Abstract
Phosphodiesterases catalyze the hydrolysis of cyclic nucleotides and maintain physiologic levels of intracellular concentrations of cyclic adenosine and guanosine mono-phosphate (cAMP and cGMP, respectively). Increased cAMP signaling has been associated with adrenocortical tumors and Cushing syndrome. Genetic defects in phosphodiesterase 11A (PDE11A) may lead to increased cAMP signaling and are found to predispose to the development of adrenocortical, prostate, and testicular tumors. A previously reported Pde11a knockout (Pde11a−/−) mouse line was studied and found to express PDE11A mRNA and protein still, albeit at reduced levels; functional studies in various tissues showed increased cAMP levels and reduced PDE11A activity. Since patients with PDE11A defects and Cushing syndrome have PDE11A haploinsufficiency, it was particularly pertinent to study this hypomorphic mouse line. Indeed, Pde11a−/− mice failed to suppress corticosterone secretion in response to low dose dexamethasone, and in addition exhibited adrenal subcapsular hyperplasia with predominant fetal-like features in the inner adrenal cortex, mimicking other mouse models of increased cAMP signaling in the adrenal cortex. We conclude that a previously reported Pde11a−/− mouse showed continuing expression and function of PDE11A in most tissues. Nevertheless, Pde11a partial inactivation in mice led to an adrenocortical phenotype that was consistent with what we see in patients with PDE11A haploinsufficiency.
Keywords: cyclic AMP, cortisol, adrenocortical hyperplasia, PDE11A gene
1. INTRODUCTION
Cyclic nucleotide phosphodiesterases (PDEs) catalyze the hydrolysis of the phosphodiester bond in the second messenger cyclic adenosine mono-phosphate (cAMP) and guanosine monophosphate (cGMP). Eleven families of PDEs have been distinguished based on aminoacid sequence and biochemical and inhibitors profiles (Makhlouf, et al., 2006). The phosphodiesterase 11A (PDE11A) family consists of four isoforms (PDE11A1, −2, −3, and −4), and was the last identified PDE (Fawcett, et al., 2000). PDE11A expression has been extensively documented in human tissues, as well as in mouse prostate and testis (Fawcett, et al., 2000; Yuasa, et al., 2000; Loughney, et al., 2005; Wayman, et al., 2005; Lakics, et al., 2010).
Human PDE11A defects were first linked to adrenal disease in 2006, after a genome-wide study identified the chromosome 2q31.2 PDE11A gene locus as the most closely linked to Cushing Syndrome (CS) in a cohort of patients with micronodular adrenal hyperplasia (MAH) (Horvath, et al., 2006a; Horvath, et al., 2006b). Soon thereafter, PDE11A genetic variants were found to predispose to a variety of adrenal lesions beyond MAH (Libe, et al., 2008), and testicular and prostate tumors (Horvath, et al., 2009; Faucz, et al., 2011; Libe, et al., 2011).
A Pde11a−/−mouse model was reported with mainly an infertility phenotype (Wayman, et al., 2005); subsequently, another Pde11a−/−mouse was reported and studied for PDE11A’s linkage to psychiatric diseases and its expression in the ventral hippocampus (Kelly, et al., 2010). Neither of the two studies looked at adrenocortical function or abnormalities. In the present study, we were able to obtain the first animal model of Pde11a deficiency (Wayman, et al., 2005), and investigate its endocrine and other phenotypic abnormalities, focusing, in particular, on the adrenal cortex. Our results show that Pde11a−/− mice failed to suppress corticosterone secretion in response to low dose dexamethasone and in addition exhibed adrenal subcapsular hyperplasia with predominant fetal-like features in the inner adrenal cortex.
2. MATERIALS & METHODS
2.1. Mouse strain, dissections and phenotype analysis
Pde11a−/− mice were obtained from Wayman et al. (Wayman, et al., 2005) and were maintained on a C57BL/6 mixed genetic background. Pde11a−l− mice were generated using a neomycin cassette replacing 16 bases of the catalytic domain in exon 12 of the human PDE11A gene, because of its high homology to the murine sequence, as the complete murine Pde11a sequence was unknown at the time (Wayman, et al., 2005). We studied wild type (WT, Pde11a+/+; n=43), Pde11a+/− (n=44) and Pde11a−l− (n=39) mice for 18 months. Male and female mice were euthanized at 6-, 12- and 18-months of age. We also examined early embryonic development, at embryonic day (e) 9.5, 12.5, 14.5, 15.5 as well as postnatal day 1 and 10. All mice were genotyped and phenotyped. Mice were fed and maintained in the same in-house animal facility, under standardized conditions spanning the two year study. For mRNA studies and protein studies, dissected tissue was snap-frozen and stored at −80°C until ready for processing. Tissue from each major organ (brain, lung, liver, kidney, adrenal gland, testis, prostate and ovary) was formalin-fixed and paraffin embedded for routine histological analysis using haematoxylin and eosin (H&E) staining and other routine staining, as outlined below. Most histopathological analysis was performed in our laboratory and at Molecular Histology, Inc., (Gaithersburg, MD, USA) or at the Division of Veterinary Resources (NIH, Bethesda, MD, USA). Blood was collected from mice, via cardiac puncture, following anaesthetization with halothane, and was used for analyzing blood biochemistry and complete blood counts; serum was collected and frozen at −80C. All animal experiments were approved by the National Institute of Health Animal Ethics Committee (ASP#18–033).
2.2. Mouse genotyping
Mouse genomic DNA was extracted from tail biopsies,and analyzed by PCR. The primers used for all PCR genotyping were: 5’-TCGGAACTGGCCATCGATGACATCC-3’, 5’-CTAACACCCTGCAGGATTCTCATGAC-3’ and 5’-GGGCCAGCTCATTCCTCCCACTCAT-3’. Amplification by PCR was performed in a DNA Thermal Cycler. PCR conditions were as follows: 16 cycles 95° - 30 sec, 62°−58° - 30 sec, 72° 45 sec and then 20 cycles 95° - 30 sec, 58° - 30 sec, 72° 45 sec. Amplicons were analyzed by gel electrophoresis on a 2% agarose gel; the wild-type allele (Pde11a+/+) generated a 281bp product, whereas the null mutation (Pde11a−/−) generated a 479bp product; mice heterozygous (Pde11a+/−) contain both the wild type and null allele. (Supplementary Figure 1).
2.3. RNA extraction
Total RNA was isolated from 30–50mg of frozen tissue (brain, lung, liver, kidney, adrenal gland, testis, prostate and ovary) using Trizol (Invitrogen, Carlsbad, CA). RNA was resuspended in diethyl pyrocarbonate-treated (DEPC) water. The concentration and purity of RNA was determined spectrophotometrically by UV absorbance (260/280nm), treated with DNAse (Invitrogen, Carlsbad, CA) and stored at −80C, until use.
2.4. RT-PCR
cDNA was synthesized using oligo (dT)s (Invitrogen, Carlsbad, CA) and the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Synthetized cDNA from Pde11a+/+ and Pde11a−/− sample from brain, adrenal, testis were analized by RT-PCR using standard oligonucleotide primers spaning two consecutive exons in different areas of the transcripts. The primers spaning exons 9–10 and exons 12–13 were 5’-CCTTGATGTGCTGTCGTACC-3’ and 5’-GGAACCCAGCAGTAGTTAGCA-3’ respectively; primer spaning exons 14–15 and exons 18–19 were 5’-CCTTCAGAGTGAGGGTCACAA-3’ and 5’-GGTTCCGGTCAAAAATAGCA-3’ respectively; primer spaning exons 1–2 and exons 10–11 were 5’-CCAGGACCGAAGATTCAATG −3’ and 5’-GTTTAAGGCAGCCAACATCC-3’respectively. Amplification by PCR was performed in Thermal Cycler. PCR conditions were as follows: 12 cycles 94° - 30 sec, 64°−58° - 30 sec, 72° 45 sec and then 23 cycles 94° - 30 sec, 58° - 30 sec, 72° 45 sec. Amplicons were analyzed by gel electrophoresis on a 2% agarose gel (Figures 2-A1–3). cDNA from Pde11a+/+ and Pde11a−/− sample from brain, adrenal, testis and lung were also analized by qRT-PCRperformed on the Applied Biosystems 7500 real-time PCR system. TaqMan probes (Applied Biosystems, Carlsbad, CA, USA) were used for qRT-PCR whereby reaction and analysis was carried out as per manufacturer guidelines. Mouse Gapdh (Applied Biosystems, Catalog number Mm99999915_g1) was used as the endogenous control. The following TaqMan probes were used: Pde11a (Applied Bioystem Catalog number Mm01327347_m1); Akr1b7 (Applied Bioystem Catalog number Mm00477605_m1), Cyp17 (Applied Bioystem Catalog number Mm00484040_m1), Akr1c18 (Applied Bioystem Catalog number Mm00506289_m1). Each sample was analyzed in triplicate.
Figure 2: Pde11a mRNA integrity in Pde11a−/− sample and its expression in the studied mice.
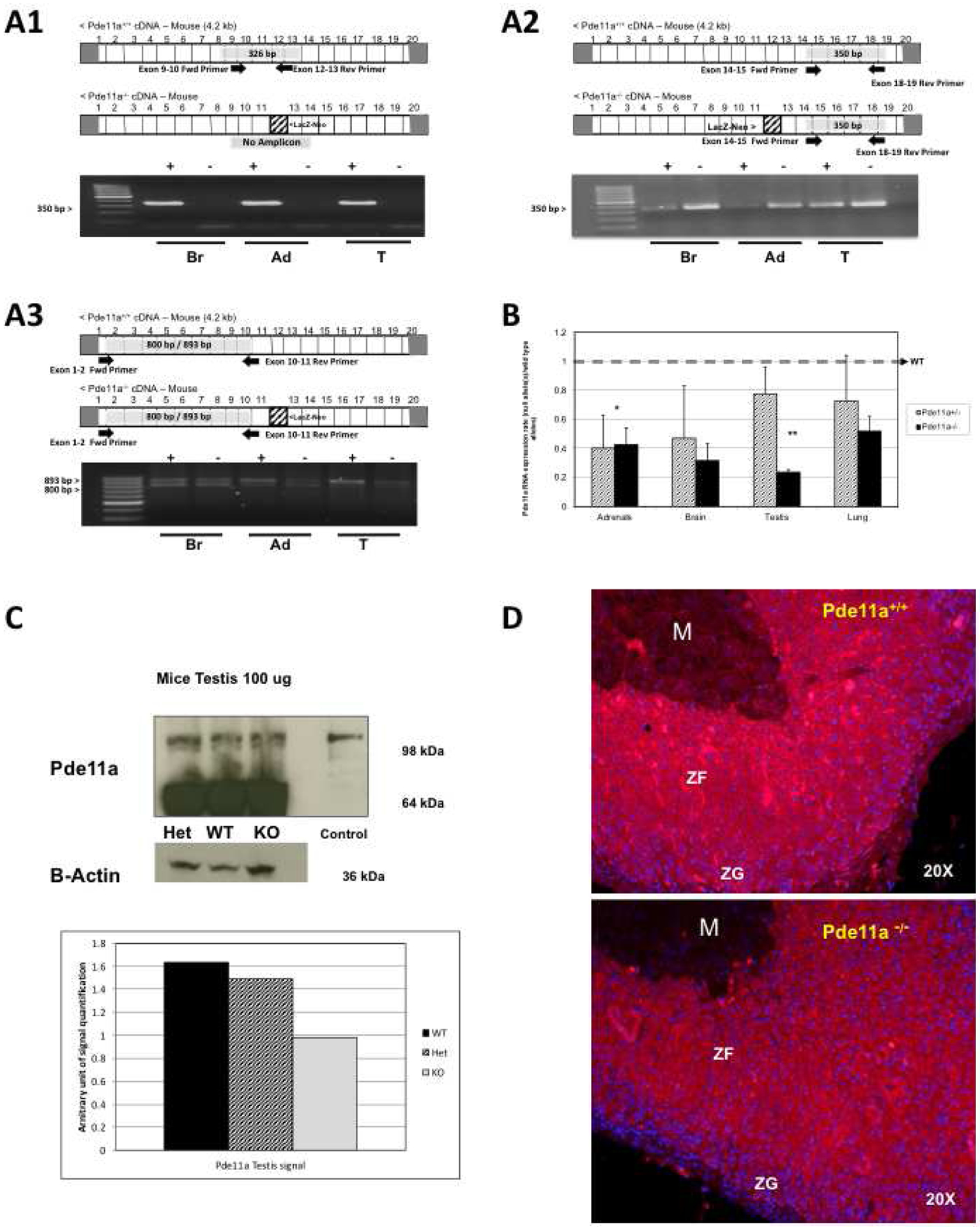
(A) Products of RT-PCR using primers spanning different regions along the Pde11a transcript of KO and Pde11a+/+ mice: A-1) primer spaning exons 9–10 and exons 12–13; A-2) primers spaning exons 14–15 and exons 18–19; A-3) primers spanning exons 1–2 and 10–11: Amplicons were analyzed by gel electrophoresis on a 2% agarose gel. Amplification in Pde11a−/− mice indicates that Pde11a−/− RNA transcript is intact from exon 1 throught 19, is not degraded and incorporate the LacZ-Neo cassette (B) qRT-PCR levels of Pde11a in adrenal, brain, testis and lungs from heterozygote (Het) Pde11a+/, and knock-out (KO) Pde11a−/− mice against Pde11a+/+ mice; clearly Pde11a mRNA is decreased in the genetically modified animals but not absent in the KO mice, *p value for KO sample 0.05, **p value KO= 0.001; (C) Protein expression of Pde11a, assessed by western blot (WB) analysis, in testis amd adrenal, as an example and quantified in testis by optical density of WB bands; once again, Pde11a protein in testis of the KO mouse is present and is lower than in the Pde11a+/+ animal but not absent. (D) Pde11a immunostainig (red) merged with nuclear staining (DAPI – blue) in Pde11a+/+ and Pde11a−/− adrenals, immunoractivity is rubust in zona fasciculata of both genotypes but reduced in Pde11a−/− adrenal. Abbreviations: Br, brain, Ad, adrenal, T, testis, M, medulla, ZF zona fasciculata, ZG, zona glumerolosa.
2.5. Immunohistochemistry
Analysis of the expression of Pde11a, from early embryonic development through to adulthood, was analyzed by immunohistochemistry and performed in collaboration with Phylogeny (Colombus, Ohio). Tissues were obtained from mouse embryos at embryonic day (e) 9.5, 12.5, 14.5, 15.5 as well as postnatal stages 1 and 10, and adult mice. Embryos and organs from postnatal stages were flash frozen and stored at −80°C. Frozen tissues were cut into 8–10μm sections, fixed for 60 minutes in 4% formaldehyde, washed in phosphate buffered saline (PBS; 0.01M, pH 7.4), rinsed in distilled water (to remove salts) and air-dried for a few days. Sections were fixed in formalin (4% PFA for 15 minutes and washed in PBS 0.01M, pH 7.4), incubated with the PDE11A antibody overnight at 4°C. The anti-Pde11a antibody (diluted 1:75; Abcam ab14624), was a rabbit polyclonal one to human PDE11A synthetic peptide (aa 454–468). Staining was performed using standard peroxidase methods as per Phylogeny (Colombus, Ohio). Detection of the primary antibody was performed using an anti-rabbit antibody coupled to biotin (111-065-144, Jackson Immuno Research Laboratory) followed by streptavidin-HRP amplification (016-030-084, Jackson Immuno Research Laboratory). HRP activity was detected with 3,3’-diaminobenzidinetetrahydrochloride (DAB) (SK-4105, Vector Labs). Normal rabbit serum was used as control in order to detect non-specific immunoreaction.
Analysis of the effect of loss of PDE11A on the adrenal was performed by immunofluorescence. All mouse adrenals were treated similarly. They were rapidly removed postmortem and immediately fixed in ice-cold 4% formaldehyde in PBS (0.01M, pH 7.4) for 24h, and embedded in paraffin following standard procedures for sectioning. Whole adrenals were bisected along the coronal plane. Sets of serial sections were cut at 5μm thickness. Sections were mounted onto poly L-ornithine-coated (Sigma, St Louis, MO) glass slides. Each section-mounted slide underwent antigen retrieval, performed with slides immersed in citric acid buffer solution (pH 6.6) and steamed for 20 minutes, to normalize staining across sections. Each section was immunostained using the following primary antibodies: Pde11a (Fabgenix Cat. PD11–112AP), and rabbit anti-20αHsd (a generous gift from Dr. Weinstein, Ben Gurion University of the Negev - Israel). For general morphology, sections were stained with H&E.
Immunofluorescence involved rehydration of sections in successive 2 min Histoclear (National Diagnostic, Atlanta-Georgia, USA. Catalog number HS-200) and ethanol rinses (100, 95, 75 and 50%) followed by washing in PBS (0.01M, pH 7.4) for 5 min each prior to antigen retrieval. Sections were incubated in blocking solution, using 10% normal goat serum (Jackson Immuno Research) made in PBS (0.01M, pH 7.4) for all antibodies, except for goat anti-Akr1b7 whereby sections stained for this antibody were blocked with 10% normal donkey serum (Jackson Immuno Research) made in PBS (0.01M, pH 7.4). All sections were blocked at room temperature in a humidified chamber (1 hour). Single-labeled immunofluorescence was performed by incubating the sections overnight at 4°C with primary antibodies, specified above. Following primary antibody incubation, sections were washed in PBS (0.01M, pH 7.4) containing 0.1% Triton-X and incubated with the appropriate fluorophore-conjugated secondary antibody: goat anti-rabbit Alexa-Fluor 555 (1:400, Molecular Probes, Life Technologies, OR, USA) and donkey anti-rabbit Alexa-Fluor 555 (1:500, Molecular Probes, Life Technologies, OR, USA). Sections were washed in PBS (0.01M, pH 7.4) containing 0.1% Triton-X and mounted with DAPI Prolong Gold (Molecular Probes, Life Technologies, OR, USA).
2.6. Adrenal zonation and histologic analysis
Histology of adrenal gland specimens from 43 Pde11a+/+, 44 Pde11a+/−, and 39 Pde11a−/− mice were analyzed following H&E staining by an experienced pathologist to determine any histological changes in the capsule, glomerulosa, fasciculata or medulla. Adrenal subcapsular hyperplasia with predominant fetal like features in the inner adrenal cortex mimicking other animal models of increase cAMP signaling in the adrenal cortex was recorded. Further correlation by immunostaining for the X-zone (anti-20α HSD antibody) was done.
The presence of foamy cells in the adrenal cortex, as well as subcapsular hyperplasia and persistence hyperplasia of the X-zone was studied in every specimen and counted in each genotype group. The presence or absence of the X-zone is an all or none phenomenon; thus, we recorded its presence or absence. Each animal was examined for the presence of X-zone in their adrenal gland after staining by 20a-HSD, which is specifically expressed in fetal corticsl cells.
2.7. Assays for cAMP levels and binding activity, and protein kinase A (PKA) activity
Levels of cAMP were measured in tissue extracts using the cAMP 3H Biotrak Assay System (Amersham Biosciences). Samples were homogenized in ethanol, centrifuged (1000g, 10 min), and the supernatant dried and resuspended in buffer, according to manufacturer’s instructions. Three samples were prepared for each sample group.
PKA enzymatic activity was measured in tissue following as previously described (Nesterova, et al., 1975; Rohlff, et al., 1993; Nesterova, et al., 2008). Briefly, tissue was processed in 10mM Tris-HCl, pH 7.5, 1mM EDTA, and 0.1mM dithiothreitol (DTT) protease inhibitor cocktail I (EMD Biosciences, Darmstadt, Germany). About 10mg protein of the tissue extracts were added to the reaction mixture (50μl) containing 0.025mM [g-32P] ATP, 5mM kemptide, 10mM MgCl2 ± 5μM cAMP, and μM PKA inhibitor (PKI). The mixture was incubated for 15 min at 30°C, spotted on phosphocellulose filters, and washed for three times using 0.1% phosphoric acid. The filters were air dried before analysis by a liquid scintillation counter. Basal levels of PKA activity correspond to the non-stimulated enzyme. Total activity reflects activation following cAMP addition. Free PKA activity represents the difference between activation without addition of cAMP and presence of PKI.
2.8. PDE activity
cAMP-binding total PDE activity was assayed using titrated cAMP (Poch, 1971). PDE activity was measured by cAMP-specific activity, whereas PDE is a dual-specificity PDE, binding c-GMP as well. A non-specific cyclic nucleotide PDE inhibitor, 3-isobutyl-1-methylxanthine (IBMX, a non-selective PDE inhibitor; PDE1, −2, −7, −10 and −11 but not PDE8), was used in order to study the degree of PDE inhibition in Pde11a+/+ and Pde11a−/− mice adrenal and testis lysated samples. In addition, BC11–38, a novel specific PDE11A inhibitor, was also used in order to detect the degree of PDE11 contribution to the total measured PDE activity in the same sample. As previously describbed (Ceyhan, et al., 2012), we used 500μM IBMX and 20μM BC11–38 in order to measure the effect of inhibition in each assay. Testis was used as control for the PDE assay because testes express more PDEs than adrenal and as described, the contribution of Pde11 is high. Giving these conditions, it is possible to measure difference in PDE activity between the two genotypes, measure small difference after adding IBMX (probably because the contribution of uninhibited PDEs) and, detect significant difference after BC11–38 because the high expressed Pde11 in testis.
2.9. Dexamethasone suppression test
Two groups of mice, Pde11a+/+ (n=8; 4 male and female) and Pde11a−/− (n=8; 4 male and female), aged between 8–10 month old were treated with low-dose (0.062mg/100g body weight) and high-dose (0.25mg/100g body weight) dexamethasone (Sigma, St. Louis, MO), over a 6 day period based on Liddle’s protocol in humans (Stratakis, et al., 1999). Dexamethasone was diluted in NaCl 0.9% standard saline physiologic solution. Low-dose was intraperitoneally injected beginning on day 2 at 1800h, and thereafter every 12 hours until day 4, when dexamethasone high-dose was started, again at 1800h and every 12 hours thereafter until day 6. Blood was collected, via tail vein bleeds, on days 1, 4 and 6. The day 1 before the first intraperitoneally dexamethasone dose injected, day 4 before the first high dose and at day 6 at the end. This way we measured corticosterone and ACTH at baseline, post-low-dose and post-high-dose dexamethasone. Corticosterone and ACTH plasma levels were determined in mice of each group using a commercial ELISA kit (Alpco, Salem, NH). Cortisol measurements were done and compared between Pde11a−/− and Pde11a+/+ mice because, like in other animals where there is persistence of the X-zone, cortisol (in addition to corticosterone) may be produced by 17a hydroxylase that is expressed in the fetal X-zone (but not in adult rodent adrenal cortex). Since Pde11a partial deficiency led to persistence of the X-zone, we wanted to determine if this zone was biochemically active and produced cortisol. Plasma cortisol levels were measured using a commercial ELISA kit for cortisol (Alpco Salem, NH), as per the manufacturer’s instructions.
2.10. Statistical analysis
Statistical analysis was performed using χ2, Fisher’s exact test, and t-test, where appropriate. For all statistical comparisons p < 0.05 was considered statistically significant. Data were analyzed using SPSS Software. Where applicable, data are represented as mean ± SEM.
3. RESULTS
3.1. Pde11a is widely expressed in adult mice but not as highly in the adrenal glands
We examined the expression of Pde11a in Pde11a+/+ mice across development at embryonic day (e)9.5, 12.5, 14.5, 15.5) and in adult animals ages 6-, 12- and 18-months old. (Figure 1, Supplementary Figure 2, Supplementary Table 1). Pde11a immunoreactivity is widely present in adult mice but at lower intensity in the adrenal glands. There was no significant Pde11a immunoreactivity in fetal adrenal cortex. At embryonic age (e)14.5 and (e)15.5 days, Pde11a protein was expressed in the hippocampus, dorsal root ganglia, choroids plexus, small intestine and the developing tooth. Higher Pde11a immunostaining was found in the epithelium of the small intestine villi, thyroid follicular cells, spermatocytes within specific sets of seminiferous tubules in the testes and epithelial cells in the prostate, as shown previously by Wayman et al (Wayman, et al., 2005). Moderate Pde11a levels were measured in neuronal cells of the grey matter and Purkinje cells of the cerebellum, while weak signal was observed in choroid plexus cells, cerebral cortex, hippocampus, hypothalamus, thalamus and cranial ganglia. Moderate immunoreactivity was observed in the remaining gut region and epithelial cells, liver hepatocytes, exocrine pancreatic acini (but not endocrine pancreas), epithelium within lung bronchi, epithelium in renal tubules and cells in glomerulus, epithelial cells in the uterus, odontoblast and ameloblast cells in the teeth. The immunoreactivity in the pituitary gland and skeletal muscles was almost undetectable. Transient Pde11a expression pattern (E14.5-P1) is evident within chondrification centers, such as the rudimental ear cochlea tympanic ring and calvaria, where protein expression does not occur during adulthood (Supplementay Figure 2).
Figure 1: Pde11a immunostaining in Adrenal of wild type mouse.
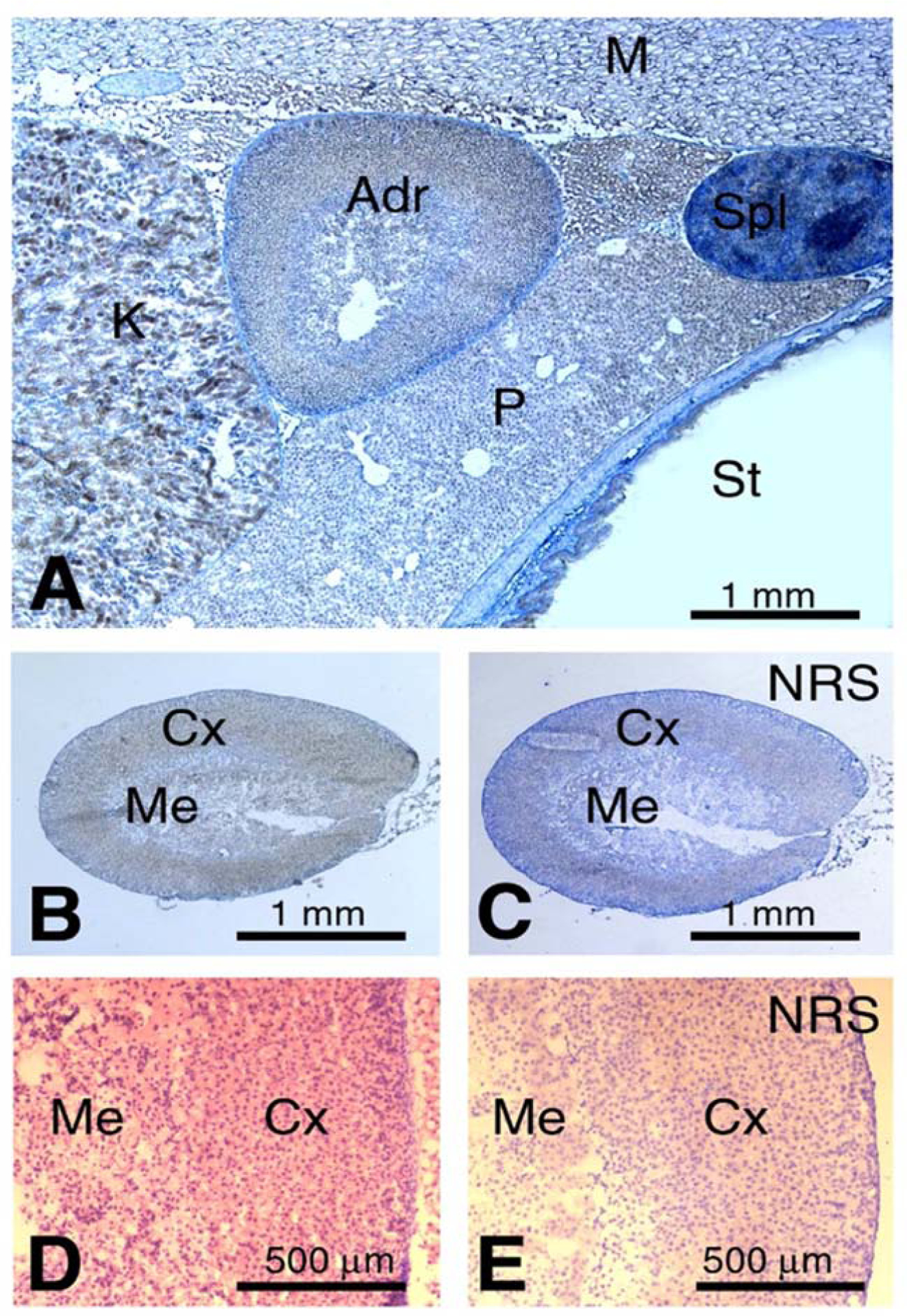
(A) Anatomical view of the adrenal gland together with adjacent kidney, pancreas, spleen, stomach and skeletal muscles; B) Weak, diffuse immunoreaction is seen in the adrenal cortex and, less, in the medulla. This is evident when compared to control staining shown in (C); C) Control staining for (B) performed with normal rabbit serum; D) Detail of the contrast between the immunoreaction in the cortex and medulla at higher magnification; E) Adrenal assayed with the negative control (normal rabbit serum); Adr - adrenal gland; Cx - cortex; K - kidney; M - muscle; Me - medulla; NRS - normal rabbit serum; P - pancreas; Spl - spleen; St - stomach.
3.2. Pde11a−/− mice established by Wayman et al. (Wayman, et al., 2005) do not carry a complete deletion of Pde11a
Mice tissues providing sufficient protein lysate were used to examine mRNA and protein expression (brain, adrenal, testis and lung). To determine the integrity of Pde11a mRNA in Pde11a−/− mice we analyzed a variety of tissues, by RT-PCR, Pde11a amplicon using primers spaning exons 9–10 and 12–13 produced a product of 326bp size in Pde11a+/+ samples and as expected no product in Pde11a−/− having LacZ-Neo cassette disrupting exon 12. (Figure 2 A1); Pde11a amplicon using primers spaning exons 14–15 and 18–19 produced a product of 350bp size in Pde11a+/+ as well as on Pde11a−/− (Figure 2 A2); finally, Pde11a amplicon using primer spaning exons 1–2 and 10–11 produced two produts sizes of 893bp and 800bp (two Isoforms are described in mice, Pde11a-201 and the Pde11a-203 lacking 93bp from exon 9) (Figure 2 A3). This results provide evidence that Pde11a−/− RNA transcript is intact from exon 1 throught 19 and, is not degraded and incorporate LacZ-Neo cassette.
Further, we assessed the degree of expression of Pde11a in three genotypes. Using primers for the 5’ region of the mouse Pde11a gene, we quantified by qRTPCR total Pde11a mRNA of Pde11a+/+, Pde11a+/− and Pde11a−/− animals. Total Pde11a mRNA was significantly reduced in Pde11a−/− and Pde11a+/− mice, compared to the Pde11a+/+ animals but not completeled deleted (Figure 2B). Pde11a protein was also reduced – as an example, testicular and adrenal Pde11a protein is shown, normalized against ß-actin (Figure 2C). Finally, analysis of the effect of loss of Pde11a on the adrenal was performed by immunofluorescence, Pde11a immunoreactivity is higher in zona fasciculata and present in both genotype, albeit reduced in Pde11a−/− adrenal cortex (Figure 2D). These results indicated that despite correct identification of Pde11a−/− vs. Pde11a+/− (heterozygote, HET) vs. Pde11a+/+ by primers described by Wayman et al (Wayman, et al., 2005) (Supplementary Figure 1), the Pde11a gene was not completely deleted in Pde11a−/− tissues; the complete transcript of Pde11a, including the Neo-cassette was still present and Pde11a protein still expressed in the tissues examined from the Pde11a−/− mice described by Wayman et al., albeit at reduced amounts vs. Pde11a+/+.
3.3. PDE activity and cAMP levels
PDE activity was measured in tissue lysates from adrenal glands from 6-mo old Pde11a+/+ and Pde11a−/− mice at baseline conditions (no inhibitor, control) and in the presence of IBMX (a non-selective PDE inhibitor) and BC11–38 (a novel specific PDE11 inhibitor). Since Pde11a is highly expressed in testis and his activity has been previously very well characterized in this mouse model, we decided to use testicular lysates as an external control for this study on adrenal PDE activity using inhibitors. In adrenal glands (Figure 3A), PDE activity was significantly decreased in both Pde11a+/+ (505.3 vs 1532.6 Arbitrary Units, p=0.01) and Pde11a−/− (369.6 vs 1681.6 Arbitrary Unitis, p<0.01) adrenals following IBMX treatment. Treatment with BC11–38 did not attenuate PDE activity in Pde11a+/+ or in Pde11a−/− (1629.7 vs 1532.6 Arbitrary Unitis, p=0.83 and 1770.6 vs 1681.6 Arbitrary Unitis, p=0.78) adrenal glands. In testis (Figure 3B) overall PDE activity was higher in Pde11a+/+ than in Pde11a−/− (441.9 vs 374.5 Arbitrary Unitis, p=0.01). Treatment with IBMX or BC11–38 resulted in a significant attenuation in PDE activity in Pde11a+/+ testis lysates (382.8 and 351.6 respectively vs 442 Arbitrary Unitis, p=0.02 and p 0.001 respectively), however, there was no significant difference in Pde11a−/− testis following BC11–38 or IBMX treatment when compared with no inhibitor control (p=043 and p=0.16 respectively). In the adrenal, as previously described, Pde11a expression is low, therefore technically the PDE assays is not sensitive enough to determine difference in wild type and even less in knockout tissue. This data shows the impact of selective inhibition for PDE11 is best appreciated in Pde11a+/+ testis and is blunted in Pde11a−/− as expected. The cAMP levels were significantly higher in adrenal glands of Pde11a−/− mice compared to adrenals of wild type animals (p=0.0038, Figure 3C).
Figure 3: Measurements of PDE11A activity.
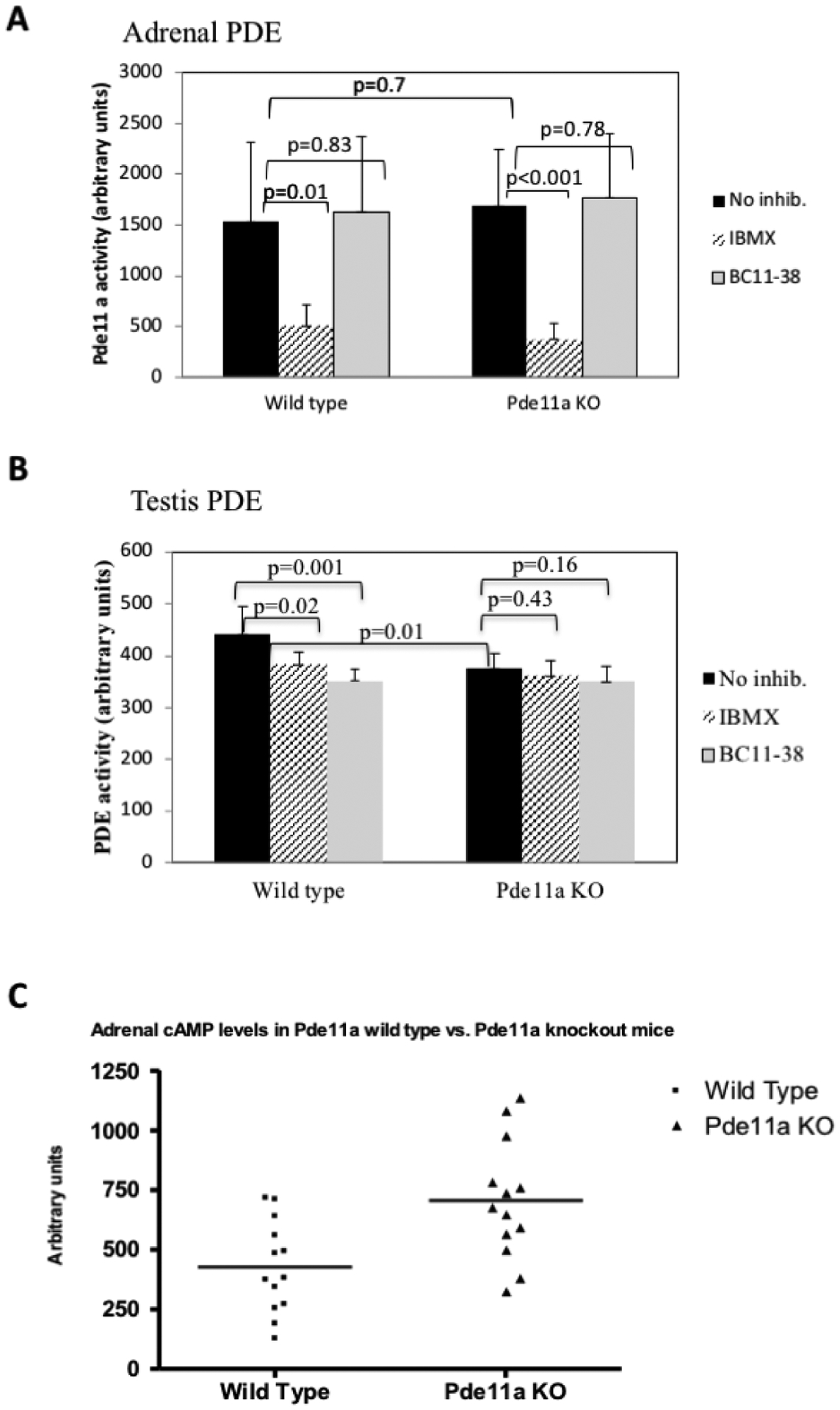
Measurements of PDE11A activity in adrenal (A) and testis (B) using PDE inhibitors, on non-specific for PDEs (IBMX) and on that is specific for PDE11A (BC11–38). In testis of wild type mice there is inhibiton of PDE11A activity by both inhibitors whereas in Pde11a knockout (Pde11a−/−) animals, the inhinbition by the specific inhibitor (BC11–38) is blunted demonstrating decreased substrate (PDE11A) in Pde11a−/− animals. In the adrenal, there is no inhibition of PDE11A activity by the specific inhibitor (BC11–38) in Pde11a−/− or wild type (Pde11a+/+) animals. (C) cAMP levels in Pde11a+/+ compared to Pde11a−/− mice. cAMP levels are higher in the adrenals of the knockout animals (p<0.001).
3.4. Dexamethasone suppression testing and PKA activity
We subjected Pde11a+/+ and Pde11a−/− to dexamethasone suppression assessing corticosterone as well as cortisol and ACTH levels, as per the test performed in humans (Stratakis, et al., 1999). Low dose dexamethasone (0.062 mg/100g of body weight) treatment resulted in the suppression of corticosterone secretion in Pde11a+/+ (0.297 ng/ml) but not in Pde11a−/− (0.424 ng/ml) mice. On the other hand, high dose dexamethasone (0.25 mg/100g body weight) treatment resulted in the suppression of corticosterone in both Pde11a+/+ (0.141 ng/ml) and Pde11a−/− (0.193 ng/ml) mice (Figure 4A). ACTH levels were suppressed as expected, however following high dose dexamethasone treatment in Pde11a−/− mice, suppression did not reach statistical significance. ACTH levels at baseline and after high-dexamethasone were 136.0 vs. 0 pg/ml in Pde11a+/+, p<0.01 and 303.8 vs. 64.4 pg/ml Pde11a−/−, p=0.3 (Figure 4B). PKA activity, both at baseline (Figure 4C) and after the addition of cAMP (Figure 4D), was generally higher in Pde11a−/− mice compared to Pde11a+/+ animals; however, the difference was not significant neither following the addition of cAMP (p=0.14) nor at baseline (p=0.3). No cortisol was detected in Pde11a−/− mice.
Figure 4: Corticosterone and ACTH responses to low and high dose dexamethasone and Adrenal PKA activity.
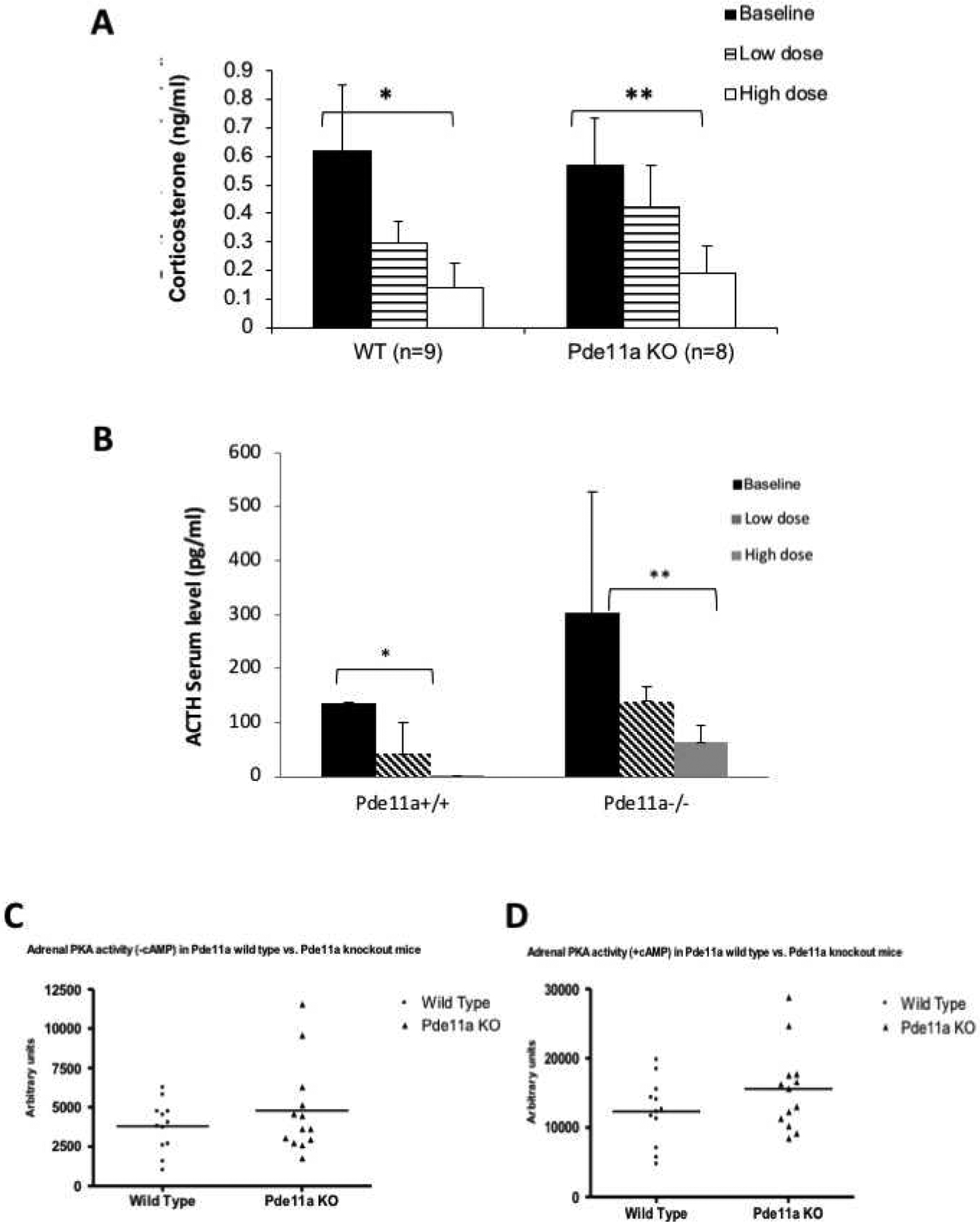
(A) Both wild type (Pde11a+/+) and knockout (Pde11a−/−) animals respond with suppression of their corticosterone levels to dexamethasone; however, the Pde11a−/− mice fail to suppress significantly in response to low dose dexamethasone; they suppressed, comparable to Pde11a+/+, in response to high-dose dexamethasone. Overall, there are no significant differences between Pde11a+/+ and Pde11a−/− mice. *p = 0.001 and p < 0.001 in low and high doses respectively. **p = 0.08 and p < 0.001 in low and high doses respectively. (B) ACTH responses to low and high dose dexamethasone: ACTH levels were suppressed as expected, however following high dose dexamethasone treatment in Pde11a−/− mice, suppression did not reach statistical significance (*p < 0.01, **p = 0.3) (C) Total adrenal PKA activity at baseline and after the addition of cAMP (D) in Pde11a+/+ and Pde11a−/− animals; knockout mice have higher PKA activity, but the difference only approached tendency to be significant after the addition of cAMP (p = 0.14).
3.5. Pde11a−/− mice showed adrenal hyperplasia and zonation defects
The adrenal cortex of 12-month old Pde11a+/+ had significantly more foamy cells than Pde11a−/− mice (Figure 5A); subcapsular hyperplasia (Figure 5B), as well as hyperplasia of the X-zone (Figure 5C) were predominant in Pde11a−/− adrenals (52% vs. 30% of all mice together, p=0.05). The X-zone was absent in all but one of the wild type female mice. In contrast, about half of heterozygous and half of knockout females had changes consistent with X-zone. In males, number of mice with changes consistent with X-zone were the same in all genotypes without statistically significant difference in number. For reasons that we can not explain, the transition from fetal to definitive adrenal cortex is better appreciated in female mice than in males (Supplementary Table 2). These changes were present at 6 months of age but became significant at 12 months. Those changes were statistically predominant in Pde11a−/− adrenals. Similar changes were seen in few Pde11a+/+ adrenals however none of them were confirmed with X-zone markers. To further investigate, and confirm, the hyperplasia seen in the X-zone, we stained for the adrenal zonation markers 20αHSD (a marker of the X-zone) and Akr1b7 (a marker for the zona fasciculata) (Figure 6A). We indeed confirmed persistence of the X-zone of the adrenal cortex in female Pde11a−/− mice, when compared to those from Pde11a+/+ animals; we even detected resurgence of the X-zone in primiparous mice (Figure 6B). In RNA expression, there was significant upregulation of Cyp17 expression in Pde11a−/− mice, consistent with X-zone persistence and/or reemergence (Figure 6C); this overexpression was mostly driven by female mice Cortisol levels were below the assay’s detection limits, despite the significant overexpression of Cyp17.
Figure 5: Histological appearance, as shown by H&E staining, of adrenal glands from 12-month old.
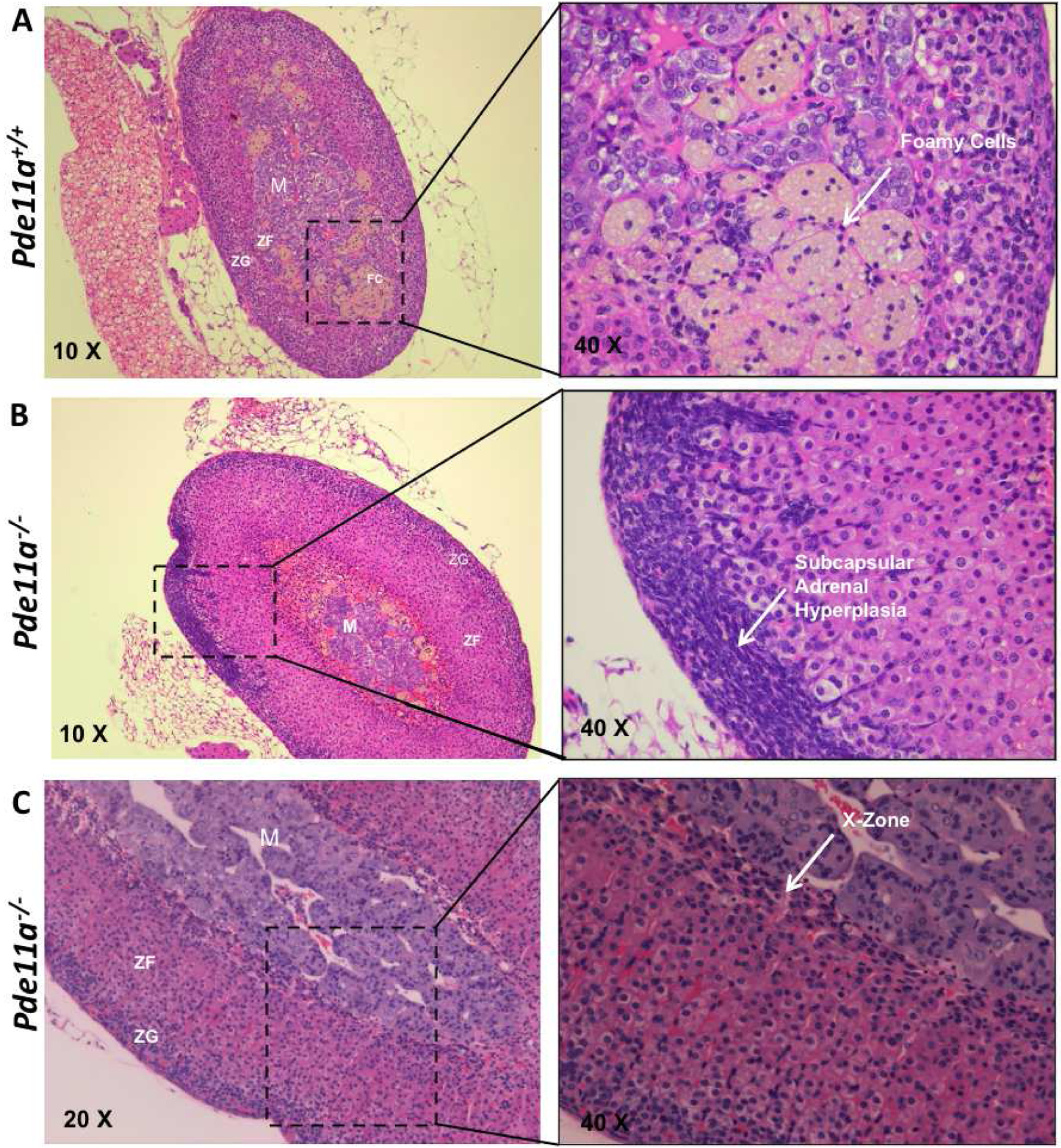
(A) Pde11a+/+ and (B & C) Pde11a−/− mice. Arrow point in (A), the presence of foamy cells in the adrenal cortex, more frequently observed in Pde11a+/+; in (B), the presence of subcapsular adrenal hyperplasia in female Pde11a−/− mice; in (C), the persistence of the X- or fetal-zone in female Pde11a−/−mice. Abbreviations: M, medulla; ZF, zona fasiculata; ZG, zona glomerulosa.
Figure 6: Persistent X-zone and mRNA expression levels of some markers in wild type (Pde11a+/+) and knockout (Pde11a−/−) animals.
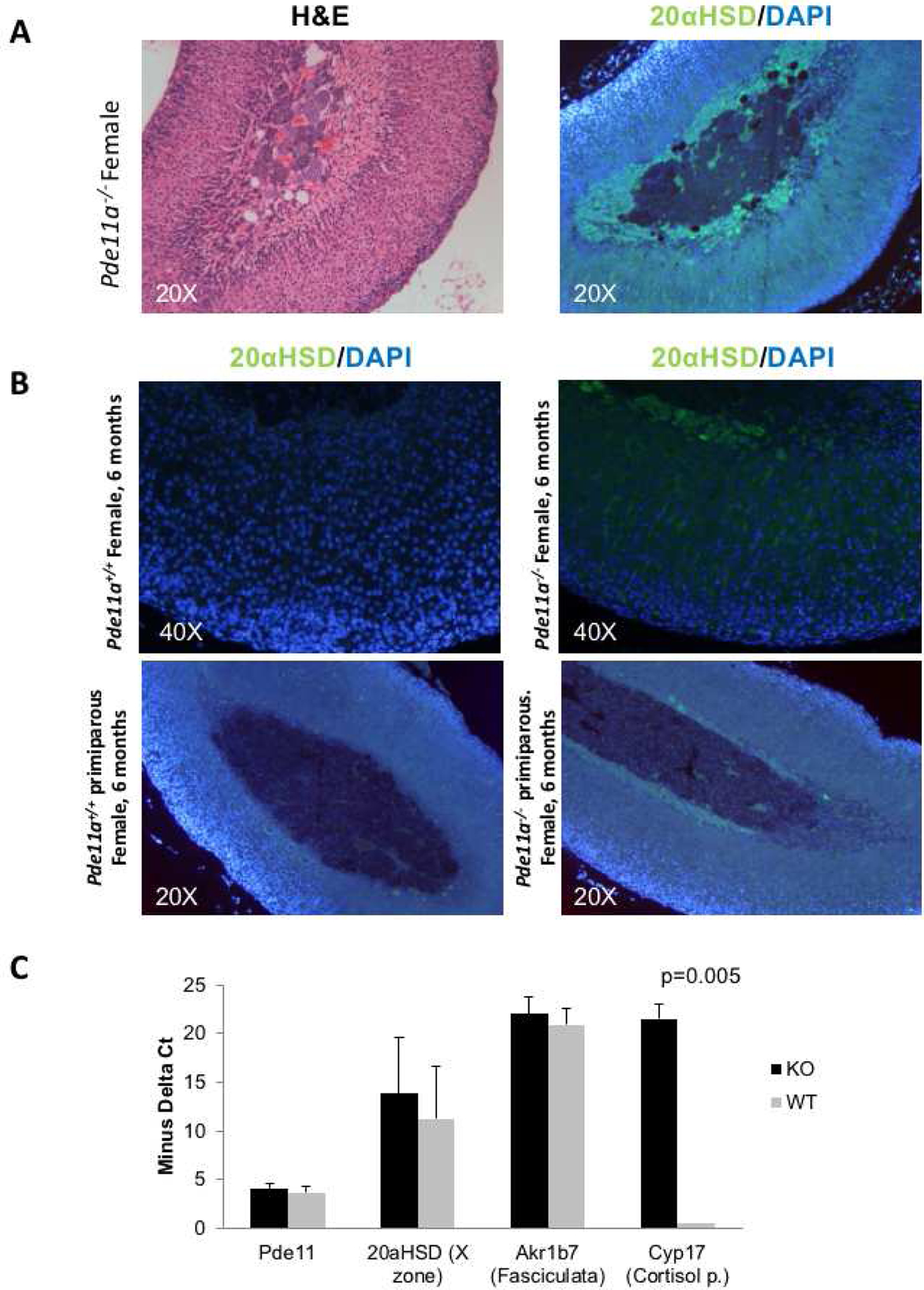
Persistent X-zone as shown by immunostaining with the X-zone-specific marker 20α-hydroxy-steroid dehydrogenase (20αHSD) (green) in Pde11a−/− mice. A. H&E and staining of the corresponding sections with an antibody specific for 20αHSD; B. Presence of the X zone in 2 animals and its absence in their age-matched littermates (one pair at 6 months and the other at 16 months); all are animals that had at least one pregnancy. C. mRNA expression levels of Pde11a, 20αHSD, Akr1b7 and Cyp17 in wild type and Pde11a−/− animals at 6 months of age; Cyp17 is substantially higher in Pde11a−/− animals.
3.6. Other phenotype differences between Pde11a−/− and Pde11a+/+ mice
There were no other significant consistent phenotypic differences found between Pde11a+/+, Pde11a+/− and Pde11a−/− animals. Total body weight and life span did not differ between genotypes. Serum biochemical profiles, including renal and liver function test and blood cell count did not differ between genotypes. No tumors or other histological difference between genotypes were found in dissected tissues from each organ analyzed after hematoxylin/eosin staining by an experienced pathologist.
4. DISCUSSION
We and others have shown that PDE11A genetic variants in humans predispose to adrenal, testicular and prostate tumors (Horvath, et al., 2006a; Horvath, et al., 2006b; Libe, et al., 2008; Horvath, et al., 2009; Faucz, et al., 2011; Libe, et al., 2011). It was a natural extension of this work to look at the phenotype of animal models with PDE11A deficiency, after first identifying widespread Pde11A expression in developing and adult mice (Supplentary Figure 2, Supplementary Table 1, Figure 1). There have been at least two mouse models with Pde11a genetic silencing described: the mice we studied here (Wayman, et al., 2005) and a more recent model described by Kelly et al. (Kelly, et al., 2010). The latter mouse has been studied extensively for its behavioral abnormalities (Hegde, et al., 2016a; Hegde, et al., 2016b; Kelly, 2017; Pilarzyk, et al., 2019). We obtained the mouse reported by Wayman et al. (Wayman, et al., 2005) and we were surprised to detect mRNA expression of Pde11a. We decided to fully characterize these mice because PDE11A expression and activity were less than the corresponding Pde11a+/+ littermates in several tissues, including the adrenal gland, brain, testis and lung (Figure 2B). The hypomorphic Pde11a−/− mouse presented us with the opportunity to study in vivo effects of decreased PDE11A activity along the lines of what human patients with inactivating PDE11A variants may have in their tissues. The data are consistent with what more than a decade of research has shown for PDE11A deficiency: despite some abnormalities in biochemistry (Figure 3) and adrenal gland histology (Figure 5 and 6, Supplementary Table 2) and spermatogenesis (Wayman, et al., 2005), adrenocortical steroid hormone production is basically normal (Figure 4) and these mice lived normal life spans without any obvious differences in tumor formation or other abnormalities in any of the tissues we examined, up to the age of 18 months.
PDE11A partial deficiency just like this gene’s haploinsufficiency by inactivating variants maybe considered more of a risk and/or predisposing factor than a causative one for the phenotypes it has been linked to: adrenocortical tumors (Horvath, et al., 2006a; Horvath, et al., 2006b; Libe, et al., 2008; Carney JA, et al., 2010) prostate cancer (Horvath, et al., 2006a; Horvath, et al., 2006b; Libe, et al., 2008; Faucz, et al., 2011) and testicular germ cell tumors (Horvath, et al., 2009; Mirabello, et al., 2012; Pathak, et al., 2015).
In patients with Carney complex (CNC) and causative PRKAR1A mutations that were shown to increase cAMP signaling (Kirschner, et al., 2000), the concurrent presence of PDE11A-inactivating variants clearly enhanced the severity of the CNC-associated adrenocortical and testicular tumor phenotypes (Libe, et al., 2011).
It is tempting to speculate that even though PDE11A inactivation leads to increased cAMP levels, as this study and a number of others in humans and mice have shown (Horvath, et al., 2006a; Horvath, et al., 2006b; Libe, et al., 2008; Kelly, et al., 2010), the effects of this increase are mitigated by the complexity of downstream signals and the presence of a multitude of other PDEs in most tissues (Levy, et al., 2011; Azevedo, et al., 2014; Szarek & Stratakis, 2014; Leal, et al., 2015). Nevertheless, increased cAMP signaling at the tissue level leads to abnormalities such as the ones described by Wayman et al. (Wayman, et al., 2005) and the ones we described here: adrenocortical hyperplasia and, in particular, X-zone persistence and/or reemergence is more prevalent in female animals (Figure 5 and 6). These histologic abnormalities have been seen in at least two other mouse models of increased cAMP signaling in the adrenal cortex that tested the effects of PRKAR1A-inactivation (Griffin, et al., 2004a; Griffin, et al., 2004b; Sahut-Barnola, et al., 2010). Female animals were more affected in both genotypes, as well as the only other mouse model of a cAMP-specific PDE which examined adrenocortical function, that of Pde8 deficiency (Tsai, et al., 2011).
PDE11A as a risk factor has been implicated in phenotypes linked to abnormal adrenocortical function, such as depression and related mental health issues and abnormal behaviors (Hegde, et al., 2016a; Hegde, et al., 2016b; Kelly, 2017), suicide (Coon, et al., 2013), and hypertension (Ohlsson, et al., 2016). Most recently it has been linked to asthma and other respiratory diseases possibly due to abnormal cAMP signaling (Sakornsakolpat, et al., 2019; Mu, et al., 2020) and sleep dysregulation (Jones, et al., 2019). Along with other PDEs, it is found frequently mutated in pediatric adrenocortical cancer (Pinto, et al., 2020) and may be involved in predisposition to other malignancies, including breast and ovarian cancer (Oliver, et al., 2019; Shahi, et al., 2019). The present study shows a hypomorphic Pde11a−/− mouse has a very modest phenotype in the adrenal gland beyond what was reported before in the testis (Wayman, et al., 2005). These data underline the need for additional animal modeling of PDE11A, one that perhaps involves tissue-specific genetic manipulation and/or crossing with other models of predisposing gene(s) to further delineate this gene’s involvement in disease pathogenesis.
Supplementary Material
Highlights.
Genetic defects in PDE11A may predispose adrenocortical tumors development.
Pde11a−/− mice failed to suppress corticosterone secretion in response to low dose dexamethasone.
Phenotype of Pde11a inactivated in mice is consistent with patients with PDE11A haploinsufficiency.
5. ACKNOWLEDGEMENTS
This work was funded by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD20892, USA. We are grateful to Dr. Charles Hoffman, Boston College, Boston, MA, USA who provided us with the BC11-38 PDE11A-specific inhibitor. We wish to thank Mr. Spyros Koliovasilis, Mr. Alejo Sierra and Dr. Anelia Horvath, who - helped during the first phases of this project. We also want to thank Dr. Charles S. Hoffman, Biology Department at Boston College, Boston, MA, USA, who provided to us the novel BC11-38 specific PDE11A inhibitor.
Disclosure statement: Dr. Stratakis holds patents on the function of the PRKAR1A, PDE11A, and GPR101 genes and related issues; his laboratory has also received research funding on GPR101 and its involvement in acromegaly and/or gigantism, abnormal growth hormone secretion and its treatment by Pfizer, Inc.; Dr. Faucz holds patent on the GPR101 gene and/or its function; The other authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Azevedo MF, Faucz FR, Bimpaki E, Horvath A, Levy I, de Alexandre RB, Ahmad F, Manganiello V and Stratakis CA. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev, 35 (2014), 195–233. 10.1210/er.2013-1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA, Gaillard RC Bertherat J, Stratakis CA. Familial micronodular adrenocortical disease, Cushing syndrome, and mutations of the gene encoding phosphodiesterase 11A4 (PDE11A). Am J Surg Pathol. 34,4 (2010): 547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan O, Birsoy K and Hoffman CS. Identification of biologically active PDE11-selective inhibitors using a yeast-based high-throughput screen. Chem Biol, 19 (2012), 155–163. 10.1016/j.chembiol.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Coon H, Darlington T, Pimentel R, Smith KR, Huff CD, Hu H, Jerominski L, Hansen J, Klein M, Callor WB, Byrd J, Bakian A, Crowell SE, McMahon WM, Rajamanickam V, Camp NJ, McGlade E, Yurgelun-Todd D, Grey T and Gray D. Genetic risk factors in two Utah pedigrees at high risk for suicide. Transl Psychiatry, 3 (2013), e325 10.1038/tp.2013.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucz FR, Horvath A, Rothenbuhler A, Almeida MQ, Libe R, Raffin-Sanson ML, Bertherat J, Carraro DM, Soares FA, Molina Gde C, Campos AH, Alexandre RB, Bendhack ML, Nesterova M and Stratakis CA. Phosphodiesterase 11A (PDE11A) genetic variants may increase susceptibility to prostatic cancer. J Clin Endocrinol Metab, 96 (2011), E135–140. 10.1210/jc.2010-1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA and Phillips SC. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci U S A, 97 (2000), 3702–3707. 10.1073/pnas.050585197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos S, Robinson-White A, Lenherr S, Weinberg FD, Claflin E, Meoli E, Cho-Chung YS and Stratakis CA. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res, 64 (2004a), 8811–8815. 10.1158/0008-5472.CAN-04-3620 [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos SG, Robinson-White A, Lenherr SM, Weinberg FD, Claflin ES, Batista D, Bourdeau I, Voutetakis A, Sandrini F, Meoli EM, Bauer AJ, Cho-Chung YS, Bornstein SR, Carney JA and Stratakis CA. A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet, 41 (2004b), 923–931. 10.1136/jmg.2004.028043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Capell WR, Ibrahim BA, Klett J, Patel NS, Sougiannis AT and Kelly MP. Phosphodiesterase 11A (PDE11A), Enriched in Ventral Hippocampus Neurons, is Required for Consolidation of Social but not Nonsocial Memories in Mice. Neuropsychopharmacology, 41 (2016a), 2920–2931. 10.1038/npp.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Ji H, Oliver D, Patel NS, Poupore N, Shtutman M and Kelly MP. PDE11A regulates social behaviors and is a key mechanism by which social experience sculpts the brain. Neuroscience, 335 (2016b), 151–169. 10.1016/j.neuroscience.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libe R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I and Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet, 38 (2006a), 794–800. 10.1038/ng1809 [DOI] [PubMed] [Google Scholar]
- Horvath A, Giatzakis C, Robinson-White A, Boikos S, Levine E, Griffin K, Stein E, Kamvissi V, Soni P, Bossis I, de Herder W, Carney JA, Bertherat J, Gregersen PK, Remmers EF and Stratakis CA. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res, 66 (2006b), 11571–11575. 10.1158/0008-5472.CAN-06-2914 [DOI] [PubMed] [Google Scholar]
- Horvath A, Korde L, Greene MH, Libe R, Osorio P, Faucz FR, Raffin-Sanson ML, Tsang KM, Drori-Herishanu L, Patronas Y, Remmers EF, Nikita ME, Moran J, Greene J, Nesterova M, Merino M, Bertherat J and Stratakis CA. Functional phosphodiesterase 11A mutations may modify the risk of familial and bilateral testicular germ cell tumors. Cancer Res, 69 (2009), 5301–5306. 10.1158/0008-5472.CAN-09-0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, van Hees VT, Mazzotti DR, Marques-Vidal P, Sabia S, van der Spek A, Dashti HS, Engmann J, Kocevska D, Tyrrell J, Beaumont RN, Hillsdon M, Ruth KS, Tuke MA, Yaghootkar H, Sharp SA, Ji Y, Harrison JW, Freathy RM, Murray A, Luik AI, Amin N, Lane JM, Saxena R, Rutter MK, Tiemeier H, Kutalik Z, Kumari M, Frayling TM, Weedon MN, Gehrman PR and Wood AR. Genetic studies of accelerometerbased sleep measures yield new insights into human sleep behaviour. Nat Commun, 10 (2019), 1585 10.1038/s41467-019-09576-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP. A Role for Phosphodiesterase 11A (PDE11A) in the Formation of Social Memories and the Stabilization of Mood. Adv Neurobiol, 17 (2017), 201–230. 10.1007/978-3-319-58811-7_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Logue SF, Brennan J, Day JP, Lakkaraju S, Jiang L, Zhong X, Tam M, Sukoff Rizzo SJ, Platt BJ, Dwyer JM, Neal S, Pulito VL, Agostino MJ, Grauer SM, Navarra RL, Kelley C, Comery TA, Murrills RJ, Houslay MD and Brandon NJ. Phosphodiesterase 11A in brain is enriched in ventral hippocampus and deletion causes psychiatric disease-related phenotypes. Proc Natl Acad Sci U S A, 107 (2010), 8457–8462. 10.1073/pnas.1000730107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS and Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet, 26 (2000), 89–92. 10.1038/79238 [DOI] [PubMed] [Google Scholar]
- Lakics V, Karran EH and Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology, 59 (2010), 367–374. 10.1016/j.neuropharm.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Leal LF, Szarek E, Faucz F and Stratakis CA. Phosphodiesterase 8B and cyclic AMP signaling in the adrenal cortex. Endocrine, 50 (2015), 27–31. 10.1007/s12020-015-0621-y [DOI] [PubMed] [Google Scholar]
- Levy I, Horvath A, Azevedo M, de Alexandre RB and Stratakis CA. Phosphodiesterase function and endocrine cells: links to human disease and roles in tumor development and treatment. Curr Opin Pharmacol, 11 (2011), 689–697. 10.1016/j.coph.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libe R, Fratticci A, Coste J, Tissier F, Horvath A, Ragazzon B, Rene-Corail F, Groussin L, Bertagna X, Raffin-Sanson ML, Stratakis CA and Bertherat J. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin Cancer Res, 14 (2008), 4016–4024. 10.1158/1078-0432.CCR-08-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libe R, Horvath A, Vezzosi D, Fratticci A, Coste J, Perlemoine K, Ragazzon B, Guillaud-Bataille M, Groussin L, Clauser E, Raffin-Sanson ML, Siegel J, Moran J, Drori-Herishanu L, Faucz FR, Lodish M, Nesterova M, Bertagna X, Bertherat J and Stratakis CA. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab, 96 (2011), E208–214. 10.1210/jc.2010-1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K, Taylor J and Florio VA. 3’,5’-cyclic nucleotide phosphodiesterase 11A: localization in human tissues. Int J Impot Res, 17 (2005), 320–325. 10.1038/sj.ijir.3901317 [DOI] [PubMed] [Google Scholar]
- Makhlouf A, Kshirsagar A and Niederberger C. Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res, 18 (2006), 501–509. 10.1038/sj.ijir.3901441 [DOI] [PubMed] [Google Scholar]
- Mirabello L, Kratz CP, Savage SA and Greene MH. Promoter methylation of candidate genes associated with familial testicular cancer. Int J Mol Epidemiol Genet, 3 (2012), 213–227. [PMC free article] [PubMed] [Google Scholar]
- Mu G, Xiang Q, Zhang Z, Liu C, Zhang H, Liu Z, Pang X, Jiang J, Xie Q, Zhou S, Wang Z, Hu K, Wang Z, Jiang S, Qin X and Cui Y. PNPT1 and PCGF3 variants associated with angiotensin-converting enzyme inhibitor-induced cough: a nested case-control genome-wide study. Pharmacogenomics, (2020), 10.2217/pgs-2019-0167 [DOI] [PubMed] [Google Scholar]
- Nesterova M, Bossis I, Wen F, Horvath A, Matyakhina L and Stratakis CA. An immortalized human cell line bearing a PRKAR1A-inactivating mutation: effects of overexpression of the wild-type Allele and other protein kinase A subunits. J Clin Endocrinol Metab, 93 (2008), 565–571. 10.1210/jc.2007-1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterova MV, Sashchenko LP, Vasiliev VY and Severin ES. A cyclic adenosine 3’,5’-monophosphate-dependent histone kinase from pig brain. Purification and some properties of the enzyme. Biochim Biophys Acta, 377 (1975), 271–281. 10.1016/0005-2744(75)90309-5 [DOI] [PubMed] [Google Scholar]
- Ohlsson T, Lindgren A, Engstrom G, Jern C and Melander O. A stop-codon of the phosphodiesterase 11A gene is associated with elevated blood pressure and measures of obesity. J Hypertens, 34 (2016), 445–451; discussion 451. 10.1097/HJH.0000000000000821 [DOI] [PubMed] [Google Scholar]
- Oliver J, Quezada Urban R, Franco Cortes CA, Diaz Velasquez CE, Montealegre Paez AL, Pacheco-Orozco RA, Castro Rojas C, Garcia-Robles R, Lopez Rivera JJ, Gaitan Chaparro S, Gomez AM, Suarez Obando F, Giraldo G, Maya MI, Hurtado-Villa P, Sanchez AI, Serrano N, Orduz Galvis AI, Aruachan S, Nunez Castillo J, Frecha C, Riggi C, Jauk F, Gomez Garcia EM, Carranza CL, Zamora V, Torres Mejia G, Romieu I, Castaneda CA, Castillo M, Gitler R, Antoniano A, Rojas Jimenez E, Romero Cruz LE, Vallejo Lecuona F, Delgado Enciso I, Martinez Rizo AB, Flores Carranza A, Benites Godinez V, Mendez Catala CF, Herrera LA, Chirino YI, Terrazas LI, Perdomo S and Vaca Paniagua F. Latin American Study of Hereditary Breast and Ovarian Cancer LACAM: A Genomic Epidemiology Approach. Front Oncol, 9 (2019), 1429 10.3389/fonc.2019.01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, Stewart DR, Faucz FR, Xekouki P, Bass S, Vogt A, Zhang X, Boland J, Yeager M, Loud JT, Nathanson KL, McGlynn KA, Stratakis CA, Greene MH and Mirabello L. Rare inactivating PDE11A variants associated with testicular germ cell tumors. Endocr Relat Cancer, 22 (2015), 909–917. 10.1530/ERC-15-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarzyk K, Klett J, Pena EA, Porcher L, Smith AJ and Kelly MP. Loss of Function of Phosphodiesterase 11A4 Shows that Recent and Remote Long-Term Memories Can Be Uncoupled. Curr Biol, 29 (2019), 2307–2321 e2305. 10.1016/j.cub.2019.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto EM, Faucz FR, Paza LZ, Wu G, Fernandes ES, Bertherat J, Stratakis CA, Lalli E, Ribeiro RC, Rodriguez-Galindo C, Figueiredo BC and Zambetti GP. Germline Variants in Phosphodiesterase Genes and Genetic Predisposition to Pediatric Adrenocortical Tumors. Cancers (Basel), 12 (2020), 10.3390/cancers12020506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch G. Assay of phosphodiesterase with radioactively labeled cyclic 3’,5’-AMP as substrate. Naunyn Schmiedebergs Arch Pharmakol, 268 (1971), 272–299. 10.1007/BF00997262 [DOI] [PubMed] [Google Scholar]
- Rohlff C, Clair T and Cho-Chung YS. 8-Cl-cAMP induces truncation and down-regulation of the RI alpha subunit and up-regulation of the RII beta subunit of cAMP-dependent protein kinase leading to type II holoenzyme-dependent growth inhibition and differentiation of HL-60 leukemia cells. J Biol Chem, 268 (1993), 5774–5782. [PubMed] [Google Scholar]
- Sahut-Barnola I, de Joussineau C, Val P, Lambert-Langlais S, Damon C, Lefrancois-Martinez AM, Pointud JC, Marceau G, Sapin V, Tissier F, Ragazzon B, Bertherat J, Kirschner LS, Stratakis CA and Martinez A. Cushing’s syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet, 6 (2010), e1000980 10.1371/journal.pgen.1000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakornsakolpat P, McCormack M, Bakke P, Gulsvik A, Make BJ, Crapo JD, Cho MH and Silverman EK. Genome-Wide Association Analysis of Single-Breath DlCO. Am J Respir Cell Mol Biol, 60 (2019), 523–531. 10.1165/rcmb.2018-0384OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi RB, De Brakeleer S, Caljon B, Pauwels I, Bonduelle M, Joris S, Fontaine C, Vanhoeij M, Van Dooren S, Teugels E and De Greve J. Identification of candidate cancer predisposing variants by performing whole-exome sequencing on index patients from BRCA1 and BRCA2-negative breast cancer families. BMC Cancer, 19 (2019), 313 10.1186/s12885-019-5494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, Chrousos GP and Papanicolaou DA. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med, 131 (1999), 585–591. 10.7326/0003-4819-131-8-199910190-00006 [DOI] [PubMed] [Google Scholar]
- Szarek E and Stratakis CA. Phosphodiesterases and adrenal Cushing in mice and humans. Horm Metab Res, 46 (2014), 863–868. 10.1055/s-0034-1389916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LC, Shimizu-Albergine M and Beavo JA. The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland. Mol Pharmacol, 79 (2011), 639–648. 10.1124/mol.110.069104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman C, Phillips S, Lunny C, Webb T, Fawcett L, Baxendale R and Burgess G. Phosphodiesterase 11 (PDE11) regulation of spermatozoa physiology. Int J Impot Res, 17 (2005), 216–223. 10.1038/sj.ijir.3901307 [DOI] [PubMed] [Google Scholar]
- Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T and Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem, 275 (2000), 31469–31479. 10.1074/jbc.M003041200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


