Abstract
Introduction
Women living with HIV are required to transition into the prevention of mother‐to‐child transmission of HIV (PMTCT) services when they become pregnant and back to ART services after delivery. Transition can be a vulnerable time when many women are lost from HIV care yet there is little guidance on the optimal transition approaches to ensure continuity of care. We reviewed the available evidence on existing approaches to transitioning women into and out of PMTCT, outcomes following transition and factors influencing successful transition.
Methods
We searched PubMed and SCOPUS, as well as abstracts from international HIV‐focused meetings, from January 2006 to July 2020. Studies were included that examined three points of transition: pregnant women already on ART into PMTCT (transition 1), pregnant women living with HIV not yet on ART into treatment services (transition 2) and postpartum women from PMTCT into general ART services after delivery (transition 3). Results were grouped and reported as descriptions of transition approach, comparison of outcomes following transition and factors influencing successful transition.
Results & discussion
Out of 1809 abstracts located, 36 studies (39 papers) were included in this review. Three studies included transition 1, 26 transition 2 and 17 transition 3. Approaches to transition were described in 26 studies and could be grouped into the provision of information at the point of transition (n = 8), strengthened communication or linkage of data between services (n = 4), use of transition navigators (n = 12), and combination approaches (n = 4). Few studies were designed to directly assess transition and only nine compared outcomes between transition approaches, with substantial heterogeneity in study design, setting and outcomes. Four themes were identified in 25 studies reporting on factors influencing successful transition: fear, knowledge and preparedness, clinic characteristics and the transition requirements and process.
Conclusions
This review highlights that, despite the need for women to transition into and out of PMTCT services for continued ART in many settings, there is very limited evidence on optimal transition approaches. Ongoing operational research is required to identify sustainable and acceptable transition approaches and service delivery models that support continuity of HIV care during and after pregnancy.
Keywords: transition, transfer, prevention of mother‐to‐child transmission, linkage, continuity of care, antiretroviral therapy
1. INTRODUCTION
Sustained engagement in antiretroviral therapy (ART) services during and after pregnancy is critical for the prevention of mother‐to‐child transmission (PMTCT) of HIV and to optimize maternal health. High rates of loss to follow‐up from ART services have been noted among pregnant and postpartum women living with HIV(WLHIV) [1, 2].
The first programmatic guidance for ART for women’s own health was released by the World Health Organisation (WHO) in 2006 [3]. This prompted the scale‐up of the provision of ART to women diagnosed with HIV during pregnancy who met the CD4 cell count requirements. If eligible, women were usually referred from antenatal care (ANC) to general ART services for treatment. In addition to missing eligible women due to poor access and delays with CD4 testing, many identified as ART eligible did not successfully transition to initiate ART prior to delivery [4, 5]. Integrated ANC and ART services (ANC/ART) were subsequently recommended, greatly improving the uptake of ART during pregnancy [6]. Similarly, continued integrated maternal ART and child health services postpartum, including early infant HIV diagnosis, have been associated with improved maternal and child health outcomes [7, 8, 9]. In the current context of universal ART, WLHIV should remain on effective ART for life, with the addition of PMTCT and routine maternal health services during periods of pregnancy and breastfeeding. However, even with universal ART and integrated ANC, maternal ART and child health services, most PMTCT services are not designed to keep mothers in care indefinitely and women will transition from PMTCT to general ART clinics at some point after delivery. Women already established on ART when they conceive need to transition into PMTCT services either in a stand‐alone ANC clinic or an integrated ANC/ART clinic for the duration of pregnancy with transition back to general ART care postpartum. A framework to describe the transitions for continued ART among women moving into and out of PMTCT is depicted in Figure 1. This cycle may occur multiple times for WLHIV, particularly in settings with high fertility rates.
Figure 1.
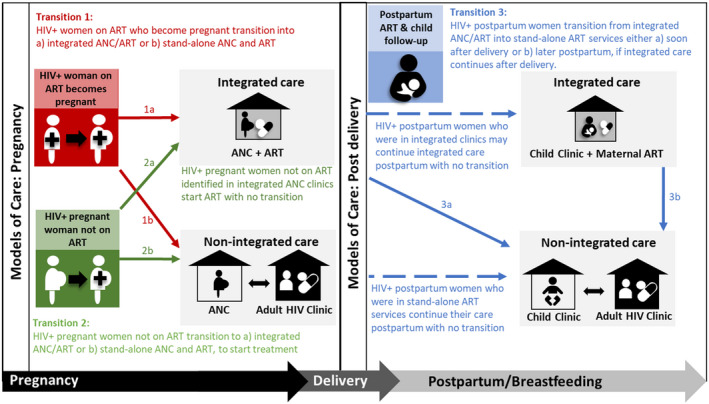
Transition points, models of care and timing of transition into and out of PMTCT for pregnant and postpartum women. Dashed lines represent continued care with no transition needed. ART, antiretroviral therapy; ANC, antenatal care.
Approaches to these transitions are not consistent and few countries have specific guidelines on transitions for HIV care during and after pregnancy [10, 11]. Although integrated ANC/ART services for pregnant women are recommended by the WHO [12], this model has not been adopted in all countries and some pregnant women still attend separate ART and ANC clinics. Furthermore, the timing of postpartum transition from PMTCT to general HIV services is variable and ranges from about six to ten weeks postpartum in South Africa and parts of Malawi, to up to two years postpartum in Kenya, Zimbabwe and Mozambique [11, 13, 14, 15, 16, 17, 18]. Successful transition and continuity of care for women on ART is critical to ensure their long‐term health, however, it is not clear how this step in the HIV care cascade is addressed in many settings.
This review aimed to synthesize the available evidence on existing approaches to transitioning women into and out of PMTCT services for continued ART. We examined approaches described in the literature and the evidence for which transition approaches improve outcomes of linkage to ART initiation, linkage to continued ART or retention in care following a transition. In addition, we examined factors that may influence successful transition into or out of PMTCT services.
2. METHODS
This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [19]. A comprehensive electronic search was conducted online in PubMed and SCOPUS. Conference abstracts from major international HIV‐focused meetings (Conference on Retroviruses and Opportunistic Infections, International AIDS Society Conference on HIV Science, International AIDS Conference, International Workshops on HIV Pediatrics, Women and HIV, Adolescents and HIV) were also searched, but none were suitable for inclusion.
Search terms and MeSH (medical subject heading) equivalents were searched using the search strategy: (HIV OR “human immunodeficiency virus”) AND (“Antiretroviral therapy” OR ART OR “antiretroviral treatment” OR “anti‐retroviral therapy” OR antiretroviral OR ARV OR “prevention of mother‐to‐child transmission” OR PMTCT OR “HIV care”) AND (pregnancy OR pregnant OR antenatal OR perinatal OR maternal OR postpartum OR postnatal) AND (transition OR transfer OR refer OR link OR linked OR linkage OR “model of care” OR integrated OR integration OR cascade OR continuum).
English language publications from January 2006 through July 2020 were included with no restrictions placed on study design or geographic location. While 2003 WHO guidance included pregnant and breastfeeding women, scale‐up of lifelong ART in the context of PMTCT was not included until 2006 [3]. Studies launched in 2005, but with most data collection in the review period were also included. Studies that did not contain information or data specific to the transition of women into or out of PMTCT services were excluded. As such, studies reporting on outcomes in integrated services with no transition were not included. Investigators were not contacted for further details. Letters, editorials, commentaries and review articles were excluded, but reference lists were checked to identify missed papers. Where more than one published paper described the same study cohort, multiple papers were included if unique information was presented in each, otherwise only the paper with the most complete data on transition approach was included.
Titles and abstracts of the studies were merged and de‐duplicated in Mendeley and screened independently by two reviewers. Full texts of all studies meeting inclusion criteria were reviewed independently by both reviewers. Reviewer disagreement was resolved through third party discussion, as needed.
Studies not focused on transition, but that described an approach to transition in the methods or results were also included. Models of care transitioned between (stand‐alone ART vs. ART integrated ANC, HIV and/or childcare) were also extracted. Since most studies included were not primarily focused on transition but only described transition or outcomes after transition in the context of other primary research questions, the quality of evidence was not included in this review.
Through discussion and consensus with all authors, three possible transition points into or out of PMTCT services for continued maternal ART were defined (Figure 1). The location of childcare is included in the figure; while not the focus of this review, it is strongly linked to maternal postpartum HIV care. Transition 1 is when women on ART in general HIV services transition into integrated ANC/ART or stand‐alone services when they conceive. Transition 2 is when pregnant women not yet on ART (either newly diagnosed or known HIV‐positive) transition into either integrated ANC/ART services or stand‐alone ART services to initiate treatment. Transition 3 is after delivery when women who have been in integrated ANC/ART services transition into general ART services either soon after delivery or later postpartum if integrated care is continued after delivery. Depending on the model of care, a transition may not be needed. For instance women in non‐integrated care during pregnancy remain in stand‐alone ART services postpartum.
The number of studies reporting on transitions at each of these points was enumerated and results were grouped and described as follows:
2.1. Description of transition approaches
All studies that described approaches to transition into or out of PMTCT services for ART were included and thematically grouped into similar strategies. Outcomes following a point of transition (where reported) were also described. Possible outcomes included: (1) successful linkage to care after transition, (2) ART initiation during pregnancy after transition from ANC to general ART services (transition 2 only) and (3) retention in care after transition.
2.2. Comparison of outcomes between two or more transition approaches
We included all studies that compared any of the above‐mentioned outcomes after the transition between two or more transition approaches, including proportions of women experiencing the outcome and relative measures of association, where available. Due to the very small number of studies comparing outcomes between different approaches to transition and the heterogeneity in outcome type, timing and definition, no pooled estimates were calculated.
2.3. Factors influencing successful transition
All studies, both quantitative and qualitative, exploring barriers or facilitators to transitioning into or out of PMTCT services were included with factors grouped by theme. We described factors reported to influence the transition process or successful transition, but factors related to other outcomes such as ART initiation and retention were excluded.
3. RESULTS
The initial search located 1809 abstracts (Figure 2). After removing duplicates (n = 449), review and commentary articles (n = 60) and unrelated titles and abstracts (n = 1030), 269 full‐text articles remained. After review, 224 were excluded as they did not pertain to transition into or out of PMTCT for ART, were study protocols, or were secondary papers from cohorts already included. Among these were 53 papers that mentioned that a transition took place, but did not present relevant data. Thus, 39 papers met the inclusion criteria: 22 quantitative, 11 qualitative and 6 mixed methods studies. Two studies reported relevant transition data in two or more papers; thus 36 unique studies were included.
Figure 2.
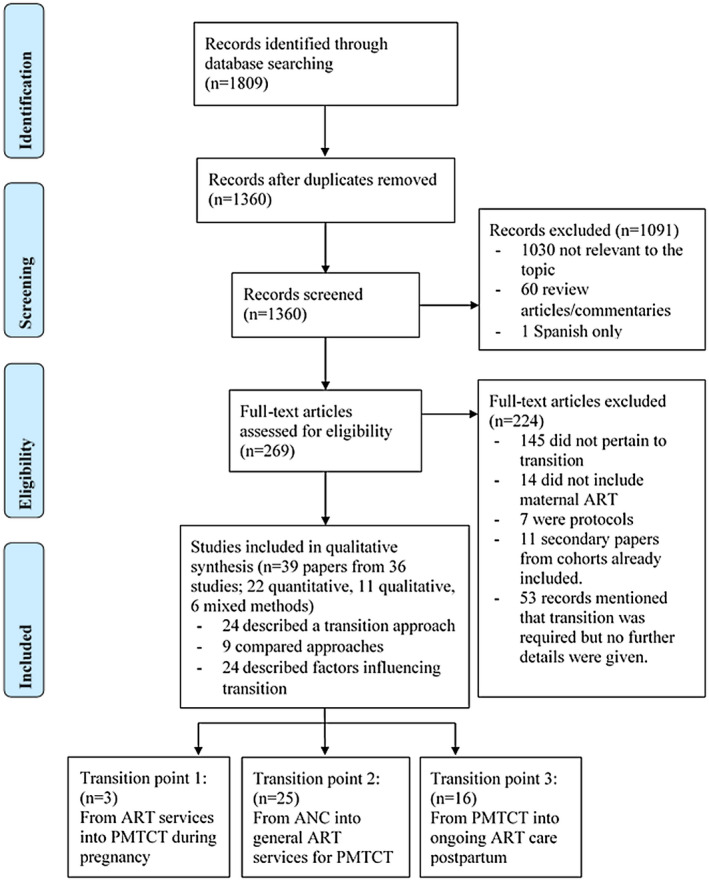
PRISMA flow diagram summarising the literature search. Note each included study may appear in multiple results groups and transition points. ANC, antenatal care; ART, antiretroviral therapy; PMTCT, prevention of mother‐to‐child transmission of HIV.
A summary of all included studies is presented in Table S1, arranged by the points of transition reported in each study. The study periods ranged from 2005 to 2018 and included data from Botswana, Cambodia, Cote de’Ivoire, Democratic Republic of Congo, India, Kenya, Malawi, Mozambique, Myanmar, Nigeria, Rwanda, South Africa, Tanzania, Uganda, the United States and Zambia. Overall, three studies include transition 1, 25 transition 2 and 16 transition 3. Twenty‐four studies described factors that may influence transition into or out of maternal ART.
3.1. Description of transition approaches
In 24 studies that described a transition approach, the approaches were grouped broadly into (1) information provided at the point of transition (transfer letters and counselling), (2) strengthened communication or data linkage between services, (3) transition navigators or (4) a combination of approaches. The approach description along with outcomes after transition (where reported) is presented in Table 1.
Table 1.
Description of approaches to transitioning women into or out of PMTCT for continued ART (24 studies)
| First author, year | Study period | Location | Description of transition approach | Linked to the new clinic after any transition | Initiated ART (only for transition point 2) | Retained in HIV care after any transition | ||
|---|---|---|---|---|---|---|---|---|
| Information given at the point of transition (transfer letters and counselling or instructions) | ||||||||
| Transition point 2 | Stinson, 2013 [20] | 2008 | South Africa, urban (three clinics, 658 women) | Pregnant women eligible for ART in ANC were provided with a transfer letter to either the adult HIV clinic on the same premises as ANC or to a distal, stand‐alone HIV clinic | Not reported |
|
Not reported | |
| Mugasha, 2014 (rural group) [21] | 2012 | Uganda, rural (three clinics, 267 women) | Pregnant women eligible for ART in a rural ANC clinic were provided with a transfer letter and given directions to the adult HIV clinic | 25% enrolled in the HIV clinic by six weeks postpartum | Not reported | Not reported | ||
| Price, 2014 [22] | 2011 to 2013 | Malawi, rural (nine clinics, 395 women) | Pregnant women in clinics not offering integrated ANC and ART were provided with a transfer letter to an adult HIV clinic | Outcomes not disaggregated by women who transferred to an ART clinic and those who received integrated care | Outcomes not disaggregated by women who transferred to an ART clinic and those who received integrated care | Not reported | ||
| Myer, 2015 (standard of care group) [23] | 2010 to 2013 | South Africa, urban (one clinic, 1615 women) | Pregnant women eligible for ART in ANC were transferred to their nearest adult HIV clinic with a transfer letter and counselling to attend the clinic | Not reported | 23% initiated ART during pregnancy | Not reported | ||
| Transition point 3 | Otieno, 2010 [24] | 2005 | Kenya, urban (four clinics, 239 women) | Women who had received integrated ANC and ART during pregnancy, and integrated childcare and ART postpartum during breastfeeding were provided with a transfer letter to attend their nearest adult HIV clinic at two years postpartum | 74% reported attending the transfer clinic (median 17 months post‐transfer) | NA | 63% of those who linked to the new clinic retained at a median of 17 months after the transition | |
| Phillips, 2015 [13] | 2013 | South Africa, urban (one clinic, 279 women) | Women who had received integrated ANC and ART during pregnancy were transferred to their nearest adult HIV clinic at six weeks postpartum with a standard transfer letter and counselling on attending the ART clinic | 74% linked to an ART clinic within a year of transfer | NA | Not reported | ||
| Cheshi, 2019 [25] | 2014 | Nigeria (two hospitals, 372 women) |
Women were referred from PMTCT to continue ART at ART services by either written referral letter or oral referral. The referral may have been done by a doctor, nurse or counsellor |
15% of women did not link to ART services | Not applicable | Not reported | ||
| Phillips, 2018 [26] and Phillips, 2020 [27] | 2013 to 2016 | South Africa, urban (one clinic, 617 women) | Women who had received integrated ANC and ART during pregnancy were either transferred to an adult HIV clinic at six weeks postpartum or they received integrated childcare and ART until cessation of breastfeeding and then were transferred to an adult HIV clinic. At transfer women received a standard transfer letter. | 79% linked to continued ART care (82% of those transferred six weeks postpartum and 74% of those transferred after cessation of breastfeeding) | NA |
75% of those who linked to a new clinic retained through 24 months on ART (59% in care through 24 months overall) 57% and 45% experienced no 180‐day gap in care by 36 months postpartum |
||
| Strengthened communication or data linkage between services | ||||||||
| Transition point 1 | Rawizza, 2015 [28] | 2015 | Nigeria (31 clinics, 31 504 women) | Pregnant women who were established on ART and became pregnant had their ART identifier, maternal PMTCT identifier and baby identifier linked to facilitate linkage between different services. | Not reported | Not reported | Not reported | |
| Transition point 2 | Gupta, 2016 [29] | 2014 | India, urban and rural (578 testing sites, 70 HIV clinics, 1118 women) | Pregnant women who tested HIV positive in ANC were entered into an electronic line listing to track service attendance through pregnancy and up to 18 months postpartum. Each testing centre (578 included) had their own listing of pregnant women which was shared with the HIV clinics (70 included). | Not reported | 91% of women started ART in pregnancy. | Not reported | |
| Kyaw, 2019 [30] | 2012 to 2017 | Myanmar, urban (five clinics, 303 women) | A nurse allocated as PMTCT focal person for Township Health Department and a medical doctor assigned by the district as responsible for PMTCT and for documenting linkage to care. The doctor collects a weekly list of women newly registered from the PMTCT focal person and searches for them in the electronic HIV clinic databases to ensure linkage. | Not reported | 84% of women started ART. Median 20 (10 to 51) days between diagnosis and referral. | Not reported | ||
| Transition navigator | ||||||||
| Transition point 1 and 2 | Geldsetzer, 2019 [31] | 2012 to 2014 | Tanzania, urban (36 wards) | Community health workers actively identify pregnant women in the community through home visits, refer to ANC and PMTCT, and follow‐up to check if women linked to care. | Results were not disaggregated for women living with and without HIV | Not reported | Not reported | |
| Transition point 2 | Stover, 2019 [32] | 2014 to 2015 | Mozambique (39 communities) | Community improvement team set up to share information about antenatal care and actively identify pregnant women in the community. The community group provides a referral form and informs the antenatal clinic when a woman is identified who then monitor whether she has attended or not. | Results were not disaggregated for women living with and without HIV | 100% of women initiated ART. No change in ART initiations over time | Not reported | |
| Tsague, 2010 [33] | 2006 to 2008 | Rwanda (urban and rural) |
Pregnant women eligible for ART in ANC were either escorted to the adult HIV clinic on the same premises as ANC or were transported to a stand‐alone adult HIV clinic. |
|
|
Not reported | ||
| Ferguson, 2014 [5] | 2010 | Kenya, urban (1 district hospital, 100 women) | Pregnant women eligible for ART in ANC were accompanied by the ANC nurse to the adult HIV clinic on the same premises as ANC. | 53% of women registered at the HIV clinic | Not reported | Not reported | ||
| Myer, 2015 (enhanced linkage group) [23] | 2010 to 2013 | South Africa, urban (one clinic, 1615 women) | Pregnant women eligible for ART in ANC were accompanied by a patient navigator to enrol at the adult HIV clinic on the same premises as ANC. | Not reported | 45% initiated ART during pregnancy | Not reported | ||
| Kojima, 2017 [34] | 2008 to 2011 | India, rural (three mobile and 1 standing clinic) | Pregnant women eligible for ART in ANC were accompanied by a health provider to the tertiary care facility where ART was provided. | Not reported | Not reported | Not reported | ||
| Suryavanshi, 2018 [35] | 2015 to 2016 | India, urban and rural (four districts, 60 outreach workers) | Pregnant women eligible for ART in ANC were accompanied by outreach workers to the adult HIV clinic. The outreach workers also provided appointment reminders and follow‐up through 18 months postpartum. | Not reported | Not reported | Not reported | ||
| Transition point 3 | Hackett, 2019 [36] | 2011 to 2017 | USA, urban (one clinic, 275 women) |
A multidisciplinary perinatal care coordination team meet monthly to determine case plans for women from prenatal care through transition to HIV care postpartum. Specifically:
|
|
|
||
| Transition point 2 and 3 | Killam, 2010 [37] | 2007 to 2008 | Zambia, urban (eight clinics, 1566 women) | Women eligible for ART in ANC were either transitioned to an adult HIV clinic in pregnancy or received integrated ANC and ART with transition to an adult HIV clinic at six weeks postpartum. At the transfer, women were escorted by a peer educator to the ART clinic on the same premises as the ANC clinic. Outcomes after transition not assessed in the integrated care group. | Only assessed among women who transitioned during pregnancy, 25% of whom enrolled in the HIV clinic |
|
Among women who started ART, 91% of women who had transitioned during pregnancy were retained after 90 days compared to 88% of women who received integrated ANC and ART. | |
| Anderson, 2017 [38] | 2005 to 2013 | USA (849 births) | Voluntary enrolment in a perinatal case management program. Medical case managers assist with navigating the complex health system and provide psychosocial support. | 42.9% linked to HIV care within 90 days of delivery | Not reported | 52.7% retained in care at one year postpartum | ||
| All transition points | Besada, 2018 [39] | 2015 | Cote D’Ivoire, DRC, Malawi, Uganda (urban and rural) | Community cadres were employed to assist pregnant and postpartum women with navigating to the required pregnancy, PMTCT and postnatal services across any required transitions. | Not reported | Not reported | Not reported | |
| Combination of approaches | ||||||||
| Transition point 2 | Weigel, 2012 [40] | 2006 to 2009 | Malawi, urban (one hospital and two clinics, 612 women) |
Pregnant women eligible for ART during ANC were transferred to adult HIV clinics. Over time the following approaches were employed:
|
|
|
|
|
| Mugasha, 2014 (urban group) [21] | 2012 | Uganda, urban (two clinics, 743 women) |
Pregnant women eligible for ART in urban ANC clinics were transferred to an adult HIV clinic. They were:
|
41% enrolled in the HIV clinic by six weeks postpartum | Not reported | Not reported | ||
| Saleem, 2014 [41] | 2012 | Uganda (11 clinics, 48 women, 11 providers) |
Pregnant women eligible for ART in ANC were transferred to adult HIV clinics with:
|
Not reported | Not reported | Not reported | ||
| Transition point 3 | Myer, 2017 [42] | 2015 | South Africa, urban (one clinic, 129 women) |
Women who had received integrated ANC and ART during pregnancy were transferred to an adult HIV clinic soon after delivery. They were:
|
Not reported | Not applicable | Not reported | |
If studies had multiple arms, we have only included here the arms or components that required a transition. ANC, antenatal care; ART, antiretroviral therapy; NA, not applicable; PMTCT, prevention of mother‐to‐child transmission of HIV.
3.2. Information provided at the point of transition
Eight studies described the use of letters to facilitate transition: four from ANC to HIV services to start ART (transition 2) in South Africa [20, 23], Malawi [22] and Uganda [21], and four from integrated ANC/ART services to a general ART clinic after delivery (transition 3) in South Africa [13, 26], Nigeria [25] and Kenya [24]. None provided the content of the transfer letter. Three studies reported that counselling or instructions on the transition process were provided at the point of transition with few details on pre‐transition counselling. In Nigeria, women received a written or a verbal referral from either a doctor, nurse, counsellor or laboratory staff [25]. Overall, 15% of women did not link to continued ART postpartum. The Ugandan study specified that, in addition to a transfer letter, women transitioning to an HIV clinic to start ART in a rural area were given directions to the clinic [21]. In this study, 25% of women successfully transitioned to the general ART clinic to initiate ART before six weeks postpartum. One Kenyan [24] and two South African [13, 26] studies assessing transition 3 reported 74% to 79% of women successfully linked from PMTCT to general ART clinics to continue care postpartum. Among women who successfully linked to the ART clinic, 63% was retained at a median of 17 months after transition in the Kenyan study [24] and 75% was retained through 24 months on ART in South Africa [26]. In a follow‐up to the South Africa study reporting on retention overall, 45% to 57% of women remained in care with no gap through 36 months postpartum [27]. ART initiation during pregnancy after transition 2 ranged from 23% to 45% following transition from ANC to a general ART clinic with a transfer letter and counselling provided at the point of transition in two South African studies [20, 23].
3.3. Strengthened communication or data linkage between services
Linking patient data between different services was used to assist the transition between clinics in studies in India [29], Myanmar [30] and Nigeria [28]. In Myanmar, direct linkage was established through a PMTCT focal nurse for ANC facilities and a district PMTCT doctor assigned to document linkage to HIV care during pregnancy [30]. During the study period 84% of women started ART with the median 20 days between diagnosis and referral. In India, a stand‐alone electronic line‐listing of all WLHIV presenting for ANC was shared between clinics to facilitate tracing of women through pregnancy and up to 18 months postpartum [29]. In Nigeria, pregnant women already enrolled on ART received unique PMTCT patient identifiers to allow records to be linked across all services [28]. PMTCT identifiers were linked to adult ART and infant patient identifiers in the electronic medical record.
3.4. Transition navigators
There were 11 studies describing the use of transition navigators conducted in India [34, 35], South Africa [23], Rwanda [33], Zambia [37], Tanzania [31], Mozambique [32], Kenya [5], the United States [36, 38], and a multisite study in Cote D’Ivoire, Democratic Republic of Congo, Malawi and Uganda [39]. Studies from Kenya and India described an approach where healthcare providers escorted women from ANC to HIV clinics which were either on the same premises or nearby [5, 34]. Other studies described the navigator as a “peer educator” [37], “patient navigator” [23], “outreach worker” [35] or “lay health worker” [39] who held additional responsibilities including counselling, sending appointment reminders, following‐up with women, and community engagement. In Rwanda, women diagnosed with HIV in ANC were escorted to either an ART clinic on the same premises or a general ART clinic with transport provided [33]. Linkage to care after transition from ANC to HIV services ranged from 25% in Zambia [37], 53% in Kenya [5] and 67% in Rwanda [33]. Most did not report retention outcomes however in Zambia retention was high among women who successfully started ART (91% at 90 days after ART initiation).
Two studies described community‐based approaches to actively identify pregnant women in the community and ensure linkage to antenatal and HIV care [31, 32]. The Tanzanian study did not report outcomes for WLHIV specifically, but in Mozambique, 100% of women identified by the community team successfully initiated ART [32]. Two US studies described the implementation of case managers [38] and a perinatal care coordination team [36] to strengthen transition from PMTCT during pregnancy to postpartum HIV care. Using voluntary enrolment into a case management programme, 43% of women linked to HIV within 90 days of delivery and 53% was in care one year postpartum [38]. Following the implementation of a multidisciplinary care coordination team, 79% of women was linked to care within 90 days of delivery [36].
3.5. Combination approaches
Four studies described the use of a combination of transition approaches. Two Ugandan studies examined the combination of a transition navigator with counselling and a transfer letter to facilitate transition 2 [21, 41]. A South African study of postpartum women transitioning to ART services after delivery described women being accompanied to the nearby ART clinic by a PMTCT counsellor, plus counselling and a transfer letter [42]. Lastly, a study in Malawi described the evolution of approaches to transitioning pregnant women from ANC to ART care to start treatment [40], including transfer letters, transport provision to new clinic, patient held care record, information leaflets with directions and signposting for the ART clinic, a memorandum of understanding between the ANC and the ART clinics to clarify roles, and linked health information systems. The incremental value of each approach was not assessed, but from 2006 to 2009, the investigators observed a reduction in median time to linkage to the ART clinic (from 41 to 15 days), increased proportions of pregnant women successfully initiating ART, from 17% to 74%, and improved six‐month retention after ART initiation, from 17% to 65% [40].
Although outcomes of different transition approaches varied substantially and there was heterogeneity in study methods and reported outcomes, successful linkage to an ART clinic to start treatment in pregnancy (transition 2) ranged from 25% using transfer letters [21] or peer navigation [37], to 56% using a combination of approaches [40] and 67% with escort to the ART clinic on the same premises as ANC [33]. Successful linkage to general ART services from PMTCT postpartum (transition 3) was only reported in studies employing a transfer letter and counselling approach with over 70% of women linking to ongoing care in all three studies [13, 24, 26].
3.6. Comparison of outcomes between two or more transition approaches
Seven studies compared outcomes between two or more transition approaches (Table 2), four focused on approaches to transition point 2 (ANC into ART services during pregnancy) [20, 21, 23, 33] and three focused on transition 3 [25, 36, 38]. In Rwanda, women were escorted from ANC to either an ART clinic on the same premises or a stand‐alone ART clinic (transport provided) [33]. Women transferred to the clinic on the same premises were almost twice as likely to successfully link (67% vs. 35%; risk ratio [RR] 1.9 95% CI 1.5 to 2.3). A South African study in which only a transfer letter was provided compared ART initiation among pregnant women eligible for treatment transferred to an ART clinic on the same premises (38%), to those transferred to a more distal ART clinic (45%), and to those receiving ART integrated in ANC (55%, global p = 0.003) [20].
Table 2.
Summary of studies comparing two or more approaches to transition women into or out of PMTCT for ART services
| First author, year | Study period | Location | Transition models/approaches | Outcome definition | Results |
|---|---|---|---|---|---|
| Transition point 2 (HIV + pregnant women not on ART transition to integrated ANC/ART or stand‐alone ART and ART services to start treatment) | |||||
| Tsague, 2010 [33] | 2006 to 2008 | Rwanda (urban and rural) |
|
Enrolment in the ART clinic |
Pregnant women transferred to the HIV clinic on the same premises were twice as likely to enrol at the ART clinic compared to those transferred to a stand‐alone ART clinic (RR 1.9, 95% CI 1.5 to 2.3) |
| Stinson, 2013 [20] | 2008 | South Africa (urban) |
|
ART initiation during pregnancy | The proportion of ART eligible women who initiated ART in pregnancy was 55% in the integrated antenatal/ART clinic, 38% when referred to a proximal ART clinic, and 45% when referred to a distal ART clinic (p = 0.003) |
| Mugasha, 2014 [21] | 2012 | Uganda (urban and rural) |
|
Linkage to HIV care before six weeks postpartum | The proportion of pregnant women who linked to the HIV clinic was 41% if the urban group and 25% in the rural group. |
| Myer, 2015 [23] | 2010 to 2013 | South Africa (urban) |
|
ART initiation during pregnancy | The proportion of women who initiated ART in pregnancy was 23% in the group that only received a transfer letter and 45% in the group that was accompanied by a patient navigator. 78% of women successfully initiated ART in the integrated antenatal ART group (p < 0.001). |
| Transition point 3 (HIV + postpartum women transition from integrated PMTCT into non‐integrated ART services soon after delivery or later postpartum) | |||||
| Cheshi, 2019 [25] | 2014 | Nigeria (urban and rural) |
|
Linked to ART service within six months after referral from PMTCT. |
No difference in successful linkage between oral and written referral (OR 1.3 95% CI 0.8 to 2.4). Referral by a doctor increased the odds of successful linkage compared to referral by a nurse, counsellor or lab staff (OR 2.0 95% CI 1.0 to 4.0) |
| Hackett, 2019 [36] | 2011 to 2017 | USA (urban) |
|
Link to outpatient HIV care within 90 days of delivery. Retention in care 12 months postpartum |
79% of women who were part of the perinatal care coordination team approach linked to care within 90 days of delivery compared to 29% of women prior to implementation (aOR 9.46 95% CI 4.46 to 20.02) Retention at 12 months was not significantly higher after implementation (55%) compared to before implementation (45%). aOR 1.35 95% CI 0.68 to 2.71. |
| Anderson, 2017 [38] | 2005 to 2013 | USA |
|
Linked to HIV care within 90 days of delivery Retention at one year postpartum |
42.9% of women with a case manager and 35.4% of women with no case manager linked to HIV care within 90 days of delivery (OR 1.36 95% CI 1.03 to 1.80; aOR 1.21 95% CI 0.88 to 1.65) 52.7% of women with a case manager and 34.2% of women with no case manager were retained 12 months postpartum (OR 2.06 95% CI 1.56 to 2.71; aOR 1.59 95% CI 1.17 to 2.16) |
| Phillips, 2020 [27] | 2013 to 2018 | South Africa (urban) |
|
Experiencing a gap in care (180 days with no evidence of HIV care) after transition and after delivery. |
Similar trajectories of time to 180‐day gap after transition in both groups (log‐rank p = 0.068) with gaps frequently occurring soon after transition. By 36 months postpartum, 57% (95% CI 51 to 64%) of women transferred out at six weeks had had a gap in care compared to 45% (95% CI 39% to 52%) of women transferred after weaning. |
| Transition points 2 (HIV + pregnant women not on ART transition to integrated ANC/ART or stand‐alone ART and ART services to start treatment) and 3 (HIV + postpartum women transition from integrated PMTCT into non‐integrated ART services soon after delivery or later postpartum) | |||||
| van Lettow, 2014 [15] | 2012 to 2013 | Malawi (urban and rural) |
|
Retention six and twelve months after ART initiation, among women who had initiated ART |
Retention six months after ART initiation: 80% of women who started ART after antenatal referral to an ART clinic were retained, compared to 89% of women who started ART within ANC and transferred later. Retention 12 months after ART initiation: 80% of women who started ART after antenatal referral to an ART clinic were retained, compared to 87% of women who started ART within ANC and transferred later. |
ANC, antenatal care; ART, antiretroviral therapy; NA, not applicable; PMTCT, prevention of mother‐to‐child transmission of HIV.
Another South African study compared ART initiation among pregnant women transferred to a nearby ART clinic using either transfer letters or patient navigators to ART initiation among pregnant women in integrated ANC/ART with no transition [23]. Among women with patient navigators, 45% of ART‐eligible women initiated treatment during pregnancy compared to 23% with transfer letters (hazard ratio [HR] 3.05 95% CI 2.11 to 4.40), but the highest ART initiation was among women in integrated care, among whom78% initiated ART and who were seven times more likely to start ART compared to women with just transfer letters (HR 7.6 95% CI 5.5 to 10.4). A study in Uganda in 2012 compared linkage to HIV care from ANC after transition in rural and urban areas [21]. In urban areas where women received a transfer letter and were accompanied by a peer navigator, 41% successfully linked to the HIV clinic, compared to 25% in rural areas where women received a referral letter and instructions on how to get to the ART clinic without a peer navigator.
At transition 3, a Nigerian study found no difference in linkage to ART within six months of postpartum referral among women who received a written versus verbal referral [25]. Women referred by a doctor had two‐fold higher odds of successful linkage compared to those referred by a nurse, counsellor or laboratory staff (OR 2.0 95% CI 4.46 to 20.02). Women who had a perinatal case manager in the United States were slightly more likely to link to HIV care within 90 days postpartum (42.9% versus 35.4%, OR 1.36 CI 1.03 to 1.80) and significantly more likely to be retained at 12 months postpartum (52.7% vs. 34.2%, OR 2.06 95% CI 1.56 to 2.71) compared to women with no case manager [38]. Also in the United States, the implementation of a perinatal care coordination team increased linkage to HIV care within 90 days postpartum from 29% to 79% (aOR 9.46 95% CI 4.46 to 20.02) [36].
In addition, one study compared outcomes between transition point 2 and integrated ANC/ART care with postpartum transition to general ART services (transition 3) [15], and another compared early versus late transition from integrated PMTCT to ART services postpartum [27]. A survey of models of care in Malawi compared outcomes of pregnant women after transition 2, to outcomes among women after transition 3 at six weeks postpartum [15]. Although ART initiation was substantially higher when ART was integrated into ANC, retention in ART care at six and twelve months after ART initiation was similar: 89% and 87% of women who transferred postpartum were retained at six and twelve months, respectively, compared to 80% retention at both six and twelve months among women who transferred to start ART during pregnancy [15]. When comparing transition to ART services at six weeks postpartum to transition after cessation of breastfeeding in South Africa, gaps in care were observed soon after transition in both groups with similar trajectories [27]. By 36 months postpartum, 57% of women transferred at six weeks and 45% of women transferred after weaning had experienced a gap in care.
3.7. Factors influencing transition into or out of PMTCT services
Twenty‐four studies reported barriers or facilitators to transition into or out of PMTCT services: 13 focused on transition 2, 9 on transition 3, one on both transitions 2 and 3, and one on transitions 1 and 2. Factors reported were grouped into four themes: fear, knowledge and preparedness, clinic characteristics, and transition process/requirements of the health system (Figure 3).
Figure 3.
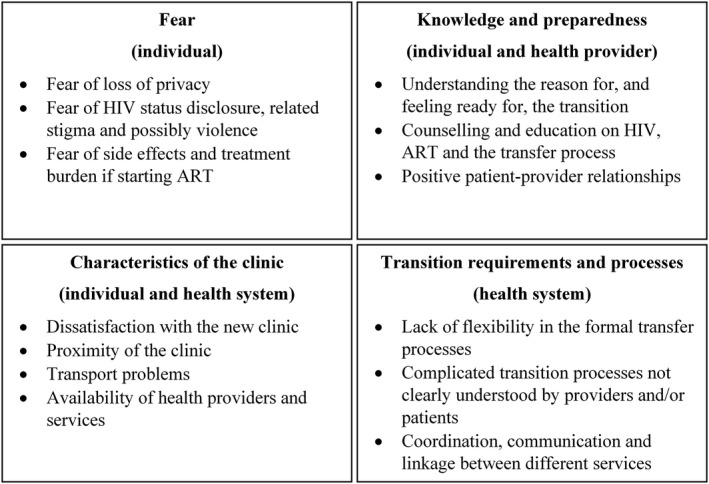
Summary of factors influencing transition into or out of PMTCT for ART services. ANC, antenatal care; ART, antiretroviral therapy; NA, not applicable; PMTCT, prevention of mother‐to‐child transmission of HIV.
3.8. Fear
Fear was frequently cited by women as a barrier to successful transition between services at transition 2 and 3, including fear of consequences from HIV status disclosure, lack of confidentiality and experiencing stigma when moving to a new clinic [4, 21, 25, 41, 43, 44, 45]. Fear of stigma and disclosure were also associated with not successfully transitioning in quantitative studies [22, 24, 46]. Women in South Africa expressed reluctance to leave integrated PMTCT services to continue ART and fear about needing to transfer [47]. Stigma and fear of disclosure were also highlighted as barriers by providers in the context of linking pregnant adolescents to HIV services in Kenya [48] and as reasons for not using referral letters provided during community identification of pregnant women in Tanzania [31].
Women in two studies reported fear of physical violence from partners if they found out about their HIV status [24, 41], while having adequate support from a partner or family member was reported as an enabler of transition [5, 21]. Women transitioning from ANC to ART clinics to start treatment reported fear of medication side effects and the burden of being on treatment as additional barriers [22, 24, 41].
3.9. Knowledge & preparedness
Another common theme reported at both antenatal and postpartum transitions was lack of knowledge and preparedness for the transition. Some women reported not feeling ready and lacking understanding of why they needed to transition to a new clinic [4, 5, 24, 49]. A Nigerian study found 12% of women referred from PMTCT to ART services postpartum did not know why they were being referred [25]. In some studies, poor knowledge of HIV, distrust of ART and cultural beliefs including a preference for herbal treatments were reported as transition barriers [4, 22, 50]. A quantitative study in the United States found that women newly diagnosed with HIV in pregnancy were less likely to link to HIV care in pregnancy than women who already knew their status [51].
The provision of counselling and education around the transition process and other topics relating to HIV care and treatment were reported as facilitators of successful transition by women [24, 41] and healthcare providers [21]. Providers in Uganda noted that the large volume of women requiring care limited their ability to provide adequate counselling before transitioning women from ANC to ART services [21]. Poor communication and bad interactions between patients and providers were also noted as barriers to linkage to ART services in Kenya [5].
3.10. Characteristics of the clinic
Some factors reported to influence transition were related to the characteristics of the receiving clinic. Some women expressed dissatisfaction with the chosen clinic, including location and anticipated waiting times (reported at transition 2 [4, 35] and 3 [24]). Even the transition to a separate clinic on the same premises was reported as a barrier to linkage to HIV care and ART initiation during pregnancy [20, 40, 41]. Transport problems including cost and distance were commonly reported as barriers to transition [4, 5, 21, 24, 35, 52, 53]; providing transport or covering costs was found to facilitate successful transition in Kenya [5]. Not having HIV treatment providers available at the clinic every day was another barrier for women starting ART in pregnancy [41]. Access to ANC and HIV care, or child health services and HIV care, in the same clinic and preferably on the same day was reported to be a facilitator of successful transition [41, 47, 54].
3.11. Transition requirements and process
The administrative requirements and transition process within the health system were both barriers and facilitators of transition. Some studies lacked details, but reported gaps or inadequacies in the referral process as barriers to transition [22, 24]. Watson‐Jones et al reported that healthcare providers had insufficient knowledge of the guidelines for transitioning women to HIV care during pregnancy (transition 2) in Tanzania and that referral letters were not always provided [4]. A Kenyan study also found that pregnant women transitioning to ART clinics were not enrolled if they did not present with a formal transfer letter which was not consistently provided to them [54]. In the same study, women reported difficulty navigating the required transition process and inadequate directions to new clinics and clinic hours information.
Travel and migration were reported as barriers to linking to HIV care in pregnancy (transition 2) [4, 5] and continuing HIV care postpartum (transition 3) [24]. Women in Kenya reported that they were refused care at HIV clinics if they were not permanent residents in the area [5]. Improved coordination between ANC and ART services was reported to be needed in order to trace patients between services at all points of transition [21, 25, 41, 53]. The ‘Linked Response’ intervention in Cambodia was reported to improve systems to trace women through required transitions of care [53] along with improved communication between community interventions and health facilities [53]. Patients and providers in two Ugandan studies reported that being escorted from ANC to the HIV clinic (transition 2) and the presence of peer mothers was helpful for transition [21, 41].
4. DISCUSSION
In the current context of universal ART, all WLHIV should initiate ART and remain on treatment for life. However, this has not eliminated the need to transition the location of care to receive ART services during and after pregnancy. We believe this to be the first systematic examination of the available evidence on the transition of pregnant and postpartum women into and out of PMTCT services for continued ART. It highlights that, while many WLHIV are required to transition into and/or out of ART services during and after pregnancy, there has been very little research into what approaches and strategies can make this critical process more successful. Although some studies have described approaches to transition, there are very limited data rigorously evaluating the impact of strategies, including examinations of different models of care. Much of the existing literature focuses on the transition from HIV diagnosis in ANC to an HIV clinic to initiate ART (transition 2) with less attention paid to postpartum transitions from PMTCT into HIV care (transition 3) and we were only able to find one study focusing on the transition of pregnant women already on ART into PMTCT (transition 1). Limited available research provides insights into the barriers to transition, including individual, healthcare provider and health system factors, which can be used to inform the development of transition approaches and guidelines.
The limited data and heterogeneity between studies precluded pooled analyses, however, it is notable that, among the studies reporting outcomes, many women did not successfully link to a new clinic following the transition. Although the definition of successful linkage varied across studies, it was higher in almost all studies of postpartum linkage to ongoing ART (transition 3) in South Africa (74% and 77%), Kenya (74%) and the United States (29%, 79% and 43%) [13, 24, 26, 36, 38] compared to studies evaluating linkage to initiate ART during pregnancy (transition 2). Studies reporting the transition from ANC to ART services during pregnancy showed the successful linkage from 25% in Zambia and Uganda to 67% in Rwanda [21, 33, 37]. Studies that reported retention in care after a successful transition observed that once women had transitioned to a new clinic, the majority (>60% across studies) were retained, underscoring that the transition is the vulnerable point [15, 24, 26, 40]. Similarly, a study comparing transition point 3 with transition at six weeks postpartum versus after weaning found similar trajectories in time to loss from care in both groups after transition [27].
In studies comparing two or more transition approaches, women transferred to HIV services on the same premises as ANC or within very close proximity were more likely to successfully transition than those transferred to stand‐alone clinics, but were still at risk for loss [20, 33]. The use of health navigators appears to improve linkage and engagement in HIV care among pregnant and postpartum women and other populations [55, 56, 57, 58]. A perinatal care coordination team also increased linkage to continued HIV care postpartum in the United States [36], although the resources required for this approach may be a barrier to implementation in high‐burden settings.
We chose not to restrict this review to the era of Universal ART in order to capture transition approaches that may have been successful for lifelong ART prior to this. It is clear that Universal ART has not removed the need for these transitions, but rather that the points of transition have shifted. Fewer women require transition to initiate ART during pregnancy, but a transition to continued ART care postpartum is likely. The postpartum transition has seldom been included in the literature, likely due to difficulties linking women across clinics and relatively short study follow‐up periods. With the expansion of Option B+ for PMTCT and universal ART for all people living with HIV, there are also increasing numbers of women already on ART at conception. Only one study was identified focusing on the transition from general ART services into ANC and PMTCT (transition 1) with few details of the process [28], whereas three studies described strategies using community health workers to actively identify pregnant women and link them to ANC and HIV care [31, 32] or to assist with the navigation of any transitions of care [39]. None of these studies reported on outcomes following transition. Further research is needed to understand the current practices for providing HIV care to pregnant women already on ART, whether transition into integrated ANC/ART services should be recommended, and how best to approach this transition to ensure continuity of care. Alternative models of care such as the provision of ANC within existing ART services also warrant exploration.
The identified factors influencing transition ranged from psychosocial factors to structural factors relating to the transition process. Fear of social consequences was a commonly cited barrier that has been previously recognized [59, 60, 61]. Women in many parts of the world are dependent on their partners and/or families for both social and economic support. Pregnant women may be particularly hesitant to risk the loss of support and may fear other consequences, such as partner violence, as a result of disclosure of their HIV status [61]. The ANC setting may provide a safe space to access HIV care under the umbrella of pregnancy and child health services and transition to stand‐alone HIV services may be particularly daunting [62, 63]. Integrated ANC, ART and child health services may provide supportive care that can achieve optimal outcomes for women however more research is needed to identify patient‐friendly models of care for pregnant and postpartum WLHIV.
Other barriers and facilitators of transition included characteristics of the health facility and system. Although the WHO recommends interventions using case managers or navigators to enhance linkage between services, a review of PMTCT policy and policy implementation in Malawi, South Africa and Tanzania, found that most Malawian, but few other facilities implemented this and none had explicit guidance in their national policy [11]. There is a clear need for guidelines on the transition process and greater understanding by health providers and clients. Better coordination and communication between health facilities could facilitate successful transition between clinics or services. Linking of data systems between services has the potential to make the transition process more seamless and the tracing of women more efficient [28, 29, 53]. Unfortunately, transition outcomes using health information systems strengthening approaches have not been evaluated to date. In addition, better information should be provided to WLHIV on all aspects of the transition process.
We note several limitations. While very broad search terms were used in two databases, it is possible that relevant papers in other databases were missed. We also did not include protocols or trial registries so ongoing work has not been considered. This review considered maternal transitions for ART and did not go into detail on other integrated services such as family planning, or the exact model of ART care before or after PMTCT. These transitions occur within the context of numerous overlapping challenges women experience [64, 65]. Addressing barriers to transition through optimal transition approaches will not necessarily result in sustained long‐term engagement in HIV care, but it could reduce the overall burden of barriers to engagement in care at a time when women may be particularly vulnerable. As approaches to transition are developed, consideration should be made of other barriers that might be present at the time, for instance transitioning a woman to general ART care immediately after delivery may be hampered by the disruptions of the early postpartum period.
5. CONCLUSIONS
In summary, transitions of care are inevitable along the PMTCT care cascade and supporting these transitions is essential for continuity of maternal HIV care. In the context of Universal lifelong ART where women may move into and out of PMTCT multiple times, guidelines to ensure women start and remain on ART through these transitions are needed. This review is the first to examine the evidence base for approaches to transition into and out of PMTCT that are being employed, and it highlights the lack of evidence on optimal approaches to transition. Given the growing numbers of women on ART, the lack of evidence on transitioning pregnant women from general ART into PMTCT is particularly notable. The existing approaches as well as the barriers and facilitators of transition that have been identified can be used to develop more rigorous transition guidelines. In addition, ongoing operational research is needed to investigate service delivery models that fit with women’s lives and facilitate lifelong retention in HIV care.
COMPETING INTEREST
The authors declare that they have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
EJA, CAT, AG and SM conceptualized and designed the review. TKP conducted the initial searches and TKP and PM screened all abstracts and conducted the data abstraction. TKP drafted the manuscript. EJA, CAT, AG, BN, SM and PM critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Supporting information
Table S1. Summary of 36 studies included in this review, arranged by points of transition discussed: transition 1 only( women on ART in adult services transition into PMTCT services when they become pregnant, n = 1), transition 2 only (women diagnosed with HIV during pregnancy, or known HIV positive but not in ART, transition into ART services, n = 19), transition 3 only (postpartum women transition from PMTCT services into ongoing ART services, n = 10), transition 1 and 2 (n = 1), transition 2 and 3 (n = 4), and all three transitions (n = 1)
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of Demi Myer and Sandisiwe Noholoza who assisted with the review conference abstracts and updated literature searches. Funding for this project was provided through the Presidents Emergency Funds for AIDS Relief (PEPFAR) by the US Centers for Disease Control and Prevention (CDC) through Cooperative Agreement number U2GGH00994. TKP was supported by a VECD Global Health Fellowship, funded by the Office of AIDS Research (OAR) and the Fogarty International Center (FIC) of the NIH (D43 TW009337). The views expressed are solely those of the authors and do not necessary represent the views of the NIH.
Phillips, T. K. , Teasdale, C. A. , Geller, A. , Ng'eno, B. , Mogoba, P. , Modi, S. and Abrams, E. J. Approaches to transitioning women into and out of prevention of mother‐to‐child transmission of HIV services for continued ART: a systematic review. J Int AIDS Soc. 2020; 24(1):e25633
Contributor Information
Tamsin K Phillips, Email: tk.phillips@uct.ac.za.
Chloe A Teasdale, Email: chloe.teasdale@sph.cuny.edu.
Amanda Geller, Email: xym3@cdc.gov.
Bernadette Ng'eno, Email: uyt0@cdc.gov.
Pheposadi Mogoba, Email: phepo.mogoba@uct.ac.za.
Surbhi Modi, Email: bkt1@cdc.gov.
Elaine J Abrams, Email: eja1@cumc.columbia.edu.
REFERENCES
- 1. Knettel BA, Cichowitz C, Ngocho JS, Knippler ET, Chumba LN, Mmbaga BT, et al. Retention in HIV care during pregnancy and the postpartum period in the option B+ era. J Acquir Immune Defic Syndr. 2018;77:427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onoya D, Sineke T, Brennan AT, Long L, Fox MP. Timing of pregnancy, postpartum risk of virologic failure and loss to follow‐up among HIV‐positive women. AIDS. 2017;31:1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: towards universal access. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 4. Watson‐Jones D, Balira R, Ross DA, Weiss HA, Mabey D. Missed opportunities: poor linkage into ongoing care for HIV‐positive pregnant women in Mwanza, Tanzania. PLoS One. 2012;7:e40091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferguson L, Grant AD, Lewis J, Kielmann K, Watson‐Jones D, Vusha S, et al. Linking women who test HIV‐positive in pregnancy‐related services to HIV care and treatment services in Kenya: a mixed methods prospective cohort study. PLoS One. 2014;9:e89764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suthar AB, Hoos D, Beqiri A, Lorenz‐Dehne K, McClure C, Duncombe C. Integrating antiretroviral therapy into antenatal care and maternal and child health settings: a systematic review and meta‐analysis. Bull World Health Organ. 2013;91:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neza G, Mwizerwa W, Odhiambo J, Hedt‐Gauthier BL, Hirschhorn LR, Mugwaneza P, et al. A novel combined mother‐infant clinic to optimize post‐partum maternal retention, service utilization, and linkage to services in HIV care in rural Rwanda. Int J MCH AIDS. 2017;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao N‐Y, et al. Integration of postpartum healthcare services for HIV‐infected women and their infants in South Africa: a randomised controlled trial. PLoS Med. 2018;15:e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aliyu MH, Blevins M, Audet CM, Kalish M, Gebi UI, Onwujekwe O, et al. Integrated prevention of mother‐to‐child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north‐central Nigeria: a cluster‐randomised controlled trial. Lancet HIV. 2016;3:e202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cawley C, McRobie E, Oti S, Njamwea B, Nyaguara A, Odhiambo F, et al. Identifying gaps in HIV policy and practice along the HIV care continuum: Evidence from a national policy review and health facility surveys in urban and rural Kenya. Health Policy Plan. 2017;32:1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones H, Wringe A, Todd J, Songo J, Frances Gómez‐Olivé X, et al. Implementing prevention policies for mother‐to‐child transmission of HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016. Bull World Health Organ. 2019;97(3):200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
- 13. Phillips T, McNairy ML, Zerbe A, Myer L, Abrams EJ. Postpartum transfer of care among HIV‐infected women initiating antiretroviral therapy during pregnancy. J Acquir Immune Defic Syndr. 2015;70:e102–9. [DOI] [PubMed] [Google Scholar]
- 14. Tweya H, Gugsa S, Hosseinipour M, Speight C, Ngambi W, Bokosi M, et al. Understanding factors, outcomes and reasons for loss to follow‐up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Heal. 2014;19:1360–6. [DOI] [PubMed] [Google Scholar]
- 15. van Lettow M, Bedell R, Mayuni I, Mateyu G, Landes M, Chan AK, et al. Towards elimination of mother‐to‐child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc. 2014;17:18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dzangare J, Takarinda KC, Harries AD, Tayler‐Smith K, Mhangara M, Apollo TM, et al. HIV testing uptake and retention in care of HIV‐infected pregnant and breastfeeding women initiated on ‘Option B+’ in rural Zimbabwe. Trop Med Int Heal. 2016;21:202–9. [DOI] [PubMed] [Google Scholar]
- 17. Atanga PN, Ndetan HT, Achidi EA, Meriki HD, Hoelscher M, Kroidl A. Retention in care and reasons for discontinuation of lifelong antiretroviral therapy in a cohort of Cameroonian pregnant and breastfeeding HIV‐positive women initiating ‘Option B+’ in the South West Region. Trop Med Int Heal. 2017;22:161–70. [DOI] [PubMed] [Google Scholar]
- 18. Clouse K, Mongwenyana C, Musina M, Bokaba D, Long L, Maskew M, et al. Acceptability and feasibility of a financial incentive intervention to improve retention in HIV care among pregnant women in Johannesburg, South Africa. AIDS Care. 2018;30:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stinson K, Jennings K, Myer L. Integration of antiretroviral therapy services into antenatal care increases treatment initiation during pregnancy: a cohort study. PLoS One. 2013;8:e63328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mugasha C, Kigozi J, Kiragga A, Muganzi A, Sewankambo N, Coutinho A, et al. Intra‐facility linkage of HIV‐positive mothers and HIV‐exposed babies into HIV chronic care: rural and urban experience in a resource limited setting. PLoS One. 2014;9:e115171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price AJ, Kayange M, Zaba B, Chimbwandira FM, Jahn A, Chirwa Z, et al. Uptake of prevention of mother‐to‐child‐transmission using Option B+ in northern rural Malawi: a retrospective cohort study. Sex Transm Infect. 2014;90:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Myer L, Phillips T, Manuelli V, McIntyre J, Bekker L‐GL‐G, Abrams EJEJ. Evolution of antiretroviral therapy services for HIV‐infected pregnant women in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2015;69:e57–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otieno PA, Kohler PK, Bosire RK, Brown ER, Macharia SW, John‐Stewart GC. Determinants of failure to access care in mothers referred to HIV treatment programs in Nairobi. Kenya. AIDS Care. 2010;22:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheshi FL, Nguku PM, Waziri NE, Sabitu K, Ayemoba OR, Umar TO, et al. Strengthening service integration for effective linkage of HIV‐positive mothers to antiretroviral treatment: a cross‐sectional study in two military health facilities in Kaduna, Nigeria, 2014. Pan Afr Med J. 2019;32:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phillips TK, Clouse K, Zerbe A, Orrell C, Abrams EJ, Myer L. Linkage to care, mobility and retention of HIV‐positive postpartum women in antiretroviral therapy services in South Africa. J Int AIDS Soc. 2018;21:e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phillips TK, Mogoba P, Brittain K, Gomba Y, Zerbe A, Myer L, et al. Long‐Term Outcomes of HIV‐Infected Women Receiving Antiretroviral Therapy After Transferring Out of an Integrated Maternal and Child Health Service in South Africa. J Acquir Immune Defic Syndr. 2020;83:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rawizza HE, Chang C, Chaplin B, Ahmed I, Meloni S, Oyebode T, et al. Loss to Follow‐Up within the Prevention of Mother‐to‐Child Transmission Care Cascade in a Large ART Program in Nigeria. Curr HIV Res. 2015;13:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta RS, Hegde A, Mulik T, Yewale K, Babu PKA, Pardeshi K, et al. Descriptive study of the utility of individual tracking tool in program monitoring for prevention of mother‐to‐child transmission of HIV, Maharashtra. India. Curr Opin HIV AIDS. 2016;11:S30–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kyaw KWY, Mon AA, Phyo KH, Kyaw NTT, Kumar AMV, Lwin TT, et al. Initiation of antiretroviral therapy or antiretroviral prophylaxis in pregnant women living with HIV registered in five townships of Mandalay, Myanmar: A cross sectional study. BMC Pregnancy Childbirth. 2019;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geldsetzer P, Mboggo E, Larson E, Lema IA, Magesa L, Machumi L, et al. Community health workers to improve uptake of maternal healthcare services: A cluster randomized pragmatic trial in dar es salaam, tanzania. PLoS Med. 2019;16:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stover KE, Shrestha R, Tsambe I, Mathe PP. Community‐Based Improvements to Increase Identification of Pregnant Women and Promote Linkages to Antenatal and HIV Care in Mozambique. J Int Assoc Provid AIDS Care. 2019;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsague L, Tsiouris FO, Carter RJ, Mugisha V, Tene G, Nyankesha E, et al. Comparing two service delivery models for the prevention of mother‐to‐child transmission (PMTCT) of HIV during transition from single‐dose nevirapine to multi‐drug antiretroviral regimens. BMC Public Health. 2010;10:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kojima N, Krupp K, Ravi K, Gowda S, Jaykrishna P, Leonardson‐Placek C, et al. Implementing and sustaining a mobile medical clinic for prenatal care and sexually transmitted infection prevention in rural Mysore. India. BMC Infect Dis. 2017;17:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suryavanshi N, Mave V, Kadam A, Kanade S, Sivalenka S, Kumar VS, et al. Challenges and opportunities for outreach workers in the Prevention of Mother to Child Transmission of HIV (PMTCT) program in India. PLoS One. 2018;13:e0203425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hackett S, Badell ML, Meade CM, Davis JM, Blue J, Curtin L, et al. Improved perinatal and postpartum human immunodeficiency virus outcomes after use of a perinatal care coordination team. Open Forum Infect Dis. 2019;6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Killam WP, Tambatamba BC, Chintu N, Rouse D, Stringer E, Bweupe M, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV‐infected pregnant women: a stepped‐wedge evaluation. AIDS. 2010;24:85–91. [DOI] [PubMed] [Google Scholar]
- 38. Anderson EA, Momplaisir FM, Corson C, Brady KA. Assessing the Impact of Perinatal HIV Case Management on Outcomes Along the HIV Care Continuum for Pregnant and Postpartum Women Living With HIV, Philadelphia 2005–2013. AIDS Behav. 2017;21:2670–2681. [DOI] [PubMed] [Google Scholar]
- 39. Besada D, Goga A, Daviaud E, Rohde S, Chinkonde JR, Villeneuve S, et al. Roles played by community cadres to support retention in PMTCT Option B+ in four African countries: a qualitative rapid appraisal. BMJ Open. 2018;8:e020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weigel R, Hosseinipour MC, Feldacker C, Gareta D, Tweya H, Chiwoko J, et al. Ensuring HIV‐infected pregnant women start antiretroviral treatment: an operational cohort study from Lilongwe. Malawi. Trop Med Int Health. 2012;17:751–9. [DOI] [PubMed] [Google Scholar]
- 41. Saleem H, Kyeyagalire R, Lunsford SS. Patient and provider perspectives on improving the linkage of HIV‐positive pregnant women to long‐term HIV care and treatment in eastern Uganda. African J AIDS Res. 2014;13:45–51. [DOI] [PubMed] [Google Scholar]
- 42. Myer L, Iyun V, Zerbe A, Phillips TK, Brittain K, Mukonda E, et al. Differentiated models of care for postpartum women on antiretroviral therapy in Cape Town, South Africa: a cohort study. J Int AIDS Soc. 2017;20:21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buregyeya E, Naigino R, Mukose A, Makumbi F, Esiru G, Arinaitwe J, et al. Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy Childbirth. 2017;17:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winestone LE, Bukusi EA, Cohen CR, Kwaro D, Schmidt NC, Turan JM. Acceptability and feasibility of integration of HIV care services into antenatal clinics in rural Kenya: A qualitative provider interview study. Glob Public Health. 2012;7:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lubaga M, Musenze I, Gukiina J, Dhafa G, Badaza R, Bakwesegha CJ, et al. Sex inequality, high transport costs, and exposed clinic location: reasons for loss to follow‐up of clients under prevention of mother‐to‐child HIV transmission in eastern Uganda: a qualitative study. Patient Prefer Adherence. 2013;:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turan B, Stringer KL, Onono M, Bukusi EA, Weiser SD, Cohen CR, et al. Linkage to HIV care, postpartum depression, and HIV‐related stigma in newly diagnosed pregnant women living with HIV in Kenya: a longitudinal observational study. BMC Pregnancy Childbirth. 2014;14:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pellowski JA, Weber AZ, Phillips TK, Brittain K, Zerbe A, Abrams EJ, et al. “You must leave but I didn’t want to leave”: qualitative evaluation of the integration of ART into postnatal maternal and child health services in Cape Town, South Africa. AIDS Care ‐ Psychol Socio‐Medical Asp AIDS/HIV. 2019;0121: 10.1080/09540121.2019.1659913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luseno WK, Iritani BJ, Maman S, Mbai I, Ongili B, Otieno FA, et al. “If the mother does not know, there is no way she can tell the adolescent to go for drugs”: Challenges in promoting health and preventing transmission among pregnant and parenting Kenyan adolescents living with HIV. Child Youth Serv Rev. 2019;103:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trafford Z, Gomba Y, Colvin CJ, Iyun VO, Phillips TK, Brittain K, et al. Experiences of HIV‐positive postpartum women and health workers involved with community‐based antiretroviral therapy adherence clubs in Cape Town. South Africa. BMC Public Health. 2018;18:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Panditrao M, Darak S, Jori V, Kulkarni S, Kulkarni V. Barriers associated with the utilization of continued care among HIV‐infected women who had previously enrolled in a private sector PMTCT program in Maharashtra. India. AIDS Care. 2015;27:642–648. [DOI] [PubMed] [Google Scholar]
- 51. FitzHarris LF, Hollis ND, Nesheim SR, Greenspan JL, Dunbar EK. Pregnancy and linkage to care among women diagnosed with HIV infection in 61 CDC‐funded health departments in the United States, 2013. AIDS Care ‐ Psychol Socio‐Medical Asp AIDS/HIV. 2017;29:858–865. [DOI] [PubMed] [Google Scholar]
- 52. Dryden‐Peterson S, Lockman S, Zash R, Lei Q, Chen JY, Souda S, et al. Initial Programmatic Implementation of WHO Option B in Botswana Associated With Increased Projected MTCT. JAIDS J Acquir Immune Defic Syndr. 2015;68:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. White J, Delvaux T, Chhea C, Saramony S, Ouk V, Saphonn V. The Linked Response: Lessons Emerging from Integration of HIV and Reproductive Health Services in Cambodia. AIDS Res Treat. 2013;2013:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Helova A, Akama E, Bukusi EA, Musoke P, Nalwa WZ, Odeny TA, et al. Health facility challenges to the provision of Option B+ in western Kenya: a qualitative study. Health Policy Plan. 2017;32:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Govindasamy D, Meghij J, Negussi EK, Baggaley RC, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre‐ART (HIV) care and initiation of ART in low‐ and middle‐income settings ‐ a systematic review. J Int AIDS Soc. 2014;17:19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ambia J, Mandala J. A systematic review of interventions to improve prevention of mother‐to‐child HIV transmission service delivery and promote retention. J Int AIDS Soc. 2016;19:20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cataldo F, Sam‐Agudu NA, Phiri S, Shumba B, Cornelius LJ, Foster G. The Roles of Expert Mothers Engaged in Prevention of Mother‐to‐Child Transmission (PMTCT) Programs. JAIDS J Acquir Immune Defic Syndr. 2017;75:S224–S232. [DOI] [PubMed] [Google Scholar]
- 58. Nachega JB, Adetokunboh O, Uthman OA, Knowlton AW, Altice FL, Schechter M, et al. Community‐Based Interventions to Improve and Sustain Antiretroviral Therapy Adherence, Retention in HIV Care and Clinical Outcomes in Low‐ and Middle‐Income Countries for Achieving the UNAIDS 90–90‐90 Targets. Curr HIV/AIDS Rep. 2016;13:241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hunter‐Adams J, Zerbe A, Philips T, Rini Z, Myer L, Petro G, et al. The dimensionality of disclosure of HIV status amongst post‐partum women in Cape Town. South Africa. African J AIDS Res. 2017;16:101–107. [DOI] [PubMed] [Google Scholar]
- 60. Crankshaw TL, Voce A, King RL, Giddy J, Sheon NM, Butler LM. Double disclosure bind: complexities of communicating an HIV diagnosis in the context of unintended pregnancy in Durban. South Africa. AIDS Behav. 2014;18(Suppl 1):S53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brittain K, Mellins CA, Remien RH, Phillips T, Zerbe A, Abrams EJ, et al. Patterns and Predictors of HIV‐Status Disclosure Among Pregnant Women in South Africa: Dimensions of Disclosure and Influence of Social and Economic Circumstances. AIDS Behav. 2018;:12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ngarina M, Popenoe R, Kilewo C, Biberfeld G, Ekstrom AM. Reasons for poor adherence to antiretroviral therapy postnatally in HIV‐1 infected women treated for their own health: experiences from the Mitra Plus study in Tanzania. BMC Public Health. 2013;13:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fords GM, Crowley T, van der Merwe AS. The lived experiences of rural women diagnosed with the human immunodeficiency virus in the antenatal period. SAHARA‐J J Soc Asp HIV/AIDS. 2017;14:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eshun‐Wilson I, Rohwer A, Hendricks L, Oliver S, Garner P. Being HIV positive and staying on antiretroviral therapy in Africa: A qualitative systematic review and theoretical model. PLoS One. 2019;14:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, et al. A Systematic Review of Individual and Contextual Factors Affecting ART Initiation, Adherence, and Retention for HIV‐Infected Pregnant and Postpartum Women. PLoS One. 2014;9:e111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of 36 studies included in this review, arranged by points of transition discussed: transition 1 only( women on ART in adult services transition into PMTCT services when they become pregnant, n = 1), transition 2 only (women diagnosed with HIV during pregnancy, or known HIV positive but not in ART, transition into ART services, n = 19), transition 3 only (postpartum women transition from PMTCT services into ongoing ART services, n = 10), transition 1 and 2 (n = 1), transition 2 and 3 (n = 4), and all three transitions (n = 1)


