Abstract
Oncologic imaging is an important facet of abdominal imaging that radiologists encounter nearly every day. Many oncology clinical trials utilize response evaluation criteria in solid tumors (RECIST) version 1.1 which divides tumor sites into target and non-target lesions. Although RECIST v1.1 provides clear instructions regarding the use of imaging in clinical trials, errors in response assessment still occur using these criteria. This is especially true of response assessment with regards to non-target lesions which involve rules which are less well-defined and somewhat subjective. This pictorial essay will review RECIST v1.1 guidelines and common non-target lesion errors which can occur at baseline and follow-up response assessment.
Keywords: Non-target lesion, RECIST v1.1, Response assessment, Target lesion
In 1981 the World Health Organization published criteria to standardize tumor measurements using imaging to assess tumor response in clinical trials [1]. Response Evaluation Criteria in Solid Tumors (RECIST) was developed in 2000 to address shortcomings with WHO criteria and better standardize response assessment [2–5]. The development of RECIST resulted in objective criteria to determine response to therapy and allowed comparison of data obtained at multiple sites and in different trials [6]. RECIST underwent several modifications and in 2009 the revised version (RECIST v1.1) was published. RECIST v1.1 provides a standardized process to assess therapy response that requires mastery of specific criteria and definitions [7].
The baseline assessment for a clinical trial using RECIST v1.1 requires that all sites of tumor are accounted for using a combination of target and non-target lesions. Target lesions must be measurable in at least one dimension. The longest diameter of tumor must be greater than or equal to 1 cm and for metastatic lymph nodes the short axis must equal or exceed 1.5 cm. There is a limit to the number of target lesions which may be selected at baseline. Target lesions should not exceed 5 total and no more than 2 lesions per organ. If there are excess lesions after the maximum number of target lesions has been reached, these excess lesions should be assigned as non-target lesions. Non-target lesions also include sites of tumor too small to qualify as target lesions and non-measurable metastatic disease (ascites, leptomeningeal disease, etc).
Specific RECIST v1.1 criteria are applied at follow-up time points after the baseline exam to evaluate the response to therapy (Table 1). For target lesions, this involves the sum of diameters of the various selected lesions. This numerical approach provides an unambiguous assessment, although there are certainly issues relying on size alone to assess therapy response (e.g., tumor may show necrosis which indicates response but the size is unchanged). Non-target lesions do not require measurements but they should be described using standardized language so the RECIST v1.1 response is clear. At our institutions, radiologists describe each non-target lesion as being present (corresponding to non-complete response (CR)/non-progressive disease (PD), absent (corresponding to CR) or unequivocal progression (corresponding to PD). The overall responses of the target and non-target lesions are combined to give an overall response rate (Table 2). Figure 1 shows a RECIST v1.1 worksheet used by radiologists and clinical trial staff to track lesions over time and assess response. A worksheet like this can be an invaluable tool to ensure RECIST v1.1 rules are followed and it also provides a quick overview of the response to therapy over time.
Table 1.
RECIST v1.1 response evaluation categories for target and non-target lesions [7]
| Target lesions | Non-target lesions |
|---|---|
| Complete response (CR) | Complete response (CR) |
| Disappearance of all target lesions, pathologic lymph nodes must measure short axis < 1 cm | Disappearance of all non-target lesions, all lymph nodes must measure short axis < 1 cm |
| Partial response (PR) | Non-CR/Non-PD |
| At least 30% decrease in sum of diameters of target lesions (relative to baseline sum) | Persistence of one or more non-target lesions |
| Stable disease (SD) | Progressive disease (PD) |
| Not enough tumor shrinkage to qualify as PR, not enough tumor growth to qualify as progression | Unequivocal progression of existing non-target lesions or any new lesions |
| Progressive disease (PD) | |
| At least 20% increase in sum of diameters of target lesions (relative to smallest sum), sum must show absolute increase of at least 5 mm, or any new lesions |
Table 2.
Time point overall response, patients with target and non-target lesions [7]
| Target lesions | Non-target lesions | Overall responses |
|---|---|---|
| CR | CR | CR |
| CR | Non-CR/non-PD | PR |
| PR | Non-PD | PR |
| SD | Non-PD | SD |
| PD | Any | PD |
| Any | PD | PD |
CR Complete response, PR partial response, SD stable disease, PD progressive disease
Fig. 1.
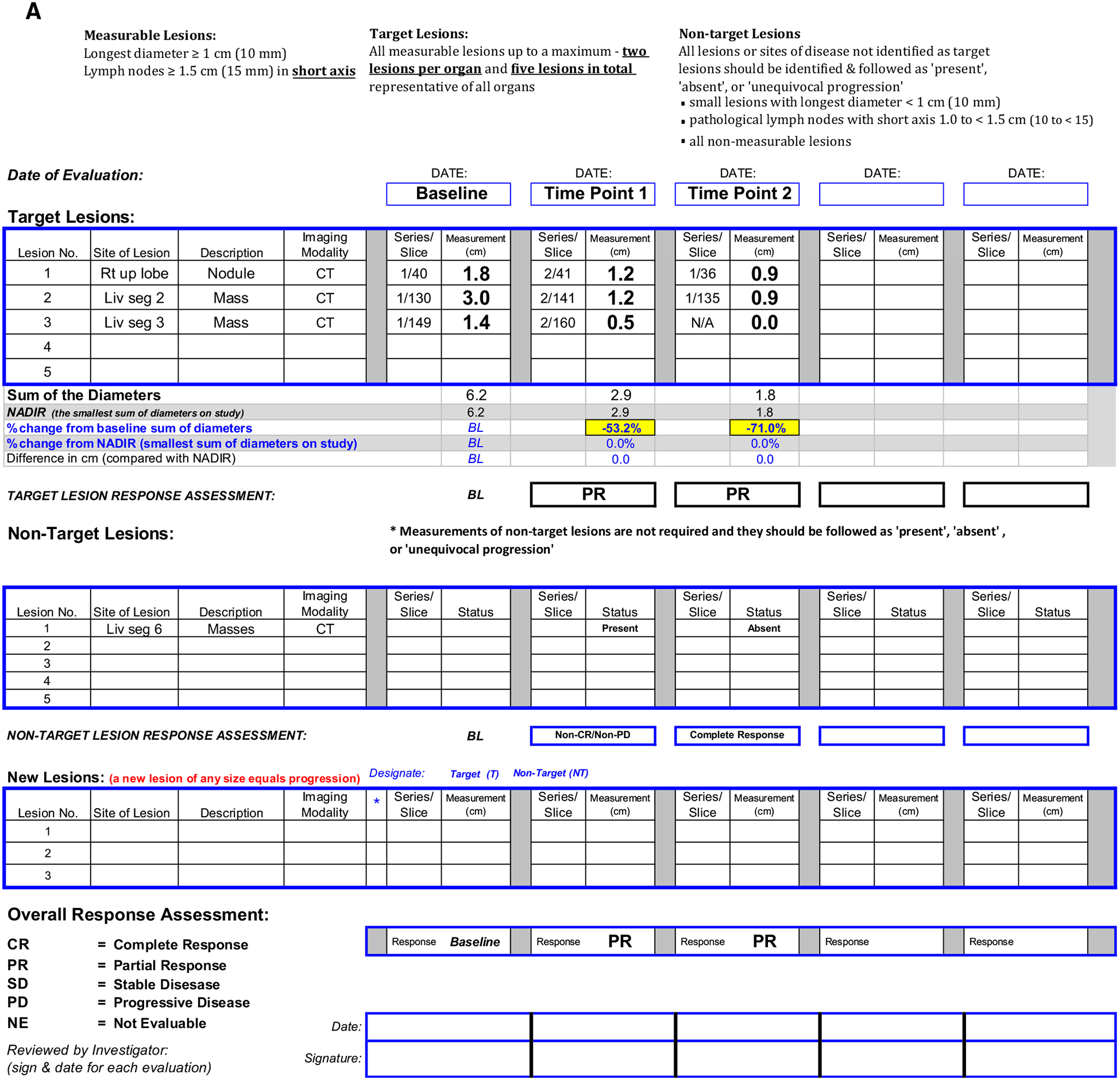
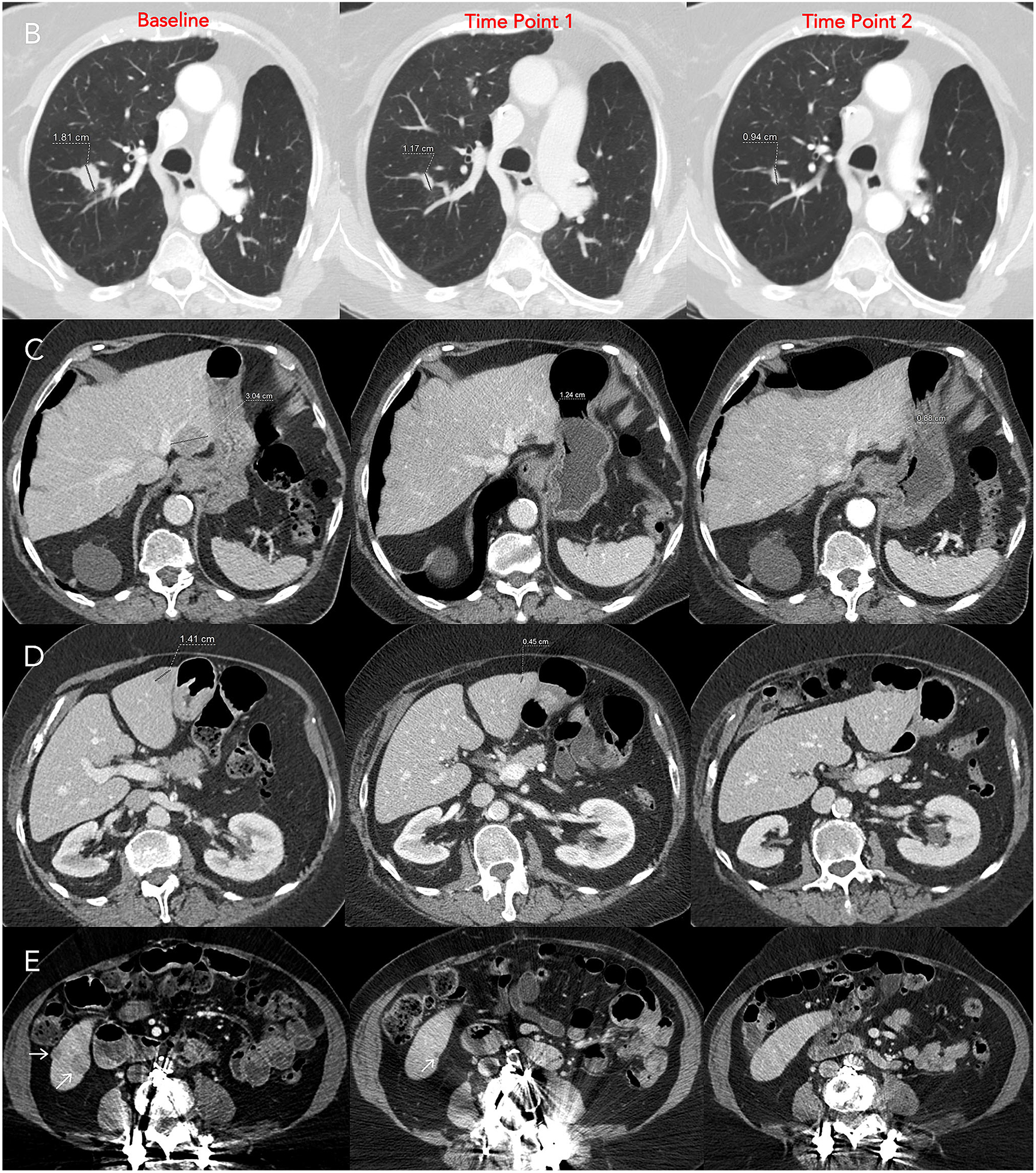
A Clinical trial worksheet using RECIST v1.1 criteria. B Target lesion 1, right upper lobe nodule, C Target lesion 2, segment 2 liver mass, D Target lesion 3, segment 3 liver mass, E Non-target lesion 1, segment 6 liver masses. Target lesions are assessed by summing the diameters at baseline and follow-up time points. In this case, the shrinkage of target lesions results in a partial response at both follow-up time points. Since two target liver lesions are assigned, the maximum number of target lesions in the liver has been reached. Therefore, the remaining liver lesions are assigned as non-target lesion 1.
A key point with regards to accurate selection of target and non-target lesions is the concept of ‘measurable’ tumor. Sites of tumor chosen as target lesions should be those which can be measured repeatedly in a reproducible fashion. Poorly defined masses should not be used as target lesions because this could lead to significant measurement variability from scan to scan. This concept of measurable tumor can be a helpful distinction because radiologists tend to pick the largest tumors as target lesions regardless of how “measurable” they are (Fig. 2). The assessment of measurability also introduces a level of subjectivity with non-target lesions (i.e., there can be disagreement about whether a lesion is non-measurable or not).
Fig. 2.
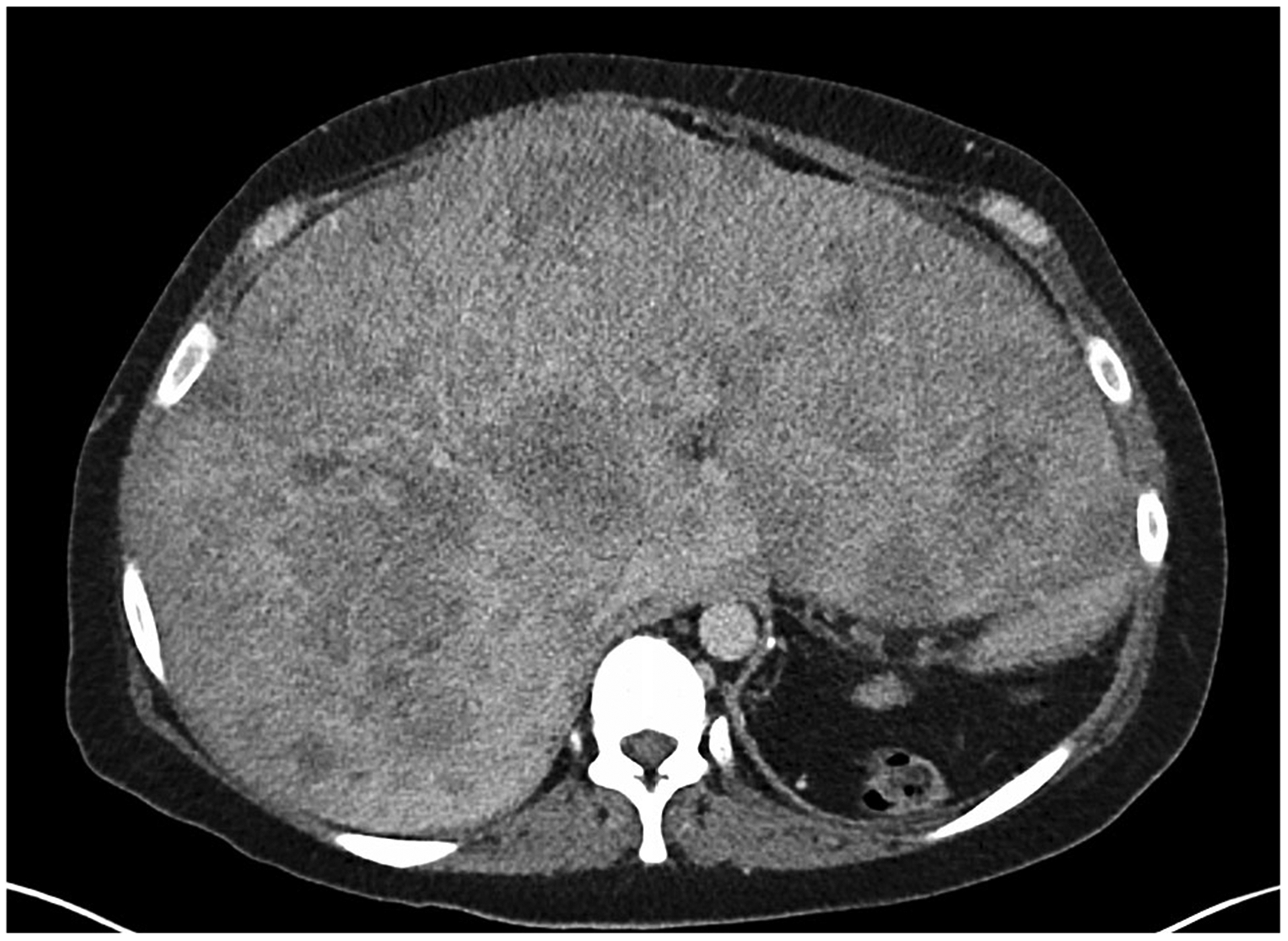
Axial post-contrast CT image from a patient with metastatic breast cancer. The metastases are poorly defined and while they could be measured it is doubtful the measurements would be reproducible from scan to scan and radiologist to radiologist.
Since non-target lesions do not require measurements over time, response assessment is a more subjective process, especially the concept of ‘unequivocal progression’. RECIST v1.1 guidelines point out that progression by non-target lesions alone (i.e., without concomitant progression by target lesions) should be an uncommon occurrence. When the patient only has non-measurable disease (a situation sometime encountered in phase III trials), the guidelines suggest target lesion criteria for progressive disease should be applied to non-target lesions; there should be a subjective increase in overall non-target tumor volume of 73%, which corresponds to increase in diameter of 20% [7]. No specific objective guidance is given with regards to patients with both measurable and non-measurable disease. However, one must remember that if PD is assigned based on non-target lesions, it may lead to discontinuation of therapy. Therefore, PD by non-target lesions should only be assigned if there is a significant increase in non-target tumor bulk to the point that progression is obvious to all observers (thus the terminology ‘unequivocal progression’).
In the authors’ experience, errors with non-target lesions are encountered more frequently than errors involving target lesions. These non-target lesion errors occur in two settings: baseline lesion assignment and response assessment. Common errors at baseline lesion assignment include: not accounting for all sites of tumor, classifying benign lesions as tumor, grouping incorrectly or lack of non-target lesion grouping, and assigning small (< 1 cm short axis) lymph nodes as non-target lesions. Common response assessment errors include incorrectly classifying lymph node response when short axis decreases to < 1 cm, and classifying progression with only limited size change of non-target lesions.
Baseline errors
Not accounting for all sites of tumor
The principal point in RECIST v1.1 guidelines is that all sites of tumor must be accounted for using target and non-target lesions. In spite of this, it is not an infrequent occurrence that some sites of tumor are not accounted for at baseline assessment, usually secondary to incorrect assignment of non-target lesions. For example, when a patient has multiple pulmonary metastases (Fig. 3), a common mistake is to measure two nodules as target lesions and ignore the rest or select 1–2 nodules to serve as non-target lesions. This causes problems with response assessment over time. If only target lesions are selected, there is no standardized way to account for the response of the other pulmonary metastases over time (i.e., as they are not delineated as non-target tumor they are not accounted for with RECIST and do not factor into the overall response rate). Similarly, selecting one or two non-target nodules out of many pulmonary metastases does not account for the bulk of the tumor in the lungs.
Fig. 3.
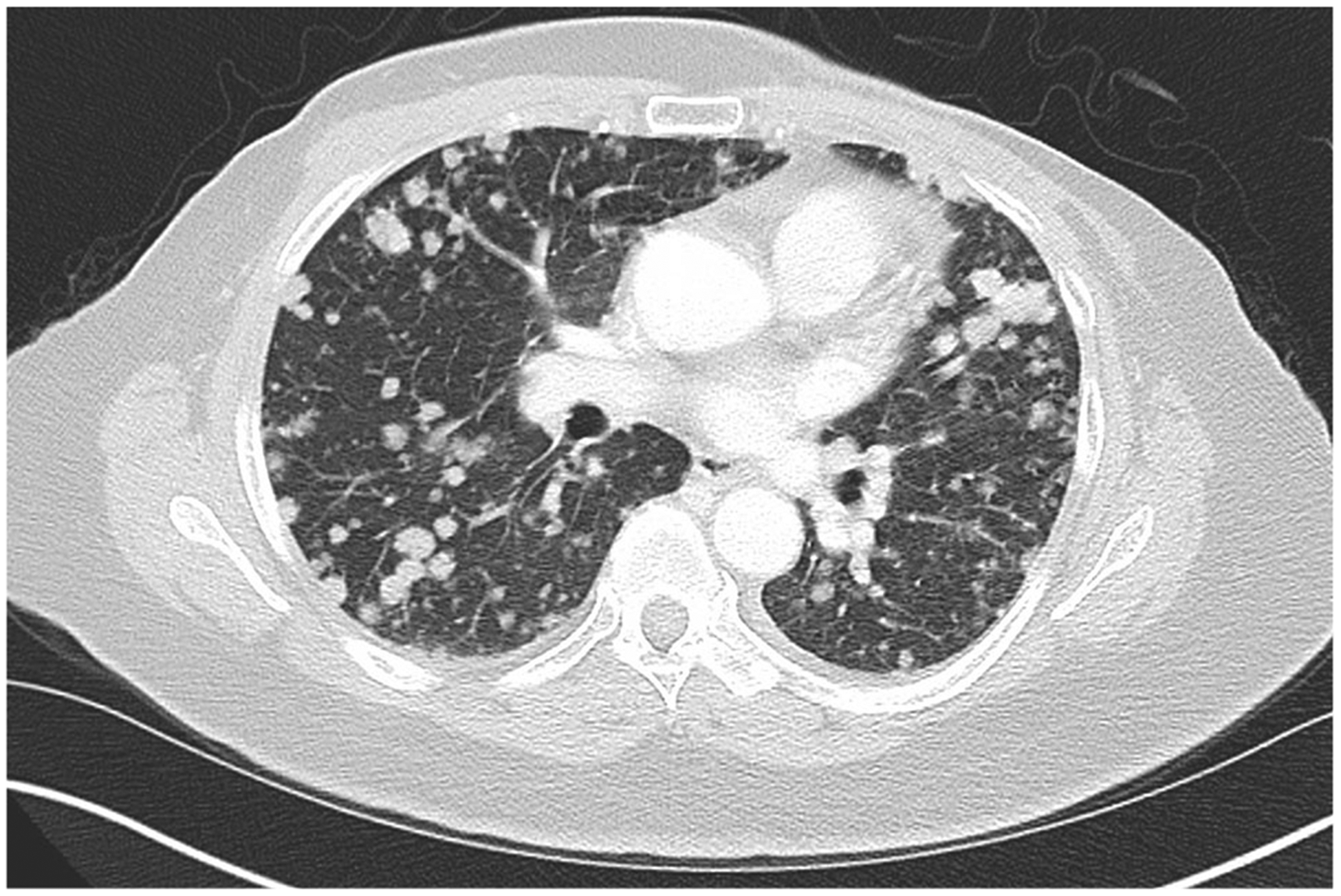
Axial post-contrast CT image from a patient with metastatic sarcoma and multiple pulmonary metastases. In this case only two target lesion pulmonary nodules were measured with no non-target lesions delineated. This does not account for the patient’s tumor burden. The lung nodules not used as target lesions should be accounted for as a single non-target lesion and described as “multiple lung metastases”.
It is important to remember that once the maximum number of target lesions are assigned (5 total lesions or 2 per organ) the remainder of measurable tumor sites should be assigned as non-target lesions. This can be confusing since measurable tumor is being classified as a non-target lesion, however, if one is familiar with the relevant rules of RECIST v1.1 (account for all sites of tumor, do not exceed target lesion number limits) the reasoning for this becomes clear. Since these lesions are followed as non-target lesions, they only require subjective assessment at follow-up imaging time points, even though they are measurable. However, in the setting of progression solely by non-target lesions, some trials or sponsors may require measurements of relevant non-target lesions to prove there is a significant increase over time.
Classifying benign lesions as tumor
Patients on clinical trials frequently have fairly widespread metastatic disease. In those cases, pitfalls that might not normally be problematic can cause issues since radiologists can be more focused on tumor identification (for appropriate target and non-target delineation), rather than lesion characterization. Remember, not every tiny pulmonary nodule is a metastasis, nor is every drop of fluid (pleural/pericardial effusions, ascites) malignant. One must resist the tendency to assign every detectable lesion as tumor without affording it the same scrutiny that might be applied in a case not on a clinical trial. If a benign lesion is assumed to be tumor and assigned as a non-target lesion (or target lesion for that matter) the patient will never achieve a complete response because that lesion will never resolve. If a lesion is indeterminate at baseline assessment prior imaging should be reviewed to see if it can be definitively characterized (Fig. 4). Consultation with the oncologists and clinical trial staff involved with the trial can be very helpful in problematic cases. For example, an indeterminate lesion could be left unassigned and then reanalyzed at follow-up imaging. The etiology might become apparent over time and relevant revisions could be made to baseline target/non-target lesions. Alternatively, all involved in the trial might determine it is critical to characterize a lesion and the radiologists’ expertise can be important to recommend appropriate further imaging or determine the feasibility of image-guided biopsy.
Fig. 4.
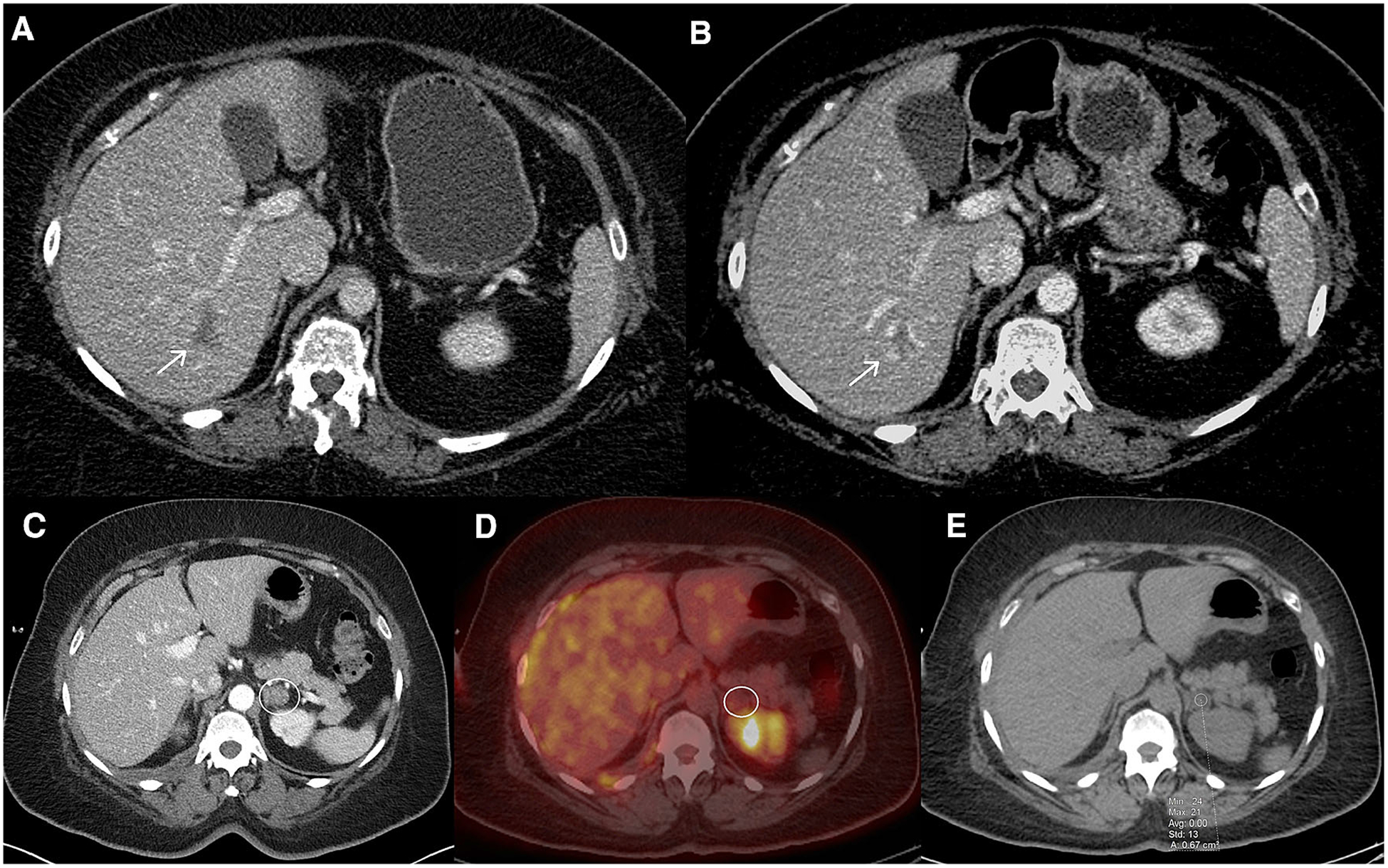
A, B Axial post-contrast images from a patient with endometrial cancer at different time points. A shows a lesion which initially was diagnosed as a metastasis (arrow). However, a CT scan from a year before was available B which was acquired with different contrast timing. The lesion (arrow) on the prior exam shows the peripheral, interrupted, nodular enhancement of a cavernous hemangioma. C, D, E Axial images from contrast-enhanced abdomen CT and a PET/CT in a different patient with metastatic lung cancer. The left adrenal nodule which was called a metastasis on contrast-enhanced abdomen CT (C; oval). However, it is relatively low density even after contrast. A subsequent PET/CT D, E confirmed this is an adenoma (no hypermetabolic activity, density (HU=0.0) on non-contrast CT compatible with adenoma).
Grouping incorrectly or lack of non-target lesion grouping
All tumor sites must be accounted for when using RECIST v1.1. Although there are limits to the maximum number of target lesions (5 overall; 2 per organ), there are no specific limits for the number of non-target lesions. This leads to a potential dilemma since there could be large numbers of non-target lesions. How can numerous sites of tumor be documented and followed in an efficient manner? To alleviate this issue, RECIST v1.1 guidelines dictate that lesions in single organs or lymph node chains can be grouped as a single non-target lesion. For example, if there are multiple liver metastases which are smaller than 1 cm they could be followed as a single non-target lesion. At follow-up imaging the liver lesions are analyzed and a global assessment is assigned for that non-target lesion using the proper RECIST v1.1 terminology (CR, non-CR/non-PD, PD). Likewise, lymph nodes in specific anatomic regions can be grouped as one non-target lesion, e.g., mediastinal nodes, axillary nodes, etc (Fig. 5).
Fig. 5.
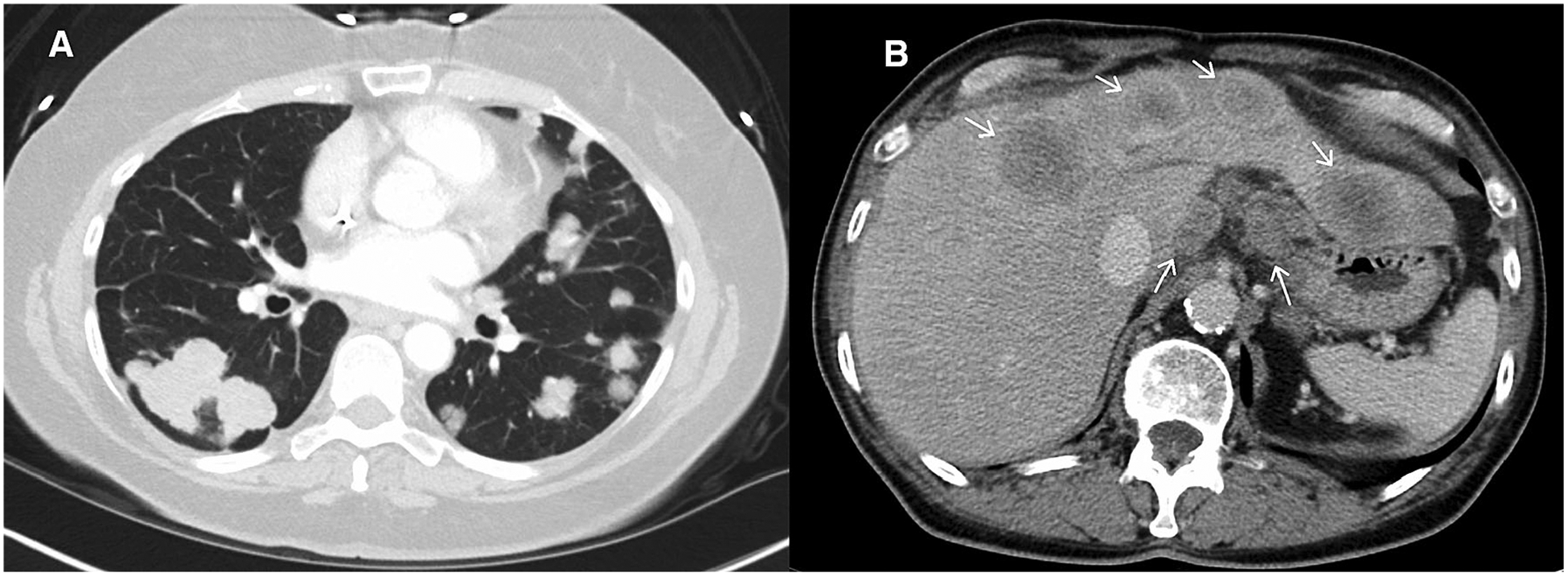
Axial post-contrast CT images from a patient with metastatic colon cancer. A shows multiple pulmonary metastases and B shows hepatic metastases and gastrohepatic ligament lymphadenopathy. If these were all accounted for as non-target lesions proper assignment would be: (1) Multiple lung metastases, (2) Hepatic metastases, and (3) Gastrohepatic nodes (assuming short axis measures ≥1 cm).
Assigning small lymph nodes (< 1 cm short axis) as non-target lesions
RECIST v1.1 guidelines dictate that complete response for a metastatic lymph node occurs when the short axis measurement falls below 1 cm. Therefore, lymph nodes should not be assigned as non-target lesions unless the short axis is 1 cm or greater. This is a potential weakness of RECIST v1.1 since small nodes can certainly harbor tumor (Fig. 6). However, the guidelines provide a way to recognize that tumor in lymph nodes may be eradicated and the lymph node will still be visualized on computed tomography. This is different from a liver metastasis or a lung nodule, which may completely resolve and thereby indicate that a complete response was achieved. If a lymph node with a short-axis less than 1 cm is erroneously listed as a non-target lesion, response criteria cannot be accurately applied because by definition a non-target lymph node achieves CR with short axis less than 1 cm (Fig. 7).
Fig. 6.
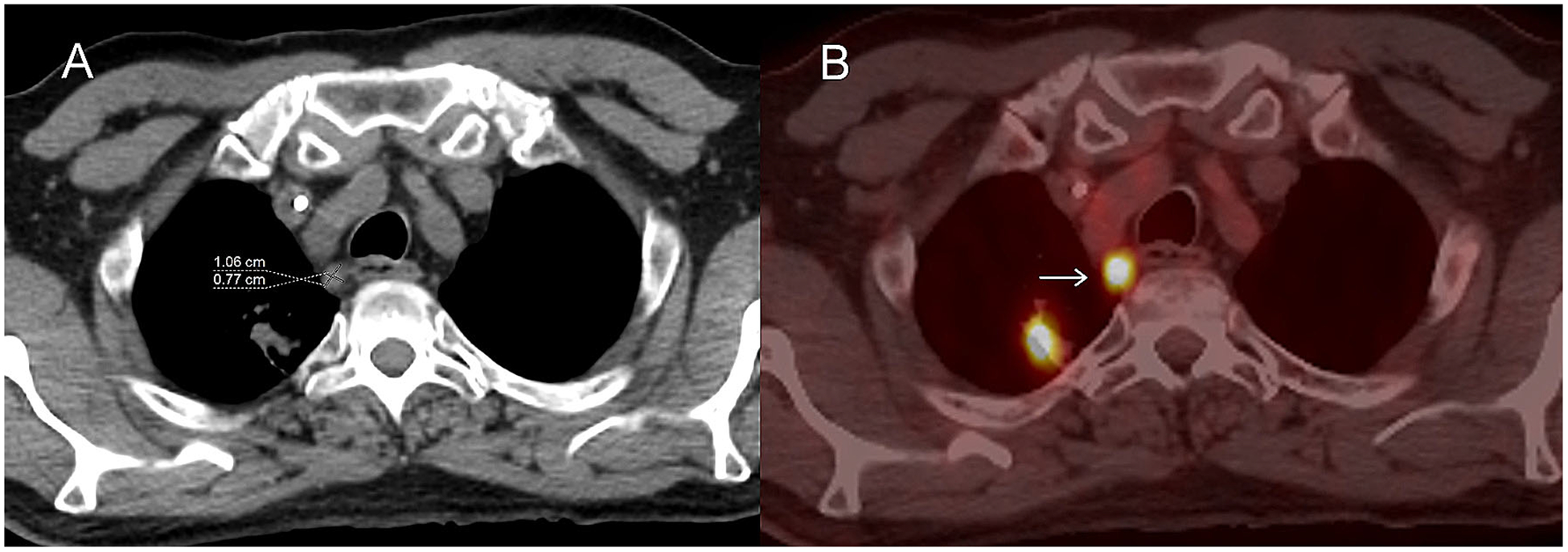
Axial images from an FDG PET/CT in a patient with metastatic lung cancer. Non-contrast CT image A shows a 0.8 cm short axis mediastinal lymph node which is hypermetabolic on fused axial PET/CT (B; arrow) and probably a metastatic lymph node. Although this is a site of disease it does not qualify as a non-target lesion because the short-axis is less than 1 cm.
Fig. 7.
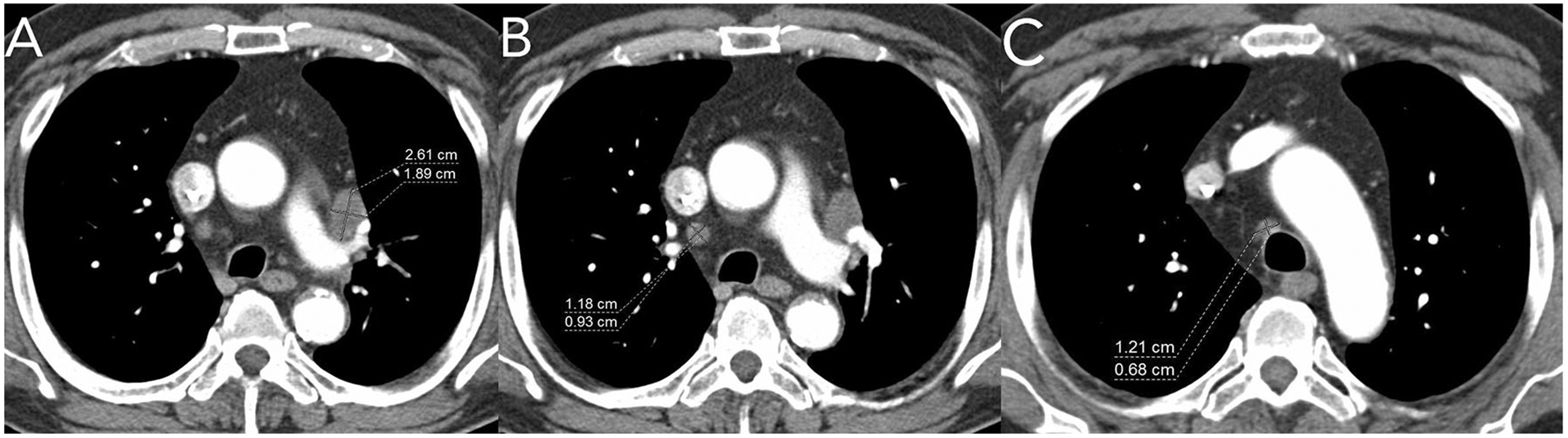
Axial post-contrast CT images from a patient with metastatic lung cancer. A shows a lymph node which was used as a target lesion. A non-target lesion of “other enlarged mediastinal lymph nodes” was also incorrectly delineated for the trial. The other nodes B, C do not meet size criteria for non-target lesions—they measure less than 1cm short-axis.
Response assessment errors
Incorrectly classifying lymph node response when short axis decreases to < 1 cm
RECIST v1.1 complete response for lymph nodes occurs when the node short axis falls below 1 cm. This seems like a simple principle but it can become problematic when multiple enlarged nodes are accounted for as a single non-target site of disease (e.g., ‘multiple mediastinal lymph nodes’). Great care must be taken when grouping lymph nodes so that only nodes of sufficient size are regarded as non-target lesions and these nodes must be scrutinized at follow-up so complete response is appropriately classified. For example, if there are multiple metastatic lymph nodes in a certain anatomic region at baseline, it can be helpful to mark nodes on the scan or list in the report nodes which exceed 1cm short-axis. That way at follow-up assessment it reminds the next reader to apply the 1 cm short-axis criteria to assign the proper response (CR, non-CR/non-PD, PD) to those specific nodes.
Classifying progression with only limited size change of non-target lesions
Progression by non-target lesions is more subjective as threshold size changes are not quantitatively specified (as they are for target lesions) (Fig. 8), but qualitatively determined as “unequivocal progression”. RECIST v1.1 points out that progression on the basis of non-target lesions in the absence of progressive disease by target lesions is possible but this should be an uncommon occurrence. It is imperative to properly assess non-target lesion response because if progressive disease is assigned for any non-target lesion the overall response becomes one of overall progression, regardless of the target lesion measurements. If a patient has measurable disease, RECIST v1.1 states the following regarding progression by non-target lesions, “In this setting, to achieve ‘unequivocal progression’ on the basis of the non-target disease, there must be an overall level of substantial worsening in non-target disease such that, even in presence of SD or PR in target disease, the overall tumour burden has increased sufficiently to merit discontinuation of therapy. A modest ‘increase’ in the size of one or more non-target lesions is usually not sufficient to quality for unequivocal progression status.” (Fig. 9) [7].
Fig. 8.
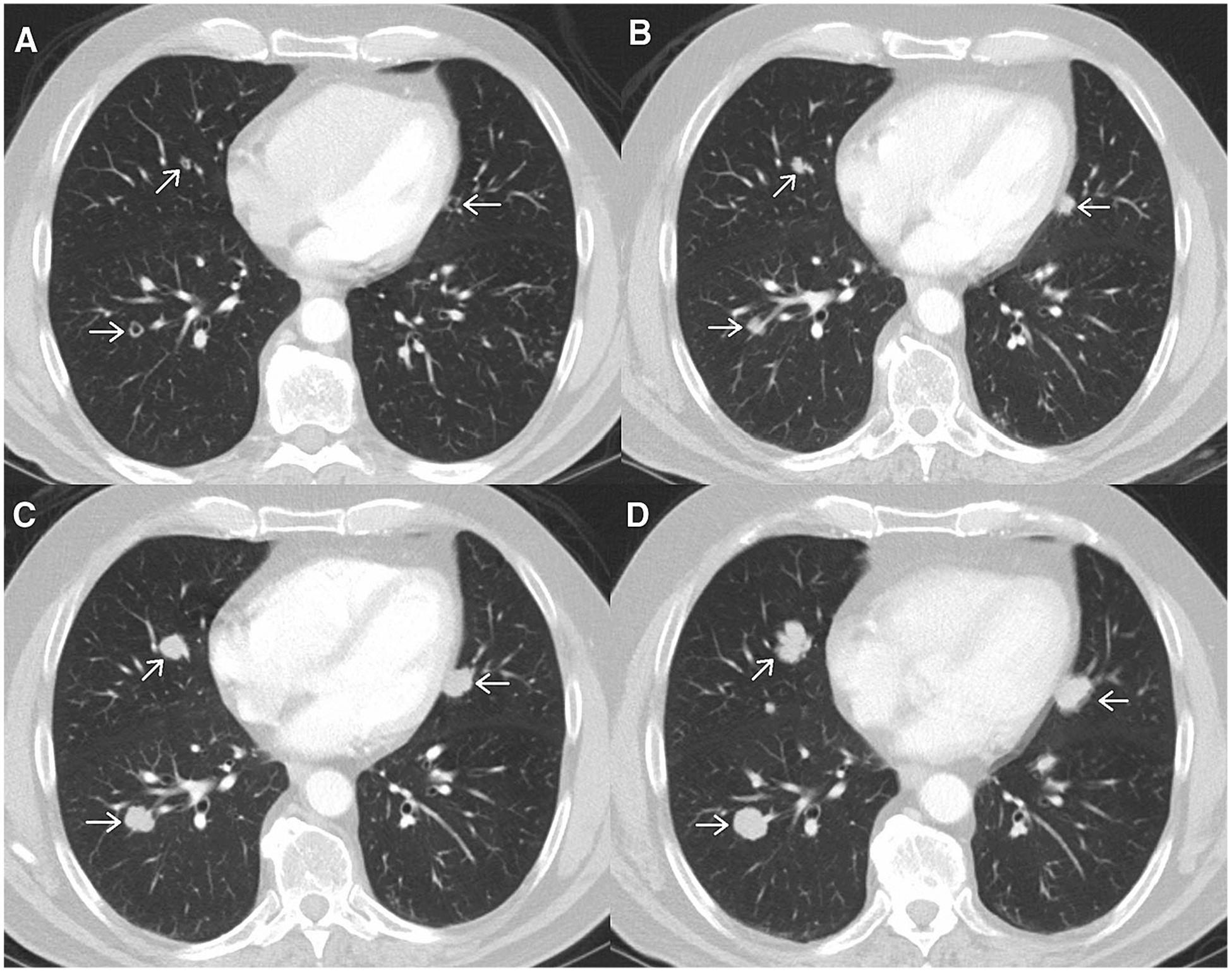
Axial post-contrast images from a patient with metastatic melanoma. Initial scan A shows multiple small nodules which can be accounted for as a non-target lesion of “multiple small pulmonary metastases”. Follow-up scans performed at two month intervals B, C, D show gradual increase in size of the nodules. This illustrates a potential difficulty arising when assessing non-target lesions per RECIST v1.1, different radiologists may disagree about the exact time point when “unequivocal progression” occurs.
Fig. 9.
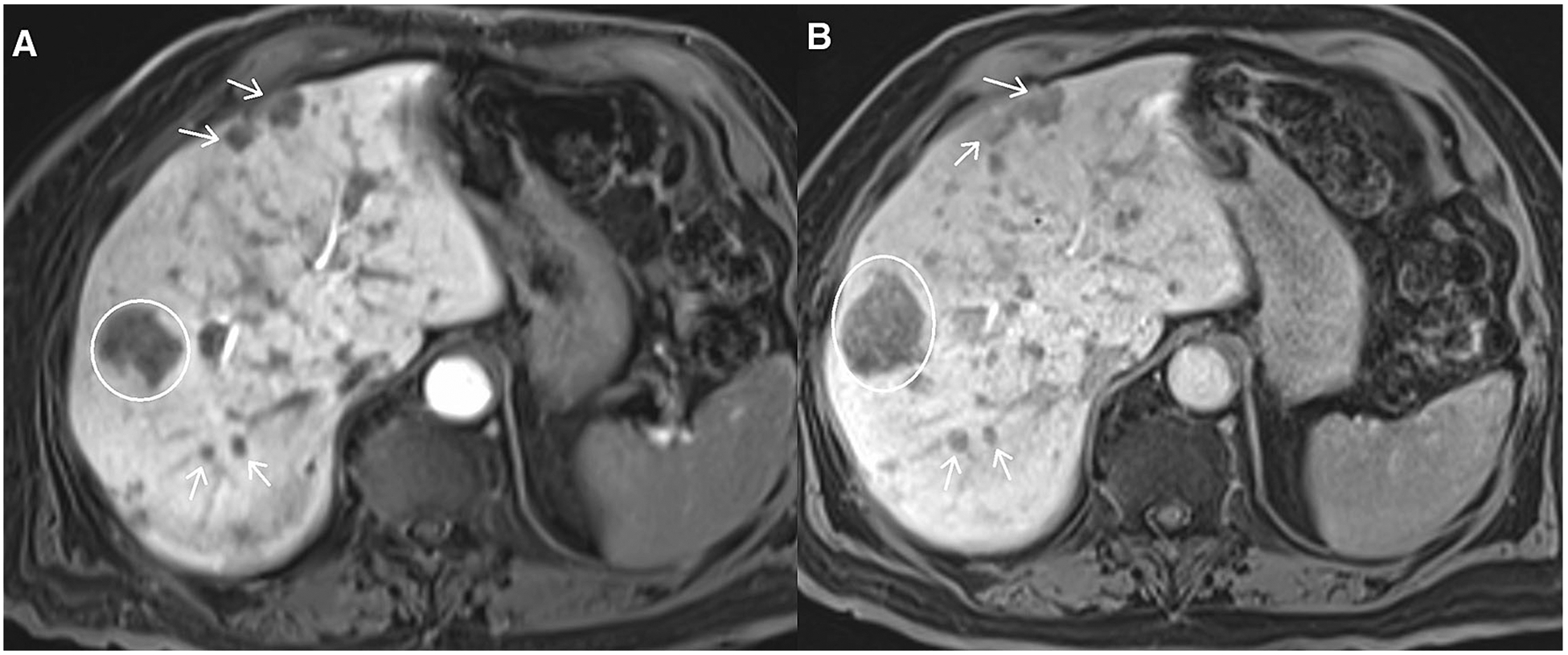
Axial post-contrast images from abdomen MRI using hepatobiliary contrast agent, multiple neuroendocrine tumor metastases to liver. A depicts a dominant metastasis (circle) and other smaller metastases (arrows). B shows that the dominant metastasis (circle) is larger but other smaller metastases (arrows) show little change. It would be incorrect to classify B as unequivocal progression based solely on the increase in size of one metastasis. When progression occurs by non-target lesions, it should reflect an increase in overall non-target tumor volume, not just a change in size of one isolated metastasis.
Summary
RECIST v1.1 provides a standardized approach for using cross-sectional imaging to evaluate therapy response. Radiologists must be familiar with the rules that govern classification of target vs. non-target lesions, as well as therapy response assessment using RECIST v1.1 guidelines. Familiarity with these rules and various possible pitfalls will help radiologists in the accurate analysis of RECIST v1.1 cases.
Funding
No funding was utilized for this work.
References
- 1.Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47(1):207–214 [DOI] [PubMed] [Google Scholar]
- 2.Therasse P, Arbuck SG, Eisenhauer EA, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216 [DOI] [PubMed] [Google Scholar]
- 3.Green S, Weiss GR (1992) Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Investig New Drugs 10(4):239–253 [DOI] [PubMed] [Google Scholar]
- 4.Warr D, McKinney S, Tannock I (1984) Influence of measurement error on assessment of response to anticancer chemotherapy: proposal for new criteria of tumor response. J Clin Oncol 2(9):1040–1046. 10.1200/JCO.1984.2.9.1040 [DOI] [PubMed] [Google Scholar]
- 5.Padhani AR, Ollivier L (2001) The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: implications for diagnostic radiologists. Br J Radiol 74(887):983–986. 10.1259/bjr.74.887.740983 [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Eisenhauer EA, Verweij J (2006) RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 42(8):1031–1039. 10.1016/j.ejca.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]


