Abstract
Pea protein isolate (PPI, from Pisum sativum L.) was fermented with six different lactic acid bacteria strains for 24 h and 48 h. The fermented samples were analyzed regarding their retronasal aroma and taste, their protein solubility, emulsifying and foaming capacity. Changes in the molecular weight distribution were analyzed to monitor potential effects of fermentation on the main allergenic protein fractions of PPI. After 24-h fermentation, PPI's characteristic aroma attributes and bitter taste decreased for all fermented PPI. However, after 48-h fermentation, cheesy aroma, and acid and salty tastes were increased. The PPI fermented with L. plantarum showed the most neutral taste and the panel's highest preference; instead, fermentation with L. fermentum led to a fecal aroma and was the least preferred. The protein solubility and emulsifying capacity decreased after PPI fermentation, while foaming capacity remained constant in comparison to the untreated PPI. The electrophoretic results showed a reduction in the intensity of the allergenic protein fractions; however, these changes might be attributed to the reduced protein solubility rather than to a high proteolytic effect of the strains. Fermentation of PPI for 24 h and 48 h might not be a suitable method for the production of highly functional pea proteins. Further modification methods have to be investigated in the future.
Keywords: Pea protein isolate, Lactic acid fermentation, Aroma profile, Functional properties, Allergenic protein fractions, Molecular weight distribution
Graphical abstract
Highlights
-
•
Fermentation with lactic acid bacteria changed the characteristic aroma profile of pea protein isolates.
-
•
Bitterness of pea protein isolates is reduced after 24-h and 48-h fermentation.
-
•
The functional properties are negatively affected after fermentation.
-
•
Electrophoretic results indicate a reduced intensity of the main allergenic protein fractions.
1. Introduction
The food industry is looking for functional and appealing plant-based ingredients to meet the growing demand for alternative protein sources. Peas (Pisum sativum L.) are an attractive raw material for vegetable food products due to their extensive plantation and good availability (Cernay et al., 2016). Furthermore, peas are rich in proteins featuring all essential amino acids. However, the use of pea proteins in the food industry is limited due to present green and grassy sensory attributes resulting from compounds such as aldehydes, ketones, and alcohols (Heng, 2005). A large part of the components responsible for the characteristic off-flavors of peas can be traced back to oxidation and enzymatic degradation products of unsaturated fatty acids during harvest, storage (Roland, Pouvreau, Curran, van de Velde and de Kok, 2017) and further processing (Azarnia et al., 2011; Heng et al., 2004; Lan et al., 2019). Fermentation has been widely used to improve sensory properties of different cereal and legume products (Ferri et al., 2016; Kaczmarska et al., 2018; Meinlschmidt et al., 2016a; Schlegel et al., 2019). During fermentation, biochemical changes occur, such as degradation and formation of organic substances developing a more intense aroma profile (Adewumi, 2019; Cabuk et al., 2018). To the best of our knowledge, only one study has investigated a 48-h lactic acid fermentation of pea protein extracts to improve the aroma profile while reducing present off-flavors (Schindler et al., 2012).
Metabolic enzymes and metabolites released during fermentation could affect the protein functionality. Few studies have investigated the functional properties of fermented pea proteins. Cabuk et al. (2018) and Kumitch et al. (2020) fermented protein-enriched pea flours with lactic acid bacteria (LAB) and fungi; they found a negative influence of fermentation on protein solubility, emulsifying and foaming capacity. These properties are relevant for several food products such as vegetable milk alternatives, ice cream, and mayonnaise. Therefore, the control and selection of appropriate microorganisms are essential for the later application potential of the fermented pea protein products.
Additionally, there are indications for a reduction in the allergic potential of fermented food products and ingredients from legumes such as soy protein isolates (Chen et al., 2020; Meinlschmidt et al., 2016b; Zhou et al., 2013). Peas are known for their low allergenic potential; however, Sanchez-Monge et al. (2004) identified two main pea allergens, increasing pea allergy awareness. Other studies investigated the incidence of pea allergies and demonstrated cross-reactivity with different nuts and legumes (Codreanu-Morel et al., 2019; Dreyer et al., 2014; Lavine and Ben-Shoshan, 2019; Richard et al., 2015). To our knowledge, only one study has focused on reducing the allergenic potential of pea flour by fermentation (Barkholt et al., 1998). They showed that 48-h fermentation with LAB could reduce the antigenicity to 10% compared to the unfermented pea flour. Thus, the fermentation of pea protein isolate (PPI) could present an important approach to reduce its allergenic potential.
The main objective of the present study was to investigate the impact of lactic acid fermentation on the sensory profile of PPI. In addition, the effects on the functional properties and on the degradation of allergenic proteins were investigated to consider the value of fermented PPI as food ingredient with lower allergenic potential.
2. Materials and methods
2.1. Materials
Pea seeds (Pisum sativum L., cultivar Navarro) were provided by Norddeutsche Pflanzenzucht Hans-Georg-Lembke KG (Germany). Broad Range™ Unstained Protein Standard, 4–20% Criterion™ TGX stain-free™ precast polyacrylamide gels, Coomassie Brilliant Blue R-250 were from Bio-Rad Laboratories GmbH (Germany). Sodium dihydrogen phosphate, sodium dodecyl sulfate, sodium tetraborate decahydrate, o-phthaldialdehyde, and sodium monohydrogen phosphate were purchased from Sigma-Aldrich (Germany). All chemicals used in this study were of analytical grade unless otherwise indicated.
2.2. Production of pea protein isolate
Peas were dehulled and split using an underflow peeler (Streckel & Schrader KG, Germany) and separated using an airlift system (Alpine Hosakawa AG, Germany). The split pea seeds were milled by an impact mill (Gebrüder Jehmlich GmbH using a REKORD A) at maximum peripheral speed of 135 m/s with a 0.5 mm sieve. The isolation of pea protein was performed according to Arteaga, Apéstegui Guardia, Muranyi, Eisner, and Schweiggert-Weisz (2020a). In brief, an aqueous alkaline extract (pH 8.0) of the pea flour was prepared in DI water while stirring constantly for 60 min. The protein extract was adjusted to pH 4.5 for isoelectric precipitation of the proteins. The precipitated proteins were separated, neutralized, pasteurized (70 ± 2 °C) for 2 min and spray-dried.
2.3. Fermentation
2.3.1. Strains, media, growth conditions and preparation
Six microorganisms were selected according to literature regarding their ability to improve the sensory profile of legumes and their proteolytic activity (Barkholt et al., 1998; Ben-Harb et al., 2019; Schindler et al., 2012). All microorganisms were cultivated for 48 h in 150 mL MRS-broth at their individual conditions (Table 1). The liquid preculture (1 mL) was serially diluted in Ringer solution (1:10 v/v) and incubated in MRS-Agar plates for 48 h at the optimal conditions of each microorganism to determine the number of colony-forming units (CFU). The CFU enabled the calculation of the aliquots required for fermentation (8 Log CFU/mL). The required aliquot was centrifuged for 10 min at 9000 rpm. The pellets were used for inoculation.
Table 1.
Microorganisms and growth conditions.
| Microorganism | Abbreviation | Specie No. | Growth/Culture conditions |
||
|---|---|---|---|---|---|
| T (°C) | Type | Medium | |||
| Lactobacillus plantarum | L. plantarum | DSM-20174 | 30 | Anaerobe | MRS |
| Lactobacillus perolens | L. perolens | DSM-12744 | 30 | Aerobe | MRS |
| Lactobacillus fermentum | L. fermentum | DSM-20391 | 37 | Aerobe | MRS |
| Lactobacillus casei | L. casei | DSM-20011 | 30 | Aerobe | MRS |
| Leuconostoc mesenteroides subsp. cremoris | Lc. cremoris | DSM-20200 | 30 | Aerobe | MRS |
| Pediococcus pentosaceus | P. pentosaceus | DSM-20336 | 30 | Anaerobe | MRS |
DSM: Deutsche Sammlung von Mikroorganismen (German Collection of Microorganisms); T: temperature; MRS: De Man, Rogosa and Sharpe.
2.3.2. Fermentation of PPI dispersions
A 9% (w/v) PPI dispersion was prepared in sterile DI water and homogenized for 7 min using an Ultraturrax (IKA® Werke GmbH & Co KG, Germany). The dispersion was pasteurized at 80 °C for 30 min in a thermostatically controlled reactor. Before inoculation, the dispersion was cooled down to the respective temperature (Table 1) and 0.5% (w/v) glucose was added. Aliquots of 990 mL were transferred to sterile 2 L Schott-Duran bottles, where the fermentation took place. The dispersions were inoculated and the fermentations were carried out for 24 h and 48 h under strain-specific conditions (Table 1) without stirring. The anaerobe fermentation was performed by closing the bottle lid completely, whereas the aerobe fermentation was done with semi-opened lid. Aliquots of each sample were taken to determine changes in viable cell count prior to inactivation at 90 °C for 10 min, neutralization, and lyophilization. All fermentations were performed in duplicate. The fermentation times were selected based on previous studies, in which 48-h fermentation improved aroma profile and reduced antigenicity (Barkholt et al., 1998; Schindler et al., 2012).
2.3.3. Growth determination
Liquid aliquots were taken after 5 min (0 h), 24 h and 48 h of inoculation of the PPI. The viable cell counts were determined on MRS-Agar plates by serial dilutions as described in section 2.3.1.
2.3.4. Determination of pH
The pH was measured every 30 min during 48 h of fermentation using a disinfected WTW ProfiLine pH 3310 pH electrode (Xylem Analytics Germany GmbH, Germany). The pH measurements were performed on an additional bottle with the same conditions for each microorganism.
2.3.5. D-Glucose and D-/L-lactic acid
The determinations of D-glucose and D-/L-lactic acid were performed using Enzymatic BioAnalysis test kits from R- BIOPHARM AG (Germany). The samples were prepared according to the manufacturer's instructions.
2.4. Chemical composition
The dry matter content (105 °C), ash content (950 °C) and protein content (N x 6.25) were performed in duplicate and according to AOAC Official Methods (AOACa, 2003; AOACb, 2003) by means of a thermogravimetric method (TGA 701, Leco Instruments, Germany) and Dumas combustion method (TruMac N, Leco Instruments, Germany).
2.5. Sensory analysis
2.5.1. Sample preparation
Dispersions of the PPI and dispersions of the 24 h and 48 h fermented samples (2%, w/w) were prepared with tap water. The respective samples were adjusted to pH 7.0 with 1 mol/L NaOH. The samples were coded using three-digit random numbers.
2.5.2. Sample evaluation
The sensory evaluation was conducted according to DIN 10967-1-1999. First, for the selection of the main attributes, an eight-member trained panel evaluated attributes regarding retronasal aroma and taste of the PPI and 48-h fermented samples. The panel was trained to identify legume aroma profile attributes; the aroma attributes were compared to specific aroma compounds provided in aroma pens. Each sample (20 mL) was presented at room temperature in glass cups and random order. Attributes selected by more than five assessors were chosen for further sensory analysis.
Second, for sensory analysis of the PPI and all fermented samples, 20 mL of each sample were presented at room temperature in glass cups and random order. The sensory analysis was divided into two sessions, where six fermented samples and the unfermented isolate were presented per session. Water and plain crackers were provided for palate cleansing in between. The panelists assessed the intensities of the attributes on a 0 (attribute not perceivable) to 10 (very strong perception of the attribute) ranging scales. The overall intensity (0 = not perceivable) to 10 (10 = very strong perception) and the indication of preference using a hedonic scale (0 = dislike, 5 = neutral, 10 = like) were assessed.
2.5.3. Principal component analysis (PCA)
The results of sensory evaluation were assessed using PCA covariance matrix to analyze the aroma attributes. The PCA was performed using OriginPro 2018b.
2.6. Functional properties
All functional experiments were performed in duplicate.
2.6.1. Protein solubility
The protein solubility was performed according to Morr et al. (1985) at different pH (pH 3.0 – pH 8.0). The protein content was determined using the Biuret method (550 nm) from the Approved Methods of Analysis (AACC, 2000) with bovine serum albumin (BSA) as calibration standard.
2.6.2. Foaming properties
The foaming capacity and foam stability were analyzed according to Phillips et al. (1987) using a whipping machine (Hobart N50, Hobart GmbH, Germany).
2.6.3. Emulsifying capacity
The emulsifying capacity was determined according to Wang and Johnson (2001) using an 1L-reactor equipped with a stirrer and an Ultraturrax (IKA-Werke GmbH & Co. KG, Germany). The oil was added gradually (10 mL/min) until a phase inversion occurred (<10 μS/cm). The volume of added oil was used to calculate the emulsifying capacity (mL oil/g sample).
2.7. Determination of protein degradation
2.7.1. Molecular weight distribution
The molecular weight distribution was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing and reducing conditions according to Laemmli (1970) with slight modifications and described in detail in García Arteaga, Apéstegui Guardia et al. (2020). Briefly, protein solutions of 5 μg/μL based on the dry matter content were prepared in treatment buffer. For reducing conditions, the samples were heated prior to centrifugation. The supernatants were mixed with treatment buffer. For the electrophoresis, an aliquot of 5 μL of the sample mixture was added into the gel pocket of the Bio-Rad 4–20% Criterion™ TGX Stain-Free™ Precast Gels. The Broad Range™ Unstained Protein Standard (Bio-Rad Laboratories, Germany) was used as molecular weight marker. Gels were run for 30 min and stained using Coomassie Brilliant Blue R-250 as described by Garcia et al. (2020b). Finally, gel images were obtained using an EZ Imager (Bio-Rad Laboratories, Germany). Protein band intensities were calculated using the Image Lab Software (Bio-Rad Laboratories, Germany).
2.7.2. Degree of hydrolysis
The degree of hydrolysis (DH) was performed according to the o-phthaldialdehyde (OPA) method (Nielsen et al., 2001). The DH value was calculated based on the total number of peptide bonds per protein equivalent (htot), and the number of hydrolyzed bonds (h) using the following equation:
| DH = h / htot · 100% |
The constant values used for α (degree of dissociation of the α-amino group), β (slope of calibration through linear regression) and htot factor were 1.0, 4.0, and 8.0, respectively, according to theoretical general values for unexamined raw material (Nielsen et al., 2001). The sample preparation was performed in duplicate with each preparation measured in triplicate.
2.8. Statistical analysis
PPI fermentation was performed in duplicate for each microorganism. All other experiments were performed in duplicate unless otherwise stated. Complete raw data can be found in Mendeley Data files (García Arteaga, Leffler, Muranyi, Eisner and Schweiggert-Weisz, 2020b). The results, expressed as mean values ± standard deviations, were analyzed by one-way analysis of variance (ANOVA). Kruskal-Wallis was used when the ANOVA assumptions were not satisfied. The mean values were compared using Tukey's post-hoc test. The relationship among functional properties, bitterness, protein band intensities and DH was analyzed using the Pearson correlation coefficient. All statistical analyses were performed using OriginPro 2018b and were considered statistically significant at P < 0.05.
3. Results and discussion
3.1. Chemical composition
The unfermented PPI contained 84.9% ± 1.4 protein, 95.5% ± 0.3 dry matter, and 5.0% ± 0.2 ash content. The average content of protein, dry matter, and ash in the fermented samples was 80.1% ± 1.8, 96.8% ± 0.6, and 6.8% ± 0.4, respectively. Only the PPI fermented with Lc. cremoris for 24 h showed a significant lower protein content (75.7% ± 3.7) compared to the unfermented PPI. This might suggest that PPI was a good source of nitrogen for Lc. cremoris which would increase the conversion to lactic acid and further by-products (Coelho et al., 2011). The significant increase in the ash content from all samples might be attributed to the increase in salts resulting from the neutralization of the samples.
3.2. Microbial growth
The growth of the selected microorganisms was evaluated through the total viable cell counts (Log CFU/mL, Table 2A), changes in the pH, the consumption of glucose and the production of D-/L-lactic acid (Table 2B).
Table 2.
(A) Viable cell count (Log CFU/mL) and (B) D- and L-Lactic acid concentrations after 0 h, 24 h and 48 h fermentation of pea protein isolate (PPI).
A
| Log CFU/mL |
|||
|---|---|---|---|
| 0 h | 24 h | 48 h | |
| L. plantarum | 8.87 ± 0.05a | 9.02 ± 0.01a | 9.13 ± 0.25a |
| L. perolens | 8.17 ± 0.07b | 8.34 ± 0.26bc | 8.89 ± 0.11bc∗ |
| L. fermentum | 7.38 ± 0.14c | 8.29 ± 0.04c | 8.35 ± 0.01bd∗ |
| L. casei | 8.50 ± 0.02ab | 8.89 ± 0.01ab | 8.90 ± 0.02ac |
| Lc. cremoris | 8.29 ± 0.00b | 8.29 ± 0.04c | 8.29 ± 0.19d |
| P. pentosaceus | 8.08 ± 0.12b | 8.37 ± 0.00bc | 8.55 ± 0.06bcd |
| D-Lactic acid (g/L) |
L-Lactic acid(g/L) |
|||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | |
| L. plantarum | 0.00 ± 0.00a | 1.85 ± 0.23a | 2.75 ± 0.15a | 0.03 ± 0.02a | 1.48 ± 0.13a | 2.20 ± 0.19a |
| L. perolens | 0.00 ± 0.00a | 0.01 ± 0.02b | 0.02 ± 0.01b | 0.18 ± 0.11a | 4.38 ± 1.41b | 5.26 ± 0.62b |
| L. fermentum | 0.00 ± 0.00a | 0.79 ± 0.19c | 0.51 ± 0.25c | 0.06 ± 0.03a | 1.47 ± 0.20a | 0.99 ± 0.27c |
| L. casei | 0.00 ± 0.00a | 0.29 ± 0.08bd | 0.60 ± 0.34c | 0.36 ± 0.15a | 4.52 ± 0.14b | 4.73 ± 0.20b |
| Lc. cremoris | 0.02 ± 0.03a | 0.60 ± 0.22cd | 0.66 ± 0.02c | 0.01 ± 0.01a | 2.75 ± 0.22c | 2.39 ± 0.05a |
| P. pentosaceus | 0.00 ± 0.00a | 0.62 ± 0.10cd | 0.78 ± 0.06c | 0.08 ± 0.05a | 1.78 ± 0.27ac | 1.89 ± 0.15ac |
Results are expressed as means ± standard deviation (CFU n = 2, Glucose and Lactic acid n = 4). Means marked with different letters indicate significant differences between the fermented samples within fermentation times in the Log CFU/mL and within microorganisms in the lactic acid values (Tukey, P < 0.05). Means marked with an asterisk (∗) indicate significant differences between the initial (0 h) and end (48 h) times of fermentation within one microorganism.
Log CFU/mL. With an exception of Lc. cremoris, which remained constant with 8.29 Log CFU/mL during the 48-h fermentation, all other microorganisms were able to grow in the PPI, although they showed a low growth rate. In contrast, the fermented sample with L. fermentum showed the highest increase after 24 h of fermentation from 7.38 Log CFU/mL to 8.29 Log CFU/mL and continued to increase after 48 h (8.35 Log CFU/mL). The samples fermented with L. perolens and L. casei showed an increase to 8.34 Log CFU/mL and 8.89 Log CFU/mL after 24 h, respectively, and to 8.89 Log CFU/mL and 8.90 Log CFU/mL after 48 h, respectively. After 24-h fermentation with P. pentosaceus, the sample showed an increase to 8.37 Log CFU/mL and to 8.55 Log CFU/mL after 48 h. Lastly, the sample fermented with L. plantarum showed an increase to 9.02 Log CFU/mL and 9.13 Log CFU/mL after 24 h and 48 h, respectively. The ability of LAB to grow in substrates depend on the nutrients present (Ciani et al., 2013). Pea proteins are known to contain low amounts of methionine and tryptophan; the latter is an important nutrient for the growth of L. plantarum, Lc. cremoris, P. pentosaceus, and L. fermentum (Corsetti et al., 2016; Holzapfel et al., 2006; Liu, 2016; Verce et al., 2020). This might explain the reduced growth of Lc. cremoris, L. plantarum, and P. pentosaceus; the growth of L. fermentum might be explained by its ability to adapt to non-optimal growth conditions by means of the arginine deiminase pathway (Vrancken et al., 2009).
Although the growth rates of the individual microorganisms were rather low, the microorganisms continued to metabolize, as shown below by the decrease in pH, the decrease in glucose and the increase in lactic acid described below as well as by the changes in the molecular weight distribution and degree of hydrolysis (described in section 3.5).
pH value. The PPI solutions showed an average initial pH of 6.5. Fermentation with L. casei lowered the pH of the sample to 4.6 after 12 h of fermentation, while all other strains were below pH 5.0 after 24 h. After 24-h fermentation, the pH of the samples fermented with L. perolens and L. casei remained constant at pH 4.7 and pH 4.5, respectively. Fermentation with L. plantarum and L. pentosaceus for 48 h reduced the pH down to pH 4.6 and to pH 4.8, respectively. Samples fermented with Lc. cremoris and L. fermentum after 24 h showed a pH of 4.7 and 4.8, respectively; which increased after 48 h to pH 4.8 and 5.8, respectively. This increase might suggest an alkalization due to the decarboxylation and/or deamination of the released amino acids into alcohols, ammonia or aldehydes (Ben-Harb et al., 2019; Liu, 2016).
Glucose content. Glucose was used by all microorganisms as a fast energy source. In particular, L. perolens, L fermentum, and L. casei metabolized the entire amount of added glucose after 24 h of fermentation. In the samples fermented with L. plantarum, Lc. cremoris and P. pentosaceus, residual amounts of glucose were detected after 24 h; however, after 48 h of fermentation, all fermented samples showed a complete depletion of the glucose.
Lactic acid content. The results obtained after fermentation with L. plantarum, L. fermentum, L. casei, Lc. cremoris and P. pentosaceus for the production of D- and L-lactic acid are consistent with the literature, as these LAB are known to produce both D- and L-lactic acid (Chun et al., 2017; Corsetti et al., 2016; González-Vara et al., 1996; Raccach, 1987; Verce et al., 2020).
The fermentation of PPI with L. perolens showed the highest production of L-lactic acid after 24 h and 48 h with 4.38 g/L and 5.26 g/L, respectively, followed by the fermentation with L. casei (4.52 g/L and 4.73 g/L, respectively). The high production of lactic acid by these two strains might suggests an adequate ability to grow in PPI solutions. Fermentation with Lc. cremoris showed a production of 2.39 g/L of L-lactic acid and 0.66 g/L of D-lactic acid after 48 h.
Fermentation of PPI with L. plantarum showed a production of D- and L-lactic acid of 2.75 g/L and 2.20 g/L, respectively, after 48 h, whereas fermentation with P. pentosaceus increased D- and L-lactic acid concentrations up to 0.78 g/L and 1.89 g/L, respectively. These LAB are known to produce larger concentrations of L-lactic acid under anaerobic conditions (Corsetti et al., 2016; Raccach, 1987). However, as oxygen was not removed prior to fermentation, the residual oxygen content could have contributed to microaerobic fermentation, which slowed down the production of lactic acid, and promoted D-lactic acid and acetate production by L. plantarum (Raccach, 1987).
Fermentation with L. fermentum showed the lowest L-lactic acid concentration after 24-h (1.47 g/L) and after 48-h (0.99 g/L) fermentation. These low concentrations could indicate that this specific strain produces mainly other by-products as soon as the carbohydrate substrates are depleted. A comparative genomic analysis of 28 strains of L. fermentum by Verce et al. (2020) revealed the production of acetate, ethanol, glycerol, diacetyl (2,3-butanedione), and 2,3-butanediol besides lactic acid production. With a pKa of 14.9, 2,3-butanediol is considered a strong base and it is also known to hinder the production of acid compounds (Ciani et al., 2013; Ji et al., 2011), which could be related to both, the low amounts of lactic acid and the increase of pH after 48 h fermentation.
3.3. Sensory analysis
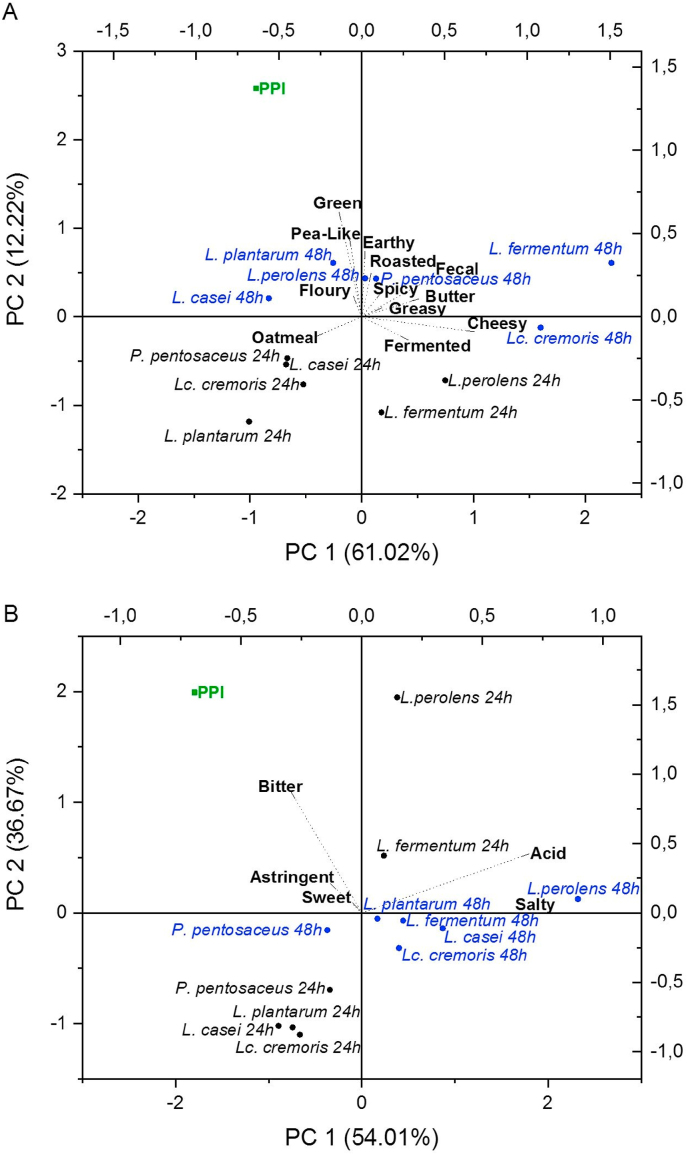
Throughout fermentation, microorganisms metabolize the substrate resulting in the production of different volatile and non-volatile compounds characteristic for the fermented products. The identified attributes (and specific compounds compared to aroma pens) were: pea-like (isopropyl-methoxypyrazine), green (hexanal), earthy (geosmin), roasted (furaneol/acetylpyridine), buttery (2,3-butanedione), cheesy (3-methylbutanoic acid), greasy (2-nonenal), spicy (sotolone), oatmeal, fermented, floury and fecal. A principal component analysis was applied to analyze relationships between samples and sensory attributes. Fig. 1 shows the biplot of the principal components 1 and 2 using the standardized scores of the PPI and fermented samples.
Fig. 1.
Biplot of retronasal aroma (A) and taste (B) of the unfermented pea protein isolate (PPI) and PPI fermented for 24 h (black) and 48 h (blue) with different microorganism strains. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Aroma. For the retronasal aroma attributes (Fig. 1A), the first two components of the PCA explained 73.2%. The sensory attribute with the strongest influence on PC1 was cheesy (0.68), whereas green showed the strongest influence on PC2 (0.62). The unfermented PPI scored the highest in the PC2 (2.58) and was in the nearest proximity to the green attribute, which is known to be one primary off-flavor of peas (Roland et al., 2017; Schindler et al., 2012). Aroma profiles of samples fermented for 24 h and 48 h were distinct from the unfermented PPI and each other.
The PPI solutions fermented for 24 h were found in the negative quadrants of the PC2 independently of the microorganism. The attributes of these samples were farther away from the unfermented PPI, which suggests greater differences in aroma. The lowest pea-like aroma was achieved after 24 h fermentation with L. perolens, L. casei, L. plantarum, and L. fermentum. Fermentation with L. plantarum for 24 h also masked other aroma attributes of the unfermented PPI (−1.00/-1.18) such as green and earthy. The PPI fermented with L. perolens for 24 h showed the highest buttery aroma, which could be attributed to the metabolism of L. perolens. Back et al. (1999) reported that L. perolens produced notably high concentrations of diacetyl, which might explain the pronounced buttery aroma in this study. Fermentation with P. pentosaceus reduced characteristic aromas from peas such as pea-like, green, and earthy, and showed the highest production of the floury attribute.
The 48-h fermentation of PPI resulted in less variation in PC2, whereas in PC1, differences were more pronounced, especially in samples fermented with L. fermentum (2.23) and Lc. cremoris (1.60). The fermentation of PPI with L. fermentum for 48 h was characterized by a fecal aroma usually produced by the catabolism of aromatic amino acids and the generation of undesirable compounds such as p-cresol, indole, and skatole (Ganesan and Weimer, 2017; Ibrahim, 2016). PPI fermented with Lc. cremoris for 48 h showed the most intense cheesy aroma, for which this microorganism is used in cheese production (Liu, 2016). The characteristic pea off-flavors were probably masked after fermentation due to the production of other aroma attributes such as buttery and cheesy (Schindler et al., 2012).
Taste. Regarding the taste attributes, PC1 and PC2 represented 90.7% of the total variance (Fig. 1B). The bitter taste showed the strongest influence on PC2 (0.87), whereas acid accounted for the strongest influence on PC1 (0.69). Fig. 1B shows clusters of the fermented samples after 24 h and 48 h, with an exception for the samples fermented with L. perolens and L. fermentum for 24 h. The unfermented PPI scored the highest in the PC2 (1.99), which can be attributed to the characteristic bitter taste from peas. In contrast, samples fermented with L. plantarum and Lc. cremoris for 24 h showed lower bitter intensities as well as low intensities of other taste attributes. The fermentation of PPI for 24 h with L. perolens led to strong bitter and acid tastes. After 48-h fermentation, L. casei showed the lowest bitter taste intensity, which might be attributed to its strong activity peptidase against bitter peptides (Arora and Lee, 1990; El Abboudi et al., 1992). The proteolytic effects during fermentation depend on the LAB species, the specific strains, the individual proteins and their cleavage sites. As a result, smaller peptides, responsible for the bitter taste in unfermented samples, might have been degraded, leading to changes in the taste profile (Saha and Hayashi, 2001).
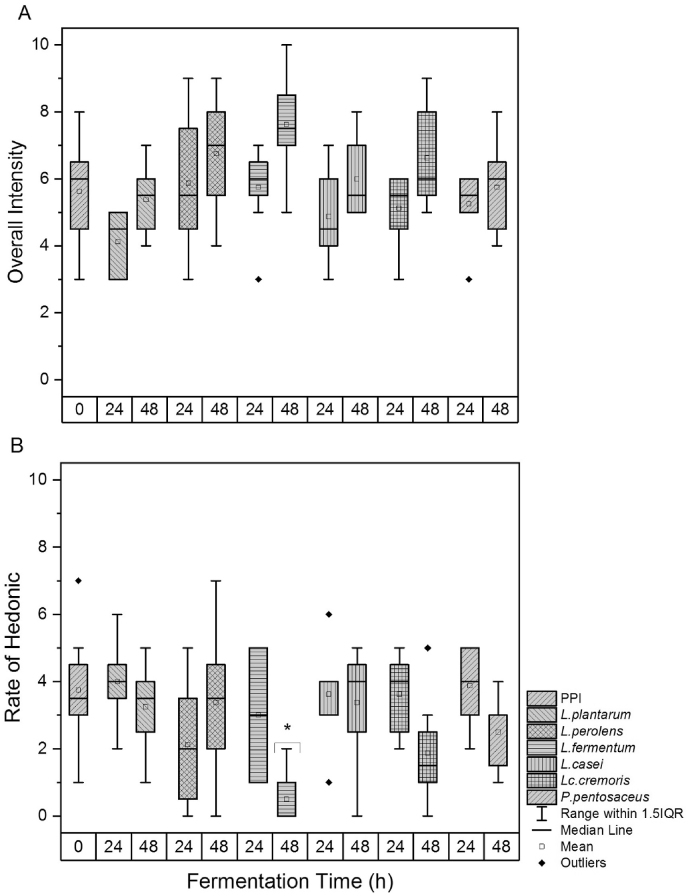
Overall intensity. The highest overall intensity was perceived in the PPI solution fermented with L. fermentum for 48 h, followed by the 48-h fermented PPI with Lc. cremoris, and L. perolens (Fig. 2A). In contrast, L. plantarum produced the lowest overall intensity after 24 h fermentation.
Fig. 2.
Overall intensity (A) and rate of hedonic (B) of the unfermented pea protein isolate (PPI) and PPI fermented for 24 h and 48 h with different microorganism strains. Bars marked with an asterisk (∗) indicate significant differences between the individual sample and the unfermented PPI (Tukey, P < 0.05).
Preference. Fig. 2B shows the trend for the preference of each sample. All microorganisms reduced the bitter and astringent attributes; however, the sample preference was not significantly improved. The sample fermented with L. plantarum for 24 h was rated slightly higher than the unfermented PPI, whereas the preference for the PPI fermented with L. fermentum for 48 h was significantly reduced. As previously mentioned, the low preference for the latter was most likely related to the production of fecal aroma attributes. The preference for samples fermented for 24 h was higher than the ones fermented for 48 h. The lower acceptance after longer times of fermentation might be attributed to the possible production of acetate and 2–3 butanediol and other undesired aroma compounds such as p-cresol, indole, and skatole. There was a negative correlation (−0.79, P < 0.05) between the overall intensity and the preference among fermented samples. This suggests that the samples were less preferred by the panelists when they had higher overall intensities.
3.4. Functional properties
3.4.1. Protein solubility
Table 3 shows the pH-dependent protein solubility profiles of the unfermented and fermented PPI samples for both fermentation times. The maximum protein solubility of unfermented PPI was shown at pH 8.0 (48.3), whereas the minimum protein solubility at pH 5.0 (7.1%). After 24-h and 48-h fermentation, all samples showed a significant improvement in protein solubility at pH 5.0 but a significant decrease at pH 3.0, 7.0, and 8.0. At pH 4.5, the protein solubility increased significantly after 24-h fermentation with Lc. cremoris and P. pentosaceus and after 48 h with L. fermentum and L. casei. Fermentation with L. fermentum for 48 h improved the protein solubility significantly at pH 6.0. The PPI fermented with L. plantarum for 24 h showed the highest protein solubility at pH 7.0 (17.8%), whereas fermentation with Lc. cremoris reached the highest protein solubility at pH 8.0 (18.4%). In contrast, after 48- h fermentation, the sample fermented with L. fermentum showed the highest protein solubility at pH 7.0 (17.2%), while the one with P. pentosaceus did at pH 8.0 (17.5%).
Table 3.
Protein solubility (%) of unfermented pea protein isolate (PPI) and PPI fermented for 24 h and 48 h with different microorganism strains.
| Protein Solubility (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Time |
pH |
||||||
| (h) | 3.0 | 4.5 | 5.0 | 6.0 | 7.0 | 8.0 | |
| PPI | 0 | 36.3 ± 3.3 | 7.9 ± 1.6 | 7.1 ± 0.3 | 12.3 ± 1.0 | 43.4 ± 4.0 | 48.3 ± 2.8 |
| L. plantarum | 24 | 12.7 ± 1.6∗ | 10.6 ± 1.2 | 11.9 ± 0.4∗ | 14.7 ± 1.8 | 17.8 ± 1.5∗ | 17.0 ± 2.4∗ |
| L. perolens | 10.8 ± 2.6∗ | 10.1 ± 1.2 | 10.6 ± 0.7∗ | 14.4 ± 1.8 | 14.8 ± 2.5∗ | 13.5 ± 2.4∗ | |
| L. fermentum | 12.4 ± 1.0∗ | 10.4 ± 2.0 | 11.4 ± 1.2∗ | 15.3 ± 2.1 | 16.8 ± 3.3∗ | 16.1 ± 1.1∗ | |
| L. casei | 12.8 ± 1.9∗ | 8.8 ± 1.1 | 11.6 ± 1.9∗ | 12.8 ± 1.9 | 14.0 ± 2.0∗ | 15.6 ± 1.2∗ | |
| Lc. cremoris | 13.4 ± 1.0∗ | 11.5 ± 1.6∗ | 12.7 ± 2.0∗ | 15.3 ± 2.1 | 16.9 ± 1.9∗ | 18.4 ± 1.7∗ | |
| P. pentosaceus | 11.2 ± 0.7∗ | 11.3 ± 2.0∗ | 11.5 ± 1.3∗ | 13.6 ± 1.6 | 15.1 ± 1.4∗ | 15.7 ± 1.5∗ | |
| L. plantarum | 48 | 12.6 ± 1.4∗ | 10.8 ± 1.0 | 11.3 ± 1.4∗ | 14.9 ± 0.8 | 15.0 ± 1.5∗ | 16.8 ± 0.9∗ |
| L. perolens | 11.4 ± 1.1∗ | 9.8 ± 0.7 | 12.7 ± 0.9∗ | 14.1 ± 2.0 | 15.4 ± 2.6∗ | 14.8 ± 2.1∗ | |
| L. fermentum | 13.7 ± 0.6∗ | 11.9 ± 0.7∗ | 13.4 ± 1.2∗ | 17.4 ± 3.3∗ | 17.2 ± 2.4∗ | 16.0 ± 1.0∗ | |
| L. casei | 11.4 ± 1.5∗ | 11.8 ± 1.7∗ | 11.9 ± 1.6∗ | 13.0 ± 1.7 | 14.9 ± 1.8∗ | 14.1 ± 1.7∗ | |
| Lc. cremoris | 12.3 ± 0.5∗ | 10.9 ± 0.9 | 11.2 ± 1.2∗ | 13.5 ± 1.3 | 14.9 ± 0.5∗ | 16.7 ± 2.2∗ | |
| P. pentosaceus | 12.5 ± 0.2∗ | 10.5 ± 0.7 | 12.0 ± 0.5∗ | 11.3 ± 2.7 | 16.8 ± 2.2∗ | 17.5 ± 2.6∗ | |
Results are expressed as means ± standard deviation (n = 4). Means marked with an asterisk (∗) indicate significant differences between the individual sample and the unfermented pea protein isolate (PPI) (Tukey, P < 0.05).
Fermentation of pea flour, soy and lupin protein isolates has shown similar effects on the protein solubility in other studies (Cabuk et al., 2018; Kumitch et al., 2020; Meinlschmidt et al., 2016b; Schlegel et al., 2019). The decline of protein solubility by fermentation might be related to different factors such as 1) changes in the protein surface, leading to exposure of hydrophobic groups and protein-protein interactions, 2) changes in the surface charge of the samples, and 3) increase in biomass due to microorganisms’ growth. These factors might induce interactions and aggregation between proteins, microbial cells, lactic acid, and other compounds produced during fermentation and neutralization of the samples. In particular, the hydrophobicity of the LAB cell surfaces might play a role in the interaction with hydrophobic proteins and by-products leading to the precipitation of these agglomerates (Daeschel and McGuire, 1998; Marín et al., 1997).
3.4.2. Foaming capacity
The foaming capacity of proteins depends on different physicochemical characteristics such as surface tension and hydrophobicity, electrostatic repulsion, and molecular weight (Zayas, 1997). The unfermented and fermented PPI were unable to form foams. The lack of foam formation by the unfermented PPI might be attributed to the alkaline extraction method (Stone et al., 2015). In addition, the possible agglomeration between LAB cells, proteins, and by-products during fermentation could have reduced protein-air-water interactions preventing the formation of foams. To our knowledge, there are no studies regarding the functional properties of fermented PPI; however, studies on pea protein enriched-flour reported no effect or even a decrease in foaming capacity after fermentation (Cabuk et al., 2018; Kumitch et al., 2020).
3.4.3. Emulsifying capacity
The fermentation of the PPI significantly decreased the emulsifying capacity of the pea proteins. Unfermented PPI showed an emulsifying capacity of 548 mL/g ± 33. The fermented samples with the highest emulsifying capacity were those fermented with L. plantarum for 24 h and 48 h with 370 mL/g ± 62 and 385 mL/g ± 24, respectively. The PPI fermented with L. perolens showed the lowest emulsifying capacity with 204 mL/g ± 27 and 180 mL/g ± 4 after 24 h and 48 h, respectively. Samples fermented with L. fermentum and P. pentosaceus showed emulsifying capacities of 320 mL/g ± 17 and 348 mL/g ± 11 after 24 h, respectively, and 275 mL/g ± 19 and 322 mL/g ± 18 after 48-h fermentation. Fermentation with L. casei for 48 h increased the emulsifying capacity significantly (300 mL/g ± 4) compared to the 24-h sample (219 mL/g ± 19). Lc. cremoris fermented samples showed emulsifying capacities of 290 mL/g ± 21 and 310 mL/g ± 11 after 24-h and 48-h fermentation, respectively. Other authors reported a reduction in emulsifying capacity with longer fermentation times of different legume preparations (Cabuk et al., 2018; Kumitch et al., 2020; Meinlschmidt et al., 2016b; Schlegel et al., 2019). A positive correlation (0.78, P < 0.05) was found between the protein solubility (pH 7.0) and the emulsifying capacity, thus, low emulsifying capacities might be attributed to the agglomeration of the proteins and the interaction of by-products. These agglomerates could prevent the hydrophobic interactions between protein and oil molecules and reduce the amphiphilic character of the proteins.
3.5. Proteolysis of PPI
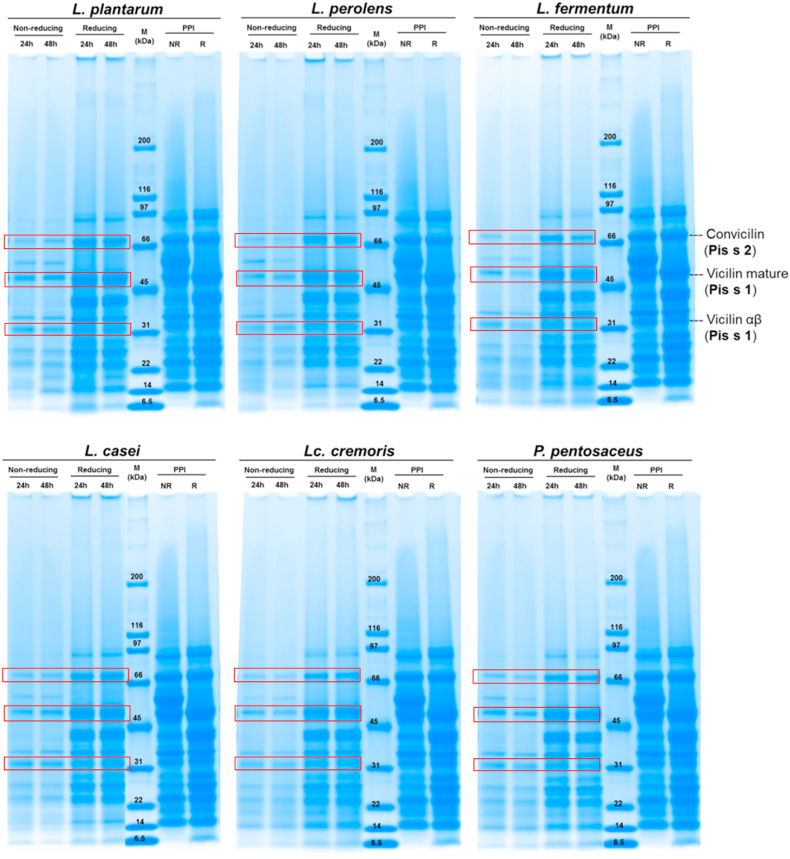
Fig. 3 shows the electrophoretic results of the unfermented and fermented PPI. The protein fractions of the unfermented PPI ranged from 91 to 6.5 kDa for both conditions (reduced and non-reduced). Sanchez-Monge et al. (2004) identified fractions of 67 kDa (convicilin, Pis s 2), and 47 kDa (mature vicilin, Pis s 1) as the main allergens; in addition, they found that the 32 kDa proteolytic fraction (αβ) from the mature vicilin was also a major allergen. In the present study, these fractions were found in both the unfermented and fermented samples.
Fig. 3.
Molecular weight distribution of the unfermented pea protein isolate (PPI) and PPI fermented for 24 h and 48 h with different microorganism strains by SDS-PAGE under non-reducing (NR) and reducing (R) conditions. M = molecular weight standard indicated in kilo Dalton (kDa).
Protein fractions of the fermented samples ranged from 70 to 6.5 kDa and 90–6.5 kDa under non-reducing and reducing conditions, respectively. Vicilin and convicilin fractions lack disulfide bonds; thus, allergen fractions were expected to remain in the PPI solutions under both conditions. However, mature vicilin can undergo post-translational cleavage resulting in different fragments, one of them being the major allergen at 32 kDa, which can be further cleaved (Tzitzikas et al., 2006). Protein volume intensities of each allergenic protein fraction as detected by the Image Lab Software are shown in Table 4. The unfermented PPI under non-reducing conditions showed protein volume intensities of 303, 320, and 142 for Pis s 2, Pis s 1, and Pis s 1 αβ, respectively. Under reducing conditions, the unfermented PPI showed intensities of 272, 313, and 98 for Pis s 2, Pis s 1, and Pis s 1 αβ, respectively.
Table 4.
Protein band volume intensities of the main pea allergens of the unfermented pea protein isolate (PPI) and PPI fermented for 24 h and 48 h analyzed by SDS-PAGE under non-reducing and reducing conditions.
| Volume (Int) |
||||
|---|---|---|---|---|
| Time |
Pis s2 |
Pis s1 |
Pis s1 (αβ) |
|
| A) NON-REDUCING | (h) | (~70 kDa) | (~50 kDa) | (~32 kDa) |
| PPI | 0 | 303 ± 70 | 320 ± 72 | 142 ± 27 |
| L. plantarum | 24 | 76 ± 45∗ | 158 ± 40∗ | 115 ± 26 |
| L. perolens | 40 ± 14∗ | 96 ± 2∗ | 68 ± 8 | |
| L. fermentum | 47 ± 14∗ | 108 ± 32∗ | 86 ± 42 | |
| L. casei | 61 ± 10∗ | 142 ± 29∗ | 134 ± 10 | |
| Lc. cremoris | 39 ± 6∗ | 99 ± 7∗ | 84 ± 17 | |
| P. pentosaceus | 55 ± 12∗ | 139 ± 13∗ | 79 ± 36 | |
| L. plantarum | 48 | 90 ± 32∗ | 151 ± 11∗ | 105 ± 40 |
| L. perolens | 64 ± 10∗ | 127 ± 4∗ | 85 ± 28 | |
| L. fermentum | 35 ± 22∗ | 75 ± 16∗ | 62 ± 28 | |
| L. casei | 71 ± 7∗ | 146 ± 10∗ | 143 ± 13 | |
| Lc. cremoris | 39 ± 6∗ | 97 ± 6∗ | 74 ± 22 | |
| P. pentosaceus | 52 ± 3∗ | 123 ± 14∗ | 81 ± 24 | |
| Volume (Int) | ||||
| Time | Pis s2 | Pis s1 | Pis s1 (αβ) | |
| B) REDUCING | (h) | (~70 kDa) | (~50 kDa) | (~32 kDa) |
| PPI | 0 | 272 ± 35 | 313 ± 79 | 98 ± 9 |
| L. plantarum | 24 | 159 ± 15 | 228 ± 14 | 55 ± 14 |
| L. perolens | 148 ± 5 | 224 ± 26 | 56 ± 6 | |
| L. fermentum | 161 ± 36 | 279 ± 24 | 60 ± 1 | |
| L. casei | 158 ± 29 | 259 ± 2 | 73 ± 4 | |
| Lc. cremoris | 160 ± 31 | 276 ± 18 | 80 ± 3 | |
| P. pentosaceus | 178 ± 39 | 275 ± 20 | 76 ± 1 | |
| L. plantarum | 48 | 146 ± 9 | 233 ± 40 | 60 ± 24 |
| L. perolens | 129 ± 13 | 223 ± 14 | 66 ± 14 | |
| L. fermentum | 131 ± 58∗ | 221 ± 24 | 32 ± 1 | |
| L. casei | 163 ± 5 | 260 ± 13 | 73 ± 11 | |
| Lc. cremoris | 158 ± 40 | 273 ± 15 | 61 ± 1 | |
| P. pentosaceus | 165 ± 43 | 267 ± 2 | 70 ± 2 | |
Results are expressed as means ± standard deviation (n = 2). Means marked with an asterisk (∗) indicate significant differences between the individual fermented sample and the unfermented pea protein isolate (PPI) (Tukey, P < 0.05).
Effect of fermentation on Pis s 2 protein fraction. Under non-reducing conditions, fermentation significantly reduced the protein band intensity of Pis s 2 after 24 h and 48 h compared to the PPI. After 24-h fermentation, isolates fermented with Lc. cremoris showed the lowest intensity under non-reducing conditions, whereas the ones with L. plantarum showed the highest. However, under reducing conditions, only PPI fermented with L. fermentum for 48 h showed a significant reduction in intensity. Under reducing conditions, fermentation for 24 h with L. perolens showed the lowest protein band intensity, whereas P. pentosaceus showed the highest. Longer fermentation (48 h) with L. fermentum showed a further reduction of this protein fraction intensity in both conditions. In contrast, fermentation for 48 h with L. plantarum, L. perolens, and L. casei showed an increase in intensity of this fraction under non-reducing conditions.
Effect of fermentation on Pis s 1 protein fraction. Pis s 1 mature vicilin and its proteolytic fraction showed a reduced intensity after fermentation with the different LAB. Under non-reducing conditions, all fermented samples showed a significant reduction in protein band intensities at both fermentation times. After 24 h fermentation, the highest reduction in mature vicilin was achieved by L. perolens under both conditions. However, after fermentation for 48 h, PPI fermented with L. fermentum showed the lowest protein band intensities under both conditions. Regarding the proteolytic fraction (αβ) of Pis s 1, the lowest protein band intensities were shown by PPI fermented with L. perolens under non-reducing and with L. plantarum under reducing conditions after 24 h. On the other hand, L. fermentum showed the lowest intensity after 48 h fermentation under both conditions.
Under both conditions, the allergen fractions of fermented samples were less intense than of the unfermented PPI, especially under non-reducing conditions. However, changes in the intensity of the protein fractions might be attributed to low protein solubility of the fermented sample and not to a high proteolytic effect during fermentation. Pearson correlations were calculated to support the latter assumption, where strong correlations (>0.80, P < 0.05) were found between the protein solubility at pH 7.0 and the protein band intensities of Pis s 2 (both conditions) and Pis s 1 (non-reducing). The difference in protein band intensities might be attributed to each microorganism and their 1) release of proteolytic enzymes, 2) production of biomass, and 3) specificity for the substrate. Some authors have investigated the effect of fermentation on allergens from different plant substrates, and they have found a reduction in immunogenicity with different microorganisms (Barkholt et al., 1998; Licandro et al., 2020; Meinlschmidt et al., 2016b). Further immunological analyses such as Western-Blot or ELISA are necessary to understand the effect of the lactic acid fermentation on main pea allergen fractions.
Effect of fermentation on the degree of hydrolysis. The proteolytic activity was also measured by means of the total amount of hydrolyzed peptide bonds. The degree of hydrolysis (DH) started with 1.73% for the unfermented PPI (Table 5). The DH of the fermented samples ranged between 1.70 -3.02% after 24 h fermentation and between 2.19-3.75% after 48 h fermentation. A significant increase was observed after 24-h fermentation with L. perolens, L. fermentum, L. casei, and P. pentosaceus; furthermore, after fermentation for 48 h, all microorganisms showed a significant increase in the DH. The lowest DH was shown after fermentation with Lc. cremoris (1.70%, 2.68%) and L. plantarum (1.74%, 2.19%) after 24 h and 48 h fermentation. Fermentation with L. perolens showed the highest DH and increased significantly after 24 h (3.02%) and 48 h (3.75%). Although fermented samples with the lowest and highest DH are consistent with the growth of viable total cell counts, statistical correlations were not found. Compared to the functional properties and SDS-PAGE results, higher DH were expected. However, the overall low DH might be attributed to the raw material, the strains of each microorganism, and the determination method. The agglomeration of the proteins or interactions of the by-products and the OPA reagent might have concealed the primary amino groups affecting the measurement.
Table 5.
Degree of hydrolysis of unfermented pea protein isolate (PPI) and PPI fermented for 24 h and 48 h.
| Sample | Degree of Hydrolysis (%) |
||
|---|---|---|---|
| Fermentation Time (h) | |||
| 0 | 24 | 48 | |
| L. plantarum | 1.73 ± 0.05a | 1.74 ± 0.22a | 2.19 ± 0.16b |
| L. perolens | 1.73 ± 0.05a | 3.02 ± 0.18b | 3.75 ± 0.18c |
| L. fermentum | 1.73 ± 0.05a | 2.43 ± 0.27b | 3.21 ± 0.15c |
| L. casei | 1.73 ± 0.05a | 2.61 ± 0.25b | 2.78 ± 0.10b |
| Lc. cremoris | 1.73 ± 0.05a | 1.70 ± 0.06a | 2.68 ± 0.12b |
| P. pentosaceus | 1.73 ± 0.05a | 2.02 ± 0.13b | 2.80 ± 0.08c |
Results are expressed as means ± standard deviation (n = 4). Means marked with different letters within one row indicate significant differences (Tukey, P < 0.05).
3.6. General remarks
Despite the low growth rate and low degree of hydrolysis, significant changes were found in the production of lactic acid, the functional properties and the electrophoretic results. On the one hand, temperature treatments used during processing, such as pasteurization, during fermentation and inactivation might have affected the protein structure of the proteins. The decrease of pH during fermentation could also have contributed to a partial denaturation of the proteins. Either by temperature or by pH, the unfolding of proteins exposes hydrophobic regions, which causes an increase in protein-protein interactions and the formation of aggregates. On the other hand, neutralizing the fermented samples with NaOH might have resulted in the formation of sodium lactate. This compound is high-soluble (Yen et al., 2010), and it might have competed for interaction with water molecules before potential soluble proteins increased their net charge. Moreover, these interactions might lead to agglomeration and thus, reduced protein solubility and in consequence reduced emulsifying and foaming capacities. Certain degrees of aggregation are known to improve emulsion and foaming capacities (Peng et al., 2016). However, a higher number of aggregates might conceal the hydrophobic moieties from interaction with oil and air, respectively, hence hindering an optimal orientation of the proteins towards oil- and air-interfaces and reducing emulsifying and foam capacities. Limited hydrolysis through reduced fermentation times might improve functional properties by a lower degree of denaturation and fewer interactions between proteins, metabolites and LAB cell surfaces, which would allow the smaller peptides to interact with the solvent and oil-water and air-water interfaces.
4. Conclusion
The present study investigated the effects of fermentation with six different LAB on the sensory profile, functional properties, and changes in the molecular weight distribution as well as in the allergenic protein fractions of PPI. Overall, fermentation of PPI reduced aroma attributes that characterize PPI, such as pea-like, green, and earthy. The aroma properties of the fermented samples depended mainly on the LAB used, their specific metabolism and the associated release of acids and other metabolites. Similarly, changes in the bitterness of the samples depended on the microorganisms, suggesting that some LAB might have higher activity against bitter peptides. PPI fermentation for 24 h resulted in higher acceptance compared to the 48-h fermented samples, which suggest that longer times of fermentation might induce the production of further compounds that are no longer attractive for consumers. Regarding the aroma profile, fermentation of PPI with L. plantarum for 24 h achieved the most neutral retronasal aroma, low bitter taste, lowest overall intensity, and highest preference among all fermented samples.
The fermentation of PPI significantly decreased the functional properties. These results might be attributed to an agglomeration of the proteins and their interaction with by-products released during fermentation. Regarding the effects on the allergenic protein fractions and molecular weight distribution, the samples need to be further investigated by immunological in vitro and in vivo assays to be able to draw a more precise conclusion about the reduction of the allergenic potential of the modified pea protein isolates.
This study aimed to investigate whether fermentation is a suitable method to improve the sensory profile and functional properties of pea protein isolates to be used as food ingredients. Unfortunately, the selected microorganisms and fermentation times were not suitable for producing good-tasting and highly functional ingredients. Shorter fermentation times and other microorganisms should be additionally investigated. Furthermore, other methods, such as enzymatic hydrolysis before or after fermentation, might be worth investigating.
Funding
This work was supported by the Fraunhofer Future Foundation, Germany.
CRediT authorship contribution statement
Verónica García Arteaga: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Sophia Leffler: Investigation. Isabel Muranyi: Methodology, Writing - review & editing, Supervision. Peter Eisner: Resources, Writing - review & editing, Supervision. Ute Schweiggert-Weisz: Resources, Writing - review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Luisa Murer and Eva Müller for their valuable contribution to this work. We greatly appreciate the sensory panel of the Fraunhofer Institute for Process Engineering and Packaging IVV, Freising, Germany, for the sensory evaluation.
References
- AACC . 11th Ed. 2000. Method 46–15.01. Crude protein – 5-minute Biuret method for wheat and other grains. Am. Assoc. Cereal Chem. [DOI] [Google Scholar]
- Adewumi G.A. Health-promoting fermented foods. In: Melton L., Shahidi F., Varelis P., editors. Encyclopedia of Food Chemistry. Academic Press; Oxford: 2019. pp. 399–418. [Google Scholar]
- AOACa . Method 923.03. Ash of flour. In: Horwitz W.A., editor. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC) International. (Ed.) AOAC International; Gaithersburg, Md: 2003. [Google Scholar]
- AOACb . Method 968.06. Protein (crude) in animal feed. In: Horwitz W.A., editor. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC) International. (Ed.) AOAC International; Gaithersburg, MD: 2003. [Google Scholar]
- Arora G., Lee B.H. Comparative studies on peptidases of lactobacillus casei subspecies. J. Dairy Sci. 1990;73(2):274–279. doi: 10.3168/jds.S0022-0302(90)78670-5. [DOI] [PubMed] [Google Scholar]
- Azarnia S., Boye J.I., Warkentin T., Malcolmson L., Sabik H., Bellido A.S. Volatile flavour profile changes in selected field pea cultivars as affected by crop year and processing. Food Chem. 2011;124(1):326–335. doi: 10.1016/j.foodchem.2010.06.041. [DOI] [Google Scholar]
- Back W., Bohak I., Ehrmann M., Ludwig W., Pot B., Kersters K., Schleifer K.H. Lactobacillus perolens sp. nov., a soft drink spoilage bacterium. Syst. Appl. Microbiol. 1999;22(3):354–359. doi: 10.1016/S0723-2020(99)80042-3. [DOI] [PubMed] [Google Scholar]
- Barkholt V., Jorgensen P.B., Sorensen D., Bahrenscheer J., Haikara A., Lemola E.…Frokiaer H. Protein modification by fermentation: effect of fermentation on the potential allergenicity of pea. Allergy. 1998;53(46 Suppl. l):106–108. doi: 10.1111/j.1398-9995.1998.tb04976.x. [DOI] [PubMed] [Google Scholar]
- Ben-Harb S., Saint-Eve A., Panouille M., Souchon I., Bonnarme P., Dugat-Bony E., Irlinger F. Design of microbial consortia for the fermentation of pea-protein-enriched emulsions. Int. J. Food Microbiol. 2019;293:124–136. doi: 10.1016/j.ijfoodmicro.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Cabuk B., Stone A.K., Korber D.R., Tanaka T., Nickerson M.T. Effect of lactobacillus plantarum fermentation on the surface and functional properties of pea protein-enriched flour. Food Technol. Biotechnol. 2018;56(3):411–420. doi: 10.17113/ftb.56.03.18.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernay C., Pelzer E., Makowski D. A global experimental dataset for assessing grain legume production. Scientific data. 2016;3 doi: 10.1038/sdata.2016.84. 160084-160084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.M., Al K.F., Craven L.J., Seney S., Coons M., McCormick H.…Burton J.P. Nutritional, microbial, and allergenic changes during the fermentation of cashew 'cheese' product using a quinoa-based rejuvelac starter culture. Nutrients. 2020;12(3) doi: 10.3390/nu12030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun B.H., Kim K.H., Jeon H.H., Lee S.H., Jeon C.O. Pan-genomic and transcriptomic analyses of Leuconostoc mesenteroides provide insights into its genomic and metabolic features and roles in kimchi fermentation. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-12016-z. 11504-11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani M., Comitini F., Mannazzu I. Fermentation. In: Fath B., editor. Encyclopedia of Ecology. second ed. Elsevier; Oxford: 2013. pp. 310–321. [Google Scholar]
- Codreanu-Morel F., Morisset M., Cordebar V., Larré C., Denery-Papini S. L’allergie au pois. Rev. Fr. Allergol. 2019;59(3):162–165. doi: 10.1016/j.reval.2019.02.197. [DOI] [Google Scholar]
- Coelho L.F., de Lima C.J.B., Bernardo M.P., Contiero J. d(−)-Lactic acid production by leuconostoc mesenteroides B512 using different carbon and nitrogen sources. Appl. Biochem. Biotechnol. 2011;164(7):1160–1171. doi: 10.1007/s12010-011-9202-6. [DOI] [PubMed] [Google Scholar]
- Corsetti A., Ciarrocchi A., Prete R. Reference Module in Food Science. Elsevier; 2016. Lactic acid bacteria: lactobacillus spp.: lactobacillus plantarum. [Google Scholar]
- Daeschel M.A., McGuire J. Interrelationships between protein surface adsorption and bacterial adhesion. Biotechnol. Genet. Eng. Rev. 1998;15:413–438. doi: 10.1080/02648725.1998.10647964. [DOI] [PubMed] [Google Scholar]
- Dreyer L., Astier C., Dano D., Hosotte M., Jarlot-Chevaux S., Sergeant P., Kanny G. Consommation croissante d’aliments contenant du pois jaune : un risque d’allergie ? Rev. Fr. Allergol. 2014;54(1):20–26. doi: 10.1016/j.reval.2013.11.007. [DOI] [Google Scholar]
- El Abboudi M., El Soda M., Pandian S., Barreau M., Trépanier G., Simard R.E. Peptidase activities in debittering and nondebittering strains of lactobacilli. Int. Dairy J. 1992;2(1):55–64. doi: 10.1016/0958-6946(92)90044-M. [DOI] [Google Scholar]
- Ferri M., Serrazanetti D.I., Tassoni A., Baldissarri M., Gianotti A. Improving the functional and sensorial profile of cereal-based fermented foods by selecting Lactobacillus plantarum strains via a metabolomics approach. Food Res. Int. 2016;89:1095–1105. doi: 10.1016/j.foodres.2016.08.044. [DOI] [Google Scholar]
- Ganesan B., Weimer B.C. Chapter 19 - amino acid catabolism and its relationship to cheese flavor outcomes. In: McSweeney P.L.H., Fox P.F., Cotter P.D., Everett D.W., editors. Cheese. fourth ed. Academic Press; San Diego: 2017. pp. 483–516. [Google Scholar]
- García Arteaga V., Apéstegui Guardia M., Muranyi I., Eisner P., Schweiggert-Weisz U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innovat. Food Sci. Emerg. Technol. 2020 doi: 10.1016/j.ifset.2020.102449. [DOI] [Google Scholar]
- García Arteaga V., Leffler S., Muranyi I., Eisner P., Schweiggert-Weisz U. Sensory profile, functional properties and molecular weight distribution of fermented pea protein isolate. Mendeley Data. 2020;V1 doi: 10.17632/j72fn7rg8c.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Vara A., Pinelli D., Rossi M., Fajner D., Magelli F., Matteuzzi D. Production of l(+) and d(−) lactic acid isomers by Lactobacillus casei subsp. casei DSM 20011 and Lactobacillus coryniformis subsp. torquens DSM 20004 in continuous fermentation. J. Ferment. Bioeng. 1996;81(6):548–552. doi: 10.1016/0922-338X(96)81478-4. [DOI] [Google Scholar]
- Heng L. Wageningen University; Wageningen: 2005. Flavour Aspects of Pea and its Protein Preparations in Relation to Novel Protein Foods.wur.nl/WebQuery/wurpubs/343588 Retrieved from library. [Google Scholar]
- Heng L., van Koningsveld G.A., Gruppen H., van Boekel M., Vincken J.P., Roozen J.P., Voragen A.G.J. Protein-flavour interactions in relation to development of novel protein foods. Trends Food Sci. Technol. 2004;15(3–4):217–224. doi: 10.1016/j.tifs.2003.09.018. [DOI] [Google Scholar]
- Holzapfel W.H., Franz C.M.A.P., Ludwig W., Back W., Dicks L.M.T. The genera pediococcus and tetragenococcus. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., editors. The Prokaryotes: Volume 4: Bacteria: Firmicutes, Cyanobacteria. Springer US; New York, NY: 2006. pp. 229–266. [Google Scholar]
- Ibrahim Salam A. Reference Module in Food Science Elsevier; 2016. Lactic acid bacteria: lactobacillus spp.: other species. [DOI] [Google Scholar]
- Ji X.-J., Huang H., Ouyang P.-K. Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnol. Adv. 2011;29(3):351–364. doi: 10.1016/j.biotechadv.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Kaczmarska K.T., Chandra-Hioe M.V., Frank D., Arcot J. Aroma characteristics of lupin and soybean after germination and effect of fermentation on lupin aroma. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2018;87:225–233. doi: 10.1016/j.lwt.2017.08.080. [DOI] [Google Scholar]
- Kumitch H.M., Stone A.K., Nickerson M.T., Korber D.R., Tanaka T. Effect of fermentation time on the physicochemical and functional properties of pea protein-enriched flour fermented by Aspergillus oryzae and Aspergillus Niger. Cereal Chem. 2020;97(2):416–428. doi: 10.1002/cche.10257. [DOI] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature. 1970;227(5259):680–&. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lan Y., Xu M., Ohm J.-B., Chen B., Rao J. Solid dispersion-based spray-drying improves solubility and mitigates beany flavour of pea protein isolate. Food Chem. 2019;278:665–673. doi: 10.1016/j.foodchem.2018.11.074. [DOI] [PubMed] [Google Scholar]
- Lavine E., Ben-Shoshan M. Anaphylaxis to hidden pea protein: a Canadian pediatric case series. J. Allergy Clin. Immunol. Pract. 2019;7(6):2070–2071. doi: 10.1016/j.jaip.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Licandro H., Ho P.H., Nguyen T.K.C., Petchkongkaew A., Nguyen H.V., Chu-Ky S.…Waché Y. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Contr. 2020;110:106957. doi: 10.1016/j.foodcont.2019.106957. [DOI] [Google Scholar]
- Liu S.Q. Reference Module in Food Science Elsevier; 2016. Lactic acid bacteria: leuconostoc spp. [DOI] [Google Scholar]
- Marín M.L., Benito Y., Pin C., Fernández M.F., García M.L., Selgas M.D., Casas C. Lactic acid bacteria: hydrophobicity and strength of attachment to meat surfaces. Lett. Appl. Microbiol. 1997;24(1):14–18. doi: 10.1046/j.1472-765X.1997.00334.x. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt P., Schweiggert-Weisz U., Eisner P. Microbial fermentation of soy protein hydrolysates with focus on the debittering effect and degradation of major soy allergens. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;(71):202–212. doi: 10.1016/j.lwt.2016.03.026. [DOI] [Google Scholar]
- Meinlschmidt P., Ueberham E., Lehmann J., Schweiggert-Weisz U., Eisner P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016;205:229–238. doi: 10.1016/j.foodchem.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Morr C.V., German B., Kinsella J.E., Regenstein J.M., Vanburen J.P., Kilara A.…Mangino M.E. A collaborative study to develop a standarized food protein solubility procedure. J. Food Sci. 1985;50(6):1715–1718. doi: 10.1111/j.1365-2621.1985.tb10572.x. [DOI] [Google Scholar]
- Nielsen P.M., Petersen D., Dambmann C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001;66(5):642–646. doi: 10.1111/j.1365-2621.2001.tb04614.x. [DOI] [Google Scholar]
- Peng W.W., Kong X.Z., Chen Y.M., Zhang C.M., Yang Y.X., Hua Y.F. Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocolloids. 2016;52:301–310. doi: 10.1016/j.foodhyd.2015.06.025. [DOI] [Google Scholar]
- Phillips L.G., Haque Z., Kinsella J.E. A method for the measurement of foam formation and stability. J. Food Sci. 1987;52(4):1074–1077. doi: 10.1111/j.1365-2621.1987.tb14279.x. [DOI] [Google Scholar]
- Raccach M. Pediococci and biotechnology. CRC Crit. Rev. Microbiol. 1987;14(4):291–309. doi: 10.3109/10408418709104442. [DOI] [PubMed] [Google Scholar]
- Richard C., Jacquenet S., Sergeant P., Moneret-Vautrin D.A. Cross-reactivity of a new food ingredient, dun pea, with legumes, and risk of anaphylaxis in legume allergic children. Euro. Annals Allergy Clin. Immunol. 2015;47(4):118–125. [PubMed] [Google Scholar]
- Roland W.S.U., Pouvreau L., Curran J., van de Velde F., de Kok P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2017;94(1):58–65. doi: 10.1094/CCHEM-06-16-0161-Fl. [DOI] [Google Scholar]
- Saha B.C., Hayashi K. Debittering of protein hydrolyzates. Biotechnol. Adv. 2001;19(5):355–370. doi: 10.1016/s0734-9750(01)00070-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Monge R., Lopez-Torrejon G., Pascual C.Y., Varela J., Martin-Esteban M., Salcedo G. Vicilin and convicilin are potential major allergens from pea. Clin. Exp. Allergy. 2004;34(11):1747–1753. doi: 10.1111/j.1365-2222.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- Schindler S., Zelena K., Krings U., Bez J., Eisner P., Berger R.G. Improvement of the aroma of pea (Pisum sativum) protein extracts by lactic acid fermentation. Food Biotechnol. 2012;26(1):58–74. doi: 10.1080/08905436.2011.645939. [DOI] [Google Scholar]
- Schlegel K., Leidigkeit A., Eisner P., Schweiggert-Weisz U. Technofunctional and sensory properties of fermented lupin protein isolates. Foods. 2019;8(12) doi: 10.3390/foods8120678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A.K., Karalash A., Tyler R.T., Warkentin T.D., Nickerson M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015;76:31–38. doi: 10.1016/j.foodres.2014.11.017. [DOI] [Google Scholar]
- Tzitzikas E.N., Vincken J.P., de Groot J., Gruppen H., Visser R.G. Genetic variation in pea seed globulin composition. J. Agric. Food Chem. 2006;54(2):425–433. doi: 10.1021/jf0519008. [DOI] [PubMed] [Google Scholar]
- Verce M., De Vuyst L., Weckx S. Comparative genomics of Lactobacillus fermentum suggests a free-living lifestyle of this lactic acid bacterial species. Food Microbiol. 2020;89:103448. doi: 10.1016/j.fm.2020.103448. [DOI] [PubMed] [Google Scholar]
- Vrancken G., Rimaux T., Wouters D., Leroy F., De Vuyst L. The arginine deiminase pathway of Lactobacillus fermentum IMDO 130101 responds to growth under stress conditions of both temperature and salt. Food Microbiol. 2009;26(7):720–727. doi: 10.1016/j.fm.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Wang C., Johnson L.A. Functional properties of hydrothermally cooked soy protein products. J. Am. Oil Chem. Soc. 2001;78(2):189–195. doi: 10.1007/s11746-001-0242-y. [DOI] [Google Scholar]
- Yen H.-W., Chen T.-J., Pan W.-C., Wu H.-J. Effects of neutralizing agents on lactic acid production by Rhizopus oryzae using sweet potato starch. World J. Microbiol. Biotechnol. 2010;26(3):437–441. doi: 10.1007/s11274-009-0186-0. [DOI] [Google Scholar]
- Zayas J.F. In Functionality of Proteins in Food. Springer Berlin Heidelberg; Berlin, Heidelberg: 1997. Foaming properties of proteins; pp. 260–309. [Google Scholar]
- Zhou Y., Wang J.S., Yang X.J., Lin D.H., Gao Y.F., Su Y.J.…Zheng J.J. Peanut allergy, allergen composition, and methods of reducing allergenicity: a review. Int. J. Food Sci. 2013;2013:909140. doi: 10.1155/2013/909140. [DOI] [PMC free article] [PubMed] [Google Scholar]