Highlights
-
•
Composite scores provide reliable metrics of domain function in multicenter cohort.
-
•
Visuo-spatial domain composite scores relate to anatomic changes in AD spectrum.
-
•
Domain scores relate to network-specific resting-state connectivity in AD spectrum.
Keywords: Visuo-spatial cognitive deficits, Resting-state functional connectivity, Alzheimer’s disease spectrum, Cognitive domain score, Cortical atrophy, Multicenter cohort
Abstract
Background
Cognitive decline has been found to be associated with gray matter atrophy and disruption of functional neural networks in Alzheimer’s disease (AD) in structural and functional imaging (fMRI) studies. Most previous studies have used single test scores of cognitive performance among monocentric cohorts. However, cognitive domain composite scores could be more reliable than single test scores due to the reduction of measurement error. Adopting a multicentric resting state fMRI (rs-fMRI) and cognitive domain approach, we provide a comprehensive description of the structural and functional correlates of the key cognitive domains of AD.
Method
We analyzed MRI, rs-fMRI and cognitive domain score data of 490 participants from an interim baseline release of the multicenter DELCODE study cohort, including 54 people with AD, 86 with Mild Cognitive Impairment (MCI), 175 with Subjective Cognitive Decline (SCD), and 175 Healthy Controls (HC) in the AD-spectrum. Resulting cognitive domain composite scores (executive, visuo-spatial, memory, working memory and language) from the DELCODE neuropsychological battery (DELCODE-NP), were previously derived using confirmatory factor analysis. Statistical analyses examined the differences between diagnostic groups, and the association of composite scores with regional atrophy and network-specific functional connectivity among the patient subgroup of SCD, MCI and AD.
Result
Cognitive performance, atrophy patterns and functional connectivity significantly differed between diagnostic groups in the AD-spectrum. Regional gray matter atrophy was positively associated with visuospatial and other cognitive impairments among the patient subgroup in the AD-spectrum. Except for the visual network, patterns of network-specific resting-state functional connectivity were positively associated with distinct cognitive impairments among the patient subgroup in the AD-spectrum.
Conclusion
Consistent associations between cognitive domain scores and both regional atrophy and network-specific functional connectivity (except for the visual network), support the utility of a multicentric and cognitive domain approach towards explicating the relationship between imaging markers and cognition in the AD-spectrum.
1. Introduction
Cognitive decline in Alzheimer’s disease (AD) has been associated with regional brain metabolic decline in fluorodeoxyglucose-positron emission tomography (FDG-PET) studies (Landau et al., 2011, Grothe et al., 2016, Ottoy et al., 2019), as well as structural and functional disruption of neural networks in structural and functional imaging studies (Agosta et al., 2012, Balachandar et al., 2015, Balachandar et al., 2017, Gardini et al., 2015). Blood-oxygen-level-dependent (BOLD) imaging during a defined resting state has been shown to reflect consistent functional networks, such as the default mode (DMN), visual (VIS) and executive networks (EN) (Rosazza and Minati, 2011). Different to task related functional MRI (fMRI), resting state fMRI is not confounded by the ability of patients to understand and memorize the instructions for fulfilling a specific task, rendering it advantageous for the study of people with cognitive decline (Cole et al., 2010). Additionally, conclusive evidence across the literature supports the use of resting-state connectivity as a biomarker of AD (Badhwar et al., 2017).
Resting-state fMRI studies in the AD-spectrum have progressively explored a large number of networks, including the DMN, EN, VIS, salience (SAL), language (LAN) and limbic (LIM) networks (Agosta et al., 2012, Balachandar et al., 2017, Zhou et al., 2010, Gour et al., 2014, Badhwar et al., 2017). A majority of studies which focused on the DMN found consistently decline of DMN connectivity in MCI and AD patients (Greicius et al., 2004, Agosta et al., 2012, Balachandar et al., 2015, Tam et al., 2015, Zhou et al., 2015). The same was observed for the VIS (Balachandar et al., 2015, Balachandar et al., 2017) in which overall a decrease in connectivity has been reported for AD patients. Studies on the SAL, on the other hand, reported increase in connectivity of the SAL for MCI and AD patients (Zhou et al., 2010, Wang et al., 2016). Similarly, an increase in functional connectivity of the LIM has also been reported for MCI and AD patients (Gour et al., 2014, Badhwar et al., 2017). For the EN, an increase in functional connectivity of the EN has mainly been observed in AD patients (Agosta et al., 2012, Balachandar et al., 2015). However, to the best of our knowledge, no study has so far reported any significant differences between HC and AD-spectrum patients for the connectivity of the LAN.
So far, most rs-fMRI studies linking pattern of cognitive decline with changes of functional networks have been conducted in monocentric cohorts, with observed case numbers substantially smaller than obtainable in a multicentric cohort. These studies have reported links between changes in functional connectivity and performance on cognitive tests of either global cognition (Ranasinghe et al., 2014, Zhou et al., 2015), or specific cognitive functions such as executive (Ranasinghe et al., 2014), memory (Dong et al., 2012, Ranasinghe et al., 2014, Balachandar et al., 2015, Gardini et al., 2015, Zhou et al., 2015, Brueggen et al., 2016) and visuo-spatial functions (Ranasinghe et al., 2014, Balachandar et al., 2017). More specifically, decreased connectivity in the VIS has majorly been associated with visuo-spatial deficits (Balachandar et al., 2017). In the study by Balachandar et al. (2017) involving 23 AD patients categorized as having mild or severe visuo-spatial deficits based on their performance on selected tests of visuo-spatial function, patients with severe visuo-spatial deficits showed more reduced connectivity in the bilateral lingual gyri and left supracalcarine gyrus areas of the VIS. The authors, however, noted that a larger sample size would be required to confirm their findings. Further notable networks in which decreased rsFC has been associated with deficits in the respective cognitive domains in monocentric cohorts include the EN (Agosta et al., 2012, Ranasinghe et al., 2014) and DMN (Greicius et al., 2004, Dong et al., 2012). Additionally, a number of studies have also probed the possible associations between regional structural atrophy and cognitive deficits in both healthy elderly cohorts (Chee et al., 2009, Bruno et al., 2016, Cacciaglia et al., 2018) and AD-spectrum patients (Di Paola et al., 2007, Mitolo et al., 2013, Ranasinghe et al., 2014, Smits et al., 2014, Cacciaglia et al., 2018), using varying measures of cognition. Associations of course, do not only depend on the imaging methods employed but also on the way cognitive performance is being quantified.
Cognitive domain composite scores may allow to more comprehensively study structural and functional underpinnings of cognitive changes in AD compared to single test scores. They may be more reliable than single test scores due to the reduction of measurement error (Wolfsgruber et al., 2017) at the same time they allow restricting the number of comparisons and ensuing type 1 error (Clark et al., 2016). Three prominent approaches to obtaining cognitive composite scores include: (1) using tests of composite scores such as the Preclinical Alzheimer Cognitive Composite (PACC) (Donohue et al., 2014, Mormino et al., 2017) in neuropsychological assessment. As reported in the study by Mormino et al. (2017), results showed the ability of the PACC to capture both early and late cognitive decline during the preclinical stages of Alzheimer’s disease. However, certain domains such as visuo-spatial function do not appear to be adequately covered by this composite score; (2) transforming raw scores of single tests to z-scores using the means and standard deviations (SDs), and further averaging the z-scores across single tests (Smits et al., 2014, Clark et al., 2016). This method, however beneficial, does not take into account the degree to which the different tests are similar in measuring the same construct; (3) combining single tests (indicators) into latent variables based on literature, and further obtaining confirmatory factor score estimates of these latent variables using the multivariate regression method (Grice, 2001, Dowling et al., 2010, Park et al., 2012, Wolfsgruber et al., 2017). This superior method of choice as already applied in the Wisconsin Registry for Alzheimer's Prevention (WRAP), Alzheimer’s Disease Neuroimaging Initiative (ADNI), and DZNE – Longitudinal Cognitive Impairment and Dementia (DELCODE) studies, especially has the advantage of taking close methodological relatedness of some indicators into account, by specifying residual correlations for these indicators and thereby avoiding overfitting the data.
In the current study, we provided a comprehensive description of the structural and functional correlates of the key cognitive domains of AD as determined by factor scores with a focus on visuo-spatial function. Patients with AD are known to be prone to experiencing impairment in visuo-spatial function during early stages of the disease (Quental et al., 2013). Impairments in visuo-spatial function in AD have mainly been attributed to posterior cortical atrophy (PCA) (Benson et al., 1988, Crutch et al., 2012), that affects the parieto-occipital areas which are equally relevant regions within the visual network (Beckmann et al., 2005, Castellazzi et al., 2014). These impairments in visuo-spatial function are not only precursors of MCI conversion to AD as has been indicated in (Didic et al., 2013) where performance on visual recognition predicted conversion to AD with a sensitivity of 80% and a specificity of 90.9%, but also relate to spatial disorientation (Henderson et al., 1989, Tetewsky and Duffy, 1999, Monacelli et al., 2003) which greatly impairs the daily life of patients. Nonetheless, there appears to be a relative lack of sufficient research into the visuo-spatial domain in the AD spectrum, in comparison to more broadly studied domains such as memory and executive functions (Park et al., 2012, Wang et al., 2015).
Using a multicentric and cognitive domain approach, our aim was to investigate associations between cognitive domain composite scores and both gray matter volume, and network-specific resting-state functional connectivity (rsFC), in patients in the AD-spectrum, ranging from memory clinic patients with subjective cognitive decline (SCD) through people with MCI to people with AD dementia, as well as in healthy controls. We tested two major hypotheses; firstly, that poorer cognitive domain composite scores would be associated with reduced regional gray matter volume, and secondly, that poorer cognitive domain composite scores would be associated with reduced rsFC of the related resting-state functional network.
2. Material and methods
2.1. Participants
We used data from an interim baseline release of the first n = 700 participants of the multicenter DELCODE study, conducted by the German Center for Neurodegenerative Diseases (DZNE) (Jessen et al., 2018). After proper quality control at the leading imaging site, we obtained data of 569 participants. However, only 490 participants; 54 Alzheimer’s disease Dementia (AD), 86 Mild Cognitive Impairment (MCI), 175 Subjective Cognitive Decline (SCD) and 175 Healthy Controls (HC) were included in this study, which had both structural MRI, rs-fMRI and factor scores from the nine study centers (Jessen et al., 2018). The patient group consisted of the AD, MCI and SCD subgroup (Table 1). The DELCODE exclusion criteria ensured that no persons were included who had a current major depressive episode, past or present major psychiatric disorders, neurological diseases other than AD, or unstable medical conditions (Jessen et al., 2018).
Table 1.
Patient demographics and clinical characteristics (mean +/- standard deviation).
| HC (n = 175) | SCD (n = 175) | MCI (n = 86) | AD (n = 54) | |
|---|---|---|---|---|
| Sex (% female) Age (years) Education (years) GDS MMSE (/30) |
58 69.0 ± 5.3 14.7 ± 2.7 0.6 ± 1.1 29.4 ± 0.8 |
49 71.2 ± 5.8 14.7 ± 3.2 1.9 ± 1.9 29.2 ± 1.0 |
41 72.5 ± 5.2 13.8 ± 2.9 2.1 ± 1.9 27.9 ± 1.7 |
57 73.6 ± 6.4 13.5 ± 3.3 2.2 ± 1.8 23.5 ± 3.3 |
The patient group includes a total of 315 patients with subjective cognitive decline (n = 175), mild cognitive impairment (n = 86), and alzheimer’s dementia (n = 54). Numbers show means and standard deviations.
HC = Healthy Controls, SCD = Subjective Cognitive Decline, MCI = Mild Cognitive Impairment, AD = Alzheimer’s Dementia, GDS = Geriatric Depression Scale, MMSE = Mini-Mental State Examination.
SCD was defined as a persistent self-perceived cognitive decline in the absence of objective cognitive impairment as measured by the CERAD test battery, lasting at least for 6 months and being unrelated to an acute event (Jessen et al., 2014). The MCI patients met the core clinical criteria for MCI according to National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroup guidelines (Albert et al., 2011). The AD patients had a clinical diagnosis of probable AD dementia according to the NIA-AA workgroups guidelines (McKhann et al., 2011). The HC participants had no objective cognitive impairment in cognitive tests, no history of neurological or psychiatric disease and did not report self-perceived cognitive decline. All participants or their representatives provided written informed consent. The study protocol was approved by the local institutional review boards and ethical committees of the participating centers. It was conducted in accord with the Helsinki Declaration of 1975.
2.2. Neuropsychological assessment
All participants (including healthy controls and patients) underwent a clinical assessment of their cognitive status, including the Mini Mental State Examination (MMSE) (Folstein et al., 1975) and an extensive neuropsychological testing battery (Jessen et al., 2018). The neuropsychological test battery included tests which assess executive function, visuo-spatial ability, memory, working memory and language function (Jessen et al., 2018). Confirmatory factor analysis (CFA) based cognitive domain composite scores (Table 2) were derived from these tests using robust maximum likelihood (MLR) estimation (Wolfsgruber et al., 2017, Wolfsgruber et al., 2020). The variance and mean of the latent factors were fixed to one and zero respectively, following the assignment of indicator variables to latent factors, which was guided by previous CFAs on similar test batteries of the ADNI and WRAP cohort studies focusing on preclinical and prodromal AD (Grice, 2001, Dowling et al., 2010, Park et al., 2012, Wolfsgruber et al., 2017). Resulting values were normally distributed (Shapiro-Wilk). Further to this, a 5-factor structure with intercorrelated factors of learning & memory, language ability, executive functions and mental processing speed, working memory and visuo-spatial abilities was tested, taking into account the close methodological relatedness of some indicators by specifying residual correlations. Factor score estimates of the latent variables were then extracted using the multivariate regression method (Grice, 2001, Dowling et al., 2010, Park et al., 2012, Wolfsgruber et al., 2017). The presence of depression among participants was assessed by means of the Geriatric Depression Scale (GDS) (Gauggel and Birkner, 1999).
Table 2.
Cognitive domain composite scores (mean +/- standard deviation).
| Cognitive function | HC | SCD | MCI | AD | p |
|---|---|---|---|---|---|
| Visuo-spatial Executive Working memory Memory Language |
0.39 ± 0.3 0.52 ± 0.4 0.39 ± 0.4 0.61 ± 0.3 0.52 ± 0.3 |
0.28 ± 0.4 0.34 ± 0.5 0.31 ± 0.6 0.36 ± 0.4 0.35 ± 0.5 |
−0.39 ± 0.7 −0.51 ± 0.7 −0.46 ± 0.7 −0.59 ± 0.6 −0.58 ± 0.6 |
−1.49 ± 1.3 −1.86 ± 0.8 −1.52 ± 0.7 −1.98 ± 0.6 −1.85 ± 0.7 |
< 0.001 < 0.05 < 0.001 < 0.001 < 0.05 |
p values indicate the significance of one-way-ANOVA comparing the patient subgroup to HC.
2.3. Image acquisition and preprocessing
The data were acquired from nine Siemens 3.0 Tesla MRI scanners (4 Verio, 1 Skyra, 3 TimTrio and 1 Prisma system) using identical acquisition parameters and harmonized instructions. To ensure high image quality throughout the acquisition phase, all scans had to pass a semi-automated quality check during the study conduction, so that protocol deviations could be reported to the study sites, and the acquisition at the respective site could be adjusted. Functional MRI was based on a T2*-weighted echo-planar imaging (EPI) sequence using a 64 × 64 image matrix with 47 axial slices (thickness 3.5 mm, no gap) and interleaved acquisition. Of 180 acquired EPIs, the first 10 time points were excluded resulting in 170 EPI volumes for the analysis. The field of view was 224 × 224 × 165 mm, isotropic voxel size of 3.5 mm, echo time 30 ms, repetition time 2,580 ms, flip angle 80◦, and parallel imaging acceleration factor 2. The sequence took 7 min 54 s. High-resolution T1-weighted anatomical images were obtained using a sagittal magnetization-prepared rapid gradient echo (MPRAGE) sequence (field of view 256 × 256 mm, matrix size 256 × 256, isotropic voxel size 1 mm, echo time 4.37 ms, flip angle 7°, repetition time 2500 ms, number of slices 192, parallel imaging acceleration factor 2). The duration of the sequence was 5 min 8 s.
Data processing was carried out using Data Processing Assistant for Resting-State fMRI Advanced (DPARSFA 4.3) (Chao-Gan and Yu-Feng, 2010). The T1-weighted anatomical images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using the Statistical Parametric Mapping (SPM12) (Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/) New Segment toolbox implemented in Matlab 2015a (Mathworks, Natwick). The T1- weighted GM and WM partitions were normalized to the Montreal Neurological Institute (MNI) reference coordinate system using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) algorithm (Ashburner, 2007) and the default brain template included in CAT12 (Kurth et al., 2015) as target. Functional MRI preprocessing included removal of the first ten volumes of each fMRI scan, slice timing correction to the temporal middle, and realignment to the mean volume. The anatomical T1-weighted image for each participant was coregistered to the mean functional image such that the deformation fields generated by DARTEL from the anatomical T1-weighted images could be used to project the functional scans from each subjects’ native image space into the MNI reference space. Subsequently, we applied temporal bandpass filtering (0.01–0.1 Hz), and spatial smoothing with an 8 mm isotropic full-width-at-half-maximum (FWHM) Gaussian kernel. The rsFC maps were calculated using the FSL melodic toolbox (Version 5.0.9, FMRIB, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl/), resulting in 20 independent component analysis (ICA) maps. The resulting maps were then evaluated by experts to identify the four resting-state networks of interest, namely the VIS, EN, DMN and LAN networks, based on their spatial patterns as reported earlier (Beckmann et al., 2005, Smith et al., 2009, Castellazzi et al., 2014). We further derived the subject-level rsFC z-maps using FSL’s dual regression, which generated subject-specific versions of the spatial maps and associated time series. Technically, this was realized by a decomposition of each subject's 4D dataset using the group-spatial-maps to give a set of time courses, and then afterwards, decomposition of those time courses and the same 4D dataset to get a subject-specific set of spatial maps, one per functional network (Beckmann et al., 2009, Nickerson et al., 2017).
As a final step, network-specific explicit masks were derived, in order to clearly define the areas of cerebral activations which actually belong to each resting-state network. These masks were obtained from the group-based independent component maps of all study participants, by thresholding them based on the highest 10th percentile of intensities, leading to liberal masks for each of the four resting-state (VIS, EN, DMN, LAN) networks of interest. For clarity, we would like to point out here that our executive, visual and default mode ICA-derived networks corresponded to the executive control, visual, and default mode components mentioned in (Rosazza and Minati, 2011). As for the language network, the spatial pattern of our language network corresponded to the temporo-parietal and lateralized fronto-parietal components mentioned in (Rosazza and Minati, 2011). The temporo-parietal component is characterized by the engagement of regions typically associated to language processing, while the lateralized fronto-parietal components has been associated to different functions, one of which is language (Rosazza and Minati, 2011).
2.4. Statistical analysis
Statistical analyses were performed using SPM12 and R-statistics (R Core Team, 2018), respectively. SPM12 was used for voxel-based analyses, including t-test and multiple linear regressions, while R-statistics was used for analysis of variance (ANOVA) and post-hoc tests on the cognitive composite scores.
One way ANOVA and Tukey honest significant difference post-hoc tests were used to compare cognitive domain composite scores across diagnostic groups as done in (Ranasinghe et al., 2014). As a significance threshold, the one way ANOVA was tested at a family-wise confidence significance level of p < 0.05.
In voxel-wise analysis, two-sample t-tests with age, sex, education and study site included as nuisance variables, were used to compare both gray matter volumes, as well as the extent of functional connectivity of the different identified resting-state networks between the healthy control and individual patient groups. This statistical approach as already applied previously in (Ranasinghe et al., 2014, Brueggen et al., 2019) was applied to study extensive differences between each diagnostic group and the control group while controlling for the previously mentioned covariates of age, sex, education and study site. The directions of the comparisons were hypothesis-driven (Brain volume: HC > SCD/MCI/AD, Functional connectivity: HC > SCD/MCI/AD). A False Discovery Rate (FDR) of p < 0.01 was applied to control for multiple comparisons.
Voxel-wise multiple regressions were used to explore the relationship between cognitive domain composite scores and both brain volume and functional connectivity. In the case of the volumetric analyses, we adopted an unbiased whole brain voxel-wise approach as similarly done in (Cacciaglia et al., 2018) by regressing each cognitive function (executive, visuo-spatial, memory, working memory and language) composite score on the gray matter volume estimates. In the case of the rsFC analysis, we specifically regressed each cognitive domain composite score on the respective resting-state functional connectivity network (executive, visual, default mode and language), known to be associated with each function based on previous literature (Rosazza and Minati, 2011). In so doing, executive and working memory scores were regressed on the EN, visuo-spatial scores on the VIS, memory scores on the DMN and language scores on the LAN.
The regression models were controlled for age, sex, education, diagnosis and study site. In the volumetric analysis, the total intracranial volume was included as a global value. For the rsFC analysis, the network-specific explicit masks described in the previous subsection were applied. As our focus was on visuo-spatial function, we tested further post-hoc models investigating the association of the sub-tests of the visuo-spatial cognitive domain when positive associations were found at the composite level. As a significance threshold for the volumetric analysis, an FDR of p < 0.05 was applied a priori. However, this led to very extensive effects across the whole brain so that we decided post hoc to use a more strict significance threshold for the volumetric findings with an FDR of p < 0.01. In the case of the FC analysis, an FDR of p < 0.05 was applied to control for multiple comparisons. In addition, for exploratory purposes, we also report volumetric and rsFC results after applying a liberal statistical significance level of p < 0.001 and p < 0.01 respectively, uncorrected for multiple comparisons. Effect sizes (Cohen’s d) are reported for all voxel clusters.
3. Results
3.1. Cognitive performance across diagnostic groups
As expected, we found that the SCD subgroup performed significantly better than all other patient subgroups on all cognitive domains (p < 0.05, one-way-ANOVA, Tukey post hoc). In turn, the MCI subgroup also showed significantly better composite scores than the AD subgroup on all cognitive functions (p < 0.05, one-way-ANOVA, Tukey post hoc). The AD subgroup showed the worst performance across the different cognitive domains (Table 2). Albeit performing in the normal range in the single cognitive tests (as required by the definition of SCD), the SCD cases performed significantly worse than the healthy controls in executive, memory, and language composite scores (Table 2).
All patient subgroups (SCD, MCI & AD) were significantly different from each other (p < 0.001, one-way-ANOVA, Tukey post hoc). Bold text further indicates the patient subgroups that were statistically different from healthy controls (HC) after Tukey post hoc comparison between the patient groups of subjective cognitive decline (SCD), mild cognitive impairment (MCI) and Alzheimer’s disease (AD), with the significance threshold set to 0.05, for the visuo-spatial, executive, working memory, memory, and language domains respectively.
3.2. Regional gray matter atrophy associated with distinct cognitive impairments in AD spectrum
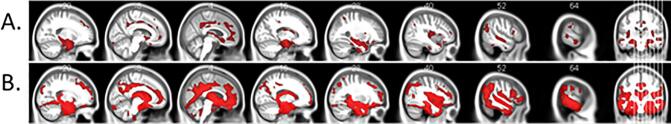
When comparing each patient group to healthy controls, we found that the MCI (p < .01, FDR corrected, 0.40 ≤ d ≤ 0.90, Fig. 1A) and AD (p < .01, FDR corrected, 0.40 ≤ d ≤ 1.70, Fig. 1B) subgroups, but not the SCD subgroup significantly differed from the control group in regard to hippocampus volumes. Additionally, the MCI subgroup also showed atrophy in the inferior frontal gyri, right superior temporal gyrus, left anterior cingulate gyrus, left middle frontal gyrus and right inferior parietal lobule. In the AD cases, further atrophy was found in the right premotor cortex, right prefrontal cortex, right putamen and superior parietal lobule (Fig. 1B). The SCD subgroup also did not differ from the HC subgroup when a lenient significance threshold of p < .001 uncorrected for multiple comparisons was applied.
Fig. 1.
Regional gray matter volume significantly differed between diagnostic groups in AD-spectrum, for the (A) MCI and (B) AD diagnostic groups respectively. Voxel-wise multiple comparisons are thresholded with p < 0.01, FDR corrected, cluster size ≥ 50 voxels, 0.40 ≤ d ≤ 1.70. Red voxels show clusters of significantly reduced gray matter volume in patients with MCI and AD compared to HC subgroup. Statistical maps are superimposed on a rendering of the Montreal Neurological Institute template brain. MNI coordinates and corresponding t values are provided in Supplementary Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
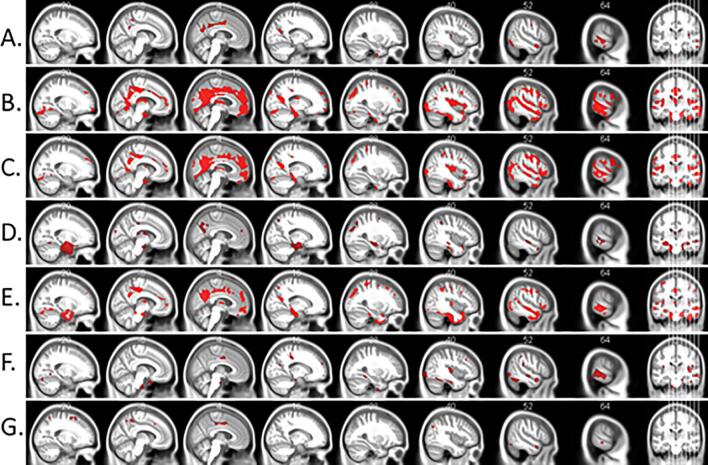
Furthermore, across the whole sample of SCD, MCI and AD controlling for age, sex, site, and diagnosis, we found significant associations between the volume estimates and cognitive measures. For visuo-spatial function, lower performance was associated with reduced gray matter volume in the middle temporal gyri, right temporal pole, right anterior cingulate gyrus, inferior parietal lobules, left inferior occipital gyrus, left premotor cortex, right fusiform gyrus and left superior parietal lobule (p < .01, FDR corrected, 0.41 ≤ d ≤ 0.60, Fig. 2A). In the case of executive function, lower performance was associated with reduced gray matter volume in the right posterior cingulate gyrus, left inferior frontal gyrus, prefrontal cortices, left premotor cortex, left middle frontal gyrus, left primary motor cortex, left superior parietal lobule, right inferior occipital gyrus and right inferior parietal lobule (p < .01, FDR corrected, 0.38 ≤ d ≤ 0.70, Fig. 2B). For working memory function, lower performance was associated with reduced gray matter volume in the left prefrontal cortex, inferior temporal gyri, right inferior occipital gyrus, left premotor cortex, middle frontal gyri, right inferior occipital gyrus, right inferior parietal lobule and left posterior cingulate gyrus (p < .01, FDR corrected, 0.38 ≤ d ≤ 0.65, Fig. 2C). When considering memory function, lower scores were associated with reduced gray matter volume in the middle temporal gyri, superior parietal lobule, inferior occipital gyrus, inferior parietal lobules, left insular cortex, right fusiform gyrus, left anterior and posterior cingulate gyri and right inferior temporal gyrus (p < .01, FDR corrected, 0.40 ≤ d ≤ 0.75, Fig. 2D). And lastly, for language function, lower performance was associated with reduced gray matter volume in the temporal poles, left posterior cingulate gyrus, left premotor cortex, inferior parietal lobules, prefrontal cortices, right insular cortex and left supplementary motor area (p < .01, FDR corrected, 0.39 ≤ d ≤ 0.70, Fig. 2E).
Fig. 2.
Regional gray matter volume is associated with the (A) visuo-spatial, (B) executive, (C) working memory, (D) memory, and (E) language domains respectively. Figure (F) and (G) shows the association of gray matter volume with the clock drawing and clock copy subtest of visuospatial function respectively. Voxel-wise multiple comparisons are thresholded at p < .01, FDR corrected for only figures a-e, cluster size ≥ 50 voxels, 0.38 ≤ d ≤ 0.70. Figures F and G are displayed at p < .001, 0.38 ≤ d ≤ 0.50 uncorrected for multiple comparisons. Red voxels show clusters of significant association between gray matter volume and cognitive domain scores. Statistical maps are superimposed on a rendering of the Montreal Neurological Institute template brain. MNI coordinates and corresponding t values are provided in Supplementary Table 2. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When applying a more lenient significance threshold, we additionally found performance on the clock copy (Fig. 2G) and clock drawing (Fig. 2F) tests to be the only sub-measures of visuo-spatial function significantly associated with gray matter atrophy; lower performance was similarly associated with reduced gray matter volume in the parietal, occipital and temporal areas, similarly as in the case of the visuo-spatial composite scores (p < .001, uncorrected, 0.38 ≤ d ≤ 0.50).
3.3. Patterns of network-specific resting-state functional connectivity associated with distinct cognitive impairments in AD spectrum
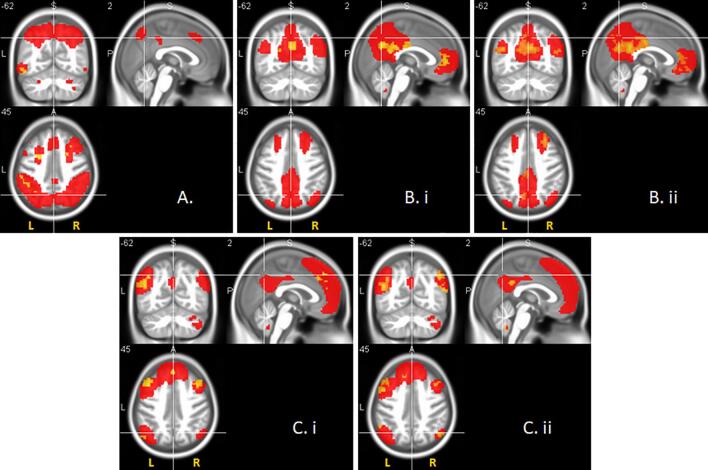
When comparing each patient subgroup to healthy controls in the extent of rsFC for each network, we found that for the visual network, neither of the patient subgroups differed significantly from the healthy control subgroup, not even at a more lenient threshold of p < 0.01, uncorrected. For the executive network we found that only the AD subgroup (p < .05, FDR corrected, 0.39 ≤ d ≤ 0.60) significantly differed from the healthy control subgroup (Fig. 3A). For the default mode network we found that both the MCI (p < .05, FDR corrected, 0.38 ≤ d ≤ 0.60, Fig. 3B.i) and AD (p < .05, FDR corrected, 0.38 ≤ d ≤ 0.80, Fig. 3B.ii) subgroup, but not the SCD subgroup differed significantly from the healthy control subgroup. When applying a more lenient significance threshold, we also found that for the language network, both the MCI (p < .01, uncorrected, 0.30 ≤ d ≤ 0.50, Fig. 3C.i) and AD (p < .01, uncorrected, 0.31 ≤ d ≤ 0.65, Fig. 3C.ii) subgroups, but not the SCD subgroup differed significantly from the healthy control subgroup.
Fig. 3.
Network-specific resting-state functional connectivity significantly differed between diagnostic groups in AD-spectrum, for the (A) executive, (B) default mode and (C) language networks respectively. Significance is reported at p < .05 FDR corrected for the executive and default mode networks, and at p < .01 uncorrected for the language network accordingly. Cluster size ≥ 20 voxels, 0.30 ≤ d ≤ 0.80. Red voxels represent group resting-state networks, yellow voxels show clusters of significant difference between the patient and healthy control subgroups on network-specific functional connectivity. Statistical comparison was restricted to the corresponding networks only by functional masks determined from the whole sample (see Section 2.3). Statistical maps are superimposed on a rendering of the Montreal Neurological Institute template brain. MNI coordinates and corresponding t values are provided in Supplementary Table 3. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
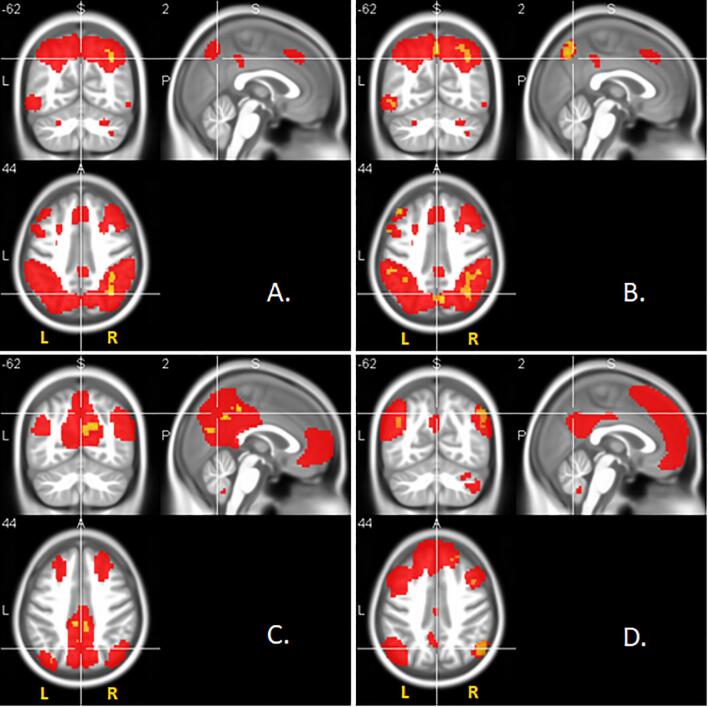
Considering the whole patient sample of SCD, MCI and AD controlling for age, sex, site, and diagnosis, we found significant associations between the cognitive composite measures and the connectivity estimates. Poorer cognitive performance scores for executive function were associated with reduced rsFC of areas within the executive network (p < .05, FDR corrected, 0.35 ≤ d ≤ 0.50, Fig. 4A). We also found similar positive outcomes for the association of memory scores with connectivity of areas within the default mode network (p < .05, FDR corrected, 0.33 ≤ d ≤ 0.50, Fig. 4C). When applying a more lenient significance threshold, we found that poorer cognitive performance scores for working memory were also associated with reduced rsFC of areas within the executive network (p < .01, uncorrected, 0.28 ≤ d ≤ 0.45, Fig. 4B). The language scores were also associated with the rsFC of areas within the language network (p < .01, uncorrected, 0.24 ≤ d ≤ 0.50, Fig. 4D). No significant effects were found for the association of the visuo-spatial function scores and the rsFC of the visual network.
Fig. 4.
Network-specific resting-state functional connectivity is associated with the (A) executive, (B) working memory, (C) memory, and (D) language functions respectively. Significance is reported at p < .05 FDR corrected for the executive and memory functions, and at p < .01 uncorrected for the working memory and language functions accordingly. Cluster size ≥ 20 voxels, 0.24 ≤ d ≤ 0.50. Red voxels represent group resting-state networks, yellow voxels show clusters of significant association between network-specific functional connectivity and cognitive domain scores. Association was restricted to the corresponding networks only by functional masks determined from the whole sample (see Section 2.3). Statistical maps are superimposed on a rendering of the Montreal Neurological Institute template brain. MNI coordinates and corresponding t values are provided in Supplementary Table 4. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study, we tested differences in gray matter volumes, CFA-derived cognitive domain scores, as well as the extent of functional connectivity of the different identified resting-state networks across diagnostic groups involving 490 cases from the AD spectrum of the DELCODE multicenter study. We also examined associations of cognitive domain composite scores with gray matter volume and network-specific rsFC among our patient subgroup of SCD, MCI and AD. Overall, we found that cognitive performance, atrophy patterns and functional connectivity significantly differed between diagnostic groups in the AD-spectrum. Additionally, regional gray matter atrophy was positively associated with visuospatial and other cognitive impairments within the AD-spectrum. Patterns of network-specific resting-state functional connectivity (except the visual network) were also positively associated with distinct cognitive impairments within the AD-spectrum.
The current study makes an important contribution towards providing a comprehensive overview of the neural correlates of domain-specific cognitive decline in the AD-spectrum. Furthermore, extending Teipel et al., 2017, Teipel et al., 2018 who primarily focused on the effect of multisite acquisition on rsFC and group separation, the current study further explored differences in cognitive performance, and also tested the association between composite cognitive function and alterations in specific rsFC in the AD-spectrum. Most importantly, our study extends previous rsFC studies (Ranasinghe et al., 2014, Smits et al., 2014, Balachandar et al., 2015) by leveraging the combined advantage of a more representative and large multicenter sample, and composite measures of the most relevant cognitive domains in AD-spectrum. We provide evidence for the viability of using CFA-derived cognitive composite scores in investigating structural atrophy and alterations in rsFC in the AD-spectrum. CFA-derived cognitive composite scores as already obtained in previous studies such as the ADNI and WRAP studies (Dowling et al., 2010, Park et al., 2012), provide a more reliable metric of cognitive function than single test measures (Wolfsgruber et al., 2017, Clark et al., 2016). Nonetheless, the association of CFA-derived cognitive domain composite scores with rsFC of underlying networks in a multricentric cohort has to the best of our knowledge not been previously studied.
Consistent with previous rsFC studies with smaller sample sizes (Ranasinghe et al., 2014, Smits et al., 2014, Balachandar et al., 2015), we showed differences between our healthy control and patient groups in a larger cohort. Nonetheless, difference of rs-FC between groups was spatially restricted, where areas of difference between groups were mainly found in small clusters. These differences, however, had moderate to large effect sizes. A possible explanation for the spatially restricted effects could owe to the notion that resting-state networks are made up of spatially distinct brain regions with underlying structures, some of which may be more susceptible to disease related alterations than the others (Greicius et al., 2004, Cai et al., 2017). For example, Greicius et al. (2004) studied hippocampal connectivity in relation to other brain regions within the DMN in AD patients, and reported that a deficit of functional connectivity was evident in the posterior cingulate and in the hippocampi, but not in other regions such as the mesial prefrontal cortex and lateral parietal cortex which also belong to the default mode network (Rosazza and Minati, 2011). Additionally, contrary to our expectation, none of the diagnostic groups showed significant differences for the connectivity of the visual network. A possible explanation for this can be found in the notion that the recruitment of the visual network may be more task dependent or external-stimuli-driven, as evidenced in a number of functional MRI studies focusing on the visual network (Stevens et al., 2010, Yang et al., 2015, Ruiz-Rizzo et al., 2018). For instance, Stevens et al. (2010) reported that VIS connectivity during resting-state fMRI was influenced by a prior visual stimuli exposure, suggesting that significant VIS activation leading to a possible difference between controls and patients, could more likely be observed when participants are subjected to a visual task or to visual stimuli in general.
When we assessed the association of CFA-derived cognitive domain composite scores with network-specific rsFC, we found consistent outcomes for the association of the executive domain scores with areas within the executive network, and the memory domain scores with areas within the default mode network, in agreement with previous studies which applied single test measures of cognitive function (Ranasinghe et al., 2014, Balachandar et al., 2015, Gardini et al., 2015, Zhou et al., 2015, Brueggen et al., 2016). Less consistent associations were found for the association of the working memory domain scores with areas within the executive network, and the language domain scores with areas within the language network. However, we could not find positive associations between visuo-spatial domain scores and the rsFC of the visual network. The finding of more consistent associations for the executive and memory domain is not surprising, as these domains are known from previous studies to be highly affected earlier in the disease process at the onset of AD (Seeley et al., 2009, Zhou et al., 2012, Quental et al., 2013, Lim et al., 2014, Rajan et al., 2015).
The lack of a significant association of the visuo-spatial domain and the visual network in the current study could be the result of a more pronounced executive control influence of the single subtests (clock drawing, clock copying & CERAD figure copying) included in the visuo-spatial cognitive domain score. Performance on the clock drawing test as reported by Cosentino et al. (2004) particularly appears to place an executive control demand on participants, thereby recruiting cognitive resources other than visuo-spatial resources. To test for this possibility, we performed post-hoc analysis, using the same method as performed earlier for the functional analysis. When associating the visuo-spatial domain scores with the rsFC of the executive network and default mode network, we expectedly found significant associations (p < .01, uncorrected), which buttresses a prominent role of executive dysfunction for visuospatial performance in the AD spectrum (Supplementary Fig. 1). Furthermore, our MCI and AD diagnostic subgroups differed significantly from the HC subgroup in extent of atrophy and rsFC patterns as expected, with the exception of the SCD subgroup. This agrees with previous studies which even after applying machine learning approaches to identifying the SCD subgroup have also reported classification accuracy below that of the MCI and AD subgroups (Liu et al., 2018, Yan et al., 2019), thereby further highlighting the difficulty in characterization and discrimination of the SCD subgroup relative to the HC subgroup. Nonetheless, some studies which overcome this particular limitation of the current study, have identified abnormal AD biomarkers in CSF and also brain correlates of AD pathology in SCD compared to HC (Wolfsgruber et al., 2020).
Our findings agree with the majority of previous studies that assessed the associations of regional gray matter volume with either single test measures of cognitive function, or composite measures of cognitive domains derived by averaging across standardized scores of single tests of cognitive function in both healthy elderly cohorts (Chee et al., 2009, Bruno et al., 2016, Cacciaglia et al., 2018) and patients (Di Paola et al., 2007, Mitolo et al., 2013, Ranasinghe et al., 2014, Smits et al., 2014, Cacciaglia et al., 2018). These previous and our findings differ from a previous MEG and MRI study Ranasinghe et al. (2014) that found no correlation between gray matter atrophy and cognitive performance, however, the number of cases n = 27 in this previous study was very low. We found consistent associations for the volumetric analysis with the related composite scores of domain specific cognitive functions, which is indicative of the utility of our approach to deriving measures of cognitive function. When considering the pattern of atrophy for visuo-spatial function, we found that higher cognitive domain scores were associated with more gray matter volume in parietal, occipital and temporal regions. When we extended the association of atrophy patterns to the single measures of visuo-spatial function, we interestingly found that in the case of the clock drawing and clock copy test scores, the parietal and temporal areas similarly showed effects as was the case with the visuo-spatial domain score. The parietal, occipital and temporal regions are known to form part of the dorsal and ventral pathways responsible for the processing of visual stimuli (Mishkin et al., 1983), hence, atrophy in such regions have been shown to be associated with poorer visuospatial abilities in patients with MCI (Mitolo et al., 2013) and AD (Smits et al., 2014).
A possible limitation of the current study is the data-driven approach employed in generating the rsFC networks using ICA, in contrast to a seed-based approach (Brueggen et al., 2016) in which regions of interest (ROI) are defined a priori. Here, it might be possible that the resulting independent components we defined as rsFC networks based on evidence in the literature such as (Castellazzi et al., 2014, Rosazza and Minati, 2011) slightly differ from a few other studies, as we generated in total ~20 components compared to for instance generating ~44 components (Tie et al., 2014), which could lead to additional brain regions being considered in our study for certain networks. This indicates that despite the advantage of data-driven approaches in the automatic derivation of resting-state networks, heterogeneity in data-driven approaches still remains an open question.
In conclusion, the current study provides a comprehensive description of the structural and functional correlates of the key cognitive domains of AD, with a focus on the visuo-spatial domain. Our findings provide evidence for CFA-derived cognitive domain composite scores as considerable proxy measures of the cognitive deficits associated with regional gray matter volume in the AD spectrum. The same is the case for the cognitive deficits associated with network-specific resting-state functional connectivity, however, with the exception of visuo-spatial cognitive deficits. We recommend the use of CFA-derived composite scores in future studies as they provide a more comprehensive measure of cognitive functions. The methodological approach applied by Balachandar et al. (2017) could be adopted, in terms of dividing the participant sample into those with severe or mild visuo-spatial deficits based on their visuo-spatial domain scores, while assessing the association with the rsFC of the visual network. This would provide the possibility to do within-diagnostic-group comparisons of the extent of rsFC with the extent of visuo-spatial deficit. As regards measuring visuo-spatial function, such future studies could then employ the usage of automated or simulated tests of visuo-spatial function, such as the computer-aided Visuo-spatial Cognitive-Performance Test (VCP-Test) (Matsubayashi et al., 1991), to provide performance metrics that are independent of rater abilities. Additionally, more robust measures of visuo-spatial function such as the Rey Complex Figure Test (Meyers and Meyers, 1995), could be considered while deriving the CFA-derived composite scores. More research focusing especially on the association of the rsFC of the visual network and visuo-spatial domain is required to confirm or extend these initial findings.
CRediT authorship contribution statement
Chimezie O. Amaefule: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Visualization. Martin Dyrba: Methodology, Data curation, Investigation, Writing - original draft. Steffen Wolfsgruber: Formal analysis, Investigation. Alexandra Polcher: Investigation. Anja Schneider: Investigation. Klaus Fliessbach: Investigation. Annika Spottke: Investigation. Dix Meiberth: Investigation. Lukas Preis: Investigation. Oliver Peters: Investigation. Enise I. Incesoy: Investigation. Eike Spruth: Investigation. Josef Priller: Investigation. Slawek Altenstein: Investigation. Claudia Bartels: Investigation. Jens Wiltfang: Investigation. Daniel Janowitz: Investigation. Katharina Bürger: Investigation. Christoph Laske: Investigation. Matthias Munk: Investigation. Janna Rudolph: Investigation. Wenzel Glanz: Investigation. Laura Dobisch: Investigation, Data curation. John D. Haynes: Investigation. Peter Dechent: Investigation. Birgit Ertl-Wagner: Investigation. Klaus Scheffler: Investigation. Ingo Kilimann: Investigation. Emrah Düzel: Investigation. Coraline D. Metzger: Investigation. Michael Wagner: Investigation. Frank Jessen: Investigation. Stefan J. Teipel: Conceptualization, Investigation, Writing - original draft, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was funded through the European Regional Development Fund (EFRE), reference number TBI-V-1-100-VBW-035. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102533.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agosta F., Pievani M., Geroldi C., Copetti M., Frisoni G.B., Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging. 2012;33:1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Badhwar A., Tam A., Dansereau C., Orban P., Hoffstaedter F., Bellec P. Resting-state network dysfunction in Alzheimer's disease: a systematic review and meta-analysis. Alzheimer's & Dementia. 2017;8:73–85. doi: 10.1016/j.dadm.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar R., Bharath S., John J.P., Joshi H., Sadanand S., Saini J., Kumar K.J., Varghese M. Resting-state functional connectivity changes associated with visuospatial cognitive deficits in patients with mild Alzheimer disease. Dement. Geriatr. Cogn. Disord. 2017;43:229–236. doi: 10.1159/000457118. [DOI] [PubMed] [Google Scholar]
- Balachandar R., John J.P., Saini J., Kumar K.J., Joshi H., Sadanand S., Aiyappan S., Sivakumar P.T., Loganathan S., Varghese M., Bharath S. A study of structural and functional connectivity in early Alzheimer's disease using rest fMRI and diffusion tensor imaging. Int. J. Geriatric Psychiatry. 2015;30:497–504. doi: 10.1002/gps.4168. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. London Series B. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage. 2009;47:S148. [Google Scholar]
- Benson D.F., Davis R.J., Snyder B.D. Posterior cortical atrophy. Arch. Neurol. 1988;45:789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Brueggen K., Dyrba M., Cardenas-Blanco A., Schneider A., Fliessbach K., Buerger K., Janowitz D., Peters O., Menne F., Priller J., Spruth E., Wiltfang J., Vukovich R., Laske C., Buchmann M., Wagner M., Röske S., Spottke A., Rudolph J., Metzger C.D., Kilimann I., Dobisch L., Düzel E., Jessen F., Teipel S.J. Structural integrity in subjective cognitive decline, mild cognitive impairment and Alzheimer's disease based on multicenter diffusion tensor imaging. J. Neurol. 2019 doi: 10.1007/s00415-019-09429-3. [DOI] [PubMed] [Google Scholar]
- Brueggen K., Kasper E., Dyrba M., Bruno D., Pomara N., Ewers M., Duering M., Bürger K., Teipel S.J. The primacy effect in amnestic mild cognitive impairment: associations with hippocampal functional connectivity. Front. Aging Neurosci. 2016;8:244. doi: 10.3389/fnagi.2016.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D., Ciarleglio A., Grothe M.J., Nierenberg J., Bachman A.H., Teipel S.J., Petkova E., Ardekani B.A., Pomara N. Hippocampal volume and integrity as predictors of cognitive decline in intact elderly. NeuroReport. 2016;27:869–873. doi: 10.1097/WNR.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciaglia R., Molinuevo J.L., Sánchez-Benavides G., Falcón C., Gramunt N., Brugulat-Serrat A., Grau O., Gispert J.D. Episodic memory and executive functions in cognitively healthy individuals display distinct neuroanatomical correlates which are differentially modulated by aging. Hum. Brain Mapp. 2018;39:4565–4579. doi: 10.1002/hbm.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Chong T., Peng Y., Shen W., Li J., von Deneen K.M., Huang L. Altered functional brain networks in amnestic mild cognitive impairment: a resting-state fMRI study. Brain imaging and behavior. 2017;11:619–631. doi: 10.1007/s11682-016-9539-0. [DOI] [PubMed] [Google Scholar]
- Castellazzi G., Palesi F., Casali S., Vitali P., Sinforiani E., Wheeler-Kingshott C.A.M., D'Angelo E. A comprehensive assessment of resting state networks: bidirectional modification of functional integrity in cerebro-cerebellar networks in dementia. Front. Neurosci. 2014;8:223. doi: 10.3389/fnins.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M.W.L., Chen K.H.M., Zheng H., Chan K.P.L., Isaac V., Sim S.K.Y., Chuah L.Y.M., Schuchinsky M., Fischl B., Ng T.P. Cognitive function and brain structure correlations in healthy elderly East Asians. NeuroImage. 2009;46:257–269. doi: 10.1016/j.neuroimage.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Clark L.R., Racine A.M., Koscik R.L., Okonkwo O.C., Engelman C.D., Carlsson C.M., Asthana S., Bendlin B.B., Chappell R., Nicholas C.R., Rowley H.A., Oh J.M., Hermann B.P., Sager M.A., Christian B.T., Johnson S.C. Beta-amyloid and cognitive decline in late middle age: findings from the Wisconsin Registry for Alzheimer's Prevention study. Alzheimer's & Dementia. 2016;12:805–814. doi: 10.1016/j.jalz.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S., Jefferson A., Chute D.L., Kaplan E., Libon D.J. Clock drawing errors in dementia. Cognitive Behavioral Neurol. 2004;17:74–84. doi: 10.1097/01.wnn.0000119564.08162.46. [DOI] [PubMed] [Google Scholar]
- Crutch S.J., Lehmann M., Schott J.M., Rabinovici G.D., Rossor M.N., Fox N.C. Posterior cortical atrophy. Lancet Neurol. 2012;11:170–178. doi: 10.1016/S1474-4422(11)70289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M., Macaluso E., Carlesimo G.A., Tomaiuolo F., Worsley K.J., Fadda L., Caltagirone C. Episodic memory impairment in patients with Alzheimer's disease is correlated with entorhinal cortex atrophy. A voxel-based morphometry study. J. Neurol. 2007;254:774–781. doi: 10.1007/s00415-006-0435-1. [DOI] [PubMed] [Google Scholar]
- Didic M., Felician O., Barbeau E.J., Mancini J., Latger-Florence C., Tramoni E., Ceccaldi M. Impaired visual recognition memory predicts Alzheimer's disease in amnestic mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2013;35:291–299. doi: 10.1159/000347203. [DOI] [PubMed] [Google Scholar]
- Dong L., Shen Y., Lei X., Luo C., Li Q., Wu W., Yao D., Li C. The heterogeneity of aging brain: altered functional connectivity in default mode network in older adults during verbal fluency tests. Chin. Med. J. 2012;125:604–610. [PubMed] [Google Scholar]
- Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G., Weiner M., Aisen P.S. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling N.M., Hermann B., La Rue A., Sager M.A. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer's disease. Neuropsychology. 2010;24:742–756. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gardini S., Venneri A., Sambataro F., Cuetos F., Fasano F., Marchi M., Crisi G., Caffarra P. Increased functional connectivity in the default mode network in mild cognitive impairment: a maladaptive compensatory mechanism associated with poor semantic memory performance. J. Alzheimer's disease. 2015;45:457–470. doi: 10.3233/JAD-142547. [DOI] [PubMed] [Google Scholar]
- Gauggel S., Birkner B. Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS) Z. Klinische Psychol. Psychotherapie. 1999;28:18–27. [Google Scholar]
- Gour N., Felician O., Didic M., Koric L., Gueriot C., Chanoine V., Confort-Gouny S., Guye M., Ceccaldi M., Ranjeva J.P. Functional connectivity changes differ in early and late-onset Alzheimer's disease. Hum. Brain Mapp. 2014;35:2978–2994. doi: 10.1002/hbm.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. PNAS. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice J.W. Computing and evaluating factor scores. Psychol. Methods. 2001;6:430–450. [PubMed] [Google Scholar]
- Grothe, M.J., Heinsen, H., Amaro, E., Grinberg, L.T., Teipel, S.J., 2016. Cognitive Correlates of Basal Forebrain Atrophy and Associated Cortical Hypometabolism in Mild Cognitive Impairment. Cerebral cortex (New York, N.Y.: 1991) 26, 2411–2426. [DOI] [PMC free article] [PubMed]
- Henderson V.W., Mack W., Williams B.W. Spatial disorientation in Alzheimer's disease. Arch. Neurol. 1989;46:391–394. doi: 10.1001/archneur.1989.00520400045018. [DOI] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G., Dubois B., Dufouil C., Ellis K.A., van der Flier W.M., Glodzik L., van Harten A.C., de Leon M.J., McHugh P., Mielke M.M., Molinuevo J.L., Mosconi L., Osorio R.S., Perrotin A., Petersen R.C., Rabin L.A., Rami L., Reisberg B., Rentz D.M., Sachdev P.S., de La Sayette V., Saykin A.J., Scheltens P., Shulman M.B., Slavin M.J., Sperling R.A., Stewart R., Uspenskaya O., Vellas B., Visser P.J., Wagner M. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimer's & Dementia. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Spottke A., Boecker H., Brosseron F., Buerger K., Catak C., Fliessbach K., Franke C., Fuentes M., Heneka M.T., Janowitz D., Kilimann I., Laske C., Menne F., Nestor P., Peters O., Priller J., Pross V., Ramirez A., Schneider A., Speck O., Spruth E.J., Teipel S., Vukovich R., Westerteicher C., Wiltfang J., Wolfsgruber S., Wagner M., Düzel E. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer's disease (DELCODE) Alzheimer's Res. Therapy. 2018;10:15. doi: 10.1186/s13195-017-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F., Gaser C., Luders E. A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM) Nat. Protoc. 2015;10:293–304. doi: 10.1038/nprot.2015.014. [DOI] [PubMed] [Google Scholar]
- Landau S.M., Harvey D., Madison C.M., Koeppe R.A., Reiman E.M., Foster N.L., Weiner M.W., Jagust W.J. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.Y., Maruff P., Pietrzak R.H., Ames D., Ellis K.A., Harrington K., Lautenschlager N.T., Szoeke C., Martins R.N., Masters C.L., Villemagne V.L., Rowe C.C. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease. Brain. 2014;137:221–231. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- Liu, T., Wang, Y., Yan, T., Liu, Y., Xu, R., Li, J., Xie, Y., 2018. Preclinical Stages of Alzheimer's Disease Classification by a Rs-fMRI Study, in: Proceedings, 2018 11th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics. CISP-BMEI 2018 : 13-15 October 2018, Beijing, China. 2018 11th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Beijing, China. 10/13/2018 - 10/15/2018. IEEE, [Piscataway, New Jersey], pp. 1–6.
- Matsubayashi K., Kawamoto A., Shimada K., Kimura S., Takeuchi K., Ozawa T., Ogura H. Computer-aided visuospatial cognitive-performance test. Nihon Ronen Igakkai zasshi. Japanese J. Geriatrics. 1991;28:182–187. doi: 10.3143/geriatrics.28.182. [DOI] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., Mohs R.C., Morris J.C., Rossor M.N., Scheltens P., Carrillo M.C., Thies B., Weintraub S., Phelps C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J.E., Meyers K.R. PAR Inc.; 1995. Rey Complex Figure Test and Recognition Trial: Professional MANUAL. [Google Scholar]
- Mishkin M., Ungerleider L.G., Macko K.A. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- Mitolo M., Gardini S., Fasano F., Crisi G., Pelosi A., Pazzaglia F., Caffarra P. Visuospatial memory and neuroimaging correlates in mild cognitive impairment. J. Alzheimer's Dis.: JAD. 2013;35:75–90. doi: 10.3233/JAD-121288. [DOI] [PubMed] [Google Scholar]
- Monacelli A.M., Cushman L.A., Kavcic V., Duffy C.J. Spatial disorientation in Alzheimer's disease: the remembrance of things passed. Neurology. 2003;61:1491–1497. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- Mormino E.C., Papp K.V., Rentz D.M., Donohue M.C., Amariglio R., Quiroz Y.T., Chhatwal J., Marshall G.A., Donovan N., Jackson J., Gatchel J.R., Hanseeuw B.J., Schultz A.P., Aisen P.S., Johnson K.A., Sperling R.A. Early and late change on the preclinical Alzheimer's cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimer's & Dementia. 2017;13:1004–1012. doi: 10.1016/j.jalz.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson L.D., Smith S.M., Öngür D., Beckmann C.F. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front. Neurosci. 2017;11:115. doi: 10.3389/fnins.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoy J., Niemantsverdriet E., Verhaeghe J., de Roeck E., Struyfs H., Somers C., Wyffels L., Ceyssens S., van Mossevelde S., van den Bossche T., van Broeckhoven C., Ribbens A., Bjerke M., Stroobants S., Engelborghs S., Staelens S. Association of short-term cognitive decline and MCI-to-AD dementia conversion with CSF, MRI, amyloid- and 18F-FDG-PET imaging. NeuroImage. Clin. 2019;22 doi: 10.1016/j.nicl.2019.101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L.Q., Gross A.L., McLaren D.G., Pa J., Johnson J.K., Mitchell M., Manly J.J. Confirmatory factor analysis of the ADNI Neuropsychological Battery. Brain Imaging Behavior. 2012;6:528–539. doi: 10.1007/s11682-012-9190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quental N.B.M., Brucki S.M.D., Bueno O.F.A. Visuospatial function in early Alzheimer's disease–the use of the Visual Object and Space Perception (VOSP) battery. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan K.B., Wilson R.S., Weuve J., Barnes L.L., Evans D.A. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology. 2015;85:898–904. doi: 10.1212/WNL.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe K.G., Hinkley L.B., Beagle A.J., Mizuiri D., Dowling A.F., Honma S.M., Finucane M.M., Scherling C., Miller B.L., Nagarajan S.S., Vossel K.A. Regional functional connectivity predicts distinct cognitive impairments in Alzheimer’s disease spectrum. NeuroImage : Clinical. 2014;5:385–395. doi: 10.1016/j.nicl.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C., Minati L. Resting-state brain networks: literature review and clinical applications. Neurol. Sci. 2011;32:773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- Ruiz-Rizzo A.L., Neitzel J., Müller H.J., Sorg C., Finke K. Distinctive correspondence between separable visual attention functions and intrinsic brain networks. Front. Hum. Neurosci. 2018;12:89. doi: 10.3389/fnhum.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. PNAS. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits L.L., Tijms B.M., Benedictus M.R., Koedam E.L.E., Koene T., Reuling I.E., Barkhof F., Scheltens P., Pijnenburg Y.A., Wattjes M.P., van der Flier W.M. Regional atrophy is associated with impairment in distinct cognitive domains in Alzheimer's disease. Alzheimer's Dementia. 2014;10:S299–S305. doi: 10.1016/j.jalz.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Stevens, W.D., Buckner, R.L., Schacter, D.L., 2010. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cerebral cortex (New York, N.Y. : 1991) 20, 1997–2006. [DOI] [PMC free article] [PubMed]
- Tam A., Dansereau C., Badhwar A., Orban P., Belleville S., Chertkow H., Dagher A., Hanganu A., Monchi O., Rosa-Neto P., Shmuel A., Wang S., Breitner J., Bellec P. Common effects of amnestic mild cognitive impairment on resting-state connectivity across four independent studies. Front. Aging Neurosci. 2015;7:242. doi: 10.3389/fnagi.2015.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S.J., Metzger C.D., Brosseron F., Buerger K., Brueggen K., Catak C., Diesing D., Dobisch L., Fliebach K., Franke C., Heneka M.T., Kilimann I., Kofler B., Menne F., Peters O., Polcher A., Priller J., Schneider A., Spottke A., Spruth E.J., Thelen M., Thyrian R.J., Wagner M., Düzel E., Jessen F., Dyrba M. Multicenter resting state functional connectivity in prodromal and dementia stages of Alzheimer's disease. J. Alzheimer's Dis.: JAD. 2018;64:801–813. doi: 10.3233/JAD-180106. [DOI] [PubMed] [Google Scholar]
- Teipel S.J., Wohlert A., Metzger C., Grimmer T., Sorg C., Ewers M., Meisenzahl E., Klöppel S., Borchardt V., Grothe M.J., Walter M., Dyrba M. Multicenter stability of resting state fMRI in the detection of Alzheimer's disease and amnestic MCI. NeuroImage. Clinical. 2017;14:183–194. doi: 10.1016/j.nicl.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetewsky S.J., Duffy C.J. Visual loss and getting lost in Alzheimer's disease. Neurology. 1999;52:958–965. doi: 10.1212/wnl.52.5.958. [DOI] [PubMed] [Google Scholar]
- Tie Y., Rigolo L., Norton I.H., Huang R.Y., Wu W., Orringer D., Mukundan S., Golby A.J. Defining language networks from resting-state fMRI for surgical planning–a feasibility study. Hum. Brain Mapp. 2014;35:1018–1030. doi: 10.1002/hbm.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zhou B., Yao H., Zhan Y., Zhang Z., Cui Y., Xu K., Ma J., Wang L., An N., Zhang X., Liu Y., Jiang T. Aberrant intra- and inter-network connectivity architectures in Alzheimer's disease and mild cognitive impairment. Sci. Rep. 2015;5:14824. doi: 10.1038/srep14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhang M., Han Y., Song H., Guo R., Li K. Differentially disrupted functional connectivity of the subregions of the amygdala in Alzheimer's disease. J. X-Ray Sci. Technol. 2016;24:329–342. doi: 10.3233/XST-160556. [DOI] [PubMed] [Google Scholar]
- Wolfsgruber S., Kleineidam L., Guski J., Peters O., Buerger K., Ewers M., Priller J., Laske C., Teipel S.J., Fließbach K., Schneider A., Spottke A., Jessen F., Wagner M. Latent-factor structure of the delcode study neuropsychological test battery. Alzheimer's & Dementia. 2017;13:P1136–P1137. [Google Scholar]
- Wolfsgruber S., Kleineidam L., Guski J., Polcher A., Frommann I., Roeske S., Spruth E.J., Franke C., Priller J., Kilimann I., Teipel S., Buerger K., Janowitz D., Laske C., Buchmann M., Peters O., Menne F., Casan M.F., Wiltfang J., Bartels C., Düzel E., Metzger C., Glanz W., Thelen M., Spottke A., Ramirez A., Kofler B., Fließbach K., Schneider A., Heneka M., Brosseron F., Meiberth D., Jessen F., Wagner M. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology. 2020 doi: 10.1212/WNL.0000000000010142. [DOI] [Google Scholar]
- Yan T., Wang Y., Weng Z., Du W., Liu T., Chen D., Li X., Wu J., Han Y. Early-stage identification and pathological development of Alzheimer's disease using multimodal MRI. J. Alzheimer's Disease: JAD. 2019;68:1013–1027. doi: 10.3233/JAD-181049. [DOI] [PubMed] [Google Scholar]
- Yang Y., Deng H., Xing G., Xia X., Li H. Brain functional network connectivity based on a visual task: visual information processing-related brain regions are significantly activated in the task state. Neural Regener. Res. 2015;10:298–307. doi: 10.4103/1673-5374.152386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Yao H., Wang P., Zhang Z., Zhan Y., Ma J., Xu K., Wang L., An N., Liu Y., Zhang X. Aberrant functional connectivity architecture in Alzheimer's disease and mild cognitive impairment: a whole-brain, data-driven analysis. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/495375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Gennatas E.D., Kramer J.H., Miller B.L., Seeley W.W. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Greicius M.D., Gennatas E.D., Growdon M.E., Jang J.Y., Rabinovici G.D., Kramer J.H., Weiner M., Miller B.L., Seeley W.W. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.