Highlights
The recent progress about zinc-ion batteries was systematically summarized in detail, including the merits and limits of aqueous and nonaqueous electrolytes, various cathode materials, zinc anode, and solid-state zinc-ion batteries.
Current challenges and perspectives to future research directions are also provided.
Keywords: Zinc-ion batteries, Electrolyte, Cathode, Zinc anode, Flexible device
Abstract
The increasing demands for environmentally friendly grid-scale electric energy storage devices with high energy density and low cost have stimulated the rapid development of various energy storage systems, due to the environmental pollution and energy crisis caused by traditional energy storage technologies. As one of the new and most promising alternative energy storage technologies, zinc-ion rechargeable batteries have recently received much attention owing to their high abundance of zinc in natural resources, intrinsic safety, and cost effectiveness, when compared with the popular, but unsafe and expensive lithium-ion batteries. In particular, the use of mild aqueous electrolytes in zinc-ion batteries (ZIBs) demonstrates high potential for portable electronic applications and large-scale energy storage systems. Moreover, the development of superior electrolyte operating at either high temperature or subzero condition is crucial for practical applications of ZIBs in harsh environments, such as aerospace, airplanes, or submarines. However, there are still many existing challenges that need to be resolved. This paper presents a timely review on recent progresses and challenges in various cathode materials and electrolytes (aqueous, organic, and solid-state electrolytes) in ZIBs. Design and synthesis of zinc-based anode materials and separators are also briefly discussed.
Introduction
Energy crisis and environmental pollution have become two of the most serious issues in the present time [1–4]. For hundreds of years, fossil fuels (petroleum, coal, etc.) have dominated the energy supply for the needs of humanity [5–8]. However, usage of fossil fuels can lead to numerous environmental issues, especially air pollution, which is caused by the emissions of sulfur dioxide, nitrous oxide, carbon dioxide, and other gases containing volatile organic compounds [9, 10]. Meanwhile, the development of environment-friendly energy sources such as solar cell and wind electricity is seriously restrained by their intermittent production and inability for storing energy [11, 12]. To overcome the above-mentioned challenges, electrical energy storage (EES) offers an effective way to improve the reliability and scalability of the grid [13–15]. In recent years, much progress has been made by developing new energy technologies, especially rechargeable batteries [16–19]. Since the first secondary cell (lead–acid battery) was invented about 150 years ago, numerous kinds of rechargeable batteries have been designed, such as nickel zinc battery, nickel metal hydride, and lithium-ion batteries (LIBs) [20, 21]. For several decades, lithium-ion batteries have been widely applied as commercial energy storage devices owing to their advantages of high efficiency in delivering energy, high voltage, and long cycling life [22, 23]. However, many issues such as high cost and safety problems seriously hinder the large-scale applications of lithium-ion batteries [24, 25]. In recent years, a lot of research work focused on aqueous rechargeable batteries using naturally abundant monovalent ions (Na+, K+) and multivalent cations (Zn2+, Mg2+, Al3+) as charge carriers [26–29]. The aqueous zinc-ion batteries (ZIBs) are very appealing owing to the unique properties of zinc anode, including the low cost, rich resources of zinc metals, high chemical/physical stability, environmental friendliness, and high safety [28, 30–35].
A typical ZIB consists of a cathode for hosting Zn ions, zinc metal anode, electrolyte, and a separator to separate cathode and anode, which is quite similar to the structure of a LIB. Zinc ions are moving between cathode and anode during charging and discharging processes. Since Volta et al. used zinc metal as electrode in battery in 1999 for the first time, Zn metal has been deemed as an ideal anode material in types of primary and secondary Zn cells due to many excellent properties, especially high capacity of Zn metal anode, nontoxicity, relatively low redox potential (− 0.76 V vs. standard hydrogen electrode (SHE)), high safety, and low cost. Therefore, such Zn metal has been applied in various batteries, such as Ni–Zn batteries, MnO2–Zn batteries, Zn-ion batteries, and Zn–air batteries. Among them, Zn–MnO2 batteries are dominant as primary batteries owing to the properties of low cost and high energy density [36, 37]. Later effort further expands the alkaline Zn–MnO2 batteries to secondary battery field, while, as electrode in rechargeable batteries, the continuous formation of zinc dendrite in zinc metal and the irreversible reaction lead to limited cycling life and low discharge capacity. Several decades ago, Yamamoto et al. first designed aqueous zinc-ion batteries by replacing the alkaline electrolyte with aqueous mild acid zinc sulfate electrolyte [38]. Unlike the case in alkaline Zn–MnO2 batteries, the formation of by-products (ZnO, Zn (OH)2, etc.) on zinc metal is very few. Also, compared with Mg metal, another advantage of zinc metal is that zinc can be dissolved more easily in electrolyte and deposited more easily on metal anode. In recent years, a lot of research work has been focused on both zinc metal anode and cathode materials, especially manganese-based oxides, vanadium-based oxides, polyanion compounds, sustainable quinone analogs, and Prussian blue analogs. This research work has made significant progress on the development of ZIBs [39–44].
Furthermore, the rapid development of various wearable electronic devices keeps promoting the exploration on portable energy storage devices with superior electrochemical performance and desirable mechanical flexibility [45, 46]. As a promising candidate, rechargeable solid-state zinc-ion storage systems also have increasingly attracted research interests [30, 47]. It should be noted that safety problem requires serious consideration in the wearable and flexible devices while they maintain high energy storage capabilities, because these electronics may undergo continuous mechanical force or damage such as strike or being bended, and directly in contact with human body [48]. In comparison with liquid electrolytes, solid-state electrolytes show a noticeable advantage for avoiding the electrolyte leakage. Furthermore, solid-state electrolytes show high stability, desirable flexibility, and can effectively control the formation of zinc dendrite and the dissolution of active electrode materials in zinc-ion storage systems [49]. To date, electrolytes based on zinc sulfate (ZnSO4) and zinc triflate (Zn(CF3SO3)2) in conjunction with polymer hosts such as ZnSO4–gelatin, Zn(CF3SO3)2–polyethylene oxide, and Zn(CF3SO3)2–polyvinyl alcohol have been reported [50–53]. However, these polymer electrolytes suffer from insufficient mechanical strength, undesirable ionic conductivity, and fast degradation. By employing these electrolytes, the batteries display relatively narrow voltage window and undesirable cycling stability, posing challenges for the gel electrolyte selection and preparation method. Additionally, an intrinsically self-healable ZIB is proposed by the utilization of hydrogel electrolyte, in order to effectively enhance the recoverability of the devices against various shape deformations [51]. More importantly, the exploration of desirable solid-state electrolytes with a wide working range from subzero to high temperature is still lacking and researchers need to put more efforts to promote its development, which can boost the practical applications of flexible ZIBs used in airplanes, aerospace, or ocean vehicles. Consequently, the careful design on the suitable solid-state electrolytes with high stability, excellent zinc-ion conductivity, superior mechanical properties, and easy fabrication is highly required for the further development of flexible and safe rechargeable zinc-ion based energy storage devices, which could effectively prevent electrolyte leakage issue and achieve industrial production of batteries with desirable structures [30].
Recently, Fan et al. have analyzed and summarized the Zn-ion storage mechanisms of various cathode materials [54]. And Liu et al. summarized the design strategies for vanadium-based cathodes for aqueous ZIBs [13]. Though many review articles have been published in recent years [53], few review papers discuss various electrolytes in the ZIBs in details despite that electrolyte is a crucial component in ZIBs and there have been rapid new developments concerning it lately. The main difference in this review is that we systematically present recent progresses and challenges of all the components in ZIBs in detail, including various cathode materials, aqueous and nonaqueous electrolytes, solid-state ZIBs, and Zn anodes. We also provide opinions on current limitations of ZIBs and perspectives for future research directions. The review is divided into six parts: (1) research background, recent development and research progress of ZIBs based on (2) aqueous electrolytes, (3) summarization of representative cathode materials, (4) solid-state zinc-ion batteries, (5) design of zinc anodes and separators, and (6) current challenges and perspectives for future research directions.
Zinc-Ion Batteries based on Aqueous Electrolytes
Aqueous rechargeable batteries are regarded as promising candidate for large-scale energy storage due to their high safety nature, low cost, and environmental friendliness [55–57]. Moreover, compared with organic electrolyte, the aqueous electrolytes can provide two times higher ionic conductivities (~ 1 S cm−1) due to the higher mobility of ions in water environment [56]. In addition, the rechargeable zinc-ion batteries employing divalent ions can provide higher specific capacity as well as energy density than monovalent ions due to the double electrons involved in redox reactions. Because the most common anode material in ZIBs is zinc metal and the battery capacity is more limited by cathode than anode, a vast variety of cathode materials have been explored for applications in high-performance ZIBs, as detailed below.
Manganese Oxide Cathode Materials
For thousands of years, manganese-based materials have been widely used by humans due to their abundant resources in the crust. Manganese oxides have remarkable diversity of atomic structures and multivalent phases due to different oxidation states of Mn: + 2, + 3, and + 4. Therefore, manganese oxides can be widely applied as catalysts, battery materials, and deoxidizer in steel making, etc. Moreover, manganese oxides are the first material researched and reported as cathode in the field of ZIBs due to its high valence state and specific phases which can readily accommodate inserted ions. To better understand manganese oxides and their crystal structures and the Mn-based materials applied in ZIBs, we summarize the recent studies about manganese oxides below.
γ-MnO2
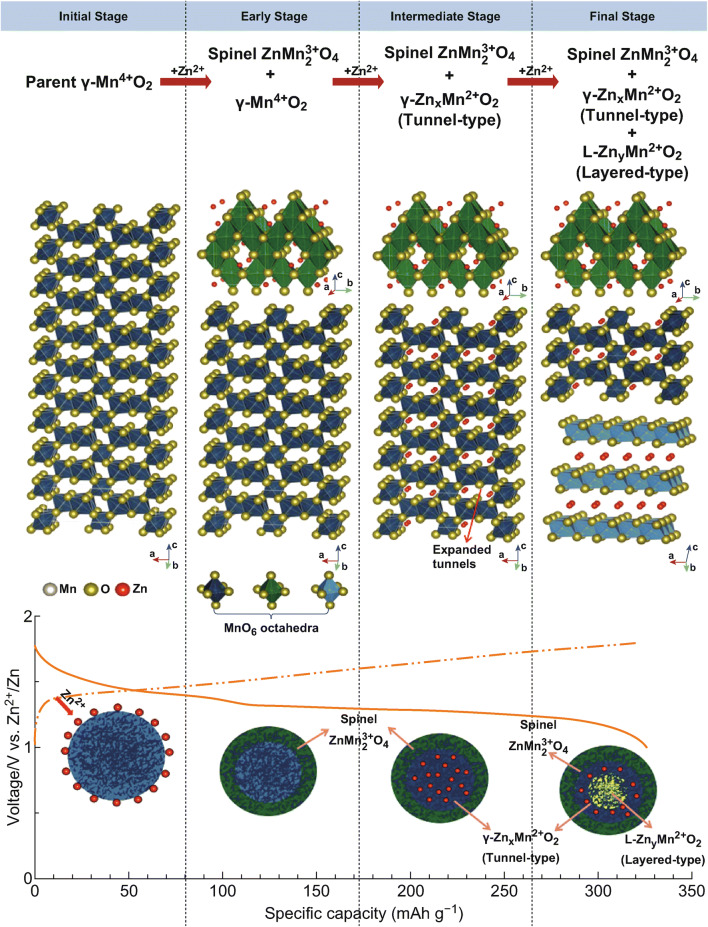
Kim et al. investigated the phase evolution in a mesoporous γ-MnO2 material during the intercalation of zinc ions via in situ Synchrotron XANES and XRD [58]. The results confirm that with the intercalation of zinc ions, the typical tunnel-type γ-MnO2 gradually transferred to spinel-type ZnMn2O4 and two new intermediary Mn(II) phases, namely tunnel-type γ-ZnxMnO2 and layered-type L-ZnyMnO2. With the extraction of zinc ions, most of these phases return back to the γ-MnO2 phase, indicating reversible phase transformation during electrochemical reaction (Fig. 1). At the current density of 0.05 mA cm−2, the mesoporous γ-MnO2 cathode can deliver an initial discharge capacity of 285 mAh cm−2.
Fig. 1.
Schematic illustration of the transformation process of γ-MnO2 cathode with Zn2+ ion insertion [58]. With permission from American Chemical Society
α-MnO2
The α-MnO2 is also a hot topic to be applied as cathode in ZIBs due to its large and stable 2 × 2 tunnels structure, which can accommodate the intercalated Zn2+ ions. The α-MnO2 was first studied as cathode of ZIBs by Zeng et al. in 2009 with the initial capacity of 210 mAh g−1 [59]. The capacity can be almost 100% maintained after 100 cycles at current density of 6 °C, showing reversible zinc storage and robust 2 × 2 tunnel structure during electrochemical reactions. However, the electrochemical reaction mechanism for MnO2/Zn batteries is still a discussion topic until now. Currently, three mechanisms are proposed in the aqueous α-MnO2/Zn batteries: zinc intercalation/deintercalation, conversion reaction, and H+ and Zn2+ co-insertion.
Zinc intercalation/deintercalation mechanism Due to the small radius of zinc ions, various compounds with layered or tunnel structure enable the intercalation of zinc ions. In 2012, Kang et al. investigated electrochemical mechanism of α-MnO2/Zn batteries with aqueous electrolyte [32]. They confirmed that ZnMn2O4 was formed after the insertion of Zn2+ ion into the tunnels of α-MnO2 and reversibly returned to α-MnO2 after the extraction via ex situ XRD, and they proposed the zinc intercalation/deintercalation mechanism as below [32]:
| 1 |
| 2 |
Afterward, some studies focus on the phase transformation after the intercalation of zinc ions in tunnels of α-MnO2. For example, Oh et al. revealed that the electrochemical reaction process of α-MnO2/Zn involves a reversible phase transition between tunneled (α-MnO2) and layered Zn–buserite, instead of spinel structure ZnMn2O4 [33].
Conversion reaction mechanism In 2016, Liu et al. significantly improved the electrochemical performance of Zn/MnO2 battery by adding MnSO4 additive in mild ZnSO4 aqueous electrolyte [60]. They found that the MnSO4 additive can suppress the Mn2+ dissolution into the electrolyte, leading to improved rate performance and capacity retention capability. In addition, to understand the electrochemical behavior, they further investigated the morphology and phase evolution of α-MnO2 material via TEM and STEM-EDS mapping. The results reveal that the electrochemical mechanism of MnO2 is based on the conversion reaction between MnOOH and MnO2 instead of Zn2+ ion intercalation/deintercalation in/out of MnO2, which is formulated as below [60]:
Cathode:
| 3 |
| 4 |
| 5 |
| 6 |
The MnOOH phase is confirmed by XRD at the fully discharged state. Instead of intercalation of zinc ions, the reaction is induced by the reaction between α-MnO2 and inserted protons. Moreover, another electrochemical mechanism of H+ and Zn2+ co-insertion was reported.
The H+ and Zn2+ co-insertion mechanism It is possible that the open tunnel α-MnO2 enables the co-insertion of H+ and Zn2+ ions. To understand the mechanism, Wang et al. [61] developed Zn/MnO2 batteries and studied consequent H+ and Zn2+ insertion processes by using electroanalytical technology combined with XRD, SEM, and TEM. The two insertion processes happened at two different voltage regions. The galvanostatic intermittent titration technique (GITT) results show that the H+ insertion process happens at region I with low overvoltage of 0.08 V, and the Zn2+ insertion process takes place at region II with much higher overvoltage of 0.6 V. The significant difference of H+ and Zn2+ insertion processes is mainly attributed to the different resistances for H+ and Zn2+ ion diffusion. The EIS results show that the ohmic resistances of these two regions are close, while the charge transfer resistance of region II is much larger than that in region I. Moreover, the bivalent zinc ions have much larger radius and stronger electrostatic interactions with the host atoms than H+ ions, leading to slower Zn2+ diffusion. It can be also observed in ZIBs with other cathodes, such as V2O5·nH2O [65] and NaV3O8·1.5H2O [78]. Despite that three different Zn2+ storage mechanisms in MnO2/Zn batteries have been proposed, more efforts are still needed to further identify the electrochemical reaction mechanism and further enhance the battery performance.
Many strategies have been applied to improve the performance of MnO2/Zn batteries. For example, to slow down the manganese dissolution during electrochemical cycling, Liang et al. introduced potassium ions and oxygen defects in MnO2 [62]. The incorporated K+ ions can stabilize the Mn-based cathodes, and the oxygen defects can improve electrical conductivity and diffusion rate for zinc ions. Therefore, the K0.8Mn8O16/Zn batteries show improved capacity at high rate. At a high current density of 1 A g−1, the K0.8Mn8O16/Zn batteries can provide a high specific capacity over 300 mAh g−1 and a high energy density of 398 Wh kg−1 for over 1000 cycles, showing outstanding durability and energy density. Composite nanostructure design is another effective strategy to boost the performance of MnO2/Zn batteries. For example, Xia et al. designed the manganese dioxide with polyaniline preintercalated in the interlayer space. The intercalated polyaniline can strengthen the layered structure of manganese dioxide in nanoscale size [63]. Furthermore, the combination of PANI-reinforced layered structure and nanoscale particle size (~ 10 nm) can efficiently hinder the phase transformation induced by the insertion of hydrated H+/Zn2+ ions, maintaining the structural stability during the electrochemical reaction. The co-insertion process of hydrated H+ and Zn2+ ions was also carefully examined and clarified a self-regulating mechanism involving generation/dissolution of electrolyte (zinc hydroxide sulfate). The PANI-reinforced MnO2 achieves a high capacity of 125 mAh g−1 for over 5000 cycles, showing long cycling life with high capacity.
ZnMn2O4
Inspired by the success of LiMn2O4, spinel MFe2O4 (M = Zn, Ni, or Cu), Co3O4, ZnCo2O4, and ZnMn2O4 have also been explored as electrode material for batteries. Manthiram et al.’s study investigated the reaction mechanism of the insertion process of Zn2+ ions in spinel compositions ZnMn2−xNixO4 (x = 0, 0.5, and 1) [64]. The zinc ions can be extracted from the structure in acid condition during the Mn3+ disproportionation reaction, while, with the increase in Ni content, the extraction of Zn2+ will decrease. The researching results appear that the spinel-structured materials are not quite suitable for the intercalation of zinc ions, while, with a certain content of defects (vacancies), the Zn-ion diffusion can be much easier due the lower electrostatic repulsion. Inspired by the understanding that the creation of defects in spinel materials can open additional pathways for the transportation of divalent ions. Chen and his co-workers prepared the cation-defective ZnMn2O4 spinel as the host material for intercalation of Zn2+ cations [19]. They applied ZnMn2O4/carbon composite as the cathode material and studied the electrochemical reaction mechanism via XRD, Raman, FTIR, NMR, and electrochemical measurements. They demonstrated that the abundant cation vacancies and small nanoscale size can facilitate the charge transfer and Zn2+ insertion into ZnMn2O4 spinel structures. The ~ 100% Zn plating/stripping efficiency enables long cycling life with high capacity. At high current of 500 mA/g, the ZnMn2O4 spinel carbon composite material can supply the specific capacity of 150 mAh g−1 for 500 cycles with retention of 94%.
Vanadium-Based Cathode Materials
V2O5
Building by sharing edges and corners of square pyramids chains, the vanadium pentoxide shows square pyramid-layered structure. More importantly, square pyramid layer of α-V2O5 can include water molecules or ions such as Na and Zn ions into the interlayers, which may change the layered structure and significantly affect the discharge/charge processes and electrochemical performances of ZIBs.
In 2018, Cheng et al. investigated the Zn storage mechanism in commercial V2O5 cathode material using Zn(CF3SO3)2 aqueous electrolyte [65]. It is reported that Zn2+ cations can reversibly insert/extract through the layered structure of commercial V2O5 bulks. Moreover, it is interesting to find that the bulk V2O5 morphology gradually develops into porous nanosheet structure after cycling, which is caused by the exfoliation during charging and discharging processes (Fig. 2). As a result, the V2O5 porous nanosheets can deliver a very high reversible capacity of 372 mAh g−1 at current density of 5 A g−1 for over 4000 cycles. In addition, the co-intercalated H2O molecules can enhance the transportation of Zn2+ ions. Subsequently, Mai et al. systematically studied the critical role of structural H2O on Zn2+ intercalation into the layered structure of V2O5·nH2O [66]. They found that water molecules can dramatically work as a “lubricant” to promote the fast transportation of zinc ions. Specifically, the structural water can function like a charge screening media in redox reactions during cycling, which can not only increase the interlayer distance, but also decrease electrostatic interactions of H2O-solvated Zn2+ ions in the V2O5 framework. This result was confirmed by the solid-state magic-angle spinning results. Benefited from the “lubricating” effect of structural water, V2O5·nH2O can deliver a very high energy density of ~ 144 Wh kg−1 at 0.3 A g−1, which is comparable with LiCoO2/graphite batteries.
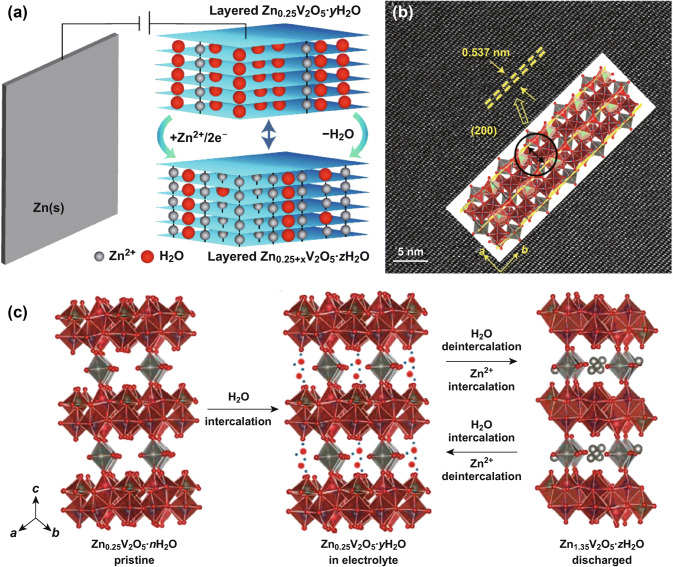
Fig. 2. a.
Schematic of aqueous ZIBs based on Zn0.25V2O5 cathode. Due to the water molecules between the V2O5 layers, the interlayer space expands. b The related HRTEM image of the Zn0.25V2O5·nH2O nanobelts. c Schematics showing the co-intercalation of water molecules accompanying Zn2+ in/out of the interlayer space of V2O5 layers during charging and discharging process [67]. With permission from Springer Nature
The critical role of structural water molecules and interlayer-doped ions is also verified by many researchers. For example, Linda et al. first studied Zn0.25V2O5·nH2O as the positive electrode for Zn cells in 2016 (Fig. 2a) [67]. They found that water molecules intercalated in the interlayers can buffer the high charge density of zinc ions and reduce the activation energy for charge transfer at the interface of cathode material (Fig. 2b). The indigenous Zn ions in Zn0.25V2O5·nH2O crystals can stabilize the layered structure, leading to the long cycling stability. Moreover, the stable layered structure can release the stress generated by the insertion/extraction of zinc ions during charging/discharging process and also short the pathways for the transportation of zinc ions (Fig. 2c). At the rate of 1 °C, the Zn0.25V2O5·nH2O can provide the capacity up to 300 mAh g−1, with energy density of ~ 450 Wh l−1 and capacity retention of more than 80% for over 1000 cycles.
VxOy Cathode Materials
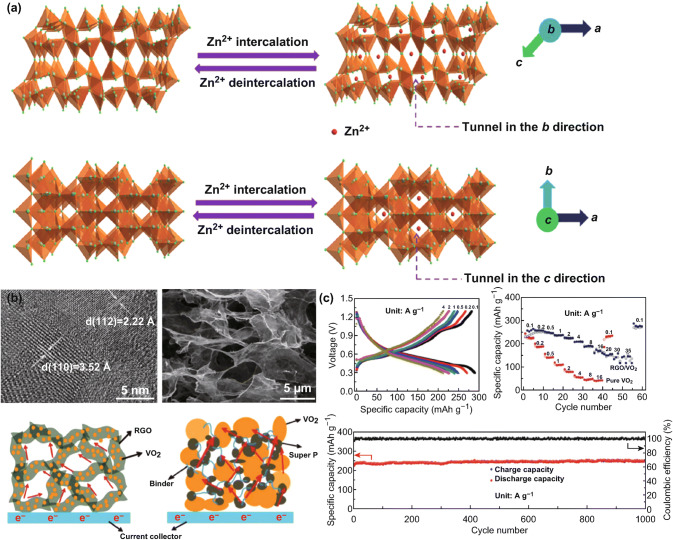
Constructed by distorted VO6 octahedra by sharing corners and edges, vanadium dioxide has a special tunnel-like framework. The big tunnel-like framework can facilitate the transportation for the diffusion of inserted ions. In recent years, VO2 (B) has been widely studied as potential electrode material in organic electrolytes with monovalent ions, while the investigation of VO2 (B) in divalent/multivalent ion batteries is almost blank. In 2018, Yang et al. first introduced VO2 (B) as cathode material in aqueous ZIBs [68]. They investigated the electrochemical mechanism by in situ XRD combined electrochemical analyses. And the results demonstrate that VO2 (B) nanofibers have an intercalation pseudocapacitance behavior. The pseudocapacitance behavior is owing to the unique tunnel-like framework, which can not only provide efficient pathways for transporting Zn2+ ions, but also has strong mechanical stability during the intercalation/deintercalation of Zn2+ ions. Therefore, the VO2 (B) nanofibers show remarkable electrochemical performance. At the high current density of 300 °C, VO2 (B) nanofibers show the reversible capacity as high as 171 mAh g−1, high energy density of 297 Wh/kg, and power densities of 180 W kg−1. In addition, Niu et al. designed the freestanding reduced graphene oxide/vanadium dioxide (RGO/VO2) composite films to further improve the performance of VO2 as cathode for zinc-ion batteries. Zn/RGO/VO2 batteries exhibit an energy density of 65 Wh kg−1 even at a high power density of 7.8 kW kg−1 (Fig. 3). The superior performance attributes to the porous structure of RGO/VO2 composite film, which can provide efficient transportation of electrons and ions during cycling. Moreover, porous network can also release the stress and accommodate the volume change during the intercalation/deintercalation of Zn2+ into/from the VO2 crystals. In recent years, Wang et al. found an interesting phase transformation phenomenon during electrochemical process of VO2 [69]. They demonstrated that the monoclinic VO2 gradually transfers to bilayered V2O5·nH2O, which is induced by the initial insertion/extraction of zinc ions in VO2 during cycling. The phase transformation leads to significantly enlarged interlayer spacing and enhanced structural stability, enabling an improved battery performance: at the current density of 100 mA g−1 (corresponding to a specific energy density of 271.8 Wh kg−1), it can deliver a capacity of 274 mAh g−1 with an excellent capacity retention of 79% for over 10,000 cycles, showing a promising application as cathode material of ZIBs.
Fig. 3. a.
Schematic illustration of Zn2+ intercalation/deintercalation in VO2 crystals. b HRTEM and SEM images of RGO-VO2; schematic view of the pathways for electron transportation in different samples (RGO-VO2 and VO2-super P). c Electrochemical performance of RGO-VO2 composite film, including charge/discharge profiles, rate capability, and long-term cycling test at 4 A g−1 [67]. With permission from Elsevier and Wiley
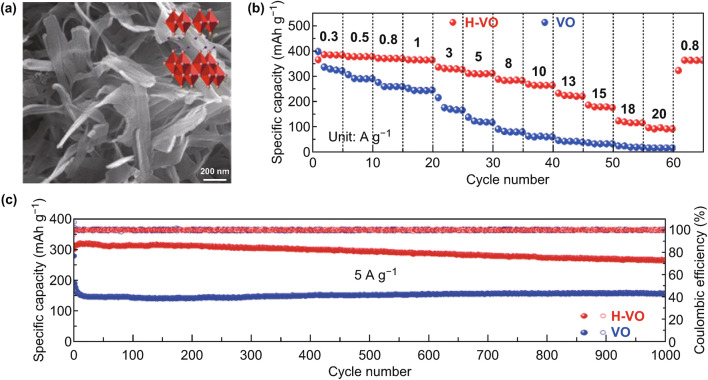
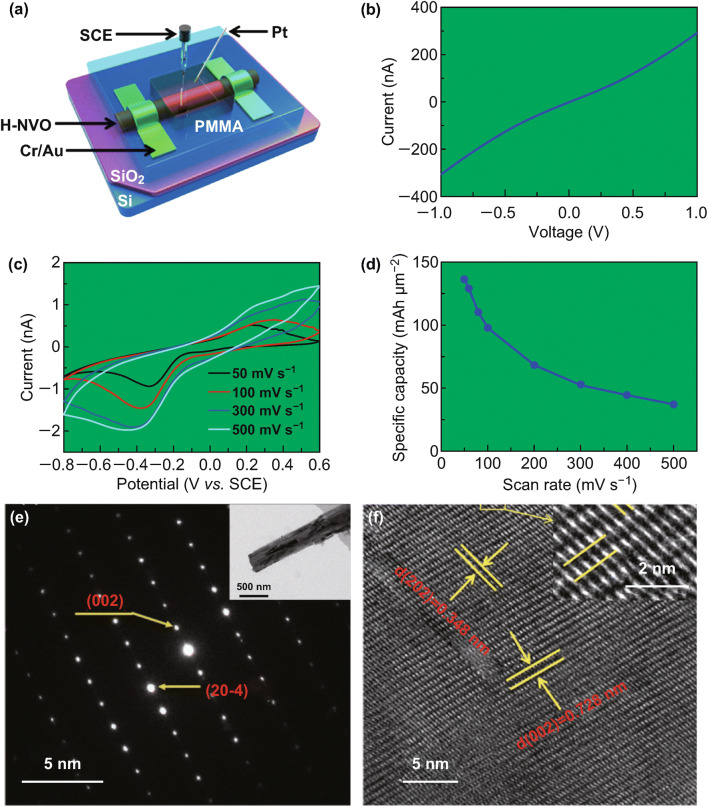
Interlayer-expanded V6O13·nH2O (H–VO) nanosheets were also prepared and evaluated as cathode material for aqueous ZIBs [70]. Benefiting from the enlarged interplanar spacing with abundant accessible channels and the ultra-thin nanosheet architecture with short diffusion pathway for Zn2+ migration, the H–VO cathode presents superior electrochemical property (Fig. 4). The as-synthesized H–VO nanosheets exhibit a very high and reversible capacity of 395 mAh g−1 at a current density of 0.1 A g−1, an exceptionally high rate capability up to 20 A g−1 with a decent capacity of 97 mAh g−1, and over 87% capacity retention after 1000 cycles, while the annealed V6O13 (VO) without lattice water shows much inferior electrochemical behaviors. This demonstrates the essential role of crystalline water in enhancing the electrochemical performances of vanadium compounds as cathode in aqueous ZIBs.
Fig. 4.
a SEM image of V6O13·nH2O (insert: the corresponding atomic structure). b Rate capabilities of V6O13·nH2O and V6O13 electrodes. c Long-term cycling performances of V6O13·nH2O and V6O13 cathodes at 5 A g−1 [70]. With permission from American Chemical Society
Choi et al. further elucidated the importance of water co-insertion with Zn2+ by the combination of density functional theory (DFT) calculations and experiments, rendering the high performance of V6O13 material as aqueous ZIB cathode [71]. Such intercalation mechanism showed that the co-inserted water can facilitate Zn2+ diffusion through reducing effective charge and thus provide electrostatic shielding. Benefiting from the hydrated intercalation, V6O13 presented a reversible capacity of 360 mAh g−1 at 0.2 A g−1, high rate capability up to 24 A g−1 with a decent capacity of 145 mAh g−1, and satisfactory cycling stability with 92% capacity retention after 2000 cycles.
Orthorhombic-structured V3O7·H2O nanowires were also studied as the cathode for ZIBs. Wei et al. designed aqueous ZIBs that composed with V3O7·H2O/rGO composite as the cathode and Zn-coated rGO as the anode [72]. The reduced graphene boosts the conductivity of both cathode and anode materials, leading to excellent electrochemical performance. It can deliver a high power density of 8400 W kg−1 at energy density of 77 Wh kg−1.
MxVyOz Vanadate Cathode Materials
Enlarging the interlayer spacing via preintercalation of cations is an effective way to improve the electrochemical performance of electrodes for aqueous ZIBs. In recent years, vanadium-based nanowires intercalated with different cations (MxVyOz, M = H, Li, Na, K, Zn, Ca, Ag, Mn, etc.) have been widely studied. For example, Mai et al. designed the aqueous ZIBs based on H2V3O8 nanowire cathode. H2V3O8 nanowires can deliver a high capacity of 423.8 mAh g−1 at 0.1 A g−1, with capacity retention as high as 94.3% for over 1000 cycles [73]. The remarkable performance is owing to the layered structure of H2V3O8 with large interlayer spacing, which facilitates the transportation of zinc ions due to lower resistance and enables the intercalation/deintercalation of zinc ions with a slight change on structure. Moreover, Wang et al. further enhance the performance via designing H2V3O8 nanowires/GO composite [74]. The composite exhibits superior zinc-ion storage capability. At the current density of 1/3 °C, the composite shows a high capacity of 394 mAh g−1. At the high rate of 20 °C, the composite can deliver the capacity of 270 mAh g−1 (high power density of 2215 W kg−1) with a retention of 87% for over 2000 cycles, exhibiting remarkable performance at high rate. The excellent performance is owing to the synergistic merits of H2V3O8 nanowires and highly conductive graphene framework.
Lithium-ion intercalation is also an effective way to improve the electrochemical performance of V2O5. Liang et al. synthesized LixV2O5·nH2O by the chemical intercalation of Li+ into the interlayer of V2O5·nH2O through the combination of hydrothermal and annealing processes [75]. The Li+ ions in V2O5·nH2O enlarge its interlayer spacing, thus facilitating the Zn2+ ion diffusion. Therefore, compared with the poor performance of the V2O5·nH2O electrode without preintercalation of lithium ions, the Li+ intercalated V2O5·nH2O electrode can deliver a high initial capacity of 304 mAh g−1 at current density of 5 A g−1, with stable retained capacity of 232 mAh g−1 after 500 cycles. Another material, Li1+xV3O8 (LVO), is also an interesting candidate to facilitate the intercalation/deintercalation of multivalent ions with large radius owing to the layered-type structure and vanadium in high oxidation states. Kim and his co-workers first applied the layered-type LiV3O8 as cathode for ZIBs and studied the detailed phase evolution during the intercalation of Zn ions via simulation techniques, in situ XRD and electrochemistry analysis [76]. The results reveal that after the intercalation of zinc ions, ZnLiV3O8 phase gradually transferred to reversible solid–solution ZnyLiV3O8 (y > 1) phase. The unique phenomenon leads to improved electrochemical performance. At the current density of 133 mA g−1, it can deliver a high specific capacity of 172 mAh g−1 for over 65 cycles with 75% capacity retention and 100% Coulombic efficiency. Another similar material, K2V6O16·2.7H2O (KVO), was also investigated by Kim and his group members. They studied the K2V6O16·2.7H2O nanorods as cathode materials in ZIBs for the first time [77]. The K2V6O16·2.7H2O compound has a unique structural arrangement of V3O8 layers of VO6 and V2O8 units with interstitial hydrated K ions. Moreover, ex situ XRD and XPS results showed that the diffusion behavior of Zn2+ ions in and out of the layered KVO structure is dominated by the redox reaction of vanadium. At the specific power of 72 W kg−1, the KVO nanorods can deliver a reversible high specific energy of 172.1 Wh kg−1 with ~ 82% capacity retention for over 500 cycles, indicating high energy density and long-term cyclability.
Na0.33V2O5 is composed by quadruple octahedra chains, which are linked by double chains of square pyramids via sharing their corners [78]. Due to the limited space for intercalated Zn2+ ions, Na0.33V2O5 framework suffers a huge structural stress after the intercalation of Zn2+ ions. After the intercalation of zinc ions, a new ZnxNa0.33V2O5 phase appeared due to the crystal distortion with Zn2+ intercalation. Therefore, a large capacity fading for about 100 mAh g−1 was observed during the initial two cycles due to the “dead sites” for inserted zinc ions. Sodium ions in sodium vanadium oxides are believed to act as pillars to stabilize the framework to keep the reversible phase transform during cycling. In the most recent years, many researchers focused on the development of sodium vanadium oxides with stable ion storage properties. For example, Chen and his co-workers further studied the electrochemical mechanism of NaV3O8·1.5H2O by ex situ Fourier transform infrared spectroscopy and nuclear magnetic resonance [79]. Their results confirm that accompanying with insertion/extraction of zinc ion, simultaneous proton insertion/extraction also exists during the electrochemical processes, which is different from conventional rechargeable batteries. This unique co-intercalation behavior ensures the remarkable electrochemical performance. At the current density of 50 mA g−1, it can deliver a high reversible specific capacity of 380 mAh g−1 (corresponding to energy density of 300 Wh kg−1) with capacity retention of 82% for over 1000 cycles, showing excellent zinc storage capability.
The significant role of structured water in Na2V6O16 enhancing the electrochemical performances is further confirmed by Mai et al. [80] They designed a highly durable zinc-ion battery system with a Na2V6O16·1.63H2O nanowire cathode with an aqueous Zn(CF3SO3)2 electrolyte. Compared with only 17% of capacity retention of NaV3O8 nanowires at 5000 mA g−1 after 4000 cycles, the Na2V6O16·1.63H2O nanowires exhibit a capacity retention of 90% for over 6000 cycles at current density of 5000 mA g−1. Moreover, they assembled a single-nanowire-based zinc-ion battery to investigate the intrinsic Zn2+ storage mechanism at nanoscale (Fig. 5). The single-nanowire zinc-ion battery verifies the high electrical conductivity and current carrying capacity of Na2V6O16·1.63H2O. The layered structure of Na1.1V3O7.9@rGO is firstly employed as cathode for aqueous zinc-ion battery by Liang et al. [81]. The designed pilotaxitic Na1.1V3O7.9 nanoribbons/graphene composite shows improved performance. In addition, to confirm the structural stability of Na0.33V2O5 nanowires after long cycles, He et al. performed TEM characterization on the electrodes after cycling. Figure 5e, f shows the SAED pattern, HRTEM image of Na0.33V2O5 electrode after 100 cycles. The results confirm that the nanowire morphology and single crystalline structure are well maintained after long cycles. Another cation, Mn(II), can also act as robust structural pillars to stabilize the layered structure of hydrated vanadate [82]. Cao et al. reported manganese expanded hydrated vanadate as a cathode for ZIBs. The chemical insertion of Mn(II) cations can expand the interplanar spacing to 12.9 Å, leading to the reduced electrostatic interactions. In addition, the expanded interplanar spacing can facilitate fast intercalation of Zn ions at higher current densities, leading to significantly enhanced reversibility and cycling stability. At a specific current of 4 A g−1, manganese expanded hydrated vanadate can deliver a high specific capacity of 260 mAh g−1 for over 2000 cycles with a high capacity retention of 92%.
Fig. 5.
a Schematic view of single-nanowire ZIB, b the transport property, c the CV curves tested at different scan rates ranging from 50 to 500 mV s−1, d the specific capacitances at different scan rates [80], e the SAED pattern; the inset shows the TEM image and f HRTEM image of Na0.33V2O5 electrode after 100 cycles [78]. With permission from American Chemical Society and Wiley
Alshareef et al. first reported another new material, layered Ca0.24V2O5 bronze, as the cathode material for aqueous ZIBs [83]. The prepared Ca0.24V2O5 is very suitable as cathode material for stable aqueous Zn2+ ion storage. At 0.2 C, the Ca0.24V2O5 structure can deliver a high capacity of 340 mAh g−1. At power density of 53.4 W kg−1, the Zn/Ca0.24V2O5 cells show a remarkable energy density of 267 Wh kg−1 for over 5000 cycles, exhibiting very high energy density and long cycling stability. Also, the low cost of Ca, Zn, V enables Ca0.24V2O5 a viable cathode for aqueous ZIBs in large-scale applications. Another novel material, Ag0.4V2O5, was synthesized by Liang et al. and first applied as cathode for ZIBs. They studied the phase evolution after the discharging process and demonstrated the displacement/intercalation mechanism [84]. Specifically, after the initial discharging, most of Ag+ in Ag0.4V2O5 is replaced by Zn2+, forming Zn2(V3O8)2. The formed Zn2(V3O8)2 can accommodate more inserted Zn2+ ions. The generated highly conductive Ag0 matrix within the material can support high electronic conductivity. Therefore, the prepared Ag0.4V2O5 material exhibits good rate capability and long cycling stability. At the high rate of 20 A g−1, it can still provide the stable capacity of 144 mAh g−1 for over 4000 cycles.
A series of layered ammonium vanadates, including NH4V4O10, NH4V3O8, and (NH4)2V3O8 with corresponding interlayer distance of 0.98, 0.79, and 0.56 nm, respectively, were examined as cathode materials for aqueous ZIBs [85]. Owing to the largest interlayer spacing of 0.98 nm and intercalated NH4+ as strong “pillars,” NH4V4O10 showed high energy density of 374.3 Wh kg−1 with the power density of 9000 W kg−1, and negligible capacity loss over 1000 cycles at 10 A g−1. Notably, the cycling stability of NH4V4O10 cathode was tested under high (50 °C) and low temperature (0 °C) at 5 A g−1, exhibiting a high reversible capacity of 377 mAh/g at 50 °C and decent capacity of 179 mAh g−1 at 0 °C, indicating impressive electrochemical properties working in a wide temperature range.
Prussian Blue Materials
Prussian blue is a kind of prototype material with open framework structure, which contains zeolitic water and possesses special physical and chemical properties. In the typical Prussian blue material (KFe3+Fe2+(CN)6), the Fe3+ ions and Fe2+ ions are octahedrally connected with the nitrogen ends and carbon ends of the CN− groups, respectively. One half of the open sites in framework structure are occupied by K+ ions. With the intercalation of more K+ ions, some of the Fe3+ ions are reduced to Fe2+. The color changes from blue to colorless. The yielded product is called Everitt’s salt. With the K+ ions extracted from Prussian blue, Fe2+ ions are oxidized. The color turns to yellow, and the product is called Prussian yellow.
CuHCF and ZnHCF Cathode Materials
As a typical Prussian blue analog-based material (PBA), copper hexacyanoferrate (CuHCF) has a well-studied open framework. Mantia et al. first applied CuHCF as a positive electrode in aqueous ZIBs. They found that CuHCF can provide an extremely high average potential of 1.73 V. At a current density of 1 °C, CuHCF can supply 90% of the theoretical capacity for 100 cycles with retention of 96.3% [86]. The battery also shows a high rate capability, delivering 96.1, 90, and 78 mAh g−1 at current density of 2.5, 5, and 10 °C, respectively. Moreover, Wang et al. prepared CuHCF nanocubes for application as cathode electrode in aqueous ZIBs [87]. They found that the intercalation/deintercalation of Zn2+ ions were controlled by the solid-phase diffusion of Zn2+ in/out of the CuHCF electrode. Mantia et al. further studied the long-term cycling stability of CuHCF in kinds of zinc-ion electrolytes [88]. They discovered that the anions and the concentration of zinc ions played a significant role for the stability of the cathode electrode. Basically, the stability of CuHCF electrode is higher with the lower concentration of electrolyte. This is due to the phase transition of the CuHCF, instead of the dissolution of the active material.
Liu et al. carefully studied the effect of different insertion cations (Na+, K+, and Zn2+) on the electrochemical reaction of ZnHCF [89]. ZnHCF can exist stably in the condition of aqueous ZnSO4 electrolyte, while ZnHCF would be dissolved in aqueous Na2SO4/K2SO4 electrolytes. Meanwhile, they discovered that the intercalation potential was highly dependent on ionic radius. Specifically, larger ionic radii can lead to higher charging/discharging potential. The ZnHCF-based ZIBs show an extremely high average operation voltage of 1.7 V, leading to a high specific energy density of 100 Wh kg−1.
Other Cathode Materials
The large family of polyanionic materials has been widely studied as cathodes for monovalent metal ion batteries (Li+ and Na+ ion batteries), due to their high redox voltage, plenty of vacancies to accommodate the inserted metal ions, as well as stable framework that is favorable for long cycling performance. In 2018, Wang et al. first designed a Zn/LiV2(PO4)3 battery and verified that the robust polyanion crystal structure could also enable the reversible intercalation of Zn2+ ions (Fig. 6) [91]. They demonstrated that the intercalated Zn2+ ions can be delocalized by multiple atoms through the p–d hybridization between the V-d and O-p orbitals. The Zn/LiV2(PO4)3 batteries can deliver the high voltage of 1.7 V and support both high power density and high energy density for over 4000 cycles. Huang et al. first introduced Na3V2(PO4)3 as cathode material for ZIBs in 2016 [43, 91]. They fabricated Na3V2(PO4)3 with carbon nanosheets wrapped around as intercalation host for zinc cations. The Zn/Na3V2(PO4)3 batteries exhibited excellent rate capability and long cycling life. At a current density of 0.5 °C, Na3V2(PO4)3 can deliver the capacity of 97 mAh g−1 for over 100 cycles. They claimed an ion occupying variation mechanism for the intercalation of zinc ions in polyanion crystal structure, which is also confirmed by CV and XRD results. Moreover, Chen et al. studied the electrochemical reaction mechanism of both Li3V2(PO4)3 and Na3V2(PO4)3 as cathodes materials in ZIBs [92]. They found that the crystal structure of both Li3V2(PO4)3 and Na3V2(PO4)3 is very stable in zinc-ion electrolyte. They further studied the effects of the pH value of aqueous electrolyte on electrochemical performance. They discovered that aqueous electrolyte with weak acidic pH value (4.0–4.5) can support the optimized electrochemical performance. Jiang et al. further investigated the electrochemical performance of Na3V2(PO4)2F3 as cathode electrode in ZIBs [93]. To avoid the formation of dendrite on Zn anode, they coated the zinc metal with a thin carbon film. The designed Zn/Na3V2(PO4)2F3 batteries can support a high average potential (1.62 V), high energy density (97.5 Wh kg−1) and a long cycling life for over 4000 cycles with capacity retention of 95%.
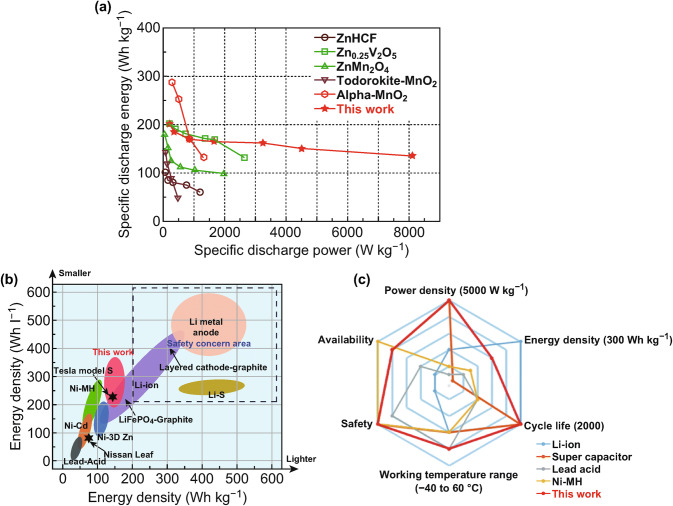
Fig. 6.
Comprehensive electrochemical performance for LiV2(PO4)3. a Comparison of LiV2(PO4)3 cathode with other researched cathode materials for ZIBs. b Gravimetric (Wh kg−1) and volumetric (Wh L−1) energy densities for different battery systems. c The spider chart for the itemized comparison of Zn/LiV2(PO4)3 cell with other commercial systems [90]. With permission from The Royal Society of Chemistry
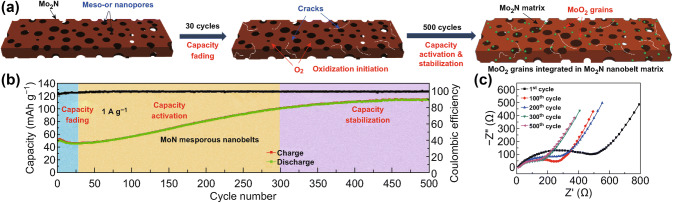
There have also been some studies on Mo-based electrode materials in ZIBs. For instance, MoO3 and MoS2 cathodes were found to deliver high initial discharge specific capacities up to 200 and 110 mAh g−1, respectively [94]. Inspired by the work of Mo-based electrodes in lithium-ion batteries, Xu et al. adopted electrochemical activation method to prepare the MoO2/Mo2N composite nanobelts [95]. They discovered that the MoO2 grains gradually in situ formed in the Mo2N matrix during the continually electrochemical activation cycling. The combination of MoO2 and Mo2N can overcome the intrinsic low conductivity and structural degradation of Mo-based materials (Fig. 7a). Specifically, the Mo2N matrix can protect the inner MoO2 grains from structural degradation during the intercalation of zinc ions. And the small MoO2 grains can accommodate more intercalated zinc ions. The synergic effects of MoO2 and Mo2N lead to significantly improved electrochemical performance. At the high rate of 1 A g−1, the formed MoO2/Mo2N composite nanobelts can still deliver a high discharge capacity of 113 mAh g−1 for over 1000 cycles, exhibiting excellent performance at high rate (Fig. 7b, c). Moreover, the electrochemical activation phenomenon was also observed in MnO-based ZIBs. As reported by Kang et al., the charging process leads to gradual formation of porous MnO2 nanosheets surrounding MnO particles [96]. The activated layered MnO2 nanosheets show significantly improved electrochemical performance. At a specific current of 0.1 A g−1, the specific capacity of the electrode can be enhanced to 330 mAh g−1.
Fig. 7.
a Schematic of formation of MoO2/Mo2N composite materials during the electrochemical activation process. b The capacity during the electrochemical activation cycling process. c The Nyquist plots at different cycling times [95]. With permission from Elsevier
Xu et al. further studied MoS2 with defects induced for application as cathode material in ZIBs with both experimental and theoretical methods [97]. They found that MoS2−x nanosheets with tremendous sulfur vacancies can accommodate preferential intercalation of Zn2+ ions, showing much higher capacity than the MoS2 without defects. Material characterization and theoretical modeling results revealed that interlayer sulfur vacancies and edge sites of MoS2 nanosheets can accommodate the intercalated Zn2+ ions, leading to a significantly enhanced capacity than MoS2 without defects. At the current density of 1 A g−1, the defect-engineered MoS2−x can deliver a high capacity of 88.6 mAh g−1 for over 1000 cycles (capacity retention of 87.8%). Subsequently, Zhi et al. prepared MoS2 with expanded interlayer spacing as promising cathode for flexible ZIBs [98]. They investigated the Zn2+ intercalation/deintercalation process via XRD and Raman techniques. At the current density of 0.1 A g−1, the E-MoS2 electrode can deliver a high specific capacity of 202.6 mAh g−1 for 600 cycles. Moreover, they further developed a quasi-solid Zn/E-MoS2 battery by using polyacrylamide (PAM) polymer as electrolyte. The quasi-solid Zn/E-MoS2 battery exhibits good performance under kinds of deformations, demonstrating potential to be applied in next-generation flexible devices. Recently, aqueous zinc–iodine batteries are emerging as an attractive electrochemical energy storage technology due to their low cost, abundant resource, and high energy density [99–102]. Similar to the widely studied lithium–sulfur batteries, zinc–iodine batteries have the similar problems of low reversibility and limited cycling, which is caused by the poor conductivity and soluble active species in the electrolytes. To address these issues, many efforts have been devoted to design iodine/carbon composite materials. For example, Pan et al. prepared microporous carbon with iodine encapsulated inside via solid–liquid conversion reactions [99]. The resulted zinc–iodine/C battery can provide a high capacity of 174.4 mAh g−1 at 1 C for over 3000 cycles. Yuan et al. designed the composite material with I2 confined in porous carbon cloth. With fully utilizing the active I2 material in the composite architecture, the zinc–iodine batteries yield a high energy density of ~ 151 Wh kg−1 with long cycling life for 1500 cycles [100].
Zinc-Ion Batteries based on Nonaqueous Electrolytes
Initially, there was research focused on using room temperature ionic liquid electrolytes (trifluoromethanesulfonic imide, trifluoromethanesulfonic imide, etc.) as electrolytes in zinc-ion batteries owing to their very low vapor pressure, extremely high chemical/physical stability, and high ionic transportation. However, the discharge capacities and cycling lifetime of batteries based on these electrolytes are not satisfactory. To achieve an ideal electrolyte with good electrode compatibility, much work concerning acetonitrile electrolytes has been carried out [103–106]. For example, Zn(ClO4)2 acetonitrile electrolyte was employed together with NiHCF as cathode in ZIBs [103]. At a rate of 0.2 °C, the battery can deliver a discharge capacity of 55.6 mAh g−1 with an average potential of 1.19 V. Gewirth et al. then explored a new series of spinels, ZnNixMnxCo2−2xO4, as cathode electrodes for ZIBs with organic electrolyte [104]. The full cells were fabricated with these spinels paired with zinc metal anode. At a specific current of 42 mA g−1, the batteries can supply a specific capacity of 174 mAh g−1 for 200 cycles, corresponding to an energy density as high as 305 Wh kg−1. The XRD and EDS results verified the reversible intercalation/deintercalation of Zn2+ ions in spinel materials. Meanwhile, the ex situ XPS results confirmed that the reversible conversion between Mn4+/Mn3+, Ni4+/Ni3+/Ni2+, Co4+/Co3+ oxidation states took place in spinel cathodes during the electrochemical process. All the results indicate that co-substitution of Mn and Ni for Co in ZnCo2O4 is an efficient way to promote the intercalation of zinc ions and improve the capacity. As such, the acetonitrile–Zn(CF3SO3)2 electrolyte shows a high anodic stability and relatively low overpotential, leading to an excellent Coulombic efficiency of the Zn anode, while V-based materials usually show low specific capacity and poor rate performance in organic electrolytes [106]. This is because the insertion of Zn ions needs a desolvation penalty at the electrode/electrolyte interface. In the water-based solution, the co-intercalation of water molecules can effectively facilitate the insertion of Zn ions and the penalty is very low. However, the co-intercalation with Zn2+ ions in nonaqueous solutions is difficult to be realized due to large radius of the solvation molecules, which is the main reason that ZIBs consisting of nonaqueous electrolytes often show poor kinetics.
Summarization of Representative Cathode Materials
Table 1 summarizes recent developments of some representative cathode materials, including the electrolyte components, testing voltage and current, discharge capacity, and cycling life. And Fig. 8 displays the specific capacity versus the discharge potential for various cathodes. As we can see in Table 1 and Fig. 8, manganese oxides show high operation voltages and acceptable rate capabilities. However, manganese oxides suffer from limited cycling life, due to the dissolution of Mn2+ ions during cycling caused by the Jahn–Teller effect during the phase transformation with the intercalation of zinc ions. Mn2+ additives in the electrolyte can hinder the dissolution of MnO2 electrodes. But appropriate concentration still needs to be examined to balance the Mn2+ dissolution and the re-oxidation. Compared with manganese oxides, vanadium oxides exhibit much lower discharge potential, but show enhanced rate performance and prolonged cycling life. The stable layered framework and structural water molecules in the V-based cathodes facilitate fast diffusion of zinc ions, leading to their high rate capability and long cycling life. However, the average operation voltage of vanadium oxides is only about 0.8 V in aqueous ZIBs, which seriously restricts its practical applications. The discharge potential of vanadium oxides can be increased by introducing polyanions or fluorines. For example, M3V2(PO4)3 (M = Li, Na) and Na3V2(PO4)2F3 show much higher discharge potential of about 1.5 V. However, these additional groups lead to increased molecular mass and decreased specific capacities (only 113.5 mAh g−1 for M3V2(PO4)3 (M = Li, Na), and 50 mAh g−1 for Na3V2(PO4)2F3). Therefore, further explorations should be focused on enhancing both the operating voltage and specific capacity for vanadium-based cathodes. Compared with manganese oxides and vanadium-based cathodes, the electrochemical performance of PBAs is poor. Even though the PBAs can support a high average operation voltage up to 1.5 V [89], they suffer low specific capacities (50–80 mAh g−1) and limited cycling life (~ 200 cycles). The poor performance of PBAs is attributed to the randomly distributed Fe(CN)6 vacancies that can break the electronic conduction between Fe–CN–M bonds and thus result in poor rate capability. Therefore, it is important to reduce the lattice defects to improve the performance. Moreover, herein we also summarize the ZIBs with aqueous and nonaqueous electrolytes. The anions and solvents in the electrolytes are significant for the diffusion of charge carriers and stabilizing electrode materials. ZnSO4 and Zn(CF3SO3)2 solutions are commonly used as electrolytes in aqueous ZIBs due to their excellent electrochemical performances. However, the acidic condition may damage the long-term stability of the Zn metal anode. Compared to ZIBs with aqueous electrolytes, ZIBs with organic electrolytes exhibit higher operation voltages and moderate discharge capacities, but they show much poorer rate performance and limited cycling life, which probably attributes to faster ionic diffusion rate, and higher reversibility of metal deposition/dissolution in mild aqueous electrolytes.
Table 1.
Summary of electrochemical properties of cathode materials for ZIBs
| Cathode | Electrolyte | Operating voltage (V) | Current rate | Capacity (mAh g−1) | Cycle performance | Refs. |
|---|---|---|---|---|---|---|
| α-MnO2 nanorods | Aqueous Zn(CF3SO3)2 | 0.8–1.8 | 5 A/g | 115.9 | 97.7% retention after 4000 cycles | [35] |
| α-MnO2/rGO | Aqueous ZnSO4 | 1.0–1.9 | 3 A/g | 145.3 | 94% retention after 3000 cycles | [40] |
| γ-MnO2 | Aqueous ZnSO4 | 1.0–1.8 | 0.5 mA/cm2 | 158 mAh/cm2 | 37% retention after 40 cycles | [58] |
| α-MnO2 nanofibers | Aqueous ZnSO4 | 1.0–1.85 | 1520 mA/g | 160 | 92% retention after 5000 cycles | [60] |
| MnO2 | Aqueous ZnSO4 | 1.0–1.8 | 1885 mA/g | 50–70 | 10,000 cycles | [61] |
| PANI-intercalated MnO2 | Aqueous ZnSO4 | 1.0–1.8 | 2 A/g | 125 | 5000 cycles | [63] |
| V2O5 | Aqueous Zn(CF3SO3)2 | 0.2–1.6 | 5 A/g | 372 | 91.1% retention after 4000 cycles | [65] |
| Bilayer V2O5·nH2O | aqueous Zn(CF3SO3)2 | 0.2–1.6 | 6 A/g | ~ 200 | 71% retention after 900 cycles | [66] |
| Zn0.25V2O5·nH2O nanobelts | Aqueous ZnSO4 | 0.5–1.4 | 2400 mA/g | 260 | 80% retention after 1000 cycles | [67] |
| VO2 (B) | Aqueous Zn(CF3SO3)2 | 0.3–1.5 | 100 mA/g | 357 | 50 cycles | [68] |
| VO2 | Aqueous Zn(CF3SO3)2 | 0.7–1.7 | 10 A/g | 133 | 79% retention after 10,000 cycles | [69] |
| V6O13·nH2O | Aqueous Zn(CF3SO3)2 | 0.2–1.4 | 5 A/g | ~ 150 | 1000 cycles | [70] |
| V6O13 | Aqueous Zn(CF3SO3)2 | 0.2–1.5 | 4 A/g | ~ 240 | 92% retention after 2000 cycles | [71] |
| V3O7·H2O/rGO | Aqueous ZnSO4 | 0.3–1.5 | 1500 mA/g | 245 | 79% retention after 1000 cycles | [72] |
| VO2/rGO | Aqueous Zn(CF3SO3)2 | 0.3–1.3 | 4 A/g | 240 | 99% retention after 1000 cycles | [42] |
| VS2 flake | Aqueous ZnSO4 | 0.4–1.0 | 200 mA/g | 125 | 99.7% retention after 250 cycles | [47] |
| H2V3O8 nanowires | Aqueous Zn(CF3SO3)2 | 0.2–1.6 | 5 A/g | 173.6 | 94.3% retention after 1000 cycles | [73] |
| H2V3O8 nanowires/GO | Aqueous Zn(CF3SO3)2 | 0.2–1.6 | 6 A/g | 270 | 87% retention after 2000 cycles | [74] |
| Li+ intercalated V2O5·nH2O | Aqueous ZnSO4 | 0.4–1.4 | 10 A/g | 192 | 1000 cycles | [75] |
| LiV3O8 | Aqueous ZnSO4 | 0.6–1.2 | 133 mA/g | ~140 | 65 cycles | [76] |
| K2V6O16·2.7H2O nanorod | Aqueous Zn(CF3SO3)2 | 0.4–1.4 | 6 A/g | 188 | 82% retention after 500 cycles | [77] |
| Na0.33V2O5 | Aqueous Zn(CF3SO3)2 | 0.2–1.6 | 1.0 A/g | 218.4 | 93% retention after 1000 cycles | [78] |
| NaV3O8 | Aqueous ZnSO4 | 0.3–1.25 | 4 A/g | 165 | 82% retention after 1000 cycles | [79] |
| Na2V6O16·1.63H2O | Aqueous Zn(CF3SO3)2 | 0.2–1.6 | 5 A/g | 158 | 90% retention after 6000 cycles | [80] |
| Na1.1V3O7.9@rGO | Aqueous Zn(CF3SO3)2 | 0.4–1.4 | 300 mA/g | 171 | 100 cycles | [81] |
| Ca0.25V2O5·nH2O | Aqueous ZnSO4 | 0.6–1.6 | ~ 20 A/g | ~ 70 | 96% retention after 3000 cycles | [83] |
| Ag0.4V2O5 | Aqueous ZnSO4 | 0.4–1.4 | 20 A/g | 144 | 4000 cycles | [84] |
| NH4V4O10 | Aqueous ZnSO4 | 0.4–1.4 | 10 A/g | 255.5 | 1000 cycles | [85] |
| ZnHCF | Aqueous ZnSO4 | 0.8–1.9 | 300 mA/g | 68 | 85% retention after 200 cycles | [41] |
| CuHCF | Aqueous ZnSO4 | 0.45–1.4 | 60 mA/g | ~50 | 100 cycles | [86] |
| CuHCF | Aqueous ZnSO4 | 0.2–1.1 | 10 C | ~40 | ~80% retention after 1000 cycles | [88] |
| ZnHCF | Aqueous ZnSO4 | 0.8–1.9 | 300 mA/g | 52.5 | 81% retention after 100 cycles | [89] |
| LiV2(PO4)3 | Aqueous Zn(OTf)2 | 0.2–1.9 | 1500 mA/g | ~110 | 78.8% retention after 4000 cycles | [90] |
| Na3V2(PO4)3/C cathode | CH3COOLi + Zn(CH3COO)2 | 0.8–1.7 | 50 mA/g | 84 | 68% retention after 200 cycles | [91] |
| M3V2(PO4)3/Zinc (M = Li, Na) | Li2SO4–ZnSO4 aqueous electrolyte | 0.7–2.1 | 0.2 °C | 113.5 | 84.1% retention after 200 cycles | [92] |
| Na3V2(PO4)2F3 | Aqueous Zn(CF3SO3)2 | 0.8–1.9 | 1 A/g | 50 | 4000 cycles | [93] |
| Na3V2(PO4)3/C | Aqueous Zn(CF3SO3)2 | 0.8–1.7 | 50 mA/g | 97 | 74% retention after 100 cycles | [43] |
| MoO2/Mo2N nanobelts | Aqueous Zn(CF3SO3)2 | 0.25–1.35 | 1 A/g | 113 | 78.8% retention after 1000 cycles | [95] |
| MoS2 | Aqueous Zn(CF3SO3)2 | 0.25–1.25 | 1 A/g | 88.6 | 87.8% retention after 1000 cycles | [97] |
| MoS2 | Aqueous ZnSO4 | 0.3–1.5 | 1 A/g | 161.7 | 97.7% retention after 500 cycles | [98] |
| Quinones | Aqueous Zn(CF3SO3)2 | 0.2–1.8 | 500 mA/g | About 120 | 87% retention after 1000 cycles | [44] |
| Polyaniline | Aqueous Zn(CF3SO3)2 | 0.5–1.5 | 5 A/g | 82 | 92% retention after 3000 cycles | [52] |
| NiHCF | Zn(ClO4)2 acetonitrile electrolyte | 0.7–1.8 | 11.2 mA/g | ~56 | 20 cycles | [103] |
| ZnNixMnxCo2–2xO4 Spinel | Zn(OTf)2 in MeCN | 0.8–2.15 | 42 mA/g | 180 | 81.66% retention after 200 cycles | [104] |
| ZnAl0.67Co1.33O4 | Zn(OTf)2 in MeCN | 1.4–2.2 | 32 mA/g | 134 | 85.1% retention after 100 cycles | [105] |
| V3O7·H2O nanobelts | ZnOTf) in acetonitrile | 0.5–1.8 | 3000 mA/g | ~ 270 | 80% retention after 200 cycles | [106] |
Fig. 8.
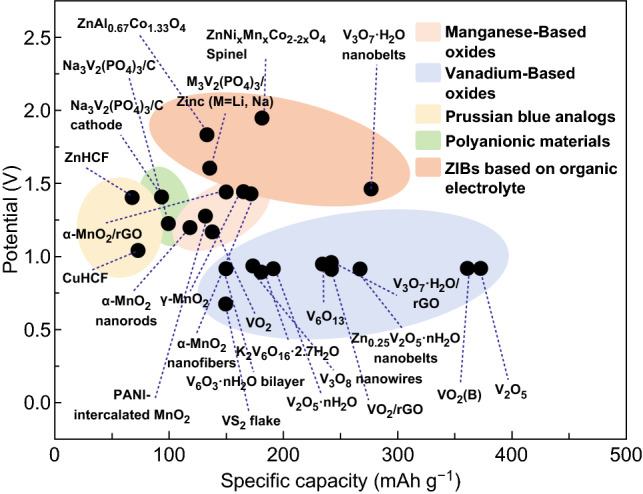
Specific capacity versus discharge potential of various cathode materials for ZIBs
Solid-state Zinc-Ion Batteries
The ever-increasing demands for portable and wearable electronics have stimulated research interests in flexible rechargeable batteries with excellent electrochemical performances and low cost. As a promising new energy storage system, solid-state zinc-ion batteries (SZIBs) exhibit a series of noticeable advantages, such as high safety without electrolyte leakage, good flexibility, and low cost. Though much work has been done on zinc-ion batteries with liquid electrolytes, progress on SZIBs is still limited due to the lack of high zinc-ion conducting solid-state electrolytes. Therefore, it is imperative to explore the physicochemical properties of suitable solid-state electrolytes and the zinc-ion migration mechanism inside the solid-state electrolytes, which may provide more insight into the realizations of practical SZIBs [30].
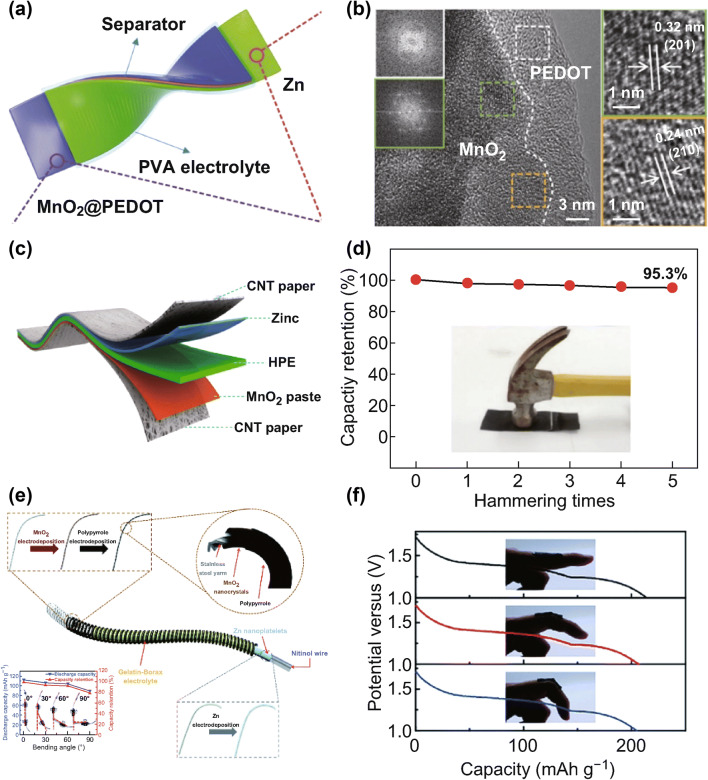
In 2017, Tong et al. first developed flexible quasi-solid-state Zn–MnO2/PEDOT battery with high performance via using PVA/ZnCl2/MnSO4 gel electrolyte and MnO2/PEDOT cathode (Fig. 9a, b) [107]. The as-fabricated battery exhibits a high capacity of 282.4 mAh g−1 at 0.37 A g−1, durable cycling up to 300 cycles with capacity retention of 77.7%, and a decent energy density of 504.9 Wh kg−1 with a power density of 8.6 kW kg−1. Moreover, this flexible battery can maintain similar discharge properties and show no capacity loss under bending or twisting, demonstrating excellent mechanical strength. However, it should be noted that the quasi-solid-state battery usually presents relatively lower rate capability compared to the aqueous system, attributed to the low ionic conductivity and high charge transfer resistance of the PVA/ZnCl2/MnSO4 gel electrolyte.
Fig. 9.
a Schematic showing the flexible quasi-solid-state Zn-MnO2@PEDOT batteries. b HRTEM images of MnO2@PEDOT sample [107]. c Schematic illustration of the structure of the solid-state ZIB and d the hammering test [108]. e Schematic illustration of the structure of the wire battery, inset showing the charging–discharging curves corresponding to the wire batteries bending at different angles [109]. f Galvanostatic discharge curves of Zn–MnO2 batteries bending at different curvatures [110]. With permission from The Royal Society of Chemistry
Zhi et al. also developed a wearable solid-state zinc-ion battery with high safety via utilizing novel gelatin–PAM-based solid-state polymer electrolyte with ZnSO4 and MnSO4, α-MnO2@CNT cathode, and flexible Zn foil anode (Fig. 9c) [108]. The solid-state polymer electrolyte was prepared by filling gelatin–PAM chains in the PAN fiber network. Such highly porous interconnected framework of the solid-state polymer electrolyte displays an extremely high ionic conductivity of 1.762 × 10−2 S cm−1 and desirable mechanical strength of 7.76 MPa. Benefiting from the solid-state electrolyte with high ionic conductivity, the zinc-ion battery cell exhibits an impressive reversible capacity of 306 mAh g−1 at a specific current of 61.6 mA g−1 and decent capacity retention of 97% up to 1000 cycles at 2772 mA g−1. Even under various severe conditions such as bending, hammering (Fig. 9d), cutting, washing, combustion, weight loading, sewing, and drilling, the designed solid-state zinc-ion batteries show desirable electrochemical behavior and high stability, demonstrating great potential as new generation energy storage system to power flexible and wearable electronics.
Zhi et al. further designed a smart wire-shaped flexible zinc-ion battery with shape memory function. The designed batteries show improved electrochemical performances with unique shape memory function by utilizing the temperature-initiated shape memory effect (Fig. 9e) [109]. The gel polymer electrolyte is fabricated by employing gelatin and borax in aqueous solution of ZnSO4/MnSO4, and it presents higher ionic conductivity of 2.0 × 10−2 S cm−1 compared to the bare gelatin gel electrolyte with 1.39 × 10−2 S cm−1. Owing to the introduction of borax as a cross-linker into the gelatin gel electrolyte, the ionic conductivity, water retention capability, and mechanical strength of the gelatin–borax gel electrolyte are effectively improved, thereby enhancing the electrochemical performances of the zinc-ion battery. Such flexible wire battery exhibits a specific capacity of 174.2 mAh g−1 at 0.5 °C and durable cycling performance up to 1000 cycles with corresponding Coulombic efficiencies over 99%. Additionally, its electrochemical performance can be well preserved when bent to 90° or consecutively bent to 45° for over 500 cycles, indicating its superior resistance against mechanical deformation.
Searching for a stable sulfate-tolerant polymer electrolyte without polymer precipitation, Li et al. explored a xanthan biopolymer in combination with aqueous ZnSO4/MnSO4 solution to fabricate a very stable gum bio-electrolyte, which was then assembled in a rechargeable Zn/MnO2 battery for electrochemical characterizations [110]. Owing to its favorable molecular structure composed of α,β-1,4-linked glucan backbone with trisaccharide side chains connected with backbone residues by α-1,3 linkages, the xanthan gum shows high salt tolerance stability. The gum bio-electrolyte is highly conductive (1.46 × 10−2 S cm−1 at room temperature and 2.5 × 10−3 S cm−1 at − 8 °C), and its ionic conductivity remains unchanged even after 1-year storage, indicating its ability of working in a wide temperature range for the long term. As a result, this battery presented a specific capacity of 260 mAh g−1 at 1 °C, high rate capability, good cycling performance with 90% capacity retention after 330 cycles at 1 °C, and even maintained a decent capacity of 127 mAh g−1 up to 1000 cycles at 5 °C. Moreover, the battery cell exhibits outstanding durability under bending and twisting (Fig. 9f) and effectively prohibits the zinc dendrite growth during continuous charge–discharge cycles, suggesting that the gum bio-electrolyte is a very promising candidate for flexible energy storage systems.
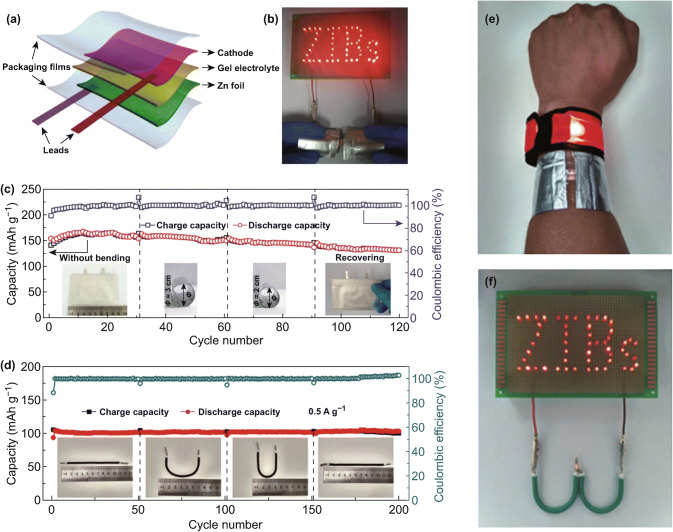
Inspired by Zhi et al.’s work above, Chen et al. investigated a Zn/NaV3O8·1.5H2O (NVO) cell by using a quasi-solid-state electrolyte that consists of gelatin and aqueous ZnSO4 solution (Fig. 10a) [50]. Although the preparation method is simple, the gel electrolyte shows degraded ionic conductivity compared to the corresponding aqueous part. Even so, the flexible quasi-solid-state Zn/NVO battery still delivers a good rate capability of 288, 228, 160, 115, and 80 mAh g−1 at 0.1, 0.2, 0.5, 1, and 2 A g−1, respectively. Furthermore, such flexible energy storage system can maintain the capacities under various bending states during charge–discharge process with only a slight capacity loss, indicating high stability of the quasi-solid-state cell (Fig. 10c). In addition, two quasi-solid-state Zn/NVO cells under bending condition in series can light up the LED array of 52 bulbs, suggesting that they can be great candidates for practical flexible energy storage devices, as shown in Fig. 10b.
Fig. 10.
a Schematic diagram of a quasi-solid-state Zn/NVO battery [50], b LED array with 52 bulbs powered by two quasi-solid-state Zn/NVO battery under bending condition, c cycling performance under various bending states at 0.5 A g−1 of the flexible quasi-solid-state Zn/NVO battery. d cycling performance of soft-packaged and cable-type quasi-solid-state batteries under various bending states at 0.5 A g−1. e A wrist strap powered by two soft-packaged quasi-solid-state batteries in series, f an LED array powered by two cable-type quasi-solid-state batteries in series [52]. With permission from Elsevier and The Royal Society of Chemistry
Chen et al. also developed a flexible quasi-solid-state batteries by using polyaniline (PANI) as cathode, PVA/Zn(CF3SO3)2 gel as electrolyte, and Zn metal as anode (Fig. 10d–f) [52]. Such soft-packaged quasi-solid-state Zn/PANI battery delivers a reversible capacity 109 mAh g−1 in the first cycle at 0.5 A g−1 and 91.7% capacity can be retained after 200 cycles, while the cable-type battery displays a capacity of 106 mAh g−1 for the first discharge process at 0.5 A g−1 and 91.5% capacity retention can be achieved over 200 cycles (Fig. 10d). The highly stable electrochemical performances of both soft-packaged and cable-type Zn/PANI batteries under different bending states hold great promise for flexible electronic applications. Moreover, two soft-packaged and two cable-type flexible cells can successfully light up a wristwatch and an LED array under bending condition, respectively (Fig. 10e, f).
There has been another report concerning quasi-solid-state zinc-ion battery with high rate capability using a layered zinc orthovanadate array as cathode, a Zn array as anode, and a gel electrolyte composed of fumed silica with aqueous ZnSO4 solution [111]. This battery displays superior electrochemical behaviors and ultra-stable flexibility. Specifically, the flexible cell exhibits a highly reversible capacity of 204 mAh g−1 with an initial high Coulombic efficiency of 95% at 0.5 °C, corresponding to the two-electron transfer process. Due to the nanoarray structure in both electrodes providing short fast ion migration pathways and introduced insertion pesudocapacitance, the quasi-solid-state battery delivers an excellent rate capability of 160 mAh g−1 at 10 °C and 101 mAh g−1 even at 50 °C. Furthermore, the quasi-solid-state ZIB shows long-term cycling stability for 2000 cycles at 20 °C and considerable energy density of 115 Wh kg−1 with a power density of 5.1 kW kg−1 based on the total masses of cathode, anode, and current collectors. Additionally, the battery reveals no obvious capacity fading with over 96% capacity retention under continuous 100 bending cycles, showing its high tolerance of bending without deterioration of the discharge performance.
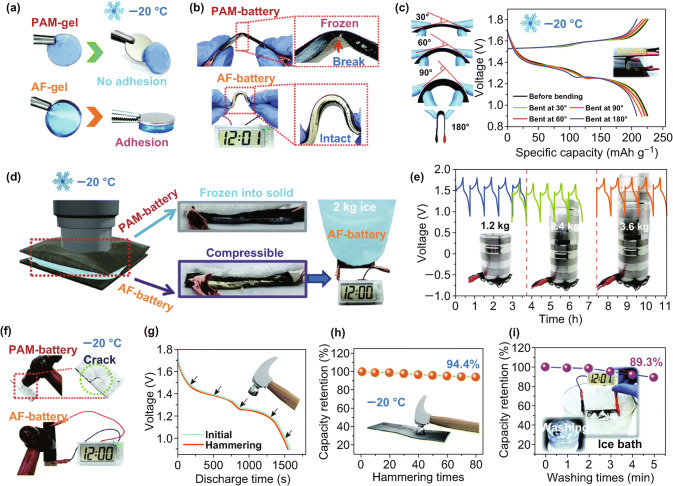
Recently, Zhi et al. obtained a flexible self-healing solid-state aqueous rechargeable NiCo/Zn battery by employing NiCo hydroxide as cathode, flexible Zn/carbon cloth as anode, and Fe3+-cross-linking sodium polyacrylate (PANa-Fe3+) hydrogel as solid-state electrolyte [112]. This self-healing electrolyte displays enhanced healing property and good ionic conductivity owing to the Fe3+ cross-linkers forming ionic bonds between the PANa chains, as well as the favorable compatibility of PANa-Fe3+ with aqueous solution of Zn(CH3COO)2 + KOH. Hence, the assembled NiCo/Zn batteries with such intrinsically self-healing PANa-Fe3+ electrolytes show autonomically self-healing ability with over 87% capacity retention after four cycles of cutting/healing. When the two broken parts of the cell were brought to connect, the watch was on again without weakening the brightness, indicating excellent healing performance and high potential applications in broken electronics. Moreover, a flexible Zn/MnO2 battery with exceptionally electrochemical performances has been achieved and can operate at subzero temperatures (Fig. 11) [113]. Hydrogel electrolytes are attractive for flexible ZIBs by virtue of their safety and high physical/chemical stability. However, the freezing issue of hydrogel electrolytes under subzero temperatures would lead to severe capacity fading and elasticity degradation, in addition to loss of mechanical robustness and flexibility under cold condition. In this work, a strong hydrogen bonding with water anchored the water units within the polymer electrolyte was designed, rendering superior anti-freezing property even at − 20 °C. The constructed Zn/MnO2 flexible battery maintained high electrochemical performances, impressive durability, and flexibility even being compressed, bent, or washed in an ice bath at − 20 °C (Fig. 11e–i). Such an excellent polymer electrolyte allows flexible ZIBs to be used under extreme conditions, such as aerospace or submarines.
Fig. 11. a.
Comparisons of the adhesion force of the PAM gel and AF gel at − 20 °C. b Bending test of the PAM battery and AF battery at − 20 °C. c Charge–discharge profile of the AF battery under bending test at 0.2 A g−1. d PAM battery and AF battery holding an ice block; the AF battery can still power an electrical watch at − 20 °C. e Cycling test of the AF battery under various heavy loads at − 20 °C. f Hammering tests of the PAM battery and AF battery after 1 day of cooling at − 20 °C. g Discharge curves and h cycling test of the AF battery under hammering tests. i Capacity retention of the AF battery washing in an ice bath [113]. With permission from The Royal Society of Chemistry
Design of Zinc Anodes and Separators
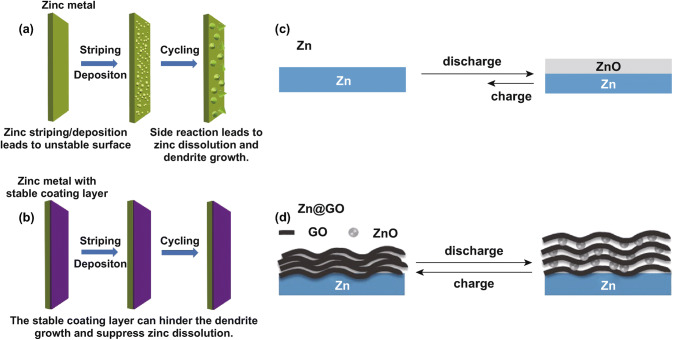
Most studies in ZIBs employ commercial zinc foil as the anode for investigation of ZIBs. Although zinc metal is considered as a promising anode for aqueous ZIBs owing to its intrinsic safety, low toxicity, and high theoretical capacity, metallic zinc inevitably suffers from passivation, irreversibility, corrosion, and growth of dendrite during plating/stripping. Especially in alkaline aqueous electrolytes, passivation problems caused by the formation of ZnO layer can limit the surface contact between electrolyte and zinc anode and seriously reduce the conductivity of the anode [114, 115]. Moreover, the growth of zinc dendrites can continuously consume water during cycling and irreversibly produce by-products (Zn(OH)2−4, etc.), leading to very low Coulombic efficiency, poor capacity, limited cycling life of ZIBs. Zinc dendrite still tends to form especially at high current densities. It is believed that the formation of zinc dendrite is due to the uneven Zn2+ distribution on flat metallic Zn foil. The sharp end of dendrite tips can act as a charge center in the electric field, supporting further growth of dendrites [54]. Moreover, with the growth of zinc dendrite, the surface area of zinc anode increases, while the corrosion and other surface-dependent reactions increase with the larger surface area of zinc anode, leading to continuously consuming of zinc anode and a faster fading of the battery performance. To overcome these problems, many efforts have been devoted to designing the composite nanostructure of the Zn metal anode, adding additives in electrolyte, or changing the concentration of zinc salts in electrolyte.
Composite nanostructure design is deemed as an effective strategy to restrict dendrite growth, suppress the formation of by-products, and constrain the corrosion of zinc anode [116]. For example, Yan et al. designed lasagna-like nanostructured Zn anode, in which ZnO nanoparticles are wrapped with graphene oxide nanosheets [117]. The composite anode can provide much higher volumetric capacity and capacity retention when compared with pure ZnO. Moreover, Wang et al. deposited zinc on conductive graphite felt via electrodeposition method [118]. The graphite felt substrate can facilitate fast electron transport and efficient zinc plating in various specific directions, leading to enhanced cycling stability.
In addition to the above composite nanostructure design of Zn anodes, adding additives in electrolyte is also an important strategy to constrain the corrosion and dendrite formation of Zn metal anode. For instance, as reported by Mantia et al., the electrolyte’s additive can decrease the grain growth rate and thus affect the morphology of zinc electrodeposition and suppress the formation of dendrites [119]. Unfortunately, at the same time, it can also decrease the kinetics of reaction and cycling stability of ZIBs due to induced higher overpotential. Mantia and his co-workers studied the effect of branched polyethyleneimine (BPEI) as an electrolyte’s additive [119]. They found that the introduction of BPEI can adjust the morphology of the deposited Zn and decrease the size of the crystals, leading to the improved efficiency and stability. Moreover, Chen and his co-workers found that the addition of Na2SO4 in ZnSO4 aqueous electrolyte can effectively hinder the growth of Zn dendrites [79]. This is because sodium ions with a lower reduction potential can form a positively charged electrostatic shield on Zn ions, avoiding the growth of Zn dendrites. In addition, Chen’s group systemically studied the additives in gel electrolyte [120]. They first applied fumed silica as additive in gel electrolyte and found that the fumed silica can significantly reduce dendrite formation. However, the adding of fumed silica can also increase the corrosion on Zn anode. Therefore, they further introduce pyrazole in electrolyte and found that the pyrazole can serve as a good additive to inhibit both zinc corrosion and dendrite growth. The optimized composition 5% fumed silica + 0.2% pyrazole finally exhibits improved performance with enhanced open-circuit voltage, rate capability, and cycling stability.
Highly concentrated neutral Zn-ion electrolyte is another effective method to suppress the dendrite growth. To completely eliminate the formation of Zn dendrites, Wang et al. developed a unique aqueous electrolyte at high concentrations composed of 1 M Zn(TFSI)2 and 20 M LiTFSI, proved to achieve 100% Coulombic efficiency and no dendrite formation during Zn plating/stripping [121]. In addition, Chen and his co-workers developed Zn/VOPO4 batteries using a water-in-salt electrolyte to realize reversible oxygen redox chemistry in a high voltage region [122]. Since Zn metal anodes show high corrosion current and low positive corrosion potential in 1 M Zn(Tr)2 electrolyte, they applied highly concentrated 21 M LiTFSI/1 M Zn(Tr)2 water-in-salt electrolyte in ZIBs, which can suppress the corrosion of zinc and also constrain the dissolution of VOPO4 cathode due to the limited activity of water. The water-in-salt electrolyte facilitates the reversible oxygen redox reaction in Zn/VOPO4 batteries, leading to increased discharge potential to 1.56 V and significantly improved power density from 160 to 217 Wh kg−1, which is comparable with lithium-ion batteries. This work of water-in-salt electrolyte is scientifically enlightening for future research. However, the electrolyte with high Zn and Li salts is quite expensive and largely hinders its potential for grid-scale production.
It is known that the passivation and dendrite growth on zinc anode surface could be reduced in acidic electrolyte [61]. However, acidic electrolytes will cause the corrosion on Zn anode, seriously affecting long-term cyclability. Surface coating on zinc metal foil is an effective way to alleviate the corrosion of zinc anode and thus improve the performance. As shown in Fig. 12a, b, stable coating layer on zinc anode can slow down zinc dissolution and dendrite growth. For example, Zhao et al. applied atomic layer deposition method to coat the zinc metal plate with thin TiO2 layer to prevent corrosion during electrochemical reactions [123]. They demonstrated that TiO2 layer can effectively protect the inner zinc from corrosion and suppress Zn(OH)2 by-product formation, leading to improved capacity retention and prolonged cycling life. In addition, Zhi et al. coated Zn anode with a uniform porous nano-CaCO3 layer to enhance the electrostripping/electroplating stability [124]. The porous uniform coatings can guide the Zn plating reaction on the entire Zn anode surface, effectively avoiding the corrosion and the growth of large protrusions/dendrites. Liu et al. coated zinc metal anode with graphene oxide (GO) nanosheets via casting method (Fig. 12c, d) [125]. The GO nanosheets can not only prevent the Zn intermediates from dissolving into aqueous electrolyte, but also improve the surface electron conductivity, leading to the prolonged cycling life and rate capability. Moreover, synthesis of zinc anode with organic additives is another strategy to reduce corrosion. For example, Chen et al. prepared novel zinc anode via electroplating with various organic additives [126]. They found that organic additives can change the surface crystallographic orientation, surface density, and texture of the produced zinc anode. Based on the linear polarization results, compared with the commercial pure zinc metal, the corrosion rate of the prepared Zn-SDS is 30 times lower, showing significantly improved corrosion tolerance. However, there are still very few studies on mechanisms of suppressing corrosion and passivation of zinc anode by now. Therefore, more research efforts should be made to manipulate the Zn2+ chemistry based on the Zn anode to overcome the above-mentioned corrosion and dendrite growth problems.
Fig. 12.
Schematic illustration of the zinc metal anode with stable coating layer. a Plating and stripping of zinc ions lead to unstable surface. Side reaction during continuous cycling can lead to passivation and dendrite growth. b A thin coating layer leads to a stable deposition/stripping process, hindering dendrite growth, and by-product formation. Schematic of morphological changes about c the formation of ZnO passivation layer on Zn anode during charging and discharging. d GO on Zn surface can suppress the formation of ZnO passivation and slow down the dissolution of Zn metal [124]. With permission from Elsevier
Separator is also critical in ZIBs for practical applications, serving to prevent short circuit by separating the cathode and anode. Currently, most ZIB studies employ filter paper or glass fiber membrane as separators in the battery cells. The systematic explorations on the separator materials are still missing, and it would be helpful to have more research in this regard to realize large-scale utilization of ZIBs. Recently, Liu et al. have constructed a cross-linked polyacrylonitrile-based membrane that is mechanically robust and cost effective, for application as the separator in rechargeable ZIBs [127]. Based on the synthetic merits, Zn/Zn symmetric cells based on this separator exhibit superior long cyclability of over 350 cycles with efficient dendrite growth suppression and lower polarization, which provides an important solution for future design of separators.
Conclusions
In summary, we provide a timely review on the recent developments in zinc-ion batteries, including the cathode materials, liquid-based electrolytes, and solid-state electrolytes. The preparation methods of the electrode materials, fundamental electrochemical reaction mechanisms, corresponding battery performances, and their correlations are discussed in detail. Continuous efforts are being made for exploring a vast variety of cathode materials. However, there remain many essential electrochemical details that are yet to clarify. For example, the electrochemical reaction mechanisms of Mn-based cathodes in ZIBs are still disputable, with three different mechanisms of Zn2+ migration, proton migration, and Zn2+/H+ co-migration being reported. Moreover, the combination of valence state change of manganese ion during cations insertion and corresponding typical voltage plateaus still need to be examined carefully, which may shed more light on the electrochemical mechanisms and thus provide effective guidance for constructing Mn-based electrode/Zn batteries with improved performances. Compared to the Mn-based electrodes, V-based cathodes display a higher specific capacity and longer cycling performances, but the lower discharge plateaus ~ 0.7 V is a distinct drawback. In addition, V-based materials may continuously dissolve in aqueous acidic Zn2+ electrolyte (ZnSO4 or Zn(CF3SO3)2 electrolyte). Therefore, developing effective strategies to prevent the vanadium dissolution is necessary, such as adjustment of the electrolyte pH or growth of protective layers on the surface of the V-based cathodes. For the PBA cathodes in ZIBs, removing interstitial water content and reducing crystal defects help to increase the robustness of their crystalline structure and further improve their electrochemical performances.
As to the liquid-based electrolytes, their electrochemical behaviors and compatibility with both cathode and Zn anode are essential for the overall electrochemical properties. Typically, aqueous Zn(CF3SO3)2 and ZnSO4 electrolytes are the most commonly used solutions in ZIBs, owing to their better electrochemical performances than other zinc salts. It should be noted that Zn(CF3SO3)2 is much more expensive than ZnSO4 (with over 18 times higher price), which is not a cost effective electrolyte choice in large-scale ZIB production, while ZnSO4 is accompanied with the formation of unfavorable by-product of hydroxide sulfate. Therefore, it is imperative to investigate the by-product caused by ZnSO4 electrolyte and the interface fluctuation between the Zn anode and ZnSO4 electrolyte, which may facilitate industrial production of ZIBs based on low-cost ZnSO4 electrolytes. For the solid-state electrolyte, most reported polymer electrolytes suffer from low ionic conductivity, insufficient mechanical strength, and fast degradation. Therefore, studies on the chemical conjunction between Zn-based electrolyte and polymer hosts and their joint electrochemical properties will play important roles in advancing solid-state electrolytes with high stability, broad voltage window, and improved long-term cycling performance. Furthermore, the systematic studies of solid-state polymer electrolytes operating in subzero or high temperature are lacking, which hinder the developments of ZIBs for harsh conditions, and more efforts should be made to explore and obtain electrolytes working in a wide temperature range. Despite much progress that has been achieved in the area of ZIBs, there remain challenges as summarized above. Additional fundamental studies are urgently needed, which are crucial for maturing the battery technology and realizing practical zinc-ion batteries for wide applications.
Overall, the combination of environment-friendly property, high safety, high energy density, and long cycling life makes ZIBs high potential candidates for future energy storage devices. Moreover, compared with current LIBs (about $300 per kWh), the price of ZIBs ($65 per kWh) is much lower, which is even comparable with lead acid batteries. The low cost makes ZIBs very promising for practical large-scale applications. The current manufacturing can be leveraged to large-scale commercialization for ZIBs. However, the major challenges of ZIBs still reside in the high energy efficiency cathode, stable Zn anode, and cheap electrolytes. In the near future, adequate zinc anodes, metal oxide cathodes (probably MnO2-, V2O5-based materials), and electrolytes can be designed with more mature technology. The commercialization of rechargeable ZIBs will potentially be scaled up.
Acknowledgements
W.W. Xu and Y. Wang acknowledge the Economic Development Assistantship and the Research Enhancement Awards program sponsored by LaSPACE for financial support. The authors also thank Jianwei Lai from Department of Mechanical Engineering and Prof. Qinglin Wu from School of Renewable Natural Resources in Louisiana State University for the discussions and suggestions.
References
- 1.Huang J, Guo Z, Ma Y, Bin D, Wang Y, Xia Y. Recent progress of rechargeable batteries using mild aqueous electrolytes. Small Methods. 2018;3(1):1800272. doi: 10.1002/smtd.201800272. [DOI] [Google Scholar]
- 2.Derraik J. The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 2002;44(9):842–852. doi: 10.1016/S0025-326X(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 3.Torres M, Barros M, Campos S, Pinto E, Rajamani S, Sayre R, Colepicolo P. Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol. Environ. Saf. 2008;71(1):1–15. doi: 10.1016/j.ecoenv.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Dincer I. Renewable energy and sustainable development: a crucial review. Renew. Sustain. Energy Rev. 2000;4(2):157–175. doi: 10.1016/S1364-0321(99)00011-8. [DOI] [Google Scholar]
- 5.McGlade C, Ekins P. The geographical distribution of fossil fuels unused when limiting global warming to 2 °C. Nature. 2015;517(7533):187–190. doi: 10.1038/nature14016. [DOI] [PubMed] [Google Scholar]
- 6.Abas N, Kalair A, Khan N. Review of fossil fuels and future energy technologies. Futures. 2015;69:31–49. doi: 10.1016/j.futures.2015.03.003. [DOI] [Google Scholar]
- 7.Das V, Padmanaban S, Venkitusamy K, Selvamuthukumaran R, Blaabjerg F, Siano P. Recent advances and challenges of fuel cell based power system architectures and control—a review. Renew. Sustain. Energy Rev. 2017;73:10–18. doi: 10.1016/j.rser.2017.01.148. [DOI] [Google Scholar]
- 8.Owusu P, Asumadu-Sarkodie S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016;3(1):1167990. doi: 10.1080/23311916.2016.1167990. [DOI] [Google Scholar]
- 9.Lelieveld J, Evans J, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 10.Kampa M, Castanas E. Human health effects of air pollution. Environ. Pollut. 2008;151(2):362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Yusoff S. Renewable energy from palm oil—innovation on effective utilization of waste. J. Clean. Prod. 2006;14(1):87–93. doi: 10.1016/j.jclepro.2004.07.005. [DOI] [Google Scholar]
- 12.Diaz-Gonzalez F, Sumper A, Bellmunt O, Robles R. A review of energy storage technologies for wind power applications. Renew. Sustain. Energy Rev. 2012;16(4):2154–2171. doi: 10.1016/j.rser.2012.01.029. [DOI] [Google Scholar]
- 13.Wan F, Niu ZQ. Design strategies of vanadium-based aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2019;58:2–12. doi: 10.1002/anie.201903941. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Cong T, Yang W, Tan C, Li Y, Ding Y. Progress in electrical energy storage system: a critical review. Prog. Nat. Sci. 2009;19(3):291–312. doi: 10.1016/j.pnsc.2008.07.014. [DOI] [Google Scholar]
- 15.Ren G, Ma G, Cong N. Review of electrical energy storage system for vehicular applications. Renew. Sustain. Energy Rev. 2015;41:225–236. doi: 10.1016/j.rser.2014.08.003. [DOI] [Google Scholar]
- 16.Goodenough J, Park K. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 2013;135(4):1167–1176. doi: 10.1021/ja3091438. [DOI] [PubMed] [Google Scholar]
- 17.Ma Z, MacFarlane DR, Kar M. Mg cathode materials and electrolytes for rechargeable Mg batteries: a Review. Batter. Supercaps. 2019;2(2):115–127. doi: 10.1002/batt.201800102. [DOI] [Google Scholar]
- 18.Xie ZQ, Lai JW, Zhu XP, Wang Y. Green synthesis of vanadate nanobelts at room temperature for superior aqueous rechargeable zinc-ion batteries. ACS Appl. Energy Mater. 2018;1(11):6401–6408. doi: 10.1021/acsaem.8b01378. [DOI] [Google Scholar]
- 19.Zhang N, Cheng FY, Liu YC, Zhao Q, Lei KX, Chen CC, Liu XS, Chen J. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 2016;138(39):12894–12901. doi: 10.1021/jacs.6b05958. [DOI] [PubMed] [Google Scholar]
- 20.Rydh CJ, Karlström M. Life cycle inventory of recycling portable nickel–cadmium batteries. Resour. Conserv. Recycl. 2002;34(4):289–309. doi: 10.1016/S0921-3449(01)00114-8. [DOI] [Google Scholar]
- 21.Majeau-Bettez G, Hawkins TR, Strømman A. Life cycle environmental assessment of lithium-ion and nickel metal hydride batteries for plug-in hybrid and battery electric vehicles. Environ. Sci. Technol. 2011;45(10):4548–4554. doi: 10.1021/es103607c. [DOI] [PubMed] [Google Scholar]
- 22.Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 2011;4(9):3243–3262. doi: 10.1039/C1EE01598B. [DOI] [Google Scholar]
- 23.Lu L, Han X, Li J, Hua J, Ouyang M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources. 2013;226:272–288. doi: 10.1016/j.jpowsour.2012.10.060. [DOI] [Google Scholar]
- 24.Nitta N, Wu F, Lee JT, Yushin G. Li-ion battery materials: present and future. Mater. Today. 2015;18(5):252–264. doi: 10.1016/j.mattod.2014.10.040. [DOI] [Google Scholar]
- 25.Scrosati B, Garche J. Lithium batteries: status, prospects and future. J. Power Sources. 2010;195(9):2419–2430. doi: 10.1016/j.jpowsour.2009.11.048. [DOI] [Google Scholar]
- 26.Han M, Gonzalo E, Singh G, Rojo T. A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries. Energy Environ. Sci. 2015;8(1):81–102. doi: 10.1039/C4EE03192J. [DOI] [Google Scholar]
- 27.Pramudita J, Sehrawat D, Goonetilleke D, Sharma N. An initial review of the status of electrode materials for potassium-ion batteries. Adv. Energy Mater. 2017;7(24):1602911. doi: 10.1002/aenm.201602911. [DOI] [Google Scholar]
- 28.Fang G, Zhou J, Pan A, Liang S. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett. 2018;3(10):2480–2501. doi: 10.1021/acsenergylett.8b01426. [DOI] [Google Scholar]
- 29.Yang Q, Huang ZD, Li XL, Liu ZX, Li HF, et al. A wholly degradable, rechargeable Zn–Ti3C2 MXene capacitor with superior anti-self-discharge function. ACS Nano. 2019;13(7):8275–8283. doi: 10.1021/acsnano.9b03650. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Fu J, Zhong C, Wu T, Chen Z, Hu W, Amine K, Lu J. Batteries: recent advances in flexible zinc-based rechargeable batteries. Adv. Energy Mater. 2019;9(1):1970001. doi: 10.1002/aenm.201970001. [DOI] [Google Scholar]
- 31.Ming J, Guo J, Xia C, Wang W, Alshareef HN. Zinc-ion batteries: materials, mechanisms, and applications. Mater. Sci. Eng. R. 2019;135:58–84. doi: 10.1016/j.mser.2018.10.002. [DOI] [Google Scholar]
- 32.Xu C, Li B, Du H, Kang F. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew. Chem. Int. Ed. 2012;51(4):933–935. doi: 10.1002/anie.201106307. [DOI] [PubMed] [Google Scholar]
- 33.Lee B, Lee HR, Kim H, Chung KY, Cho BW, Oh SH. Elucidating the intercalation mechanism of zinc ions into α-MnO2 for rechargeable zinc batteries. Chem. Commun. 2015;51(45):9265–9268. doi: 10.1039/C5CC02585K. [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Mo FN, Liu ZX, Ma LT, Li XL, et al. Activating C-coordinated iron of iron hexacyanoferrate for Zn hybrid-ion batteries with 10000-cycle lifespan and superior rate capability. Adv. Mater. 2019;31(32):1901521. doi: 10.1002/adma.201901521. [DOI] [PubMed] [Google Scholar]
- 35.Xu WN, Zhao KN, Huo WC, Wang YZ, Yao G, et al. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy. 2019;62:275–281. doi: 10.1016/j.nanoen.2019.05.042. [DOI] [Google Scholar]
- 36.Cheng F, Chen J, Gou X, Shen P. High-power alkaline Zn-MnO2 batteries using γ-MnO2 nanowires/nanotubes and electrolytic zinc powder. Adv. Mater. 2005;17(22):2753–2756. doi: 10.1002/adma.200500663. [DOI] [Google Scholar]
- 37.Yang C, Lin S. Improvement of high-rate capability of alkaline Zn–MnO2 battery. J. Power Sources. 2002;112(1):174–183. doi: 10.1016/S0378-7753(02)00354-3. [DOI] [Google Scholar]
- 38.Yamamoto T, Shoji T. Rechargeable Zn|ZnSO4|MnO2-type cells. Inorg. Chim. Acta. 1986;117(2):L27–L28. doi: 10.1016/S0020-1693(00)82175-1. [DOI] [Google Scholar]
- 39.Yuan C, Zhang Y, Pan Y, Liu X, Wang G, Cao D. Investigation of the intercalation of polyvalent cations (Mg2+, Zn2+) into λ-MnO2 for rechargeable aqueous battery. Electrochim. Acta. 2014;116:404–412. doi: 10.1016/j.electacta.2013.11.090. [DOI] [Google Scholar]
- 40.Wu B, Zhang G, Yan M, Xiong T, He P, He L, Xu X, Mai L. Graphene scroll-Coated-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small. 2018;14(13):1703850. doi: 10.1002/smll.201703850. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Chen L, Zhou X, Liu Z. Morphology-dependent electrochemical performance of zinc hexacyanoferrate cathode for zinc-ion battery. Sci. Rep. 2015;5:18263. doi: 10.1038/srep18263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai X, Wan F, Zhang L, Cao H, Niu Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019;17:143–150. doi: 10.1016/j.ensm.2018.07.022. [DOI] [Google Scholar]
- 43.Li G, Yang Z, Jiang Y, Jin C, Huang W, Ding X, Huang Y. Towards polyvalent ion batteries: a zinc-ion battery based on NASICON structured. Nano Energy. 2016;25:211–217. doi: 10.1016/j.nanoen.2016.04.051. [DOI] [Google Scholar]
- 44.Zhao Q, Huang W, Luo Z, Liu L, Lu Y, et al. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018;4(3):1761. doi: 10.1126/sciadv.aao1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng W, Shu L, Li Q, Chen S, Wang F, Tao X. Fiber-based wearable electronics: a review of materials, fabrication, devices, and applications. Adv. Mater. 2014;26(31):5310–5336. doi: 10.1002/adma.201400633. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Kumar R, Bandodkar A, Wang J. Advanced materials for printed wearable electrochemical devices: a review. Adv. Electron. Mater. 2017;3(1):1600260. doi: 10.1002/aelm.201600260. [DOI] [Google Scholar]
- 47.Wang ZQ, Hu JT, Han L, Wang ZJ, Wang HB, et al. A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy. 2019;56:92–99. doi: 10.1016/j.nanoen.2018.11.038. [DOI] [Google Scholar]
- 48.Liu Z, Mo F, Li H, Zhu M, Wang Z, Liang G, Zhi C. Advances in flexible and wearable energy-storage textiles. Small Methods. 2018;2(11):1800124. doi: 10.1002/smtd.201800124. [DOI] [Google Scholar]
- 49.Lu W, Xie C, Zhang H, Li X. Zinc dendrite growth in zinc-based batteries. Chemsuschem. 2018;11(23):3996–4006. doi: 10.1002/cssc.201801657. [DOI] [PubMed] [Google Scholar]
- 50.Peng Z, Wei QL, Tan SS, He P, Luo W, An QY, Mai LQ. Novel layered iron vanadate cathode for high-capacity aqueous rechargeable zinc batteries. Chem. Commun. 2018;54(32):4041–4044. doi: 10.1039/C8CC00987B. [DOI] [PubMed] [Google Scholar]
- 51.Silva M, Smith MJ, Lightfoot P. Characterisation of a Zn triflate-based polymer electrolyte. Portugaliae Electrochim. Acta. 1999;17:3–10. doi: 10.4152/pea.199901003. [DOI] [Google Scholar]
- 52.Wan F, Zhang L, Wang X, Bi S, Niu Z, Chen J. An aqueous rechargeable zinc-organic battery with hybrid mechanism. Adv. Funct. Mater. 2018;28(45):1804975. doi: 10.1002/adfm.201804975. [DOI] [Google Scholar]
- 53.Li H, Ma L, Han C, Wang Z, Liu Z, Tang Z, Zhi C. Advanced rechargeable zinc-based batteries: recent progress and future perspectives. Nano Energy. 2019;62:550–587. doi: 10.1016/j.nanoen.2019.05.059. [DOI] [Google Scholar]
- 54.Song M, Tan H, Chao D, Fan HJ. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018;28(41):1802564. doi: 10.1002/adfm.201802564. [DOI] [Google Scholar]
- 55.Alias N, Mohamad A. Advances of aqueous rechargeable lithium-ion battery: a review. J. Power Sources. 2015;274:237–251. doi: 10.1016/j.jpowsour.2014.10.009. [DOI] [Google Scholar]
- 56.Kim H, Hong J, Park K, Kim H, Kim S, Kang K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014;114(23):11788–11827. doi: 10.1021/cr500232y. [DOI] [PubMed] [Google Scholar]
- 57.Beck F, Rüetschi P. Rechargeable batteries with aqueous electrolytes. Electrochim. Acta. 2000;45(15–16):2467–2482. doi: 10.1016/S0013-4686(00)00344-3. [DOI] [Google Scholar]
- 58.Alfaruqi M, Mathew V, Gim J, Kim S, Song J, Baboo JP, Choi SH, Kim J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015;27(10):3609–3620. doi: 10.1021/cm504717p. [DOI] [Google Scholar]
- 59.Xu C, Du H, Li B, Kang F, Zeng Y. Reversible insertion properties of zinc ion into manganese dioxide and its application for energy storage. Electrochem. Solid-State Lett. 2009;12(4):A61–A65. doi: 10.1149/1.3065967. [DOI] [Google Scholar]
- 60.Pan H, Shao Y, Yan P, Cheng Y, Han K, et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy. 2016;1(5):16039. doi: 10.1038/nenergy.2016.39. [DOI] [Google Scholar]
- 61.Sun W, Wang F, Hou S, Yang C, Fan X, et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017;139(29):9775–9778. doi: 10.1021/jacs.7b04471. [DOI] [PubMed] [Google Scholar]
- 62.Fang GZ, Zhu CY, Chen MH, Zhou J, Tang BY, et al. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery. Adv. Funct. Mater. 2019;29(15):1808375. doi: 10.1002/adfm.201808375. [DOI] [Google Scholar]
- 63.Huang J, Wang Z, Hou M, Dong X, Liu Y, Wang Y, Xia Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018;9:2908. doi: 10.1038/s41467-018-04949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knight J, Therese S, Manthiram A. Chemical extraction of Zn from ZnMn2O4-based spinels. J. Mater. Chem. A. 2015;3(42):21077–21082. doi: 10.1039/C5TA06482A. [DOI] [Google Scholar]
- 65.Zhang N, Dong Y, Jia M, Bian X, Wang Y, et al. Rechargeable aqueous Zn–V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018;3(6):1366–1372. doi: 10.1021/acsenergylett.8b00565. [DOI] [Google Scholar]
- 66.Yan M, He P, Chen Y, Wang S, Wei Q, et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018;30(1):1703725. doi: 10.1002/adma.201703725. [DOI] [PubMed] [Google Scholar]
- 67.Kundu D, Adams B, Duffort V, Vajargah S, Nazar L. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy. 2016;1(10):16119. doi: 10.1038/nenergy.2016.119. [DOI] [Google Scholar]
- 68.Ding J, Du Z, Gu L, Li B, Wang L, Wang S, Gong Y, Yang S. Ultrafast Zn2+ intercalation and deintercalation in vanadium dioxide. Adv. Mater. 2018;30(26):1800762. doi: 10.1002/adma.201800762. [DOI] [PubMed] [Google Scholar]
- 69.Wei T, Li Q, Yang G, Wang CJ. An electrochemically induced bilayered structure facilitates long-life zinc storage of vanadium dioxide. J. Mater. Chem. A. 2018;6(17):8006–8012. doi: 10.1039/C8TA02090F. [DOI] [Google Scholar]
- 70.Lai J, Zhu H, Zhu X, Koritala H, Wang Y. Interlayer-expanded V6O13·nH2O architecture constructed for advanced rechargeable aqueous zinc ion battery. ACS Appl. Energy Mater. 2019;2(3):1988–1996. doi: 10.1021/acsaem.8b02054. [DOI] [Google Scholar]
- 71.Shin J, Choi D, Lee H, Jung Y, Choi J. Hydrated intercalation for high-performance aqueous zinc ion batteries. Adv. Energy Mater. 2019;9(14):1900083. doi: 10.1002/aenm.201900083. [DOI] [Google Scholar]
- 72.Shen C, Li X, Li N, Xie K, Wang J, Liu X, Wei B. Graphene-boosted, high-performance aqueous Zn-ion battery. ACS Appl. Mater. Interfaces. 2018;10(30):25446–25453. doi: 10.1021/acsami.8b07781. [DOI] [PubMed] [Google Scholar]
- 73.He P, Quan Y, Xu X, Yan M, Yang W, An Q, He L, Mai L. High-performance aqueous zinc-ion battery based on layered H2V3O8 nanowire cathode. Small. 2017;13(47):1702551. doi: 10.1002/smll.201702551. [DOI] [PubMed] [Google Scholar]
- 74.Pang Q, Sun C, Yu Y, Zhao K, Zhang Z, et al. H2V3O8 nanowire/graphene electrodes for aqueous rechargeable zinc ion batteries with high rate capability and large capacity. Adv. Energy Mater. 2018;8(19):1800144. doi: 10.1002/aenm.201800144. [DOI] [Google Scholar]
- 75.Yang Y, Tang Y, Fang G, Shan L, Guo J, et al. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018;11(11):3157–3162. doi: 10.1039/C8EE01651H. [DOI] [Google Scholar]
- 76.Alfaruqi M, Mathew V, Song J, Kim S, Islam S, et al. Electrochemical zinc intercalation in lithium vanadium oxide: a high-capacity zinc-ion battery cathode. Chem. Mater. 2017;29(4):1684–1694. doi: 10.1021/acs.chemmater.6b05092. [DOI] [Google Scholar]
- 77.Sambandam B, Soundharrajan V, Kim S, Alfaruqi M, Jo J, Kim S, Mathew V, Sun Y, Kim J. K2V6O16·2.7H2O nanorod cathode: an advanced intercalation system for high energy aqueous rechargeable Zn-ion batteries. J. Mater. Chem. A. 2018;6(32):15530–15539. doi: 10.1039/C8TA02018C. [DOI] [Google Scholar]
- 78.He P, Zhang G, Liao X, Yan M, Xu X, An Q, Liu J, Mai L. Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries. Adv. Energy Mater. 2018;8(10):1702463. doi: 10.1002/aenm.201702463. [DOI] [Google Scholar]
- 79.Wan F, Zhang L, Dai X, Wang X, Niu Z, Chen J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018;9:1656. doi: 10.1038/s41467-018-04060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu P, Zhu T, Wang X, Wei X, Yan M, et al. Highly durable Na2V6O16·1.63H2O nanowire cathode for aqueous zinc-ion battery. Nano Lett. 2018;18(3):1758–1763. doi: 10.1021/acs.nanolett.7b04889. [DOI] [PubMed] [Google Scholar]
- 81.Cai Y, Liu F, Luo Z, Fang G, Zhou J, Pan A, Liang S. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode. Energy Storage Mater. 2018;13:168–174. doi: 10.1016/j.ensm.2018.01.009. [DOI] [Google Scholar]
- 82.Liu CF, Neale Z, Zheng JQ, Jia XX, Huang JJ, et al. Expanded hydrated vanadate for high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2019;12:2273–2285. doi: 10.1039/C9EE00956F. [DOI] [Google Scholar]
- 83.Xia C, Guo J, Li P, Zhang X, Alshareef H. Highly stable aqueous zinc-ion storage using a layered calcium vanadium oxide bronze cathode. Angew. Chem. Int. Ed. 2018;57(15):3943–3948. doi: 10.1002/anie.201713291. [DOI] [PubMed] [Google Scholar]
- 84.Shan L, Yang Y, Zhang W, Chen H, Fang G, Zhou J, Liang S. Observation of combination displacement/intercalation reaction in aqueous zinc-ion battery. Energy Storage Mater. 2019;18:10–14. doi: 10.1016/j.ensm.2018.08.008. [DOI] [Google Scholar]
- 85.Tang B, Zhou J, Fang G, Liu F, Zhu C, Wang C, Pan A, Liang S. Engineering the interplanar spacing of ammonium vanadates as a high-performance aqueous zinc-ion battery cathode. J. Mater. Chem. A. 2019;7(3):940–945. doi: 10.1039/C8TA09338E. [DOI] [Google Scholar]
- 86.Trócoli R, Mantia F. An aqueous zinc-ion battery based on copper hexacyanoferrate. Chemsuschem. 2015;8(3):481–485. doi: 10.1002/cssc.201403143. [DOI] [PubMed] [Google Scholar]
- 87.Jia Z, Wang B, Wang Y. Copper hexacyanoferrate with a well-defined open framework as a positive electrode for aqueous zinc ion batteries. Mater. Chem. Phys. 2015;149:601–606. doi: 10.1016/j.matchemphys.2014.11.014. [DOI] [Google Scholar]
- 88.Kasiri G, Trócoli R, Hashemi A, Mantia F. An electrochemical investigation of the aging of copper hexacyanoferrate during the operation in zinc-ion batteries. Electrochim. Acta. 2016;222:74–83. doi: 10.1016/j.electacta.2016.10.155. [DOI] [Google Scholar]
- 89.Zhang L, Chen L, Zhou X, Liu Z. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: the zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 2015;5(2):1400930. doi: 10.1002/aenm.201400930. [DOI] [Google Scholar]
- 90.Wang F, Hu E, Sun W, Gao T, Ji X, et al. A rechargeable aqueous Zn2+-battery with high power density and a long cycle-life. Energy Environ. Sci. 2018;11(11):3168–3175. doi: 10.1039/C8EE01883A. [DOI] [Google Scholar]
- 91.Li GL, Yang Z, Jiang Y, Zhang WX, Huang YH. Hybrid aqueous battery based on Na3V2(PO4)3/C cathode and zinc anode for potential large-scale energy storage. J. Power Sources. 2016;308:52–57. doi: 10.1016/j.jpowsour.2016.01.058. [DOI] [Google Scholar]
- 92.Zhao H, Hu C, Cheng H, Fang J, Xie Y, et al. Novel rechargeable M3V2(PO4)3//zinc (M = Li, Na) hybrid aqueous batteries with excellent cycling performance. Sci. Rep. 2016;6:25809. doi: 10.1038/srep25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li W, Wang K, Cheng S, Jiang K. A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Storage Mater. 2018;15:14–21. doi: 10.1016/j.ensm.2018.03.003. [DOI] [Google Scholar]
- 94.Liu W, Hao J, Xu C, Mou J, Dong L, et al. Investigation of zinc ion storage of transition metal oxides, sulfides, and borides in zinc ion battery systems. Chem. Commun. 2017;53(51):6872–6874. doi: 10.1039/C7CC01064H. [DOI] [PubMed] [Google Scholar]
- 95.Xu W, Zhao K, Wang Y. Electrochemical activated MoO2/Mo2N heterostructured nanobelts as superior zinc rechargeable battery cathode. Energy Storage Mater. 2018;15:374–379. doi: 10.1016/j.ensm.2018.06.028. [DOI] [Google Scholar]
- 96.Wang JJ, Wang JG, Liu HY, You ZY, Wei CG, Kang FY. Electrochemical activation of commercial MnO microsized particles for high-performance aqueous zinc-ion batteries. J. Power Sources. 2019;438:226951. doi: 10.1016/j.jpowsour.2019.226951. [DOI] [Google Scholar]
- 97.Xu W, Sun C, Zhao K, Cheng X, Rawal S, Xu Y, Wang Y. Defect engineering activating (Boosting) zinc storage capacity of MoS2. Energy Storage Mater. 2019;16:527–534. doi: 10.1016/j.ensm.2018.09.009. [DOI] [Google Scholar]
- 98.Li H, Yang Q, Mo F, Liang G, Liu Z, et al. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-ion batteries. Energy Storage Mater. 2018;19:94–101. doi: 10.1016/j.ensm.2018.10.005. [DOI] [Google Scholar]
- 99.Xie CX, Zhang HM, Xu WB, Wang W, Li XF. A long cycle life, self-healing zinc-iodine flow battery with high power density. Angew. Chem. Int. Ed. 2018;130(35):11341–11346. doi: 10.1002/ange.201803122. [DOI] [PubMed] [Google Scholar]
- 100.Li B, Nie ZM, Vijayakumar M, Li GS, Liu J, Sprenkle V, Wang W. Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery. Nat. Commun. 2015;6:6303. doi: 10.1038/ncomms7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan HL, Li B, Mei DH, Nie ZM, Shao YY, et al. Controlling solid–liquid conversion reactions for a highly reversible aqueous zinc–iodine battery. ACS Energy Lett. 2017;2(12):2674–2680. doi: 10.1021/acsenergylett.7b00851. [DOI] [Google Scholar]
- 102.Bai C, Cai F, Wang L, Guo S, Liu X, Yuan Z. A sustainable aqueous Zn–I2 battery. Nano Res. 2018;11(7):3548–3554. doi: 10.1007/s12274-017-1920-9. [DOI] [Google Scholar]
- 103.Chae M, Heo JW, Kwak HH, Lee H, Hong S. Organic electrolyte-based rechargeable zinc-ion batteries using potassium nickel hexacyanoferrate as a cathode material. J. Power Sources. 2017;337:204–211. doi: 10.1016/j.jpowsour.2016.10.083. [DOI] [Google Scholar]
- 104.Pan C, Zhang R, Nuzzo R, Gewirth A. ZnNixMnxCo2−2xO4 spinel as a high-voltage and high-capacity cathode material for nonaqueous Zn-ion batteries. Adv. Energy Mater. 2018;8(22):1800589. doi: 10.1002/aenm.201800589. [DOI] [Google Scholar]
- 105.Pan CS, Nuzzo RG, Gewirth AA. ZnAlxCo2−xO4 Spinels as cathode materials for non-aqueous Zn batteries with an open circuit voltage of ≤ 2 V. Chem. Mater. 2017;29(21):9351–9359. doi: 10.1021/acs.chemmater.7b03340. [DOI] [Google Scholar]
- 106.Kundu DP, Vajargah SH, Wan LW, Adams B, Prendergast D, Nazar LF. Aqueous vs. nonaqueous Zn-ion batteries: consequences of the desolvation penalty at the interface. Energy Environ. Sci. 2018;11(4):881–892. doi: 10.1039/C8EE00378E. [DOI] [Google Scholar]
- 107.Zeng YX, Zhang XY, Meng Y, Yu MH, Yi JN, et al. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn–MnO2 battery. Adv. Mater. 2017;29(26):1700274. doi: 10.1002/adma.201700274. [DOI] [PubMed] [Google Scholar]
- 108.Li H, Han C, Huang Y, Huang Y, Zhu M, et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 2018;11(4):941–951. doi: 10.1039/C7EE03232C. [DOI] [Google Scholar]
- 109.Wang Z, Ruan Z, Liu Z, Wang Y, Tang Z, et al. A flexible rechargeable zinc-ion wire-shaped battery with shape memory function. J. Mater. Chem. A. 2018;6(18):8549–8557. doi: 10.1039/C8TA01172A. [DOI] [Google Scholar]
- 110.Zhang S, Yu N, Zeng S, Zhou S, Chen M, Di J, Li Q. An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries. J. Mater. Chem. A. 2018;6(26):12237–12243. doi: 10.1039/C8TA04298E. [DOI] [Google Scholar]
- 111.Chao D, Zhu C, Song M, Liang P, Zhang X, et al. A high-rate and stable quasi-solid-state zinc-ion battery with novel 2D layered zinc orthovanadate array. Adv. Mater. 2018;30(32):1803181. doi: 10.1002/adma.201803181. [DOI] [PubMed] [Google Scholar]
- 112.Huang Y, Liu J, Wang J, Hu M, Mo F, Liang G, Zhi C. An intrinsically self-healing NiCo||Zn rechargeable battery with a self-Healable ferric-ion-crosslinking sodium polyacrylate hydrogel electrolyte. Angew. Chem. Int. Ed. 2018;57(31):9810–9813. doi: 10.1002/anie.201805618. [DOI] [PubMed] [Google Scholar]
- 113.Mo F, Liang G, Meng Q, Liu Z, Li H, Fan J, Zhi C. A flexible rechargeable aqueous zinc manganese-dioxide battery working at − 20 °C. Energy Environ. Sci. 2019;12(2):706–715. doi: 10.1039/C8EE02892C. [DOI] [Google Scholar]
- 114.McKubre MCH, Macdonald DD. The dissolution and passivation of zinc in concentrated aqueous hydroxide. J. Electrochem. Soc. 1981;128(3):524–530. doi: 10.1149/1.2127450. [DOI] [Google Scholar]
- 115.Gu P, Zheng MB, Zhao QX, Xiao X, Xue HG, Pang H. Rechargeable zinc–air batteries: a promising way to green energy. J. Mater. Chem. A. 2017;5(17):7651–7666. doi: 10.1039/C7TA01693J. [DOI] [Google Scholar]
- 116.Li HF, Liu ZX, Liang GJ, Huang Y, Huang Y, et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano. 2018;12(4):3140–3148. doi: 10.1021/acsnano.7b09003. [DOI] [PubMed] [Google Scholar]
- 117.Yan Y, Zhang YM, Wu YT, Wang ZZ, Mathur A, et al. A lasagna-inspired nanoscale ZnO anode design for high-energy rechargeable aqueous batteries. ACS Appl. Energy Mater. 2018;1(11):6345–6351. doi: 10.1021/acsaem.8b01321. [DOI] [Google Scholar]
- 118.Wang LP, Li NW, Wang TS, Yin YX, Guo YG, Wang CR. Conductive graphite fiber as a stable host for zinc metal anodes. Electrochim. Acta. 2017;244:172–177. doi: 10.1016/j.electacta.2017.05.072. [DOI] [Google Scholar]
- 119.Hashemi AB, Kasiri G, Mantia FL. The effect of polyethyleneimine as an electrolyte additive on zinc electrodeposition mechanism in aqueous zinc-ion batteries. Electrochim. Acta. 2017;258:703–708. doi: 10.1016/j.electacta.2017.11.116. [DOI] [Google Scholar]
- 120.Hoang TKA, Doan TNL, Cho JH, Su JYJ, Lee C, Lu CY, Chen P. Sustainable gel electrolyte containing pyrazole as corrosion inhibitor and dendrite suppressor for aqueous Zn/LiMn2O4 battery. Chemsuschem. 2017;10(13):2816–2822. doi: 10.1002/cssc.201700441. [DOI] [PubMed] [Google Scholar]
- 121.Wang F, Borodin O, Gao T, Fan X, Sun W, et al. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018;17(6):543–549. doi: 10.1038/s41563-018-0063-z. [DOI] [PubMed] [Google Scholar]
- 122.Wan F, Zhang Y, Zhang LL, Liu DB, Wang CD, Song L, Niu ZQ, Chen J. Reversible oxygen redox chemistry in aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2019;58(21):7062–7067. doi: 10.1002/anie.201902679. [DOI] [PubMed] [Google Scholar]
- 123.Zhao KN, Wang C, Yu Y, Yan M, Wei Q, et al. Ultrathin surface coating enables stabilized zinc metal anode. Adv. Mater. Interfaces. 2018;5(16):1800848. doi: 10.1007/s10008-017-3589-0. [DOI] [Google Scholar]
- 124.Kang LT, Cui MW, Jiang FY, Gao YF, Luo HJ, Liu JJ, Liang W, Zhi CY. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Adv. Energy Mater. 2018;8(25):1801090. doi: 10.1002/aenm.201801090. [DOI] [Google Scholar]
- 125.Zhou Z, Zhang Y, Chen P, Wu Y, Yang H, et al. Graphene oxide-modified zinc anode for rechargeable aqueous batteries. Chem. Eng. Sci. 2019;194:142–147. doi: 10.1016/j.ces.2018.06.048. [DOI] [Google Scholar]
- 126.Sun KEK, Hoang TKA, Doan TNL, Yu Y, Zhu X, Tian Y, Chen P. Suppression of dendrite formation and corrosion on zinc anode of secondary aqueous batteries. ACS Appl. Mater. Interfaces. 2017;9(11):9681–9687. doi: 10.1021/acsami.6b16560. [DOI] [PubMed] [Google Scholar]
- 127.Lee B, Cui S, Xing X, Liu H, Yue X, Petrova V, Lim H, Chen R, Liu P. Dendrite suppression membranes for rechargeable zinc batteries. ACS Appl. Mater. Interfaces. 2018;10(45):38928–38935. doi: 10.1021/acsami.8b14022. [DOI] [PubMed] [Google Scholar]