Abstract
Objectives
Canakinumab is an IL-1β antibody that neutralises the activity of IL-1β. This study examined the efficacy and safety of canakinumab in patients with moderate COVID-19-related pneumonia.
Design
This study aimed to evaluate the reduction in duration of hospitalisation with adequate oxygen status. Forty-eight patients with moderate COVID-19-related pneumonia were asked to participate in the prospective case-control study: 33 patients (cases) signed informed consent and received canakinumab (Cohort 1) and 15 patients (Controls) refused to receive the experimental drug and received institutional standard of care (Cohort 2).
Results
Hospital discharge within 21 days was seen in 63% of patients in Cohort 1 vs. 0% in Cohort 2 (median 14 vs. 26 days, respectively; p < 0.001). There was significant clinical improvement in ventilation regimes following administration of canakinumab compared with Cohort 2 (Stuart-Maxwell test for paired data, p < 0.001). Patients treated with canakinumab experienced a significant increase in PaO2:FiO2 (p < 0.001) and reduction in lung damage by CT (p = 0.01), along with significant decreases in immune/inflammation markers that were not observed in Cohort 2. Only mild side-effects were seen in patients treated with canakinumab; survival at 60 days was 90.0% (95% CI 71.9–96.7) in patients treated with canakinumab and 73.3% (95% CI 43.6–89.1) for Cohort 2.
Conclusions
Treatment with canakinumab in patients with COVID-19-related pneumonia rapidly restored normal oxygen status, decreased the need for invasive mechanical ventilation, and was associated with earlier hospital discharge and favourable prognosis versus standard of care.
Keywords: COVID-19, Pneumonia, SARS-CoV-2, Canakinumab
Introduction
SARS coronaviruses (SARS-CoV) are a large family of viruses that normally cause mild-to-moderate upper respiratory tract illnesses (Trivedi et al., 2019). The novel coronavirus SARS-CoV-2 is currently causing a global pandemic, as declared by the World Health Organization on 11 March 2020. Sepsis, respiratory failure, acute respiratory distress syndrome (ARDS), and multiorgan failure are the common complications of an apparently aberrant host immune response (Huang et al., 2020). Huang et al. reported the clinical features of patients infected by COVID-19 and suggested that hyperstimulation of cytokines–such as interferon gamma, interleukin (IL)-1, IL-6, and tumour necrosis factor α–is associated with the severity and clinical complexity of the infection (Huang et al., 2020). Indeed, it is becoming increasingly evident that these complications are related to the so-called cytokine storm observed in some severe patients, involving release of cytokines including IL-6, IL-18 and interferon (Mehta et al., 2020, Zhang et al., 2020). The clinical complications impede alveolar gas exchange and trigger dissemination of systemic thrombosis. Consistently, infection of cells in the lower respiratory tract by COVID-19 can give rise to severe ARDS, with consequent release of additional pro-inflammatory cytokines such as IL-1β (Conti et al., 2020, Toldo et al., 2020).
To date, therapeutic options for severe COVID-19 remain limited. Antiviral drugs such as lopinavir/ritonavir have shown no benefits compared to standard care (Cao et al., 2020). Recently, remdesivir was reported to have modest clinical benefits in patients affected by COVID-19 (Beigel et al., 2020). Thus, new treatment strategies are urgently needed to achieve a significant impact on prognosis of COVID-19. The suspicion that overzealous immune responses in some COVID-19 patients have given rise to interest in anti-cytokine therapy to mitigate the cytokine storm (Megna et al., 2020, Zhou et al., 2020). Accordingly, immune-modulatory agents such as anti-IL-6, anti-IL-6R and anti-IL-1 antibodies are under investigation. Recently, the anti-IL-1R agent anakinra and anti-IL-6R agent tocilizumab have shown promising results in patients with COVID-19 and ARDS (Campochiaro et al., 2020, Cavalli et al., 2020, Colaneri et al., 2020). Canakinumab is a high-affinity human monoclonal antihuman IL-1β antibody of the IgG1/κ isotype designed to block the interaction of IL-1β with its receptor, thus neutralising its activity (Goh et al., 2014).
This study investigated the clinical efficacy of canakinumab in patients with moderate COVID-19 pneumonia. It aimed to evaluate the reduction in hospital duration with adequate oxygen status. In addition, it focused on survival without the need for invasive mechanical ventilation, adverse events, modulation of biological parameters, and chest computed tomography (CT) response.
Methods
Study design
The main focus was to obtain information on the activity and safety of canakinumab in patients with moderate COVID-19-related pneumonia. This prospective, case-control, single-center, observational study carried out at the Azienda Socio-Sanitaria Territoriale di Cremona (ASST), Cremona (Italy) was approved by the ASST of Cremona Institutional Review Board and is without a trial registration number. All study participants gave written informed consent for participation. Studies were conducted in conformity with applicable local requirements and regulations regarding the protection of the rights and welfare of human subjects that were enrolled. The authors conceived the study, collected all data, vouched for the accuracy and completeness of the data, and made the decision, after the final version, to submit the manuscript for publication. The first draft of the manuscript was conceived and prepared by the first author who was the principal investigator of the study. The study drug–canakinumab–was provided by Novartis Farma, SpA.
Patient population
Forty-eight patients with moderate COVID-19-related pneumonia were enrolled in this prospective case-control study between 01–25 April 2020 at a single institution. Thirty-three patients were treated with canakinumab (Cohort 1) and 15 patients continued with the standard of care (Cohort 2). All patients who were enrolled met criteria defined by the vademecum of the Lombardy Section of the Italian Society of Infectious and Tropical Diseases (Lombardy Section Italian Society and Tropical, 2020). For diagnosis, throat swabs were obtained under aseptic conditions and analysed by real-time RT-PCR; chest X-ray and/or CT with signs of pneumonia (e.g. lateral multiple lobular and sub-segmental areas of ground-glass opacity or consolidation) were also considered for diagnosis. Criteria for diagnosis of moderate conditions are described in the supplemental materials. Clinical features – including body temperature, concentration of oxygen inhalation and oxygen saturations – were recorded daily during treatment. Clinical improvement was based on an ordinal clinical improvement scale that compared with baseline ventilation status of patients with respect to last follow-up.
Scores on the scale were defined as follows: 0, not receiving supplemental oxygen; (1) high-flow nasal cannula; (2) Venturi mask oxygen therapy; (3) mask with reservoir bag; (4) non-invasive ventilation (NIV) and continuous positive airway pressure (CPAP); and (5) intubation. The cumulative incidence of clinical improvement was defined by either a decrease of ≥1 point on the 6-point ordinal scale or live discharge.
Whole blood white cell counts along with markers of inflammation (C-reactive protein (CRP), IL-6, fibrinogen, d-dimer, and ferritin) were monitored. Most patients had undergone spiral chest CT upon admission and before discharge using a 64-row spiral Revolution EVO (GE Healthcare) in a whole-lung, low-dosage exposure mode, scanning with 1.25-mm slices. The software used for CT scan analysis was Thoracic VCAR, using a modified protocol with a suitable threshold to detect solid/sub-solid pulmonary changes. Lung involvement was automatically reported as a percentage by two independent radiologists. The mean of the scores was used for subsequent analysis. Safety and adverse events were monitored using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Treatment
Canakinumab (150 mg) was administered by subcutaneous injection on day 1 and on day 7, as approved for auto-inflammatory conditions (EMA authorisation 30 January 2020). The schedule was empirically interrupted in three cases after the first dose, given the substantial clinical and radiological benefits that were observed. All patients meeting eligibility criteria who had signed a consent form and who had begun experimental treatment were evaluated as a case group (Cohort 1). All eligible patients who initiated treatment were evaluated for adverse events. Patients hospitalised in the same period who refused to be treated with the experimental drug (Cohort 2) were used as a control group.
Statistical analysis
Two independent statisticians blinded to each other analysed the data. Continuous variables were expressed as median (range) or mean (standard deviation), according to data distribution checked with the Shapiro–Wilk test. Categorical variables were expressed as absolute frequencies and percentages. Homogeneity of baseline characteristics between the two cohorts were assessed through t-test or Mann–Whitney test. One-way ANOVA or Friedman test for repeated measures were performed to evaluate differences in clinical parameters over time for both cohorts (separately). Pairwise post-hoc contrasts were considered and p-values adjusted for multiple comparisons (Holm method). Type of ventilation before and after treatment was compared using the Stuart-Maxwell test. Spearman’s linear coefficient was calculated to assess correlation between variables of interest. Linear mixed-effect models (LMEM) for repeated measures were used to investigate changes in values over time with respect to group assignment. The method used to treat missing data was complete data analysis: the percentage of missing values across variables of interest over time varied between 0%–27% for Cohort 1 (at maximum nine missing values for variables) and between 0%–13% for patients in Cohort 2 (at maximum two missing values for variables). Missing values can be considered as completely random due to the emergency situation. Overall survival was defined as the time from date of admission to hospital to death, and estimated with the Kaplan-Meier method. The last follow-up update was on 27 May 2020. The log-rank test was conducted to assess between-group differences. All statistical analyses were performed using R version 4.0.0. Statistical significance was set as p < 0.05.
Results
Patient characteristics
From 01 to 25 April 2020, 48 patients with moderate COVID-19 and pneumonia were included: 33 were treated with canakinumab (Cohort 1) and 15 (Cohort 2) received the institutional standard of care. All patients were managed outside of the ICU. Baseline characteristics are summarised in Table 1 . Median age was similar in Cohort 1 (70 years, range 29–89) and Cohort 2 (69 years, range 44–85). The majority of patients were male (76% for Cohort 1 and 87% in Cohort 2). The range of comorbidities and presenting symptoms were broadly similar in both groups. Patients in both groups had received treatment with antivirals, hydroxychloroquine and antibiotics (see Supplementary Appendix) before entering the study. For treating COVID-19-related pneumonia, patients in Cohort 1 received canakinumab and heparin and those in Cohort 2 received high-dose (10,000 IU) heparin only.
Table 1.
Demographic characteristics of patients enrolled in the prospective interventional study.
| Characteristic | Cohort 1 |
Cohort 2 |
p-value | ||
|---|---|---|---|---|---|
| (n = 33) | (n = 15) | ||||
| Median age, years | 70 (29–89) | 69 (44–85) | 0.38 | ||
| Age category, N (%) | 0.54 | ||||
| <50 years | 1 (3%) | 1 (6%) | |||
| 50–70 years | 13 (39%) | 4 (27%) | |||
| ≥70 years | 19 (58%) | 10 (67%) | |||
| Gender | 0.47 | ||||
| Male | 25 (76%) | 13 (87%) | |||
| Female | 8 (24 %) | 2 (13%) | |||
| Comorbidities | |||||
| Cardiovascular disease | 18 (54%) | 8 (53%) | 0.94 | ||
| Pulmonary disorder | 0 | 0 | |||
| Cancer | 1 (3%) | 0 | |||
| Metabolic disordersa | 9 (27%) | 3 (20%) | |||
| Neurological disorder | 10 (30%) | 3 (20%) | |||
| Concomitant Therapies, N (%) | Beforeb | Afterb | Beforec | Afterc | 0.75d |
| Antivirals | 23 (70%) | 0 | 12 (80%) | 0 | |
| Hydroxychloroquine | 30 (91%) | 1 (3%) | 14 (93%) | 2 (13%) | |
| Antibiotics | 25 (76%) | 2 (6%) | 8 (53%) | 2 (13%) | |
| Heparin | 33 (100%) | 33 (100%) | 14 (93%) | 14 (93%) | |
| Corticosteroids | 17 (52%) | 4 (12%) | 4 (27%) | 0 | |
| Hospital days | |||||
| Median (min–max) | 14 (5–40) | 26 (21–42) | <0.001 | ||
| Hospital days, N (%) | |||||
| <14 | 11 (33%) | 0 (0%) | |||
| 14–21 | 10 (30%) | 0 (0%) | <0.001 | ||
| ≥21 | 12 (36%) | 15 (100%) | |||
| Symptoms, N (%) | 0.85 | ||||
| Fever | 29 (89%) | 10 (67%) | |||
| Cough | 13 (39%) | 8 (53%) | |||
| Dyspnoea | 23 (70%) | 10 (67%) | |||
| Fatigue | 4 (12%) | 2 (13%) | |||
| Gastrointestinal | 4 (12%) | 1 (6%) | |||
Type 2 diabetes, hypercholesterolaemia.
Before and after treatment with canakinumab.
Same time frame in which cases were treated with canakinumab.
p-values refer to between-group comparison of concomitant therapies after administration of canakinumab.
Ventilation, oxygenation status and chest CT
At baseline, most patients receiving canakinumab (75.8%) were on non-invasive ventilation such as CPAP (30.4%) or supplemental oxygen (with nasal prongs) (45.4%); the remaining 24.2% had NIV. In Cohort 2, 46.7% of patients had supplemental oxygen, 40% had CPAP and 13.3% had NIV.
At 10 days after the last administration of canakinumab, no clinical need for NIV and a reduction (15.1%) in the use of CPAP with an improvement of use of supplemental oxygen by 63.7% were observed; 21.2% restored breathing with ambient air. The improvement in ventilation regimes following administration of canakinumab was statistically significant in Cohort 1 (Stuart-Maxwell test for paired data, p < 0.001). In Cohort 2, which received high-dose heparin only, there were no significant changes in clinical benefit and/or reduction in ventilation needed at pre-treatment and post-treatment times (p = 0.28). While 13.3% of patients still needed NIV, 26.7% were maintained on CPAP, and supplemental oxygen was needed for 53.3% of patients. At the end of the same period of observation, 6.7% of patients were no longer in need of supplemental oxygen.
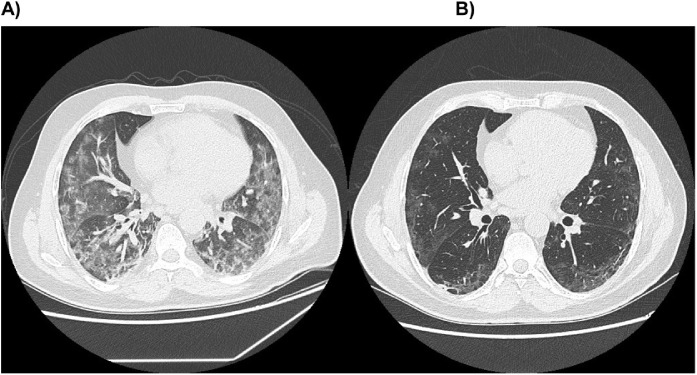
This study also compared the PaO2:FiO2 ratio and chest CT before starting canakinumab and at 7–10 days after the second injection of the anti-IL1β antibody. The same comparison was performed in Cohort 2 during the same time frame of patients receiving canakinumab. The ventilation approaches in the two cohorts were comparable at baseline; however, more patients needed less invasive types of ventilation after administration of canakinumab. As shown in Table 2 , compared with baseline, patients treated with canakinumab experienced a significant increase in the PaO2:FiO2 ratio (p < 0.001) and a reduction in lung damage evaluated by CT (p = 0.01). Cohort 2 had clinical benefit only in the PaO2:FiO2 ratio (p = 0.05). No significant correlation was observed between the change (Δ) in PaO2:FiO2 ratio, or Δ in lung damage in controls (Table S1). Figure 1 shows the changes in lung damage related to COVID-19 induced by canakinumab.
Table 2.
Changes in PaO2/FiO2 ratio and lung damage evaluated by chest CT (%) in Cohort 1 (a) and Cohort 2 (b) during the same time period before treatment with canakinumab and at 7–10 days after the second administration of canakinumab. (c) Comparison between the changes measured between before the first administration of canakinumab and at follow-up between the two cohorts with regards to the PaO2/FiO2 ratio and lung damage on chest CT scan (%).
| a) Cohort 1 | |||
|---|---|---|---|
| Cohort 1 | Before first administration | At follow-up | P-value |
| (n = 33 at baseline) | |||
| PaO2:FiO2 ratio | |||
| Mean (min–max) | 142.2 (59.0) | 257.4 (105.8) | <0.001 |
| Lung damage (%) | |||
| Mean (SD) | 48.5 (21.1) | 38.6 (18.5) | 0.01 |
| b) Cohort 2 | |||
|---|---|---|---|
| Cohort 2 | Before first administration | At follow-up | P-value |
| (n = 15 at baseline) | |||
| PaO2:FiO2 ratio | |||
| Mean (SD) | 148.1 (58.4) | 197.1 (46.8) | 0.05 |
| Lung damage (%) | |||
| Mean (SD) | 37.9 (15.4) | 35.7 (10.0) | 0.68 |
| c) Comparison between the changes measured between before the first administration of canakinumab and at follow-up between the two cohorts with regards to the PaO2/FiO2 ratio and lung damage on chest CT scan (%). | |||
|---|---|---|---|
| Variable | Cohort 1 | Cohort 2 | P-value |
| Change in PaO2:FiO2 ratio | |||
| Mean (SD) | 99.9 (62.2) | 50.5 (63.3) | 0.03 |
| Change in lung damage (%) | |||
| Mean (SD) | −17.1% (31.1%)a | 11.7% (58.3%) | 0.03 |
Matched-pair analysis was available for 23 patients.
Figure 1.
Changes in lung damage before ((A) Diffuse ground glass opacities with peripheral nodular consolidation) and after administration ((B) Less peripheral ground glass opacities) of canakinumab.
Modulation of immune-inflammatory markers
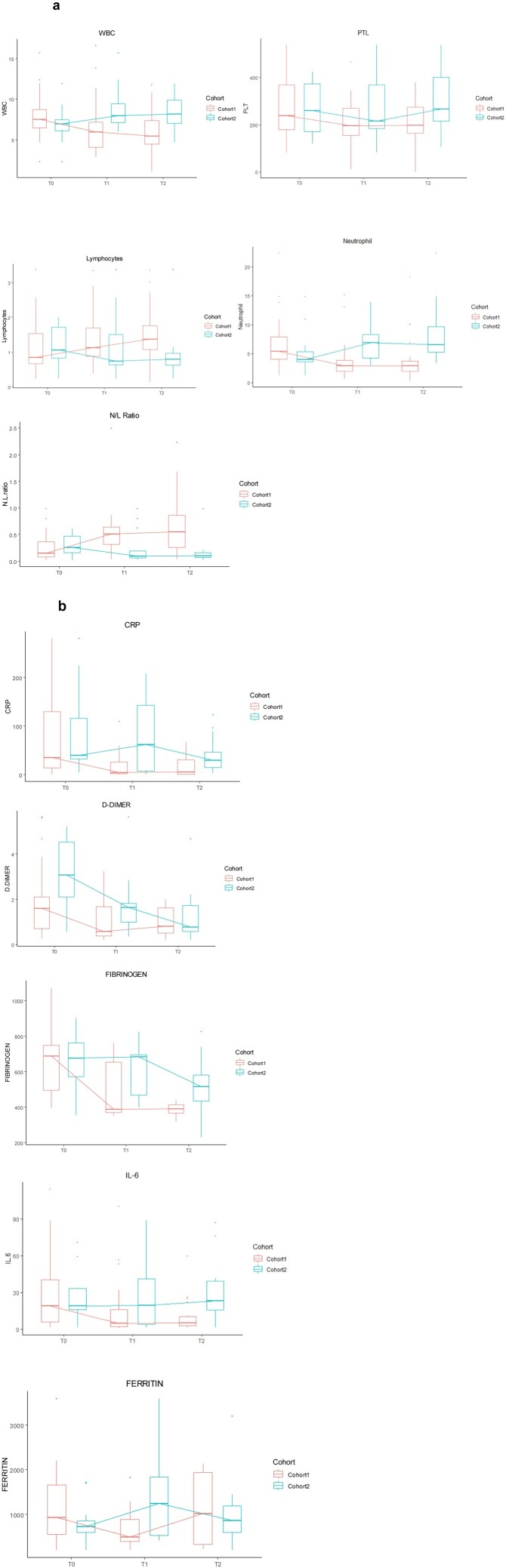
Laboratory immune and inflammatory markers were tested prior to administration of canakinumab at days 1 and 7 and at 7–10 days after the second injection of the antibody (Table S2). Canakinumab led to a reduction in white blood cells (p < 0.001), platelets (p = 0.005) and neutrophils (p < 0.001), and an increase in lymphocytes (p = 0.01), over the time of measurement, while no significant variations were found for the same parameters in Cohort 2 (Figure 2 a). The changes over time in immune and inflammatory markers are reported in Tables S3a and S3b, and in Tables S4a and S4b, respectively. Moreover, the neutrophil/lymphocyte ratio, considered as a surrogate marker of response to treatment in other settings such as cancer (Kim et al., 2019), showed a significant increase (p < 0.001) over time. This trend was not observed in Cohort 2, where only an increase in neutrophil count was seen pre-treatment and before the second administration (p = 0.05).
Figure 2.
(a) Changes over time in immune response-related markers: comparison between Cohort 1 and Cohort 2 (T0: basal; T1 after first administration of canakinumab; T2 after 7–10 days from the second administration of canakinumab). The N/L ratio is also reported. (b) Changes over time in immune-inflammatory-related biomarkers: comparison between Cohort 1 and Cohort 2.
With regards to inflammatory markers, canakinumab treatment was associated with prompt reductions in serum CRP (p < 0.001), IL-6 (p < 0.03) and ferritin (p < 0.001). In Cohort 2, only a reduction in the level of d-dimer was noted (p = 0.05) (Figure 2b), which was likely due to the higher dose of heparin used compared with Cohort 1.
Effect of canakinumab on laboratory markers
Effects of time, irrespective of treatment, were found in WBC, neutrophils and at the limit of statistical significance in CRP. Interactions between time and treatment were significant for WBC, lymphocytes, neutrophils, platelets, CRP, and the neutrophils/lymphocyte ratio (Table S5 and Figure 2a).
Survival, clinical outcomes and adverse events
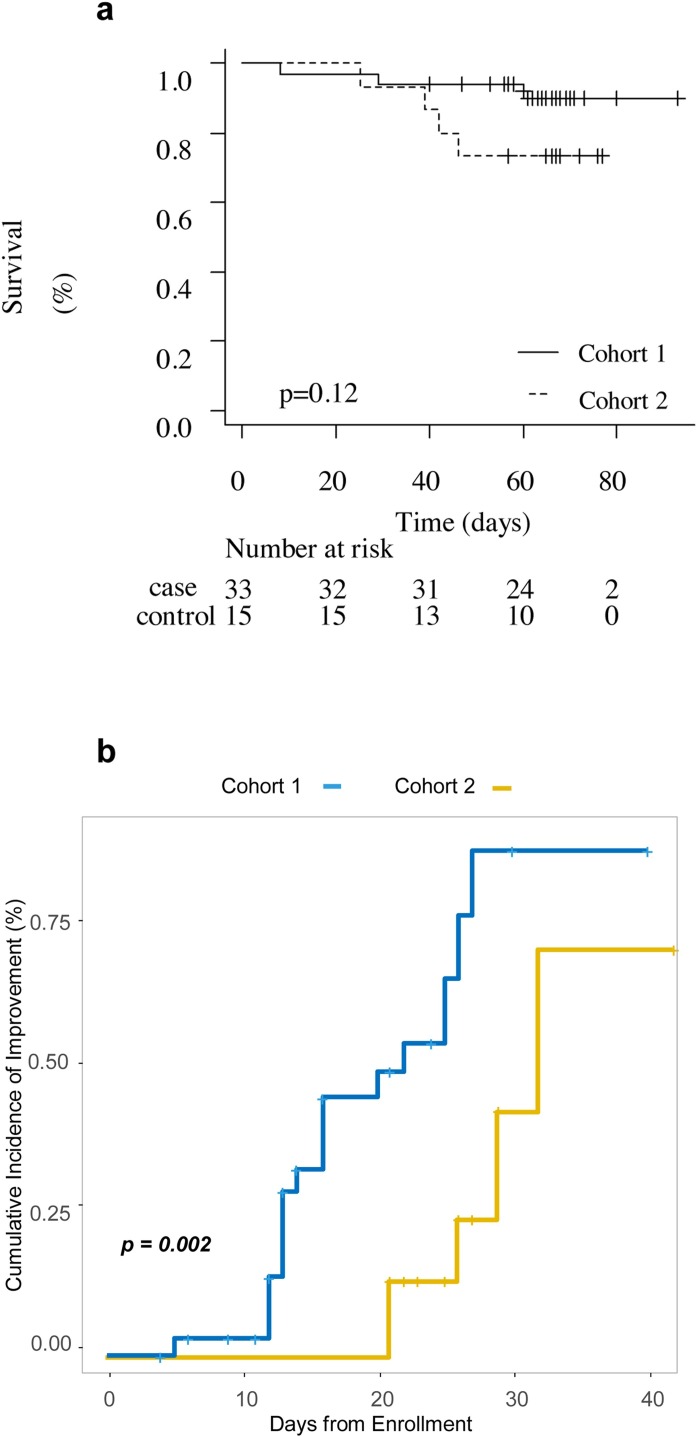
Clinical outcomes of the 33 patients treated with canakinumab were compared with those of the 15 patients who received standard treatment only. An improvement in survival in Cohort 1 was observed. The survival rate at 60 days was 90.0% (95% CI 71.9–96.7) in the canakinumab-treated cohort vs. 73.3% (95% CI 43.6–89.1) in Cohort 2 (Figure 3a). Moreover, 63% of patients in Cohort 1 were hospitalised for <21 days compared with 0% in Cohort 2 (median 14 vs. 26 days, respectively; p < 0.001) (Table 1). Of the 30 patients who received two administrations of canakinumab (three patients received only one subcutaneous injection due to very rapid improvement in ventilation), 21 (72%) experienced an improvement in respiratory function. The Kaplan–Meier curves for the time to improvement on the ordinal clinical improvement scale are shown in Figure 3b. The univariate hazard ratio for clinical improvement in Cohort 1 compared with Cohort 2 was 4.20 (95% CI 1.56–11.32, p = 0.005).
Figure 3.
(a) Overall survival in Cohort 1 and Cohort 2. (b) Cumulative incidence of clinical improvement from hospital admission to hospital discharge.
Causes of death in patients receiving canakinumab were pulmonary thromboembolism (n = 2) and multiorgan failure (n = 2). Causes of death in patients in Cohort 2 were sepsis (n = 1), multiorgan failure (n = 1) and pulmonary thromboembolism (n = 2).
In both cohorts, heparin, independently of the dose, was well tolerated without any serious adverse events. Four patients in Cohort 1 experienced injection site reactions, while two experienced nausea (Grade 1) and one patient referred headache (Grade 1). These symptoms were not recorded in patients in Cohort 2.
Discussion
In the absence of standard of care, existing therapies can potentially be repurposed to treat COVID-19. This pandemic has favoured the off-label or compassionate use of investigational agents such as remdesivir, immune-modulating compounds or convalescent plasma (Lima et al., 2020, Manhas et al., 2020) that are approved or licensed for other indications. To date, the use of other immunomodulatory agents such as tocilizumab and anakinra for the treatment of the cytokine storm related to COVID-19 has highlighted the potential importance of modulating this pathway in preventing worsening of disease (Campochiaro et al., 2020, Cavalli et al., 2020, Colaneri et al., 2020). It has also been reported that tocilizumab reduces admissions to the ICU and mortality in patients with COVID-19 (Klopfenstein et al., 2020).
A recent retrospective analysis of 10 patients with COVID-19-pneumonia who were safely treated with canakinumab (Ucciferri et al., 2020) showed that all patients recovered within 45 days, along with an improvement in oxygenation and a decrease in the systemic inflammatory response. The PaO2:FiO2 ratio increased in 82% of patients, while improvement in the oxygen-support category was observed in 61% of cases. Overall, mortality was 13.6%.
This study reports, in a real-world setting, the activity of canakinumab for the treatment of patients with moderate COVID-19 pneumonia. The recent data about the intense inflammasome formation characterising the lungs of patients with fatal COVID-19 disease due to ARDS support a rational role of canakinumab in managing COVID-19-related complications (Conti et al., 2020, Toldo et al., 2020). Moreover, it found that hypoxaemia and lung CT opacity both rapidly improved in most patients after treatment with canakinumab (heavily pre-treated with antivirals and hydroxychloroquine) compared with Cohort 2, suggesting that IL-1β blockade could play an important role in modulating the cytokine storm. Of note, canakinumab was also associated with significant time to clinical improvement vs. Cohort 2.
An increase in the percentage of lymphocytes with a concomitant increase in neutrophils is considered to be an important indicator for immune response in clinical settings (Zahorec, 2001). Levels of both CRP and IL-6 decreased during therapy with canakinumab (p = 0.02; Figure S1), along with a slight reduction in markers related to complements C3 and C4 (data not shown), supporting the role of canakinumab in modulating the immune response/cytokine storm. It is likely that canakinumab is more efficacious before the patient’s condition progresses from mild to severe (e.g. increased lung opacity, increased IL-6, CRP, and hypoxaemia), as early treatment may effectively control the deterioration of symptoms and complications related to the massive cytokine storm observed in patients with severe forms of COVID-19. However, the long-term safety of IL-1 inhibition remains to be investigated. Specifically, canakinumab produces sustained IL-1 suppression and therefore vigilance is necessary to monitor for adverse events.
All of the current patients were treated with hydroxychloroquine, lopinavir/ritonavir or remdesivir, alone or in combination, before starting canakinumab. The variety of treatment schedules used was related to the absence of defined treatment protocols during the first few weeks of the COVID-19 epidemic. The current analysis evaluated the potential therapeutic efficacy of canakinumab/heparin versus heparin alone without the interference of other drugs, which were suspended before starting the study, accordingly to SIMIT guidelines (SIMIT, 2021).
Even considering the emergency situation when the study was performed, there were several limitations to this study. The number of patients was relatively limited, as in many similar studies with preliminary but encouraging findings. The uncontrolled nature of the study mandates caution in interpretation of the results: it was a prospective, non-randomised study and some bias may have occurred. However, based on these results, a randomised controlled trial with canakinumab in COVID-19-pneumonia is warranted in outpatients with manageable disease. A randomised phase III clinical trial with intravenous canakinumab in hospitalised patients with COVID-19-induced pneumonia is on-going (NCT04362813), but this study will assess the efficacy of a higher dose (administered IV and by body weight).
Canakinumab appears to decrease the need for invasive mechanical ventilation and improve clinical symptoms in patients with COVID-19. Blockade of the cytokine storm with canakinumab prevents clinical deterioration of patients with COVID-19 pneumonia, thereby favouring earlier hospital discharge and better prognosis.
Ethical approval
This investigator-driven, single-center, prospective, observational study carried out at the Azienda Socio-Sanitaria Territoriale di Cremona (ASST), Cremona (Italy) was approved by the ASST of Cremona Institutional Review Board and is without a trial registration number. All study participants gave written informed consent for participation.
Conflict of interest
None declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. Maurizio Scaltrici is funded by the National Cancer Institute (NCI), USA under the MSK Cancer Center Support Grant/Core Grant (P30 CA008748).
Author contributions
Research design: AP, AM, BG, FM, MGP, ST, DG. Clinical research and data collection: DG, GB, FM, AC, ST, LR, AF, AM, LP, GG, EV, MGP, MF, RT, IZ, GC, AM, MC, AG, VDG, CC, CC, OB, MS, AM, AP. Statistical analysis, data analysis and pharmacology data interpretation: SV, FG, MS, DG. Writing the paper: SV, FG, MS, DG.
Acknowledgments
This work was supported by Novartis, which provided the study drug, and the “Direzione Strategica” of Azienda Socio, Sanitaria di Cremona, Regione Lombardia, Italy, which supports clinicians and research during the SARS-CoV2 outbreak. The authors also thank Health Publishing & Services srl who provided medical writing support funded by Novartis Farma SpA. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. Maurizio Scaltriti is funded by the National Cancer Institute (NCI) under the MSK Cancer Center Support Grant/Core Grant (P30 CA008748). Special thanks to all patients, caregivers, nurses, volunteers, and to the members of the Cremona COVID-19 Study Group (CreSCo): Pan A, Bosio G, Giorgi-Pierfranceschi, M, Testa S, Abruzzi L, Mukh A, Accardo G, Ajiour A, Ananadiou S, Aschedamini S, Barbini U, Barletta G, Beccara L, Baglivo F, Baratta V, Betti M, Bondi G, Bonvecchio A, Borsella A, Buffoli F, Buselli P, Cammelli L, Caresana G, Castellini M, Cataldo M, Cavalli C, Cavalli I, Cavalli M, Cimardi L, Cocco N, Coluccello A, Benedetta Conti C, Crescini G, Cristadoro D, Cuzzoli A, Danzi G, De Gennaro F, De Marco, Dizioli P, Drera B, Ferrari L, Ferraresi A, Foramitti A, Fornabaio C, Fornaciari M, Fumarola B, Gardini M, Generali D, Gianotti G, Giuliano G, Grappa E, Grassia R, Harizaj F, Iannaccone D, Invernici F, Lasusa T, Liberto A, Ombi S, Machiavelli A, Maestrelli M, Magaldi C, Mainardi E, Malberti F, Maninetti L, Martinelli E, Milanetti M, Milesi M, Molteni A, Morandini R, Muri M, Nardecchia L, Paoletti O, Passalacqua R, Pasquali C, Pasquali N, Coluzzi L, Pezzarossa E, Pezzetti F, Pianta L, Pini S, Poli N, Quarta Colosso M, Ranocchia S, Ribola A, Rigolli A, Rizzi E, Rizzi N, Romanini L, Riccardi A, Rizzardi S, Ruggeri P, Ruggeri P, Sagradi F, Sergio P, Silla A, Stifani I, Tala M, Tamayo L, Tamburelli F, Telli S, Tisi M, Toffolon F, Tonidandel L, Torres A, Ungari M, Verwij F, Viola E, Virzì G, Vismarra M, Zambolin G, Zadeh S, Zeliani C, Zoncada A, Canino R.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2020.12.073.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19–preliminary report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F. Tocilizumab for treatment of Severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8(5) doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Goh A.X., Bertin-Maghit S., Ping Yeo S., Ho A.W., Derks H., Mortellaro A. A novel human anti-interleukin-1beta neutralizing monoclonal antibody showing in vivo efficacy. MAbs. 2014;6(3):765–773. doi: 10.4161/mabs.28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Kim T.H., Yoon H.K., Lee A. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting neoadjuvant chemotherapy response in breast cancer. J Breast Cancer. 2019;22(3):425–438. doi: 10.4048/jbc.2019.22.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Zayet S., Lohse A., Balblanc J.C., Badie J., Royer P.Y. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020;50(5):397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima W.G., Brito J.C.M., Overhage J., Nizer W. The potential of drug repositioning as a short-term strategy for the control and treatment of COVID-19 (SARS-CoV-2): a systematic review. Arch Virol. 2020;165(8):1729–1737. doi: 10.1007/s00705-020-04693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardy Section Italian Society I., Tropical D. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143–152. [PubMed] [Google Scholar]
- Manhas S., Anjali A., Mansoor S., Sharma V., Ahmad A., Rehman M.U. Covid-19 pandemic and current medical interventions. Arch Med Res. 2020;51(6):473–481. doi: 10.1016/j.arcmed.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megna M., Napolitano M., Fabbrocini G. May IL-17 have a role in COVID-19 infection? Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMIT. Available at: http://www.simit.org/medias/1569-covid19-vademecum-13-03-202.pdf. [Accessed 2 May 2020].
- Toldo S., Bussani R., Nuzzi V., Bonaventura A., Mauro A.G., Cannata A. Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res. 2020;2020(October) doi: 10.1007/s00011-020-01413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi S.U., Miao C., Sanchez J.E., Caidi H., Tamin A., Haynes L. Development and evaluation of a multiplexed immunoassay for simultaneous detection of serum IgG antibodies to six human coronaviruses. Sci Rep. 2019;9(1):1390. doi: 10.1038/s41598-018-37747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucciferri C., Auricchio A., Di Nicola M., Potere N., Abbate A., Cipollone F. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020;2(8) doi: 10.1016/S2665-9913(20)30167-3. e457-ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- Zhang B., Zhou X., Qiu Y., Song Y., Feng F., Feng J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.