Abstract
Glucocorticoids for primary therapy of acute GVHD have limited responses. A phase I/II trial tested 4 weeks of deacetylase inhibitor panobinostat started within 48 h of glucocorticoids (1 mg/kg/day prednisone or equivalent) as primary treatment for patients with either classic acute GVHD (n = 16) or acute GVHD overlapping with chronic (n = 6). Four patients received 2.5 mg/m2 IV three times a week (TIW). Subsequent to discontinuation of IV panobinostat, patients received oral doses (PO). Two patients treated with 10 mg TIW (PO level 1) had progressive GVHD, after which patients were treated with 5 mg TIW (PO level −1; n = 16); 31/41 adverse events were possibly related, including thrombocytopenia (n = 13), leukopenia (n = 7), hypercholesterolemia (n = 3), hypertriglyceridemia (n = 5), anemia (n = 1), fatigue (n = 1), and hepatobiliary disorder (n = 1). GVHD responses were complete (n = 12) or partial (n = 3), with 1 progression at PO level −1. T-regulatory cells increased at day 8, CD4/CD8 and monocytes exhibited enhanced H3 acetylation, and CD4 or CD8 numbers remained unchanged with a decreased interleukin 12p40 plasma level. Panobinostat in combination with prednisone is safe and warrants further testing in GVHD.
Introduction
Graft-versus-host disease (GVHD) is the major complication of allogeneic hematopoietic cell transplantation (HCT) [1, 2]. Glucocorticoids are the current standard initial treatment for acute GVHD, with complete responses (CRs) in 25–41% of patients, correlating with the degree of GVHD severity [3]. Response to primary therapy is of central importance, as it predicts survival after HCT. There is no consensus on optimal secondary treatment for patients unresponsive to glucocorticoids, due to the paucity of controlled studies and overall poor study results [4–10]. New immunosuppressive agents are required to control acute GVHD. Improved GVHD therapy would result in a higher remission rate and better survival rates.
Nonselective pan-histone deacetylase inhibitors (HDACi’s) have several properties that are valuable in the management of GVHD, including reduction of pro-inflammatory cytokines [11, 12], increased numbers and enhanced suppressive function of regulatory T cells [13], enhanced immunomodulatory properties of human dendritic cells [14], and direct antitumor activity [15, 16]. HDACi’s decrease GVHD mortality without affecting graft-versus-leukemia effects in murine transplantation models [17, 18]. Furthermore, Choi et al. studied the safety and efficacy of vorinostat for GVHD prevention in patients undergoing HCT, with encouraging results [19–21].
Panobinostat (PANO) is a nonselective HDACi approved for the treatment of relapsed multiple myeloma [22, 23]. PANO in murine models demonstrates accelerated liver GHVD with increased systemic Th1 cytokines [24]. These preclinical findings have not been observed in human trials testing PANO in the setting of maintenance therapy post HCT [25] and/or epigenetic therapy in combination with donor lymphocyte infusions [26]. Moreover, PANO is known to reduce inflammatory cytokines from human dendritic cells, including interleukin (IL) 6 and the p40 cytokines, which are biologically relevant mediators of GVHD [27, 28]. Therefore, we conducted a phase I study to test the safety and preliminary clinical activity of PANO in combination with glucocorticoids in the upfront treatment of GVHD.
The primary objective of the phase I trial was to define a safe dose of PANO in combination with 1 mg/kg/day prednisone, when used as primary therapy for acute GVHD. Herein, we report results from 12 phase-I patients treated intravenously (IV) or orally (PO) and 10 subsequent patients who were enrolled at the maximum tolerated dose (MTD).
Subjects and methods
Overview of trial design
This phase I to II trial (NCT01111526) was designed to define a safe dose and explore the efficacy of PANO in combination with glucocorticoids for GVHD primary therapy. Patients either had classic acute GVHD, acute GVHD with late onset at ≥100 days without chronic GVHD, or acute GVHD overlapping with chronic GVHD manifestations (acute GVHD features with chronic manifestations ≥100 days post transplant). Follow-up was 12 months, and drug safety was conducted until 36 days after enrollment. The primary endpoint for the phase II portion was to evaluate GVHD response at day 36 of PANO. Additional endpoints included chronic GVHD by National Institutes of Health criteria, flares, proportion of patients discontinuing immunosuppression, survival, and allied correlative studies. We completed phase I (n = 12) and enrolled 10 of the 46 patients planned for phase II. The trial was closed to accrual prematurely, due to slower-than-anticipated enrollment.
Inclusion, exclusion, and discontinuation criteria
Adults aged ≥18 years with new-onset acute GVHD requiring systemic glucocorticoid therapy were enrolled. Acute GVHD diagnosis and severity scoring were based on the Consensus Criteria GVHD grading system [29], with minor modifications in liver and gastrointestinal staging. Liver modifications included aspartate aminotransferase and/or alanine aminotransferase (>3 × upper limit of normal (ULN) for grade 1; 3.1–5.0 × ULN for grade 2; 5.1–20.0 × ULN for grade 3; and >20 × ULN for grade 4) or alkaline phosphatase with gammaglutamyl transpeptidase besides bilirubin above the ULN (>ULN—2.5 × ULN for grade 1; >2.6–5 × ULN for grade 2; >5.1–20 × ULN for grade 3; >20 × ULN for grade 4). Most participants were outpatients, limiting the information on accurate stool volumes; however, patient-reported increase in number of stool episodes per day over baseline was captured. Chronic GVHD scoring adhered to the National Institutes of Health Consensus Criteria on Diagnosis and Staging of Chronic GVHD [30].
Patients excluded as follows: pregnant or breast feeding; on mechanical ventilation support; active or uncontrolled life-threatening viral or fungal infection; investigational drugs; use of HDACs, heat-shock protein 90 inhibitors, or valproic acid within 30 days; receipt of cytochrome P450 3A4 inhibitors, with the exceptions of tacrolimus, voriconazole, posaconazole, and cyclosporine, which were allowed; impaired cardiac function or clinically significant cardiac diseases, including congenital long QT syndrome; sustained ventricular tachyarrhythmia (controlled atrial arrhythmia was eligible); any history of ventricular fibrillation or torsade de pointes; bradycardia (<50 beats per minute); patients whose average of three corrected QT intervals by Fredericia formula was >470 ms on screening electrocardiogram; bifascicular block; myocardial infarction or unstable angina within ≤6 months or other clinically significant heart disease; HIV positive; and/or noncompliance.
Study patients began PANO therapy within 48 h of the initiation of corticosteroid therapy for acute GVHD. Study drug PANO was discontinued in the event of GVHD treatment failure (defined as progressive GVHD within 7 days, grade III–IV GVHD persisting for at least 7 days, or grade II GVHD persisting for at least 14 days), significant toxicity, an abnormal laboratory result that could not be corrected, protocol violation, patients withdrawn/lost to follow-up, or death.
Treatment plan
Prednisone was uniformly initiated at 1 mg/kg/day or methylprednisolone at 0.8 mg/kg/day, for at least 14 days. Prednisone tapering after 14 days was not required. In the case of GVHD progression, PANO was discontinued.
Phase I was to examine escalating doses of IV PANO, with dose-level cohorts of 2.5 mg/m2 (IV level 1) or 5 mg/m2 (IV level 2) for 4 weeks dosage based on prior drug experience). Due to discontinuation of IV formulation after enrolling 4 patients, the protocol was amended to examine escalating doses of PO PANO in cohorts of patients starting at PO dose level 1, which was 10 mg three times a week (TIW) for 4 weeks. A de-escalation dose of 5 mg PO TIW for 4 weeks (PO level −1) was also planned, in case excess toxicity or safety concerns occurred at dose level 1 (PO level 1). All patients were treated with either micafungin (n = 5) or azoles (n = 17) prophylaxes, with no adjustments in PANO dosage.
Phase I trial characteristics
The phase I trial followed a traditional (3 + 3) escalation design. The dose limiting toxicity (DLT) and toxicity observation period was 36 days. DLT was defined as the occurrence of Common Terminology Criteria for Adverse Events grade 3 or greater toxicity that was unexpected with HCT or absolute neutrophil count (ANC) <750/μL and platelets <10,000/μL for transfusion-independent patients. Patients in the phase I MTD cohort were carried forward to the phase II component.
Response definitions
CR was resolution of GVHD; partial response (PR) improvement in 1 or more organs, without deterioration in any others; progressive disease was deterioration in ≥1 organ; and stable disease was the absence of any difference sufficient to meet the minimal criteria for improvement or deterioration in any organ (adjusted from Martin P et al.) [31].
Data and safety monitoring
The trial was conducted with the approval of the University of South Florida IRB. Patients signed the informed consent. Adverse events were reported to IRB, Protocol Monitoring Committee, and/or the Food and Drug Administration.
Pharmacodynamics
T-cell subsets
Fluorescence-activated cell-sorting analyses were performed on whole blood with labeled antibodies to CD3, CD4, CD8, CD25, CD127, and Foxp3. Samples were drawn prior to PANO administration (baseline) and on days 8 (±1), 15 (±1), and 29 (±1) after PANO. Absolute cell counts were calculated using BD TruCOUNT tubes (BD Bioscience).
Histone 3 acetylation of blood cell subsets
Thawed cells were incubated with the viability marker zombie yellow and then stained for CD3/CD4/CD8 or CD14/CD45/CD11c, using labeled antibodies. After methanol permeabilization, cells were stained for acetylated histone 3 (Lys 9).
Activity of HDAC
We used a luminescent assay to measure the relative activity of HDAC class I and II enzymes from peripheral blood mononuclear cells, in accordance with manufacturer instructions (DACGlo I/II Screening System, Promega). Results were displayed as percentage changes from baseline to 8 and 15 days after the initiation of PANO.
Cytokine measurement
Plasma samples were tested by Quansys Biosciences (Logan, Utah), using QPlex Array kits for human cytokines for the detection of IL7, IL17, IL10, transforming growth factor beta, interferon, tumor necrosis factor alpha (TNF), TNF receptor 1, and IL12p40.
Statistical methods
Following a standard 3 + 3 design, escalation to each higher dose level was permitted when 0 out of 3 or no more than 1 out of 6 patients in a dose-level cohort experienced DLT (number treated per level was increased to 6, if 1 out of 3 experienced DLTs). A total of 6 patients was planned for the MTD cohort and subsequent patients (n = 10) were enrolled at this dose level.
For pharmacodynamics T-cell analysis, since the normality assumption by Shapiro-Wilk test was not met, the change from baseline was assessed by the Wilcoxon signed-rank test. HDAC enzymatic activity and cytokine levels were done in triplicates and analyzed using paired Student t tests comparing baseline to results over time using Prism software. P < .05 was considered significant.
Results
Baseline patient characteristics
Four of the patients treated during phase I received the IV formulation of PANO, including 3 on IV dose level 1 (2.5 mg/m2) and 1 on IV dose level 2 (5 mg/m2). After the manufacturer discontinued IV formulation, we treated 2 patients at PO dose level 1 (10 mg PO TIW for 4 weeks). PANO was discontinued in both patients after 3–4 doses due to GVHD progression. Due to concern for a role of PANO in GVHD progression, the next patients were treated at dose level −1 PO (5 mg PO TIW for 4 weeks). Sixteen patients in total were treated at dose level −1 PO, including 6 patients in phase I and 10 in the phase II extension component of the trial.
Baseline patient characteristics are summarized in Table 1. HCTs were from matched siblings (n = 6), 8/8 human leukocyte antigen allele-matched unrelated donors (n = 13), or 7/8 human leukocyte antigen allele-matched unrelated donors (n = 3). All patients received filgrastim-mobilized peripheral blood stem cells. The median age of recipients was 53 years (range, 34–76 years) and 68% were male; patients were conditioned prior to HCT with either a myeloablative (n = 11) or reduced-intensity (n = 11) regimen, as defined by Bacigalupo et al. [32].
Table 1.
Baseline patient characteristics
| Intravenousa | Oralb | Total N = 22 |
|||
|---|---|---|---|---|---|
| Level 1 n = 3 | Level 2 n = 1 | Level 1 n = 2 | Level −1 n = 16 | ||
| Age, y | |||||
| Median | 59.4 | 53 | 43.3 | 53 | 53 |
| Range | 45.1–62.6 | NA | 39.4–47.3 | 34–76 | 34–76 |
| Sex, % | |||||
| Male | 33 | 100 | 100 | 68 | 68 |
| Race, no. | |||||
| White | 2 | 1 | 1 | 13 | 17 |
| Hispanic | 1 | 0 | 1 | 3 | 5 |
| Disease category, no. | |||||
| MDS | 1 | 0 | 0 | 4 | 5 |
| CLL | 1 | 0 | 0 | 1 | 2 |
| Myeloma | 1 | 0 | 0 | 0 | 1 |
| FL NHL | 0 | 1 | 0 | 0 | 1 |
| CML | 0 | 0 | 1 | 0 | 1 |
| IMF | 0 | 0 | 1 | 3 | 4 |
| AML | 0 | 0 | 0 | 7 | 7 |
| ALL | 0 | 0 | 0 | 1 | 1 |
| Conditioning, no. | |||||
| Pento/BU/rituxan | 1 | 1 | 0 | 0 | 2 |
| BU/FLU/AUC 5300 | 1 | 0 | 1 | 9 | 11 |
| Flu/Mel | 1 | 0 | 1 | 3 | 5 |
| BU/FLU/AUC 3500 | 0 | 0 | 0 | 4 | 4 |
| GVHD prophylaxis, No. | |||||
| TAC/MTX | 1 | 1 | 0 | 5 | 7 |
| TAC/MTX/ATG | 1 | 0 | 0 | 0 | 1 |
| TAC/MMF | 1 | 0 | 1 | 1 | 3 |
| TAC/SIR | 0 | 0 | 1 | 7 | 8 |
| TAC/SIR/ATG | 0 | 0 | 0 | 2 | 2 |
| TAC/SIR/UST | 0 | 0 | 0 | 1 | 1 |
| Donor, no. | |||||
| MUD 8/8 | 1 | 1 | 1 | 10 | 13 |
| MRD | 1 | 0 | 1 | 4 | 6 |
| MMUD 7/8 | 1 | 0 | 0 | 2 | 3 |
| Graft source PBSC, no. | 3 | 1 | 2 | 16 | 22 |
ALL acute lymphoblastic leukemia, AML acute myelogenous leukemia, ATG antithymocyte globulin, AUC area under the curve, BU busulfan, CML chronic myeloid leukemia, CLL chronic lymphocytic leukemia, FL NHL follicular lymphoma, FLU fludarabine, GVHD graft-versus-host disease, IMF idiopathic myelofibrosis, MDS myelodysplastic syndrome, Mel melphalan, MMF mycophenolate mofetil, MMUD mismatched unrelated donor, MRD matched related donor, MTX methotrexate, MUD matched unrelated donor, NA not applicable, PBSC peripheral blood stem cell, Pento pentostatin, SIR sirolimus, TAC tacrolimus, UST ustekinumab
Patients who received intravenous panobinostat
Patients who received oral panobinostat
Acute GVHD types and stages at initiation of PANO therapy are presented in Table 2A and 2B. Classic acute GVHD (n = 16) had onset on day 37 (range, 26–98 days) after transplantation. Late acute GVHD (n = 6) had onset on day 318 (range, 109–981 days) after transplantation. Patients had an overall GVHD score grade II (n = 15, 68%) or III (n = 7, 32%), with 10 (45%) patients having multiple organ sites concurrently involved at therapy initiation. Based on institutional practices, most patients received GVHD prophylaxis with either tacrolimus and sirolimus (n = 8, 36%) or tacrolimus and methotrexate (n = 7, 32%).
Table 2A.
Acute and overlap GVHD characteristics at initiation of therapy
| Intravenousa | Oralb | Total (N = 22) | |||
|---|---|---|---|---|---|
| Level 1 (n = 3) | Level 2 (n = 1) | Level 1 (n = 2) | Level − 1 (n = 16) | ||
| Acute | 3 | 1 | 2 | 10 | 16 (72.7) |
| GVHD*, no. (%) | |||||
| Onset post BMT, days | |||||
| Median | 37 | 26 | 59.5 | 48 | 37 |
| Range | 27–37 | NA | 26–93 | 32–98 | 26–98 |
| Acute GVHD overlapping with chronic GVHDc, no. (%) | |||||
| Onset post | 0 | 0 | 0 | 6 | 6 (27.3) |
| BMT, days | |||||
| Median | 423 | 423 | |||
| Range | 109–981 | 109–981 | |||
| Baseline immunosuppression, no. (%) | |||||
| TAC/MTX | 1 | 1 | 0 | 5 | 7 (32) |
| TAC/ | 1 | 0 | 0 | 0 | 1 (4.5) |
| MTX/ATG | |||||
| TAC/SIR | 0 | 0 | 1 | 7 | 8 (36) |
| TAC/SIR/ | 0 | 0 | 0 | 2 | 2(9) |
| ATG | |||||
| TAC/SIR/ | 0 | 0 | 0 | 1 | 1 (4.5) |
| UST | |||||
| TAC/MMF | 1 | 0 | 1 | 1 | 3 (14) |
| Overall acute GVHD score, no. (%) | |||||
| Grade 2 | 1 | 1 | 1 | 12 | 15 (68) |
| Grade 3 | 2 | 0 | 1 | 4 | 7 (32) |
| Antifungal prophylaxis, no. | |||||
| 0 | 0 | 0 | 4 | 4 | |
| Mycafungin | |||||
| Azole | 3 | 1 | 2 | 12 | 18 |
ATG antithymocyte globulin, GVHD graft-versus-host disease, MMF mycophenolate mofetil, MTX methotrexate, NA not applicable, SIR sirolimus, TAC tacrolimus, UST ustekinumab
Patients who received intravenous panobinostat
Patients who received oral panobinostat
At the time of trial enrollment. For late acute GVHD overlapping with chronic, we report the grading of acute manifestations only
Table 2B.
Organ-specific GVHD staging by patient
| Staging Category | Patients (panobinostat dose level, sequential patient number) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV 1 | IV 2 | PO 1 | PO −1 | |||||||||||||||||||
| 1 | 2a | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | |
| Skin, stageb | ||||||||||||||||||||||
| Day 1 | 2 | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||||
| Day 8 | 2 | 2 | 4 | 1 | 1 | 2 | 2 | 3 | 3 | 3 | 1 | 2 | 2 | 3 | 3 | |||||||
| Day 15 | 2 | … | … | 2 | 2 | 2 | … | 1 | 2 | 3 | ||||||||||||
| Day 22 | 2 | … | … | 2 | 1 | 1 | … | 1 | 1 | |||||||||||||
| Day 26 | 1 | … | … | … | 1 | 1 | ||||||||||||||||
| Day 33 | 1 | … | … | … | 1 | |||||||||||||||||
| Gut, stage | ||||||||||||||||||||||
| Day 1 | 3 | 3 | 1 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Day 8 | 1 | 1 | ||||||||||||||||||||
| Day 15 | … | … | … | |||||||||||||||||||
| Day 22 | … | … | … | |||||||||||||||||||
| Day 26 | … | … | … | |||||||||||||||||||
| Day 33 | … | … | … | |||||||||||||||||||
| Liver, stage | ||||||||||||||||||||||
| Day 1 | 1 | 2 | 1 | 1 | 3 | 1 | 3 | |||||||||||||||
| Day 8 | 3 | 1 | 4 | 1 | 3 | |||||||||||||||||
| Day 15 | … | … | 1 | … | 1 | 2 | ||||||||||||||||
| Day 22 | … | … | … | 1 | ||||||||||||||||||
| Day 26 | … | … | 1 | … | 1 | |||||||||||||||||
| Day 33 | … | … | 1 | … | 1 | |||||||||||||||||
| Overall acute GVHD, grade | ||||||||||||||||||||||
| Day 1 | I | III | III | II | II | III | II | II | III | II | II | III | II | III | II | II | II | II | II | III | III | II |
| Day 8 | I | I | IV | III | I | II | I | II | II | IV | II | I | II | I | I | III | ||||||
| Day 15 | I | … | … | I | II | I | … | I | II | I | III | |||||||||||
| Day 22 | I | … | … | I | I | I | … | I | I | |||||||||||||
| Day 26 | I | … | … | I | … | I | I | |||||||||||||||
| Day 33 | I | … | … | I | … | I | ||||||||||||||||
| Overall response, category | ||||||||||||||||||||||
| Day 1 | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL | BL |
| Day 8 | PR | CR | CR | PR | PD | PD | PR | PR | CR | PR | PR | CR | SD | PD | CR | PR | PR | PR | PR | PR | SD | CR |
| Day 15 | PR | CR | CR | CR | … | … | CR | CR | CR | PR | PR | CR | PR | … | CR | PR | CR | PR | PR | CR | PR | CR |
| Day 22 | PR | CR | CR | CR | … | … | CR | CR | CR | PR | PR | CR | PR | … | CR | CR | CR | CR | PR | CR | PR | CR |
| Day 26 | PR | CR | CR | CR | … | … | CR | CR | CR | CR | PR | CR | CR | … | CR | CR | CR | CR | PR | CR | PR | CR |
| Day 33 | PR | CR | CR | CR | … | … | CR | CR | CR | CR | PR | CR | CR | … | CR | CR | CR | CR | CR | CR | PR | CR |
BL baseline, CR complete response, GVHD graft-versus-host disease, PD progressive disease, PR partial response, SD stable disease, IV intravenous, PO oral
On day 335, patient 2 had skin, gut, liver, and GVHD grade 0 and complete response
Blank cells signify a value of zero. Ellipses signify data not applicable as had GVHD progression therefore panobinostat was discontinued (n = 3)
Preliminary assessment of efficacy, glucocorticoid tapering, and other outcomes
GVHD responses in the 3 patients treated at IV level 1 were CR in 2 and PR in 1; the patient treated at IV dose level 2 achieved CR. Patients treated at PO level 1 had either skin or liver GVHD progression in 1/3 organs compromised. Changes in acute GVHD score-response assessments for PO level −1 are presented in Table 2B. Responses were observed in overall grades II and III acute GVHD across individual organ sites and in both classic (n = 10) and late-onset (n = 6) subtypes of acute GVHD. At 36 days after PANO initiation, CRs were observed in 12 patients (75%), PRs in 3 patients (19%), and progressive disease in 1 patient (6%), with most achieving responses at 21 days of treatment. All patients completed PANO treatment course unless progressed (n = 3).
For patients treated with IV formulation (n = 4), a median of 172 days (range, 60–455 days) was required to decrease prednisone to physiological doses of 0.05 mg/kg/day, and it was completely discontinued at a median of 461 days (range,129–634 days). For patients treated at PO level −1, a median of 83 days (range, 48–412 days) was required to decrease prednisone to 0.05 mg/kg/day, and it was completely discontinued at a median 117 days (range, 49–442 days) in evaluable patients (n = 12). These data show a large percentage reduction from the starting prednisone dose, especially at the recommended dose of 5 mg PO TIW for 4 weeks (level −1 PO). After glucocorticoid discontinuation, only 1 patient had GVHD flare, requiring additional immunosuppressive medications until the patient died 26 months after enrollment.
Evaluable patients treated for classic acute GVHD with IV PANO had maximum National Institutes of Health chronic GVHD scores of 0 (n = 1) or 1 (n = 3) at follow-up within 1 year of PANO treatment. Five evaluable patients treated at PO level −1 had maximum scores of 1 (n = 3), 2 (n = 1), or 3 (n = 1) within 1 year follow-up. Of these 5 patients, at study completion 2 had chronic GVHD resolution, 1 had overall GVHD improvement (score 3 to 2), and 2 remained stable at score 1.
Overall survival at 365 days after PANO initiation was 100% in IV-treated patients (n = 4). Both patients treated at level 1 PO died from GVHD. Patients treated at level −1 PO had an overall survival rate of 70% 1 year after enrollment (n = 11 out of the cohort of 16), with all remaining patients continuing on immunosuppressive therapies 365 days after enrollment. Causes of death were sepsis (n = 1), malignancy relapse (n = 2), GVHD (n = 1), and cardiogenic shock (n = 1). No deaths were deemed to be study therapy related.
Pharmacodynamics studies on T-cell subsets
Therapy was associated with no changes in lymphocyte counts (CD3, CD4, and CD8). Regulatory T-cell counts were significantly increased over baseline 8 days after PANO administration (Fig. 1a).
Fig. 1.
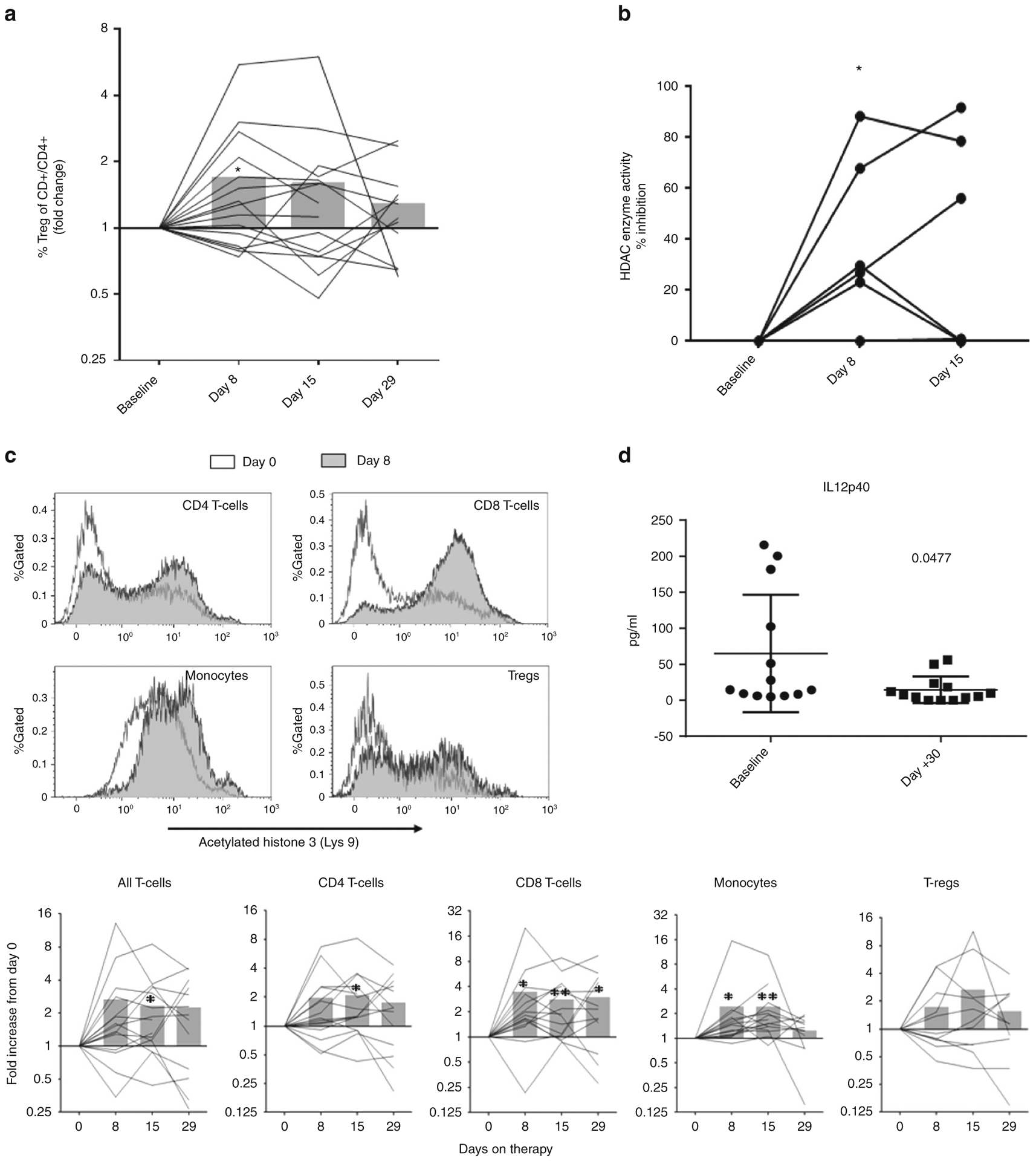
Pharmacodynamics studies. a Fold change of T regulatory cells (T-regs CD25+/CD3+/CD4+) measured by flow cytometry and compared to baseline at days 8, 15, and 29 after panobinostat administration: *P = 0.05. b Luminescent assay measuring HDAC class I/II enzyme activity displayed as percentage change from baseline: *P = 0.03 T-test. c Representative histogram illustrating histone 3 acetylation on lymphocyte subsets and monocytes (top). Fold increase histone 3 acetylation compared to baseline (day 0) in lymphocytes/monocytes at different time points after panobinostat. *P = 0.05, **P = 0.005. d IL12p40 plasma cell levels
Inhibition of HDAC enzymatic activity testing showed a median 40% inhibition (23–88%) above baseline as early as 8 days post PANO (n = 6/7; Fig. 1b). PANO resulted in statistically significant increases in fold change in H3 acetylation in CD4 and CD8 T cells and monocytes and a trend in regulatory T-cell H3 acetylation, suggesting HDAC inhibition in different lymphocyte subsets (Fig. 1c).
Only IL12p40 inflammatory cytokine levels were significantly decreased from baseline levels 30 days after treatment commenced (Fig. 1d).
Safety: dose-limiting toxicity, adverse events, and infectious complications
Two patients treated on PO dose level 1 developed GVHD progression; subsequent patients were treated on oral dose level −1. This dose was subsequently designated as the MTD and was recommended for the treatment of newly enrolled patients. No DLT events occurred during the study.
Total adverse events on trial are listed in Table 3. Of 41 total adverse events, 31 were deemed possibly related to PANO, with thrombocytopenia (n = 13) being the most common myelotoxicity. Other adverse events included leukopenia (n = 7), hypercholesterolemia (n = 3), hypertriglyceridemia (n = 5), anemia (n = 1), fatigue (n = 1), and hepatobiliary disorder (n = 1). Total infections or viral reactivations within 36 days follow-up are described in Table 4.
Table 3.
Summary of adverse events
| Adverse event | Grade 1, no. (%)a |
Grade 2, no. (%) |
Grade 3, no. (%) |
Grade 4, no. (%) |
Grade 5, no. (%) |
|---|---|---|---|---|---|
| Intravenous administration, level 1 (n = 3/3b) | |||||
| Thrombocytopenia | 3c (13.5) | ||||
| Leukopenia | 2 (9) | ||||
| Hypertriglyceridemia | 1 (4.5) | 1 (4.5) | |||
| Hypercholesterolemia | 1 (4.5) | ||||
| Intravenous administration, level 2 (n = 1/1b) | |||||
| Thrombocytopenia | 1 (4.5) | ||||
| Anemia | 1 (4.5) | ||||
| Hypercholesterolemia | 1 (4.5) | ||||
| Hypertriglyceridemia | 1 (4.5) | ||||
| Oral administration, level 1 (n = 0/2b) | |||||
| Toxic epidermal necrolysis | 1 (4.5) | ||||
| Thrombocytopenia | 2 (9) | ||||
| Anemia | 1 (4.5) | ||||
| Portal hypertension (sinusoidal obstruction syndrome) | 1 (4.5) | ||||
| Oral administration, level − 1 (n = 15/16b) | |||||
| Thrombocytopenia | 1 (4.5) | 2 (13.5) | 3 (13.5) | 1 (4.5) | |
| Leukopenia | 3 (13.5) | 2 (9) | |||
| Anemia | 1 (4.5) | ||||
| Hyperglycemia | 1 (4.5) | ||||
| Hyponatremia | 1 (4.5) | ||||
| Fatigue | 1 (4.5) | ||||
| Hypercholesterolemia | 1 (4.5) | ||||
| Hypertriglyceridemia | 2 (9) | 1 (4.5) | |||
| Hyperglycemia | 1 (4.5) | ||||
| Ischemia cerebrovascular | 1 (4.5) | ||||
| Pericardial effusion with cardiac tamponade | 1(4.5) | ||||
| Hepatobiliary disorders (suspect drug toxicity) | 1 (4.5) | ||||
| Total adverse events (N = 41) | 10 (25) | 14 (34) | 14 (34) | 2(5) | 1 (2) |
Individual adverse events observed on trial (assessed per Common Terminology Criteria for Adverse Events version 4.0), from time of treatment initiation through follow-up. Individual adverse events are presented with the percentage of total study patients (N = 22) who experienced them
Number of patients who experienced adverse events/number of patients in treatment subcategory
Adverse events deemed possibly related to PANO therapy are presented in bold type (n = 31/41 or 76% of total adverse events)
Table 4.
Summary of infections/viral reactivations on trial (N = 22) for all patients within 36 days follow-up period
| Site | Organism | Grade | Study sequence number |
|---|---|---|---|
| Nasopharynx | Influenza A | 2 | 9 |
| Metapneumovirus | 2 | 16 | |
| Oral | Candida | 2 | 19 |
| Surgical | Submandibular abscess (Streptococcus constellatus viridans group) | 3 | 8 |
| Blood | Viral reactivations: | ||
| Cytomegalovirus | 2 | 2, 3, 9 | |
| Epstein-Barr virus | 2 | 10,19 | |
| Bacteremia: | |||
| Coagulase negative staphylococcus (epidermidis) | 2 | 2 | |
| Coagulase negative staphylococcus (capitis) | 2 | 3 | |
| Coagulase negative staphylococcus | 2 | 3 | |
| Klebsiella pneumoniae | 2 | 9 | |
| Urinary Tract Infection | E. coli | ||
| Viral | Varicella-zoster virus | 2 | 11 |
| Pericarditis (suspected) | 5 | 17 | |
| BK hemorrhagic cystitis | 2 | 12 |
Discussion
Due to HDACi’s immune modulation, we tested PANO combined with corticosteroid as initial therapy for acute GVHD. Our data support the safety of PANO in the primary therapy of acute GVHD, with no DLTs observed. Due to the unknown contribution of PANO to GVHD progression in 2 patients treated at PO level 1, dose was de-escalated and led to the definition of MTD. All patients at MTD completed treatment, except for 1 patient with progressive GVHD.
The adverse events observed on trial were largely the expected complications of HCT and/or prednisone therapy, with 76% of adverse events attributed to PANO. Most adverse events were related to myelotoxicity and dyslipidemia, as previously described, with no observed QT prolongation [33, 34]. Observed hematological toxicities were mild with deterioration of ≤2 CTCAE V4 grades from baseline and reversible in most patients. Despite PANO’s gastrointestinal toxicity, none of the patients developed worsening diarrhea, which could be attributed to the low dosage. Additional immune suppression in the setting of prednisone therapy raised concerns for potential increases in infection risk. Such concerns were not realized, and overall survival was acceptable compared to historical data. Nevertheless, only limited conclusions can be drawn given the sample size.
While the phase I component of this trial was not adequately powered to address efficacy, we note that therapy was associated with encouraging preliminary response rates at 36 days, compared to historical controls (approximately 40%), but general application remains speculative [35–37]. Only 1 patient had GVHD progression requiring an additional line of systemic immune-suppressive therapy beyond first-line treatment. While specific comparisons are limited in availability, responses were seen in both grades 2 and 3 acute GVHD across varied sites of organ involvement and in both acute and late-onset acute GVHD subtypes. As a surrogate of effective acute GVHD control, a major reduction in prednisone dose was achieved by a median of 3 months, and it was discontinued completely at a median of 4 months of therapy, although all evaluable patients continued on other forms of immunosuppression 1 year after enrollment.
Pharmacodynamic studies demonstrated that PANO at 5 mg PO TIW was able to hit histone target(s). IL12p40 levels were compromised, which has been implicated in interferon gamma induction and Th1 differentiation [38, 39].
This clinical trial established the MTD of PANO and demonstrated safety and toxicity profiles that fit well to expected HCT complications and PANO side effects. PANO MTD is currently being evaluated in a prospective phase II study for GVHD prevention in addition to tacrolimus and rapamycin for matched HCT (NCT02588339).
Acknowledgements
We thank Erika Elmer, Michele Burton, Jermaine Castro, and Carmen Patton for trial coordination/data acquisitions. We thank Sonya Smyk (Moffitt Cancer Center) for editorial assistance. Novartis Pharmaceuticals Corporation supported this trial and provided PANO.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129:22–29. 10.1182/blood-2016-08-686659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nassereddine S, Rafei H, Elbahesh E, Tabbara I. Acute graft versus host disease: a comprehensive review. Anticancer Res. 2017;37:1547–55. [DOI] [PubMed] [Google Scholar]
- 3.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–63. 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y-B, Perales M-A, Li S, Kempner M, Reynolds C, Brown J, et al. Phase I multicenter trial of brentuximab vedotin for steroid refractory acute graft-vs.-host disease (GVHD). Blood 2017. 10.1182/blood-2017-03-772210 [DOI] [PubMed] [Google Scholar]
- 5.Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Multicenter open-label phase 2 study of ibrutinib in chronic graft versus host disease (cgvhd) after failure of corticosteroids. Blood 2016;128:LBA–3. [Google Scholar]
- 6.Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multi-center survey. Leukemia. 2015;29:2062–8. 10.1038/leu.2015.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koreth J, Kim HT, Jones KT, Lange PB, Reynolds CG, Chammas MJ, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128:130–7. 10.1182/blood-2016-02-702852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler C, Miklos D, Kim HT, Treister N, Woo S-B, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–62. 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R, Chen B, Dreger P, Schmitt M, Schmitt A. Extracorporeal photopheresis for steroid-refractory chronic graft-versus-host disease after allogeneic hematopoietic stemcell transplantation: a systematic review and meta-analysis. Cancer Transl Med. 2015;1:201–8. 10.4103/2395-3977.172859. [DOI] [Google Scholar]
- 10.Alousi AM, Weisdorf DJ, Logan BR, Bolaños-Meade J, Carter S, DiFronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7. 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA. 2002;99:2995–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111:539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. [DOI] [PubMed] [Google Scholar]
- 14.Nencioni A, Beck J, Werth D, Grunebach F, Patrone F, Ballestrero A, et al. Histone deacetylase inhibitors affect dendritic cell differentiation and immunogenicity. Clin Cancer Res. 2007;13: 3933–41. 10.1158/1078-0432.ccr-06-2903. [DOI] [PubMed] [Google Scholar]
- 15.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. [DOI] [PubMed] [Google Scholar]
- 16.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–6. [DOI] [PubMed] [Google Scholar]
- 17.Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA. 2004; 101:3921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng C, Gries M, Ziegler J, Lokshin A, Mascagni P, Lentzsch S, et al. Reduction of graft-versus-host disease by histone deacetylase inhibitor suberonylanilide hydroxamic acid is associated with modulation of inflammatory cytokine milieu and involves inhibition of STAT1. Exp Hematol. 2006;34:776–87. [DOI] [PubMed] [Google Scholar]
- 19.Choi SW, Braun T, Chang L, Ferrara JLM, Pawarode A, Magenau JM, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SW, Gatza E, Hou G, Sun Y, Whitfield J, Song Y, et al. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood. 2015;125:815–9. 10.1182/blood-2014-10-605238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SW, Braun T, Henig I, Gatza E, Magenau J, Parkin B, et al. Vorinostat plus tacrolimus/methotrexate to prevent GVHD following myeloablative conditioning unrelated donor HCT. Blood 2017. 10.1182/blood-2017-06-790469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.San-Miguel JF, Hungria VTM, Yoon S-S, Beksac M, Dimopoulos MA, Elghandour A, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–206. 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 23.Richardson PG, Laubach JP, Lonial S, Moreau P, Yoon S-S, Hungria VTM, et al. Panobinostat: a novel pan-deacetylase inhibitor for the treatment of relapsed or relapsed and refractory multiple myeloma. Expert Rev Anticancer Ther. 2015;15:737–48. 10.1586/14737140.2015.1047770. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Iclozan C, Liu C, Xia C, Anasetti C, Yu X-Z. LBH589 enhances T-cell activation in vivo and accelerates graft-versus-host disease in mice. Biol Blood Marrow Transplant. 2012;18: 1182 10.1016/j.bbmt.2012.06.002.e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bug G, Burchert A, Wagner E-M, Kroeger N, Jedlickova Z, Gueller S. et al. Phase I/II study of the deacetylase inhibitor panobinostat as maintenance therapy after an allogeneic stem cell transplantation in patients with high-risk MDS or AML: the Panobest-Trial. Blood. 2015;126:4344. [Google Scholar]
- 26.Cornelissen JJ, van Norden Y, van Gelder M, Breems DA, Maertens J, Jongen-Lavrencic M. et al. Early post-transplant epigenetic therapy by panobinostat and decitabine followed by donor lymphocyte infusion (DLI): interim results of the HOVON-116 Phase I/II Feasibility Study in poor-risk AML recipients of allogeneic stem cell transplantation (alloHSCT). Blood. 2016;128:832. [Google Scholar]
- 27.Tawara I, Koyama M, Liu C, Toubai T, Thomas D, Evers R, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17:77–88. 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song W, Tai YT, Tian Z, Hideshima T, Chauhan D, Nanjappa P, et al. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia. 2011;25:161–8. http://www.nature.com/leu/journal/v25/n1/suppinfo/leu2010244s1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J. et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 30.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2005;11:945–56. 10.1016/j.bbmt.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 31.Martin P, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum F, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–72. [PubMed] [Google Scholar]
- 32.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeAngelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T. et al. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia. 2013;27:1628–36. 10.1038/leu.2013.38. [DOI] [PubMed] [Google Scholar]
- 34.Khot A, Dickinson M, Prince HM. Panobinostat in lymphoid and myeloid malignancies. Expert Opin Investig Drugs. 2013;22: 1211–23. 10.1517/13543784.2013.815165. [DOI] [PubMed] [Google Scholar]
- 35.Levine JE, Logan B, Wu J, Alousi AM, Ho V, Bolaños-Meade J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16:1693–9. 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94. [DOI] [PubMed] [Google Scholar]
- 37.Martin PJ, Bachier CR, Klingemann H-G, McCarthy PL, Szabolcs P, Uberti JP, et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a joint statement. Biol Blood Marrow Transplant. 2009;15:777–84. 10.1016/j.bbmt.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welniak LA, Blazar BR, Wiltrout RH, Anver MR, Murphy WJ. Role of interleukin-12 in acute graft-versus-host disease(1). Transplant Proc. 2001;33:1752–3. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z, Xie F, Zhang X, Yang J, Wang R, Yang R, et al. Update on the association between interleukin-12 p40 gene polymorphism and risk of psoriasis: a meta-analysis. Dermatol Sin. 2016; 34:126–30. 10.1016/j.dsi.2016.01.001. [DOI] [Google Scholar]


