Highlights
This review gives a thinking based on the generic mechanisms rather than simply dividing them as different types of combination of materials, which is unique and valuable for understanding and developing the novel hybrid materials in the future.
The hybrid materials, their sensing mechanism, and their applications are systematically reviewed. Critical thinking and ideas regarding the orientation of the development of hybrid material-based gas sensor in the future are also discussed.
Keywords: Gas sensor, Hybrid, Chemi-resistor, Functional nanomaterials
Abstract
Chemi-resistive sensors based on hybrid functional materials are promising candidates for gas sensing with high responsivity, good selectivity, fast response/recovery, great stability/repeatability, room-working temperature, low cost, and easy-to-fabricate, for versatile applications. This progress report reviews the advantages and advances of these sensing structures compared with the single constituent, according to five main sensing forms: manipulating/constructing heterojunctions, catalytic reaction, charge transfer, charge carrier transport, molecular binding/sieving, and their combinations. Promises and challenges of the advances of each form are presented and discussed. Critical thinking and ideas regarding the orientation of the development of hybrid material-based gas sensor in the future are discussed.
Introduction
Monitoring and recording chemical stimulus or variations in the environment are increasingly important in future production and daily life of human health [1]. Achieving this goal relies on the availability of high-performance sensor units that are capable of detecting gas analytes, such as volatile/semi-volatile organic compounds (VOCs/SVOCs) highly rich regarding critical information for the detection, monitoring and closed-loop control in many fields, including medicine, food industry, environmental monitoring, public security, and agricultural production [2, 3].
An ideal gas sensor requires high responsivity, good selectivity, fast response/recovery, great stability/repeatability, room-working temperature, low cost, and easy-to-fabricate for practical applications [4–6]. To meet those requirements, many types of gas sensors with different transduction forms, e.g., chemi-resistor, field-effect transistor (FET), solid-state electrochemical sensor (SSES), quartz-crystal microbalance (QCM), gas capacitor, surface acoustic wave (SAW), have been well studied and developed. Among them, since the 1960s [7], a chemi-resistor that contains an active sensing layer bridging a pair of electrodes became a promising candidate due to its advantages [4–6, 8–12] including easy-to-fabricate, use of very small quantity (milligram level) active materials, wide adoption of sensitive materials, and simple sensing data, which ensure its success in certain commercialization opportunities [13–15]. However, it is rare to find chemi-resistors that can meet these specific requirements.
An emerging approach in chemi-resistors to meet these needs relies on hybrid materials, viz. materials that integrate 2+ single constituents at the nanometer or molecular level [16–36], to achieve new and/or enhanced sensing properties. In this progress report, we review the advances of the hybrid material-based gas sensors concisely and comprehensively. The hybrid materials-based chemi-resistive gas sensors are distinguished, understood, and introduced based on the generic mechanisms rather than simply dividing them as different types of combination of materials. Then, the report, in detail, focuses on the research and development (R&D) aspects of hybrid gas sensors, while presenting and discussing the sensing performances of different types of hybrid materials, and associated enhanced sensing mechanisms. Promises and challenges toward the future development of each elements are deeply thought and discussed. Critical thinking and ideas regarding the orientation of the development of hybrid material-based gas sensor in the future are also discussed.
The Need for Hybrid Functional Nanomaterials for Sensing Applications
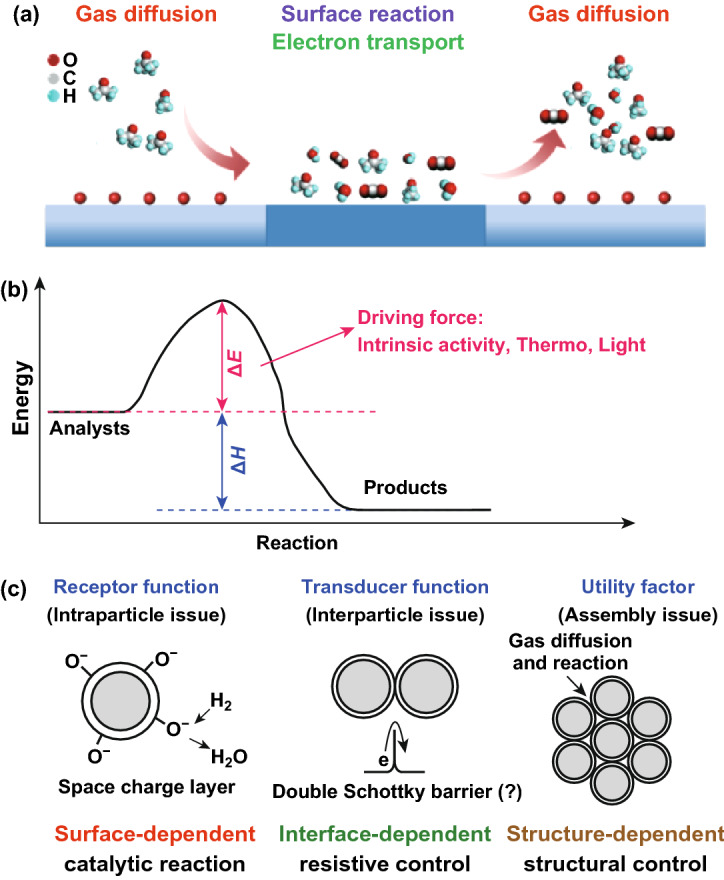
Chemi-resistors for gas sensing include three main processes: diffusion/molecule capture unit, surface reaction unit (including charge transfer), and charge carrier transport unit (Fig. 1a) [37]. To date, most of these sensors and/or sensor arrays utilize sensing elements that are based on single material or transduction mechanism, of which intrinsic sensing activity or additional thermal/photonic energy are usually employed as the driving force to stimulate the sensing effects of target gases (Fig. 1b). The hindrances are unavoidable at several levels: (i) not satisfying long-term stability and sensitivity of organic chemi-resistors due to the high affinity of conductive polymers (CP), such as polyaniline (PANI), polypyrrole (PPy), and polythiophene (PTh), toward volatile organic compounds (VOCs) and humidity existed in the atmosphere; (ii) high operating temperatures (usually > 200 °C), baseline drift, limited selectivity, and oxidation/decomposition of VOCs in the case of inorganic materials (especially metal oxide materials, e.g., ZnO, SnO, TiO2, SnO2)-based chemi-resistor. A reliable solution for these drawbacks is the design and utilization of new gas sensitive materials based on hybrid inorganic–inorganic [8], organic–organic [12], and inorganic–organic materials [8, 9, 12, 16–36].
Fig. 1.
Schematic illustration of a three gas-sensing units and b sensing reactions. c The enhanced gas-sensing mechanisms for hybrid chemi-resistive nanomaterials. The upper part of c was modified from reference. Reproduced with permission [38]. Copyright 2009, Elsevier
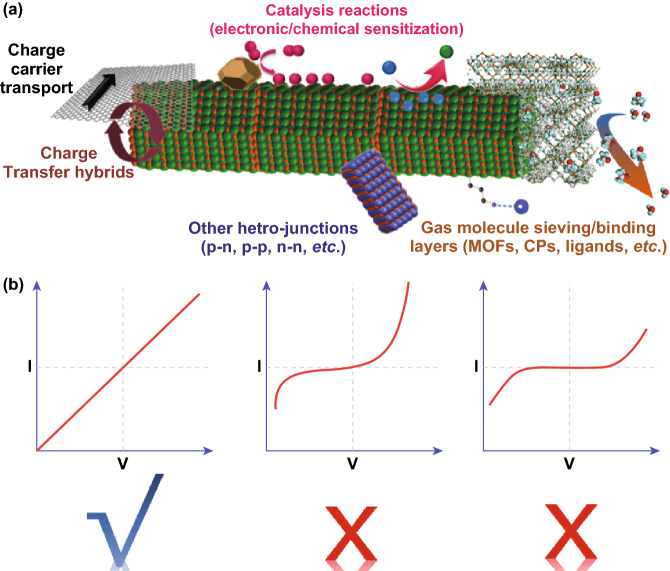
Using hybrid materials as sensitive transducer offers several obvious advantages, compared with the single constituent. First, the inexhaustible abundance of hybrid materials (in both the complex constituents and novel nanostructures) makes it possible to involve an almost infinite continuum of variable factors (surface-dependent factor, interface-dependent factor, and structure-dependent factor) to generate new sensing behaviors (Fig. 1c) [38–49]. Second, with hybrid material, more chemical/physical processes with different enhanced mechanisms could be introduced to precisely design, regulate, and enhance the sensing performance mainly through catalytic reaction with analyte [50–58], charge transfer [59–63], charge carrier transport [64–66] manipulation/construction of heterojunctions [39, 67], molecular binding/sieving [68–73], and their combinations [74–77].
Hybrid Chemi-Resistive Gas Sensors
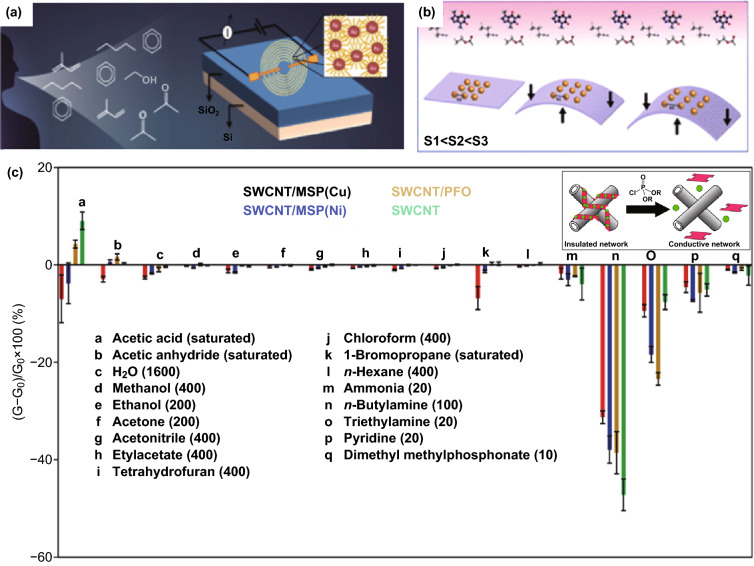
Hybrid materials can perform improved sensing characteristics via one or a combination from five typical hybridizing forms which are categorized into three sensing-dependent factors (Fig. 2a). The first combination relies on catalysis reactions (normally noble metal catalysts, e.g., Pt [78], Pd [79], Au [50], and Ag [51]) between analyte gas and decorated catalysts on host semi-conductive materials (categorized as surface-dependent factor). The second relies on a fast charge transfer process, viz carrier withdrawal or donation, electron acceptor or acceptor between guest additives and the host material (e.g., carbon nanotubes (CNTs)), reduced graphene oxide (rGO) (categorized as interface-dependent factor) [63]. The third relies on regulating the charge carrier transport in a conductive/semi-conductive materials (e.g., single-wall carbon nanotube (SWCNT)-metallo-supramolecular polymer (MSP), gold nanoparticles (GNPs)-thiols, N,N’-diphenyl perylene tetracarboxylic diimide (PTCDI-Ph)/para-sexiphenyl (p-6P)) upon exposure to gas analytes (categorized as interface- and structure-dependent factor) [66, 80, 81]. The fourth relies on manipulation/construction of the heterojunctions such as n–n, p–n, p–p, p–n–p heterogeneous semi-conductive materials (categorized as interface-dependent factor) [39]. The last one relies on semiconductors coated by gas molecular sieving/binding layers or ligands/complexes for selective gas detection (categorized as surface- and structure-dependent factor) [72, 76, 82]. In the following section, we provide more details on each of these combinations. It should be noted that, according to the understanding of authors on chemi-resistors (1. measuring the resistors directly; 2. measuring the current when the device is applied a constant bias voltage; 3. measuring the partial voltage on the device in parallel with a constant resistance when the resistance and device is applied a constant bias voltage), we will review here only resistive-change sensing devices in which the contact of sensitive materials and electrodes is ohmic (good linearity of I–V curves under DC bias, Fig. 2b); therefore, we exclude devices in which the I–V curves under DC bias are nonlinear despite that they exhibit similar resistance changes (e.g., chemical diodes, proton/ions types).
Fig. 2.
a Schematic illustration of five typical forms of hybrid functional nanomaterials for chemi-resistive gas sensing; b typical I–V curves of different sensors under DC bias (from left to right, chemi-resistors, chemical diodes, and proton/ions types)
Hybrid Gas Sensors Based on Catalytic Effects
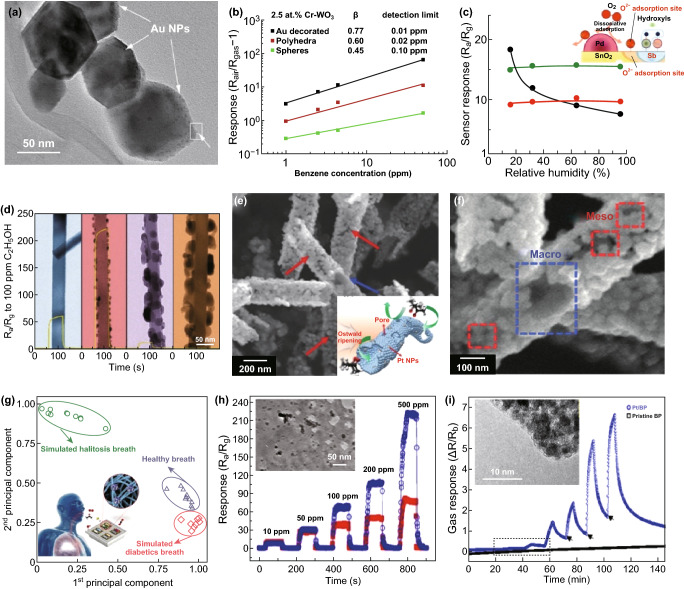
Catalytic effects of hybrid functional nanomaterials contribute to high response, fast speed, and low operating temperature via chemical/electronic sensitization, which is usually accompanied by synergistic effects, complementary behavior, and porous structures [50, 52, 83–86]. In addition, the exposed facets (morphologies) of matrix nanograins (facet-dependent chemical activity) and catalytic additives can greatly enhance the sensing properties of hybrid nanomaterials [87, 88]. For example, introduction of Cr dopants to WO3 polydedra can not only control the specific exposed facets and activation energy, but bring catalytic effects to the matrix [50]. The combining effects led to improved sensitivity and reduced operating temperature. Further hybridization with catalytic Au nanocrystals—to form Au/Cr–WO3 hybrids—contributed to high sensitivity, fast speed, and reduced working temperature to acetone and benzene due to Au/Cr co-catalysts-enhanced surface reaction (Fig. 3a, b). The advantages of co-catalysts can improve even further the hybrid materials. A recent example of this approach is Pd/Sb nanocrystals modification of SnO2 that Sb and Pd functioned as anti-humidity and catalytic sites, respectively, which remarkably reduced humidity interference and improved responses toward H2 (Fig. 3c) [89, 90].
Fig. 3.
a SEM images of WO3 octahedron, b 2.5 at.% Cr–WO3 truncated octahedron and 10.0 at.% Cr–WO3 cuboid. Reproduced with permission [50]. Copyright 2015, American Chemical Society. c Humidity dependence of sensor response to 200 ppm hydrogen at 350 °C, using (black) undoped SnO2, (red) 0.1 mol % Sb-doped SnO2, and (green) 0.1 mol.% Pd-loaded and Sb-doped SnO2 nanoparticles. Reproduced with permission [89]. Copyright 2016, American Chemical Society. d TEM images of pure SnO2, 5Ag–SnO2, 10Ag–SnO2, and 50Ag–SnO2 NW after heating at 450 °C for 2 h and the corresponding response-recovery curves to ethanol gas. Reproduced with permission [51]. Copyright 2011, American Chemical Society. e, f SEM images of Pt–PS–SnO2 NTs (the inset is a schematic illustration). Reproduced with permission [52]. Copyright 2016, Wiley–VCH. g Pattern recognition by PCA using dataset from sensor arrays of PtM-decorated meso-WO3 NFs evaluating real and simulated (diabetes and halitosis) breath. Reproduced with permission [53]. Copyright 2017, Wiley–VCH. h TEM image of Pd nanoparticles@ZnO NSs and the corresponding response-recovery curves to acetone gas (red ZnO NSs). Reproduced with permission [54]. Copyright 2012, American Chemical Society. i Gas response of Pt/BP and pristine BP to various H2 concentrations (the inset shows the TEM images of Pt/BP). Reproduced with permission [55]. Copyright 2017, American Chemical Society. (Color figure online)
The catalytic effects of loaded catalysts on host-sensitive materials are associated with the contact between catalyst and gas. A gas diffusion-favoured structure can provide additional exposed surface areas and fast speed via a combination of surface- and structure-dependent factors. The introduction of catalytic Ag NCs via e-beam evaporation and calcination into quasi-1D heterostructures significantly enhances the response and selectivity to ethanol (Fig. 3d) [51, 91]. For even further performance, quasi-1D nanostructures with both porosity and sensitive nanobuilding blocks, namely mesoporous 1D nanofibers/tubes (meso-NF/NTs), have been reported [41, 92–94]. By introducing sacrificial polymeric colloids and protein-templated catalysts to the solutions, meso- and macro-porous Pt-decorated SnO2 NTs have been fabricated by electrospinning and sintering in sequence (Fig. 3e, f) [52]. The combined effects of porous nanostructures, fully depleted sensing areas and uniformly distributed Pt nanocatalysts on SnO2 NTs allow a highly selective detection of acetone (R5ppm = 192). Similarly, bimetallic PtM (M = Pd, Rh, and Ni) catalysts can be introduced to meso-WO3 NFs that are then highly selective detectors of acetone and H2S gas [53]. Sensors array combined with pattern recognition methods (so-called e-nose) based on three different PtM-decorated meso-WO3 NFs can accurately detect and discriminate the breath of a simulated biomarker through principal component analysis (PCA, Fig. 3g).
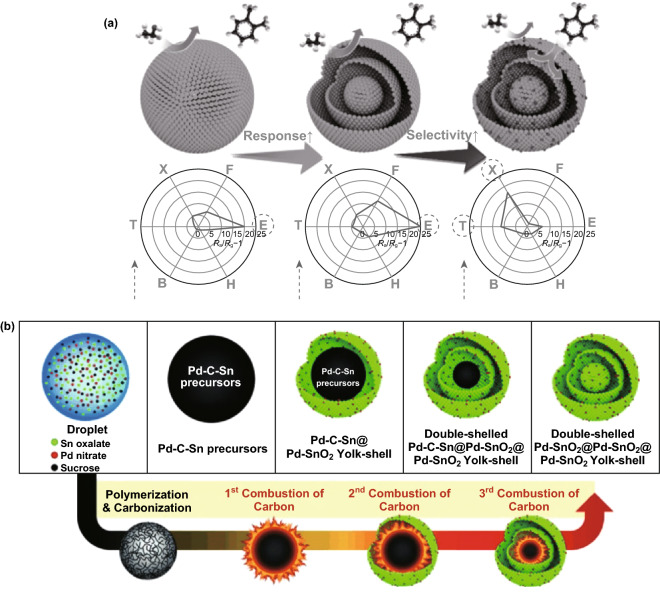
On the basis of intensive works on porous 2D ZnO nanostructures [37, 95, 96], Pd NCs have been deposited on porous 2D ZnO nanoplates (host materials) transformed by Zn5(CO3)2(OH)6 nanoplates to form 2D Pd/ZnO hybrid nanoplates (Fig. 3h); these acquire enhanced sensing properties [54]. Similarly, catalytic Pt can be used to decorate the surface of BP, which enables RT detection of H2 by Pt/BP at RT (Fig. 3i) [55]. When the structure of the host materials is further upgraded to 3D hierarchical porous (hp) nanostructures, a good gas diffusion platform is obtained with a large loading area of catalysts. By taking the advantage of the opals/polymeric beads or mesoporous silica/carbon/polymers templating method, hp-MOX thin films (3D hp nanostructures) with certain additives (catalysts) have been developed [97–102]. In this simple approach, hp-SnO2-inverted opal thin films loaded with mono-dispersed Pt catalyst (of uniform size of ~5 nm) were prepared (Fig. 4a) [103]. The improved sensing responses of Pt-doped SnO2-inverted opal thin films were achieved due to increased porosity, electronic sensitization, and synergism (Fig. 4b). Similarly, hp–Pt–WO3 or hp–Cr–WO3-inverted opal thin films have been successfully prepared (Fig. 4c) [104]. Due to the catalytic activation of N–H bond dissociation and effective gas diffusion within the macro-porous structures, excellent NH3 responses were obtained for the hp–Pt–WO3 sensor. As another example, well-controlled self-assembly of block copolymers such as poly(ethylene oxide)-blockpolystyrene (PEO–b–PS) could generate a perfect template with a highly ordered structure. Mesoporous WO3/Pt with a highly ordered and porous structure (inset of Fig. 4e) could be obtained by using this template and a 2-step pyrolysis process (Fig. 4d, including first treated in inert atmosphere and finally calcinated in air) [56]. Contributed to high surface areas (112–128 m2 g−1), large pore size (13 nm), and well-dispersed catalytic Pt NCs (~ 4 nm), the WO3/Pt-0.5 sensor had the highest response and fastest response-recovery speeds (Fig. 4e). Another category of nanostructures of host materials is multi-shell, yolk–shell, and multi-yolk–shell with hollow nanochamber [83–86]. With the hollow host nanochamber (SnO2) loading catalytic Pd through spray pyrolysis (Fig. 5a) [57], and showing the formation of double-shelled Pd–SnO2@Pd–SnO2@Pd–SnO2 yolk–shell spheres (Fig. 5b) [57], both sensitivity and selectivity were enhanced due to the unique hierarchical porous structure and uniformly exposed Pd catalysts. The representative works are summarized in Table 1.
Fig. 4.
a Schema of the one-step preparation of Pt-doped SnO2-inverted opal films, and b responses comparison of different sensors as a function of CO concentration at 350 °C (insets are the corresponding HRTEM micrographs). Reproduced with permission [103]. Copyright 2010, American Chemical Society. c Responses comparison of different sensors as a function of working temperature to 74 ppm NH3 gas (the inset is the corresponding HRTEM micrograph of Pt-WO3-inverted opal films). Reproduced with permission [104]. Copyright 2011, American Chemical Society. d Synthesis of ordered mesoporous WO3/Pt hybrids. e Gas response of WO3/Pt-0.5 and WO3/Pt-0 to different gases (hydrogen, CO, methane, ethanol, ammonia, acetone, benzene, and toluene) at 100 ppm and 125 °C in 55–60% RH (the inset is an FESEM image of the crystalline WO3/Pt-0.5 viewed from the top surface). Reproduce with permission [56]. Copyright 2018, Wiley–VCH
Fig. 5.
a Gas responses of dense SnO2 spheres, SnO2 yolk–shell spheres, and Pd-loaded SnO2 yolk–shell spheres to various analytical gases at 350-450 °C (B: benzene, H: H2, E: C2H5OH, F: HCHO, X: o-xylene, T: toluene). b Scheme showing the formation of double-shelled Pd-SnO2@Pd-SnO2@Pd-SnO2 yolk–shell spheres. Reproduced with permission [57]. Copyright 2014, The Royal Society of Chemistry
Table 1.
Representative works based on catalytic effects
| Materials | Gas detection | Detection range | Work temperature | Refs. |
|---|---|---|---|---|
| WO3/Pt | CO | 100–500 ppm | 125 °C | [56] |
| SiO2/In2O3 | NOx | 970 ppb–97 ppm | RT | [220] |
| Pd/WO3 | Acetone | 50 ppb–500 ppm | 300 °C | [221] |
| PdO/ZnFe2O4 | Acetone | 5–300 ppm | 275 °C | [222] |
| Au/LaFeO3 | Acetone | 2.5–40 ppm | 100 °C | [223] |
| Au NPs/ZnO | Athanol | 5–60 ppm | RT | [224] |
| Sm2O3/SnO2 | Acetone | 0.1–200 ppm | 250 °C | [225] |
| Pd/SWNT | CH4 | 6–100 ppm | RT | [226] |
| Pt/SnO2 NTs | CH3COCH3 | 10–100 ppb | 350 °C | [52] |
| Pd@SnO2 | H2S | 5–100 ppm | 290 °C | [227] |
| CuO/Pd | H2S | 1–100 ppm | 20-100 °C | [228] |
As aforementioned, greatly improved sensitivity and speed have been realized by well design on the dispersion of catalysts/co-catalysts and gas diffusion-favored structures of hybrid gas sensors based on catalytic effects. The remaining problems of operating temperature and selectivity might be resolved by further combination with charge transfer (Sect. 3.2) and molecule sieving layers (Sect. 3.5), respectively. Moreover, the newly developed single atomic metal and/or metal cluster-based catalysts with better catalytic effect might bring new understandings and chance in such areas [105–108].
Hybrid Gas Sensors Based on Charge Transfer Effects
Charge transfer happens between decorations and the host materials (good conductivity), which could vary the conductivity of the hybrid materials. This process improves sensitivity to the analysts at low temperature or even at room temperature (RT), accompanying fast response and recovery properties [4]. Discrete and uniform SnO2 NCs-decorated multi-walled CNTs (MWCNTs) (Fig. 6a) [59] gave high performances (response of ~ 180% to 100 ppm of NO2) at RT due to the abundance of active sites and easy electron transfer under the assistance of the well-matched work functions of SnO2 and MWCNTs. Liu et al. [109] used rGO instead of the carbon nanotubes. This researcher successfully synthesized SnO2 QDs/rGO hybrids by a one-step solvent thermal reaction at 180 °C (oleic acid and oleylamine as capping agents) (Fig. 6b) [109]. Due to co-effects of excellent gas adsorption of QDs, effective charge transfer between SnO2–rGO interfaces and the superb transport capability of rGO, the sensor responses in 2 s with fully recovery properties upon exposure to 33 to 50 ppm of H2S at RT (Fig. 6c). Meanwhile, the SnO2/rGO-based sensor showed an obvious enhanced response compared with the responses of pure SnO2- or rGO-based sensors toward H2S at 22 °C, in which the rGO acted as a host transducer material. By combining both advantages, 2D MoS2 sheets were hybridized with 2D graphene to form rGO/MoS2 aerogel with large surface areas, porous structure, and high electrical conductivity (Fig. 6d) [61]. Efficient and rapid charge transfer across the interface ensured enhancement and fast detection of NO2 than bare rGO or MoS2 (Fig. 6e). Ascribing to the high specific surface area of porous Cu2O nanowires networks and improved conductivity via effective charge transfer, rGO–Cu2O mesocrystals had much higher sensitivity to NO2 at RT, surpassing the performance of stand-alone systems of Cu2O and rGO sheets (Fig. 6f) [62].
Fig. 6.
a SEM image of the sensor after assembly of SnO2 nanocrystals onto the MWCNTs (inset is the HRTEM image of a MWCNT uniformly coated with SnO2 nanocrystals). Reproduced with permission [59]. Copyright 2009, Wiley–VCH. b TEM image of SnO2 QDs/rGO hybrids; and c sensor responses to 50 ppm of different gas at RT. Reproduced with permission [109]. Copyright 2016, American Chemical Society. d Enlarged TEM image demonstrating the MoS2 coating of the few-layer graphene scaffold. e Sensor response to 0.5 ppm NO2 at different microheater temperatures, improving response and recovery time. Reproduced with permission [61]. Copyright 2016, Wiley–VCH. f Sensitivities of NO2 sensor for the three devices (inset is a schema of the mechanism of NO2 sensing of rGO-Cu2O). Reproduced with permission [62]. Copyright 2012, American Chemical Society. g Schema of the sensing mechanism of rGO-PANI hybrids. h NH3 responses of PANI, rGO and their hybrids at different concentrations. i Repeated NH3 responses of PANI, rGO and their hybrids at 10 ppm. Reproduced with permission [63]. Copyright 2016, Royal Society Chemistry
CPs could also be applied in charge transfer hybrids by replacing the inorganic components. For instance, graphene was combined with PANI to form a hybrid thin film that had improved, reversible, and stable NH3 sensing (Fig. 6g, h). The fast electron transfer between hybrids and NH3, assisted by π–π interactions of PANI and rGO with low electron transfer energy barrier, led to more electrons transfer from PANI to rGO; this effectively improved the responsivity and response time (Fig. 6i) [63]. Up to date, the detecting gases are limited to strong reducing/oxidizing molecules such as NO2, NH3, and H2S, which hinders the widely application of such hybrid gas sensors. The critical points to overcome this shortcoming may be depicted as: (1) the chemical/electronic modification of the reported charge transfer based hybrids to improve the sensitivity and expand the types of detectable gases (i.e., Pt–SnO2/rGO, details in Sect. 3.6); (2) the development on the new candidate of chemi-resistive decorations with desired absorption–desorption process and well-tailored energy level/energy band gap structure, which always shows low thermal activation energy (< 0.5 eV, i.e., electronic conductive metal–organic frameworks (EC-MOFs), for details see Sect. 3.5). The representative works are summarized in Table 2.
Table 2.
Representative works based on charge transfer effects
| Materials | Gas detection | Detection range | Work temperature | Refs. |
|---|---|---|---|---|
| CuxO/multilayer graphene | NOx | 97 ppb–97 ppm | RT | [229] |
| rGO/NiO | NO2 | 0.25–60 ppm | RT | [230] |
| ZnO QDs/graphene | HCHO | 25–100 ppm | RT | [231] |
| SnO2/rGO | H2S | 10–100 ppm | RT | [109] |
| Graphite/polyaniline | NH3 | 50–1600 ppm | RT | [232] |
| SnO2/graphene | CH4 | 1000–10,000 ppm | 150 °C | [233] |
| SnO2 CQD/MWCNT | H2S | 3.3–100 ppm | 70 °C | [234] |
| rGO/TiO2–Nb | CO | 100–1000 ppm | 380 °C | [235] |
| Fe3O4@RGO | NO2 | 50 ppb–50 ppm | RT | [236] |
Hybrid Gas Sensors Based on Regulation of Charge Transport
Different from charge transfer that simply uses high charge transport capability of the highly conductive component, hybrid gas sensors based on regulation of charge carrier transport can manipulate the sensing properties by changing carrier concentrations, transportation mode, and/or pathways of charge transport.
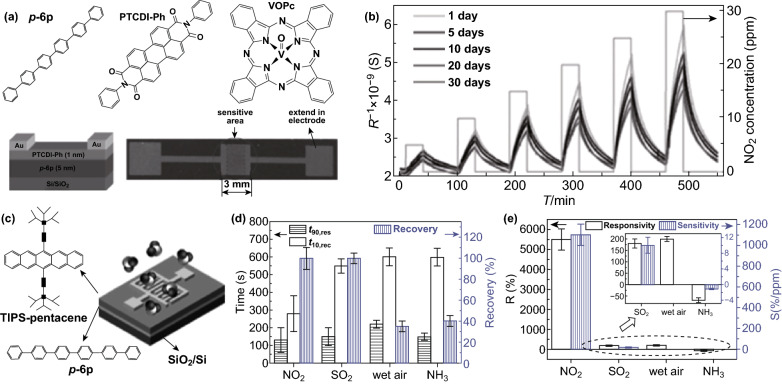
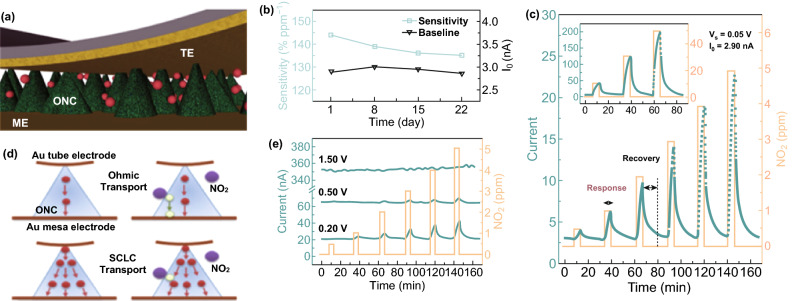
A simple and effective way of enhancing responsivity relies on controlling the charge transport by tuning carrier concentrations. For example, a PTCDI-Ph/p-6P ultrathin film was fabricated with a thickness of only 6 nm, of which 5 nm was attributed to (p-6p/p type) asymmetric thickness and 1 nm to (PTCDI-Ph/n type) (Fig. 7a) [64]. Electrons in the PTCDI-Ph were deprived by NO2, which simultaneously released the restricted hole in p-6p, and thus influenced the transportation of p-6p; this generated a NO2 sensing signal at RT (Fig. 7b). Chi et al. [64] thermal-deposited a high-quality crystalline terrace-like TIPS-pentacene film on p-6P that can easily be positively charged (Fig. 7c). The efficient charge transport ability and low original carrier concentration gave superb NO2 sensing in terms of both response/recovery speed (Fig. 7d) and responsivity/sensitivity (Fig. 7e) [64]. Impressively, when the transport direction of charge carriers changes from horizontal to vertical in a vertical diode (containing top/down electrodes and VOPc/F16CuPc layers, Fig. 8a), the sensor responded remarkably well to 0.5–5 ppm NO2 at RT (Fig. 8c), with an acceptable sensing stability (Fig. 8b) and wide linear working region [65]. More interestingly, when the voltage bias is raised from 0.2 to 1.5 V, the sensing ability weakened dramatically (Fig. 8d), being ascribed to the transportation change from ohmic to space charge limited current (SCLC) mode (Fig. 8e) [65], which may give guidance as to how to choose the bias to control the charge transportation for gas sensors to get them to work under the best conditions.
Fig. 7.
a Sensor device configuration and molecular structures of the materials. b The relative response of 1 nm PTCDI-Ph/5 nm p-6P film to NO2 pulses. The relative response curve is plotted as a function of time as the devices become exposed to different NO2 concentrations. Reproduced with permission [66]. Copyright 2013, Wiley–VCH. c Sensor device configuration and molecular structures of the materials. d The t90,res, t10,rec and relative recovery after 10-min N2 pulse of the responses to different gases. e Responsivity (R) and sensitivity (S) to different gases. Reproduced with permission [64]. Copyright 2017, Wiley–VCH
Fig. 8.
a Schema of the cross section of the tube/organic/mesa structure. VOPc nanopyramids (green cones) provide sufficient space for NO2 gas molecules to penetrate the structure. The red particles denote NO2 gas molecules. b Stability test in ambient atmosphere: sensitivity (left axis) and baseline (right axis) over time. c Response of periodic NO2 exposure. The device is operated under 0.05 V bias applied to the tube electrode. d Schematic diagram of charge transport and response to NO2 under ohmic transport (upper panel) and SCLC transport (lower panel) conditions. e Response of organic nanocrystal diode sensor (device 1) at 0.20, 0.50, and 1.50 V bias applied to the tube electrode. Reproduced with permission [65]. Copyright 2018, Wiley–VCH. (Color figure online)
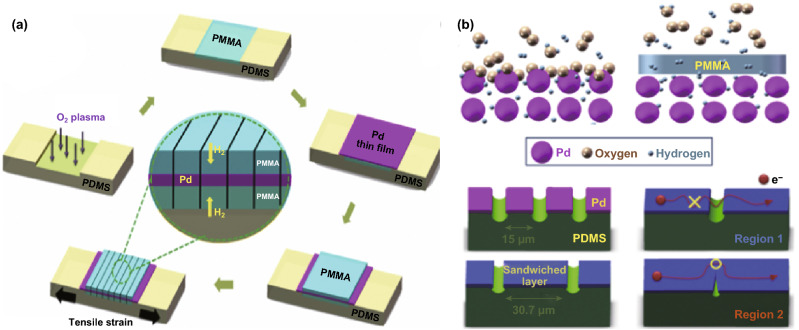
Another strategy to manipulate the properties of sensors based on charge transport is by regulating the conductive pathways of conductive-insulate hybrid materials via physical cracks, chemical bindings, or the phase of the component. More specifically, this approach relies indeed on regulating the electron hopping barrier, the interspace between conductive materials, phase-change or their combination. Insulating polymers can be combined with sensitive inorganic materials to fabricate highly sensitive and selective chemi-resistors (Fig. 9a). Hybrid thin films as sandwiched PMMA/Pd/PMMA (PMMA = poly(methyl methacrylate)) were prepared on a flexible substrate using sputtering and spin coating in sequence (Fig. 9a) [110]. Hybrid thin films with nanogaps formed by 25% mechanical stretching have very selectivity and sensitivity in detecting H2 against O2, ascribed to the selective penetration of H2 in PMMA membranes and the density reduction of the cracks formed in the trilayer of the hybrid thin films (Fig. 9b). Adoption of similar principles, but with higher effects, relies on films of GNPs coated with monolayers of thiols [111]. These structures were a good solution for VOCs sensing due to the swelling and shrinkage of molecular chains interacting with VOCs (Fig. 10a) [81]. Assembly of the GNP-based chemi-resistors with a wide variety of functional groups creates sensor arrays with different resistances that can be further varied after interacting with VOCs [81]. The transport of electrons, expressed in electrical resistance, can be dually regulated by controlling the interspaces between GNPs after applying strain to the GNPs-based film, which further influence sensitivities (Fig. 10b) [112]. CNTs are also used as the host with the surface coverage of molecular (MSPs) (inset of Fig. 10c) [80]. Such MSPs could create sensory devices with a dosimetric (time- and concentration-integrated) increase in electrical conductivity triggered by electrophilic chemical substances (Fig. 10c).
Fig. 9.
a Schematic diagram of the fabrication of nanogap sensors using PMMA/Pd/PMMA trilayer films on a PDMS substrate. b Schematic diagrams indicating the origins of the difference between the H2 detection limits of the Pd nanogap and the PMMA/Pd/PMMA hybrid nanogap sensors. Reproduced with permission [110]. Copyright 2014, Elsevier
Fig. 10.
a Schema of the working principles of gold NPs thiol-coated thiols gas sensor. Reproduced with permission [81]. Copyright 2014, Elsevier. b Schema of a strain regulated gold NPs-based gas sensor. Reproduce with permission [112] . Copyright 2015, American Chemical Society. c Response of SWCNT-based chemi-resistive sensors after 50-s exposure to different vapors in N2 (concentration in ppm in parentheses). The inset is the schema of the working mechanism. Reproduced with permission [80]. Copyright 2015, American Chemical Society
In summary, for cases where the resistance decreased upon exposure to target gas, the depression of the off current via carrier concentrations reduction, transportation mode changes and/or physical cracks might be the most effective way to realize high sensitivity. Simultaneously, as presented above, the component used for the controlling charge transport of host materials can further contribute to improved speed, long-term stability, and excellent selectivity. The representative works are summarized in Table 3.
Table 3.
Representative works based on regulation of charge transport
| Materials | Gas detection | Detection range | Work temperature | Refs. |
|---|---|---|---|---|
| TIPS-pentacene | NO2 | 0.2–20 ppm | RT | [64] |
| PMMA/Pd/PMMA | H2 | 600–6000 ppm | RT | [110] |
| PANI/SWCNT | NH3 | 1–100 ppm | RT | [237] |
| Oleylamine/Pt | Organic contamination | < 0.3 ppm | RT | [238] |
| Ionic liquids/CNT | Heptanal | 200 ppm | RT | [239] |
| Toluene | 1000 ppm | |||
| Ethanol | 1000 ppm | |||
| CNTs/hexa-peri-hexabenzocoronene bilayers | Decane | ~ 10 ppb | RT | [240] |
| Octane | ~ 15 ppb | |||
| Hexane | ~ 10 ppb | |||
| Ethanol | ~ 10 ppb | |||
| GNPs | Nonanal, styrene, ethanol, propionitrile | 50–1000 ppb | RT | [112] |
Hybrid Gas Sensors Based on Heterojunctions
Heterojunction is defined as the interface between two dissimilar semiconductors (one is the host, and the other one is the guest) that form a junction (n–n, p–p, p–n) linked with energy band structure due to the alignment of their fermi level. Notably, although the broad definition of heterojunctions covers all types of composites forming a junction in the interface, it is not clear enough for the well understanding of the complicated sensing mechanisms of composites sensing materials. Therefore, in this work, the narrow definition of manipulating/constructing heterojunctions is used, which excludes cases of catalytic effects, charge transfer, and charge carrier transport. According to the definition, the junction changes the interface potential energy barriers and regulates the transfer and/or injection of electrons and holes in a precise manner when it interacts with gas analytes. For example, n–n heterojunctions made of In2O3 hollow spheres (acetone-sensitive host) coated with CeO2 nanoparticles (humidity-sensitive guest) were synthesized and characterized as a chemi-resistive film. Exposing the layer to various gas analytes has shown selective detection of acetone in the presence of water, taking advantage of the chemical interaction between CeO2, In2O3, and water vapor, which greatly reduces the interfering effects of humidity (Fig. 11a) [113]. By modulating interface potential energy barrier between n–n junctions, as in the case of Fe2O3/TiO2 tube-like quasi-1D nanostructures synthesized through a multi-step hydrolysis (Ostwald ripening & thermal reduction), the corresponding sensing performance could be greatly improved (Fig. 11b) [114]. Combining modulation of electron transfer over the energy barrier at the perfect SnO2/ZnO heterojunction—fabricated by atomic layer deposition—and UV light generated electron–hole pairs, the sensitivity to NO2 could be improved using the SnO2/ZnO core-sheath nanowires (Fig. 11c) [115]. By introducing narrow band gap into the junction, such as in the case of In2O3 NCs to ZnO, a good response at visible-light conditions to gas analytes (e.g., formaldehyde) at RT (R100 ppm = 419%) [54] was attainable.
Fig. 11.
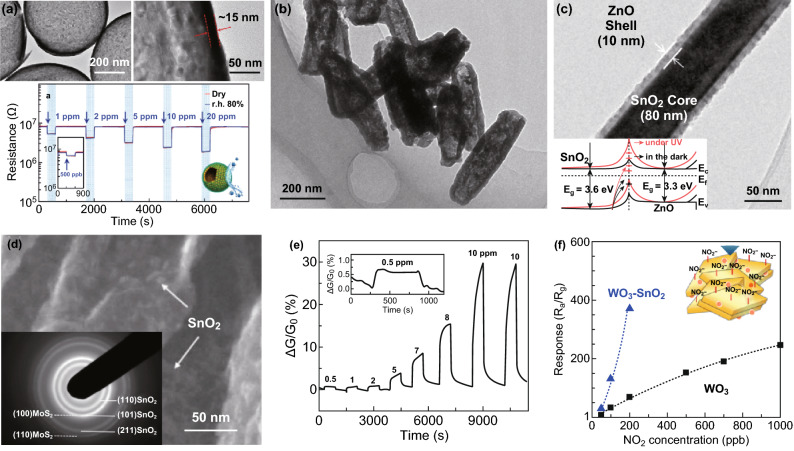
a TEM images and dynamic sensing transients of Ce–In2O3 hollow spheres exposed to 0.5–20 ppm of acetone at 450 °C in dry (red) and RH 80% (blue). Reproduced with permission [113]. Copyright 2016, Wiley–VCH. b TEM image of 1D Fe2O3/TiO2 tube-like nanostructures. Reproduced with permission [114]. Copyright 2012, American Chemical Society. c Low-magnification TEM image of SnO2/ZnO core–shell nanowires (the inset is the corresponding energy band diagram of the SnO2/ZnO system with/without UV light). Reproduced with permission [115]. Copyright 2013, American Chemical Society. d High-resolution SEM image showing that SnO2 NCs decorate on the MoS2 nanosheets (the inset is the corresponding SAED patterns), and e dynamic sensing response of the MoS2/SnO2 nanohybrids to different NO2 concentrations (the inset is the enlarged sensing response curve for 0.5 ppm NO2). Reproduced with permission [116]. Copyright 2012, Wiley–VCH. f Schematic of the sensing mechanism of WO3–SnO2 nanoplates and the corresponding responses comparison toward NO2 gas. Reproduced with permission [117]. Copyright 2014, American Chemical Society. (Color figure online)
The unique morphology (good compatibility with the devices), nanoscale thickness, and high surface area of 2D nanostructures make them promising as the host materials for chemi-resistive gas sensors. Hybrids of SnO2 NCs-decorated MoS2 nanosheet (MoS2/SnO2) were synthesized via hydrolysis-pyrolysis processes (Fig. 11d) with air stability [116]. The SnO2 NCs not only enhanced the stability of MoS2 nanosheets in dry air, but served as strong dopants for MoS2, leading to the changes of conduction channels in the MoS2 nanosheets (Fig. 11e). For further improvements in the sensing performance, introduction of porosity, such as in the case of WO3 lamella-based films loaded with mono-dispersed SnO2 QDs (~ 4 nm) (Fig. 11f), could reach high levels [117]. Experimental results show that the porous lamellar-structured WO3–SnO2 hybrid films could achieve high response to NO2 gas, ascribing the effective insertion of QDs into lamella stacks as a strong electronic sensitization.
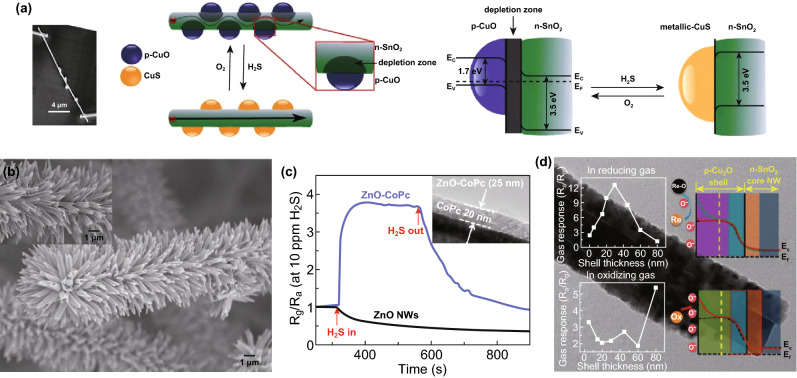
Compared with n–n heterojunctions, p–n heterojunctions provide a stronger manipulation on interface potential energy barriers, build-in electric field and additional catalytic effects in some unique cases. For example, exposing p–CuO nanoparticles loaded on CuO–SnO2 p–n nanowires to H2S transformed it to highly conductive CuS (Fig. 12a) [67], resulting in depleted region change (the p–n junction breakup) and second-order effects (the oxidation of H2S by absorbed oxygen) after p-CuO was reversibly generated and removed on the SnO2 surface. Without generating new chemical compounds, simply tuning the thickness of in situ oxidation layer, rich Te–Te or TeO2/TeO2 bridging point contacts and additional p–n heterojunctions (Te/SnO2) contributed to further excellent sensing performances (to CO and NO2) of the brush-like heterostructures (Fig. 12b) [118]. The nanorods of p-type coating layer can be replaced by continuous layer to form core-sheath hybrids processing radial modulation of potential energy barriers, for instance, both n-ZnO/p-CoPc (cobalt phthalocyanines, Fig. 12c) [119] and n-SnO2/p-Cu2O (Fig. 12d) [120]. Core-sheath NRs have better sensitivity of the target gases.
Fig. 12.
a SEM image of a single hybrid NW and the schematic illustration of gas-sensing mechanism of CuO–SnO2 p–n nanowires. Reproduced with permission [67]. Copyright 2013, Elsevier. b SEM images of the as-grown Te-coated SnO2 brush-like products prepared at source temperatures of 560 °C. Reproduced with permission [118]. Copyright 2014, American Chemical Society. c The dynamic response-recovery curves for ZnO and n-ZnO/p-CoPc to 10 ppm of H2S (the inset is the TEM image of n-ZnO/p-CoPc). Reproduced with permission [119]. Copyright 2015, American Chemical Society. d TEM image of 1D SnO2/CuO nanostructures (left insets are responses to reducing and oxidizing gas as a function of shell thickness, respectively; right insets are the corresponding sensing mechanism). Reproduced with permission [120]. Copyright 2015, American Chemical Society
Conductive polymers (CPs, e.g., PPy, poly(3,4-ethylenedioxythiophene) (PEDOT), PANI) that are p-type components of diverse types of p-n heterojunctions can work at RT or low operating temperature with different working principles. First, PPy-ZnSnO4, p–n hybrid nanoparticles, can enhance the NH3 sensing performance (3–4 times higher) compared with pure PPy and ZnSnO4 (Fig. 13a) [121]. The concentration of NH3 can be quantitatively detected (Fig. 13b) with shorter time of response (26 s) and recovery (24 s) (Fig. 13c). The overall improved performance has been ascribed to the p–n heterojunction, in which the holes at high concentration in PPy and the electrons in Zn2SnO4 diffuse into each other to form a built-in electric field of a depletion layer (Fig. 13d). Interaction between Zn2SnO4–PPy and NH3 broadens the depletion layer, which determines the response, and the speed of response/recovery. When the p–n junction was reinforced as dual p–n junctions (p–n–p) in the hybrids of the hollow In2O3 nanofibers (NFs) and PANI (Fig. 13e), the performances were further enhanced (Fig. 13f) [122]. For CP-based chemi-resistive heterojunctions, unsatisfied sensitivity (response) and long-term stability will be two challenging issues for researchers.
Fig. 13.
a NH3 response to PPy-Zn2SnO4, PPy, and Zn2SnO4. b Curve of concentration versus response. c Curve of a single response and recovery to NH3. d Schema of sensing mechanism. Reproduced with permission [121]. Copyright 2018, Elsevier. e Schema of sensing mechanism. f Gas response of PANI, solid In2O3/PANI and hollow In2O3/PANI. Reproduced with permission [122]. Copyright 2016, Springer
Obvious advantages of heterojunction-type chemi-resistive hybrids-based gas sensor can be summarized as: (1) higher sensitivity due to manipulations of the potential energy barrier formed by band bending of different components (e.g., Fe2O3/TiO2 tube-like quasi-1D nanostructures (n–n) [114], n-ZnO/p-CoPc [119], and n-SnO2/p-Cu2O [120] core-sheath NRs); (2) improved selectivity to some gases (e.g., CuO–SnO2 p–n nanowires to H2S [67]); (3) promising, although limited so far, to anti-interference gas (e.g., CeO2–In2O3 hollow spheres with anti-humidity properties [113]); (4) avoiding UV-introduced ozone and performance degradation (for example, when narrow-band guest material hybrids with semi-conductive host materials, e.g., CdS-ZnO [123, 124], ZnO-CdS [125], CdSe-ZnO [126], the room operation temperature can be achieved by the visible-light-driven gas sensing). The representative works are summarized in Table 4.
Table 4.
Representative works based on heterojunctions
| Materials | Gas detection | Detection range | Work temperature | Refs. |
|---|---|---|---|---|
| α-Fe2O3/SnO2 | Acetone | 10–2000 ppm | 250 °C | [241] |
| ZnO/SnO2 | NO2 | 200–2000 ppb | RT | [242] |
| SnO2/SnS2 | NO2 | 1–8 ppm | 80 °C | [243] |
| SnO2/α-Fe2O3 | Ethanol | 20–100 ppm | 225 °C | [244] |
| ZnO/ZnFe2O4 | Acetone | 5–700 ppm | 250 °C | [245] |
| α-Fe2O3/NiO | Toluene | 5–100 ppm | 300 °C | [246] |
| SnO2/SnS2 | NH3 | 10–500 ppm | RT | [247] |
| TiO2 QDs/NiO | NO2 | 5–60 ppm | RT | [248] |
| ZnO/MoS2 | Acetone | 10–500 ppb | 350 °C | [249] |
| ZnO/ZnCo2O4 | Acetone | 50–300 ppm | 175 °C | [250] |
| Si/SnO2 | H2S | 10–50 ppm | 100 °C | [251] |
| SnO2@PANI | NH3 | 10 ppb–100 ppm | RT | [252] |
| NiO@SnO2 | H2S | 0.1–50 ppm | 250 °C | [253] |
| SnS2/SnS | NO2 | 0.125–8 ppm | RT | [254] |
| SnO2/Sn3O4 | NO2 | 20 ppb–50 ppm | 150 °C | [255] |
| ZnO/ZnCo2O4 | Acetone | 10–100 ppm | 275 °C | [256] |
| In2O3/ZnO | HCHO | 5–100 ppm | RT | [257] |
In summary, the critical points to achieve better performance in heterojunction-based gas sensor are depicted as: (1) maximum effective contact areas of the interfaces via surface and structure design; (2) matched band structure to facilitate the manipulation of potential energy barrier; (3) additional capability of catalysis of guest additives to host materials; (4) visible-light-driven photocatalytic abilities and good charge carriers separations for light-driven n–n or n–p hybrids; (5) selectivity-improvement-purposed heterojunction design based on specific interaction (e.g., Pd–H2, CuO–H2S, PPy–NH3) or n–p response-type reversion (Co3O4–SnO2 p–n junctions for H2) [127].
Hybrid Gas Sensors Enhanced by Molecular Probing and Sieving Effect
Functionalization, coating or doping in/on the sensing materials, i.e., introduction of the sensing probe, was demonstrated as an effective way of improving the selectivity and specificity through a one-lock one-key binding or structure similarity-based combination. Both the inorganic and organic probes have been well developed. The sieving of interferon, especially the humidity in the environment, is an alternative way of improving specificity.
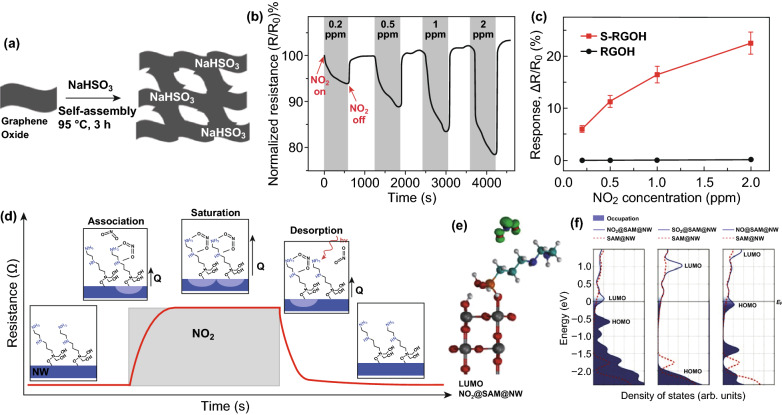
A 3D sulfonated rGO hydrogel (S-RGOH)-based gas sensor combining chemical functionalization and porous structures was synthesized in a one-step hydrothermal reaction (Fig. 14a) [68]. Addition of a NaHSO3 probe dramatically enhanced the response (Fig. 14c) of NO2 with fast recovery (Fig. 14b), assisted by the porous structures of the graphene host. Self-assembled monolayers (SAMs) with suitable alignment of the gas–SAM frontier molecular orbitals (Fig. 14e) with respect to the SAM–NW Fermi level (Fig. 14f); this led to high selectivity and sensitivity to analyte gas [70]. SnO2 NWs were modified with amine-terminated SAM and applied as light-driven chemi-resistors working at RT, achieving good NO2 sensing performance, the schematic mechanism of which can be found in Fig. 14d. This concept was extended to porous MOX nanostructures for further enhancements of their sensing properties. APTES-modified porous WO3 nanotubes (P-WO3 NTs (10%)@APTES) performed the best sensitivity and selectivity (Fig. 14a), which can be ascribed to the large surface area and high gas diffusion rate provided by P-WO3, and selective reaction between NO2 and surface SAM with APTES (Fig. 15b, c). The existence of SAM on the surface of inorganic materials (except 2D nanomaterials) limits the working temperature, which greatly weakens the sensing performance, although it could be resolved by UV irradiation. Using conductive polymer as the host material with surface SAM functionalization by the “1-stone 2 birds” strategy was promising and novel (Fig. 15d, e) [72]. Superb sensing performances were achieved by combining RT sensitivity of CP and good selectivity of SAM (Fig. 15f) [72].
Fig. 14.
a Schema of the synthesis of 3D chemically modified graphene hydrogel. b Dynamic response of the 3D S-RGOH sensor versus time after exposure to 0.2–2 ppm NO2. c Plots of the quantitative responses of the S-RGOH and RGOH sensors versus NO2 concentration. Reproduced with permission [68]. Copyright 2017, Wiley–VCH. d, e Schematic illustration of the NO2 sensing mechanism by en-APTAS 1-modified NWs. f Density of states (DOS) of the en-APTAS 1-modified SnO2 with adsorbed NO2, SO2, and NO. The Fermi levels of the different systems are set at 0 eV. For comparison, the DOS of the en-APTAS 1-modified SnO2 NW without an adsorbed gas molecule (dashed red line) is shown in each graph. Reproduced with permission [70]. Copyright 2014, Wiley–VCH
Fig. 15.
a Selectivity comparison of different gas sensors at the corresponding operating temperature. Schematic illustration of the NO2 sensing mechanism of sensors based on b P-WO3 NTs (10%) and c P-WO3 NTs (10%)@APTES. Reproduced with permission [71]. Copyright 2018, Royal Society of Chemistry. d The schema of silane-coated PEDOT:PSS sensor. e The molecular chain of different silane-coated on PEDOT:PSS. f Response to VOCs. Reproduced with permission [72]. Copyright 2018, Royal Society of Chemistry
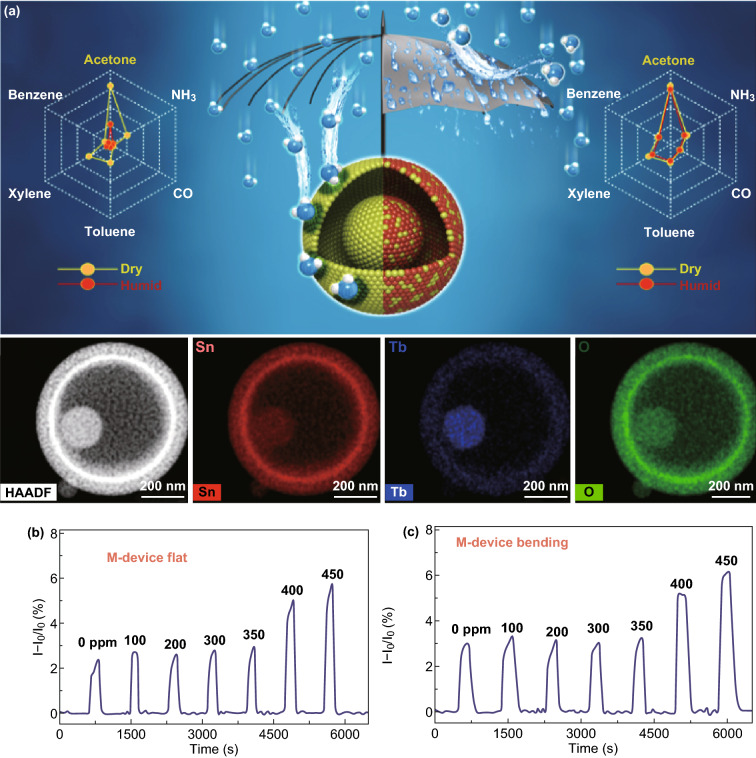
Figure 16a shows the low cross-sensitivity to humidity and other interferon gases by refreshing the regenerative surface involving the interaction between facile redox fair (Tb3+/Tb4+) and surface OH group (or water vapor) on SnO2. This 5 Tb–SnO2-based chemi-resistor achieved high response to acetone exposure [128]. The oleic acid SAM also was effective in screening the effect of humidity (< 350 ppm) when it was layered on PANI surface (Fig. 16b, c) [82]. Although it is not enough for practical application, this demonstration is still valuable in pointing to a promising way to eliminate the interferon of humidity.
Fig. 16.
a Polar plots of the gas responses of pure SnO2 and 5 Tb–SnO2 sensors exposed to different gases (at 20 ppm) in dry (yellow) and RH 80% (red) atmospheres, and EDS elemental mapping of Sn, Tb, and O in 5 Tb–SnO2. Reproduced with permission [128]. Copyright 2018, American Chemical Society. b, c Response curves to 1-hexanal at different humidity in bending and flat states. Reproduced with permission [82]. Copyright 2018, Wiley–VCH. (Color figure online)
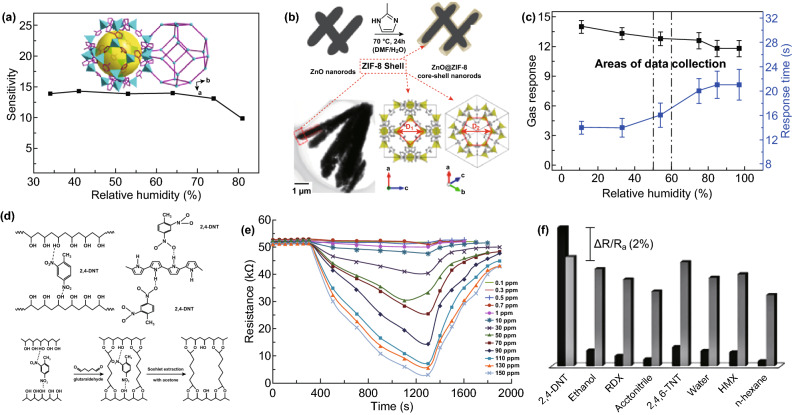
Recently, MOF materials are great opportunity in generating sub-nanometer or nanometer pores with high uniformity. Neat MOFs chemi-resistors were prepared based on hydrophobic MOF (ZIF-67), which showed selective response to VOCs, with slightly interfering effects of humidity (Fig. 17a) [129, 130]. ZnO@ZIF-8 core-sheath NRs powders were synthesized by hydrothermal reaction using a self-template strategy (Fig. 17b) [131]. The chemi-resistive gas sensor based on the thick film of ZnO@ZIF-8 hybrids had satisfactory sensitivity and response time to 100 ppm formaldehyde, even under interfering humidity (Fig. 17c). Mixing the CP with molecularly imprinted polymer (MIP) molecular (Fig. 17d) was another effective approach to improve not only the responsivity, but selectivity (Fig. 17f) [132]. Responses of interferon (2,4,6-TNT) and analyte (2,4-DNT) were suppressed and enhanced, respectively, although they are of very similar molecular structure and functional group [132]. However, the speed of response and recovery showed no obvious change (Fig. 17e) [132]. Instead of using functional MOFs as filter film coating on sensing materials to provide additional selectivity and/or sensitization, EC-MOFs are novel emerging materials with regular porosity and conductivity, which are promising for chemi-resistors with high sensitivity and selectivity [76, 133–158]. Unlike MOX and MOX-MOFs, which still need additional thermal or photonic energy as the trigger source to activate the sensing reaction, EC-MOFs can be directly used as sensitive materials based on their regular micro-porosity, selective frameworks, high electronic conductivity, and RT activity [139, 144–146, 159–166]. Therefore, EC-MOFs are promising components for hybrid gas sensors and will be powerful competitors for the new generation of gas sensors.
Fig. 17.
a Effect of environmental humidity on sensitivity of ZIF-67 sensor (inset is the SOD-type structure of ZIF-67). Reproduce with permission [129]. Copyright 2014, American Chemical Society. b Schematic diagram of the ZnO@ZIF-8 NRs synthesized with ZnO NRs as a template; and c gas response and response time for 100 ppm formaldehyde as a function of the relative humidity. Reproduced with permission [131]. Copyright 2016, American Chemical Society. d Schema of the interaction of 2,4-DNT with the hybrid ingredients (PVA, PPy, and MIP). e Response of a fabricated chemi-resistor sensor coated with PVA/PPy/MIP hybrids with respect to the 2,4- DNT explosive vapor. f Column curves of PVA/PPy/MIP hybrids to different organic compounds. Reproduced with permission [132]. Copyright 2018, Wiley–VCH
As mentioned above, the introduction of molecular probing and sieving effect can effectively overcome the poor selectivity problem of chemi-resistors. Up to date, only a few organic/inorganic probe or porous materials with molecule sieving effects have been applied to chemi-resistors to realize simple guest–matrix interaction (e.g., -NH2 group with NO2, –NO2 group with NH3, NaHSO3 with NO2) or molecule rejection (e.g., anti-humidity, gas molecules with large kinetic diameter). Such cases are ultra-small fraction of the state-of-art gas molecule adsorption and separation areas. More guest–matrix interactions (e.g., van der Waals interactions, hydrogen bond, π–π interactions, weak acid–base interactions) and gas separation design (e.g., channel traffic effects, framework flexibility), that have been well studied on rGO, polymers (e.g., metal-induced ordered microporous polymers (MMPs), covalent-organic frameworks (COFs), conjugated mesoporous/microporous polymers (CMPs)), MOFs, can be introduced to chemi-resistors for advanced sensing performances. The representative works are summarized in Table 5.
Table 5.
Representative works based on molecular probing and sieving effect
| Materials | Gas detection | Detection range | Work temperature | Refs. |
|---|---|---|---|---|
| ZnO@ZIF-71 | Benzene | 10–200 ppm | 250 °C | [258] |
| ZnO@ZIF-CoZn | Acetone | 0.25–100 ppm | 260 °C | [259] |
| ZnO@ZIF-8 | H2 | 5–50 ppm | 250 °C | [260] |
| ZnO@ZIF-8 |
Propene Ethene |
250 ppm | RT | [261] |
| Polyoxometalate @ZIF-8@ZnO | HCHO | 25–200 ppm | RT | [262] |
| ZnO@ZIF-8 | HCHO | 10–200 ppm | 300 °C | [263] |
| ZnO@ZIF-8 | H2 | 10–50 ppm | 300 °C | [264] |
Hybrid Gas Sensors Based on Combined Mechanisms
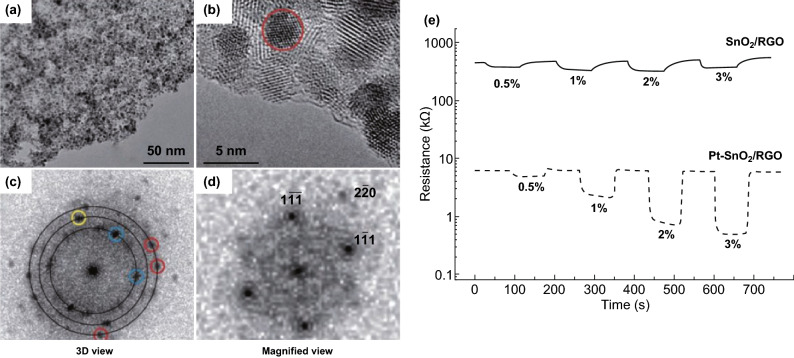
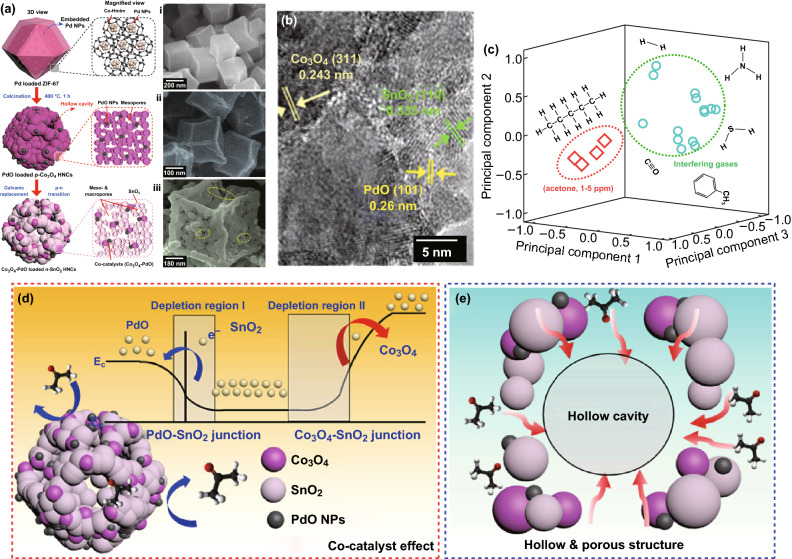
In many cases, multi-forms working on the hybrid materials can simultaneously and dramatically improve sensitivity and responsivity. When the heterojunction barrier (the SnO2/rGO heterostructure interface) was combined with catalytic Pt, sensitivity of the SnO2/rGO hybrids to H2 was greatly enhanced (Fig. 18a–e) [74]. Hydrogen ranging from 0.5 to 3% in air could be quantitatively detected at near RT with response and recovery times of 3–7 and 2–6 s, respectively. Furthermore, when the catalytic effect was co-working with the p-n heterojunctions and porous structure (Fig. 19a), Co3O4–PdO loaded on n-SnO2 hybrid hollow nanocubes (Fig. 19b) reached selectivity and response amplitudes for the detection of acetone superior to those MOF-derived metal oxide sensing layers previously reported. Accordingly, the sensor arrays (Co3O4-loaded n-SnO2 HNCs and Co3O4–PdO-loaded n-SnO2 HNCs) can clearly distinguish 1 ppm humid acetone molecules among the seven interfering analytes (Fig. 19c). The reason is electron migration from n-SnO2 to PdO or p-Co3O4 in the multi-junctions significantly influencing the electron depletion regions, which leads to the superb sensitivity (Fig. 19d, e).
Fig. 18.
a TEM and b HRTEM images of Pt-SnO2/rGO. Power spectrum of c the whole HRTEM image and d the region of the HRTEM image indicated by the red circle. e Hydrogen-sensing performance at 50 °C of the materials under investigation: variation in the resistance of the rGO/SnO2 (solid line) and rGO/SnO2/Pt sensors (dashed line) with pulses of hydrogen at 0.5, 1, 2, and 3%. Reproduced with permission [74]. Copyright 2012, Wiley–VCH
Fig. 19.
a Schematic illustration and SEM images of synthetic process for the n-SnO2 HNCs functionalized with Co3O4 and PdO. b HRTEM lattice spacing image of SnO2–PdO-loaded p-Co3O4 HNCs. c Pattern recognition based on PCA using sensor arrays (Co3O4-loaded n-SnO2 HNCs and Co3O4–PdO-loaded n-SnO2 HNCs). Schematic diagrams of d gas-sensing reaction derived from co-catalyst effect, and e magnified sensing mechanism on the porous surface of HNC. Reproduced with permission [75]. Copyright 2017, Wiley–VCH
When the catalytic effect co-worked with the molecular sieving effect, improved selectivity (anti-interferon) with enhanced sensitivity can be achieved by coating a layer of hydrophobic and thermally catalytic bimetallic ZIF-CoZn thin film on ZnO to form core-sheath MOX@MOFs nanowire arrays (NWAs) (Fig. 20a) [76]. The bimetallic ZIF-CoZn MOF sheathes gave good thermal stability (ZIF-8(Zn)) and excellent thermal catalytic ability on ZnO (ZIF-67(Co)), as well as hydrophobic channels. By combining their advantages, the ZnO@ZIF-CoZn preparation showed greatly enhanced performance on selectivity (good anti-humidity, Fig. 20b) and also on its response, response and recovery behavior and working temperature (Fig. 20c). More complicated hybrid nanostructures containing MOX, plasmonic/catalytic NMs, and hydrophobic MOFs, i.e., the dual-functional Au@ZnO@ZIF-8 Janus structure (Fig. 20d, e), have been fabricated [77]. Au@ZnO@ZIF-8 hybrids had enhanced selective adsorption, detection and oxidation of HCHO and prevented interference from gases such as H2O and toluene (Fig. 20f), where Au NRs helped to generate charge carriers on a ZnO surface under visible-light irradiation. The representative works are summarized in Table 6.
Fig. 20.
a Schematic illustration of ZnO@ZIF-CoZn core-sheath NWAs sensor; b Response-recovery curves to acetone at different concentrations in dry air and in 10 ppm acetone with different relative humidity and at 260 °C; and c temperature-dependent responses of ZnO@5 nm ZIF-CoZn. Reproduced with permission [76]. Copyright 2016, Wiley–VCH. d Plots of Au@ZnO and Au@ZnO@ZIF-8 size versus time; insets are the representative TEM images of the products at specific times. e Kinetics of HCHO adsorption (solid symbols). f Proposed mechanism of oxidation of HCHO into HCOOH. Reproduced with permission [77]. Copyright 2018, Springer
Table 6.
Representative works based on combined mechanisms
| Materials | Gas detection | Detection range | Work temperature | Refs. |
|---|---|---|---|---|
| Co3O4/PEI-CNTs | CO | 5–1000 ppm | RT | [265] |
| HC(NH2)2SnI3/SnO2/Pt | HCHO | 5–100 ppm | 80 °C | [266] |
| PdO/SnO2/CuO | CO | 100–2000 ppm | 200 °C | [267] |
| Pd/ZnO/In2O3 | H2 | 50–172 ppb | 350 °C | [268] |
| rGO/ZnO/Pd | CH4 | 25–500 ppm | RT | [269] |
| Pt/ZnO/g-C3N4 | Ethanol | 0.5–50 ppm | 250 °C | [270] |
| NO2 | 0.5–15 ppm | 150 °C | ||
| Au/Cu2O/ZnO | NO2 | 5–1000 ppb | RT | [271] |
| Ag/SnO2/rGO | Ethanol | 100–2000 ppm | 280 °C | [272] |
| TiO2/InVO4 | NH3 | 10–1000 ppm | 250 °C | [273] |
| Pd–SnO2/rGO | CH4 | 800–1200 ppm | RT | [274] |
| Au@In2O3@PANI | NH3 | 0.5–100 ppm | RT | [275] |
| Au–ZnO@ZIF-DMBIM | Acetone | 0.0001–1000 ppm | RT | [203] |
| SnO2/α-Fe2O3/Pt | Styrene | 0.25–1.25 ppm | 206 °C | [276] |
Summary and Perspective
Summary of Hybrid Gas-Sensitive Materials
The current progress report reviews advances and the advantages of the chemi-resistive hybrid nanomaterials compared with the single constituent, according to five main sensing mechanisms: manipulating/constructing heterojunctions, catalytic reaction, charge transfer, charge transport, molecular binding/sieving, and their combinations. Table 7 lists typical chemi-resistive materials for hybrid gas sensors categorized by types of materials and conductivity.
Table 7.
Typical chemi-resistive materials for hybrid gas sensors
| Types of chemi-resistive materials | Types of conductivity | ||
|---|---|---|---|
| n | p | n/p | |
| Inorganic compounds | ZnO, SnO2,TiO2, MoO3, WO3, In2O3, V2O5, Ta2O5, Nb2O5, RuO2, MoS2, ZnSnO3 | NiO, Co3O4, TeO2, CuS, Cr2O3, Sb2O3, CuO, Cu2O, Mn2O3, CeO2, PdO, Ag2O, Bi2O3, CoPc. WS2, MoSe2, LaFeO3 [277] | Fe2O3 |
| Organic compounds | PTCDI-Ph | PPy, PEDOT, PANI, p-6P, Ti3C2Tx [278] PADS [279] | Polyphenylene [280] |
| MOFs | CuHITP | Cu-HHTP, NiHITP, NH2-UiO-66 [281],Cu-HHTP-THQ [204] | |
| Others | CNTs, BP, rGO | ||
Applications of Chemi-Resistive Sensor-Based e-nose
As the first commercial gas sensor, metal oxide-based chemi-resistors still occupy a leading role in both fundamental researches and commercial devices. Various commercial chemi-resistive gas sensors based on single or hybrid materials have been developed for the detection toward target gases (toxic, flammable, VOCs, explosive, H2, etc.) ranging from sub-ppm to saturated vapor, which are widely used in fields including environment monitoring, medical care, food industry, agriculture production, and public security. The versatile commercial chemi-resistive gas sensors are introduced but not limited as follows.
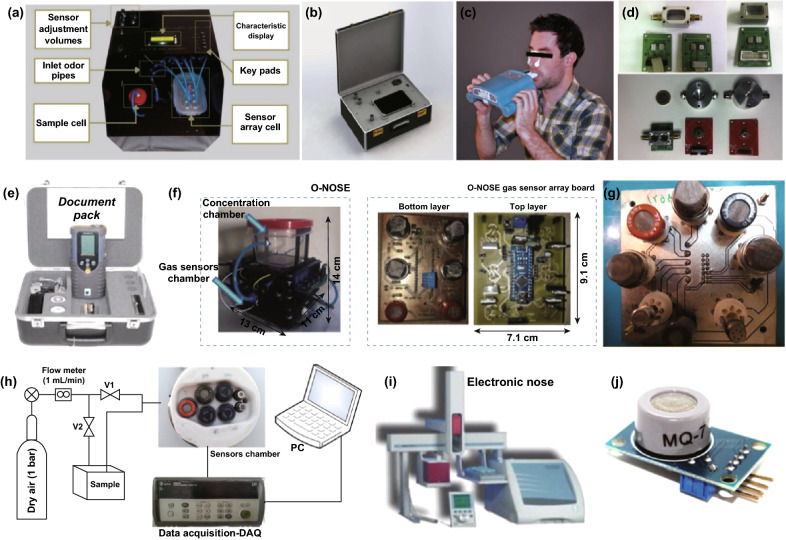
Some e-nose systems comprised of chemi-resistive sensor arrays have succeeded in the application of medical care. Commercial e-nose PEN3 (Airsense Analytics GmbH, Schwerin, Germany) made up of a gas sampling unit and a sensor array (10 different metal oxide thick film sensors (MOS)) can screen colorectal cancer (CRC) and polyps [167]. Another 14 commercial gas sensor-integrated e-nose system could generate characteristic “breath fingerprints” by exhalation components and could diagnose the lung disease through pattern recognition of a “breath fingerprint.” Those sensors categorized as MOS, hot wire gas, catalytic combustion gas, and electrochemical gas sensors are produced by the manufacturers, Hanwei (Fig. 21a), Figaro (Fig. 21b) [168], Winsen, Nemoto and Alphasense [169]. Aeonose in (Fig. 21c) [170] is a CE-certified, handheld, and battery-powered e-nose device designed by a Zutphen Company in Netherlands. The aeonose comprises three micro-hotplate metal oxide sensors and a pump to detect gastric cancer from exhaled breath [171]. Sunshine Haick Ltd. have successfully designed the sensor arrays to diagnose lung and gastric cancer via pattern analysis of exhaled VOCs, which has made great and has a perfect perspective. Other representative commercial e-nose in clinical diagnosis of complex regional pain, diabetics, head and neck cancer, dyskinesia, and prostate cancer are summarized in Table 8.
Fig. 21.
Schematic of the diffferent commercial e-nose. a Photographic image of Hanwei e-nose. Reproduced with permission [212]. Copyright 2017, Springer. b E-nose system “NOS.E” produced by Figaro Engineering Inc. [168]. Copyright 2018, IEEE. c Aeonose to diagnosis prostate cancer. Reproduced with permission [170]. Copyright 2018, European Association of Urology. d E-nose produced by institute of Physics Technology and Information, Spanish Council for Scientific Research. Reproduce with permission [213]. Copyright 2018, Elsevier Ltd. e The picture of Cyranose 320. Reproduce with permission [214]. From Chang and Heinemann, Copyright 2018, ASABE. f The sensors manufactured by Hanwei Sensors. Reproduced with permission [215]. Copyright 2019, Elsevier Ltd. g E-nose produced by Figaro Engineering, Inc., Hanwei company and FIS Inc. Reproduced with permission [216]. Copyright 2018, Elsevier Ltd. h E-nose based on MOS TGS and FIS sensors. Reproduced with permission [217]. Copyright 2018, sensors. I Fox 3000 electronic nose system. Reproduced with permission [218]. Copyright 2017, Elsevier Ltd. j MQ-7 (TORO) sensor model [219]
Table 8.
Applications of electronic nose instruments for disease diagnosis
| Diseases | Objective | E-nose configuration | Sensor type | Sensor arrays | Multivariate data analysis | Refs. |
|---|---|---|---|---|---|---|
| Armpit body odor | Detection and classification of human body odor | Tagushi (TGS) gas sensor from Figaro Engineering Inc. | MOS | 5 | PCA | [282] |
| Bile acid diarrhea (BAD) | Identify BAD in volatile organic compounds | The FOX 4000 e-nose from Alpha MOS, Toulouse, France | MOS | 18 | LDA | [283] |
| Lung cancer | Diagnosing lung cancer in exhaled breath | Cyranose 320 from Smiths Detection Inc., Edgewood, MD, USA | CP | 32 | LDA,KNN, PNN, NB, and SVM | [284] |
| Head/neck and lung carcinomas | Discriminating head and neck carcinoma from lung carcinoma | Aeonose from Zutphen, the Netherlands | MOS | 3 | PARAFAC and TUCKER | [285] |
| Prostate Cancer | The detection of prostate cancer from exhaled breath | Aeonose from Zutphen, the Netherlands | MOS | 3 | ANN | [170] |
| Complex Regional Pain | Diagnosing complex regional pain syndrome | Aeonose from Zutphen, the Netherlands | MOS | 3 | ANN | [286] |
| Mycobacterium tuberculosis | The detection of mycobacterium tuberculosis | ModelBH114-Bloodhound sensors from Leeds, UK | CP | 14 | PCA | [287] |
| Patients breath | The VOCs from breath | E-nose from Sunshine Haick medical Co. | GNPs capped with thiols | 8–20 | PCA, LDA | [112, 288] |
MOS metal oxide semiconductor, CP conducting polymer, GNPs gold nanoparticles
Commercial e-nose has acted as an indispensable instrument for the rapid, accurate, and overall-process assessments of food health and quality aim at adulterated counterpart, contamination and spoilage [172]. An PEN-2 e-nose (WMA Airsense Analysentechnik GmbH, Schwerin, Germany) composed of 10 different metal oxide sensors was utilized to monitor the adulteration of milk with water or reconstituted milk powder [173]. Also, the PEN-2 is used to monitoring the change in volatile production of mandarin during different picking-date [174]. Meanwhile, PEN-2 is used to characterize espresso coffees brewed with different thermal profiles [13]. MOS sensors manufactured by Figaro (Figaro Inc., Japan) were used to recognize odors emitted from different stages in a waste water treatment plant [175]. Tagushi gas sensor based on metal oxide semiconductor from Figaro Engineering Inc. is used to classify the tea aroma [176]. Cyranose 320 in Fig. 21e that consists of an array of 32 thin-film carbon-black conducting polymer sensors was used to identify odor emitted from dairy operations. The portable e-nose based on thin-film semiconductor (SnO2) sensors (Hanwei Sensors) in Fig. 21f can perform early detection of wine spoilage thresholds in routine tasks of wine quality control. An e-nose system (Fig. 21g) was used to detect detergent powder in raw milk. Representative applications are summarized in Table 9.
Table 9.
Applications of electronic nose for monitoring foods and beverage
| Product | Objective | E-nose configuration | Sensor type | Sensor arrays | Multivariate data analysis | Refs. |
|---|---|---|---|---|---|---|
| Black tea | Monitoring of black tea fermentation process | Tagushi (TGS) gas sensor from Figaro Engineering Inc. | MOS | 8 | PCA, 2NM MDM | [289] |
| Pork | Measurement of total volatile basic nitrogen (TVB-N) in pork meat | Tagushi (TGS) gas sensor from Figaro Engineering Inc. | MOS | 11 | PCA and BP-ANN | [290] |
| Green tea | Identification of coumarin-enriched Japanese green teas and their particular flavor | E-nose device (FF-2A Fragrance & Flavor Analyzer, Shimadzu, Japan) | OSS | 10 | PCA, CA | [14] |
| Milk | Aroma profiling of milk adulteration | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA, LDA and 4ANN | [291] |
| Meat | Analysis adulteration of minced mutton with pork | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | CDA, BDA, PLS, MLR, BPNN | [292] |
| Ham | Differentiation of hams marked | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA | [293] |
| Cherry tomato Juice | Classification with overripe tomato juice | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA, CA | [294] |
| Tea | Characterizing the degree of invasion of tea trees | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA and MLP | [295] |
| Sausage | Evaluation of lipid oxidation of Chinese-style sausage | A PEN-3 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | SVM, ANN, PLSDA, MLR | [296] |
| Mango | Quality rapid determination of mango | E-nose from HANWEI Electronics Co. | MOS | 8 | PCA and SR | [297] |
| Chicken fat | Rapid measuring of oxidized chicken fat | The FOX 4000 e-nose from Alpha MOS, Toulouse, France | MOS | 18 | APLSR and ANOVA | [298] |
| Honey | Identify the botanical origin of honeys | The FOX 3000 e-nose from Alpha MOS, Toulouse, France | MOS | 18 | PCA, DFA, LS-SVM and PLS | [299] |
| Orange juice | Classification of Valencia orange juices | The FOX 3000 e-nose from Alpha MOS, Toulouse, France | MOS | 12 | PCA, FDA | [300] |
| Wine | Geographical origin confirmation | The FOX 3000 e-nose from Alpha MOS, Toulouse, France | MOS | 12 | LDA | [301] |
| Coffee | Study the aromatic profile of espresso coffee as a function of the grinding grade and extraction time | αFOX from Alpha MOS, Toulouse, France | MOS | 6 | PCA | [302] |
| Cheese | Authenticity of cheese marked with Picorino | EOS 507 from Sacmi Imola S.C., Imola, Bologna, Italy | MOS | 6 | PCA, ANN | [303] |
| Tea | Classification of pure Sri Lanka tea | Model 3320 applied sensor lab emission analyzer from applied Sensor Co., Linkoping, Sweden | MOSFET | 10 | PCA, PLS | [304] |
| MOS | 12 | |||||
| Honey | Botanical origin and adulteration with cane sugar | Cyranose 320 from Intelligent Optical Systems Inc., CA, USA | CP | 32 | PCA, LDA | [305] |
OSS oxide semiconductor sensors, MOSFET metal oxide semiconductor field-effect transistor
The application of commercial e-nose to monitor volatile compounds in the environment both indoor and outdoor provides a reliable solution. Single semiconductor gas sensor GGS 10331 (produced by Umwelt Sensor Technik) was made with a semiconductor sensing layer on Al2O3 substrate to predict the concentration of ammonia under humidity interference [177]. Tagushi (TGS) gas sensor (Figaro Engineering Inc.) was applied to detect NH3, CO, H2, C2H6O, C4H10, C3H8, CH4, alcohol, and solvent vapors and the accuracy was 100% [178]. MQ-7 (Fig. 21j) is a commercial electronic nose for monitoring CO. Portable electronic noses in Fig. 21d were used to classify pollutants in water. Similarly, the commercial e-nose is widely used to identify the toxic wastes, soil/water pollution, indoor volatile organic compounds, etc. Table 10 summarizes the recent applications of e-nose for monitoring environment.
Table 10.
Applications of electronic nose in environmental monitoring
| Target gases | Objective | E-nose configuration | Sensor type | Sensor arrays | Multivariate data analysis | Refs. |
|---|---|---|---|---|---|---|
| Ethanol, acetone | The detection of ethanol and acetone in outdoor courtyard | Tagushi (TGS) gas sensor from Figaro Engineering Inc. (TGS2600, TGS2602,TGS2611TGS2620) | MOS | 4 | MoGC and K-NNC | [306] |
| Air | The quantification of VOC at indoor environments | Figaro TGS2602 air contaminant sensor | MOS | 14 | PLS-2 calibration models | [307] |
| Air | The detection of NH3, CO, H2, C2H6O, C4H10, C3H8, CH4, Alcohol | A sensor array composed of TGS826,TGS2442,TGS2600,TGS2602TGS2610,TGS2611,TGS2620 | MOS | 7 | LDA, PCA, DT, KNN | [178] |
| Air | The detection of xylene | TGS2620 | MOS | 1 | N/A | [308] |
| Air | The detection of n-hexane, acetone, and toluene | e-nose consists of TGS813, TGS2106, TGS2444, TGS244, TGS822, TGS2602, TGS2201, TGS2201 | MOS | 8 | PLS and PCR | [309] |
| Air | The detection of methane, hydrogen, carbon monoxide | A gas e-nose system based on TGS2611, TGS821, TGS2442 | MOS | 3 | ANNs | [310] |
| Inorganic analytes emissions | Analysis in landfill and industrial area chemical emissions monitoring | E-nose from Alphasense Inc. | MOS | 7 | LDA | [311] |
| Toxic wastes | The detection of ammonia | GGS10331 from Umwelt Sensor Technik | MOS | 1 | PCA and PLS | [177] |
| Air | The detection of NO2 | MiCS-2714 produced by SGX Sensortech | MOS | 1 | N/A | [312] |
| Odorless gases | The detection of CO and CH4 | MQ sensors from Hanwei Electronics Co. | MOS | 6 | ANN and LSR | [219] |
E-nose is widely used in agricultural to analyze growth, classify seeds, detect the maturity, monitor quality, which promoted agricultural modernization and saved labor [2]. Eight MOS sensors produced by FIS (Osaka, Japan), MQ (Hanwei, China), and TGS (Figaro Engineering Inc.) were applied for classifying cumin, caraway, and other seeds [179]. Similarly, e-nose based on MOS TGS and FIS sensors (Fig. 21h) were distinguished Iranian Rosa damascena essential oils. An e-nose FOX 4000 (Alpha MOS, Toulouse, France) was chosen to analyze ginseng at different stages [180]. An e-nose FOX 3000 (Fig. 21i) was applied to characterize and classify seven Chinese robusta coffee cultivars. Commercially available chemical sensors intended for agriculture are summarized in Table 11.
Table 11.
Applications of electronic nose in agriculture
| Product | Objective | E-nose configuration | Sensor type | Sensor arrays | Multivariate data analysis | Refs. |
|---|---|---|---|---|---|---|
| Sesame oil | Detection adulteration in sesame oil | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA, FLT, Step-LDA, SFW, PNN, BPNN, GRNN | [313] |
| Olive oils | The detection of olive oils | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA | [314] |
| Red raspberries | The aromatic characteristics of red raspberries | A PEN-2 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA | [315] |
| Compost maturity | The monitoring of composting process produces | A PEN-3 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA | [316] |
| Hyssopus officinalis | Discriminate the accessions | A PEN-3 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA and HCA | [317] |
| Rice | Estimation of the age and amount of brown rice plant | A PEN-3 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA, LDA, PNN, and BPNN | [318] |
| Jujubes | Characterization of different varieties of Chinese jujubes | A PEN-3.5 e-nose from Win Muster Airsense Analytics Inc., Schwerin, Germany | MOS | 10 | PCA and LDA | [319] |
| Virgin olive oil | Adulteration with hazelnut oil | The FOX 4000 e-nose from Alpha MOS, Toulouse, France | MOS | 18 | PCA and PLS | [320] |
| Ginseng | Discrimination of American ginseng and Asian ginseng | The FOX 4000 e-nose from Alpha MOS, Toulouse, France | MOS | 18 | PCA and PLS | [321] |
| Flax seed oil | Differently processed oils for fraud detection | The FOX 3000 e-nose from Alpha MOS, Toulouse, France | MOS | 18 | PCA | [322] |
| Lonicera japonica | Quality control of Lonicera Japonica stored for different period of time | The FOX 3000 e-nose from Alpha MOS, Toulouse, France | MOS | 12 | LDA, PCA, and RBF-ANN | [323] |
| Tomato | Comparison of different stages of tomato | The FOX 4000 e-nose from Alpha MOS, Toulouse, France | MOS | 18 | PCA and ANOVA | [324] |
| White pepper | The chemical and flavor qualities of white pepper | α-Gemini from Alpha M.O.S. SA, Toulouse, France | MOS | 6 | PCA | [325] |
| Virgin olive oil | Confirmation of geographical origin and authentication | Model 3320 applied sensor lab emission analyzer from applied Sensor Co., Linkoping, Sweden | MOSFET | 10 | PCA, CP-ANN | [326] |
| MOS | 12 | |||||
| Asphalt odor | Asphalt odor patterns in hot mix asphalt production | Cyranose 320 from Intelligent Optical Systems Inc., CA, USA | CP | 32 | Polar plots, PCA | [327] |
| Plant Pest and Disease | The discrimination of plant pest and disease | Model ST214 from Scensive Technologies Ltd., Normanton, UK | OCP | 13 | PCA, DFA, CA | [328] |
| Odors emissions | Monitoring of odors from a composting plan | EOS from Sacmi Group, Imola, Italy | MOS | 6 | kNN | [329] |
OCP organic conducting polymer
Some commercial e-noses are attempted to detect explosives with ultra-low saturated concentration (parts-per-billion or below) [181]. Figaro Engineering Inc. produce an e-nose comprised of eight MOS sensors to discriminate and quantify different chemical warfare agents mimics [182]. More expectations in applications are also possible in the future.
Challenges and Perspectives
Although excellent advances in both e-nose system and chemi-resistive sensory unit have been reached in the field during the last few years, there is still room for improvements. Below is a summary of the main rules for improving the performance of hybrid material-based gas sensors (details see the summary paragraph of each section):
For sensors based on heterojunctions (potential energy barrier manipulation), the more uniform and the larger the contact area of heterojunctions and charge transfer hybrids, the higher the response, resulting in faster speed/lower operating temperature, e.g., Fe2O3/TiO2 tube-like quasi-1D nanostructures (n–n) [114], n-ZnO/p-CoPc [119], CeO2–In2O3 hollow spheres with anti-humidity properties [113], and CdS–ZnO [123, 124].
For sensors based on catalytic effect assistance, the higher the potential energy barrier tuning, the higher the response, e.g., Pd/Sb–SnO2 [89, 90].
For sensors based on charge transfer, the more dispersion uniformity and the smaller size of catalysts on host-sensing materials, the higher the response, giving faster speed and lower operating temperatures, e.g., SnO2 QDs/rGO hybrids [109], rGO/MoS2 aerogel [61], and PANI/rGO [63].
For sensors based on regulation of charge carrier transport, the thinner and the more defect-rich of the hybrid film (e.g., suppression of original gas-off current in current-increased gas sensor), the higher and faster the responses obtained, e.g., PTCDI-Ph/p-6P ultrathin film [64], sandwiched PMMA/Pd/PMMA [110], and MSP-covered CNTs [80].
For sensors based on molecular binding/sieving, the more selective and uniform dispersion of molecular binding/sieving guests, the higher the selectivity, e.g., APTES-modified porous WO3 nanotubes [72], the oleic acid SAM-modified PANI [82], the ZnO@ZIF-CoZn NWAs [76] P-O3 NTs (10%)@APTES [71].
Improving the performance requires better understanding of the mechanism. Recently, most sensing mechanism represented in the research articles is “possible mechanism” based on the results of comparative tests instead of direct observations. Exactly, the latter one is more trustable and solid results to support the mechanism study, e.g., in situ FTIR [183, 184], in situ Kelvin probe [185], in situ STM [186, 187], in situ TEM [188, 189]). In addition, Theoretically studies (such as DFT simulation) [190], are also helpful for researchers to understand the interaction between the gas analyte and sensitive materials, the succedent electronic structure changes, or band gap regulation in heterojunction, or charge transfer, etc., which can guide the orientation of materials design [70, 190–195]. Otherwise, learning theoretical studies toward hybrid catalyst designs can inspire the further researches on hybrid gas sensing due to the similar surface physical/chemical science, band gap theories, and charge transfer process [196–201].
Controlling the fluidic behavior of gas [202], enhancing the anti-interferon ability by loading novel porous sieving materials (e.g., MOF, COF) [203–206], screening the cross-talk (such as deformation [207, 208]) by special micro-/nanostructures, deeply mining the features of sensing signal [209] (e.g., response, area of peak, and speed), and enhancing catalysis effect using small NPs, clusters, or even single-atom catalyst [108] are the long-term challenges of hybrid gas-sensing materials to adapt the applications under real-world conditions [210, 211]. The advances in knowledge in all our endeavors can be a foundation and useful experience for sensing technology, surface science, catalysis, fluidic mechanics, and microelectronics.
Acknowledgements
This research was funded by the Phase-II Grand Challenges Explorations award from the Bill, Melinda Gates Foundation (Grant ID: OPP1109493), International Research Fellow of the Japan Society of the Promotion of Science (JSPS, Postdoctoral Fellowships for Research in Japan (Standard), P18334), the National Natural Science Foundation of China (21801243), and the Natural Science Foundation of Shaanxi province (2018JM6045, 2018JM1046). Research funding was received from Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration (SHUES2019A02).
Footnotes
Yingying Jian and Wenwen Hu contributed equally to this review.
Contributor Information
Hossam Haick, Email: hhossam@technion.ac.il.
Mingshui Yao, Email: mingshuiyao@gmail.com.
Weiwei Wu, Email: wwwu@xidian.edu.cn.
References
- 1.Kotha HD, Gupta VM. IoT application: a survey. Int. J. Eng. Technol. 2018;7(2.7):891–896. doi: 10.14419/ijet.v7i2.7.11089. [DOI] [Google Scholar]
- 2.Hu W, Wan L, Jian Y, Ren C, Jin K, et al. Electronic noses: from advanced materials to sensors aided with data processing. Adv. Mater. Technol. 2018;4(2):1800488. doi: 10.1002/admt.201800488. [DOI] [Google Scholar]
- 3.Dung TT, Oh Y, Choi S-J, Kim I-D, Oh M-K, Kim M. Applications and advances in bioelectronic noses for odour sensing. Sensors. 2018;18(1):103. doi: 10.3390/s18010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Liu X, Neri G, Pinna N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2015;28(5):795–831. doi: 10.1002/adma.201503825. [DOI] [PubMed] [Google Scholar]
- 5.Kim I-D, Rothschild A, Tuller HL. Advances and new directions in gas-sensing devices. Acta Mater. 2013;61(3):974–1000. doi: 10.1016/j.actamat.2012.10.041. [DOI] [Google Scholar]
- 6.Fennell JF, Liu SF, Azzarelli JM, Weis JG, Rochat S, Mirica KA, Ravnsbæk JB, Swager TM. Nanowire chemical/biological sensors: status and a roadmap for the future. Angew. Chem. Int. Ed. 2015;55(4):1266–1281. doi: 10.1002/anie.201505308. [DOI] [PubMed] [Google Scholar]
- 7.Ihokura K, Watson J. The Stannic Oxide Gas Sensor principles and Applications. London: CRC Press; 2017. [Google Scholar]
- 8.Korotcenkov G, Cho BK. Metal oxide composites in conductometric gas sensors: achievements and challenges. Sens. Actuat. B: Chem. 2017;244:182–210. doi: 10.1016/j.snb.2016.12.117. [DOI] [Google Scholar]
- 9.Kaushik A, Kumar R, Arya SK, Nair M, Malhotra BD, Bhansali S. Organic-inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015;115(11):4571–4606. doi: 10.1021/cr400659h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stassen I, Burtch N, Talin A, Falcaro P, Allendorf M, Ameloot R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017;46(11):3185–3241. doi: 10.1039/C7CS00122C. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Xiao A-S, Zou B, Zhang H-X, Yan K-L, Lin Y. Advances of metal–organic frameworks for gas sensing. Polyhedron. 2018;154:83–97. doi: 10.1016/j.poly.2018.07.028. [DOI] [Google Scholar]
- 12.Andre RS, Sanfelice RC, Pavinatto A, Mattoso LHC, Correa DS. Hybrid nanomaterials designed for volatile organic compounds sensors: a review. Mater. Des. 2018;156:154–166. doi: 10.1016/j.matdes.2018.06.041. [DOI] [Google Scholar]
- 13.Buratti S, Benedetti S, Giovanelli G. Application of electronic senses to characterize espresso coffees brewed with different thermal profiles. Eur. Food Res. Technol. 2017;243(3):511–520. doi: 10.1007/s00217-016-2769-y. [DOI] [Google Scholar]
- 14.Yang Z, Dong F, Shimizu K, Kinoshita T, Kanamori M, Morita A, Watanabe N. Identification of coumarin-enriched Japanese green teas and their particular flavor using electronic nose. J. Food Eng. 2009;92(3):312–316. doi: 10.1016/j.jfoodeng.2008.11.014. [DOI] [Google Scholar]
- 15.Dutta R, Hines EL, Gardner JW, Kashwan KR, Bhuyan M. Tea quality prediction using a tin oxide-based electronic nose: an artificial intelligence approach. Sens. Actuat. B: Chem. 2003;94(2):228–237. doi: 10.1016/S0925-4005(03)00367-8. [DOI] [Google Scholar]
- 16.Soler-Illia GJAA, Azzaroni O. Multifunctional hybrids by combining ordered mesoporous materials and macromolecular building blocks. Chem. Soc. Rev. 2011;40(2):1107–1150. doi: 10.1039/C0CS00208A. [DOI] [PubMed] [Google Scholar]
- 17.Tan JC, Cheetham AK. Mechanical properties of hybrid inorganic–organic framework materials: establishing fundamental structure–property relationships. Chem. Soc. Rev. 2011;40(2):1059–1080. doi: 10.1039/C0CS00163E. [DOI] [PubMed] [Google Scholar]
- 18.Rogez G, Massobrio C, Rabu P, Drillon M. Layered hydroxide hybrid nanostructures: a route to multifunctionality. Chem. Soc. Rev. 2011;40(2):1031–1058. doi: 10.1039/C0CS00159G. [DOI] [PubMed] [Google Scholar]
- 19.Laberty-Robert C, Vallé K, Pereira F, Sanchez C. Design and properties of functional hybrid organic–inorganic membranes for fuel cells. Chem. Soc. Rev. 2011;40(2):961–1005. doi: 10.1039/C0CS00144A. [DOI] [PubMed] [Google Scholar]
- 20.Le Bideau J, Viau L, Vioux A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011;40(2):907–925. doi: 10.1039/C0CS00059K. [DOI] [PubMed] [Google Scholar]
- 21.Lebeau B, Innocenzi P. Hybrid materials for optics and photonics. Chem. Soc. Rev. 2011;40(2):886–906. doi: 10.1039/C0CS00106F. [DOI] [PubMed] [Google Scholar]
- 22.Brun N, Ungureanu S, Deleuze H, Backov R. Hybrid foams, colloids and beyond: from design to applications. Chem. Soc. Rev. 2011;40(2):771–788. doi: 10.1039/B920518G. [DOI] [PubMed] [Google Scholar]
- 23.Kanamori K, Nakanishi K. Controlled pore formation in organotrialkoxysilane-derived hybrids: from aerogels to hierarchically porous monoliths. Chem. Soc. Rev. 2011;40(2):754–770. doi: 10.1039/C0CS00068J. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez C, Belleville P, Popall M, Nicole L. Applications of advanced hybrid organic–inorganic nanomaterials: from laboratory to market. Chem. Soc. Rev. 2011;40(2):696–753. doi: 10.1039/C0CS00136H. [DOI] [PubMed] [Google Scholar]
- 25.Hu L-C, Shea KJ. Organo–silica hybrid functional nanomaterials: how do organic bridging groups and silsesquioxane moieties work hand-in-hand? Chem. Soc. Rev. 2011;40(2):688–695. doi: 10.1039/C0CS00219D. [DOI] [PubMed] [Google Scholar]
- 26.Pardo R, Zayat M, Levy D. Photochromic organic–inorganic hybrid materials. Chem. Soc. Rev. 2011;40(2):672–687. doi: 10.1039/C0CS00065E. [DOI] [PubMed] [Google Scholar]
- 27.Yuan J, Xu Y, Müller AHE. One-dimensional magnetic inorganic–organic hybrid nanomaterials. Chem. Soc. Rev. 2011;40(2):640–655. doi: 10.1039/C0CS00087F. [DOI] [PubMed] [Google Scholar]
- 28.Tran-Thi T-H, Dagnelie R, Crunaire S, Nicole L. Optical chemical sensors based on hybrid organic–inorganic solgel nanoreactors. Chem. Soc. Rev. 2011;40(2):621–639. doi: 10.1039/C0CS00021C. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann F, Fröba M. Vitalising porous inorganic silica networks with organic functions: PMOs and related hybrid materials. Chem. Soc. Rev. 2011;40(2):608–620. doi: 10.1039/C0CS00076K. [DOI] [PubMed] [Google Scholar]
- 30.Vallet-Regí M, Colilla M, González B. Medical applications of organic–inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011;40(2):596–607. doi: 10.1039/C0CS00025F. [DOI] [PubMed] [Google Scholar]
- 31.Oh J-M, Park D-H, Choy J-H. Integrated bio-inorganic hybrid systems for nano-forensics. Chem. Soc. Rev. 2011;40(2):583–595. doi: 10.1039/C0CS00051E. [DOI] [PubMed] [Google Scholar]
- 32.Schubert U. Cluster-based inorganic–organic hybrid materials. Chem. Soc. Rev. 2011;40(2):575–582. doi: 10.1039/C0CS00009D. [DOI] [PubMed] [Google Scholar]
- 33.Mehdi A, Reye C, Corriu R. From molecular chemistry to hybrid nanomaterials. Design and functionalization. Chem. Soc. Rev. 2011;40(2):563–574. doi: 10.1039/B920516K. [DOI] [PubMed] [Google Scholar]
- 34.Carlos LD, Ferreira RAS, de Zea Bermudez V, Julián-López B, Escribano P. Progress on lanthanide-based organic–inorganic hybrid phosphors. Chem. Soc. Rev. 2011;40(2):536–549. doi: 10.1039/C0CS00069H. [DOI] [PubMed] [Google Scholar]
- 35.Clemente-León M, Coronado E, Martí-Gastaldo C, Romero FM. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011;40(2):473–497. doi: 10.1039/C0CS00111B. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez C, Shea KJ, Kitagawa S. Recent progress in hybrid materials science. Chem. Soc. Rev. 2011;40(2):471–472. doi: 10.1039/C1CS90001C. [DOI] [PubMed] [Google Scholar]
- 37.Yao M, Hu P, Cao Y, Xiang W, Zhang X, Yuan F, Chen Y. Morphology-controlled ZnO spherical nanobelt-flower arrays and their sensing properties. Sens. Actuat. B: Chem. 2013;117:562–569. doi: 10.1016/j.snb.2012.11.088. [DOI] [Google Scholar]
- 38.Yamazoe N, Shimanoe K. New perspectives of gas sensor technology. Sens. Actuat. B: Chem. 2009;138(1):100–107. doi: 10.1016/j.snb.2009.01.023. [DOI] [Google Scholar]
- 39.Miller DR, Akbar SA, Morris PA. Nanoscale metal oxide-based heterojunctions for gas sensing: a review. Sens. Actuat. B: Chem. 2014;204:250–272. doi: 10.1016/j.snb.2014.07.074. [DOI] [Google Scholar]
- 40.Kim H-J, Lee J-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: overview. Sens. Actuat. B: Chem. 2014;192:607–627. doi: 10.1016/j.snb.2013.11.005. [DOI] [Google Scholar]
- 41.Li T, Zeng W, Wang Z. Quasi-one-dimensional metal-oxide-based heterostructural gas-sensing materials: a review. Sens. Actuat. B: Chem. 2015;221:1570–1585. doi: 10.1016/j.snb.2015.08.003. [DOI] [Google Scholar]
- 42.Zhao Z, Tian J, Sang Y, Cabot A, Liu H. Structure, synthesis, and applications of TiO2 nanobelts. Adv. Mater. 2015;27(16):2557–2582. doi: 10.1002/adma.201405589. [DOI] [PubMed] [Google Scholar]
- 43.Wagner T, Haffer S, Weinberger C, Klaus D, Tiemann M. Mesoporous materials as gas sensors. Chem. Soc. Rev. 2013;42(9):4036–4053. doi: 10.1039/C2CS35379B. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Liu X, Neri G, Pinna N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016;28(5):795–831. doi: 10.1002/adma.201503825. [DOI] [PubMed] [Google Scholar]
- 45.Choi S-J, Kim I-D. Recent developments in 2D nanomaterials for chemiresistive-type gas sensors. Electron. Mater. Lett. 2018;14(3):221–260. doi: 10.1007/s13391-018-0044-z. [DOI] [Google Scholar]
- 46.Senesac L, Thundat TG. Nanosensors for trace explosive detection. Mater. Today. 2008;11(3):28–36. doi: 10.1016/s1369-7021(08)70017-8. [DOI] [Google Scholar]
- 47.Xu CN, Tamaki J, Miura N, Yamazoe N. Grain-size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuat. B: Chem. 1991;3(2):147–155. doi: 10.1016/0925-4005(91)80207-z. [DOI] [Google Scholar]
- 48.Xu JQ, Pan QY, Shun YA, Tian ZZ. Grain size control and gas sensing properties of ZnO gas sensor. Sens. Actuat. B: Chem. 2000;66(1–3):277–279. doi: 10.1016/s0925-4005(00)00381-6. [DOI] [Google Scholar]
- 49.Hongsith N, Wongrat E, Kerdcharoen T, Choopun S. Sensor response formula for sensor based on ZnO nanostructures. Sens. Actuat. B: Chem. 2010;144(1):67–72. doi: 10.1021/am5081277. [DOI] [Google Scholar]
- 50.Yao M, Li Q, Hou G, Lu C, Cheng B, et al. Dopant-controlled morphology evolution of WO3 polyhedra synthesized by RF thermal plasma and their sensing properties. ACS Appl. Mater. Interfaces. 2015;7(4):2856–2866. doi: 10.1021/am5081277. [DOI] [PubMed] [Google Scholar]
- 51.Hwang I-S, Choi J-K, Woo H-S, Kim S-J, Jung S-Y, Seong T-Y, Kim I-D, Lee J-H. Facile control of C2H5OH sensing characteristics by decorating discrete Ag nanoclusters on SnO2 nanowire networks. ACS Appl. Mater. Interfaces. 2011;3(8):3140–3145. doi: 10.1021/am200647f. [DOI] [PubMed] [Google Scholar]
- 52.Jang J-S, Choi S-J, Kim S-J, Hakim M, Kim I-D. Rational design of highly porous SnO2 nanotubes functionalized with biomimetic nanocatalysts for direct observation of simulated diabetes. Adv. Funct. Mater. 2016;26(26):4740–4748. doi: 10.1002/adfm.201600797. [DOI] [Google Scholar]
- 53.Kim S-J, Choi S-J, Jang J-S, Cho H-J, Koo W-T, Tuller HL, Kim I-D. Exceptional high-performance of Pt-based bimetallic catalysts for exclusive detection of exhaled biomarkers. Adv. Mater. 2017;29(36):1700737. doi: 10.1002/adma.201700737. [DOI] [PubMed] [Google Scholar]
- 54.Xiao Y, Lu L, Zhang A, Zhang Y, Sun L, Huo L, Li F. Highly enhanced acetone sensing performances of porous and single crystalline ZnO nanosheets: high percentage of exposed (100) facets working together with surface modification with Pd nanoparticles. ACS Appl. Mater. Interfaces. 2012;4(8):3797–3804. doi: 10.1021/am3010303. [DOI] [PubMed] [Google Scholar]
- 55.Cho S-Y, Koh H-J, Yoo H-W, Jung H-T. Tunable chemical sensing performance of black phosphorus by controlled functionalization with noble metals. Chem. Mater. 2017;29(17):7197–7205. doi: 10.1021/acs.chemmater.7b01353. [DOI] [Google Scholar]
- 56.Ma J, Ren Y, Zhou X, Liu L, Zhu Y, et al. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: gas sensing performance and mechanism study. Adv. Funct. Mater. 2018;28(6):1705268. doi: 10.1002/adfm.201705268. [DOI] [Google Scholar]
- 57.Hong YJ, Yoon J-W, Lee J-H, Kang YC. One-pot synthesis of Pd-loaded SnO2 yolk-shell nanostructures for ultraselective methyl benzene sensors. Chem. Eur. J. 2014;20(10):2737–2741. doi: 10.1002/chem.201304502. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Guo Z, Xu W-H, Yao H-B, Li M-Q, et al. Templating synthesis of SnO2 nanotubes loaded with Ag2O nanoparticles and their enhanced gas sensing properties. Adv. Funct. Mater. 2011;21(11):2049–2056. doi: 10.1002/adfm.201002701. [DOI] [Google Scholar]
- 59.Lu G, Ocola LE, Chen J. Room-temperature gas sensing based on electron transfer between discrete tin oxide nanocrystals and multiwalled carbon nanotubes. Adv. Mater. 2009;21(24):2487–2491. doi: 10.1002/adma.200803536. [DOI] [Google Scholar]
- 60.Guan L, Wang S, Gu W, Zhuang J, Jin H, Zhang W, Zhang T, Wang J. Ultrasensitive room-temperature detection of NO2 with tellurium nanotube based chemiresistive sensor. Sens. Actuat. B: Chem. 2014;196:321–327. doi: 10.1016/j.snb.2014.02.014. [DOI] [Google Scholar]
- 61.Long H, Harley-Trochimczyk A, Pham T, Tang Z, Shi T, Zettl A, Carraro C, Worsley MA, Maboudian R. High surface area MoS2/graphene hybrid aerogel for ultrasensitive NO2 detection. Adv. Funct. Mater. 2016;26(28):5158–5165. doi: 10.1002/adfm.201601562. [DOI] [Google Scholar]
- 62.Deng S, Tjoa V, Fan HM, Tan HR, Sayle DC, Olivo M, Mhaisalkar S, Wei J, Sow CH. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012;134(10):4905–4917. doi: 10.1021/ja211683m. [DOI] [PubMed] [Google Scholar]
- 63.Guo Y, Wang T, Chen F, Sun X, Li X, Yu Z, Wan P, Chen X. Hierarchical graphene–polyaniline nanocomposite films for high-performance flexible electronic gas sensors. Nanoscale. 2016;8(23):12073–12080. doi: 10.1039/C6NR02540D. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, Huang L, Zhu X, Zhou X, Chi L. An ultrasensitive organic semiconductor NO2 sensor based on crystalline TIPS-pentacene films. Adv. Mater. 2017;29(38):1703192. doi: 10.1002/adma.201703192. [DOI] [PubMed] [Google Scholar]
- 65.Jalil AR, Chang H, Bandari VK, Robaschik P, Zhang J, et al. Fully integrated organic nanocrystal diode as high performance room temperature NO2 sensor. Adv. Mater. 2016;28(15):2971–2977. doi: 10.1002/adma.201506293. [DOI] [PubMed] [Google Scholar]
- 66.Ji S, Wang H, Wang T, Yan D. A high-performance room-temperature NO2 sensor based on an ultrathin heterojunction film. Adv. Mater. 2013;25(12):1755–1760. doi: 10.1002/adma.201204134. [DOI] [PubMed] [Google Scholar]
- 67.Shao F, Hoffmann MWG, Prades JD, Zamani R, Arbiol J, et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sens. Actuat. B: Chem. 2013;181:130–135. doi: 10.1016/j.snb.2013.01.067. [DOI] [Google Scholar]
- 68.Wu J, Tao K, Guo Y, Li Z, Wang X, et al. A 3D chemically modified graphene hydrogel for fast, highly sensitive, and selective gas sensor. Adv. Sci. 2017;4(3):1600319. doi: 10.1002/advs.201600319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin H, Huynh T-P, Haick H. Self-healable sensors based nanoparticles for detecting physiological markers via skin and breath: toward disease prevention via wearable devices. Nano Lett. 2016;16(7):4194–4202. doi: 10.1021/acs.nanolett.6b01066. [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann MWG, Daniel Prades J, Mayrhofer L, Hernandez-Ramirez F, Jaervi TT, Moseler M, Waag A, Shen H. Highly selective SAM-nanowire hybrid NO2 sensor: insight into charge transfer dynamics and alignment of frontier molecular orbitals. Adv. Funct. Mater. 2014;24(5):595–602. doi: 10.1002/adfm.201301478. [DOI] [Google Scholar]
- 71.Liu W, Xu L, Sheng K, Chen C, Zhou X, et al. APTES-functionalized thin-walled porous WO3 nanotubes for highly selective sensing of NO2 in a polluted environment. J. Mater. Chem. A. 2018;6(23):10976–10989. doi: 10.1039/c8ta02452a. [DOI] [Google Scholar]
- 72.Jiang Y, Tang N, Zhou C, Han Z, Qu H, Duan X. A chemiresistive sensor array from conductive polymer nanowires fabricated by nanoscale soft lithography. Nanoscale. 2018;10(44):20578–20586. doi: 10.1039/C8NR04198A. [DOI] [PubMed] [Google Scholar]
- 73.Esser B, Schnorr JM, Swager TM. Selective detection of ethylene gas using carbon nanotube-based devices: utility in determination of fruit ripeness. Angew. Chem. Int. Ed. 2012;51(23):5752–5756. doi: 10.1002/anie.201201042. [DOI] [PubMed] [Google Scholar]
- 74.Russo PA, Donato N, Leonardi SG, Baek S, Conte DE, Neri G, Pinna N. Room-temperature hydrogen sensing with heteronanostructures based on reduced graphene oxide and tin oxide. Angew. Chem. Int. Ed. 2012;51(44):11053–11057. doi: 10.1002/anie.201204373. [DOI] [PubMed] [Google Scholar]
- 75.Jang J-S, Koo W-T, Choi S-J, Kim I-D. Metal organic framework-templated chemiresistor: sensing type transition from p-to-n using hollow metal oxide polyhedron via galvanic replacement. J. Am. Chem. Soc. 2017;139(34):11868–11876. doi: 10.1021/jacs.7b05246. [DOI] [PubMed] [Google Scholar]
- 76.Yao MS, Tang WX, Wang GE, Nath B, Xu G. MOF thin film-coated metal oxide nanowire array: significantly improved chemiresistor sensor performance. Adv. Mater. 2016;28:5229–5234. doi: 10.1002/adma.201506457. [DOI] [PubMed] [Google Scholar]
- 77.Wang D, Li Z, Zhou J, Fang H, He X, Jena P, Zeng J-B, Wang W-N. Simultaneous detection and removal of formaldehyde at room temperature: Janus Au@ZnO@ZIF-8 nanoparticles. Nano-Micro Lett. 2018;10(1):4. doi: 10.1007/s40820-017-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu J, Zhao C, Zhang J, Peng Y, Xie E. Enhanced gas sensing performance of electrospun Pt-functionalized NiO nanotubes with chemical and electronic sensitization. ACS Appl. Mater. Interfaces. 2013;5(15):7410–7416. doi: 10.1021/am4017347. [DOI] [PubMed] [Google Scholar]
- 79.Xiao L, Xu S, Yu G, Liu S. Efficient hierarchical mixed Pd/SnO2 porous architecture deposited microheater for low power ethanol gas sensor. Sens. Actuat. B: Chem. 2018;255:2002–2010. doi: 10.1016/j.snb.2017.08.216. [DOI] [Google Scholar]
- 80.Ishihara S, Azzarelli JM, Krikorian M, Swager TM. Ultratrace detection of toxic chemicals: triggered disassembly of supramolecular nanotube wrappers. J. Am. Chem. Soc. 2016;138(26):8221–8227. doi: 10.1021/jacs.6b03869. [DOI] [PubMed] [Google Scholar]
- 81.Nakhleh MK, Amal H, Awad H, Gharra AL, Abu-Saleh N, Jeries R, Haick H, Abassi Z. Sensor arrays based on nanoparticles for early detection of kidney injury by breath samples. Nanomed. Nanotechnol. 2014;10(8):1767–1776. doi: 10.1016/j.nano.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Deng Y, Sun J, Jin H, Khatib M, Li X, et al. Chemically modified polyaniline for the detection of volatile biomarkers of minimal sensitivity to humidity and bending. Adv. Healthc. Mater. 2018;7(15):1800232. doi: 10.1002/adhm.201800232. [DOI] [PubMed] [Google Scholar]
- 83.Sun XM, Li YD. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004;43(5):597–601. doi: 10.1002/anie.200352386. [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Lou Z, Fei T, Zhang T. Zinc oxide core-shell hollow microspheres with multi-shelled architecture for gas sensor applications. J. Mater. Chem. 2011;21(48):19331–19336. doi: 10.1039/C1JM13354C. [DOI] [Google Scholar]
- 85.Rai P, Yoon J-W, Jeong H-M, Hwang S-J, Kwak C-H, Lee J-H. Design of highly sensitive and selective Au@NiO yolk-shell nanoreactors for gas sensor applications. Nanoscale. 2014;6(14):8292–8299. doi: 10.1039/c4nr01906g. [DOI] [PubMed] [Google Scholar]
- 86.Rai P, Yoon J-W, Kwak C-H, Lee J-H. Role of Pd nanoparticles in gas sensing behaviour of Pd@In2O3 yolk-shell nanoreactors. J. Mater. Chem. A. 2016;4(1):264–269. doi: 10.1039/c5ta08873a. [DOI] [Google Scholar]
- 87.Han X, Jin M, Xie S, Kuang Q, Jiang Z, Jiang Y, Xie Z, Zheng L. Synthesis of tin dioxide octahedral nanoparticles with exposed high-energy 221 facets and enhanced gas-sensing properties. Angew. Chem. Int. Ed. 2009;48(48):9180–9183. doi: 10.1002/anie.200903926. [DOI] [PubMed] [Google Scholar]
- 88.Han X-G, He H-Z, Kuang Q, Zhou X, Zhang X-H, Xu T, Xie Z-X, Zheng L-S. Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J. Phys. Chem. C. 2009;113(2):584–589. doi: 10.1021/jp808233e. [DOI] [Google Scholar]
- 89.Suematsu K, Sasaki M, Ma N, Yuasa M, Shimanoe K. Antimony-doped tin dioxide gas sensors exhibiting high stability in the sensitivity to humidity changes. ACS Sensor. 2016;1(7):913–920. doi: 10.1021/acssensors.6b00323. [DOI] [Google Scholar]
- 90.Ma N, Suematsu K, Yuasa M, Shimanoe K. Pd size effect on the gas sensing properties of Pd-loaded SnO2 in humid atmosphere. ACS Appl. Mater. Interfaces. 2015;7(28):15618–15625. doi: 10.1021/acsami.5b04380. [DOI] [PubMed] [Google Scholar]
- 91.Lin Y, Deng P, Nie Y, Hu Y, Xing L, Zhang Y, Xue X. Room-temperature self-powered ethanol sensing of a Pd/ZnO nanoarray nanogenerator driven by human finger movement. Nanoscale. 2014;6(9):4604–4610. doi: 10.1039/c3nr06809a. [DOI] [PubMed] [Google Scholar]
- 92.Guo L, Chen F, Xie N, Kou X, Wang C, et al. Ultra-sensitive sensing platform based on Pt–ZnO–In2O3 nanofibers for detection of acetone. Sens. Actuat. B: Chem. 2018;272:185–194. doi: 10.1016/j.snb.2018.05.161. [DOI] [Google Scholar]
- 93.Wang Z, Li Z, Jiang T, Xu X, Wang C. Ultrasensitive hydrogen sensor based on Pd0-loaded SnO2 electrospun nanofibers at room temperature. ACS Appl. Mater. Interfaces. 2013;5(6):2013–2021. doi: 10.1021/am3028553. [DOI] [PubMed] [Google Scholar]
- 94.Choi S-J, Koo W-T, Kim S-J, Jang J-S, Tuller HL, Kim I-D. Heterogeneous sensitization of metal-organic framework driven metal@metal oxide complex catalysts on oxide nanofiber scaffold toward superior gas sensors. J. Am. Chem. Soc. 2016;138(40):13431–13437. doi: 10.1021/jacs.6b09167. [DOI] [PubMed] [Google Scholar]
- 95.Yao M, Hu P, Han N, Ding F, Yin C, Yuan F, Yang J, Chen Y. ZnO micro-windbreak for enhanced gas diffusion. Sens. Actuat. B: Chem. 2013;186:614–621. doi: 10.1016/j.snb.2013.06.057. [DOI] [Google Scholar]
- 96.Jing Z, Zhan J. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008;20(23):4547–4551. doi: 10.1002/adma.200800243. [DOI] [Google Scholar]
- 97.Lai X, Li J, Korgel BA, Dong Z, Li Z, Su F, Du J, Wang D. General synthesis and gas-sensing properties of multiple-shell metal oxide hollow microspheres. Angew. Chem. Int. Ed. 2011;50(12):2738–2741. doi: 10.1002/anie.201004900. [DOI] [PubMed] [Google Scholar]
- 98.Shimizu Y, Hyodo T, Egashira M. Meso-to macro-porous oxides as semiconductor gas sensors. Catal. Surv. Asia. 2004;8(2):127–135. doi: 10.1023/b:cats.0000027014.79515.87. [DOI] [Google Scholar]
- 99.Sun F, Cai W, Li Y, Jia L, Lu F. Direct growth of mono- and multilayer nanostructured porous films on curved surfaces and their application as gas sensors. Adv. Mater. 2005;17(23):2872–2877. doi: 10.1002/adma.200500936. [DOI] [Google Scholar]
- 100.Song F, Su H, Han J, Lau WM, Moon W-J, Zhang D. Bioinspired hierarchical tin oxide scaffolds for enhanced gas sensing properties. J. Phys. Chem. C. 2012;116(18):10274–10281. doi: 10.1021/jp2118136. [DOI] [Google Scholar]
- 101.Waitz T, Wagner T, Sauerwald T, Kohl C-D, Tiemann M. Ordered mesoporous In2O3: synthesis by structure replication and application as a methane gas sensor. Adv. Funct. Mater. 2009;19(4):653–661. doi: 10.1002/adfm.200801458. [DOI] [Google Scholar]
- 102.Rossinyol E, Prim A, Pellicer E, Arbiol J, Hernandez-Ramirez F, et al. Synthesis and characterization of chromium-doped mesoporous tungsten oxide for gas-sensing applications. Adv. Funct. Mater. 2007;17(11):1801–1806. doi: 10.1002/adfm.200600722. [DOI] [Google Scholar]
- 103.D’Arienzo M, Armelao L, Cacciamani A, Mari CM, Polizzi S, et al. One-step preparation of SnO2 and Pt-doped SnO2 as inverse opal thin films for gas sensing. Chem. Mater. 2010;22(13):4083–4089. doi: 10.1021/cm100866g. [DOI] [Google Scholar]
- 104.D’Arienzo M, Armelao L, Mari CM, Polizzi S, Ruffo R, Scotti R, Morazzoni F. Macroporous WO3 thin films active in NH3 sensing: role of the hosted Cr isolated centers and Pt nanoclusters. J. Am. Chem. Soc. 2011;133(14):5296–5304. doi: 10.1021/ja109511a. [DOI] [PubMed] [Google Scholar]
- 105.Jones J, Xiong H, DeLaRiva AT, Peterson EJ, Pham H, et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science. 2016;353(6295):150–154. doi: 10.1126/science.aaf8800. [DOI] [PubMed] [Google Scholar]
- 106.Liu P, Zhao Y, Qin R, Mo S, Chen G, et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science. 2016;352(6287):797–800. doi: 10.1126/science.aaf5251. [DOI] [PubMed] [Google Scholar]
- 107.Therrien AJ, Hensley AJ, Marcinkowski MD, Zhang R, Lucci FR, et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 2018;1(3):192. doi: 10.1038/s41929-018-0028-2. [DOI] [Google Scholar]
- 108.Wang A, Li J, Zhang T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018;2:65–81. doi: 10.1038/s41570-018-0010-1. [DOI] [Google Scholar]
- 109.Song Z, Wei Z, Wang B, Luo Z, Xu S, et al. Sensitive room-temperature H2S gas sensors employing SnO2 quantum wire/reduced graphene oxide nanocomposites. Chem. Mater. 2016;28(4):1205–1212. doi: 10.1021/acs.chemmater.5b04850. [DOI] [Google Scholar]
- 110.Jang B, Lee KY, Noh J-S, Lee W. Nanogap-based electrical hydrogen sensors fabricated from Pd-PMMA hybrid thin films. Sens. Actuator. B: Chem. 2014;193:530–535. doi: 10.1016/j.snb.2013.11.080. [DOI] [Google Scholar]
- 111.Peng G, Tisch U, Adams O, Hakim M, Shehada N, et al. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009;4:669. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 112.Kahn N, Lavie O, Paz M, Segev Y, Haick H. Dynamic nanoparticle-based flexible sensors: diagnosis of ovarian carcinoma from exhaled breath. Nano Lett. 2015;15(10):7023–7028. doi: 10.1021/acs.nanolett.5b03052. [DOI] [PubMed] [Google Scholar]
- 113.Yoon J-W, Kim J-S, Kim T-H, Hong YJ, Kang YC, Lee J-H. A new strategy for humidity independent oxide chemiresistors: dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small. 2016;12(31):4229–4240. doi: 10.1002/smll.201601507. [DOI] [PubMed] [Google Scholar]
- 114.Zhu C-L, Yu H-L, Zhang Y, Wang T-S, Ouyang Q-Y, et al. Fe2O3/TiO2 tube-like nanostructures: synthesis, structural transformation and the enhanced sensing properties. ACS Appl. Mater. Interfaces. 2012;4(2):665–671. doi: 10.1021/am201689x. [DOI] [PubMed] [Google Scholar]
- 115.Park S, An S, Mun Y, Lee C. UV-enhanced NO2 gas sensing properties of SnO2-Core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Interfaces. 2013;5(10):4285–4292. doi: 10.1021/am400500a. [DOI] [PubMed] [Google Scholar]
- 116.Cui S, Wen Z, Huang X, Chang J, Chen J. Stabilizing MoS2 nanosheets through SnO2 nanocrystal decoration for high-performance gas sensing in air. Small. 2015;11(19):2305–2313. doi: 10.1002/smll.201402923. [DOI] [PubMed] [Google Scholar]
- 117.Kida T, Nishiyama A, Hua Z, Suematsu K, Yuasa M, Shimanoe K. WO3 nano lamella gas sensor: porosity control using SnO2 nanoparticles for enhanced NO2 sensing. Langmuir. 2014;30(9):2571–2579. doi: 10.1021/la4049105. [DOI] [PubMed] [Google Scholar]
- 118.Her Y-C, Yeh B-Y, Huang S-L. Vapor-solid growth of p-Te/n-SnO2 hierarchical heterostructures and their enhanced room-temperature gas sensing properties. ACS Appl. Mater. Interfaces. 2014;6(12):9150–9159. doi: 10.1021/am5012518. [DOI] [PubMed] [Google Scholar]
- 119.Kumar A, Samanta S, Singh A, Roy M, Singh S, et al. Fast response and high sensitivity of ZnO nanowires—cobalt phthalocyanine heterojunction based H2S sensor. ACS Appl. Mater. Interfaces. 2015;7(32):17713–17724. doi: 10.1021/acsami.5b03652. [DOI] [PubMed] [Google Scholar]
- 120.Kim JH, Katoch A, Kim SH, Sang SK. Chemiresistive sensing behavior of SnO2 (n)–Cu2O (p) core-shell nanowires. ACS Appl. Mater. Interfaces. 2015;7(28):15351–15358. doi: 10.1021/acsami.5b03224. [DOI] [PubMed] [Google Scholar]
- 121.Zhang D, Wu Z, Zong X, Zhang Y. Fabrication of polypyrrole/Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sens. Actuat. B: Chem. 2018;274:575–586. doi: 10.1016/j.snb.2018.08.001. [DOI] [Google Scholar]
- 122.Pang Z, Nie Q, Wei A, Yang J, Huang F, Wei Q. Effect of In2O3 nanofiber structure on the ammonia sensing performances of In2O3/PANI composite nanofibers. J. Mater. Sci. 2017;52(2):686–695. doi: 10.1007/s10853-016-0362-1. [DOI] [Google Scholar]
- 123.Villani M, Calestani D, Lazzarini L, Zanotti L, Mosca R, Zappettini A. Extended functionality of ZnO nanotetrapods by solution-based coupling with CdS nanoparticles. J. Mater. Chem. 2012;22(12):5694–5699. doi: 10.1039/C2JM16164H. [DOI] [Google Scholar]
- 124.Zhai J, Wang D, Peng L, Lin Y, Li X, Xie T. Visible-light-induced photoelectric gas sensing to formaldehyde based on CdS nanoparticles/ZnO heterostructures. Sens. Actuat. B: Chem. 2010;147(1):234–240. doi: 10.1016/j.snb.2010.03.003. [DOI] [Google Scholar]
- 125.Zhai J, Wang L, Wang D, Li H, Zhang Y, He DQ, Xie T. Enhancement of gas sensing properties of CdS nanowire/ZnO nanosphere composite materials at room temperature by visible-light activation. ACS Appl. Mater. Interfaces. 2011;3(7):2253–2258. doi: 10.1021/am200008y. [DOI] [PubMed] [Google Scholar]
- 126.Chizhov A, Rumyantseva M, Vasiliev R, Filatova D, Drozdov K, Krylov I, Abakumov A, Gaskov A. Visible light activated room temperature gas sensors based on nanocrystalline ZnO sensitized with CdSe quantum dots. Sens. Actuat. B: Chem. 2014;205:305–312. doi: 10.1016/j.snb.2014.08.091. [DOI] [Google Scholar]
- 127.Huo L, Yang X, Liu Z, Tian X, Qi T, Wang X, Yu K, Sun J, Fan M. Modulation of potential barrier heights in Co3O4/SnO2 heterojunctions for highly H2-selective sensors. Sens. Actuat. B: Chem. 2017;244:694–700. doi: 10.1016/j.snb.2017.01.061. [DOI] [Google Scholar]
- 128.Kwak C-H, Kim T-H, Jeong S-Y, Yoon J-W, Kim J-S, Lee J-H. Humidity-independent oxide semiconductor chemiresistors using terbium-doped SnO2 yolk-shell spheres for real-time breath analysis. ACS Appl. Mater. Interfaces. 2018;10(22):18886–18894. doi: 10.1021/acsami.8b04245. [DOI] [PubMed] [Google Scholar]
- 129.Chen E-X, Yang H, Zhang J. Zeolitic imidazolate framework as formaldehyde gas sensor. Inorg. Chem. 2014;53(11):5411–5413. doi: 10.1021/ic500474j. [DOI] [PubMed] [Google Scholar]
- 130.Chen E-X, Fu H-R, Lin R, Tan Y-X, Zhang J. Highly selective and sensitive trimethylamine gas sensor based on cobalt imidazolate framework material. ACS Appl. Mater. Interfaces. 2014;6(24):22871–22875. doi: 10.1021/am5071317. [DOI] [PubMed] [Google Scholar]
- 131.Tian H, Fan H, Li M, Ma L. Zeolitic imidazolate framework coated ZnO nanorods as molecular sieving to improve selectivity of formaldehyde gas sensor. ACS Sensor. 2016;1(3):243–250. doi: 10.1021/acssensors.5b00236. [DOI] [Google Scholar]
- 132.Koudehi MF, Pourmortazavi SM. Polyvinyl alcohol/polypyrrole/molecularly imprinted polymer nanocomposite as highly selective chemiresistor sensor for 2,4-DNT vapor recognition. Electroanalysis. 2018;30(10):2302–2310. doi: 10.1002/elan.201700751. [DOI] [Google Scholar]
- 133.Tan K, Zuluaga S, Fuentes E, Mattson EC, Veyan JF, Wang H, Li J, Thonhauser T, Chabal YJ. Trapping gases in metal-organic frameworks with a selective surface molecular barrier layer. Nat. Commun. 2016;7:13871. doi: 10.1038/ncomms13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li H, Sadiq MM, Suzuki K, Ricco R, Doblin C, et al. Magnetic metal-organic frameworks for efficient carbon dioxide capture and remote trigger release. Adv. Mater. 2016;28(9):1839–1844. doi: 10.1002/adma.201505320. [DOI] [PubMed] [Google Scholar]
- 135.Liao P-Q, Huang N-Y, Zhang W-X, Zhang J-P, Chen X-M. Controlling guest conformation for efficient purification of butadiene. Science. 2017;356(6343):1193–1196. doi: 10.1126/science.aam7232. [DOI] [PubMed] [Google Scholar]
- 136.Vermoortele F, Maes M, Moghadam PZ, Lennox MJ, Ragon F, et al. p-Xylene-selective metal-organic frameworks: a case of topology-directed selectivity. J. Am. Chem. Soc. 2011;133(46):18526–18529. doi: 10.1021/ja207287h. [DOI] [PubMed] [Google Scholar]
- 137.Zhang L, Cui P, Yang H, Chen J, Xiao F, et al. Metal–organic frameworks as promising photosensitizers for photoelectrochemical water splitting. Adv. Sci. 2016;3(1):1500243. doi: 10.1002/advs.201500243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao M, Yuan K, Yun W, Li G, Guo J, Lin G, Hu W, Zhao H, Tang Z. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nature. 2016;539(7627):76. doi: 10.1038/nature19763. [DOI] [PubMed] [Google Scholar]
- 139.Campbell MG, Sheberla D, Liu SF, Swager TM, Dincă M. Cu3(hexaiminotriphenylene)2: an electrically conductive 2D metal–organic framework for chemiresistive sensing. Angew. Chem. Int. Ed. 2015;127(14):4423–4426. doi: 10.1002/anie.201411854. [DOI] [PubMed] [Google Scholar]
- 140.Sheberla D, Bachman JC, Elias JS, Sun CJ, Shao-Horn Y, Dinca M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017;16(2):220–224. doi: 10.1038/nmat4766. [DOI] [PubMed] [Google Scholar]
- 141.Yamada T, Sadakiyo M, Kitagawa H. High proton conductivity of one-dimensional ferrous oxalate dihydrate. J. Am. Chem. Soc. 2009;131(9):3144–3145. doi: 10.1021/ja808681m. [DOI] [PubMed] [Google Scholar]
- 142.Xu G, Otsubo K, Yamada T, Sakaida S, Kitagawa H. Superprotonic conductivity in a highly oriented crystalline metal-organic framework nanofilm. J. Am. Chem. Soc. 2013;135(20):7438–7441. doi: 10.1021/ja402727d. [DOI] [PubMed] [Google Scholar]
- 143.Sun L, Campbell MG, Dincă M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 2016;55(11):3566–3579. doi: 10.1002/anie.201506219. [DOI] [PubMed] [Google Scholar]
- 144.Xu G, Guo GC, Yao MS, Fu ZH, Wang GE. The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications: 14. Weinheim: Wiley; 2016. pp. 421–462. [Google Scholar]
- 145.Hmadeh M, Lu Z, Liu Z, Gándara F, Furukawa H, Wan S, et al. New porous crystals of extended metal-catecholates. Chem. Mater. 2012;24(18):3511–3513. doi: 10.1021/cm301194a. [DOI] [Google Scholar]
- 146.Talin AA, Centrone A, Ford AC, Foster ME, Stavila V, et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science. 2014;343(6166):66–69. doi: 10.1126/science.1246738. [DOI] [PubMed] [Google Scholar]
- 147.Huang X, Sheng P, Tu ZY, Zhang FJ, Wang JH, et al. A two-dimensional π–d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour. Nat. Commun. 2015;6:7408. doi: 10.1038/ncomms8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takaishi S, Hosoda M, Kajiwara T, Miyasaka H, Yamashita M, et al. Electroconductive porous coordination polymer Cu[Cu(pdt)2] composed of donor and acceptor building units. Inorg. Chem. 2009;48(19):9048–9050. doi: 10.1021/ic802117q. [DOI] [PubMed] [Google Scholar]
- 149.Kambe T, Sakamoto R, Hoshiko K, Takada K, Miyachi M, et al. π-Conjugated nickel bis(dithiolene) complex nanosheet. J. Am. Chem. Soc. 2013;135:2462–2465. doi: 10.1021/ja312380b. [DOI] [PubMed] [Google Scholar]
- 150.Takaishi S, Hosoda M, Kajiwara T, Miyasaka H, Yamashita M, et al. Electroconductive porous coordination polymer Cu[Cu (pdt)2] composed of donor and acceptor building units. Inorg. Chem. 2008;48(19):9048–9050. doi: 10.1021/ic802117q. [DOI] [PubMed] [Google Scholar]
- 151.Ribas X, Dias JC, Morgado J, Wurst K, Santos IC, et al. Alkaline side-coordination strategy for the design of nickel (II) and nickel (III) bis (1, 2-diselenolene) complex based materials. Inorg. Chem. 2004;43(12):3631–3641. doi: 10.1021/ic049860x. [DOI] [PubMed] [Google Scholar]
- 152.Erickson KJ, Leonard F, Stavila V, Foster ME, Spataru CD, et al. Thin film thermoelectric metal-organic framework with high Seebeck coefficient and low thermal conductivity. Adv. Mater. 2015;27(22):3453–3459. doi: 10.1002/adma.201501078. [DOI] [PubMed] [Google Scholar]
- 153.Park SS, Hontz ER, Sun L, Hendon CH, Walsh A, Van Voorhis T, Dinca M. Cation-dependent intrinsic electrical conductivity in isostructural tetrathiafulvalene-based microporous metal-organic frameworks. J. Am. Chem. Soc. 2015;137(5):1774–1777. doi: 10.1021/ic049860x. [DOI] [PubMed] [Google Scholar]
- 154.Cui J, Xu Z. An electroactive porous network from covalent metal-dithiolene links. Chem. Commun. 2014;50(30):3986–3988. doi: 10.1039/c4cc00408f. [DOI] [PubMed] [Google Scholar]
- 155.Panda T, Banerjee R. High Charge Carrier Mobility in two dimensional indium (III) isophthalic acid based frameworks. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2014;84(2):331–336. doi: 10.1007/s40010-014-0152-6. [DOI] [Google Scholar]
- 156.Chen D, Xing H, Su Z, Wang C. Electrical conductivity and electroluminescence of a new anthracene-based metal-organic framework with π-conjugated zigzag chains. Chem. Commun. 2016;52(10):2019–2022. doi: 10.1039/C5CC09065B. [DOI] [PubMed] [Google Scholar]
- 157.Darago LE, Aubrey ML, Yu CJ, Gonzalez MI, Long JR. Electronic conductivity, ferrimagnetic ordering, and reductive insertion mediated by organic mixed-valence in a ferric semiquinoid metal–organic framework. J. Am. Chem. Soc. 2015;137(50):15703–15711. doi: 10.1021/jacs.5b10385. [DOI] [PubMed] [Google Scholar]
- 158.Sun L, Hendon CH, Minier MA, Walsh A, Dincă M. Million-fold electrical conductivity enhancement in Fe2(DEBDC) versus Mn2(DEBDC) (E = S, O) J. Am. Chem. Soc. 2015;137(19):6164–6167. doi: 10.1021/jacs.5b02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sheberla D, Sun L, Blood-Forsythe MA, Er SL, Wade CR, Brozek CK, Aspuru-Guzik AN, Dincă M. High electrical conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a semiconducting metal-organic graphene analogue. J. Am. Chem. Soc. 2014;136:8859–8862. doi: 10.1021/ja502765n. [DOI] [PubMed] [Google Scholar]
- 160.Kambe T, Sakamoto R, Kusamoto T, Pal T, Fukui N, et al. Redox control and high conductivity of nickel bis(dithiolene) complex π-nanosheet: a potential organic two-dimensional topological insulator. J. Am. Chem. Soc. 2014;136:14357–14360. doi: 10.1021/ja507619d. [DOI] [PubMed] [Google Scholar]
- 161.Hermosa C, Alvarez JV, Azani MR, Gomez-Garcia CJ, Fritz M, et al. Intrinsic electrical conductivity of nanostructured metal-organic polymer chains. Nat. Commun. 2013;4:1709. doi: 10.1038/ncomms2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takaishi S, Tobu Y, Kitagawa H, Goto A, Shimizu T, Okubo T, Mitani T, Ikeda R. The NOR observation of Spin-Peierls transition in an antiferromagnetic MX-chain complex NiBr(chxn) (2) Br-2. J. Am. Chem. Soc. 2004;126(6):1614–1615. doi: 10.1021/ja039857x. [DOI] [PubMed] [Google Scholar]
- 163.Heintz RA, Zhao H, Ouyang X, Grandinetti G, Cowen J, Dunbar KR. New insight into the nature of Cu (TCNQ): solution routes to two distinct polymorphs and their relationship to crystalline films that display bistable switching behavior. Inorg. Chem. 1999;38(1):144–156. doi: 10.1021/ic9812095. [DOI] [Google Scholar]
- 164.Campbell MG, Liu SF, Swager TM, Dinca M. Chemiresistive sensor arrays from conductive 2D metal-organic frameworks. J. Am. Chem. Soc. 2015;137(43):13780–13783. doi: 10.1021/jacs.5b09600. [DOI] [PubMed] [Google Scholar]
- 165.Smith MK, Jensen KE, Pivak PA, Mirica KA. Direct self-assembly of conductive nanorods of metal–organic frameworks into chemiresistive devices on shrinkable polymer films. Chem. Mater. 2016;28(15):5264–5268. doi: 10.1021/acs.chemmater.6b02528. [DOI] [Google Scholar]
- 166.Smith MK, Mirica KA. Self-organized frameworks on textiles (SOFT): conductive fabrics for simultaneous sensing, capture, and filtration of gases. J. Am. Chem. Soc. 2017;139(46):16759–16767. doi: 10.1021/jacs.7b08840. [DOI] [PubMed] [Google Scholar]
- 167.Altomare DF, Porcelli F, Picciariello A, Pinto M, Di Lena M, et al. The use of the PEN3 e-nose in the screening of colorectal cancer and polyps. Tech. Coloproctol. 2016;20(6):405–409. doi: 10.1007/s10151-016-1457-z. [DOI] [PubMed] [Google Scholar]
- 168.T.W. Zhang, T.Liu, M. Zhang, Y. Zhang, H. Li et al., NOS.E: a new fast response electronic nose health monitoring system, in 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC)2018, pp. 4977–4980 (2018). 10.1109/EMBC.2018.8513416 [DOI] [PubMed]
- 169.Li W, Liu H, Xie D, He Z, Pi X. Lung cancer screening based on type-different sensor arrays. Sci. Rep. 2017;7(1):1969. doi: 10.1038/s41598-017-02154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Waltman CG, Marcelissen TAT, van Roermund JGH. Exhaled-breath testing for prostate cancer based on volatile organic compound profiling using an electronic nose device (aeonose): a preliminary report. Eur. Urol. Focus. 2018 doi: 10.1016/j.euf.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 171.Schuermans VNE, Li Z, Jongen A, Wu Z, Shi J, Ji J, Bouvy ND. Pilot study: detection of gastric cancer from exhaled air analyzed with an electronic nose in Chinese patients. Surg. Innov. 2018;25(5):429–434. doi: 10.1177/1553350618781267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sanaeifar A, ZakiDizaji H, Jafari A, Guardia MDL. Early detection of contamination and defect in foodstuffs by electronic nose: A review. TrAC Trend. Anal. Chem. 2017;97:257–271. doi: 10.1016/j.trac.2017.09.014. [DOI] [Google Scholar]
- 173.Yu H, Wang J, Xu Y. Identification of adulterated milk using electronic nose. Sens. Mater. 2007;19:275–285. doi: 10.1007/978-0-387-71720-3_15. [DOI] [Google Scholar]
- 174.Gómez AH, Wang J, Hu G, Pereira AG. Electronic nose technique potential monitoring mandarin maturity. Sens. Actuat. B: Chem. 2006;113(1):347–353. doi: 10.1016/j.snb.2005.03.090. [DOI] [Google Scholar]
- 175.Blanco-Rodriguez A, Camara VF, Campo F, Becheran L, Duran A, et al. Development of an electronic nose to characterize odours emitted from different stages in a wastewater treatment plant. Water Res. 2018;134:92–100. doi: 10.1016/j.watres.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 176.Borah S, Hines EL, Leeson MS, Iliescu DD, Bhuyan M, Gardner JW. Neural network based electronic nose for classification of tea aroma. Sens. Instrum. Food Qual. Saf. 2007;2(1):7–14. doi: 10.1007/s11694-007-9028-7. [DOI] [Google Scholar]
- 177.Wozniak L, Kalinowski P, Jasinski G, Jasinski P. FFT analysis of temperature modulated semiconductor gas sensor response for the prediction of ammonia concentration under humidity interference. Microelectron. Reliab. 2018;84:163–169. doi: 10.1016/j.microrel.2018.03.034. [DOI] [Google Scholar]
- 178.Ali AAS, Farhat A, Mohamad S, Amira A, Bensaali F, Benammar M, Bermak A. Embedded platform for gas applications using hardware/software co-design and RFID. IEEE Sens. J. 2018;18(11):4633–4642. doi: 10.1109/jsen.2018.2822711. [DOI] [Google Scholar]
- 179.Ghasemi-Varnamkhasti M, Amiri ZS, Tohidi M, Dowlati M, Mohtasebi SS, Silva AC, Fernandes DDS, Araujo MCU. Differentiation of cumin seeds using a metal-oxide based gas sensor array in tandem with chemometric tools. Talanta. 2018;176:221–226. doi: 10.1016/j.talanta.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 180.Cui S, Wang J, Yang L, Wu J, Wang X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC-MS combined with chemometrics. J. Pharm. Biomed. Anal. 2015;102:64–77. doi: 10.1016/j.jpba.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 181.Gardner J, Yinon J. Electronic Noses and Sensors for the Detection of Explosives. Netherlands: Springer; 2004. [Google Scholar]
- 182.Olguín C, Laguarda-Miró N, Pascual L, García-Breijo E, Martínez-Mañez R, Soto J. An electronic nose for the detection of Sarin, Soman and Tabun mimics and interfering agents. Sens. Actuat. B: Chem. 2014;202:31–37. doi: 10.1016/j.snb.2014.05.060. [DOI] [Google Scholar]
- 183.Wang B, Huynh T-P, Wu W, Hayek N, Do TT, et al. A highly sensitive diketopyrrolopyrrole-based ambipolar transistor for selective detection and discrimination of xylene isomers. Adv. Mater. 2016;28(21):4012–4018. doi: 10.1002/adma.201505641. [DOI] [PubMed] [Google Scholar]
- 184.Pavelko RG, Daly H, Hübner M, Hardacre C, Llobet E. Time-resolved DRIFTS, MS, and resistance study of SnO2 materials: the role of surface hydroxyl groups in formation of donor states. J. Phys. Chem. C. 2013;117(8):4158–4167. doi: 10.1021/jp312532u. [DOI] [Google Scholar]
- 185.Oprea A, Bârsan N, Weimar U. Work function changes in gas sensitive materials: fundamentals and applications. Sens. Actuat. B: Chem. 2009;142(2):470–493. doi: 10.1016/j.snb.2009.06.043. [DOI] [Google Scholar]
- 186.Feng H, Tan S, Tang H, Zheng Q, Shi Y, et al. Temperature- and coverage-dependent kinetics of photocatalytic reaction of methanol on TiO2 (110) − (1 × 1) surface. J. Phys. Chem. C. 2016;120(10):5503–5514. doi: 10.1021/acs.jpcc.5b12010. [DOI] [Google Scholar]
- 187.Phillips KR, Jensen SC, Baron M, Li S-C, Friend CM. Sequential photo-oxidation of methanol to methyl formate on TiO2(110) J. Am. Chem. Soc. 2013;135(2):574–577. doi: 10.1021/ja3106797. [DOI] [PubMed] [Google Scholar]
- 188.Wu J, Shan H, Chen W, Gu X, Tao P, Song C, Shang W, Deng T. In situ environmental TEM in imaging gas and liquid phase chemical reactions for materials research. Adv. Mater. 2016;28(44):9686–9712. doi: 10.1002/adma.201602519. [DOI] [PubMed] [Google Scholar]
- 189.Kishita K, Kamino T, Watabe A, Kuroda K, Saka H. In situ TEM observation of solid-gas reactions. J. Phys: Conf. Ser. 2008;126(1):012085. doi: 10.1088/1742-6596/126/1/012085. [DOI] [Google Scholar]
- 190.Yoosefian M, Raissi H, Mola A. The hybrid of Pd and SWCNT (Pd loaded on SWCNT) as an efficient sensor for the formaldehyde molecule detection: a DFT study. Sens. Actuat. B: Chem. 2015;212:55–62. doi: 10.1016/j.snb.2015.02.004. [DOI] [Google Scholar]
- 191.Dobrokhotov VV, McIlroy DN, Norton MG, Berven CA. Transport properties of hybrid nanoparticle-nanowire systems and their application to gas sensing. Nanotechnology. 2006;17(16):4135–4142. doi: 10.1088/0957-4484/17/16/024. [DOI] [PubMed] [Google Scholar]
- 192.Wang L, Chai R, Lou Z, Shen G. Highly sensitive hybrid nanofiber-based room-temperature CO sensors: experiments and density functional theory simulations. Nano Res. 2017;11(2):1029–1037. doi: 10.1007/s12274-017-1718-9. [DOI] [Google Scholar]
- 193.Omidvar A, Anafcheh M, Hadipour NL. Computational studies on carbon nanotube-graphene nanoribbon hybrids by density functional theory calculations. Sci. Iran. 2013;20(3):1014–1017. doi: 10.1016/j.scient.2013.05.018. [DOI] [Google Scholar]
- 194.Guo Z, Liao N, Zhang M, Xue W. Theoretical approach to evaluate graphene/PANI composite as highly selective ammonia sensor. Appl. Surf. Sci. 2018;453:336–340. doi: 10.1016/j.apsusc.2018.05.108. [DOI] [Google Scholar]
- 195.Fu H, Yang X, An X, Fan W, Jiang X, Yu A. Experimental and theoretical studies of V2O5@TiO2 core-shell hybrid composites with high gas sensing performance towards ammonia. Sens. Actuat. B: Chem. 2017;252:103–115. doi: 10.1016/j.snb.2017.05.027. [DOI] [Google Scholar]
- 196.Tian J, Zhao Z, Kumar A, Boughton RI, Liu H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: a review. Chem. Soc. Rev. 2014;43(20):6920–6937. doi: 10.1039/c4cs00180j. [DOI] [PubMed] [Google Scholar]
- 197.Friedmann D, Hakki A, Kim H, Choi W, Bahnemann D. Heterogeneous photocatalytic organic synthesis: state-of-the-art and future perspectives. Green Chem. 2016;18(20):5391–5411. doi: 10.1039/c6gc01582d. [DOI] [Google Scholar]
- 198.Gao C, Wang J, Xu H, Xiong Y. Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 2017;46(10):2799–2823. doi: 10.1039/c6cs00727a. [DOI] [PubMed] [Google Scholar]
- 199.Rajeshwar K, Osugi ME, Chanmanee W, Chenthamarakshan CR, Zanoni MVB, Kajitvichyanukul P, Krishnan-Ayer R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol., C. 2008;9(4):171–192. doi: 10.1016/j.jphotochemrev.2008.09.001. [DOI] [Google Scholar]
- 200.Qu Y, Duan X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013;42(7):2568–2580. doi: 10.1039/c2cs35355e. [DOI] [PubMed] [Google Scholar]
- 201.Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009;38(1):253–278. doi: 10.1039/b800489g. [DOI] [PubMed] [Google Scholar]
- 202.Broza YY, Zhou X, Yuan M, Qu D, Zheng Y, et al. Disease detection with molecular biomarkers: from chemistry of body fluids to nature-inspired chemical sensors. Chem. Rev. 2019;119(22):11761–11817. doi: 10.1021/acs.chemrev.9b00437. [DOI] [PubMed] [Google Scholar]
- 203.Yao M-S, Cao L-A, Tang Y-X, Wang G-E, Liu R-H, et al. Gas transport regulation in a MO/MOF interface for enhanced selective gas detection. J. Mater. Chem. A. 2019;7(31):18397–18403. doi: 10.1039/C9TA05226G. [DOI] [Google Scholar]
- 204.Yao M, Zheng J-J, Wu A-Q, Xu G, Nagarkar SS, Zhang G, Tsujimoto M, Sakaki S, Horike S, Otake K-I. Dual-ligand porous coordination polymer chemiresistor with modulated conductivity and porosity. Angew. Chem. Int. Ed. 2020;59(1):172–176. doi: 10.1002/anie.201909096. [DOI] [PubMed] [Google Scholar]
- 205.Yao MS, Xiu JW, Huang QQ, Li W-H, Wu WW, et al. Van der Waals heterostructured MOF-on-MOF thin films: cascading functionality to realize advanced chemiresistive sensing. Angew. Chem. Int. Ed. 2019;58(42):14915–14919. doi: 10.1002/anie.201907772. [DOI] [PubMed] [Google Scholar]
- 206.Fang X, Zong B, Mao S. Metal-organic framework-based sensors for environmental contaminant sensing. Nano-Micro Lett. 2018;10(4):64. doi: 10.1007/s40820-018-0218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wu W, Wang B, Segev-Bar M, Dou W, Niu F, et al. Free-standing and eco-friendly polyaniline thin films for multifunctional sensing of physical and chemical stimuli. Adv. Funct. Mater. 2017;27(40):1703147. doi: 10.1002/adfm.201703147. [DOI] [Google Scholar]
- 208.Kwon OS, Park SJ, Lee JS, Park E, Kim T, et al. Multidimensional conducting polymer nanotubes for ultrasensitive chemical nerve agent sensing. Nano Lett. 2012;12(6):2797–2802. doi: 10.1021/nl204587t. [DOI] [PubMed] [Google Scholar]
- 209.Yan K, Zhang D. Feature selection and analysis on correlated gas sensor data with recursive feature elimination. Sens. Actuat. B: Chem. 2015;212:353–363. doi: 10.1016/j.snb.2015.02.025. [DOI] [Google Scholar]
- 210.Cui S, Mao S, Lu G, Chen J. Graphene coupled with nanocrystals: opportunities and challenges for energy and sensing applications. J. Phys. Chem. Lett. 2013;4(15):2441–2454. doi: 10.1021/jz400976a31d. [DOI] [Google Scholar]
- 211.Mao S, Lu G, Chen J. Nanocarbon-based gas sensors: progress and challenges. J. Mater. Chem. A. 2014;2(16):5573–5579. doi: 10.1039/c3ta13823b. [DOI] [Google Scholar]
- 212.Estakhroyeh HR, Rashedi E, Mehran M. Design and construction of electronic nose for multi-purpose applications by sensor array arrangement using IBGSA. J. Intell. Robot. Syst. 2017;92(2):205–221. doi: 10.1007/s10846-017-0759-3. [DOI] [Google Scholar]
- 213.Herrero JL, Lozano J, Santos JP, Suarez JI. On-line classification of pollutants in water using wireless portable electronic noses. Chemosphere. 2016;152:107–116. doi: 10.1016/j.chemosphere.2016.02.106. [DOI] [PubMed] [Google Scholar]
- 214.Chang F, Heinemann P. Prediction of human responses to dairy odor using an electronic nose and neural networks. Trans. ASABE. 2018;61(2):399–409. doi: 10.13031/trans.12177. [DOI] [Google Scholar]
- 215.Rodriguez Gamboa JC, Albarracin E ES, da Silva AJ, de Andrade-Lima LL, Ferreira TAE. Wine quality rapid detection using a compact electronic nose system: application focused on spoilage thresholds by acetic acid. 2019;Lwt 108:377–384. doi: 10.1016/j.lwt.2019.03.074. [DOI] [Google Scholar]
- 216.Tohidi M, Ghasemi-Varnamkhasti M, Ghafarinia V, Saeid Mohtasebi S, Bonyadian M. Identification of trace amounts of detergent powder in raw milk using a customized low-cost artificial olfactory system: a novel method. Measurement. 2018;124:120–129. doi: 10.1016/j.measurement.2018.04.006. [DOI] [Google Scholar]
- 217.Gorji-Chakespari A, Nikbakht AM, Sefidkon F, Ghasemi-Varnamkhasti M, Brezmes J, Llobet E. Performance comparison of fuzzy ARTMAP and LDA in qualitative classification of iranian rosa damascena essential oils by an electronic nose. Sensors (Basel) 2016;16(5):636. doi: 10.3390/s16050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dong W, Zhao J, Hu R, Dong Y, Tan L. Differentiation of Chinese robusta coffees according to species, using a combined electronic nose and tongue, with the aid of chemometrics. Food Chem. 2017;229:743–751. doi: 10.1016/j.foodchem.2017.02.149. [DOI] [PubMed] [Google Scholar]
- 219.Shahid A, Choi JH, Rana A, Kim HS. Least squares neural network-based wireless E-Nose system using an SnO2 sensor array. Sensors (Basel) 2018;18(5):1446. doi: 10.3390/s18051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yao L, Kan K, Lin Y, Song J, Wang J, Gao J, Shen P, Li L, Shi K. Si doped highly crystalline mesoporous In2O3 nanowires: synthesis, characterization and ultra-high response to NOx at room temperature. RSC Adv. 2015;5(20):15515–15523. doi: 10.1039/c4ra14354j. [DOI] [Google Scholar]
- 221.He K, Jin Z, Chu X, Bi W, Wang W, Wang C, Liu S. Fast response–recovery time toward acetone by a sensor prepared with Pd doped WO3 nanosheets. RSC Adv. 2019;9(49):28439–28450. doi: 10.1039/c9ra04429a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lv L, Wang Y, Cheng P, Zhang B, Dang F, Xu L. Ultrasonic spray pyrolysis synthesis of three-dimensional ZnFe2O4-based macroporous spheres for excellent sensitive acetone gas sensor. Sens. Actuat. B: Chem. 2019;297:126755. doi: 10.1016/j.snb.2019.126755. [DOI] [Google Scholar]
- 223.Shingange K, Swart H, Mhlongo GH. Ultrafast detection of low acetone concentration displayed by Au-loaded LaFeO3 nanobelts owing to synergetic effects of porous 1D morphology and catalytic activity of Au nanoparticles. ACS Omega. 2019;4(21):19018–19029. doi: 10.1021/acsomega.9b01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hsu C-L, Jhang B-Y, Kao C, Hsueh T-J. UV-illumination and Au-nanoparticles enhanced gas sensing of p-type Na-doped ZnO nanowires operating at room temperature. Sens. Actuat. B: Chem. 2018;274:565–574. doi: 10.1016/j.snb.2018.08.016. [DOI] [Google Scholar]
- 225.Zhang Y, Zhou L, Liu Y, Liu D, Liu F, et al. Gas sensor based on samarium oxide loaded mulberry-shaped tin oxide for highly selective and sub ppm-level acetone detection. J. Colloid Interface Sci. 2018;531:74–82. doi: 10.1016/j.jcis.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 226.Lu Y, Li J, Han J, Ng HT, Binder C, Partridge C, Meyyappan M. Room temperature methane detection using palladium loaded single-walled carbon nanotube sensors. Chem. Phys. Lett. 2004;391(4–6):344–348. doi: 10.1016/j.cplett.2004.05.029. [DOI] [Google Scholar]
- 227.Li H, Xu J, Zhu Y, Chen X, Xiang Q. Enhanced gas sensing by assembling Pd nanoparticles onto the surface of SnO2 nanowires. Talanta. 2010;82(2):458–463. doi: 10.1016/j.talanta.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 228.Kim J-Y, Lee J-H, Kim J-H, Mirzaei A, Kim HW, Kim SS. Realization of H2S sensing by Pd-functionalized networked CuO nanowires in self-heating mode. Sens. Actuat. B: Chem. 2019;299:126965. doi: 10.1016/j.snb.2019.126965. [DOI] [Google Scholar]
- 229.Yang Y, Tian C, Wang J, Sun L, Shi K, Zhou W, Fu H. Facile synthesis of novel 3D nanoflower-like Cu(x)O/multilayer graphene composites for room temperature NO(x) gas sensor application. Nanoscale. 2014;6(13):7369–7378. doi: 10.1039/c4nr00196f. [DOI] [PubMed] [Google Scholar]
- 230.Zhang J, Zeng D, Zhao S, Wu J, Xu K, Zhu Q, Zhang G, Xie C. Room temperature NO2 sensing: what advantage does the rGO-NiO nanocomposite have over pristine NiO? Phys. Chem. Chem. Phys. 2015;17(22):14903–14911. doi: 10.1039/c5cp01987g. [DOI] [PubMed] [Google Scholar]
- 231.Huang Q, Zeng D, Li H, Xie C. Room temperature formaldehyde sensors with enhanced performance, fast response and recovery based on zinc oxide quantum dots/graphene nanocomposites. Nanoscale. 2012;4(18):5651–5658. doi: 10.1039/c2nr31131c. [DOI] [PubMed] [Google Scholar]
- 232.Wang H, Nie S, Li H, Ali R, Fu J, et al. 3D hollow quasi-graphite capsules/polyaniline hybrid with a high performance for room-temperature ammonia gas sensors. ACS Sens. 2019;4(9):2343–2350. doi: 10.1021/acssensors.9b00882. [DOI] [PubMed] [Google Scholar]
- 233.Kooti M, Keshtkar S, Askarieh M, Rashidi A. Progress toward a novel methane gas sensor based on SnO2 nanorods-nanoporous graphene hybrid. Sens. Actuator. B: Chem. 2019;281:96–106. doi: 10.1016/j.snb.2018.10.032. [DOI] [Google Scholar]
- 234.Liu H, Zhang W, Yu H, Gao L, Song Z, et al. Solution-processed gas sensors employing SnO2 quantum dot/MWCNT nanocomposites. ACS Appl. Mater. Interfaces. 2016;8(1):840–846. doi: 10.1021/acsami.5b10188. [DOI] [PubMed] [Google Scholar]
- 235.Liang F, Chen S, Xie W, Zou C. The decoration of Nb-doped TiO2 microspheres by reduced graphene oxide for enhanced CO gas sensing. J. Phys. Chem. Solids. 2018;114:195–200. doi: 10.1016/j.jpcs.2017.11.001. [DOI] [Google Scholar]
- 236.Zou C, Hu J, Su Y, Shao F, Tao Z, et al. Three-dimensional Fe3O4@reduced graphene oxide heterojunctions for high-performance room-temperature NO2 sensors. Front. Mater. 2019;6:00195. doi: 10.3389/fmats.2019.00195. [DOI] [Google Scholar]
- 237.Jiang T, Wan P, Ren Z, Yan S. Anisotropic polyaniline/SWCNT composite films prepared by in situ electropolymerization on highly oriented polyethylene for high-efficiency ammonia sensor. ACS Appl. Mater. Interfaces. 2019;11(41):38169–38176. doi: 10.1021/acsami.9b13336. [DOI] [PubMed] [Google Scholar]
- 238.Ekaterina Dovgolevsky GK, Tisch U, Haick H. Monolayer-capped cubic platinum nanoparticles for sensing nonpolar analytes in highly umid atmospheres. Am. Chem. Soc. 2010;114(33):14042–14049. doi: 10.1021/jp105810w. [DOI] [Google Scholar]
- 239.Park CH, Schroeder V, Kim BJ, Swager TM. Ionic liquid-carbon nanotube sensor arrays for human breath related volatile organic compounds. ACS Sens. 2018;3(11):2432–2437. doi: 10.1021/acssensors.8b00987. [DOI] [PubMed] [Google Scholar]
- 240.Zilberman Y, Tisch U, Shuster G, Pisula W, Feng X, Mullen K, Haick H. Carbon nanotube/hexa-peri-hexabenzocoronene bilayers for discrimination between nonpolar volatile organic compounds of cancer and humid atmospheres. Adv. Mater. 2010;22(38):4317–4320. doi: 10.1002/adma.201001275. [DOI] [PubMed] [Google Scholar]
- 241.Sun P, Cai Y, Du S, Xu X, You L, et al. Hierarchical α-Fe2O3/SnO2 semiconductor composites: hydrothermal synthesis and gas sensing properties. Sens. Actuat. B: Chem. 2013;182:336–343. doi: 10.1016/j.snb.2013.03.019. [DOI] [Google Scholar]
- 242.Lu G, Xu J, Sun J, Yu Y, Zhang Y, Liu F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuat. B: Chem. 2012;162(1):82–88. doi: 10.1016/j.snb.2011.12.039. [DOI] [Google Scholar]
- 243.Gu D, Li X, Zhao Y, Wang J. Enhanced NO2 sensing of SnO2/SnS2 heterojunction based sensor. Sens. Actuat. B: Chem. 2017;244:67–76. doi: 10.1016/j.snb.2016.12.125. [DOI] [Google Scholar]
- 244.Peng Sun CW, Liu J, Zhou X, Li X, Hu X, Lu G. Hierarchical assembly of α-Fe2O3 nanosheets on SnO2 hollow nanospheres with enhanced ethanol sensing properties. ACS Appl. Mater. Interfaces. 2015;7(34):19119–19125. doi: 10.1021/acsami.5b04751. [DOI] [PubMed] [Google Scholar]
- 245.Li X, Wang C, Guo H, Sun P, Liu F, Liang X, Lu G. Double-shell architectures of ZnFe2O4 nanosheets on ZnO hollow spheres for high-performance gas sensors. ACS Appl. Mater. Interfaces. 2015;7(32):17811–17818. doi: 10.1021/acsami.5b04118. [DOI] [PubMed] [Google Scholar]
- 246.Wang C, Cheng X, Zhou X, Sun P, Hu X, Shimanoe K, Lu G, Yamazoe N. Hierarchical α-Fe2O3/NiO composites with a hollow structure for a gas sensor. ACS Appl. Mater. Interfaces. 2014;6(15):12031–12307. doi: 10.1021/am501063z. [DOI] [PubMed] [Google Scholar]
- 247.Xu K, Li N, Zeng D, Tian S, Zhang S, Hu D, Xie C. Interface bonds determined gas-sensing of SnO2–SnS2 hybrids to ammonia at room temperature. ACS Appl. Mater. Interfaces. 2015;7(21):11359–12368. doi: 10.1021/acsami.5b01856. [DOI] [PubMed] [Google Scholar]
- 248.Wu C, Zhang J, Wang X, Xie C, Shi S, Zeng D. Effect of heterointerface on NO2 sensing properties of in situ formed TiO2 QDs-decorated NiO nanosheets. Nanomaterials. 2019;9(11):1628. doi: 10.3390/nano9111628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chang X, Li X, Qiao X, Li K, Xiong Y, Li X, Guo T, Zhu L, Xue Q. Metal-organic frameworks derived ZnO@MoS nanosheets core/shell heterojunctions for ppb-level acetone detection: ultra-fast response and recovery. Sens. Actuat. B: Chem. 2020;304:127430. doi: 10.1016/j.snb.2019.127430. [DOI] [Google Scholar]
- 250.Alali KT, Liu J, Liu Q, Li R, Zhang H, Aljebawi K, Liu P, Wang J. Enhanced acetone gas sensing response of ZnO/ZnCo2O4 nanotubes synthesized by single capillary electrospinning technology. Sens. Actuat. B: Chem. 2017;252:511–522. doi: 10.1016/j.snb.2017.06.034. [DOI] [Google Scholar]
- 251.Bang JH, Choi MS, Mirzaei A, Oum W, Han S, Kim SS, Kim HW. Porous Si/SnO2 nanowires heterostructures for H2S gas sensing. Ceram. Int. 2020;46(1):604–611. doi: 10.1016/j.ceramint.2019.09.010. [DOI] [Google Scholar]
- 252.Li S, Liu A, Yang Z, He J, Wang J, et al. Room temperature gas sensor based on tin dioxide@ polyaniline nanocomposite assembled on flexible substrate: ppb-level detection of NH3. Sens. Actuat. B: Chem. 2019;299:126970. doi: 10.1016/j.snb.2019.126970. [DOI] [Google Scholar]
- 253.Liu L, Wang Y, Dai Y, Li G, Wang S, Li T, Zhang T, Qin S. In situ growth of NiO@SnO2 hierarchical nanostructures for high performance H2S sensing. ACS Appl. Mater. Interfaces. 2019;11(47):44829–44836. doi: 10.1021/acsami.9b13001. [DOI] [PubMed] [Google Scholar]
- 254.Sun Q, Wang J, Hao J, Zheng S, Wan P, Wang T, Fang H, Wang Y. SnS2/SnS p–n heterojunctions with an accumulation layer for ultrasensitive room-temperature NO2 detection. Nanoscale. 2019;11(29):13741–13749. doi: 10.1039/c9nr02780g. [DOI] [PubMed] [Google Scholar]
- 255.Zeng W, Liu Y, Mei J, Tang C, Luo K, Li S, Zhan H, He Z. Hierarchical SnO2–Sn3O4 heterostructural gas sensor with high sensitivity and selectivity to NO2. Sens. Actuat. B: Chem. 2019;301:127010. doi: 10.1016/j.snb.2019.127010. [DOI] [Google Scholar]
- 256.Zhou X, Feng W, Wang C, Hu X, Li X, Sun P, Shimanoe K, Yamazoe N, Lu G. Porous ZnO/ZnCo2O4 hollow spheres: synthesis, characterization, and applications in gas sensing. J. Mater. Chem. A. 2014;2(41):17683–17690. doi: 10.1039/c4ta04386c. [DOI] [Google Scholar]
- 257.Han L, Wang D, Cui J, Chen L, Jiang T, Lin Y. Study on formaldehyde gas-sensing of In2O3-sensitized ZnO nanoflowers under visible light irradiation at room temperature. J. Mater. Chem. 2012;22(25):12915–12920. doi: 10.1039/c2jm16105b. [DOI] [Google Scholar]
- 258.Zhou T, Sang Y, Wang X, Wu C, Zeng D, Xie C. Pore size dependent gas-sensing selectivity based on ZnO@ZIF nanorod arrays. Sens. Actuat. B: Chem. 2018;258:1099–1106. doi: 10.1016/j.snb.2017.12.024. [DOI] [Google Scholar]
- 259.Yao MS, Tang WX, Wang GE, Nath B, Xu G. MOF thin film-coated metal oxide nanowire array: significantly improved chemiresistor sensor performance. Adv. Mater. 2016;28(26):5229–5234. doi: 10.1002/adma.201506457. [DOI] [PubMed] [Google Scholar]
- 260.Wu X, Xiong S, Mao Z, Hu S, Long X. A designed ZnO@ZIF-8 core-shell nanorod film as a gas sensor with excellent selectivity for H2 over CO. Chemistry. 2017;23(33):7969–7975. doi: 10.1002/chem.201700320. [DOI] [PubMed] [Google Scholar]
- 261.Nair SS, Illyaskutty N, Tam B, Yazaydin AO, Emmerich K, et al. ZnO@ZIF-8: Gas sensitive core-shell hetero-structures show reduced cross-sensitivity to humidity. Sens. Actuat. B: Chem. 2020;304:127184. doi: 10.1016/j.snb.2019.127184. [DOI] [Google Scholar]
- 262.Wang P, Zou X, Tan H, Wu S, Jiang L, Zhu G. Ultrathin ZIF-8 film containing polyoxometalate as an enhancer for selective formaldehyde sensing. J. Mater. Chem. C. 2018;6(20):5412–5419. doi: 10.1039/c8tc00987b. [DOI] [Google Scholar]
- 263.Tian H, Fan H, Li M, Ma L. Zeolitic imidazolate framework coated ZnO nanorods as molecular sieving to improve selectivity of formaldehyde gas sensor. ACS Sensor. 2015;1(3):243–250. doi: 10.1021/acssensors.5b00236. [DOI] [Google Scholar]
- 264.Drobek M, Kim JH, Bechelany M, Vallicari C, Julbe A, Kim SS. MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces. 2016;8(13):8323–8328. doi: 10.1021/acsami.5b12062. [DOI] [PubMed] [Google Scholar]
- 265.Dang L, Zhang G, Kan K, Lin Y, Bai F, Jing L, Shen P, Li L, Shi K. Heterostructured Co3O4/PEI–CNTs composite: fabrication, characterization and CO gas sensors at room temperature. J. Mater. Chem. A. 2014;2(13):4558–4565. doi: 10.1039/c3ta15019d. [DOI] [Google Scholar]
- 266.Zhang X, Sun Y, Fan Y, Liu Z, Zeng Z, Zhao H, Wang X, Xu J. Effects of organotin halide perovskite and Pt nanoparticles in SnO2-based sensing materials on the detection of formaldehyde. J. Mater. Sci.-Mater. Electron. 2019;30(23):20624–20637. doi: 10.1007/s10854-019-02428-0. [DOI] [Google Scholar]
- 267.Javanmardi S, Nasresfahani S, Sheikhi MH. Facile synthesis of PdO/SnO2/CuO nanocomposite with enhanced carbon monoxide gas sensing performance at low operating temperature. Mater. Res. Bull. 2019;118:110496. doi: 10.1016/j.materresbull.2019.110496. [DOI] [Google Scholar]
- 268.Lee JH, Kim JH, Kim JY, Mirzaei A, Kim HW, Kim SS. ppb-Level selective hydrogen gas detection of Pd-functionalized In2O3-loaded ZnO nanofiber gas sensors. Sensors (Basel) 2019;19(19):4276. doi: 10.3390/s19194276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xia Y, Wang J, Xu L, Li X, Huang S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuat. B: Chem. 2020;304:127334. doi: 10.1016/j.snb.2019.127334. [DOI] [Google Scholar]
- 270.Tian H, Fan H, Ma J, Liu Z, Ma L, Lei S, Fang J, Long C. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 2018;341:102–111. doi: 10.1016/j.jhazmat.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 271.Lu W-C, Kumar SS, Chen Y-C, Hsu C-M, Lin H-N. Au/Cu2O/ZnO ternary nanocomposite for low concentration NO2 gas sensing at room temperature. Mater. Lett. 2019;256:126657. doi: 10.1016/j.matlet.2019.126657. [DOI] [Google Scholar]
- 272.Wei Y, Yi G, Xu Y, Zhou L, Wang X, et al. Synthesis, characterization, and gas-sensing properties of Ag/SnO2/rGO composite by a hydrothermal method. J. Mater. Sci.-Mater. Electron. 2017;28(22):17049–17057. doi: 10.1007/s10854-017-7630-y. [DOI] [Google Scholar]
- 273.Zhou Y, Ding Q, Li J, Yang Q, Wu T, et al. TiO2/InVO4 n–n heterojunctions for efficient ammonia gas detection and their sensing mechanisms. J. Mater. Sci. 2019;54(21):13660–13673. doi: 10.1007/s10853-019-03868-z. [DOI] [Google Scholar]
- 274.Nasresfahani S, Sheikhi MH, Tohidi M, Zarifkar A. Methane gas sensing properties of Pd-doped SnO2/reduced graphene oxide synthesized by a facile hydrothermal route. Mater. Res. Bull. 2017;89:161–169. doi: 10.1016/j.materresbull.2017.01.032. [DOI] [Google Scholar]
- 275.Li S, Diao Y, Yang Z, He J, Wang J, et al. Enhanced room temperature gas sensor based on Au-loaded mesoporous In2O3 nanospheres@polyaniline core-shell nanohybrid assembled on flexible PET substrate for NH3 detection. Sens. Actuator. B: Chem. 2018;276:526–533. doi: 10.1016/j.snb.2018.08.120. [DOI] [Google Scholar]
- 276.Liu B, Li Y, Gao L, Zhou F, Duan G. Ultrafine Pt NPs-decorated SnO2/α-Fe2O3 hollow nanospheres with highly enhanced sensing performances for styrene. J. Hazard. Mater. 2018;358:355–365. doi: 10.1016/j.jhazmat.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 277.Chen M, Wang H, Hu J, Zhang Y, Li K, et al. Near-room-temperature ethanol gas sensor based on mesoporous Ag/Zn–LaFeO3 nanocomposite. Adv. Mater. Interfaces. 2018;6(1):1801453. doi: 10.1002/admi.201801453. [DOI] [Google Scholar]
- 278.Lee E, VahidMohammadi A, Prorok BC, Yoon YS, Beidaghi M, Kim D-J. Room temperature gas sensing of two-dimensional titanium carbide (MXene) ACS Appl. Mater. Interfaces. 2017;9(42):37184–37190. doi: 10.1021/acsami.7b11055. [DOI] [PubMed] [Google Scholar]
- 279.Zhou J, Lin H, Cheng X-F, Shu J, He J-H, et al. Ultrasensitive and robust organic gas sensors through dual hydrogen bonding. Mater. Horiz. 2019;6:554–562. doi: 10.1039/C8MH01098F. [DOI] [Google Scholar]
- 280.Vossmeyer T, Guse B, Besnard I, Bauer RE, MüLLEN K, Yasuda A. Gold nanoparticle/polyphenylene dendrimer composite films: preparation and vapor-sensing properties. Adv. Mater. 2002;14(3):238–242. doi: 10.1002/1521-4095(20020205)14:3<238::AID-ADMA238>3.0.CO;2-#. [DOI] [Google Scholar]
- 281.DMello ME, Sundaram NG, Singh A, Singh AK, Kalidindi SB. An amine functionalized zirconium metal–organic framework as an effective chemiresistive sensor for acidic gases. Chem. Commun. 2019;55(3):349–352. doi: 10.1039/C8CC06875E. [DOI] [PubMed] [Google Scholar]
- 282.Wongchoosuk C, Lutz M, Kerdcharoen T. Detection and classification of human body odor using an electronic nose. Sensors (Basel) 2009;9(9):7234–7249. doi: 10.3390/s90907234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Covington JA, Westenbrink EW, Ouaret N, Harbord R, Bailey C, et al. Application of a novel tool for diagnosing bile acid diarrhoea. Sensors (Basel) 2013;13(9):11899–11912. doi: 10.3390/s130911899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Thriumani R, Zakaria A, Hashim YZH, Jeffree AI, Helmy KM, et al. A study on volatile organic compounds emitted by in vitro lung cancer cultured cells using gas sensor array and SPME-GCMS. BMC Cancer. 2018;18(1):362. doi: 10.1186/s12885-018-4235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.van Hooren MR, Leunis N, Brandsma DS, Dingemans AC, Kremer B, Kross KW. Differentiating head and neck carcinoma from lung carcinoma with an electronic nose: a proof of concept study. Eur. Arch. Otorhinolaryngol. 2016;273(11):3897–3903. doi: 10.1007/s00405-016-4038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bijl EJ, Groeneweg JG, Wesselius DW, Stronks DL, Huygen F. Diagnosing complex regional pain syndrome using an electronic nose, a pilot study. J. Breath Res. 2019;13(3):036004. doi: 10.1088/1752-7163/aaf9c1. [DOI] [PubMed] [Google Scholar]
- 287.Pavlou AK, Magan N, Jones JM, Brown J, Klatser P, Turner AP. Detection of Mycobacterium tuberculosis (TB) in vitro and in situ using an electronic nose in combination with a neural network system. Biosens. Bioelectron. 2004;20(3):538–544. doi: 10.1016/j.bios.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 288.Amal H, Leja M, Funka K, Skapars R, Sivins A, et al. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut. 2016;65(3):400–407. doi: 10.1136/gutjnl-2014-308536. [DOI] [PubMed] [Google Scholar]
- 289.Bhattacharyya N, Seth S, Tudu B, Tamuly P, Jana A, Ghosh D, Bandyopadhyay R, Bhuyan M. Monitoring of black tea fermentation process using electronic nose. J. Food Eng. 2007;80(4):1146–1156. doi: 10.1016/j.jfoodeng.2006.09.006. [DOI] [Google Scholar]
- 290.Huang L, Zhao J, Chen Q, Zhang Y. Nondestructive measurement of total volatile basic nitrogen (TVB-N) in pork meat by integrating near infrared spectroscopy, computer vision and electronic nose techniques. Food Chem. 2014;145:228–236. doi: 10.1016/j.foodchem.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 291.Tohidi M, Ghasemi-Varnamkhasti M, Ghafarinia V, Bonyadian M, Mohtasebi SS. Development of a metal oxide semiconductor-based artificial nose as a fast, reliable and non-expensive analytical technique for aroma profiling of milk adulteration. Int. Dairy J. 2018;77:38–46. doi: 10.1016/j.idairyj.2017.09.003. [DOI] [Google Scholar]
- 292.Tian X, Wang J, Cui S. Analysis of pork adulteration in minced mutton using electronic nose of metal oxide sensors. J. Food Eng. 2013;119(4):744–749. doi: 10.1016/j.jfoodeng.2013.07.004. [DOI] [Google Scholar]
- 293.Laureati M, Buratti S, Giovanelli G, Corazzin M, Lo Fiego DP, Pagliarini E. Characterization and differentiation of Italian Parma, San Daniele and Toscano dry-cured hams: a multi-disciplinary approach. Meat Sci. 2014;96(1):288–294. doi: 10.1016/j.meatsci.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 294.Hong X, Wang J, Qiu S. Authenticating cherry tomato juices: discussion of different data standardization and fusion approaches based on electronic nose and tongue. Food Res. Int. 2014;60:173–179. doi: 10.1016/j.foodres.2013.10.039. [DOI] [Google Scholar]
- 295.Sun Y, Wang J, Cheng S. Discrimination among tea plants either with different invasive severities or different invasive times using MOS electronic nose combined with a new feature extraction method. Comput. Electron. Agric. 2017;143:293–301. doi: 10.1016/j.compag.2017.11.007. [DOI] [Google Scholar]
- 296.Gu X, Sun Y, Tu K, Pan L. Evaluation of lipid oxidation of Chinese-style sausage during processing and storage based on electronic nose. Meat Sci. 2017;133:1–9. doi: 10.1016/j.meatsci.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 297.Shao L, Wei L, Zhang X, Hui G, Zhao Z. Fabrication of electronic nose system and exploration on its applications in mango fruit (M-indica cv. Datainong) quality rapid determination. J. Food Meas. Charact. 2017;11(4):1969–1977. doi: 10.1007/s11694-017-9579-1. [DOI] [Google Scholar]
- 298.Song S, Yuan L, Zhang X, Hayat K, Chen H, Liu F, Xiao Z, Niu Y. Rapid measuring and modelling flavour quality changes of oxidised chicken fat by electronic nose profiles through the partial least squares regression analysis. Food Chem. 2013;141(4):4278–4288. doi: 10.1016/j.foodchem.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 299.Huang L, Liu H, Zhang B, Wu D. Application of electronic nose with multivariate analysis and sensor selection for botanical origin identification and quality determination of honey. Food Bioprocess Tech. 2014;8(2):359–370. doi: 10.1007/s11947-014-1407-6. [DOI] [Google Scholar]
- 300.Steine C, Beaucousin F, Siv C, Peiffer G. Potential of semiconductor sensor arrays for the origin authentication of pure Valencia orange juices. J. Agr. Food Chem. 2001;49(7):3151–3160. doi: 10.1021/jf0014664. [DOI] [PubMed] [Google Scholar]
- 301.Berna AZ, Trowell S, Clifford D, Cynkar W, Cozzolino D. Geographical origin of Sauvignon Blanc wines predicted by mass spectrometry and metal oxide based electronic nose. Anal. Chim. Acta. 2009;648(2):146–152. doi: 10.1016/j.aca.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 302.Severini C, Ricci I, Marone M, Derossi A, De Pilli T. Changes in the aromatic profile of espresso coffee as a function of the grinding grade and extraction time: a study by the electronic nose system. J. Agric. Food Chem. 2015;63(8):2321–2327. doi: 10.1021/jf505691u. [DOI] [PubMed] [Google Scholar]
- 303.Cevoli C, Cerretani L, Gori A, Caboni MF, Gallina Toschi T, Fabbri A. Classification of Pecorino cheeses using electronic nose combined with artificial neural network and comparison with GC-MS analysis of volatile compounds. Food Chem. 2011;129(3):1315–1319. doi: 10.1016/j.foodchem.2011.05.126. [DOI] [PubMed] [Google Scholar]
- 304.Kovács Z, Dalmadi I, Lukács L, Sipos L, Szántai-Kőhegyi K, Kókai Z, Fekete A. Geographical origin identification of pure Sri Lanka tea infusions with electronic nose, electronic tongue and sensory profile analysis. J. Chemomet. 2010;24(3–4):121–130. doi: 10.1002/cem.1280. [DOI] [Google Scholar]
- 305.Zakaria A, Shakaff AY, Masnan MJ, Ahmad MN, Adom AH, et al. A biomimetic sensor for the classification of honeys of different floral origin and the detection of adulteration. Sensors (Basel) 2011;11(8):7799–7822. doi: 10.3390/s110807799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.V.H. Bennetts, E. Schaffernicht, V.P. Sese, A.J. Lilienthal, M. Trincavelli, A novel approach for gas discrimination in natural environments with open sampling systems, in IEEE Sensors 2014 Proceedings (2014). 10.1109/icsens.2014.6985437 [DOI]
- 307.Wolfrum EJ, Meglen RM, Peterson D, Sluiter J. Metal oxide sensor arrays for the detection, differentiation, and quantification of volatile organic compounds at sub-parts-per-million concentration levels. Sens. Actuat. B: Chem. 2006;115(1):322–329. doi: 10.1016/j.snb.2005.09.026. [DOI] [Google Scholar]
- 308.Zhou X, Wang Y, Wang Z, Yang L, Wu X, Han N, Chen Y. Synergetic p + n field-effect transistor circuits for ppb-level xylene detection. IEEE Sens. J. 2018;18(9):3875–3882. doi: 10.1109/jsen.2018.2818710. [DOI] [Google Scholar]
- 309.Szulczyński B, Gębicki J, Namieśnik J. Monitoring and efficiency assessment of biofilter air deodorization using electronic nose prototype. Chem. Pap. 2017;72(3):527–532. doi: 10.1007/s11696-017-0310-9. [DOI] [Google Scholar]
- 310.Mumyakmaz B, Karabacak K. An E-Nose-based indoor air quality monitoring system: prediction of combustible and toxic gas concentrations. Turk. J. Electr. Eng. Comput. Sci. 2015;23:729–740. doi: 10.3906/elk-1304-210. [DOI] [Google Scholar]
- 311.S. De Vito, E. Massera, G. Di Francia, C. Ambrosino, P. Di Palma, V. Magliulo, Sensors and Microsystems, Chapter 59, 373–377 (2011). http://doi.org/10.1007/978-94-007-1324-6_59
- 312.Urasinska-Wojcik B, Vincent TA, Chowdhury MF, Gardner JW. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuat. B: Chem. 2017;239:1051–1059. doi: 10.1016/j.snb.2016.08.080. [DOI] [Google Scholar]
- 313.Hai Z, Wang J. Electronic nose and data analysis for detection of maize oil adulteration in sesame oil. Sens. Actuat. B: Chem. 2006;119(2):449–455. doi: 10.1016/j.snb.2006.01.001. [DOI] [Google Scholar]
- 314.Jolayemi OS, Tokatli F, Buratti S, Alamprese C. Discriminative capacities of infrared spectroscopy and e-nose on Turkish olive oils. Eur. Food Res. Technol. 2017;243(11):2035–2042. doi: 10.1007/s00217-017-2909-z. [DOI] [Google Scholar]
- 315.Giovanelli G, Limbo S, Buratti S. Effects of new packaging solutions on physico-chemical, nutritional and aromatic characteristics of red raspberries (Rubus idaeus L.) in postharvest storage. Postharvest Biol. Technol. 2014;98:72–81. doi: 10.1016/j.postharvbio.2014.07.002. [DOI] [Google Scholar]
- 316.Lopez R, Giraldez I, Palma A, Jesus Diaz M. Assessment of compost maturity by using an electronic nose. Waste Manag. 2016;48:174–180. doi: 10.1016/j.wasman.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 317.Hajdari A, Giorgi A, Beretta G, Gelmini F, Buratti S, et al. Phytochemical and sensorial characterization of Hyssopus officinalis subsp aristatus (godr.) Nyman (Lamiaceae) by GC-MS, HPLC-UV-DAD, spectrophotometric assays and e-nose with aid of chemometric techniques. Eur. Food Res. Technol. 2018;244(7):1313–1327. doi: 10.1007/s00217-018-3046-z. [DOI] [Google Scholar]
- 318.Xu S, Zhou Z, Lu H, Luo X, Lan Y, Zhang Y, Li Y. Estimation of the age and amount of brown rice plant hoppers based on bionic electronic nose use. Sensors. 2014;14(10):18114–81130. doi: 10.3390/s141018114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Chen Q, Song J, Bi J, Meng X, Wu X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC-MS coupled with E-nose. Food Res. Int. 2018;105:605–615. doi: 10.1016/j.foodres.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 320.Mildner-Szkudlarz S, Jeleń HH. The potential of different techniques for volatile compounds analysis coupled with PCA for the detection of the adulteration of olive oil with hazelnut oil. Food Chem. 2008;110(3):751–761. doi: 10.1016/j.foodchem.2008.02.053. [DOI] [Google Scholar]
- 321.Cui S, Wu J, Wang J, Wang X. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography-mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017;41(1):85–95. doi: 10.1016/j.jgr.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wei CQ, Liu WY, Xi WP, Cao D, Zhang HJ, et al. Comparison of volatile compounds of hot-pressed, cold-pressed and solvent-extracted flaxseed oils analyzed by SPME-GC/MS combined with electronic nose: major volatiles can be used as markers to distinguish differently processed oils. Eur. J. Lipid Sci. Technol. 2015;117(3):320–330. doi: 10.1002/ejlt.201400244. [DOI] [Google Scholar]
- 323.Xiong Y, Xiao X, Yang X, Yan D, Zhang C, et al. Quality control of Lonicera japonica stored for different months by electronic nose. J. Pharm. Biomed. Anal. 2014;91:68–72. doi: 10.1016/j.jpba.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 324.Liu T, Zhu W, Huang J, Chen H, Nie R, Li C-M. Comparison of the nutritional as well as the volatile composition of in-season and off-season Hezuo 903 tomato at red stage. Eur. Food Res. Technol. 2016;243(2):203–214. doi: 10.1007/s00217-016-2736-7. [DOI] [Google Scholar]
- 325.Liu H, Zeng FK, Wang QH, Wu HS, Tan LH. Studies on the chemical and flavor qualities of white pepper (Piper nigrum L.) derived from five new genotypes. Eur. Food Res. Technol. 2013;237(2):245–251. doi: 10.1007/s00217-013-1986-x. [DOI] [Google Scholar]
- 326.Cosio MS, Ballabio D, Benedetti S, Gigliotti C. Geographical origin and authentication of extra virgin olive oils by an electronic nose in combination with artificial neural networks. Anal. Chim. Acta. 2006;567(2):202–210. doi: 10.1016/j.aca.2006.03.035. [DOI] [Google Scholar]
- 327.Autelitano F, Giuliani F. Analytical assessment of asphalt odor patterns in hot mix asphalt production. J. Clean. Prod. 2018;172:1212–1223. doi: 10.1016/j.jclepro.2017.10.248. [DOI] [Google Scholar]
- 328.Laothawornkitkul J, Moore JP, Taylor JE, Possell M, Gibson TD, Hewitt CN, Paul ND. Discrimination of plant volatile signatures by an electronic nose: a potential technology for plant pest and disease monitoring. Environ. Sci. Technol. 2008;42(22):8433–8439. doi: 10.1021/es801738s. [DOI] [PubMed] [Google Scholar]
- 329.L. Capelli, S. Sironi, IEEE, Monitoring odour emissions from an oil & gas plant: electronic nose performance testing in the field, in 2017 ISOCS/IEEE International Symposium on Olfaction and Electronic Nose (ISOEN). 10.1109/ISOEN.2017.7968862 [DOI]