Abstract
Introduction
Although enhanced recovery after surgery (ERAS) components include both anesthesia and surgical care processes, it is unclear whether a multidisciplinary approach to implementing ERAS care processes improves clinical outcomes. The addition of multidisciplinary care with anesthesiology-related components to an existing ERAS protocol for radical cystectomy at a US comprehensive cancer center provided an opportunity to compare short- and long-term outcomes.
Methods
We retrospectively compared the outcomes of 116 consecutive patients who underwent cystectomy after implementation of a multidisciplinary ERAS protocol with those of a historical control group of 143 consecutive patients who had been treated with a surgical ERAS protocol. Length of stay, return of bowel function, rate of blood transfusion, nausea, pain, and readmission rates were examined.
Results
Implementation of a multidisciplinary ERAS protocol was associated with better postsurgical symptom control, as indicated by lower rates of patient-reported nausea (P < .05). Multivariate Poisson regression analysis showed a decrease in estimated intraoperative transfusions (P ≤ .001) after adjusting for the effects of potential confounding variables. There were no statistically significant differences noted in length of stay, return of bowel function, 30- and 90-day complications, or readmissions.
Conclusion
This is the first study to investigate the effects of adding anesthesia ERAS components to an existing surgical ERAS protocol for radical cystectomy. We found that with the addition of anesthesia-related interventions, there was a decrease in transfusions and nausea.
Introduction
The standardization of care processes through clinical pathways improves outcomes, increases patient safety, and improves physician efficiency [1]. The enhanced recovery after surgery (ERAS) study group, which was assembled in Denmark in 2000, developed a perioperative pathway based on evidence-based surgical and anesthesia practices. Previous studies have consistently shown that patients managed with ERAS pathways have shorter lengths of hospital stay and fewer postoperative complications following major surgery [2]. Although ERAS pathways were first implemented in colorectal surgery [2], they have also been linked to decreased lengths of stay in pancreatic [3, 4], gynecologic [5], head and neck cancer [6], and urologic surgery [7, 8].
Each ERAS pathway traditionally comprises a large number of individual components. Although all components are supported by evidence [9], it is difficult to quantify the individual contribution of each intervention to the overall effectiveness of ERAS protocols. Furthermore, few of the reported ERAS studies have audited compliance to the protocol. Of those that have, they found that increased compliance reduces length of stay and complications [10].
Although it is difficult to isolate each intervention’s contribution to outcomes, we have been able to distinguish surgical- and anesthesia-related components, due to the implementation of a multidisciplinary ERAS (mdERAS) program following the use of a surgical ERAS (sERAS) protocol at our institution (both are described in detail below), making this the first study to examine outcomes after adding anesthesia components to an existing surgical ERAS protocol. To date, most ERAS studies have focused on length of stay and complications, but more granular aspects of postoperative care and outcomes have received far less attention. Given these gaps, the objectives of this study were to compare nausea and pain in the immediate postoperative period, the incidence of transfusion, and traditional outcomes such as length of stay and return of bowel function between cohorts of patients who experienced each ERAS protocol.
Patients and methods
After obtaining approval from an Institutional Review Board, a database was queried for open radical cystectomy cases from a single high-volume US comprehensive cancer center. A total of 259 were identified, involving 143 patients who had been treated consecutively with a standard sERAS protocol from May 2014 to June 2015 (the study control group) and 116 who had been treated consecutively from July 2015 to July 2016 after the implementation of a mdERAS program that expanded pre- and intraoperative anesthesiology care processes. All patients were treated in the same institution, and the data were collected and recorded consistently, making direct comparisons of outcomes between the groups valid, despite the asynchronous time frames of treatment.
Study variables
Clinical and pathologic demographic variables included age, sex, American Society of Anesthesiology (ASA) class, pathologic stages, use of neoadjuvant chemotherapy, preoperative hemoglobin, epidural use, and types of diversion. Outcome variables included estimated blood loss, total intravenous fluids, length of stay, return of bowel function, 30- and 90-day readmission, intraoperative and postoperative transfusion. Immediate postoperative outcomes of interest included assessment of postoperative pain, nausea, and length of stay in the post-anesthesia care unit (PACU). Pain was assessed by a 0–10 numeric pain rating scale performed every 15 min. Evaluation of presence and severity of nausea was conducted by nursing inquiry. Both demographic and outcome variables were derived from explicit abstraction from medical records.
ERAS protocols
The sERAS protocol encompassed surgeon-directed preoperative administration of the μ-opioid receptor blocker alvimopan, no mechanical bowel preparation, early feeding, avoidance of nasogastric decompression, and early ambulation.
The mdERAS protocol added anesthesiologist participation and allowed for limited fasting time, epidural analgesia, multimodal analgesia, and goal-directed fluid therapy (Table 1). Patients were instructed to drink clear liquids up to 2 h before surgery, thereby avoiding the fasting state. Thoracic epidurals were placed preoperatively, and a dilute local anesthetic was used during the procedure to allow for minimal narcotic administration in the intraoperative period. Multimodal analgesia consisted of 600 mg of preoperative gabapentin and 1000 mg acetaminophen. Acetaminophen was given every 6 h during the intraoperative and postoperative periods. Goal-directed fluid therapy consisted of using a fluid management algorithm with a minimally invasive volume status monitor to guide fluid resuscitation. The fluid resuscitation algorithm employed a balanced crystalloid solution for maintenance fluids and an isosmotic colloid for fluid bolus [11–13].
Table 1.
Compliance to anesthetic interventions in mdERAS group
| Intervention | Compliance rate |
|---|---|
| Limited NPO status | Unable to obtain |
| Epidural | 60% (70/116) |
| Goal-directed fluid therapy | 98% (114/116) |
| Multimodal analgesia | 100% (116/116) |
Statistical analyses
Descriptive statistics for all demographic variables were stratified by ERAS group. Continuous variables were tested using Wilcoxon rank-sum tests. Categorical variables were summarized in frequency tables with counts and percentages and were tested using Pearson χ2 tests for normally distributed variables and the Fisher’s exact test where necessary. Bivariate comparisons were conducted on all outcomes of interest between ERAS groups. For PACU lengths of stay and peak pain scores, generalized linear regression modeling was conducted. Logistic regression was conducted with regard to the nausea variable. Multivariate Poisson regression was used to investigate the effects of ERAS on intraoperative transfusion after adjusting for other variables. Multivariate models were then built for all three outcomes, using a backwards elimination procedure that required a P value of < .05 to enter the model and < .15 to stay in the model. All statistical analyses were conducted in SAS 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Of the 259 patients included, clinical and demographic variables (Tables 2, 3) were similar in both groups. The mean age at the time of radical cystectomy was 68.6 years and similar in sERAS and mdERAS groups (69.3 vs. 67.8, respectively; P = .15). Likewise, there were no significant differences in sex distribution (P = .95) or ASA status (P = .31). Patients in the control group had a slightly higher starting hemoglobin (12.7 vs. 12.1 g/dL; P = .02). Total intraoperative fluids (4000 vs. 3600 mL; P = .03) were less with the mdERAS group. In addition, with the mdERAS approach, epidural use (35 vs. 60.3%; P = < .001) was substantially higher. There was no difference in the distribution of pathologic stage or diversion type between the two groups.
Table 2.
Study variables: patient demographics
| Patient demographic | sERAS (n = 143) | mdERAS (n = 116) | P valuea |
|---|---|---|---|
| Age, year | |||
| Mean, SD | 69.3 (10.6) | 67.8 (10.02) | .15b |
| Median, range | 71 (63–77) | 69 (58–76) | |
| Sex, n (%) | |||
| Male | 118 (82.5) | 87 (75) | .95c |
| Female | 25 (17.5) | 29 (25) | |
| ASA class, n (%) | |||
| ASA 1 | 1 (0.7) | 0(0) | .31 |
| ASA 2 | 49 (34.3) | 39 (33.6) | |
| ASA 3 | 85 (59.4) | 75 (64.7) | |
| ASA 4 | 8 (5.6) | 2(1.7) | |
| Type of urinary diversion, n (%) | |||
| Ileal conduit | 118 (82.5) | 92 (79.3) | .72 |
| Pouch | 7 (4.9) | 5 (4.3) | |
| Neobladder | 18 (12.6) | 19 (16.4) | |
| Epidural, n (%) | |||
| Yes | 50 (35) | 70 (60.3) | <.001 |
| No | 93 (65) | 46 (39.7) | |
| Preoperative hemoglobin, g/dL | |||
| Mean, SD | 12.70 (1.89) | 12.12 (1.95) | .02 |
| Median, range | 12.80 (11.3–14) | 12.05 (10.7–13.4) |
Bold values indicate statistically significant
ASA American Society of Anesthesiology class, mdERAS multidisciplinary enhanced recovery after surgery group, sERAS surgical enhanced recovery after surgery group, SD standard deviation
sERAS versus mdERAS groups
P values for continuous variables calculated using Wilcoxon rank-sum test
P values for categorical variables calculated using the Fisher’s exact test
Table 3.
Outcome variables
| Outcome | sERAS (n = 143) | mdERAS (n = 116) | P valuea |
|---|---|---|---|
| Total intraoperative fluid, mL | |||
| Mean, SD | 4176.54 (1355.06) | 3888.3 (1289.53) | .03b |
| Median, range | 4000 (3300–4608) | 3600 (3000–4500) | |
| Blood loss, mL | |||
| Mean, SD | 914.86 (642.50) | 982.33 (636.99) | .15 |
| Median, range | 800 (450–1200) | 800 (575–1200) | |
| Postoperative transfusion units | |||
| Mean, SD | 0.54 (0.97) | 0.74 (1.53) | .29 |
| Median, range | 0 (0–1) | 0 (0–1) | |
| Intraoperative transfusion units | |||
| Mean, SD | 1.35 (1.77) | 1.11 (1.71) | .31 |
| Median, range | 0 (0–2) | 0 (0–2) | |
| Length of stay, days | |||
| Mean, SD | 7.73 (4.60) | 7.79 (7.17) | .8 |
| Median, range | 6 (5–8) | 6 (5–8) | |
| Time to first flatus, days | |||
| Mean, SD | 3.30 (1.49) | 3.46 (1.23) | .08 |
| Median, range | 3(3–4) | 3(3–4) | |
| Readmission in 30 days, n (%) | |||
| No | 122 (85.3) | 102 (87.9) | .34c |
| Yes | 21 (14.7) | 14 (12.1) | |
| Readmission in 90 days, n (%) | |||
| No | 112(78.3) | 98 (84.5) | .14 |
| Yes | 31 (21.7) | 18 (15.5) | |
| Complications (30 day) | |||
| Clavien ≥ 3 | 9 (6.3) | 4 (3.4) | .30 |
| Complications (90 day) | |||
| Clavien ≥ 3 | 5 (3.5) | 7 (6.0) | .38 |
Bold value indicates statistically significant
mdERAS multidisciplinary enhanced recovery after surgery group, sERAS surgical enhanced recovery after surgery group, SD standard deviation
sERAS versus mdERAS groups
P values for continuous variables calculated using Wilcoxon rank-sum test
P values for categorical variables calculated using the Fisher’s exact test
The mean number of intraoperative transfusions for the entire cohort was 1.2 (standard deviation [SD] 1.8), while the mean number of postoperative units transfused was 0.6 (SD 1.3). There was no difference in the total number of combined intraoperative and postoperative transfused units between the two groups (P = .66). Univariate analyses showed that estimated blood loss, sex, pathology stage, preoperative hemoglobin, and postoperative transfusion were all individually statistically associated with intraoperative transfusion units. When controlling for sex, estimated blood loss, postoperative transfusion units, preoperative hemoglobin, and the interaction between postoperative transfusion units and administration of an epidural, multivariate Poisson regression analysis demonstrated that use of mdERAS was associated with a lower mean number of intraoperative transfusion units (P < .001; Table 4 and Fig. 1). When taking these variables into account, the estimated number of units transfused was 0.97 (SD 0.09) and 0.58 (SD 0.12) for the sERAS and mdERAS groups, respectively. Interestingly, despite the standardization of protocol via fluid resuscitation algorithm, there was no difference in the variances of transfusion rates between the sERAS and mdERAS groups (P = .77).
Table 4.
Results of multivariate poisson regression analysis for intraoperative transfusion
| Parameter | Estimate | Standard error | Wald 95% confidence limits | P value | |
|---|---|---|---|---|---|
| ERAS status | |||||
| mdERAS | |||||
| sERAS | 0.4974 | 0.1189 | 0.2645 | 0.7304 | <.001 |
| Estimated blood loss, mL | 1.3910 | 0.1058 | 1.1837 | 1.5983 | <.001 |
| Preoperative hemoglobin, g/dL | −0.3429 | 0.0349 | −0.4113 | −0.2744 | <.001 |
| Sex | |||||
| Male | |||||
| Female | 0.3365 | 0.1235 | 0.1244 | 0.6086 | .003 |
| Postoperative transfusion units | −0.1839 | 0.0804 | −0.3416 | −0.0262 | .03 |
Bold values indicate statistically significant
mdERAS multidisciplinary enhanced recovery after surgery, sERAS surgical enhanced recovery after surgery
Fig. 1.
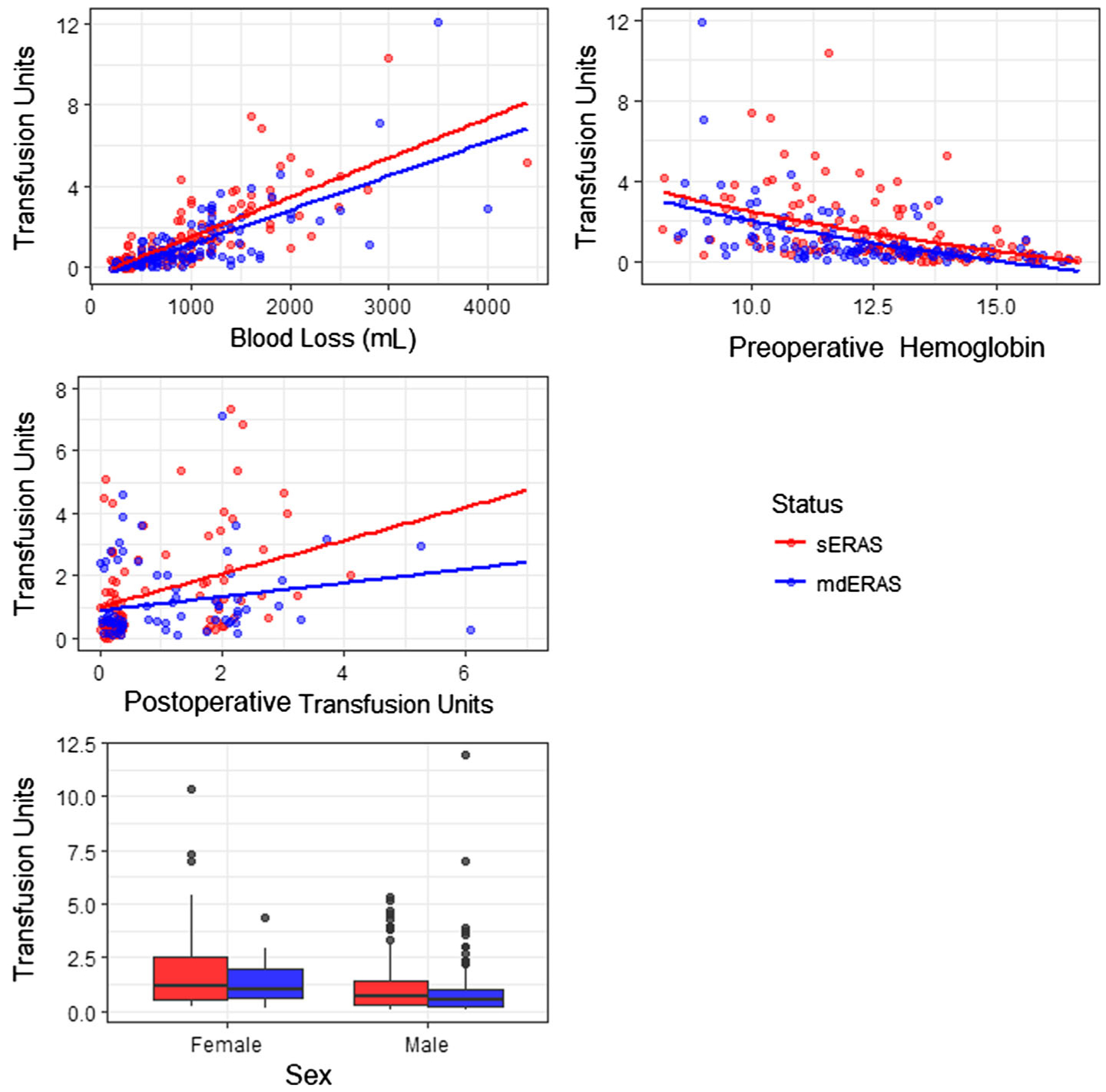
Expected number of transfusion units by model covariates, stratified by group
Immediate postoperative outcomes were also examined in the PACU. Multivariate analyses demonstrated improvement only in nausea (Table 5), which was significantly reduced (8.1 vs. 3.1%; P = .05). Despite this, mean PACU length of stay did not differ between the before and after groups (128 vs. 130 min; P = .89). Adjusted analysis showed that in the immediate postoperative period, patients in the sERAS group were nearly three times more likely to report nausea than patients managed with the multidisciplinary ERAS protocol (odds ratio 2.7, 95% confidence interval, 1.1–6.7). Univariate analysis of peak pain scores did not uncover a significant difference between the sERAS and mdERAS groups (4.4 vs. 4.1; P = .66). Further analysis demonstrated nausea, PACU length of stay, epidural, and age were good predictors of peak pain scores. However, pain scores were not significantly different between groups on adjusted analyses (P = .89). In addition, there was not a statistically significant difference in the variance of the peak pain scores between groups.
Table 5.
Univariate and multivariate results for multidisciplinary ERAS group variables
| Outcome | Univariate analysis results | Multivariate analysis results | ||
|---|---|---|---|---|
| Wald χ2 | P value | Wald χ2 | P value | |
| PACU length of stay | 0.07 | .79 | 0.03 | .87 |
| Nausea | 3.81 | .05 | 5.01 | .02 |
| Peak pain score | 0.48 | .49 | 0.02 | .89 |
Bold value indicates statistically significant
PACU post-anesthesia care unit
There was no difference in time to first flatus between the groups, with a mean of 3.4 days (SD 1.4) for the entire cohort. In addition, hospital stay was similar between the sERAS and mdERAS groups, with a median length of stay of 6 days (range 5–8). Readmission rates at 30 and 90 days were similar and showed no statistical significance (P = .34 and P = .14, respectively).
Discussion
Our study demonstrates the value of a multidisciplinary approach in the execution of an ERAS protocol. It is still unknown which components are most significant; however, this is the first study to isolate anesthesia variables after implementation of surgical interventions.
We did not see a significant difference in time to first flatus and length of hospital stay clinical outcomes, which may have been due to the incorporation of alvimopan in the sERAS protocol. Indeed, previous studies have reported accelerated gastrointestinal recovery after radical cystectomy with alvimopan [14]. As is well established, the major driver of length of hospital stays is recovery of bowel function. We therefore did not expect to see a large difference in these clinical outcomes, as the gains may already have been achieved in the control group.
Compliance with anesthetic interventions was high in both categories with multimodal analgesia and goal-directed fluid therapy being administered to 100 and 98% of the mdERAS group, respectively. Despite the use of a restrictive fluid hydration algorithm with the use of a vasopressor drip during euvolemia, we only demonstrated a 10% decrease in total intravenous fluid. The anesthetic interventions did have a significant impact on nausea, which is supported by a large body of evidence that correlates use of multimodal analgesia and goal-directed fluid therapy with a decreased incidence of nausea [15, 16]. Alleviating nausea can be viewed as a relevant clinical outcome as it may lead to a decrease in PACU length of stay despite not being able to demonstrate this in the mdERAS group.
Interestingly, although we would expect pain control to improve with anesthetic intervention, peak pain scores in the immediate postoperative periods were similar in both groups. Even with a statistically significant increase in epidural usage in the mdERAS group, patients experienced equivalent pain control. This may be attributed to the large percentage of patients (40%) in the mdERAS group who did not receive an epidural, due to patient refusal or current anticoagulation use or because they were not deemed a candidate by the anesthesiologist.
Previous studies using ERAS and restrictive fluid strategy protocols have demonstrated decreases in transfusions [17, 18]. These studies also found statistically significant decreases in blood losses, which explain the lower transfusion rates. Our study uniquely demonstrated a decrease in the intraoperative transfusion rate after adjusting for confounding factors, such as blood loss and preoperative hemoglobin. Similarly, a recent analysis from the National Surgical Quality Improvement Program database showed a consistent decline in length of stay and transfusion rates after implementation of ERAS in radical cystectomy [19]. What remains to be determined is the explanation for a decrease in blood transfusion when blood loss remains the same. Most have attributed decreases in transfusion rates to changes in operating room culture and the standardization of teams and protocols. Standardization of operating room teams has been shown to reduce procedure duration and improve teamwork and the safety climate [20]. Recently, Jaeger et al. [21] showed that standardization can also lead to lower readmission rates when implemented in anesthesia teams. When standardization is combined with a change in transfusion culture, a lower transfusion rate may be achievable.
Even though we were not able to improve clinical outcomes such as length of stay, anesthetic interventions should still be considered to be a crucial portion of a complete ERAS protocol. Analgesic techniques can provide long-term benefits that may not be captured in traditional outcomes such as length of stay, return of bowel function, and complications. Epidural analgesia has been associated with reducing the severity of residual postoperative pain and optimizing quality of life for up to 3 months after surgery [22]. Similarly, the use of multimodal analgesia has been associated with decreased chronic pain syndromes after breast and thoracic surgeries [23, 24].
Limitations of this study include its retrospective nature, the implementation of multiple interventions, and a lack of patient-reported outcomes. Nevertheless, this is the first study to examine the effects of the anesthetic portion of an ERAS protocol. Although our study found that there was a decrease in transfusions and nausea with the addition of anesthesia-related interventions, further prospective work is needed to examine the interventions’ impact on patient-reported outcomes, such as quality of life and quality of recovery.
Acknowledgements
We thank Sonya J. Smyk for editorial support.
Footnotes
Conflict of interest The authors declare no real or potential conflicts of interest.
References
- 1.Wood DL, Brennan MD, Chaudhry R et al. (2008) Standardizing care processes to improve quality and safety of patient care in a large academic practice: the Plummer Project of the Department of Medicine, Mayo Clinic. Health Serv Manage Res 21(4):278–280 [DOI] [PubMed] [Google Scholar]
- 2.Miller TE, Thacker JK, White WD et al. (2014) Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg 118(5):1052–1061 [DOI] [PubMed] [Google Scholar]
- 3.Bai X, Zhang X, Lu F et al. (2016) The implementation of an enhanced recovery after surgery (ERAS) program following pancreatic surgery in an academic medical center of China. Pancreatology 16:665–670 [DOI] [PubMed] [Google Scholar]
- 4.Morgan KA, Lancaster WP, Walters ML et al. (2016) Enhanced recovery after surgery protocols are valuable in pancreas surgery patients. J Am Coll Surg 222(4):658–664 [DOI] [PubMed] [Google Scholar]
- 5.Relph S, Bell A, Sivashanmugarajan V, Munro K et al. (2014) Cost effectiveness of enhanced recovery after surgery programme for vaginal hysterectomy: a comparison of pre and post-implementation expenditures. Int J Health Plan Manag 29(4):399–406 [DOI] [PubMed] [Google Scholar]
- 6.Coyle MJ, Main B, Hughes C et al. (2016) Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clin Otolaryngol 41(2):118–126 [DOI] [PubMed] [Google Scholar]
- 7.Di Rollo D, Mohammed A, Rawlinson A (2015) Enhanced recovery protocols in urological surgery: a systematic review. Can J Urol 22(3):7817–7823 [PubMed] [Google Scholar]
- 8.Tyson MD, Chang SS (2016) Enhanced recovery pathways versus standard care after cystectomy: a meta-analysis of the effect on perioperative outcomes. Eur Urol 70:995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerantola Y, Valerio M, Persson B et al. (2013) Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced recovery after surgery (ERAS) society recommendations. Clin Nutr 32:879–887 [DOI] [PubMed] [Google Scholar]
- 10.Ghodoussipour S, Cameron B, Wang C et al. (2017) Is compliance to an enhanced recovery protocol after radical cystectomy associated with improved postoperative outcomes? J Urol 197:e1127–e1128 [Google Scholar]
- 11.Pillai P, McEleavy Gaughan M et al. (2011) A double-blind randomized controlled clinical trial to assess the effect of doppler optimized intraoperative fluid management on outcome following radical cystectomy. J Urol 186:2201–2206 [DOI] [PubMed] [Google Scholar]
- 12.McGee WT (2009) A simple physiologic algorithm for managing hemodynamics using stroke volume and stroke volume variation: physiologic optimization program. J Int Care Med 24:352–360 [DOI] [PubMed] [Google Scholar]
- 13.Ramsingh DS, Sanghvi C, Gamboa J et al. (2013) Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: a randomized controlled trial. J Clin Monit Comput 27:249–257 [DOI] [PubMed] [Google Scholar]
- 14.Lee CT, Chang SS, Kamat AM et al. (2014) Alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo-controlled trial. Eur Urol 66:265–272 [DOI] [PubMed] [Google Scholar]
- 15.Grant MC, Lee H, Page AJ et al. (2016) The effect of preoperative gabapentin on postoperative nausea and vomiting: a meta-analysis. Anesth Analg 122:976–985 [DOI] [PubMed] [Google Scholar]
- 16.Giglio MT, Marucci M, Testini M et al. (2009) Goal-directed hemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 103:637–646 [DOI] [PubMed] [Google Scholar]
- 17.Pang KH, Groves R, Venugopal S et al. (2017) Prospective implementation of enhanced recovery after surgery protocols to radical cystectomy. Eur Urol. 10.1016/j.eururo.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 18.Wuethrich PY, Studer UE, Thalmann GN et al. (2014) Intraoperative continuous norepinephrine infusion combined with restrictive deferred hydration significantly reduces the need for blood transfusion in patients undergoing open radical cystectomy: results of a prospective randomized trial. Eur Urol 66(2):352–360 [DOI] [PubMed] [Google Scholar]
- 19.Johnson SC, Smith ZL, Golan S et al. (2017) Temporal trends in perioperative morbidity for radical cystectomy using the National Surgical Quality Improvement Program database. Urol Oncol 35(11):659.e13–659.e19 [DOI] [PubMed] [Google Scholar]
- 20.Stepaniak PS, Heij C, Buise MP et al. (2012) Bariatric surgery with operating room teams that stayed fixed during the day: a multicenter study analyzing the effects on patient outcomes, teamwork and safety climate, and procedure duration. Anesth Analg 115:1384–1392 [DOI] [PubMed] [Google Scholar]
- 21.Jaeger MT, Siemens DR, Wei X et al. (2017) Association between anesthesiology volumes and early and late outcomes after cystectomy for bladder cancer: a population based study. Anesth Analg 125:147–155 [DOI] [PubMed] [Google Scholar]
- 22.Capdevila X, Moulard S, Plasse C et al. (2017) Effectiveness of epidural analgesia, continuous surgical site analgesia, and patient-controlled analgesia morphine for postoperative pain management and hyperalgesia, rehabilitation, and health-related quality of life after open nephrectomy: a prospective, randomized, controlled study. Anesth Analg 124:336–345 [DOI] [PubMed] [Google Scholar]
- 23.Amr YM, Yousef AA (2010) Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain 26:381–385 [DOI] [PubMed] [Google Scholar]
- 24.Solak O, Metin M, Esme H et al. (2007) Effectiveness of gabapentin in the treatment of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 32:9–12 [DOI] [PubMed] [Google Scholar]


