Abstract
Objective
We evaluated our experience treating patients with localized extraskeletal myxoid chondrosarcomas (EMC) to evaluate outcomes and relapse rates in order to better inform treatment decisions for these rare soft tissue sarcomas.
Methods
We reviewed the records of 41 consecutive patients with localized EMC treated at our institution from 1990 to 2016. Most patients (n=33, 80%) received combined modality therapy (CMT) with surgery and RT, whereas only 8 (20%) underwent surgery alone. The Kaplan-Meier method was used to estimate rates of overall survival (OS), disease-specific survival (DSS), local control (LC), and distant metastatic free survival (DMFS).
Results
Median follow-up time was 94 months (range, 8–316). The 10-year local control (LC), distant metastatic free survival (DMFS), disease specific survival (DSS), and overall survival rates were 90%, 69%, 85%, and 66%, respectively. There were 5 patients (12%) with local relapse at a median time of 75 months (range 13–176). On univariate analysis, the only significant factor associated with poorer LC was the use of surgery alone (10-year LC 63% vs. 100% for CMT, P=0.004), which remained the only factor also significant on multivariable analysis (P=0.02, HR 12.7, 95% CI 1.4 −115.3). Thirteen patients (32%) developed DM at a median time of 28 months (range, 3–154). Interestingly, local recurrence was the only factor associated with poorer DMFS on multivariate analysis (P=0.04, HR 3.9, 95% CI 1.1–14.7).
Conclusions
For patients with EMC, surgery alone was associated with a higher risk of local recurrence. Therefore, we recommend optimal local therapeutic strategies upfront with both surgery and RT to reduce the risk of local and ultimately distant recurrence.
Keywords: extraskeletal, chondrosarcoma, extraskeletal myxoid chondrosarcoma, EMC, radiation therapy
INTRODUCTION
Extraskeletal myxoid chondrosarcomas (EMC) are rare translocation-associated soft tissue sarcomas (STS) of uncertain lineage that commonly present as a painless enlarging mass in the proximal extremities of middle aged male patients.1–4 Most EMC tumors harbor a characteristic chromosomal translocation t(9;22), which results in EWSR1 fusing to NR4A3, giving rise to gene products that alter cellular growth and differentiation.5–7 Histologically, these unusual tumors are characterized by multinodular collections of eosinophilic cells arranged in cords and strands separated by a myxoid stroma.2,8,9 The tumor cells were originally thought to resemble developing chondroblasts, but despite their classification as a chondrosarcoma, there is no evidence of cartilaginous differentiation.2,9 Therefore, the cell line of origin remains uncertain.
Historically, EMCs were regarded as low grade tumors. However, longer term follow up has demonstrated that while EMCs have fairly indolent growth patterns, biologically they behave more like an intermediate to high grade STS because of their high propensity for recurrence and metastases. Despite this high relapse risk, patients with EMC have generally favorable survival outcomes. Studies have demonstrated local recurrence rates ranging between 35% to 48% and distant recurrence rates of up to 50%.1,3,4,10 The indolent nature of EMC and the historical low grade classification has potentially led to the underutilization of combined modality local therapy (CMT) for patients with this disease contributing to the high risk of local relapse. In comparison to EMC, local relapse rates for other STS managed with CMT, including both surgery and RT, are notably lower.11–14 Therefore, the high local recurrence risk of patients with EMC suggest improved local control may be achievable by adding RT to surgical resection routinely.
Based on the available literature, there is a paucity of data to guide local management strategies for patients with EMC. Many of the studies consist of case reports, pathologic descriptions, or small cohorts comprised of patients with both metastatic and localized disease, which limits interpretation. Therefore, we present a homogenous cohort of patients with localized extraskeletal myxoid chondrosarcomas to evaluate outcomes and relapse rates in order to better inform treatment decisions for these rare tumors.
MATERIAL AND METHODS
We identified 41 consecutive patients with localized, non-metastatic histologically confirmed EMC treated at the University of Texas MD Anderson Cancer Center (MDACC) during the period from 1990 through 2016. The medical charts were then retrospectively reviewed after institutional review board approval. Patients underwent a complete history, physical examination, and staging before treatment. All diagnoses were confirmed at the time of presentation by sarcoma pathologists at MDACC.
Classically, EMC arise in soft tissue and thus are commonly treated using the STS sarcoma treatment paradigm at our institution. Patients are typically presented at a multidisciplinary tumor board for treatment discussion. For patients presenting with gross disease, they commonly underwent preoperative RT and resection. If patients presented after an outside excision, the decision for re-resection was made by the surgeon and multidisciplinary team based on margin status, tumor location, and impact of additional surgery on function. In cases where re-excision was not recommended, postoperative RT was performed.
Radiation delivery techniques varied as treatment era and anatomic location guided modality selection at the discretion of the treating physician. Most patients received photon-based RT (2D or 3D n=22; IMRT n=7), whereas three patients with superficial targets were treated with electrons and one received protons.
Recommendations regarding the use of neoadjuvant/adjuvant systemic therapy are typically based on perceived risk of dissemination and presence of active disease. Only three patients received cytotoxic neoadjuvant chemotherapy with differing regimens of which two contained doxorubicin and one was single agent ifosfamide.
For patients that develop metastatic disease, most are initially closely observed for a period of time given the slowly progressive nature of EMC. Treatment commonly consists of local therapies for patients with low volume metastatic disease or eventual initiation of systemic therapy once the burden of disease increases. Once systemic therapy was initiated for patients in our cohort with disseminated disease, most first-line regimens consisted of tyrosine kinase inhibitors (pazopanib n=4; sunitinib n=1), whereas two patients received gemcitabine/taxotere, and one trabectedin.
Follow-up and Statistical Analysis
Follow-up with a history and physical and restaging imaging studies of the primary site and lungs were performed typically every 3–4 months for the first two years, every 6 months for years 3–5 and then at least annually. Given the potential for late relapses, long-term follow up for patients with EMC is required. Local control (LC) was defined as no evidence of tumor recurrence in the primary tumor bed based on serial cross-sectional imaging with magnetic resonance imaging (MRI) or computed tomography (CT). Distant metastases were detected most commonly with CT chest, though several patients were followed with chest x-ray.
Descriptive statistics were used to evaluate baseline characteristics, and differences between proportions of categorical data were analyzed by using Fisher’s exact test and chi-squared analyses as appropriate. Survival times were calculated from the completion date of local therapy (the latest date of either surgery or last day of radiation therapy) to the first occurrence of the event of interest. The Kaplan-Meier method was used to estimate overall survival (OS), disease-specific survival (DSS), LC, and distant metastatic free survival (DMFS). Log-rank tests were used to assess for significance of differences between curves. Multivariate analyses were conducted using the Cox proportional hazards model. Significant (P ≤ 0.05) estimated hazard ratios (HR) and 95% confidence intervals (CI) are reported. IBM SPSS Statistics 22 was used for data analysis.
RESULTS
Patient and Tumor Characteristics
Patient and tumor characteristics are listed in Table 1. The median age was 53 years (range, 20–84 years), and 83% (n=34) were male. Anatomically, most tumors were located in the lower extremity (n=22, 54%; subsites: thigh n=14, knee n=2, leg n=2, angle/foot n=3, groin n=1) or upper extremity (n=10, 24%; subsites: shoulder n=1, upper arm n=3, elbow n=1, forearm n=3) with a limited number in the trunk (n=8, 20%; subsites: chest wall n=2, intrathoracic n=1; superficial abdomen n=1, pelvis n=2, buttocks n=4) or neck (2%, n=1). The median maximal tumor dimension was 7.5 cm (range, 2–38 cm) with a majority of tumors >5cm (n=32, 78%).
Table 1.
Patient and Tumor Characteristics for Patients
| Variable | Value or No. (%) (n=41) |
|---|---|
| Follow-up time living patients, months | |
| Median | 94 |
| Range | 8–316 |
| Age, years | |
| Median | 53 |
| Range | 20–84 |
| ≤ 65 | 34 |
| > 65 | 7 |
| Sex | |
| Female | 7 |
| Male | 34 |
| Tumor Location | |
| Head and neck | 1 |
| Upper Extremities | 10 |
| Trunk | 8 |
| Lower Extremities | 22 |
| Maximum Tumor Dimension, cm | |
| Median | 7.5 |
| Range | 2–38 |
| Tumor size | |
| ≤ 5 cm | 9 |
| > 5 cm | 32 |
| Final Margin Status | |
| Positive/Uncertain | 6 |
| Negative | 35 |
| Treatment Approach | |
| Postoperative RT | 10 |
| Preoperative RT | 23 |
| Surgery alone | 8 |
| Radiation Dose, Gy | |
| Median | 50 |
| Range | 50–65 |
| < 60 | 23 |
| ≥ 60 | 10 |
| Chemotherapy | |
| Neo/Adj | 3 |
Abbreviations: RT, radiation therapy; Neo/Adj, neoadjuvant or adjuvant
Treatment
Twenty-seven patients (66%) presented to MDACC with gross disease, whereas 14 patients (34%) presented after an outside excision had already been performed, of which 12 patients had a positive/uncertain margins and 2 had negative margins. Final margin status was negative in 35 patients (85%) and positive/uncertain in 6 patients (15%).
All but 4 patients (90%) were recommended to undergo either initial or repeat surgical resection at MDACC, and the 4 patients that did not undergo additional surgery received postoperative RT. The only patient or treatment factor that was associated with use of surgery alone was tumor location in the lower extremity (n=7, P=0.03; age P=0.42; sex P=0.58; tumor size P=0.23; margins P=0.71).
The majority of patients (n=33, 80%) received combined modality local therapy with both surgery and RT, whereas 8 patients received surgery alone (20%). The 23 patients (56%) who received preoperative RT received a median dose of 50 Gy (range, 50–50.4 Gy). The 10 patients (24%) who were treated with postoperative RT received a median dose of 60 Gy (range, 60–65 Gy). A total of only 3 patients (7%) were treated with chemotherapy either neoadjuvantly or adjuvantly.
Survival
The median follow-up time from the completion of local therapy for patients alive at last follow-up was 94 months (range, 8–316 months).
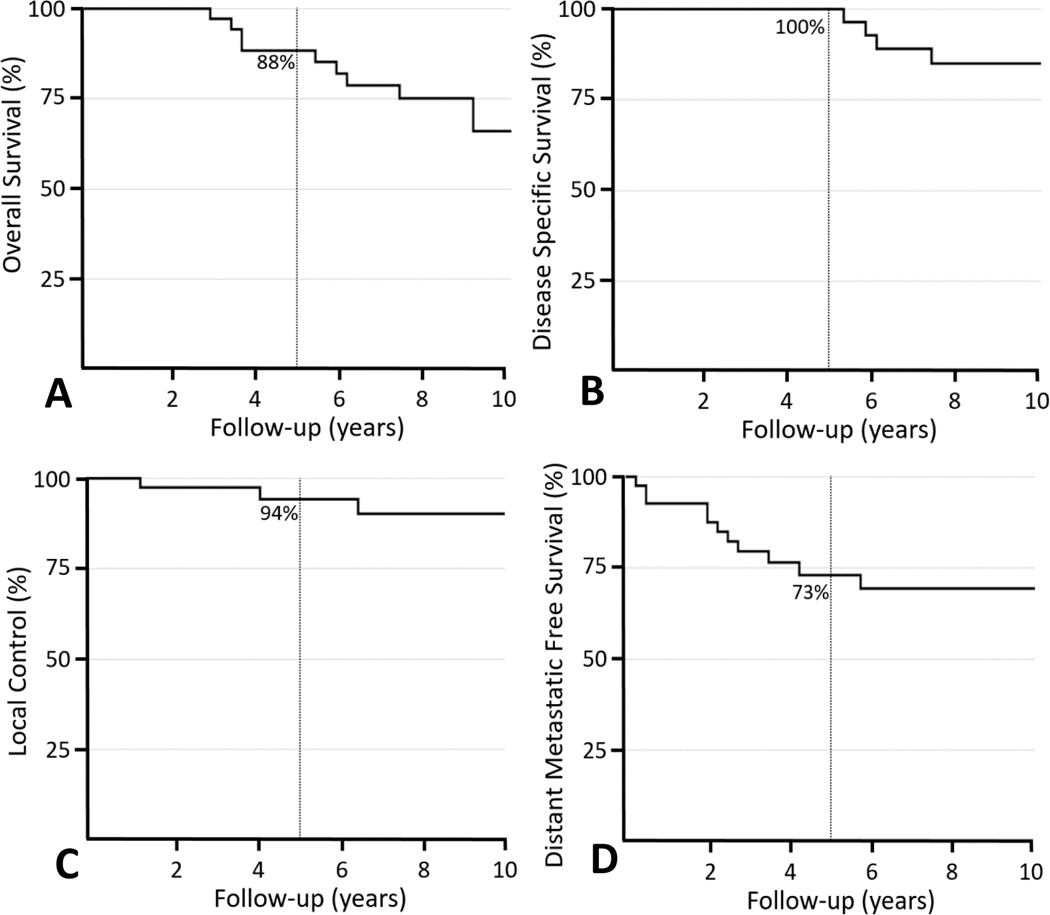
The 5-year and 10-year OS rates were 88% and 66%, respectively (Figure 1A). There were 5 deaths (12%) that were attributable to disease with a median survival of 74 months (range, 65–122 months) which resulted in a 5- and 10-year DSS of 100% and 85%, respectively (Figure 1B). All died with distant metastases.
Figure 1.
Kaplan-Meier curves showing 5-year outcomes for patients with extraskeletal myxoid chondrosarcomas: (A) overall survival (88%) (B) disease-specific survival (100%), (C) local control (94%), and (D) distant metastatic free survival (73%).
There were no disease- or treatment-related factor associated with DSS on univariate analysis (Table 2). However, distant recurrence was associated with a poorer 10-year DSS (62% vs. 100% if no DM, P=0.002) without influencing 10-year OS (62% vs. 68% if no DM, P=0.52).
Table 2.
Univariate Analyses for distant metastatic free survival, disease specific survival and local control
| Variable | DMFS at 5y | DMFS P value | DSS at 10y | DSS P Value | LC at 10y | LC P Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 78 | 0.67 | 100 | 0.67 | 100 | 0.73 |
| Male | 80 | 80 | 87 | |||
| Age, years | ||||||
| ≤ 65 | 66 | 0.43 | 87 | 0.57 | 89 | 0.70 |
| >65 | 86 | 75 | 100 | |||
| Tumor Location | ||||||
| Head and neck | 100 | 0.48 | 100 | 0.96 | 100 | 0.35 |
| Trunk | 50 | 100 | 100 | |||
| Upper Extremities | 88 | 80 | 100 | |||
| Lower Extremities | 70 | 80 | 82 | |||
| Tumor Size | ||||||
| > 5 cm | 59 | 0.02 | 80 | 0.15 | 87 | 0.60 |
| ≤ 5 cm | 100 | 100 | 100 | |||
| Final Margin Status | ||||||
| Positive/Uncertain | 67 | 0.25 | 83 | 0.91 | 80 | 0.12 |
| Negative | 70 | 86 | 93 | |||
| Treatment Plan | ||||||
| Surgery alone | 75 | 0.55 | 100 | 0.42 | 63 | 0.01 |
| Postoperative RT | 50 | 76 | 100 | |||
| Preoperative RT | 76 | 83 | 100 | |||
| Combined local therapy | ||||||
| Yes | 67 | 0.91 | 80 | 0.19 | 100 | 0.004 |
| No | 75 | 100 | 63 | |||
| Chemotherapy | ||||||
| Neo or adjuvant | 67 | 0.53 | 100 | 0.39 | 67 | 0.32 |
| None | 69 | 83 | 94 |
Abbreviations: DMFS, distant metastatic free survival; DSS, disease specific survival; OS, overall survival; RT, radiation therapy
Patterns of Disease Recurrence
Fifteen patients (37%) developed disease recurrence for an actuarial 5-year and 10-year disease free survival rate of 73 and 60%, respectively. The median time to any failure was 15 months (range, 3–176 months).
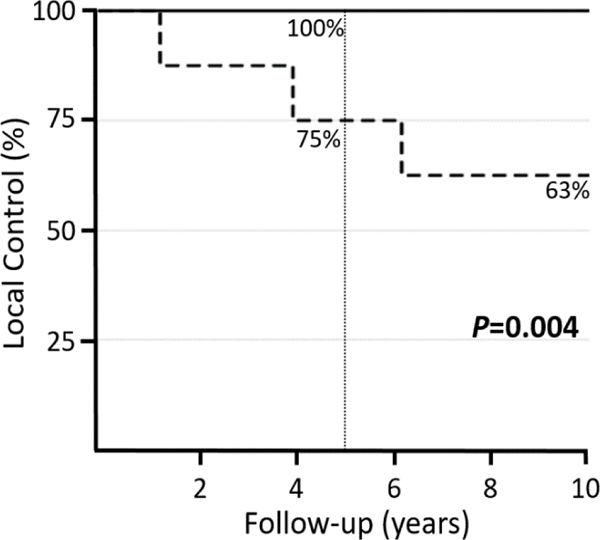
The actuarial 5-year and 10-year LC rates were 94% and 90% (Figure 1C). There were 5 patients (12%) with local relapse at a median time of 75 months (range 13–176 months). Four of those patients underwent surgery alone. The median time to local relapse occurred earlier in patients receiving surgery alone compared to the one patient who received CMT (61 months vs. 143 months). Margin status, tumor size, prior treatment, nor tumor location were associated with local failure (Table 2). The only factor that was associated with a lower 10-year LC rate was receipt of surgery alone (63% vs. 100% in patients receiving combined modality therapy, P=0.004) (Figure 2). A multivariate model was constructed to assess for local recurrence adjusting for margin status, tumor size, prior treatment, and use of surgery alone. The only factor that emerged as significantly associated with poorer LC was use of surgery alone (P=0.02, HR 12.7, 95% CI 1.4 −115.3) compared to combined modality therapy.
Figure 2.
Kaplan-Meier curve shows superior local control for patients receiving combined surgery and radiation therapy (solid line) compared to surgery alone (dotted line) (5-year 100% vs. 75%; 10-year 100% vs. 63%).
Two patients (5%) developed nodal relapse. The actuarial 5-year and 10-year nodal control rates were 97% and 91%, respectively.
Thirteen patients (32%) developed distant metastases - of which all but one patient (bone) recurred within the lung (n=12, 92%) – at a median time of 28 months (range 3–154 months). The actuarial 5- and 10-year rates of DMFS were 73% and 69%, respectively (Figure 1D). Two factors were associated with poorer DMFS on univariate analysis including tumor size >5cm (5-yr 59% vs. 100% ≤5cm, P=0.02) and local relapse (5-yr 60% vs 74% no local relapse, P=0.05). However, on multivariable analysis accounting for tumor size, margin status, use of chemotherapy, and local relapse, local recurrence was the only factor associated with poorer DMFS (P=0.04, HR 3.9, 95% CI 1.1–14.7).
Outcomes After Relapse
For the 15 patients who experienced tumor recurrence, the median DSS from the time of relapse was 57 months (range, 25–241 months) with only 5 patients having died of disease. The 5- and 10-year DSS after relapse of 75% and 51%, respectively.
Among the 5 patients that locally recurred, the 5-year LC rate after treatment of the initial local relapse was 75%. Two of the patients underwent salvage CMT with surgery and RT after receiving surgery alone for their initial presentation; both were without local recurrence at last follow-up. The other 3 patients underwent salvage surgery alone, of which one (33%) had a second local recurrence.
For the 13 patients who recurred distantly, 5 patients (38%) underwent surgical resection of metastases, and 8 patients (62%) received salvage systemic therapy. Neither salvage chemotherapy (P=0.24) nor surgery (P=0.15) were associated with improved DSS after distant tumor relapse. However, one patient was ultimately rendered NED and the other 7 living patients have active metastatic disease, most of which are very slowly progressing.
DISCUSSION
Extraskeletal myxoid chondrosarcoma represents a rare and biologically-unique, translocation-associated STS diagnosis. In this series, we present a modern and more homogenous cohort, compared to previous studies, of patients with localized EMC treated at a single institution. With long-term follow up, we observed an overall low-risk of local recurrence, likely attributable to our use of combined modality local therapy with surgery and RT. In fact, patients who were treated with CMT had improved local control compared to patients receiving surgery alone. Interestingly, our data also showed that local relapse was the only factor associated with poorer distant metastatic free survival. Therefore, optimizing local control at initial presentation is critical for minimizing the risk of developing distant metastases. Despite the high risk of tumor relapse, disease progression is relatively indolent leading to prolonged survival outcomes living with disease.
Previous reports have observed a high propensity for local relapse among patients with EMC with the majority of rates ranging between 30% to 48%.1,3,4,10,15 In contrast, we observed a comparatively lower 10-year local recurrence risk of only 10%, likely related to the frequent use of CMT. One of the larger studies by Meis-Kindblom and colleagues was predominantly a pathologic characterization of 117 cases of EMC.10 They reported a 48% local relapse rate among the 83 cases with available data. Unfortunately, further characterization of this cohort was not performed, but most patients received surgery alone with limited use of CMT (n=14/117, 12%). A similarly high local recurrence was reported by Drilon and colleagues with an observed rate of 37% in patients who were treated with curative intent.1 Interestingly, the risk of local relapse they reported was the same that we observed in patients receiving surgery alone (10-year local relapse rate 37%).
Our comparatively more modern series captures patients after increasing awareness of the biology and relapse risk of EMC. Therefore, like other STS, our treatment strategy for patients with EMC has commonly been to recommend both surgery and RT. Our data supports this practice. The only factor associated with poorer LC was the use of surgery alone compared to CMT (HR 12.7, P=0.02). This is the first study to our knowledge to provide clear evidence that standard local therapy STS paradigms should be recommended for patients with EMC. Previous reports indicate RT has been underutilized for this histology. One plausible explanation is that early reports with limited follow up did not recognize the high risk of disease recurrence.2 Therefore, EMC were treated more like low grade STS using surgery alone. Additionally, despite its name, EMC is genetically and histolocially distinct from conventional chondrosarcoma of bone and in fact, is classified by the WHO as a tumor of uncertain lineage.8 Unfortunately however, his tumor name has likely influenced local management patterns. Based on our data in this study, and similar to data related to other extraskeletal osteogenic tumors, the bone sarcoma treatment pathways do not apply for soft tissue origins.14,16 Ultimately, for patients with EMC, RT should not be omitted due to misconceptions of tumor grade or extrapolations related to primary bone tumor paradigms.
Our data showed that local relapse was the only factor associated with DMFS (HR 3.9, P=0.04) suggesting that patients who locally recur are at high risk of developing distant disease; optimizing LC with surgery and RT can help reduce this. A study by Kemmerer and colleagues also demonstrated the importance of RT for patients with EMC.17 They recently reported a series of 172 patients identified in the SEER database; the use of RT was associated with a cause specific survival (CSS) benefit (P=0.02). If a more granular analysis was achievable with SEER data, the authors may have been able to conclude that the CSS benefit associated with the use of RT was related to a lower rate of distant metastases.
Regardless of local therapy, EMC biologically still has a high risk of distant relapse. We observed a distant recurrence rate of 32% that was similar to the reported literature ranging between 26% and 46%.1,3,4,10 The only tumor- or treatment-related factor associated with DMFS on univariate analysis was tumor size, whereas the primary driver of distant metastasis in our cohort was local relapse. Few patients in our series received systemic therapy. This is likely related to the recognition that EMC has limited response to cytotoxic chemotherapy.4,18 A previous report by Patel and colleagues observed no objective responses in 10 patients receiving doxorubicin- or dacarbazine-based regimens.18 Similarly, Drilon and colleagues reported no radiologic complete or partial responses in 21 patients receiving chemotherapy; the best responses were stable disease for limited lengths of time with a median time to progression of 5 months.1
Despite the high risk of disease recurrence and limited efficacy of systemic therapy, patients with EMC have prolonged survival. Previous studies have demonstrated a 10-year OS of 65–70%, similar to the 10-year OS rate we observed of 66%.1,10 Fewer patients, however, are succumbing to EMC disease progression with a 10-year DSS of 85%. In our series, local or distant disease recurrence was not significantly association with OS, which reinforces that patients are living a long time with their disease. These outcomes differ from patients with other STS histologies that have a poorer DSS, particularly when they develop distant metastases. Because of the prolonged survival of patients with EMC, emphasis should be placed on maintaining a high quality of life. Local control should be a primary goal given that multiple interventions have a negative impact on quality of life and can lead to significant morbidity. Even for patients who present with metastatic disease, given the length of time patients live with disease, it may still be important to consider combined modality local therapy for patients with good performance status in an expert multidisciplinary setting.
This study represents one of the most homogenous and well-characterized cohorts of patients with localized EMC in the literature. However, as with all retrospective studies, there are innate biases that may limit interpretation. We attempted to minimize selection bias; however, this cannot be fully controlled for due to the retrospective nature of the study. Additionally, while it is a relatively large cohort of patients with this disease, the rarity of this histology resulted in a small sample size, which ultimately limits statistical analyses. Despite the limitations, this study provides meaningful data to guide therapeutic decision-making for patients with EMC.
Extraskeletal myxoid chondrosarcoma is a biologically unique soft tissue sarcoma that benefits from combined modality local therapy of both RT and surgery. We observed a higher risk of local relapse in patients who received surgery alone. Furthermore, patients who relapsed locally were significantly more likely to go on to develop distant metastases. Therefore, we recommend optimizing local therapeutic strategies upfront with both surgery and RT to reduce the risk of local and ultimately distant recurrence. Despite high distant relapse rates, disease progression is indolent and patients have prolonged survival; thus, particular attention should be paid to maintaining a high quality of life. Future studies should focus on better characterizing the molecular attributes of EMC and on improving systemic therapy strategies to control systemic disease.
Acknowledgments
Funding: No funding was provided for this study.
Footnotes
Disclaimers: The authors declare no conflicts of interest.
REFERENCES
- 1.Drilon AD, Popat S, Bhuchar G, et al. : Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer 113:3364–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enzinger FM, Shiraki M: Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol 3:421–35, 1972 [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi S, Wada T, Nagoya S, et al. : Extraskeletal myxoid chondrosarcoma: a Multi-Institutional Study of 42 Cases in Japan. Cancer 97:1285–92, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Saleh G, Evans HL, Ro JY, et al. : Extraskeletal myxoid chondrosarcoma. A clinicopathologic study of ten patients with long-term follow-up. Cancer 70:2827–30, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Filion C, Labelle Y: Identification of genes regulated by the EWS/NR4A3 fusion protein in extraskeletal myxoid chondrosarcoma. Tumour Biol 33:1599–605, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Panagopoulos I, Mertens F, Isaksson M, et al. : Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer 35:340–52, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Stenman G, Andersson H, Mandahl N, et al. : Translocation t(9;22)(q22;q12) is a primary cytogenetic abnormality in extraskeletal myxoid chondrosarcoma. Int J Cancer 62:398–402, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Davis EJ, Wu YM, Robinson D, et al. : Next generation sequencing of extraskeletal myxoid chondrosarcoma. Oncotarget 8:21770–21777, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao R, Lao IW, Wang L, et al. : Clinicopathologic and radiologic features of extraskeletal myxoid chondrosarcoma: a retrospective study of 40 Chinese cases with literature review. Ann Diagn Pathol 23:14–20, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Meis-Kindblom JM, Bergh P, Gunterberg B, et al. : Extraskeletal myxoid chondrosarcoma: a reappraisal of its morphologic spectrum and prognostic factors based on 117 cases. Am J Surg Pathol 23:636–50, 1999 [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan B, Davis AM, Turcotte R, et al. : Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359:2235–41, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Yang JC, Chang AE, Baker AR, et al. : Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16:197–203, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Zagars GK, Ballo MT, Pisters PW, et al. : Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 97:2530–43, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bishop AJ, Livingston JA, Araujo DM, et al. : Extraskeletal Osteosarcomas: A Case Made for Combined Modality Local Therapy With Radiation and Surgery. Am J Clin Oncol 42:238–242, 2019 [DOI] [PubMed] [Google Scholar]
- 15.McGrory JE, Rock MG, Nascimento AG, et al. : Extraskeletal myxoid chondrosarcoma. Clin Orthop Relat Res:185–90, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. : The clinical approach towards chondrosarcoma. Oncologist 13:320–9, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kemmerer EJ, Gleeson E, Poli J, et al. : Benefit of Radiotherapy in Extraskeletal Myxoid Chondrosarcoma: A Propensity Score Weighted Population-based Analysis of the SEER Database. Am J Clin Oncol 41:674–680, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Patel SR, Burgess MA, Papadopoulos NE, et al. : Extraskeletal myxoid chondrosarcoma. Long-term experience with chemotherapy. Am J Clin Oncol 18:161–3, 1995 [DOI] [PubMed] [Google Scholar]