Abstract
Near-infrared photoimmunotherapy (NIR-PIT) is a recently developed hybrid cancer therapy that directly kills cancer cells as well as producing a therapeutic host immune response. Conventional immunotherapies, such as immune-activating cytokine therapy, checkpoint inhibition, engineered T cells and suppressor cell depletion, do not directly destroy cancer cells, but rely exclusively on activating the immune system. NIR-PIT selectively destroys cancer cells, leading to immunogenic cell death that initiates local immune reactions to released cancer antigens from dying cancer cells. These are characterized by rapid maturation of dendritic cells and priming of multi-clonal cancer-specific cytotoxic T cells that kill cells that escaped the initial direct effects of NIR-PIT. The NIR-PIT can be applied to a wide variety of cancers either as monotherapy or in combination with conventional immune therapies to further activate anti-cancer immunity. A global Phase 3 clinical trial (https://clinicaltrials.gov/ct2/show/NCT03769506) of NIR-PIT targeting the epidermal growth factor receptor (EGFR) in patients with recurrent head and neck cancer is underway, employing RM1929/ASP1929, a conjugate of anti-EGFR antibody (cetuximab) plus the photo-absorber IRDye700DX (IR700). NIR-PIT has been given fast-track recognition by regulators in the USA and Japan. A variety of imaging methods, including direct IR700 fluorescence imaging, can be used to monitor NIR-PIT. As experience with NIR-PIT grows, additional antibodies will be employed to target additional antigens on other cancers or to target immune-suppressor cells to enhance host immunity. NIR-PIT will be particularly important in patients with localized and locally advanced cancers and may help such patients avoid side-effects associated with surgery, radiation and chemotherapy.
Keywords: multi-clonal immune response, imaging biomarker, immunogenic cell death, immunotherapy, regulatory cells
Near-infared photoimmunotherapy of cancer
Background
The three major cancer therapies, surgery, radiation and chemotherapy (1, 2), have dominated oncologic therapy since the beginning of modern medicine. These cancer therapies aim to eradicate cancer cells but do so at the expense of normal cells, leading to severe and sometimes lethal side-effects. Indeed, success with these treatment modalities is measured by the so-called ‘therapeutic index’, which compares the potential benefits to the potential risks. However, the off-target effects of these therapies can have profound effects on quality of life and actually reduce the helpful effects of the host immune system (3). For example, standard surgical procedures frequently remove regional lymph nodes, which could play a potential role of systemic host immunity against cancer, together with tumors. Additionally, radiation and chemotherapy sometimes preferentially kill lymphocytes much earlier than cancer cells because of the increased radiation sensitivity and high proliferation rate of lymphocytes (4, 5).
Ever since researchers realized the value of the immune system in the fight against cancer, there have been various approaches to enhancing the host immune response. Cancer immunity typically begins with dendritic cell (DC) activation whereby tumor antigens are recognized, processed and presented to T cells. DCs selectively activate CD8+ T cells that can react with cancer-specific antigens, then proliferate and differentiate into cytotoxic effector T cells capable of destroying cancer cells (6). Various strategies have been employed to amplify this immune response to cause enhanced anti-tumor activity.
To achieve efficient tumor killing, it is important that a sufficient number of cancer-specific T cells are present in the tumor microenvironment (TME). Cytokine therapies (7) such as systemic administration of T-cell-activating type 1 cytokines like IL-2 and IL-15 (8, 9) have been used to enhance T-cell activation, proliferation and effector function. Even when sufficient numbers of cancer cell-reacting T cells are produced, cancer cells can escape from host immunity by MHC class 1 down-regulation or PD-L1 expression, or by accommodating immune-suppressor cells (10–12).
To be effective, cancer-specific T cells have to be resident in the TME and maintain their cytotoxicity. However, there are multiple endogenous T-cell suppressor systems that counteract T-cell activation in the TME. One of these uses immune checkpoints, on T cells (PD-1) and tumors (PD-L1) to inhibit T-cell responses. Binding of PD-1 expressed on activated T cells to PD-L1 expressed on cancer cells results in loss of effector function, thus defeating anti-cancer immunity (13). Another inhibitory mechanism is based on CTLA4, a receptor that negatively regulates T-cell activation by blocking the activation signal from DCs (14). Immune-checkpoint inhibitors (15) such as anti-PD-1, anti-PD-L1 and anti-CTLA4 monoclonal antibodies (mAbs) aim to counteract T-cell suppression by interfering with these cell–cell interactions.
Additionally, some T cells negatively regulate T-cell effector function in the TME. Depletion of negative regulatory T cells (Tregs) (16) and myeloid-derived suppressor cells (MDSCs) (17) has been shown to improve anti-tumor immune responses (18, 19).
There are also therapeutic strategies that target a specific cancer antigens with engineered T cells. Chimeric antigen receptor (CAR)-T cells are designed to attack the target cancer cells, which express a specific surface antigen, by modifying the antigen receptor on T cells (20). This therapy requires production of transfected T cells outside the body, followed by cell transfer back to the patient. Yet another approach is to use cancer vaccines that provoke an anti-cancer immune response based on the introduction of a cancer-specific antigen (21).
Collectively, these therapies have created an exciting new direction for cancer therapy and are increasingly used clinically. However, one persistent limitation of the current immunotherapies is that they are inherently systemic and, while desirable effects may occur in the tumor, undesirable side-effects such as autoimmune reactions can occur in other, normal, organs (22). Additionally, such therapies are useful only in a minority of patients, probably because therapeutic effects rely either on existing lymphocytes that have been exposed to cancer, or on a single target antigen that can be hidden, allowing for immune escape. It is still poorly understood which biomarkers are predictive of immune responses. In some cases, the immune therapy has its desired effect but the effect is too mild to take on the bulk of cancer cells within an established tumor (13).
Theoretically, an ideal cancer therapy would both directly destroy cancer cells to minimize residual cancer cells as well as activate the local host immune response to wipe out remaining cancer cells. Such a therapy would be highly selective for cancer cells but have minimal or no off-target effects in the TME.
Design strategy of near-infrared photoimmunotherapy
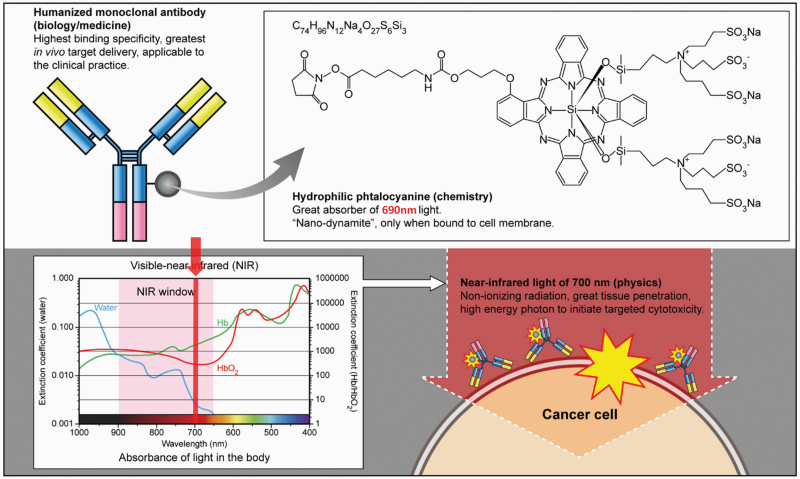
Near-infrared photoimmunotherapy (NIR-PIT) is a therapy that induces direct cancer killing via immunogenic cell death (ICD), thus activating the anti-cancer immune system locally in the TME (23). NIR-PIT is designed to selectively destroy target cells. The specificity comes from the mAb, which is conjugated to the photo-activating, phthalocyanine-based chemical, IRDye700DX (IR700). Selective cytotoxicity is induced in a modest number of antibody–IR700-bound cells, only when exposed to non-thermal doses of NIR at 690 nm, the activating wavelength for IR700 (Fig. 1). Upon absorption of this NIR light, a photo-induced ligand-release reaction occurs, which leads to dramatic physical and chemical changes in the conjugated antibody–antigen complex (24). This, in turn, leads to cell membrane micro-perforations that quickly coalesce into blebs followed by cell rupture.
Fig. 1.
Mechanism of NIR-PIT.
By choosing tumor-specific antigens [such as epidermal growth factor receptor (EGFR) or human epidermal growth factor receptor 2 (HER2) but many others will work], this therapy specifically destroys cancer cells while minimally harming any adjacent normal cells particularly tumor-infiltrating immune T cells or blood vessels. Furthermore, IR700 is a water-soluble photo-absorbing dye with no phototoxic or cytotoxic properties of its own; therefore, free unbound IR700 is safe and is rapidly excreted in the urine. The safety of NIR-PIT derives from two properties: first, the antibody–photo-absorber conjugate (APC) binds predominantly to specifically targeted cancer cells; and, second, the APC is only activated in areas exposed to NIR light. Therefore, NIR-PIT can be highly targeted to the tumor without damaging adjacent normal cells (25, 26).
This unique design has led to much success in pre-clinical experiments. Indeed, NIR-PIT has been shown efficacious against bladder (27–29), prostate (30, 31), gastric (32, 33), primary lung (34) and breast cancers (35), as well as epidermoid (23, 36), hepatocellular (37) and head and neck cancers (38, 39), glioblastoma (40, 41), melanoma (42), mesothelioma (43) and B-cell Lymphoma (44). Far from treating only primary tumors, NIR-PIT has also shown efficacy against metastatic lung cancers [both for solitary (45) and disseminated (46) metastases], peritoneally disseminated gastric (33, 47) and ovarian cancers (48) and pleural disseminated non-small cell (49) and small cell (50) lung cancer. As for hematological malignancies, NIR-PIT can successfully treat B-cell lymphomas by targeting CD20 and T-cell lymphomas by targeting CD25 in local masses (44), yet it is difficult for NIR-PIT to treat leukemias that do not form any local mass. However, bone cortex would not be the critical problem for NIR-PIT because 690 nm of NIR light can penetrate through human bone cortex (51). Therefore, if located, the bone marrow lesions of leukemias could be treated with NIR-PIT.
Immunological consequences after NIR-PIT
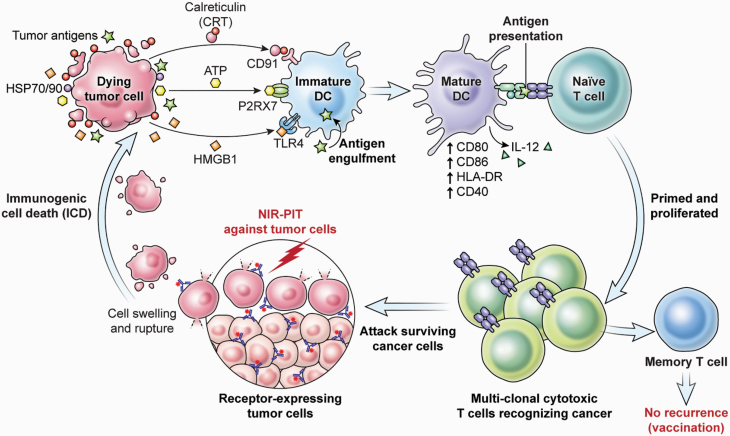
Destroying cancer cells without damaging normal cells nor compromising the host immune system is a significant benefit of NIR-PIT. Additionally, NIR-PIT induces ICD; that is, it initiates host immunity against targeted cancer cells (52). NIR-PIT-treated cancer cells release death signals including calreticulin, ATP and HMGB1, which can activate adjacent immature DCs even in tumor beds. These signals promote maturation of immature DCs, which engulf cancer-specific antigens that are released from the ruptured tumor cell, and these mature DCs prime and educate naive T cells to become cancer-specific CD8+ T cells (Fig. 2) (53). Such newly primed cancer-specific CD8+ T cells proliferate and attack other cancer cells, resulting in an amplified host anti-tumor immune response. This consequential process could convert some non-immunogenic tumors into immunogenic tumors by recognizing massively released neo-antigens.
Fig. 2.
NIR-PIT-induced ICD and immunological consequences.
This anti-tumor immune activation occurs first in the treated tumor site but eventually extends to other cancer sites because immune cells migrate throughout the body, resulting in a systemic immune response. Therefore, although NIR-PIT is a local therapy, the effect of NIR-PIT can be systemic, and may have an effect on distant metastatic sites. Indeed, some tumor-bearing mice and cancer patients achieve complete remission after a single therapy of cancer-cell-targeted NIR-PIT (53).
An important feature of host immune activation induced by NIR-PIT is that this therapy simultaneously activates the immune system against multiple antigens released from ruptured cancer cells (53). Most current targeted immunotherapies, including cancer vaccines or CAR-T therapies, identify a single target molecule on which to base the therapy. Having multiple clones of anti-tumor T cells, each responding to a unique antigen, results in a more comprehensive response to tumors expressing a broad spectrum of cancer-specific neo-antigens (54, 55).
NIR-PIT has demonstrated a profound immune response in humans. First-in-human Phase 1 and Phase 2 clinical trials of NIR-PIT with cetuximab–IR700 (RM1929) (56) targeting EGFR in patients with recurrent and advanced head and neck squamous cell cancer were successfully completed in 2016 and in late 2017, respectively. Several complete remissions and multiple significant partial remissions were reported in these studies (57, 58). The results far exceeded those of pre-clinical models in immune-deficient host xenograft models. Once the models were transferred to syngeneic models, a robust immune response was demonstrated. There is considerable evidence that this same response is seen in humans.
Further activation of host immunity
Recognizing that NIR-PIT not only selectively destroys tumor cells but also activates anti-cancer immunity, how can the immune effect be modulated to further improve therapeutic efficacy?
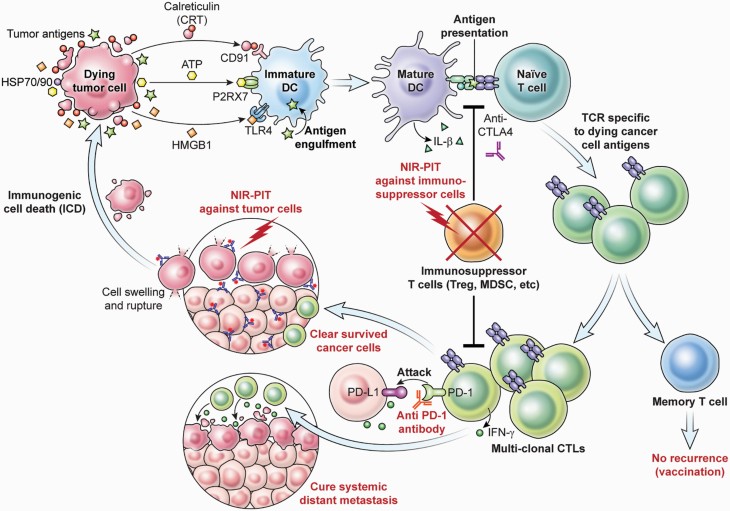
Conventional cancer immunotherapies—including type 1 cytokines such as IL-2 and IL-15 (8, 9), immune-checkpoint inhibitors such as anti-CTLA4 (59, 60) or anti-PD1/anti-PD-L1 antibodies (61, 62), and depletion of immune-suppressor cells such as Tregs or MDSCs (16, 17)—have been employed as anti-cancer strategies. Such therapies can activate and enhance effector function of existing CD8+ T cells, and they could potentially enhance the effect of ICD after NIR-PIT.
It may also be possible to selectively remove inhibitory immune cells from the local TME with NIR-PIT. For example, CD25-targeted or CCR4-targeted NIR-PIT can be used to eliminate Tregs, and CXCR2-targeted NIR-PIT can eliminate MDSCs from the TME. Such local suppressor cell depletion accompanied by tumor-targeted NIR-PIT could amplify the anti-cancer effects of NIR-PIT while minimizing systemic immunological side-effects compared with current therapies.
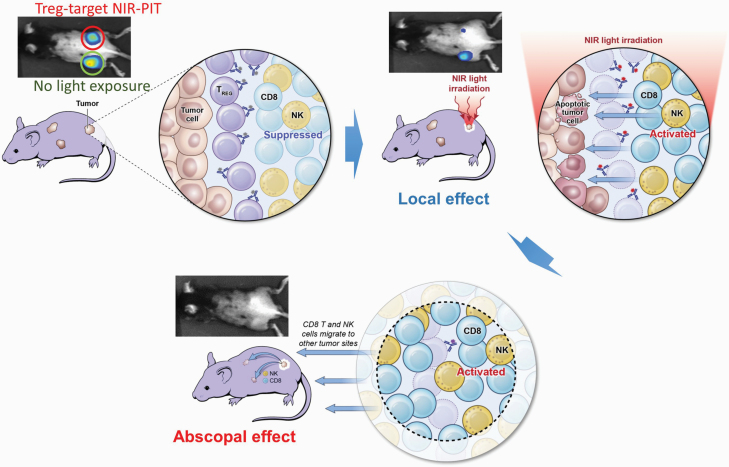
Localized Treg depletion with anti-CD25 NIR-PIT results in cancer cell killing (63). In the treated tumor beds, CD8+ T and NK cells were fully activated, and they released IFN-γ within a few hours of Treg depletion. Interestingly, this local Treg depletion produced therapeutic effects in non-treated tumors of the same cell type, grown in distant sites in the same animal, showing that anti-tumor immune activation was systemic even though the treatment was local (Fig. 3). Since NIR-PIT only kills local populations of Tregs, re-population of Tregs from the systemic circulation might hamper long-lasting immunity. In our experience, Tregs re-populate tumor beds approximately 1 week after NIR-PIT (53). However, the CD8+ T-cell/Treg ratio remains high for several weeks. Therefore, a CD8+ T-cell dominant TME would maintain an activated anti-cancer host immunity that could be enhanced by checkpoint inhibitors. If necessary, repeated cycles of NIR-PIT can be used to eliminate Treg cells from the tumor bed for extended periods because NIR-PIT is not limited in the number of times it can be administered. Ideally, Treg-targeted NIR-PIT could be combined with cancer-cell-targeted NIR-PIT. Animal models of this approach have suggested superior local and systemic tumor responses compared with either approach alone (64). Similarly, when checkpoint inhibitors are combined with cancer-targeted NIR-PIT, efficacy is also enhanced (53).
Fig. 3.
Local negative Treg cell depletion by NIR-PIT that targets CD25 in the tumor bed induces rapid tumor cell killing in the treated tumor and abscopal effects to distant untreated tumors.
During treatment of cancer-targeted NIR-PIT, tumors start shrinking and disappear several days after NIR light exposure. Initial tumor regression is a result of NIR-PIT’s direct cytotoxicity, but the sustained response is a result of newly primed CD8+ T cells proliferating and differentiating into cytotoxic T cells. In some cases, tumors completely respond without recurrence, meaning long-term immune memory was successfully established. Strong T-cell priming by an activating cytokine is known to promote not only T-cell activation but also the memory T-cell formation necessary for long-term immunity (65, 66). Thus, the combination of tumor-targeted NIR-PIT and IL-2/IL-15 cytokine therapy is predicted to enhance both T-cell activation and long-time immunity. In animal models, after complete tumor eradication with NIR-PIT, it is often impossible to re-introduce the same tumor in the mouse, indicating that long-term immunity has been established (Fig. 4).
Fig. 4.
Combination therapy of cancer-targeting NIR-PIT with immunoactivation therapies.
Imaging evaluation of NIR-PIT
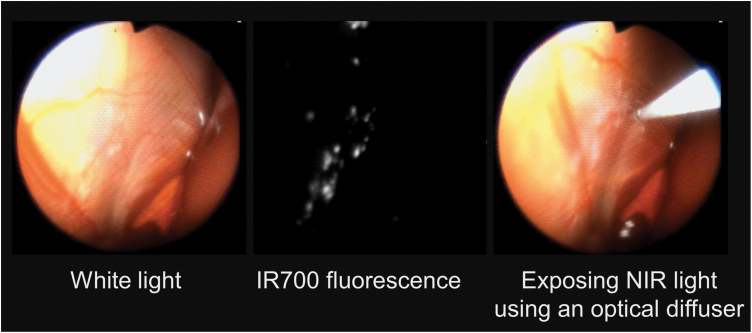
The therapeutic effects of NIR-PIT can be monitored with several imaging modalities. One method, designed for imaging during therapy, is IR700 fluorescence imaging, which can be utilized to confirm that the conjugate has bound to the tumor. Upon activation with 690nm NIR light, IR700 fluorescence disappears due to the formation of dimers and oligomers of phthalocyanine cores and/or precipitation of conjugated proteins after a photochemical ligand-release reaction (24). This photobleaching leads to loss of fluorescence and indicates that maximal light exposure has been achieved. Thus, IR700 fluorescence can be used during light exposure as an indicator of proper light dosimetry (24). A customized system incorporating an excitation NIR light source and a fluorescence camera system has been designed and implemented for intraoperative imaging of NIR-PIT (Fig. 5) (47).
Fig. 5.
NIR-PIT via a IR700 fluorescence endoscopy system.
Once the NIR-PIT treatment is complete, several other imaging methods can be used to assess efficacy. For example, if a tumor takes up glucose before treatment, it will immediately lose that ability after treatment. This is because, within minutes to hours of treatment, most of the cancer cells die rapidly releasing intracellular ATP, the loss of which disables biological functions including transporters and metabolic pathways. Thus, 18F-fluorodeoxy glucose positron emission tomography (18F-FDG-PET) serves as an excellent method of assessing acute therapeutic effects of NIR-PIT treatment. Changes on PET occur much earlier than physically observable tumor size changes (67).
For superficial lesions or where endoscopy is used, there are two additional optical methods of assessing NIR-PIT. Fluorescence lifetime imaging (68) and bioluminescence imaging (BLI) (69) can be used to assess acute PIT therapeutic effects. For now, these methods are only used in pre-clinical studies. By measuring fluorescence lifetime shortening of IR700, acute necrotic cell death or ICD can be evaluated. In the case of BLI, similar to 18F-FDG-PET, loss of ATP and cell destruction leads to a reduced BLI signal. However, BLI requires the transfection of tumor cells with a luciferase gene in order to catalyze the reaction whereby the substrate luciferin produces light. Thus, it is strictly limited to pre-clinical applications.
NIR-PIT-induced super-enhanced permeability and retention
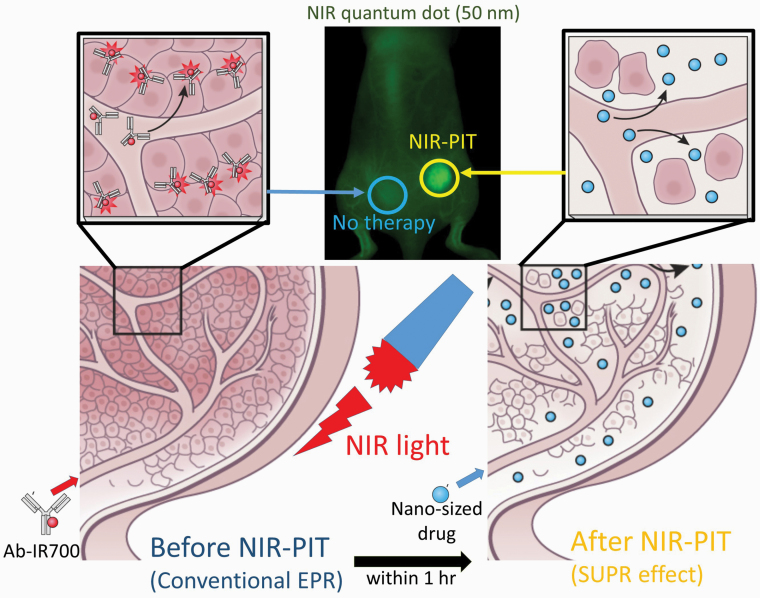
Another unique aspect of NIR-PIT is that immediately after the administration of NIR light, the vascular permeability in the tumor bed is greatly enhanced, especially for nano-sized molecules. This phenomenon has been termed super-enhanced permeability and retention (SUPR) to contrast it with enhanced permeability and retention (EPR) that is a common feature of tumor vasculature (70). The SUPR effect dramatically increases leakage into the tumor up to 24-fold compared with EPR effects (Fig. 6). The SUPR effect lasts only for a few hours after NIR-PIT but then begins to normalize.
Fig. 6.
Mechanism of NIR-PIT-induced SUPR effects.
The SUPR effect is caused by killing of perivascular cancer cells, as these are the first cells exposed to the APC. This killing creates a space between the vessel wall and the remaining tumor mass, reducing vascular resistance, enlarging vessels, increasing tumor blood volume and decreasing blood velocity. Consequently, there is improved delivery of nano-sized therapeutic agents into the treated tumor bed where they can remain and be effective for several days. Therefore, a combination of NIR-PIT and nano-sized anti-cancer agents is more effective than either of the therapies alone and can eliminate tumors left over after the initial NIR-PIT treatments.
In a study employing FDA-approved liposome-encapsulated daunorubicin (DaunoXome®) or nanoparticle albumin-bound paclitaxel (nab-paclitaxel; Abraxane®) in mouse xenograft models of cancer, there were significantly better therapeutic effects after NIR-PIT than with either therapy alone (71). The SUPR effect also allows for enhanced delivery of antibodies and APCs with increased leakage into tumor beds after the initial NIR-PIT treatment. Mouse xenograft cancer models showed that multiple applications of light slowed regrowth and increased the cure rate (72). Antibody–drug conjugates (ADCs), with photoactivatable drug-release systems can also be incorporated in the treatments by (i) performing NIR-PIT and inducing SUPR effects, then (ii) delivering large amounts of ADCs through the SUPR effect and (iii) re-exposing the tumor site to a second dose of NIR light, thus releasing the drug (73). Low-molecular-weight anti-cancer agents that bind to proteins also behave similarly to nano-sized agents and, thus, demonstrate enhanced delivery after NIR-PIT.
SUPR effects can also provide an opportunity for an imaging biomarker of successful NIR-PIT. For instance, by using nano-sized imaging agents such as iron oxide nanoparticles can be used in magnetic resonance imaging (MRI) after NIR-PIT. Quantum dots could be used for fluorescence imaging. Additionally protein-binding small molecular contrast agents such as gadofosveset (Ablavar®) could be used in MRI after NIR-PIT (74). Indocyanine Green could also be used for fluorescence imaging after NIR-PIT (75). Thus, the SUPR effect can be used as a pharmacodynamic biomarker of NIR-PIT.
Summary
NIR-PIT is a new type of cancer therapy that will add to the methods currently used to treat cancer. It features highly selective cancer cell killing with ICD that activates a robust host immune response. Although NIR-PIT is a local treatment, the induced immune activation is potentially systemic, thus providing a greater-than-expected impact for a ‘local’ therapy. A number of imaging methods have been developed to monitor this therapy and assess acute tumor response. By combining cancer-targeted and immune-suppressor-targeted NIR-PIT, an even more robust immune response can be generated. Adjuvant therapies such as cytokine therapy and checkpoint inhibitors may also amplify the immune effect. With its wide range of flexible applications and multiple ways of enhancing its effects, NIR-PIT has great potential to become a valuable cancer therapy.
Funding
This research was supported by the Intramural Research Program of the National Institutes for Health, National Cancer Institute, Center for Cancer Research (ZIA BC 011513). For AR, this research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Chabner B. A. and Roberts T. G. Jr. 2005. Timeline: chemotherapy and the war on cancer. Nat. Rev. Cancer 5:65. [DOI] [PubMed] [Google Scholar]
- 2. Chen H. H. W. and Kuo M. T. 2017. Improving radiotherapy in cancer treatment: promises and challenges. Oncotarget 8:62742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang D. H., Weaver M. T., Park N. J., Smith B., McArdle T. and Carpenter J. 2009. Significant impairment in immune recovery after cancer treatment. Nurs. Res. 58:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackall C. L., Fleisher T. A., Brown M. R. et al. 1994. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 84:2221. [PubMed] [Google Scholar]
- 5. Dainiak N. 2002. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 30:513. [DOI] [PubMed] [Google Scholar]
- 6. Chen D. S. and Mellman I. 2013. Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1. [DOI] [PubMed] [Google Scholar]
- 7. Waldmann T. A. 2018. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 10:a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang T., Zhou C. and Ren S. 2016. Role of IL-2 in cancer immunotherapy. Oncoimmunology 5:e1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson T. O. and Schluns K. S. 2017. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 190:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garrido F., Algarra I. and García-Lora A. M. 2010. The escape of cancer from T lymphocytes: immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol. Immunother. 59:1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagaraj S. and Gabrilovich D. I. 2008. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 68:2561. [DOI] [PubMed] [Google Scholar]
- 12. Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T. and Minato N. 2002. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99:12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang Y., Li Y. and Zhu B. 2015. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 6:e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowshanravan B., Halliday N. and Sansom D. M. 2018. CTLA-4: a moving target in immunotherapy. Blood 131:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kyi C. and Postow M. A. 2016. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy 8:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viehl C. T., Moore T. T., Liyanage U. K. et al. 2006. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann. Surg. Oncol. 13:1252. [DOI] [PubMed] [Google Scholar]
- 17. Gabrilovich D. I. 2017. Myeloid-derived suppressor cells. Cancer Immunol. Res. 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fleming V., Hu X., Weber R. et al. 2018. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front. Immunol. 9:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dominguez G. A., Condamine T., Mony S. et al. 2017. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin. Cancer Res. 23:2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. June C. H. and Sadelain M. 2018. Chimeric antigen receptor therapy. N. Engl. J. Med. 379:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mougel A., Terme M. and Tanchot C. 2019. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front. Immunol. 10:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farhood B., Najafi M. and Mortezaee K. 2019. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiol. 234:8509. [DOI] [PubMed] [Google Scholar]
- 23. Mitsunaga M., Ogawa M., Kosaka N., Rosenblum L. T., Choyke P. L. and Kobayashi H. 2011. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 17:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sato K., Ando K., Okuyama S. et al. 2018. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: a mechanism of cancer cell cytotoxicity after near-infrared photoimmunotherapy. ACS Cent. Sci. 4:1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi H. and Choyke P. L. 2019. Near-infrared photoimmunotherapy of cancer. Acc. Chem. Res. 52:2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi H., Griffiths G. L. and Choyke P. L. 2020. Near-infrared photoimmunotherapy: photoactivatable antibody-drug conjugates (ADCs). Bioconjug. Chem. 31:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Railkar R., Krane L. S., Li Q. Q. et al. 2017. Epidermal growth factor receptor (EGFR)-targeted photoimmunotherapy (PIT) for the treatment of EGFR-expressing bladder cancer. Mol. Cancer Ther. 16:2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siddiqui M. R., Railkar R., Sanford T. et al. 2019. Targeting epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) expressing bladder cancer using combination photoimmunotherapy (PIT). Sci. Rep. 9:2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiss B., van den Berg N. S., Ertsey R. et al. 2019. CD47-targeted near-infrared photoimmunotherapy for human bladder cancer. Clin. Cancer Res. 25:3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagaya T., Nakamura Y., Okuyama S. et al. 2017. Near-infrared photoimmunotherapy targeting prostate cancer with prostate-specific membrane antigen (PSMA) antibody. Mol. Cancer Res. 15:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lütje S., Heskamp S., Franssen G. M. et al. 2019. Development and characterization of a theranostic multimodal anti-PSMA targeting agent for imaging, surgical guidance, and targeted photodynamic therapy of PSMA-expressing tumors. Theranostics 9:2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shirasu N., Yamada H., Shibaguchi H., Kuroki M. and Kuroki M. 2014. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. Int. J. Cancer 135:2697. [DOI] [PubMed] [Google Scholar]
- 33. Kazuhide S., Peter L. C., and Hisataka K. 2015. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS ONE 9:e113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagaya T., Nakamura Y., Sato K. et al. 2017. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget 8:8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tadanobu N., Kazuhide S., Toshiko H., Yuko N., Peter L. C., and Hisataka K. 2015. Near infrared photoimmunotherapy targeting EGFR positive triple negative breast cancer: optimizing the conjugate-light regimen. PLoS ONE 10:e0136829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sano K., Nakajima T., Choyke P. L. and Kobayashi H. 2014. The effect of photoimmunotherapy followed by liposomal daunorubicin in a mixed tumor model: a demonstration of the super-enhanced permeability and retention effect after photoimmunotherapy. Mol. Cancer Ther. 13:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanaoka H., Nagaya T., Sato K. et al. 2015. Glypican-3 targeted human heavy chain antibody as a drug carrier for hepatocellular carcinoma therapy. Mol. Pharm. 12:2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watanabe S., Noma K., Ohara T. et al. 2019. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol. Ther. 20:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagaya T., Nakamura Y., Okuyama S. et al. 2017. Syngeneic mouse models of oral cancer are effectively targeted by anti-cd44-based NIR-PIT. Mol. Cancer Res. 15:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jing H., Weidensteiner C., Reichardt W. et al. 2016. Imaging and selective elimination of glioblastoma stem cells with theranostic near-infrared-labeled CD133-specific antibodies. Theranostics 6:862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burley T. A., Mączyńska J., Shah A. et al. 2018. Near-infrared photoimmunotherapy targeting EGFR-Shedding new light on glioblastoma treatment. Int. J. Cancer 142:2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei W., Jiang D., Ehlerding E. B. et al. 2019. CD146-targeted multimodal image-guided photoimmunotherapy of melanoma. Adv. Sci. (Weinh.). 6:1801237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagaya T., Nakamura Y., Sato K. et al. 2016. Near infrared photoimmunotherapy with an anti-mesothelin antibody. Oncotarget 7:23361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagaya T., Nakamura Y., Sato K., Harada T., Choyke P. L. and Kobayashi H. 2016. Near infrared photoimmunotherapy of B-cell lymphoma. Mol. Oncol. 10:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sato K., Nagaya T., Nakamura Y., Harada T., Choyke P. L. and Kobayashi H. 2015. Near infrared photoimmunotherapy prevents lung cancer metastases in a murine model. Oncotarget 6:19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sato K., Nagaya T., Mitsunaga M., Choyke P. L. and Kobayashi H. 2015. Near infrared photoimmunotherapy for lung metastases. Cancer Lett. 365:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nagaya T., Okuyama S., Ogata F., Maruoka Y., Choyke P. L. and Kobayashi H. 2019. Near infrared photoimmunotherapy using a fiber optic diffuser for treating peritoneal gastric cancer dissemination. Gastric Cancer 22:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato K., Hanaoka H., Watanabe R., Nakajima T., Choyke P. L. and Kobayashi H. 2015. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol. Cancer Ther. 14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sato K., Nagaya T., Choyke P. L. and Kobayashi H. 2015. Near infrared photoimmunotherapy in the treatment of pleural disseminated NSCLC: preclinical experience. Theranostics 5:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Isobe Y., Sato K., Nishinaga Y. et al. 2020. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine 52:102632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakamura Y. A., Okuyama S., Furusawa A. et al. 2019. Near-infrared photoimmunotherapy through bone. Cancer Sci. 110:3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogawa M., Tomita Y., Nakamura Y. et al. 2017. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 8:10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nagaya T., Friedman J., Maruoka Y. et al. 2019. Host immunity following near-infrared photoimmunotherapy is enhanced with PD-1 checkpoint blockade to eradicate established antigenic tumors. Cancer Immunol. Res. 7:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brown C. E. and Mackall C. L. 2019. CAR T cell therapy: inroads to response and resistance. Nat. Rev. Immunol. 19:73. [DOI] [PubMed] [Google Scholar]
- 55. Chen D. S. and Mellman I. 2017. Elements of cancer immunity and the cancer-immune set point. Nature 541:321. [DOI] [PubMed] [Google Scholar]
- 56. Kochuparambil S. T., McDonald D., Fidler M. J., Stenson K., Vasan N., Razaq M. A. 2017. Study of RM-1929 and photoimmunotherapy in patients with recurrent head and neck cancer. Ann. Oncol. 28: v372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cognetti D. M., Johnson J. M., Curry J. M. et al. 2019. Results of a phase 2a, multicenter, open-label, study of RM-1929 photoimmunotherapy (PIT) in patients with locoregional, recurrent head and neck squamous cell carcinoma (rHNSCC). J. Clin.Oncol. 37:6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gillenwater A. M., Cognetti D., Johnson J. M. et al. 2018. RM-1929 photo-immunotherapy in patients with recurrent head and neck cancer: results of a multicenter phase 2a open-label clinical trial. J. Clin. Oncol. 36:6039. [Google Scholar]
- 59. Seidel J. A., Otsuka A. and Kabashima K. 2018. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao Y., Yang W., Huang Y., Cui R., Li X. and Li B. 2018. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell. Physiol. Biochem. 47:721. [DOI] [PubMed] [Google Scholar]
- 61. Wu X., Gu Z., Chen Y. et al. 2019. Application of PD-1 blockade in cancer immunotherapy. Comput. Struct. Biotechnol. J. 17:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salmaninejad A., Valilou S. F., Shabgah A. G. et al. 2019. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J. Cell. Physiol. 234:16824. [DOI] [PubMed] [Google Scholar]
- 63. Sato K., Sato N., Xu B. et al. 2016. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 8:352ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maruoka Y., Furusawa A., Okada R. et al. 2020. Combined CD44- and CD25-targeted near-infrared photoimmunotherapy selectively kills cancer and regulatory T cells in syngeneic mouse cancer models. Cancer Immunol. Res. 8:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Williams M. A., Tyznik A. J. and Bevan M. J. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khan S. H., Martin M. D., Starbeck-Miller G. R., Xue H. H., Harty J. T. and Badovinac V. P. 2015. The timing of stimulation and IL-2 signaling regulate secondary CD8 T cell responses. PLoS Pathog. 11:e1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sano K., Mitsunaga M., Nakajima T., Choyke P. L. and Kobayashi H. 2013. Acute cytotoxic effects of photoimmunotherapy assessed by 18F-FDG PET. J. Nucl. Med. 54:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nakajima T., Sano K., Mitsunaga M., Choyke P. L. and Kobayashi H. 2012. Real-time monitoring of in vivo acute necrotic cancer cell death induced by near infrared photoimmunotherapy using fluorescence lifetime imaging. Cancer Res. 72:4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maruoka Y., Nagaya T., Nakamura Y. et al. 2017. Evaluation of early therapeutic effects after near-infrared photoimmunotherapy (NIR-PIT) using luciferase-luciferin photon-counting and fluorescence imaging. Mol. Pharm. 14:4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sano K., Nakajima T., Choyke P. L. and Kobayashi H. 2013. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano. 7:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hanaoka H., Nakajima T., Sato K. et al. 2015. Photoimmunotherapy of hepatocellular carcinoma-targeting glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine (Lond.) 10:1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mitsunaga M., Nakajima T., Sano K., Choyke P. L. and Kobayashi H. 2012. Near-infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugate. Bioconjug. Chem. 23:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nagaya T., Gorka A. P., Nani R. R. et al. 2018. Molecularly targeted cancer combination therapy with near-infrared photoimmunotherapy and near-infrared photorelease with duocarmycin-antibody conjugate. Mol. Cancer Ther. 17:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nakamura Y., Bernardo M., Nagaya T. et al. 2016. MR imaging biomarkers for evaluating therapeutic effects shortly after near infrared photoimmunotherapy. Oncotarget 7:17254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ali T., Nakajima T., Sano K., Sato K., Choyke P. L. and Kobayashi H. 2014. Dynamic fluorescent imaging with indocyanine green for monitoring the therapeutic effects of photoimmunotherapy. Contrast Media Mol. Imaging 9:276. [DOI] [PMC free article] [PubMed] [Google Scholar]