Abstract
Radiotherapy has long been the mainstay of treatment for patients with head and neck cancer and has traditionally involved a stage-dependent strategy whereby all patients with the same TNM stage receive the same therapy. We believe there is a substantial opportunity to improve radiotherapy delivery beyond just technological and anatomical precision. In this Series paper, we explore several new ideas that could improve understanding of the phenotypic and genotypic differences that exist between patients and their tumours. We discuss how exploiting these differences and taking advantage of precision medicine tools—such as genomics, radiomics, and mathematical modelling—could open new doors to personalised radiotherapy adaptation and treatment. We propose a new treatment shift that moves away from an era of empirical dosing and fractionation to an era focused on the development of evidence to guide personalisation and biological adaptation of radiotherapy. We believe these approaches offer the potential to improve outcomes and reduce toxicity.
Introduction
Radiotherapy is one of the most potent and frequently used treatment options against cancer. More than 500 000 patients with cancer in the USA receive radiotherapy each year, either alone or in combination with systemic therapy, or surgery, or both.1 The field of radiotherapy in oncology has evolved substantially during the past century. Radiation oncologists have taken advantage of the engineering, physics, and computational advances to more accurately target tumours. The development of intensity-modulated radiotherapy, image-guided radiotherapy, stereotactic radiosurgery, stereotactic body radiotherapy, intraoperative radiotherapy, particle therapies, and brachytherapy are all examples of high-precision radiotherapy techniques that allow the delivery of high radiation doses to tumours with lower doses to the surrounding organs. The result is typically lower complications with better quality of life compared with use of conventional radiotherapy techniques. These advances might also result in improved functional outcomes, as seen in stereotactic body radiotherapy for non-small cell lung cancer.2,3 This focus on anatomical personalisation has permitted radiation oncologists to substantially improve outcomes for many patients with head and neck cancers, which occupy or are adjacent to vital structures required for voice, speech articulation, swallowing, vision, hearing, salivation, and cosmetic integrity.
Despite these successes, personalised radiotherapy treatment has been insufficient in a number of important ways. For example, how much radiation needs to be delivered to an individual patient or to a subregion of a specific tumour? When the same type of tumour, with the same stage, and in the same location, is treated in different patients, some patients are cured and others are not—despite identical doses and fractionation. The basic premise that these patients had the same tumour, and therefore require identical treatment, is clearly false. Just as every person is different, every person’s cancer might be very different and therefore require a very different course of radiotherapy than their apparent counterpart. Yet, clinicians have been unable to make the distinctions required to biologically personalise radiotherapy treatment. The underlying explanation of why some patients are cured, and others are not, has been elusive and needs further exploration.
In this Series paper, we explore some ideas that might help improve the understanding of the phenotypic and genotypic differences that exist between patients and their tumours. We discuss how exploiting these differences might allow the creation of personalised radiotherapy treatment plans that are biology-based and might further improve outcomes and reduce toxicity. Our group has already described a framework4 for integrating genomic information into clinical practice. These kinds of advances, along with similar advances in precision medicine in drug therapy, have the potential to improve outcomes more than the advances in one method alone. We propose to explore how we can move away from a one-size-fits-all approach to radiotherapy treatment for patients with head and neck cancer, and to develop evidence with which to guide personalisation and biological adaptation of radiotherapy to improve outcomes and reduce toxicity.
Biology
A fundamental principle of personalised medicine is to develop therapeutic strategies that address the biological heterogeneity characteristic of cancer. In medical oncology, genomic-based strategies have been developed to identify patients unlikely to benefit from cytotoxic chemotherapy and for targeted drug development and selection (ie, exome sequencing, fusion genes). However, in radiation oncology, similar strategies are still not used in the clinical management of patients.
The rationale is strong for the incorporation of a biology-based strategy into clinical decisions in radiation oncology, and such strategies will substantially affect clinical outcome for patients treated with radiotherapy. A biology-based strategy can inform multiple radiotherapy clinical measurements including total dose, fractionation scheme, physical dose distribution within the tumour, and a range of daily radiotherapy doses. Additionally, the protracted approach to radiotherapy delivery could provide opportunities to further inform treatment parameters based on serum and imaging biomarker changes that occur during treatment. Radiation oncology should depart from the one size fits all empirical approach and embrace the clinical potential that might reside in an approach designed to customise radiotherapy to the inherent biological differences within cancer.
To develop a genomic strategy that could affect the clinical practice of radiation oncology, our group developed a biomarker discovery strategy that focused on the identification of pan-tissue radiotherapy-specific biomarkers. Based on this strategy, we developed the radiosensitivity index, a gene-expression molecular signature, as a molecular estimate for cellular survival at 2 Gy, a classic cellular measure of intrinsic radiosensitivity.5 The radiosensitivity index was first validated by showing that it predicted the experimental survival value of independent cell lines better than by chance (p=0·02). Additionally, we showed that the radiosensitivity index predicted tumour response to preoperative radiotherapy in two independent cohorts of patients with rectal and oesophageal cancer (sensitivity 80%, specificity 82%, area under the curve=0·84).6 Importantly, the radiosensitivity index has been systematically validated as a predictor of clinical outcome in multiple independent radiotherapy-treated cohorts across nine different disease sites, including head and neck cancer.7,8 Notably, the radiosensitivity index functions as a radiotherapy-specific pan-tissue biomarker to predict response, and is not predictive of outcome in patients treated without radiotherapy. Although promising, the radiosensitivity index will need to be validated by other groups or by prospective clinical trials.
In patients with head and neck cancer, the radiosensitivity index has been shown to distinguish clinical outcomes between patients who are sensitive and resistant to radiation therapy who have previously been treated within prospective clinical trials with concurrent cisplatin-based chemoradiation.8 Patients with a radiosensitivity index-sensitive signature had improved locoregional control compared with those who were radiosensitivity index-resistant at 2 years (86% vs 61%, respectively; p=0·05).8 Thus, subsets of patients who would derive larger therapeutic benefit from radiotherapy could probably be identified using a radiosensitivity index.
Patients with head and neck cancer and a range of radiosensitivity index values were enrolled in the umbrella Total Cancer Care protocol at Moffitt Cancer Center (figure 1). The radiosensitivity index values for patients with head and neck cancer were heterogeneous—the difference between the most sensitive and most resistant samples was more than 3 times.4 This variability in individual tumour radiosensitivity implies that a uniform strategy to clinical practice can be improved by integrating approaches that will tailor radiotherapy treatment to the biological differences within tumours.
Figure 1: Range of radiosensitivity index values in oropharyngeal and non-oropharyngeal squamous cell carcinomas of the head and neck.
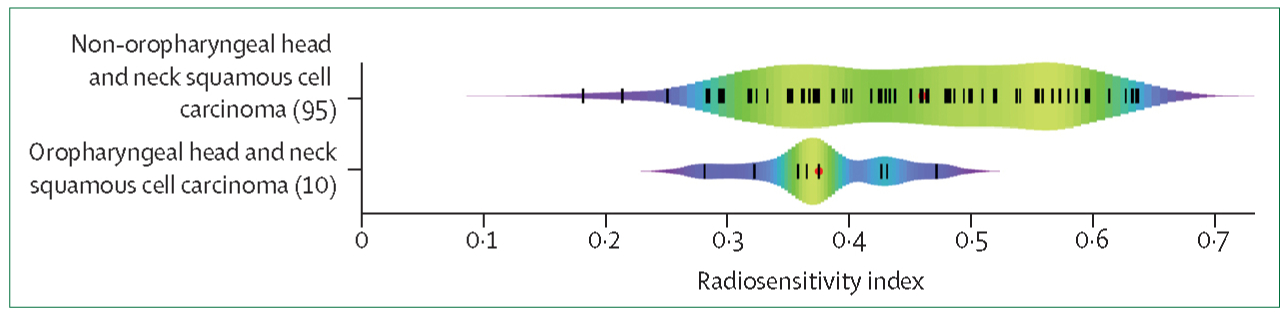
Adapted with permission from Scott and colleagues.4
To integrate biological differences into clinical radiotherapy parameters, we developed the genomic-adjusted radiation dose, a genome-based model for adjusting optimum radiation dose. The genomic-adjusted radiation dose is derived using the linear quadratic model, the individual patient radiosensitivity index, and the radiation dose or fractionation schedule planned for each patient.4 To our knowledge, this model provides the first opportunity to select radiotherapy dose to match tumour radiosensitivity, which allows for adjustments to dose based on tumour biology. Importantly, the model also allows for the development of genomic-guided clinical trials, an approach that potentially represents a new shift in radiation oncology treatment. To our knowledge, we are currently developing the first clinical trial of genomically-guided dose prescription in radiation oncology for patients with non-metastatic human papillomavirus (HPV)-positive squamous cell carcinoma of the oropharynx. The clinical promise in the technological advances in radiotherapy delivery might be fully realised when they are used to exploit the biological differences that determine intrinsic radiosensitivity.
Our group has recently described a more detailed roadmap for using the genomic-adjusted radiation dose in the determination of radiation dose into clinical practice.4 These ideas can be extended into the multidisciplinary care model and help improve our understanding of how to optimally combine radiation, surgical, and systemic therapy. For example, the genomic-adjusted radiation dose values might suggest that a patient who would ordinarily have had either surgery or radiotherapy as standard of care, might be effectively treated with a deintensification of radiotherapy dose. Alternatively, in less radiosensitive tumours, the genomic-adjusted radiation dose might suggest the radiation dose required to treat a patient is beyond technical feasibility, or the tolerance of the adjacent normal tissue. In this situation, the patient might be more effectively treated with primary surgery. Even in less radiosensitive tumours, radiation dose could be escalated or used concomitantly with systemic therapy for curative intent. Furthermore, genes or gene networks identified with the radiosensitivity index might be targets for manipulation via drug therapy. If radiosensitivity can be modified with targeted, potentially less toxic drug therapy, patients previously thought not to benefit from radiotherapy could be effectively treated with personalised combined therapy. These ideas will require further study and validation in clinical trials before they are integrated into multidisciplinary care.
Other extant genomic signatures could help personalise radiotherapy. For example, at least three genomic signatures can predict hypoxia.9–11 These signatures have been shown to be prognostic for outcome in an independent dataset.12 Hypoxia signatures might help identify subregions of the tumour for radiation dose escalation. Alternatively, hypoxia signatures might identify patients who would benefit from surgery rather than radiotherapy, or a combination of the two, as necessary. Additionally, patients shown to have hypoxic tumours might benefit from an increased dose of radiotherapy or hypoxia pretargeting through the use of, for example, hypoxia-activated prodrugs.13 Although hypoxia-activated prodrugs have failed in late-stage clinical trials as mono therapies or in combination with chemotherapies, some studies14,15 have shown that hypoxia-activated prodrugs in combination with radiotherapy are promising. Another signature might correlate with immune activation, which could be interesting with the advent of potent immunotherapeutics that are now being combined with radiotherapy.16
Discussion of approaches to personalise the clinical parameters of radiotherapy is not complete without addressing normal tissue toxicity, potentially the biggest barrier to uniform radiotherapy dose escalation. The Radiogenomics Consortium has been pursuing the hypothesis that toxicity from radiation is genetically predetermined.17–19 Toxicity is a major problem in the treatment of patients with head and neck cancer, and certainly a genomic-based approach to toxicity prediction would be of clinical value.
Radiomics
Radiomics is an emerging field that involves throughput conversion of quantitative automated imaging features into mineable data.20 This approach is predicated on the rationale that biomedical images of tumours are influenced by their underlying pathophysiology and hence, its aim is to provide comprehensive quantification of the tumour phenotypes that can be incorporated into classifier models.21 The predictive power of radiomics has opened new opportunities for individual treatment, especially in the context of imaging-reliant modern radiation oncology practice.22 Standard of care images provide 3D information of entire tumours and surroundings, longitudinally and adaptively, before and during the course of therapy.
Vital spatial and temporal anatomical information provided by imaging informs radiotherapy target delineation, planning, tumour motion, treatment delivery, and response monitoring. The most commonly used imaging methods are MRI, CT, and PET. With advances in radiomics, tumour characterisation is not just limited to anatomy, but it can also reveal cellular and genomic level information that is quantifiable as imaging phenotypes.23–25 Head and neck cancers present a unique set of diagnostic and therapeutic challenges because of their anatomical complexity and heterogeneity. Each individual head and neck cancer (even of similar American Joint Committee on Cancer [AJCC] stage) has a distinct phenotypic trait based on homogeneity, shape, infiltration, and intrinsic radiosensitivity. Even tumours that are similar clinically, radiographically, or pathologically have different intratumoural heterogeneity, which is one of the key challenges to precision medicine (figure 2). Biopsies provide phenotypic and genotypic information, but are limited by the fact that they are acquired at a single timepoint and from a single anatomical location. Hence, biopsies do not accurately represent the overall pathophysiological landscape of temporal changes.26 By contrast, radiomics might be able to provide enough information for a virtual 3D biopsy where the entire tumour can be sampled non-invasively and repeatedly.
Figure 2: Radiomic process in two patients with squamous cell carcinoma of the base of tongue and tonsil treated with definitive radiotherapy.
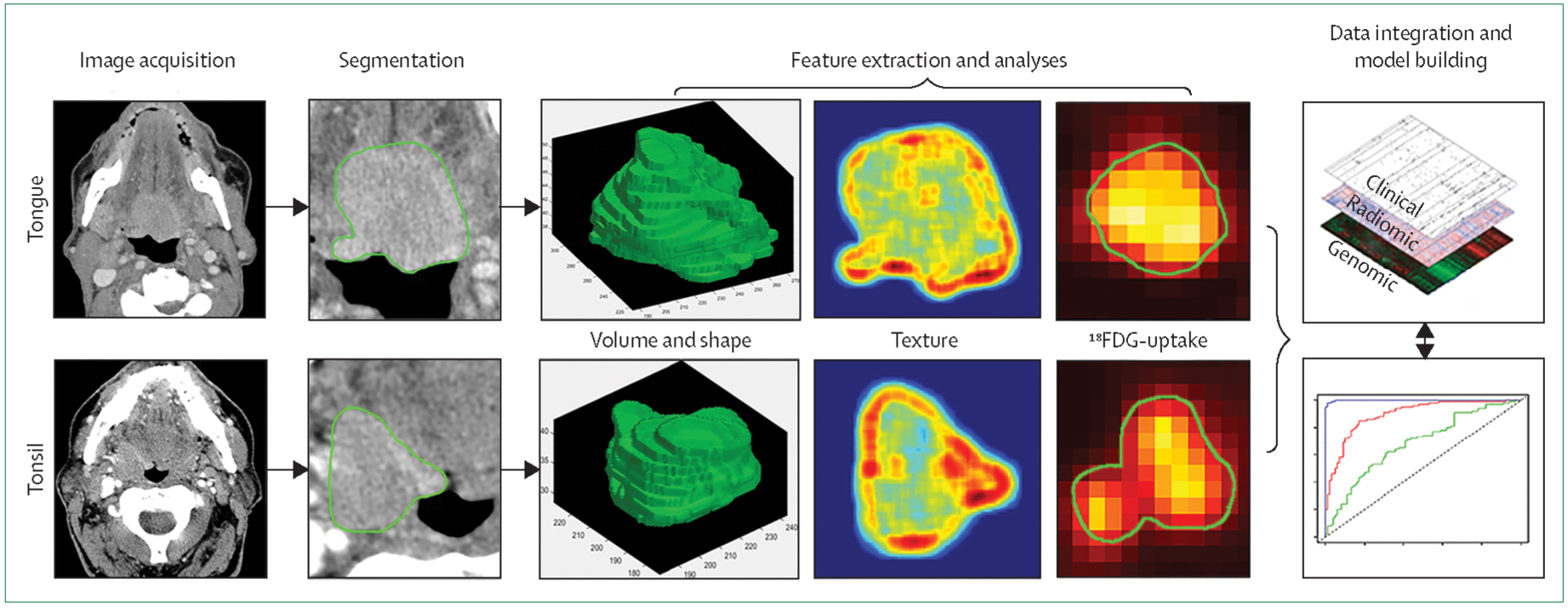
Standard of care images are used for tumour delineation (segmentation) creating a volume of interest (third column). Quantitative features are then extracted from the volume of interest. The fourth column shows Hounsfield units heterogeneity and the fifth column shows standard uptake values, revealing significant intratumoural heterogeneity. These features can later be combined with clinical and genomic data to generate a predictive model (or decision support system) to guide therapy personalisation.
Adaptation of the radiation dose inside a tumour to address pathophysiological and response heterogeneity is an unmet medical need. Targeting different regions that have variable radiosensitivity with the same raditation dose does not fully exploit radiotherapy’s precise dose-depositing capabilities that can optimise each patient’s outcome. Radiomics texture analyses provide preliminary evidence for describing distinctive tumour phenotypes that are driven by underlying genotypes. Particularly in head and neck cancer, distinguishing between HPV-positive and HPV-negative oropharyngeal and even non-oropharyngeal cancers is possible based on contrast-enhanced CT scan images.27,28 Radiomics also allows the identification of radioresistant subclones within tumours, such that radiation plans can be personalised to selectively boost these subclones (or habitats) to higher tumouricidal doses. One such approach is guided-selective radiation dose escalation for hypoxic regions. In this approach, PET-labelled nitroimidazole compounds (eg, 18F-labelled misonidazole, fluoroazomycin arabinoside, or HX-4) can be used to precisely determine spatial location of intratumoural resistant hypoxic voxels in which dose can be escalated selectively (ie, dose painting).29–31 On the basis of clinical outcomes, higher 18F-FDG-avid regions within a tumour might depict greater tumour to stroma cell ratio, and could also be a possible target for subvolume dose boosting.32 Of course, the role of radiomics in providing prognostic information in regard to tumour heterogeneity will need to be validated in a large number of patients with uniform investigations (eg, using CT or MRI).
Radiomic signatures can hold predictive and prognostic information to guide personalised radiotherapy (eg, dose or fraction, dose painting, and total maximum dose). In a relatively large study33 of patients treated with radiotherapy, an imaging biomarker containing four radiomic features (tumour image intensity, shape, texture, and wavelet decomposition) was found to be significantly prognostic of intratumour heterogeneity across different cancers, including head and neck cancer. This study33 analysed 231 patients with head and neck cancer, and was validated across two independent multi-institutional datasets. Additional validation of a prognostic signature was done successfully in 542 patients with oropharyngeal cancer.34 In another study,35 features specific to the prognostic performance of head and neck cancer were identified. Advanced textural features seen in PET have been shown to predict outcomes better than conventional standardised uptake value (SUV) measures, such as SUV maximum and mean.36 Temporal changes of tumour images during treatment also depict prognostic significance. In one study,37 a rapid drop in 18F-FDG uptake correlated with improved locoregional control and overall survival, and the authors concluded that imaging at 10–20 Gy (1–2 weeks into radiotherapy) was the best timepoint for using 18F-FDG PET to monitor response during therapy. Diffusion-weighted imaging, which is an emerging MRI technique that is sensitive to cell density, has been shown to be predictive of response and overall outcome in patients with head and neck squamous cell carcinoma who were treated with radiotherapy.38 Increased pretreatment apparent diffusion coefficient values were associated with adverse prognosis.38 Therefore, the application of radiomics in radiation oncology might be a powerful tool to adapt radiotherapy to individual patients to maximise tumour eradication once the field matures and radiomic biomarkers are properly identified and validated.
Integrative mathematical oncology
Although different fractionation protocols have been tested in prospective clinical trials, why some tumour phenotypes respond to altered fractionation compared with others needs to be further elucidated, as well as understanding how to select the most appropriate fractionation schedule for an individual patient. Innovative models based on cell biology and interactions of a tumour with its individual environment could lead to personalised adaptive radiotherapy protocols that improve tumour control and decrease normal tissue damage. The uniqueness of each patient at presentation due to the intrinsic properties of tumour and normal tissues creates a highly patient-specific set of circumstances, which makes it difficult to predict individual patients’ responses to radiotherapy. However, progress in integrated mathematical oncology, a powerful approach that uses experimental and clinical data to build calibrated quantitative models, makes this analysis approachable.39 A study40 that combined experimental and mathematical modelling derived optimised radiotherapy dose and fractionation protocols for platelet derived growth factor-driven glioblastoma. Using a parsimonious mathematical model informed with experimental data for dose-dependent radiation response, the authors simulated response to the conventional 2 Gy per day radiation fractionation protocol and compared it with hypofractionation and hyperfractionation regimens as well as arbitrary dose schedules with identical total dose. The mathematical model predicted that doses delivered at varying frequencies could offer prolonged growth delays—results that were subsequently verified in animal studies.40
A clinically applicable mathematical model41 postulates that tumours are actually composites of proliferating and growth arrested cells, and that overall radiation response depends on their respective proportions. From two radiological scans routinely obtained at patient diagnosis and radiation treatment simulation, the change in tumour volume over time can be estimated, which gives crucial insights into the proportion of proliferating and quiescent cells. Tumours in patients with a high proliferation saturation index have low proliferating cell fractions and are hypothesised to be treatment refractory, whereas tumours with a low proliferation saturation index are more proliferative and thus hypothesised to be more radiosensitive. Simulations of radiation-induced death after the linear quadratic model42,43 predict patient-specific gross tumour volume reduction during radiotherapy (figure 3).
Figure 3: Proliferation saturation index-dependent radiotherapy response.
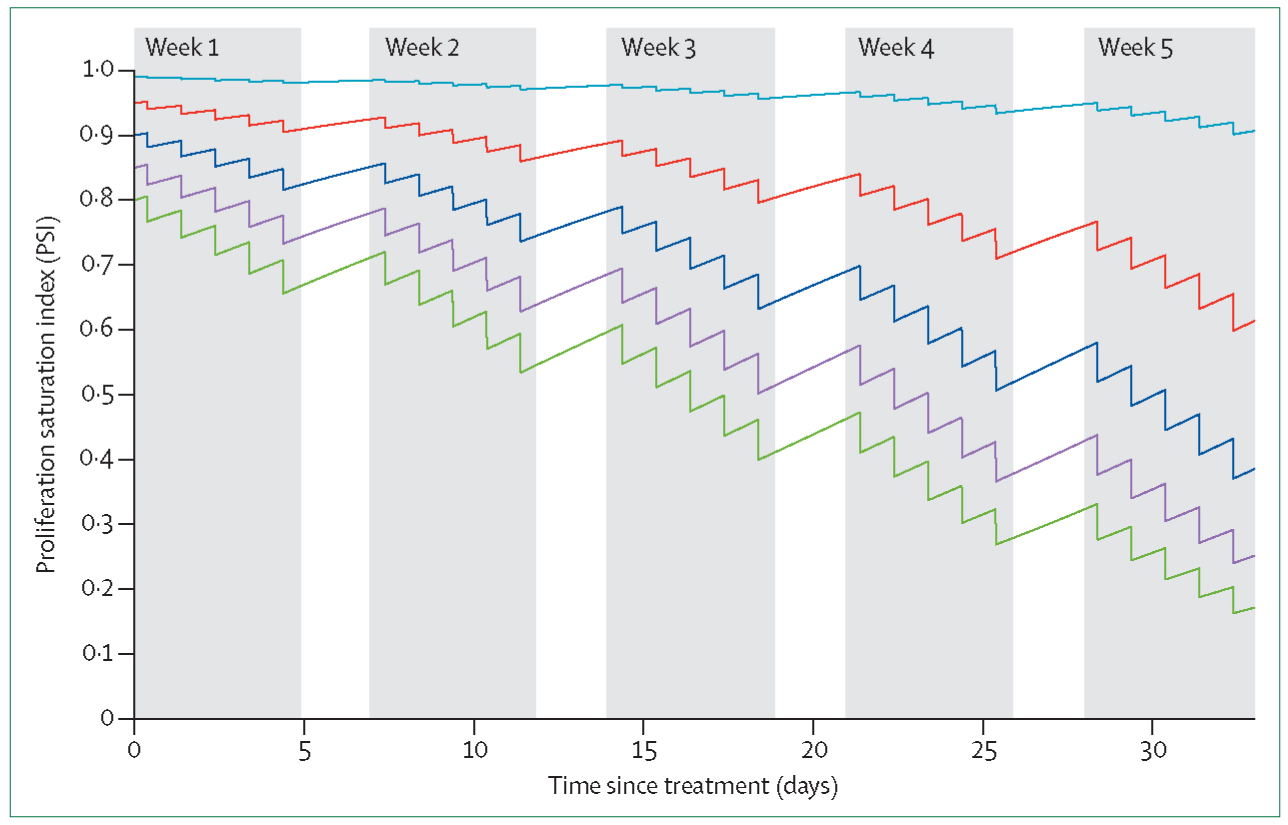
Tumour growth and standard fractionation radiotherapy is simulated for five tumours with different proportions of proliferating and quiescent cells (proliferation saturation index) at the beginning of treatment. Reproduced from Prokopiou and colleagues.41
From retrospective longitudinal tumour volume evolution during radiotherapy—obtainable from cone-beam CT, CT on rails, or MRI—the shape of the response curves that correlate with a complete response after radiatiotherapy can be obtained. To prospectively predict treatment response, pretreatment proliferation saturation index data can be used to inform a mathematical model of tumour growth and simulate radiation response. Longitudinally collected radiological data during therapy can be used to calibrate and validate patient-specific tumour growth and treatment response parameters. Such a calibrated model can then forecast actionable gross tumour volume response curves during the remainder of therapy, and ultimately predict outcomes. If predicted responses from patient-specific therapy deviates from the shape of response that correlates with the achievement of a complete response, virtual trials that simulate alternative dose and dose fractionation protocols in silico could identify treatment adaptations that shift the patient’s response curve to a more favourable outcome. Treatment adaptations could lead to dose escalations or dose de-escalations where possible. Other adaptations on the basis of simulated and observed tumour radiosensitivity include dose hyperfractionation or hypofractionation protocols and, ultimately, dynamic protocol adaptations to maximise individual responses. Control theory as well as global optimisation procedures and heuristic methods can be applied to mathematically identify optimal fractionation protocols on a per patient basis.
Biological adaptation
Generally, existing approaches to adaptive radiotherapy have been predicated upon changing the volume receiving radiation in response to tumour shrinkage. This strategy has not found widespread acceptance due to the variability in tumour response as well as the uncertainty regarding the identification of the best timepoint for volumetric adaptation.44 Additionally, organs at risk might also shift anatomically as the tumour responds to treatment, or due to patient factors (eg, weight loss), thus affecting the incremental gain in dose reduction to normal tissues.45,46
A large number of trials are investigating adaptation of dose-based radiotherapy on biological features for patients with head and neck cancer, as we describe. A number of de-escalation approaches (surgery, radiotherapy, and systemic therapy) exist for HPV-positive oropharyngeal cancer. For example, on the basis of the proportion of patients with HPV-positive oropharyngeal cancer who achieved a complete clinical response to induction chemotherapy, the Eastern Cooperative Oncology Group reduced the dose of radiotherapy to 54 Gy from 69.96 Gy (NCT01084083). Similarly, another cooperative group trial is testing a reduction in radiotherapy dose from 60 Gy to 50 Gy in patients with intermediate-risk features following a minimally invasive operation for HPV-positive oropharyngeal cancer (NCT01898494). NRG Oncology, a new National Clinical Trials Network (NCTN) group created through the coordinated efforts of the National Surgical Adjuvant Breast and Bowel Project (NSABP), the Radiation Therapy Oncology Group (RTOG), and the Gynecologic Oncology Group (GOG), is testing a reduction of dose from 70 Gy to 60 Gy with or without chemotherapy for low-risk HPV-positive oropharyngeal cancers (NCT02254278). Other groups are testing forms of escalation therapy, especially with the incorporation of immunotherapy, in patients with head and neck cancer with non-oropharyngeal primary cancer, or HPV-negative oropharyngeal primary cancer. For example, NRG Oncology is testing the addition of pembrolizumab to cisplatin and radiotherapy for high-risk patients with head and neck cancer (NCT02775812).
However, other more significant opportunities for treatment adaptation might exist that are based on genomics, radiomics, or mathematical modelling. In this framework, adaptation could change not only the volume of treatment, but also the total dose, fractionation, fraction size, or the addition or subtraction of systemic therapy, or possibly even surgery. To respond in this manner, information is needed that helps make a decision for whether adaptation and tailoring of treatment is required. A very simple indication for treatment adaptation would be to monitor the dynamics of a tumour’s response to radiotherapy. Data suggest that a response quantified by a nodal decrease of 40% or more at 4 weeks was associated with 100% locoregional control at 2 years in patients with head and neck cancer.47 If a patient’s tumour is not responding as predicted, mathematical models could have untapped potential to adapt the total dose, fractionation, and fraction size to increase predicted response over the remaining treatment time. These adaptations can be initially based on measurement of volume change by cone-beam CT, MRI, or other imaging methods. But there could be significant underlying radiomic data that can be used to adapt treatment planning. Sub-volumes based on radiomic features within the tumour itself might be more advantageous in treatment planning as they can receive different total doses or fraction sizes. This treatment heterogeneity across the tumour could be monitored in a similar manner via the most advantageous imaging methods.
Immunotherapy
Recently, a shift in the way head and neck cancer is treated, initiated by responses by potent immune checkpoint inhibitors, has rekindled preclinical and clinical exploration of strategies to harness the immunogenicity of radiotherapy. The immunological effect of radiotherapy is of particular relevance in head and neck cancers; a substantial proportion of head and neck cancers are viral-associated.48 HPV-associated squamous cell carcinoma or Epstein-Barr virus-driven nasopharyngeal carcinoma express viral antigens that are distinctly recognised as foreign by the host immune system. In fact, favourable prognosis associated with HPV-positive squamous cell carcinoma might be associated with immune recognition.49 Similarly, the enhanced radio sensitivity observed with head and neck cancers located in predominantly lymphoid tissues such as tonsils could be attributable to antitumour immune responses. In the context of effective immunotherapy for head and neck cancer, the capacity of radiotherapy to induce immune activation is of pivotal importance. The benefit of therapeutic synergy between radiotherapy and cancer immunotherapy is two-fold. First, cancer immunotherapy might enhance the efficacy of radiotherapy as a locoregional method because previous preclinical studies have suggested that the therapeutic efficacy of radiotherapy depends on a functional immune system.50–55 Multiple clinical trials are underway to investigate whether PD-1 blockade might enhance locoregional control by radiotherapy in head and neck cancers (NCT02775812, 02952586, and 02764593). Second, radiotherapy could serve as an in situ vaccine to augment the efficacy of systemic immunotherapy.56
The question of whether radiotherapy can augment the efficacy of immunotherapy is currently being addressed in a phase 2 trial of nivolumab alone or in combination with stereotactic body radiotherapy in patients with metastatic head and neck squamous cell carcinoma (NCT02684253). The key question remains regarding the optimal dose fractionation of radiotherapy to maximally stimulate the immune system. The best evidence thus far derives from preclinical studies, which suggest hypofractionated doses between 7 and 8 Gy to be the most immunogenic doses,57,58 although these values have to be verified in a clinical trial setting. However, the inherent heterogeneity of tumours might dictate variability in the optimal dose fractionation to induce immune activation dependent on the individual tumour biology. To select the optimal patient and optimal radiation dose fractionation for a given patient, we need to develop a predictive biomarker for immunotherapy and radiotherapy synergy. Messina and others have developed a chemokine signature that could provide such a starting point.16
Ideally, immunosensitivity and radiosensitivity across a tumour would be correlated with radiomic features to inform mathematical models, creating signatures with the potential to trigger specific treatment adaptation to personalise treatment. However, substantial work needs to be done to investigate the best timepoints, triggers, and responses to tumour changes that can be tested in a prospective manner.
Sample clinical trial
Patients with HPV-positive oropharyngeal cancer represent a significant opportunity for personalised radiotherapy as a first foray into precision radiotherapy. We have developed a clinical trial that uses genomics and treatment response to personalise radiotherapy dose delivered to each patient. Retrospective analyses of multiple clinical trials59–61 have shown that HPV-associated oropharyngeal cancers are sensitive to radiotherapy. Multiple trials are ongoing, testing a variety of de-escalation approaches for this disease. However, all these trials share the same empirical, one size fits all approach. By contrast, we propose using the radiosensitivity index and genomic-adjusted radiation dose to identify the dose needed to provide a cure for each individual patient. For safety, we would limit the range of doses at both the low end (54 Gy) and high end (82 Gy).62,63 As a second safety measure, we propose using a midtreatment MRI or cone-beam CT to verify that the response is as predicted by the genomic-adjusted radiation dose. As previously mentioned, previous data46 suggest that a nodal response of more than 40% at 4 weeks is associated with a 100% rate of locoregional control at 2 years. Thus, we propose that if the genomic-adjusted radiation dose predicts a lower than standard dose of radiotherapy and the response at 4 weeks is more than 40%, only the genomically predicted dose of radiotherapy should be delivered. If, however, the genomic-adjusted radiation dose predicts a higher than standard dose of radiotherapy, and the response at 4 weeks is less than 40%, then the higher dose of radiotherapy should be delivered (table). Should the predicted dose and response be non-concordant, a standard dose of radiotherapy should be used. We believe this approach would allow patient safety to be maintained while testing the hypothesis.
Table:
Proposed personalised radiotherapy dose clinical trial algorithm
| GARD ≤ standard dose | GARD > standard dose | |
|---|---|---|
| Response after 20 fractions ≥40% | GARD (≥54 Gy) | Standard dose |
| Response after 20 fractions <40% | Standard dose | GARD (≤82 Gy) |
Standard dose is the institutional dose used for treating the particular stage disease (66–70 Gy). GARD=genomic-adjusted radiation dose.
Hopefully, once we establish the usefulness of the genomic-adjusted radiation dose for HPV-positive oropharyngeal cancer, we can retrospectively investigate the use of mathematical modelling, other potential genomic signatures, and radiomics, to begin integrating these tools into other aspects of patient personalisation, including fraction size, fractionation, volumetric adaptive therapy, systemic therapy, and surgical therapy.
Conclusion
We believe treatment personalisation for patients with head and neck cancer offers a substantial opportunity to improve radiotherapy outcomes beyond geometric and anatomical precision. The era of empirical dosing and radiotherapy fractionation should be replaced by a more personalised approach. By taking advantage of precision medicine tools—such as genomics, radiomics, and mathematical modelling—we can investigate ways to personalise and adapt radiotherapy for each patient in our goal to improve cancer outcomes while reducing complications.
Search strategy and selection criteria.
We did not perform a formal literature search for this article. Most articles were selected manually at the discretion of the authors from a review of the contents of high-impact general medical and cancer journals that were published in the past 15 years. Only articles published in English were considered.
Acknowledgments
This work has been supported in part by the IRAT Core Facility at the H Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292).
Footnotes
This is the second in a Series of four papers about head and neck cancer
Declaration of interests
JFT-R declares patents associated with the radiosensitivity index and genomic-adjusted radiation dose, and is the cofounder and shareholder of Cvergenx, which holds commercial rights to the radiosensitivity index and genomic-adjusted radiation dose. All other authors declare no competing interests.
References
- 1.Pan HY, Haffty BG, Falit BP, et al. Supply and demand for radiation oncology in the United States: updated projections for 2015 to 2025. Int J Rad Biol Phys 2016: 96: 493–500. [DOI] [PubMed] [Google Scholar]
- 2.Liu H-W, Gabos Z, Ghosh S, Roberts B, Lau H, Kerba M. Outcomes in stage I non-small cell lung cancer following the introduction of stereotactic body radiotherapy in Alberta—A population-based study. Radiother Oncol J Eur Soc Ther Radiol Oncol 2015; 117: 71–76. [DOI] [PubMed] [Google Scholar]
- 3.Koshy M, Malik R, Mahmood U, Husain Z, Sher DJ. Stereotactic body radiotherapy and treatment at a high volume facility is associated with improved survival in patients with inoperable stage I non-small cell lung cancer. Radiother Oncol 2015; 114: 148–54. [DOI] [PubMed] [Google Scholar]
- 4.Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol 2017; 18: 202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres-Roca JF, Eschrich S, Zhao H, et al. Prediction of radiation sensitivity using a gene expression classifier. Cancer Res 2005; 65: 7169–76. [DOI] [PubMed] [Google Scholar]
- 6.Pramana J, Van den Brekel MWM, van Velthuysen M-LF, et al. Gene expression profiling to predict outcome after chemoradiation in head and neck cancer. Int J Radiat Oncol 2007; 69: 1544–52. [DOI] [PubMed] [Google Scholar]
- 7.Torres-Roca JF, Fulp WJ, Caudell JJ, et al. Integration of a radiosensitivity molecular signature into the assessment of local recurrence risk in breast cancer. Int J Radiat Oncol Biol Phys 2015; 93: 631–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys 2009; 75: 489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toustrup K, Sørensen BS, Nordsmark M, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 2011; 71: 5923–31. [DOI] [PubMed] [Google Scholar]
- 10.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet 2009; 10: 821–32. [DOI] [PubMed] [Google Scholar]
- 11.Eustace A, Mani N, Span PN, et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin Cancer Res 2013; 19: 4879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tawk B, Schwager C, Deffaa O, et al. Comparative analysis of transcriptomics based hypoxia signatures in head- and neck squamous cell carcinoma. Radiother Oncol J Eur Soc Ther Radiol Oncol 2016; 118: 350–58. [DOI] [PubMed] [Google Scholar]
- 13.Meng F, Evans JW, Bhupathi D, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther 2012; 11: 740–51. [DOI] [PubMed] [Google Scholar]
- 14.Larue RTHM, Van De Voorde L, Berbée M, et al. A phase 1 “window-of-opportunity” trial testing evofosfamide (TH-302), a tumour-selective hypoxia-activated cytotoxic prodrug, with preoperative chemoradiotherapy in oesophageal adenocarcinoma patients. BMC Cancer 2016; 16: 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peeters SGJA, Zegers CML, Biemans R, et al. TH-302 in combination with radiotherapy enhances the therapeutic outcome and is associated with pretreatment [18F]HX4 hypoxia PET imaging. Clin Cancer Res 2015; 21: 2984–92. [DOI] [PubMed] [Google Scholar]
- 16.Messina JL, Fenstermacher DA, Eschrich S, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep 2012; 2: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett GC, Coles CE, Elliott RM, et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: a prospective analysis study. Lancet Oncol 2012; 13: 65–77. [DOI] [PubMed] [Google Scholar]
- 18.Andreassen CN, Rosenstein BS, Kerns SL, et al. Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother Oncol 2016; 121: 431–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerns SL, Dorling L, Fachal L, et al. Meta-analysis of genome wide association studies identifies genetic markers of late toxicity following radiotherapy for prostate cancer. EBioMedicine 2016; 10: 150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambin P, van Stiphout RGPM, Starmans MHW, et al. Predicting outcomes in radiation oncology--multifactorial decision support systems. Nat Rev Clin Oncol 2013; 10: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriksson E, Kjellen E, Wahlberg P, Ohlsson T, Wennerberg J, Brun E. 2-Deoxy-2-[18F] fluoro-D-glucose uptake and correlation to intratumoral heterogeneity. Anticancer Res 2007; 27: 2155–59. [PubMed] [Google Scholar]
- 24.Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging 2011; 38: 987–91. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Knopp MV. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol 2011; 2011: 732848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366: 883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buch K, Fujita A, Li B, Kawashima Y, Qureshi MM, Sakai O. Using texture analysis to determine human papillomavirus status of oropharyngeal squamous cell carcinomas on CT. AJNR Am J Neuroradiol 2015; 36: 1343–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita A, Buch K, Li B, Kawashima Y, Qureshi MM, Sakai O. Difference between HPV-positive and HPV-negative non-oropharyngeal head and neck cancer: texture analysis features on CT. J Comput Assist Tomogr 2016; 40: 43–47. [DOI] [PubMed] [Google Scholar]
- 29.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012; 9: 674–87. [DOI] [PubMed] [Google Scholar]
- 30.Servagi-Vernat S, Differding S, Sterpin E, et al. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: a planning study. Acta Oncol 2015; 54: 1008–16. [DOI] [PubMed] [Google Scholar]
- 31.Thorwarth D, Alber M. Implementation of hypoxia imaging into treatment planning and delivery. Radiother Oncol 2010; 97: 172–75. [DOI] [PubMed] [Google Scholar]
- 32.Jeong J, Setton JS, Lee NY, Oh JH, Deasy JO. Estimate of the impact of FDG-avidity on the dose required for head and neck radiotherapy local control. Radiother Oncol 2014; 111: 340–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aerts HJWL Velazquez ER, Leijenaar RTH, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leijenaar RTH, Carvalho S, Hoebers FJP, et al. External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma. Acta Oncol 2015; 54: 1423–29. [DOI] [PubMed] [Google Scholar]
- 35.Parmar C, Leijenaar RTH, Grossmann P, et al. Radiomic feature clusters and prognostic signatures specific for lung and head & neck cancer. Sci Rep 2015; 5: 11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatt M, Majdoub M, Vallières M, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med 2015; 56: 38–44. [DOI] [PubMed] [Google Scholar]
- 37.Hentschel M, Appold S, Schreiber A, et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur J Nucl Med Mol Imaging 2011; 38: 1203–11. [DOI] [PubMed] [Google Scholar]
- 38.Noij DP, Pouwels PJW, Ljumanovic R, et al. Predictive value of diffusion-weighted imaging without and with including contrast-enhanced magnetic resonance imaging in image analysis of head and neck squamous cell carcinoma. Eur J Radiol 2015; 84: 108–16. [DOI] [PubMed] [Google Scholar]
- 39.Anderson ARA, Quaranta V. Integrative mathematical oncology. Nat Rev Cancer 2008; 8: 227–34. [DOI] [PubMed] [Google Scholar]
- 40.Leder K, Pitter K, Laplant Q, et al. Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell 2014; 156: 603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prokopiou S, Moros EG, Poleszczuk J, et al. A proliferation saturation index to predict radiation response and personalize radiotherapy fractionation. Radiat Oncol 2015; 10: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sachs RK, Hlatky LR, Hahnfeldt P. Simple ODE models of tumor growth and anti-angiogenic or radiation treatment. Math Comput Model 2001; 33: 1297–305. [Google Scholar]
- 43.Fowler JF. Linear quadratics is alive and well. Int J Radiat Oncol Biol Phys 2008; 72: 957. [DOI] [PubMed] [Google Scholar]
- 44.Brouwer CL, Steenbakkers RJHM, Langendijk JA, Sijtsema NM. Identifying patients who may benefit from adaptive radiotherapy: Does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol 2015; 115: 285–94. [DOI] [PubMed] [Google Scholar]
- 45.Kager PM, van Weerdenburg SCC, van Kranen SR, et al. Geometric changes of parotid glands caused by hydration during chemoradiotherapy. Radiat Oncol 2015; 10: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raghavan G, Kishan AU, Cao M, Chen AM. Anatomic and dosimetric changes in patients with head and neck cancer treated with an integrated MRI- tri-(60)Co teletherapy device. Br J Radiol 2016; 20160624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart R, Hu KS, Li Z, et al. Use of cone beam CT to assess midtreatment nodal response to chemoradiation therapy in oropharyngeal squamous cell carcinomas: implications for adaptive radiation therapy. Int J Radiat Oncol Biol Phys 2015; 93: 302–03. [Google Scholar]
- 48.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010; 11: 781–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16ink4a expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27: 1992–98. [DOI] [PubMed] [Google Scholar]
- 50.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst 1979; 63: 1229–35. [PubMed] [Google Scholar]
- 51.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124: 687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S, Ramakrishnan R, Lavilla-Alonso S, et al. Radiation-induced autophagy potentiates immunotherapy of cancer via up-regulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol Immunother 2014; 63: 1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimoto Y, Suzuki Y, Mimura K, et al. Radiotherapy-induced antitumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PloS One 2014; 9: e92572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouquet F, Pal A, Pilones KA, et al. TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 2011; 17: 6754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114: 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009; 10: 718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15: 5379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012; 83: 1306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ang K, Pajak T, Wheeler R, et al. A phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas (RTOG 0129): report of efficacy and toxicity. Int J Radiat Oncol 2010; 77: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lassen P, Primdahl H, Johansen J, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol 2014; 113: 310–16. [DOI] [PubMed] [Google Scholar]
- 61.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32: 2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marur S, Lee J-W, Cmelak A, et al. ECOG 1308: a phase II trial of induction chemotherapy followed by cetuximab with low dose versus standard dose IMRT in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx (OP). 2012 ASCO annual meeting Chicago, IL; June 1–5, 2012 Abstr 5566. [Google Scholar]
- 63.Madani I, Duprez F, Boterberg T, et al. Maximum tolerated dose in a phase I trial on adaptive dose painting by numbers for head and neck cancer. Radiother Oncol 2011; 101: 351–55. [DOI] [PubMed] [Google Scholar]


