Abstract
Background.
Talimogene laherparepvec (TVEC) is an oncolytic herpes virus used as intralesional therapy for patients with unresectable stage IIIB through IV melanoma. We reviewed the standard of care treatment of TVEC at a single institution.
Methods.
All patients treated with TVEC for advanced melanoma were retrospectively evaluated from 2015 to 2018. Patient demographics, clinicopathologic characteristics, treatment response, and toxicity were reviewed.
Results.
Twenty-seven patients underwent therapy with TVEC. Median age was 75 years, and 63% of patients were female. Seventeen (63.0%) patients underwent injections on the lower extremity, four (14.8%) on the upper extremity, four (14.8%) on the head and neck, and two (7.4%) on the trunk. Median number of injections was five. Median follow-up was 8.6 months. Of the 27 patients, 23 patients met the criteria for response analysis with at least 8 weeks follow-up. Ten (43.5%) patients experienced a complete response (CR), three (13.1%) experienced a partial response (PR), and five (21.7%) had stable disease (SD) for an overall response rate of 56.5% (CR + PR) and a disease control rate of 78.3% (CR + PR + SD). Adverse events were mostly limited to mild constitutional symptoms within 48 h of injection. Two patients developed cellulitis treated with oral antibiotics, and one patient underwent excision of a lesion for ulceration and bleeding during therapy.
Discussion.
TVEC is an effective and well-tolerated intralesional therapy for patients with unresectable stage IIIB through IV melanoma. A CR was achieved in almost half of patients treated. Disease control is seen in the vast majority.
The heterogeneous presentation of melanoma continues to pose a significant therapeutic challenge. Up to 10% of patients with melanoma may develop recurrent locoregional disease, often presenting as in-transit metastasis defined as disease within the dermal lymphatics between the primary tumor site and regional draining nodal basin.1–3 In addition to its impact on survival, a recent patient-reported outcomes study by Weitman et al.4 found advanced locoregional melanoma to have significant adverse effects on patients’ quality of life and emotional well-being. Fortunately, treatment options for these patients have evolved over the past decade, including systemic immunotherapies and targeted therapies, isolated limb infusion (ILI) and perfusion, and intralesional therapies.5–14
Talimogene laherparepvec (TVEC) (AMGEN Inc, Thousand Oaks, CA) is an FDA-approved modified oncolytic herpes simplex virus type 1 (HSV-1) used as intralesional therapy for patients with unresectable stage IIIB through IV melanoma. With TVEC, the neurovirulence factors in the HSV-1 virus, the ICP34.5 loci, have been removed. Additionally, the virus has been modified to include the capacity to express granulocyte macrophage colony-stimulating factor (GM-CSF).15,16 This allows for preferential replication within tumor cells resulting in cell lysis. Additionally, the release of virally-derived GM-CSF along with antigens derived from ruptured tumor cells can induce a systemic tumor-specific immune response which may lead to regression of distant uninjected lesions.15
In the phase III, randomized, controlled OncovexGM-CSF Pivotal Trial in Melanoma (OPTiM) trial, TVEC showed a significant improvement in durable response rate over the control GM-CSF (16.3% TVEC vs. 2.1%, p < 0.001).17 Furthermore, when substratifying patients with only stage IIIB, IIIC, and IVM1a disease, there was a significant improvement in overall survival with TVEC versus GM-CSF (41.1 months TVEC vs. 21.5 months GM-CSF, p < 0.001).17 As the role of intralesional therapy for advanced locoregional melanoma continues to evolve, we present our experience with the standard of care use of TVEC at a single institution.
METHODS
After Institutional Review Board approval, we performed a retrospective single institution review of all patients who underwent TVEC therapy as standard of care from 2015 to 2018. Any patients who were treated with TVEC as part of a clinical trial were excluded. Patient demographics (age, gender, ECOG status), clinicopathologic characteristics (prior treatment, disease stage according to the AJCC 7th edition, regional nodal involvement at time of injection, all lesions injected, number of injection cycles performed), response to treatment [complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD)], and toxicity were reviewed.18
All injections were performed in the outpatient setting. Patients were injected according to the manufacturer (AMGEN Inc, Thousand Oaks, CA) package insert guidelines and recommendations, with the initial dose given at 106 plaque-forming units (PFU) per milliliter. Three weeks later, patients received a second injection cycle at a concentration of 108 PFU per milliliter, which was subsequently repeated every 2 weeks in perpetuity, until either progression of disease, complete response, or intolerance to therapy. The initial dose was at a lower concentration to allow for seroconversion of herpes virus-naïve patients. For patients with numerous lesions, the largest lesions were injected first until the maximum cumulative dose of 4 mL was given or all lesions had been injected. Subsequent doses were used to treat newest and largest lesions first until a maximum of 4 mL was used or there were no further lesions to inject. All injected lesions were cutaneous, subcutaneous, or palpable nodes, and therefore no image-guided injections were performed.
Response to therapy was characterized as best overall response (ORR) and was measured using the revised World Health Organization (WHO) Handbook criteria.19 Responses were determined by changes in measurement of the largest lesion that were maintained for at least 4 weeks after meeting initial response criteria, and stability of disease was required for at least 8 weeks before meeting criteria for SD.20 If patients underwent surgical resection of disease after initiation of TVEC, response assessment was determined before resection. All patients who experienced a CR or underwent resection to no evidence of disease were followed with clinical exams ± imaging until further disease developed.
Statistical Analysis
The primary endpoint was best overall response (ORR). Secondary endpoints were time to response and overall survival (OS). A Chi square test (or Fisher’s exact test) was used to determine significant associations between clinicopathologic characteristics and response to therapy. Survival analysis was calculated using the Kaplan–Meier method.
RESULTS
Patient Demographics and Clinicopathologic Characteristics
At time of analysis, 27 patients were treated with TVEC outside of a clinical trial for advanced locoregional melanoma. The median age was 75 (range 51–94) years, and 62.9% of patients were female. Multiple patients received prior regional and/or systemic therapies as four patients previously underwent ILI, five patients were previously treated with systemic immunotherapy, and four patients had previously been treated with both ILI and immunotherapy. The median time from ILI/immunotherapy to TVEC was 3.8 (range 1.0–16.3) months in 8 of these 13 patients whose prior treatment dates were available in the medical record. The majority (81.5%) of patients were treated for stage III disease, whereas five (18.5%) patients were treated for stage IV disease. Fifteen (55.6%) patients had all lesions injected. The median number of injection cycles performed was 5 (range 2–14) cycles (Table 1).
TABLE 1.
Patient demographics and clinicopathologic characteristics included in the study
| N | % | |
|---|---|---|
| Age (years) | ||
| < 75 | 13 | 48 |
| ≥ 75 | 14 | 52 |
| Gender | ||
| Female | 17 | 63 |
| Male | 10 | 37 |
| ECOG | ||
| 0 | 13 | 48 |
| 1 | 14 | 52 |
| Prior treatment | ||
| Isolated limb infusion | 4 | 15 |
| Immunotherapy | 5 | 19 |
| Isolated limb infusion and immunotherapy | 4 | 15 |
| None | 14 | 52 |
| Disease stage | ||
| IIIb | 9 | 33 |
| IIIc | 13 | 48 |
| IVa | 4 | 15 |
| IVb | 0 | 0 |
| IVc | 1 | 4 |
| Response by disease stage | ||
| IIIb | ||
| CR | 5 | 22 |
| PR | 1 | 4 |
| SD | 0 | 0 |
| PD | 0 | 0 |
| IIIc | ||
| CR | 4 | 17 |
| PR | 1 | 4 |
| SD | 4 | 17 |
| PD | 4 | 17 |
| IV | ||
| CR | 1 | 4 |
| PR | 1 | 4 |
| SD | 1 | 4 |
| PD | 1 | 4 |
| Regional nodal involvement | ||
| Yes | 4 | 15 |
| No | 23 | 85 |
| All lesions injected | ||
| Yes | 15 | 56 |
| No | 12 | 44 |
| No. of TVEC cycles | ||
| ≤ 5 | 17 | 63 |
| > 5 | 10 | 37 |
Response to Therapy
Median follow-up for the entire cohort was 8.6 (range 2.8–20.5) months. Four patients were not included in the treatment response analysis: three patients did not have at least 8 weeks follow-up at time of analysis; one patient died at an outside hospital after one initial injection (106 PFU per mL) from a myocardial infarction secondary to atrial fibrillation with rapid ventricular response. Of the remaining 23 patients, 10 (43.5%) patients experienced a CR, 3 (13.0%) patients experienced a PR, and 5 (21.7%) patients had SD for an overall response rate of 56.5% (CR + PR) and a disease control rate of 78.3% (CR + PR + SD). Five (21.7%) patients experienced disease progression (Fig. 1). Of the five patients with disease progression, four patients were transitioned to immune checkpoint inhibitors, whereas one patient declined further therapy.
FIG. 1.
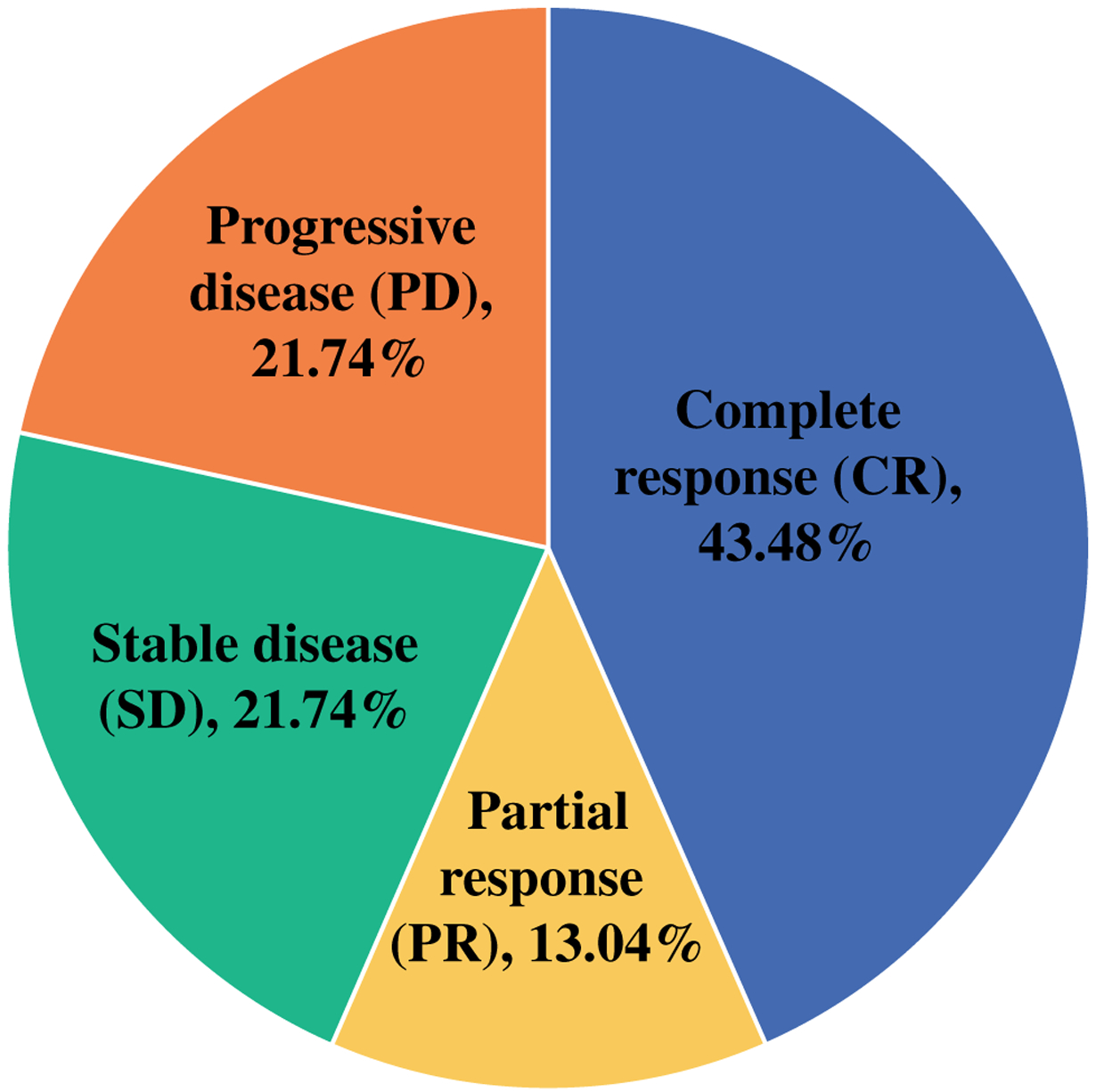
Response to therapy
The median time to response was 2.1 (range 0.7–2.6) months. In those patients who experienced a CR, the median time to CR was 2.8 months (6 cycles; Fig. 2a, b). At time of analysis, only one of the patients who experienced a CR had disease recurrence (at 3.7 months). In the 18 patients with disease control, 3 patients with stable disease and one patient with a partial response underwent resection of all residual disease. Surgical pathology of these excisions showed partial tumor necrosis and dense lymphocytic infiltrate. A CR was achieved in 6 (46.2%) of the 13 patients who had all their lesions injected. There was a significant association with disease stage and response to therapy, with 100%, 39%, and 50% of patients responding to therapy with stage IIIB, IIIC, and IV, respectively (p = 0.041). Six (26.1%) of the 23 patients included in the response analysis received prior immunotherapy, and only 2 of these 6 patients responded to therapy. There was no significant association observed between prior immunotherapy and response to TVEC (p = 0.341).
FIG. 2.
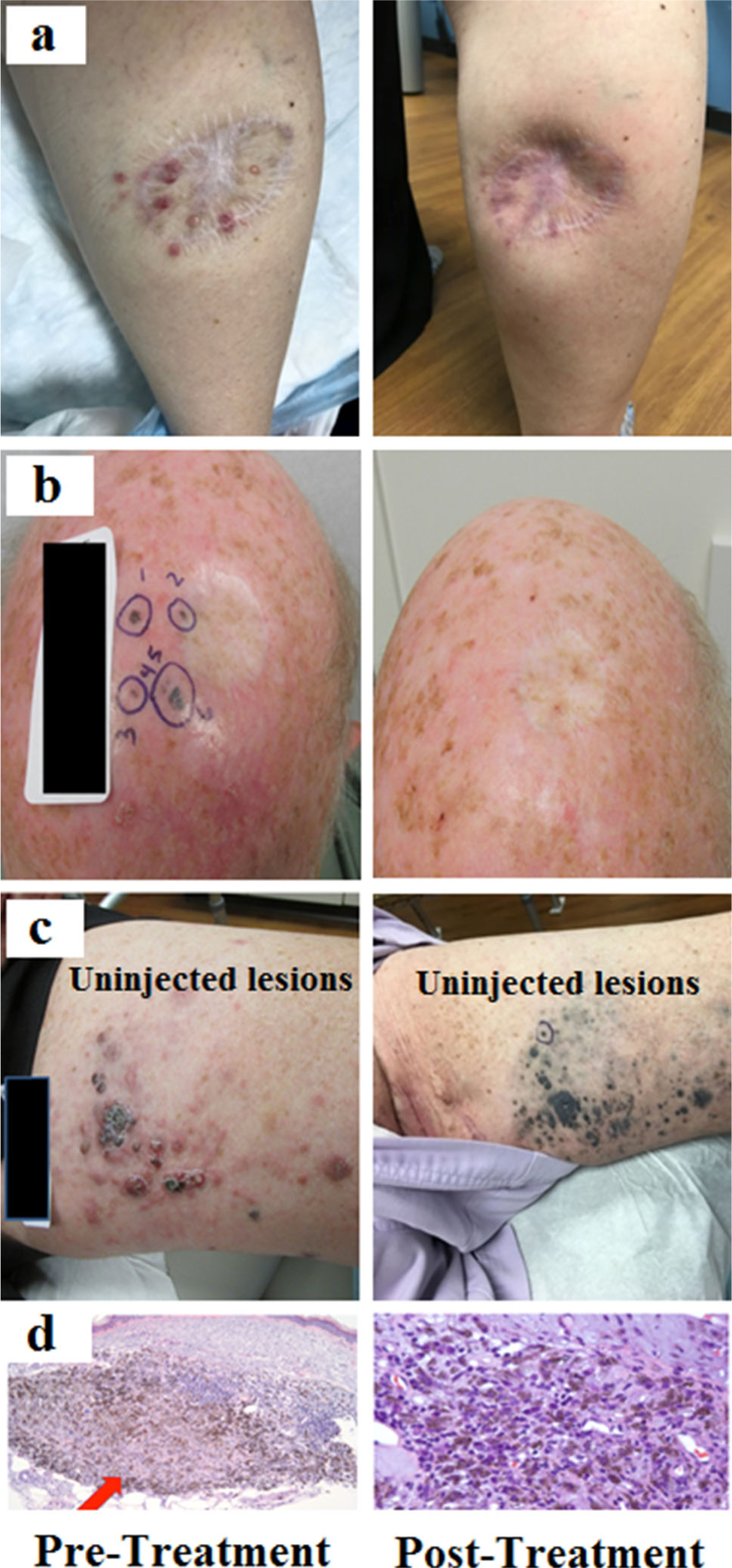
Clinical images pre- and posttherapy demonstrating complete response (a and b) and bystander response (c) to therapy along with pathologic images of a complete response (d). (Top row images previously published in: Miura JT, Zager JS. Intralesional therapy as a treatment for locoregionally metastatic melanoma. Expert Rev Anticancer Ther. 2018;18(4):399–408)
Survival
At time of analysis, four (14.8%) patients had died of disease, and one (3.7%) patient died of other causes. Median OS for those included in the response analysis was not reached at a median follow up of 8.6 months, and the OS was 80.0% at 1 year as shown in Fig. 3.
FIG. 3.
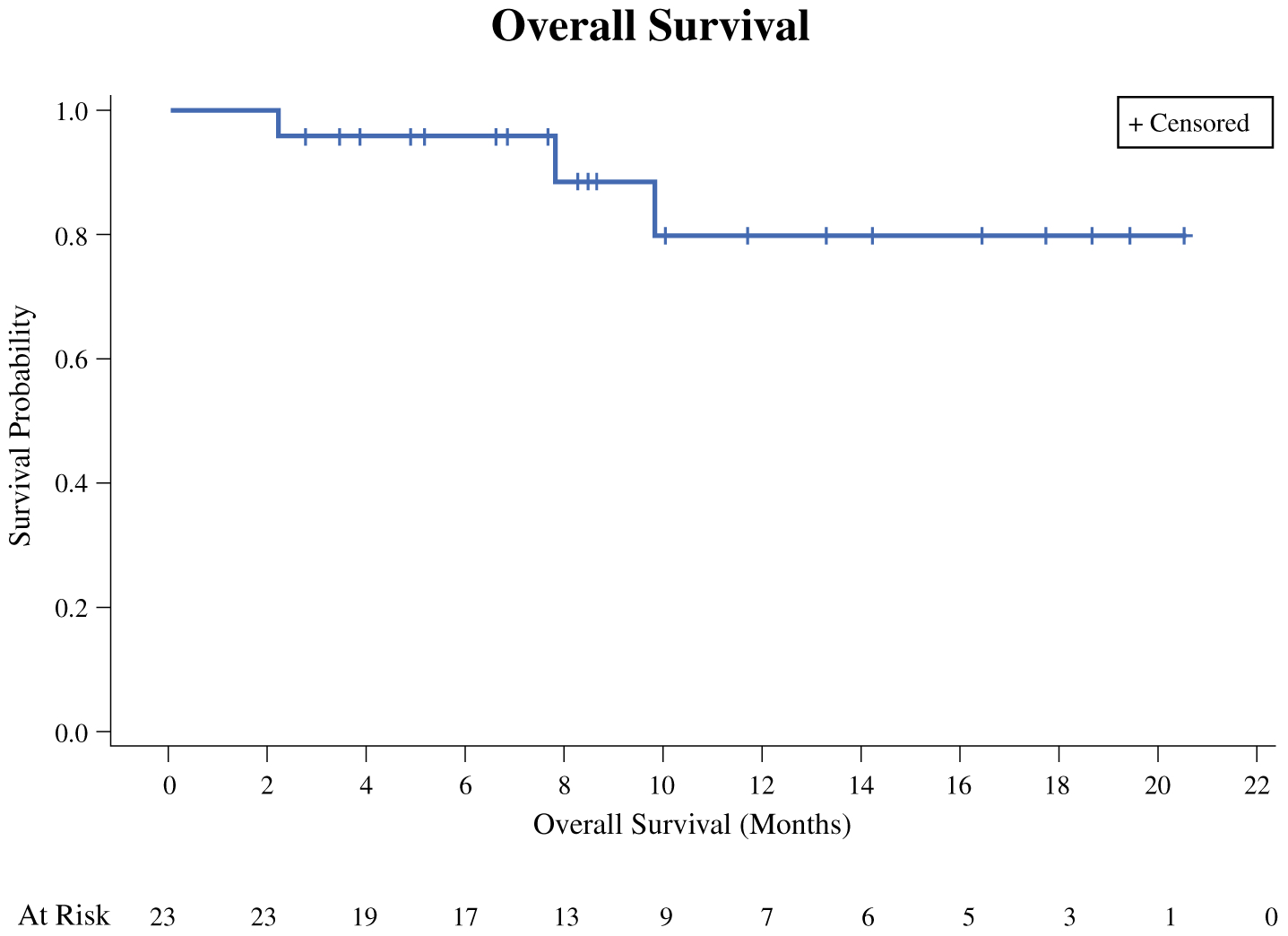
Kaplan–Meier estimate for overall survival
Treatment-Related Toxicity
The treatments were generally well-tolerated, and the majority of the treatments were without significant patient-reported adverse events. Mild constitutional symptoms, such as malaise, low-grade fever, and muscle aches, were reported in nine (33.3%) patients. Two patients developed cellulitis at the injection site, both of which resolved with the administration of oral antibiotics in the outpatient setting. One patient’s injected lesion ulcerated and developed intermittent bleeding requiring a nonemergent palliative resection.
DISCUSSION
Locoregional melanoma can pose a significant therapeutic challenge. While resection of recurrent disease with clear margins is preferred, surgery often is not effective or even feasible when disease is advanced and multifocal.21 As an intralesional therapy, TVEC is directly delivered to the precise sites of the disease, concentrating the treatment effect to the site of disease while theoretically minimizing systemic toxicity. This retrospective single institution review is the largest published series to date evaluating the standard of care use of TVEC and demonstrates TVEC is a safe and effective therapy for patients with advanced locoregional melanoma.
The ORR in this study was 56.5%, with nearly half of the patients achieving a CR. This result is significantly higher than the observed ORR in the OPTiM trial (26.4%) and slightly higher than the response rate previously observed with PV-10 (51%).17,22 However, the OPTiM trial included a significantly greater number of patients with stage IV disease (70%), including M1b and M1c disease (45%), whereas this current response analysis of 23 patients included only 4 patients (17.4%) with stage IV disease, all M1a. This is consistent with our finding that patients with stage IIIB disease were significantly more likely to have a response to therapy compared with patients with stage IIIC and IV disease (p = 0.041).
Although the follow-up time at this point is limited at 8.6 months, only one of the ten patients who experienced a CR in this study had evidence of disease recurrence at time of analysis, similar to the OPTiM trial which reported a majority of the responders (71.8%) continuing to have an ongoing response at time of final analysis.17 While the true incidence of bystander effect was not able to be accurately characterized from this retrospective analysis, four of ten patients who experienced a CR did not have all of their lesions directly injected. Furthermore, one patient experienced flattening of painful, inflamed thigh disease after undergoing injections to the distal lower extremity, and subsequent biopsy of the uninjected thigh lesions revealed numerous melanophages with no viable melanoma (Fig. 2c).
The response rate in this analysis is comparable to those seen with isolated limb infusion (59–75%).8,23,24 Immune checkpoint inhibitors, such as anti-CTLA-4 and anti-PD1 agents, report response rates from 10 to 40% in metastatic melanoma with single agent use, and up to 60% with combination therapy, albeit often with significant systemic toxicity.5,6,11,12 Targeted therapies, such as vemurafenib and dabrafenib, have shown response rates up to 70% in patients with BRAF-mutated tumors when used with a MEK inhibitor.25 However, the true response rates of these systemic agents in the specific patient population of advanced locoregional melanoma remains unclear.
Given the multiple treatment options available for patients with advanced locoregional melanoma, patient selection for specific treatment modalities is an area of active interest. Unless there is rapidly progressing disease, our preference is to reserve systemic therapy for future use in the event of subsequent disease progression. For patients presenting to our institution with disease that is amenable to both intralesional therapy and isolated limb infusion, we have utilized both treatment modalities based upon patient preference (Fig. 4). Patients who are travelling from further distances may opt for isolated limb infusion, because less treatment visits are required compared with intralesional therapy that requires injections every 2 weeks. Patients who are best served by avoiding general anesthesia opt for intralesional therapy.
FIG. 4.
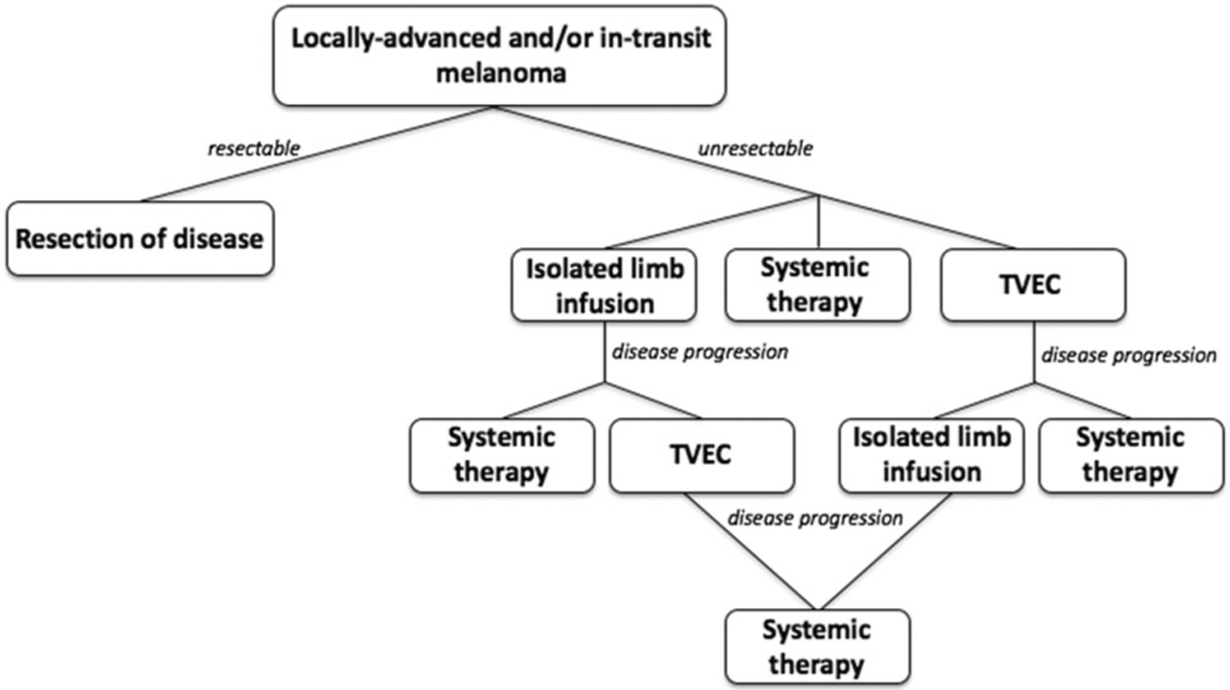
Flowchart showing typical treatment pathway for locally advanced and in-transit melanoma
We believe intralesional therapy is an excellent option for patients with advanced locoregional melanoma, especially in patients where all lesions are able to be directly injected. The combination of intralesional therapy with immune checkpoint inhibitors has been increasingly employed and may be ideal for patients with injectable locoregionally advanced disease and synchronous distant sites. Furthermore, multiple previous preclinical in vivo studies have shown a synergistic effect with the combined use of these modalities.26,27 A phase 1 trial showed response rates of 75% with TVEC and ipilimumab for patients with Stage IIIB-IVM1a and 50% in all patients.28 Clinical trials are currently underway in other countries evaluating the concurrent use of TVEC with immune checkpoint inhibitors (ipilimumab and pembrolizumab).
The limitations of this study are consistent with the inherent flaws of a retrospective study, including nonsystematic reporting of adverse events as all patients received therapy outside of a clinical trial setting. We suspect adverse events were underreported in this study given the majority of toxicity seen with therapy is mild and unlikely to be documented in routine clinic documents. However, prior prospective trials have shown favorable toxicity profiles with TVEC, mostly limited to mild injection site toxicities, which are consistent with our findings.17 Another limitation of this study is the relative small number of patients included. While prior treatment did not show a significant correlation to response to therapy in our study, it is possible this is a result of the power of the study. Attempting to identify predictors of response in larger future retrospective or prospective studies will continue to be of high interest as the landscape of advanced melanoma management continues to evolve.
CONCLUSIONS
TVEC is a safe and effective option for patients with advanced locoregional melanoma. The majority of patients respond to therapy, with almost half of patients achieving a CR. Response rates appear to be durable as only one patient with a CR had recurred at time of final analysis.
Footnotes
DISCLOSURES
JSZ, consultant, Amgen; speaker bureau, Amgen; research funding, Amgen; AAS, research funding, Amgen; MCP, research funding, Amgen.
REFERENCES
- 1.Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual, 8th edn Berlin: Springer; 2017. [Google Scholar]
- 2.Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol. 1999;6(3):315–21. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12(8):587–96. [DOI] [PubMed] [Google Scholar]
- 4.Weitman ES, Perez M, Thompson JF, et al. Quality of life patient-reported outcomes for locally advanced cutaneous melanoma. Melanoma Res. 2018;28(2):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. [DOI] [PubMed] [Google Scholar]
- 7.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Sem Surg Oncol. 1998;14(3):238–47. [DOI] [PubMed] [Google Scholar]
- 8.O’Donoghue C, Perez MC, Mullinax JE, et al. Isolated limb infusion: a single-center experience with over 200 infusions. Ann Surg Oncol. 2017;24(13):3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read TA, Smith A, Thomas J, et al. Intralesional PV-10 for the treatment of in-transit melanoma metastases: results of a prospective, non-randomized, single center study. J Surg Oncol. 2018;117(4):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura JT, Zager JS. Intralesional therapy as a treatment for locoregionally metastatic melanoma. Expert Rev Anticancer Ther. 2018;18(4):399–408. [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32. [DOI] [PubMed] [Google Scholar]
- 12.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1. [DOI] [PubMed] [Google Scholar]
- 13.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlman KB, Xia J, Hutchinson K, et al. BRAF(L597) mutations in melanoma are associated with sensitivity to MEK inhibitors. Cancer Discov. 2012;2(9):791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohlhapp FJ, Kaufman HL. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22(5):1048–54. [DOI] [PubMed] [Google Scholar]
- 16.Hercus TR, Thomas D, Guthridge MA, et al. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114(7):1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8. [DOI] [PubMed] [Google Scholar]
- 18.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization; 1979. [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. [DOI] [PubMed] [Google Scholar]
- 21.Couture J Melanoma: the management of local recurrence and in-transit metastasis. Canadian J Surg. 1982;25(6):698–700. [PubMed] [Google Scholar]
- 22.Thompson JF, Agarwala SS, Smithers BM, et al. Phase 2 study of intralesional PV-10 in refractory metastatic melanoma. Ann Surg Oncol. 2015;22(7):2135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208(5):706–15. (discussion 715–7). [DOI] [PubMed] [Google Scholar]
- 24.Kroon HM, Coventry BJ, Giles MH, et al. Australian multicenter study of isolated limb infusion for melanoma. Ann Surg Oncol. 2016;23(4):1096–103. [DOI] [PubMed] [Google Scholar]
- 25.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95(17):10067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(22):2619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


