Highlights
Lead-based halide perovskite materials have revealed excellent properties in optoelectronic applications. However, the material stability and the toxicity of lead still hinder their large-scale commercial applications.
Lead-free halide double perovskite materials possess the characteristics of environmental friendliness, exceptional stability and tunable optoelectronic properties.
A limited number of halide double perovskites have been synthesized, and extremely few have been developed for optoelectronic applications. Continuing effort is needed to explore more halide double perovskites and modulate the properties for their further applications.
Keywords: Halide double perovskite, Optoelectronic applications, Efficiency, Stability, Toxicity
Abstract
Lead-based halide perovskites have emerged as excellent semiconductors for a broad range of optoelectronic applications, such as photovoltaics, lighting, lasing and photon detection. However, toxicity of lead and poor stability still represent significant challenges. Fortunately, halide double perovskite materials with formula of A2M(I)M(III)X6 or A2M(IV)X6 could be potentially regarded as stable and green alternatives for optoelectronic applications, where two divalent lead ions are substituted by combining one monovalent and one trivalent ions, or one tetravalent ion. Here, the article provides an up-to-date review on the developments of halide double perovskite materials and their related optoelectronic applications including photodetectors, X-ray detectors, photocatalyst, light-emitting diodes and solar cells. The synthesized halide double perovskite materials exhibit exceptional stability, and a few possess superior optoelectronic properties. However, the number of synthesized halide double perovskites is limited, and more limited materials have been developed for optoelectronic applications to date. In addition, the band structures and carrier transport properties of the materials are still not desired, and the films still manifest low quality for photovoltaic applications. Therefore, we propose that continuing efforts are needed to develop more halide double perovskites, modulate the properties and grow high-quality films, with the aim of opening the wild practical applications.
Introduction
Halide perovskites with the generic formula AM(II)X3 (A: CH3NH3+, CH(NH2)+2, Cs+; M(II): Pb2+, Sn2+; X: I−, Br−, Cl−) can be divided into two broad categories according to the A-location cation: organic–inorganic hybrid and all-inorganic halide perovskites. Initially, halide perovskites of CH3NH3PbX3 (X=Br, I) were utilized as light sensitizer to replace dyes in dye-sensitized solar cells with liquid electrolyte in 2009 [1]. However, due to the underdeveloped efficiency ~ 3.8% and stability, the initial perovskite solar cells (PSCs) did not capture widespread attention. Until 2012, a solid hole transport layer was substituted for the liquid electrolyte to develop all-solid-state PSCs. Most strikingly, the efficiency and stability were simultaneously distinctly improved [2], which stimulated “perovskite fever” [3–5]. Recently, the certified efficiency of PSCs has risen to 23.7%, which is comparable to conventional silicon solar cells [6]. The rocketing improvement of efficiency is attributed to the suitable direct bandgap, strong absorption coefficient, long-range charge diffusion length, balanced electron–hole mobility, high dielectric constant, excellent carrier mobility and small exciton binding energy of halide perovskites [7–10]. Besides solar cells, these materials have been applied in other optoelectronic applications, such as light-emitting diodes (LEDs) [11, 12], lasers [13], photodetectors [14, 15] and X-ray detectors [16].
Although efficiency of PSCs has been progressively grown, there are still huge barriers which limit the commercial applications. For instance, the long-term stability over 10 years is the most critical obstacle to restrict the commercialization [17]. Currently, the PSCs cannot remain stable more than 1 year outdoors, whereas silicon solar cells are usually guaranteed to work for at least 25 years. Moisture, temperature, oxygen and extreme light levels all cause PSCs to decompose. Moisture is the worst problem because reaction with the water forms hydrates to destroy the crystal structures [18]. To improve the stability of PSCs, six main solutions have been adopted: (1) regulating the crystal structures to improve phase stability by doping [19], (2) reducing crystal defects to limit penetrating channels from external environment [20, 21], (3) designing new stable halide perovskite materials [22, 23], (4) using stable inorganic charge transport layers [24], (5) adopting 2D halide perovskite materials [25, 26] and (6) packaging the devices [27]. Based on the above solutions, cell lifetimes have extended from a few days to months. Nevertheless, there is still a long way to go toward long-time stability over tens of years. Also, toxic lead is still necessary to achieve high performance. Lead pollution will do serious harm to human health, such as fatigue, muscle weakness, clumsiness and clouded consciousness [28]. Extensive efforts have been paid to design new non-/low-toxic and stable halide perovskites for solar cells as well as other optoelectronic applications [29–31]. Sn2+ and Ge2+ ions could be expected to replace Pb2+ ions in perovskites. However, the Sn2+ and Ge2+ cations tend to undergo oxidation due to the high-energy-lying 5 s orbitals, rendering the corresponding perovskite extremely unstable in ambient atmosphere [30, 31]. Similarly, Bi3+ and Sb3+ ions with the similar isoelectronic structure have been substituted for Pb2+ ions to develop stable and lead-free perovskites. However, inherently low-dimensional structures of the A3M(III)2X9 perovskites result in less impressive performance [22, 23]. Afterward, doping bivalent metal ions in moderation not only decreases lead content, but also enhances the efficiency and stability, such as Sr2+ [32], Co2+ [33] and Zn2+ [34]. The main reason is that the metal ion-based doping can moderately improve the quality of perovskite films. Simultaneously, doping a certain type of heterovalent metal ions can engineer the band structure of perovskite to enhance the performance, such as Ag+ [35], Sb3+ [36] and Bi3+ [37]. However, the doping method cannot thoroughly solve any issues of stability or toxicity.
Recently, halide double perovskites (A2M(I)M(III)X6, A2M(IV)X6) have been proposed as stable and green alternatives to lead halide perovskites, where two toxic lead ions are substituted by combining one monovalent and one trivalent ions, or one tetravalent ion and one vacancy site (marked as “□”) to yield the same overall charge balance as the conventional perovskites, as shown in Fig. 1a [38]. The formula of A2M(IV)X6 is considered as vacancy-ordered halide double perovskites, which is the analogy of A2M(I)M(III)X6. Because of the cubic structure to extend three dimensions with corner-sharing metal halide octahedra, halide double perovskites have attracted extensive attention as promising optoelectronic candidates. In this review, we highlight lead-free halide double perovskite materials and their related optoelectronic applications including photodetectors, X-ray detectors, photocatalyst, LEDs and solar cells. The synthesized halide double perovskites exhibited pleasurable stability. But only a limited number of halide double perovskites have been synthesized, and extremely few have been developed for optoelectronic applications. In addition, the band structures and carrier transport properties of the materials are still not desired, and the films still manifest low quality for photovoltaic applications. It is universally acknowledged that significant effort is needed to discover more candidates and modulate the properties for their further applications.
Fig. 1.
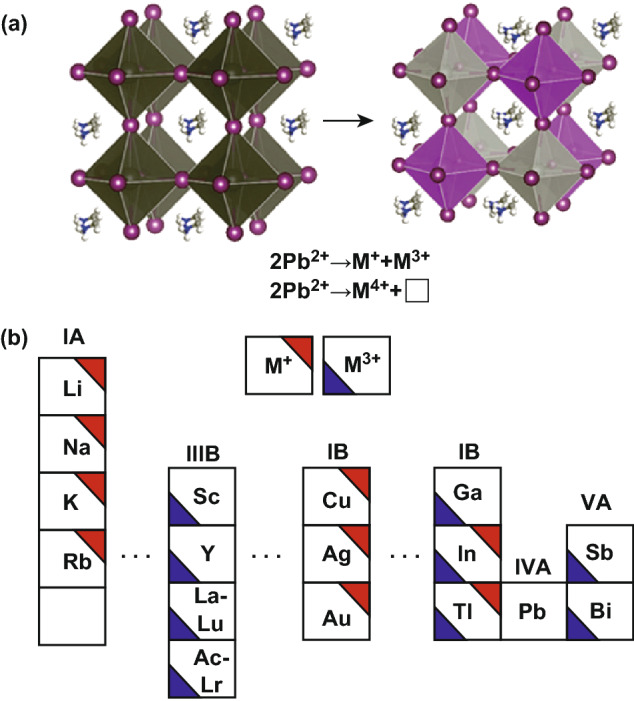
a Schematic illustration of the transformation from APbX3 to A2M(I)M(III)X6 or A2M(IV)X6, where two toxic Pb2+ ions are substituted by combining M+ and M3+ ions (or M4+ ion). “□” denotes M-site vacancy. b Elements of M-location cations with M+ and M3+ in the periodic table for halide double perovskites. When any M-site elements are localized at IA or IIIA groups of the periodic table, the materials have direct bandgaps
Halide Double Perovskite Materials
Besides stability, the band structures and carrier transport properties of the materials predetermine to a large extent the specific applications and their performance. In halide perovskites with the chemical formula APbX3, the valence and conduction bands are predominantly made up of the Pb-6p and X-5p orbitals. In addition, the size of the A-location cation affects the PbX6 octahedra to distort/tilt, which can slightly modify the band structures [39]. Similarly, the band structures of the halide double perovskites are mainly decided by the M(I)-, M(III)- (or M(IV)-) and X-site atoms. The elements of M-location cations with monovalent and trivalent in the periodic table toward halide double perovskites are shown in Fig. 1b. The elements in IA, IB and IIIA groups can occupy the M+ sites, and the elements in IIB, IIIA and VA groups are found at the M3+ sites. When any M-site elements of the halide double perovskites are localized at IA or IIIA groups of the periodic table, the materials have direct bandgaps. The In and Tl elements in IIIA group possess valencies +1 and +3. The vacancy-ordered halide double perovskites (A2M(IV)X6) all have direct bandgaps. Theoretically, there are a lot of halide double perovskites, though the exact formula must be considered two aspects of the Goldschmidt’s rule and thermodynamic stability [40]. However, only a limited number of halide double perovskites have been synthesized so far, as listed in Table 1 including their synthetic methods, where MA is CH3NH3.
Table 1.
Summary of the prepared halide double perovskites
| Materials | Morphology | Synthetic method | References |
|---|---|---|---|
|
Cs2NaMCl6 (M=Am, Bk, Tl, Bi, Lu, etc.) |
Powder or single crystal | Evaporating HCl solution to dryness, or heating anhydrous chlorides, or precipitating from cold HCl solution, or growing crystals from dilute HCl solution by cooling evaporation | [41–47] |
| Cs2AgBiCl6 | Powder |
Melt crystallization Precipitation from heated acid solution |
[48, 49] |
| Nanocrystal |
Hot injection Antisolvent recrystallization |
[50, 51] | |
| Cs2AgBiBr6 | Powder |
Melt crystallization Precipitation from heated acid solution |
[48] |
| Single crystal | Cooling crystallization | [52, 54, 55] | |
| Nanocrystal |
Hot injection Antisolvent recrystallization |
[50, 51, 56] | |
| Cs2(Ag1−aBi1−b)TlxBr6 | Single crystal | Cooling crystallization | [57] |
| Cs2Ag(Bi1−xMx)Br6 (M=In, Sb) | Single crystal | Melt crystallization | [58] |
| Cs2AgBiI6 | Nanocrystal |
Antisolvent recrystallization Anion exchange |
[50, 51] |
| Cs2AgInCl6 |
Single crystal Powder |
Cooling crystallization Precipitating from cold HCl solution |
[59–61] |
| Mn-doped Cs2AgInCl6 | Microcrystal | Hot injection | [62] |
| Cs2NaBiI6 | Microcrystal | Hydrothermal method | [64] |
| Cs2SnI6 |
Microcrystal Nanocrystal |
Hot injection | [74–76] |
| Bi-doped Cs2SnCl6 | Single crystal | Cooling crystallization | [77] |
| Cs2PdBr6 | Single crystal | Cooling crystallization | [78] |
| Nanocrystal | Antisolvent recrystallization | [79] | |
| Cs2PdI6 | Nanocrystal | Anion exchange | [79] |
|
A2TiBr6 (A=K, Rb, Cs) |
Powder | Molten salt | [80] |
|
Cs2TiIxBr6−x (x = 0, 2, 4, 6) |
Powder | Melt crystallization | [81] |
| (MA)2KBiCl6 | Powder | Evaporating HCl solution to dryness | [38] |
| (MA)2TlBiBr6 | Single crystal | Hydrothermal method | [82] |
| (MA)2AgBiBr6 | Powder | Evaporating HBr solution to dryness | [83] |
| (MA)2AgSbI6 | Powder | Melt crystallization | [84] |
| (MA)2AgBiI6 | Powder | Melt crystallization | [85] |
|
(MA)2KGdCl6 (MA)2KYCl6 |
Powder | Evaporating HCl solution to dryness | [86] |
| (MA)2AgInBr6 | Single crystal | MAPbBr3-induced crystallization | [87] |
| (MA)2SnI6 | Powder | Mixed iodides | [88] |
All-Inorganic Halide Double Perovskites
Initially, all-inorganic halide double perovskite of Cs2NaAmCl6 was prepared in 1968 by evaporating HCl solution containing cations to dryness [41]. Afterward, a few all-inorganic halide double perovskites (Cs2NaM(III)Cl6) were fabricated by the same method [42–44]. In those days, ferroelectric phase transition was considered particularly attracted for all-inorganic halide double perovskites [45–47], which was observed on the cooling Cs2NaBiCl6 [47].
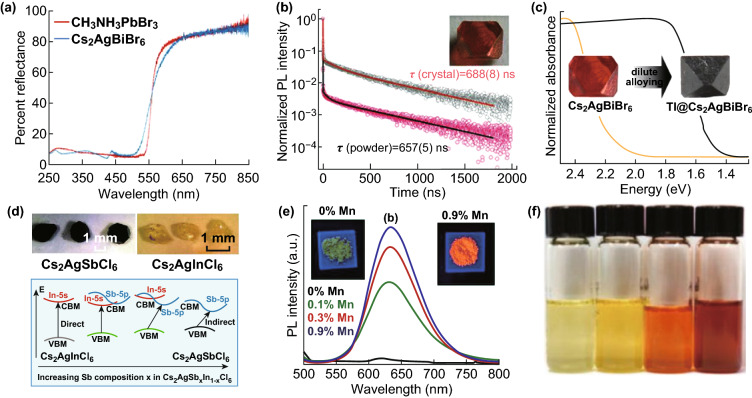
In light of the obsessive interest in halide perovskites, halide double perovskites have attained great concern in recent 2 years. In 2016, three different groups almost simultaneously reported Cs2AgBiX6 (X=Cl or Br) as a promising alternative to the lead halide perovskites, which crystallizes in cubic symmetry and shows light absorption at the visible range of the spectrum [48, 49, 52]. Woodward et al. [48] measured diffuse reflectance to reveal bandgaps of 2.19 and 2.77 eV for Cs2AgBiBr6 and Cs2AgBiCl6, respectively, as shown in Fig. 2a. The band structure calculation indicates that the interaction between Ag 4d orbitals and 3p/4p orbitals of the halide ions modifies valence band, leading to an indirect bandgap. Both compounds are stable when exposed to air; however, Cs2AgBiBr6 degrades over a period of weeks when exposed to ambient air and light. Meanwhile, Karunadasa et al. [52] tried to synthesize highly thermal and moisture-stable Cs2AgBiBr6 single crystal with an indirect bandgap of 1.95 eV and photoluminescence (PL) lifetime of ~ 660 ns, as shown in Fig. 2b, which is very encouraging for photovoltaic applications. Then, Giustino et al. [49] designed and synthesized Cs2AgBiCl6 with bandgaps between 1.95 and 3.04 eV. However, the bandgap of Cs2AgBiX6 (X=Cl, Br) is indirect and slightly large, not ideal for thin-film photovoltaic applications. To engineer the bandgaps, Karunadasa et al. [52, 57] tried to incorporate Tl as a dilute impurity into their reported Cs2AgBiBr6 single crystals. After incorporating Tl, the color changed to opaque black from translucent orange, which reflects the reduction of the bandgaps, as shown in Fig. 2c. The Tl content can be tuned across the series Cs2(Ag1−aBi1−b)TlxBr6 (0.003 < x = a + b < 0.075), and Cs2(Ag1−aBi1−b)TlxBr6 (x = 0.075) displays low indirect and direct bandgaps of 1.40 and 1.57 eV, respectively. Importantly, time-resolved photoconductivity measurements reveal long-lived carriers with microsecond lifetimes in the alloyed material, suggesting that carriers can be efficiently extracted in a solar cell [53]. The alloyed perovskite is the first halide double perovskite to show comparable bandgap and carrier lifetime to those of CH3NH3PbI3, but unfortunately the content of Tl is still toxic [53, 57]. In 2017, Yan et al. [58] used Cs2AgBiBr6 as a host to engineer the bandgap through alloying of In3+ and Sb3+. Cs2Ag(Bi1−xMx)Br6 (M=In, Sb) accommodates up to 75% In3+ with increased bandgap, and up to 37.5% Sb3+ with reduced bandgap, that is, enabling ~ 0.41 eV bandgap modulation through introduction of the two metals, with smallest value of 1.86 eV for Cs2Ag(Bi0.625Sb0.375)Br6. Band structure calculations indicate that opposite bandgap shift directions associated with Sb/In substitution arise from different atomic configurations for these atoms. Similarly, McQueen et al. [59] designed indirect and direct bandgap transitions using alloy strategy, as shown in Fig. 2d. The synthesized Cs2AgSbCl6 and Cs2AgInCl6 single crystals have indirect and direct bandgaps, respectively. When increasing Sb composition x in Cs2AgSbxIn1−xCl6, the compounds gradually transit from direct to indirect bandgap. Later on, Giustino et al. [60] identified that Cs2InAgCl6 has direct bandgap of 3.3 eV and the compound is found to be photosensitive and turns reversibly from white to orange under ultraviolet (UV) illumination. Typically, Cs2InAgCl6 exhibits a wide bandgap which limits its application in the visible region. In 2018, Nag et al. [62] imparted the visible-light emission property in direct bandgap Cs2AgInCl6 by doping Mn2+ ions, as shown in Fig. 2e. Cs2AgInCl6 host absorbs UV light and then transfers the excitation energy to Mn d electrons. For X-site in perovskites, the Bohr radii gradually increase from F to I elements, resulting in increasing tightly bound nature and bandgaps [63]. Obviously, the X elements in the synthesized halide double perovskites are usually Cl or Br, and the bandgaps are relatively large. Gamelin et al. [51] synthesized Cs2AgBiX6 (X=Cl, Br) colloidal nanocrystals by a hot-injection approach, which were converted to new materials including Cs2AgBiI6 with a narrow bandgap about 1.75 eV through anion exchange. Figure 2f shows the photograph of dilute toluene solutions of (left to right) Cs2AgBiBr6, Cs2AgBiBr5.2I0.8, Cs2AgBiBr1.6I4.4 and Cs2AgBiI6 nanocrystals, and the dark red color of Cs2AgBiI6 reflects that the absorption extends throughout the visible region. Recently, Ma et al. [64] reported first time the synthesis of novel halide double perovskite material of Cs2NaBiI6 and determined its crystal structure by XRD and XPS tests and optical properties by UV–Vis absorption spectra. Cs2NaBiI6 has a low direct bandgap of 1.66 eV and exhibits high stability against moisture and oxygen in ambient air.
Fig. 2.
a Diffuse reflectance spectra of Cs2AgBiBr6 and CH3NH3PbBr3. Reproduced with permission from Ref. [48]. b Time-resolved room-temperature PL and fits for the PL decay time (τ) in powder and single-crystal samples. The inset is the photograph of a Cs2SgBiBr6 single crystal. Reproduced with permission from Ref. [52]. c Apparent bandgaps of Cs2AgBiBr6 and Cs2(Ag1−aBi1−b)TlxBr6 (x = a + b = 0.075) single crystals extracted by linear fits to α2 vs. E (direct gap) and α1/2 vs. E plots (indirect gap). Reproduced with permission from Ref. [57]. d Photographs of Cs2AgSbCl6 and Cs2AgInCl6 single crystals (top), and band diagram. A change in the character of the conduction band minimum (CBM) from s orbital derived to p orbital derived while having the valence band maximum (VBM) primarily Ag-d states results in a transition from direct to indirect bandgap. Reproduced with permission from Ref. [59]. e PL spectra of Mn-doped Cs2AgInCl6 with different Mn contents, after excitation with 340 nm light. Insets show photographs of luminescence from powder samples under UV light. Reproduced with permission from Ref. [62]. f Photograph of dilute toluene solutions of Cs2AgBiBr6, Cs2AgBiBr5.2I0.8, Cs2AgBiBr1.6I4.4 and Cs2AgBiI6 nanocrystals. Reproduced with permission from Ref. [51]
To seek halide double perovskites theoretically, Zhang et al. [65–69] designed a series of all-inorganic halide double perovskites through first-principles calculations for the last 2 years. Photovoltaic-functionality-directed material screening process involves totally sixty-four candidate materials to identify 11 Sb- and Bi-based optimal materials containing intrinsic thermodynamic stability, suitable bandgaps, small carrier effective masses and low excitons binding energies as promising photovoltaic materials. When the monovalent ion is Tl+ or In+, the materials have direct bandgap, among which Cs2InSbCl6 and Cs2InBiCl6 have the bandgap about 1.0 eV, and show the theoretical maximum performance comparable to that of CH3NH3PbI3. However, the Tl is still toxic and the In+ is unstable by spontaneous oxidation into In3+ [70]. They simultaneously designed trivalent In3+ ion with monovalent Ag+ or Cu+ ion to find halide double perovskites [69]. Among them, Rb2CuInCl6, Rb2AgInBr6 and Cs2AgInBr6 have direct bandgaps of 1.36, 1.46 and 1.50 eV, respectively, and theoretical spectroscopic limited maximal efficiency comparable to CH3NH3PbI3.
It is noteworthy that there is another type of halide double perovskite as vacancy-ordered A2M(IV)X6 (M(IV)=Sn, Ti, Pd, Te, etc.), which not only proposes direct bandgaps, but also intrinsic stability and non-/low toxicity [71–73]. In 2014, Kanatzidis et al. [74] prepared Cs2SnI6 microcrystal by a hot-injection method. Cs2SnI6 nanocrystal was prepared with controlled shapes by the same method [75, 76]. The bandgap of the Cs2SnI6 was found to be varied in the range of 1.36–1.67 eV. Quantum confinement effect has been observed for the nanoparticles of dimension below 8 nm. In 2018, Tang et al. [77] fabricated Bi-doped Cs2SnCl6 single crystals, where the photoluminescence was observed from Bi3+ ions. In 2017, Snaith et al. [78] reported Cs2PdBr6 single crystal, which exhibits long-lived photoluminescence, direct bandgap of 1.6 eV and long-term stability. In 2018, Kuang et al. [79] used antisolvent recrystallization method to prepare Cs2PdBr6 nanocrystals with an average particle diameter of 2.8 nm. Such Cs2PdBr6 nanocrystals not only display high stability against light illumination (one sun for more than 1000 h), moisture (70% for 2 months) and high temperature (120 °C for 600 h) conditions but also possess intriguing optical and ultrafast photophysical properties with a narrow direct bandgap of 1.69 eV. Further, a fast anion exchange method is adopted to prepare the Cs2PdI6 nanocrystals. Another Ti-based vacancy-ordered halide double perovskites can start back in the early 1960s, and K2TiBr6, Rb2TiBr6 and Cs2TiBr6 were prepared by using fused SbBr3 as the solvent [80]. However, the studies of them in optoelectronics have not been performed until 2018, and Zhou et al. [81] synthesized a series of Cs2TiIxBr6−x (x = 0, 2, 4, 6) powers using the melt crystallization method, of which the bandgap can be tuned continuously from 1.02 to 1.78 eV. The present vacancy-ordered halide double perovskites possess several desirable properties, including ultra-stability, suitable direct bandgap, excellent optical absorption and benign defect.
Hybrid Halide Double Perovskites
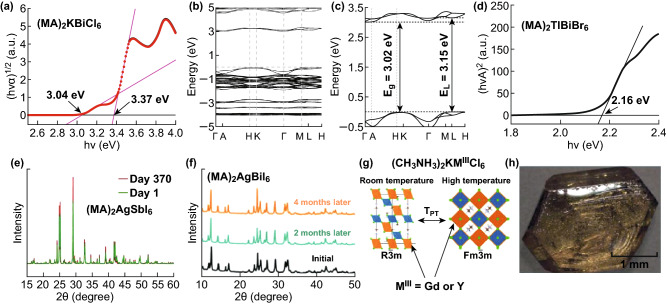
To date, there have been synthesized nine hybrid halide double perovskites to the best of our knowledge, which are (MA)2KBiCl6 [38], (MA)2TlBiBr6 [82], (MA)2AgBiBr6 [83], (MA)2AgSbI6 [84], (MA)2AgBiI6 [85], (MA)2KGdCl6 [86], (MA)2KYCl6 [86], (MA)2AgInBr6 [87], and (MA)2SnI6 [88], as listed in Table 1. In 2016, the first hybrid halide double perovskite (MA)2KBiCl6 was synthesized by evaporating HCl solution to dryness [38]. In the Tauc plot from the reflectance spectrum, there are two edges with values of 3.04 and 3.37 eV in Fig. 3a, in accordance with the theoretically calculated indirect bandgap of 3.02 eV and direct bandgap of 3.15 eV as shown in Fig. 3b, c. Further, density functional theory screening of (MA)2MBiX6 (M=K, Cu, Ag, Tl; X=Cl, Br, I) shows that systems with bandgaps similar to those of the CH3NH3PbX3 lead compounds can be expected for M=Cu, Ag, Tl. Motivated by these findings, (MA)2TlBiBr6, isoelectronic with CH3NH3PbBr3, was synthesized and found to have a direct bandgap of 2.16 eV, as shown in Fig. 3d [82]. However, despite its interesting electronic properties, the severe toxicity of Tl precludes (MA)2TlBiBr6 from being a practical alternative to the Pb analog. Subsequently, they synthesized a hybrid halide double perovskite, (MA)2AgBiBr6, that has a low indirect bandgap of 2.02 eV and is relatively stable and nontoxic [83]. The material is stable in air and moisture and exhibits higher decomposition temperature than that of MAPbBr3. In 2017, the same group synthesized (MA)2AgSbI6 [84] and (MA)2AgBiI6 [85] with indirect bandgap of ~ 2 eV and exhibited high stability in air, as the XRD patterns shown in Fig. 3e, f. Though Sb and Bi are in the same group, there are still some differences between them. Compared with Bi atom, Sb atom has a smaller mass and ion radius and the smaller ion radius of Sb leads to structural distortion of (MA)2AgSbI6, while the structure of (MA)2AgBiI6 is the orthogonal phase. Besides, due to the seriously relativistic effects in heavy metal atom Bi, the impact of spin–orbit coupling on bandgap of (MA)2AgBiI6 is more pronounced. Simultaneously, (MA)2KGdCl6 and (MA)2KYCl6 have been synthesized by a solution evaporation method, which adopt a rhombohedral structure with R͞3 m symmetry [86]. Both phases exhibit a rhombohedral-to-cubic phase transition on heating to ~ 435 K as shown in Fig. 3g. Density functional calculations on the rhombohedral phase indicate that both materials have large direct bandgaps about 5 eV and mechanical stability. In 2018, (MA)2AgInBr6 single crystal [87], as shown in Fig. 3h, is obtained through the use of Pb2+ (from CH3NH3PbBr3) to modulate the soluble intermediates and force the formation. In the same year, (MA)2SnI6 powder was obtained by mixing SnI4 with CH3NH3I powder at room temperature [88]. The powder was evaporated in a tungsten boat at 120 °C in a vacuum chamber to deposit the (MA)2SnI6 films. The films have a direct bandgap of 1.81 eV with a strong absorption coefficient of ~ 7 × 104 cm−1. In addition, the films were n type with a carrier concentration of ~ 2 × 1015 cm−3 and an electron mobility of ~ 3 cm2/V/s. Moreover, the conductivity was increased by a factor of 4 under simulated solar illumination (100 mW cm−2). These results indicate that (MA)2SnI6 is a lead-free optical semiconductor suitable for solar cell applications. The synthesized hybrid halide double perovskites indicate substantially better stability, unlike CH3NH3PbI3. However, to the best of our knowledge, there are no reports about applications of hybrid halide double perovskites.
Fig. 3.
a Tauc plot (assumed indirect bandgap), b-c calculated band structure of (MA)2KBiCl6 and the enlarged view of the band structure near the bandgap. Reproduced with permission from Ref. [38]. d Tauc plot (assumed direct bandgap) of (MA)2TlBiBr6. Reproduced with permission from Ref. [82]. e XRD patterns of fresh (MA)2AgSbI6 compared to (MA)2AgSbI6 after 370 days of exposure to air. Reproduced with permission from Ref. [84]. f Air stability of (MA)2AgBiI6. Reproduced with permission from Ref. [85]. g Schematic illustration of rhombohedral-to-cubic phase transition on high-temperature heating for (MA)2KGdCl6 and (MA)2KYCl6. Reproduced with permission from Ref [86]. h Photograph of a (MA)2AgBiBr6 single crystal. Reproduced with permission from Ref. [87]
Optoelectronic Applications
Photodetectors
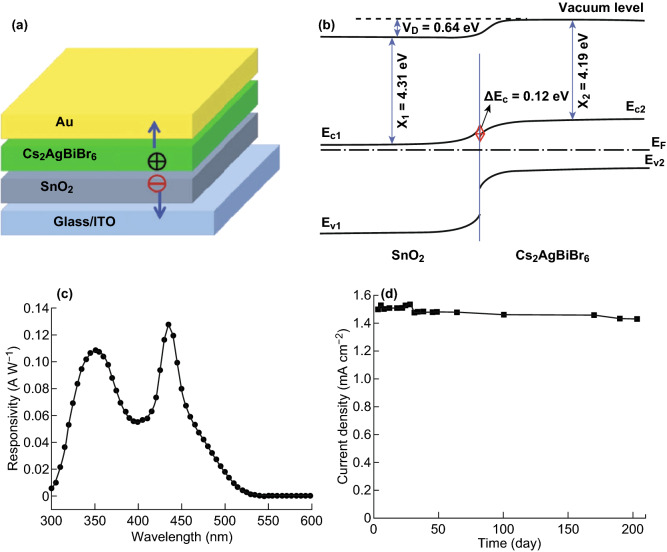
In 2017, Tang et al. [61] successfully prepared high-quality Cs2AgInCl6 single crystals with a low trap state density ((8.6 ± 1.9) × 108 cm−3) by a hydrothermal method, which were further applied in UV detectors. The obtained results experimentally verified the existence of parity-forbidden transition [89] and identified that the oxygen was effective on the optical properties. By eliminating oxygen contamination in vacuum, Cs2AgInCl6 single crystal-based UV detector showed best performance with visible blind, high ON–OFF ratio (~ 500), fast photoresponse (~ 1 ms), low dark current (~ 10 pA at 5 V bias) and high detectivity (~ 1012 Jones). If the Cl is replaced by Br in Cs2AgInX6 compounds, the bandgaps reduce and absorption range can be extended to visible region. Shi et al. [90] used a one-step spin-coating method to prepare Cs2AgBiBr6 thin films for photodetectors. The device exhibits high responsivity of 7.01 A W−1, ON–OFF ratio of 2.16 × 104, specific detectivity of 5.66 × 1011 Jones, EQE of 2146% and demonstrates remarkable stability against the water and oxygen degradation. Wang et al. [91] fabricated highly efficient and stable self-powered UV and deep-blue detector based on Cs2AgBiBr6/SnO2 heterojunction, as shown in Fig. 4a. The photogenerated carriers in Cs2AgBiBr6 film can be separated at the Cs2AgBiBr6/SnO2 heterojunction interface by its built-in field, as illustrated in Fig. 4b. The device is self-powered with two responsivity peaks at 350 and 435 nm, which is suitable for UV (320–400 nm) and deep-blue light detection, as shown in Fig. 4c. A high responsivity of 0.11 A W−1 at 350 nm and a quick response time of less than 3 ms are obtained, which are significantly higher than those of other semiconductor oxide heterojunction-based UV detectors. More importantly, the photocurrent shows no noticeable degradation after more than 6 months of storage in ambient conditions without encapsulation, as shown in Fig. 4d. In addition, the aforementioned vacancy-ordered double perovskites of Cs2PdBr6 single crystal and Cs2SnI6 nanocrystal were successfully applied in stable and fast photodetectors [75, 78]. Consequently, the halide double perovskite-based photodetectors displayed high efficiency and stability and environmental friendliness, which are potential alternatives for practical applications.
Fig. 4.
a Configuration diagram of Cs2AgBiBr6-based photodetector. b Band scheme diagram of Cs2AgBiBr6/SnO2 heterojunction. c Responsivity of photodetector at zero bias. d Long-term stability test of Cs2AgBiBr6-based photodetector. Reproduced with permission from Ref. [91]
X-ray Detectors
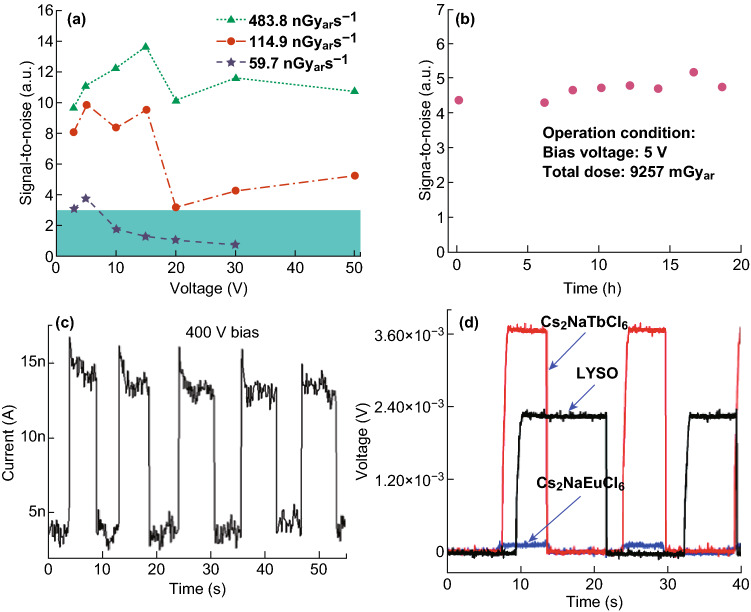
In 2017, Tang et al. [54] also prepared Cs2AgBiBr6 single crystals by controlling cooling rate in a solution for sensitive X-ray detectors with a low detection limit. The Cs2AgBiBr6 single crystals can directly convert X-rays into electrical signals due to the high average atomic number, high carrier drift length per unit electric field, low ionization energy and high resistivity. The optimized device exhibited high sensitivity of 105 µC/Gyair/cm2, low detection limit of 59.7 nGyair s−1 under an external bias of 5 V and demonstrated long-term operational stability, as shown in Fig. 5a, b, all of which are crucial for potential applications in X-ray security screening systems and medical diagnostics. Subsequently, Yu et al. [92] used composite films of Cs2AgBiBr6 embedded in a polymer matrix for X-ray detectors. The polymer with hydroxyl functional groups can greatly improve the uniformity of the composite films, and large area dense films can be obtained by a simple drop-casting process. X-ray detectors based on the composite films exhibit a sensitivity of 40 µC/Gyair/cm2 under an external bias of 400 V, as shown in Fig. 5c, and tolerate a 5% tensile/compressive strain in the composite films without performance degradation. Pixelated X-ray detectors fabricated on the same composite film can realize X-ray imaging and resolve a proof-of-concept geometric pattern. Recently, Tang et al. [93] have used lanthanide series as trivalent metals to obtain highly stable halide double perovskites (Cs2NaLnCl6, Ln=Tb or Eu) with high scintillation light yield. The crystals exhibit typical f–f transitions of lanthanide cations, while Cs2NaTbCl6 exhibits strong green photoluminescence and Cs2NaEuCl6 exhibits red photoluminescence. Under X-ray radiations, the light yield of Cs2NaTbCl6 reaches 46,600 photons MeV−1, much higher than that of the commercially used (Lu,Y)2SiO5:Ce3+ crystals (LYSO, 28,500 photons MeV−1), as shown in Fig. 5d. Lanthanide-based halide double perovskites open up a new route toward radiation detections and potential medical imaging.
Fig. 5.
a Signal-to-noise ratio of the device derived by calculating the standard deviation of the X-ray photocurrent. The red dashed line represents a SNR of 3, and thus the detection limit is 59.7 nGyair s−1 at 5 V bias, as indicated by the purple star surrounded by the red dashed circle. b Operational stability of our Cs2AgBiBr6 single-crystal X-ray detector. Testing conditions: continued 138.7 μGyair s−1 X-ray irradiation with constant 5 V bias, tested in ambient air without any encapsulation. Reproduced with permission from Ref. [54]. c Transient responses of the detector at a constant 400 V. Reproduced with permission [92]. d The generated voltage of multiplier tubes by scintillation light of Cs2NaTbCl6 (red line), Cs2NaEuCl6 (blue line) and (Lu,Y)2SiO5:Ce3+ (LYSO, black line), respectively (color online). Reproduced with permission from Ref. [93]. (Color figure online)
Photocatalyst
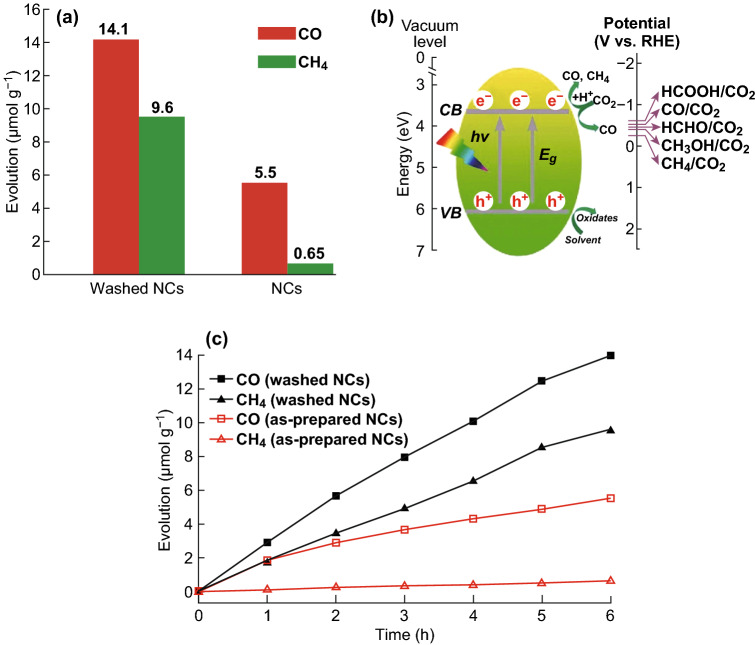
In 2018, Kuang et al. [56] fabricated Cs2AgBiBr6 nanocrystals (NCs) via a simple hot-injection method for photocatalytic CO2 reduction. The Cs2AgBiBr6 NCs can maintain their structure stability in low-polarity solutions (up to 3 weeks) and phase uniformity against 55% relative humidity for 90 days, light-soaking stability toward 70 mW cm−2 for 500 h or 100 °C heating for 300 h, thus declaring the impressive stability in moisture, light and temperature. Photocatalytic CO2 was conducted in ethyl acetate in a Pyrex glass bottle under simulated solar light (AM 1.5G, 150 mW cm−2) illumination. After the constant irradiation for 6 h, the pristine Cs2AgBiBr6 NCs could afford the evolution of CO and CH4 with 5.5 and 0.65 µmol g−1, respectively. Meanwhile, the electron consumption attained 16.2 µmol g−1, as shown in Fig. 6a. On the contrary, the washed Cs2AgBiBr6 NCs have boosted the evolution of R(CO) and R(CH4) to 14.1 and 9.6 µmol g−1, presenting a 6.5-fold enhancement in the electron consumption. A tentative mechanism for the photocatalytic CO2 reduction over the Cs2AgBiBr6 NCs is proposed in Fig. 6b, in which the Cs2AgBiBr6 NCs have a suitable conduction band to drive CO2 reduction. The time-dependent evolution of CO and CH4 using the as-prepared and -washed NCs by absolute ethanol as photocatalysts under light irradiation is shown in Fig. 6c, where the evolution of CO and CH4 rose nearly linearly with irradiation time and the washed NCs exhibited higher efficiency for CO2 conversion. Therefore, the novel Cs2AgBiBr6 NCs were successfully used to conduct the CO2 reduction reactions with high selectivity and stability, which hold great potential in the further photochemical applications.
Fig. 6.
a Comparison of photocatalytic CO2 reduction performance of the as-prepared Cs2AgBiBr6 NCs and washed NCs. b Schematic diagram of the photoreduction of CO2 on the surface of Cs2AgBiBr6 NCs. c Time course of CO and CH4 evolutions over as-prepared and -washed Cs2AgBiBr6 NCs. Reproduced with permission from Ref. [56]
LEDs
Tang et al. [77] used the synthesized Bi-doped Cs2SnCl6 as blue emissive phosphors, where Bi3+ is the luminescent dopant. Hybrid density functional theory calculations suggest the preferred formation of [BiSn + VCl] defect complex, responsible for the optical absorption and the associated blue emission. The Bi-doped Cs2SnCl6 also shows impressive thermal and water stability due to its inorganic nature and the formation of protective BiOCl layer. A significant boost of the photoluminescence (emission peak: 455 nm, PLQY: 78.9%) was observed upon doping. This is the highest PLQY ever reported for all-inorganic lead-free perovskites and even comparable to the highest value of lead perovskites with blue emissions. Bi-doped Cs2SnCl6 showed great potential as blue phosphors, and its LED exhibited a warm white high light emission with a correlated color temperature of 4486 K and a Commission Internationale de I’Eclairage coordinate of (0.36; 0.37) when integrated with yellow phosphor. Recently, Tang et al. [94] broke the parity-forbidden transition of Cs2AgInCl6 by alloying Na+ cations, which leads to efficient white emission via radiative recombination of self-trapped excitons. The Bi3+ incorporation is believed to improve crystal perfection and promote exciton localization [95]. The optimally alloyed Cs2(Ag0.60Na0.40)InCl6 with 0.04% Bi3+ doping emits warm white light with (86 ± 5) % quantum efficiency and works for over 1000 h. Therefore, halide double perovskites hold great potential for display and lighting applications and merit further study to realize their full potential.
Solar Cells
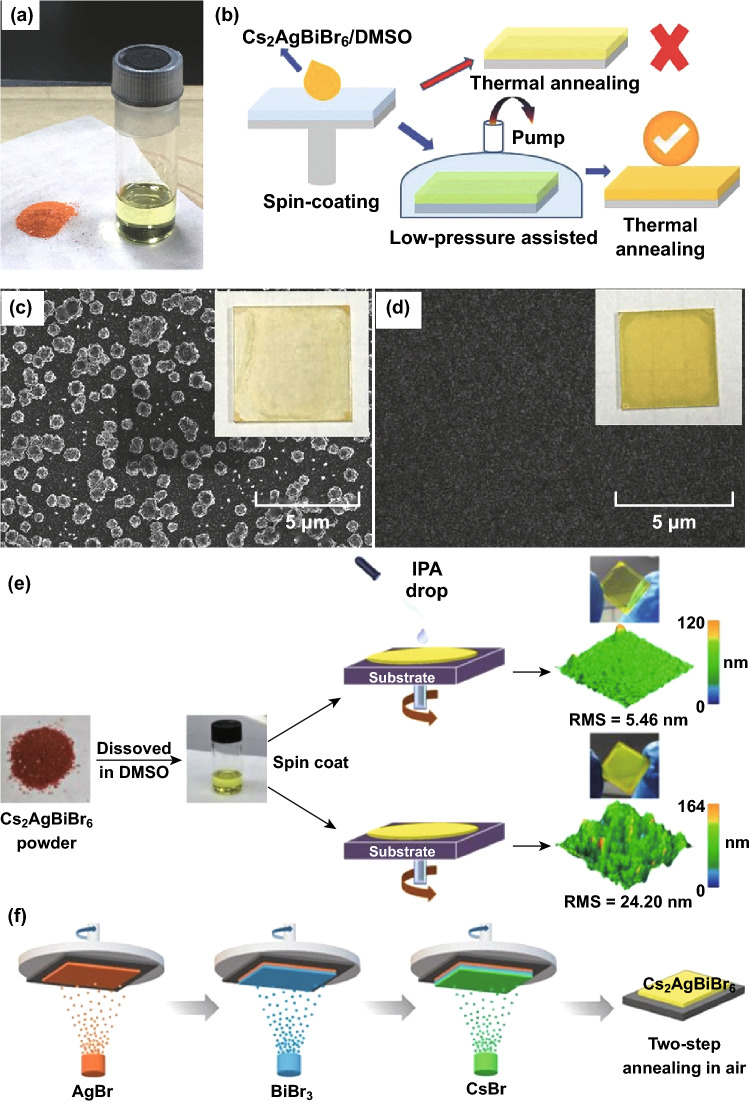
To date, Cs2AgBiBr6 is most applied in halide double perovskite-based solar cells [96–100]. In 2017, Bein et al. [96] fabricated Cs2AgBiBr6 films by a spin-coating method and incorporated them into solar cells for the first time. After optimized synthesis conditions, the Cs2AgBiBr6-based solar cells revealed power conversion efficiency (PCE) of 2.43% and excellent stability upon exposure without encapsulation. Inadequately, there is a thick agglomerated morphology of the Cs2AgBiBr6 films with micrometer-sized grains on the surface, and the hysteresis of the devices is serious. In 2018, Wang et al. [97] developed planar heterojunction solar cells with high-quality Cs2AgBiBr6 film by low-pressure-assisted solution processing under ambient conditions, as shown in Fig. 7a, b, borrowing Cs2AgBiBr6/SnO2 heterojunction from their reported detectors [91]. Orange Cs2AgBiBr6 powder was dissolved in DMSO solution to form a light yellow transparent solution and then fabricate the film using spin-coating technique, as presented in Fig. 7a. The spin-coated film was quickly moved to a low-pressure chamber pumped to 20 Pa, in which the transparent film would gradually turn to light yellow, as shown in Fig. 7b. Next, the as-prepared film was annealed and attained smooth morphology as shown in Fig. 7d. The optimized Cs2AgBiBr6 film achieved 1.44% PCE of the solar cells with P3HT hole conductor layer. On the contrary, the traditional thermal annealing method generated rough Cs2AgBiBr6 film in Fig. 7c, and the corresponding devices usually showed a poor PCE (< 0.1%). Furthermore, these devices also showed hysteresis phenomenon. Wu et al. [98] used anti-solvent dropping technology and post-annealing process to realize high-quality Cs2AgBiBr6 film with ultra-smooth morphology, microsized grains and high crystallinity (shown in Fig. 7e), which was applied in inverted planar heterojunction solar cells. The device shows PCE up to 2.23% with free hysteresis. Subsequently, Grancini et al. [99] realized hysteresis-free mesoporous double-perovskite solar cells by fine-tuning the material deposition parameters, enabling the growth of a highly uniform and compact Cs2AgBiBr6 film, and by engineering the device interfaces by screening different molecular and polymeric hole-transporting materials. Chlorobenzene was the anti-solution in the film formation before the annealing step. Recently, Liu et al. [100] utilized a sequential vapor deposition method to fabricate Cs2AgBiBr6 films for solar cells, as shown in Fig. 7f. The two-step annealing process produces films with better quality in terms of crystallization and film uniformity. The solar cells with planar device structure show an optimized PCE of 1.37%, which can be maintained at 90% after 240 h of storage under ambient condition. In addition, Ma et al. [64] incorporated their synthesized Cs2NaBiI6 into solar cells, which shows great stability and reproducibility.
Fig. 7.
a Image of Cs2AgBiBr6 powder (left) and solution in DMSO (right). b The film fabrication process diagram. c, d SEM images of film obtained by traditional thermally annealed and low-pressure-assisted process, respectively, inset: film photograph, size, 25 × 25 mm2. Reproduced with permission from Ref. [97]. e Schematic illustration of the spin-coating process with and without anti-solvent dropping protocol; the morphology of the as-prepared film can be improved after IPA dropping. Reproduced with permission from Ref. [98]. f Scheme of sequential vapor deposition processing of Cs2AgBiBr6 double perovskite. Reproduced with permission from Ref. [100]
It is greatly significative that the vacancy-ordered A2M(IV)X6 halide double perovskites have direct suitable bandgaps, which have been successfully applied in solar cells [74, 101–104]. In 2014, Kanatzidis et al. [74] first time introduced Cs2SnI6 as a hole-transporting material in dye-sensitized solar cells. In 2016, Cao et al. [101] discovered that B-γ-CsSnI3 film can spontaneously convert to an air-stable Cs2SnI6 film in air and at room temperature, which can be adopted as lead-free solar cell light absorber owing to its direct bandgap of 1.48 eV and high absorption coefficient (over 105 cm−1 from 1.7 eV). A planar PSC using the Cs2SnI6 film as the light absorber achieved PCE nearly 1% in air. Later on, Cao et al. [102] synthesized Cs2SnI6 powder through a modified solution process and demonstrated its application as absorbing layer in mesoporous solar cell with a configuration of FTO/ZnO compact layer/nanorods/Cs2SnI6/P3HT/Ag. With careful control of ZnO nanorod length and pore size to ensure high loading of the Cs2SnI6 absorber, the PCE achieved was nearly 1%. The bandgap tuning achieved by substitution of Br is anticipated to enhance the open-circuit voltage of Cs2SnI6-based solar cell. The compounds are Cs2SnI6−xBrx for a range of x that provide the desired bandgaps from 1.3 to 2.9 eV with x < 3 being suitable for solar cell design. The cells show a PCE of 2.1% for the case of the x = 2 compound [103]. Recently, Cs2TiBr6 thin films were prepared through a facile low-temperature vapor-based method and incorporated into planar heterojunction PSCs [104]. The Cs2TiBr6 thin films exhibit favorable bandgap of 1.8 eV, long and balanced carrier diffusion lengths, suitable energy levels and superior intrinsic and environmental stability, which result in a stable solar cell with efficiency up to 3.3%.
Conclusion and Perspective
Herein, we intend to summarize the most recent developments regarding halide double perovskite materials and the related applications. Due to distinguished stability and tunable properties, halide double perovskites have enormous potential leading to high-performance, stable and environmentally friendly optoelectronic devices for practical applications. Although significant progress has been achieved in halide double perovskites, there are still many challenges to be addressed.
Multitude of halide double perovskite materials to be existed is theoretically predicted, but a very limited number have been explored experimentally. In addition, synthesized strategies to those materials remain limited, which are mainly divided into solid-state and wet-chemical routes. The solid-state route is processed to heat anhydrous haloids as melt crystallization. Wet-chemical route includes hot injection, antisolvent recrystallization, hydrothermal methods, etc. The single crystals are classically obtained by the hydrothermal method through controlling the cooling rate from hot solution. Because a few iodine-based double perovskite materials are not easily obtained by direct combination of reactants, anion exchange and induced crystallization have been taken [51, 87]. Accordingly, we propose two aspects to make efforts: borrowing the present routes to explore the theoretical predictions and discovering viable routes to more halide double perovskite materials.
The present halide double perovskite materials exhibit excellent stability in moisture, heat and light, unlike the fashionable Pb-based perovskite materials. For instance, (MA)2AgSbI6 powder is relatively unaltered after being exposed to air for 370 days and the thermal stability can be up to 260 °C [84]. As a general rule, all-inorganic halide perovskites possess better stability [105–107]. Cs2AgBiBr6 single crystal is stable up to 430 °C, and differential thermal analysis indicates no phase transitions within this temperature range [42]. Cs2NaBiI6 exhibits superior stability against the moisture and the oxygen in the ambient air, which can be washed with water [53]. However, there is lack of proven stability under complicated environmental conditions or with long term over 10 years. In addition, the underlying cause of stability under different conditions remains covered. Hence, more attempts need to place emphasis on enhancing long-term and complicated environmental stability, as well as discovering the stability mechanisms.
Hitherto, extremely few all-inorganic and no hybrid halide double perovskite materials have been developed for optoelectronic applications. More halide double perovskite materials are desirable to be developed and widely applied. Moreover, the optoelectronic devices based on single-crystal halide double perovskite materials enjoy pleasurable performance, comparable to that of Pb-based perovskite analogs, such as Cs2AgBiBr6 single-crystal X-ray detectors [54]. However, the device performance of halide double perovskite films is still lower than that of the Pb-based perovskite analogs. The underlying causes can be ascribed to the underdeveloped electronic structures, material properties, film qualities of halide double perovskite materials and device architectures. It is difficult to develop a synthetic route to obtain uniform thin films of the correct phase and composition. Typically, Cs2AgBiBr6 [96], Cs2SnI6-xBrx [103] and Cs2TiBr6 [104] absorbers in PSCs have achieved optimized efficiency of 2.4, 2.1 and 3.3%, respectively, far below that of the Pb-based perovskite analogs. To enhance Cs2AgBiBr6-film quality, anti-solvent dropping and low-pressure-assisted solution methods have been adopted [97, 98]. Interface engineering of device architectures was taken to achieve hysteresis-free Cs2AgBiBr6-based PSCs [99]. However, the performance is still unsatisfactory because Cs2AgBiBr6 has a relatively large indirect bandgap. Various strategies can be attempted to engineer the bandgap and modify the device structures to improve the device performance, such as chemical doping and alloying approaches. Meanwhile, chemical doping and alloying approaches are expected to bring original effects for applications. As an example, Bi-doped Cs2SnCl6 can be applied as blue emissive phosphors, where Bi3+ is the luminescent dopant [77].
In summary, we are very much optimistic that the current astonishing achievements will encourage more researchers to overcome the above challenges in the future.
Acknowledgements
This work was financially supported by the Ministry of Education of China (IRT1148), the National Natural Science Foundation of China (U1732126, 11804166, 51602161, 51372119), the National Synergetic Innovation Center for Advanced Materials (SICAM), the China Postdoctoral Science Foundation (2018M630587), the Priority Academic Program Development of Jiangsu Higher Education Institutions (YX03001) and the Natural Science Foundation of NJUPT (NY217091).
Contributor Information
Jianping Yang, Email: yangjp@njupt.edu.cn.
Xing’ao Li, Email: iamxali@njupt.edu.cn.
References
- 1.Kojima A, Teshima K, Shirai Y, Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009;131(17):6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- 2.Kim HS, Lee CR, Im JH, Lee KB, Moehl T, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9% Sci. Rep. 2012;2:6022–6025. doi: 10.1038/srep00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Chen Q, Li G, Luo S, Song TB, et al. Interface engineering of highly efficient perovskite solar cells. Science. 2014;345(6196):542–546. doi: 10.1126/science.1254050. [DOI] [PubMed] [Google Scholar]
- 4.Jeon NJ, Noh JH, Yang WS, Kim YC, Ryu S, Seo J, Seok SI. Compositional engineering of perovskite materials for high-performance solar cells. Nature. 2015;517:476–480. doi: 10.1038/nature14133. [DOI] [PubMed] [Google Scholar]
- 5.Luo D, Yang W, Wang Z, Sadhanala A, Hu Q, et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science. 2018;360(6396):1442. doi: 10.1126/science.aap9282. [DOI] [PubMed] [Google Scholar]
- 6.National Renewable Energy Laboratory (NREL) (2018). https://www.nrel.gov/pv/assets/pdfs/pv-efficiencies-chart.20181214.pdf
- 7.Lee MM, Teuscher J, Miyasaka T, Murakumi TN, Snaith HJ. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science. 2012;338(6107):643–647. doi: 10.1126/science.1228604. [DOI] [PubMed] [Google Scholar]
- 8.Stranks SD, Eperon GE, Grancini G, Menelaou C, Alcocer MJP, Leijtens T, Hertz LM, Petrozza A, Snaith HJ. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science. 2013;342(6156):341–344. doi: 10.1126/science.1243982. [DOI] [PubMed] [Google Scholar]
- 9.Xing GC, Mathews N, Sun SY, Lim SS, Lam YM, Gratzel M, Mhaisalkar S, Sum TC. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science. 2013;342(6156):344–347. doi: 10.1126/science.1243167. [DOI] [PubMed] [Google Scholar]
- 10.Yang WS, Park BW, Jung EH, Jeon NJ, Kim YC, et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science. 2017;356(6345):1376–1379. doi: 10.1126/science.aan2301. [DOI] [PubMed] [Google Scholar]
- 11.Stranks SD, Snaith HJ. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotech. 2015;10(5):391–402. doi: 10.1038/nnano.2015.90. [DOI] [PubMed] [Google Scholar]
- 12.Cho H, Jeong SH, Park MH, Kim YH, Wolf C, Lee CL, et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science. 2015;350(6265):1222–1225. doi: 10.1126/science.aad1818. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Fu Y, Meng F, Wu X, Gong Z, et al. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015;14(6):636–642. doi: 10.1038/nmat4271. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Yang B, Zheng K, Yang S, Li Y, Deng W, He R. Formamidinium lead bromide (FAPbBr3) perovskite microcrystals for sensitive and fast photodetectors. Nano-Micro Lett. 2018;10(3):43. doi: 10.1007/s40820-018-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu L, Hu R, Liu W, Ma Y, Zhang R, Yang J, Li X. Screen printing large-area organometal halide perovskite thin films for efficient photodetectors. Mater. Res. Bull. 2018;98:322. doi: 10.1016/j.materresbull.2017.10.039. [DOI] [Google Scholar]
- 16.Chen Q, Wu J, Ou X, Huang B, Almutlaq J, et al. All-inorganic perovskite nanocrystal scintillators. Nature. 2018;561(7721):88. doi: 10.1038/s41586-018-0451-1. [DOI] [PubMed] [Google Scholar]
- 17.Luo H, Lin X, Hou X, Pan L, Huang S, Chen X. Efficient and air-stable planar perovskite solar cells formed on graphene-oxide-modified PEDOT:PSS hole transport layer. Nano-Micro Lett. 2017;9(4):39. doi: 10.1007/s4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, You J. Make perovskite solar cells stable. Nature. 2017;544(7649):155. doi: 10.1038/544155a. [DOI] [PubMed] [Google Scholar]
- 19.Saliba M, Matsui T, Domanski K, Seo JY, Ummadisingu A, et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science. 2016;354(6309):5557. doi: 10.1126/science.aah5557. [DOI] [PubMed] [Google Scholar]
- 20.Ball JM, Petrozza A. Defects in perovskite-halides and their effects in solar cells. Nat. Energy. 2016;1(11):16149. doi: 10.1038/nenergy.2016.149. [DOI] [Google Scholar]
- 21.Cheng J, Zhang H, Zhang S, Ouyang D, Huang Z, Nazeeruddin MK, Hou J, Choy WC. Highly efficient planar perovskite solar cells achieved by simultaneous defect engineering and formation kinetic control. J. Mater. Chem. A. 2018;6(46):23865. doi: 10.1039/C8TA08819E. [DOI] [Google Scholar]
- 22.Hebig JC, Kühn I, Flohre J, Kirchartz T. Optoelectronic properties of (CH3NH3)3Sb2I9 thin films for photovoltaic applications. ACS Energy Lett. 2016;1(1):309–314. doi: 10.1021/acsenergylett.6b00170. [DOI] [Google Scholar]
- 23.Singh T, Kulkarni A, Ikegami M, Miyasaka T. Effect of electron transporting layer on bismuth-based lead-free perovskite (CH3NH3)3Bi2I9 for photovoltaic applications. ACS Appl. Mater. Interfaces. 2016;8(23):14542–14547. doi: 10.1021/acsami.6b02843. [DOI] [PubMed] [Google Scholar]
- 24.You J, Meng L, Song TB, Guo TF, Yang YM, et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotech. 2016;11(1):75–81. doi: 10.1038/nnano.2015.230. [DOI] [PubMed] [Google Scholar]
- 25.Liao W, Zhao D, Yu Y, Grice CR, Wang C, et al. Lead-free inverted planar formamidinium tin triiodide perovskite solar cells achieving power conversion efficiencies up to 6.22% Adv. Mater. 2016;28(42):9333–9340. doi: 10.1002/adma.201602992. [DOI] [PubMed] [Google Scholar]
- 26.Tsai H, Nie W, Blancon JC, Stoumpos CC, Asadpour R, et al. High-efficiency two-dimensional ruddlesden-popper perovskite solar cells. Nature. 2016;536(7616):312–316. doi: 10.1038/nature18306. [DOI] [PubMed] [Google Scholar]
- 27.Matteocci F, Cinà L, Lamanna E, Cacovich S, Divitini G, Midgley PA, Ducati C, Carlo AD. Encapsulation for long-term stability enhancement of perovskite solar cells. Nano Energy. 2016;30:162–172. doi: 10.1016/j.nanoen.2016.09.041. [DOI] [Google Scholar]
- 28.Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip. Toxicol. 2012;5(2):47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang L, Gao P. Lead-free hybrid perovskite absorbers for viable application: can we eat the cake and have it too. Adv. Sci. 2018;5(2):1700331. doi: 10.1002/advs.201700331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao F, Stoumpos CC, Chang RPH, Kanatzidis MG. Anomalous band gap behavior in mixed Sn and Pb perovskites enables broadening of absorption spectrum in solar cells. J. Am. Chem. Soc. 2014;136(22):8094–8099. doi: 10.1021/ja5033259. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamoorthy T, Ding H, Yan C, Leong WL, Baikie T, et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A. 2015;3(47):23829–23832. doi: 10.1039/C5TA05741H. [DOI] [Google Scholar]
- 32.Wang H, Zhang H, Chueh CC, Zhao T, Mao C, Chen W, Jenac AKY. Enhanced crystallization and performance of formamidinium lead triiodide perovskite solar cells through PbI2-SrCl2 modulation. Mater. Today Energy. 2018;7:239. doi: 10.1016/j.mtener.2017.10.002. [DOI] [Google Scholar]
- 33.Klug MT, Osherov A, Haghighirad AA, Stranks SD, Brown PR, et al. Tailoring metal halide perovskites through metal substitution: influence on photovoltaic and material properties. Energy Environ. Sci. 2017;10(1):236. doi: 10.1039/C6EE03201J. [DOI] [Google Scholar]
- 34.Jin J, Li H, Chen C, Zhang B, Xu L, Dong B, Song H, Dai Q. Enhanced performance of perovskite solar cells with zinc chloride additives. ACS Appl. Mater. Interfaces. 2017;9(49):42875–42882. doi: 10.1021/acsami.7b15310. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q, Chen L, Ye FY, Zhao T, Tang F, et al. Ag-incorporated organic-inorganic perovskite films and planar heterojunction solar cells. Nano Lett. 2017;17(5):3231–3237. doi: 10.1021/acs.nanolett.7b00847. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Shang M, Wang P, Huang X, Xu J, Hu Z, Zhu Y, Han L. n-Type doping and energy states tuning in CH3NH3Pb1–xSb2x/3I3 perovskite solar cells. ACS Energy Lett. 2016;1(3):535–541. doi: 10.1021/acsenergylett.6b00241. [DOI] [Google Scholar]
- 37.Hu Y, Qiu T, Bai F, Miao X, Zhang S. Enhancing moisture-tolerance and photovoltaic performances of FAPbI3 by bismuth incorporation. J. Mater. Chem. A. 2017;5(48):25258–25265. doi: 10.1039/C7TA08824H. [DOI] [Google Scholar]
- 38.Wei F, Deng Z, Sun S, Xie F, Kieslich G, Evans DM, Carpenter MA, Bristowe PD, Cheetham AK. The synthesis, structure and electronic properties of a lead-free hybrid inorganic-organic double perovskite (MA)2KBiCl6 (MA = methylammonium) Mater. Horiz. 2016;3(4):328–332. doi: 10.1039/C6MH00053C. [DOI] [Google Scholar]
- 39.Matthews PD, Lewis DJ, O’Brien P. Updating the road map to metal-halide perovskites for photovoltaics. J. Mater. Chem. A. 2017;5(33):17135–17150. doi: 10.1039/C7TA04544A. [DOI] [Google Scholar]
- 40.Giustino F, Snaith HJ. Toward lead-free perovskite solar cells. ACS Energy Lett. 2016;1(6):1233–1240. doi: 10.1021/acsenergylett.6b00499. [DOI] [Google Scholar]
- 41.Bagnall KW, Laidler JB, Stewart MAA. Americium chloro-complexes. J. Chem. Soc. A. 1968;0:133–136. doi: 10.1039/J19680000133. [DOI] [Google Scholar]
- 42.Morss LR, Fuger J. Preparation and crystal structures of dicesium berkelium hexachloride and dicesium sodium berkelium hexachloride. Inorg. Chem. 1969;8(7):1433–1439. doi: 10.1021/ic50077a013. [DOI] [Google Scholar]
- 43.Morss LR, Siegal M, Stenger L, Edelstein N. Preparation of cubic chloro complex compounds of trivalent metals: Cs2NaMCl6. Inorg. Chem. 1970;9(7):1771–1775. doi: 10.1021/ic50089a034. [DOI] [Google Scholar]
- 44.Morrs LR, Robinson WR. Crystal structure of Cs2NaBiCl6. Acta Crystallogr. B. 1972;28(2):653–654. doi: 10.1107/S0567740872002948. [DOI] [Google Scholar]
- 45.Prokert F, Aleksandrov KS. Neutron scattering studies on phase transition and phonon dispersion in Cs2NaBiCl6. Phys. Status Solidi B. 1984;124(2):503. doi: 10.1002/pssb.2221240208. [DOI] [Google Scholar]
- 46.Smit WMA, Dirksen GJ, Stufkens DJ. Infrared and Raman spectra of the elpasolites Cs2NaSbCl6 and Cs2NaBiCl6: evidence for a pseudo Jahn-Teller distorted ground state. J. Phys. Chem. Solids. 1990;51(2):189–196. doi: 10.1016/0022-3697(90)90092-T. [DOI] [Google Scholar]
- 47.Flerov IN, Gorev MV, Aleksandrov KS, Tressaud A, Grannec J, Couzi M. Phase transitions in elpasolites (ordered perovskites) Mater. Sci. Eng. 1998;24(3):81–151. doi: 10.1016/S0927-796X(98)00015-1. [DOI] [Google Scholar]
- 48.McClure ET, Ball MR, Windl W, Woodward PM. Cs2AgBiX6 (X = Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 2016;28(5):1348–1354. doi: 10.1021/acs.chemmater.5b04231. [DOI] [Google Scholar]
- 49.Volonakis G, Filip MR, Haghighirad AA, Sakai N, Wenger B, Snaith HJ, Giustino F. Lead-free halide double perovskites via heterovalent substitution of noble metals. J. Phys. Chem. Lett. 2016;7(7):1254–1259. doi: 10.1021/acs.jpclett.6b00376. [DOI] [PubMed] [Google Scholar]
- 50.Bekenstein Y, Dahl JC, Huang J, Osowiecki WT, Swabeck JK, Chan EM, Yang P, Alivisatos AP. The making and breaking of lead-free double perovskite nanocrystals of cesium silver-bismuth halide compositions. Nano Lett. 2018;18(6):3502. doi: 10.1021/acs.nanolett.8b00560. [DOI] [PubMed] [Google Scholar]
- 51.Creutz SE, Crites EN, De Siena MC, Gamelin DR. Colloidal nanocrystals of lead-free double-perovskite (elpasolite) semiconductors: synthesis and anion exchange to access new materials. Nano Lett. 2018;18(2):1118. doi: 10.1021/acs.nanolett.7b04659. [DOI] [PubMed] [Google Scholar]
- 52.Slavney AH, Hu T, Lindenberg AM, Karunadasa HI. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic application. J. Am. Chem. Soc. 2016;138(7):2138–2141. doi: 10.1021/jacs.5b13294. [DOI] [PubMed] [Google Scholar]
- 53.Bi Y, Hutter EM, Fang Y, Dong Q, Huang J, Savenije TJ. Charge carrier lifetimes exceeding 15 μs in methylammonium lead iodide single crystals. J. Phys. Chem. Lett. 2016;7(5):923–928. doi: 10.1021/acs.jpclett.6b00269. [DOI] [PubMed] [Google Scholar]
- 54.Pan W, Wu H, Luo J, Deng Z, Ge C, et al. Cs2AgBiBr 6 single-crystal X-ray detectors with a low detection limit. Nat. Photonics. 2017;11(11):726–732. doi: 10.1038/s41566-017-0012-4. [DOI] [Google Scholar]
- 55.Hoye RLZ, Eyre L, Wei F, Brivio F, Sadhanala A, et al. Fundamental carrier lifetime exceeding 1 µs in Cs2AgBiBr 6 double perovskite. Adv. Mater. Interfaces. 2018;5(15):1800464. doi: 10.1002/admi.201800464. [DOI] [Google Scholar]
- 56.Zhou L, Xu YF, Chen BX, Kuang DB, Su CY. Synthesis and photocatalytic application of stable lead-free Cs2AgBiBr 6 perovskite nanocrystals. Small. 2018;14(11):1703762. doi: 10.1002/smll.201703762. [DOI] [PubMed] [Google Scholar]
- 57.Slavney AH, Leppert L, Bartesaghi D, Gold-Parker A, Toney MF, Savenije TJ, Neaton JB, Karunadasa HI. Defect-induced band-edge reconstruction of a bismuth-halide double perovskite for visible-light absorption. J. Am. Chem. Soc. 2017;139(14):5015–5018. doi: 10.1021/jacs.7b01629. [DOI] [PubMed] [Google Scholar]
- 58.Du KZ, Meng W, Wang X, Yan Y, Mitzi DB. Bandgap engineering of lead-free double perovskite Cs2AgBiBr 6 through trivalent metal alloying. Angew. Chem. Int. Ed. 2017;56(28):8158–8274. doi: 10.1002/anie.201703970. [DOI] [PubMed] [Google Scholar]
- 59.T.T. Tran, J.R. Panella, J.R. Chamorro, J.R. Morey, T.M. McQueen Designing indirect-direct bandgap transitions in double perovskites. Mater. Horiz. 2017;4(4):688–693. doi: 10.1039/C7MH00239D. [DOI] [Google Scholar]
- 60.Volonakis G, Haghighirad AA, Milot RL, Sio WH, Filip MR, et al. Cs2InAgCl6: a new lead-free halide double perovskite with direct band gap. J. Phys. Chem. Lett. 2017;8(4):772–778. doi: 10.1021/acs.jpclett.6b02682. [DOI] [PubMed] [Google Scholar]
- 61.Luo J, Li S, Wu H, Zhou Y, Li Y, et al. Cs2AgInCl6 double perovskite single crystals: parity forbidden transitions and their application for sensitive and fast UV photodetectors. ACS Photonics. 2017;5(2):398–405. doi: 10.1021/acsphotonics.7b00837. [DOI] [Google Scholar]
- 62.Nandha N, Nag A. Synthesis and luminescence of Mn-doped Cs2AgInCl6 double perovskites. Chem. Comm. 2018;54(41):5205–5208. doi: 10.1039/C8CC01982G. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka K, Takahashi T, Ban T, Kondo T, Uchida K, Miura N. Comparative study on the excitons in lead-halide-based perovskite-type crystals CH3NH3PbBr3 CH3NH3PbI3. Solid State Commun. 2003;127(9–10):619–623. doi: 10.1016/S0038-1098(03)00566-0. [DOI] [Google Scholar]
- 64.Zhang C, Gao L, Teo S, Guo Z, Xu Z, Zhao S, Ma T. Design of a novel and highly stable lead-free Cs2NaBiI6 double perovskite for photovoltaic application. Sustainable Energy Fuels. 2018;2(11):2419. doi: 10.1039/C8SE00154E. [DOI] [Google Scholar]
- 65.Zhao XG, Yang JH, Fu Y, Yang D, Xu Q, Yu L, Wei SH, Zhang L. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 2017;139(7):2630–2638. doi: 10.1021/jacs.6b09645. [DOI] [PubMed] [Google Scholar]
- 66.Li T, Zhao X, Yang D, Du MH, Zhang L. Intrinsic defect properties in halide double perovskites for optoelectronic applications. Phys. Rev. Appl. 2018;10(4):41001. doi: 10.1103/PhysRevApplied.10.041001. [DOI] [Google Scholar]
- 67.Zhao XG, Yang D, Ren JC, Sun Y, Xiao Z, Zhang L. Rational design of halide double perovskites for optoelectronic applications. Joule. 2018;2(9):1662. doi: 10.1016/j.joule.2018.06.017. [DOI] [Google Scholar]
- 68.Xu Q, Yang D, Lv J, Sun YY, Zhang L. Perovskite solar absorbers: materials by design. Small Methods. 2018;2(5):1700316. doi: 10.1002/smtd.201700316. [DOI] [Google Scholar]
- 69.Zhao XG, Yang D, Sun Y, Li T, Zhang L, Yu L, Zunger A. Cu-In halide perovskite solar absorbers. J. Am. Chem. Soc. 2017;139(19):6718–6725. doi: 10.1021/jacs.7b02120. [DOI] [PubMed] [Google Scholar]
- 70.Xiao ZW, Du KZ, Meng WW, Wang JB, Mitzi DB, Yan YF. Intrinsic instability of Cs2In(I)M(III)X6 (M=Bi, Sb; X=Halogen) double perovskites: a combined density functional theory and experimental study. J. Am. Chem. Soc. 2017;139(17):6054–6057. doi: 10.1021/jacs.7b02227. [DOI] [PubMed] [Google Scholar]
- 71.Brik MG, Kityk IV. Modeling of lattice constant and their relations with ionic radii and electronegativity of constituting ions of A2XY6 cubic crystals (A=K, Cs, Rb, Tl; X=tetravalent cation, Y=F, Cl, Br, I) J. Phys. Chem. Solids. 2011;72(11):1256–1260. doi: 10.1016/j.jpcs.2011.07.016. [DOI] [Google Scholar]
- 72.Maughan AE, Ganose AM, Bordelon MM, Miller EM, Scanlon DO, Neilson JR. Defect tolerance to intolerance in the vacancy-ordered double perovskite semiconductors Cs2SnI6 and Cs2TeI6. J. Am. Chem. Soc. 2016;138(27):8453–8464. doi: 10.1021/jacs.6b03207. [DOI] [PubMed] [Google Scholar]
- 73.Maughan AE, Ganose AM, Candia AM, Granger JT, Scanlon DO, Neilson JR. Anharmonicity and octahedral tilting in hybrid vacancy-ordered double perovskites. Chem. Mater. 2018;30(2):472–482. doi: 10.1021/acs.chemmater.7b04516. [DOI] [Google Scholar]
- 74.Lee B, Stoumpos CC, Zhou N, Hao F, Malliakas C, Yeh CY, Marks TJ, Kanatzidis MG, Chang RPH. Air-stable molecular semiconducting iodosalts for solar cell applications: Cs2SnI6 as a hole conductor. J. Am. Chem. Soc. 2014;136(43):15379–15385. doi: 10.1021/ja508464w. [DOI] [PubMed] [Google Scholar]
- 75.Ghosh S, Paul S, De SK. Control synthesis of air-stable morphology tunable Pb-free Cs2SnI6 perovskite nanoparticles and their photodetection properties. Part. Part. Syst. Char. 2018;35(9):1800199. doi: 10.1002/ppsc.201800199. [DOI] [Google Scholar]
- 76.Wang A, Yan X, Zhang M, Sun S, Yang M, Shen W, Pan X, Wang P, Deng Z. Controlled synthesis of lead-free and stable perovskite derivative Cs2SnI6 nanocrystals via a facile hot-injection process. Chem. Mater. 2016;28(22):8132–8140. doi: 10.1021/acs.chemmater.6b01329. [DOI] [Google Scholar]
- 77.Tan Z, Li J, Zhang C, Li Z, Hu Q, et al. Highly efficient blue-emitting Bi-doped Cs2SnCl6 perovskite variant: photoluminescence induced by impurity doping. Adv. Funct. Mater. 2018;28(29):1801131. doi: 10.1002/adfm.201801131. [DOI] [Google Scholar]
- 78.Sakai N, Haghighirad AA, Filip MR, Nayak PK, Nayak S, et al. Solution-processed cesium hexabromopalladate(IV), Cs2PdBr 6, for optoelectronic applications. J. Am. Chem. Soc. 2017;139(17):6030–6033. doi: 10.1021/jacs.6b13258. [DOI] [PubMed] [Google Scholar]
- 79.Zhou L, Liao JF, Huang ZG, Wang XD, Xu YF, Chen HY, Kuang DB, Su CY. All-inorganic lead-free Cs2PdX6 (X=Br, I) perovskite nanocrystals with single unit cell thickness and high stability. ACS Energy Lett. 2018;3(10):2613–2619. doi: 10.1021/acsenergylett.8b01770. [DOI] [Google Scholar]
- 80.Guenther KF. The preparation of some alkali hexabromotitanates (IV) Inorg. Chem. 1964;3(12):1788–1789. doi: 10.1021/ic50022a033. [DOI] [Google Scholar]
- 81.Ju MG, Chen M, Zhou Y, Garces HF, Dai J, et al. Earth-abundant nontoxic titanium (IV)-based vacancy-ordered double perovskite halides with tunable 1.0 to 1.8 eV bandgaps for photovoltaic applications. ACS Energy Lett. 2018;3(2):297–304. doi: 10.1021/acsenergylett.7b01167. [DOI] [Google Scholar]
- 82.Deng Z, Wei F, Sun S, Kieslich G, Cheetham AK, Bristowe PD. Exploring the properties of lead-free hybrid double perovskites using a combined computational-experimental approach. J. Mater. Chem. A. 2016;4(31):12025–12029. doi: 10.1039/C6TA05817E. [DOI] [Google Scholar]
- 83.Wei FX, Deng ZY, Sun SJ, Zhang FH, Evans DM, et al. Synthesis and properties of a lead-free hybrid double perovskite: (CH3NH3)2AgBiBr 6. Chem. Mater. 2017;29(3):1089–1094. doi: 10.1021/acs.chemmater.6b03944. [DOI] [Google Scholar]
- 84.Li YJ, Wu T, Sun L, Yang RX, Jiang L, et al. Lead-free and stable antimony-silver-halide double perovskite (CH3NH3)2AgSbI6. RSC Adv. 2017;7(56):3517–35180. doi: 10.1039/C7RA06130G. [DOI] [Google Scholar]
- 85.Cheng P, Wu T, Li Y, Jiang L, Deng W, Han K. Combining theory and experiment in the design of a lead-free ((CH3NH3)2AgBiI6) double perovskite. New J. Chem. 2017;41:9598–9601. doi: 10.1039/C7NJ02365K. [DOI] [Google Scholar]
- 86.Deng Z, Wei F, Brivio F, Wu Y, Sun S, Bristowe PD, Cheetham AK. Synthesis and characterization of the rare-earth hybrid double perovskites: (CH3NH3)2KGdCl6 and (CH3NH3)2KYCl6. J. Phys. Chem. Lett. 2017;8(20):5015–5020. doi: 10.1021/acs.jpclett.7b02322. [DOI] [PubMed] [Google Scholar]
- 87.T.T. Tran, M.A. Quintero, K.E. Arpino, Z.A. Kelly, J.R. Panella, X. Wang, T.M. McQueen Chemically controlled crystal growth of (CH3NH3)2AgInBr 6. CrystEngComm. 2018;20:5929–5934. doi: 10.1039/C8CE00702K. [DOI] [Google Scholar]
- 88.Funabiki F, Toda Y, Hosono H. Optical and electrical properties of perovskite variant (CH3NH3)2SnI6. J. Phys. Chem. C. 2018;122(20):10749. doi: 10.1021/acs.jpcc.8b01820. [DOI] [Google Scholar]
- 89.Meng W, Wang X, Xiao Z, Wang J, Mitzi DB, Yan YJ. Parity-forbidden transitions and their impact on the optical absorption properties of lead-free metal halide perovskites and double perovskites. J. Phys. Chem. Lett. 2017;8(13):2999–3007. doi: 10.1021/acs.jpclett.7b01042. [DOI] [PubMed] [Google Scholar]
- 90.Lei LZ, Shi ZF, Li Y, Ma ZZ, Zhang F, et al. High-efficiency and air-stable photodetectors based on lead-free double perovskite Cs2AgBiBr 6 thin films. J. Mater. Chem. C. 2018;6(30):7982–7988. doi: 10.1039/C8TC02305K. [DOI] [Google Scholar]
- 91.Wu C, Du B, Luo W, Liu Y, Li T, et al. Highly efficient and stable self-powered ultraviolet and deep-blue photodetector based on Cs2AgBiBr 6/SnO2 heterojunction. Adv. Optical Mater. 2018 doi: 10.1002/adom.201800811. [DOI] [Google Scholar]
- 92.Li H, Shan X, Neu JN, Geske T, Davis M, Mao P, Xiao K, Siegrist T, Yu Z. Lead-free halide double perovskite-polymer composites for flexible X-ray imaging. J. Mater. Chem. C. 2018;6:11961–11967. doi: 10.1039/C8TC01564C. [DOI] [Google Scholar]
- 93.Hu Q, Deng Z, Hu M, Zhao A, Zhang Y, Tan Z, Niu G, Wu H, Tang J. X-ray scintillation in lead-free double perovskite crystals. Sci. China Chem. 2018;61:1. doi: 10.1007/s11426-018-9308-2. [DOI] [Google Scholar]
- 94.Luo J, Wang X, Li S, Liu J, Guo Y, et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature. 2018;563:541–545. doi: 10.1038/s41586-018-0691-0. [DOI] [PubMed] [Google Scholar]
- 95.Moser F, Lyu S. Luminescence in pure and I-doped AgBr crystals. J. Lumin. 1971;3(6):447–458. doi: 10.1016/0022-2313(71)90025-1. [DOI] [Google Scholar]
- 96.Greul E, Petrus ML, Binek A, Docampo P, Bein T. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A. 2017;5(37):19972–19981. doi: 10.1039/C7TA06816F. [DOI] [Google Scholar]
- 97.Wu C, Zhang Q, Liu Y, Luo W, Guo X, et al. The dawn of lead-free perovskite solar cell: highly stable double perovskite Cs2AgBiBr6 film. Adv. Sci. 2018;5(3):1700759. doi: 10.1002/advs.201700759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao W, Ran C, Xi J, Jiao B, Zhang W, Wu M, Hou X, Wu Z. Quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2% efficiency. ChemPhysChem. 2018;19(14):1696–1700. doi: 10.1002/cphc.201800346. [DOI] [PubMed] [Google Scholar]
- 99.Pantaler M, Cho KT, Queloz VIE, Benito IG, Fettkenhauer C, et al. Hysteresis-free lead-free double perovskite solar cells by interface engineering. ACS Energy Lett. 2018;3(8):1781–1786. doi: 10.1021/acsenergylett.8b00871. [DOI] [Google Scholar]
- 100.Wang M, Zeng P, Bai S, Gu J, Li F, Yang Z, Liu M. High-quality sequential-vapor-deposited Cs2AgBiBr6 thin films for lead-free perovskite solar cells. Solar RRL. 2018 doi: 10.1002/solr.201800217. [DOI] [Google Scholar]
- 101.Qiu X, Cao B, Yuan S, Chen X, Qiu Z, et al. room unstable CsSnI3 to air-stable Cs2SnI6: a lead-free perovskite solar cell light absorber with bandgap of 1.48 eV and high absorption coefficient. Sol. Energy Mater. Sol. Cells. 2017;159:227–234. doi: 10.1016/j.solmat.2016.09.022. [DOI] [Google Scholar]
- 102.Qiu X, Jiang Y, Zhang H, Qiu Z, Yuan S, Wang P, Cao B. Lead-free mesoscopic Cs2SnI6 perovskite solar cells using different nanostructured ZnO nanorods as electron transport layers. Phys. Status Solidi (RRL) 2016;10(8):587–591. doi: 10.1002/pssr.201600166. [DOI] [Google Scholar]
- 103.Lee B, Krenselewski A, Baik SI, Seidman DN, Chang RPH. Solution processing of air-stable molecular semiconducting iodosalts, Cs2SnI6-xBrx, for potential solar cell applications. Sustainable Energy Fuels. 2017;1(4):710–724. doi: 10.1039/C7SE00100B. [DOI] [Google Scholar]
- 104.Chen M, Ju MG, Carl AD, Zong Y, Grimm RL, et al. Cesium titanium (IV) bromide thin films based stable lead-free perovskite solar cells. Joule. 2018;2(3):558–570. doi: 10.1016/j.joule.2018.01.009. [DOI] [Google Scholar]
- 105.Akkerman QA, Gandini M, Di Stasio F, Rastogi P, Palazon F, et al. Strongly emissive perovskite nanocrystal inks for high-voltage solar cells. Nat. Energy. 2016;2(2):16194. doi: 10.1038/nenergy.2016.194. [DOI] [Google Scholar]
- 106.Liu Z, Sun B, Liu X, Han J, Ye H, Shi T, Tang Z, Liao G. Efficient carbon-based CsPbBr3 inorganic perovskite solar cells by using Cu-phthalocyanine as hole transport material. Nano-Micro Lett. 2018;10(2):34. doi: 10.1007/s40820-018-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Wu X, Chu Y, Zhou J, Zhou B, Huang J. Hybrid field-effect transistors and photodetectors based on organic semiconductor and CsPbI3 perovskite nanorods bilayer structure. Nano-Micro Lett. 2018;10(4):57. doi: 10.1007/s40820-018-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]