 mM La(NO3)3 and 2 mM hexamethylenetetramine (HMTA) was prepared in a glass vial with a 4.5 cm2 opening area. After standing the aqueous solution in air for 40 min, 10 µL chloroform solution of mixed surfactants containing (~ 0.9 vol %) stearic acid (SA) solution and (~ 0.1 vol %) oleylamine (OAM) solution was spread on the water surface. The SA solution had a concentration of 1.8 mol L−1. The OAM solution had a concentration of 1.8 mol L−1 and mixed with hydrochloric acid to a concentration of 0.01 mol L−1. This two-layer solution was exposed in atmosphere for 10 min to allow the chloroform to evaporate. Subsequently, the glass vial was screw-capped and placed in a 45 °C convection oven for 5 h for La2O3 nanosheets to grow. The La2O3 nanosheets were directly scooped onto a substrate and dried naturally in air. Secondly, the La2O3 nanosheets on the substrate were annealed in Ar atmosphere at 400 °C for 1 h, yielding 2.27-nm La2O3@NP-NS for further characterization and properties measurements. Si substrates were used for SEM and AFM characterization. Fifty-nanometer Au-coated Si substrates were used for XPS characterization. Holy carbon grids were used for TEM. The ILE-synthesized La2O3 samples were firstly transferred on holy carbon grids for TEM measurement, and these ILE-synthesized La2O3 samples on TEM grid were then annealed in Ar atmosphere for TEM analysis of La2O3@NP-NS samples. Fluorine-doped tin oxide (FTO) glass substrates were used for electrocatalytic OER measurement.
Synthesis of Thicker La2O3 Nanosheets
La2O3@NP-NS (8.68 nm) and La2O3 (28.26 nm) nanosheets were synthesized through the same ILE and subsequent Ar annealing process as described above, where the reaction temperature during ILE process was increased to 60 and 80 °C for 5 h, respectively.
La2O3 Nanoparticles–FTO Electrode
La2O3 NPs were synthesized under the same ILE process at a temperature of 80 °C for 5 h. The NPs were collected from the bulk solution by centrifuge. As-received La2O3 NPs (2 mg) was ultrasonically dispersed into an ethanol solution to form a catalytic ink. This ethanol solution contained 0.5 mL of ethanol and 50 μL of a Nafion® solution (5 wt% in aliphatic alcohol from Sigma-Aldrich). La2O3 NPs–FTO electrode was prepared by depositing 3 μL catalytic ink onto surface of FTO, which corresponded to a loading of 0.02 mg of catalyst per cm2. The ink was dried at room temperature in air.
IrO2–FTO, RuO2–FTO, and Commercial La2O3–FTO Electrode
Commercial IrO2, RuO2, or La2O3 powder (10 mg, from Sigma-Aldrich) was ultrasonically dispersed into an ethanol solution to form a catalytic ink. This ethanol solution contained 0.5 mL of ethanol and 50 μL of a Nafion® solution (5 wt% in aliphatic alcohol from Sigma-Aldrich). IrO2–FTO or RuO2–FTO electrode was prepared by depositing 10 μL catalytic ink onto surface of FTO, which corresponded to a loading of 0.926 mg of catalyst per cm2. The ink was dried at room temperature in air.
Material Characterizations
A Zeiss LEO 1530 field-emission scanning electron microscope was used to characterize morphologies of La2O3 nanosheets. The atomic force microscopy (AFM) tomography images were obtained using an XE-70 Park System. X-ray photoelectron spectroscopy (XPS) spectrum was obtained by a Thermo Scientific K-alpha XPS instrument with a 100 μm spot size, with the flood gun turned on during the measurements. The crystal structure was investigated by A FEI TF30 transmission electron microscopy (TEM) operated at 300 kV.
Electrochemical Testing
The OER performance was done on an Autolab PGSTAT302N workstation with a standard three-electrode system, which was used to record the catalytic activity of samples in N2-saturated 1 M NaOH solution (PH = 13.6).The as-prepared La2O3@NP-NS on FTO substrate directly acted as the working electrode (mass loading: 0.0014 mg cm−2 for 2.27-nm La2O3@NP-NC, 0.0055 mg cm−2 for 8.68 nm La2O3@NP-NS, and 0.0186 mg cm−2 for 28.3 nm La2O3 nanosheets), the saturated Ag/AgCl was used as the reference electrode, and a platinum wire was used as the counter electrode. The OER polarization curves were recorded at a scan rate of 5 mV s−1 1 M NaOH solution after purging with nitrogen for 30 min. All the potentials in this study are reported versus RHE. The electrochemical impedance spectroscopy (EIS) measurements were collected with frequencies ranging from 10 kHz to 0.1 Hz under an AC potential of 5 mV. For the stability test, the electrode was firstly activated in N2-saturated 1 M NaOH solution for 25 cycles and then recorded the first CV polarization curves at a sweep rate of 5 mV s−1. After continuous tests for 11 or 27 h, the final CV polarization curves were recorded at a sweep rate of 5 mV s−1 again. The overpotential (η) was calculated by the following relationship: η = E (vs. Ag/AgCl) + 0.21 V + 0.0592 × pH − 1.23 V − iRu, where i is the current, and Ru equals to the Rct value resolved from Nyquist plots. TOF can be calculated as TOF = (j × A)/(4 × F × n), where j (mA cm−2) is the current density at a particular overpotential, A is the area of the working electrode, F is the Faraday constant (96,500 C mol−1), and n is the number of moles of the active materials. The mass activity can be calculated as: mass activity = j/m, where m is the mass loading of the working electrode (mg cm−2) and j is the measured current density (mA cm−2) at given potential. Electrochemical active surface area (ECSA) was calculated by the equation ECSA = Cdl/Cs, where Cs is the specific capacitance of the samples. Herein, Cs of 0.04 mF cm−2 was used according to the previously reported value of metal oxide/hydroxides in NaOH solution [38]. Cdl was calculated from the slope of the line in the plot of capacitive current density (jdl) versus scan rates v (V/s). A is surface area of electrode. jdl = (Cdl × ν)/A, and cyclic voltammograms (CVs) were recorded in the double-layer regime (1 M NaOH, 0 to 0.1 V vs. Ag|AgCl) by varying scan rates ranging from 10 to 60 mV s−1.
Results and Discussion
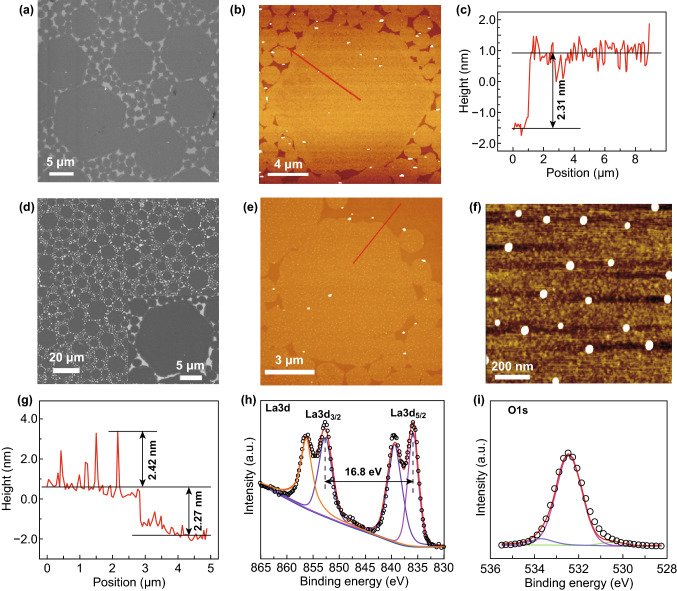
The La2O3@NP-NS was synthesized by a solution-based ILE process at the water–air interface under a monolayer of mixed surfactants followed by Ar annealing as shown in Fig. S1. Firstly, the La2O3 nanosheets were synthesized by ILE process. This aqueous solution contained 0.2 mM La(NO3)3 and 2 mM HMTA as precursors and had a weak alkaline environment because HMTA decomposed into HCHO and ammonium hydroxide at 45 °C. Under weak alkaline conditions, La3+ could easily combine with OH− to form Ce(OH)3, which would then be dehydrated to La2O3 after subsequent drying in the air. The as-synthesized La2O3 nanosheets exhibited a hexagonal shape with a bi-model size distribution (Fig. 1a). All the nanosheets appeared to be hexagonal, and their size exhibited a bi-model distribution. Larger nanosheets had a size of 15.7 ± 2.4 μm; while small ones were mostly around 1.8 ± 0.9 μm. This bi-model distribution was possibly due to the ILE mechanism where small nuclei or nanosheets tend to merge into big nanosheets driven by the reduction in surface energy [39]. Directly transferred La2O3 nanosheets covered almost the entire Si substrate surface, and no overlap was observed. AFM topography image (Fig. 1b) revealed that all the nanosheets had a very flat surface (surface roughness of 0.5 nm) with a uniform thickness of 2.31 nm (Fig. 1c). XPS analysis (Fig. S2a) showed that the spin–orbit splitting between La 3d3/2 and La 3d5/2 peaks was 16.8 eV, which was the typical characteristic of La2O3 phase [40–42]. TEM image (Fig. S3) revealed the amorphous structure of as-synthesized La2O3 nanosheets. In addition, the thickness of the La2O3 nanosheets could be tuned by the synthesis temperatures (Fig. S4). As the synthesis temperature rose from 45 to 60, and 90 °C, the nanosheet thickness increased from 2.31 to 8.73, and 28.30 nm, respectively. All the nanosheets still exhibited similar bi-model hexagonal geometry with excellent surface flatness.
Figure 1.
Morphology and elemental information of La2O3 nanosheets and La2O3@NP-NS. a SEM image of La2O3 nanosheets on a silicon substrate. b AFM topography scan of La2O3 nanosheets. c Height profiles derived from the red line in b. d SEM image of La2O3@NP-NS on a silicon substrate at low magnification. Inset is high-magnification SEM image of La2O3@NP-NS. e AFM topography scan of La2O3@NP-NS. f High-magnification AFM topography scan of La2O3@NP-NS. g Height profiles derived from the red line in e. h La 3d and i O 1s XPS spectrum of La2O3@NP-NS
The La2O3@NP-NS was obtained by annealing the as-synthesized La2O3 nanosheets in Ar at 400 °C for 1 h (Fig. S1). As shown by a low-magnification SEM image (Fig. 1d), the La2O3@NP-NS exhibited the same morphology and size distribution after the annealing. No cracks or dissociations could be observed. AFM image discovered that numerous fine NPs appeared on the nanosheet surface with a fairly uniform distribution (Fig. 1e). The surface density of the NPs was about 20 per μm2. Enlarged AFM image (Fig. 1f) showed that these NPs mostly had a spherical shape, anchored on the surface of nanosheets, and their sizes were in the range from 29 to 46 nm. The nanosheets still had a thickness of 2.27 nm (Fig. 1g), while the NPs were extruded above surface of nanosheet surface from 1 to 3 nm.
The elemental composition and chemical state of La2O3@NP-NS were then investigated by XPS. As shown in Fig. 1h, the La 3d spectrum was split into La 3d3/2 and La 3d5/2, located at 852.7 and 835.9 eV, respectively, with two satellite peaks at 856.3 and 839.4 eV, indicating the existence of La3+. Typical characteristic 16.8 eV between La 3d3/2 and La 3d5/2 peaks of La2O3 phase was still shown. In addition, the O 1s peaks at 532.5 and 530.5 eV could be assigned to the La–O bond in the La2O3 phase (Fig. 1i) [28, 43], further evidencing that the composition of La2O3@NP-NS was La2O3. By comparing the XPS results before and after Ar annealing, no obvious change was found for both La peaks and O peaks, suggesting both nanosheet and NPs had the same La2O3 chemistry. Thicker La2O3 nanosheets (8.73 and 28.30 nm) also showed the same morphology change after annealing (Fig. S5) with the same chemistry (Fig. S6). There was also a large quantity of NPs appeared uniformly on the surface of 8.68 nm nanosheets (Fig. S5c and inset). However, no NPs could be observed from the 28.26 nm nanosheets, possibly because the large thickness completely buried the NPs inside (Fig. S5d).
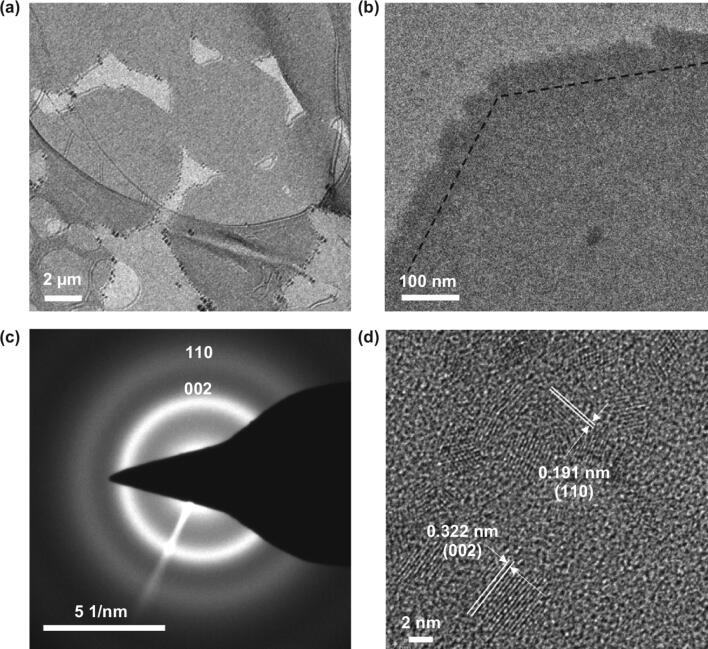
The crystal structure of La2O3 is hexagonal structure. There are two types of oxygen atoms in each hexagonal unit cell: One (denoted O4) is coordinated with four La atoms and the other (denoted O6) with six La atoms, while each La atom is coordinated with seven oxygen atoms: Four of them are O4, and the other three are O6 [44]. The crystal structure of La2O3@NP-NS was then studied by TEM. Low-magnification TEM image showed a uniform contrast of all hexagonal La2O3@NP-NS (Fig. 2a). A zoom-in image (Fig. 2b) at the corner area revealed that the angle between two adjacent edges of nanosheets was about 120° as marked by the dotted lines. The overall nanosheet showed a uniform contrast but no clearly observable lattices. The very front of all the edges was mostly wavy. Both features indicate amorphous or polycrystallinity structure of the nanosheet. Selected area electron diffraction (SAED) pattern collected from multiple nanosheets surfaces (Fig. 2c) showed wide and diffusive diffraction rings, suggesting that the La2O3@NP-NS indeed had a significant amount of an amorphous phase with small crystallites. The spread of interplanar spacing measured from the ring diameters was associated with the (002) and (110) planes of hexagonal-structured La2O3, confirming its polycrystalline structure [45]. HRTEM image (Fig. 2d) revealed the NP-NS hybrid structure. All the NPs had a crystalline phase with a size ranging from 6 to 32 nm, in good agreement with AFM observations. The interplanar spacing from the NPs was identified to be 0.324 and 0.192 nm, corresponding to the (002) and (110) planes of hexagonal La2O3 [45]. No crystal lattices could be observed from the nanosheet region, suggesting the nanosheet was still remained as an amorphous phase providing a uniform matrix supporting the crystalline NPs. These structure analyses indicated that during annealing in the Ar, the amorphous La2O3 nanosheets could firstly begin to crystallize and many tiny crystalline domains would thus form in the largely amorphous nanosheets; as the annealing time progressed, these tiny crystalline domains would then grow in both lateral and vertical size, gradually be extruded above surface of nanosheets and eventually grow into nanoparticles embedded in the nanosheets. Besides, Fig. S7 reveals the structure of crystallites distributed in the amorphous nanosheets region for 28.26 nm nanosheets, which confirmed that large thickness completely buried the NPs inside the 28.26 nm nanosheets after annealing.
Figure 2.
Structural characterization of 2.27-nm La2O3@NP-NS. a TEM image of hexagonal La2O3@NP-NS on a holy carbon TEM grid. b TEM image taken from a corner of a large La2O3@NP-NS. c SAED pattern of La2O3@NP-NS. d HRTEM image of La2O3@NP-NS
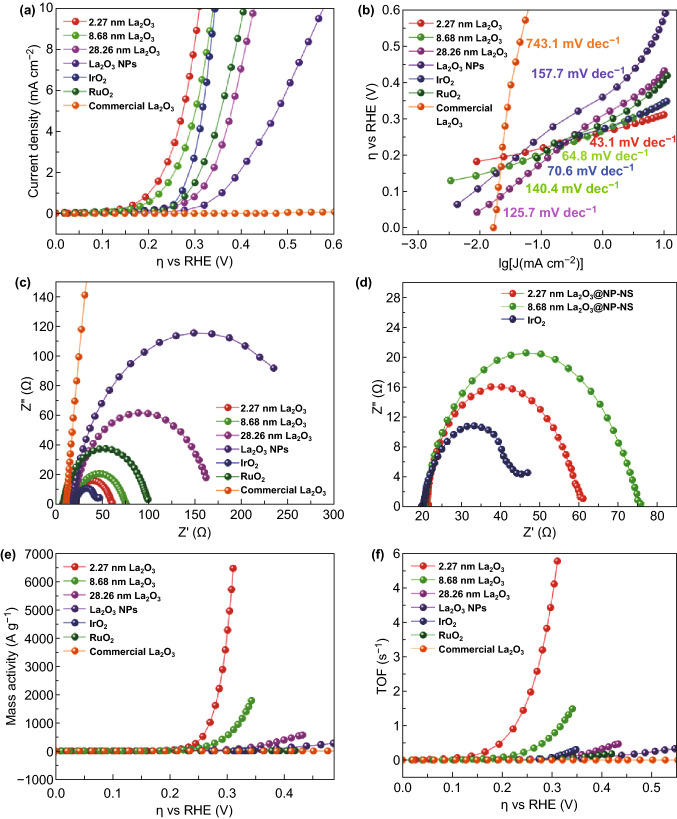
To investigate the electrocatalytic performance of La2O3@NP-NS for OER, the OER polarization curves of 2.27-nm La2O3@NP-NS on FTO substrate were probed in alkaline electrolyte (1 M NaOH). Through XPS analysis (Fig. S8), the La2O3@NP-NS grown on FTO substrate has the same La2O3 chemistry with that on Si substrate. As a comparison, the OER activities of thicker La2O3@NP-NS nanosheets, La2O3 NPs, commercial IrO2, RuO2, and La2O3 powders were also characterized under the same conditions. As shown in Fig. 3a, the 2.27-nm La2O3@NP-NS exhibited the smallest overpotential of 310 mV at a current density of 10 mA cm−2. Thicker nanosheets exhibited higher overpotential of 343 and 429 mV for 8.68 and 28.26 nm nanosheets, respectively. The very low overpotential from the 2.27-nm nanosheets outperformed the state-of-the-art noble metals oxides, e.g., IrO2 (343 mV) and RuO2 (408 mV) at the same current density. Besides, commercial La2O3 powders exhibited a very high overpotential of 1330 mV (Fig. S12a), confirming the significant morphological advantage of the 2D nanosheets.
Figure 3.
Electrocatalytic OER performance comparison of 2.27-nm La2O3@NP-NS (red), 8.68 nm La2O3@NP-NS (green), and 28.26 nm La2O3 nanosheets (magenta), ILE synthesized La2O3 NPs (violet), commercial IrO2 (blue), RuO2 (olive), and La2O3 powder (orange). a OER polarization curves measured in 1 M NaOH solution. b Tafel plots. c Nyquist plots measured in 1 M NaOH solution at a potential of 310 mV versus RHE. d Enlarged Nyquist plots. e Mass activity determined from current density as a function of ŋ. f TOF determined from j as a function of η. (Color figure online)
Tafel plots from La2O3@NP-NS with different thicknesses and other comparison samples were then characterized to evaluate and compare their reaction rate constants and electrocatalytic kinetics. As illustrated in Fig. 3b, Tafel slope of the 2.27-nm La2O3@NP-NS was calculated to be only 43.1 mV dec−1, significantly lower than that of thicker nanosheets (Table 1). In addition, the low Tafel slope of the 2.27-nm La2O3@NP-NS was also superior to the benchmark IrO2, RuO2 (Table 1), and some recently reported novel OER catalysts, such as CeOx/CoOx (66 mV dec−1) [46], NiO/CoN PINWs (44.5 mV dec−1) [47], CoxNi1−xFe2O4 (46.4 mV dec−1) [48], Ni0.75V0.25-LDH (50 mV dec−1) [49], and ZnCo2O4/N-CNT (70.6 mV dec−1) [50]. Such a low Tafel slope value from the 2.27-nm La2O3@NP-NS suggests that the reaction rate constant and charge transfer rate were greatly improved as the nanosheets thickness reduced to the ultrathin nanometer scale. These improved reaction rate constant and charge transfer rate could be attributed to 2D electron gas, which was possibly presented at the interface of NP-NS in the 2D La2O3 nanohybrids and contributed to their improved electrocatalytic properties by enhancing electron mobility and conductivity of nanohybrids [51].
Table 1.
OER performance comparison between the samples synthesized in this work
| Samples | Over potential (mV) @10 mA cm−2 | Tafel slope (mV dec−1) | Rct (Ω) | Mass activity (A g−1) @310 mV | TOF (s−1) @310 mV |
|---|---|---|---|---|---|
| 2.27 nm La2O3@NP-NS | 310 | 43.1 | 38 | 6666.7 | 5.79 |
| 8.68 nm La2O3@NP-NS | 343 | 64.8 | 57 | 713 | 0.88 |
| 28.26 nm La2O3 | 429 | 125.7 | 143 | 51 | 0.055 |
| ILE synthesized La2O3 NPs | 580 | 157.7 | 272 | 17.4 | 0.012 |
| Commercial IrO2 powder | 343 | 70.4 | 25.4 | 4.4 | 0.0026 |
| Commercial RuO2 powder | 408 | 140.6 | 89.6 | 2.05 | 7.23 × 10−4 |
| Commercial La2O3 powder | 1330 | 743.1 | 47,598 | 0.048 | 4.87 × 10−5 |
EIS was further investigated to reveal the charge transfer resistance (Rct) of La2O3@NP-NS and to understand its role in the enhanced OER performance. As shown in Fig. 3c, Nyquist plot of the 2.27-nm La2O3@NP-NS had a much smaller semicircle compared to thicker nanosheets. From the Nyquist plots, Rct of the 2.27-nm La2O3@NP-NS was found only about 38 Ω (Fig. 3d), which was lower than those of thicker nanosheets, La2O3 nanoparticles, and RuO2 powders, and only slightly larger than that of IrO2 powder (Table 1). The largely reduced Rct was possibly due to the greatly shortened charge transfer distance between the catalysis surface and substrate as a result of the ultrasmall thickness [30].
One major advantage of the ultrathin nanosheet for electrochemical catalysis is its ultrahigh ratio of active catalytic sites. Figure 3e demonstrates the calculated mass activity plots. A remarkable mass activity of 6666.7 A g−1 was obtained from the 2.27-nm La2O3@NP-NS at overpotential 310 mV. It was one to more than two orders of magnitudes higher than other morphologies of La2O3 (Table 1) at the same overpotential. Such a large mass activity evidenced the unique merit of the ultrathin 2D geometry, which involved an extremely low mass and rendered considerably large exposed surface area allowing a very high ratio of catalytically active sites within the material. Besides, the NPs extruded above surface of nanosheet should also contribute to high ratio of active catalytic sites. Furthermore, this mass activity was three orders of magnitude higher than benchmark IrO2 (4.4 A g−1) and RuO2 (2.05 A g−1), and five orders of magnitude higher than commercial La2O3 at the same overpotential, suggesting that our 2.27-nm La2O3@NP-NS could be very competitive electrochemical catalysts for industrial applications. The electrochemical active surface area (ECSA) was also calculated based on cyclic voltammograms curves (Fig. S13) to estimate the exposure of active sites. As shown in Fig. S14, the values of ECSA for 2.27 (0.025) and 8.68 nm (0.021 cm2) La2O3@NP-NS were close and about twice as much as 28.26 nm La2O3 nanosheets, which could be because the NPs extruded above surface of nanosheet also contributed to active catalytic sites. Moreover, the ECSA value of commercial La2O3 powder (0.31 cm2) was much bigger than that of 2.27-nm La2O3@NP-NS, but it exhibited poor OER performance, which could attribute to good conductivity caused by ultrathin thickness, but poor intrinsic OER catalytic activity of commercial La2O3 powder [32].
The turnover frequency (TOF) was another critical OER parameter that evaluates the number of O2 molecules formed per active metal site per second. As shown in Fig. 3f, the TOF of 2.27-nm La2O3@NP-NS reached up to 5.79 s−1 at the overpotential of 310 mV, which was about one to five orders of magnitudes higher than those comparison samples (Table 1). The extremely high TOF could be attributed to the largely facilitated electron transfer from the ultrathin nanosheet to the FTO substrate as well as the very large mass activity. The significant enhancement in both mass activity and TOF confirmed the ultrathin La2O3@NP-NS could provide a highly efficient OER performance with a minimal use of rare-earth elements.
To further figure out the origin of the active sites for La2O3@NP-NS, electrocatalytic OER performances of 2.31 nm La2O3 nanosheets before Ar annealing were also investigated and compared to that of 2.27-nm La2O3@NP-NS, as shown in Fig. S15. It was found that the OER performance of 2.31 nm La2O3 nanosheets was greatly enhanced after Ar annealing at 400 °C for one hour. The overpotential was shifted toward a lower overpotential by 59 mV after annealing, with corresponding Tafel slope reducing from 95.4 to 43.1 mV dec−1. Moreover, mass activity increased greatly from 3238 to 6666.7 A g−1. All these results confirmed that the catalytic active sites of La2O3@NP-NS were attributed to precipitated La2O3 nanoparticles after Ar annealing, as well as ultrathin nanosheet region.
To better understand the OER performance of the ultrathin La2O3@NP-NS hybrid structure, the OER characteristic data (e.g., overpotential, mass loading, mass activity, and TOF) from other representative state-of-the-art OER catalysts were collected and compared (Table S1). While the overpotential from this work was generally superior or at least comparable to those Ni-, Co-, Mn-, or Fe-based OER catalysts, the biggest advantage of our nanosheets was obviously the extremely large mass activity. As a result of the ultrasmall thickness and uniform coverage over a large area, the mass loading of our La2O3@NP-NS was only 0.0014 mg cm−2, which brought the mass activity to more than three orders of magnitude higher than typical OER catalysts such as CoFe–LDHs [52], Co3O4 [53], and Ni@NC [54].
For nanostructured catalysts, the stability is vital due to the largely increased atom mobility on nanoscale surfaces. Impressively, the ultrathin La2O3@NP-NS exhibited superb stability with 90% retention of the initial current density after 11 h of continuous OER operation in 1 M NaOH at η = 345 mV (Fig. 4a). SEM image showed that before (Fig. S16a) and after (Fig. S16b) the 11-hour continuous OER test, the coverage of nanosheets was nearly the same and no obvious damage could be observed; XPS analysis (Fig. S17a) revealed that the La2O3@NP-NS almost retained their chemistry. In addition, the OER polarization curve showed only a slight decay after the OER test (Fig. 4b), which confirmed appropriate function of the La2O3@NP-NS coating during the long-term operation. The high stability of the ultrathin La2O3@NP-NS nanosheets might be attributed to the amorphous–crystallites hybrid structure. The amorphous La2O3 matrix could stabilize the small La2O3 NPs from aggregation by limiting the atomic diffusion, and on the other hand, the anchored La2O3 NPs could act as spacers to prevent the 2D La2O3 nanosheets from restacking during electrochemical cycling [37, 51]. Besides, the stability test of 2.27-nm La2O3@NP-NS with longer time was also investigated. As shown in Fig. S18a, the stability test survived for 27 h because there was a sharp decrease in the current density at the time range from 25 to 27 h. SEM images (Fig. S18c, d) revealed that the majority of La2O3@NP-NS fell off and only part of samples was adsorbed on the FTO substrate after 27 h stability test, which resulted in the decrease in current density during stability test and confirmed that OER performance truly came from the ultrathin La2O3@NP-NS. Long-hour immersion in alkaline solution may weaken the adsorption of La2O3@NP-NS on FTO substrate, which could cause the La2O3@NP-NS to fall off from the substrate during stability test.
Figure 4.
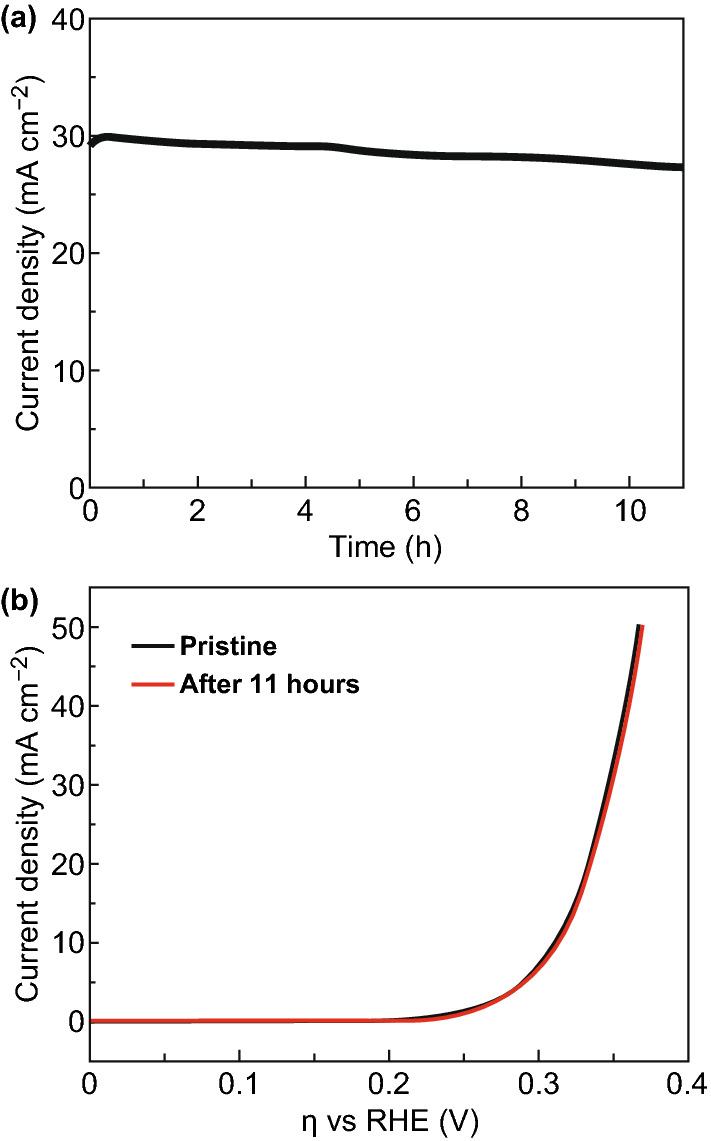
Electrochemical stability tests. a Current density measured at η = 345 mV (vs. RHE) as a function of time. b OER polarization curves of 2.27-nm La2O3@NP-NS before and after 11-h OER
Conclusions
In conclusion, La2O3@NP-NS was synthesized by a facile solution-based ILE approach in a large area. Outstanding electrocatalytic OER performance was observed when the nanosheet thickness was reduced to 2.27 nm. The 2.27-nm La2O3@NP-NS exhibited a low overpotential of 310 mV at 10 mA cm−2, a small Tafel slope of 43.1 mVdec−1, and charge transfer resistance of 38 Ω. A high mass activity of 6666.7 A g−1 and turnover frequency of 5.79 s−1 were also obtained from the 2.27-nm La2O3@NP-NS at an overpotential of 310 mV. This very large mass activity was more than three orders of magnitude higher than benchmark IrO2 (4.4 A g−1) and RuO2 (2.05 A g−1), and five orders of magnitude higher than commercial La2O3 (0.048 A g−1) at the same overpotential. The nanosheets also exhibited an impressive stability for over 11-h continuous operation with only 10% decay. The excellent electrocatalytic OER activity could be attributed to the ultrathin thickness that enables overall short out-of-plane charge diffusion length and facilitated electron transfer from the nanosheet surface to the substrate. This development opens up a promising strategy for the development of highly efficient electrocatalysts with largely reduced loading of catalytic materials. Creating the hybrid ultrathin 2D morphologies could potentially become an ultimate solution to preserve precious elements in advanced and sustainable electrocatalyst design in many energy and environmental systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Y.G. thanks Jun Li and Lazarus German for helpful discussion. This work is supported by Army Research Office (ARO) under Grant W911NF-16-1-0198, the National Science Foundation (DMR-1709025), and China Scholarship Council.
Contributor Information
Zhiqiang Cao, Email: caozq@dlut.edu.cn.
Yanchao Mao, Email: ymao@zzu.edu.cn.
Xudong Wang, Email: xudong.wang@wisc.edu.
References
- 1.Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature. 2012;488:294–302. doi: 10.1038/nature11475. [DOI] [PubMed] [Google Scholar]
- 2.Dresselhaus M, Thomas I. Alternative energy technologies. Nature. 2001;414:332–337. doi: 10.1201/b17893. [DOI] [PubMed] [Google Scholar]
- 3.Wang YY, Xie C, Zhang ZY, Liu DD, Chen R, et al. In situ exfoliated, n-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction. Adv. Funct. Mater. 2018;28:1703363. doi: 10.1002/adfm.201703363. [DOI] [Google Scholar]
- 4.Jin YS, Wang HT, Li JJ, Yue X, Han YJ, et al. Porous MoO2 nanosheets as non-noble bifunctional electrocatalysts for overall water splitting. Adv. Mater. 2016;28:3785–3790. doi: 10.1002/adma.201506314. [DOI] [PubMed] [Google Scholar]
- 5.Tahir M, Pan L, Idrees F, Zhang X, Wang L, et al. Electrocatalytic oxygen evolution reaction for energy conversion and storage: a comprehensive review. Nano Energy. 2017;37:136–157. doi: 10.1016/j.nanoen.2017.05.022. [DOI] [Google Scholar]
- 6.Mao YC, Ning C, Zhang N, Hu Y, Li MY, et al. Enhancing photoelectrochemical performance of TiO2 nanowires through a facile acid treatment method. J. Electrochem. Soc. 2018;165:799–803. doi: 10.1149/2.0431813jes. [DOI] [Google Scholar]
- 7.Mao YC, Cheng YG, Wang JQ, Yang H, Li MY, et al. Amorphous NiO electrocatalyst overcoated ZnO nanorod photoanodes for enhanced photoelectrochemical performance. New J. Chem. 2016;40:107–112. doi: 10.1039/c5nj01815c. [DOI] [Google Scholar]
- 8.Wang XT, Ouyang T, Wang L, Zhong JH, Ma T, et al. Redox-inert Fe3+ in octahedral sites of Co–Fe spinel oxides with enhanced oxygen catalytic activity for rechargeable Zn-air batteries. Angew. Chem. Int. Ed. 2019;58:13291–13296. doi: 10.1002/ange.201907595. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang T, Ye YQ, Wu CY, Xiao K, Liu ZQ, et al. Heterostructures composed of N-doped carbon nanotubes encapsulating Co and β-Mo2C nanoparticles as bifunctional electrodes for water splitting. Angew. Chem. Int. Ed. 2019;58:4923–4928. doi: 10.1002/anie.201814262. [DOI] [PubMed] [Google Scholar]
- 10.Su H, Wang XT, Hu JX, Ouyang T, Xiao K, et al. Co-Mn spinel supported self-catalysis induced N-doped carbon nanotubes with high efficiency electron transport channels for zinc-air batteries. J. Mater. Chem. A. 2019;7:22307–22313. doi: 10.1039/C9TA08064C. [DOI] [Google Scholar]
- 11.Guo DH, Shibuya RK, Akiba C, Saji S, Kondo T, et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science. 2016;351:361–365. doi: 10.1126/science.aad0832. [DOI] [PubMed] [Google Scholar]
- 12.Xia BY, Yan Y, Li N, Wu HB, Lou XWD, Wang X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy. 2016;1:15006. doi: 10.1038/nenergy.2015.6. [DOI] [Google Scholar]
- 13.Cheng WR, Zhao X, Su H, Tang F, Che W, et al. Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis. Nat. Energy. 2019;4:115–122. doi: 10.1038/s41560-018-0308-8. [DOI] [Google Scholar]
- 14.Zheng S, Zheng L, Zhu Z, Chen J, Kang J, Huang Z, Yang D. MoS2 nanosheet arrays rooted on hollow rGO spheres as bifunctional hydrogen evolution catalyst and supercapacitor electrode. Nano-Micro Lett. 2018;10:62. doi: 10.1007/s40820-018-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma TY, Dai S, Jaroniec M, Qiao SZ. Graphitic carbon nitride nanosheet-carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2014;53:7281–7285. doi: 10.1002/anie.201403946. [DOI] [PubMed] [Google Scholar]
- 16.Walter MG, Warren EL, McKone JR, Boettcher SW, Mi Q, et al. Solar water splitting cells. Chem. Rev. 2010;110:6446–6473. doi: 10.1021/cr1002326. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Chen C, Baiyee ZM, Shao Z, Ciucci F, et al. Nonstoichiometric oxides as low-cost and highly-efficient oxygen reduction/evolution catalysts for low-temperature electrochemical devices. Chem. Rev. 2015;115:9869–9921. doi: 10.1021/acs.chemrev.5b00073. [DOI] [PubMed] [Google Scholar]
- 18.Zhang BW, Jiang K, Wang HT, Hu S. Fluoride-induced dynamic surface self-reconstruction produces unexpectedly efficient oxygen-evolution catalyst. Nano Lett. 2019;19:530–537. doi: 10.1021/acs.nanolett.8b04466. [DOI] [PubMed] [Google Scholar]
- 19.Zhang RR, Zhang YC, Pan L, Shen GQ, Mahmood N, et al. Engineering cobalt defects in cobalt oxide for highly efficient electrocatalytic oxygen evolution. ACS Catal. 2018;8:3803–3811. doi: 10.1021/acscatal.8b01046. [DOI] [Google Scholar]
- 20.Li XN, Liu H, Chen ZZ, Wu QM, Yu ZY, et al. Enhancing oxygen evolution efficiency of multiferroic oxides by spintronic and ferroelectric polarization regulation. Nat. Commun. 2019;10:1409. doi: 10.1038/s41467-019-09191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Y, Qiu M, Kim MG, Liu P, Nam G, et al. Atomically dispersed nickel-nitrogen-sulfur species anchored on porous carbon nanosheets for efficient water oxidation. Nat. Commun. 2019;10:1392. doi: 10.1038/s41467-019-09394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang ZF, Song JJ, Du YH, Xi SB, Dou S, et al. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy. 2019;4:329–338. doi: 10.1038/s41560-019-0355-9. [DOI] [Google Scholar]
- 23.Hu L, Xiong TZ, Liu R, Hu YW, Mao YC, et al. Co3O4@Cu-based conductive metal–organic framework core-shell nanowire electrocatalysts enable efficient low-overall-potential water splitting. Chem. Eur. J. 2019;25:6575–6583. doi: 10.1002/chem.201900045. [DOI] [PubMed] [Google Scholar]
- 24.Hu L, Hu Y, Liu R, Mao Y, Balogun MSJT, Tong Y. Co-based MOF-derived Co/CoN/Co2P ternary composite embedded in N- and P-doped carbon as bifunctional nanocatalysts for efficient overall water splitting. Int. J. Hydrogen Energy. 2019;44:11402–11410. doi: 10.1016/j.ijhydene.2019.03.157. [DOI] [Google Scholar]
- 25.Wang FX, Hu L, Liu R, Yang H, Xiong TZ, et al. Hybrid implanted hybrid hollow nanocube electrocatalyst facilitates efficient hydrogen evolution activity. J. Mater. Chem. A. 2019;7:11150–11159. doi: 10.1039/c9ta00931k. [DOI] [Google Scholar]
- 26.Suntivich J, May KJ, Gasteiger HA, Goodenough JB, Shao-Horn Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science. 2011;334:1383–1385. doi: 10.1126/science.1212858. [DOI] [PubMed] [Google Scholar]
- 27.Montini T, Melchionna M, Monai M, Fornasiero P. Fundamentals and catalytic applications of CeO2-Based materials. Chem. Rev. 2016;116:5987–6041. doi: 10.1021/acs.chemrev.5b00603. [DOI] [PubMed] [Google Scholar]
- 28.Kang JG, Kim YI, Cho DW, Sohn Y. Synthesis and physicochemical properties of La(OH)3 and La2O3 nanostructures. Mat. Sci. Semicon. Proc. 2015;40:737–743. doi: 10.1016/j.mssp.2015.07.050. [DOI] [Google Scholar]
- 29.Yadav AA, Lokhande AC, Pujaria RB, Kimb JH, Lokhande CD. The synthesis of multifunctional porous honey comb-like La2O3 thin film for supercapacitor and gas sensor applications. J. Colloid Interface Sci. 2016;484:51–59. doi: 10.1016/j.jcis.2016.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Shifa TA, Wang F, Liu Y, He J. Heterostructures based on 2d materials: a versatile platform for efficient catalysis. Adv. Mater. 2018;31(45):1804828. doi: 10.1002/adma.201804828. [DOI] [PubMed] [Google Scholar]
- 31.Jin H, Guo C, Liu X, Liu J, Vasileff A, et al. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 2018;118:6337–6408. doi: 10.1021/acs.chemrev.7b00689. [DOI] [PubMed] [Google Scholar]
- 32.Tian P, Yu YH, Yin X, Wang XD. A wafer-scale 1 nm Ni(OH)2 nanosheet with superior electrocatalytic activity for the oxygen evolution reaction. Nanoscale. 2018;10:5054–5059. doi: 10.1039/c7nr09042k. [DOI] [PubMed] [Google Scholar]
- 33.Deng D, Novoselov KS, Fu Q, Zheng N, Tian Z, et al. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016;11:218–230. doi: 10.1038/nnano.2015.340. [DOI] [PubMed] [Google Scholar]
- 34.Yu M, Zhou S, Wang Z, Zhao J, Qiu J. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy. 2018;44:181–190. doi: 10.1016/j.nanoen.2017.12.003. [DOI] [Google Scholar]
- 35.Tang C, Wang HS, Wang HF, Zhang Q, Tian GL, et al. Spatially confined hybridization of nanometer-sized NiFe hydroxides into nitrogen-doped graphene frameworks leading to superior oxygen evolution reactivity. Adv. Mater. 2015;27:4516–4522. doi: 10.1002/adma.201501901. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Wang H, Xie L, Liang Y, Hong G, et al. MoS2 Nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011;133:7296–7299. doi: 10.1021/ja201269b. [DOI] [PubMed] [Google Scholar]
- 37.Mujtaba J, Sun HY, Huang GY, Mølhave K, Liu YG, et al. Nanoparticle decorated ultrathin porous nanosheets as hierarchical Co3O4 nanostructures for lithium ion battery anode materials. Sci. Rep. 2016;6:20592. doi: 10.1038/srep20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charles CLM, Suho J, Jonas CP, Thomas FJ. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013;135:16977. doi: 10.1021/ja407115p. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Seo JH, Luo G, Starr MB, Li Z, et al. Nanometre-thick single-crystalline nanosheets grown at the water–air interface. Nat. Commun. 2016;7:10444. doi: 10.1038/ncomms10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Guan B, Maclennan A, Hu YF, Li DD, et al. Porous waxberry-like MnO2/La2O3 microspheres for high performance asymmetric supercapacitor. Electrochim. Acta. 2017;241:395–405. doi: 10.1016/j.electacta.2017.04.175. [DOI] [Google Scholar]
- 41.Honma T, Benino Y, Fujiwara T, Komatsu T. Electronic polarizability, optical basicity, and interaction parameter of La2O3 and related glasses. J. Appl. Phys. 2002;91:2942–2950. doi: 10.1063/1.1436292. [DOI] [Google Scholar]
- 42.Yang C, Fan H, Qiu S, Xi Y, Fu Y. Microstructure and dielectric properties of La2O3 films prepared by ion beam assistant electron-beam evaporation. J. Non-Cryst. Solids. 2009;355:33–37. doi: 10.1016/j.jnoncrysol.2008.09.029. [DOI] [Google Scholar]
- 43.Sunding MF, Hadidi K, Diplas S, Løvvik OM, Norby TE, et al. XPS characterisation of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures. J. Electron. Spectrosc. 2011;184:399–409. doi: 10.1016/j.elspec.2011.04.002. [DOI] [Google Scholar]
- 44.Li B, Metiu H. DFT studies of oxygen vacancies on undoped and doped La2O3 surfaces. J. Phys. Chem. C. 2010;114:12234–12244. doi: 10.1021/jp103604b. [DOI] [Google Scholar]
- 45.Huang P, Zhao Y, Zhang J, Zhu Y, Sun Y. Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction. Nanoscale. 2013;5:10844–10848. doi: 10.1039/c3nr03617k. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Shin K, Kawashima K, Youn DH, Lin J, et al. Enhanced activity promoted by CeOx on a CoOx electrocatalyst for the oxygen evolution reaction. ACS Catal. 2018;8:4257–4265. doi: 10.1021/acscatal.8b00820. [DOI] [Google Scholar]
- 47.Yin J, Li YX, Lv F, Fan QH, Zhao YQ, et al. Porous nanowires as efficient bifunctional catalysts for Zn–air batteries. ACS Nano. 2017;11:2275–2283. doi: 10.1021/acsnano.7b00417. [DOI] [PubMed] [Google Scholar]
- 48.Maruthapandian V, Mathankumar O, Saraswathy V, Subramanian B, Muralidharan O. Study of the oxygen evolution reaction catalytic behavior of CoxNi1–xFe2O4 in alkaline medium. ACS Appl. Mater. Interfaces. 2017;9:13132–13141. doi: 10.1021/acsami.6b16685. [DOI] [PubMed] [Google Scholar]
- 49.Fan K, Chen H, Ji Y, Huang H, Claesson PM, et al. Nickel–vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016;7:11981. doi: 10.1038/ncomms11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu ZQ, Cheng H, Li N, Ma TY, Su YZ. ZnCo2O4 quantum dots anchored on nitrogen-doped carbon nanotubes as reversible oxygen reduction/evolution electrocatalysts. Adv. Mater. 2016;28:3777–3784. doi: 10.1002/adma.201506197. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Ma C, Zhang Q, Wang W, Pan P, et al. 2D electron gas and oxygen vacancy induced high oxygen evolution performances for advanced Co3O4/CeO2 nanohybrids. Adv. Mater. 2019;31:1900062. doi: 10.1002/adma.201900062. [DOI] [PubMed] [Google Scholar]
- 52.Li P, Wang MY, Duan XX, Zheng LR, Cheng XP, et al. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides. Nat. Commun. 2019;10:1711. doi: 10.1038/s41467-019-09666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Ouyang B, Xu J, Jia G, Chen S, et al. Rapid synthesis of cobalt nitride nanowires: highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. Int. Ed. 2016;55:8670–8674. doi: 10.1002/anie.201604372. [DOI] [PubMed] [Google Scholar]
- 54.Ren J, Antonietti M, Fellinger TP. Efficient water splitting using a simple Ni/N/C paper electrocatalyst. Adv. Energy Mater. 2015;5:1401660. doi: 10.1002/aenm.201401660. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.