Abstract
Outcomes in patients with secondary acute myeloid leukemia (sAML) (including therapy related myeloid neoplasms and AML with myelodysplasia related changes (MRC)) are poor. Patients treated with hypomethylating agents (HMAs) for antecedent hematological malignancy (AHM) have suboptimal responses to induction chemotherapy upon transformation to AML. We investigated outcomes after various induction strategies in patients with sAML who had prior HMA exposure. We identified 242 patients with sAML who had prior HMA treatment for AHM and later received induction chemotherapy upon AML transformation and divided into 3 cohorts based on induction regimen: (A) CLAG/M (B) 7 + 3 and (C) CPX-351. The CR/CRi rate was 53% in cohort A, 32% in cohort B and 41.2% in cohort C (p = 0.005 between cohort A and B) (p = 0.329 between cohorts A and C) (p = 0.402 between cohorts B and C). The early death rates were not significantly different among the three cohorts (p = 0.200). In patients who received ≤4 cycles of HMAs prior to AML transformation, response rates to CPX-351 were higher (64.3%) with a trend toward better overall survival (OS) (19.9 vs. 5.5 months) compared to > 4 cycles (p = 0.092). There was no significant difference in median OS among the 3 groups: cohort A (7.27 months), cohort B (7.63 months) and cohort C (7.07 months) (p = 0.887). We demonstrate that CLAG/M and CPX-351 yield higher CR/CRi rates compared to 7 + 3 in patients with sAML after HMA failure. Median OS remains poor and did not differ among the 3 groups, illustrating the unmet need for more efficacious therapy for sAML patients following HMA failure.
Keywords: Acute myeloid leukemia, Secondary AML, Induction chemotherapy, Hypomethylating agents
1. Introduction
Acute myeloid leukemia with myelodysplasia related changes (AML-MRC) represents a disease category with overall poor outcomes. Traditionally, AML-MRC includes patients harboring multilineage dysplasia (defined as detection of 50% or more dysplastic cells in at least 2 cell lines), history of prior myelodysplastic syndrome (MDS), or presence of MDS-related cytogenetic abnormalities. Definition of AML-MRC was recently updated in 2016 and excludes conventional favorable risk mutations of nucleophosmin 1 (NPM1) or biallelic CEBPA [1]. Sixty to seventy percent of patients with AML-MRC have had an antecedent myeloid malignancy, most commonly MDS [2,3]. AML-MRC arising from MDS typically occurs in older patients and has a poor prognosis with lower rates of complete remission (CR) and inferior survival compared to other subtypes of AML [4].
For patients with high-grade MDS, hypomethylating agents (HMAs), decitabine and azacitidine, are frequently used because of their proven benefit of delaying progression to AML and improving overall survival [5–7]. Treatment options are limited and responses are suboptimal in those patients who progress to AML after HMA therapy [8,9]. Since its approval in August 2017, CPX-351 represents a new standard of care for patients with newly diagnosed AML-MRC or therapy related AML (t-AML) who are fit for intensive chemotherapy. The approval was based on a randomized phase 3 clinical trial which compared the standard induction strategy of “7 + 3” with the liposomal formulation of cytarabine and daunorubicin (CPX-351) in secondary AML (sAML) (including AML-MRC and t-AML) that yielded superior survival and remission rates with CPX-351 [10]. Prior to CPX-351, retrospective data demonstrated that induction with cladribine, cytarabine, filgrastim, and mitoxantrone (CLAG-M) yields significantly higher response rates (64%) than 7 + 3 (cytarabine and anthracycline) (29%) in sAML patients who had prior HMA exposure [11]. A separate retrospective study assessed responses and survival among AML patients after HMA failure did not show any difference among high-dose cytarabine containing induction regimens when compared to 7 + 3 or purine analog containing regimens [12].
Data specifically addressing the question of which induction regimen is most effective after HMA failure for antecedent hematologic malignancy (AHM) remain limited. Moreover, comparison of the new standard of care, CPX-351, to traditional chemotherapy regimens (7 + 3 and high dose cytarabine containing regimens) in this subpopulation has not been performed. Therefore, this analysis aimed to investigate outcomes after treatment with CPX-351, high-dose cytarabine and cladribine regimens, and 7 + 3 in patients with sAML after failing HMA therapy for an antecedent myeloid malignancy.
2. Methods
2.1. Patient selection
Patients included in this study received at least 1 cycle of azacitidine or decitabine for an antecedent myeloid malignancy, including a histologically proven diagnosis of MDS (International Prognostic Scoring System [IPSS] intermediate-1 or higher) or chronic myelomonocytic leukemia (CMML) as defined by World Health Organization 2008 classification [13,14]. Upon transformation to AML, all patients received at least induction regimen as discussed in the “remission induction strategies” section. Patients were ≥18 years of age. At the time of transformation to AML, clinical variables were collected including age, bone marrow blast percentage, complete blood count with differential, karyotype, induction chemotherapy regimen, response to induction, vital status, date of death or last contact, and allogeneic hematopoietic stem cell transplant (allo-HSCT) status. This study was approved by the institutional review board at Moffitt Cancer Center/University of South Florida. Patient data were obtained and combined to create a uniform cohort from the following institutions: Moffitt Cancer Center, Roswell Park Comprehensive Cancer Center, and Memorial Sloan Kettering Cancer Center.
2.2. Remission induction strategies
After confirmation of sAML, patients received one of many induction chemotherapy regimens. We classified the regimens into 3 cohorts, based on the initial induction regimen a patient received: Cohort A) CLAG/M including cladribine (5 mg/m2/day on days 2–6), cytarabine (2000 mg/m2/day per day for days 2–6), granulocyte colony stimulating factor (G-CSF) on days 1–6 if WBC < 10,000 at the time of treatment initiation, and in some cases mitoxantrone (10 mg/m2/day on days 2–4); Cohort B) 7 + 3 including combinations of cytarabine (100–200 mg/m2) and anthracycline (daunorubicin 60 mg/m2 or idarubicin 12 mg/m2); Cohort C) CPX-351 (100 units/m2 (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) on day 1,3,5).
2.3. Outcome and response assessments
Reponses to remission induction therapy was assessed according to the International Working Group (IWG) response criteria for AML [15]. The primary study objective was to compare the overall response rates (complete response (CR)+complete response with incomplete blood count recovery (CRi)) for the three different cohorts. CR and CRi were assessed by bone marrow biopsy and aspirate and peripheral blood counts at the time of the bone marrow biopsy. Secondary objectives included assessment of median overall survival (mOS), 30-day mortality rate and rate of reinduction. mOS was calculated from the time of induction chemotherapy to death or last follow-up. Reinduction was defined as patients requiring a second cycle of intensive chemotherapy due to residual AML noted in the bone marrow evaluation after the initial induction. Additional secondary outcomes included 30-day all-cause mortality from the time of treatment initiation and the number of responding patients who proceeded to allo-HSCT.
2.4. Statistical analysis
Baseline characteristics included both continuous and categorical variables. Continuous variables were summarized utilizing means, medians, and standard deviations. Categorical variables were summarized with numbers and percentages. Two-sided chi-square analyses and t-tests were conducted at the 5% level of significance to compare baseline characteristics. The Kaplan-Meier method was used to estimate mOS, and the log-rank test was used to compare the two groups. All data were analyzed using SPSS version 20.0 (IBM Corp., Armonk, N.Y., USA) statistical software. All p-values reported are two-sided.
3. Results
A total of 241 patients were included in this study. All patients were treated between 2012 and 2018 at three academic cancer centers. Cohort A (CLAG/M) included 114 patients, cohort B (7 + 3) included 93 patients, and cohort C (CPX-351) included 34 patients. Of the cohort C patients, 21 were treated at Moffitt Cancer Center, 11 were treated at Memorial Sloan Kettering and 2 were treated at Roswell Park Comprehensive Cancer Center. Baseline characteristics are outlined in Table 1. No statistically significant differences were identified among the three cohorts with the exception of a higher baseline white blood cell count (WBC) in cohort C, and a higher baseline platelet count in the CLAG/M cohort. Table 2 summarizes the best response achieved with hypomethylating agent therapy prior to transformation to AML in all cohorts. No significant difference in the responses to HMA therapy was noted between cohorts.
Table 1.
Baseline characteristics for the entire cohort.
| Clinical parameter | CLAG/M | 7 + 3 | CPX-351 | p-value |
|---|---|---|---|---|
| n = 114 | n = 93 | n = 34 | ||
| Age | 65 (33–82) | 66 (26–81) | 69 (36–82) | Ns |
| Gender | ||||
| Male | 78 (68.4%) | 59 (63.4%) | 18 (52.9%) | 0.4937 |
| Female | 36 (31.6%) | 34 (36.56 %) | 16 (47.1%) | |
| Karyotype | ||||
| Favorable | 0 | 0 | 0 | 0.1944 |
| Intermediate | 62/107 (57.9%) | 51/78 (65.4%) | 22/31 (71.0%) | |
| Poor | 45/107 (42.1%) | 27/78 (34.6%) | 9/31 (29.0%) | |
| WBC (mean) | 4.401 (0.47–34.50) | 4.16 (0.88–18.0) | 8.52 (0.40–50.35) | 0.0013 |
| Hb | 9.36 (2.30–14.30) | 9.66 (4.0–14.7) | 8.9 (6.0–13.0) | 0.3869 |
| ANC | 2.04 (0.00–28.8) | 1.75 (0.07–6.5) | 3.3 (0–25.2) | 0.0902 |
| Platelets | 126 (8–1233) | 102 (2–834) | 78 (2–350) | 0.0497 |
| BM blasts (%) | 33.3% (20–96%) | 36.9% (20–93%) | 34.7 (9–76) | 0.1797 |
| HMA | ||||
| Azacitidine | 110 (88.7%) | 79 (84.9 %) | 28 (82.4%) | 0.5991 |
| Decitabine | 14 (11.3%) | 14 (15.1 %) | 6 (17.6%) | |
| Median number of HMA cycles | 6 (1–47) | 4 (1–72) | 5 (1–36) | Ns |
Table 2.
Best response achieved with prior hypomethylating agent treatment.
| Response | CLAG/M n = 114 | 7 + 3 n = 93 | CPX-351 n = 34 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Primary refractory disease (defined as progression of disease at ≤4 cycles) | 41 (36.0%) | 41 (44.1%) | 14 (41.2%) |
| Responders to intensive chemotherapy | 23 (20.2%) | 11 (11.8%) | 9 (26.5 %) |
| Stable disease, including hematologic improvement | 49 (43.0%) | 39 (41.9%) | 8 (23.5 %) |
| Responders to intensive chemotherapy | 23 (20.2%) | 18 (19.4%) | 1 (2.9%) |
| Partial remission | 6 (5.3%) | 3 (3.2%) | 0 (0%) |
| Responders to intensive chemotherapy | 4 (3.5%) | 0 (0%) | 0 (0%) |
| Complete response (including CR marrow) | 18 (15.8%) | 11 (11.8%) | 12 (35.3%) |
| Responders to intensive chemotherapy | 10 (8.8%) | 1 (0.01 %) | 4 (11.8%) |
3.1. Overall responses with each induction strategy
The overall response rates (CR/CRi) were assessed in all three cohorts. The CR/CRi rates were 53% in cohort A (n = 60), 32% in cohort B (n = 30), and 41.2% in cohort C (n = 14). The difference in CR/CRi rates lacked statistical significance when comparing CPX-351 treated patients to CLAG/M or 7 + 3 (p = 0.402 when comparing cohorts A and C; p = 0.329 when comparing cohorts B and C), however there was a significant increase in the rate of CR/CRi when comparing CLAG/M to 7 + 3 (p = 0.005) (Table 3). Five patients (13%) required a second induction (reinduction) in cohort A because of an inadequate response to the initial induction. This compared favorably to cohorts B and C, in which 63% (n = 35) and 36.8% (n = 7), respectively, required reinduction (p < 0.001). Sixty percent (n = 3) of patients in cohort A attained a subsequent response after reinduction compared to 31% (n = 11) in cohort B and 28.6% in cohort C, however the difference did not reach statistical significance (p = 0.43).
Table 3.
Overall outcomes and responses to induction treatments.
| Responses to treatment | CLAG/M | 7 + 3 | CPX-351 | p value |
|---|---|---|---|---|
| n = 114 | n = 93 | n = 34 | ||
| CR/CRi | 60 (53%) | 30 (32%) | 14 (41.2 %) | 0.002 |
| No response | 40 (35%) | 56 (60%) | 19 (55.9 %) | |
| Treatment related mortality (< 30 days) | 14 (12%) | 7 (8%) | 1 (2.9%) | 0.200 |
| Allogeneic stem cell transplant (alloSCT) (of CR/CRi) | 24 (40.0%) | 11 (36.7%) | 7 (50.0 %) | 0.192 |
| Second induction | 5 (13%) | 35 (63%) | 7 (36.8 %) | <0.001 |
| CR/CRi after reinduction | 3 (60%) | 11 (31%) | 2 (28.6 %) | 0.276 |
The rate of allo-HSCT between the three cohorts was assessed as well. Overall, 40% of the responding patients were bridged to allo-HSCT in the CLAG/M cohort compared to 50% in the CPX-351 arm and 36.7% in 7 + 3 arm (p = 0.19).
The median follow-up for each cohort was 52 months in cohort A, 53 months in cohort B and 49 months in cohort C. The mOS was not significantly different among the three groups, at 7.63 months with 7 + 3, 7.27 months with CLAG/M, and 7.07 months with CPX-351 (p = 0.89) (Fig. 1). The mOS was significantly higher in the responders compared to nonresponders with 6-month (p < 0.0001) and 12-month (p = 0.0011) landmark analysis (Fig. 2A). In the landmark analysis, the mOS for responders alive at 6 months was 20.7 months with 7 + 3, 12.9 months with CLAG/M, and 22.0 months with CPX-351 (p = 0.77). In the patients who proceeded to allo-HSCT, mOS was 26.9 months in cohort A, 25.03 months in cohort B, and not reached in cohort C, however there was no statistical difference (p = 0.62).
Fig. 1.
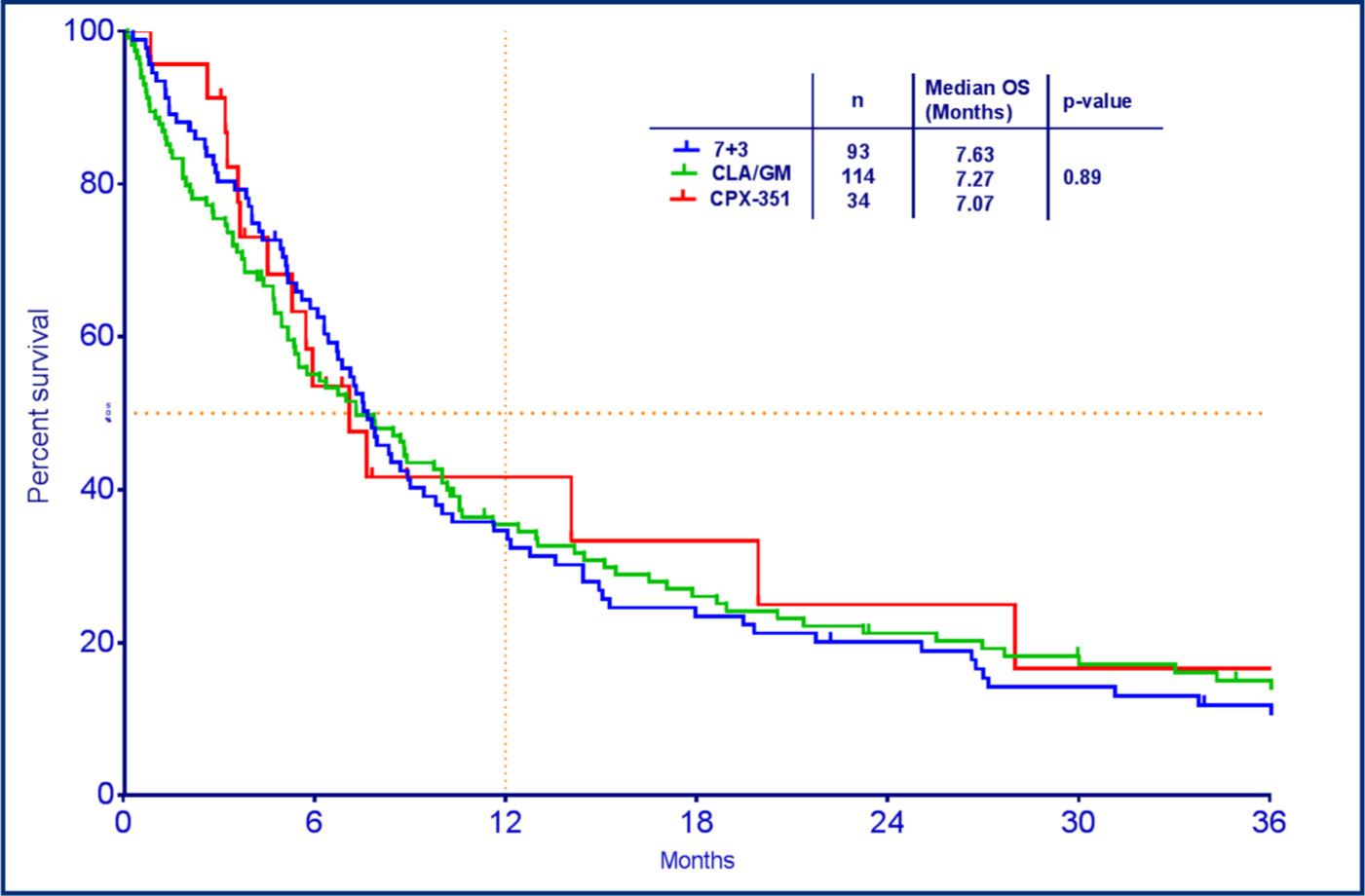
Median overall survival based on induction regimen.
Fig. 2.
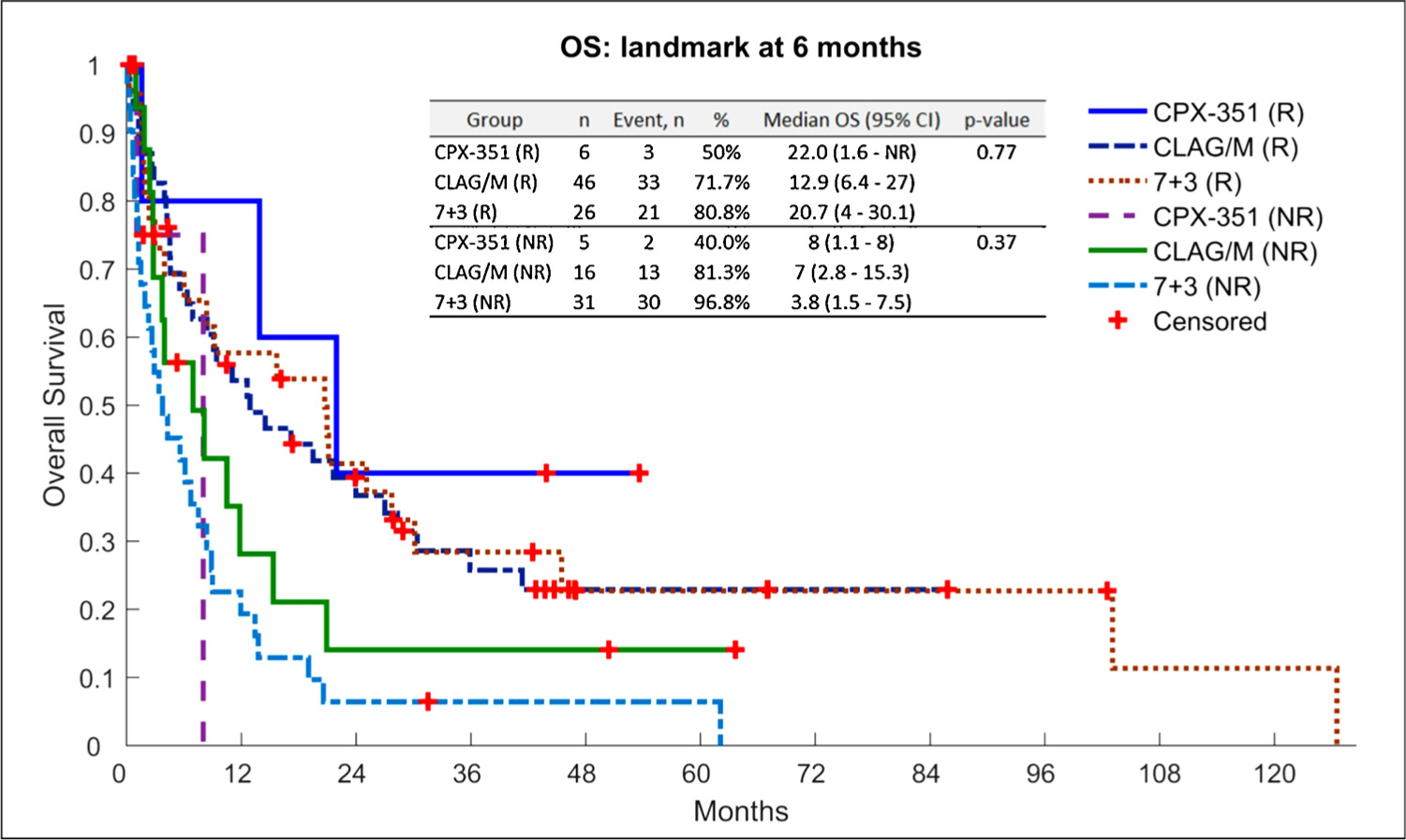
Landmark survival analysis (6-month) of patients based on induction regimen.
3.2. Impact of prior hypomethylating agent therapy
The impact on overall survival of duration of hypomethylating agent prior to AML progression was assessed (Table 4). The total cohort of 241 patients was dichotomized into patients who received ≤4 cycles of HMA (HMA ≤ 4) and those receiving > 4 cycles of HMA (HMA > 4). Among HMA ≤ 4 subgroup, 46 patients received 7 + 3, 46 patients received CLAG/M, and 14 patients received CPX-351. In this group of patients, the CR/CRi rate was 39.1% with 7 + 3, 56.5% with CLAG/M and 64.3% with CPX-351, which lacked statistical significance (p = 0.35). On the contrary, in the HMA > 4 cohort, the CR/CRi rate was 25.5% with 7 + 3, 25% with CPX-351 and significantly higher at 50.0% with CLAG/M treatment (p = 0.0068). Rates of CR/CRi were not significantly different based on duration of HMA therapy when patients were treated with CLAG/M or 7 + 3. However, there was a significant difference in response rates in the CPX-351 treated patients based on the number of cycles of HMAs received (64.3% with HMA ≤ 4 vs. 25% with HMA > 4, p = 0.023). A trend was seen toward higher mOS in the CPX-351 cohort with ≤4 cycles of prior HMAs compared to those with > 4 cycles (19.9 months vs. 5.47 months, p = 0.092).
Table 4.
Overall outcomes and responses to induction treatments based on the duration of HMA exposure.
| Responses to treatment | CLAG/M | 7 + 3 | CPX-351 | |||
|---|---|---|---|---|---|---|
| HMA ≤ 4 cycles (n = 46) | HMA > 4 cycles (n = 68) | HMA ≤ 4 cycles (n = 46) | HMA > 4 cycles (n = 47) | HMA ≤ 4 cycles (n = 14) | HMA > 4 cycles (n = 20) | |
| CR/CRi | 26 (565%) | 34 (50.0%) | 18 (39.1%) | 12 (25.5%) | 9 (64.3%) | 5 (25%) |
| No response | 17 (37.0%) | 25 (36.8%) | 25 (54.3%) | 31 (66.0%) | 5 (35.7%) | 14 (70.0%) |
| Death | 3 (6.5%) | 9 (13.2%) | 3 (6.5%) | 4 (8.5%) | 0 (0%) | 1 (5.0%) |
| p value | 0.568 | 0.188 | 0.035 | |||
| Reinduction (of nonresponders) | 2(11.8%) | 3 (12%) | 15 (60.0%) | 20 (64.5%) | 2 (40.0%) | 5 (25%) |
| CR/CRi after reinduction | 2 (100 %) | 1 (33.3 %) | 4 (26.7 %) | 7 (35 %) | 1 (50.0 %) | 1 (20.0 %) |
| Allogeneic stem cell transplant | 22 (47.8%) | 24 (35.3%) | 15 (32.6%) | 4 (8.51%) | 5 (35.7%) | 2 (10.0%) |
| Median OS | 9.97 months | 5.47 months | 7.47 months | 8.67 months | 19.9 months | 5.47 months |
| p value | 0.275 | 0.706 | 0.092 | |||
4. Discussion
Since the approval of CPX-351, the standard of care for patients with secondary AML has shifted to utilizing CPX-351 as first-line therapy. However, for patients with prior HMA therapy, the median overall survival ranges from three to six months [16–18]. The phase 3 trial of CPX-351 versus 7 + 3 included a subset of patients treated with prior HMA therapy and showed no difference in outcomes when treated with CPX-351 (n = 62) compared to 7 + 3 (n = 71), with a mOS of 5.65 months vs. 5.90 months, respectively (HR 0.86, 95% CI 0.59–1.26). Remission rates were comparable between the two arms with 37.1% achieving CR/CRi after treatment with CPX-351 and 28.2% with 7 + 3 (OR 1.50, 95% CI 0.73–3.12). Several studies have suggested that high-dose cytarabine based regimens could perhaps achieve higher response rates in this patient population [11,12]. Jaglal et al. previously published that the CLAG/M regimen results in higher CR/CRi rates of 64% compared to 29% with 7 + 3 with the mOS favoring CLAG/M as well (202 days vs. 86 days, p = 0.025). However, CPX-351 has not previously been compared to CLAG/M.
In this retrospective study, CLAG/M induction for sAML that failed therapy with an HMA resulted with a higher CR/CRi rate and improved overall survival when compared with standard 7 + 3 therapy as previously demonstrated by Jaglal et al. [11]. Importantly, response rates were similar between CPX-351 and CLAG/M induction regimens thus CPX-351 represents a viable treatment option in this subgroup of patients. The remission rates between 7 + 3 and CPX-351 were not statistically different (32% vs. 41.2%, p = 0.53). On the contrary, the need for a second induction was highest, at 63% among patients receiving 7 + 3, whereas only 13% with CLAG/M and 36.8% with CPX-351 required two inductions (p < 0.001). Upon second induction, a trend toward higher response rates was noted in the CLAG cohort (60%) compared to 31% with 7 + 3 and 28.6% in the CPX-351 cohort, however this was not statistically significant (p = 0.276). In general, all three induction modalities were well tolerated with similar 30-day treatment related mortality ranging from 2.9%–12% (p = 0.2).
Despite the significant differences in CR/CRi rates in this study, the mOS was not different between the three cohorts, further supporting the need for optimization of treatments for this specific subgroup of patients. The median OS was 7.07 months with CPX-351, 7.27 months with CLAG/M and 7.63 months with 7 + 3 (p = 0.89) suggesting overall dismal outcomes despite novel therapies. Survival was significantly longer for patients who achieved CR/CRi compared to nonresponders (p < 0.0001). When landmark analysis was performed at 6-months and 12-months, survival was superior among responders compared to nonresponders.
The percentage of responding patients who proceeded to allo-HSCT was similar between the three cohorts, with 50% in the CPX-351 treated group, 40% after CLAG/M, and 36.7% in the 7 + 3 cohort (p = 0.19). The mOS was greater in patients who proceeded to allo-HSCT compared with those who did not undergo a transplant. Looking specifically at the patients who underwent an allogeneic transplant, the mOS was not reached in the CPX-351 cohort, compared to 25.03 months with 7 + 3, and 26.9 months with CLAG/M, however this did not reach statistical significance (p = 0.62). Despite observing relatively greater proportion of patients achieving a response when treated with CLAG/M (although no significantly different than CPX-351), rate of patients proceeding with allo-HSCT was similar. This could potentially be attributed to patient preference and overall performance status of the patient hampering candidacy for allo-HSCT however such information was not captured and represents one of the limitations of this retrospective study.
The overall mechanism of resistance to HMA in high-grade MDS is unclear. Moreover, the reason that this particular subset of patients classically shows significant resistance to AML directed chemotherapy also needs further elucidation. One hypothesis is that hypomethylating agents have a similar metabolic pathway to cytarabine which could potentially explain resistance to conventional cytarabine containing regimens [19]. In addition to functioning as epigenetic modifying agents, HMAs also have some cytotoxic activity, and prior HMA exposure might select for clones resistant to cell-cycle directed chemotherapies. This study analyzed responses to induction regimens after failure of > or ≤4 cycles of HMA therapy and noted a trend toward higher responses to CPX-351 when ≤4 cycles of HMA were administered. When patients received > 4 cycles of HMA therapy, response to CPX-351 and 7 + 3 was significantly less (25% and 25.5%, respectively) than CLAG/M therapy (p = 0.007). Higher response rates with CLAG/M in this cohort however failed to translate into greater survival, and thus the significance of achieving CR/CRi after a certain number of HMA cycles prior to induction is unclear.
Although the results of this study may provide guidance for treatment decisions, they do have limitations. As with any retrospective study, one significant limitation includes the lack of randomization for the initial treatment selection. Given relatively recent approval of CPX-351 compared to prior use of 7 + 3 and CLAG/M, number of patients included in the CPX-351 arm is relatively small compared to the other two arms and does pose limitations when drawing conclusions. Furthermore, molecular data is lacking for the CLAG/M and 7 + 3 cohorts, and it is now clear that the molecular landscape may have impacted outcomes to chosen therapy.
In conclusion, CPX-351 serves as a viable treatment option for this difficult to treat group of patients and provides similar outcomes to CLAG/M. Results of this study suggest that CLAG/M or CPX-351 may provide superior outcomes to 7 + 3 in patients with sAML after HMA failure. Our data also demonstrate that prior HMA exposure ≥4 cycles is associated with a lower likelihood of response to CPX-351, potentially suggesting CLAG/M as a preferred option in this subgroup, although mOS is not thereby improved. The role of allo-HSCT for sAML patients with prior HMA exposure is again reaffirmed by these observations. The results of this study need to be confirmed in a randomized, prospective fashion. In spite of many new advances in the treatment of AML, identifying effective therapies for this challenging subset of AML patients remains difficult. Clinical trials using new agents which can produce deep remissions and allow more patients the opportunity to proceed to a potentially curative allogeneic stem cell transplant remain a significant focus in AML research.
Acknowledgements
We thank our colleagues from Memorial Sloan Kettering as well as Roswell Park Comprehensive Cancer Center who provided insight and expertise that greatly assisted the research. We thank Jongphil Kim, PhD for assistance with statistical analysis and all authors for their comments that greatly improved the manuscript. This study is not funded by any external source.
References
- [1].Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW, The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia, Blood 127 (20) (2016) 2391–2405, 10.1182/blood-2016-03-643544 Epub 2016/04/14, [DOI] [PubMed] [Google Scholar]
- [2].Stolzel F, Pfirrmann M, Aulitzky WE, Kaufmann M, Bodenstein H, Bornhauser M, Rollig C, Kramer M, Mohr B, Oelschlagel U, Schmitz N, Soucek S, Thiede C, Ehninger G, Schaich M, Study Alliance L, Risk stratification using a new prognostic score for patients with secondary acute myeloid leukemia: results of the prospective AML96 trial, Leukemia 25 (3) (2011) 420–428, 10.1038/leu.2010.279 Epub 2010/12/08. [DOI] [PubMed] [Google Scholar]
- [3].Leone G, Mele L, Pulsoni A, Equitani F, Pagano L, The incidence of secondary leukemias, Haematologica 84 (10) (1999) 937–945 Epub 1999/10/06. [PubMed] [Google Scholar]
- [4].Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, Head DR, Appelbaum FR, Willman CL, Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study, Blood. 89 (9) (1997) 3323–3329 Epub 1997/05/01. [PubMed] [Google Scholar]
- [5].Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR, International Vidaza High-Risk MDSSSG. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study, Lancet Oncol. 10 (3) (2009) 223–232, 10.1016/S1470-2045(09)70003-8 Epub 2009/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Font P, Azacitidine for the treatment of patients with acute myeloid leukemia with 20%−30% blasts and multilineage dysplasia, Adv. Ther 28 (Suppl. 3) (2011) 1–9, 10.1007/s12325-011-0002-8 Epub 2011/04/02. [DOI] [PubMed] [Google Scholar]
- [7].Kantarjian H, Issa Jp, Rosenfeld Cs, Bennett Jm, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R 3rd, Shen L, Nimer Sd, Leavitt R, Raza A, Saba H, Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study, Cancer 106 (8) (2006) 1794–1803, 10.1002/cncr.21792 Epub 2006/03/15. [DOI] [PubMed] [Google Scholar]
- [8].Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J, Estey E, Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome, Cancer 106 (5) (2006) 1090–1098, 10.1002/cncr.21723 Epub 2006/01/26. [DOI] [PubMed] [Google Scholar]
- [9].Gajewski JL, Ho WG, Nimer SD, Hirji KF, Gekelman L, Jacobs AD, Champlin RE, Efficacy of intensive chemotherapy for acute myelogenous leukemia associated with a preleukemic syndrome, J. Clin. Oncol 7 (11) (1989) 1637–1645, 10.1200/JCO.1989.7.11.1637 Epub 1989/11/01. [DOI] [PubMed] [Google Scholar]
- [10].Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, Stuart RK, Strickland SA, Hogge D, Solomon SR, Stone RM, Bixby DL, Kolitz JE, Schiller GJ, Wieduwilt MJ, Ryan DH, Hoering A, Banerjee K, Chiarella M, Louie AC, Medeiros BC, CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia, J. Clin. Oncol 36 (26) (2018) 2684–2692, 10.1200/JCO.2017.77.6112 Epub 2018/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jaglal MV, Duong VH, Bello CM, Al Ali NH, Padron E, Fernandez HF, List AF, Lancet JE, Komrokji RS, Cladribine, cytarabine, filgrastim, and mitoxantrone (CLAG-M) compared to standard induction in acute myeloid leukemia from myelodysplastic syndrome after azanucleoside failure, Leuk. Res 38 (4) (2014) 443–446, 10.1016/j.leukres.2013.12.010 Epub 2014/01/21. [DOI] [PubMed] [Google Scholar]
- [12].Ball B, Komrokji RS, Ades L, Sekeres MA, DeZern AE, Pleyer L, Vey N, Almeida A, Germing U, Cluzeau T, Platzbecker U, Gore SD, Fenaux P, Prebet T, Evaluation of induction chemotherapies after hypomethylating agent failure in myelodysplastic syndromes and acute myeloid leukemia, Blood Adv 2 (16) (2018) 2063–2071, 10.1182/bloodadvances.2018015529 Epub 2018/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD, The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes, Blood 114 (5) (2009) 937–951, 10.1182/blood-2009-03-209262 Epub 2009/04/10. [DOI] [PubMed] [Google Scholar]
- [14].Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J, International scoring system for evaluating prognosis in myelodysplastic syndromes, Blood 89 (6) (1997) 2079–2088 Epub 1997/03/15. [PubMed] [Google Scholar]
- [15].Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis SoRCTO, Reporting Standards for Therapeutic Trials in Acute Myeloid L, Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia, J. Clin. Oncol 21 (24) (2003) 4642–4649, 10.1200/JCO.2003.04.036 Epub 2003/12/16. [DOI] [PubMed] [Google Scholar]
- [16].Prebet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, Dreyfus F, Rauzy OB, Recher C, Ades L, Quesnel B, Beach CL, Fenaux P, Vey N, Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure, J. Clin. Oncol 29 (24) (2011) 3322–3327, 10.1200/JCO.2011.35.8135 Epub 2011/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jabbour E, Garcia-Manero G, Batty N, Shan J, O’Brien S, Cortes J, Ravandi F, Issa JP, Kantarjian H, Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy, Cancer 116 (16) (2010) 3830–3834, 10.1002/cncr.25247 Epub 2010/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Prebet T, Gore SD, Thepot S, Esterni B, Quesnel B, Beyne Rauzy O, Dreyfus F, Gardin C, Fenaux P, Vey N, Outcome of acute myeloid leukaemia following myelodysplastic syndrome after azacitidine treatment failure, Br. J. Haematol 157 (6) (2012) 764–766, 10.1111/j.1365-2141.2012.09076.x Epub 2012/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qin T, Castoro R, El Ahdab S, Jelinek J, Wang X, Si J, Shu J, He R, Zhang N, Chung W, Kantarjian HM, Issa JP, Mechanisms of resistance to decitabine in the myelodysplastic syndrome, PLoS One 6 (8) (2011) e23372, , 10.1371/journal.pone.0023372 Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]


