Abstract
Tumor-infiltrating CD8 T cells are associated with improved patient survival and response to immunotherapy in various cancers. Persistent antigen leads to CD8 T-cell exhaustion, where proliferation/self-renewal and killing are divided within distinct subsets of CD8 T cells in the tumor. CD8 T-cell responses in chronic antigen settings must be maintained for long periods of time, suggesting that mechanisms that regulate chronic CD8 T-cell responses may differ from those in acute settings. Currently, factors that regulate the maintenance of stem-like CD8 T cells in the tumor or their differentiation into terminally differentiated cells are unknown. In this review, we discuss the role of dendritic cells in the activation and differentiation of CD8 T-cell subsets within secondary lymphoid tissue and tumors. In addition, we examine changes in CD4 T-cell differentiation in response to chronic antigens and consider how subset-specific mechanisms could assist the stem-like and terminally differentiated CD8 T-cell subsets. Finally, we highlight how tumor-infiltrating CD4 T cells and dendritic cells interact with CD8 T cells within organized lymphoid-like areas in the tumor and propose a CD8 T-cell differentiation model that requires the collaboration of CD4 T cells and dendritic cells. These organized interactions coordinate the anti-tumor response and control disease progression by mechanisms that regulate CD8 T-cell differentiation, which permit the maintenance of an effective balance of stem-like and terminally differentiated CD8 T cells.
Keywords: CD4 T cells, CD8 T-cell differentiation, dendritic cells, lymphoid-like niches, T-cell exhaustion
Niches containing DCs and CD4 cells control CD8 T cell anti-cancer responses
Introduction
Immune responses to pathogens are shaped by the coordinated ability of immune cells to discriminate between self and non-self, providing a clear paradigm of when and how to respond to exogenous antigens. An effective response to cancer, however, deviates from this model as the tumor antigens are self-derived. Cancer cells develop from the accumulation of mutations in oncogenes and tumor suppressor genes that routinely regulate crucial cellular functions, including growth rate and survival, leading to cell immortality (1, 2). Although originally disputed, given that cancer cells have self-origin, the immune system plays an indispensable role in tumor control and rejection (1, 3–5).
An increasing number of studies report that CD8 T-cell infiltration in tumors is an independent predictor of favorable patient prognosis and response to therapy across multiple cancer types (6–10). Although their role in mediating the anti-tumor response is clear, what regulates the CD8 T-cell responses in the tumor is poorly understood.
Given that CD4 T cells are essential helpers for CD8 T-cell responses during viral and bacterial infections, we examine different CD4 T-cell phenotypes infiltrating tumors and discuss how each CD4 subset-specific effector function assists in the organization, maintenance and differentiation of CD8 T cells. Additionally, we discuss the possibility of an organized model that allows for CD4 T cells and dendritic cells (DCs) to collaborate and contribute to CD8 T-cell differentiation in the tumor.
Intratumoral CD8 T cells mediate tumor growth and disease progression
CD8 T cells are critical for tumor control in various cancer types. In fact, tumor-infiltrating CD8 T cells independently predict patients’ survival and response to checkpoint blockade therapy (11–15). The evidence for a functional role of CD8 T cells to control tumor growth is compelling, as multiple groups report infiltrating activated CD8 T cells in the tumor prior to treatment to be predictive of response to immunotherapy in melanoma, non-small cell lung, renal cell and colorectal cancers (16–19). Effector CD8 T cells contribute to tumor control via the cytolytic lysis of their target tumor cells; however, the antigen specificity of these responsive cells in human tumors remains poorly defined.
T cells recognize self- and neo-antigens in cancer
The nature of the antigens recognized by tumor-specific T cells was first reported in 1989, where mouse CD8 T cells were responsive towards a self-peptide that had been mutated in cancer cells (20, 21). This observation was followed by a large number of studies that identified distinct classes of tumor antigens on the basis of their tissue of origin that can be presented to T cells via major histocompatibility complex (MHC) molecules (22). Antigens that can induce T-cell responses in cancer can be broadly classified into those that are tumor-specific (mutational and cancer-germline antigens), also known as neoantigens, and those that are derived from non-mutated self-proteins (differentiation and overexpressed antigens) (21).
Although several groups have identified T cells specific for mutational tumor antigens (23–28), significant challenges remain for ‘neoantigen’-targeted therapy, given that these antigens make up a small percentage of the overall T-cell response. The limitation of using neoantigens that can be targeted by T cells is highlighted by recent studies in melanoma, lung and colorectal tumors. CD8 T cells reacting to neoantigen epitopes are predicted to be at a rate of less than 0.5% of the total T-cell response in the tumor (24, 28). A recent study screened ~1200 potential tumor neoantigens with only 2 positive hits in a cohort of 24 patients, only accounting for 0.2% of the CD8 T-cell response in the tumor (25). Similarly, in a cohort of ~20 000 human cancer genomes less than 0.005% of the CD8 T-cell response is directed towards neoantigens (29). These data suggest that the majority of the T-cell response targets non-mutated self-antigens that vary across individual patients (5).
Thus, although antigen specificity may be limiting and variable in the majority of cancers, the reliance on a CD8 anti-tumor response for disease control is consistent across all cancer types. Given the importance of having a CD8 T-cell response in the tumor, irrespective of antigen specificity, it is crucial to understand the phenotype of infiltrating CD8 T cells, as well as the signals that sustain this long-term response in the tumor.
Two subsets of exhausted CD8 T cells control the response to chronic viral infections and cancer
During an acute infection, naive CD8 T cells undergo effector differentiation, followed by population contraction and the formation of a minor population of memory cells following antigen clearance (Fig. 1A). Tumors and chronic viral infections, on the other hand, persist for long periods of time. Chronic antigen exposure results in responsive (virus-specific or tumor infiltrating) CD8 T cells to undergo substantial transcriptional and functional changes, leading them into a state known as T-cell exhaustion (Fig. 1C). CD8 T-cell exhaustion was first described in the chronic lymphocytic choriomeningitis virus (LCMV) mouse model, where antigen-specific CD8 T cells progressively lost the ability to proliferate and kill target cells, while acquiring sustained expression of inhibitory receptors (15, 30).
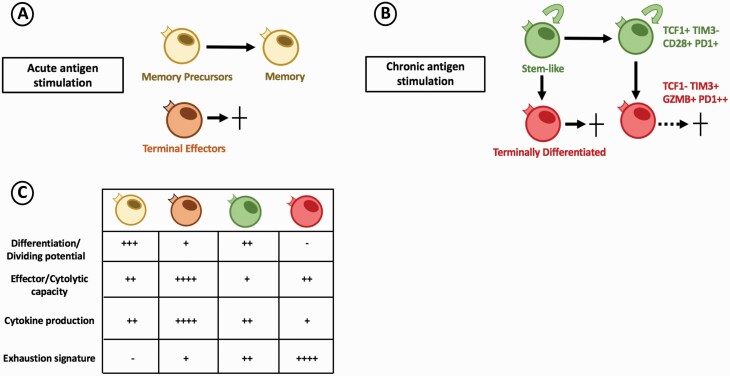
Fig. 1.
CD8 T-cell exhaustion model in chronic antigen stimulation. (A) At the peak of the T-cell response during acute antigen stimulation, responding CD8 T cells differentiate into a heterogenous effector pool composed of terminal effector and memory precursor cells. Upon antigen clearance, terminal effectors undergo cell death, while memory precursors survive and become functional memory cells. (B) Under chronic antigen stimulation, responding CD8 T cells differentiate into two subsets of PD-1-expressing exhausted CD8 T cells. The stem-like CD8 T-cell (green) maintains self-renewal and proliferative potential helping to sustain the pool of antigen-specific CD8 T cells. Stem-like cells also give rise to terminally differentiated (red) CD8 T cells which contribute to antigen (viral or tumor) control and progressively lose cytolytic function resulting in cell death. (C) Responding CD8 T-cell populations differ in phenotype and function during acute and chronic antigen stimulation.
This homogenous model of CD8 T-cell exhaustion has recently been challenged by several groups, where several distinct subsets of exhausted CD8 T cells were described in chronic LCMV, highlighting the heterogeneity that exists within the virus-specific CD8 T-cell population (31–35). Broadly, these subsets can be divided into stem-like cells that are capable of self-renewal and further differentiation and their progeny, terminally differentiated cells that retain some cytolytic capacity (15, 31–34). Importantly, both the stem-like and terminally differentiated CD8 T cells differ in transcription and function from canonical effector and memory CD8 T cells from acute viral infections (15, 36). Although both of these subsets of exhausted cells express the checkpoint molecule programmed cell death protein 1 (PD-1), they differ in phenotype and function during the response to chronic antigen. The stem-like cells can be defined by their high expression of co-stimulatory molecules, including CD28, and transcription cell factor 1 (TCF1). TCF1 regulates the transcriptional program of the stem-like CD8 T cells and is also shared with hematopoietic stem cells, which are known for their ability to be maintained in an undifferentiated state (37). In contrast, the terminal differentiated cells have higher expression of checkpoint molecules, including TIM3 and CD244, as well as cytotoxic molecules, including granzyme B (GZMB), and perforin (15). These findings established a new model of T-cell exhaustion, where proliferation/self-renewal and cytolytic function are divided within distinct subsets of the responding CD8 T cells (Fig. 1B). It is important to point out that, although T-cell exhaustion prevents optimal viral clearance or full eradication of tumors, the terminally differentiated cells retain some cytolytic function. Thus, although these cells are considered hypofunctional, they are crucial to control viral load without severe immunopathology. Therefore, the stem-like CD8 T cells sustain the CD8 response in chronic antigen settings by self-renewal and by generating shorter-lived terminally differentiated cytotoxic CD8 T cells. This elegant compartmentalization of roles allows for an organized and continuous response of CD8 T cells in chronic antigen settings.
Given that chronic antigen exposure is one of the key drivers of T-cell exhaustion, its understanding is also of particular relevance in cancer. This model of CD8 T-cell exhaustion is not restricted to chronic viral infections, as the stem-like and terminally differentiated CD8 T-cell subsets were also found to infiltrate tumors and their respective functions are critical for tumor control and response to therapy in various cancers (11, 14, 33, 37). Although the proportions of stem-like and terminally differentiated CD8 T cells vary between patients and cancer types, these cells make up the majority of the responsive CD8 T-cell pool in human and mouse tumors (11, 38–40). Several groups have now reported the frequency of stem-like CD8 T cells within tumors to be associated with better clinical outcomes in cancer patients (11, 38). In fact, the frequency of stem-like CD8 T cells in the tumor is predictive of the magnitude of the bulk-activated (PD-1+) CD8 T-cell response. Stem-like CD8 T cells also provide the proliferative burst after blockade of PD-1 (31), making their maintenance also essential in the response to immunotherapy. The benefit of having stem-like cells in the tumor has also been reported in immunotherapy human clinical studies, where patients with higher infiltration of TCF1-expressing CD8 T cells in the tumor had a better response to therapy in various cancer types (38, 41, 42). These observations suggest that the presence of the stem-like CD8 T-cell population within the tumor is crucial for (i) maintenance of the pool of responsive CD8 T cells to the persistent tumor antigens, (ii) continued differentiation into terminally differentiated cells that retain some cytolytic capacity and (iii) response to immunotherapy. It is thus critical that the CD8 T-cell terminal differentiation process continues within the tumor without losing the stem-like CD8 T-cell population. Therefore, understanding the cellular components and signals required at each step (activation, maintenance and terminal differentiation) of the CD8 T-cell response will have a significant impact on patients’ survival and potential targets for therapeutic interventions. This raises important questions about the CD8 T-cell response in the tumor. What other cell populations help the CD8 T-cell exhausted subsets in the tumor? How can activated stem-like CD8 T cells endure consistent antigen exposure and be maintained in a peripheral tumor tissue? What signals drive stem-like CD8 T-cell differentiation? What mechanisms regulate the balance of maintenance of stem-like CD8 T cells and terminally differentiated cells in the tumor?
DCs support the CD8 T-cell response in the tumor
DCs are critical to induce effective CD8 T-cell effector responses during viral or bacterial infections, thus holding great potential to also modulate CD8 T-cell responses in tumors. DCs are known for their ability to interact with CD8 T cells in secondary lymphoid tissues and regulate their antigen-specific activation and effector differentiation. Virus-specific stem-like CD8 T cells in the chronic LCMV model are preferentially located within splenic T-cell zones, where DCs also reside in large numbers (31). The restricted presence of stem-like CD8 T cells within secondary lymphoid tissues in chronic viral infections makes the presence of stem-like CD8 T cells within tumors surprising. These data suggest that, in order for these cells to be sustained in the tumor, other cells normally present in secondary lymphoid tissues may be critical for their maintenance in peripheral tissues.
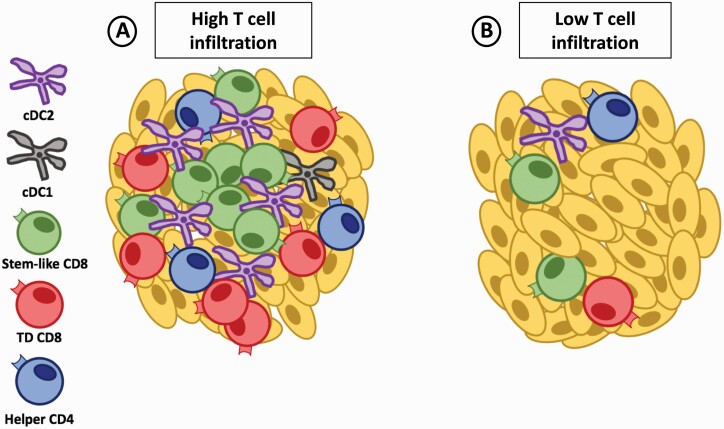
DCs, given their consistent presence in the tumor, represent a cell population that could support the CD8 T-cell response. In support of this notion, various groups report that DC infiltration correlates with the magnitude of CD8 T-cell population in tumors (11, 43, 44). Additionally, our group recently reported that DCs were the only myeloid population to positively correlate with and predict the presence and magnitude of stem-like CD8 T cells within prostate and kidney tumors (11). In addition, the stem-like CD8 T cells were located within immune cell rich structures in the tumor, which could serve as a ‘niche’ that allows for organized interactions between T cells and DCs outside of lymphoid tissues (Fig. 2A). Since tumors without immune niches have lower total T-cell and DC infiltration, the interaction between these cells could be crucial to maintain the stem-like CD8 T-cell population and drive their terminal differentiation in the tumor (Fig. 2B).
Fig. 2.
APC niches support the maintenance of stem-like CD8 T cells in the tumor. (A) Tumors with high T-cell infiltration have dense regions of lymphoid-like niches rich in cDC2 and helper CD4 T cells that help to maintain stem-like CD8 T cells. The stem-like CD8 T cells preferentially reside within these lymphoid-like niches while the terminally differentiated (TD) CD8 T cells migrate to the periphery away from the DC-rich environment to undergo their cytolytic function. (B) Tumors with low T-cell infiltration lack dense regions of lymphoid-like structures in the tumor and progressively lose the CD8 T-cell pool potentially because of a failure to maintain the stem-like CD8 T-cell subset.
The importance of DC populations both in mouse tumor models and in human disease is evident, but how they help to promote the T-cell response or maintain it within the tumor still needs to be fully described. Understanding DC–CD8 T-cell interactions in tumors may therefore be critical to understand all the components that contribute to an effective anti-tumor response.
Roles of DC subsets in the anti-tumor response
DCs are composed of several subsets, including conventional/classical dendritic (cDC) 1 cells (cDC1s), cDC2s and plasmacytoid DCs. cDC1s have been extensively studied in tumors because of their ability to cross-present exogenous antigens and preferentially activate CD8 T cells in secondary lymphoid tissues (45, 46). Their importance has been reported in various tumor models, as their absence is associated with increased tumor burden and reduced T-cell responses (43, 44). It has also been shown that cDC1s need the chemokine receptor CCR7 in order to migrate into tumor-draining lymph nodes (TDLNs) to activate tumor-specific CD8 T cells (47). These data are also supported in human studies, where a higher cDC1 signature correlates with better overall survival of patients in a wide range of cancers (43).
Activated CD8 T cells may also in turn recruit cDC1s in the LN, as studies in viral models have shown that activated CD8 T cells promote cDC1 aggregation by producing a variety of chemokines, such as XCL1 (48). The recruitment of cDC1s and the cytokines produced in response to viruses, including type I interferons, leads to the up-regulation of co-stimulatory molecules and pro-inflammatory cytokines on DCs that induce a more potent CD8 T-cell activation (48). In tumor models, the lack of type I interferon signaling on cDC1s greatly reduced the CD8 T-cell anti-tumor response, highlighting the importance of soluble factors that activate DCs that then promote CD8 T-cell activation or differentiation (49). Understanding signals that promote cDC1 activation in tumors, how they activate tumor-specific CD8 T cells and how this can be improved is crucial in the study of both T-cell and DCs in tumors.
Even though cDC1s have been linked to tumor control and better overall survival of patients, they are present in scarce numbers among the DC populations within both murine and human tumors (43, 50). cDC2s, instead, make up a large proportion of the DCs that are resident within the tumor (43, 50). Conventionally, cDC2s present antigen on MHC class II (MHC-II) and are thought to preferentially activate CD4 T cells (46), but their role in the tumor response is a new area of research. cDC2s are a heterogenous population and can acquire different phenotypes, as was recently shown in a study analyzing the cDC2 compartment in humans (51). Subsets of cDC2s were found to express monocyte markers (CD14 or CD163) and importantly these subsets were found to produce more pro-inflammatory cytokines, than the conventional cDC2s. The production of certain pro-inflammatory cytokines, including type 1 interferons, could be important to drive CD8 terminal differentiation in the tumor, mimicking the environment that induces potent effector T-cell responses in acute viral and bacterial infections. Recent work has shown in breast cancer that CD14-expressing cDC2s are present within the tumor and correlate with infiltration by tissue-resident CD8 cells, suggesting a potential connection of cDC2s and CD8 T cells within tumors (52).
Most studies have focused on the role of cDC1s and how they can promote the CD8 T-cell anti-tumor response. This population of DCs represents a small proportion of the DCs infiltrating tumors, suggesting other DC subsets most likely play a role in the T-cell response within the tumor. Although more remains to be learned about pro-inflammatory cDC2s, these cells have functional features that could promote CD8 T-cell differentiation in the tissue. Therefore, subsets of DCs (cDC1s and cDC2s) potentially have different roles in the activation and differentiation of tumor-specific CD8 T cells. A plausible explanation may be that the cDC1 population exerts their primary role in the TDLN, whereas the cDC2 population may play a larger role in maintaining the CD8 T-cell response within the tumor. The different subsets of conventional DCs and their possible functional role in the anti-tumor response are summarized in Table 1.
Table 1.
Summary of the phenotype and function of cDCs found in human and mouse TDLNs and tumors: cDC1s, cDC2s and inflammatory cDC2s
| Conventional DC subset | Phenotype | Phenotype | Proposed function | Proposed function |
|---|---|---|---|---|
| Human | Mouse | TDLN | Tumor | |
| cDC1 | CD11c+, HLA-DR+, CD141+, CLEC9a+ | CD11c+, MHC-II+, XCR1+, CD8a+, CD103+/− | Priming/primary activation of CD8 T cells Cross-presentation |
Migration from tumor to deliver tumor antigens to TDLN Associated with tumor control and better patient survival |
| cDC2 | CD11c+, HLA-DR+, CD1c+, CLEC10a+ pro-inflammatory subset: CD14+ CD163+ |
CD11c+, MHC-II+, CD11b+, CD16− | Priming/activation of CD4 T cells | Most abundant population within tumors Lineage differentiation of CD4 T cells via diverse cytokine secretion Support stem-like CD8 T cells within lymphoid-like niches in the tumor Pro-inflammatory cytokine secretion from CD14+ CD163+ cDC2s |
The function and primary location of each of these DCs are expanded upon in the main text.
Niches rich in antigen-presenting cells support the maintenance of stem-like CD8 T cells in the tumor
Given the compelling evidence that stem-like CD8 T cells are one of the key components for a productive anti-tumor response, it is important to understand how these cells are maintained in the tumor. We can gain insight about mechanisms that help to maintain the stem-like CD8 T-cell subset from the mouse model of chronic LCMV infection and, given its similarities with cancer, we can use it to understand the CD8 T-cell response in tumors.
Possible insights into mechanisms that sustain the stem-like CD8 subset come from differences in location and positioning between the stem-like cells and terminally differentiated cells throughout the course of the infection. Stem-like CD8 T cells are almost exclusively positioned in lymphoid tissues, whereas terminally differentiated cells are localized primarily in non-lymphoid peripheral tissues (18, 25). Additionally, the stem-like subset appears to preferentially reside within the splenic white pulp in the presence of DCs, whereas terminally differentiated cells migrate to the periphery or the splenic red pulp where viral loads are highest (31, 53). Weak co-stimulation and low antigen loads in the spleen have been shown to protect stem-like CD8 T cells from terminal differentiation (54); thus, the presence of antigen-presenting cell (APC)-rich lymphoid structures may be key in sustaining the T-cell response in chronic antigen settings. These data suggest that stem-like CD8 T cells may need organized structures to maintain the balance of self-renewal and their terminal differentiation by restricting their exposure to high levels of antigen.
A similar organization may exist within tumors to help support the stem-like cells outside lymphoid tissues. Recent work from our group in patients with kidney, prostate or bladder cancer showed the positioning of stem-like CD8 T cells to be restricted to organized secondary-lymphoid-like zones within the tumors that were densely populated by APCs and other lymphocyte populations (11). Similarly, stem-like CD8 T cells have also been described to primarily be located within tertiary lymphoid structures in melanoma or lung cancer patients (55, 56). Importantly, the presence of these ‘immune protective’ structures was positively correlated with a benefit in patients’ survival, as without these immune-populated niches the stem-like and total CD8 T-cell populations failed to be maintained and patients rapidly progressed after surgery (Fig. 2). Given that T-cell responses usually require complex and high levels of organization within secondary lymphoid tissues, the presence of lymphoid-like structures with a rich presence of APCs in the tumor suggests that these structures could allow for the continued interaction of DCs and CD8 T cells within the tumor. Thus, the maintenance and continued differentiation of the stem-like CD8 T-cell population in the tumor may be an organized and collaborative effort between various immune cells as represented in Fig. 2(A) and as further discussed in the following sections.
CD4 T-cell populations in chronic viral infections and cancer—CD4 T-cell subsets take center stage
As discussed in previous sections, consistent differentiation of stem-like CD8 T cells into terminally differentiated cells without losing the stem population within tumors is essential to maintain an efficient anti-tumor response. In addition to DCs, CD4 T cells play an important role in controlling the CD8 response in chronic viral infections. Thus, examining the functional relationship between CD4 T cells and the stem-like CD8 T-cell subset in the tumor may help us better understand what sustains and drives CD8 T-cell terminal differentiation in the tumor.
CD4 T-cell help is essential for the survival and effector differentiation of virus-specific CD8 T cells in chronic viral infections (39, 54, 57–60). CD8 T cells directly require CD4 T-cell factors that sustain CD8 T-cell differentiation and effector activity. Depletion of CD4 T cells during chronic LCMV infection results in an inability to clear the infection, leading to lifelong viremia (57). Similarly, the progression of human immunodeficiency virus (61) infection to AIDS and the associated CD8 T-cell dysfunction is associated with the loss of CD4 T cells in the periphery and secondary lymphoid organs (62).
Although CD4 T cells have been extensively studied in cancer, their function in the tumor remains unclear given that different subsets may play conflicting roles in the anti-tumor response. When taken together, bulk-activated CD4 T cells infiltrating tumors appear to positively impact the tumor response, as they enhance CD8 T-cell cytotoxicity and reduce disease burden (10, 39, 63–67). Although CD4 T cells are not required for initial activation of CD8 T cells, CD4 T-cell help appears to enhance the maintenance and function of effector CD8 T cells in the tumors (68, 69). The adoptive transfer of helper CD4 T cells in combination with tumor-specific CD8 T cells was most effective in sustaining CD8 effector functions and tumor regression in a melanoma model (64). In agreement, recent studies found a critical role for CD4 T-cell epitopes in enhancing the CD8 T-cell response in human tumors (10, 68, 69). The expression of MHC-II restricted antigens was essential to induce an effective CD8 T-cell response that resulted in tumor clearance, as well as render a non-responsive tumor into a checkpoint therapy responsive cell line (69). Importantly, CD4 T cells alone were not sufficient for tumor control, suggesting that they function to enhance the CD8 response at the tumor site. These observations indicate that helper subsets of CD4 T cells may play a functional role at the tumor site and positively contribute to the CD8 T-cell anti-tumor response. In addition, CD4 T cells appear to also be located within organized immune-rich areas within tumors (Figs 2 and 3) where they could interact with CD8 T cells and DCs to undertake their helper functions (65, 66, 70).
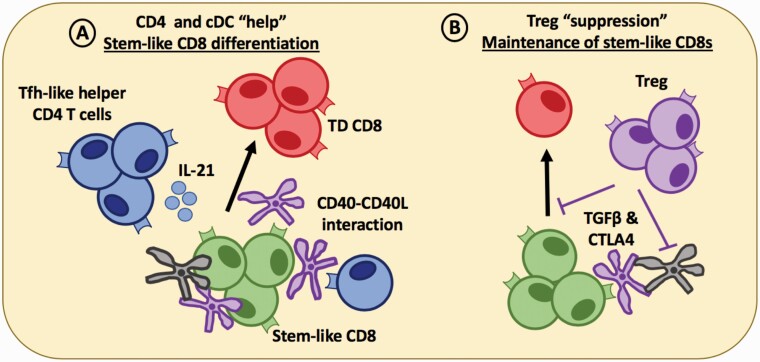
Fig. 3.
CD4 T cells relay help-signals via DCs that support CD8 T-cell differentiation in the tumor. (A) Within lymphoid-like niches in the tumor, helper Tfh-like CD4 T cells (blue) which also reside within APC-rich zones in the tumor, can license cDC1 (black) or cDC2 (purple) via CD40–CD40L interactions and secrete soluble factors, such as IL-21 that can help drive terminal differentiation (TD) of stem-like CD8 T cells. (B) Treg cells can secrete immunoregulatory cytokines, such as TGF-β to maintain the stem-like CD8 T-cell population in a quiescent state until the differentiation signal is delivered. Tregs can also block the co-stimulatory signals delivered to the stem-like CD8 T cells via CTLA4 interacting with CD80/86 on cDCs within the tumor. The combination of these signals may act to sustain a stem-like CD8 T-cell population that can be maintained for long periods of time within the tumor while driving the production of terminally differentiated CD8 T cells that maintain cytolytic capacity to control disease progression.
CD4 T-cell differentiation and the complete diversity of the CD4 T-cell subsets generated in response to tumor antigens is a growing area of study. Recent single-cell analysis from various tumor types in human patients revealed a variety of CD4 T-cell populations infiltrating the tumor (67, 71–73). In this section, we summarize what is currently known about the role of each subset of CD4 T cells in cancer and their potential role in regulating the balance of stem-like and terminally differentiated CD8 T cells in the tumor. Given that data on the diversity and role of helper CD4 T-cell populations in cancer are scarce, we can also consider what we know about CD4 populations and their contributions in other chronic antigen settings.
T-regulatory cells
Much of the CD4 T-cell research in cancer has been focused on T-regulatory (Treg) cells, which are defined as the CD4 population that negatively impacts the anti-tumor immune response in mouse tumor models and human patients (74, 75). As a prominent CD4 T-cell population in the tumor, Tregs represent ~15–30% of the infiltrating CD4 T cells in human patients (76, 77). Tregs generally hinder the anti-tumor response, as these cells are associated with reduced CD8 T-cell function, greater tumor burden and poor clinical prognosis. In fact, depletion of Tregs in vivo induces a more effective CD8 T-cell anti-tumor response, followed by rejection of the tumor in various murine tumor models (78–81).
The suppressive nature of Tregs is attributed to the constitutively high expression of the high-affinity IL-2 receptor (CD25), and inhibitory CTLA4 and TIGIT molecules. Additionally, these cells secrete high levels of immunoregulatory soluble factors including IL-10 and transcription growth factor β (TGF-β) that differentially regulate T cells and DCs in peripheral tissues (74, 75). How these soluble factors regulate the maintenance of stem-like CD8 T cells or their differentiation in cancer is not completely understood. Thus, we can use what we know from chronic viral infection models to speculate into how they may regulate the anti-tumor response.
IL-10 is an immunoregulatory cytokine that promotes the maintenance of CD8 T-cell exhaustion and attenuates effector CD8 T-cell responses in chronic viral infections (82). Although elevated levels of plasma/serum IL-10 are reported in cancer patients, the role of this cytokine in tumors is still unclear as both stimulatory and suppressive actions are reported, thus requiring further examination (83–85).
TGF-β is a cytokine with diverse immunoregulatory homeostatic functions (86); however the elevated blood levels during chronic LCMV infection, as well as in HIV-infected (61) or hepatitis C virus (HCV)-infected patients are associated with weak T-cell responses and viral persistence (86–89). TGF-β signaling via apoptosis-dependent pathways regulates the magnitude of the virus-specific CD8 T-cell pool in chronic LCMV infection (90). Although TGF-β regulates cell death, consistent signaling via the TGF-β receptor was not associated with further reduced function in the exhausted CD8 T-cell populations (90, 91). Although the role of TGF-β in chronic antigen settings is not fully understood, TGF-β signaling may be an important component that controls the CD8 effector response in cancer, thus preventing excessive pathophysiology and burnout of responsive CD8 T cells.
Recent data suggest that TGF-β, via its primary receptor (TGFBRI), may serve to maintain CD8 T cells in a ‘quiescent’ state prior to receiving all the signals required for full CD8 T-cell differentiation (92). Inhibitory TGF-β signaling is dominant during CD8 T-cell activation to low-affinity antigens and, in environments of poor immunogenicity, TGFBRI down-regulation does not occur, which prevents complete differentiation into an effector state (92). This suppressive role of TGF-β is particularly relevant in the tumor microenvironment, where TGF-β levels are elevated (93–95) and the majority of tumor antigens are of medium–low affinity (4, 24, 28). The maintenance of CD8 T cells of medium to low affinity during chronic antigen exposure is also common among viral infections in human and mice, whereas high-affinity CD8 T cells are deleted from the responsive pool (96, 97). In chronic LCMV and cytomegalovirus (CMV) infection, it was recently described that the TCR repertoire is drastically curtailed compared to acute infection, further showing that the repertoire is selected, and the diversity is reduced during chronic antigen stimulation (98, 99). TGF-β, therefore, could be one of the signals that maintain stem-like CD8 T cells in a quiescent state in the tumor, helping to sustain the anti-tumor response for long periods of time. These observations are supported by various cancer studies, where expression of cytolytic molecules was noted after reduction of the TGF-β signaling in CD8 T cells (95, 100, 101).
When attempting to promote effective anti-tumor immune responses, we must consider how the balance of stem-like and terminally differentiated CD8 T cells would be affected. Treg cells suppressing CD8 T-cell differentiation via the secretion of TGF-β suggests that their presence in the tumor niche may be an important regulatory step to maintain the balance of stem-like CD8 T cells and terminally differentiated cells in the tumor (Fig. 3).
T-follicular-helper-like CD4 T cells
Naive CD4 T cells have the ability to differentiate into various helper lineages with differing effector functions and the ability to produce selective cytokines and chemokines (102). CD4 T-cell differentiation during chronic infections is diverted away from the typical T-helper 1 (Th1)-biased response to acute viral infections and, instead, skews the response towards a T-follicular-helper (Tfh) phenotype as the infection persists (59, 103). Concomitantly, the majority of virus-specific CD4 T cells at late timepoints during chronic LCMV infection express CXCR5, produce high levels of IL-21 and have a reduced capacity for Th1-associated cytokine production (103). Tfh cells are known to express cellular factors or to secrete selective cytokines, for example CD40 ligand (CD40L) or IL-21, that are known to recruit CD8 T cells and enhance their effector functions.
Although B-cell responses contribute to viral clearance, the Tfh bias of CD4 T cells has been shown to be beneficial for CD8 T-cell maintenance and effector functions. IL-21 has been reported as one of the critical soluble factors that sustains the CD8 T-cell response during chronic viral infections (39, 54, 58). Specifically, lack of IL-21 signaling in virus-specific CD8 T cells resulted in an accumulation of stem-like CD8 T cells and prevented them from giving rise to the terminally differentiated subset. Although stem-like cells are required to sustain the virus-specific CD8 response, their continued differentiation into terminally differentiated TIM3+ cells are needed to control the virus. In addition to promoting stem-like differentiation, IL-21 was reported to drive CD8 T cells to a more functional and less ‘exhausted’ effector state, characterized by expression of the chemokine receptor CX3CR1 and highly associated with viral control (35, 39).
Additionally, it has been proposed that the Tfh CD4 lineage bias may benefit the host by reduction of Th1-dependent immunopathology (59). These data are suggestive of a connection between the lineage differentiation changes within the CD4 T-cell compartment and the factors needed to both sustain CD8 T-cell responses in chronic antigen settings and promote differentiation of stem-like CD8 T cells. These observations can be related back to potential mechanisms of tumor-infiltrating Tfh-like CD4 cells that could benefit the CD8 T-cell tumor response.
Among the diverse populations infiltrating tumors, a Tfh-like cell with high expression of PD-1 and CXCL13 was found within various human cancers (67, 71–73). Tfh-like cells appear to benefit the anti-tumor response, as high infiltration of TCF1+ CXCL13+ CD4 T cells in melanoma or colorectal cancer patients was associated with increased survival and immunoprotective CD8 T-cell responses (104–106). Tfh cells are known for their role in synchronizing germinal-center responses for high-affinity antibody production (107) rendering their presence in the tumor unexpected. However, given that soluble factors known to be secreted by Tfh cells drive stem-like CD8 T-cell differentiation in chronic viral infections, it may suggest that a common Tfh differentiation program exists that may benefit the CD8 T-cell response.
One possibility is that Tfh-like cells positively influence the anti-tumor response by organizing lymphoid-like or tertiary lymphoid structures in the tumor tissue that maintain the stem-like CD8 T cells in peripheral tissues. In support of this hypothesis, the lymphoid-like structures reported by our group within kidney and prostate tumors had high infiltration of TCF1-expressing non-CD8 cells that could fit the CD4 Tfh-like phenotype within these structures. Additionally, Tfh cells produce a variety of chemokines, in particular CXCL13, which could recruit and organize CXCR5-expressing stem-like CD8 T cells in the tumor.
Another possibility includes the secretion of IL-21 to induce terminal CD8 T-cell differentiation in the tertiary lymphoid-like structures within the tumor (Fig. 3). Evidence in support of this proposed mechanism in cancer comes from a recent paper describing a critical role for IL-21 for CD8 T-cell differentiation into a transitory (CX3CR1+) exhausted population of terminally differentiated cells expressing high levels of cytotoxic molecules (39). Importantly, this population of terminally differentiated cells was also critical for controlling tumor growth in a murine melanoma model. IL-21 has also been described as a potent inducer of tumor regression activity in adoptively transferred human CD8 T cells (108). Differentiation biases, as well as the presence of a Tfh-like cell in tumors, suggests that the signals and soluble factors needed to sustain an effective CD8 T-cell response in chronic antigen settings are different from acute infections. Tfh-like dependent factors, including IL-21 and CXCL13, may play a positive role in organizing stem-like CD8 T cells and driving their differentiation in the tumor (Fig. 3). Although an interesting hypothesis, much more remains to be learned about Tfh-like cells and their role in cancer.
Th1 cells and other helper CD4 T-cell subsets in cancer
Recent single-cell analysis reported the infiltration of cytotoxic Th1-like cells and Th1 cells expressing high levels of checkpoint molecules in various human cancers (67, 71–73). The presence of Th1 and Th1-like cytotoxic CD4 T cells is positively associated with improved outcomes in cancer patients (67, 109). Although their lineage differentiation is still unclear, some evidence indicates that cytolytic Th1-like cells are driven by the transcription factors eomesodermin (Eomes) and/or T-box expressed in T cells (Tbet) and have anti-tumor activity via the production of IFN-γ and secretion of granzyme and perforin molecules.
In addition to Th1 cells and cytotoxic CD4 T cells, Th17 cells have also been suggested to be beneficial in tumor responses. Although their presence in tumors is rare, in vitro polarized Th17 cells can outperform Th1 and naive cells in adoptive cell therapy, as they exhibit a stem-like phenotype and enhanced cytotoxicity (110, 111).
Overall, these data suggest that various CD4 helper subsets help to support the CD8 response in the tumor either via direct mechanisms or indirectly via the production of soluble factors. Further research must be conducted to determine the differentiation bias of CD4 T cells in cancer and the mechanisms by which each subset helps to support CD8 T cells and their differentiation in cancer.
CD4 T cells relay help-signals via DCs that support CD8 T-cell differentiation in the tumor
In addition to soluble factors secreted by CD4 T cells to enhance the CD8 T-cell response, DC ‘licensing’ has been proposed as an alternative mechanism to enhance CD8 T-cell function and promote their differentiation. In the absence of innate pathogen-derived signals, an antigen-specific contact between DCs and CD4 T cells may be needed to enhance the antigen presentation and co-stimulatory signals from DCs that will be relayed to CD8 T cells for their full activation/differentiation (65). Intravital imaging revealed that this form of CD4 T-cell help is delivered via cell–cell contact through CD40–CD40L interactions, where CD4 and CD8 T cells interact with the same DC after their initial independent activation (112). These secondary signals up-regulate the expression of co-stimulatory ligands CD80/CD86 and CD70 on the DC, as well as increasing the production of stimulating cytokines (65, 113).
In support of this mechanism, various studies have shown that CD70 and CD80/CD86 up-regulation via CD40 or Toll-like receptor (TLR) stimulation on DCs is sufficient to induce potent CD8 T-cell effector responses in the absence of CD4 T cells during viral infections (112, 114). This can also be recapitulated in a therapeutic tumor-vaccination model, where CD4 T-cell help contributed to CD70 expression on DCs and optimized CD8 T-cell effector and cytolytic functions (63). In cancer, the absence of pathogen-derived signals and the suppressive nature of the tumor microenvironment reduce the antigen-presenting and co-stimulatory capacities of DCs (115).
In the tumor lymphoid-like structures, DC licensing could be a critical mechanism that promotes up-regulation of co-stimulatory molecules on DCs, rendering CD4 T-cell help as a potential mechanism for effective CD8 terminal differentiation in the tumor. Tfh-like cells express high levels of CD40L and could serve as the helper CD4 T-cell to deliver this signal in the tumor. In this differentiation model, effective CD8 anti-tumor responses are therefore dependent on signals received at a second step in the tumor where CD4 T-cell help is needed for DC licensing or for secretion of soluble factors that promote the transition of stem-like to terminally differentiated CD8 T cells (Fig. 3). CD4 ‘help’ in cancer would thus require DCs, CD4 T cells and CD8 T cells to interact in an organized manner in the tumor. As previously mentioned, intravital microscopy and immune profiling of various tumors have described APCs and activated populations of CD8 and CD4 T-cell residing in structured lymphoid-like structures that could serve as the ideal location for the delivery of these secondary signals that drive terminal differentiation of the stem-like CD8 T cells.
Taken together, the tumor and chronic viral infection data provide a basis for the hypothesis that CD4 T-cell subsets may play a broader and positive role to support the anti-tumor response.
Conclusion—CD8 T-cell differentiation occurs in the tumor
The CD8 T-cell response is critical to disease control and to responses to checkpoint blockade therapy. On the basis of the data examined, we discuss the possibility of a tumor CD8 T-cell differentiation model that incorporates the help of DCs, CD4 T cells and organized lymphoid-like structures in tumors. The stem-like CD8 T-cell population must continuously proliferate and give rise to terminally differentiated cells to sustain an efficient response in the tumor. The stem-like CD8 T-cell therefore acts as an undifferentiated exhausted precursor and resides within lymphoid-like structures in the tumor. We propose that stem-like CD8 T cells are maintained in a quiescent state in a protective lymphoid-like ‘niche’ within the tumor, similar to the splenic white pulp waiting for secondary differentiation signals to be delivered.
In addition, we propose that stem-like CD8 T cells require a secondary signal for effector differentiation in the tumor. This secondary differentiation signal may come from a collaborative effort from CD4 and the DCs residing within the same ‘niche’. The lack of innate signals and the immunosuppressive environment may require DC licensing by helper CD4 T cells via CD40–CD40L signaling within the tumor. Additionally, secretion of IL-21 from helper CD4 T cells may further drive stem-like CD8 differentiation. The combination of these signals may act to sustain a stem-like CD8 T-cell population that can be maintained for long periods of time within the tumor while driving the production of terminally differentiated cells that are essential for disease and tumor control (Fig. 3). Further investigation into these mechanisms is needed to fully understand how the T-cell response in cancer.
Funding
This work was funded by NCI on grant number: R00 CA197804.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Dunn, G P, Old, L J and Schreiber, R D. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22:329. [DOI] [PubMed] [Google Scholar]
- 2. Gerstung, M, Jolly, C, Leshchiner, Iet al. . 2020. The evolutionary history of 2,658 cancers. Nature 578:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rao, S, Gharib, K and Han, A. 2019. Cancer immunosurveillance by T cells. Int. Rev. Cell Mol. Biol. 342:149. [DOI] [PubMed] [Google Scholar]
- 4. Houghton, A N and Guevara-Patiño, J A. 2004. Immune recognition of self in immunity against cancer. J. Clin. Invest. 114:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Houghton, A N, Gold, J S and Blachere, N E. 2001. Immunity against cancer: lessons learned from melanoma. Curr. Opin. Immunol. 13:134. [DOI] [PubMed] [Google Scholar]
- 6. Galon, J, Costes, A, Sanchez-Cabo, Fet al. . 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960. [DOI] [PubMed] [Google Scholar]
- 7. Azimi, F, Scolyer, R A, Rumcheva, Pet al. . 2012. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 30:2678. [DOI] [PubMed] [Google Scholar]
- 8. Gooden, M J, de Bock, G H, Leffers, N, Daemen, T and Nijman, H W. 2011. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br. J. Cancer 105:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbst, R S, Soria, J C, Kowanetz, Met al. . 2014. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiraoka, K, Miyamoto, M, Cho, Yet al. . 2006. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br. J. Cancer 94:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansen, C S, Prokhnevska, N, Master, V Aet al. . 2019. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tumeh, P C, Harview, C L, Yearley, J Het al. . 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daud, A I, Loo, K, Pauli, M Let al. . 2016. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Invest. 126:3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller, BC, Sen, DR, Al Abosy, Ret al. . 2019. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hashimoto, M, Kamphorst, A O, Im, S Jet al. . 2018. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu. Rev. Med. 69:301. [DOI] [PubMed] [Google Scholar]
- 16. Wolchok, J D, Kluger, H, Callahan, M Ket al. . 2013. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reck, M, Rodríguez-Abreu, D, Robinson, A Get al. . 2016. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375:1823. [DOI] [PubMed] [Google Scholar]
- 18. Overman, M J, Lonardi, S, Wong, K Y Met al. . 2018. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 36:773. [DOI] [PubMed] [Google Scholar]
- 19. Overman, M J, McDermott, R, Leach, J Let al. . 2017. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18:1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lurquin, C, Van Pel, A, Mariamé, Bet al. . 1989. Structure of the gene of tum-transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell 58:293. [DOI] [PubMed] [Google Scholar]
- 21. Schreiber, R D, Old, L J and Smyth, M J. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565. [DOI] [PubMed] [Google Scholar]
- 22. Coulie, P G, Van den Eynde, B J, van der Bruggen, P and Boon, T. 2014. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14:135. [DOI] [PubMed] [Google Scholar]
- 23. Gee, M H, Han, A, Lofgren, S Met al. . 2018. Antigen identification for orphan T cell receptors expressed on tumor-infiltrating Lymphocytes. Cell 172:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khodadoust, M S, Olsson, N, Wagar, L Eet al. . 2017. Antigen presentation profiling reveals recognition of lymphoma immunoglobulin neoantigens. Nature 543:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simoni, Y, Becht, E, Fehlings, Met al. . 2018. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557:575. [DOI] [PubMed] [Google Scholar]
- 26. Carreno, B M, Magrini, V, Becker-Hapak, Met al. . 2015. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schumacher, T N, Scheper, W and Kvistborg, P. 2019. Cancer neoantigens. Annu. Rev. Immunol. 37:173. [DOI] [PubMed] [Google Scholar]
- 28. McGranahan, N, Furness, A J, Rosenthal, Ret al. . 2016. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351:1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forbes, S A, Beare, D, Gunasekaran, Pet al. . 2015. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 43(Database issue):D805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zajac, A J, Blattman, J N, Murali-Krishna, Ket al. . 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Im, S J, Hashimoto, M, Gerner, M Yet al. . 2016. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He, R, Hou, S, Liu, Cet al. . 2016. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 537:412. [DOI] [PubMed] [Google Scholar]
- 33. Utzschneider, D T, Charmoy, M, Chennupati, Vet al. . 2016. T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 45:415. [DOI] [PubMed] [Google Scholar]
- 34. Leong, Y A, Chen, Y, Ong, H Set al. . 2016. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol. 17:1187. [DOI] [PubMed] [Google Scholar]
- 35. Hudson, W H, Gensheimer, J, Hashimoto, Met al. . 2019. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McLane, L M, Abdel-Hakeem, M S and Wherry, E J. 2019. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37:457. [DOI] [PubMed] [Google Scholar]
- 37. Wu, T, Ji, Y, Moseman, EAet al. . 2016. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 1:eaai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siddiqui, I, Schaeuble, K, Chennupati, Vet al. . 2019. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50:195. [DOI] [PubMed] [Google Scholar]
- 39. Zander, R, Schauder, D, Xin, Get al. . 2019. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 51:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beltra, J C, Manne, S, Abdel-Hakeem, M Set al. . 2020. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sade-Feldman, M, Yizhak, K, Bjorgaard, S Let al. . 2019. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 176:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kurtulus, S, Madi, A, Escobar, Get al. . 2019. Checkpoint blockade immunotherapy induces dynamic changes in PD-1-CD8+ Tumor-Infiltrating T cells. Immunity 50:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Broz, M L, Binnewies, M, Boldajipour, Bet al. . 2014. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 26:938. [DOI] [PubMed] [Google Scholar]
- 44. Spranger, S, Dai, D, Horton, B and Gajewski, T F. 2017. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Theisen, D J, Davidson, J T4th, Briseño, C Get al. . 2018. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 362:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dudziak, D, Kamphorst, A O, Heidkamp, G Fet al. . 2007. Differential antigen processing by dendritic cell subsets in vivo. Science 315:107. [DOI] [PubMed] [Google Scholar]
- 47. Roberts, E W, Broz, M L, Binnewies, Met al. . 2016. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in Melanoma. Cancer Cell 30:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brewitz, A, Eickhoff, S, Dähling, Set al. . 2017. CD8+ T cells orchestrate pDC-XCR1+ dendritic cell spatial and functional cooperativity to optimize priming. Immunity 46:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diamond, M S, Kinder, M, Matsushita, Het al. . 2011. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208:1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laoui, D, Keirsse, J, Morias, Yet al. . 2016. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat. Commun. 7:13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dutertre, C A, Becht, E, Irac, S Eet al. . 2019. Single-cell analysis of human mononuclear phagocytes reveals subset-defining markers and identifies circulating inflammatory dendritic cells. Immunity 51:573. [DOI] [PubMed] [Google Scholar]
- 52. Bourdely, P, Anselmi, G, Vaivode, Ket al. . 2020. Transcriptional and functional analysis of CD1c+ human dendritic cells identifies a CD163+ subset priming CD8+CD103+ T cells. Immunity 53:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Im, S J, Konieczny, B T, Hudson, W H, Masopust, D and Ahmed, R. 2020. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc. Natl Acad. Sci. USA 117:4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Snell, L M, MacLeod, B L, Law, J Cet al. . 2018. CD8+ T cell priming in established chronic viral infection preferentially directs differentiation of memory-like cells for sustained immunity. Immunity 49:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cabrita, R, Lauss, M, Sanna, Aet al. . 2020. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 580:E1. [DOI] [PubMed] [Google Scholar]
- 56. Dieu-Nosjean, M C, Giraldo, N A, Kaplon, H, Germain, C, Fridman, W H and Sautès-Fridman, C. 2016. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 271:260. [DOI] [PubMed] [Google Scholar]
- 57. Matloubian, M, Concepcion, R J and Ahmed, R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elsaesser, H, Sauer, K and Brooks, D G. 2009. IL-21 is required to control chronic viral infection. Science 324:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vella, L A, Herati, R S and Wherry, E J. 2017. CD4+ T cell differentiation in chronic viral infections: The Tfh perspective. Trends Mol. Med. 23:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morou, A, Brunet-Ratnasingham, E, Dubé, Met al. . 2019. Altered differentiation is central to HIV-specific CD4+ T cell dysfunction in progressive disease. Nat. Immunol. 20:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sidique, N, Kohli, A, Shivakumar, Bet al. . 2011. HIV/HCV-coinfected natural viral suppressors have better virologic responses to PEG-IFN and ribavirin than ARV-treated HIV/HCV patients. J. Acquir. Immune Defic. Syndr. 58:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Douek, D C, Picker, L J and Koup, R A. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265. [DOI] [PubMed] [Google Scholar]
- 63. Ahrends, T, Spanjaard, A, Pilzecker, Bet al. . 2017. CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 47:848. [DOI] [PubMed] [Google Scholar]
- 64. Antony, P A, Piccirillo, C A, Akpinarli, Aet al. . 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Borst, J, Ahrends, T, Bąbała, N, Melief, C J M and Kastenmüller, W. 2018. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18:635. [DOI] [PubMed] [Google Scholar]
- 66. Kennedy, R and Celis, E. 2008. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. 222:129. [DOI] [PubMed] [Google Scholar]
- 67. Oh, D Y, Kwek, S S, Raju, S Set al. . 2020. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 181:1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marzo, A L, Kinnear, B F, Lake, R Aet al. . 2000. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J. Immunol. 165:6047. [DOI] [PubMed] [Google Scholar]
- 69. Alspach, E, Lussier, D M, Miceli, A Pet al. . 2019. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Muranski, P and Restifo, N P. 2009. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr. Opin. Immunol. 21:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zheng, C, Zheng, L, Yoo, J Ket al. . 2017. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 169:1342. [DOI] [PubMed] [Google Scholar]
- 72. Guo, X, Zhang, Y, Zheng, Let al. . 2018. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24:1628. [DOI] [PubMed] [Google Scholar]
- 73. Magen, A, Nie, J, Ciucci, Tet al. . 2019. Single-cell profiling defines transcriptomic signatures specific to tumor-reactive versus virus-responsive CD4+ T cells. Cell Rep. 29:3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tanaka, A and Sakaguchi, S. 2017. Regulatory T cells in cancer immunotherapy. Cell Res. 27:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wing, J B, Tanaka, A and Sakaguchi, S. 2019. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 50:302. [DOI] [PubMed] [Google Scholar]
- 76. Quezada, S A, Peggs, K S, Curran, M A and Allison, J P. 2006. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Griffiths, R W, Elkord, E, Gilham, D Eet al. . 2007. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol. Immunother. 56:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Binnewies, M, Mujal, A M, Pollack, J Let al. . 2019. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell 177:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sharma, M, Khong, H, Fa’ak, Fet al. . 2020. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat. Commun. 11:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang, D, Quiros, J, Mahuron, Ket al. . 2018. Targeting EZH2 reprograms intratumoral regulatory T cells to enhance cancer immunity. Cell Rep. 23:3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Arce Vargas, F, Furness, A J S, Solomon, Iet al. . 2017. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 46:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wilson, E B and Brooks, D G. 2011. The role of IL-10 in regulating immunity to persistent viral infections. Curr. Top. Microbiol. Immunol. 350:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Naing, A, Papadopoulos, K P, Autio, K Aet al. . 2016. Safety, antitumor activity, and immune activation of pegylated recombinant human Interleukin-10 (AM0010) in patients with advanced solid tumors. J. Clin. Oncol. 34:3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sun, Z, Fourcade, J, Pagliano, Oet al. . 2015. IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res. 75:1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Qiao, J, Liu, Z, Dong, Cet al. . 2019. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell 35:901. [DOI] [PubMed] [Google Scholar]
- 86. Li, M O, Wan, Y Y, Sanjabi, S, Robertson, A K and Flavell, R A. 2006. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24:99. [DOI] [PubMed] [Google Scholar]
- 87. Sanjabi, S, Oh, SA, Li, MO. 2017. Regulation of the immune response by TGF-beta: from conception to autoimmunity and infection. Cold Spring Harb. Perspect. Biol. 9:a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Reinhold, D, Wrenger, S, Kähne, T and Ansorge, S. 1999. HIV-1 Tat: immunosuppression via TGF-beta1 induction. Immunol. Today 20:384. [DOI] [PubMed] [Google Scholar]
- 89. Taniguchi, H, Kato, N, Otsuka, Met al. . 2004. Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J. Med. Virol. 72:52. [DOI] [PubMed] [Google Scholar]
- 90. Tinoco, R, Alcalde, V, Yang, Y, Sauer, K and Zuniga, E I. 2009. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang, N and Bevan, M J. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tu, E, Chia, C P Z, Chen, Wet al. . 2018. T cell receptor-regulated TGF-β type I receptor expression determines T cell quiescence and activation. Immunity 48:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bruna, A, Darken, R S, Rojo, Fet al. . 2007. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 11:147. [DOI] [PubMed] [Google Scholar]
- 94. Chang, H L, Gillett, N, Figari, I, Lopez, A R, Palladino, M A and Derynck, R. 1993. Increased transforming growth factor beta expression inhibits cell proliferation in vitro, yet increases tumorigenicity and tumor growth of Meth A sarcoma cells. Cancer Res. 53:4391. [PubMed] [Google Scholar]
- 95. Gorelik, L and Flavell, R A. 2001. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat. Med. 7:1118. [DOI] [PubMed] [Google Scholar]
- 96. Janbazian, L, Price, D A, Canderan, Get al. . 2012. Clonotype and repertoire changes drive the functional improvement of HIV-specific CD8 T cell populations under conditions of limited antigenic stimulation. J. Immunol. 188:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ogonek, J, Verma, K, Schultze-Florey, Cet al. . 2017. Characterization of high-avidity cytomegalovirus-specific T cells with differential tetramer binding coappearing after allogeneic stem cell transplantation. J. Immunol. 199:792. [DOI] [PubMed] [Google Scholar]
- 98. Chang, YM, Wieland, A, Li, ZRet al. . 2020. T cell receptor diversity and lineage relationship between virus-specific CD8 T cell subsets during chronic LCMV infection. J Virol. doi: 10.1128/JVI.00935-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schober, K, Voit, F, Grassmann, Set al. . 2020. Reverse TCR repertoire evolution toward dominant low-affinity clones during chronic CMV infection. Nat. Immunol. 21:434. [DOI] [PubMed] [Google Scholar]
- 100. Mempel, T R, Pittet, M J, Khazaie, K, Weninger, W, Weissleder, R, von Boehmer, H and von Andrian, U H. 2006. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 25:129. [DOI] [PubMed] [Google Scholar]
- 101. Thomas, D A and Massagué, J. 2005. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8:369. [DOI] [PubMed] [Google Scholar]
- 102. DuPage, M and Bluestone, J A. 2016. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat. Rev. Immunol. 16:149. [DOI] [PubMed] [Google Scholar]
- 103. Fahey, L M, Wilson, E B, Elsaesser, H, Fistonich, C D, McGavern, D B and Brooks, D G. 2011. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 208:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gu-Trantien, C, Migliori, E, Buisseret, Let al. . 2017. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2:2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li, H, van der Leun, A M, Yofe, Iet al. . 2019. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human Melanoma. Cell 176:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Becht, E, de Reyniès, A, Giraldo, N Aet al. . 2016. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin. Cancer Res. 22:4057. [DOI] [PubMed] [Google Scholar]
- 107. Crotty, S. 2019. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hinrichs, C S, Spolski, R, Paulos, C Met al. . 2008. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111:5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liakou, C I, Kamat, A, Tang, D Net al. . 2008. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl Acad. Sci. USA 105:14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bailey, S R, Nelson, M H, Majchrzak, Ket al. . 2017. Human CD26high T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat. Commun. 8:1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Muranski, P, Borman, Z A, Kerkar, S Pet al. . 2011. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Eickhoff, S, Brewitz, A, Gerner, M Yet al. . 2015. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell 162:1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Grewal, I S and Flavell, R A. 1996. The role of CD40 ligand in costimulation and T-cell activation. Immunol. Rev. 153:85. [DOI] [PubMed] [Google Scholar]
- 114. Bennett, S R, Carbone, F R, Karamalis, F, Flavell, R A, Miller, J F and Heath, W R. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478. [DOI] [PubMed] [Google Scholar]
- 115. Wculek, S K, Cueto, F J, Mujal, A M, Melero, I, Krummel, M F and Sancho, D. 2020. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20:7. [DOI] [PubMed] [Google Scholar]