Abstract
Chronic stress is a risk-factor for the development of mood and stress-related disorders. Clinical evidence indicates that probiotics can influence the stress response and mood. The Sisu study investigated whether Lacticaseibacillus paracasei Lpc-37® (Lpc-37®) could modulate stress, mood and well-being. Prior to a two-week run-in period, 120 healthy adults (18-45 y) were stratified for sex and chronic stress and randomized to either 1.75 × 1010 colony forming units (CFU) of Lpc-37 or placebo (1:1) per day for 5 weeks. The primary objective was the effect of Lpc-37 on heart rate (HR) in response to the Trier Social Stress Test (TSST). Secondary objectives were assessed by biomarkers and self-report scales over the study. The primary hypothesis was not met in either the Intention-to-Treat (ITT) or Per Protocol (PP) population, but Lpc-37 reduced the increase in HR in participants with low chronic stress (LCS) and increased HR in participants with high chronic stress (HCS) during the TSST. Supporting significant efficacy in the PP population (n = 113), Lpc-37 reduced perceived stress following intervention. More significant effects were identified within the subgroups where Lpc-37 reduced exhaustion during the TSST and normalized cortisol levels at 8pm in participants with LCS, reduced perceived stress also in females, and increased perceived health and sleep-related recovery in participants with HCS. Adverse events (AEs) were similar between groups, there were no severe AEs, and vital signs remained unchanged. Overall, Lpc-37 reduced perceived stress compared to placebo. Other beneficial effects within biomarkers related to stress indicate that the effects of Lpc-37 may be differentially dependent on sex and chronic stress. (ClinicalTrials.gov: NCT03494725).
Keywords: Trier Social Stress Test, Stress, Anxiety, Lacticaseibacillus paracasei Lpc-37, Probiotic
Highlights
-
•
The Trier Social Stress Test was used to investigate the effect of Lpc-37 on stress
-
•
The effects of Lpc-37 on response to acute stress may be dependent on daily stress
-
•
Lpc-37 reduced perceived stress in the general population compared to placebo
-
•
Lpc-37 also reduced perceived stress in female, but not male participants
-
•
Other beneficial effects of Lpc-37 may be dependent on daily/chronic stress
1. Introduction
Everyday life can be demanding with many sources of stress. While short-term stress is a beneficial adaption process to stressors (McEwen, 2007), chronic stress is a major risk-factor for the development of a wide range of physical and mental disorders (Chrousos, 2009). According to the American Psychological Association (APA) Stress in America report of 2018, nearly 75% of adults reported experiencing at least one physical or emotional symptom of stress in the past month and almost 50% reported higher average stress levels than their perceived healthy levels of stress within the past month (American Psychological Association, 2018). Understanding the risks to our health and ways to reduce daily stress are therefore paramount.
Overwhelming evidence now indicates that the health benefits of the gut microbiome extend far beyond the gut. Here, host-microbe interactions influence the release of several immunological and neurological signaling molecules, and microbial by-products which communicate along the bi-directional pathway of the microbiota-gut-brain axis through central, enteric and autonomic nervous systems as well as the hypothalamic pituitary adrenal (HPA) axis (Rea et al., 2020). The gut microbiome therefore exerts a regulatory function upon neuroinflammation, neurodevelopment and the neuroendocrine stress response (El Aidy et al., 2014; Foster et al., 2016; Kelly et al., 2015), influencing brain physiology, psychological responses and ultimately, behavior. Infiltrating the realms of psychology and psychiatry, the APA have recognized the gut microbiome as a novel paradigm for studying the psychobiological underpinnings of mental illness (Liu, 2017). Recent clinical data supports the hypothesis that the stress response can be influenced through targeted modification of the gut microbiota (Cryan et al., 2019; Foster et al., 2017). Probiotics are one such means of targeting the gut microbiota to deliver health benefits. The physiological and psychological benefits of probiotics on stress and mood outcomes have been described in pre-clinical trials using different models, with some translation to clinical trials in different populations ranging from healthy participants (Allen et al., 2016; Marotta et al., 2019; Messaoudi et al., 2011a, 2011b), to subjects under various stress levels (Benton et al., 2007; Chahwan et al., 2019; Chong et al., 2019; Pinto-Sanchez et al., 2017; Sawada et al., 2017; Slykerman et al., 2017). Of note, one recent study demonstrated that the neurocognitive benefits of a multispecies probiotic became evident only when the participants were stressed, highlighting the need to carefully characterize study populations (Papalini et al., 2019).
Lacticaseibacillus paracasei Lpc-37® (Lpc-37®), formerly known as Lactobacillus paracasei Lpc-37®, has proven effective in preventing chronic stress-associated behaviors from developing in two recent pre-clinical experiments of the same model (Stenman et al., 2020). The Sisu study investigated the a priori hypotheses that Lpc-37 could reduce the expected increase in physiological markers of stress such as heart rate (HR) and blood pressure (BP) in response to an acute stress; the Trier Social Stress Test (TSST), and furthermore to normalize the cortisol awakening response (CAR) and evening cortisol levels, improve psychological test scores both in response to the TSST and over the study, and improve sleep, productivity and overall well-being following a five-week intervention compared to placebo. The primary objective was the effect of Lpc-37 on HR in response to the TSST and was chosen mainly due to the suspected mode of action: the vagus nerve activity responsible for the gut-brain interaction. A biomarker for the autonomic nervous system (ANS), HR has also repeatedly been shown to be affected by the TSST. Since the clinical effects of Lpc-37 on outcomes of stress and anxiety were unknown, one single primary objective and endpoint was selected. Although HR was expected to increase in response to the TSST, chronic psychosocial factors have affected HR reactivity to acute psychological stress with mixed results (Chida and Hamer, 2008). To control for chronic stress while investigating the effect of Lpc-37 on the ANS response to acute stress, the population was stratified into low and high chronic stress using cut off values previously defined in an age-related population using the Trier Inventory for Chronic Stress (TICS) (Schulz and Schlotz, 1999). Secondary objectives were measured throughout the study and were assessed by biomarkers and self-report scales. The results serve as an indication that the study design is suitable to investigate clinical stress-related effects of probiotics and confirm that Lpc-37 is a safe and effective probiotic to beneficially impact several outcomes related to physiological and psychological stress in healthy adults.
2. Materials and methods
2.1. Study design
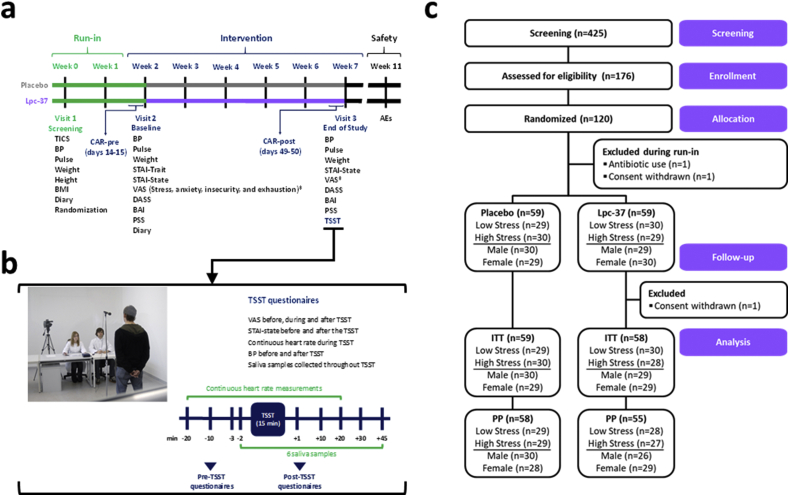
The Sisu study was a randomized, double-blind, placebo-controlled, two-arm (allocation ratio 1:1) and parallel groups clinical trial. The study design included a two-week run-in period between Visit 1 (V1) and Visit 2 (V2) when randomized participants were not permitted to consume products containing concentrated sources of probiotics and/or prebiotics. This was followed by a five-week intervention with the investigational products (IP)s between V2 and Visit 3 (V3). Randomized participants were provided with saliva collection kits and instructed to collect saliva at home during two consecutive working days before V2 and V3 and provided with training and access to an online daily diary from V1 to V3. A detailed outline of the investigation steps at each visit are shown in Fig. 1a and b. The primary objective evaluated the efficacy of Lpc-37 on HR before, during and after the TSST (V3). Secondary objectives evaluated the efficacy of Lpc-37 before, during and after the TSST, before and after intervention, and throughout the study period by salivary cortisol analyses, HR, BP, self-report scales, validated inventories and diary entries. Prior to recruitment, the protocol, participant information and the informed consent form (ICF) were reviewed and approved by the Independent Ethics Committee (IEC) of the Chamber of Physicians of the State of Rhineland-Palatinate on March 13, 2018 and the study was registered at ClinicalTrials.gov (NCT 03494725). The study was conducted at a single site at daacro GmbH and Co. KG (Trier, Germany) in accordance with the Declaration of Helsinki (World Medical Association, 2013), and the guidelines for good clinical practice (GCP) (ICH Expert Working Group, 1996), following all applicable laws and regulations for clinical research in Germany. During the study, a minor amendment was added to the ICF to include follow-up calls for ongoing adverse events (AEs) at V3 and was approved by the IEC. Clinical monitoring was performed by an external Clinical Research Associate.
Fig. 1.
a. Sisu study design. b. Study-specific Trier Social Stress Test (TSST) procedures. c. CONSORT flow diagram. Abbreviations: AEs, Adverse Events; BAI, Beck Anxiety Inventory; BMI, Body Mass Index; BP, Blood Pressure; CAR, Cortisol Awakening Response; CONSORT, Consolidated Standards of Reporting Trials; DASS, Depression Anxiety Stress Scale; ITT, Intention to Treat; PP, Per Protocol; PSS, Perceived Stress Scale; STAI, State Trait Anxiety Inventory; TICS, Trier Inventory for Chronic Stress; VAS, Visual Analog Scale.
2.2. Study participants
Participants were recruited from daacro's in-house database (www.werdeproband.de). A total of 120 eligible participants signed the ICF and were randomized into the study at V1. The full description of eligibility criteria is included in Supplementary Methods. Randomization was managed by Oy 4Pharma Ltd. (Turku, Finland) and performed using block randomization according to a computer-generated randomization list, with concealed allocation. All randomized participants were assigned to one of two study groups (verum or placebo). Within each study group, the randomization was stratified for sex and prolonged perceived stress levels using the TICS (Schulz and Schlotz, 1999; Schulz et al., 2004). Chronic stress was determined using the Screening Subscale for Chronic Stress, a subscale of the TICS. The classification of low chronic stress (LCS) and high chronic stress (HCS) depended on whether the participant's score was above or below the age-related median score for the frequency of stressful events perceived within the last three months. Participants with a score ≤13 were stratified into the LCS subgroup and participants with a score ≥14 were stratified into the HCS subgroup (Schulz et al., 2004). A detailed description of the TICS is included in Supplementary Methods.
2.3. IPs
The verum (batch 1103180371) consisted of Lpc-37 at a dose of 1.75 × 1010 colony forming units (CFU), microcrystalline cellulose, magnesium stearate and silicon dioxide in one capsule per day. The matching placebo (batch 1103180369) was the same formulation without Lpc-37, in one capsule per day. Both IPs were identical in appearance and taste and a five-week supply plus some extra capsules was provided to participants at V2. Participants were instructed to consume one capsule of their assigned IP each morning, at least 30 min before breakfast or their first meal of the day, with a glass of plain water.
DuPont Nutrition & Biosciences, Danisco USA Inc. (Madison, WI, USA) produced, packaged and labelled the IPs with individual randomization numbers per capsule bottle as per the unblinded randomization list provided by 4Pharma (Turku, Finland). The identity of the IPs was blinded to participants, site staff, the principal investigator (PI) and all sponsor personnel involved in the trial. The PI, site staff, data manager, biostatistician and all sponsor personnel involved in the trial remained blinded to the group assignments until after the database was locked and the blind data review (BDR) was completed. The integrity of the sealed individual blinded envelopes was inspected during routine interim monitoring visits.
IP compliance was documented by participants each day in the online diary and percentage compliance was calculated by counting the number of remaining capsules in the bottles returned at V3: 35/(40 – number of capsules returned)*100, where 35 was the number of expected capsules to have been taken over the five-week intervention and 40 was the number of capsules provided. All participants had completed the intervention before the expiration date of the IPs.
2.4. Study outcomes
2.4.1. TSST
The TSST is a protocol for inducing an acute and physiological stress, including an endocrine reaction to experimental psychosocial stress in humans (Allen et al., 2017; Dickerson and Kemeny, 2004). All participants completed the TSST (Kirschbaum et al., 1993) at V3 which consisted of the following four components: introduction, preparation, interview and mental arithmetic task. The TSST is described in detail by Kudielka et al. (Kudielka et al., 2007a, 2007b; Kudielka and Wust, 2010) and a study specific description of the TSST is included in Supplementary Methods. Specific procedures measured before, during and after the TSST are described below and outlined in Fig. 1b.
2.4.2. Primary outcome: HR in response to the TSST
The primary outcome was change in HR in response to the TSST. Efficacy was defined as a lower increase in HR in response to the TSST following intervention with Lpc-37, compared to placebo. A Polar watch device (M400, Polar Electro GmbH, Büttelborn, Germany) worn by participants collected HR measurements every second throughout a 55 min test period. Mean values were calculated per group before, during and after the TSST: 10 min sitting pre-TSST; 10 min standing pre-TSST; 5 min during the TSST introduction and preparation; 5 min during the interview; 5 min during the mental arithmetic task; 10 min standing post-TSST; 10 min sitting post-TSST.
2.4.3. Secondary outcomes: TSST-related outcomes
2.4.3.1. Salivary cortisol and alpha amylase (AA)
Individual saliva samples were collected from each participant 2 min before and 1-, 10-, 20-, 30- and 45-min after the TSST. Saliva was collected using Salivette® Cortisol, code blue collection tubes (Sarstedt, Nuembrecht, Germany). Briefly, participants gently moved the swab from the Salivette® in the mouth for approximately 1 min to stimulate salivation and to ensure the swab was soaked thoroughly in saliva. The swab containing the absorbed saliva was then returned to the Salivette® and the cap was replaced. All saliva samples collected during the TSST were stored frozen at −20 °C until analysis. Salivary cortisol levels were determined using a high sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, PA, USA). Salivary AA levels were determined using a kinetic enzyme assay kit (Salimetrics). All samples were analyzed at daacro.
2.4.3.2. BP
BP measurements were taken from each participant 3 min before and 1 min after the TSST. Systolic and diastolic BP were obtained using an automated device (OMRON M10-IT, OMRON Medizintechnik Handelsgesellschaft mbH, Mannheim, Germany).
2.4.3.3. State-trait anxiety inventory (STAI; X1 Form)
Participants rated their state anxiety levels using the STAI-X1 Form at 10 min before and 1 min after the TSST. The STAI-X1 Form is a subscale of the STAI self-report questionnaire that measures the presence and severity of current symptoms of anxiety and the propensity to be anxious (Spielberger and Gorsuch, 1983). It comprises 20 items which assess momentary anxiety characterized by tension, solitude, nervousness, uneasiness and fear of future situations. Participants rated how they felt on a scale ranging from 1 = “not at all” to 4 = “very much so”. The total score for the STAI-X1 Form was obtained by summing the scores of all 20 items. The range of the total score for anxiety is 20–80, wherein the higher the score, the higher the anxiety.
2.4.3.4. Perceived -stress, -anxiety, -emotional insecurity and -exhaustion
Participants rated their individual perception of stress, anxiety, emotional insecurity and exhaustion, using separate visual analog scales (VAS) (Bond and Lader, 1974; Aitken, 1969). These psychological measures were taken 10 min before the TSST, between the interview and mental arithmetic TSST tasks, and 1 min after the TSST. Participants marked a spot on the line representing their perceived stress, anxiety, emotional insecurity and exhaustion; where 0 = “feeling not at all” and 100 = “feeling highly stressed/anxious/insecure/exhausted”. Scores were determined with millimeter precision and reported as percentage ranging from 0 to 100. The VAS is a useful and suitable tool to measure perceived psychological reactions to the TSST (Hellhammer and Schubert, 2012).
2.4.4. Secondary outcomes: baseline and end of study-related outcomes
2.4.4.1. CAR and 8pm cortisol
Individual saliva samples were collected from each participant on two consecutive working days before V2 and V3. Participants were provided with saliva collection kits containing Salivette® Cortisol, code blue collection tubes (Sarstedt, Germany) and instructions on how to collect saliva samples at home. The method for saliva sample collection using the swabs from the Salivette® was the same as briefly described in section 2.4.3.1. Saliva samples for the CAR were collected at 0-, 30-, 45- and 60-min post-awakening and one sample was collected at 8pm that evening. Participants stored the saliva samples in either their refrigerator or freezer at home and were instructed to bring the samples with them to the study site at their next scheduled visit. Saliva samples were stored at −20 °C until analysis. Salivary cortisol levels were determined using a high sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics). Mean values were calculated for each time point for the two measuring days. The CAR was summarized using the following variables: area under the curve with respect to increase (AUCi), area under the curve with respect to ground (AUCg), peak value (maximum value of the two-day mean of the four CAR samples) and mean increase (two-day mean of cortisol at awakening subtracted from two-day mean peak value). The two AUC measurements aggregated the change in cortisol levels over the time course of the CAR and were calculated as previously described (Pruessner et al., 2003). Efficacy for the CAR variables AUCg, AUCi, cortisol at awakening and 8pm cortisol levels were defined in terms of a normalization, i.e. number of participants with normal test values (between first and third quantile of reference measures relative to a gender specific control data base) and numbers of participants with low or high values were compared before and after the intervention. The normative database was generated using assay kits manufactured by Salimetrics, including n = 1746 participants (n = 1296 women and n = 450 men), established in 2017.
2.4.4.2. BP
BP measurements were taken for each participant upon arrival at the site at V2 and V3, as described in 2.4.3.2.
2.4.4.3. Self-report questionnaires and VAS
Participants completed a battery of four questionnaires (Perceived Stress Scale (PSS), Beck Anxiety Inventory (BAI), 42-Item Depression, Anxiety and Stress Scale (DASS-42), the STAI-X1 Form (as described in 2.4.3.3) and four VAS’ (as described in 2.4.3.4), to investigate the effects of Lpc-37 on self-reported symptoms and perception of anxiety, stress, depression, emotional insecurity and exhaustion following five-weeks of intervention. In all cases the German language versions were used.
2.4.4.3.1. PSS
The PSS is a widely used psychological instrument for measuring the degree to which people perceived their lives as stressful within the last month (Cohen et al., 1983). The PSS comprises of 14 items that are answered on a 5-point scale from 0 = “never” to 4 = “very often”. The total score was calculated by summing the scores of the 14 items.
2.4.4.3.2. BAI
The BAI is a self-rating scale designed to measure anxiety in adults and youths within the last week (Beck et al., 1988). It comprises of 21 items that are answered on a 4-point scale from 0 = “not at all” to 3 = “severely – it bothered me a lot”. The total score was calculated by summing the scores of the 21 items.
2.4.4.3.3. DASS-42
The DASS-42 is a 42 item questionnaire that collects information about negative emotional states of depression, anxiety and stress during the past week (Lovibond, 1998; Lovibond and Lovibond, 1996). These three subscales include 14 items ranging from 0 = “did not apply to me at all” to 3 = “applied to me very much or most of the time”. Scores for depression, anxiety and stress were calculated by summing the scores for the relevant items within each subscale.
2.4.5. Secondary outcomes: online diary-related outcomes measured throughout the study
Following randomization at V1, participants received an individual access to the online diary and were instructed to complete the diary everyday between 3am and 12pm, during the run-in period and throughout the intervention period. If entries were not performed in a timely manner, the study team received an e-mail notification. The respective participants were then contacted the following day by a study team member and asked to provide the information. The online diary collected information on perceived productivity, perceived health, sleep quality (sleep disruptions, both binary and reported number of sleep disruptions (count), sleep duration and sleep related-recovery) and perceived mood.
2.5. Vital signs and assessment of safety
The safety objectives of this study were to evaluate if vital signs (BP and HR), body mass index (BMI) and the incidence and intensity of AEs were comparable between the groups. Systolic and diastolic BP and HR were obtained at V1 using an automated device (OMRON M10-IT) to determine eligibility. At V2 and V3, BMI, BP and HR were obtained following participant arrival at site. AEs were assessed at each visit with open, standardized questions such as “Have you had any health problems since you were last questioned?”. Additionally, participants were asked to record any occurring AE as follows: description of the event, onset (date and time), resolution (date and time), whether the AE was ongoing at the end of the study, intensity (mild, moderate, severe), therapy of event, action taken, and outcome. The PI classified causality (definitely, probably, possibly, unlikely, not related, not assessable) and whether it constituted a serious adverse event (SAE) or not. Any AEs still ongoing at study completion on V3 were followed up to 30 days after V3.
2.6. Sample size calculation and statistical analyses
The sample size was computed for a repeated measurement ANOVA with two groups and seven repeated measurements (power = 0.85, α = 0.05, f = 0.1). The calculation resulted in a group size of 56 participants each, which was rounded up to 60 participants per study group to account for attrition. Subgroup analyses were performed for the different strata, i.e. female, male, HCS and LCS. For the subgroup analyses, which relied on 50% of the total sample size, this resulted in a power = 0.55 for the parameters assumed for the sample size calculation (α = 0.05, f = 0.1).
For all endpoints, analyses were performed for the Intention-to-Treat (ITT) and Per Protocol (PP) populations, separately. For the PP analyses, individual decisions on exclusion of participants or data points were made during the BDR, resulting in different Ns for different endpoints. A detailed description of the methodology to define the PP population is included in Supplementary Methods. Table S1a lists the number of participants in the ITT and PP population per endpoint, statistical model, and transformation criteria and Table S1b lists the number of participants in the PP population per endpoint along with reasons for exclusion.
Endpoints with more than two measurements were analyzed using linear mixed models. Mixed models were built up gradually, first testing how many time polynomials should be included, then testing possible covariates (gender, chronic stress, STAI trait, BMI, weight, age), and lastly adding the effect of study group and time × group interaction terms (e.g. time 1 × group, time 2 × group). Models were built including time and intercept as random factors. In case of convergence difficulties, time was dropped from the random effects. Type II F-tests were conducted using Satterthwaite's degrees of freedom method. Endpoints with two measurements (before and after TSST or intervention) were analyzed using repeated measures ANOVAs including relevant covariates (see above).
If the assumptions of a statistical analysis were violated despite efforts of transformation, alternative parametric or non-parametric tests were used. P-values in section 3. Results describe efficacy for a study group based on the interaction between study group and time for all parametric tests and based on group difference between change scores for non-parametric tests. All P-values <0.05 were considered as statistically significant and in some cases P-values ≥0.05 and < 0.10 are reported as trends where interesting. The results described in the main text focus on the PP population because they more accurately represent those participants who strictly followed the protocol, however significant P-values found only within the ITT (and not the PP) population are also reported. Fisher's exact test on frequency of compliance in percent between groups was used to compare compliance of IP between the groups. Statistical analyses were conducted using R Version 3.5.2 (R Core Team, 2018).
3. Results
3.1. Participants and baseline characteristics
A total of 425 volunteers were telephone screened, of which 176 were eligible and invited to a screening visit (V1). Of those, 120 participants met the inclusion/exclusion criteria and were enrolled in the study between April and October 2018. Two participants were excluded during the run-in period (use of antibiotics and withdrawn consent) and one participant withdrew consent during the intervention period. A total of 117 participants completed the study. Fig. 1c displays the CONSORT flow diagram with detailed disposition of participants. There were no marked differences in baseline and demographic characteristics between the groups in the general population (Table 1) or in the subgroups (Table S2).
Table 1.
Demographics and other baseline characteristics for randomized participants (n = 120).
| Placebo |
Lpc-37 |
|
|---|---|---|
| Mean (SD) | Mean (SD) | |
| TICS (score) | 15.32 (8.65) | 15.08 (9.28) |
| Age (years) | 23.25 (4.20) | 23.73 (4.27) |
| Height (cm) | 173.58 (9.33) | 175.58 (8.86) |
| Weight (kg) | 69.79 (12.15) | 71.13 (11.05) |
| BMI (kg/m2) | 23.02 (2.67) | 22.97 (2.30) |
| Systolic BP (mmHg) | 120.72 (13.47) | 120.75 (12.09) |
| Diastolic BP (mmHg) | 74.88 (8.53) | 74.22 (7.25) |
| Heart rate (bpm) | 71.03 (12.43) | 72.27 (13.71) |
Abbreviations: BMI, Body Mass Index; BP, Blood Pressure; n, number of participants; SD, Standard Deviation; TICS, Trier Inventory for Chronic Stress.
The PP population was identified before database lock, after the BDR and included all randomized participants that satisfied the inclusion/exclusion criteria and had no major protocol deviations (n = 113; Lpc-37, n = 55; placebo, n = 58). For individual endpoints, participants were excluded if they showed deviations that might have affected that endpoint (Tables S1a and S1b). The ITT population included all randomized participants that satisfied the inclusion/exclusion criteria with data available for all endpoints for 117 participants (Lpc-37; n = 58 and placebo; n = 59). The safety population included all participants that received at least one dose of IP and contained 118 participants (Lpc-37; n = 59 and placebo; n = 59).
3.2. IP compliance, IP stability and blinding
All participants satisfied the criterion of >80% compliance. Mean compliance in the ITT population was 100.4% for Lpc-37 and 99.9% for placebo (P = 0.987). While the target dose of Lpc-37 was 1 × 1010 CFU/capsule, the certificate of analysis recorded the initial dose as 1.75 × 1010 CFU/capsule. Both the presence of Lpc-37 and absence of contaminants and genetic variants was confirmed by genomic sequencing of the IP (DuPont Nutrition & Biosciences, Danisco USA Inc.). From IP bottles stored at the study site until all participants had completed the study, the final dose of Lpc-37 was determined to be 1.68 × 1010 CFU/capsule. The randomization code was not broken for any participant during the study.
3.3. Stress reactivity - physiological response to the TSST
3.3.1. Primary outcome: the effects of Lpc-37 on HR are dependent on chronic stress
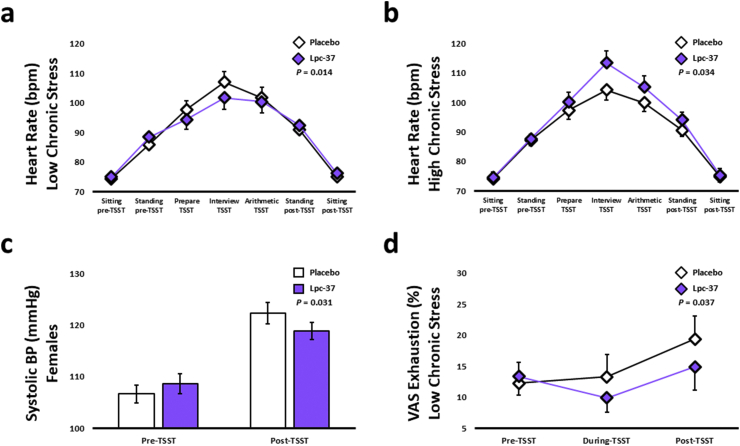
As expected, there was a significant change in HR in both groups in response to TSST-induced acute stress (P < 0.001). HR increased by 43.7% in the Lpc-37 group from sitting pre-TSST to interview TSST and by 42.1% in the placebo group (Table 2). There was no significant effect of Lpc-37 on HR in the general population (Table 2). The HR-increase in response to acute stress was significantly lower in participants with LCS (Fig. 2a; P = 0.014), but significantly higher in participants with HCS (Fig. 2b; P = 0.034) in the Lpc-37 group compared to the placebo group. There were no effects of Lpc-37 on HR in either male or female participants (Table S3).
Table 2.
Summary measures in response to the Trier Social Stress Test (TSST) for participants in the Per Protocol population.
| Sitting pre-TSST−20 min |
Standing pre-TSST−10 min |
Pre-TSST−3 min |
Pre-TSST−2 min |
Interview TSST |
Arithmetic TSST |
Post-TSST+1 min |
Standing post-TSST +10 min |
Sitting post-TSST+20 min |
Post-TSST+30 min |
Post-TSST+45 min |
P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
Heart Rate (bpm) | ||||||||||||
| Placebo (n = 57) | 74.34 (9.04) | 86.69 (10.74) | 97.62 (16.23) | – | 105.66 (18.86) | 100.81 (17.20) | – | 90.81 (12.11) | 74.97 (9.86) | – | – | 0.757T |
| Lpc-37 (n = 55) |
74.84 (10.20) |
88.15 (11.13) |
97.34 (17.15) |
– |
107.56 (21.56) |
102.77 (19.57) |
– |
93.32 (14.08) |
75.88 (11.11) |
– |
– |
|
|
Salivary Cortisol (nmol/L) | ||||||||||||
| Placebo (n = 57) | – | – | – | 4.82 (2.60) | – | – | 6.85 (3.50) | 8.97 (5.84) | 9.21 (6.59) | 7.71 (5.06) | 6.16 (3.79) | 0.566T |
| Lpc-37 (n = 55) |
– |
– |
– |
4.79 (2.62) |
– |
– |
6.96 (3.73) |
9.48 (5.75) |
9.89 (6.51) |
8.04 (5.36) |
6.21 (3.17) |
|
|
Salivary Alpha Amylase (U/ml) | ||||||||||||
| Placebo (n = 57) | – | – | – | 161.67 (110.89) | – | – | 270.55 (174.85) | 158.85 (91.21) | 141.49 (93.00) | 138.48 (90.31) | 148.15 (105.60) | 0.815T |
| Lpc-37 (n = 55) |
– |
– |
– |
154.04 (98.17) |
– |
– |
246.29 (153.62) |
146.53 (86.80) |
130.11 (82.45) |
125.19 (79.67) |
141.13 (92.94) |
|
|
Systolic BP (mmHg) | ||||||||||||
| Placebo (n = 58) | – | – | 114.33 (14.07) | – | – | – | 129.19 (14.33) | – | – | – | – | 0.274 |
| Lpc-37 (n = 55) |
– |
– |
115.11 (12.53) |
– |
– |
– |
127.47 (13.67) |
– |
– |
– |
– |
|
|
Diastolic BP (mmHg) | ||||||||||||
| Placebo (n = 58) | – | – | 78.41 (8.32) | – | – | – | 88.36 (9.72) | – | – | – | – | 0.345 |
| Lpc-37 (n = 55) |
– |
– |
79.13 (7.83) |
– |
– |
– |
90.38 (7.17) |
– |
– |
– |
– |
|
|
STAI-State (score) | ||||||||||||
| Placebo (n = 58) | – | 36.83 (9.48) | – | – | – | – | 43.60 (10.00) | – | – | – | – | 0.755 |
| Lpc-37 (n = 55) |
– |
36.09 (8.45) |
– |
– |
– |
– |
42.38 (10.91) |
– |
– |
– |
||
|
VAS Stress (score) | ||||||||||||
| Placebo (n = 58) | – | 18.52 (21.73) | – | – | 51.51 (28.10) | – | 32.85 (23.66) | – | – | – | – | 0.327T |
| Lpc-37 (n = 55) |
– |
19.89 (20.61) |
– |
– |
47.71 (27.08) |
– |
31.72 (24.25) |
– |
– |
– |
– |
|
|
VAS Insecurity (score) | ||||||||||||
| Placebo (n = 58) | – | 17.19 (21.37) | – | – | 52.19 (27.16) | – | 23.69 (23.58) | – | – | – | – | 0.364T |
| Lpc-37 (n = 55) |
– |
14.47 (16.96) |
– |
– |
45.08 (28.92) |
– |
23.92 (23.87) |
– |
– |
– |
– |
|
|
VAS Anxiety (score) | ||||||||||||
| Placebo (n = 58) | – | 8.50 (14.94) | – | – | 22.47 (23.51) | – | 11.74 (18.46) | – | – | – | – | 0.251T |
| Lpc-37 (n = 55) |
– |
6.80 (10.95) |
– |
– |
20.85 (23.61) |
– |
10.68 (15.19) |
– |
– |
– |
– |
|
|
VAS Exhaustion (score) | ||||||||||||
| Placebo (n = 58) | - | 19.79 (21.88) | – | – | 21.30 (22.47) | – | 25.68 (26.07) | - | - | - | - | 0.101T |
| Lpc-37 (n = 55) | – | 21.18 (21.49) | – | – | 19.20 (21.11) | – | 22.12 (22.46) | – | – | – | – | |
Abbreviations: BP, Blood Pressure; n, number of participants; SD, Standard Deviation; STAI; State-Trait Anxiety Inventory; TSST, Trier Social Stress Test; VAS, Visual Analog Scale.
T Outcome was subjected to transformation to meet model assumptions.
Fig. 2.
Trier Social Stress Test (TSST) related outcomes: a. Heart rate in the low chronic stress subgroup (Mean ± SE). b. Heart rate in the high chronic stress subgroup (Mean ± SE). c. Systolic blood pressure in the female subgroup (Mean ± SE). d. Visual analog scale (VAS) exhaustion in the low chronic stress subgroup (Mean ± SE). Abbreviations: BP, Blood Pressure; TSST, Trier Social Stress Test; VAS, Visual Analog Scale.
3.3.2. Lpc-37 had no effect on salivary cortisol or AA, but reduced the acute stress induced increase in systolic BP in females
Both salivary cortisol and AA levels significantly changed in both groups in response to the TSST (P < 0.001). There was no significant effect of Lpc-37 on either salivary cortisol or AA levels in the general population (Table 2), or in any of the subgroups (Table S3).
The TSST resulted in a significant increase in both systolic (P < 0.001) and diastolic (P < 0.001) BP in both groups. There were no significant effects of Lpc-37 on either systolic or diastolic BP in response to the TSST (Table 2). In female participants, systolic BP increased significantly less in the Lpc-37 group, compared to the placebo group (Fig. 2c; P = 0.031), with no significant difference in diastolic BP between groups (Table S3). There were no significant effects of Lpc-37 on systolic or diastolic BP in the other subgroups (Table S3). Results for the effects of Lpc-37 on the physiological response to the TSST in the ITT population are included in Tables S4 and S5.
3.4. Stress reactivity - psychological response to the TSST
3.4.1. Lpc-37 reduced perceived exhaustion in participants with LCS, but had no effect on state anxiety or perceived -stress, -anxiety or -insecurity
The TSST resulted in a significant increase in state anxiety in both groups (P < 0.001). There were no significant effects of Lpc-37 on state anxiety in the general population (Table 2), or in any of the subgroups (Table S3).
Perceived -stress, -insecurity and -anxiety significantly changed in both groups, in response to the TSST (P < 0.001), while perceived exhaustion did not. There were no significant effects of Lpc-37 on any of the four outcome measures in the general population (Table 2). In participants with LCS, the increase in perceived exhaustion was significantly lower in the Lpc-37 group compared to the placebo group (Fig. 2d; P = 0.037). There were no significant effects of Lpc-37 on perceived exhaustion in the other subgroups (Table S3). Furthermore, there were no significant effects of Lpc-37 on perceived -stress, -insecurity or -anxiety in any of the subgroups (Table S3). Results for the effects of Lpc-37 on the psychological response to the TSST in the ITT population are included in Tables S4 and S5.
3.5. Physiological biomarkers of stress – changes over intervention
3.5.1. Lpc-37 normalized 8pm cortisol levels in participants with LCS, and reduced diastolic BP in participants with HCS
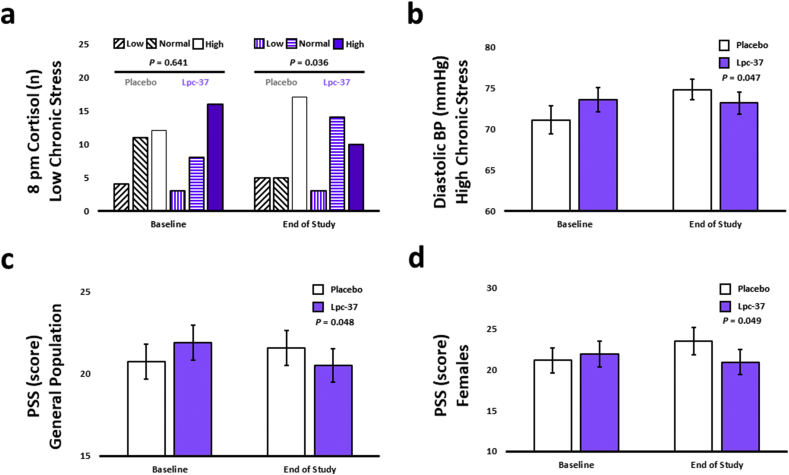
For all four variables; AUCg, AUCi, cortisol at awakening and cortisol at 8pm, there were no significant differences between the groups at baseline in the distribution of participants in different cortisol test value categories (low, normal, high) within the general population (Table 3) or in any of the subgroups (Table S6). For the variables AUCg, AUCi and cortisol at awakening, Lpc-37 had no significant impact on the distribution of participants in different cortisol test value categories at the end of study (Table 3). There was however an increase of 40.0%, and a decrease of 21.7% of participants in the normal test value category for cortisol at 8pm following intervention with Lpc-37 and placebo, respectively, at the end of study (Table 3; P = 0.082), highlighting a marginally favorable effect of Lpc-37 on 8pm cortisol levels. In addition, in participants with LCS, there was an increase of 75.0%, and a decrease of 54.5% of participants in the normal test value category for cortisol at 8pm following intervention with Lpc-37 and placebo, respectively, at the end of the study (Fig. 3a; P = 0.036) indicating a significant effect favoring the Lpc-37 group on 8pm cortisol levels. For the variable AUCg, in participants with HCS, there was an increase of 23.5% in the placebo group and a decrease of 26.3% in the Lpc-37 group of participants in the normal test value category at the end of the study (Table S6; P = 0.058). There were no differences between the groups at the end of study in the distribution of participants in different cortisol test value categories for the other subgroups for the variables AUCg and cortisol at 8pm; for any of the subgroups for the variables AUCi and cortisol at awakening (Table S6).
Table 3.
Number of participants by cortisol test value category at baseline and end of study for participants in the Per Protocol population.
| Baseline |
End of Study |
|||||||
|---|---|---|---|---|---|---|---|---|
| Low | Normal | High | P | Low | Normal | High | P | |
|
AUCg(n) | ||||||||
| Placebo (n = 55) | 12 | 30 | 13 | 0.270 | 7 | 35 | 13 | 0.442 |
| Lpc-37 (n = 53) |
6 |
36 |
11 |
11 |
28 |
14 |
||
|
AUCi(n) | ||||||||
| Placebo (n = 55) | 22 | 28 | 5 | 0.413 | 15 | 36 | 4 | 1.000 |
| Lpc-37 (n = 53) |
16 |
34 |
3 |
15 |
34 |
4 |
||
|
Cortisol at awakening (n) | ||||||||
| Placebo (n = 55) | 16 | 26 | 13 | 0.425 | 12 | 34 | 9 | 0.265 |
| Lpc-37 (n = 53) |
14 |
31 |
8 |
19 |
26 |
8 |
||
|
Cortisol at 8pm (n) | ||||||||
| Placebo (n = 55) | 6 | 23 | 26 | 0.718 | 7 | 18 | 30 | 0.082 |
| Lpc-37 (n = 53) | 4 | 20 | 29 | 3 | 28 | 22 | ||
Abbreviations: AUCg, Area Under the Curve with respect to ground; AUCi, Area Under the Curve with respect to increase; High, above 75% quantile; Low, under 25% quantile; n, number of participants; Normal, between 25% and 75% quantile.
Fig. 3.
Baseline and end of study related outcomes: a. 8pm cortisol in the low chronic stress subgroup (Low, under 25% quantile; Normal, between 25 and 75% quantiles; High, above 75% quantile). b. Diastolic BP in the high chronic stress subgroup (Mean ± SE). c. PSS in the general population (Mean ± SE). d. PSS in the female subgroup (Mean ± SE). Abbreviations: BP, Blood Pressure; PSS, Perceived Stress Scale; TSST, Trier Social Stress Test; VAS, Visual Analog Scale.
Results for the effects of Lpc-37 on the distribution of participants in different cortisol test value categories following intervention in the ITT population are included in Tables S7 and S8.
There were no significant effects of Lpc-37 on either systolic or diastolic BP following intervention (Table 4). In participants with HCS, diastolic BP increased significantly less in the Lpc-37 group from baseline to end of study compared to the placebo group (Fig. 3b; P = 0.047). There were no significant effects of Lpc-37 on systolic BP in any of the subgroups or on diastolic BP in participants with LCS, or male and female participants (Table S9). Results for the effects of Lpc-37 on systolic and diastolic BP following intervention in the ITT population are included in Tables S10 and S11.
Table 4.
Summary measures at baseline and end of study for participants in the Per Protocol population.
| Baseline |
End of Study |
P |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
|
Systolic BP (mmHg) | |||
| Placebo (n = 58) | 119.66 (13.82) | 122.86 (14.14) | 0.871missT |
| Lpc-37 (n = 55) |
119.60 (14.21) |
121.87 (14.28) |
|
|
Diastolic BP (mmHg) | |||
| Placebo (n = 58) | 71.68 (9.16) | 74.62 (6.39) | 0.327miss |
| Lpc-37 (n = 55) |
71.89 (7.74) |
73.18 (7.45) |
|
|
STAI-State (score) | |||
| Placebo (n = 58) | 34.33 (7.73) | 35.33 (8.37) | 0.715T |
| Lpc-37 (n = 55) |
33.65 (6.80) |
35.18 (8.38) |
|
|
PSS (score) | |||
| Placebo (n = 57) | 20.72 (7.97) | 21.56 (8.16) | 0.048 |
| Lpc-37 (n = 55) |
21.89 (7.90) |
20.49 (7.51) |
|
|
DASS Depression (score) | |||
| Placebo (n = 58) | 5.21 (6.38) | 5.10 (5.61) | 0.221T |
| Lpc-37 (n = 55) |
4.60 (4.94) |
4.15 (5.52) |
|
|
DASS Anxiety (score) | |||
| Placebo (n = 58) | 3.07 (4.58) | 3.45 (5.08) | 0.224V 0.933B 0.117EOS |
| Lpc-37 (n = 55) |
2.60 (3.35) |
2.44 (3.59) |
|
|
DASS Stress (score) | |||
| Placebo (n = 58) | 9.41 (7.87) | 10.09 (8.17) | 0.248T |
| Lpc-37 (n = 55) |
9.76 (7.92) |
8.91 (7.14) |
|
|
BAI (score) | |||
| Placebo (n = 58) | 5.85 (5.73) | 6.33 (7.26) | 0.099T |
| Lpc-37 (n = 55) |
5.51 (4.46) |
4.75 (4.39) |
|
|
VAS Stress (score) | |||
| Placebo (n = 58) | 19.34 (21.44) | 20.67 (21.63) | 0.436T |
| Lpc-37 (n = 55) |
19.11 (22.97) |
23.32 (23.18) |
|
|
VAS Insecurity (score) | |||
| Placebo (n = 58) | 15.91 (19.60) | 17.30 (20.15) | 0.355V 0.234B 0.344EOS |
| Lpc-37 (n = 55) |
13.58 (21.41) |
16.44 (19.67) |
|
|
VAS Anxiety (score) | |||
| Placebo (n = 58) | 7.58 (14.05) | 7.85 (13.40) | 0.204V 0.362B 0.584EOS |
| Lpc-37 (n = 55) |
7.29 (15.13) |
9.26 (16.48) |
|
|
VAS Exhaustion (score) | |||
| Placebo (n = 58) | 23.19 (21.08) | 18.45 (21.31) | 0.609T |
| Lpc-37 (n = 55) | 29.56 (27.63) | 24.66 (22.78) | |
Abbreviations: BAI, Beck Anxiety Inventory; BP, Blood Pressure; DASS, Depression Anxiety Stress Scale; n, number of participants; PSS, Perceived Stress Scale; SD, Standard Deviation; STAI; State-Trait Anxiety Inventory; VAS, Visual Analog Scale.
V Model assumptions for ANOVA were violated. Change score = baseline vs end of study.
miss Inferential statistics is not based on the same data set as descriptive statistics as records with missing data had to be excluded.
T Outcome was subjected to transformation to meet model assumptions.
B Model assumptions for ANOVA were violated. P value at baseline.
EOS Model assumptions for ANOVA were violated. P value at end of study.
3.6. Psychological markers of stress – changes over intervention
3.6.1. Lpc-37 reduced perceived stress in the general population and females
PSS scores increased in the placebo group (+0.84 points; +4.1%) and decreased in the Lpc-37 group (−1.40 points; −6.4%) from baseline to end of study in the general population indicating a significant effect of Lpc-37 toward reducing perceived stress compared to placebo (Fig. 3c; P = 0.048). In female participants, Lpc-37 significantly reduced perceived stress (−1.00 point; −4.6%) following intervention compared to placebo (+2.36 points; +11.2%; Fig. 3d; P = 0.049). There were no significant effects of Lpc-37 on perceived stress in the other subgroups (Table S9).
BAI scores increased in the placebo group (+0.48 points; +8.2%) and decreased in the Lpc-37 group (−0.76 points; −13.8%) from baseline to end of study, indicating a marginally favorable effect of Lpc-37 toward reducing anxiety compared to placebo (Table 4; P = 0.099). There were no significant effects of Lpc-37 on anxiety in any of the subgroups (Table S9).
There was no significant effect of Lpc-37 on DASS-depression, -anxiety and -stress scores, following intervention in either the general population (Table 4) or in any of the subgroups (Table S9).
There was no significant effect of Lpc-37 on VAS-stress -anxiety, -insecurity and -exhaustion in the general population (Table 4). Further, there were no significant effects of Lpc-37 on VAS-stress -anxiety and -exhaustion in any of the subgroups (Table S9) and no significant effects of Lpc-37 on VAS-insecurity in participants with LCS, HCS and female participants. In male participants, the difference for the change score was marginally significant with VAS-insecurity scores decreasing in the placebo group and increasing in the Lpc-37 group from baseline to end of study (Table S9; P = 0.063). This result became significant in the ITT population (Table S11; P = 0.031).
There was no significant effect of Lpc-37 on STAI-state anxiety in either the general population (Table 4), or in any of the subgroups (Table S9). Results for the effects of Lpc-37 on the psychological markers of stress following intervention in the ITT population are included in Tables S10 and S11.
3.7. Online diary measures of health and well-being. Lpc-37 increased perceived health and sleep-related recovery in participants with HCS
In the general population, Lpc-37 tended to increase perceived productivity scores compared to the placebo group throughout the study (Table 5; P = 0.054). Furthermore, Lpc-37 tended to increase perceived productivity in male participants (Table S12; P = 0.092). There were no significant effects of Lpc-37 on perceived productivity in the other subgroups (Table S12). In the ITT population, Lpc-37 significantly increased perceived productivity in participants with HCS compared to placebo (Table S14; P = 0.037).
Table 5.
Summary online diary measures for participants in the Per Protocol population.
| Week 1 run-in |
Week 2 run-in |
Week 3 treatment |
Week 4 treatment |
Week 5 treatment |
Week 6 treatment |
Week 7 treatment |
P | ||
|---|---|---|---|---|---|---|---|---|---|
|
Perceived Productivity (score) | |||||||||
| Placebo (n = 47) | Mean (SD) | 7.15 (1.07) | 7.29 (1.03) | 7.30 (1.01) | 7.34 (1.18) | 7.43 (1.17) | 7.31 (1.22) | 7.32 (1.25) | 0.054 |
| Lpc-37 (n = 44) |
Mean (SD) |
6.98 (1.02) |
7.34 (1.06) |
7.53 (0.97) |
7.48 (1.19) |
7.59 (1.04) |
7.57 (1.13) |
7.50 (1.17) |
|
|
Perceived Health Status (score) | |||||||||
| Placebo (n = 47) | Mean (SD) | 7.86 (1.08) | 7.92 (1.12) | 7.92 (1.06) | 8.01 (1.05) | 7.92 (1.16) | 7.73 (1.26) | 7.75 (1.52) | 0.093V |
| Lpc-37 (n = 44) |
Mean (SD) |
7.80 (1.31) |
7.89 (1.15) |
7.88 (1.20) |
7.91 (1.18) |
8.05 (1.22) |
8.11 (1.20) |
7.91 (1.15) |
|
|
Sleep Duration (min) | |||||||||
| Placebo (n = 47) | Mean (SD) | 447.45 (38.76) | 448.13 (41.62) | 456.90 (37.08) | 459.81 (39.44) | 457.26 (42.04) | 450.16 (42.04) | 459.66 (39.71) | 0.737 |
| Lpc-37 (n = 44) |
Mean (SD) |
447.27 (47.50) |
444.01 (44.60) |
449.45 (41.47) |
450.62 (36.07) |
454.50 (39.82) |
450.88 (38.95) |
445.60 (40.02) |
|
|
Sleep Disruptions (binary) | |||||||||
| Placebo (n = 47) | Proportion (yes/total) | 0.465 | 0.426 | 0.418 | 0.310 | 0.292 | 0.331 | 0.389 | 0.061 |
| Lpc-37 (n = 44) |
Proportion (yes/total) |
0.477 |
0.435 |
0.354 |
0.367 |
0.306 |
0.279 |
0.290 |
|
|
Sleep Disruptions (count) | |||||||||
| Placebo (n = 47) | Mean of week sum (SD) | 6.09 (4.96) | 5.49 (4.82) | 5.11 (4.89) | 4.30 (6.05) | 3.53 (3.80) | 4.02 (4.68) | 5.83 (6.23) | 0.084 |
| Lpc-37 (n = 44) |
Mean of week sum (SD) |
7.30 (6.87) |
5.50 (4.62) |
4.89 (5.11) |
5.43 (9.20) |
3.52 (3.48) |
3.80 (7.40) |
4.66 (6.37) |
|
|
Sleep Related Recovery (score) | |||||||||
| Placebo (n = 47) | Mean (SD) | 6.91 (1.00) | 7.15 (1.07) | 7.27 (1.12) | 7.29 (1.18) | 7.36 (1.19) | 7.10 (1.28) | 7.28 (1.18) | 0.232T |
| Lpc-37 (n = 44) |
Mean (SD) |
6.71 (1.34) |
7.07 (1.28) |
7.32 (1.11) |
7.30 (1.30) |
7.36 (1.22) |
7.42 (1.19) |
7.31 (1.25) |
|
|
Mood Ratings (score) | |||||||||
| Placebo (n = 47) | Mean (SD) | 7.27 (1.04) | 7.49 (1.10) | 7.46 (1.13) | 7.53 (1.15) | 7.50 (1.24) | 7.40 (1.21) | 7.55 (1.22) | 0.179T |
| Lpc-37 (n = 44) | Mean (SD) | 7.31 (1.25) | 7.53 (1.21) | 7.66 (1.05) | 7.77 (1.25) | 7.73 (1.17) | 7.90 (1.10) | 7.77 (1.30) | |
Abbreviations: n, number of participants; SD, Standard Deviation.
V Model assumptions for linear mixed models were violated. ANOVA on aggregated data.
T Outcome was subjected to transformation to meet model assumptions.
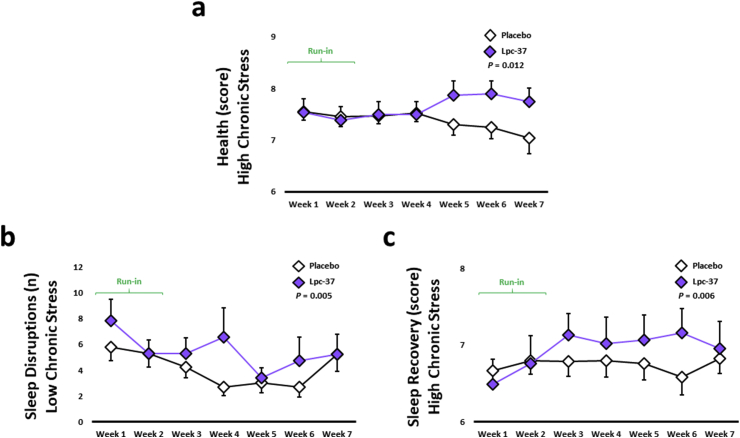
Perceived health scores tended to increase in the Lpc-37 group compared to the placebo group throughout the study (Table 5; P = 0.093). In participants with HCS, Lpc-37 significantly increased perceived health scores, compared to placebo throughout the study (Fig. 4a; P = 0.012). There were no significant effects of Lpc-37 on perceived health in the other subgroups (Table S12).
Fig. 4.
Online diary related outcomes: a. Perceived health in the high chronic stress subgroup (Mean ± SE). b. Number of sleep disruptions in the low chronic stress subgroup (Mean ± SE). c. Sleep related recovery in the high chronic stress subgroup (Mean ± SE).
Lpc-37 tended to reduce sleep disruptions (binary) throughout the study period, compared to placebo (Table 5; P = 0.061), but had no significant effect on sleep disruptions (count) (Table 5). In participants with LCS, there was a larger decrease observed in the placebo group compared with the Lpc-37 group for sleep disruptions (count), although both groups displayed the same sleep disruptions at the end of study (Fig. 4b; P = 0.005). There were no significant effects of Lpc-37 on sleep disruptions (binary) in any of the subgroups and on sleep disruptions (count) in participants with HCS, or male and female participants (Table S12). For sleep duration and sleep-related recovery, the interaction between treatment group and time was not significant throughout the study in the general population (Table 5). In participants with HCS, Lpc-37 significantly increased sleep-related recovery scores compared to placebo (Fig. 4c; P = 0.006). There were no significant effects of Lpc-37 on sleep-related recovery scores in the other subgroups and on sleep duration in any of the subgroups (Table S12).
There was no significant effect of Lpc-37 on mood ratings throughout the study in either the general population (Table 5), or in any of the subgroups (Table S12).
Results for the effects of Lpc-37 on the online diary measures of health and well-being in the ITT population are included in Tables S13 and S14.
3.8. Safety parameters
Concerning the safety objectives of this study, no significant differences were observed in either systolic or diastolic BP, HR, weight, and BMI between randomized participants in the study groups at V3. Causality of all AEs reported by the participants were rated as “unlikely” or “not related” by the PI and the study physicians for both groups. Moreover, the maximum severity of these events was “moderate”. Thus, no SAEs were recorded in this study for either group. Only two AEs were lost to follow-up, but all other AEs were resolved, and no action was necessary (i.e. study interruption or withdrawal). In total, 111 AEs were reported in the placebo group from 74 participants (Table S15) and 100 AEs were reported in the Lpc-37 group from 71 participants (Table S16) over the duration of the study. There were no significant differences in the frequencies of the most frequently occurring AEs; common cold, sore throat, headache or stomach ache between the groups. The number of participants was too small for all other AEs to estimate statistical differences between the groups. The distribution of AEs was similar between the groups.
4. Discussion
Exposure to stress can impact the gut microbial profile and in turn, experimental alteration of the gut microbiota can influence the stress response (Foster et al., 2017). Manipulation of the gut microbiota through probiotic intervention is therefore a novel approach to influence stress, mood and well-being. Previously, Lpc-37 prevented stress-associated behaviors and an anxious phenotype from developing in mice from two experiments using the same chronic stress model (Stenman et al., 2020). The results of this clinical trial point to different directions with respect to efficacy of Lpc-37 on physiological and psychological outcomes when analyzed over the study and in response to an acute stressor (summarized in Table 6 for the PP and Table S17 for the ITT).
Table 6.
Summary of effects of Lpc-37 for participants in the Per Protocol population and subgroups.
| All data | Low chronic stress | High chronic stress | Male | Female | |
|---|---|---|---|---|---|
| TSST-related endpoints† | |||||
| Heart Rate (bpm) | ↓ | ↑ | |||
| Systolic BP (mmHg) | ↓ | ||||
| VAS Exhaustion (score) | ↓ | ||||
| Baseline and End of Study‡ | |||||
| Cortisol Normalization AUCg | (↓)EOS | ||||
| Cortisol Normalization at 8pm | (↑)EOS | ↑ EOS | |||
| Diastolic BP (mmHg) | ↓ | ||||
| PSS (score) | ↓ | ↓ | |||
| BAI (score) | (↓) | ||||
| DASS-anxiety (score) | (↓)EOS* | ||||
| VAS Insecurity (score) | (↑)V | ||||
| VAS Anxiety (score) | ↑V,B | ||||
| Online diary measures∅ | |||||
| Perceived Productivity (score) | (↑) | (↑) | |||
| Perceived Health Status (score) | (↑) | ↑ | |||
| Sleep Disruptions (binary) | (↓) | (↑)* | |||
| Sleep Disruptions (count) | (↑)* | ↑ | (↑)* | ||
| Sleep Related Recovery (score) | ↑ | ||||
The data in Table 6 report p-values according to the following criteria: a) significant p-values (P < 0.05) and b) marginal/trend p-values (P < 0.1). All p-values in Table 6 describe effects for the Lpc-37 group based on the interaction between treatment group and time. Abbreviations: AUCg, Area Under the Curve with respect to ground; BAI, Beck Anxiety Inventory; BP, Blood Pressure; DASS, Depression, Anxiety, Stress Scale; PSS, Perceived Stress Scale; TSST, Trier Social Stress Test; VAS, Visual Analog Scale.
B Baseline.
EOS End of study.
V Change score = baseline vs end of study.
* Due to the patterns presented by the groups, the effect cannot be confirmed.
↑ Increase for the Lpc-37 group as compared to the placebo group.
↓ Decrease for the Lpc-37 group as compared to the placebo group.
† Based on the mean difference over the duration of the TSST.
‡ Based on the mean difference from baseline to end of study.
∅ Based on the mean difference over the entire study period.
↑,↓ P < 0.05.
(↑,↓) P < 0.1.
The primary objective of this study was selected based on previous studies which demonstrated that the TSST elicits a significant increase in HR (Kirschbaum et al., 1993; Hellhammer and Schubert, 2012; Hellhammer et al., 2014). While HR was expected to increase in response to the TSST, chronic psychosocial factors have been shown to affect cardiovascular reactivity to acute stress, with some studies demonstrating an association between HCS and blunted cardiovascular reactivity (Fries et al., 2005; Teixeira et al., 2015). Such conditions have also been associated with a host of negative behavioral outcomes (Carroll et al., 2017). For this reason, the study population was stratified to investigate the impact of chronic stress on HR as a biomarker of the ANS response to acute stress. In the general population, Lpc-37 had no effect on HR in response to the TSST, however significant effects were observed within the subgroups. While Lpc-37 reduced the increase in HR in response to acute stress in participants with LCS, the opposite was seen in participants with HCS. The exact mechanisms for these effects are unknown but could suggest that the effect of Lpc-37 on HR may be differentially dependent on chronic stress. Although cardiovascular reactivity was not blunted per se in the HCS population, the effect of Lpc-37 could be more pronounced in a clinically stressed population. The ANS is just one component of the microbiota-gut-brain axis and perhaps there is some mechanism mediated through the gut and influenced through probiotic intervention which beneficially influences the response to acute stress differently, dependent on underlying stress. This hypothesis based on the results described herein is purely exploratory and should be investigated in future studies. To our knowledge this is the first time a probiotic has demonstrated different effects on HR under different conditions of chronic stress.
Lpc-37 also significantly reduced perceived exhaustion/fatigue in response to the TSST in participants with LCS. This psychological response could indeed be associated with the reduced HR in response to the acute stress also seen within this subgroup. Furthermore, Lpc-37 significantly decreased both diastolic and systolic BP in participants with HCS and females, respectively. It has previously been shown in mildly hypertensive patients that consumption of a fermented milk drink containing Lacticaseibacillus casei strain Shirota (LcS) and gamma-aminobutyric acid (GABA) decreased both systolic and diastolic BP after four weeks and up to twelve weeks of intervention (Inoue et al., 2003). Furthermore, the incidence of hypertension among community-living normotensive elderly participants consuming the same fermented milk drink containing LcS (without GABA), three times or more per week over a five-year interval was lower than those consuming the drink less than three times per week (Aoyagi et al., 2017). The effects of Lpc-37 on biomarkers of the ANS response to stress indicate one pathway through which microbiota-gut-brain signaling could be influenced by Lpc-37.
The secondary objectives of this study included a range of outcomes to measure the stress response following intervention with Lpc-37. The PSS is a global measure of subjective stress, not restricted to any one specific life event or clinical condition and is suitable for use across diverse populations and settings. Other probiotic interventions have proven relatively unsuccessful in reducing self-reported perceived stress using this scale (Messaoudi et al., 2011a; Chung et al., 2014; Ostlund-Lagerstrom et al., 2016; Siegel and Conklin, 2020). While post-hoc analyses from Messaoudi and colleagues demonstrated a within-group effect of the probiotic formulation, the result was expressed as a percentage of change in PSS score from baseline to follow-up and was only found in a subset of participants with low levels of 24-h urinary-free cortisol at baseline (Messaoudi et al., 2011b). In a repeated measures design, Allen and colleagues demonstrated that Bifidobacterium longum 1714 reduced PSS score compared between groups using AUC measurements (Allen et al., 2016). Participants in the Sisu study reported significantly lower PSS scores following intervention with Lpc-37 and this result was reflected in absolute scores from baseline to end of study. Perceived stress was also reduced in females taking Lpc-37. Interestingly, covariate analyses revealed that females had higher stress (DASS-stress), sleep disruptions (binary), and lower sleep-related recovery scores compared to males, indicating that sex is an underlying factor influencing the stress response. Thus, female participants in this study could be considered more stressed than males.
The custom-designed online diary proved a successful tool for gathering exploratory data throughout the study. The diary results have alluded to some mechanistic insights into the significant effect of Lpc-37 on perceived stress. Participants in the general population and males consuming Lpc-37 had a marginally significant increase in productivity. In participants with HCS within the ITT population, those consuming Lpc-37 had a significant increase in productivity. These results indicate Lpc-37 could increase feelings of productivity. Probiotic interventions have proven to support various aspects of work-place healthiness (Tubelius et al., 2005), a healthy immune system (Turner et al., 2017; Weizman et al., 2005), and prevent the onset of symptoms in participants exposed to stress (Sawada et al., 2017; Culpepper et al., 2016; Kato-Kataoka et al., 2016). Interestingly, while these studies suggest a link between probiotics and productivity, none have measured the individual perception of such. The association between productivity and chronic stress is of major relevance as workplace stress and burn-out are increasingly prevalent (Street and Lacey, 2019). Perhaps while reducing perceived stress, Lpc-37 might be beneficial in targeting stress-associated dips in productivity. In addition, Lpc-37 marginally increased perceived health throughout the study, becoming significant in participants with HCS. These results suggest some potential pathways through which Lpc-37 may influence symptoms of stress, be it through increasing perceived productivity or health, or vice versa.
The TSST successfully induced an endocrine stress response in both the HPA axis (cortisol) and sympatho-adreno-medullary system (AA), however there was no effect of Lpc-37 on either system's acute stress response. Lpc-37 marginally normalized the 8pm cortisol levels, i.e. more participants in the normal-test value category in the Lpc-37 group at the end of study. This trend became significant in participants with LCS and is worth exploring in future studies. Vreeberg and colleagues previously reported that depressed participants in a large community-based study had higher evening cortisol levels when compared to non-depressed participants (Vreeburg et al., 2009). Therefore, there is some indication that evening cortisol directly correlates with stress-associated disorders. Some studies have found an impact of probiotics on the cortisol response in stressed participants (Chong et al., 2019), in particular in the response to exam stress (Sawada et al., 2017; Kato-Kataoka et al., 2016; Andersson et al., 2016; Takada et al., 2016). Manipulation of the gut microbiota can therefore alter the neuroendocrine stress response through the HPA axis.
The gut microbiome has been implicated in sleep disturbances (Benedict et al., 2016), and some studies support the role of probiotics in improving sleep patterns in humans (Marotta et al., 2019; Takada et al., 2016; Yamamura et al., 2009). While participants taking Lpc-37 tended to have less reported sleep disruptions (binary), those with LCS had significantly higher self-reported sleep disruptions (count) throughout the intervention. Lpc-37 increased sleep-related recovery – or – how rested participants with HCS felt after a night sleep. In a recent meta-analysis, probiotics had a significant effect on the Pittsburgh Sleep Quality Index-total score but had no significant effect on other subjective sleep scales or objective parameters of sleep (Irwin et al., 2020). Therefore, the effects of Lpc-37 on sleep observed in this study should be considered exploratory, and future study designs with Lpc-37 to explore the effect of this strain on sleep should include more comprehensive measures of sleep quality and efficiency. Indeed, stress is closely linked with sleep disruption which plays a central role in mediating psychiatric disorders (Simon et al., 2020).
4.1. Limitations
Although considered the gold standard in clinical experimental stress research, perhaps the most obvious limitation of the TSST (Allen et al., 2017), was its single administration and lack of comparative baseline data. The TICS was used to stratify the population according to chronic stress over the past three months and while this inventory has delivered helpful results in previous TSST studies (Hellhammer et al., 2010, 2012, 2014; Schult et al., 2010), it may not differentiate enough to fully depict the large variety of chronic stress as a predecessor for physical and mental health problems. Finally, while Lpc-37 did have a beneficial impact on many endpoints in this study, the mechanisms are largely unknown and will be explored in future studies.
5. Conclusion and future perspectives
The intake of Lpc-37 for five weeks significantly reduced perceived stress. In addition, Lpc-37 tended to improve many other biomarkers related to stress in the general population and other significant beneficial effects were identified within the subgroups. Concerning safety, there were no SAEs and only mild to moderate AEs were recorded throughout the study, with no significant differences between the groups. The occurrence of AEs was therefore not connected to any study group. Vital signs remained unaffected at the end of the study. Thus, the findings from this study do not raise any concerns over the safety of Lpc-37. In the sample studied, the mean scores for the screening scale of the TICS were still in a relatively normal range, even for the HCS subgroups. Therefore, one could speculate that the reported effects of Lpc-37 in participants with HCS would be enhanced in participants under more pronounced chronic stress. Considering the unexpected findings that Lpc-37 decreased HR in response to the TSST in participants with LCS, but increased the same biomarker for the ANS response to stress in participants with HCS, future probiotic intervention studies should include elaborated psychobiological diagnostics for chronic stress and could combine the TSST innovative methods assessing the psychological and physiological response to an acute stressor. Such an approach would decipher whether the effects of probiotics are somewhat dependent on daily/chronic stress. Finally, Lpc-37 maintained stability and did not fall below the target dose throughout the study, thereby there are no stability concerns for Lpc-37.
CRediT authorship contribution statement
Elaine Patterson: Conceptualization, Methodology, Writing - original draft, Visualization, Project administration. Síle M. Griffin: Writing - original draft, Visualization, Project administration. Alvin Ibarra: Conceptualization, Methodology, Writing - original draft, Visualization. Emilia Ellsiepen: Methodology, Software, Formal analysis, Data curation, Writing - original draft. Juliane Hellhammer: Conceptualization, Methodology, Resources, Writing - original draft, Supervision.
Declaration of competing interest
Lacticaseibacillus paracasei Lpc-37 is a commercial product of DuPont Nutrition & Biosciences. Lpc-37® is a registered trademark or trademark of DuPont de Nemours, Inc. or its affiliated companies. Elaine Patterson, Alvin Ibarra and Síle M. Griffin are employees of DuPont Nutrition & Biosciences, the manufacturer of Lacticaseibacillus paracasei Lpc-37 and were employed by DuPont Nutrition & Biosciences during the study. Juliane Hellhammer and Emilia Ellsiepen are employees of daacro GmbH and Co. KG, a company that was hired by DuPont Nutrition & Biosciences to conduct the study.
Acknowledgments
We wish to acknowledge Rita Stiemke from DuPont Nutrition & Biosciences for the production and quality control of the IPs. Special thanks to Nadin Meyer and Simone Kugel for study management, Nicole Weber for protocol writing, Dominik Seithel for data management, Christof Zintel for programing the online diary and Helma Veerman for her relentless telephone screening. We wish to acknowledge Monica Mota for monitoring the study and ensuring quality was maintained throughout. Very special thanks to Johanna Maukonen and Arthur Ouwehand for their support and comments on the manuscript. Finally, we wish to extend our gratitude to all the Sisu study participants who made this study possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100277.
Funding sources
This study was fully sponsored by DuPont Nutrition & Biosciences, Danisco Sweeteners Oy.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aitken R.C. Measurement of feelings using visual analogue scales. Proc. Roy. Soc. Med. 1969;62(10):989–993. [PMC free article] [PubMed] [Google Scholar]
- Allen A.P., Hutch W., Borre Y.E. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry. 2016;6(11):e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.P., Kennedy P.J., Dockray S., Cryan J.F., Dinan T.G., Clarke G. The trier social stress test: principles and practice. Neurobiol Stress. 2017;6:113–126. doi: 10.1016/j.ynstr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association . American Psychological Association; 2018. Stress in America: Generation Z. Stress in AmericaTM Survey. [Google Scholar]
- Andersson H., Tullberg C., Ahrne S. Oral administration of Lactobacillus plantarum 299v reduces cortisol levels in human saliva during examination induced stress: a randomized, double-blind controlled trial. Internet J. Microbiol. 2016;2016:8469018. doi: 10.1155/2016/8469018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi Y., Park S., Matsubara S. Habitual intake of fermented milk products containing Lactobacillus casei strain Shirota and a reduced risk of hypertension in older people. Benef. Microbes. 2017;8(1):23–29. doi: 10.3920/BM2016.0135. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56(6):893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Benedict C., Vogel H., Jonas W. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab. 2016;5(12):1175–1186. doi: 10.1016/j.molmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D., Williams C., Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007;61(3):355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- Bond A., Lader M. The use of analogue scales in rating subjective feelings. Br. J. Med. Psychol. 1974;47(3):211–218. [Google Scholar]
- Carroll D., Ginty A.T., Whittaker A.C., Lovallo W.R., de Rooij S.R. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci. Biobehav. Rev. 2017;77:74–86. doi: 10.1016/j.neubiorev.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahwan B., Kwan S., Isik A., van Hemert S., Burke C., Roberts L. Gut feelings: a randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019;253:317–326. doi: 10.1016/j.jad.2019.04.097. [DOI] [PubMed] [Google Scholar]
- Chida Y., Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol. Bull. 2008;134(6):829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chong H.X., Yusoff N.A.A., Hor Y.Y. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef. Microbes. 2019;10(4):355–373. doi: 10.3920/BM2018.0135. [DOI] [PubMed] [Google Scholar]
- Chrousos G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Chung Y.-C., Jin H.-M., Cui Y. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. Journal of functional foods. 2014;10:465–474. [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cryan J.F., O'Riordan K.J., Cowan C.S.M. The microbiota-gut-brain Axis. Physiol. Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Culpepper T., Christman M.C., Nieves C., Jr. Bifidobacterium bifidum R0071 decreases stress-associated diarrhoea-related symptoms and self-reported stress: a secondary analysis of a randomised trial. Benef. Microbes. 2016;7(3):327–336. doi: 10.3920/BM2015.0156. [DOI] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- El Aidy S., Dinan T.G., Cryan J.F. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 2014;5:146. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.A., Lyte M., Meyer E., Cryan J.F. Gut microbiota and brain function: an evolving field in neuroscience. Int. J. Neuropsychopharmacol. 2016;19(5) doi: 10.1093/ijnp/pyv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Hesse J., Hellhammer J., Hellhammer D.H. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Hellhammer J., Schubert M. The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology. 2012;37(1):119–124. doi: 10.1016/j.psyneuen.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hellhammer J., Waladkhani A.R., Hero T., Buss C. Effects of milk phospholipid on memory and psychological stress response. Br. Food J. 2010 [Google Scholar]
- Hellhammer J., Hero T., Franz N., Contreras C., Schubert M. Omega-3 fatty acids administered in phosphatidylserine improved certain aspects of high chronic stress in men. Nutr. Res. 2012;32(4):241–250. doi: 10.1016/j.nutres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Hellhammer J., Vogt D., Franz N., Freitas U., Rutenberg D. A soy-based phosphatidylserine/phosphatidic acid complex (PAS) normalizes the stress reactivity of hypothalamus-pituitary-adrenal-axis in chronically stressed male subjects: a randomized, placebo-controlled study. Lipids Health Dis. 2014;13:121. doi: 10.1186/1476-511X-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH Expert Working Group . ICH; Geneva: 1996. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6 (R1) 1996. [Google Scholar]
- Inoue K., Shirai T., Ochiai H. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003;57(3):490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- Irwin C., McCartney D., Desbrow B., Khalesi S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2020 doi: 10.1038/s41430-020-0656-x. [DOI] [PubMed] [Google Scholar]
- Kato-Kataoka A., Nishida K., Takada M. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes. 2016;7(2):153–156. doi: 10.3920/BM2015.0100. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Wust S. Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13(1):1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Wust S., Kirschbaum C., Hellhammer D.H. Trier social stress test. Encyclopedia Stress. 2007;3:776–781. [Google Scholar]
- Kudielka B.M., Hellhammer D.H., Kirschbaum C. Ten years of research with the trier social stress test (TSST)–revisited. In: Harmon-Jones E., Winkielman P., editors. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. Guilford Press; New York: 2007. pp. 56–83. [Google Scholar]
- Liu R.T. The microbiome as a novel paradigm in studying stress and mental health. Am. Psychol. 2017;72(7):655–667. doi: 10.1037/amp0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond P.F. Long-term stability of depression, anxiety, and stress syndromes. J. Abnorm. Psychol. 1998;107(3):520–526. doi: 10.1037//0021-843x.107.3.520. [DOI] [PubMed] [Google Scholar]
- Lovibond S.H., Lovibond P.F. Psychology Foundation of Australia; 1996. Manual for the Depression Anxiety Stress Scales. [Google Scholar]
- Marotta A., Sarno E., Del Casale A. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front. Psychiatr. 2019;10:164. doi: 10.3389/fpsyt.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Messaoudi M., Lalonde R., Violle N. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Messaoudi M., Violle N., Bisson J.F., Desor D., Javelot H., Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microb. 2011;2(4):256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- Ostlund-Lagerstrom L., Kihlgren A., Repsilber D., Bjorksten B., Brummer R.J., Schoultz I. Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutr. J. 2016;15(1):80. doi: 10.1186/s12937-016-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalini S., Michels F., Kohn N. Stress matters: randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol Stress. 2019;10:100141. doi: 10.1016/j.ynstr.2018.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Sanchez M.I., Hall G.B., Ghajar K. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459 e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rea K., Dinan T.G., Cryan J.F. Gut microbiota: a perspective for psychiatrists. Neuropsychobiology. 2020;79(1):50–62. doi: 10.1159/000504495. [DOI] [PubMed] [Google Scholar]
- Sawada D., Kawai T., Nishida K., Kuwano Y., Fujiwara S., Rokutan K. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. Journal of Functional Foods. 2017;31:188–197. [Google Scholar]
- Schult J., Hero T., Hellhammer J. Effects of powdered fertilized eggs on the stress response. Clin. Nutr. 2010;29(2):255–260. doi: 10.1016/j.clnu.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Schulz P., Schlotz W. Trierer Inventar zur Erfassung von chronischem Sre (TICS): skalenkonstruktion, teststatistische Überprüfung und Validierung der Skala Arbeitsüberlastung. Diagnostica. 1999 [Google Scholar]
- Schulz P., Schlotz W., Becker P. TICS; Hogrefe: 2004. Trierer Inventar Zum Chronischen Stress. [Google Scholar]
- Siegel M.P., Conklin S.M. Acute intake of B. longum probiotic does not reduce stress, anxiety, or depression in young adults: a pilot study. Brain, Behavior, & Immunity-Health. 2020:100029. doi: 10.1016/j.bbih.2019.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E.B., Rossi A., Harvey A.G., Walker M.P. Overanxious and underslept. Nat Hum Behav. 2020;4(1):100–110. doi: 10.1038/s41562-019-0754-8. [DOI] [PubMed] [Google Scholar]
- Slykerman R.F., Hood F., Wickens K. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017;24:159–165. doi: 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L. Consulting Psychologists Press; 1983. State-trait Anxiety Inventory for Adults: Manual and Sample: Manual, Instrument and Scoring Guide. [Google Scholar]
- Stenman L.K., Patterson E., Meunier J., Roman F.J., Lehtinen M.J. Strain specific stress-modulating effects of candidate probiotics: a systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020;379:112376. doi: 10.1016/j.bbr.2019.112376. [DOI] [PubMed] [Google Scholar]
- Street T.D., Lacey S.J. Accounting for employee health: the productivity cost of leading health risks. Health Promot. J. Aust. 2019;30(2):228–237. doi: 10.1002/hpja.200. [DOI] [PubMed] [Google Scholar]
- Takada M., Nishida K., Kataoka-Kato A. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neuro Gastroenterol. Motil. 2016;28(7):1027–1036. doi: 10.1111/nmo.12804. [DOI] [PubMed] [Google Scholar]
- Teixeira R.R., Diaz M.M., Santos T.V. Chronic stress induces a hyporeactivity of the autonomic nervous system in response to acute mental stressor and impairs cognitive performance in business executives. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0119025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubelius P., Stan V., Zachrisson A. Increasing work-place healthiness with the probiotic Lactobacillus reuteri: a randomised, double-blind placebo-controlled study. Environ. Health. 2005;4:25. doi: 10.1186/1476-069X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.B., Woodfolk J.A., Borish L. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection - a randomised controlled trial. Benef. Microbes. 2017;8(2):207–215. doi: 10.3920/BM2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg S.A., Hoogendijk W.J., van Pelt J. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch. Gen. Psychiatr. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Weizman Z., Asli G., Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115(1):5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Yamamura S., Morishima H., Kumano-go T. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur. J. Clin. Nutr. 2009;63(1):100–105. doi: 10.1038/sj.ejcn.1602898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.