Abstract
We evaluated pain status change and associations with subsequent opioid/marijuana use among 1208 adult survivors of childhood cancer. Pain status and opioid/marijuana were self-reported at baseline and follow-up evaluation (mean interval = 4.2 years). Over time, 18.7% of survivors endorsed persistent/increasing significant pain; 4.8% and 9.0% reported having used opioids and marijuana at follow-up. Persistent/increased (vs none/decreased) pain, persistent/increased (vs none/decreased) anxiety, and lack of health insurance increased odds of subsequent opioid use by 7.69-fold (95% confidence interval [CI] = 3.71 to 15.95), 2.55-fold (95% CI = 1.04 to 6.24), and 2.50-fold (95% CI = 1.07 to 5.82), respectively. Persistent/increased (vs none/decreased) depression increased odds of subsequent marijuana use by 2.64-fold (95% CI = 1.10 to 6.33).
Pain is reported by up to 60% of survivors of childhood cancer (1), who are 1.4 times more likely to use opioids for pain compared with their siblings (2). However, long-term use of opioids is not recommended, because these agents do not eliminate pain and may increase risks of misuse and abuse as well as overdose-related death (3,4). Marijuana is increasingly used as an alternative to opioids but efficacy has not been established (5). Recent evidence indicates that marijuana use is associated with elevated pain severity and interference and decreased self-efficacy with pain management (6). We evaluated the change of pain status and psychological distress in associations with subsequent opioid or marijuana use among adult survivors of childhood cancer. We hypothesized that increased pain would increase subsequent opioid or marijuana use.
Data were collected from 1208 survivors of childhood cancer who participated in the St. Jude Lifetime Cohort Study (2007-2016). Eligible participants included survivors who received cancer treatment at St. Jude Children’s Research Hospital, were at least 10 years from initial diagnosis, aged 18 years or older at participation, and underwent a baseline (T1) and a follow-up (T2) clinical assessment (Supplementary Figure 1, available online). Cancer diagnoses and treatment data were abstracted from medical records. Sociodemographics, pain status, psychological distress, and opioid and marijuana use were self-reported. Survivors provided written informed consent for the participation of this study. The hospital’s institutional review board approved the study protocol.
Pain status was measured using the Medical Outcomes Study SF-36’s bodily pain scale; each participant was categorized as having significant (t scores <40) or nonsignificant pain (≥40) (1,2). Change in significant pain status was categorized as persistent/increased if significant pain was reported at both T1 and T2 or changed from nonsignificant at T1 to significant at T2 and categorized as none/decreased if nonsignificant pain was reported at both T1 and T2 or changed from significant at T1 to nonsignificant at T2. Participants listed prescribed opioids consistently taken for at least 30 days in the prior 2 years. American Hospital Formulary Service Drug Information (7) was used to classify opioid medications. Marijuana utilization was categorized as ever vs never used in the prior 30 days. Symptoms of anxiety and depression were assessed using the Brief Symptom Inventory-18, with t scores above 63 as significant (2).
Multivariable multinomial logistic regression model estimated the odds ratios (ORs) for associations of significant pain status change with T2 opioid or marijuana use vs neither substance use, adjusting for age, sex, race, education, annual household income, marital status, health insurance, years since diagnosis, cancer therapy contributing to chronic pain, anxiety/depression, and T1 opioid/marijuana use. We used the elastic net technique to select a parsimonious list of independent variables that can best explain the associations between independent and dependent variables. This method is appropriate in the setting of sparse data and involves 2 penalty terms that constrain the size of the estimated coefficients, which decreases the chance of false-positive findings (8). Statistically significant differences were decided by P values less than .05 (2-sided).
The mean age of participants at baseline was 33.6 (SD = 7.9) years, mean time since cancer diagnosis to baseline was 24.7 (SD = 7.9) years, and mean interval between T1 and T2 was 4.2 (SD = 1.3) years. Nonparticipants were likely to be younger at T1 and to have shorter follow-up time from cancer diagnosis, a diagnosis of central nervous system malignancy, lower socioeconomic status, and undergone major surgery. Survivors who completed health surveys at both T1 and T2 were less likely to use marijuana compared with survivors who completed surveys at T1 only (Supplementary Table 1, available online). Regarding pain status change, 18.7% had persistent/increased significant pain, and 81.3% had none/decreased significant pain (Supplementary Table 2, available online). For substance use, 8.5% of participants reported having opioid use for at least 30 days and 11.9% reported having marijuana use at T1 and/or T2. At T2 alone, 4.8% and 9.0% reported opioid and marijuana, respectively (Supplementary Table 3, available online).
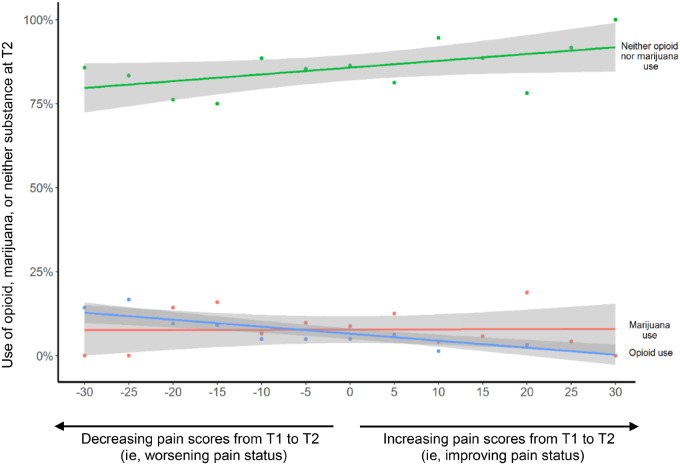
When pain was analyzed as a continuous variable, survivors with increased pain over time were more likely to report opioid than marijuana use (Figure 1). When pain was analyzed as a binary variable (significant/nonsignificant), survivors with persistent/increased significant pain had 7.69-fold higher odds of reporting opioid (95% confidence interval [CI] = 3.71 to 15.95) but not marijuana use (OR = 0.96; 95% CI = 0.42 to 2.17) at T2 vs survivors with none/decreased significant pain (Table 1). Other factors for reporting opioid use included persistent/increased vs none/decreased anxiety (OR = 2.55, 95% CI = 1.04 to 6.24), White vs non-White (OR = 8.40, 95% CI = 1.08 to 65.10), and uninsured vs insured status (OR = 2.50, 95% CI = 1.07 to 5.82). Survivors with persistent/increased depressive symptoms had 2.64-fold higher odds of reporting marijuana use at T2 (95% CI = 1.10 to 6.33).
Figure 1.
The overall trend of using opioid, marijuana, or neither in associations with the change of pain scores over time. Change of pain scores over time in associations with the use of opioid or marijuana at a follow-up time point. This figure was created based on linear regression model, where the independent variables are the change of pain scores (a continuous variable) from T1 (baseline) and T2 (follow-up), and the dependent variable is the substance status at T2. The shading in the figure represents the 95% confidence intervals for the estimated association of the pain score change from T1 and T2 with the substance use at T2. Pain status was measured by the SF-36 Bodily Pain Scale as continuous scores with lower values indicating more pain; therefore, more positive values in this figure indicate improving pain status and more negative values indicate worsening pain status from T1 and T2. T1 was defined as the time point when survivors completed baseline medical evaluation and self-reported health survey, and T2 as the time point when St. Jude Lifetime Cohort Study (SJLIFE) survivors completed a follow-up evaluation and self-reported health survey.
Table 1.
Pain status change from T1a to T2b and other factors associated with subsequent opioid or marijuana use at T2: multivariable multinomial logistic regression
| Determinants | Opioid use only at T2c,d | Marijuana use only at T2c,d |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Change of significant pain status from T1 to T2e | ||
| None/decreased | 1.00 (Referent) | 1.00 (Referent) |
| Persistent/increased | 7.69 (3.71 to 15.95) | 0.96 (0.42 to 2.17) |
| Change of significant depression status from T1 to T2f | ||
| None/decreased | 1.00 (Referent) | 1.00 (Referent) |
| Persistent/increased | 1.45 (0.60 to 3.52) | 2.64 (1.10 to 6.33) |
| Change of significant anxiety status from T1 to T2f | ||
| None/decreased | 1.00 (Referent) | 1.00 (Referent) |
| Persistent/increased | 2.55 (1.04 to 6.24) | 0.68 (0.24 to 1.97) |
| Opioid/marijuana use at T1g | ||
| None | 1.00 (Referent) | 1.00 (Referent) |
| Opioid only | 4.97 (1.98 to 12.47) | 2.00 (0.44 to 9.14) |
| Marijuana only | 1.23 (0.24 to 6.27) | 51.06 (26.04 to 100.13) |
| Age at survey | ||
| 18-29.9 y | 1.00 (Referent) | 1.00 (Referent) |
| 30-39.9 y | 0.97 (0.28 to 3.36) | 0.74 (0.35 to 1.55) |
| ≥ 40 y | 1.89 (0.57 to 6.26) | 0.41 (0.18 to 0.93) |
| Sex | ||
| Male | 1.00 (Referent) | 1.00 (Referent) |
| Female | 0.71 (0.36 to 1.40) | 0.48 (0.27 to 0.87) |
| Race/ethnicity | ||
| Non-Hispanic White | 1.00 (Referent) | 1.00 (Referent) |
| White | 8.40 (1.08 to 65.10) | 1.40 (0.60 to 3.30) |
| Educational level | ||
| Below HS | 1.11 (0.36 to 3.43) | 0.93 (0.29 to 2.96) |
| HS graduate/GED | 1.24 (0.51 to 3.03) | 1.37 (0.62 to 3.02) |
| Some college/training after HS | 1.57 (0.67 to 3.69) | 2.07 (1.02 to 4.16) |
| College graduate and above | 1.00 (Referent) | 1.00 (Referent) |
| Marital status | ||
| Married/living with a partner | 1.00 (Referent) | 1.00 (Referent) |
| Status other than married | 1.18 (0.59 to 2.37) | 1.18 (0.65 to 2.13) |
| Heath insurance | ||
| Insured | 1.00 (Referent) | 1.00 (Referent) |
| Uninsured | 2.50 (1.07 to 5.82) | 1.97 (0.96 to 4.02) |
| History of steroid therapy | ||
| No | 1.00 (Referent) | 1.00 (Referent) |
| Yes | 0.60 (0.25 to 1.48) | 0.59 (0.29 to 1.23) |
| History of vincristine therapy | ||
| No | 1.00 (Referent) | 1.00 (Referent) |
| Yes | 1.15 (0.49 to 2.69) | 1.77 (0.85 to 3.68) |
| History of major surgery | ||
| No | 1.00 (Referent) | 1.00 (Referent) |
| Yes | 1.42 (0.55 to 3.69) | 1.59 (0.73 to 3.48) |
T1: the time point when survivors completed baseline medical evaluation and self-reported health survey. CI = confidence interval; GED = general education diploma; HS = high school; OR = odds ratio.
T2: the time point when survivors completed a follow-up evaluation and self-reported health survey.
Reference group: using neither opioid nor marijuana.
Using both opioid and marijuana was excluded because of a small sample size (n = 13) and nonconverged computation.
Significant pain status: the SF-36 bodily pain t score <40.
Significant depression and anxiety status: the Brief Symptom Inventory-18 depression and anxiety t scores ≥63.
Opioid/marijuana use at T1 was included as an independent variable because drug use at T1 was statistically significantly associated with drug use at T2 (as a dependent variable), and controlling for this variable helps elucidate true associations between pain status change over time and subsequent drug use at T2.
We observed that persistent/increased significant pain was statistically significantly associated with higher odds of opioid use. Increased anxiety and depression were related to both opioid and marijuana use, suggesting survivors may be using them to self-medicate emotional distress. The higher prevalence of opioid use among uninsured survivors is consistent with general US population data (9). In a national study, more than 20% of participants reported having diverted prescribed opioids to friends and/or family members, typically those who were uninsured or could not afford opioid medication for pain management (10). It is possible that uninsured survivors had limited access to evidence-based pain interventions, leading to the use of diverted opioids for worsening pain. Of concern, lack of insurance is related to twofold greater odds of opioid misuse and use disorder in US adults (11). Therefore, expanded insurance coverage to facilitate access to safe and effective multidisciplinary pain management is urgently needed.
Several state- and national-level studies have reported associations between cannabis legalization and decreased rates of opioid prescriptions (12,13) as well as overdose deaths (14). We identified decreased marijuana and increased opioid use among survivors whose pain worsened over time. These findings do not suggest survivors are substituting marijuana for opioids, but rather those with worsening pain may rely on opioids more than marijuana for pain. Additionally, worsening depression was associated with increased odds of marijuana use. These findings underscore the importance of continued monitoring of opioid/marijuana use, pain status, and emotional distress among survivors during follow-up care to assess drug use and provide appropriate interventions to prevent drug-related adverse effects.
This study has limitations. Our findings may not generalize to other survivor populations. The current analysis relied on data of pediatric cancer survivors from a single institution with the majority clustered in the US Southeast/Midwest regions where medical use of marijuana is not legalized (ie, St. Jude Children’s Research Hospital vs those in other states). In addition, our findings may not generalize to survivors outside the United States, with different health-care and legislation systems. Second, the differences in sociodemographic and clinical factors between participants and nonparticipants might threaten external validity of findings (ie, generalizability to all survivors of childhood cancer) but not internal validity (ie, associations of pain status change from T1 to T2 with substance use at T2). Third, pain status change and subsequent opioid/marijuana were observed for only 4.2 years, which is relatively brief for participants who were 29.3 years postcancer diagnosis. Fourthly, we used self-reported data on prescribed medications, not accounting for nonprescription opioids (eg, street drugs). Fifthly, persistent/increased pain was defined as significant pain present at 2 time points over 4 years instead of pain that persisted for at least 3 consecutive months, which defines chronic pain (15).
In conclusion, persistent/increased significant pain was associated with higher prevalence of opioid use in adult survivors of childhood cancer. Future research investigating opioid and marijuana use among survivors with chronic pain and prevalence and risk factors for substance-related use disorder is warranted.
Funding
This study was supported by the US National Cancer Institute grants U01 CA195547 (to MM Hudson & LL Robison) and P30 CA021765-33 (to C Roberts [CORE]).
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Author contributions: I-CH, DLA: Concept and design. NMA, TMB, KRK, JLK, LLR, MMH: Administrative support. LLR, MMH: Provision of study materials. MGB, MJE, WLG: Collection and assembly of data. I-CH, NMA, ZL, DKS, LLR, MMH, DLA: Data analysis and interpretation. I-CH: Manuscript writing. All authors: editing and final approval of manuscript.
Disclosures: All coauthors declare no conflict of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort study. J Clin Oncol. 2013;31(33):4242–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brinkman TM, Ullrich NJ, Zhang N, et al. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7(1):104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldini A, Von Korff M, Lin EH. A review of potential adverse effects of long-term opioid therapy: a practitioner’s guide. Prim Care Companion CNS Disord. 2012;14(3):PCC.11m01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–286. [DOI] [PubMed] [Google Scholar]
- 5. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932–1954. [DOI] [PubMed] [Google Scholar]
- 6. Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health. 2018;3(7):e341–e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Society of Health-System Pharmacists. American Hospital Formulary Service® Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2019. [Google Scholar]
- 8. Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc B. 2005;67(2):301–320. [Google Scholar]
- 9. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy-Hendricks A, Gielen A, McDonald E, McGinty EE, Shields W, Barry CL. Medication sharing, storage, and disposal practices for opioid medications among US adults. JAMA Intern Med. 2016;176(7):1027–1029. [DOI] [PubMed] [Google Scholar]
- 11. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 12. Bradford AC, Bradford WD, Abraham A, Bagwell Adams G. Association between US state medical cannabis laws and opioid prescribing in the Medicare Part D population. JAMA Intern Med. 2018;178(5):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen H, Hockenberry JM. Association of medical and adult-use marijuana laws with opioid prescribing for Medicaid enrollees. JAMA Intern Med. 2018;178(5):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med. 2014;174(10):1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2019;160(1):45–1007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.