Abstract
Gait disturbances in people with Parkinson’s disease (PD) are a major cause for functional dependence and have recently been shown to be the largest risk factor for falls, institutionalization and death in PD. The use of external cues has been successful at improving gait in people with PD, but the effect of external cues on gait stability is unclear. We examined whether different forms of cueing, open-loop and closed-loop, influenced the local dynamic stability of three critical phases of gait. Forty-three adults with PD completed six, two-minute long walking trials in the following cued conditions: no cue (B), open-loop cueing, fixed auditory cue (OL), closed-loop cueing, tactile feedback delivered to wrist when the ipsilateral foot contacted with the ground (CL). Conditions were performed with and without a cognitive task. Kinematic data were recorded with inertial sensors.
Only CL cueing was associated with changes in trunk stability, and these changes were only evident during the weight transfer phase of gait. Both OL and CL caused reductions in overall gait speed, stride length, and an increase in stride time.
While CL cueing significantly influenced local dynamic stability during weight transfer, it remains unknown whether these changes are associated with more or less global stability. Future research will explore the clinical implications.
Keywords: Cueing, Dual-task, Gait stability, Parkinson’s disease, Wearable sensors, Lyapunov exponents, accelerometers
I. Introduction
Gait disturbances, continuous or episodic, are a major cause for functional dependence and have recently been shown to be the largest risk factor for falls, institutionalization, and death in people with Parkinson’s disease (PD) [1, 2]. Freezing of Gait (FoG), an intermittent failure to initiate or maintain locomotion, is among the most severe gait disturbances [3]. Even without freezing of gait, people with PD walk slowly with short stride length, decreased rhythmicity, and increased stride time variability [4].
The majority of the studies investigating gait in people with PD focused on aspects of pace, rhythm, variability and asymmetry of gait [5–9], often excluding aspects of dynamic stability of the trunk which are also impaired in people with PD [10–14]. For example, phase-dependent local dynamic stability (LDS) quantifies the rate at which local perturbations are attenuated during specific phases of the gait cycle [15]. Phase-dependent LDS during weight transfer, but not other phases, can discriminate among young and older adults [15] and between elderly fallers and non-fallers during everyday living [16]. We recently found that LDS during weight transfer is impaired in people with PD compared to older adults [10].
Although bradykinetic and hypometric spatial characteristics of gait, such as gait speed and stride length improve with dopaminergic medications, aspects of stability of gait, such as spatial or temporal variability, and balance do not change, or can even worsen, with dopaminergic medication [4, 14]. Accordingly, several studies have examined the effect of nonpharmacological interventions, such as gait and balance training, on improving mobility in people with PD [17–21]. In addition to training, external cues that are visual (lines on the ground), auditory (metronome beeps), or tactile in nature have been successful at improving gait in people with PD [6, 22].
Traditionally, sensory cues have been used in an open-loop manner (for example, constant rhythmical stimuli) rather than a closed-loop manner (intermittent stimuli triggered by each individual’s walking pattern) [23]. As the open-loop cue is delivered at a constant rate, individuals must entrain their gait patterns to the new cue at each step. The external, open-loop cue therefore serves as a target, either temporal or spatial; the individual attempts to match the target on every step. With the recent developments in wearable technology, alternatives to open-loop cueing have been explored [23]. For example, novel closed-loop, intelligent systems have been developed [23] and tested to improve gait in people with neurological disorders, such as PD. Closed-loop cueing can substitute or augment real-time biological information that would otherwise be unknown to patients, thereby complimenting internal feedback and reinforcing patients’ weak or absent sensory signals [24]. Our approach uses novel, wearable technology to provide unobtrusive, closed-loop cueing, using tactile feedback triggered by each patient’s actual motor performance [25, 26]. Specifically, the system delivers a phase-dependent tactile vibration in the internal part of the wrist at each heel strike and for the duration of the stance phase of gait [25]. A previous feasibility study in the laboratory validated and showed the efficacy of the system compared to an open-loop metronome to improve turning and FoG in forty-three participants with PD [26].
Yet, it remains unclear how gait and gait stability are influenced by different cueing strategies. Therefore, we investigated how different forms of cueing (no cue vs. open-loop vs. closed-loop) influence the local dynamic stability of three critical phases of gait (weight acceptance, early stance, and mid stance) in people with PD. We hypothesized that different forms of cueing would yield different results, and that the effects of cuing would be specific to individual phases of gait. However, we did not have specific a priori hypotheses regarding the phase or the directional of the effect.
II. Methods
A. Participants
Forty-three adults (ten females/thirty-three males) with PD participated to the study [mean (SD) age = 70 (7.3) yrs; MDS-UPDRS III = 46 (11), disease duration = 8.6 (5.6) yrs]. Twenty-two of the 43 subjects reported freezing of gait using the New Freezing of Gait Questionnaire (NFOGQ = 16.6 (6.3)) [27]. All subjects were clinically diagnosed with idiopathic PD by a movement disorders specialist. Inclusion criteria for subjects with PD were (1) between 50 and 90 years old, (2) no major musculoskeletal or peripheral disorders (other than PD) that could significantly affect their balance and gait, (3) ability to stand and walk unassisted, and (4) met criteria for idiopathic PD according to the according to the United Kingdom Parkinson’s disease Society Brain Bank clinical diagnostic criteria [28]. Individuals were excluded if they could not safely walk 20 feet or if they had dementia. Participants mean (SD) on the Montreal Cognitive Assessment (MoCA) [29] was 25(4.5), indicating intact to mildly impaired overall cognition [29]. Subjects were tested in the practical Off levodopa state, after withholding anti-parkinsonian medication for ≥12 h. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the OHSU IRB (#9903).
B. Procedures
Subjects completed six, two-minute long walking trials between lines marked 7.6m apart. The trials consisted of walking with: no cue (Baseline; B), fixed auditory open-loop cueing through a metronome (OL), tactile feedback closed-loop cueing delivered to wrist when the ipsilateral foot contacted with the ground (CL). The order of conditions was randomized for each subject. During the OL condition, an auditory tone was delivered through a portable speaker placed in the center of the laboratory so that it was possible to clearly hear the tone while performing the motor tasks. Subjects self-selected the pace of the auditory cue and were asked to synchronize each step with the auditory cue. The metronome was adjusted based on participant preference before data collection, specifically, each participant selected a metronome beat that they could comfortable keep for periods of two-minute walking (a step for each beat). During the CL condition, VibroGait, a previously described wearable system [25], delivered tactile stimuli similar to that of a vibrating cell phone (primary resonance 200–300 Hz) to the wrist while in the stance phase of gait. Briefly, the VibroGait unit consists of a novel controller (Arduino microcontroller) that uses a gyroscope to detect when the foot is on the ground and, when the foot is on the ground, activates the tactor unit to generate a vibration (in our case to the wrist).
Each feedback condition (B, CL, OL) was performed with and without a simultaneous cognitive task (reciting every other letter of the alphabet). Kinematic data were recorded with eight inertial sensors (Opal, APDM Inc.) placed over the sternum, lumbar spine, and bilaterally on the wrists, anterior distal portion of the shank, and dorsum of the feet using elastic straps. Each inertial sensor recorded acceleration, angular velocity, and magnetometer data at 128 Hz. For this analysis, only data recorded by the shank, feet, and trunk sensors were used.
C. Analysis
Data analysis was performed consistent with previous investigations on phase-dependent LDS in people with PD (see Fig 1) [10]. Briefly, raw accelerometer and gyroscope data were extracted from the trunk, bilateral shanks, and bilateral feet sensors for each trial. Trials were then parsed into straight walking bouts by removing one step before and after turns detected using an angular velocity threshold of 30°/s. Gait events of heel contact, toe-off, and mid-swing were identified using the angular velocity of the shank sensors [30]. Straight walking bouts were then divided into non-overlapping segments of five strides. Each stride was time-normalized to 130 data points to maintain equal data length across segments. Walking bouts that did not include five strides were excluded from the analysis.
Fig. 1.
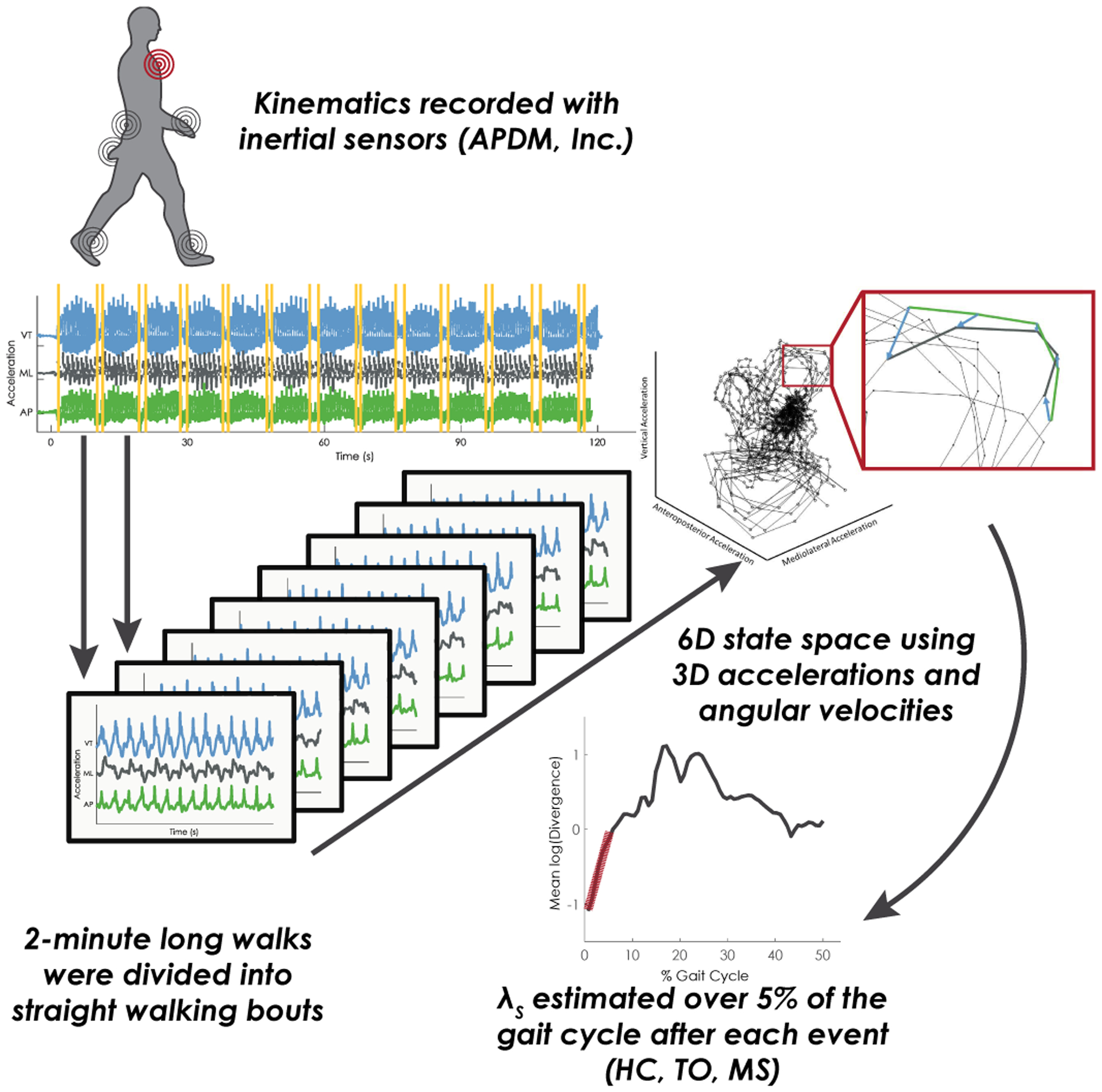
Depiction of phase-dependent local dynamic stability analysis pipeline. First, kinematic data is recorded during a two-minute long walk. Accelerometer and gyroscope data are used to segment the long-walk trials into individual short bouts of gait by removing turns. Each short bout was time-normalized using 5 strides normalized to 130 points per stride, and then the accelerations from the sternum were embedded in a 6D state space. Nearby trajectories are tracked in state space and the slope of the log-mean divergence curve is extracted for the first 5% of the gait cycle following each gait event (heel contact, HC; toe-off, TO; misswing, MS).
Phase-dependent local dynamic stability of the trunk was estimated for each walking bout at three phases of gait identified using the three gait events: weight transfer (heel contact); early swing (toe off); and late swing (mid swing). Subsequently, a 6D state-space was reconstructed using the 3D accelerations and angular velocities of the trunk sensor [31]. Points corresponding to each gait event were located within the state-space and the two nearest neighbors for each gait event were identified. The average distance of the two nearest neighboring trajectories was then tracked for one step, and mean log divergence curves were created as a function of percent of stride. The divergence exponents, λHS, λTO, λMS, corresponding to the phase-dependent local dynamic stability at weight transfer (heel contact), early swing (toe off), and late swing (mid swing) were then estimated from the slope of the mean log divergence curve from the initial gait event to the next 5% of the gait cycle. Finally, the median of λHS, λTO, and λMS was calculated across all segments for each subject.
In addition to the stability outcomes, gait speed, stride length, step time, step time standard deviation (SD), and step time asymmetry (magnitude of the difference between right and left step times) were assessed using the standard output from Mobility Lab v2, the commercial software associated with the APDM inertial sensors [32]. These outcomes were chosen to serve as descriptive characteristics of our sample and to align with pace (gait speed and stride length), rhythm (step time), variability (step time SD), and asymmetry (step time asymmetry) domains previously identified in people with PD [33].
D. Statistical Analysis
Outcome measures were examined for normality and heteroscedasticity using histogram and residual plots. Linear mixed models were implemented to test the effect of feedback (B, OL, CL) and cognitive task (single vs. dual) on λ at each phase, with baseline and single-task serving as the reference conditions. Gait speed was included as a covariate in each model. A random intercept term by participant was included to account for the within-subject correlations across conditions. The feedback*cognitive task was initially included in each model, but removed if no interaction was detected at a 0.10 significance level. Akaike Information Criteria (AIC) model fit statistics confirmed the appropriate model selection. The baseline (B) and single-task condition served as references in each model. Pairwise contrasts were implemented to test for differences between CL and OL conditions. All significance values were corrected for multiple comparisons using a false discovery rate (FDR) correction [34] and a significance level of 0.05. All statistical analysis was performed in MATLAB using the Statistical and Machine Learning Toolbox (r2018a, The MathWorks Inc.).
III. Results
Univariate statistics for each outcome are provided in Table 1. During the weight transfer phase of gait, the divergence exponent λ (λHS) was smaller during CL compared to B (Table 2) and OL (pair-wise contrast adjusted p = <0.001) conditions. Additionally, λHS was smaller during dual-task conditions compared to single task during the weight transfer phase. No differences in λ were detected across cueing or cognitive tasks for early (λTO) or late (λMS) swing (Table 2, Figure 1). Gait speed affected the divergence exponent during late swing (λMS), with faster gait speeds associated with greater divergence exponents. No significant feedback*task interaction was detected for the divergence exponents at any phase, and thus no interactions were retained in the final models.
Table 1.
Univariate means and standard deviations (SD) for each outcome during single-task (ST) and dual-task (DT) conditions.
| Baseline (B) | Tactile Biofeedback (CL) | Metronome(OL) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Gait Speed (m/s) | ST | 0.88 | 0.22 | 0.74 | 0.28 | 0.75 | 0.26 |
| DT | 0.77 | 0.22 | 0.68 | 0.28 | 0.69 | 0.26 | |
| Stride Length (m) | ST | 0.93 | 0.21 | 0.86 | 0.25 | 0.87 | 0.25 |
| DT | 0.86 | 0.22 | 0.80 | 0.26 | 0.81 | 0.25 | |
| Stride Time (s) | ST | 1.08 | 0.22 | 1.29 | 0.46 | 1.20 | 0.18 |
| DT | 1.15 | 0.27 | 1.29 | 0.41 | 1.25 | 0.30 | |
| Step Time (ms) | ST | 542 | 111 | 645 | 230 | 602 | 91 |
| DT | 573 | 136 | 646 | 206 | 626 | 150 | |
| Step Time SD (ms)† | ST | 22 | 16 | 27 | 29 | 26 | 26 |
| DT | 25 | 15 | 31 | 29 | 30 | 33 | |
| Step Time Asymmetry (ms)† | ST | 15 | 32 | 27 | 29 | 21 | 36 |
| DT | 28 | 44 | 20 | 48 | 23 | 40 | |
| Weight Transfer - λHS | ST | 0.12 | 0.04 | 0.11 | 0.04 | 0.12 | 0.04 |
| DT | 0.10 | 0.03 | 0.08 | 0.03 | 0.10 | 0.04 | |
| Early Swing – λTO | ST | 0.08 | 0.02 | 0.08 | 0.02 | 0.08 | 0.02 |
| DT | 0.08 | 0.02 | 0.08 | 0.03 | 0.08 | 0.03 | |
| Late Swing - λMS | ST | 0.12 | 0.02 | 0.12 | 0.02 | 0.11 | 0.02 |
| DT | 0.12 | 0.02 | 0.11 | 0.03 | 0.11 | 0.03 | |
Values reported as median and IQR
Table 2.
Linear mixed model results for divergence exponents during weight transfer (λHS), early swing (λTO), and late swing (λMS) phases of gait. Reference conditions for feedback and cognitive task were baseline and single task. Bold terms indicate significance at an FDR-adjusted p < 0.05 level.
| Estimate | SE | Adj. p | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|
| Weight Transfer - λHS | |||||
| Intercept | 0.098 | 0.012 | <0.001 | 0.075 | 0.122 |
| Feedback: B | Ref. | - | - | - | - |
| Feedback: CL | −0.011 | 0.003 | 0.003 | −0.018 | −0.005 |
| Feedback:OL | 0.005 | 0.003 | 0.229 | −0.002 | 0.012 |
| Task: ST | Ref. | - | - | - | - |
| Task: DT | −0.020 | 0.003 | <0.001 | −0.026 | -0.015 |
| Gait Speed | 0.029 | 0.013 | 0.057 | 0.004 | 0.054 |
| Early Swing – λTO | |||||
| Intercept | 0.068 | 0.008 | <0.001 | 0.051 | 0.084 |
| Feedback: B | Ref. | - | - | - | - |
| Feedback: CL | −0.002 | 0.003 | 0.566 | −0.008 | 0.004 |
| Feedback: OL | 0.001 | 0.003 | 0.819 | −0.005 | 0.007 |
| Task: ST | Ref. | - | - | - | - |
| Task: DT | 0.002 | 0.003 | 0.537 | −0.003 | 0.007 |
| Gait Speed | 0.017 | 0.009 | 0.124 | −0.001 | 0.035 |
| Late Swing - λMS | |||||
| Intercept | 0.083 | 0.008 | <0.001 | 0.068 | 0.098 |
| Feedback: B | Ref. | - | - | - | - |
| Feedback: CL | 0.001 | 0.002 | 0.630 | −0.003 | 0.006 |
| Feedback: OL | −0.001 | 0.002 | 0.773 | −0.005 | 0.004 |
| Task: ST | Ref. | - | - | - | - |
| Task: DT | 0.001 | 0.002 | 0.779 | −0.003 | 0.004 |
| Gait Speed | 0.041 | 0.008 | <0.001 | 0.025 | 0.057 |
The descriptive results for spatiotemporal gait measures indicated gait speed slowed during both CL and OL feedback conditions compared to B, and slowed during the DT compared to ST condition. Stride length decreased in both CL and OL conditions compared to B, and in DT compared to ST (Table 1), and did not differ between CL and OL conditions. Similar results were found for step time and step time variability, where both CL and OL conditions increased step time compared to B, and DT increased step time and step time variability compared to ST. For statistical comparison of spatiotemporal gait measures, see Supplemental Appendix.
IV. Discussion
The purpose of this study was to examine the immediate influence of cueing strategies on dynamic gait stability at distinct phases of the gait cycle. Our results support our hypothesis that cueing strategies affect gait stability differently, and that the effects of cueing may be phase-dependent. Specifically, while both OL and CL caused reductions in overall gait speed, stride length, and an increase in stride time (see Table 1 and Appendix), only CL was associated with changes in trunk stability as indicated by the divergence exponent λ. Further, changes in gait stability were only apparent during the weight transfer phase of gait, supporting our hypothesis that certain cueing strategies may be phase-specific.
Our findings suggest that the immediate application of open-loop auditory cues and closed-loop tactile cues while walking may have a different effect on trunk stability. Dynamic gait stability remained unchanged with the open-loop cueing, but gait stability during weight transfer significantly changed with closed-loop cueing. It remains unknown whether the changes in λHS are associated with more or less global stability [10, 15, 16, 31]. Here, λHS during ST walking with CL cues was more similar to λHS during the DT walking without cues (B), suggesting that CL may have imposed a cognitive demand on subjects to process the cueing stimulus that onset precisely during the start of weight transfer. Alternatively, this result could be interpreted as a shift in automaticity. DT gait is used to probe the automaticity of locomotion by reducing the cognitive involvement in regulating gait [35, 36]. Since gait stability with CL resembled DT gait, it is possible that CL reduced the cognitive control of gait through added sensory signals (e.g., the vibration) that could be integrated in standard sensorimotor, rather than cognitive-motor control loops. Future research should probe the cortical activity to explore these competing explanations. Finally, the effect of CL may be due to the closed-loop nature of the cueing. Since the CL cue was delivered precisely at initial contact, compared to inherent variability when subjects were instructed to match the metronome beat, the effect of CL on λHS may reflect the phase-locked nature of the cue.
Differences between our present study and previous results may be partly explained by: 1) the medication status at the time of cueing and disease severity – participants in our study were tested in the practical Off medication status while other studies tested people with PD On their levodopa medication. While it is known that levodopa improves spatiotemporal aspect of gait, it may affect gait variability differently [37]. Additionally, our cohort of participants included people at different stages of disease as well as both people with and without Freezing of Gait. 2) The study design itself; baseline walking was always tested first, followed by either metronome or tactile-cueing first. The presented results are relative to the phase in which participants are using cueing devices for the first time, after a short time to familiarize with them. Therefore, the increased variability, asymmetry, and slower gait might be attributable to an increase in attentional load during the task. 3) The present study is that the difference in results between OL and CL may be driven by the different sensory modality (auditory vs tactile) rather than open-loop vs closed-loop.
Future research will need to design longer walking period for the participants to evaluate the after-effect of this specific training and investigate whether gait dynamic stability measures are related to falls in people with PD. Further, we will explore the clinical implication of changes in phase-dependent stability.
In conclusion, closed-loop CL cueing elicited changes in gait stability specific to the weight transfer phase while OL cueing did not influence gait stability. While there are competing speculative interpretations of these changes, future research should examine the neural underpinnings and clinical implications of phase-dependent changes in gait stability.
Supplementary Material
Fig. 2.
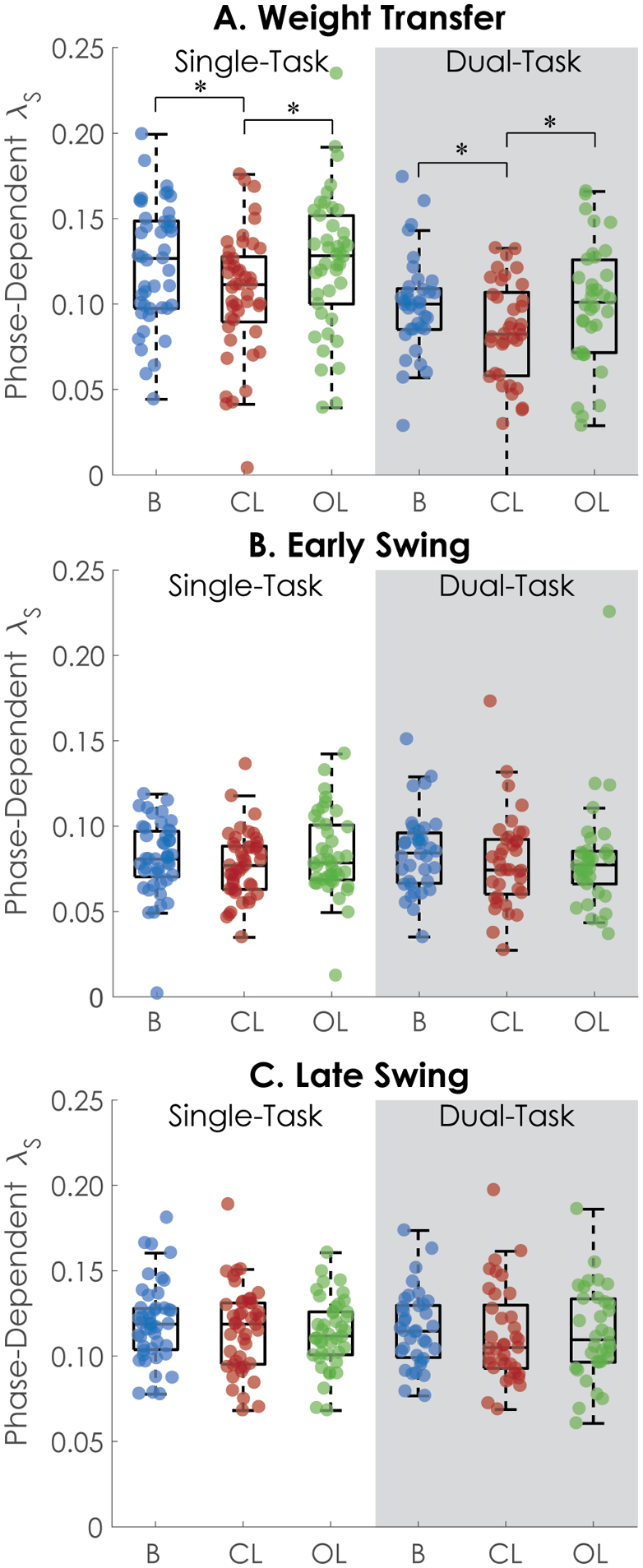
Phase-dependent local dynamic stability during A) weight transfer, B) early swing, and C) late swing. A) CL resulted in lower λ regardless of cognitive task. Dual-task conditions also had lower λ across all cueing conditions. * indicates statistically significant pair-wise difference. B-C) No differences in λ were observed between single- and dual-task or between cueing conditions during early swing or late swing conditions.
Acknowledgment
The authors are grateful to the participants in this study for their time, and to Graham Harker for data collection and project coordination.
This work was supported by Grants from National Institute of Health via Career Development Award K99 HD078492 0IAI (Mancini PI), and 5R00 HD078492 (Mancini PI).
Contributor Information
Peter C. Fino, Department of Health, Kinesiology, and Recreation at the University of Utah, Salt Lake City, UT 84112 USA
Martina Mancini, Department of Neurology at Oregon Health & Science University, Portland, OR 97239 USA.
References
- [1].Morris ME, Huxham FE, McGinley J, and Iansek R, “Gait disorders and gait rehabilitation in Parkinson’s disease,” Advances in neurology, vol. 87, pp. 347–61, 2001. [PubMed] [Google Scholar]
- [2].Hausdorff JM, “Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling,” Chaos, vol. 19, no. 2, p. 026113, June 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, and Nieuwboer A, “Freezing of gait: moving forward on a mysterious clinical phenomenon,” Lancet Neurol, vol. 10, no. 8, pp. 734–44, August 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smulders K, Dale ML, Carlson-Kuhta P, Nutt JG, and Horak FB, “Pharmacological treatment in Parkinson’s disease: Effects on gait,” Parkinsonism Relat Disord, vol. 31, pp. 3–13, October 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, and Giladi N, “Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease,” Eur J Neurosci, vol. 26, no. 8, pp. 2369–75, October 2007. [DOI] [PubMed] [Google Scholar]
- [6].Nieuwboer A et al. , “Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial,” Journal of neurology, neurosurgery, and psychiatry, vol. 78, no. 2, pp. 134–40, February 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rochester L et al. , “The Effect of External Rhythmic Cues (Auditory and Visual) on Walking During a Functional Task in Homes of People With Parkinson’s Disease,” Archives of Physical Medicine and Rehabilitation, vol. 86, no. 5, pp. 999–1006, 2005/May/01/ 2005. [DOI] [PubMed] [Google Scholar]
- [8].Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, and Brault JM, “Rhythmic auditory stimulation in gait training for Parkinson’s disease patients,” Movement Disorders, vol. 11, no. 2, pp. 193–200, 1996. [DOI] [PubMed] [Google Scholar]
- [9].Lirani-Silva E, Lord S, Moat D, Rochester L, and Morris R, “Auditory Cueing for Gait Impairment in Persons With Parkinson Disease: A Pilot Study of Changes in Response With Disease Progression,” J Neurol Phys Ther, vol. 43, no. 1, pp. 50–55, January 2019. [DOI] [PubMed] [Google Scholar]
- [10].Fino PC, Mancini M, Curtze C, Nutt JG, and Horak FB, “Gait Stability Has Phase-Dependent Dual-Task Costs in Parkinson’s Disease,” Frontiers in Neurology, vol. 9, no. 373, 2018-May-30 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gougeon MA, Zhou L, and Nantel J, “Nordic Walking improves trunk stability and gait spatial-temporal characteristics in people with Parkinson disease,” NeuroRehabilitation, vol. 41, no. 1, pp. 205–210, 2017. [DOI] [PubMed] [Google Scholar]
- [12].Pelicioni PHS et al. , “Head and trunk stability during gait before and after levodopa intake in Parkinson’s disease subtypes,” Exp Gerontol, vol. 111, pp. 78–85, October 1 2018. [DOI] [PubMed] [Google Scholar]
- [13].Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, and Horak FB, “The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease,” J Neurol Neurosurg Psychiatry, vol. 81, no. 2, pp. 171–6, February 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, and Horak FB, “Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease,” Mov Disord, vol. 30, no. 10, pp. 1361–70, September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ihlen EA, Goihl T, Wik PB, Sletvold O, Helbostad J, and Vereijken B, “Phase-dependent changes in local dynamic stability of human gait,” J Biomech, vol. 45, no. 13, pp. 2208–14, August 31 2012. [DOI] [PubMed] [Google Scholar]
- [16].Ihlen EAF, Weiss A, Beck Y, Helbostad JL, and Hausdorff JM, “A comparison study of local dynamic stability measures of daily life walking in older adult community-dwelling fallers and non-fallers,” J Biomech, vol. 49, no. 9, pp. 1498–1503, June 14 2016. [DOI] [PubMed] [Google Scholar]
- [17].Abbruzzese G, Marchese R, Avanzino L, and Pelosin E, “Rehabilitation for Parkinson’s disease: Current outlook and future challenges,” Parkinsonism & related disorders, vol. 22 Suppl 1, pp. S60–S64, 2016. [DOI] [PubMed] [Google Scholar]
- [18].Ahn S, Chen Y, Bredow T, Cheung C, and Yu F, “Effects of Non-Pharmacological Treatments on Quality of Life in Parkinson’s Disease: A Review,” Journal of Parkinson’s disease and Alzheimer’s disease, vol. 4, no. 1, pp. 10.13188/2376-922X.1000021, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ellis T and Rochester L, “Mobilizing Parkinson’s Disease: The Future of Exercise,” Journal of Parkinson’s disease, vol. 8, no. s1, pp. S95–S100, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mak MK, Wong-Yu IS, Shen X, and Chung CL, “Long-term effects of exercise and physical therapy in people with Parkinson disease,” Nature reviews. Neurology, vol. 13, no. 11, pp. 689–703, 2017. [DOI] [PubMed] [Google Scholar]
- [21].Ramazzina I, Bernazzoli B, and Costantino C, “Systematic review on strength training in Parkinson’s disease: an unsolved question,” Clinical interventions in aging, vol. 12, pp. 619–628, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rochester L et al. , “The attentional cost of external rhythmical cues and their impact on gait in Parkinson’s disease: effect of cue modality and task complexity,” J Neural Transm (Vienna), vol. 114, no. 10, pp. 1243–8, 2007. [DOI] [PubMed] [Google Scholar]
- [23].Ginis P, Nackaerts E, Nieuwboer A, and Heremans E, “Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives,” Ann Phys Rehabil Med, September 7 2017. [DOI] [PubMed] [Google Scholar]
- [24].Giggins OM, Persson UM, and Caulfield B, “Biofeedback in rehabilitation,” Journal of NeuroEngineering and Rehabilitation, vol. 10, no. 1, p. 60, 2013/June/18 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harrington W et al. , “Alleviating freezing of gait using phase-dependent tactile biofeedback,” in 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2016, pp. 5841–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mancini M, Smulders K, Harker G, Stuart S, and Nutt JG, “Assessment of the ability of open- and closed-loop cueing to improve turning and freezing in people with Parkinson’s disease,” Sci Rep, vol. 8, no. 1, p. 12773, August 24 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nieuwboer A et al. , “Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers,” Gait Posture, vol. 30, no. 4, pp. 459–63, November 2009. [DOI] [PubMed] [Google Scholar]
- [28].Gibb WR and Lees AJ, “The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease,” J Neurol Neurosurg Psychiatry, vol. 51, no. 6, pp. 745–52, June 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nasreddine ZS et al. , “The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment,” J Am Geriatr Soc, vol. 53, no. 4, pp. 695–9, April 2005. [DOI] [PubMed] [Google Scholar]
- [30].Salarian A et al. , “Gait assessment in Parkinson’s disease: toward an ambulatory system for long-term monitoring,” IEEE Trans Biomed Eng, vol. 51, no. 8, pp. 1434–43, August 2004. [DOI] [PubMed] [Google Scholar]
- [31].Ihlen EA, Weiss A, Helbostad JL, and Hausdorff JM, “The Discriminant Value of Phase-Dependent Local Dynamic Stability of Daily Life Walking in Older Adult Community-Dwelling Fallers and Nonfallers,” Biomed Res Int, vol. 2015, p. 402596, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morris R, Stuart S, McBarron G, Fino PC, Mancini M, and Curtze C, “Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease,” Physiol Meas, vol. 40, no. 9, p. 095003, September 30 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lord S, Galna B, and Rochester L, “Moving forward on gait measurement: toward a more refined approach,” Mov Disord, vol. 28, no. 11, pp. 1534–43, September 15 2013. [DOI] [PubMed] [Google Scholar]
- [34].Benjamini Y and Hochberg Y, “Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing,” Journal of the Royal Statistical Society: Series B (Methodological), vol. 57, no. 1, pp. 289–300, 1995. [Google Scholar]
- [35].Hausdorff JM, Balash J, and Giladi N, “Effects of Cognitive Challenge on Gait Variability in Patients with Parkinson’s Disease,” Journal of Geriatric Psychiatry and Neurology, vol. 16, no. 1, pp. 53–58, 2003. [DOI] [PubMed] [Google Scholar]
- [36].O’Shea S, Morris ME, and Iansek R, “Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks,” Phys Ther, vol. 82, no. 9, pp. 888–97, September 2002. [PubMed] [Google Scholar]
- [37].Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, and Hausdorff JM, “Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa,” J Neurol Sci, vol. 212, no. 1–2, pp. 47–53, August 15 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


