Abstract
Objectives:
Regional therapy for metastatic melanoma to the liver represents an alternative to systemic therapy. Hepatic progression-free survival (HPFS), progression-free survival (PFS), and overall survival (OS) were evaluated.
Materials and Methods:
A retrospective review of patients with liver metastases from cutaneous or uveal melanoma treated with yttrium-90 (Y90), chemoembolization (CE), or percutaneous hepatic perfusion (PHP) was conducted.
Results:
Thirty patients (6 Y90, 10 PHP, 12 CE, 1 PHP then Y90, 1 CE then PHP) were included. Multivariate analysis showed improved HPFS for PHP versus Y90 (P = 0.004), PHP versus CE (P = 0.02) but not for CE versus Y90. PFS was also significantly different: Y90 (54 d), CE (52 d), PHP (245 d), P = 0.03. PHP treatment and lower tumor burden were significant predictors of prolonged PFS on multivariate analysis. Median OS from time of treatment was longest, but not significant, for PHP at 608 days versus Y90 (295 d) and CE (265 d), P = 0.24. Only PHP treatment versus Y90 and lower tumor burden had improved OS on multivariate analysis (P = 0.03, 0.03, respectively).
Conclusions:
HPFS and PFS were significantly prolonged in patients treated with PHP versus CE or Y90. Median OS in PHP patients was over double that seen in Y90 or CE patients but was significant only between PHP and Y90.
Keywords: regional therapy, uveal, melanoma, survival
In 2015, an estimated 74,000 new cases of melanoma were diagnosed and ~9900 people died of the disease in the United States.1 Uveal melanoma represents about 3% of all melanoma cases (or about 2000 cases per year), and is the most common primary intraocular malignancy in adults.2 Uveal melanoma is an aggressive form of melanoma that is associated with a higher rate of hepatic metastases compared with cutaneous melanoma. Patients with uveal melanoma who develop metastases will have liver involvement in 50% to 80% of the cases, whereas only 20% of metastases from primary cutaneous melanoma involve the liver.3,4 Isolated hepatic metastasis occurs in ~50% of patients with uveal melanoma.3 Unfortunately, metastases from uveal melanoma are often refractory to systemic chemotherapy, with a reported median survival of 4 to 5 months and a 1-year survival of 10 to 15%.3,5,6 Patients with metastatic cutaneous melanoma also have a poor long-term survival of only 10% and 5-year survival for patients with unresectable hepatic metastases has been reported to be as low as 0.7
Surgical resection provides a chance for improved survival for a patient with isolated liver metastasis. When a R0 liver resection, defined as removal of all grossly macroscopic disease, can be achieved in patients with uveal melanoma, the median survival is markedly improved and has been reported to be as long as 27 months.8 In other studies that combine patients with liver metastases from cutaneous and uveal melanoma, the median survival after surgical resection has been reported as high as 39 months.9 The number of patients eligible for attempted curative resection, however, is only 2% to 13%.9,10 The emergence of immunotherapy regimens may be effective in reducing the burden of disease for some patients; however, not all patients will be candidates for these drugs. In addition, there is evidence that systemic immunotherapies are not very effective in treating metastatic ocular melanoma to the liver.11 Regional therapies, which include radioembolization, open isolated hepatic perfusion, percutaneous hepatic perfusion (PHP), and hepatic chemoembolization (CE), are all potential alternatives to systemic therapy for patients with isolated liver metastases from uveal or cutaneous melanoma that are not surgically resectable. Regional therapy allows for higher doses of chemotherapy or radiation to be delivered directly to the tumor while minimizing the risk of systemic toxicity.9,12–14
No study exists comparing the impact of the different individual methods of regional therapy on survival outcomes in patients with liver metastases from melanoma. We performed a retrospective study to evaluate the outcomes of hepatic progression-free survival (HPFS), progression-free survival (PFS), and overall survival (OS) in highly selected patients with hepatic recurrence treated with Y90, PHP, or CE.
MATERIALS AND METHODS
After obtaining Institutional Review Board approval, a single institution retrospective review of all patients treated with Y90, PHP, or CE for unresectable hepatic metastases secondary to uveal or cutaneous melanoma from 2008 to 2014 was conducted. Patients were identified for inclusion in the study by reviewing personal physician and departmental case-log databases. Demographic and clinical variables (age at diagnosis, sex, Eastern Cooperative Oncology Group status, presence of extrahepatic disease, tumor burden, and complications along with adjuvant treatments and outcomes data were retrieved from existing databases and electronic medical records. A diagnosis of melanoma was based upon pathologic review and confirmed by a board-certified dermatopathologist. All patients underwent computed tomography (CT) scans of the abdomen before undergoing treatment, during the treatment course, and as part of surveillance. The length of time between CT scans was at the discretion of the treating physician. The length of time between CT scans was at the discretion of the treating physician; and on average imaging was performed at 12 week intervals for all patients undergoing surveillance regardless of response to treatment. All images were reviewed by a single, board-certified radiologist to assess tumor burden and response to therapy or progression of disease based on Response Evaluation Criteria in Solid Tumors. Tumor burden was defined as 0% to 25%, 25% to 50%, 50% to 75%, or >75% to allow for comparison among groups given this small cohort of patients.
All patients above 18 years of age who presented for treatment of cutaneous or uveal melanoma with metastatic disease to the liver regardless of age or status of their primary tumor and who underwent regional therapy with PHP, Y90, or CE were included in the study. Patients who had stable extrahepatic disease, defined as no evidence of progression on imaging studies, or prior surgical, regional, or systemic therapy for their disease were also included in the study.
Treatment
The vast majority of cases were discussed in a multidisciplinary tumor board to determine if patients were suitable for systemic therapy, clinical trials, regional therapy, or surgical resection based on the standard practice of our cutaneous oncology department. Some patients were direct referrals to interventional radiology for specific therapy, however, in all instances these patients were referred to cutaneous oncology for discussion of treatment options before undergoing any regional therapy.
Y90 radioembolization of hepatic tumors has been proven to be a safe method of radiation delivery which minimizes the risk of systemic toxicity.15 The preferential blood supply to liver metastases is by the hepatic artery, which allows high irradiation doses to be delivered directly to the tumor and spares the normal liver parenchyma which is supplied through the portal vein. In this study all Y90 procedures were performed utilizing glass microspheres (TheraSphere; BTG International, London, UK). Patients underwent either selective or lobar liver treatment based upon volume and distribution of disease.
PHP was performed under general anesthesia by both an interventional radiologist and a surgical oncologist. This procedure has been described in detail in the literature.7 A double-balloon hepatic isolation and aspiration catheter (Delcath Systems Inc., New York, NY) was placed in the inferior vena cava to isolate hepatic venous blood, which was then filtered extracorporeally using a veno-veno bypass system before returning to systemic circulation. After ensuring there was an adequate seal without leakage of contrast, chemotherapy was then administered through the arterial catheter. Melphalan, a nonspecific alkylating agent with high first pass metabolism and high hepatic clearance rate, was infused through an arterial catheter in all cases. Intra-arterial perfusion was performed for 30 minutes and an additional 30 minutes of bypass was performed to allow for washout of any residual drug. Hemodynamic monitoring was performed throughout the case.7 PHP is not currently approved by the FDA for use in the United States. The patients included in our analysis were either on a clinical phase III trial (NCT00324727), an extended access protocol (NCT01728051) or compassionate use protocol.
CE was performed by an interventional radiologist under conscious sedation by accessing the right common femoral artery. The hepatic artery was selectively catheterized and confirmed by digital subtraction arteriography. A mixture of doxorubicin, mitomycin C, and cisplatin emulsified with ethoidized oil (Lipiodol, Guerbet LLC, Bloomington, IN) was instilled in the lobe with the greatest volume of disease. Embolic particles were then added to the emulsification to create further stasis (Embosphere microspheres, Merit Medical, South Jordan, UT). The microcatheter was removed once a repeat arteriography showed stagnation of flow within the terminal branches of the hepatic artery.
Statistical Analysis
Demographic data and clinical variables were collected and analyzed using the Fisher exact test to compare their distributions between treatment groups. The Kaplan-Meier survival estimates (KM), log-rank test, and multivariate Cox regression analysis (MVA) with time-dependent covariate were used to relate patient, tumor and treatment variables to HPFS, PFS, and OS. If a patient received >1 type of liver therapy, he or she was excluded from KM survival analysis but was included in MVA by dealing with types of liver therapies as a time-dependent covariate while adjusting for variables significantly associated with each survival of interest in univariate Cox regression analysis. HPFS and PFS were calculated at the time from first regional treatment until the first date of documented progression in the liver (HPFS) or overall progression (PFS). Overall PFS was defined as progression of disease at any site in the body, not limited to liver (ie, brain, liver, lung, nodal). OS was calculated from the date of first treatment until date of death or date of last follow-up. The patient records, tumor registry records, and the social security death index database were used to determine date of death. Statistical significance was determined by a 2-sided P-value of ≤0.05. All analyses were done in R (a language and environment for statistical computing, version 3.1.0).
RESULTS
Patient Characteristics
A total of 30 patients were included in the study (16 uveal, 13 cutaneous, 1 unknown primary melanoma). Treatments included 6 Y90, 10 PHP, 12 CE, 1 patient received Y90 after progressing post-PHP and another received PHP after progressing post-CE. Y90 was administered as either a selective (n = 1) or lobar (n = 5) treatment. In the cohort of patients receiving PHP, the median number of treatments received was 3 (range, 1 to 6). Patients in the CE cohort received only 1 treatment. There were no differences in sex, age, performance status, extrahepatic disease, tumor burden, adjuvant therapy use, prior hepatic treatment, or posttreatment complications between the groups (Table 1). Complications in our study were mostly Grade I and II as defined by the Clavien-Dindo Classification of surgical complications. Most of the complications recorded were anorexia, abdominal pain, fatigue and nausea, or emesis. Laboratory abnormalities such as thrombocytopenia and derangement in liver function tests immediately after the procedure were seen in some patients with the abnormalities coming back to baseline within a few days after treatment. There was a difference in the location of the primary tumor, with a majority of patients undergoing Y90 having uveal melanoma, whereas cutaneous melanoma was more common in the other treatment groups (Fisher’s exact test; P = 0.002) (Table 1).
TABLE 1.
Patient Demographics and Tumor Characteristics by Treatment Received
| Variables | n (%) | P | ||||
|---|---|---|---|---|---|---|
| Y90 (N = 6) | PHP (N = 10) | CE (N = 12) | PHP Then Y90 (N = 1) | CE Then PHP (N = 1) | ||
| Sex | ||||||
| Male | 4 (67) | 4 (40) | 8 (67) | 0 (0) | 0 (0) | 0.38 |
| Female | 2 (33) | 6 (60) | 4 (33) | 1 (100) | 1 (100) | |
| Age | ||||||
| 30–60 | 2 (33) | 4 (40) | 5 (42) | 1 (100) | 1 (100) | 0.71 |
| 60–90 | 4 (67) | 6 (60) | 7 (58) | 0 (0) | 0 (0) | |
| Location | ||||||
| Uveal | 5 (83) | 3 (30) | 3 (25) | 1 (100) | 0 (0) | 0.002* |
| Cutaneous | 1 (17) | 7 (70) | 9 (75) | 0 (0) | 0 (0) | |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |
| Eastern Cooperative Oncology Group | ||||||
| 0 | 1 (17) | 9 (90) | 3 (25) | 1 (100) | 1 (100) | 0.12 |
| 1 | 3 (50) | 1 (10) | 5 (42) | 0 (0) | 0 (0) | |
| 2 | 2 (33) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | |
| Missing | 0 (0) | 0 (0) | 3 (25) | 0 (0) | 0 (0) | |
| Extrahepatic disease | ||||||
| No | 5 (83) | 6 (60) | 3 (25) | 1 (100) | 0 (0) | 0.06 |
| Yes | 1 (17) | 4 (40) | 9 (75) | 0 (0) | 0 (0) | |
| Tumor burden | ||||||
| 0%−25% | 2 (33) | 6 (60) | 6 (50) | 0 (0) | 1 (100) | 0.63 |
| 25%−50% | 1 (17) | 3 (30) | 1 (8) | 1 (100) | 0 (0) | |
| 50%−75% | 1 (17) | 1 (10) | 3 (25) | 0 (0) | 0 (0) | |
| >75% | 2 (33) | 0 (0) | 2 (17) | 0 (0) | 0 (0) | |
| Adjuvant therapy | ||||||
| No | 1 (17) | 2 (20) | 5 (42) | 0 (0) | 0 (0) | 0.73 |
| Yes | 5 (83) | 8 (80) | 7 (58) | 1 (100) | 1 (100) | |
| Prior hepatic therapy | ||||||
| No | 5 (83) | 10 (100) | 11 (92) | 1 (100) | 0 (0) | 0.71 |
| Yes | 1 (17) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | |
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |
| Complication | ||||||
| No | 0 (0) | 3 (30) | 2 (17) | 0 (0) | 0 (0) | 0.53 |
| Yes | 6 (100) | 6 (60) | 10 (83) | 1 (100) | 1 (100) | |
| Missing | 0 (0) | 1 (10) | 0 (0) | 0(0) | 0 (0) | |
Statistical significance.
Hepatic Progression Free Survival, Progression Free Survival and Overall Survival
There was a significant difference in median HPFS: Y90 54 days, CE 80 days and PHP 361 days; Log-rank test, P = 0.001 (Fig. 1). MVA showed significantly improved HPFS for PHP versus Y90 (hazard ratio [HR], 0.11, 95% confidence interval [CI], 0.03, 0.49; P = 0.004), PHP versus CE (HR, 0.31, 95% CI, 0.12, 0.81; P = 0.02) but not for CE versus Y90 (HR, 0.36, 95% CI, 0.089, 1.51; P = 0.17). Tumor burden was a significant factor in HPFS. Patients with a tumor burden of 50% to 75% had a significantly worse HPFS (HR, 5.49, 95% CI, 1.24, 24.30; P = 0.03) when compared with patients with a tumor burden of 0% to 25%. Patients with a tumor burden of 25% to 50%, or >75% did not have a significantly different HPFS compared with patients with a tumor burden of 0% to 25% (Table 2).
FIGURE 1.
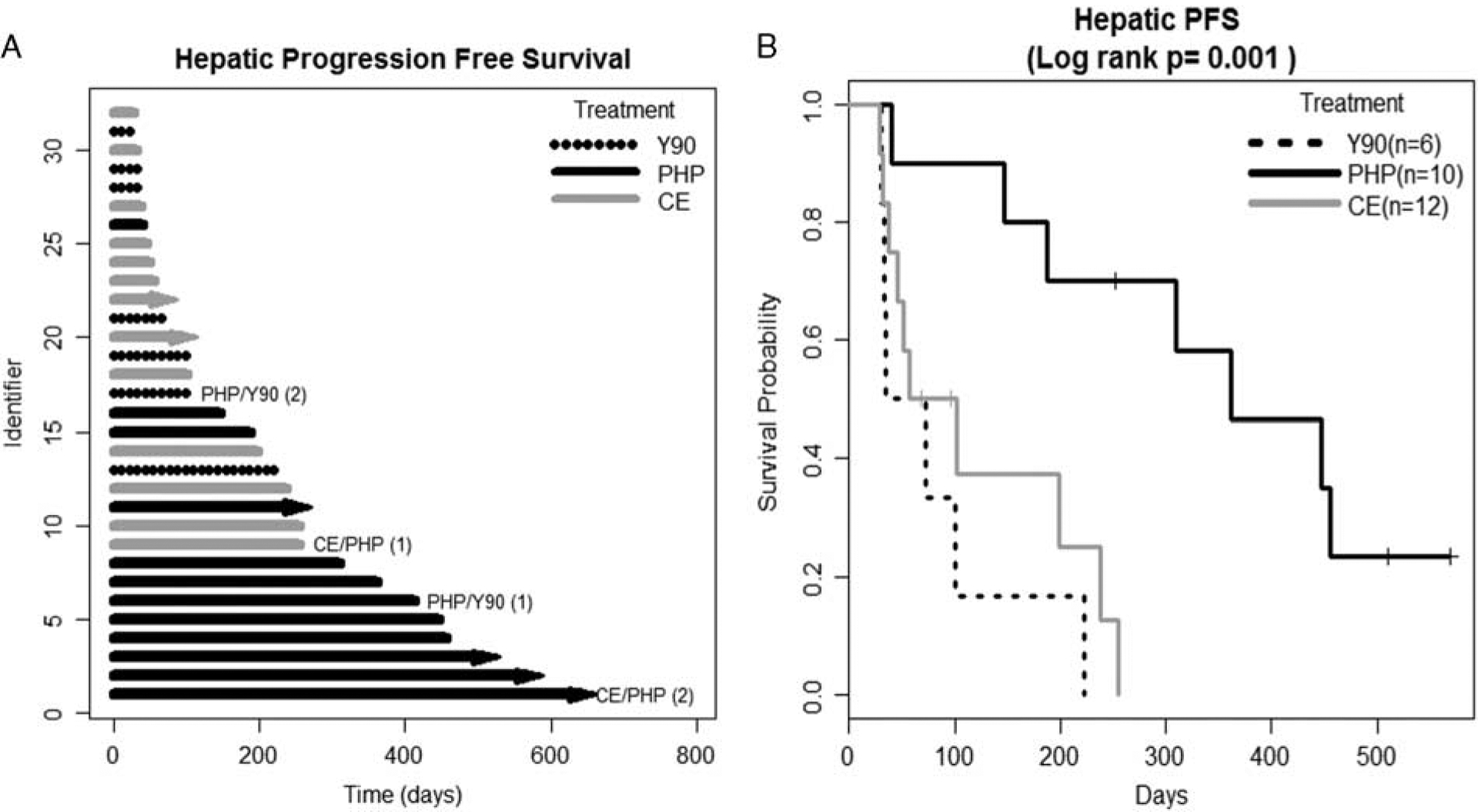
Hepatic progression free survival (HPFS). A, Swimmer’s plot. Lines with arrows represent ongoing HPFS, lines without arrows indicate that patient was censored due to progression, lost to follow-up or death. B, The Kaplan-Meier survival curve. Patients undergoing 2 forms of liver-directed therapy were removed from the Kaplan-Meier survival analysis. CE indicates chemoembolization; PHP, percutaneous hepatic perfusion; Y90, yttrium-90.
TABLE 2.
Multivariate Analysis of Factors Associated With Hepatic Progression-free Survival, Progression-free Survival, and Overall Survival
| Hepatic Progression-free Survival | Progression-free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| PHP vs. Y90 | 0.11 (0.03–0.49) | 0.004* | 0.17 (0.04–0.63) | 0.008* | 0.12 (0.02–0.78) | 0.03* |
| PHP vs. CE | 0.31 (0.12–0.81) | 0.02* | 0.37 (0.14–0.94) | 0.04* | 0.47 (0.17–1.25) | 0.13 |
| CE vs. Y90 | 0.36 (0.09–1.51) | 0.17 | 0.46 (0.13–1.65) | 0.23 | 0.26 (0.05–1.34 | 0.11 |
| TB 25–50%† | 1.07 (0.39–3.00) | 0.89 | 0.82 (0.31–2.16) | 0.69 | 0.58 (0.18–1.94) | 0.38 |
| TB 50–75%† | 5.49 (1.24–24.30) | 0.03* | 3.37 (1.00–11.37) | 0.05* | 6.42 (1.16–35.46) | 0.03* |
| TB > 75%† | 0.98 (0.25–3.90) | 0.98 | 0.65 (0.17–2.51) | 0.53 | 0.54 (0.12–2.31) | 0.40 |
Statistical significance.
Tumor burdens listed in the table are compared with a tumor burden of 0% to 25%.
CI indicates confidence interval; CE, chemoembolization; HR, hazard ratio; PHP, percutaneous hepatic perfusion; TB, tumor burden; Y90, yttrium-90.
There was a significant difference in median PFS: Y90 54 days; PHP 245 days, and CE 52 days; Log-rank test, P = 0.03 (Fig. 2). The same variables remained significant for PFS as for HPFS. MVA showed improved PFS for PHP versus Y90 (HR, 0.17, 95% CI, 0.04, 0.63; P = 0.008), PHP versus CE (HR, 0.37, 95% CI, 0.14, 0.94; P = 0.04) but not for CE versus Y90 (HR, 0.46, 95% CI, 0.13, 1.65; P = 0.23). Patients with a tumor burden of 50% to 75% had a significantly worse PFS (HR, 3.37, 95% CI, 1.00, 11.37; P = 0.05) compared with patients with 0% to 25% tumor burden, while a tumor burden of 25% to 50% or >75% did not significantly impact PFS (Table 2).
FIGURE 2.
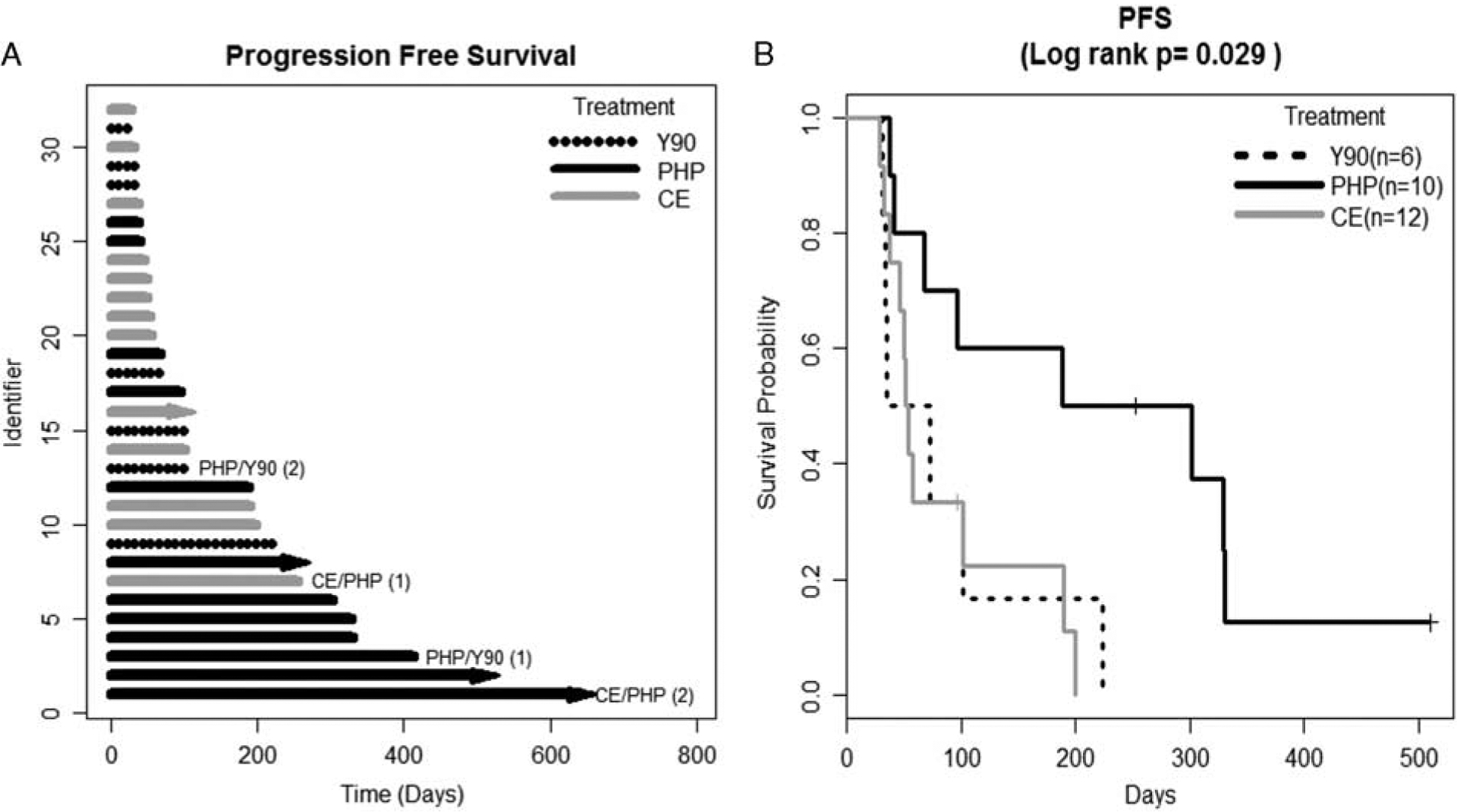
Progression-free survival (PFS). A, Swimmer’s plot. Lines with arrows represent ongoing PFS, lines without arrows indicate that patient was censored due to progression, lost to follow-up or death. B, The Kaplan-Meier survival curve. Patients undergoing 2 forms of liver-directed therapy were removed from the Kaplan-Meier survival analysis.
Median OS from time of treatment was longest for PHP at 608 days versus Y90 295 days or CE 265 days. Although PHP OS was over double that of Y90 and CE, it did not reach statistical significance (P = 0.24; Fig. 3). There was a significant difference on MVA of OS for PHP versus Y90 (P = 0.03) but not for PHP versus CE (P = 0.13) or CE versus Y90 (0.11); additionally, tumor burden of 50% to 75% remained a significant predictor of OS (HR, 6.42, 95% CI, 1.16, 35.46; P = 0.03; Table 2).
FIGURE 3.
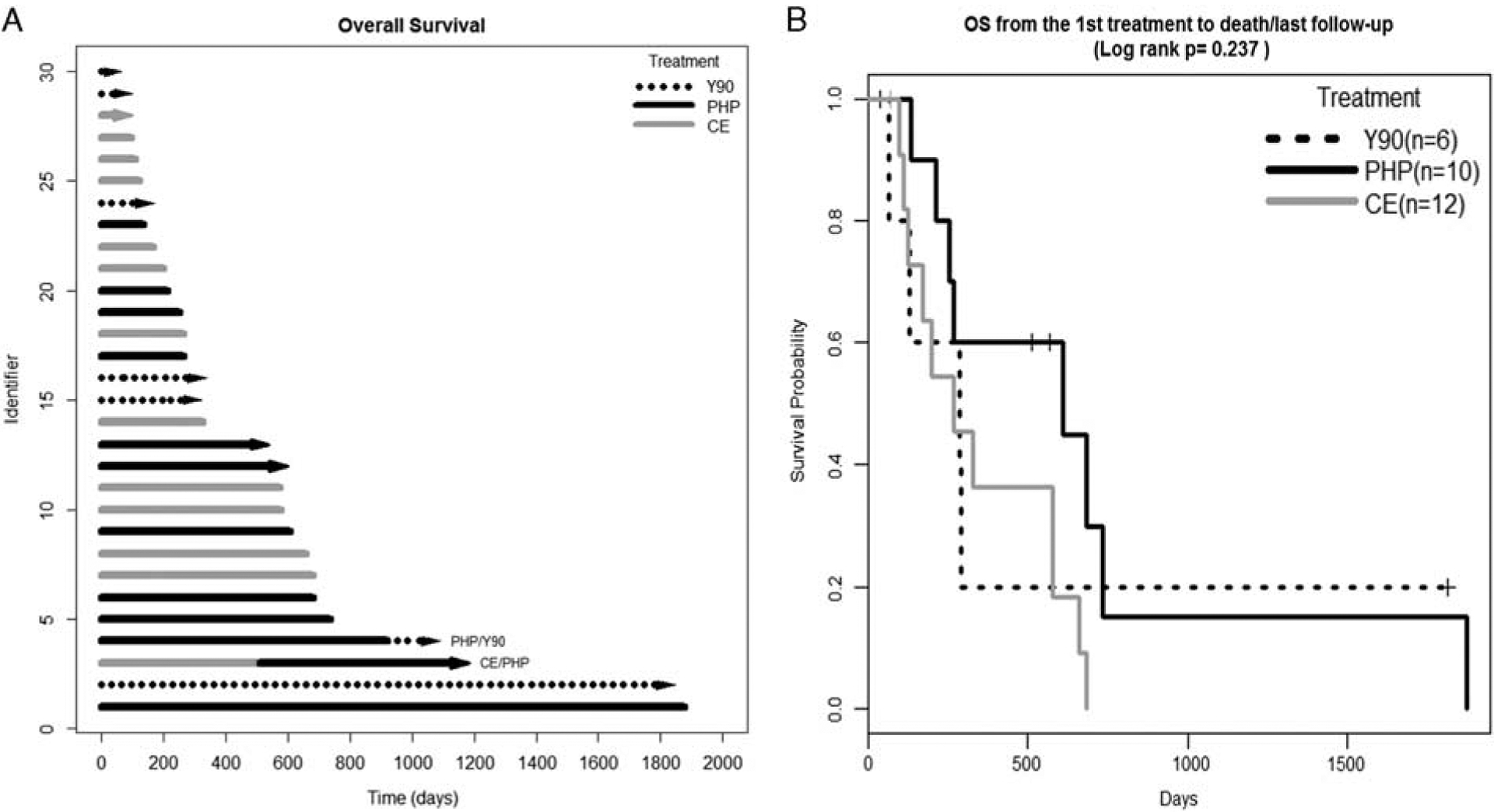
Overall survival (OS). A, Swimmer’s plot. Lines with arrows represent ongoing survival, lines without arrows indicate that patient was censored due to lost to follow-up or death. B, The Kaplan-Meier survival curve. Patients undergoing 2 forms of liver-directed therapy were removed from the Kaplan-Meier survival analysis.
The patient undergoing Y90 after PHP and the patient who received PHP after CE were not included in the KM survival analysis because of methodological limitations of KM analysis in handling correlated outcomes. These patients were included in the swimmer’s plots to demonstrate a period of survival after progression from the first treatment with use of a second modality of regional therapy as well as in the Cox regression analysis dealing with different types of therapies as a time-dependent covariate to evaluate effects of different types of therapies on survival times.
DISCUSSION
In this study, which evaluates outcomes after regional therapy for hepatic metastases in a mixed cohort of patients with cutaneous and uveal melanoma, we demonstrate that the type of regional therapy may have a potentially significant impact on HPFS and PFS. Patients treated with PHP had a significantly longer HPFS and PFS compared with patients treated with Y90 or CE, however the difference in OS was only seen for patients treated with PHP compared with Y90 despite the near doubling of median OS in days between PHP and CE.
The application of Y90 in the treatment of metastatic melanoma, although less well studied than its use for colorectal cancer and hepatocellular carcinoma, has been reported in the literature as safe and efficacious. In 3 studies, which ocular melanoma was the primary cause of liver metastases, the median OS ranged from 7.6 to 10.1 months.16–18 This supports our finding of a median OS of 9.5 months (285 d). In a study by Kennedy et al19 of 11 patients with uveal melanoma, a response rate of 77% and a 1-year survival of 80% were reported. This high response rate, however, has not yet been confirmed by additional studies.20 Gonsalves et al18 evaluated 32 patients with uveal melanoma and reported a treatment response of only 6% based on Response Evaluation Criteria in Solid Tumors criteria. Memon et al16 reported outcomes using Therasphere Y90 radioembolization in 16 patients with mixed melanoma (7 ocular, 4 cutaneous, 5 other sites). They reported a 31% response rate with a median OS of 7.6 months.
Patients with bilobar disease may require staged treatments with Y90 in which each lobe is treated separately. PHP offers the advantage of treating the entire liver through percutaneous vascular access and has less morbidity compared with traditional open isolated liver perfusion. The main advantage of PHP is that the procedure can be repeated every 6 to 8 weeks. Long-term survival data have not been widely reported.21,22 A phase III trial (NCT00324727) of 44 patients undergoing PHP compared with 49 patients undergoing best alternative care found that median HPFS was 7.0 months for patients treated with PHP compared with 1.6 months with best alternative care (P < 0.001) The authors also reported a median OS of 10.6 months after PHP compared with 10.0 months for patients treated with best alternative care (P = NS).22 A series of 10 patients with unresectable liver metastases from melanoma or sarcoma by Forster et al7 found that >90% of patients had stable disease or a partial response to treatment. The authors reported a median HPFS of 240 days with a median OS of 12.6 months. In this study our median HPFS (361 d/12 mo), PFS (245 d/8 mo), and OS (608 d/20 mo) are all longer than currently reported in the literature. A second phase 3, randomized controlled trial, NCT02678572, is currently enrolling patients with uveal melanoma and liver metastases by randomizing patients to 2 treatment groups: PHP versus best alternative care (transarterial chemoembolization, dacarbazine, ipilimumab, or pembrolizumab). The primary outcome of this study will be OS and is expected to be completed in June 2019.
CE has been well studied for liver metastases of many varying tumor types, which has prompted its use in the treatment of metastatic melanoma. Overall response rates of 0% to 39% and median OS rates of 5.0 to 8.9 months have been reported in the literature.23–27 In a series of 125 patients with ocular melanoma undergoing CE for liver metastases, only 11% of patients had a partial response, 16% had a minor responses, while 65% had stable disease and 8% had progressive disease. The median OS and PFS in this cohort were 6.7 and 3.8 months, respectively. Similar to our findings, tumor burden has been found to be a significant predictor of survival.23 In a study of 125 patients undergoing CE, Gupta and colleagues found that >75% liver involvement was associated with a short OS on MVA (> 75% vs. ≤75%, HR, 3.34, 95% CI, 1.43, 7.78; P = 0.05) with a median OS duration of only 2.4 months.
This study attempts to evaluate the impact of individual regional therapy modalities in the context of survival in a comparison study. We acknowledge that there are several limitations of this study secondary to its retrospective nature. The type of therapy administered was not randomized and was decided upon through discussion by a multidisciplinary tumor board and best judgement of the treating physician. In addition, referral patterns may have impacted treatment decision-making as patients receiving adjuvant therapy from an outside facility may have been referred specifically to receive a particular therapy. The procedures were all performed by the same 3 physicians (1 surgeon, 1 radiation oncologist, and 1 interventional radiologist), however, which may eliminate some treatment variability. The total number of patients in this series was small making the 3 treatment cohort sizes small and this may have limited our ability to detect a statistically significant difference in outcomes. Because of the rarity of uveal melanoma, accrual of a large number of patients with only this subtype of disease is very difficult. Therefore, the cohort contains a mixed population of patients with both uveal and cutaneous melanoma. The patients with a tumor burden of 50% to 75% had a statistically significant survival difference compared with patients with 0% to 25% tumor burden, but not those with 25% to 50% or >75%. Small sample size may contribute to this finding and with larger numbers tumor burden may be significant across all comparison cohorts. There was a difference, however, seen in HPFS and PFS and in OS for patients undergoing PHP compared with Y90 and these findings are important for discussing treatment options with patients and prompting additional studies to confirm these results. The survival advantage seen for PHP may be in part because the therapy can be repeated and each PHP treats the entire liver, therefore treating microscopic, radiographically occult disease with each perfusion.
CONCLUSIONS
Liver directed regional therapies are additional treatment options for patients with unresectable liver disease from metastatic uveal or cutaneous melanoma. PHP demonstrats promising results when compared with Y90 and CE. Further studies including a randomized controlled trial are needed to determine if clinically superior outcomes can be achieved with one regional therapy method compared with another. The treatment of metastatic melanoma continues to evolve with the use of targeted immunotherapies although recent evidence shows that immunotherapy is not as effective for metastatic uveal melanoma as it is for metastatic cutaneous melanoma. A new randomized phase 3 study (NCT02678572) has included immunotherapy (ipilimumab and pembrolizumab), chemoembolization, and dacarbazine into the best alternative care treatment arm which will be important for further defining the role of immunotherapy in patients with metastatic uveal melanoma. A potential future direction may include combining these new systemic modalities with regional therapy, which could potentially lead to higher response rates or OS.
Acknowledgments
J.S.Z. is on the medical advisory board for Delcath Systems and has grant and research support from Delcath Systems.
Footnotes
The other authors declare no conflicts of interest.
REFERENCES
- 1.SEER Stat Fact Sheets: Melanoma of the Skin. Statistics at a Glance. http://seer.cancer.gov/statfacts/html/melan.html. Accessed November 4, 2015.
- 2.Hu DN, Yu GP, Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46:151–166. [DOI] [PubMed] [Google Scholar]
- 3.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123: 1639–1643. [DOI] [PubMed] [Google Scholar]
- 4.Leiter U, Meier F, Schittek B, et al. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86:172–178. [DOI] [PubMed] [Google Scholar]
- 5.Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–390. [DOI] [PubMed] [Google Scholar]
- 6.Singh AD, Borden EC. Metastatic uveal melanoma. Ophthalmol Clin North Am. 2005;18:143–150. [DOI] [PubMed] [Google Scholar]
- 7.Forster MR, Rashid OR, Perez MC, et al. Chemosaturation with percutaneous hepatic perfusion for unresectable metastatic melanoma or sarcoma to the liver: a single institution experience. J Surg Oncol. 2013;109:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariani P, Piperno-Neumann S, Servois V, et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at Institut Curie. Eur J Surg Oncol. 2009;35: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 9.Agarwala SS, Eggermont AM, O’Day S, et al. Metastatic melanoma to the liver. A contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer. 2014;120:781–789. [DOI] [PubMed] [Google Scholar]
- 10.Frenkel S, Nir I, Hendler K, et al. Long-term survival of uveal melanoma patients after surgery for liver metastasis. Br J Ophthalmol. 2009;93:1042–1046. [DOI] [PubMed] [Google Scholar]
- 11.Feldman ED, Pingpank JF, Alexander HR. Regional treatment options for patients with ocular melanoma metastatic to the liver. Ann Surg Oncol. 2004;11:290–297. [DOI] [PubMed] [Google Scholar]
- 12.Olofsson R, Cahlin C, All-Ericsson C, et al. Isolated hepatic perfusion for ocular melanoma metastasis: registry data suggests a survival benefit. Ann Surg Oncol. 2014;21:466–472. [DOI] [PubMed] [Google Scholar]
- 13.Olofsson R, Ny L, Eilard MS, et al. Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases (the SCANDIUM trial): study protocol for a randomized controlled trial. Trials. 2014;15:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han D, Beasley GM, Tyler DS, et al. Minimally invasive intra-arterial regional therapy for metastatic melanoma: Isolated limb infusion and percutaneous hepatic perfusion. Expert Opin Drug Metab Toxicol. 2011;7:1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riaz A, Lewandowski RJ, Kulik L, et al. Yttrium-90 radioembolization using Therasphere in the management of primary and secondary liver tumors. Q J Nucl Med Mol Imaging. 2009;53: 311–316. [PubMed] [Google Scholar]
- 16.Memon K, Kuzel TM, Vouche M, et al. Hepatic yttrium-90 radioembolization for metastatic melanoma: a single-center experience. Melanoma Res. 2014;24:244–251. [DOI] [PubMed] [Google Scholar]
- 17.Xing M, Prajapati HJ, Dhanasekaran R, et al. Selective internal Yttrium-90 radioembolization therapy (90Y-SIRT) versus best supportive care in patients with unresectable metastatic melanoma to the liver refractory to systemic therapy: safety and efficacy cohort study. Am J Clin Oncol. 2014. [Epub ahead of print].. [DOI] [PubMed] [Google Scholar]
- 18.Gonsalves CF, Eschelman DJ, Sullivan KL, et al. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJ Am J Roentgenol. 2011;196: 468–473. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy AS, Nutting C, Jakobs T, et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest. 2009;27:682–690. [DOI] [PubMed] [Google Scholar]
- 20.Kuei A, Saab S, Cho SK, et al. Effects of Yttrium-90 selective internal radiation therapy on non-conventional liver tumors. World J Gastroenterol. 2015;21:8271–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashid OM, Sloot S, Zager JS. Regional therapy in metastatic melanoma: an update on minimally invasive intraarterial isolated limb infusion and percutaneous hepatic perfusion. Expert Opin Drug Metab Toxicol. 2014;10:1355–1364. [DOI] [PubMed] [Google Scholar]
- 22.Hughes M, Zager JS, Faries M, et al. Results of a randomized controlled multi-center phase III trial of percutaneous hepatic perfusion compared to best available care for patients with melanoma liver metastases. Ann Surg Oncol. 2015; 23:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Bedikian AY, Ahrar J, et al. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: response, survival, and prognostic factors. Am J Clin Oncol. 2010;33:474–480. [DOI] [PubMed] [Google Scholar]
- 24.Ahrar J, Gupta S, Ensor J, et al. Response, survival, and prognostic factors after hepatic arterial chemoembolization in patients with liver metastases from cutaneous melanoma. Cancer Invest. 2011;29:49–55. [DOI] [PubMed] [Google Scholar]
- 25.Patel K, Sullivan K, Berd D, et al. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a phase II study. Melanoma Res. 2005;15:297–304. [DOI] [PubMed] [Google Scholar]
- 26.Schuster R, Lindner M, Wacker F, et al. Transarterial chemoembolization of liver metastases from uveal melanoma after failure of systemic therapy: toxicity and outcome. Melanoma Res. 2010;20:191–196. [DOI] [PubMed] [Google Scholar]
- 27.Sharma KV, Gould JE, Harbour JW, et al. Hepatic arterial chemoembolization for management of metastatic melanoma. AJR Am J Roentgenol. 2008;190:99–104. [DOI] [PubMed] [Google Scholar]


