Highlights
-
•
Higher reserve scores relate to activation of the right inferior temporal gyri.
-
•
Higher reserve scores relate to activation of the left occipital fusiform gyri.
-
•
The temporal activation moderates the effect of hippocampal volume on memory.
-
•
Recruitment of the temporal lobe protects against hippocampal atrophy.
-
•
Temporal activation supports cognitive reserve to sustains memory performance.
Keywords: Cognitive reserve, Aging, Subjective cognitive decline, Mild cognitive impairment, Hippocampal atrophy, Associative memory
Abstract
Introduction
Cognitive reserve can be defined as a property of the brain that enables an individual to sustain cognitive performance in spite of age-related neural changes. This study uses brain imaging to identify which cognitive reserve mechanisms protect against the detrimental effect of hippocampal atrophy on associative memory.
Methods
The study included 108 older adults from the Quebec Consortium for the early identification of Alzheimer’s disease. They received a magnetic resonance imaging examination to measure memory-related activations and hippocampal volume. Participants also completed a reserve-proxy questionnaire, and received a comprehensive clinical assessment.
Results
Higher scores on the reserve questionnaire were associated with more activation in the right inferior temporal and left occipital fusiform gyri. The activation of the right temporal gyrus moderated the relationship between the volume of the hippocampus and face-name memory. A smaller volume was associated with weaker memory in participants with lower activation, but not in those with greater activation.
Discussion
Recruitment of the temporal lobe protects against the detrimental effect of hippocampal atrophy on associative memory and contributes to cognitive reserve.
1. Background
Cognitive aging is not uniform from one individual to another. Some people appear to be more resilient than others against the effects of age-related brain changes or neurodegenerative diseases on cognition (Katzman, 1993, Stern, 2009, Stern et al., 2018a). This suggests the existence of brain mechanisms that intervene to maintain cognitive abilities despite the presence of brain damage. Cognitive reserve has been proposed as a model to capture this phenomenon (Stern et al., 2018a). It is defined as a property of the brain that enables an individual to sustain cognitive performance in spite of age-related neural changes or diseases. A number of favorable genetic, environmental or lifestyle factors may influence these protective brain properties and affect inter-individual differences in cognitive reserve (Cabeza et al., 2018, Stern et al., 2018a). Conceptually, cognitive reserve differs from brain reserve, which refers to the status of the brain at any point in time, and from brain maintenance, which refers to the relative preservation of neural resouces over time (Stern et al., 2018a). The concepts of cognitive reserve, brain reserve and brain maintenance have been extremely fruitful as frameworks to guide research on age-related inter-individual differences and explain the impact of lifestyle factors on cognitive aging. Even though cognitive reserve is thought to rely on a set of brain properties and hence, conceptualized as a neubiological construct, its neural mechanisms remain largely unknown. The goal of this paper is to identify some of the functional brain properties that underlie cognitive reserve.
Over the past decade, functional magnetic resonance imaging (fMRI) has been used to investigate the expression of cognitive reserve in terms of functional brain responses. These studies have generally assessed whether socio-behavioral proxies, such as intellectual quotient (IQ), education or profession, are associated with a particular pattern of brain response to cognitive tasks. The findings were interpreted under three broad concepts (Steffener et al., 2011, Stern et al., 2018a): First, it was found that higher scores on cognitive reserve proxies were associated with a lower brain response in regions involved in the task. For instance, studies have reported a negative correlation between reserve proxies and brain response in the inferior frontal cortex during a working memory task (Bartrés-Faz et al., 2009). This pattern could reflect higher neural efficiency as a mechanism, meaning a more efficient use of neural resources (Cabeza et al., 2018, Stern, 2009, Tucker and Stern, 2011). Neural efficiency is an influential model in the field of inter-individual differences in intelligence, and was proposed to explain the negative relationship between intelligence quotient (IQ) and brain activation, which was measured with a range of neuroimaging methods (Haier et al., 1988; see Neubauer and Fink, 2009 for a review). Little is known about the aspect of the brain that supports neural efficiency and whether it is a reflection of the neurons themselves or the supporting structure (vasculature, myelin, glia). However, it is hypothesized that neural efficiency is indicative of differentiated brain representations (Cabeza et al., 2018). Other studies have observed that reserve proxies are associated with higher activation for more difficult tasks, whether it be due to age or task-demand, and proposed that the mechanism behind this pattern may reflect a higher neural capacity (Steffener and Stern, 2012, Stern et al., 2018a). Finally, it was suggested that compensation may occur when regions usually not recruited by the task are activated to compensate for deficits in recruiting habitual networks (Cabeza et al., 2018, Colangeli et al., 2016, Steffener and Stern, 2012, Stern, 2009, Tucker and Stern, 2011). It has been proposed that these three mechanisms are not mutually exclusive and intervene at different levels of task difficulty (Boller et al., 2017, Bosch et al., 2010, Colangeli et al., 2016, Solé-Padullés et al., 2009). The three mechanisms may reflect inter-individual differences in adaptability, defined as the ability to adjust cerebral processes to brain aging or brain injury (Steffener and Stern, 2012, Stern et al., 2018b).
As presented above, fMRI studies investigating the neural implementation of cognitive reserve typically examined the link between brain activation and reserve proxies. Although these studies provide valuable information regarding the associations between reserve proxies and brain activation, they do not directly assess whether these activations are protective. To conceptually explain cognitive reserve, proxy-related activations should not only relate to typical socio-behavioral reserve proxies, but also moderate the effect of pathological brain markers on cognition (Steffener et al., 2011). In other words, it is necessary to demonstrate that having these neurological attributes make individuals more resilient to age-related changes in the brain, compared to individuals who don’t. The very few studies that have relied on moderation analyses to examine the protective effect of proxy-related activation differences show promising effects. Benson et al. (2018) found that connectivity within the fronto-parietal network moderates the detrimental effect of white matter lesions on executive functions in healthy middle-aged and older adults. Stern et al. (2018b) reported that a task-invariant network moderated the effect of cortical thickness on fluid reasoning.
The goal of this study is to identify the type and regions of functional activation associated with reserve proxies and evaluate how these activations moderate the detrimental effect of hippocampal atrophy on associative memory. There are many reasons to study resilience against the detrimental impact of hippocampal atrophy on associative memory. Hippocampal atrophy is among the earliest brain markers of Alzheimer’s disease (AD). Associative memory depends on hippocampal integrity, which is impaired with AD and typical aging. Furthermore, cognitive reserve may be a potentially relevant concept to explain inter-individual age-related differences for both hippocampal volume and memory performance (Nyberg et al., 2012). Surprisingly, very few studies have assessed cognitive reserve in the context of associative memory in the aging spectrum, and none have investigated its moderating effect. Showing that individuals expressing a particular functional pattern do not display the typically detrimental effect of hippocampal atrophy on associative memory could identify crucial neural mechanisms underlying cognitive reserve in aging.
The approach used in this study was to first identify whether differences on a reserve proxy questionnaire were related to differences in brain responses to associative memory. Then, proxy-related activations were assessed to determine if they moderated the detrimental effect of hippocampal atrophy on an independent associative memory task administered outside the scanner in a different session. An independent measure of associative memory was used to avoid circularity and ensure that moderation was not contaminated by the effect of performance on activation. Finally, the relationship between hippocampal volume and associative memory was examined in individuals with high versus low proxy-related activation to interpret the moderation effect. The study included participants ranging from cognitively intact older adults, to older adults with subjective cognitive decline (SCD) and mild cognitive impairment (MCI). Using these three populations increased the range of hippocampal atrophy and performance values and therefore, the likelihood of finding a protective effect.
2. Methods
2.1. Participants
Participants included 108 older adults from the Quebec Consortium for the early identification of Alzheimer’s disease (CIMA-Q). CIMA-Q is a multi-center collaborative research infrastructure that tracks AD progression in older adults at risk for the disease (Belleville et al., 2019). The consortium has created a longitudinal cohort of older adults meeting criteria for SCD, MCI, dementia of the Alzheimer’s type (AD), and healthy controls (HC).
CIMA-Q participants were community-dwelling older adults, who were age 65 or over and either native French or English speakers. The initial cohort (i.e., CIMAQ-1) comprises 290 participants (Gaudreau et al., 2007). At baseline (2015–2017), 259 participants without dementia were included; 43% had received an optional fMRI examination. The study included 25 older adults with MCI, 58 older adults with SCD and 25 HC, all of whom completed the clinical, neuropsychological, and fMRI examination (Table 1). All procedures of recruitment, clinical, cognitive, neuropsychiatric measures are described in Belleville et al., 2014, Belleville et al., 2019 and can be found in Supplementary Material (S1 text). The CIMA-Q study and this present study were approved by the Research Ethics Board of the Institut universitaire de gériatrie de Montréal. All human subjects provided informed consent.
Table 1.
Characteristics of participants.
|
P values |
||||||
|---|---|---|---|---|---|---|
| HC (N = 25) | SCD (N = 58) | MCI (N = 25) | HC vs. SCD | HC vs. MCI | SCD vs. MCI | |
| Age (years)* | 71.1 (4.6) | 72.4 (5.0) | 75.6 (5.0) | n.s. | 0.005† | 0.022† |
| Sex (M/F) | 7/18 | 24/34 | 11/14 | n.s. | n.s. | n.s. |
| Education (years) | 15.6 (3.5) | 15.2 (3.3) | 15.2 (3.3)§ | n.a. | n.a. | n.a. |
| Cognitive reserve questionnaire (/30) | 17.5 (4.1) | 16.6 (4.3) | 17.2 (3.7) | n.a. | n.a. | n.a. |
| GDS (/30)* | 2.4 (3.1) | 5.7 (4.6) | 6.4 (5.3) | 0.010† | 0.008† | n.s. |
| MoCA (/30)* | 28.4 (1.4) | 27.9 (1.3) | 24.9 (2.3) | n.s. | < 0.001‡ | < 0.001‡ |
| Logical Memory | ||||||
| Immediate recall (/25)* | 14.9 (4.2) | 14.9 (4.2) | 11.7 (3.7) | n.s. | 0.013† | 0.017† |
| Delayed recall (/25)* | 15.1 (3.9) | 13.1 (4.5) | 10.9 (4.6) | n.s. | 0.003† | n.s. |
| Face-Name associative recognition (/9)* | 7.6 (0.9) | 6.8 (1.8)¶ | 6.0 (2.1) | n.s. | 0.004† | n.s. |
| fMRI task | ||||||
| Recognition (corrected old hit rate) | 0.80 (0.11) | 0.81 (0.14) | 0.74 (0.17) | n.a. | n.a. | n.a. |
| Source (corrected source hit rate) | 0.53 (0.17) | 0.57 (0.20) | 0.45 (0.21) | n.a. | n.a. | n.a. |
| Recognition discrimination index (d')* | 2.3 (0.52) | 2.28 (0.78) | 1.75 (0.84) | n.s. | 0.032† | 0.012† |
| Associative memory accuracy* | 0.60 (0.18) | 0.60 (0.19) | 0.48 (0.20) | n.s. | n.s. | 0.032† |
| Hippocampal volume* (average left and right volume) Proportional value (% ICV) |
0.29 (0.05)§ | 0.28 (0.06)# | 0.24 (0.05)§ | n.s. | 0.001† | 0.006† |
Note: Values are means (SD). Analysis of variance was performed, followed by post hoc pairwise comparisons with Bonferroni correction. A Chi-Square test of independence was performed for sex.
Abbreviations: HC = healthy controls, SCD = subjective cognitive decline, MCI = mild cognitive impairment, GDS = Geriatric Depression Scale, MoCA = Montreal Cognitive Assessment, fMRI = functional magnetic resonance imaging, ICV = intracranial volume, n.a. = No post hoc performed when the main group effect was non significant. n.s. = Non significant side by side comparison.
*Significant main group effect.
†post hoc comparison p < 0.05.
‡post hoc comparison p < 0.001.
§Data missing for one participant.
¶Data missing for three participants.
#Data missing for five participants.
Associative memory score = Corrected source hit rate / (corrected old hit rate + corrected false alarme rate).
Identification of MCI was based on NIA/AA criteria (Albert et al., 2011, McKhann et al., 2011). Participants with SCD met the criteria of the Subjective Cognitive Decline Initiative (Jessen et al., 2014, Molinuevo et al., 2017). HC were cognitively unimpaired and reported that their memory was not as good as it used to be but they were not worried about it.
2.2. Experimental and neuroimaging measures
2.2.1. Reserve proxy questionnaire
Reserve proxy was measured with the Cognitive Reserve Index questionnaire (CRIq; adapted in French by Eduardo Cisneros) (Rami et al., 2011). The questionnaire includes 15 questions covering different aspects that have been proposed as reserve proxies, such as education, profession, stimulating hobbies and physical activities. Larger scores indicate larger reserve proxies, with a maximum score of 30.
2.2.2. Associative face-name recognition
An associative face-name recognition test was used as an independent measure of associative recognition, as it has been reported to be highly sensitive to the early symptoms of AD (Belleville et al., 2017, Irish et al., 2011). Our goal was to use a memory task that measured the same associative memory process as the tasks used in the scanner, but that was sufficiently different to reduce circularity. The face-name association task was the best choice among the tasks included in the CIMA-Q protocol, because it measures associative memory and is presented visually, similarly to the fMRI object-location task. The choice was also a pragmatic one as we used CIMA-Q data. There was a significant and moderate size correlation between performance on the two tasks (r = 0.249, p < 0.05 when using accuracy on both tasks; r = 0.326, p < 0.01 when using the discriminability index for the object-location task).
In the encoding phase, participants were shown a series of nine faces of men and women, each associated with a different first name, and were instructed to remember the name that was paired with the face. Faces included younger, middle-age and older adults. Each pair was displayed one at a time on the screen for eight seconds. There was an immediate recall and a 20-minute delayed recall, where participants were asked to report the name associated with the face. This was followed by a delayed associative recognition phase, where the same nine faces were presented on the screen and paired with a correct or incorrect first name. Participants were asked to determine whether the face was paired with the same name as in the learning phase (intact), with a name that had not been presented in the learning phase (new), or with a name that was presented in the learning phase but paired with a different face (recombined) (Caillaud et al., 2019). Of the nine face-name pairs, three were new intact, three were new, and three were recombined (Maximal score = 9). Performance on the associative recognition portion was used as the dependent variable based on score distribution.
2.2.3. Brain imaging
CIMA-Q uses a comprehensive imaging protocol harmonized for manufacturers/software configurations to optimize commonality (Belleville et al., 2019, Duchesne et al., 2019). This present study uses anatomical 3D-T1-w and task-related fMRI sequences from this protocol (cf. Table S1).
Segmentation of the hippocampus and volumetric analyses were done with FreeSurfer 5.3 (Dale et al., 1999, Fischl et al., 1999). Normative hippocampal volumes were obtained by converting values into Z scores, correcting for age and sex (Potvin et al., 2016). Proportional volumes were then computed by dividing hippocampal volume by estimated intracranial volume. Since the analyses for the right and left hippocampal volumes yielded similar effects, proportional values were combined. Therefore, analyses presented in this study represent average hippocampal volumes.
The associative memory task, which was completed in the scanner in one session, consisted in encoding 78 colored pictures of common objects and their location in a four-quadrant grid. Thirty-nine gray squares were used as control items. Each stimulus was presented for three seconds with a 500 to 18500 ms inter-stimulus interval (ISI), during which a fixation target was shown in the center of the screen. The order in which the stimuli were presentated and the duration of ISI were optimized for an efficient rapid event-related design with the Optseq2 tool (http://surfer.nmr.mgh.harvard.edu/optseq/).
Participants were instructed to remember the object and its location. Participants completed a retrieval phase outside the scanner, during which 117 pictures (78 old, 39 new) were randomly presented onscreen. Participants were asked to determine whether the item was presented during the encoding phase by pressing a yes/no response button, and indicate its location on the grid with a keypad (See Fig. S1 and S2 Text for details).
2.2.4. Functional data processing
Functional data processing was done with the Statistical Parametric Mapping (SPM12) software (http://www.fil.ion.ucl.ac.uk/spm) in Matlab v9.4.0. The functional images were realigned and corrected for slice timing. Participants’ anatomical image was segmented and co-registered with the fMRI images. Images were then normalized to the MNI stereotaxic space and smoothed with an 8 mm FWHM gaussian filter kernel. A fixed-effects general linear model at the single-subject level was conducted to obtain the task activation in an event-related design approach. The events were modeled with constant epoch (3 sec.; image presentation) and convolved with the full basis set including the canonical hemodynamic response function (HRF) and its time and dispersion derivatives. They were labelled according to response in the retrieval phase (i.e., correct recognition/correct source; correct recognition/wrong source; incorrect rejection, see S2 Text). The gray squares were labelled as the control condition. Six realignment parameters were included as covariates of no interest.
2.2.5. Activations related to the task
Activations associated with the task were analyzed in the HC group to assess whether reserve-related activations were found in regions that were activated by the task or in new regions. We used the following to determine associative memory contrast: activations associated with the control condition (gray squares) were subtracted from activations associated with encoding items, which were both correctly recognized and positioned during retrieval. This is a variant of the subsequent memory effect as it identifies activation for trials in which later retrieval was successful (Maillet and Rajah, 2014, Rypma and D'Esposito, 2003). Group level analysis was considered to give a significant result if any cluster had a FWE-corrected p-value < 0.05.
2.2.6. Activation related to reserve proxy questionnaire and reproducibility analysis
Regions associated with the reserve proxy questionnaire were first identified in the HC group to avoid the confounding effect of disease on the relationship between reserve and activation. We used the associative memory contrast described above with a whole brain multiple regression in SPM12 (clusterwise FWE correction, p < 0.05).
Since this is a critical measure, the reserve-related activations identified with the original CIMA-Q sample were validated in a separate independent sample of 17 older adults tested in the same conditions to assess reproducibility. This was done as a validation process to verify that the reserve-related activation was not a characteristic of a particular sample. Participants in the reproducibility study were recruited in a later phase from the same community and in the same manner as the CIMA-Q cohort. Regions of interest (ROIs) were created based on the reserve-related activations identified with the CIMA-Q sample. These ROIs were the used in the independent sample; extracted activation values (betas) were assessed to determine if they correlated with the reserve proxy questionnaire (See S3 Text for details of procedure and Table S2 for characteristics of participants).
2.2.7. Moderation analyses
This was followed by moderation analyses measuring whether activation amplitude in the proxy-related regions moderates the detrimental effect of hippocampal atrophy on face-name associative memory. The SPSS linear regression tool was used for the moderation analysis with face-name associative recognition as a predicted score, hippocampal volume as a predictor and activation amplitude (same contrast as in Section 2.2.5) extracted with Marsbar (Brett et al., 2002) as a moderator. To obtain the moderator, the variables (hippocampal volume and temporal activation) were first mean-centered and then multiplied together. In the case of a significant moderation effect, the effect of the predictor was assessed using three moderator values: the mean, the value one standard deviation above the mean, and the value one standard deviation below the mean. These values are based on Cohen‘s Convention (Cohen and Cohen, 1983) see also (Aiken et al., 1991), and were used to measure conditional effects of hippocampal volume on face-name associative recognition. The moderation was also probed using the Jonhson-Neyman technique (Hayes, 2018, Hayes and Matthes, 2009, Process Macro (http://processmacro.org/index.html)) to determine at which specific level of activation (not centered) the effect of hippocampal volume on memory performance was significant.
3. Results
3.1. Brain activation and deactivation related to the associative memory task
Table 2 illustrates the regions showing the brain’s blood-oxygen-dependent (BOLD) signal change during the fMRI association task in HC. The task activates the bilateral occipital lobe (left and right fusiform gyrus and left inferior occipital gyrus), the right inferior temporal gyrus and left medial and inferior frontal gyrus. Two regions of deactivation were found in the right superior and middle temporal gyrus (See Fig. S2 and Table 2).
Table 2.
Memory-related activations.
| MNI Coordinates |
|||||
|---|---|---|---|---|---|
| Region Label | Extent | t-value | x | y | z |
| Correct associative recognition > Grey square control | |||||
| L Fusiform Gyrus (BA 37) | 1318 | 10.914 | −36 | −67 | −13 |
| L Inferior Occipital Gyrus | 1318 | 7.078 | −24 | −94 | −1 |
| R Fusiform Gyrus | 1405 | 10.761 | 36 | −55 | −16 |
| R Inferior Temporal Gyrus | 1405 | 9.062 | 48 | −70 | −4 |
| L Superior Medial Frontal Gyrus | 341 | 8.236 | −6 | 20 | 44 |
| L IFG (p. Orbitalis) | 787 | 7.256 | −30 | 32 | −13 |
| L IFG (p. Triangularis) | 787 | 6.510 | −48 | 29 | 23 |
| Grey square control > Correct associative recognition | |||||
| R Superior Temporal Gyrus | 670 | −7.802 | 51 | −49 | 23 |
| R Middle Temporal Gyrus | 670 | −6.189 | 63 | −43 | 8 |
Abbreviations: BA: Brondman area; MNI: Montreal Neurological Institute; Clusterwise FWE correction, p < 0.05.
3.2. Activation related to reserve proxy questionnaire and reproducibility analysis
Two regions were found to be positively correlated with the CRQ reserve proxy questionnaire in HC. The first region was in the right inferior temporal gyrus and the second in the left occipital fusiform gyrus (See Fig. S3 and Table 3).
Table 3.
Activation regions associated with the reserve-proxy questionnaire in the healthy control group.
| MNI Coordinates |
|||||
|---|---|---|---|---|---|
| Region Label | Extent | t-value | x | y | z |
| Left Fusiform and Left Cerebellum | 101 | 5.919 | −42 | −70 | −22 |
| Right Inferior Temporal (BA37) | 76 | 5.345 | 51 | −61 | −1 |
Abbreviations: BA: Brondman area; MNI: Montreal Neurological Institute (Clusterwise FWE correction, p < 0.05).
The reproducibility analysis supported the significant positive correlation between the reserve proxy questionnaire and estimate parameters in the right temporal ROI (r = 0.53; p < 0.05 see Table S3).
3.3. Moderation analyses
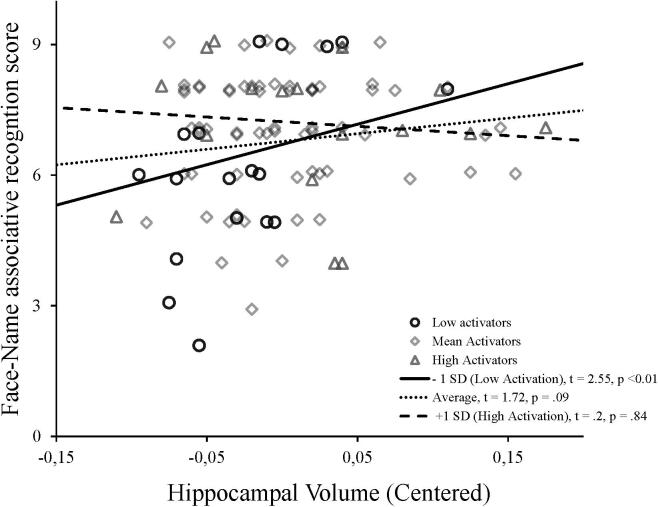
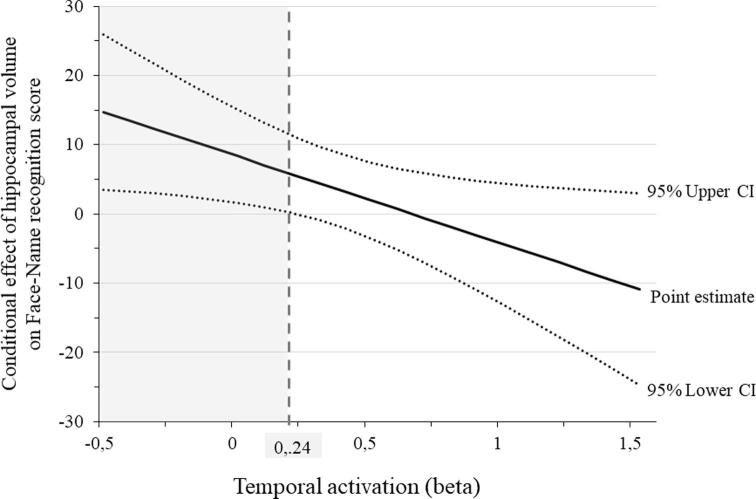
As shown in Table 4a, right temporal activation significantly moderates the effect of hippocampal volume on the face-name associative recognition score. This interaction is illustrated in Fig. 1, and was probed by testing the conditional effects of the hippocampal volume at three levels of temporal activation: one standard deviation below the mean, at the mean, and one standard deviation above the mean. As shown in Table 4b, hippocampal volume was positively and significantly related to face-name associative recognition when temporal activation was one standard deviation below the mean (β = 9.94; p < 0.01), but not when it was at the mean (β = 4.645; p = 0.09) or one standard deviation above the mean (β = −0.65; p = 0.84). The Johnson-Neyman plot showed that the positive relationship between the hippocampal volume and memory performance was significant when temporal activation was lower than 0.2415 but non-significant with higher values of activation (see Fig. 2). Thus, higher activation within the right temporal gyrus moderates the detrimental effet of smaller hippocampal volume on memory performance. There was no moderation effect for the fusiform gyrus (Table 5).
Table 4.
Moderation analysis of activation amplitude in the temporal lobe ROI and hippocampal volume on face-name associative recognition.
| a) Moderation analysis | |||
|---|---|---|---|
| Predictor | β | P value | 95% CI |
| Hippocampal volume | 4.645 | 0.087 | [−0.692; 9.982] |
| Right temporal activation | 0.603 | 0.106 | [−0.13; 1.335] |
| Hippocampal volume × right temporal activation interaction | −12.59* | 0.028 | [−23.786; −1.402] |
| b) Conditional effects of hippocampal volume on face-name performance | |||
| Temporal activation | β | P value | 95% CI |
| One SD below mean | 9.94* | 0.01 | [2.204; 17.676] |
| At the mean | 4.645 | 0.087 | [−0.692; 9.982] |
| One SD above mean | -0.65 | 0.841 | [−7.084; 5.784] |
*p < 0.05.
Fig. 1.
Illustration of the moderation effect of right temporal activation on the relationship between the hippocampal volume and face-name recognition performance. The effect of hippocampal volume on the face-name score was significant only in the low activators (1 SD below the mean).
Fig. 2.
Johnson- Neyman plot illustrating the conditional effect of hippocampal volume on face-name recognition score at each moderator level (mean activation in the right inferior temporal ROI). The vertical dotted lines represent the value of activation at which the lower and upper limits of the confidence interval crosses the zero point.
Lower activation below (≤ 0.2415; gray area) represents the significant effect (p ≤ 0.05).
Table 5.
Moderation analysis of activation amplitude in the fusiform gyrus ROI and hippocampal volume on face-name associative recognition.
| Predictor | β | P value | 95% CI |
|---|---|---|---|
| Hippocampal volume | 4.396 | 0.104 | [−0.917; 9.71] |
| Left fusiform activation | −0.146 | 0.654 | [−0.792; 0.5] |
| Hippocampal volume × left fusiform activation interaction | −2 | 0.674 | [−11.44; 7.43] |
4. Discussion
The goal of this study was to identify functional brain mechanisms supporting the hypothesis of cognitive reserve in older adults without dementia. The study focused on processes that protect against the detrimental effect of hippocampal atrophy because the hippocampus is critical for associative memory, and altered by aging and AD (Atienza et al., 2011, Chua et al., 2007, Sperling et al., 2003, Thielen et al., 2018). Our study indicates that there is a moderation effect, where a smaller hippocampal volume is associated with weaker associative memory in participants with lower activation, but not in those with greater activation. Our results indicate a positive association between reserve proxies and activation of two regions: the inferior temporal gyrus in the right hemisphere and the fusiform gyrus in the left hemisphere. A larger score on the reserve proxy questionnaire is associated with greater activation in both regions. The relationship between the reserve proxy questionnaire and the right temporal lobe region was confirmed and replicated in the reproducibility study.
When examining whether these activations moderate the relationship between hippocampal volume and face-name memory, it was found that activation of the right inferior temporal gyrus moderates the relationship between the hippocampal volume and performance on face-name memory. Smaller hippocampal volume was associated with lower face-name memory in participants with lower activation, but this association was absent in participants with greater activation. Fig. 1 indicates that older adults with small hippocampal volumes and higher activation demonstrated better face-name memory than those with lower activation as compared with those with smaller volumes and lower amplitude activation. The right inferior temporal gyrus is involved in high-level visual processes and memory, particularly for visual scenes and objects, (Kim, 2011, Riches et al., 1991) which is consistent with the cognitive processes involved in the associative tasks used. Overall, the pattern of results suggests that activation of the right inferior temporal gyrus has a protective effect and that older adults with a reduced hippocampal volume show preserved memory if they have larger activation in that region.
As mentioned above, our study was interested in associative memory because it is a process which depends on the hippocampus. Interestingly, our moderation findings show that a smaller volume is associated with weaker associative memory only in participants with lower activation, and not in those with greater activation. This suggests that while hippocampal volume may be involved in associative memory, the presence of right temporal activation can be used to support that role in people with a larger reserve. As a result, the relationship between hippocampal volume and associative memory may no longer be observed in this particular population. This stresses the importance of taking into account the effect of inter-individual differences on reserve proxies when examining brain-behavior relationships.
The left fusiform gyrus was activated by the task and has been frequently linked to both verbal and associative memory (Kim, 2011, Maillet and Rajah, 2016). Although it was found to correlate with the proxies, it was not found to be protective, meaning activation in this region did not moderate the relationship between the hippocampus and face-name association. This finding indicates that larger activation of this region is not sufficient to protect against the detrimental effects of hippocampal atrophy. Furthermore, the reproducibility study failed to observe a similar relationship between the putative reserve proxies and activation in the fusiform gyrus in a smaller independent sample. Activations associated with socio-behavioral measures are not always protective and are not direct measures of brain mechanisms that would form a cognitive reserve. They reflect a variety of dimensions, which may not all provide resilience against brain changes (Stern et al., 2018b). Socio-behavioral proxies are convenient in the absence of a comprehensive neurobiological model of reserve. However, as more evidence clarifies the brain mechanisms that support these models, research is likely to increasingly rely on more direct biological measures to determine inter-individual resilience differences.
Our results have implications for neurobiological models of reserve. The pattern observed reflects greater flexibility to engage the right inferior temporal gyrus, which is recruited by the task and typically involved with visual memory (Kim, 2011, Riches et al., 1991). Recruiting the right temporal lobe minimizes the negative effect of smaller hippocampal volume on associative memory. The right inferior temporal gyrus is part of the memory network and its connection with the hippocampus was found to predict associative memory performance (Adnan et al., 2016). Thus, older adults with higher scores on reserve proxies are better able to engage a region, which is potentially involved in the task and interconnected with the impaired hippocampus. This in turn, contributes to maintaining associative memory performance.
Observing that reserve relies on activation within the memory network is probably due to this study’s use of a memory task to elicit activation. For instance, other studies relying on executive tasks have found improved efficiency or capacity within the frontal regions (Boller et al., 2017). This may suggest that cognitive reserve involves cross-cutting adaptability processes, which take place in different networks engaged by the task. Adaptability is defined as the ability to adjust cerebral processes to brain aging or injury (Steffener and Stern, 2012, Stern et al., 2018a). One interesting question for future research is whether individuals with high reserve develop higher adaptability across all these networks or if different genetic, environmental or lifestyle factors influence different networks.
It should be noted that the effects of cognitive reserve were tested with a location-picture association, whereas moderation was assessed with a face-name association. This methodology was used to ensure relative independence between the task eliciting activation and the moderation test, and for practical reaons given that face-name association was included in the CIMAQ protocol. Given that the two tasks differ in some ways and are not perfectly correlated, it is possible that slightly different results may have been found using a more similar paradigm to test moderation effects. For instance, the lack of a moderation effect for the left fusiform gyrus may be due to the fact that face perception depends more on the right than left fusiform gyrus (Rossion et al., 2003, Rossion et al., 2000, Schiltz et al., 2010).
This study is innovative in several respects: First, very few studies with a large sample size have assessed cognitive reserve in the context of associative memory in the aging spectrum, and none have investigated moderation models in relation to the impact of hippocampal volume on associative memory. For this reason, no other study has provided supporting evidence for an activation pattern that protects against hippocampal volume loss. Relying on task-related activation is another strength of this study: the concept of cognitive reserve is used to explain the disconnection between the state of the brain and cognitive performance, which should be optimally addressed with activation related to a task. Furthermore, the underlying mechanisms are likely to depend on regions and functions involved in vulnerable cognitive processes. We used an event-related fMRI paradigm and measured a subsequent memory effect, which estimate the neural activity that supports successful associative memory encoding. In addition, using an item-related design showed that differences in activation patterns cannot be merely explained by differences in behavior.
The study also has some limitations: First, it should be acknowledged that fMRI is not a direct measure of neural activity, as it relies on BOLD contrasts, and its signal depends on the task and design (Buxton et al., 2004). It is better understood as a measure of neuronal unit function, primarily reflecting the neuronal substrate and its vascularization capacity. Younger adults were not included, which did not provide a direct measure of whether the activation pattern found in high-activation individuals is similar to that of younger adults. However, it is interesting to note that older adults typically under-recruit posterior regions in favor of anterior ones compared to younger adults (Maillet and Rajah, 2014). Furthermore, this study was cross-sectional. Longitudinal studies will be required to assess whether similar mechanisms are found when examining inter-individual differences on associative memory changes. We did not conduct beta-amyloid measurements in the group of participants with MCI and SCD and therefore, they are likely to represent a heterogenous sample with a proportion not progressing to dementia. Finally, data was combined from different groups of older adults without dementia. This was done to optimize statistical power as moderation is known to require high power (McClelland and Judd, 1993, Whisman and McClelland, 2005). Therefore this study cannot speak to potential group differences along this spectrum. This is an interesting question for future adequately powered studies because cognitive reserve effects may be more potent in a more impaired population.
In conclusion, it was found that the right inferior temporal gyrus, a region within the memory network that is highly connected with the hippocampus, supports resilience against hippocampal atrophy in older adults. This supports the notion that cognitive reserve may rely on the adaptability of regions or networks that are involved in or interconnected to the cognitive process upon which it plays its protective role.
CRediT authorship contribution statement
Sylvie Belleville: Conceptualization, Methodology, Resources, Supervision, Validation, Funding acquisition, Writing - original draft. Samira Mellah: Formal analysis, Visualization, Project administration, Data curation, Writing - original draft. Simon Cloutier: Formal analysis, Visualization, Writing - original draft. Thien Thanh Dang-Vu: Writing - original draft. Simon Duchesne: Funding acquisition, Methodology, Resources, Writing - review & editing. Samantha Maltezos: Formal analysis, Visualization, Writing - review & editing. Natalie Phillips: Writing - review & editing. Carol Hudon: Funding acquisition, Methodology, Writing - review & editing. : .
Declaration of Competing Interest
S.B: consultant for Sojecci; S.M. : none; S.C.: none; T.T.D-V: consultant for Eisai; S.D: co-founder and officer of True Positive Medical Devices Inc; S.Ma: none; N.P: none; C.H: none.
Acknowledgments
Acknowledgements
The data used for this article was obtained from the Quebec Consortium for the Early Identification of Alzheimer’s Disease (CIMA-Q; http://www.cima-q.ca/en/home/). CIMA-Q investigators contributed to the development of the project design, implementation, and acquisition of clinical, cognitive, neuroimaging and biological data. A complete list of CIMA-Q investigators is available on www.cima-q.ca. The authors would like to thank physicians, nurses, psychometricians, MRI technologists and other staff members involved in assessing participants, collecting biomaterials, data entry and quality control and the implementation of CIMA-Q. Thanks to Annie Webb for the English revision, Renée-Pier Filiou for support in the submission process. We are thankful to Dr Jason Steffener and anonymous reviewers for the thoughtful comments on an earlier version of the paper.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR) to SB [grant number154265]; the Fonds de recherche du Québec – Santé Pfizer Canada Innovation Fund to the Quebec Consortium for the Early Identification of Alzheimer’s Disease (CIMA-Q) [grant number 27239]; Fonds de recherche du Québec Cohort grant [grant number 137794]; Quebec Network for Research on Aging, a network supported by the FRQS; Fondation Courtois (NeuroMod project; grant to SB) and Fondation Institut de gériatrie de Montréal to S.B.. S.B. holds a Canada Research Chair in cognitive neurosciences of aging and cerebral plasticity (#950-232074) S.D. is a Research Scholar from the Fonds de recherche du Québec–Santé (#30801). T.T.D-V. is a Research Scholar from the Fonds de recherche du Québec–Santé (#251602), and is supported by CIHR (MOP 142191, PJT 153115, PJT 156125 and PJT 166167) and the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-06990). The Fonds de recherche du Québec – Santé is represented by one member on the CIMA-Q advisory boards but his/her role is that of an observer and hence they have no control on decisions, content or management of CIMA-Q. The funding agencies have no involvement in the design, analysis and report of the study reported in the present paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102526.
Contributor Information
Sylvie Belleville, Email: sylvie.bellevillle@umontreal.ca.
Samira Mellah, Email: samira.mellah@criugm.qc.ca.
Thien Thanh Dang-Vu, Email: TT.DangVu@concordia.ca.
Simon Duchesne, Email: simon.duchesne@fmed.ulaval.ca.
Samantha Maltezos, Email: sarantia.samantha.maltezos@mail.mcgill.ca.
Natalie Phillips, Email: natalie.phillips@concordia.ca.
Carol Hudon, Email: carol.hudon@psy.ulaval.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adnan A., Barnett A., Moayedi M., McCormick C., Cohn M., McAndrews M.P. Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Struct. Funct. 2016;221(6):2999–3012. doi: 10.1007/s00429-015-1084-x. [DOI] [PubMed] [Google Scholar]
- Aiken L.S., West S.G., Reno R.R. Sage Publications; 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienza M., Atalaia-Silva K.C., Gonzalez-Escamilla G., Gil-Neciga E., Suarez-Gonzalez A., Cantero J.L. Associative memory deficits in mild cognitive impairment: the role of hippocampal formation. Neuroimage. 2011;57(4):1331–1342. doi: 10.1016/j.neuroimage.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Bartrés-Faz D., Solé-Padullés C., Junqué C., Rami L., Bosch B., Bargalló N., Falcón C., Sanchez-Valle R., Molinuevo J.L. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol. Psychol. 2009;80(2):256–259. doi: 10.1016/j.biopsycho.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Belleville S., Fouquet C., Duchesne S., Collins D.L., Hudon C., and the CIMA-Q group Detecting early preclinical Alzheimer’s disease via cognition, neuropsychiatry, and neuroimaging: qualitative review and recommendations for testing. J. Alzheimers Dis. 2014;42(Suppl 4):S375–S382. doi: 10.3233/JAD-141470. [DOI] [PubMed] [Google Scholar]
- Belleville S., Fouquet C., Hudon C., Zomahoun H.T.V., Croteau J., Consortium for the Early Identification of Alzheimer’s disease-Quebec Neuropsychological Measures that Predict Progression from Mild Cognitive Impairment to Alzheimer’s type dementia in Older Adults: a Systematic Review and Meta-Analysis. Neuropsychol Rev. 2017;27(4):328–353. doi: 10.1007/s11065-017-9361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S., LeBlanc A.C., Kergoat M.J., Calon F., Gaudreau P., Hebert S.S., Hudon C., Leclerc N., Mechawar N., Duchesne S., Gauthier S., the Consortium for the Early Identification of Alzheimer’s disease-Quebec (CIMA-Q) The Consortium for the early identification of Alzheimer’s disease-Quebec (CIMA-Q) Alzheimers Dement (Amst) 2019;11:787–796. doi: 10.1016/j.dadm.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson G., Hildebrandt A., Lange C., Schwarz C., Kobe T., Sommer W., Flöel A., Wirth M. Functional connectivity in cognitive control networks mitigates the impact of white matter lesions in the elderly. Alzheimers Res. Ther. 2018;10(1):109. doi: 10.1186/s13195-018-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller B., Mellah S., Ducharme-Laliberté G., Belleville S. Relationships between years of education, regional grey matter volumes, and working memory-related brain activity in healthy older adults. Brain Imaging Behav. 2017;11(2):304–317. doi: 10.1007/s11682-016-9621-7. [DOI] [PubMed] [Google Scholar]
- Bosch B., Bartrés-Faz D., Rami L., Arenaza-Urquijo E.M., Fernández-Espejo D., Junqué C., Solé-Padullés C., Sanchez-Valle R., argallo N., Falcón C., Molinuevo J.L. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2010;46(4):451–461. doi: 10.1016/j.cortex.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Brett, M., Anton, J. L., Valabregue, R., & Poline, J. B. (2002). Region of interest analysis using an SPM toolbox. In 8th international conference on functional mapping of the human brain, 16(2), 497.
- Buxton R.B., Uludağ K., Dubowitz D.J., Liu T.T. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Albert M., Belleville S., Craik F.I.M., Duarte A., Grady C.L., Lindenberger U., Nyberg L., Park D.C., Reuter-Lorenz P.A., Rugg M.D., Steffener J., Rajah M.N. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018;19(11):701–710. doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud M., Hudon C., Boller B., Brambati S., Duchesne S., Lorrain D., Gagnon J.F., Maltezos S., Mellah S., Phillips N., Belleville S., the Consortium for the Early Identification of Alzheimer’s disease-Quebec Evidence of a relation between hippocampal volume, white matter hyperintensities, and cognition in subjective cognitive decline and mild cognitive impairment. J. Gerontol. B Psychol. Sci. Soc. Sci. 2019 doi: 10.1093/geronb/gbz120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua E.F., Schacter D.L., Rand-Giovannetti E., Sperling R.A. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17(11):1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Cohen J., Cohen P. Taylor & Francis Group; 1983. Applied Multiple Regression/correlation Analysis for the Behavioral Sciences. [Google Scholar]
- Colangeli S., Boccia M., Verde P., Guariglia P., Bianchini F., Piccardi L. Cognitive reserve in healthy aging and alzheimer's disease: a meta-analysis of fMRI studies. Am. J. Alzheimers Dis. Other Demen. 2016;31(5):443–449. doi: 10.1177/1533317516653826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Duchesne S., Dieumegarde L., Chouinard I., Farokhian F., Badhwar A., Bellec P. Structural and functional multi-platform MRI series of a single human volunteer over more than fifteen years. Sci. Data. 2019;6(1):245. doi: 10.1038/s41597-019-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gaudreau P., Morais J.A., Shatenstein B., Gray-Donald K., Khalil A., Dionne I. Nutrition as a determinant of successful aging: description of the Quebec longitudinal study Nuage and results from cross-sectional pilot studies. Rejuvenation Res. 2007;10(3):377–386. doi: 10.1089/rej.2007.0596. [DOI] [PubMed] [Google Scholar]
- Haier R.J., Siegel B.V., Nuechterlein K.H., Hazlett E., Wu J.C., Paek J. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. [Google Scholar]
- Hayes A.F., Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav. Res. Methods. 2009;41(3):924–936. doi: 10.3758/brm.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2018). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach.
- Irish M., Lawlor B.A., Coen R.F., O'Mara S.M. Everyday episodic memory in amnestic mild cognitive impairment: a preliminary investigation. BMC Neurosci. 2011;12:80. doi: 10.1186/1471-2202-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G., Subjective Cognitive Decline Initiative (SCD-I) Working Group A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43(1):13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54(3):2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Maillet D., Rajah M.N. Age-related differences in brain activity in the subsequent memory paradigm: a meta-analysis. Neurosci. Biobehav. Rev. 2014;45:246–257. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Maillet D., Rajah M.N. Assessing the neural correlates of task-unrelated thoughts during episodic encoding and their association with subsequent memory in young and older adults. J. Cogn. Neurosci. 2016;28(6):826–841. doi: 10.1162/jocn_a_00935. [DOI] [PubMed] [Google Scholar]
- McClelland G.H., Judd C.M. Statistical difficulties of detecting interactions and moderator effects. Psychol. Bull. 1993;114(2):376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo J.L., Rabin L.A., Amariglio R., Buckley R., Dubois B., Ellis K.A., the Subjective Cognitive Decline Initiative (SCD-I) Working Group Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13(3):296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer A.C., Fink A. Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 2009;33(7):1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Nyberg L., Lovden M., Riklund K., Lindenberger U., Backman L. Memory aging and brain maintenance. Trends Cogn. Sci. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Potvin O., Mouiha A., Dieumegarde L., Duchesne S., Alzheimer's Disease Neuroimaging I. Normative data for subcortical regional volumes over the lifetime of the adult human brain. Neuroimage. 2016;137:9–20. doi: 10.1016/j.neuroimage.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Rami L., Valls-Pedret C., Bartres-Faz D., Caprile C., Sole-Padulles C., Castellvi M. Cognitive reserve questionnaire. Scores obtained in a healthy elderly population and in one with Alzheimer’s disease. Rev Neurol. 2011;52(4):195–201. Retrieved from. [PubMed] [Google Scholar]
- Riches I.P., Wilson F.A., Brown M.W. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J. Neurosci. 1991;11(6):1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B., Dricot L., Devolder A., Bodart J.M., Crommelinck M., De Gelder B., Zoontjes R. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J. Cogn. Neurosci. 2000;12(5):793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- Rossion B., Caldara R., Seghier M., Schuller A.M., Lazeyras F., Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126(Pt 11):2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Rypma B., D'Esposito M. A subsequent-memory effect in dorsolateral prefrontal cortex. Brain Res. Cogn. Brain Res. 2003;16(2):162–166. doi: 10.1016/s0926-6410(02)00247-1. [DOI] [PubMed] [Google Scholar]
- Schiltz C., Dricot L., Goebel R., Rossion B. Holistic perception of individual faces in the right middle fusiform gyrus as evidenced by the composite face illusion. J. Vis. 2010;10(2):25.21-16. doi: 10.1167/10.2.25. [DOI] [PubMed] [Google Scholar]
- Solé-Padullés C., Bartrés-Faz D., Junqué C., Vendrell P., Rami L., Clemente I.C. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging. 2009;30(7):1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Sperling R., Chua E., Cocchiarella A., Rand-Giovannetti E., Poldrack R., Schacter D.L., Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/s1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J., Stern Y. Exploring the neural basis of cognitive reserve in aging. BBA. 2012;1822(3):467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J., Reuben A., Rakitin B.C., Stern Y. Supporting performance in the face of age-related neural changes: testing mechanistic roles of cognitive reserve. Brain Imaging Behav. 2011;5(3):212–221. doi: 10.1007/s11682-011-9125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Arenaza-Urquijo E.M., Bartres-Faz D., Belleville S., Cantilon M., Chetelat G., the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018 doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Gazes Y., Razlighi Q., Steffener J., Habeck C. A task-invariant cognitive reserve network. Neuroimage. 2018;178:36–45. doi: 10.1016/j.neuroimage.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen J.W., Hong D., Rohani Rankouhi S., Wiltfang J., Fernández G., Norris D.G., Tendolkar I. The increase in medial prefrontal glutamate/glutamine concentration during memory encoding is associated with better memory performance and stronger functional connectivity in the human medial prefrontal-thalamus-hippocampus network. Hum. Brain Mapp. 2018;39(6):2381–2390. doi: 10.1002/hbm.24008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.M., Stern Y. Cognitive reserve in aging. Curr. Alzheimer Res. 2011;8(4):354–360. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman M.A., McClelland G.H. Designing, testing, and interpreting interactions and moderator effects in family research. J. Fam. Psychol. 2005;19(1):111–120. doi: 10.1037/0893-3200.19.1.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.