Abstract
The cytokine interleukin-1β (IL-1β) is critical for antimicrobial defenses; the inflammasome pathway typically controls IL-1β release, but pathogens often evade this pathway. In this issue Donado et al. (2020) describe an alternative, two-cell model, to instruct inflammasome-independent IL-1β release.
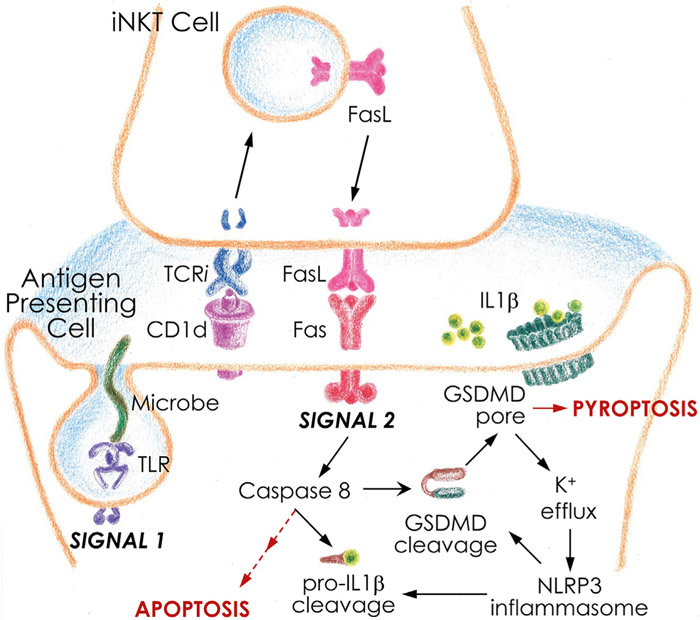
Interleukin-1β (IL-1β) is an inflammatory cytokine important in antimicrobial defenses. It causes fever and initiates the recruitment of innate cells to the site of infection. It also instructs the activation of adaptive immunity and differentiation of effector T cells. IL-1β overproduction is linked to several inherited or acquired chronic inflammatory diseases (e.g., cryopyrin-associated periodic syndrome, type 2 diabetes, rheumatoid arthritis, etc.) and is a therapeutic target for many of them (Dinarello, 2011). IL-1β release is thus tightly controlled in a cell-intrinsic two-step process (Dinarello, 2011). Step one upregulates the expression of the inactive cytosolic precursor, pro-IL-1β, in response to pathogen-derived ligands (e.g., lipopolysaccharide) or inflammatory cytokines (e.g., tumor necrosis factor). Step two processes pro-IL-1β precursor into the bioactive, secreted form, typically by the enzyme Caspase-1 (Dinarello, 2011). Caspase-1 is the effector protease in the inflammasome pathway, a multiprotein cytosolic signaling platform activated upon the loss of cellular homeostasis caused by infection or tissue damage (Broz and Dixit, 2016). Activated Caspase-1 also cleaves a pore forming protein Gasdermin D (GSDMD), which translocates to the plasma membrane, where it forms large pores to cause the death of the compromised cell (Broz and Dixit, 2016). The classical in vitro studies of the inflammasome biology have focused on these cell-intrinsic molecular events which underpin the inflammasome pathway. They, nonetheless, failed to answer one key question: how is IL-1β released when cell-intrinsic inflammasome activation is absent or is inhibited by pathogens (Shin and Brodsky, 2015)? In this issue of Cell Reports, Donado et al. (2020) describe an alternative, two-cell model for IL-1β release, where another immune cell, the invariant natural killer T cell (iNKT cell), becomes activated quickly during infection and provides the second signal to antigen-presenting cells (APCs) to instruct inflammasome-independent processing and secretion of IL-1β (Figure 1).
Figure 1. Two-Cell Model for Inflammasome-Independent IL-1β Release.
Inflammasome-dependent and -independent IL-1β maturation and secretion require two signals. Signal 1 is shared by both mechanisms, which involves microbe recognition by a TLR and the upregulation of pro-IL-1β production. Signal 2 distinguishes inflammasome-dependent and -independent mechanisms. See text for details.
iNKT cells are innate-like lymphocytes that recognize pathogen-derived or pathogen-induced glycolipid agonists presented by CD1d molecules on the surface of infected APCs (Brennan et al., 2013). iNKT cells respond rapidly and robustly to stimulation as a clonal population because they express a conserved semi-invariant T cell receptor (TCRi)–that acts more like a pattern recognition receptor–and because they have mRNAs and proteins for many immune effector molecules premade in the cytoplasm (Brennan et al., 2013; Kumar et al., 2017). Donado and colleagues dubbed iNKT cells “cellular adjuvants” of the immune system, because during the early stages of infection their main effector function is to transactivate other leukocytes, including APCs, through cytokine production and contact-dependent mechanisms (Brennan et al., 2013; Kumar et al., 2017). In the current study, Donado et al. (2020) now reveal one such contact-dependent immune cell cross-talk that instructs inflammasome-independent IL-1β release during bacterial infection (Figure 1).
To understand how iNKT cells instruct IL-1β release, Donado et al. (2020) developed an in vitro two-cell co-culture model; it consisted of purified mouse or human iNKT cells and bone marrow-or blood monocyte-derived APCs. The APCs were exposed to several bacterial activators: purified ligands (lipopeptide Pam3Cys) or commensals (Bacterioides fragilis) or intracellular pathogens (e.g., Chlamydia trachomatis). APCs so primed upregulated pro-IL1β expression but failed to activate inflammasomes for canonical IL-1β processing and release. But when iNKT cells were presented in the co-culture, they recognized, through their TCRi, the pathogen-induced changes in the CD1d-glycolipid agonist presentation. In response to those changes, NKT cells rapidly, within 2–3 h, translocated the pre-existing intracellular pool of FasL to their cell surface, to transactivate the death receptor Fas on the APCs, and the Fas-associated effector enzyme Caspase-8 (signal 2; Figure 1). Activated Caspase-8 cleaved pro-IL-1β into the bioactive form released from infected APCs. Caspase-8 also cleaved caspases-3 and −7 to drive the apoptosis of the infected APCs (Figure 1).
The study by Donado et al. (2020) clearly defines Caspase-8 as the apical enzyme in Fas-driven IL-1β release, independent of the canonical Caspase-1 or noncanonical Caspase-11 inflammasomes. However, in the co-culture model, Caspase-8 also cleaved a small fraction of GSDMD into its pore-forming fragment. The resulting GSDMD membrane pore drove potassium efflux that indirectly activated NLRP3-Caspase-1 inflammasome, to further amplify the cell death response and IL-1β secretion (Figure 1). The findings of Donado et al. (2020) are in agreement with earlier reports of Fas-Caspase-8-dependent IL-1β release (Bossaller et al., 2012). Critically, however, the current study significantly advances the field by placing the earlier findings into a physiological context. It also provides a mechanistic explanation for the source of Fas-instructive signal for IL-1β release during infection.
What is the physiological relevance of a two-cell model for IL-1β release? The two-cell back-up model for IL-1β release would clearly be important in inflammatory responses to those pathogens that fail to activate or evade inflammasomes in a cell-intrinsic manner (Shin and Brodsky, 2015). Another recent study (Jain et al., 2020) extends the findings by Donado et al. (2020) to non-infectious inflammation as well, where IL-1β is an important driver of immunopathology. Specifically, Jain et al. (2020) identified activated effector CD4+ T cells as another cellular source of FasL to instruct inflammasome-independent IL-1β release and drive inflammation in a mouse model of experimental autoimmune encephalomyelitis.
Collectively, these reports (Donado et al., 2020; Jain et al., 2020) answer an important question: how IL-1β is released when cell-intrinsic activation of inflammasomes is evaded by pathogens. As do all important studies, this study too opens new questions: What is the role for the two-cell model of IL-1β release in antimicrobial defense or sterile inflammation in vivo? Can other cells instruct bioactive IL-1β release? APCs derived from granulocyte-macrophage colony-stimulating factor-stimulated bone marrow cultures are heterogenous mixtures of macrophages and dendritic cells (Erlich et al., 2019), but macrophages appear superior in IL-1β release (Donado et al., 2020; Erlich et al., 2019). Many other cells, particularly those at epithelial barriers, express CD1d or related MHC-I like molecules (Brennan et al., 2013; Kumar et al., 2017). Do they communicate with other innate-like lymphocytes to control IL-1β release in response to pathogens or commensals? Is the release of other IL-1 family members, such as IL-18, controlled by a similar mechanism? What is the physiological purpose of cell death in this process? The anticipation of answers to these and other questions foretells exciting times ahead for inflammation biology.
REFERENCES
- Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. (2012). Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol 189, 5508–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, and Brenner MB (2013). Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol 13, 101–117. [DOI] [PubMed] [Google Scholar]
- Broz P, and Dixit VM (2016). Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol 16, 407–420. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2011). Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donado CA, Cao AB, Simmons DP, Croker BA, Brennan PJ, and Brenner MB (2020). A two-cell model for IL-1beta release mediated by death-receptor signaling. Cell Rep. 31, this issue, 107466-1–107466-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich Z, Shlomovitz I, Edry-Botzer L, Cohen H, Frank D, Wang H, Lew AM, Lawlor KE, Zhan Y, Vince JE, and Gerlic M (2019). Macro-phages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat. Immunol 20, 397–406. [DOI] [PubMed] [Google Scholar]
- Jain A, Irizarry-Caro RA, McDaniel MM, Chawla AS, Carroll KR, Overcast GR, Philip NH, Oberst A, Chervonsky AV, Katz JD, and Pasare C (2020). T cells instruct myeloid cells to produce inflammasome-independent IL-1β and cause autoimmunity. Nat. Immunol 21, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Suryadevara N, Hill TM, Bezbradica JS, Van Kaer L, and Joyce S (2017). Natural Killer T Cells: An Ecological Evolutionary Developmental Biology Perspective. Front. Immunol 8, 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, and Brodsky IE (2015). The inflammasome: Learning from bacterial evasion strategies. Semin. Immunol 27, 102–110. [DOI] [PubMed] [Google Scholar]