Abstract
APOBEC3G (A3G) is a single-stranded DNA (ssDNA) cytosine deaminase that can restrict HIV-1 infection by mutating the viral genome. A3G consists of a non-catalytic N-terminal domain (NTD) and a catalytic C-terminal domain (CTD) connected by a short linker. While the CTD catalyzes cytosine deamination, the NTD is believed to provide additional affinity for ssDNA. Structures of both A3G domains have been solved individually; however, a full-length A3G structure has been challenging. Recently, crystal structures of full-length rhesus macaque A3G variants were solved which suggested dimerization mechanisms and RNA binding surfaces, whereas the dimerization appeared to compromise catalytic activity. We determined the crystal structure of a soluble variant of human A3G (sA3G) at 2.5Å and from these data generated a model structure of wild-type A3G. This model demonstrated that the NTD was rotated 90° relative to the CTD along the major axis of the molecule, an orientation that forms a positively charged channel connected to the CTD catalytic site, consisting of NTD loop-1 and CTD loop-3. Structure-based mutations, in vitro deamination and DNA binding assays, and HIV-1 restriction assays identify R24, located in the NTD loop-1, as essential to a critical interaction with ssDNA. Furthermore, sA3G was shown to bind a deoxy-cytidine dinucleotide near the catalytic Zn2+, yet not in the catalytic position, where the interactions between deoxy-cytidines and CTD loop-1 and loop-7 residues were different from those formed with substrate. These new interactions suggest a mechanism explaining why A3G exhibits a 3' to 5' directional preference in processive deamination.
INTRODUCTION
Human APOBEC3G (A3G) is a member of the seven human APOBEC3 (A3A, A3B, A3C, A3D, A3F, A3G and A3H) family of proteins which belong to the larger APOBEC super-family[1-5]. All APOBEC [APOBEC1, APOBEC2, APOBEC3, APOBEC4 and activation-induced cytidine deaminase (AID)] proteins catalyze Zn-dependent deamination of deoxycytidine in single-stranded DNA (ssDNA) converting deoxy-cytidine to deoxy-uridine[6]. With their deaminase activity, APOBEC proteins play crucial roles in a variety of biological processes ranging from antibody diversification to defense against viral infections [7-10]. Accordingly, A3G (also A3D, A3F and A3H) is capable of restricting human immunodeficiency virus type-1 (HIV-1) and other retroviruses[7, 8, 11-16]. However, HIV-1 counteracts APOBEC3 proteins via the viral protein Vif, which targets APOBEC3 proteins for proteasomal degradation [15, 17-22]. Briefly, in the absence of Vif, A3G is encapsidated into newly forming virions in association with viral and host RNAs[7, 17-19, 23-27]. The viral RNA is reverse transcribed into negative strand (−) DNA which can then act as a template for positive strand (+) DNA synthesis. Encapsidated A3G recognizes substrate deoxy-cytidines, including hotspot sequences (5'-CCC and 5'-CC) in the newly formed negative strand (−) DNA, and catalyzes the deamination of deoxy-cytidine to deoxy-uridine. Subsequently, during positive strand (+) DNA synthesis this deoxy-uridine is used as a template, resulting in G-to-A hypermutation in the positive strand (+) DNA. Thus, mutations exerted in the proviral DNA render the virus inactive or non-functional[28-30]. In addition to lethal hypermutation, deaminase-independent mechanisms of HIV-1 restriction by APOBEC3 proteins have also been documented[31-36].
Among APOBEC3 proteins, A3B, A3D, A3F and A3G consist of two homologous Zn2+ binding domains. A3G contains a catalytically inactive N-terminal (A3G-NTD) and a catalytically active C-terminal (A3G-CTD) domain[37-39]. A3G-NTD plays an essential role in encapsidation of A3G through association with viral and host RNAs[23, 40, 41]. In addition, A3G-NTD binds to ssDNA and may stabilize the A3G:ssDNA complex thereby supporting catalysis. Vif interaction with A3G-NTD triggers the ubiquitin-mediated degradation of A3G [17-20]. A3G-CTD contains a Zn2+ binding motif HxE-x23–28-C-x2–4-C and catalyzes the deamination of deoxy-cytidine to deoxy-uridine. Mechanistically, a Zn2+ coordinated water molecule produces a hydroxide ion which attacks the C4 atom of cytosine, causing hydrogen to be transferred to the carboxylate group of a glutamic acid coordinated to Zn2+ through a water molecule and ultimately to the amine resulting in an ammonia leaving group[42-44]. Although all APOBEC3 proteins use a similar deamination mechanism, they show varying preferences for target nucleotide sequences; A3G prefers 5′-CCC and 5′-CC, whereas other A3s prefer 5′-TC[45]. Nucleotides flanking the target motifs, and secondary structures of ssDNA can also modulate A3 binding affinity[46]. Furthermore, previous studies showed that A3G deaminates multiple target sequences processively from the 3′-end to the 5′-end of a ssDNA, although the mechanism that constrains deamination to one polarity remains elusive[47].
Three dimensional structures of individual domains (NTD and CTD) of A3G have been solved by us and other laboratories using NMR and X-ray crystallography[48-55]. Overall these structures are similar and share secondary structures, including six α-helices and five β-strands, and one HxE-x23–28-C-x2–4-C Zn2+ binding motif. Recently, structures of APOBEC3 domains complexed with ssDNA have emerged. Xiao et al. published a crystal structure of rhesus macaque A3G N-terminal domain complexed with poly-T ssDNA, revealing non-catalytic binding of a single thymine[56]. Co-crystal structures of A3A[57, 58] and chimeric A3B-CTD[58] with ssDNA bound have been informative with regard to the 5′-TC target sequence preference for A3A/A3B. Our recent structure of A3G-CTD co-crystalized with substrate ssDNA provided extensive information on how A3G-CTD specifically recognizes its 5′-CCC preferred target sequence[59]. Another structure of A3G-CTD with a non-preferred adenine nucleotide bound near the catalytic pocket[60] showed possible interactions for initial DNA scanning of target sequences. Most recently, we have revealed that DNA interaction with helix-1 and loop-1 (T201-L220) of A3G-CTD distinguishes the substrate binding mode from non-substrate binding modes, and that a 2′-endo sugar conformation of the target deoxy-cytidine is important for stabilization of the substrate during catalysis[61].
Biochemical and structural studies[62-66] have suggested that human A3G forms dimeric and higher-order oligomeric structures, and RNA binding may play an important role for forming A3G oligomers. In spite of these earlier studies, single-molecule experiments indicated that monomeric forms of human A3G are more catalytically active than oligomeric forms[67]. Most recently, Morse and co-workers used optical tweezers to characterize A3G-ssDNA binding modes, and their experiments suggested that NTD binds ssDNA first, allowing CTD to search for preferred deamination target sequences, whereas oligomerization of A3G stabilizes ssDNA binding but inhibits CTD to find the target sequences[68]. Interestingly, A3G is likely to change relative orientation of NTD and CTD as molecular dynamics simulations and high-speed atomic force microscopy studies have indicated[69].
Resolving the structures of full-length APOBEC3 proteins containing both NTD and CTD has been challenging due to their poor solubility and aggregation tendency. Most recently, two soluble variants of rhesus macaque A3G (rmA3G) structures have been determined, and they revealed a dimerization mechanism and potential RNA-binding surfaces[70]. These rmA3G variant dimer structures were apparently catalytically compromised as the dimerization interface occluded the substrate-binding loop-1 of CTD[70], yet provided important structural mechanisms for blocking entry of ssDNA to the catalytic site which had been proposed by Morse and co-workers[67].
Here we present a crystal structure of soluble monomeric variant of A3G (sA3G) at 2.5 Å resolution. We generated a wild-type A3G structural model based on our soluble variant structure by replacing substituted residues with wild-type amino acids. This wtA3G structural model shows a positively-charged channel formed between NTD and CTD domains. This channel is continuous from the 3' side of a substrate ssDNA bound to A3G-CTD in our previous co-crystal structure[59]. We have identified amino acid R24, which is located in this channel, plays a significant role in ssDNA-binding and deamination. Equally interesting our sA3G crystal structure also captured a deoxy-cytidine dinucleotide near the catalytic Zn2+, which provided experimental evidence of important non-catalytic deoxy-cytidine interactions to find deamination target sequences.
RESULTS
Generation of a soluble monomeric A3G variant:
We improved protein solubility and molecular homogeneity of A3G by generating a soluble monomeric variant. Previously, we generated soluble variants of A3G-NTD (sNTD) and A3G-CTD (CTD2) by rational amino acid substitutions, and we were able to determine their NMR and crystal structures[48, 51, 59]. All substitutions in sNTD were made with consensus amino acids among relatively soluble APOBEC3 protein domains including A3G-CTD, A3A and A3B-CTD, and structural integrity was confirmed for each substitution by using 1H-15N HSQC spectra[51]. CTD2 was generated by rigorously systematic substitutions of surface residues to improve both solubility and catalytic activity[48, 59]. As a result, A3G domain structures were intact for sNTD and CTD2 (PDB ID: 2MZZ and 6BUX, respectively), and these structures retained affinity for HIV-1 Vif and catalytic activity, respectively[51, 59]. Using these soluble domain variants as starting templates, we added nine substitutions and two deletions to generate a double-domain A3G variant that was predominantly monomeric in solution, which we named soluble A3G or sA3G. The amino acid sequence of the sA3G construct compared to wild-type A3G is shown in Supplementary Fig. 1a. Among nine additional substitutions, Y19D, L27A, W34Y, V58A and Y59A had been tested during the generation of sNTD, and these substitutions improved solubility although weakened affinity for HIV-1 Vif[51]. Alanine substitutions of NTD loop-7 residues, including Y125A, F126A and W127A, helped A3G to stay as monomer in solution as previously reported[71]. In addition, we found that deletions of 47-PPL-49 (NTD loop-4) and 79-KLHR-82 (NTD loop-8) decreased protein-protein interactions between the N-terminal domains, while the structure was retained intact (monitored by 1H-15N HSQC NMR spectra, data not shown). sA3G was catalytically active as demonstrated in the deamination assays provided in Supplementary Fig. 1c. It is reasonable to see catalytic activity of the predominantly-monomeric sA3G since recent single molecule experiments have indicated that the monomeric state of A3G is the most catalytically active[67, 68]. For crystallization, we used a catalytically inactive variant in which catalytically critical E259 was substituted to alanine (E259A) in order to avoid catalyzing deoxy-cytidines and increase the chance to retain bound ssDNA, referred to as sA3G* hereafter.
Crystal structure of sA3G*:
We solved structures of sA3G* crystals obtained from three different conditions at three different resolutions 3.5 Å, 3.0 Å and 2.5 Å all in the same space group I 1 2 1. All three structures are essentially the same and a single sA3G* molecule occupied the asymmetric unit (statistics are provided in Table 1). We will use the highest resolution (2.5 Å) structure that was refined to Rwork/Rfree at 0.197/0.248 for structural analysis and figures hereafter (Fig. 1a).
Table 1. Crystallographic data collection and refinement statistics.
| PDB ID | 6WMA | 6WMB | 6WMC |
|---|---|---|---|
| Space group | I 1 2 1 | I 1 2 1 | I 1 2 1 |
| Cell dimensions | |||
| a, b, c (Å) | 59.94, 41.87, 191.54 | 60.23, 42.08, 194.21 | 60.23, 42.09, 191.91 |
| α, β, γ (°) | 90.00, 95.36, 90.00 | 90.00, 95.76, 90.00 | 90.00, 95.94, 90.00 |
| Resolution (Å) | 31.72 – 2.50 (2.64 – 2.50)* | 40.00 – 3.10 (3.21 – 3.10)* | 50.00-3.50 (3.63-3.50)* |
| Rmerge (%) | 10.70 (42.3) | 18.30 (57.7) | 15.80 (49.70) |
| Rmeas (%) | 12.80 (51.7) | 22.40 (73.3) | 19.30 (61.50) |
| Rpim (%) | 7.00 (29.2) | 12.70 (44.5) | 10.90 (35.60) |
| I/σI | 8.10 (2.7) | 5.17 (1.18) | 5.08 (1.37) |
| CC1/2 | 0.990 (0.769) | 0.942 (0.498) | 0.985 (0.718) |
| Completeness (%) | 90.10 (80.80) | 84.10 (79.20) | 89.00 (83.40) |
| Redundancy | 3.0 (2.8) | 2.7 (2.1) | 2.8 (2.6) |
| Refinement | |||
| Resolution (Å) | 31.71 – 2.50 (2.58 - 2.50)* | 35.22-3.01 (3.21-3.01)* | 47.72-3.49 (3.61-3.49)* |
| No. of reflections | 14930 | 7726 | 5588 |
| Rwork/Rfree (%) | 19.70/24.80 | 24.67/30.46 | 21.64/27.42 |
| No. of atoms | 3141 | 2960 | 2999 |
| Protein | 2929 | 2875 | 2921 |
| DNA | 54 | 38 | 38 |
| Ligand/ion | 6 (GOL), 1 (Zn2+) and 1 (Cl−1) | 1 (Zn2+) | 1 (Zn2+) |
| Water | 150 | 46 | 39 |
| B-factor | |||
| Average B-factors (Å2) | 37.46 | 73.0 | 66.3 |
| Protein/DNA | 37.50 | 73.2 | 66.5 |
| Ligand/ion | 48.60 | 103.5 | 56.9 |
| Water | 34.80 | 60.6 | 46.9 |
| R.m.s deviations | |||
| Bond lengths (Å) | 0.004 | 0.002 | 0.002 |
| Bond angles (°) | 0.74 | 0.43 | 0.51 |
Values in parentheses are for highest-resolution shell.
Figure. 1. Co-crystal structure of sA3G* and a dinucleotide.
a The asymmetric unit contains one protein (yellow) molecule comprising NTD (N-terminal domain) and CTD (C-terminal domain) with a dinucleotide (dC, blue) interacting with CTD. A purple sphere represents the Zn2+ ion in the catalytic site. N and C indicate the N- and C- terminal ends of the protein. Helices and β-strands are labeled as h and β, respectively. b Superimposition of the sA3G*-NTD structure (yellow, this study) with rhesus macaque A3G-NTD structure (green, PDB ID: 5K81). c Superimposition of the sA3G*-CTD (yellow, this study) and wild-type human A3G-CTD (raspberry, PDB ID: 4ROV) structures. d Relative orientation of NTD and CTD. Helix-6 (h6) of CTD is oriented almost perpendicular to the h6 of NTD. The linker region between the two structural domains connecting NTD-h6 and CTD-h1 is colored orange. Loop-1 (L1) of NTD is colored green, Loops (L1, L3 and L7) of CTD which are involved in binding the deamination target sequence 5′-TCCCA[59]are colored light blue. e CTDs of sA3G*and rmA3G variants (PDB IDs: 6P40 and 6P3X) are superimposed (colored red), NTDs are colored gray except helix-6 which is colored yellow (sA3G*), green (rmA3G: 6P40) and pale green (rmA3G: 6P3X). f Residues at the interface between NTD and CTD of sA3G*. NTD residues are colored green, CTD residues are colored blue, and residues located in the linker are colored orange. Gray dotted line indicates hydrogen bonding.
To evaluate the sA3G* structure, we first compared sA3G*-NTD and sA3G*-CTD separately with available closely related crystal structures of A3G-NTD and A3G-CTD, respectively. As expected, sA3G*-NTD has the same secondary structures (α helices 1-6 and β strands 1-5) as seen in a soluble-variant human A3G-NTD[51] (PDB ID: 2MZZ) and rhesus macaque A3G-NTD[56] (PDB ID: 5K81). Fig. 1b shows superimposition of the sA3G*-NTD structure (this study, yellow) and rhesus macaque A3G-NTD structure[56] (PDB ID: 5K81, green), in which the pairwise root mean square deviation (rmsd) of these two structures is 2.2 Å. It is noted that although overall structures are very similar, the Zn2+ ion is absent from the sA3G*-NTD. Previously, Zn2+ ions were missing in some APOBEC3 crystal structures of catalytic domains of A3F and rmA3G, likely due to the crystallization condition[70, 72](PDB ID: 5HX4; 6P3X). This lack of Zn2+ chelation likely caused the sA3G*-NTD helix-2 to tilt in comparison to that of rmA3G-NTD. Helix-4 is also tilted compared with that of rmA3G-NTD possibly because the N-terminus of helix-4 interacts with the CTD, which will be described in following section. Fig. 1c superimposes sA3G*-CTD (yellow) with a wild-type A3G-CTD crystal structure[54] (PDB ID: 4ROV) (raspberry). As previously shown by our co-crystal structure of A3G-CTD2 and ssDNA[59], there is no significant difference between the A3G-CTD2 structure and the wild-type A3G-CTD structure as pairwise rmsd is 0.8Å. Overall, individual NTD and CTD domains of sA3G* have conserved secondary and tertiary structures of primate A3Gs, which was reasonable because none of the amino acid substitutions in sA3G* were observed to disrupt protein folding as analyzed by 1H-15N HSQC-TROSY spectra[73].
Relative orientation of NTD and CTD:
The relationship between the relative orientation of each domain and the functionality of A3G has been an interesting question. Most recently, two soluble variants of rhesus macaque A3G (rmA3G) structures have been determined in dimeric forms, and they revealed a possible mechanism of dimerization and potential RNA-binding surfaces[70]. However, in vitro deamination assays indicated that these rmA3G variants were catalytically compromised[70]. Likely explanation for this loss of activity was that the catalytic site was altered in the E/Q variant (PDB ID: 6P3X) and loop-1 of CTD, which is critical for substrate binding, is occluded in the dimerization interface of the FKL variant (PDB ID: 6P40). Therefore, relative orientation of A3G domains in a catalytically active conformation could, in fact, be different from the orientation found in these rmA3G variant structures. The crystal structure of sA3G* shows that the NTD is rotated nearly 90° relative to the CTD along the major axis of the molecule, which can be seen by examining the orientation of helix-6 from each domain (Fig. 1d). Figure 1d also illustrates locations of ssDNA substrate-binding regions of CTD including loops-1, −3 and −7 (light blue), and NTD loop-1 (green) as it is continuous to the substrate-binding region. Figure 1e compares relative domain orientations of sA3G* (this study), the rmA3G-FKL variant (PDB ID: 6P40) and the rmA3G-E/Q variant (PDB ID: 6P3X) by superimposing their CTD structures. In rmA3G-FKL and rmA3G-Q/E structures, NTD appear to be ~30° and ~60° planarly rotated relative to CTD around a perpendicular axis at the center of two domains, respectively[70]. It is noted that NTD domains pivot at the end of NTD-helix-6, allowing wide range of relative orientation between NTD and CTD (Fig. 1e). This orientation difference is not surprising because molecular dynamics simulations predicted a wide range of relative orientations between NTD and CTD in a monomeric form of human A3G[69]. To dissect detailed contacts between NTD and CTD in the sA3G* structure, expansions of the interface between NTD and CTD are shown in Figure 1f. Clustered Van der Waals contacts are seen near the linker, as S196 and M197 (linker, orange) interact with V351 and D352 located at the end of CTD-h5 and F241 in CTD-β2′. Next to the linker, E191 located at the end of NTD-h6 forms a hydrogen bond with Y219 located at the beginning of CTD-β1. It is noted that these interface residues are all wild-type residues which had not been substituted in sA3G*. One substituted residue M188R located in NTD-h6 apparently provides additional stability to this interface by forming a hydrogen bond with the E191 (Fig. 1f). There are additional Van der Waals contacts involving residues in NTD loop-1 and loop-7 and CTD loop-3 and helix-2 including P25: P247K (−1.3 kcal/mol), L27A:L253 (−2.5kcal/mol), P129:F268 (−2.6 kcal/mol), P129:V265 (−1.6 kcal/mol), Y125:Q245 (−1.4 kcal/mol), D128:F268 (−2.1 kcal/mol), D128:V265 (−1.3kcal/mol).
5′-CC dinucleotide captured by CTD:
We found electron density fit with a dinucleotide near the catalytic site of the C-terminal domain of sA3G* (sA3G*-CTD) in all three crystals formed in different crystallization conditions. The dinucleotide was well ordered, and two deoxy-cytidines (5'-dCdC) fit best to the electron density (Supplementary Fig. 2). Since we did not add ssDNA to the solution in which crystals formed, we believe these nucleotides emanated from E. coli and remained bound to sA3G* during purification. Although only a dinucleotide is visible in the crystal structure, we cannot exclude the possibility that this dinucleotide is a part of a longer ssDNA because we may not obtain electron densities for dynamically moving nucleotides as we have evidently seen in APOBEC3 co-crystal structures including APOBEC3A and ssDNA[58, 74] (PDB ID: 5SWW; 5KGE), rhesus macaque A3G-NTD and poly-deoxy-T[56] (PDB ID: 5K83), APOBEC3F-CTD and poly-deoxy-T[75] and Pot1-fused-A3G-CTD and ssDNA[60] (PDB ID: 6BWY). It is not surprising that sA3G* was co-purified with oligo-nucleotides since A3G has non-specific affinity to both RNA and DNA[47, 62], and rhesus macaque A3G variants were apparently copurified with RNA and crystalized although nucleotides were not seen in their structures[70] (PDB ID: 6P40 and 6P3X). In addition, APOBEC3H has been copurified with variety of RNA oligomers that originated from E. coli or a plasmid and co-crystalized[76-78] (PDB ID: 5Z98; 6B0B; 5W3V). The dinucleotide appeared to be important for stable crystal formation because it interacts with two molecules of sA3G* in neighboring asymmetric units through hydrogen bonding. Details of these crystallographic symmetry interactions are provided in Supplementary Fig. 3.
Two deoxy-cytidines in the dinucleotide, numbered as C1 and C2 from the 5′ to 3′ direction (5′-C1C2), are bound near the catalytic site, but distant from Zn2+ (Fig. 2a), not positioned for deamination. The interactions between 5'-dCdC and sA3G*-CTD are different from those observed in our previous co-crystal structure of A3G-CTD2 and ssDNA substrate[59] (PDB ID: 6BUX) where the target deoxy-cytosine base was positioned deep in the catalytic pocket right next to Zn2+ and five nucleotides had extensive hydrogen bonding with protein. Figure 2b superimposes the sA3G*:5'-dCdC structure (this study, dinucleotide is colored blue) with the A3G-CTD2:ssDNA structure (6BUX, ssDNA is colored pink). C1 is positioned between the C−2 and C−1 position of the ssDNA substrate (Fig. 2b), and the Watson-Crick face of C1 interacts with sA3G*-CTD by three hydrogen bonds including a carbonyl group with the mainchain amino proton of V212 from loop-1, an amino group with the mainchain amino proton of D316 of loop-7, and N3 proton with mainchain carbonyl group through an ordered water molecule. We also observed hydrogen bonding interaction involving the C1 ribose ring O4 and NE1 atom of W211. Additionally, the C1 pyrimidine ring is stabilized by a π–π stacking interaction with the indole ring of W211 (Fig. 2a). These interactions are likely occurring in solution as well, because we observed NMR chemical shift perturbations of W211 and D316 upon titration of a short DNA oligo, 5'-AACCAA (Supplementary Fig. 4). Although the Watson-Crick face of C2 points toward Zn2+, it is distant from the C−1 position as the backbone phosphate of C2 is 3.5 Å away from the backbone phosphate of C−1 (black double-arrow-headed line in Fig. 2b). The Watson-Crick face of C2 also interacts with sA3G*-CTD through three hydrogen bonds including N3 proton with the main chain carbonyl group of R215, carbonyl group with Nδ1 of H216, and amino group with side chain hydroxy-oxygen of T218 through an ordered water molecule (Fig. 2a). Figure 2c superimposes the ssDNA bound A3G-CTD2 (6BUX) and the dinucleotide bound sA3G* without DNAs, showing amino acids with direct interactions to the deamination target sequence (6BUX, light purple; sA3G*, yellow). This comparison implies that W211, R213, H216 and Y315 would adjust their positions and sidechain rotamers in order to bind target sequence for catalysis. Ziegler et al. have reported a crystal structure of A3G-CTD bound to a non-preferred adenine nucleotide near the catalytic site[60] (PDB ID: 6BWY). Superimposition of this adenine bound A3G-CTD (6BWY, magenta) with dinucleotide-bound sA3G*-CTD (this study, blue) shows partial overlap of the adenine base with the C1 base of the dinucleotide (Fig. 2d), revealing that these two nucleotides bind to a similar position of A3G-CTD. Nonetheless, interactions between adenine and sA3G*-CTD are slightly different from those of C1 as the amino group of adenine forms hydrogen bonds to the backbone carbonyl groups of P210 and I314 (Fig. 2d). It is remarkable that the same region of A3G-CTD is recognizing nucleobase type difference through hydrogen bonding, while both cytosine and adenine bases are stabilized by partial π–π stacking interaction with indole ring of W211 (Figs. 2a and 2d). Figure 2e summarizes all interactions between the dinucleotide and sA3G*.
Figure. 2. Non-catalytic interaction between the 5′-CC dinucleotide and sA3G*.
a Interactions between dinucleotide and sA3G*-CTD. The Watson-Crick faces of both cytosines (C1 and C2) position towards loop-7 and the catalytic Zn2+ ion. sA3G* is colored yellow and shows the side chains as sticks. Atoms in the dinucleotide are colored blue, navy, red and orange for C, N, O and P respectively. Water molecules are shown as red spheres, and Zn2+ is shown as a purple colored sphere. Gray dotted lines indicate hydrogen bonding. b Superimposition of the sA3G*:dinucleotide structure (this study, dinucleotide is colored blue) with the A3G-CTD2:ssDNA structure (PDB ID: 6BUX, ssDNA is colored pink) comparing positions of nucleotides in the two structures. The double arrow-headed line points to the backbone phosphorous atoms of C2 of the dinucleotide and C−1 of the A3G-CTD2:ssDNA structure. c Superimposition of the sA3G* structure (this study, sA3G* is colored yellow) with the A3G-CTD2 structure (PDB ID: 6BUX, A3G-CTD2* is colored light purple) highlighting side-chain conformational differences of key amino acid residues for ssDNA substrate binding. DNAs are not shown. d Superimposition of the sA3G*-dinucleotide structure (this study, dinucleotide is colored blue) with the adenine bound A3G-CTD structure (PDB ID: 6BWY, adenine is colored magenta) showing overlap of the adenine base with the C1 base of the dinucleotide. Dotted lines indicate hydrogen bonds between the adenine amino group and two mainchain carbonyls; I314 and P210 of A3G-CTD (gray colored sticks). e Summary of the interactions between sA3G* and the dinucleotide.
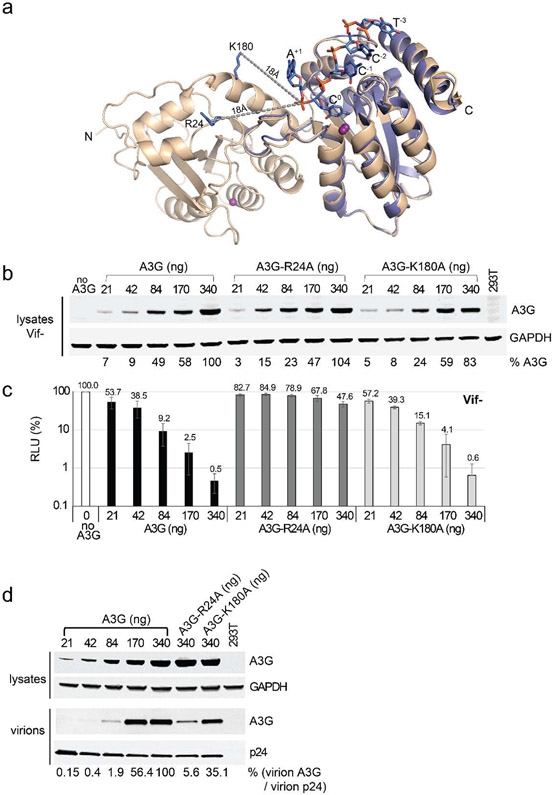
Wild-type A3G structural model and HIV-1 restriction assay suggest that R24 is important for binding ssDNA:
To gain further structural insights on ssDNA binding in wild-type A3G context, we generated a structural model of wild-type human A3G (wtA3G) based on the sA3G* structure (this study). The wtA3G structural model added a Zn2+ ion in NTD resulting that NTD-h2 is placed in the regular position seen in other APOBEC3 domain structures, whereas all other secondary structures and the relative orientation of NTD and CTD in the sA3G* structure are retained in the same position found in the sA3G* structure (a superimposition of sA3G* and wtA3G structures is shown in Supplementary Fig. 5). Superimposition of the wtA3G model and co-crystal structure of A3G-CTD2 and ssDNA (PDB ID: 6BUX) shows that two positively charged residues in NTD, R24 in loop-1 and K180 in helix-6 are within four to five nucleotides distance (~18 Å) from the adenine located at the 3′ side of a target sequence (A+1 in 5'-T−3C−2C−1C0A+1, C0 is the deamination target cytidine) bound in the catalytic site of CTD (Fig. 3a). This distance seems to support previous observations in ssDNA-binding assays and atomic force microscopy experiments in which human A3G bound a 10-nt ssDNA as strongly as a 69-nt ssDNA[47], ssDNA-binding kinetics suggested binding site was on the order of 15 nt[68], and one molecule of A3G bound less than 17-nt stretch in a 69nt ssDNA[79-81]. Therefore, we sought to explore whether R24 and/or K180 is important for HIV-1 restriction/deamination.
Figure 3. Antiviral activity and encapsidation of A3G-R24A and A3G-K180A.
a Superimposition of a wtA3G structural model (this study, wheat color) with the A3G-CTD2:ssDNA structure (PDB ID: 6BUX, lavender color, ssDNA is colored blue, orange and red for C, P and O atoms). R24 and K180 of A3G-NTD are shown as blue sticks, and gray dotted lines indicate distances between phosphorous of A+1 and R24 or K180. Zn2+ ions are shown as purple spheres. b Representative western blot showing 293T cells co-transfected with increasing amounts of N-terminal FLAG-tagged wild-type A3G, A3G-R24A, or A3G-K180A (21, 42, 84, 170, 340 ng), HDV-EGFP, and VSV-G in the absence of Vif. Average A3G expression from 4 independent experiments relative to 340 ng A3G (set to 100%) is shown after adjusting for GAPDH band intensities. c Single-cycle infectivity of normalized p24 capsid amounts harvested from transfected cells in part (a) were assayed in TZM-bl target cells. Infectivity is proportional to relative light units (RLU) produced by induction of luciferase expression (normalized to the no A3G control). Error bars represent the standard deviation from three independent experiments. d Representative western blot of 293T cell lysates and virions produced from 293T cells co-transfected with increasing amounts of N-terminal FLAG-tagged wild-type A3G (21, 42, 84, 170, 340 ng), A3G-R24A (340ng), or A3G-K180A (340 ng), HDV-EGFP, and VSV-G in the absence of Vif. Relative A3G expression was normalized to GAPDH levels in cell lysates and to capsid p24 levels in virions. The average packaging efficiency of A3G, A3G-R24A and A3G-K180A was determined by dividing the amount of A3G encapsidated in the virions by the amount of A3G expressed in the lysates, and further normalized to A3G 340 ng (100%) from two independent experiments.
We used wild-type A3G to determine the influence of R24A and K180A substitutions, called A3G-R24A and A3G-K180A hereafter, on inhibition of HIV-1 infectivity. To determine the effect of A3G-R24A and A3G-K180A on HIV-1 infectivity, VSV-G-pseudotyped virions were prepared in the presence of increasing amounts of wild-type A3G, A3G-R24A, or A3G-K180A expressing plasmids (Fig. 3b). Western blotting analyses of the transfected cells (in the absence of Vif) indicated similar steady-state expression levels of the wild-type and mutant A3Gs. Infectivity of the virions produced from the transfected cells was determined by infection of TZM-bl cells (Fig. 3c). The results indicated that expression of wild-type A3G and A3G-K180A potently inhibited HIV-1 infectivity in a dose-dependent manner, but expression of A3G-R24A exhibited reduced A3G antiviral activity; transfection with 340 ng of wild-type A3G or A3G-R24A reduced virus infectivity to 0.5% or 47.6% of the control, respectively. Previous studies regarding RNA-binding of human A3G have reported that the A3G-R24A mutation reduces virion incorporation of A3G[36, 82, 83]. We compared the virion incorporation of wild-type A3G in the presence of increasing amounts of A3G plasmid to the virion incorporation of A3G-R24A and A3G-K180A in the presence of 340 ng of the plasmids (Fig. 3d). The results confirmed that the A3G-R24A mutant was highly defective in virion incorporation. The amount of A3G-R24A that was packaged into virions when 340 ng of plasmid was transfected (virion A3G / virion p24 = 0.056 or 5.6 %) was similar to the amount packaged when 84 ng of wild-type A3G was transfected (virion A3G / virion p24 = 0.019 or 1.9 %) (Fig. 3d). It was noted that when infectivity was compared in conditions where similar amounts of A3Gs were in virions (84 ng of wild-type A3G vs 340 ng of A3G-R24A in Fig. 3d), A3G-R24A showed less restriction than wild-type A3G by 5 fold (47.6% infectivity for A3G-R24A vs 9.2% infectivity for wild-type A3G in Fig. 3c). Since this decreased restriction by A3G-R24A may or may not relate to its catalytic activity, we further studied effects of the R24A substitution for ssDNA binding and catalytic activity in vitro in the following section. The A3G-R24A and A3G-K180A mutants remained sensitive to Vif-mediated degradation (Supplementary Fig. 6a) and in the presence of Vif, virus infectivity was restored to wild-type levels (Supplementary Fig. 6b).
R24 is important for ssDNA binding and catalytic activity in vitro:
We generated full-length A3G containing wild-type NTD and soluble variant CTD2, called A3G-NTD-CTD2 hereafter, because this construct was previously shown to be catalytically active, able to restrict HIV-1 infection and sensitive to HIV-1 Vif induced degradation[59], while it is relatively soluble and homogeneous in solution which was essential for reliable quantitative measurements in our in vitro ssDNA-binding and deamination assays.
First, we hypothesized that a 16-nt ssDNA (5'-AATCCCAATTTTTTTT) containing additional deoxy-thymidines on the 3' side of a deamination target sequence would bind A3G-NTD-CTD2 stronger than a 9-nt ssDNA (5'-AATCCCAAA) because our previous co-crystal structure of A3G-CTD2 and this 9nt ssDNA[59] had revealed that TCCCA interacts with CTD and the 3' side of this target sequence was directed toward NTD. The 16-nt length was chosen because previous atomic force microscopy experiments indicated that four molecules of A3G can bind 69-nt linear ssDNA implying ≤17-nt binding site per A3G molecule[79-81]. Indeed, A3G-NTD-CTD2 showed stronger affinity (KD = 6.8 μM) for the 16-nt ssDNA than the 9-nt ssDNA (KD = 20.5 μM) using microscale thermophoresis (MST) assays (binding curves are provided in Supplementary Fig. 7). Then we compared affinities for the 5'-CCC motif and the 5'-CC motif in the 16-nt length ssDNA in order to test whether the R24A substitution affects preference of target sequence in binding because A3G prefers the CCC motif (3'-end C is most preferred for deamination) as deamination target than the CC motif[9, 45, 84]. We used 5'-AATCCCAATTTTTTTT and 5'-AAATCCAATTTTTTTT to measure affinities with GST-fused A3G-NTD-CTD2 and A3G-NTD-R24A-CTD2. We kept the GST-tag during purification because it made proteins more soluble and stable in solution without altering affinity nor specificity. Binding curves are provided in Figure 4a and apparent dissociation constant (KD) values are listed in Table 2. These data showed that R24A substitution decreased affinity for both 5'-AATCCCAATTTTTTTT (KD = 24.6 μM) and 5'-AAATCCAATTTTTTTT (KD = 132.2 μM) compared with A3G-NTD-CTD2 (4.9 μM and 31.4 μM for the 5'-CCC motif and the 5'-CC motif, respectively), although preference to the 5'-CCC motif was not affected. It is noted that the KD value of A3G-NTD-R24A-CTD2 for 5'-AATCCCAATTTTTTTT was 24.6 μM that was very similar to the KD value of A3G-NTD-CTD2 for 9-nt 5'-AATCCCAAA (20.5 μM, described above), implying R24 provides additional affinity for DNA(s) located in the 3' side of the target sequence.
Figure 4. R24 provides a key interaction for catalytic ssDNA binding.
a ssDNA binding assays. Each marker represents microscale thermophoresis (MST) measurement of mixture containing fluorescent-labeled A3G-NTD-CTD2 (50nM) and 16 nt ssDNA 5′-AATCCCAATTTTTTTT (red circle) or 5′-AATTCCAATTTTTTTT (pink circle), and mixture containing fluorescent-labeled A3G-NTD-R24A-CTD2 (50nM) and 5′-AATCCCAATTTTTTTT (blue triangle) or 5′-AATTCCAATTTTTTTT (light blue triangle). Three independent MST experiments were performed for each A3G-ssDNA combination, and bars of data points represent standard error of n=3 measurements. b Comparison of deamination speeds of A3G-NTD-CTD2 and A3G-NTD-R24A-CTD2. 1H NMR detects the formation of the 3′-cytidine deamination products of 20 nt ssDNA 5'-AATCCCAAT12 or 5'-AAATCCAAT12 as a function of time for A3G-NTD-CTD2 (red circles for TCCC and pink circles for TCC) and A3G-NTD-R24A-CTD2 (blue triangles for TCCC and light blue triangles for TCC). c Electrostatic surface distribution of the wtA3G structural model showing positive (+3, blue) and negative (−3, red) regions, and a possible substrate ssDNA binding channel (dashed orange line). The coordinates of the target sequence 5'-T−3C−2C−1C0A+1 were taken from the co-crystal structure of A3G-CTD2:ssDNA (PDB ID: 6BUX).
Table 2. Biochemical characteristics.
(a) Apparent dissociation constants (KD) were given for 16nt 5'-AATCCCAATTTTTTTT (5'-TCCC-polyT) or 5'-AATTCCAATTTTTTTT (5'-TCC-polyT) with A3G-NTD-CTD2 (WT) or A3G-NTD-R24A-CTD2 (R24A). (b) Deamination rates for the 3′-cytidine of the 20nt 5′-TCCC-polyT and 5′-TCC-polyT substrates are given as reactions / minute for A3G-NTD-CTD2 (WT) and A3G-NTD-R24A-CTD2 (R24A).
| ssDNA binding | ||
|---|---|---|
| 5'-TCCC-polyT | 5'-TCC-polyT | |
| WT | 4.9 ± 0.1 μM | 31.4 ± 3.8 μM |
| R24A | 24.6 ± 2.7 μM | 132.2 ± 2.6 μM |
| Deamination rate | ||
| 5′-TCCC-polyT | 5′-TCC-polyT | |
| WT | 4.6 ± 0.2 min−1 | 2.2 ± 0.2 min−1 |
| R24A | 3.0 ± 0.2 min−1 | 1.1 ± 0.2 min−1 |
Since the R24A substitution decreased affinity for ssDNA substrate binding, we hypothesized it also affects catalytic activity. We used 20-nt ssDNAs, including 5'-AATCCCAATTTTTTTTTTTT (5'-CCC-polyT) and 5'-AAATCCAATTTTTTTTTTTT (5'-CC-polyT) as substrates for NMR based deamination assays. Again, the 5'-CCC motif and the 5'-CC motif were compared to test whether deamination target preference has changed by the R24A substitution. Since A3G-NTD likely provides sequence non-specific DNA interaction, we expected substitutions in NTD would not alter the deamination target preference. Table 2 summarizes initial speed of reaction of A3G-NTD-CTD2 and A3G-NTD-R24A-CTD2 for each substrate (production curves are provided in Fig. 4b). A3G-NTD-R24A-CTD2 reduced reaction speed compared with A3G-NTD-CTD2 by 35% for 5′-CCC-polyT (3.0 min−1 and 4.6 min−1 for the R24A and wt, respectively) and 50% for 5′-CC-polyT (1.1 min−1 and 2.2 min−1 for the R24A and wt, respectively). Overall, our ssDNA-binding assays and deamination assays suggest that R24A substitution reduces affinity for ssDNA which resulted in reduction of catalytic speed.
As ssDNA is likely binding a positively charged surface, we examined the electrostatic surface distribution of our wtA3G structural model. We found that a positively charged channel formed by NTD loop-1 and CTD loop-3 which is connected to the 3' side of the target sequence bound to the CTD catalytic site (Fig. 4c). Amino acid R24 is located in NTD loop-1 and positions at the center of this positively-charged channel. This structural model supports previous studies by Chelico and co-workers[85] in which they proposed that the A3G-NTD interacts with DNAs located on the 3' side of a target sequence in the catalytically active orientation [see Figure 7B of the reference[85]].
DISCUSSION
Our crystal structure of a soluble A3G variant showed structural features by which catalytically inactive NTD may be involved in ssDNA binding. In addition, the 5'-CC dinucleotide captured by CTD provided insights regarding non-catalytic DNA interaction by A3G. The 5′-CC dinucleotide is positioned near the catalytic Zn2+, yet lacks specific interactions required for catalysis. Interestingly, this dinucleotide and the adenine found in the previously published co-crystal structure of A3G-CTD and ssDNA[60] occupy a similar position. As proposed by Ziegler et al., their adenine and our dinucleotide may be showing protein-DNA interactions when A3G is searching for deamination target sequences. In this searching mechanism, residues in loop-1 and loop-7 of A3G-CTD may interact with DNAs in variable ways, which enables identification of nucleobase types. This hypothesis is well supported by our most recent results using NMR[61], which indicated that loops-1, −3 and −7 of A3G-CTD are dynamically interacting with DNAs until a deamination target sequence binds tightly in the catalytic position. Morse and co-workers also suggested that A3G-CTD dynamically searches for deamination target sequences while NTD tethers to the ssDNA[68].
An important aspect of the deamination catalyzed by A3G is 3' to 5' directional preference in processive deamination of multiple target sequences on a ssDNA[9, 47, 86], and Chelico and co-workers have studied effects of F126A/W127A double mutations and H186R mutation in ssDNA binding and deamination[85, 87]. The F126A/W127A mutation made A3G predominantly monomeric[85] and increased affinity for ssDNA, whereas the H186R mutation did not alter either multimeric status or affinity for ssDNA[87]. Remarkably, both mutants showed stronger preference for 3' to 5' directionality in processive deamination than wild-type A3G[87]. The H186R mutation is a polymorphism identified in African American HIV-1 infected patients; mutation is associated with high viral loads, decline in CD4T-cells and accelerated progression of AIDS, although this mutated A3G appears to restrict the production of infectious virus particles comparable to the wild-type A3G[88, 89]. By examining H186 in our wtA3G structural model, we found that H186 sidechain is facing inside of the NTD domain structure and forming π-π stacking with Y182 located in NTD helix-6 and a hydrogen bonding network with Y182 and N153 located in NTD loop-9. The H186R mutation would lose the π-π stacking interaction and may not retain the hydrogen bonding network and integrity of the local structure.
The residues located in NTD loop-7, including Y125, F126 and W127, have been suspected to bind ssDNA partly because CTD loop-7 residues bind deamination target sequences, and the NTD and the CTD are structurally very similar, but alanine substitutions of F126/W127 indicated that they were not important for ssDNA binding or catalytic reaction[68, 85, 87]. Interestingly a co-crystal structure of rhesus macaque A3G-NTD and 10-nt poly-T ssDNA had shown that Y125 formed two direct hydrogen bonds with a thymidine[56]. Therefore, we substituted Y125 to an alanine, called A3G-NTD-Y125A-CTD2 hereafter, and measured catalytic activity. A3G-NTD-Y125A-CTD2 showed catalytic activity as strong as A3G-NTD-CTD2 (Supplementary Fig.8). Overall we can conclude that these NTD loop-7 residues are not binding ssDNA for catalysis. Although substitutions of F126/W127 and H186 strengthened the 3' to 5' directionality of processive deamination[87], it is difficult to hypothesize their possible roles in the processive deamination based on available experimental data.
Based on this study and previous structural, biochemical and biophysical studies by us and others, we propose a structural mechanism that generates the 3' to 5' directional preference in processive deamination (Fig. 5). Experimental evidence indicated that 1) monomeric A3G has the 3' to 5' directional preference[67, 68, 85, 87], 2) in the catalytically active binding, the NTD faces the 3' end, whereas the CTD faces the 5' end of a ssDNA[85], 3) W211 and D316 bind nucleotides flanking the 5' side of the deamination target cytidine during catalysis[59], 4) W211 and D316 interact with cytidines in substrate as well as non-substrate ssDNA in solution, while other hot-spot (5'-CCC) binding residues are not strongly interacting with non-substrate ssDNA [[61] and this study], 5) A3G-CTD/ssDNA complex crystal structures showed that W211 and D316 distinguish nucleobase type through hydrogen bonding[[59, 60] and this study], 6) A3G does not deaminate single cytosine nor cytidine [e.g. [84] and observations by many other laboratories], which implies that the catalytic Zn2+ is not accessible to DNA in solution as indicated by solution structures of A3G-CTD[53, 90, 91], and, 7) the dinucleotide in the sA3G* structure suggests a possible route of ssDNA which is a few Å away from the catalytic Zn2+ for the search of deamination target sequences (this study). The left column of Figure 5 illustrates catalytically active 5'-CCC recognition. If CTD is facing the 5' end of a ssDNA, which is the catalytically active orientation of molecule[85], and A3G slides 3' to 5' direction, W211 and D316 encounter the 3' cytidine (C0) that is the most preferred deamination target. A3G keeps moving in the 3' to 5' direction, and W211 and D316 recognize C−1 then C−2 sequentially, while C0 is passed down toward Zn2+ through interactions with another target-sequence binding residue Y315, H257, E258, N244, S286 and T218, and C0 finally reaches to Zn2+. The right column of Figure 5 illustrates catalytically inactive 5'-CCC interaction. In this case, A3G binds a ssDNA in the catalytically active orientation but moves in the 5' to 3' direction, with C−2 would be the first cytidine to contact W211 and D316, but C−1 and C0 are not interacting with the target sequence binding residues, E258, N244, S286 and T218, because they are deep inside of catalytic pocket and cannot reach from surface. It is noted that Furukawa et al. previously showed that the reaction rate is faster when A3G-CTD approaches the target cytidine from 3' to 5' direction than approaching from 5' to 3' direction by analyzing kinetic parameters of the deamination catalyzed by full-length A3G and A3G-CTD alone[92]. Our model gives a mechanical explanation for Furukawa’s experimental observations.
Figure 5.
Model mechanism of the 3′ to 5′ preference in processive deamination. Left column shows the scenario where A3G moves from 3′ to 5′ of ssDNA which leads the target cytidine in catalytic site. The right column shows the scenario where A3G moves from 5′ to 3′ of ssDNA, and misses the catalytic binding. Red line indicates ssDNA, three cytidines of a 5′-C−2C−1C0 motif are labeled (C0 is the target cytidine). Zn2+ molecule is gray circle, sidechains of W211 and D316 are shown as blue sticks, and blue letters, including Y, S, E, N and T, are indicating Y315, S286, E258, N244 and T218, respectively. NTD and CTD indicates the N-terminal and the C-terminal domain of A3G, respectively.
Our study indicates the importance of R24 in catalytically-active ssDNA binding. The R24 amino acid has attracted investigative interest since previous studies found that substitution of R24 reduces encapsidation of A3G, suggesting its involvement in RNA interaction and oligomerization of A3G[36, 82, 83], although Y124, F126 and W127 play central roles for those events[41, 85, 93]. Indeed, recent study of rhesus macaque A3G (rmA3G) variant structures[70] confirmed the importance of Y124/Y125/W127 in oligomerization since these residues were buried in homo-dimerization interface interacting with CTD loop-1 (PDB ID: 6P40) or helix-6 (PDB ID: 6P3X). Since CTD loop-1 is occluded in the dimerization interface, deamination target sequence can’t bind the catalytic site in the 6P40 structure. In the 6P3X structure, R24 of rmA3G is located at the center of a positively-charged patch at the homodimerization interface, but this patch does not directly connect to a catalytic site as R24 would be ~40 Å away from the closest end of a target sequence bound to CTD. Therefore, the rmA3G variant structures indicate RNA-binding/dimerization mechanisms, whereas the sA3G* structure suggests a ssDNA-binding mechanism of monomeric A3G. A3G seems to be capable of changing the relative orientation of NTD and CTD in order to achieve different biological functions[68, 69]. Antiviral activity of human A3G can be neutralized by HIV-1 Vif, but not by Vif from Simian Immunodeficiency Virus which infects the African green monkey (SIVagm), generating a barrier for cross-species transmission[94-98]. D128, P129 and D130 have been identified as key residues for Vif-induced degradation of human A3G as substitutions of these residues abrogated the degradation[94-98]. Our wtA3G structural model shows that residues Y124, Y125, W127, D128 and D130 are surface exposed, accessible to Vif interaction, but separated from the proposed ssDNA binding channel. Nevertheless, a structure of the A3G:Vif complex is ultimately required to reveal the domain orientation and atomic-level interactions between A3G and Vif.
MATERIALS AND METHODS
Plasmid generation and protein purification
sA3G was generated by combining a soluble NTD, namely sNTD[51] and a soluble CTD, namely CTD2[59] with additional nine substitutions and two deletions (Supplementary Fig. 1a shows the sequence of sA3G). Catalytically inactive variant of sA3G (sA3G*) was made by substituting E259 with alanine. pGEX6P-1 expression vector carrying sA3G* gene were transformed in BL21 (DE3) cells (Invitrogen). Cells were grown in LB media at 37°C until reaching an optical density of 0.5-0.6 at 600 nm. Then temperature was changed to 17°C and cells were induced by adding 0.2 mM IPTG at optical density 0.6-0.8. Cells were further grown overnight at 17°C. All the steps for protein purification were performed at 4°C unless specified. E. coli cells were harvested by centrifugation and resuspended in lysis buffer (50 mM sodium phosphate, pH 7.3, 150 mM NaCl, 25 μM ZnCl2, 2mM DTT and 0.002% Tween-20) and protease inhibitor (Roche, Basel, Switzerland). The suspended cells were disrupted by sonication and then cell debris was separated by centrifugation at 20,000 rpm for 30 min. Supernatant containing desired protein was applied to glutathione-Sepharose (GE Healthcare Life Science) beads, pre-equilibrated with lysis buffer and agitated for about 2 hours. The beads were washed with cleavage buffer (50 mM sodium phosphate, pH 7.5, 100 mM NaCl, 2mM DTT and 0.002% Tween-20) and incubated overnight with PreScission protease (GE Healthcare Life Science). GST-free proteins were separated from the beads by centrifugation. Supernatant having the GST-free protein was further purified by Superdex-75 size exclusion column (GE Healthcare Life Science) in FPLC buffer (20 mM Bis-Tris, pH 6.5, 100 mM NaCl, 10 uM ZnCl2, 2mM DTT and 0.002% Tween-20) using an AKTA FPLC system. Purity and concentration of the proteins were measured by gel electrophoresis and UV spectroscopy.
Crystal growth and data collection
Purified sA3G*, was concentrated to about 100 μM using Amicon Ultra-4 (Merck Millipore). Crystallization screening was performed using commercially available different crystallization screens by sitting drop vapor-diffusion method at 4°C. Crystal drops were set up by mixing 0.3 μl sample and 0.3 μl reservoir solution in sitting drop 2-well crystallization plate (Molecular Dimension) using a robot, Mosquito Crystal (TTP Labtech). Crystals appeared after two weeks in a range of conditions. Crystals formed from three different conditions including condition-1: 0.1 M BICINE (pH 9.0) and 10% w/v PEG 6000 (JCSG plus E-10 from Molecular Dimensions), condition-2: 0.1M Tris (pH 8.0) and 5.5% w/v PEG 4000 (MemGold B-12 screen from Molecular Dimensions ) and condition-3: 0.03 M Citric acid, 0.07 M BIS-TRIS propane (pH 7.6) and 20% w/v PEG 3,350 (PEGION H-4 screen from Hampton Research). Crystals were cryoprotected using reservoir solution containing 15% v/v glycerol and flash frozen in liquid nitrogen. X-ray diffraction data were collected at Southeast Regional Collaborative Access Team (SER-CAT) 22-ID and 22-BM beam lines at the Advanced Photon Source, Argon National Laboratory. The collected diffraction data were indexed, integrated and scaled using iMosflm[99] and HKL2000 program[100]. All the crystals belong to the space group I121.
Structure determination and analysis
Structures from condition-1, −2 and −3 were solved by molecular replacement using human A3G-CTD structure (PDB ID: 6BUX, DNA was removed) and rhesus macaque A3G-NTD structure (PDB ID: 5K81) as search model at 3.5 Å, 3.0 Å and 2.5 Å resolution, respectively. The molecular replacement and initial structure refinement were performed using Phaser[101] and Refmec5[102] of CCP4 program suit respectively. Model building of the protein and bound DNA were manually performed using the program Coot[103]. The final model was refined by Phenix refinement[104, 105]and model was validated with PDB validation tool and Molprobity[106]. Pairwise rms deviation were calculated using Doli[107]. We used the highest resolution (2.5 Å) structure for the structure analysis and figure preparation. Structural figures were generated using PyMOL[108]. Data collection statistics and refinement parameters of all three crystal structures are given in Table 1.
Generation of wild-type A3G model
The structure of wild-type A3G was modelled primarily from sA3G* crystal structure (this study). The crystal structures of CTD2 with ssDNA (PDB ID: 6BUX) and rhesus macaque A3G-NTD (PDB ID: 5K81) were also used in modeling to provide additional structural information for CTD and NTD separately. The wild-type model was first generated through program Modeller 9.15 using basic modeling. The model was then further optimized using the Protein Preparation Wizard[109] from Schrodinger at pH 6.5 and energy minimized with gradually reduced restraints (1000, 5, 0 force constant) on backbone and solute heavy atoms using Desmond[110]. The electrostatic distribution of wild-type A3G was calculated using PDB2PQR[111] server and Pymol with the APBS plugin, and visualized with contour levels positive (+3) and negative (−3).
Real-time NMR deamination assay
We determined initial rates of deamination reaction by using 1H nuclear magnetic resonance (NMR) spectra as previously reported[53, 112]. 20nt ssDNA substrates, including 5′-AATCCCAATTTTTTTTTTTT (C is the primary target cytidine, 5′- TCCC- polyT) and 5′-AAATCCAATTTTTTTTTTTT (C is the target cytidine, 5′- TCC-polyT) (Integrated DNA Technologies), were used to determine the reaction rates. NMR spectra were acquired at 25°C on Bruker NMR spectrometers operating at 1H Larmor frequencies of 600 MHz. To test effects of the substitution in the wild-type NTD context, sA3G-NTD was replaced by wild-type A3G-NTD in the sA3G construct called A3G-NTD-CTD2 hereafter. A3G-NTD-CTD2 was used as a template to generate substitution of R24 in NTD with alanine, called A3G-NTD-R24A-CTD2 hereafter. NMR samples contained 5% deuterium oxide with 50 nM protein, 200 μM ssDNA substrate, 100 mM NaCl, 0.002% Tween20, 1 mM DTT, 10 μM ZnCl2 and also included 50 mM sodium phosphate adjusted to pH 6.5. Concentration of deamination products were determined from integration of the H5 uracil proton peak as described previously[112]. H5 uracil proton peak was unambiguously assigned by taking 1H spectrum of expected product ssDNA which was synthesized separately for each ssDNA substrate. A series of 1H spectra were measured and the product concentrations as a function of the reaction times were used to determine the initial rate via linear regression. Reaction rates were normalized for the protein concentration and given as reactions per minute. Deamination assays were repeated 3 times independently, and the standard deviation of n=3 measurements is given as the initial rate error.
Microscale thermophoresis ssDNA binding assay
The binding affinity of A3G-NTD-CTD2 and A3G-NTD-R24A-CTD2 with 5'-AATCCCAAA (9 nt-CCC), 5'-AATCCCAATTTTTTTT (16 nt-CCC) 5'-AATTCCAATTTTTTTT (16 nt-CC) (IDT), were measured using Monolith NT.115 (Nano Temper Technologies)[113]. RED-tris-NTA fluorescent dye solution was prepared at 100 nM in the MST buffer (20 mM Bis-Tris pH 6.5, 100 mM NaCl, 1 mM DTT, 0.002% Tween20, 20 μM ZnCl2). 6xHis tag was added to the C-terminus of A3G-NTD-CTD2 and A3G-NTD-R24A-CTD2 while GST-tag was fused to the N-terminal. The His-tag was used to attach the NTA fluorescent dye for the MST experiments. The GST-tag was kept during the purification, and the GST-fused A3G-NTD-CTD2 or A3G-NTD-R24A-CTD2 was mixed with dye at final concentration of 100 nM and incubated for 30 min at room temperature followed by centrifugation at 15,000 g for 10 min. The ssDNAs were prepared to stock concentration of 16 mM, 4 mM and 8 mM for 5'-AATTCCCAAA, 5'-AATTCCCAATTTTTTTT and 5'-AATCCAATTTTTTTT in the MST buffer, respectively. To determine the binding affinity, 10 μl of ssDNA solution at 16 different concentrations, ranging from 8 mM to 0.24 μM, 2 mM to 0.96 μM and 4 mM to 0.48 μM for 5'-AATTCCCAAA, 5'-AATTCCCAATTTTTTTT and 5'-AATCCAATTTTTTTT were prepared in LoBind centrifuge tubes, respectively (Fisher Scientific), then 10 μl of fluorescent labelled protein solution (100 nM) was added to each tube. The mixtures were incubated at room temperature for 1hr to reach equilibrium. Each incubated solution was loaded into a Nano Temper MST premium coated capillary. The measurement was performed at room temperature using 40% LED power and 40% MST power. The experiment was repeated three times using freshly purified protein at each time, and data analysis was carried out using Nano Temper analysis software (MO affinity).
ssDNA binding NMR experiment
NMR spectra were acquired on 850MHz Bruker Ascend spectrometer equipped with a 5mm Z-gradient TCI cryoprobes. Samples contained a final volume of 300 μL (97% H2O/3% D2O, v/v), and spectra were taken at 293K. 15N-TROSY-HSQC with ssDNA were collected on 35 μM 15N uniformly-labelled sA3G* samples in 100 mM NaCl, 0.002% Tween20, 1 mM DTT, 10 μM ZnCl2 and 50 mM sodium phosphate adjusted to pH 6.5 with unlabeled ssDNA at 1 mM and 2 mM concentrations.
HIV-1 infection assay
Plasmid construction:
The plasmids in this study are designated with a “p” while the names of viruses and proviruses generated from these plasmids are not. pHCMV-G expresses the G glycoprotein of vesicular stomatitis virus (VSV-G)[114]. pHDV-EGFP is an HIV-1 derived vector that expresses HIV-1 Gag-Pol and enhanced green fluorescent protein (EGFP) but does not express Env, Vif, Vpr, Vpu, or Nef[115]. pVif-HA is a codon-optimized HIV-1 Vif expressing a C-terminal HA epitope tag[116]. pFLAG-A3G expresses wild-type A3G with an N-terminal FLAG epitope tag[117]. pFLAG-A3G was subjected to site-directed mutagenesis to introduce R24A or K180A (Quick Change Lightning Site-Directed Mutagenesis Kit, Agilent Technologies) to create pFLAG-A3G-R24A and pFLAG-A3G-K180A, respectively. The structures of all final plasmids were confirmed by sequencing (Macrogen).
Tissue culture and cell lines:
Human embryonic kidney 293T cells (American Type Culture Collection) and TZM-bl cells (obtained through the NIH AIDS Reagent Program [Cat. No. 8129], Division of AIDS, NIAID, NIH: TZM-bl from Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc.[118]) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (HyClone) and 1% penicillin-streptomycin stock (penicillin 50 U/ml and streptomycin 50 μg/ml, final concentration; Gibco). TZM-bl cells contain a HIV-1 Tat-inducible luciferase reporter gene that is expressed upon HIV-1 infection and Tat expression. All cells were maintained in humidified 37° C incubators with 5% CO2.
Transfection, virus production and single-cycle infection assays:
Transfections were performed using LT1 reagent (Mirus Bio) according to manufacturer’s instructions. To generate virus for infection, 4 × 105 293T cells were transfected with pHDV-EGFP (1 μg), with or without pVif-HA (2.5 μg), pHCMV-G (0.25 μg), and variable concentrations of pFLAG-A3G, pFLAG-A3G-R24A or pFLAG-A3G-K180A (21, 42, 84, 170 or 340 ng). Equivalent DNA amounts in the transfection mix were maintained by adding pcDNA3.1 empty vector. Forty-eight hours post-infection, virus was harvested, filtered through 0.45-μm filters, and stored at −80 °C. Capsid p24 amounts were determined using the HIV-1 p24 ELISA Kit (XpressBio) according to manufacturer’s instructions. Normalized p24 was used to infect 4000 TZM-bl cells in a 96-well plate, and 48-h post-infection, luciferase activity was measured using a 96-well luminometer (LUMIstar Galaxy, BMG LABTECH). Data were plotted as the percent inhibition of luciferase activity compared to the “No APOBEC3G” control. For some experiments, virus particles were isolated through a 20% sucrose cushion (15,000 rpm, 2 h, 4° C, in a Sorvall WX80+ ultracentrifuge), concentrated 50-fold, and virion encapsidation of FLAG-A3G, pFLAG-A3G-R24A and pFLAG-A3G-K180A was determined by western blotting.
Western blot analysis:
Cell lysates were prepared using CelLytic M (Sigma) solution containing Protease Inhibitor Cocktail (Roche), followed by a 10-min, 10,000 × g spin to remove cellular debris. The cell lysates were mixed with NuPAGE LDS sample buffer (Invitrogen) containing β-mercaptoethanol and heated for 5 min at 95 °C. Samples were analyzed on 4 – 20% Tris-Glycine Gels (Invitrogen) using standard western blotting techniques. Proteins were detected with primary antibodies as follows: FLAG-A3G (mouse anti-FLAG M2 monoclonal antibody, 1:5,000 dilution, Sigma catalog #F3165); Vif-HA (mouse anti-HA monoclonal antibody, 1:5,000 dilution, Sigma catalog #H3663); glyceraldehyde 3-phosphate dehydrogenase (GAPDH); rabbit anti-GAPDH antibody, 1:10,000 dilution, Abcam catalog #ab128915). Antibody against HIV-1 p24 (monoclonal, 1:10,000 dilution) was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 p24 Gag Monoclonal (#24-3) from Dr. Michael Malim (catalog #6458) [119]. An IRDye 800CW-labeled goat anti-rabbit secondary antibody (LI-COR catalog #926-32211) was used at a 1:10,000 dilution to detect rabbit primary antibodies and an IRDye 680-labeled goat anti-mouse secondary antibody (LI-COR catalog #926-68070) was used at a 1:10,000 dilution to detect mouse primary antibodies. Protein bands were visualized and quantified using an Odyssey Infrared Imaging System (LI-COR).
Supplementary Material
ACKOWLEDGEMENTS
The authors would like to thank Drs. Kylie Walters and Ann M. Sheehy for their advices and suggestions. The authors would like to acknowledge Dr. Sergey G. Tarasov and Ms. Marzena A. Dyba at the Biophysics Resources of CCR/NIH for providing instruments and technical support.
FUNDING
This work was supported by in part with grant from the U.S. National Institutes of Health R01AI150478. W.M. is supported in part by the NIH office of intramural training and education’s Intramural AIDS Research Fellowship. For A.M. V.B. and H.M., this project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, by Intramural AIDS Targeted Antiviral Program grant funding to V.K.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY
Atomic coordinates and structural factors for the reported crystal structure have been deposited in the Protein Data Bank under the accession number 6WMA, 6WMB and 6WMC.
REFERENCES
- [1].Conticello SG, Thomas CJ, Petersen-Mahrt SK, Neuberger MS. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol Biol Evol. 2005;22:367–77. [DOI] [PubMed] [Google Scholar]
- [2].Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chiu YL, Greene WC. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu Rev Immunol. 2008;26:317–53. [DOI] [PubMed] [Google Scholar]
- [4].Goila-Gaur R, Strebel K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Feng Y, Baig TT, Love RP, Chelico L. Suppression of APOBEC3-mediated restriction of HIV-1 by Vif. Front Microbiol. 2014;5:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–6. [DOI] [PubMed] [Google Scholar]
- [7].Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–50. [DOI] [PubMed] [Google Scholar]
- [8].Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–9. [DOI] [PubMed] [Google Scholar]
- [9].Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, et al. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004;11:435–42. [DOI] [PubMed] [Google Scholar]
- [10].Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. [DOI] [PubMed] [Google Scholar]
- [11].Dang Y, Wang X, Esselman WJ, Zheng YH. Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J Virol. 2006;80:10522–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].OhAinle M, Kerns JA, Malik HS, Emerman M. Adaptive evolution and antiviral activity of the conserved mammalian cytidine deaminase APOBEC3H. J Virol. 2006;80:3853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dang Y, Siew LM, Wang X, Han Y, Lampen R, Zheng YH. Human cytidine deaminase APOBEC3H restricts HIV-1 replication. J Biol Chem. 2008;283:11606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–91. [DOI] [PubMed] [Google Scholar]
- [15].Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004;78:6073–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–13. [DOI] [PubMed] [Google Scholar]
- [18].Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9:1398–403. [DOI] [PubMed] [Google Scholar]
- [19].Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–7. [DOI] [PubMed] [Google Scholar]
- [20].Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–60. [DOI] [PubMed] [Google Scholar]
- [21].Liu B, Sarkis PT, Luo K, Yu Y, Yu XF. Regulation of Apobec3F and human immunodeficiency virus type 1 Vif by Vif-Cul5-ElonB/C E3 ubiquitin ligase. J Virol. 2005;79:9579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shirakawa K, Takaori-Kondo A, Kobayashi M, Tomonaga M, Izumi T, Fukunaga K, et al. Ubiquitination of APOBEC3 proteins by the Vif-Cullin5-ElonginB-ElonginC complex. Virology. 2006;344:263–6. [DOI] [PubMed] [Google Scholar]
- [23].Svarovskaia ES, Xu H, Mbisa JL, Barr R, Gorelick RJ, Ono A, et al. Human apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) is incorporated into HIV-1 virions through interactions with viral and nonviral RNAs. J Biol Chem. 2004;279:35822–8. [DOI] [PubMed] [Google Scholar]
- [24].Zennou V, Perez-Caballero D, Gottlinger H, Bieniasz PD. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J Virol. 2004;78:12058–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kao S, Khan MA, Miyagi E, Plishka R, Buckler-White A, Strebel K. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J Virol. 2003;77:11398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Simon JH, Miller DL, Fouchier RA, Soares MA, Peden KW, Malim MH. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. Embo J. 1998;17:1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, et al. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. [DOI] [PubMed] [Google Scholar]
- [28].Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. [DOI] [PubMed] [Google Scholar]
- [29].Lecossier D, Bouchonnet F, Clavel F, Hance AJ. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science. 2003;300:1112. [DOI] [PubMed] [Google Scholar]
- [30].Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, et al. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Okada A, Iwatani Y. APOBEC3G-Mediated G-to-A Hypermutation of the HIV-1 Genome: The Missing Link in Antiviral Molecular Mechanisms. Front Microbiol. 2016;7:2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guo F, Cen S, Niu M, Saadatmand J, Kleiman L. Inhibition of tRNA(3)(Lys)-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication. J Virol. 2006;80:11710–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, et al. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res. 2007;35:7096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 2008;4:e1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pollpeter D, Parsons M, Sobala AE, Coxhead S, Lang RD, Bruns AM, et al. Deep sequencing of HIV-1 reverse transcripts reveals the multifaceted antiviral functions of APOBEC3G. Nat Microbiol. 2018;3:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Haché G, Liddament MT, Harris RS. The retroviral hypermutation specificity of APOBEC3F and APOBEC3G is governed by the C-terminal DNA cytosine deaminase domain. J Biol Chem. 2005;280:10920–4. [DOI] [PubMed] [Google Scholar]
- [38].Gooch BD, Cullen BR. Functional domain organization of human APOBEC3G. Virology. 2008;379:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, et al. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr Biol. 2005;15:166–70. [DOI] [PubMed] [Google Scholar]
- [40].Burnett A, Spearman P. APOBEC3G multimers are recruited to the plasma membrane for packaging into human immunodeficiency virus type 1 virus-like particles in an RNA-dependent process requiring the NC basic linker. J Virol. 2007;81:5000–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huthoff H, Malim MH. Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and Virion encapsidation. J Virol. 2007;81:3807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Betts L, Xiang S, Short SA, Wolfenden R, Carter CW Jr., Cytidine deaminase. The 2.3 A crystal structure of an enzyme: transition-state analog complex. J Mol Biol. 1994;235:635–56. [DOI] [PubMed] [Google Scholar]
- [43].Xiang S, Short SA, Wolfenden R, Carter CW Jr. Transition-state selectivity for a single hydroxyl group during catalysis by cytidine deaminase. Biochemistry. 1995;34:4516–23. [DOI] [PubMed] [Google Scholar]
- [44].Xiang S, Short SA, Wolfenden R, Carter CW Jr. The structure of the cytidine deaminase-product complex provides evidence for efficient proton transfer and ground-state destabilization. Biochemistry. 1997;36:4768–74. [DOI] [PubMed] [Google Scholar]
- [45].Desimmie BA, Delviks-Frankenberrry KA, Burdick RC, Qi D, Izumi T, Pathak VK. Multiple APOBEC3 restriction factors for HIV-1 and one Vif to rule them all. J Mol Biol. 2014;426:1220–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Silvas TV, Hou S, Myint W, Nalivaika E, Somasundaran M, Kelch BA, et al. Substrate sequence selectivity of APOBEC3A implicates intra-DNA interactions. Sci Rep. 2018;8:7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chelico L, Pham P, Calabrese P, Goodman MF. APOBEC3G DNA deaminase acts processively 3' --> 5' on single-stranded DNA. Nat Struct Mol Biol. 2006;13:392–9. [DOI] [PubMed] [Google Scholar]
- [48].Chen KM, Harjes E, Gross PJ, Fahmy A, Lu Y, Shindo K, et al. Structure of the DNA deaminase domain of the HIV-1 restriction factor APOBEC3G. Nature. 2008;452:116–9. [DOI] [PubMed] [Google Scholar]
- [49].Harjes E, Gross PJ, Chen KM, Lu Y, Shindo K, Nowarski R, et al. An extended structure of the APOBEC3G catalytic domain suggests a unique holoenzyme model. J Mol Biol. 2009;389:819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shandilya SM, Nalam MN, Nalivaika EA, Gross PJ, Valesano JC, Shindo K, et al. Crystal structure of the APOBEC3G catalytic domain reveals potential oligomerization interfaces. Structure. 2010;18:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kouno T, Luengas EM, Shigematsu M, Shandilya SM, Zhang J, Chen L, et al. Structure of the Vif-binding domain of the antiviral enzyme APOBEC3G. Nat Struct Mol Biol. 2015;22:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Holden LG, Prochnow C, Chang YP, Bransteitter R, Chelico L, Sen U, et al. Crystal structure of the anti-viral APOBEC3G catalytic domain and functional implications. Nature. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Furukawa A, Nagata T, Matsugami A, Habu Y, Sugiyama R, Hayashi F, et al. Structure, interaction and real-time monitoring of the enzymatic reaction of wild-type APOBEC3G. EMBO J. 2009;28:440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lu X, Zhang T, Xu Z, Liu S, Zhao B, Lan W, et al. Crystal structure of DNA cytidine deaminase ABOBEC3G catalytic deamination domain suggests a binding mode of full-length enzyme to single-stranded DNA. J Biol Chem. 2015;290:4010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xiao X, Yang H, Arutiunian V, Fang Y, Besse G, Morimoto C, et al. Structural determinants of APOBEC3B non-catalytic domain for molecular assembly and catalytic regulation. Nucleic Acids Res. 2017;45:7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xiao X, Li SX, Yang H, Chen XS. Crystal structures of APOBEC3G N-domain alone and its complex with DNA. Nat Commun. 2016;7:12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kouno T, Silvas TV, Hilbert BJ, Shandilya SMD, Bohn MF, Kelch BA, et al. Crystal structure of APOBEC3A bound to single-stranded DNA reveals structural basis for cytidine deamination and specificity. Nat Commun. 2017;8:15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shi K, Carpenter MA, Banerjee S, Shaban NM, Kurahashi K, Salamango DJ, et al. Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B. Nat Struct Mol Biol. 2017;24:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Maiti A, Myint W, Kanai T, Delviks-Frankenberry K, Sierra Rodriguez C, Pathak VK, et al. Crystal structure of the catalytic domain of HIV-1 restriction factor APOBEC3G in complex with ssDNA. Nat Commun. 2018;9:2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ziegler SJ, Liu C, Landau M, Buzovetsky O, Desimmie BA, Zhao Q, et al. Insights into DNA substrate selection by APOBEC3G from structural, biochemical, and functional studies. PLoS One. 2018;13:e0195048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Solomon WC, Myint W, Hou S, Kanai T, Tripathi R, Kurt Yilmaz N, et al. Mechanism for APOBEC3G catalytic exclusion of RNA and non-substrate DNA. Nucleic Acids Res. 2019;47:7676–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Iwatani Y, Takeuchi H, Strebel K, Levin JG. Biochemical activities of highly purified, catalytically active human APOBEC3G: correlation with antiviral effect. J Virol. 2006;80:5992–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chelico L, Pham P, Calabrese P, Goodman MF. APOBEC3G DNA deaminase acts processively 3' --> 5' on single-stranded DNA. Nat Struct Mol Biol. 2006. [DOI] [PubMed] [Google Scholar]
- [64].Chelico L, Sacho EJ, Erie DA, Goodman MF. A model for oligomeric regulation of APOBEC3G cytosine deaminase-dependent restriction of HIV. J Biol Chem. 2008;283:13780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wedekind JE, Gillilan R, Janda A, Krucinska J, Salter JD, Bennett RP, et al. Nanostructures of APOBEC3G support a hierarchical assembly model of high molecular mass ribonucleoprotein particles from dimeric subunits. J Biol Chem. 2006;281:38122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–14. [DOI] [PubMed] [Google Scholar]
- [67].Morse M, Huo R, Feng Y, Rouzina I, Chelico L, Williams MC. Dimerization regulates both deaminase-dependent and deaminase-independent HIV-1 restriction by APOBEC3G. Nat Commun. 2017;8:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Morse M, Naufer MN, Feng Y, Chelico L, Rouzina I, Williams MC. HIV restriction factor APOBEC3G binds in multiple steps and conformations to search and deaminate single-stranded DNA. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gorle S, Pan Y, Sun Z, Shlyakhtenko LS, Harris RS, Lyubchenko YL, et al. Computational Model and Dynamics of Monomeric Full-Length APOBEC3G. ACS Cent Sci. 2017;3:1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yang H, Ito F, Wolfe AD, Li S, Mohammadzadeh N, Love RP, et al. Understanding the structural basis of HIV-1 restriction by the full length double-domain APOBEC3G. Nat Commun. 2020;11:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chelico L, Prochnow C, Erie DA, Chen XS, Goodman MF. A structural model for deoxycytidine deamination mechanisms of the HIV-1 inactivation enzyme APOBEC3G. J Biol Chem. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shaban NM, Shi K, Li M, Aihara H, Harris RS. 1.92 Angstrom Zinc-Free APOBEC3F Catalytic Domain Crystal Structure. J Mol Biol. 2016;428:2307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kouno T, Luengas EM, Shigematsu M, Shandilya SMD, Zhang JY, Chen L, et al. Structure of the Vif-binding domain of the antiviral enzyme APOBEC3G. Nature Structural & Molecular Biology. 2015;22:485–U79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kouno T, Silvas TV, Hilbert BJ, Shandilya SMD, Bohn MF, Kelch BA, et al. Crystal structure of APOBEC3A bound to single-stranded DNA reveals structural basis for cytidine deamination and specificity. Nature Communications. 2017;8:15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fang Y, Xiao X, Li SX, Wolfe A, Chen XS. Molecular Interactions of a DNA Modifying Enzyme APOBEC3F Catalytic Domain with a Single-Stranded DNA. J Mol Biol. 2018;430:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Matsuoka T, Nagae T, Ode H, Awazu H, Kurosawa T, Hamano A, et al. Structural basis of chimpanzee APOBEC3H dimerization stabilized by double-stranded RNA. Nucleic Acids Res. 2018;46:10368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Shaban NM, Shi K, Lauer KV, Carpenter MA, Richards CM, Salamango D, et al. The Antiviral and Cancer Genomic DNA Deaminase APOBEC3H Is Regulated by an RNA-Mediated Dimerization Mechanism. Mol Cell. 2018;69:75–86 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bohn JA, Thummar K, York A, Raymond A, Brown WC, Bieniasz PD, et al. APOBEC3H structure reveals an unusual mechanism of interaction with duplex RNA. Nat Commun. 2017;8:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shlyakhtenko LS, Lushnikov AY, Li M, Lackey L, Harris RS, Lyubchenko YL. Atomic force microscopy studies provide direct evidence for dimerization of the HIV restriction factor APOBEC3G. J Biol Chem. 2011;286:3387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shlyakhtenko LS, Lushnikov AY, Miyagi A, Li M, Harris RS, Lyubchenko YL. Nanoscale structure and dynamics of ABOBEC3G complexes with single-stranded DNA. Biochemistry. 2012;51:6432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shlyakhtenko LS, Lushnikov AY, Miyagi A, Li M, Harris RS, Lyubchenko YL. Atomic force microscopy studies of APOBEC3G oligomerization and dynamics. J Struct Biol. 2013;184:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Huthoff H, Autore F, Gallois-Montbrun S, Fraternali F, Malim MH. RNA-dependent oligomerization of APOBEC3G is required for restriction of HIV-1. PLoS Pathog. 2009;5:e1000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fukuda H, Li S, Sardo L, Smith JL, Yamashita K, Sarca AD, et al. Structural Determinants of the APOBEC3G N-Terminal Domain for HIV-1 RNA Association. Front Cell Infect Microbiol. 2019;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Harjes S, Solomon WC, Li M, Chen KM, Harjes E, Harris RS, et al. Impact of H216 on the DNA Binding and Catalytic Activities of the HIV Restriction Factor APOBEC3G. Journal of Virology. 2013;87:7008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chelico L, Prochnow C, Erie DA, Chen XS, Goodman MF. Structural model for deoxycytidine deamination mechanisms of the HIV-1 inactivation enzyme APOBEC3G. J Biol Chem. 2010;285:16195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Suspene R, Rusniok C, Vartanian JP, Wain-Hobson S. Twin gradients in APOBEC3 edited HIV-1 DNA reflect the dynamics of lentiviral replication. Nucleic Acids Res. 2006;34:4677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Feng Y, Chelico L. Intensity of deoxycytidine deamination of HIV-1 proviral DNA by the retroviral restriction factor APOBEC3G is mediated by the noncatalytic domain. J Biol Chem. 2011;286:11415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].An P, Bleiber G, Duggal P, Nelson G, May M, Mangeat B, et al. APOBEC3G genetic variants and their influence on the progression to AIDS. J Virol. 2004;78:11070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Reddy K, Winkler CA, Werner L, Mlisana K, Abdool Karim SS, Ndung'u T, et al. APOBEC3G expression is dysregulated in primary HIV-1 infection and polymorphic variants influence CD4+ T-cell counts and plasma viral load. AIDS. 2010;24:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chen KM, Harjes E, Gross PJ, Fahmy A, Lu YJ, Shindo K, et al. Structure of the DNA deaminase domain of the HIV-1 restriction factor APOBEC3G. Nature. 2008;452:116–U16. [DOI] [PubMed] [Google Scholar]
- [91].Harjes E, Gross PJ, Chen KM, Lu YJ, Shindo K, Nowarski R, et al. An Extended Structure of the APOBEC3G Catalytic Domain Suggests a Unique Holoenzyme Model. Journal of Molecular Biology. 2009;389:819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Furukawa A, Sugase K, Morishita R, Nagata T, Kodaki T, Takaori-Kondo A, et al. Quantitative analysis of location- and sequence-dependent deamination by APOBEC3G using real-time NMR spectroscopy. Angew Chem Int Ed Engl. 2014;53:2349–52. [DOI] [PubMed] [Google Scholar]
- [93].Friew YN, Boyko V, Hu WS, Pathak VK. Intracellular interactions between APOBEC3G, RNA, and HIV-1 Gag: APOBEC3G multimerization is dependent on its association with RNA. Retrovirology. 2009;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mangeat B, Turelli P, Liao S, Trono D. A single amino acid determinant governs the species-specific sensitivity of APOBEC3G to Vif action. J Biol Chem. 2004;279:14481–3. [DOI] [PubMed] [Google Scholar]
- [95].Mehle A, Strack B, Ancuta P, Zhang C, McPike M, Gabuzda D. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J Biol Chem. 2004;279:7792–8. [DOI] [PubMed] [Google Scholar]
- [96].Schrofelbauer B, Chen D, Landau NR. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif). Proc Natl Acad Sci U S A. 2004;101:3927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bogerd HP, Doehle BP, Wiegand HL, Cullen BR. A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc Natl Acad Sci U S A. 2004;101:3770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Xu H, Svarovskaia ES, Barr R, Zhang Y, Khan MA, Strebel K, et al. A single amino acid substitution in human APOBEC3G antiretroviral enzyme confers resistance to HIV-1 virion infectivity factor-induced depletion. Proc Natl Acad Sci U S A. 2004;101:5652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AG. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr. 2011;67:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–26. [DOI] [PubMed] [Google Scholar]
- [101].Bunkoczi G, Echols N, McCoy AJ, Oeffner RD, Adams PD, Read RJ. Phaser.MRage: automated molecular replacement. Acta Crystallogr D Biol Crystallogr. 2013;69:2276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. [DOI] [PubMed] [Google Scholar]
- [104].Adams PD, Afonine PV, Bunkoczi G, Chen VB, Echols N, Headd JJ, et al. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Echols N, Grosse-Kunstleve RW, Afonine PV, Bunkoczi G, Chen VB, Headd JJ, et al. Graphical tools for macromolecular crystallography in PHENIX. J Appl Crystallogr. 2012;45:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Labarga A, Valentin F, Anderson M, Lopez R. Web services at the European Bioinformatics Institute. Nucleic Acids Research. 2007;35:W6–W11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Schrodinger, LLC. The AxPyMOL Molecular Graphics Plugin for Microsoft PowerPoint, Version 1.8. 2015.
- [109].Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. 2013;27:221–34. [DOI] [PubMed] [Google Scholar]
- [110].Bowers KJ, Chow E, Xu H, Dror RO, Eastwood MP, Gregersen BA, Klepeis JL, Kolossvary I, Moraes MA, Sacerdoti FD, Salmon JK, Shan Y and Shaw DE . In Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. Proceedings of the 2006 ACM/IEEE conference on Supercomputing2006. [Google Scholar]
- [111].Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Harjes S, Solomon WC, Li M, Chen KM, Harjes E, Harris RS, et al. Impact of H216 on the DNA binding and catalytic activities of the HIV restriction factor APOBEC3G. J Virol. 2013;87:7008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wienken CJ, Baaske P, Rothbauer U, Braun D, Duhr S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat Commun. 2010;1:100. [DOI] [PubMed] [Google Scholar]
- [114].Yee JK, Miyanohara A, LaPorte P, Bouic K, Burns JC, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci U S A. 1994;91:9564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Smith JL, Izumi T, Borbet TC, Hagedorn AN, Pathak VK. HIV-1 and HIV-2 Vif interact with human APOBEC3 proteins using completely different determinants. J Virol. 2014;88:9893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Russell RA, Pathak VK. Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F. J Virol. 2007;81:8201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Simon JH, Fouchier RA, Southerling TE, Guerra CB, Grant CK, Malim MH. The Vif and Gag proteins of human immunodeficiency virus type 1 colocalize in infected human T cells. J Virol. 1997;71:5259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Atomic coordinates and structural factors for the reported crystal structure have been deposited in the Protein Data Bank under the accession number 6WMA, 6WMB and 6WMC.