Abstract
Mitochondria make physical contact with nearly every other membrane in the cell, and these contacts have a rich variety of functions that are carried out by proteins that reside at the sites of contact. Over the past decade, tremendous insight into the identity and functions of proteins localized to mitochondria contact sites has been gained. In doing so, it has become clear that one protein or protein complex can contribute to contact site formation and function in a wide variety of ways. Thus, complex and often surprising relationships between the roles of a mitochondria contact site and its multifunctional resident proteins continue to be unraveled.
Introduction
Mitochondria contact nearly every other membrane in the cell, and the list of proteins that reside at these contact sites has rapidly grown over the past decade. While identifying mitochondrial membrane contact site (MMCS) proteins is not an easy task, elucidating the functions of these proteins has proven far more challenging [1, 2]. These functions range from simply tethering organelles to form a contact site to contributing to processes that take place at the site of contact (Figure 1). In addition, some proteins only contribute to the function, but not formation, of the contact site. Deciphering the precise functions of MMCS proteins is further complicated by the multiple roles many of these proteins have at or outside of the contact site. While our understanding of the many functions of MMCSs, which includes modulating mitochondrial morphology and dynamics, transporting lipids and metabolites, and responding to signals in the cellular environment, continues to expand, it is far from complete (Figure 1) [1, 3, 4]. In this review, we highlight a few recent examples demonstrating how the multifunctional nature of MMCS proteins facilitates communication between organelles and integrates organelle functions to maintain cellular homeostasis.
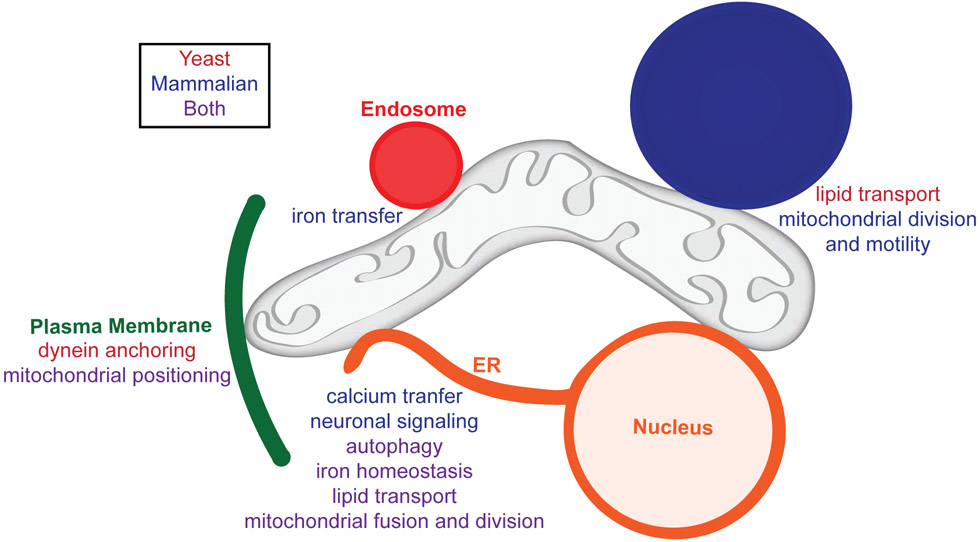
Figure 1: Mitochondria contact site proteins perform a variety of functions.
Mitochondria contact nearly every other membrane in the cell, and these contacts not only maintain mitochondrial distribution and morphology, but also function in metabolism and cellular homeostasis. MMCS proteins facilitate the transfer of molecules to and from mitochondria, creating a network of metabolic signaling. This figure highlights the multitude of functions performed by MMCS proteins in yeast and mammalian cells.
MMCSs spatially organize mitochondria
A major role for MMCS proteins is to spatially position mitochondria. However, the function of tether-based mitochondrial positioning mechanisms often extends beyond simply placing mitochondria within the cell. For example, in yeast, the protein Num1 tethers mitochondria to the plasma membrane and cortical ER forming a MMCS called MECA, for mitochondria-ER-cortex-anchor [5-7]. Num1-mediated tethering of mitochondria to the plasma membrane is required to maintain an evenly distributed mitochondrial network. Interestingly, this seemingly simple tether-based positioning role for Num1 affects more than mitochondrial morphology. Sites of Num1-mediated mitochondrial anchoring also serve as cortical attachment sites for dynein. When formation of Num1-mediated mitochondrial tethering sites is disrupted, defects in dynein-mediated spindle positioning are observed [8, 9]. Thus, the MMCS, MECA, serves to integrate mitochondrial and nuclear positioning pathways. In mammary stem cells, mitochondria-plasma membrane contact is mediated by the mitochondrial fusion protein, Mfn1, in complex with a cortically anchored kinase, PKCζ. This tethering complex serves to regulate mitochondrial inheritance, so that mitochondria with enhanced functions are inherited into the daughter cells maintaining stem cell identity [10•]. Thus, what seems to be a simple positioning function for MMCS proteins may have a far greater and unexpected impact on cellular organization and function.
More than a tether: understanding the intricate roles of MMCS proteins by examining the functions of ER-mitochondria contacts
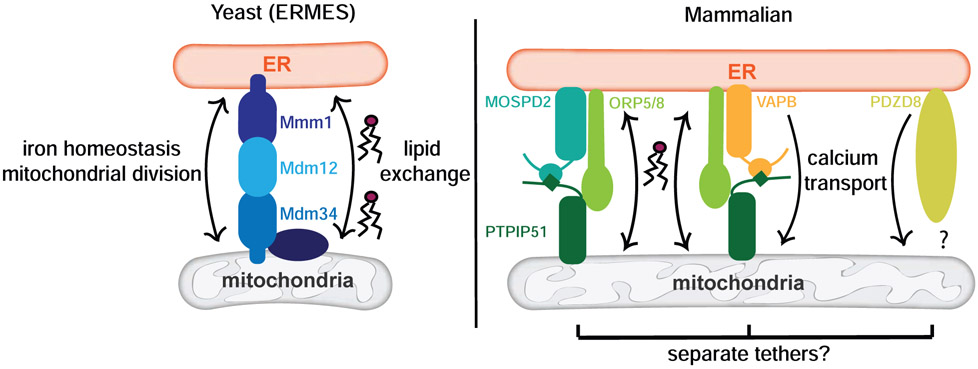
Proteins that reside at MMCSs contribute to contact site function in many ways. This is exemplified by the most well-studied MMCS proteins across species, those that reside at the interface between the ER and mitochondria. In yeast, one of the best-known contacts is the ER-Mitochondria Encounter Structure (ERMES) [11] (Figure 2). First discovered in screens for mitochondrial morphology mutants, the four core proteins of the ERMES complex are Mdm10 (mitochondrial distribution and morphology protein 10), Mdm12, Mdm34, and Mmm1 (maintenance of mitochondrial morphology protein 1). ERMES mediates contact between mitochondria and the ER at sites of mitochondrial division [12], and loss of any one of the ERMES proteins causes the collapse of the tubular mitochondrial network [11]. ERMES mutants exhibit growth defects on non-fermentable carbon sources, suggesting that the tethering complex affects mitochondrial function in addition to distribution. A functional role, beyond tethering, for ERMES is supported by the observation that Mmm1, Mdm12, and Mdm34 have a synaptotagmin-like mitochondrial lipid-binding protein (SMP) domain, suggesting these proteins bind lipid and facilitate lipid exchange between the ER and mitochondria [13, 14]. This idea is supported by recent work demonstrating that a complex of Mdm12 and Mmm1 binds phospholipids and mediates lipid transfer in vitro [15-17••]. Recent work also implicates ERMES in iron regulation. In the absence of ERMES components, mitochondrial iron levels are altered. The mechanism for ERMES-mediated iron homeostasis is unclear but appears to be independent of the contact site’s other functions [18]. Thus, despite the knowledge we have garnered, there is still much to be uncovered about the multifunctional nature of ERMES proteins.
Figure 2: ER-mitochondria contact site proteins facilitate lipid and calcium transport and iron homeostasis.
Yeast and mammalian ER-mitochondria contact sites are excellent examples of the rich variety of functions that MMCS proteins perform. In addition to tethering the ER to mitochondria in yeast, the ERMES contact site proteins are implicated in lipid exchange between the organelles as well as iron homeostasis. In mammalian cells, proteins MOSPD2 and VAPB both interact with PTPIP51 to tether mitochondria to the ER, and these tethers associate with ORP5 and ORP8 to facilitate lipid exchange. Additionally, the VAPB tether along with the newly-discovered MCS protein PDZD8 is involved in calcium transport between the ER and mitochondria.
Using bioinformatics to find metazoan proteins with homology to the SMP domains of the ERMES proteins, PDZD8 was identified [13]. Paralogous to the ERMES protein Mmm1 [19], PDZD8 also mediates contact between the ER and mitochondria, which is required for efficient calcium transport from the ER into mitochondria (Figure 2) [20]. High concentrations of calcium, usually supplied by the ER, are required for several mitochondrial functions, including activating the TCA cycle, apoptosis, and mitochondrial division. Calcium uptake by mitochondria also regulates cytosolic calcium levels. Thus, PDZD8-tethering and its impact on calcium transfer can have a myriad of effects within the cell. While a role for PDZD8 in lipid transfer between the ER and mitochondria has not been demonstrated, a recent study indicates that PDZD8 mediates contact between the ER and late endosomes/lysosomes and suggests that PDZD8 facilitates lipid exchange at these contacts [21]. Additional work is needed to untangle the many proposed roles for PDZD8 at MCSs and determine which are direct effects versus indirect consequences of PDZD8 localization and function.
ER-mitochondrial contact mediated by an interaction between VAPB, an integral ER protein, and PTPIP51, an outer mitochondrial membrane protein, has also been implicated in calcium homeostasis (Figure 2). In the absence of the VAPB-PTPIP51 interaction, ER-mitochondria contact is decreased and calcium uptake by mitochondria following release from the ER is reduced [22, 23], which is shown to stimulate autophagy [24]. Conversely, when the VAPB-PTPIP51 complex is overexpressed and ER-mitochondria contact is increased, autophagy is downregulated. Thus, this MMCS links calcium transfer to the regulation of autophagy. Recently, VAPB-PTPIP51 ER-mitochondria contacts have been shown to form at neuronal synapses, and loss of the complex results in perturbed synaptic function [25]. Which of the many functions associated with the VAPB-PTPIP51 tethering complex are required for synaptic function have yet to be determined.
Understanding the precise function of PTPIP51 at MMCS is complicated by recent work demonstrating that oxysterol-binding protein-related proteins, ORP5 and ORP8, interact with PTPIP51 at ER-mitochondria contact sites (Figure 2). Loss of ORP5 or ORP8 results in compromised mitochondrial morphology and function without altering the number of ER-mitochondria contact sites [26]. Given that recruitment of ORP5 and ORP8 to ER-mitochondria contact sites requires their functional lipid-binding domains and that ORP5 and ORP8 facilitate phosphatidylserine transport at ER-PM contact sites [27], it is likely these proteins mediate lipid transfer between the ER and mitochondria. Adding further complexity to PTPIP51 function, the protein also functions as an ER-mitochondrial tether independent of VAPB through its interaction with the ER protein MOSPD2 [28]. Whether the activities of the MOSPD2-PTPIP51 tether, the VAPB-PTPIP51 tether, and the MMCS-localized ORP5, ORP8, and PDZD8 proteins are related and integrated have yet to be determined (Figure 2). These examples highlight a common theme for MMCS proteins – one protein or protein complex can contribute to contact site function in a variety of ways.
The moonlighting roles of mitochondrial import and dynamics proteins and vesicular trafficking proteins at MMCSs
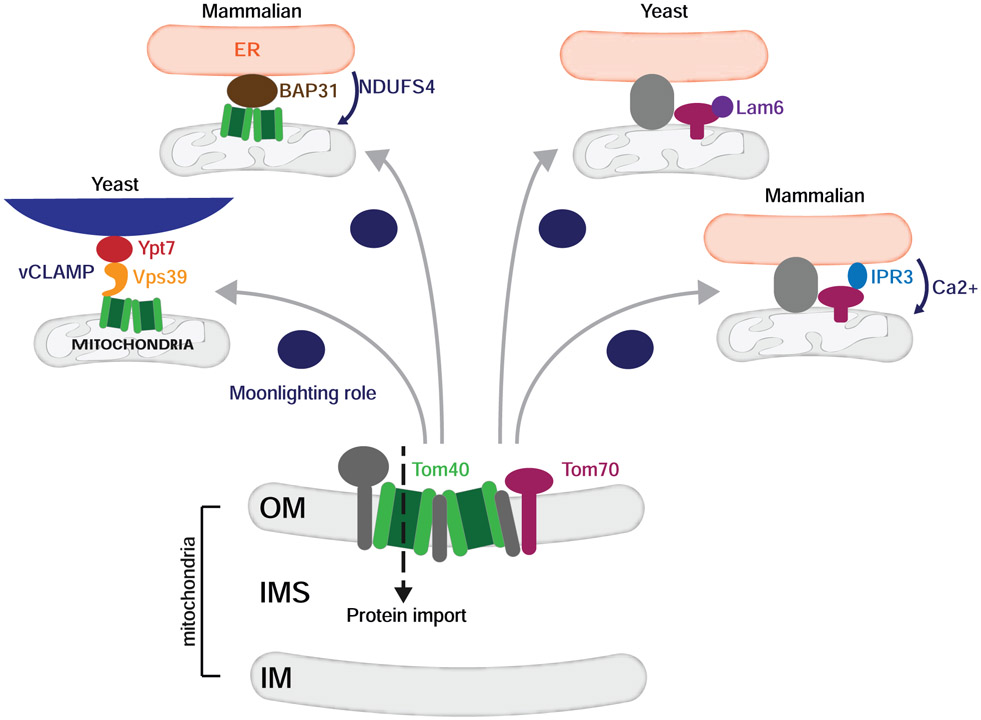
As MMCS components and their functional importance are elucidated, it is clear that proteins vital to organelle function moonlight in the formation and function of contact sites (Figure 3). This is exemplified by vCLAMP, a protein complex that drives contact between the mitochondria and vacuole in yeast [29, 30]. Formation of this contact is dependent on Vps39, a protein involved in endosome-vacuole tethering and fusion. Vps39 associates with the vacuolar GTPase, Ypt7, and an essential mitochondrial import protein, Tom40, to tether mitochondria to the vacuole [30, 31••]. While the functional importance of vCLAMP is unclear, it has been implicated in the transfer of phospholipids and maintenance of cellular fitness in stress conditions [29-31••]. A role for Tom40 in the formation and function of MMCSs extends to mammalian cells. Recent work demonstrates that Tom40 associates with BAP31, an ER resident protein, to ensure the proper translocation of NDUFS4, a component of Complex I in the electron transport chain, into the mitochondria [32]. The presence of ER stress downregulates this MMCS resulting in decreased translocation of NDUFS4 and, consequently, reduced oxygen consumption, suggesting this contact relays a readout of ER health to mitochondria.
Figure 3: Mitochondrial import proteins have moonlighting roles at mitochondria contact sites.
TOM complex proteins that primarily function in mitochondrial import also have moonlighting roles in the formation and maintenance of mitochondria contact sites. Tom40, for example, acts as the mitochondrial receptor in the yeast vacuole-mitochondria contact site (vCLAMP). Tom40 also localizes to ER-mitochondria contact sites in mammalian cells and facilitates the import of NDUFS4, a respiratory complex component. Another mitochondrial import protein, Tom70, is found at ER-mitochondria contact sites in both yeast and mammalian cells and is implicated in calcium transfer in the latter cell type.
Other mitochondrial import proteins have been found to moonlight at MMCSs. In yeast, a role for Mdm10 in the biogenesis of mitochondrial outer membrane beta barrel proteins was appreciated long before it was identified as a component of ERMES [33]. Additionally, the sterol transporter Lam6 (also known as Ltc1) is recruited to sites of ER-mitochondria contact via interactions with mitochondrial import proteins Tom70 and Tom71 [34-36]. In mammalian cells, Tom70 is concentrated at ER-mitochondria contact sites and recruits the IP3R3 complex, promoting Ca2+ transfer from the ER to mitochondria to sustain proper mitochondrial function and ultimately cellular fitness [37].
The list of proteins with well-established functions in membrane dynamics and trafficking pathways that have moonlighting roles at MMCSs continues to grow. Like its yeast homologue Ypt7, RAB7, a key player in endosomal-lysosomal trafficking in mammalian cells, is an important component of contact sites. GTP-bound lysosomal RAB7 and the RAB7 GAP TBC1D15, which is recruited by mitochondrial fission protein Fis1, regulate mitochondria-lysosome contact sites. These contact sites mark sites of mitochondrial fission and impact mitochondrial motility via the regulation of inter-mitochondrial tethering [38•, 39]. Thus, RAB7 and TBC1D15 impact the morphology and function of both lysosomes and mitochondria. In addition, components of the mitochondrial fusion machinery have also been implicated in the formation of MMCSs. In yeast, the mitochondrial fusion protein, Fzo1, functions as a mitochondria-peroxisome tether [40], and its human homologs, the Mitofusins, tether mitochondria to the ER, melanosomes, lipid droplets, and PM [10•, 41-43]. Linking proteins with canonical functions in organelle homeostasis to MMCSs likely provides a mechanism to coordinate the functions of mitochondria and the organelles they contact. Thus, the next challenge will be to determine if and how the canonical functions of these proteins impact or are impacted by the MMCSs at which they reside.
Maintaining cellular homeostasis through the integrated regulation of membrane contact sites
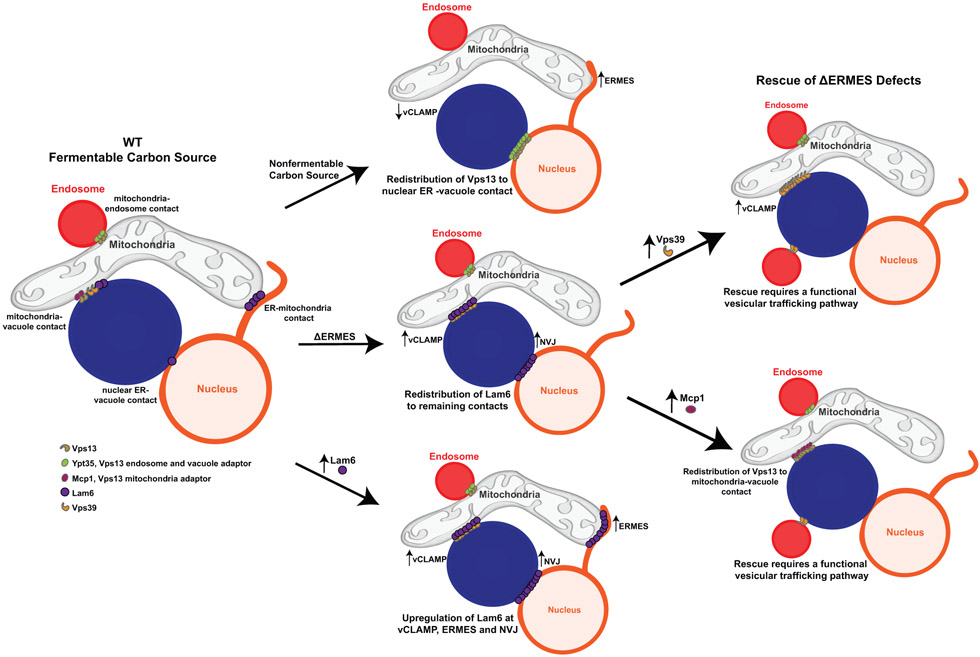
A specific MCS does not function in isolation, rather research has repeatedly shown that the formation and function of MCSs are integrated and coordinated in response to changes in the cellular environment. This is well represented by the relationship between Lam6, which resides at both ER-mitochondria and ER-vacuole contact sites, ERMES, and vCLAMP (Figure 4). When yeast cells are forced to respire, ERMES is upregulated while vCLAMP is downregulated, suggesting integrated regulation between contact sites in response to a metabolic shift [30]. Furthermore, disruption of vCLAMP leads to upregulation of ERMES and vice versa, all of which depend on the presence of Lam6 [29, 34]. Preventing Lam6 recruitment to one contact redistributes it to others, and overexpression of Lam6 results in overall contact expansion [34, 36]. Thus, Lam6 may act as a messenger between contact sites, relaying cellular changes through its protein level and recruitment to specific MCSs.
Figure 4: Interorganelle communication through contact site reorganization.
MCSs are coordinately regulated and reorganized in response to changes in the cellular environment. For example, contact site reorganization is driven by changes in growth conditions. In response to growth on non-fermentable carbon sources, vCLAMP (represented by Vps39) is downregulated, ERMES is upregulated, and Vps13 relocalizes from mitochondria-endosome and mitochondria-vacuole contacts to nuclear ER-vacuole contacts. In a second example, contact sites expansion is driven by loss of other contact sites. Loss of ERMES results in the redistribution of Lam6 to vCLAMP and nuclear ER-vacuole contacts, resulting in an upregulation of these contact sites. Finally, regulating the localization of proteins to specific contact sites provides a mechanism to regulate contact site function. The loss of ERMES can be functionally rescued by the expansion of mitochondria-vacuole contact. This can be done by the upregulation of vCLAMP (overexpression of Vps39) or the recruitment of Vps13 to mitochondria-vacuole contact by overexpressing Mcp1, the mitochondrial adaptor for Vps13. In addition, overexpression of Lam6 results in upregulation of Lam6 at vCLAMP, ERMES, and nuclear ER-vacuole contacts and subsequent expansion of these contact sites.
Vps13, a putative lipid transport protein that is found at multiple contact sites [44••,45•-48], is another player in the complex MCS relationship described above (Figure 4). Like Lam6, Vps13 is required in the absence of ERMES, and dominant suppressor mutations within Vps13 rescue the defects associated with ERMES deficiency [46, 47, 49]. Vps13 recruitment is mediated by interactions with organelle-specific proteins that recognize its DUF1162 domain [44••]. Competition between these organelle-specific proteins regulates the metabolic state-dependent recruitment of Vps13 to specific MCSs. For example, Vps13 shifts its localization from mitochondria-endosome/mitochondria-vacuole contacts in the presence of fermentable carbon sources to the nuclear ER-vacuole junction (NVJ) when cells are forced to respire [46, 47], suggesting there is functional importance to the localization of Vps13 at specific contacts. Consistently, overexpression of the Vps13 mitochondrial adaptor, Mcp1, recruits Vps13 to mitochondria and rescues the growth defect associated with loss of ERMES (Figure 4) [44••, 49]. Thus, the condition-dependent recruitment of a multi-MCS protein, such as Vps13, to a specific MCS likely provides a mechanism to regulate the activity of that contact site in a condition-specific manner.
Further functionally linking ERMES, vCLAMP, and Vps13 is the unexpected connection between the functions of these proteins/protein complexes at MMCSs and vesicular trafficking. The use of a series of mutants that separate the role of Vps39 in vCLAMP from its role in vesicular trafficking made it clear that the well-known role of Vps39 in vesicular trafficking, and not its role in vCLAMP, is required for survival in cells lacking ERMES [31••]. Consistently, several other components necessary for trafficking to the vacuole are required for cell survival in the absence of ERMES. Interestingly, the ability of the Vps13 suppressor mutants and Mcp1 overexpression to rescue defects associated with ERMES deficiency also depend on a functional vesicular trafficking pathway. Thus, there is a functional relationship between proper trafficking to the vacuole and the functions of these MMCSs (Figure 4). These unexpected revelations highlight the level of complexity that exists in ascertaining the precise functions of the many multifunctional proteins that reside at MMCSs.
Conclusion
As research continues to elucidate the many facets of MCS, it is clear that MCS formation and functions are vastly more complicated than originally thought. MMCSs function as hubs to regulate a myriad of functions and facilitate communication between mitochondria and other organelles in multiple ways. In addition, they respond to and relay changes in organelle homeostasis and the cellular environment to maintain cellular homeostasis and fitness. Many MMCSs are formed by proteins with well-established canonical roles in organelle biogenesis and function. These canonical roles may contribute to or be impacted by the formation, function, and regulation of MMCSs. In addition, MMCS proteins can have multiple functions at one contact site or function at multiple contact sites. These revelations highlight the complex relationship between MCS function, the proteins that form them, and the participating organelles and the challenges that will be faced as we continue to unravel the mechanisms of MCS formation, function, and regulation.
Acknowledgments
We would like to thank members of the Lackner Lab for helpful discussions. We would like to apologize to colleagues whose outstanding work on mitochondria-organelle contacts could not be included due to space limitations. This work has been supported in part by funding from the NIH NIGMS Training Grant T32GM008382 to AJW, the NIH NIGMS Training Grant T32GM008449 and National Science Foundation Graduate Research Fellowship DGE-1842165 to CSH, and the NIH NIGMS grant R01GM120303 to LLL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Eisenberg-Bord M, Shai N, Schuldiner M, Bohnert M: A Tether Is a Tether Is a Tether: Tethering at Membrane Contact Sites. Dev Cell 2016, 39:395–409. [DOI] [PubMed] [Google Scholar]
- [2].Scorrano L, De Matteis MA, Emr S, Giordano F, Hajnóczky G, Kornmann B, Lackner LL, Levine TP, Pellegrini L, Reinisch K et al. : Coming together to define membrane contact sites. Nat Commun 2019, 10:1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Eisenberg-Bord M, Schuldiner M: Mitochatting - If only we could be a fly on the cell wall. Biochim Biophys Acta Mol Cell Res 2017, 1864:1469–1480. [DOI] [PubMed] [Google Scholar]
- [4].Lackner LL: The Expanding and Unexpected Functions of Mitochondria Contact Sites. Trends Cell Biol 2019, 29:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Klecker T, Scholz D, Fortsch J, Westermann B: The yeast cell cortical protein Num1 integrates mitochondrial dynamics into cellular architecture. J Cell Sci 2013, 126:2924–2930. [DOI] [PubMed] [Google Scholar]
- [6].Lackner LL, Ping H, Graef M, Murley A, Nunnari J: Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc Natl Acad Sci U S A 2013, 110:E458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ping HA, Kraft LM, Chen W, Nilles AE, Lackner LL: Num1 anchors mitochondria to the plasma membrane via two domains with different lipid binding specificities. J Cell Biol 2016, 213:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kraft LM, Lackner LL: Mitochondria-driven assembly of a cortical anchor for mitochondria and dynein. J Cell Biol 2017, 216:3061–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schmit HL, Kraft LM, Lee-Smith CF, Lackner LL: The role of mitochondria in anchoring dynein to the cell cortex extends beyond clustering the anchor protein. Cell Cycle 2018, 17:1345–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu MJ, Chen YS, Kim MR, Chang CC, Gampala S, Zhang Y, Wang Y, Chang CY, Yang JY, Chang CJ: Epithelial-Mesenchymal Transition Directs Stem Cell Polarity via Regulation of Mitofusin. Cell Metab 2018, DOI: 10.1016/j.cmet.2018.11.004.•In mammary stem cells, the mitochondrial fusion protein, MFN1, and protein kinase, PKCζ, are shown to form a complex that tethers mitochondria to the cell periphery. This contact site ensures the asymmetric inheritance of fused mitochondria with enhanced function to one cell to maintain a self-renewing stem cell pool.
- [11].Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P: An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 2009, 325:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, Nunnari J: ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. Elife 2013, 2:e00422.23682313 [Google Scholar]
- [13].Kopec KO, Alva V, Lupas AN: Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics 2010, 26:1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee I, Hong W: Diverse membrane-associated proteins contain a novel SMP domain. FASEB J 2006, 20:202–206. [DOI] [PubMed] [Google Scholar]
- [15].AhYoung AP, Jiang J, Zhang J, Khoi Dang X, Loo JA, Zhou ZH, Egea PF: Conserved SMP domains of the ERMES complex bind phospholipids and mediate tether assembly. Proc Natl Acad Sci U S A 2015, 112:E3179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jeong H, Park J, Jun Y, Lee C: Crystal structures of Mmm1 and Mdm12-Mmm1 reveal mechanistic insight into phospholipid trafficking at ER-mitochondria contact sites. Proc Natl Acad Sci U S A 2017, 114:E9502–E9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kawano S, Tamura Y, Kojima R, Bala S, Asai E, Michel AH, Kornmann B, Riezman I, Riezman H, Sakae Y et al. : Structure-function insights into direct lipid transfer between membranes by Mmm1-Mdm12 of ERMES. J Cell Biol 2018, 217:959–974.••This study uses in vitro lipid transport assays with isolated mitochondria and ER membranes to demonstrate that a complex between ERMES components Mdm12 and Mmm1 mediates phospholipid transfer between membranes.
- [18].Xue Y, Schmollinger S, Attar N, Campos OA, Vogelauer M, Carey MF, Merchant SS, Kurdistani SK: Endoplasmic reticulum-mitochondria junction is required for iron homeostasis. J Biol Chem 2017, 292:13197–13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wideman JG, Balacco DL, Fieblinger T, Richards TA: PDZD8 is not the ‘functional ortholog’ of Mmm1, it is a paralog. F1000Res 2018, 7:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hirabayashi Y, Kwon SK, Paek H, Pernice WM, Paul MA, Lee J, Erfani P, Raczkowski A, Petrey DS, Pon LA, Polleux F: ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science 2017, 358:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guillén-Samander A, Bian X, De Camilli P: PDZD8 mediates a Rab7-dependent interaction of the ER with late endosomes and lysosomes. Proc Natl Acad Sci U S A 2019, 116:22619–22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].De Vos KJ, Mórotz GM, Stoica R, Tudor EL, Lau KF, Ackerley S, Warley A, Shaw CE, Miller CC: VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum Mol Genet 2012, 21:1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau KF, Vizcay-Barrena G, Lin WL, Xu YF, Lewis J et al. : ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun 2014, 5:3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gomez-Suaga P, Paillusson S, Stoica R, Noble W, Hanger DP, Miller CCJ: The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy. Curr Biol 2017, 27:371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gómez-Suaga P, Pérez-Nievas BG, Glennon EB, Lau DHW, Paillusson S, Mórotz GM, Calì T, Pizzo P, Noble W, Miller CCJ: The VAPB-PTPIP51 endoplasmic reticulum-mitochondria tethering proteins are present in neuronal synapses and regulate synaptic activity. Acta Neuropathol Commun 2019, 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Galmes R, Houcine A, van Vliet AR, Agostinis P, Jackson CL, Giordano F: ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO Rep 2016, 17:800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P: INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 2015, 349:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Di Mattia T, Wilhelm LP, Ikhlef S, Wendling C, Spehner D, Nominé Y, Giordano F, Mathelin C, Drin G, Tomasetto C, Alpy F: Identification of MOSPD2, a novel scaffold for endoplasmic reticulum membrane contact sites. EMBO Rep 2018, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Elbaz-Alon Y, Rosenfeld-Gur E, Shinder V, Futerman AH, Geiger T, Schuldiner M: A dynamic interface between vacuoles and mitochondria in yeast. Dev Cell 2014, 30:95–102. [DOI] [PubMed] [Google Scholar]
- [30].Hönscher C, Mari M, Auffarth K, Bohnert M, Griffith J, Geerts W, van der Laan M, Cabrera M, Reggiori F, Ungermann C: Cellular metabolism regulates contact sites between vacuoles and mitochondria. Dev Cell 2014, 30:86–94. [DOI] [PubMed] [Google Scholar]
- [31].González Montoro A, Auffarth K, Hönscher C, Bohnert M, Becker T, Warscheid B, Reggiori F, van der Laan M, Fröhlich F, Ungermann C: Vps39 Interacts with Tom40 to Establish One of Two Functionally Distinct Vacuole-Mitochondria Contact Sites. Dev Cell 2018, 45:621–636.e7.••Through a series of mutations in Vps39, the function of Vps39 in vesicular trafficking is separated from its function within the vCLAMP. Using these mutations, the authors elegantly demonstrate that the well-known rescue of ERMES deficiency by vCLAMP is, surprisingly, not due to the function of Vps39 within vCLAMP, but rather its canonical function in vesicular trafficking.
- [32].Namba T: BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites. Sci Adv 2019, 5:eaaw1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wiedemann N, Pfanner N: Mitochondrial Machineries for Protein Import and Assembly. Annu Rev Biochem 2017, 86:685–714. [DOI] [PubMed] [Google Scholar]
- [34].Elbaz-Alon Y, Eisenberg-Bord M, Shinder V, Stiller SB, Shimoni E, Wiedemann N, Geiger T, Schuldiner M: Lam6 Regulates the Extent of Contacts between Organelles. Cell Rep 2015, 12:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gatta AT, Wong LH, Sere YY, Calderón-Noreña DM, Cockcroft S, Menon AK, Levine TP: A new family of StART domain proteins at membrane contact sites has a role in ER-PM sterol transport. Elife 2015, 4:e07253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Murley A, Sarsam RD, Toulmay A, Yamada J, Prinz WA, Nunnari J: Ltc1 is an ER-localized sterol transporter and a component of ER-mitochondria and ER-vacuole contacts. J Cell Biol 2015, 209:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Filadi R, Leal NS, Schreiner B, Rossi A, Dentoni G, Pinho CM, Wiehager B, Cieri D, Call T, Pizzo P, Ankarcrona M: TOM70 Sustains Cell Bioenergetics by Promoting IP3R3-Mediated ER to Mitochondria Ca2+ Transfer. Curr Biol 2018, 28:369–382.e6. [DOI] [PubMed] [Google Scholar]
- [38].Wong YC, Ysselstein D, Krainc D: Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 2018, 554:382–386.•The authors identify the formation and regulation of mitochondria-lysosome contact sites in mammalian cells using EM, SIM, and high resolution confocal live cell imaging. Active GTP-bound RAB7 mediates this contact in addition to regulating both mitochondrial and lysosomal fission. This study adds another unexpected player to sites of mitochondrial fission.
- [39].Wong YC, Peng W, Krainc D: Lysosomal Regulation of Inter-mitochondrial Contact Fate and Motility in Charcot-Marie-Tooth Type 2. Dev Cell 2019, 50:339–354.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shai N, Yifrach E, van Roermund CWT, Cohen N, Bibi C, IJlst L, Cavellini L, Meurisse J, Schuster R, Zada L et al. : Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat Commun 2018, 9:1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Boutant M, Kulkarni SS, Joffraud M, Ratajczak J, Valera-Alberni M, Combe R, Zorzano A, Cantó C: Mfn2 is critical for brown adipose tissue thermogenic function. EMBO J 2017, 36:1543–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Daniele T, Hurbain I, Vago R, Casari G, Raposo G, Tacchetti C, Schiaffino MV: Mitochondria and melanosomes establish physical contacts modulated by Mfn2 and involved in organelle biogenesis. Curr Biol 2014, 24:393–403. [DOI] [PubMed] [Google Scholar]
- [43].de Brito OM, Scorrano L: Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456:605–610. [DOI] [PubMed] [Google Scholar]
- [44].Bean BDM, Dziurdzik SK, Kolehmainen KL, Fowler CMS, Kwong WK, Grad LI, Davey M, Schluter C, Conibear E: Competitive organelle-specific adaptors recruit Vps13 to membrane contact sites. J Cell Biol 2018, 217:3593–3607.••This study identified an interaction motif contained within all identified Vps13 organelle adaptors that competes for the same conserved binding region within Vps13. Excitingly, this competition between adaptors regulates Vps13 localization to different membranes.
- [45].Kumar N, Leonzino M, Hancock-Cerutti W, Horenkamp FA, Li P, Lees JA, Wheeler H, Reinisch KM, De Camilli P: VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol 2018, 217:3625–3639.•In this elegant study, the authors elucidate the structure of the N-terminal domain of Vps13 and demonstrate that the hydrophobic cavity that this domain forms is able to transfer lipids between membranes in vitro.
- [46].Lang AB, John Peter AT, Walter P, Kornmann B: ER-mitochondrial junctions can be bypassed by dominant mutations in the endosomal protein Vps13. J Cell Biol 2015, 210:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park JS, Thorsness MK, Policastro R, McGoldrick LL, Hollingsworth NM, Thorsness PE, Neiman AM: Yeast Vps13 promotes mitochondrial function and is localized at membrane contact sites. Mol Biol Cell 2016, 27:2435–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yeshaw WM, van der Zwaag M, Pinto F, Lahaye LL, Faber AI, Gómez-Sánchez R, Dolga AM, Poland C, Monaco AP, van IJzendoorn SC et al. : Human VPS13A is associated with multiple organelles and influences mitochondrial morphology and lipid droplet motility. Elife 2019, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].John Peter AT, Herrmann B, Antunes D, Rapaport D, Dimmer KS, Kornmann B: Vps13-Mcp1 interact at vacuole-mitochondria interfaces and bypass ER-mitochondria contact sites. J Cell Biol 2017, 216:3219–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]