Abstract
This study evaluated elderly patients with urothelial carcinoma of the bladder receiving neoadjuvant chemotherapy (NAC). Compared with a younger cohort of patients, NAC demonstrated equivalent toxicity and oncologic outcomes in our single-institution cohort. Although older patients had significantly poorer performance status and renal function, there were no differences in survival or response to NAC.
Introduction:
We conducted this study to determine if, in appropriately selected elderly patients receiving neoadjuvant chemotherapy (NAC), clinical outcomes including pathologic complete response/downstaging and overall survival were similar to a younger cohort.
Methods:
Chart review was performed on patients receiving NAC for urothelial carcinoma of the bladder (UCB) from 2004 to 2013. A total of 116 patients were identified that underwent NAC from 2004 to 2013 for ≥ cT2N0M0 UCB. Patients were excluded who received 2 cycles or less of chemotherapy (N = 18; 11 patients in the younger cohort, 7 in the elderly group; P = .74). Data was analyzed, and Kaplan-Meir analysis curves were used for survival and recurrence.
Results:
Forty-six elderly patients (age ≥ 70 years) (67% cisplatin-based regimen) were identified and compared with 70 (93% cisplatin-based regimen) younger patients. The estimated glomerular filtration rate, performance status, preoperative hemoglobin, and body mass index were significantly worse in elderly patients. Dose reduction and pathologic downstaging to non–muscle-invasive disease was not statistically different between older and younger patients Complete pathologic response in older patients (16%) and in the younger cohort (17%) were similar (P = .146). There was no significant difference in follow-up, recurrence, or in median overall survival between patient groups (28 months elderly vs. 35 months younger; P = .78). Age was not an independent predictor of pathologic downstaging, complete response, overall survival, or recurrence-free survival.
Conclusions:
NAC in elderly patients (≥ 70 years old) demonstrated equivalent toxicity and oncologic outcomes in our single-institution cohort. Although older patients had significantly poorer performance status and renal function, there were no differences in survival or response to NAC.
Keywords: Adjuvant chemotherapy, Cystectomy, Neoadjuvant therapy, Survival, Urinary bladder neoplasms
Introduction
As the population ages, the incidence of bladder cancer is expected to increase in coming years, and as a result, older patients will comprise a larger proportion of bladder cancer cases at diagnosis.1 Age is an important risk factor in the development of bladder cancer, after tobacco exposure.2 The median age of patients diagnosed with bladder cancer is 73 years, with a mean age of 66 years at the time of radical cystectomy (RC).3,4 Despite the increasing utilization of neoadjuvant chemotherapy (NAC) prior to RC for muscle-invasive bladder cancer (MIBC) across all populations, elderly patients may be excluded from this beneficial treatment on the basis of age alone.5,6 Age has been implicated as a negative prognostic predictor of survival in prior studies. These studies showed that elderly patients who undergo RC have poorer cancer-specific survival compared with younger patients. In addition, elderly patients are less likely to receive standard-of-care treatments and are prone to undertreatment.7–10 Despite medical comorbidities in some elderly patients, age alone should not be a deterrent toward elderly patients receiving NAC.11,12 The goal of this study was to assess pathologic response to NAC and overall survival in elderly patients compared with a younger cohort.
Materials and Methods
After obtaining institutional review board approval, electronic medical records of patients with MIBC who received NAC prior to undergoing RC from 2004 to 2013 were identified. All patients who received NAC and underwent RC and met the following criteria were included: urothelial carcinoma, muscle-invasive or greater disease (clinical T2a-T4N0 M0), and planned NAC with methotrexate, vinblastine, adriamycin, and cisplatin (MVAC) (dose dense included), gemcitabine + cisplatin (GC), or gemcitabine + carboplatin (GCarbo). Patients who received 2 cycles of chemotherapy or less were excluded from analysis (n = 18). There were 7 (13%) elderly patients excluded compared with 11 (15%) younger patients (P = .74) The 2 cohorts of patients were divided between patients < 70 years old and ≥70 years old. Variant histologies with urothelial components were included in the analysis.
All patients with MIBC were considered potential candidates for NAC. Many factors are considered when determining which patients receive NAC, including patient Eastern Cooperative Oncology Group (ECOG) performance status (PS),13 renal function (estimated glomular filtration rate [eGFR] > 60, calculated using Cockcroft-Gault Equation),14 Charlson Comorbidity Index (not age-adjusted),15 stage of disease, performance of complete visible transurethral resection of bladder tumor (TURBT) (as documented in operative report), and other clinical factors. Clinical response to NAC was determined by restaging preoperative computed tomography scans. Partial response was defined as radiographic decrease in tumor burden according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria.16 Pathologic downstaging was defined as less than pT2 final pathology. Pathologic complete response was defined as final pathology of pT0.17
For the preliminary analysis of age differences among clinical and demographic variables either the χ2 test or the Fisher exact test was used; for continuous variables, the Wilcoxon Rank Sum test was used. For the outcome analysis, significant variables at the 0.05 significance level were used to adjust for possible effects. Those variables were body mass index (BMI), ECOG PS, hemoglobin, and eGFR. We examined these variables for significant correlations with each other and found no significant correlations. Modeling approaches were then used, including logistic regression, ordinal regression, linear regression, and Cox proportional hazards regression. The variable, number of nodes removed, in the linear regression was checked for normality using the Anderson-Darling test and was adjusted with a square-root transformation prior to analysis. For all of these models, adjustments were included for the 4 variables mentioned previously. The Kaplan-Meier curves for overall survival and recurrence-free survival are also presented along with the unadjusted P-values for the log rank test. SAS and R software packages were used in the analyses. Multiple testing adjustments were not used in the analysis.
Results
Clinical and Demographic Characteristics
From 2004 to 2013, 116 patients were identified who underwent NAC (clinically node-negative and completed at least 3 cycles of chemotherapy) followed by RC. The overall median patient age was 68 years (interquartile range [IQR], 59–74 years). Forty-six patients were ≥ 70 years of age, compared with 708 patients in the younger cohort. Distribution by age is displayed in Figure 1. Of note, 5 patients in the elderly cohort were ≥ 80 years of age. Patient demographic and clinical parameters are shown in Tables 1 and 2. Gender, completeness of visible TURBT, pathologic grade, presence of lymphovascular invasion, presence of carcinoma in situ, clinical T stage at initial diagnosis, presence of hydronephrosis, and Charlson Comorbidity scores were not different between the older and younger cohorts. BMI and ECOG PS were different between the 2 cohorts (P = .04 and P ≤ .0001, respectively), with elderly patients having lower BMI and PS. eGFR prior to chemotherapy was significantly lower among elderly patients (60 vs. 74 mL/min/1.73 m2; P = .02). Finally, hemoglobin was lower in elderly patients (12.7 vs. 13.8; P < .0005).
Figure 1.
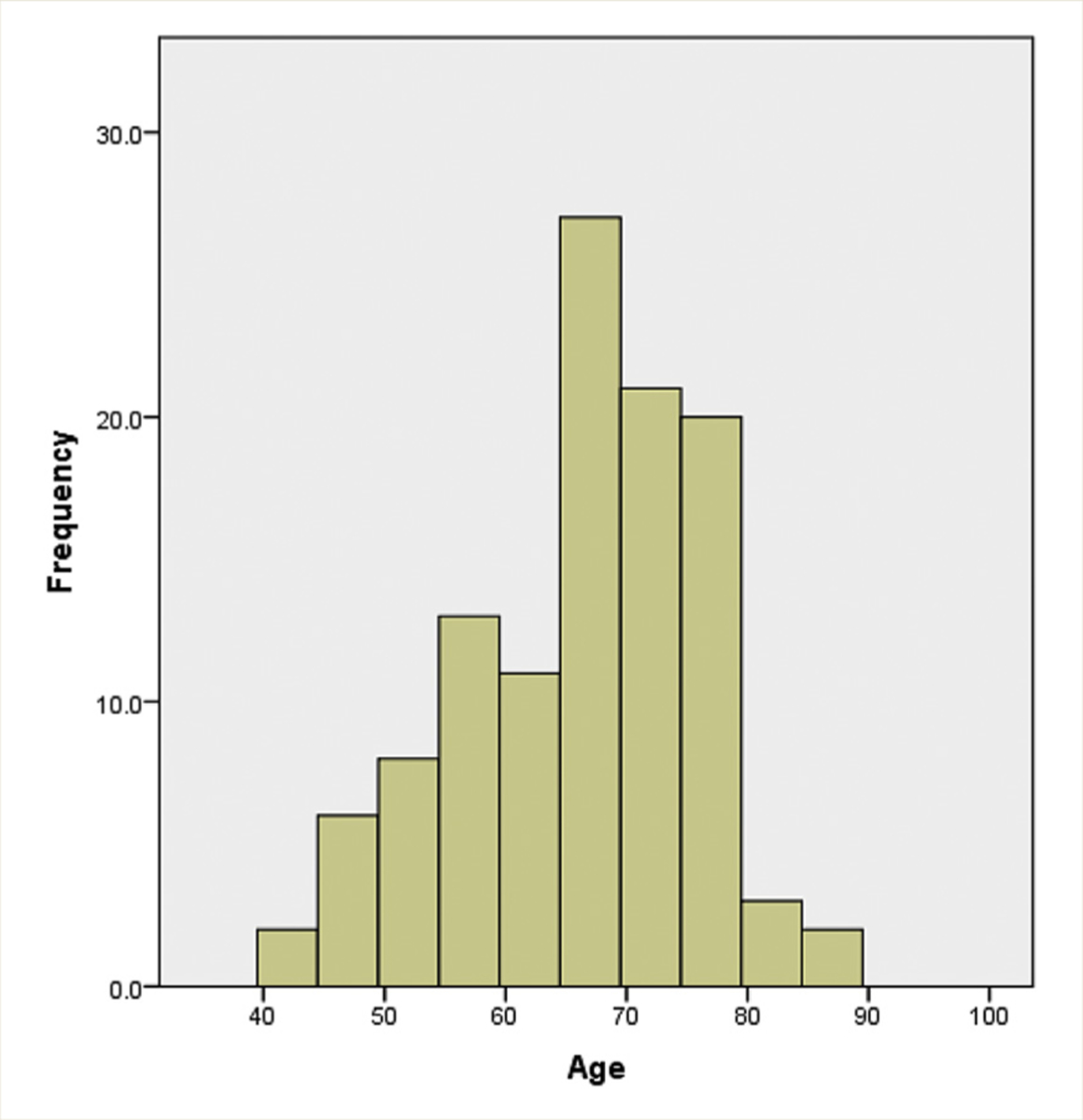
Age Distribution of the Neoadjuvant Chemotherapy Population
Table 1.
Demographic Data
| Variable | Levels | Age < 70 (n = 70) N (%) | Age ≥ 70 (n = 46) N (%) | All Ages (n = 116) N (%) | P Valuea |
|---|---|---|---|---|---|
| Median age (range) | Y | 61.5 (32.0, 69.0) | 75.0 (70.0, 85.0) | 68.0 (32.0, 85.0) | <.0001 |
| Gender | Male | 54 (77.1) | 32 (69.6) | 86 (74.1) | .3619 |
| Female | 16 (22.9) | 14 (30.4) | 30 (25.9) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Median BMI (range) | kg/m2 | 28.6 (19.8, 39.8) | 26.6 (18.2, 43.4) | 27.8 (18.2, 43.4) | .04 |
| Charlson comorbidity score | 2 | 32 (45.7) | 14 (30.4) | 46 (39.7) | .3713 |
| 3 | 23 (32.9) | 19 (41.3) | 42 (36.2) | ||
| 4 | 12 (17.1) | 9 (19.6) | 21 (18.1) | ||
| 5 | 3 (4.3) | 4 (8.7) | 7 (6.0) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| ECOG PSb | 0 | 53 (75.7) | 15 (32.6) | 68 (58.6) | <.0001 |
| 1 | 14 (20.0) | 26 (56.5) | 40 (34.5) | ||
| 2 | 3 (4.3) | 5 (10.9) | 8 (6.9) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) |
Abbreviations: BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; PS = performance status.
The χ2 test or Fisher exact test for categorical variables and the Wilcoxon Rank Sum test for continuous variables.
The test for ECOG PS used a dichotomized variable of 0 versus 1, 2.
Table 2.
Clinical Data
| Variable | Levels | Age < 70 (n = 70) N (%) | Age ≥ 70 (n = 46) N (%) | All Ages (n = 116) N (%) | P Valuea |
|---|---|---|---|---|---|
| Median hemoglobin (range) | (g/dL) | 13.8 (9.7, 113.0) | 12.7 (9.4, 15.5) | 13.2 (9.4, 113.0) | .0005 |
| Median eGFR, pre-chemotherapy (range) | mL/min/1.73 m2 | 74.3 (31.5, 114.5) | 60.2 (32.8, 119.4) | 72.0 (31.5, 119.4) | .0202 |
| Complete visible TURBT | No | 34 (48.6) | 26 (56.5) | 60 (51.7) | .4019 |
| Yes | 36 (51.4) | 20 (43.5) | 56 (48.3) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| T stage (TURBT) | 1 | 2 (2.9) | 0 (0.0) | 2 (1.7) | .1330 |
| 2 | 45 (64.3) | 22 (47.8) | 67 (57.8) | ||
| 3 | 18 (25.7) | 17 (37.0) | 35 (30.2) | ||
| 4 | 5 (7.1) | 7 (15.2) | 12 (10.3) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Clinical N stage | 0 | 70 (100.0) | 46 (100.0) | 116 (100.0) | - |
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Hydronephrosis | No | 38 (54.3) | 27 (58.7) | 65 (56.0) | .6397 |
| Yes | 32 (45.7) | 19 (41.3) | 51 (44.0) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Histology | Pure UC | 58 (82.9) | 40 (87.0) | 98 (84.5) | .6099 |
| Other | 12 (17.1) | 6 (13.0) | 18 (15.5) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| LVI on TURBT | No | 53 (75.7) | 36 (78.3) | 89 (76.7) | .7509 |
| Yes | 17 (24.3) | 10 (21.7) | 27 (23.3) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| CIS on TURBT | No | 56 (80.0) | 39 (84.8) | 95 (81.9) | .6250 |
| Yes | 14 (20.0) | 7 (15.2) | 21 (18.1) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Chemotherapy regimenb | MVAC | 5 (7.1) | 1 (2.2) | 6 (5.2) | .0299 |
| DD-MVAC | 2 (2.9) | 0 (0.0) | 2 (1.7) | ||
| Gem-cis | 58 (82.9) | 30 (65.2) | 88 (75.9) | ||
| Gem-carbo | 5 (7.1) | 15 (32.6) | 20 (17.2) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Median creatinine, pre-chemotherapy (range) | 1.00 (0.60, 2.20) | 1.10 (0.40, 2.20) | 1.00 (0.40, 2.20) | .1762 | |
| No. cycles chemotherapy | 3 | 39 (57.4) | 35 (76.1) | 74 (64.9) | .1302 |
| 4 | 25 (36.8) | 9 (19.6) | 34 (29.8) | ||
| >4 | 4 (5.9) | 2 (4.3) | 6 (5.3) | ||
| Total | 68 (59.6) | 46 (40.4) | 114 (100.0) | ||
| Change to carbo | No | 68 (97.1) | 44 (95.6) | 112 (96.6) | .6483 |
| Yes | 2 (2.9) | 2 (4.4) | 4 (3.4) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Dose reduction | No | 51 (82.3) | 32 (82.1) | 83 (82.2) | .9789 |
| Yes | 11 (17.7) | 7 (17.9) | 18 (17.8) | ||
| Total | 62 (61.4) | 39 (38.6) | 101 (100.0) |
Abbreviations: Carbo = carboplatin; CIS = carcinoma in situ; DD = dose-dense; eGFR = estimated glomular filtration rate; Gem-carbo = gemcitabine + carboplatin; Gem-cis = gemcitabine + cisplatin; LVI = lymphovascular invasion; MVAC = methotrexate, vinblastine, adriamycin, and cisplatin; TURBT = transurethral resection of bladder tumor.
The χ2 test or Fisher Exact test for categorical variables and Wilcoxon Rank Sum test for continuous variables.
The test for chemotherapy regimen used a dichotomized variable of Gem-cis versus other.
Chemotherapy
From 2004 to 2013 and in patients with muscle-invasive urothelial carcinoma, there was no difference in the overall utilization of NAC between elderly or younger patients (39% vs. 43%; P = .54; n = 627). However, as displayed in Table 2, chemotherapy regimens were different in the older and younger cohorts (P = .029). One (2%) elderly patient and 7 (10%) younger patients received MVAC regimens. The majority of patients in both cohorts received GC (83% vs. 65%; P = .01). A greater portion of elderly patients (32%) received GCarbo versus 7% of the younger cohort. There were no statistical differences in the number of cycles received between the 2 groups, with the majority of patients in both cohorts receiving 3 or more cycles (Table 3). A similar proportion of patients in each cohort received 2 cycles or less (15% elderly vs. 13% younger; P = .87), and these patients were excluded from analysis. There were 2 (4%) patients in the elderly group and 2 (3%) patients in the younger cohort that were changed from cisplatin to carboplatin secondary to toxicity, most commonly because of nephrotoxicity (P = .18). Overall toxicity during chemotherapy was not statistically different in the 2 groups including nephrotoxicity, bone marrow suppression, and thromboembolic events (Table 4). Dose reduction was necessary in 7 (18%) elderly patients and 11 (17%) younger patients and was secondary to myelosupression, pulmonary embolism, and nephrotoxicity (P = .62). Thrombocytopenia, neutropenia, and pancytopenia were not different between the 2 cohorts.
Table 3.
Pathologic Downstaging by Chemotherapy Regimen
| Chemotherapy Regimen |
< 70 Years N = 70 (%) | ≥ 70 Years N = 46 (%) | P Value |
|---|---|---|---|
| MVAC/Gem/Cis | 29 (41) | 14 (30) | .22 |
| Gem Carbo | 1 (1) | 5 (11) | .015 |
Abbreviations: Carbo = carboplatin; Cis = cisplatin; Gem = gemcitabine; MVAC = methotrexate, vinblastine, adriamycin, and cisplatin.
Table 4.
Chemotherapy-related Toxicity
| Parameter | Patients < 70 Years | Patients ≥ 70 Years | P Value |
|---|---|---|---|
| TTP/neutropenia/ pancytopenia |
16 | 12 | NS |
| Nephrotoxicity | 4 | 3 | NS |
| Sepsis | 1 | 0 | NS |
| Pulmonary embolism | 0 | 1 | NS |
Abbreviations: NS = not significant; TTP = thrombotic thrombocytopenic purpura.
Oncologic Outcomes and Survival
Pathologic complete response rates were similar in the elderly (2 [17%]) patients versus the younger (4 [17%]) patients (P = .14), despite higher rates of carboplatin-based regimens in the elderly cohort. Pathologic down-staging rates between the elderly patients (41%) versus the younger patients (44%) were not different (P = .524). When stratified by cisplatin-based chemotherapy regimen (Table 3), there was no difference in pathologic down-staging in the elderly cohort versus the younger patients (417% vs. 30%; P = .22). However, in the elderly population compared with the younger cohort, patients receiving carboplatin were more likely to have downstaging (11% vs. 1%; P = .015) (Table 3). Adjustment was performed using significantly different variables, BMI, ECOG performance score, hemoglobin, and eGFR for overall and recurrence-free survival, and there were no significant correlations with each other.
Median overall survival was similar between the 2 cohorts (unadjusted P = .223). Recurrence-free survival was not different (unadjusted P = .101) (Figures 2 and 3).
Figure 2.
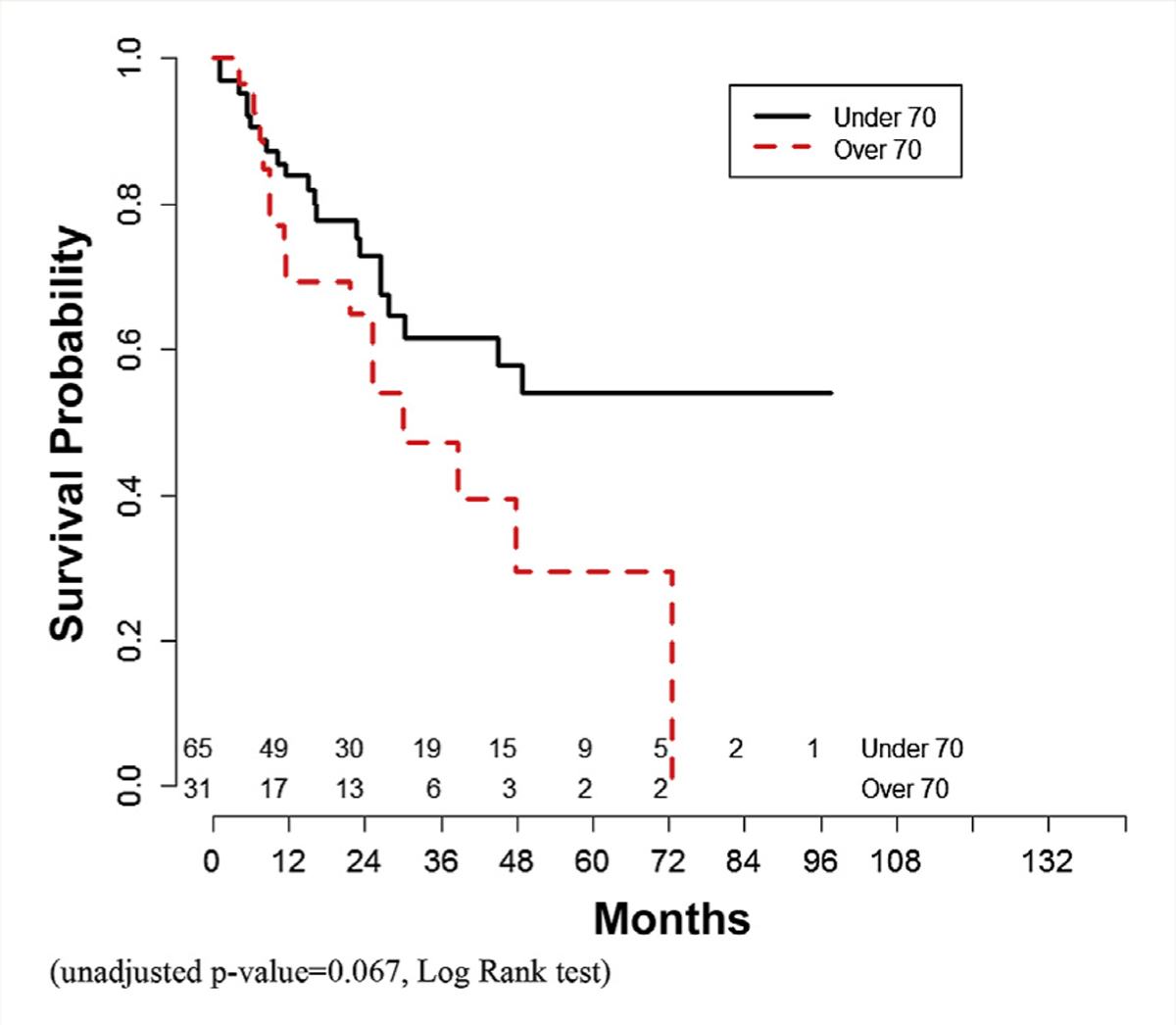
Overall Survival Curve (Unadjusted P = .067, Log Rank Test)
Figure 3.
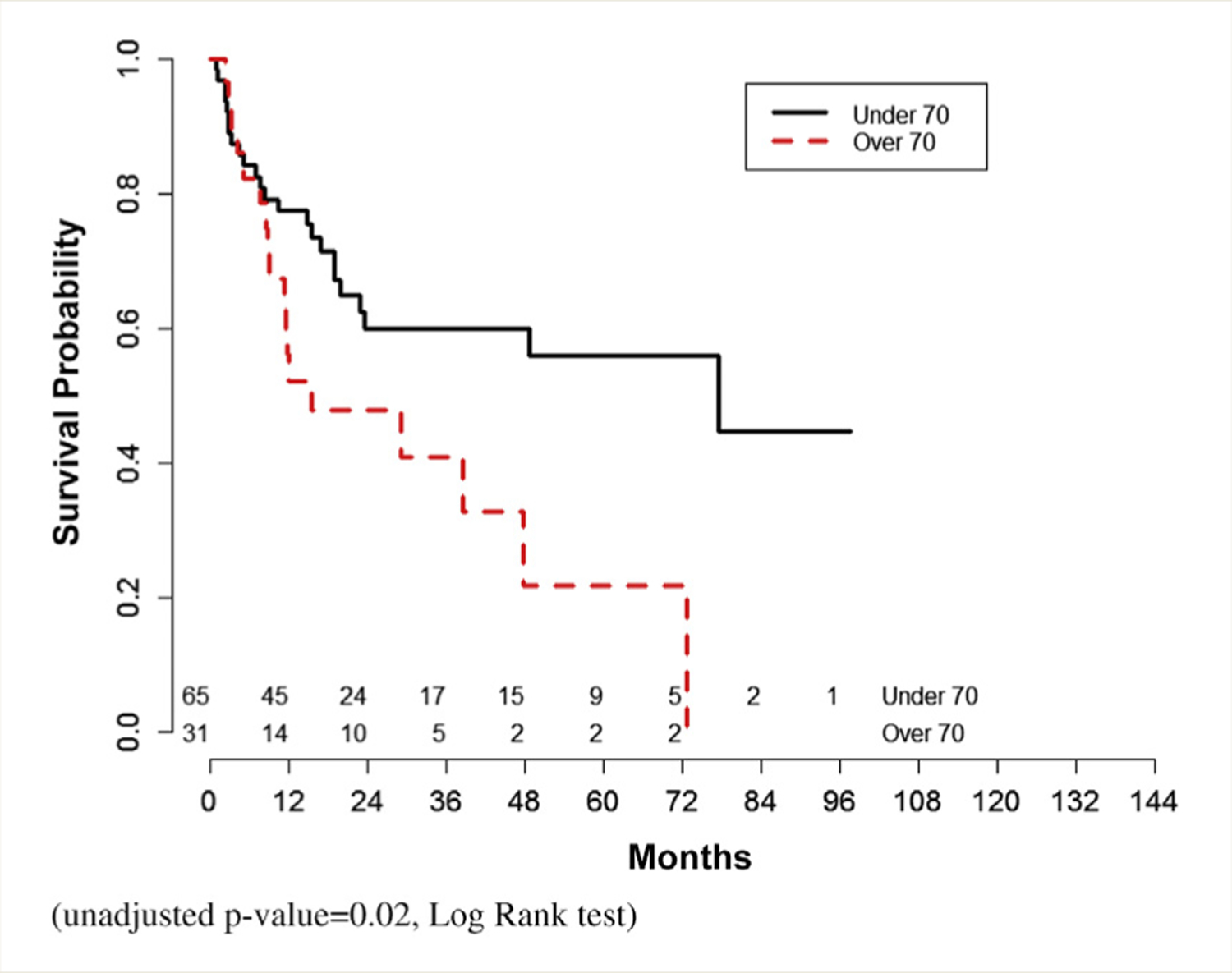
Recurrence-free Survival (Unadjusted P = .02, Log Rank Test)
Analysis was repeated excluding patients receiving carboplatin-based NAC (data not shown). There were 65 younger patients and 31 older patients available for analysis. There were no differences in complete pathologic response or downstaging between the 2 cohorts. Overall survival was also not significantly different (unadjusted P = .067). Recurrence free-survival was superior in younger patients (unadjusted P = .02).
Discussion
The current study shows that elderly patients who receive NAC have similar overall survival and pathologic response compared with younger patients. Utilization of NAC for MIBC has doubled from 2006 to 2010 based upon prior studies and carries an associated 30% pathologic complete response rate. NAC is essential in optimizing oncologic outcomes in patients with MIBC, given that one-half of all patients will develop metastatic disease.18 However, the identification of which patients will garner the most benefit from NAC remains uncertain. One of the major benefits on NAC with cisplatin-based treatment regimen is increased tolerability prior to surgery compared with postoperatively as renal function is optimized. This may be an especially important factor in optimizing treatment of MIBC in the elderly patient population with limited reserve. The 2 largest randomized trials demonstrating the benefit of NAC (European Organisation for Research and Treatment of Cancer Medical Research Council [EORTC-MRC] and Southwest Oncology Group [SWOG]) showed that age did not impact patient survival.5,19 Additionally, only 17% of the patients in the EORTC trial were > 70 years of age. SWOG stratified its patients as those ≤ 65 and those > 65 years of age, and a minority of the cohort was ≥ 65. Other retrospective studies showed poorer survival overall for all elderly patients (the majority without NAC) who underwent RC.7–9
As discussed by Chau et al, elderly patients may have been less likely to participate in these major randomized trials, and real world experience may not accurately reflect the younger studied patient population.20 A meta-analysis of the NAC trials in bladder cancer suggests that less than 50% of the patients included were older than 65 years.21 However, data from other cancers, including advanced colon cancer, suggest that, whereas elderly patients are less likely to receive chemotherapy, they may be as likely to reap the benefits of chemotherapy.22
Guancial et al discuss how elderly patients with bladder cancer are medically vulnerable and complex. Risk factors that predict chemotherapy toxicity are especially common in elderly patients, including poor renal function, hearing impairment, recent fall, limited ambulation, and decreased social and emotional health. Additionally, elderly patients are more likely to have poorer cognition, nutritional status, and social support structure. These authors recommend widespread implementation of Geriatric Assessment tools and multidisciplinary involvement in geriatricians to aid in stratifying elderly patients.23
Chronic kidney disease represents a major limitation of successful application of cisplatin-based NAC in elderly patient population. Inherent with patient aging is a decline in renal function. According to the National Comprehensive Cancer Network guidelines, there is no alternative regimen in patients who are unfit for cisplatin-based NAC.24 In the current study, the majority of elderly patients had renal function that may preclude the administration of cisplatin-based NAC and were thus administered carboplatin. Controversially, carboplatin has been substituted for cisplatin in the neoadjuvant setting, yet quality evidence to substantiate this paradigm is lacking.25 Extrapolating from data obtained in patients with metastatic bladder cancer has shown carboplatin to be inferior compared with cisplatin-based regimens.26,27
A small series of patients by Mertens et al received carboplatin-based regimens and experienced a pathologic complete response rate of 30.4%.25 Another retrospective trial compared neoadjuvant GCarbo with MVAC and found no difference in ≤ pT1 rates.28 Regimens including carboplatin were found to be inferior on multivariate analysis compared with MVAC or GC regimens in 1 large multicenter retrospective trial.29 In this study, the elderly cohort experienced similar rates of pathologic downstaging despite higher utilization of GCarbo regimens (Table 5). This is likely a reflection of a small sample size in the younger cohort and is of uncertain clinical significance. Upon excluding patients who received carboplatin, we did note an advantage in recurrence-free survival in younger patients.
Table 5.
Outcomes Data
| Variable | Levels | Age < 70 (n = 70) N (%) | Age ≥70 (n = 46) N (%) | All Ages (n = 116) N (%) | P Valuea |
|---|---|---|---|---|---|
| Clinical response | Partial response | 27 (38.6) | 18 (39.1) | 45 (38.8) | .3568 |
| Stable/progression | 43 (61.4) | 28 (60.9) | 71 (61.2) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| T stage (path) | T0, Ta, Tis, T1 | 31 (44.3) | 19 (41.3) | 50 (43.1) | .6917 |
| T2 | 11 (15.7) | 8 (17.4) | 19 (16.4) | ||
| T3 | 17 (24.3) | 9 (19.6) | 26 (22.4) | ||
| T4 | 11 (15.7) | 10 (21.7) | 21 (18.1) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Clavien complicationb | 0 | 34 (48.6) | 18 (39.1) | 52 (44.8) | - |
| 1 | 10 (14.3) | 4 (8.7) | 14 (12.1) | ||
| 2 | 16 (22.9) | 15 (32.6) | 31 (26.7) | ||
| 3 | 5 (7.1) | 2 (4.3) | 7 (6.0) | ||
| 4 | 2 (2.9) | 3 (6.5) | 5 (4.3) | ||
| 5 | 1 (1.4) | 3 (6.5) | 4 (3.4) | ||
| 6 | 0 (0.0) | 1 (2.2) | 1 (0.9) | ||
| 7 | 2 (2.9) | 0 (0.0) | 2 (1.7) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Clavien complication | (<3) | 60 (85.7) | 37 (80.4) | 97 (83.6) | .3490 |
| (≥3) | 10 (14.3) | 9 (19.6) | 19 (16.4) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| P N Stage | 0 | 53 (75.7) | 34 (73.9) | 87 (75.0) | .5446 |
| 1 | 7 (10.0) | 2 (4.3) | 9 (7.8) | ||
| 2 | 7 (10.0) | 6 (13.0) | 13 (11.2) | ||
| 3 | 3 (4.3) | 2 (4.3) | 5 (4.3) | ||
| Unknown | 0 (0.0) | 2 (4.3) | 2 (1.7) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Median no. nodes removed (range) | 16.5 (2.0, 46.0) | 15.5 (0, 39.0) | 16.0 (0, 46.0) | .1748 | |
| Complete path response (pT0) | No | 19 (27.1) | 10 (21.7) | 29 (25.0) | .1462 |
| Yes | 4 (17.4) | 2 (16.7) | 6 (17.1) | ||
| Total | 23 (65.7) | 12 (34.3) | 35 (100.0) | ||
| Downstage (<T2) | No | 39 (55.7) | 27 (58.7) | 66 (56.9) | .5240 |
| Yes | 31 (44.3) | 19 (41.3) | 50 (43.1) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Recurrence site | Distant | 12 (17.1) | 6 (13.0) | 18 (15.5) | .8303 |
| Other | 58 (82.9) | 40 (87.0) | 98 (84.5) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Recurrence site | Local | 2 (2.9) | 3 (6.5) | 5 (4.3) | .5497 |
| Other | 68 (97.1) | 43 (93.5) | 111 (95.7) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) | ||
| Salvage chemotherapy | No | 54 (77.1) | 41 (89.1) | 95 (81.9) | .0727 |
| Yes | 16 (22.9) | 5 (10.9) | 21 (18.1) | ||
| Total | 70 (60.3) | 46 (39.7) | 116 (100.0) |
Overall survival hazard ratio for age (95% confidence interval) (ref = age < 70 y) HR = 0.648 (0.311, 1.347); P = .2450.
Recurrence-free survival hazard ratio for age (95% confidence interval) (ref = age < 70 y) HR = 0.660 (0.333, 1.307); P = .2331.
Logistic, linear, ordinal and Cox proportional hazards regressions were used with adjustments for ECOG performance status, BMI, number of cycles and hemoglobin.
Clavien Complication outcome did not meet the Proportional Odds assumption for Ordinal regression, were tested a dichotomized variable.
In our study, we controlled for extent of TURBT. Wright et al emphasized the importance of complete endoscopic resection as it correlates to pT0 status in patients receiving NAC prior to RC.30 The prior study investigating the role of age on NAC and BC did not control for complete visible endoscopic resection. In our study, both the elderly and younger patients underwent similar rates of complete visible TURBT (46% younger vs. 45% elderly). Also, compared with Chau et al, we included alternative regimens including GCarbo. Finally, we had a small group of octogenarians that the previous study by Chau did not include.
In this study, there was no difference in overall survival between elderly patients and younger patients. Prior studies suggested poorer overall survival for elderly patients, and this is likely related to the underutilization of NAC. Applying standard-of-care NAC to all eligible patients, including the elderly, may optimize patient survival.
There are several limitations to our study, primarily related to its retrospective nature. First, we only included patients who underwent RC, and therefore, some patients with progression on NAC would be excluded. The indication for NAC use was not uniformly standardized for all of the patients in this study. Finally, the definition of elderly is subjective. According to the World Health Organization (WHO), elderly is defined as ≥ 65 years of age.31 We chose 70 years and older as the definition of elderly, similar to previously published studies.
Conclusions
Elderly patients receiving NAC had similar pathologic complete response, downstaging, and overall survival compared with younger patients, despite more GCarbo usage. The elderly patients had poorer PS and more chronic kidney disease. Future studies are needed to better define which patients, including well-selected elderly patients, would receive benefit from NAC. Although elderly patients received increased rates of carboplatin, there may be a subpopulation of patients who respond optimally to this regimen.
Clinical Practice Points.
Elderly patients with urothelial carcinoma of the bladder are less likely to receive standard-of-care therapy including NAC. The reason behind undertreatment is likely related to clinician concern of tolerability of chemotherapy and delay to definitive surgical treatment.
In this study, we sought to establish that in selected elderly patients with urothelial carcinoma of the bladder, NAC offers equivalent outcomes and survival benefit compared with younger patients.
Previous studies suggest that age is not an independent prognostic factor in determining which patients have pathologic downstaging.
Based upon our conclusion, clinicians should be willing in appropriately selected elderly patients to offer and deliver NAC.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74:2913–21. [DOI] [PubMed] [Google Scholar]
- 2.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013; 63:234–41. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 4.Smith AB, Deal AM, Woods ME, et al. Muscle-invasive bladder cancer: evaluating treatment and survival in the National Cancer Data Base. BJU Int 2014; 114:719–26. [DOI] [PubMed] [Google Scholar]
- 5.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349:859–66. [DOI] [PubMed] [Google Scholar]
- 6.Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 2014; 83:75–80. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen ME, Shariat SF, Karakiewicz PI, et al. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol 2007; 51:699–706, discussion: 706–8. [DOI] [PubMed] [Google Scholar]
- 8.Chamie K, Hu B, Devere White RW, Ellison LM. Cystectomy in the elderly: does the survival benefit in younger patients translate to the octogenarians? BJU Int 2008; 102:284–90. [DOI] [PubMed] [Google Scholar]
- 9.Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JW. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer 2013; 108:1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messing EM, Madeb R, Feng C, et al. Grade and stage at presentation do not predict mortality in patients with bladder cancer who survive their disease. J Clin Oncol 2009; 27:2443–9. [DOI] [PubMed] [Google Scholar]
- 11.Krabbe LM, Westerman ME, Margulis V, et al. Changing trends in utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer. Can J Urol 2015; 22:7865–75. [PubMed] [Google Scholar]
- 12.Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol 2015; 67:165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conill C, Verger E, Salamero M. Performance status assessment in cancer patients. Cancer 1990; 65:1864–6. [DOI] [PubMed] [Google Scholar]
- 14.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 17.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol 2014; 65:350–7. [DOI] [PubMed] [Google Scholar]
- 18.Ghoneim MA, Abdel-Latif M, el-Mekresh M, et al. Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J Urol 2008; 180: 121–7. [DOI] [PubMed] [Google Scholar]
- 19.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet 1999; 354:533–40. [PubMed] [Google Scholar]
- 20.Chau C, Wheater M, Geldart T, Crabb SJ. Clinical outcomes following neoadjuvant cisplatin-based chemotherapy for bladder cancer in elderly compared with younger patients. Eur J Cancer Care (Engl) 2015; 24:155–62. [DOI] [PubMed] [Google Scholar]
- 21.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005; 48:202–5, discussion: 205–6. [DOI] [PubMed] [Google Scholar]
- 22.Khattak MA, Townsend AR, Beeke C, et al. Impact of age on choice of chemotherapy and outcome in advanced colorectal cancer. Eur J Cancer 2012; 48:1293–8. [DOI] [PubMed] [Google Scholar]
- 23.Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging 2015; 10:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw 2013; 11:446–75. [DOI] [PubMed] [Google Scholar]
- 25.Mertens LS, Meijer RP, Kerst JM, et al. Carboplatin-based induction chemotherapy for nonorgan confined bladder cancerea reasonable alternative for cisplatin unfit patients? J Urol 2012; 188:1108–13. [DOI] [PubMed] [Google Scholar]
- 26.Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 1997; 80:1966–72. [DOI] [PubMed] [Google Scholar]
- 27.Witjes JA, Comperat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014; 65: 778–92. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki K, Obara W, Kato Y, Takata R, Tanji S, Fujioka T. Neoadjuvant gemcitabine plus carboplatin for locally advanced bladder cancer. Jpn J Clin Oncol 2013; 43:193–9. [DOI] [PubMed] [Google Scholar]
- 29.Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015; 67:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James AC, Lee FC, Izard JP, et al. Role of maximal endoscopic resection before cystectomy for invasive urothelial bladder cancer. Clin Genitourin Cancer 2014; 12: 287–91. [DOI] [PubMed] [Google Scholar]
- 31.Sabharwal S, Wilson H, Reilly P, Gupte CM. Heterogeneity of the definition of elderly age in current orthopaedic research. Springerplus 2015; 4:516. [DOI] [PMC free article] [PubMed] [Google Scholar]


