Abstract
Background & Aims
Chronic HBV infection cannot be cured by current therapeutics owing to their limited ability to reduce covalently closed circular (ccc)DNA levels in the livers of infected individuals. Therefore, greater understanding of the molecular determinants of cccDNA formation and persistence is required. One key issue is the extent to which de novo nucleocapsid-mediated replenishment (reimport) contributes to cccDNA levels in an infected hepatocyte.
Methods
We engineered an infectious HBV mutant with a genome encoding a stop codon at position T67 in the HBV core open reading frame (ΔHBc HBV). Importantly, ΔHBc HBV virions cannot initiate nucleocapsid synthesis upon infection. Long-term in vitro HBV infection markers were followed for up for 9 weeks in HepG2-NTCP cells (A3 clone) and HBV DNA was quantified using a newly-developed, highly-precise PCR assay (cccDNA inversion quantitative PCR).
Results
ΔHBc and wild-type (WT) HBV resulted in comparable expression of HBV surface antigen (HBsAg), which could be blocked using the entry inhibitor Myrcludex B, confirming bona fide infection via the receptor sodium taurocholate cotransporting polypeptide (NTCP). In primary human hepatocytes, Huh7-NTCP, HepG2-NTCP, and HepaRG-NTCP cells, comparable copy numbers of cccDNA were formed. cccDNA levels, transcription of viral RNA, and HBsAg secretion remained comparably stable in WT and ΔHBc HBV-infected cells for at least 9 weeks.
Conclusions
Our results imply that de novo synthesised HBc plays a minor role in transcriptional regulation of cccDNA. Importantly, we show that initially-formed cccDNA is stable in hepatocytes without requiring continuous replenishment in in vitro infection systems and contribution from de novo DNA-containing nucleocapsids is not required. Thus, short-term therapeutic targeting of capsid-reimport is likely an inefficient strategy in eliminating cccDNA in chronically infected hepatocytes.
Lay summary
The hepatitis B virus can maintain itself in the liver for a patient's lifetime, causing liver injury and cancer. We have clarified exactly how it maintains itself in an infected cell. This now means we have a better idea at how to target the virus and cure a chronic infection.
Keywords: Covalently closed circular DNA, Myrcludex B, Bulevirtide, Hepcludex, HBV persistence, Core protein, HBcAg, Antivirals, Capsid inhibitors, HBV DNA integration
Abbreviations: ALT, alanine aminotransferase; cccDNA, covalently closed circular DNA; CIs, capsid inhibitors; DHBV, duck hepatitis B virus; dpi, days post inoculation; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; mge, multiplicity of genomic equivalent; NC, naked capsids; NTCP, sodium taurocholate cotransporting polypeptide; NUCs, nucleos(t)ide analogues; ORF, open reading frame; PEG, polyethylene glycol; pgRNA, pregenomic RNA; PHH, primary human hepatocytes; rcDNA, relaxed circular DNA; SN, supernatant; vge, viral genome equivalents; VP, virions; WT, wild-type
Graphical abstract
Highlights
-
•
Covalently closed circular (ccc)DNA is key for maintaining chronic HBV infection.
-
•
Virus core protein expression is not required for cccDNA formation, stability, or transcription within 9 weeks of in vitro infection.
-
•
Our results suggest that targeting HBV core with short-term treatment is inefficient in clearing intrahepatic cccDNA.
-
•
Viral entry inhibitors or capsid inhibitors could prevent breakthrough of novel HBV variants.
Introduction
Chronic infection with hepatitis B virus (HBV) is the main cause of hepatocellular carcinoma worldwide and a major risk factor for progressive liver disease and liver cirrhosis. Together, ∼884,000 deaths each year are directly attributable to chronic HBV infection.1,2 In addition to these medical harms to physical health, people living with chronic HBV infection face considerable community stigma and discrimination as a result of its highly infectious and incurable nature.3 Most chronic HBV infections are established after neonatal exposure during birth. This life-long infection is characterised by fluctuating liver inflammation over decades, which can continuously progress to end-stage liver disease and liver cancer.4 HBV infection persists because of the maintenance of the viral covalently closed circular (ccc)DNA, a stable episomal form in the nucleus of infected cells.5
Establishment of cccDNA is an early event in virus infection, occurring within 16 h of infection.6 Circulating virions enter hepatocytes, the parenchymal cells of the liver, via cellular sodium taurocholate co-transporting polypeptide (NTCP).7,8 Viral nucleocapsids containing HBV relaxed-circular (rc)DNA genomes are transported to the nucleus.9 Here, rcDNA is converted into cccDNA, the template for all viral RNA transcripts.10 As an aside, double-stranded linear DNA (a second, less-common form of virion DNA) can occasionally integrate into the cellular genome at the site of chromosomal DNA breaks, forming stable templates for viral antigen expression (including HBV surface antigen and the HBV X protein).11
The cccDNA mini-chromosome is complexed with histones and partially co-localises with HBV core protein (HBc) in the nucleus of infected hepatocytes.12 HBc has been reported to epigenetically modulate cccDNA stability and its transcriptional activity through poorly defined mechanisms. Transcribed HBV pregenomic RNA (pgRNA) and bound viral polymerase are encapsidated, forming RNA-containing nucleocapsids composed of 120 dimers of HBc. Reverse-transcription of the pgRNA occurs within nucleocapsids, which are subsequently enveloped, secreted, and mature into infectious virions.13 Alternatively, newly synthesised nucleocapsids may enter the nucleus to replenish the pool of established cccDNA molecules during turnover (using a similar route to that of nucleocapsids during infection establishment). This route of cccDNA replenishment has been described in the related duck hepadnaviruses as ‘cccDNA amplification’.14
Chronic HBV infection cannot be cured by presently approved standard of care treatment with nucleos(t)ide analogues (NUCs), which interfere with reverse transcription of pgRNA to rcDNA. Because reverse transcription occurs only after cccDNA formation, NUCs can only indirectly affect cccDNA levels,15 through inhibition or ideally blockade of nuclear import of de novo produced nucleocapsids for cccDNA replenishment. However, only marginal reductions in cccDNA levels have been observed in NUC-treated HBV patients.16 This could indicate that reverse-transcription is only partially inhibited, allowing ongoing cccDNA replenishment or nuclear import plays a subordinate role in maintenance of the cccDNA pool.
A novel group of investigational drugs called capsid inhibitors (CIs) target HBV capsids and may thereby affect cccDNA. They are divided into 2 groups each with distinct modes of action (prevention of capsid assembly or capsid destabilisation). Owing to the multiple functions of the HBV capsid during the HBV life cycle, they could affect cccDNA through different pathways: (i) by inhibiting nucleocapsid assembly and preventing the nuclear import route of cccDNA amplification (see reviews17,18); (ii) by interference with disassembly of incoming nucleocapsids prior to cccDNA formation6,[19], [20], [21]; or (iii) by interfering with HBc-mediated cccDNA transcription and stability.19,[21], [22], [23] A key determinant of the effectiveness of CI to eliminate cccDNA is the underlying mechanism by which cccDNA is maintained in an infected cell.
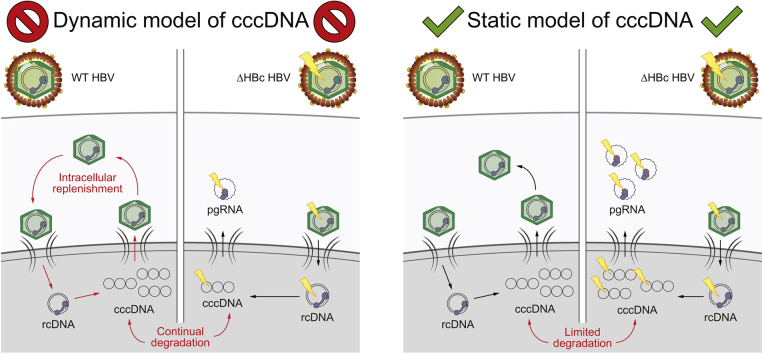
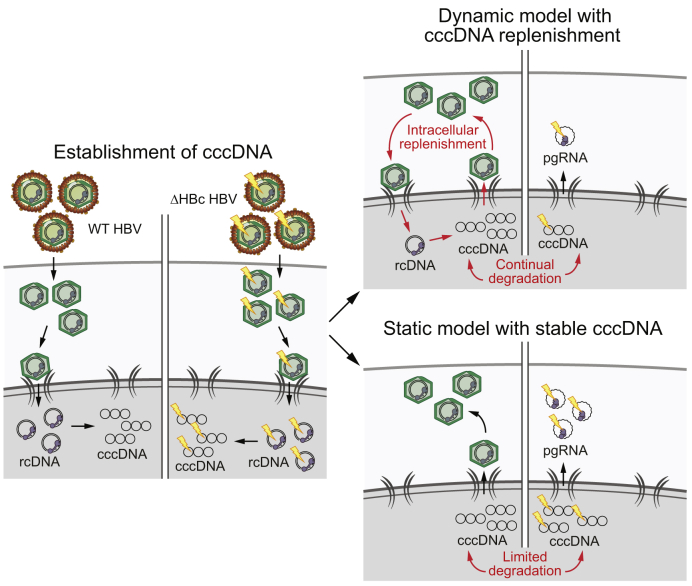
Although it is generally accepted that ‘net’ cccDNA levels remain stable over time in a HBV-infected cell, the stability of individual cccDNA molecules in the cell remains unknown. One hypothetical model holds that cccDNA levels are static after its establishment. In this model, additional cccDNA formation is repressed once the cccDNA copy numbers reached a plateau (Fig. 1). The second model is based on the idea of a continuously ongoing (dynamic) cccDNA turnover. Here, cccDNA undergoes constant degradation while de novo synthesis maintains copy numbers at an equilibrium. In general, resupply of cccDNA may occur by 2 routes: an intracellular ‘internal route’ through nuclear import of de novo synthesised cytoplasmic nucleocapsids (NTCP-receptor independent), or an extracellular ‘external route’ through reinfection of a hepatocyte with progeny virions (NTCP-dependent) (see review24).
Fig. 1.
Two potential models of cccDNA maintenance in chronic HBV infection.
After initial cccDNA formation (left), the subsequent net stable levels of cccDNA can be maintained by 2 hypothetical models. In the dynamic model of cccDNA maintenance (right), continual degradation and renewal via de novo virions maintain the observed constant levels of cccDNA in an infected cell. However, the static model does not require constant renewal and instead predicts very stable cccDNA molecules. If the dynamic model is valid, we would expect to see a difference in cccDNA levels between WT and ΔHBc HBV infections (decreased in the latter). By contrast, no difference in cccDNA levels would be expected in the static model. cccDNA, covalently closed circular DNA; HBV, hepatitis B virus; WT, wild-type.
In this study, we provide experimental evidence for the ‘static model’ of cccDNA taking advantage of a replication-deficient HBV which establishes a mutant cccDNA incapable of encoding HBc (hereby referred to as core-deficient mutant HBV or ΔHBc HBV). Using this virus to infect susceptible hepatocytes, we investigated the role of de novo nucleocapsid formation on cccDNA levels. We found strong evidence that cccDNA-driven de novo synthesis of HBc does not play a key role in: (i) establishment of the initial cccDNA pool in the first round of infection; (ii) maintenance of this cccDNA pool for >60 days after infection; and (iii) sustaining transcription of genomic and subgenomic HBV RNAs. This provides compelling support for cccDNA maintenance without the requirement of continuous replenishment and rather points at a ‘salvage pathway’ to rescue cccDNA if antigen production ceases.
Materials and methods
Virus production and characterisation
The codon at amino acid T67 site of the HBc open reading frame (ORF) in a genotype D HBV construct (pHBV1.1, a pcDNA3.1 plasmid where a HBV 1.1mer is under the control of a human cytomegalovirus promoter, previously generated and kindly provided by Dr Yi Ni25) was mutated to a TAG stop codon using overlapping PCR. Primers encoding the stop codon and flanking complementary sequences up- and downstream the T67 codon were used to amplify 2 overlapping fragments of pHBV1.1 (5ʹ-AGATATACGCGTTGACATTGATTATTGACTAG-3ʹ and 5ʹ-CCCAGGTAGCTAGCTACATTAGTTCCCC-3ʹ for the upstream product; 5ʹ-GGGGAACTAATGTAGCTAGCTACCTGGG-3ʹ and 5ʹ- GTTGTGGAATTCCACTGCATGG-3ʹ for the downstream product, stop codon underlined) by Phusion® High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA USA). The PCR product was cloned into pHBV1.1 at the MluI and EcoRI sites, resulting in the pHBV1.1-HBcstop construct.
Huh7 cells were co-transfected with pHBV1.1-HBcstop and pHBc (encoding a genotype D HBV HBc protein under a cytomegalovirus promoter, kindly provided by Dr Christine Bekker) for the production of ΔHBc HBV. As control, cells were transfected with the wild-type (WT) pHBV1.1 or only pHBV1.1-HBcstop. Cells were transfected in 10 cm plates using TransIT-LT1 transfection reagent (Mirus Bio, Madison, WI, USA), according to the manufacturer's protocol. The cell culture supernatants (SNs) were collected every 2 days until 9 days post transfection, pooled, and filtered through a Stericup with a 0.45 μm PVDF membrane (SCHVU05RE, Merck, Darmstadt, Germany).
For analysis of viral particle composition, the SN of transfected cells were concentrated ∼100-fold by precipitation with 6% w/v polyethylene glycol (PEG) 8000 (89510, Sigma-Aldrich, St Louis, MO, USA). A 50-μl sample of the concentrated virus stock solution was analysed by analytical caesium chloride density gradient and DNA dot blot, as described.26,27 Titres of naked nucleocapsids and virions were quantified using a pHBV1.1 plasmid DNA standard and the software QuantityOne (Biorad, Hercules, CA).
For virus purification, 600 ml SN was applied to a 5-ml heparin affinity chromatography column (HiTrap® Heparin High Performance column, GE Healthcare) at a flow rate of 1 ml/min. The column was washed (140 mM NaCl, 20 mM Tris-HCl, pH = 7.4) and virions were eluted by gradual increase of NaCl (up to 2140 mM NaCl over 10 column volumes). Virus-containing fractions were determined by absorbance at 280 nm UV, pooled (7.5 ml), diluted with H2O to a final NaCl concentration of 140 nM, concentrated ∼3-fold on a 100-kDa Amicon Ultra-15 centrifugal filter (UFC910024, Merck), combined with foetal bovine serum to a final concentration of 10% v/v, and stored at −80°C. HBV DNA of the inoculum was quantified using the COBAS® AmpliPrep/COBAS® TaqMan® HBV Test (Roche) by the Heidelberg University Clinic Virology Diagnostics Department (Heidelberg, Germany) and this value was used to determine viral genome equivalents (vge) per millilitre.
Cell culture and detection of HBV infection
Primary human hepatocytes (PHH), differentiated HepaRG-hNTCP cells (differentiated as per Ni et al.7), Huh7-NTCP and HepG2-NTCP cells were used for in vitro infection. PHH kindly provided by Florian Vondran (Hanover Medical School, Hanover, Germany) were isolated from liver resections of patients undergoing partial hepatectomy.28 All tissue donors gave written informed consent for the experimental use of liver specimens and the protocol was approved by the Ethics Review Committee of Hannover Medical School (#252-2008). PHH and HepaRG-NTCP cells were cultivated in William's E media supplemented with 1.5% v/v DMSO.29,30 Huh7-NTCP and HepG2-NTCP cells were maintained in DMSO-free Dulbecco's modified Eagle's medium.7
HBV infection was performed in the presence of 4% w/v PEG 8000 and 1.5% v/v (PHH and HepaRG-NTCP) or 2.5% v/v DMSO (HepG2-NTCP and Huh7-NTCP) for 16 h at 37°C. 1 µM Myrcludex B was added 30 min before and during the infection as control for infection inhibition. Following inoculation, cells were washed twice with PBS and further cultivated with an exchange of media every 2 days.
Secreted HBeAg and HBsAg (diluted with 1× PBS as indicated) was quantified via immunoassay (ADIVA Centaur HBeAg, Siemens, Munich, Germany and Architect HBsAg ELISA, Abbott Laboratories, Chicago, IL, USA) by the Heidelberg University Clinic Analytical Centre.
Integrated HBV DNA in in vitro infected cells was quantified by inverse nested PCR, as previously described.31,32
cccDNA and total HBV DNA quantification by cccDNA inversion quantitative (cinq)PCR
Total HBV DNA and cccDNA per cell were quantified by cinqPCR (which is compatible with the Genotype D HBV strain used in this project), as previously described.6 Total HBV DNA extracts (1 μg) were digested with HhaI (New England Biolabs [NEB]) which excises a fragment proximal to the DNA nick region present in HBV rcDNA (nt1801-nt2800). The 5ʹ→3ʹ single-stranded DNA exonuclease Recjf (NEB) was added to digest the single-stranded region of rcDNA, which is exposed by unstable binding of the DNA fragment between the DNA nick and the HhaI cut site.
cccDNA (lacking a DNA nick) remains stable and is impervious to Recjf activity. T4 DNA ligase (NEB) was used to induce intra-molecular ligation of cccDNA fragments (which retain intact complimentary sticky ends), but not rcDNA fragments (which have 1 sticky end digested away by Recjf). The DNA rings were then linearised by digestion with XbaI (NEB) and then amplified using digital droplet PCR with specific primers to the cccDNA fragment and RNaseP (a single-copy cellular gene). Throughout this procedure, dslDNA does not participate in the intramolecular ligation as the 5ʹ HhaI site is absent in this form.
For these inverted samples, a second HhaI digestion fragment (nt147-1231, which is identical regardless of HBV DNA form) was used to quantify total HBV DNA.
Southern blotting
Samples of 2×106 HepG2-NTCP cells/well were seeded in 6-well plates and infected on the next day with a multiplicity of genomic equivalents (mge) of 100 with WT or ΔHBc HBV as the inoculum. At 7 and 14 days post infection (dpi), cccDNA DNA was extracted and enriched using the MasterPure Complete DNA and RNA Purification kit (MC85200, Lucigen) as per the manufacturer's protocol with minor modifications (doubling of buffer volumes to ensure complete lysis). Cells were washed with PBS twice, incubated with 1.2 ml Tissue and Cell Lysis solution (MTC096H, Lucigen), and scraped off the well surface. Cell lysates were transferred to a 2 ml tube, placed on ice for 5 min, and then hydrolysed with RNase A (15 μg/ml) (MRNA092, Lucigen) at 37°C for 30 min. Then 0.6 ml of MPC buffer was added and tubes were placed on ice for 5 min, followed by centrifugation at 13,800 × g at 4°C for 10 min. SNs were transferred to a new tube and centrifuged at 13,800 × g again for 5 min. Again, the SN was transferred to a new tube and an equal volume of isopropanol was added to precipitate cccDNA-enriched DNA. The DNA was pelleted by centrifugation at 13,800 × g at 4°C for 10 min. The pellet was washed then with an equal volume of 70% v/v ethanol, air-dried and dissolved with 50 μl TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH = 7.5). Southern blot hybridisation was then performed exactly as previously described.33 Autoradiography were read by a phorphoimager and analysed by Fiji ImageJ imaging software (Open source, version 1.52i).34
Immunofluorescence for HBV antigens
Cells were fixed with 4% w/v formaldehyde in 1x PBS for 20 min and permeabilised with 0.25% v/v Triton-X in 1x PBS for 20 min. HBV core and surface antigens were detected using a 1:3,000 dilution of polyclonal rabbit anti-HBcAg antibody (B0586, Dako)29 and a 1:3,000 dilution of HBsAb (Humabs Biomed, kind gift from Davide Corti) in 1x PBS and 2% w/v IgG-free bovine serum albumin (Sigma Aldrich) overnight at 4°C. After washing with PBS, cells were overlaid with 1:500 AF488-conjugated goat anti-rabbit secondary antibody (A-11008, Invitrogen, Carlsbad, CA, USA), 1:1,000 AF555-conjugated goat anti-human secondary antibody (A-21433, Invitrogen), and 2 μg/ml Hoechst 33342 (H1399, Invitrogen) in 1x PBS and 2% w/v IgG-free bovine serum albumin (Sigma Aldrich) and then incubated in the dark for 1 h. Fluorescence microscopy images were acquired at 20x magnification. For each sample, an area the size of 5 × 5 fields of view were acquired and merged by NIS Elements Advanced software (Nikon). Images were edited and analysed with Fiji ImageJ imaging software.34
RT-qPCR for HBV transcripts
Total cellular RNA was extracted using the NucleoSpin RNA kit (Macherey-Nagel), according to the manufacturer's instructions. Cellular RNA (500 ng) was reverse-transcribed using the High Capacity cDNA Reverse Transcription kit (ThermoFisher) at 37°C for 2 h. Either HBV pgRNA and pre-core mRNA or total HBV transcripts were detected by qPCR using iTaq Universal SYBR Green Supermix (Bio-rad). PCR conditions were: initial denaturation at 95°C for 10 min; 40 cycles of 95°C for 10 s and 60°C for 30 s with fluorescence reading at the end of the cycle; then 65–95°C in 0.5°C increments for the melting curve. Absolute copy numbers of transcripts were calculated using pSHH2.1 plasmid standards, previously quantified using the COBAS® AmpliPrep/COBAS® TaqMan® HBV Test (as described above). Primers specific for pgRNA and pre-core mRNA35: sense (5ʹ-CTCCTCCAGCTTATAGACC-3ʹ); antisense (5ʹ-GTGAGTGGGCCTACAAA-3ʹ). Primers for total transcripts: sense (5ʹ-TCAGCAATGTCAACGACCGA-3ʹ); antisense (5ʹ- TGCGCAGACCAATTTATGCC-3ʹ).
Results
Production and characterisation of HBc-deficient (ΔHBc) HBV
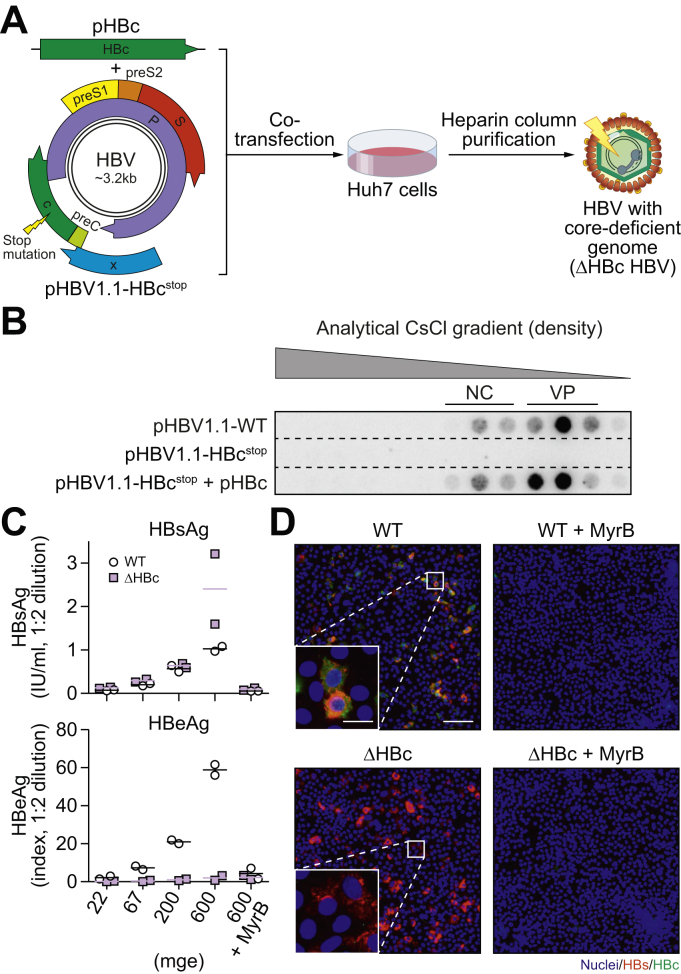
ΔHBc and WT HBV stocks were respectively generated by: (i) co-transfection of Huh7 cells with pHBV1.1-HBcstop and pHBc (Fig. 2A) and (ii) transfection with pHBV1.1. pHBV1.1 encodes both the pgRNA and the sub-genomic HBV transcripts and initiates HBV replication.25 Because pHBV1.1-HBcstop derived pgRNA contains a stop codon at amino acid T67 of the HBc ORF, generation of the ΔHBc HBV requires trans-complementation with plasmid pHBc encoding HBc.
Fig. 2.
Production and characterisation of ΔHBc HBV.
(A) Huh7 cells were either transfected with a WT HBV construct (pHBV1.1) or co-transfected with a construct containing a HBV 1.1 mer with stop mutation (lightning bolt) in the HBc ORF (pHBV1.1-HBcstop) along with a construct expressing WT HBc (pHBc). Supernatant from transfected cells was collected and purified by heparin affinity chromatography. (B) Precipitated supernatant was loaded on caesium chloride density gradients and fractions were analysed by dot blot. Naked capsids (NC) and virions (VP) were identified by the density of the fractions. (C) HepG2-NTCP cells were infected with WT or ΔHBc HBV at increasing inoculating dose. Supernatant was collected from 5 to 7 days post inoculation (dpi) and extracellular HBsAg and HBeAg were measured by ELISA. MyrB-mediated blockade of HBV infection was used as a negative control. The mean (line) of 2 independent experiments (circles/squares) is shown. (D) The numbers of HBs and HBc-positive HepG2-NTCP cells were determined by immunofluorescence 7 dpi. Cells infected at mge 200 are shown. Scale bars represent 100 μm (main figure) and 20 μm (inset). HBV, hepatitis B virus; mge, multiplicity of genomic equivalent; NTCP, sodium taurocholate cotransporting polypeptide; WT, wild-type.
SNs from the (co)-transfected Huh7 cells were analysed for HBV DNA by an analytical caesium chloride gradient and the DNA from virus-containing fractions were quantified by DNA dot blot (Fig. 2B). Comparable quantities of WT (1.2 × 108 vge/μl), as calculated by densitometry of the 3 virion-containing fractions (VP) and ΔHBc HBV (1.5 × 108 vge/μl) were obtained indicating that ΔHBc HBV can be efficiently assembled and secreted. Overexpression of HBc did not affect the ratio between naked nucleocapsids and virions. As expected, transfection with the ΔHBc over-length construct alone did neither result in secretion of HBV DNA-containing naked capsids nor virions, indicating the necessity of HBc for particle secretion. The SNs of transfected cells were pooled, purified by heparin affinity chromatography, concentrated, quantified by qPCR, and used as inocula for subsequent experiments.
ΔHBc HBV shows comparable levels of HBsAg secretion as WT HBV
We first tested the infectivity of the 2 HBV inocula at different mge (22, 67, 200, and 600) in HBV-susceptible HepG2-NTCP cells. Consistent with previous studies,7,29 inoculation at increasing mge of WT HBV resulted in a proportional increase of secreted HBs (HBsAg) and HBV e antigen (HBeAg) (Fig. 2C). As expected, ΔHBc HBV-infected HepG2-NTCP cells did not secrete HBeAg since it is encoded in the same ORF as HBc. Remarkably, secreted HBsAg upon ΔHBc HBV infection reaches similar levels as WT HBV infection. Secretion of HBeAg and HBsAg in WT HBV infection and HBsAg in ΔHBc HBV infection was blocked by Myrcludex B (MyrB), verifying that infection with both viruses exclusively followed NTCP-receptor mediated entry and that the measured HBsAg was produced from cccDNA (rather than being a left-over of the applied inoculum).
The analysis of intracellular viral antigens by immunofluorescence showed that cells infected with ΔHBc HBV also stain positive for HBsAg (red) but are deficient in expressing intracellular HBc (green) (Fig. 2D). By contrast, all HBsAg-positive cells infected with WT HBV also stained positive for HBc. Quantification of the number of HBsAg-positive cells showed comparable infection rates when using identical mge of WT and ΔHBc HBV (Fig. S1).
Taken together, these data indicate that ΔHBc HBV enters HepG2-NTCP cells via NTCP with similar efficacy to WT virus, initiating transcription and translation of HBsAg.
WT and ΔHBc HBV establish comparable levels of cccDNA in HBV-susceptible cells
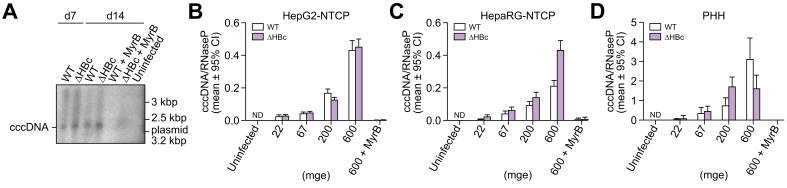
The comparable levels of HBsAg secretion in ΔHBc and WT HBV-infected HepG2-NTCP cells indicated that cccDNA formation and transcription is not constrained in infected cells lacking de novo HBc and HBeAg synthesis. To directly test this hypothesis, we infected HepG2-NTCP cells with both viruses, and then analysed intracellular cccDNA levels at 7 and 14 dpi by Southern blot (Fig. 3A). As shown previously,36 cccDNA was detectable at 7 and 14 dpi with WT virus without a significant increase within the second week. Formation of cccDNA could be blocked by MyrB, confirming receptor mediated entry as a prerequisite for cccDNA formation. Remarkably, almost identical cccDNA amounts could be detected at 7 and 14 dpi following infection with ΔHBc HBV, indicating that repair of the incoming genomic rcDNA to cccDNA proceeds in the absence of de novo capsid formation.
Fig. 3.
HBc expression is not required for the establishment of HBV cccDNA.
(A) HepG2-NTCP cells were infected with WT or ΔHBc HBV at mge 100. At 7 and 14 dpi, cccDNA was extracted and HBV DNA specific Southern blotting was performed. MyrB treatment was used to inhibit infection as control. (B) HepG2-NTCP cells, (C) differentiated HepaRG-NTCP cells, and (D) PHH were infected with WT or ΔHBc HBV with increasing inoculating doses. Total cellular DNA extracted at 7 dpi was analysed by cinqPCR to detect cccDNA levels relative to the single-copy cellular gene RNaseP. The error bars (Poisson 95% CI) represent the technical error of the ddPCR assay. No significant differences in cccDNA levels were detected between WT and ΔHBc HBV-infected cells in any cell line at any inoculating dose. Results are representative of 2 independent experiments. cccDNA, covalently closed circular DNA; dpi, days post inoculation; HBV, hepatitis B virus; mge, multiplicity of genomic equivalents; ND, not detected; NTCP, sodium taurocholate cotransporting polypeptide; PHH, primary human hepatocytes; WT, wild-type.
To prove and extend this finding, we used a recently developed novel method allowing precise quantification of cccDNA copy number relative to the cellular single-copy gene RNaseP (cinqPCR).6 Cells were infected with increasing mge of WT and ΔHBc virus and cccDNA was quantified at 7 dpi.
As depicted in Fig. 3B, comparable copy numbers of cccDNA/RNaseP gene were formed after infection with different mge of WT and ΔHBc HBV. Copy numbers increased proportionally with mge (22, 67, 200, and 600), demonstrating that the equivalence between WT and ΔHBc HBV is not a result of reaching a cccDNA saturation limit in the cell culture.
To investigate whether these findings also hold true in the most authentic in vitro infection systems, we implemented differentiated HepaRG-NTCP cells and PHH (Fig. 3C and D). In both cell types, ΔHBc HBV formed cccDNA at comparable copy numbers to WT HBV. Copy numbers proportionally increased with inoculation dose, presumably reflecting an increase in the percentage of infected hepatocytes (as previously shown by Schulze et al.29). In differentiated HepaRG-NTCP cells, cccDNA/RNAseP levels were comparable to infection in HepG2-NTCP cells (Fig. 3C), and were higher in the most authentic in vitro infection system PHH (Fig. 3D).
Taken together, these data confirm in the most authentic in vitro infection systems that cccDNA establishment requires neither the de novo synthesis of HBc nor HBeAg. Moreover, nuclear import of newly assembled rcDNA-containing HBV nucleocapsids does not significantly contribute to an increase of the cccDNA pool at least during short-term/first-round infection (at 14 dpi), suggesting that all cccDNA molecules are formed by the initial infection.
cccDNA levels of WT and ΔHBc HBV-infected HepG2-NTCP cells are equivalently maintained during long-term infection
The absence of cccDNA during the initial round of infection does not exclude the requirement of replenishment during long-term infection. We therefore took advantage of HepG2-NTCP A3 cells, which have been shown to support long-term in vitro infection7 and investigated cccDNA replenishment by nucleocapsid import over 63 days.
Following inoculation with WT or ΔHBc HBV (mge 40), comparable percentages of infected cells were observed by HBc/HBs-specific immunofluorescence at different times post-infection (Fig. 4A). Immunostaining of HBc and HBs at week 1, 4, and 9 after infection with WT HBV (upper row) revealed that ∼10% of cells were HBs-positive (Fig. S2), indicating that cells remained viable and supported HBV replication under the chosen culture conditions. As reported previously,7 viral spread was limited in this system. Infection with ΔHBc HBV resulted only in HBs-positive signals that also remained positive at 9 weeks (lower row). Owing to the mutation in HBc, no HBc-specific staining was detectable.
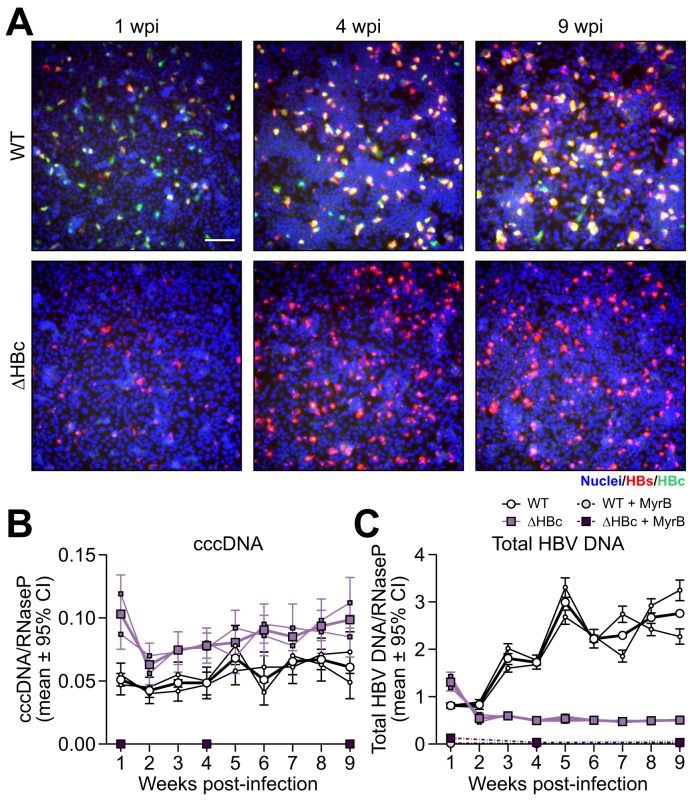
Fig. 4.
HBc expression is not required for the maintenance of cccDNA levels over months of infection.
(A) HepG2-NTCP cells were infected with WT or ΔHBc HBV at mge 40. Cells were fixed 1, 4, and 9 weeks post inoculation and immunofluorescence for HBs and HBc was performed. Scale bar represents 100 μm. Cells infected with WT or ΔHBc HBV were harvested every week for total DNA extraction. (B) cinqPCR was then performed to quantify cccDNA (left) and total HBV DNA (right) levels relative to the single-copy cellular gene RNaseP. MyrB treatment was used as control for infection inhibition (1, 4, and 9 weeks post inoculation). Two biological replicates (thin lines) were carried out. Error bars (Poisson 95% CI) represent the technical error of the ddPCR assay. The mean value of the 2 replicates is shown as a thick line. Results are representative of 2 independent experiments. cccDNA, covalently closed circular DNA; HBV, hepatitis B virus; mge, multiplicity of genomic equivalents; NTCP, sodium taurocholate cotransporting polypeptide; WT, wild-type.
We next quantified cccDNA by cinqPCR in the cell lysates of WT HBV- and ΔHBc HBV-infected HepG2-NTCP cells. As depicted in Fig. 4B, cccDNA levels were comparable following WT and ΔHBc HBV infections (with slightly higher initial levels induced by the mutant virus). Remarkably, cccDNA levels of both viruses remained unchanged over 9 weeks of infection (Fig. 4B). By contrast, total HBV DNA levels were low and stable in ΔHBc HBV-infected hepatocytes (no rcDNA synthesis without new nucleocapsids) but increased over time in WT-infected cells (continuous synthesis and release of progeny virus) (Fig. 4B). In both cases, treatment with MyrB blocked cccDNA formation and subsequent replication. Altogether these results imply that cccDNA degradation and consecutive replenishment from progeny nucleocapsids does not occur in infected resting cells within >2 months of infection. They also provide functional evidence that complete abolishment of de novo HBc synthesis has no effect on cccDNA stability in non-dividing cells.
De novo expressed HBc does not alter HBV transcription levels
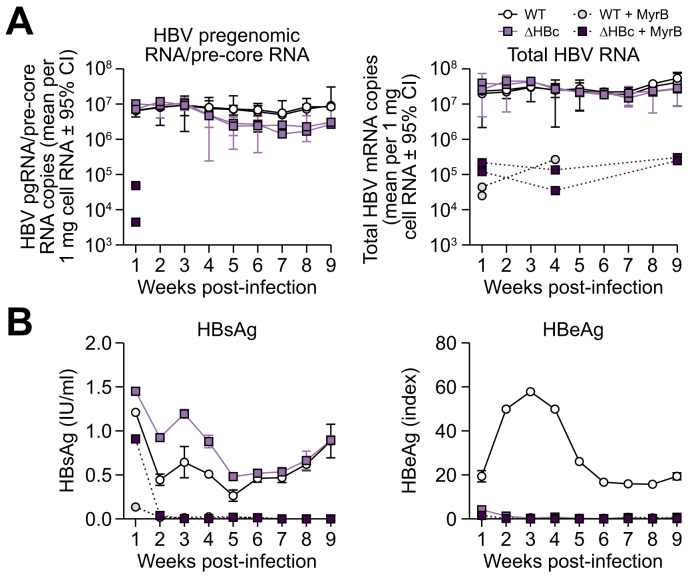
As evidenced by immunoprecipitation and electron microscopy-based studies, HBc binds to cccDNA and reportedly regulates viral RNA synthesis by epigenetic remodelling of the transcription complex.19,[21], [22], [23] To test whether HBc is functionally involved in viral RNA expression within an authentic infection model, we measured HBV RNA transcription (both pgRNA/pre-core mRNA and total HBV RNA) from WT and ΔHBc HBV-derived cccDNA using quantitative real time PCR. HepG2-NTCP A3 cells were infected with equal mge of both viruses. After establishment of cccDNA at 7 dpi RNA was extracted every week for 9 weeks. We found that WT and ΔHBc HBV expressed essentially identical levels of both pgRNA/pre-core mRNA and total HBV RNA over 9 weeks of infection (Fig. 5A). As controls for translation, we quantified HBsAg and HBeAg secretion (Fig. 5B). Consistent with the results of short-term infections, the HBsAg secretion of both viruses followed similar courses, while HBeAg was not expressed after infection with ΔHBc HBV. This also indicates that no reversion of the artificially-introduced stop codon had occurred during 9 weeks of infection. Again, all viral markers (besides HBsAg at 7 dpi, representing input) could be blocked by MyrB, verifying that cccDNA was established via authentic cell entry.
Fig. 5.
De novo HBc expression does not alter viral RNA transcription levels.
(A) HepG2-NTCP cells were infected with WT or ΔHBc HBV at 40 mge. Total cellular RNA was extracted from cells harvested each week and RT-qPCR was performed to detect HBV pgRNA/pre-core mRNA using primers targeting the pre-core region of the HBV genome (left) and total HBV RNA levels using primers targeting HBx reading frame (right). MyrB treatment (dashed lines) was used as control for infection inhibition (1, 4, and 9 weeks post inoculation). Two biological replicates were carried out (separate lines). Error bars (95% confidence interval) represent the technical error of the qPCR performed in triplicate. (B) Supernatant was collected every week and secreted HBsAg (left) and HBeAg (right) levels were measured. The mean of 2 biological replicates (±SD) is shown. Antigen results are representative of 2 independent experiments. HBV, hepatitis B virus; mge, multiplicity of genomic equivalents; NTCP, sodium taurocholate cotransporting polypeptide; WT, wild-type.
HBV DNA integration does not depend on HBc expression
We have shown that integration of HBV DNA into the host cell chromosome occurs early after infection and is not affected by NUC therapy.37 Thus, an independent measure of HBV DNA nuclear entry is the quantification of HBV DNA integrated into the host genome. Here, we used Huh7-NTCP cells, which we have previously shown to be a suitable system for quantifying HBV DNA integration.32,37 We confirmed that comparable levels of cccDNA are formed by WT or ΔHBc HBV in this system (Fig. S3).
Total cellular DNA was extracted from Huh7-NTCP cells after infection with 200 mge of WT or ΔHBc HBV and analysed by inverse nested PCR at 7 dpi (Fig. S4). We found that WT and ΔHBc HBV integrated at a geometric mean frequency (±geometric SD factor) of 4.82 × 10−5 (±2.36) and 5.97 × 10−5 (±1.57) integrations per cell (p = 0.4692, 2-tailed Mann-Whitney U test). No integrations were detected in DNA extracted from MyrB treated cells. These data underscore infrequent nuclear import of de novo produced nucleocapsids during infection.
Discussion
Our study provides strong experimental evidence that de novo HBV core protein produced from cccDNA (and, by extension, de novo produced rcDNA containing nucleocapsids) is not required to maintain HBV cccDNA copy numbers during long-term (>60 days) in vitro infection. Our data are consistent with past in vitro studies demonstrating that HBV-infected cells (including PHH) show no accumulation of cccDNA (typically 2–5 molecules/infected cell) even upon inoculation with very high mge (>500) for up to 2 weeks.29,36 Once formed, the levels of cccDNA are resistant to reverse transcriptase inhibitors and viral entry inhibitors, showing that cccDNA amplification through intracellular and extracellular means is generally limited in vitro.6,36,38 Our finding also confirms previous work that demonstrated that a more than genome length construct carrying a HBc stop mutation can be rescued by trans-complementation with a HBc expressing plasmid39 to produce replication-deficient virions.
The limited role of nuclear cycling of mature nucleocapsids is supported not only by the observations of cccDNA levels, but also by the results that HBV DNA integration rate (which similarly requires nuclear import of HBV DNA) is not affected by de novo HBV DNA production (Fig. S4). This is consistent with our37,40 and other41 previous work suggesting that integration occurs upon HBV infection establishment.
Our results however contrast with previous observations in the duck hepatitis B (DHBV) model, where reimport of mature nucleocapsids (produced from the initial wave of viral transcription following primary infection) is a key event for building up an intra-nuclear cccDNA pool with high copy numbers (typically 1–17 per cell, but greater in 10% of cells.14,42). Although others have shown the lack of nuclear import is virus-, not host-specific,43 the underlying molecular basis for these differences between avian and the human HBV remains unknown. We posit it may be linked to the evolutionary acquisition of the regulatory X-protein of HBV that counteracts cccDNA silencing, which is lacking in DHBV.44 We hypothesise that the lower cccDNA copy numbers in HBV may be compensated by X protein-mediated cccDNA stability and transcription activity. Whether there is a synergy between HBc and X protein on other epigenetic features of cccDNA remains an open question.
One important finding is that abrogation of de novo synthesis of HBc does not considerably influence transcription and stability of pregenomic and subgenomic HBV RNAs from cccDNA during long-term infection. HBV cccDNA has been observed to be physically associated with HBc in the nucleus of infected hepatocytes, leading to the hypothesis that HBc might episomally regulate cccDNA transcription.12 This hypothesis has never been proven experimentally and our results do not support HBc functionally altering cccDNA transcriptional activity. Our data suggests that de novo-expressed HBc is probably not involved in stabilising HBV cccDNA (Fig. 4) and preventing it from degradation. Finally, the lack of de novo-expressed HBc did not affect HBV transcription (Fig. 5), contrary to previous findings that were based on biochemical interaction studies22 and lack functional data obtained in authentic infection systems.
However, we cannot exclude that HBc may be altering the epigenetic make-up of cccDNA without affecting viral transcription or stability in non-dividing cultures. We also cannot determine from these experiments whether the 120 incoming core dimers per rcDNA may initially contribute to cccDNA formation and transcription.
Taking advantage of a long-term in vitro infection system, our results suggest a ‘static’ model of cccDNA maintenance in an infected hepatocyte (a stable episome without a dynamic turnover as long as the hepatocyte remains differentiated) rather than a ‘dynamic’ model where cccDNA levels depend on continuous degradation and replenishment (Fig. 1). They are also consistent with clinical experience showing that cccDNA levels do not profoundly change in NUC-treated chronic HBV patients with normal alanine aminotransferase (ALT) values, suggesting that cccDNA amplification is not active in these conditions.15
Such a dynamic has clinical implications on the emergence of NUC-resistant HBV variants in a patient. Given HBV variants must form cccDNA to be established in the liver, our data showing that internal replenishment of the cccDNA pool occurs in a limited manner suggest that any NUC-resistant variants must infect a new hepatocyte to emerge. As previously posited,45 this opens up the tantalising possibility of prophylactic prevention of NUC-resistance by treating patients with therapies that target early steps in viral infection (e.g. entry inhibitors or CIS that target incoming nucleocapsids).
It is important to note that the in vitro studies performed here do not completely rule out the possibility that cccDNA can be replenished in hepatocytes in the liver of an HBV-infected patient. However, the provided evidence indicates that cccDNA replenishment is likely a ‘salvage’ pathway to rescue an occasional loss of cccDNA (e.g. by the activity of nucleases) rather than a strong ‘driver’ for counteracting ongoing degradation. The static model of cccDNA maintenance is consistent with the clinical observation that HBV displays low quasispecies variation in patients not undergoing liver turnover, despite high levels of virus replication. Conversely, an active antiviral immune response and the resultant liver turnover is associated with greater HBV variation (see reviews24,25), presumably owing to greater turnover of cccDNA through loss via mitosis and replenishment through reinfection.
Different mechanisms to maintain cccDNA levels may be involved in a HBV-infected liver undergoing turnover (e.g. resultant from a cellular antiviral immune response). HBV cccDNA is lost with mitosis of the infected cell at least to some degree,[46], [47], [48], [49] suggesting that de novo infection is necessary for maintenance of infection in a liver undergoing turnover.24 Our data suggest that the effect of capsid assembly modulators on cccDNA in these situations is likely to be a result of suppression of virion production and new infection rather than inhibiting nuclear reimport of nucleocapsids in an infected hepatocyte.19,20,36
Thus, we posit that the reduction of cccDNA in a chronic HBV infection is most efficiently achieved with a multi-pronged approach. First, established cccDNA could be targeted by immunotherapies (e.g. pegylated interferon) stimulating either direct killing of infected hepatocytes or inducing liver turnover to promote cccDNA loss. Experimental clinical approaches to induce these ‘good’ ALT flares have been pursued (e.g. by cessation of NUC therapy50,51). The second crucial arm is the inhibition of new cccDNA formation either by prevention of ongoing hepatocyte infection (entry inhibitor therapy) or by disrupting incoming nucleocapsids (capsid inhibitors).
In conclusion, our findings provide evidence that de novo HBV capsids (and by extension, de novo generated nucleocapsids and virus genomes) play a limited role in cccDNA maintenance, stability, and cccDNA gene expression in cells with an already established HBV infection. This supports the hypothesis that cccDNA turnover is associated more with the turnover of the infected hepatocytes and that therapeutic targeting of the intracellular reimport of progeny nucleocapsids leads to limited cccDNA elimination.
Financial support
This work received funding from the German Center for Infection Research (DZIF) TTU Hepatitis Projects 5.807 and 5.704 (T.T. and S.U.), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 240245660 – SFB 1129 (TP 16) (B.Z. and S.U.) and – Projektnummer 272983813 – SFB/TRR179 (TP 15) (B.Q. and S.U.), and the Australian Centre for HIV and Hepatitis Virology Research (T.T.).
Authors' contributions
Conceived the concept of the project: S.U., T.T., B.Z.
Designed and carried out the experiments, analysed the data, generated figures and wrote the manuscript: T.T., B.Z.
Carried out Southern blot hybridisation and RT-qPCR studies, generated figures, and assisted in writing the manuscript: B.Q.
Provided funding, contributed intellectual input toward the project and experimental design, and did final editing of the manuscript: S.U.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials. Any additional data are available from the corresponding author SU upon reasonable request.
Conflict of interest
S.U. is co-applicant and co-inventor on patents protecting HBV preS-derived lipopeptides (Myrcludex B) for the use of HBV/HDV entry inhibitors. The other authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Dr Yi Ni, Dr Florian A. Lempp, Dr Kerry Mills, Dr Christine Bekker, and Dr Stefan Seitz for reagents (plasmids, cell lines, and HBV inoculum); PD Dr med. Florian WR Vondran for providing the PHH, and Lisa Walter, Anja Rippert, Franziska Schlund, and Dr Christa Kuhn for technical support. We are grateful to Miriam Kleinig for proofreading, and Prof. Dr Ralf Bartenschlager for continuous support.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2020.100195.
Supplementary data
References
- 1.WHO . World Health Organization; Geneva: 2017. Global Hepatitis Report 2017. [Google Scholar]
- 2.Polaris Observatory Collaborators Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 3.Tu T., Block J.M., Wang S., Cohen C., Douglas M.W. The lived experience of chronic hepatitis B: a broader view of its impacts and why we need a cure. Viruses. 2020;12:515. doi: 10.3390/v12050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu C.-M., Liaw Y.-F. Natural history of hepatitis B virus infection. In: Liaw Y.-F., Zoulim F., editors. Hepatitis B Virus in Human Diseases. Springer; Cham: 2016. pp. 217–247. [Google Scholar]
- 5.Seeger C., Litwin S., Mason W.S. Hepatitis B virus: persistence and clearance. In: Liaw Y.-F., Zoulim F., editors. Hepatitis B Virus in Human Diseases. Springer; Cham: 2016. pp. 123–145. [Google Scholar]
- 6.Tu T., Zehnder B., Qu B., Ni Y., Main N., Allweiss L. A novel method to precisely quantify hepatitis B virus covalently closed circular (ccc)DNA formation and maintenance. Antivir Res. 2020;181:104865. doi: 10.1016/j.antiviral.2020.104865. [DOI] [PubMed] [Google Scholar]
- 7.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Falth M. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabe B., Vlachou A., Pante N., Helenius A., Kann M. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc Natl Acad Sci U S A. 2003;100:9849–9854. doi: 10.1073/pnas.1730940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seeger C., Mason W.S. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480:672–686. doi: 10.1016/j.virol.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu T., Budzinska M.A., Shackel N.A., Urban S. HBV DNA integration: molecular mechanisms and clinical implications. Viruses. 2017;9:75. doi: 10.3390/v9040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bock C.T., Schwinn S., Locarnini S., Fyfe J., Manns M.P., Trautwein C. Structural organization of the hepatitis B virus minichromosome. J Mol Biol. 2001;307:183–196. doi: 10.1006/jmbi.2000.4481. [DOI] [PubMed] [Google Scholar]
- 13.Seitz S., Iancu C., Volz T., Mier W., Dandri M., Urban S. A slow maturation process renders hepatitis B virus infectious. Cell Host Microbe. 2016;20:25–35. doi: 10.1016/j.chom.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Wu T.T., Coates L., Aldrich C.E., Summers J., Mason W.S. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 15.Sherman M., Yurdaydin C., Sollano J., Silva M., Liaw Y.F., Cianciara J. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039–2049. doi: 10.1053/j.gastro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Werle-Lapostolle B., Bowden S., Locarnini S., Wursthorn K., Petersen J., Lau G. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Yang L., Lu M. Small molecule inhibitors of hepatitis b virus nucleocapsid assembly: a new approach to treat chronic HBV infection. Curr Med Chem. 2018;25:802–813. doi: 10.2174/0929867324666170704121800. [DOI] [PubMed] [Google Scholar]
- 18.Nijampatnam B., Liotta D.C. Recent advances in the development of HBV capsid assembly modulators. Curr Opin Chem Biol. 2019;50:73–79. doi: 10.1016/j.cbpa.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Lahlali T., Berke J.M., Vergauwen K., Foca A., Vandyck K., Pauwels F. Novel potent capsid assembly modulators regulate multiple steps of the hepatitis B virus life cycle. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00835-18. e00835-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berke J.M., Dehertogh P., Vergauwen K., Van Damme E., Mostmans W., Vandyck K. Capsid assembly modulators have a dual mechanism of action in primary human hepatocytes infected with hepatitis B virus. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00560-17. e00560-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y.H., Li Y.N., Zhao J.R., Zhang J., Yan Z. HBc binds to the CpG islands of HBV cccDNA and promotes an epigenetic permissive state. Epigenetics. 2011;6:720–726. doi: 10.4161/epi.6.6.15815. [DOI] [PubMed] [Google Scholar]
- 22.Chong C.K., Cheng C.Y.S., Tsoi S.Y.J., Huang F.Y., Liu F., Seto W.K. Role of hepatitis B core protein in HBV transcription and recruitment of histone acetyltransferases to cccDNA minichromosome. Antivir Res. 2017;144:1–7. doi: 10.1016/j.antiviral.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Belloni L., Palumbo G.A., Lupacchini L., Li L., Reddy Chirapu S., Calvo L. P0529: anti capsid drugs HAP12 and AT130 target HBV core protein nuclear functions. J Hepatol. 2015;62:S513–S514. [Google Scholar]
- 24.Tu T., Urban S. Virus entry and its inhibition to prevent and treat hepatitis B and hepatitis D virus infections. Curr Opin Virol. 2018;30:68–79. doi: 10.1016/j.coviro.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Ni Y. University of Heidelberg; Heidelberg: 2010. Characterization of the Infectivity Determinants of the Envelope Proteins of Hepatitis B Virus. [Google Scholar]
- 26.Engelke M., Mills K., Seitz S., Simon P., Gripon P., Schnolzer M. Characterization of a hepatitis B and hepatitis delta virus receptor binding site. Hepatology. 2006;43:750–760. doi: 10.1002/hep.21112. [DOI] [PubMed] [Google Scholar]
- 27.Ni Y., Sonnabend J., Seitz S., Urban S. The pre-s2 domain of the hepatitis B virus is dispensable for infectivity but serves a spacer function for L-protein-connected virus assembly. J Virol. 2010;84:3879–3888. doi: 10.1128/JVI.02528-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleine M., Riemer M., Krech T., DeTemple D., Jager M.D., Lehner F. Explanted diseased livers - a possible source of metabolic competent primary human hepatocytes. PLoS One. 2014;9:e101386. doi: 10.1371/journal.pone.0101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulze A., Mills K., Weiss T.S., Urban S. Hepatocyte polarization is essential for the productive entry of the hepatitis B virus. Hepatology. 2012;55:373–383. doi: 10.1002/hep.24707. [DOI] [PubMed] [Google Scholar]
- 30.Gripon P., Rumin S., Urban S., Le Seyec J., Glaise D., Cannie I. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu T., Jilbert A.R. Detection of hepatocyte clones containing integrated hepatitis B virus DNA using inverse nested PCR. Methods Mol Biol. 2017;1540:97–118. doi: 10.1007/978-1-4939-6700-1_9. [DOI] [PubMed] [Google Scholar]
- 32.Tu T., Urban S. Detection of low copy number integrated viral DNA formed by in vitro hepatitis B infection. J Vis Exp. 2018;141:e58202. doi: 10.3791/58202. [DOI] [PubMed] [Google Scholar]
- 33.Cai D., Nie H., Yan R., Guo J.T., Block T.M., Guo H. A southern blot assay for detection of hepatitis B virus covalently closed circular DNA from cell cultures. Methods Mol Biol. 2013;1030:151–161. doi: 10.1007/978-1-62703-484-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucifora J., Xia Y., Reisinger F., Zhang K., Stadler D., Cheng X. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu B., Ni Y., Lempp F.A., Vondran F.W.R., Urban S. T5 exonuclease hydrolysis of hepatitis B virus replicative intermediates allows reliable quantification and fast drug efficacy testing of covalently closed circular DNA by PCR. J Virol. 2018;92:e01117–e01118. doi: 10.1128/JVI.01117-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu T., Budzinska M.A., Vondran F.W.R., Shackel N.A., Urban S. Hepatitis B virus DNA integration occurs early in the viral life cycle in an in vitro infection model via sodium taurocholate cotransporting polypeptide-dependent uptake of enveloped virus particles. J Virol. 2018;92:e02007–e02017. doi: 10.1128/JVI.02007-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Y., Carpentier A., Cheng X., Block P.D., Zhao Y., Zhang Z. Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions. J Hepatol. 2017;66:494–503. doi: 10.1016/j.jhep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi Y., Gao Z., Xu G., Peng B., Liu C., Yan H. DNA polymerase kappa is a key cellular factor for the formation of covalently closed circular DNA of hepatitis B virus. PLoS Pathog. 2016;12:e1005893. doi: 10.1371/journal.ppat.1005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tu T., Mason W.S., Clouston A.D., Shackel N.A., McCaughan G.W., Yeh M.M. Clonal expansion of hepatocytes with a selective advantage occurs during all stages of chronic hepatitis B virus infection. J Viral Hepat. 2015;22:737–753. doi: 10.1111/jvh.12380. [DOI] [PubMed] [Google Scholar]
- 41.Mason W.S., Gill U.S., Litwin S., Zhou Y., Peri S., Pop O. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology. 2016;151:986–998.e4. doi: 10.1053/j.gastro.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y.Y., Zhang B.H., Theele D., Litwin S., Toll E., Summers J. Single-cell analysis of covalently closed circular DNA copy numbers in a hepadnavirus-infected liver. Proc Natl Acad Sci U S A. 2003;100:12372–12377. doi: 10.1073/pnas.2033898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kock J., Rosler C., Zhang J.J., Blum H.E., Nassal M., Thoma C. Generation of covalently closed circular DNA of hepatitis B viruses via intracellular recycling is regulated in a virus specific manner. PLoS Pathog. 2010;6:e1001082. doi: 10.1371/journal.ppat.1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decorsiere A., Mueller H., van Breugel P.C., Abdul F., Gerossier L., Beran R.K. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386–389. doi: 10.1038/nature17170. [DOI] [PubMed] [Google Scholar]
- 45.Revill P.A., Tu T., Netter H.J., Yuen L.K.W., Locarnini S.A., Littlejohn M. The evolution and clinical impact of hepatitis B virus genome diversity. Nat Rev Gastroenterol Hepatol. 2020;17:618–634. doi: 10.1038/s41575-020-0296-6. [DOI] [PubMed] [Google Scholar]
- 46.Allweiss L., Volz T., Giersch K., Kah J., Raffa G., Petersen J. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut. 2018;67:542–552. doi: 10.1136/gutjnl-2016-312162. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y., Yamamoto T., Cullen J., Saputelli J., Aldrich C.E., Miller D.S. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311–322. doi: 10.1128/JVI.75.1.311-322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dandri M., Burda M.R., Will H., Petersen J. Increased hepatocyte turnover and inhibition of woodchuck hepatitis B virus replication by adefovir in vitro do not lead to reduction of the closed circular DNA. Hepatology. 2000;32:139–146. doi: 10.1053/jhep.2000.8701. [DOI] [PubMed] [Google Scholar]
- 49.Reaiche-Miller G.Y., Thorpe M., Low H.C., Qiao Q., Scougall C.A., Mason W.S. Duck hepatitis B virus covalently closed circular DNA appears to survive hepatocyte mitosis in the growing liver. Virology. 2013;446:357–364. doi: 10.1016/j.virol.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Rinker F., Zimmer C.L., Honer Zu Siederdissen C., Manns M.P., Kraft A.R.M., Wedemeyer H. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69:584–593. doi: 10.1016/j.jhep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Honer Zu Siederdissen C., Rinker F., Maasoumy B., Wiegand S.B., Filmann N., Falk C.S. Viral and host responses after stopping long-term nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Infect Dis. 2016;214:1492–1497. doi: 10.1093/infdis/jiw412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials. Any additional data are available from the corresponding author SU upon reasonable request.