Abstract
Background.
Isolated limb infusion (ILI) is a minimally invasive procedure for delivering high-dose regional chemotherapy to patients with locally advanced or in-transit melanoma located on a limb. The current international multicenter study evaluated the perioperative and long-term oncologic outcomes for patients who underwent ILI for stage 3B or 3C melanoma.
Methods.
Patients undergoing a first-time ILI for stage 3B or 3C melanoma (American Joint Committee on Cancer [AJCC] 7th ed) between 1992 and 2018 at five Australian and four United States of America (USA) tertiary referral centers were identified. The primary outcome measures included treatment response, in-field (IPFS) and distant progression-free survival (DPFS), and overall survival (OS).
Results.
A total of 687 first-time ILIs were performed (stage 3B: n = 383, 56%; stage 3C; n = 304, 44%). Significant limb toxicity (Wieberdink grade 4) developed in 27 patients (3.9%). No amputations (grade 5) were performed. The overall response rate was 64.1% (complete response [CR], 28.9%; partial response [PR], 35.2%). Stable disease (SD) occurred in 14.5% and progressive disease (PD) in 19.8% of the patients. The median follow-up period was 47 months, with a median OS of 38.2 months. When stratified by response, the patients with a CR or PR had a significantly longer median IPFS (21.9 vs 3.0 months; p < 0.0001), DPFS (53.6 vs 12.7 months; p < 0.0001), and OS (46.5 vs 24.4 months; p < 0.0001) than the nonresponders (SD + PD).
Conclusion.
This study is the largest to date reporting long-term outcomes of ILI for locoregionally metastatic melanoma. The findings demonstrate that ILI is effective and safe for patients with stage 3B or 3C melanoma confined to a limb. A favorable response to ILI is associated with significantly longer IFPS, DPFS, and OS.
With an increasing incidence of cutaneous melanoma worldwide, there is a corresponding increase in the number of patients with a diagnosis of locoregionally recurrent or metastatic disease (satellite lesions, in-transit disease, and regional nodal involvement).1 Whereas surgical resection often is effective for limited disease confined to a limb, bulky recurrences or disseminated lesions present a clinical treatment challenge. Since the 1950s, regional chemotherapy treatments, including hyperthermic isolated limb perfusion (HILP) and isolated limb infusion (ILI), have been used to treat these patients. Both procedures establish vascular isolation of the affected extremity to deliver much higher doses of chemotherapy than would be tolerated systemically.2
First described in 1994, ILI, a minimally invasive intra-arterial alternative to HILP, is technically less complex and associated with decreased procedure-related morbidity.2,3 Previous studies of ILI have demonstrated overall response rates (ORR) ranging from 50 to 100%, with complete response (CR) rates of 23% to 44%.4–10 Although these are reasonably satisfactory results, some patients do go on to experience distant metastatic disease.
Although ILI remains a valuable treatment strategy, effective systemic therapies are currently available. In the last decade, the management of distant metastatic melanoma evolved dramatically after the introduction of new systemic therapies shown to improve overall survival (OS).11–13
Studies also have shown intra-lesional agents to be effective for patients with metastatic melanoma confined to a limb.14–16
Given the range of treatment options currently available for patients with locally advanced regional disease, understanding the long-term oncologic outcomes of each treatment option will be of value to appropriately selected patients for specific therapies. Centers from Australia (AUS) and the United States of America (USA) currently have a large experience using ILI, with long-term follow-up evaluation. The current study was conducted to analyze and report an international multicenter experience, with the aim of defining the perioperative and long-term oncologic outcomes of patients who underwent ILI for locoregionally metastatic melanoma.
PATIENTS AND METHODS
After approval by each center’s institutional review board or ethics research committee, an international multicenter study was conducted that identified all patients who underwent first-time ILIs between 1992 and 2018. Patients eligible for inclusion in the study had in-transit melanoma confined to a limb, without distant metastatic disease (American Joint Committee on Cancer [AJCC] stage 3B or 3C disease).17 A history of other therapy (HILP, intra-lesional therapy, surgical resection, or systemic therapy) before to the first ILI was not an exclusion criterion.
The clinicopathologic characteristics abstracted were age, gender, stage of disease, involved extremity (upper vs lower), and burden of disease (BOD: low ≤ 10 lesions and no lesion > 2 cm; high ≥ 10 lesions or any one lesion > 2 cm18). Additional locoregional therapy (including a repeat ILI, surgery, or intra-lesional injections) or systemic therapies (cytotoxic chemotherapy, immunotherapy, targeted therapy) were permissible after disease progression at the discretion of the treating surgeon.
All participating institutions had demonstrated prior proficiency regarding the technical aspects of the procedure.8,9 The ILI procedure was performed as previously described.8,19 In brief, the cytotoxic drug combination of melphalan (7.5 mg/L for lower extremities and 10 mg/L for upper extremities) and actinomycin-D (100 μg/L) was used. Dosage was based on preoperative limb volume measurements. The maximum melphalan dose was 100 mg for lower and 50 mg for upper extremities. The USA centers routinely corrected the melphalan dose for ideal body weight (IBW).20 Patients with metastatic disease to groin or axillary lymph nodes underwent regional lymphadenectomy under the same general anesthesia after the ILI procedure.
After the procedure, patients were monitored by physical examination and serum creatine phosphokinase (CPK) levels. Limb toxicity was assessed according to the Wieberdink grading scale.21 At the USA centers, response to treatment was determined 3 months after the procedure according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for cutaneous lesions.22 The Australian centers measured treatment response using the World Health Organization (WHO) criteria for reporting results of cancer treatment.23 The best response was captured after two observations more than 4 weeks apart.
Continuous variables are presented as medians and interquartile ranges (IQR), and categorical variables are described as frequencies.
The outcomes measured included treatment response, in-field progression-free survival (IPFS), distant progression-free survival (DPFS), and OS. Time to in-field progression, distant progression, and death were calculated from the time of ILI procedure. Log-rank tests were used to compare responders with nonresponders regarding distribution of time to event. Kaplan–Meier survival curves were used to display outcomes. Multivariate Cox proportional hazard models with stepwise backward methods were used to analyze survival. Alpha was set at 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 687 patients underwent a first-time ILI. Table 1 reflects the baseline clinicopathologic characteristics of the cohort. The median age was 71 years (interquartile range [IQR], 62–79 years), and the majority of the patients were women (60%, n = 412). Most had lower extremity ILIs (n = 610, 88%). The median Breslow thickness of the primary melanoma was 2.7 mm. At the time of ILI, distribution of AJCC stages 3B and 3C tumors were respectively 55.7% (n = 383) and 44.3% (n = 304).
TABLE 1.
Patient and tumor characteristics
| Variable | Patients |
|---|---|
| n (%) | |
| Median age: years (IQR) | 71 (62–79) |
| Sex | |
| Male | 275 (40) |
| Female | 412 (60) |
| Extremity | |
| Upper | 77 (11.2) |
| Lower | 610 (88.8) |
| Disease stage at time of ILI | |
| 3B | 383 (55.7) |
| 3C | 304 (44.3) |
| Burden of disease | |
| Low | 371 (54.2) |
| High | 313 (45.8) |
| Median Breslow thickness of primary melanoma: mm (IQR) | 2.67 (1.6–4.1) |
IQR interquartile range, ILI isolated limb infusion
Perioperative Data
The procedural details are reported in Table 2. The median hospital stay after ILI was 7 days (IQR, 5–9 days). Wieberdink grade 1 limb toxicity was experienced by 11.1% (n = 76), grade 2 by 59.8% (n = 411), grade 3 by 24% (n = 165), and grade 4 by 3.9% (n = 27) of the patients. No grade 5 limb toxicity (warranting amputation) occurred. The median peak CPK was 577 IU/L (IQR, 158–1907 IU/L) at a median of 4 days (IQR, 3–5 days) after the procedure.
TABLE 2.
Perioperative and clinical outcomes
| Variable | |
|---|---|
| Median limb volume: L (IQR) | 6.5 (5–8.1) |
| Median melphalan dose: mg (IQR) | 43 (34–50.5) |
| Median actinomycin dose: mg (IQR) | 493 (350–575) |
| Median drug circulation time: min (IQR) | 30 (20–30) |
| Median tourniquet time: min (IQR) | 54 (44–67) |
| Median postoperative CPK peak value: IU/L (IQR) | 577 (158–1907) |
| Median CPK peak postoperative day (IQR) | 4 (3–5) |
| Median hospital stay: days (IQR) | 7 (5–9) |
| Wieberdink toxicity score21: n (%) | |
| 1 (no visible effect) | 76 (11.1) |
| 2 (slight erythema and/or edema) | 411 (59.8) |
| 3 (considerable erythema and/or edema with blistering) | 165 (24) |
| 4 (epidermolysis and/or obvious damage to deep tissues with a threatened or actual compartment syndrome) | 27 (3.9) |
| 5 (severe tissue damage necessitating amputation) | 0 |
| Unknown | 8 (1.2) |
| Treatment response: n (%) | |
| Complete response | 199 (28.9) |
| Partial response | 242 (35.2) |
| Stable disease | 100 (14.5) |
| Progressive disease | 136 (19.8) |
| Unknown | 10 (1.6) |
IQR interquartile range, CPK creatine phosphokinase
Treatment Response and Survival
The ORR (complete response [CR] plus partial response [PR]) was 64.1%, and the disease control rate (DCR: CR + PR + stable disease [SD]) was 78.6% (Table 2). A CR was observed in 28.9% of the patients (n = 199), whereas progressive disease (PD) occurred in 19.8% of the patients (n = 136). During a median follow-up period of 47 months, median IPFS was 10.1 months, median DPFS was 28.6 months, and median OS was 38.2 months (Fig. 1). The stage 3C patients had a significantly worse IPFS (12.7 vs 7.1 months), DPFS (65.3 vs 15.2 months), and OS (48.4 vs 26.9 months) (all p < 0.0001) compared to stage 3B patients. The patients with a high BOD also experienced a worse IPFS (11 vs 8.3 months), DPFS (38 vs 20.9 months), and OS (51.1 vs 27.7 months) (all p < 0.0001) compared to those with low BOD.
FIG. 1.
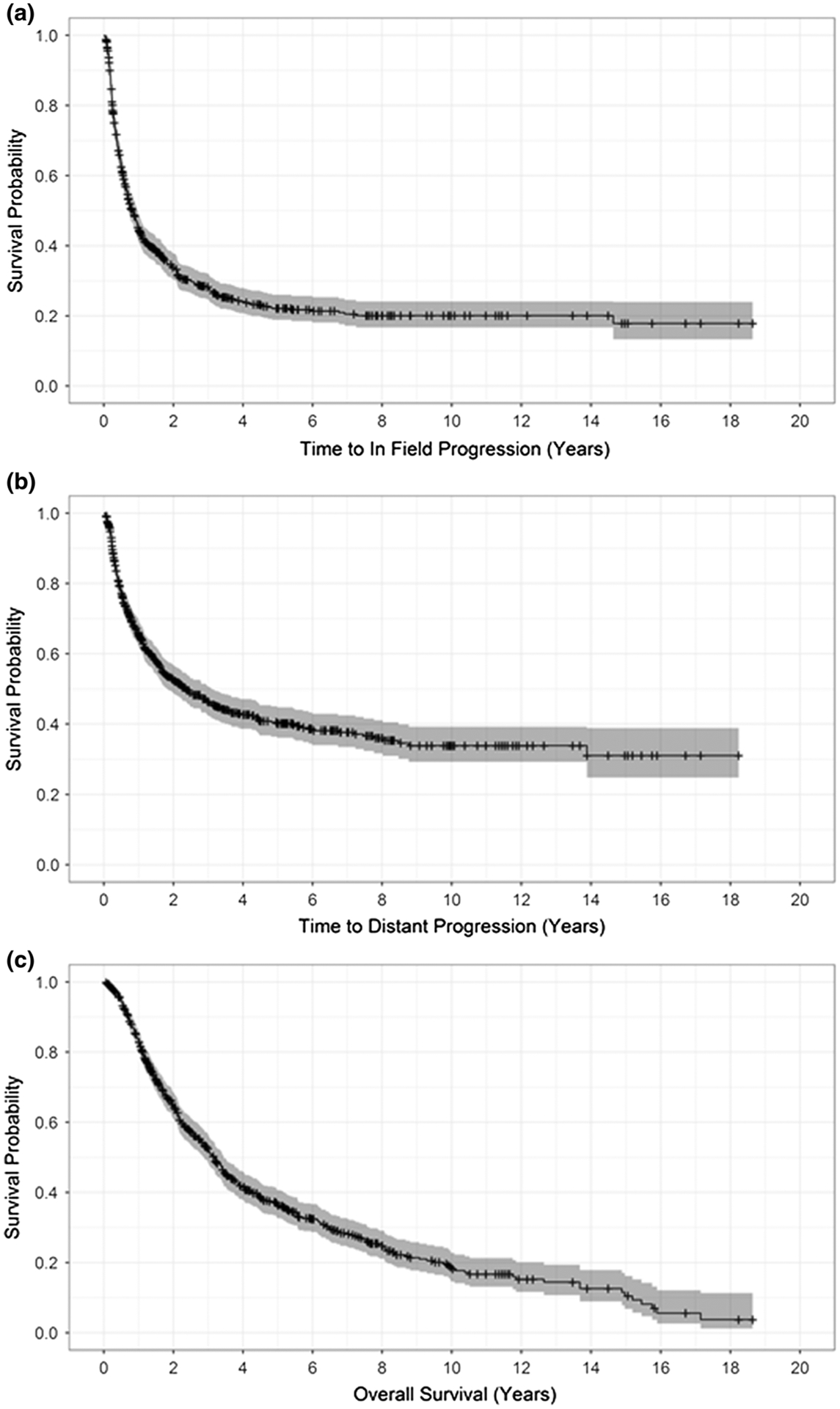
a In-field progression-free survival, b distant progression-free survival, and c overall survival (months) after isolated limb infusion for the entire cohort of 687 patients
The responders (CR + PR) had a significantly longer median IPFS (21.9 vs 3.0 months; p < 0.0001), DPFS (53.6 vs 12.7 months; p < 0.0001), and OS (46.5 vs 24.4 months; p < 0.0001) than nonresponders (SD + PD). The median IPFS by treatment response was 36.7 months for the patients with a CR, 13.5 months for those with a PR, 5.4 months for those with a SD, and 2.4 months for those with a PD (p < 0.0001, Fig. 2a). Similarly, improved DPFS was observed among the patients with a CR (CR: 101.7 months vs. PR: 26.3 months; SD: 14.7 months; PD: 10.1 months; p < 0.0001, Fig. 2b). In the multivariate models, the factors independently associated with both IPFS and DPFS were stage of disease and treatment response (Table 3).
FIG. 2.
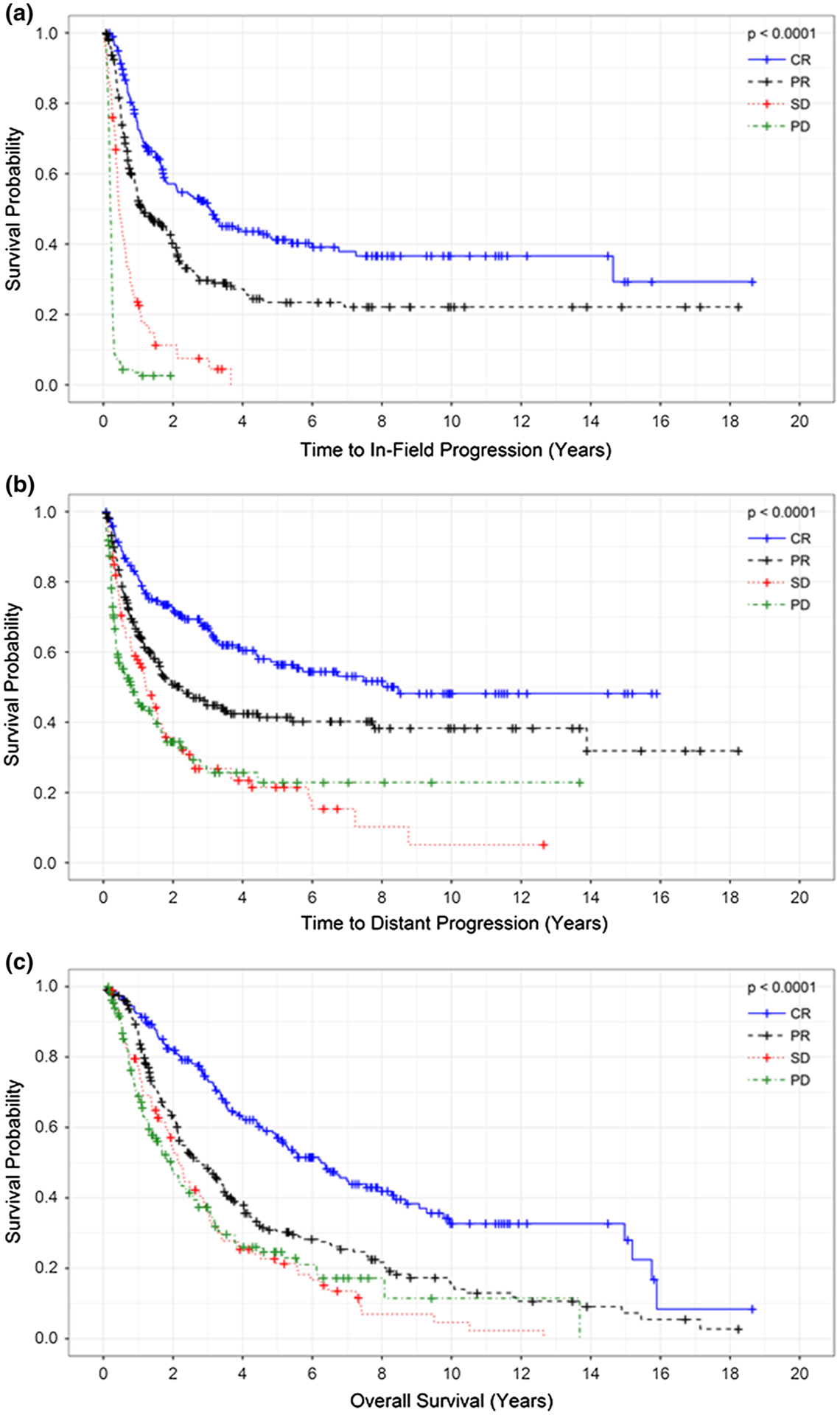
a In-field progression-free survival, b distant progression-free survival, and c overall survival (months) stratified by treatment response after isolated limb infusion. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease
TABLE 3.
Uni- and multivariate Cox proportional hazards analysis assessing variables associated with (a) in-field and (b) distant progression-free survival and (c) overall survival
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| (a) In-field progression-free survival | ||||
| Age at diagnosis | 0.99 (0.99–1.01) | 0.81 | ||
| Breslow thickness of primary melanoma | 0.99 (0.95–1.04) | 0.71 | ||
| Gender | ||||
| Male | Reference | |||
| Female | 0.9 (0.73–1.12) | 0.34 | ||
| Wieberdink toxicity | 1.05 (0.9–1.23) | 0.53 | ||
| Extremity | ||||
| Upper extremity | Reference | |||
| Lower extremity | 0.99 (0.72–1.39) | 0.99 | ||
| Stage | ||||
| 3C | Reference | Reference | 0.04 | |
| 3B | 0.83 (0.66–1.03) | 0.09 | 0.81 (0.66–0.99) | |
| Burden of disease | ||||
| Low | Reference | |||
| High | 1.05 (0.85–1.3) | 0.62 | ||
| Treatment response | ||||
| Complete response | Reference | Reference | ||
| Partial response | 1.74 (1.32–2.28) | < 0.0001 | 1.74 (1.33–2.28) | < 0.0001 |
| Stable disease | 4.09 (2.91–5.73) | < 0.0001 | 4.09 (2.95–5.68) | < 0.0001 |
| Progressive disease | 15.06 (10.86–20.87) | < 0.0001 | 15.01 (10.87–20.73) | < 0.0001 |
| (b) Distant progression-free survival | ||||
| Age at diagnosis | 0.99 (0.98–0.99) | 0.01 | 0.99 (0.98–0.99) | 0.01 |
| Breslow thickness | 0.98 (0.93–1.03) | 0.34 | ||
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.86 (0.68–1.1) | 0.22 | 0.86 (0.66–1.1) | 0.22 |
| Wieberdink toxicity | 0.92 (0.77–1.09) | 0.33 | 0.9 (0.76–1.07) | 0.24 |
| Extremity | ||||
| Upper extremity | Reference | Reference | ||
| Lower extremity | 0.81 (0.56–1.18) | 0.27 | 0.8 (0.56–1.16) | 0.24 |
| Stage | ||||
| 3C | Reference | Reference | ||
| 3B | 0.56 (0.44–0.71) | < 0.001 | 0.55 (0.44–0.7) | < 0.0001 |
| Burden of disease | ||||
| Low | Reference | |||
| High | 1.1 (0.87–1.40) | 0.43 | ||
| Treatment response | ||||
| Complete response | Reference | Reference | ||
| Partial response | 1.84 (1.35–2.5) | 0.0001 | 1.83 (1.34–2.49) | 0.0001 |
| Stable disease | 2.74 (1.89–3.96) | < 0.0001 | 2.67 (1.86–3.84) | < 0.0001 |
| Progressive disease | 3.05 (2.16–4.32) | < 0.0001 | 3.04 (2.15–4.3) | < 0.0001 |
| (c) Overall survival | ||||
| Age at diagnosis | 1.02 (1.01–1.03) | < 0.0001 | 1.02 (1.01–1.03) | < 0.0001 |
| Breslow thickness | 1.05 (1.00–1.09) | 0.04 | 1.05 (1.00–1.09) | 0.04 |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 0.83 (0.66–1.05) | 0.12 | 0.84 (0.67–1.05) | 0.13 |
| Wieberdink toxicity | 1.00 (0.85–1.19) | 0.98 | ||
| Extremity | ||||
| Upper extremity | Reference | |||
| Lower extremity | 1.06 (0.73–1.55) | 0.75 | ||
| Stage | ||||
| 3C | Reference | Reference | ||
| 3B | 0.67 (0.53–0.85) | 0.001 | 0.67 (0.53–0.84) | 0.0007 |
| Burden of disease | ||||
| Low | Reference | Reference | ||
| High | 1.35 (1.07–1.71) | 0.01 | 1.35 (1.07–1.69) | 0.01 |
| Treatment response | ||||
| Complete response | Reference | Reference | ||
| Partial response | 1.8 (1.36–2.39) | 0.0001 | 1.8 (1.36–2.39) | < 0.0001 |
| Stable disease | 2.35 (1.63–3.4) | < 0.0001 | 2.35 (1.63–3.38) | < 0.0001 |
| Progressive disease | 2.36 (1.68–3.32) | < 0.0001 | 2.36 (1.67–3.31) | < 0.0001 |
HR hazard ratio, CI confidence interval
In the unadjusted survival analysis, the patients with a CR after ILI had a median OS of 75 months compared with 33.2 months for those with a PR, 26.1 months for those with an SD, and 23.5 months for those with a PD (p < 0.0001; Fig. 2c). This improved survival after a favorable response was preserved in the multivariate analysis, which demonstrated that a CR, low BOD, stage 3B disease, and a thinner primary melanoma Breslow thickness were independently associated with improved survival (Table 3c).
DISCUSSION
This study reports the outcome for 687 patients undergoing a first-time ILI for stage 3B or 3C melanoma with a median follow-up of almost 4 years (47 months). The ORR in this large cohort was 64.1%, and the OS was 38.2 months. Although 20% of the patients experienced disease progression soon after ILI, the patients who achieved a CR (28.9%) had a significantly better survival (OS, 75 months), suggesting a durable therapeutic effect after ILI for those patients.
Multiple single and multicenter studies have indicated the efficacy of ILI for melanoma. The largest single-center experience was reported by the Sydney Melanoma Unit (SMU, currently the Melanoma Institute Australia). This experience involved 185 patients and reported a CR of 38% and an ORR of 84%.6 Although these results were not reproduced in large multicenter studies, the response rates remained favorable and consistent, with ORR ranging from 57 to 75%,7,18 and the ORR of 64.1% in the current study is compatible with these previous series.
The variance in treatment efficacy can be partially explained by the heterogeneity in the patient populations and the subtleties of the selection criteria. In the SMU experience, 11% of the patients had lower disease stages (AJCC stage 1 or 2), which possibly contributed to the higher ORR. The current study limited inclusion to patients with stage 3B or 3C disease only, capturing a cohort of patients with more advanced disease and thus likely to be less responsive to ILI, especially because BOD was identified as an independent associated factor for response.
Although the majority of patients treated by ILI respond from the first few months after treatment, the durability of response and its effect on survival remain poorly defined. In the current study, we sought to address this by performing an analysis in which patients were stratified based on response to treatment. As expected, the patients who responded to therapy (CR + PR) had both a significantly longer IPFS (21.9 vs 3.0 months) and DPFS (53.6 vs 12.7 months) than the nonresponders. Importantly, the improvement in progression-free survival, both regionally and distantly, also translated into an OS benefit (responders: 46.5 months vs nonresponders: 24.4 months), indicating that the response to ILI is associated with durable survival. Although responders did achieve a prolongation of progression-free survival, disease progression and recurrence tended to be mostly locoregional, as indicated by the shorter IPFS compared with DPFS. Therefore, repeat ILI could be considered for this subgroup of patients because prior studies have demonstrated that repeat ILIs are safe and achieve response rates similar to those for initial ILI procedures.7,24 For example, O’Donoghue et al.7 < demonstrated similar limb toxicity and ORR when comparing initial and repeat ILIs (58.4% vs 60%; p = 0.7). In contrast, nonresponders experienced disease progression early after treatment, at a median of 3 months. From a clinical perspective, early recognition of nonresponders could allow a more expeditious transition to other therapies. Although tumor biology is likely the major driver for resistance to ILI, prompt recognition of early failures could signify the need for clinicians to use other therapies, perhaps prolonging survival.
Given the multiple different treatment options currently available for locoregionally metastatic melanoma, the challenge is to select and tailor the appropriate therapy for the individual patient. Currently, the National Comprehensive Cancer Network guidelines catalog ILI, intra-lesional therapy and systemic treatment options as appropriate considerations for these patients.25 However, the lack of comparative analyses between these treatment options is a limitation of this recommendation. As a result, treatment selection is left to the clinician’s discretion.
Talimogene laherparepvec (TVEC) (AMGEN Inc., Thousand Oaks, CA, USA) is the only FDA-approved intra-lesional therapy available for patients with unresectable stage 3B or 3C melanoma, but it is not available in all countries. The FDA approval was based on the Oncovex Pivotal Trial in Melanoma (OPTiM), which treated patients with stages 3B, 3C, and 4 melanoma with TVEC and observed an ORR of 26.4%.26 In the subgroup of patients with stage 3B or 3C disease, the ORR was 52.3%.
Although ILI results in a higher ORR, individual patient factors must be considered in selecting the best treatment option when both treatment methods are available. For example, it is not uncommon for patients, such as those included in this study, to travel great distances for treatment at tertiary referral centers. In such cases, ILI may be preferred over intra-lesional therapy, which requires repeated injections at 2-week intervals. Additionally, BOD may influence treatment selection because findings have demonstrated that in the setting of a high BOD, ILI is associated with an ORR of 47%.18
In contrast, with intra-lesional agents, only a fixed volume can be delivered during each treatment session. Thus, in the setting of high BOD, it is unlikely that all sites of disease can be injected, thereby decreasing the direct treatment effect. However, intra-lesional therapies may be a more attractive option for those who cannot tolerate general anesthesia, those who have progressed after ILI, and those with lesions involving the head, neck, and truncal regions.
Another alternative to ILI currently available is systemic immunotherapy or targeted therapy. With systemic approaches, response rates reaching 60% have been reported, but the treatment can be associated with significant toxicities, including severe dose-limiting autoimmunity and even death, especially when delivered as a dual checkpoint inhibitor regimen.11
An alternative treatment strategy combining locoregional therapies with systemic agents also has been investigated. Findings have demonstrated intra-lesional agents combined with checkpoint inhibitors to be safe, but the ORR (57%) is similar to those reported after ILI or TVEC when delivered alone.27 Additionally, a phase 2 trial by Ariyan et al.28 investigated whether the addition of ipilimumab, a CTLA-4 antibody, to ILI would augment response rates and survival. For the 26 patients enrolled, an ORR of 85% (n = 22) was achieved, 62% (n = 16) of whom had a CR. Although these results are promising, 38% of the patients experienced grade 3 or 4 ipilimumab-related toxicities, with colitis/diarrhea the most commonly reported severe adverse event. In contrast, response rates after ILI as a single treatment method remain consistent, with CR rates of 35%, moderate regional toxicity, and no systemic toxicity or procedure-related mortality.8,9,29 Therefore, until new systemic agents are able to achieve durable results with less toxicity, treatment by ILI should be considered in the context of the known risks and benefits of available treatment options.
Although the current study represents the largest series to date reporting outcomes after ILI, its retrospective nature represents an inherent limitation, and the study had several other limitations. Because the study was not conducted as a prospective multicenter series, minor protocol variations between institutions were present. Nevertheless, we attempted to minimize the impact of confounders by limiting the study to a select group of patients (with stage 3B or 3C disease). In addition, only high-volume centers that routinely perform ILI were included to minimize the impact of the learning curve associated with the procedure. We were unable to capture all additional therapies that patients may have received during the course of their disease, and it is likely that patients received multiple sequential lines of therapy, which ultimately had an impact on OS. Finally, the variability between the USA and Australia centers in how response to ILI was assessed represented a possible limitation. Although the criteria assessing response were similar (World Health Organization [WHO] vs RECIST), the lack of a standardized reporting system may have affected the analysis of response rates. Nonetheless, the study did demonstrate that treatment response is independently associated with improved survival.
In conclusion, this is the largest series reporting the safety, efficacy, and long-term oncologic outcomes of ILI for locoregionally metastatic melanoma to date. We report that ILI can be safely performed and is well tolerated, with low treatment-related limb toxicity and no significant risk of amputation or mortality. A CR or PR response after ILI was associated with a longer IPFS, DPFS, and OS. As the management of locoregionally metastatic melanoma continues to evolve, criteria for the selection of patients who could benefit from ILI should remain a primary focus of ongoing investigation.
Footnotes
DISCLOSURE
There are no relevant conflicts of interest to disclose related to the subject matter in the manuscript.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JF, Lai DT, Ingvar C, et al. Maximizing efficacy and minimizing toxicity in isolated limb perfusion for melanoma. Melanoma Res. 1994;4(Suppl 1):45–50. [PubMed] [Google Scholar]
- 3.Kroon HM, Coventry BJ, Giles MH, et al. Safety and efficacy of isolated limb infusion chemotherapy for advanced locoregional melanoma in elderly patients: an Australian multicenter study. Ann Surg Oncol. 2017;24:3245–51. [DOI] [PubMed] [Google Scholar]
- 4.Mian R, Henderson MA, Speakman D, et al. Isolated limb infusion for melanoma: a simple alternative to isolated limb perfusion. Can J Surg. 2001;44:189–92. [PMC free article] [PubMed] [Google Scholar]
- 5.Lindner P, Doubrovsky A, Kam PC, et al. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–36. [DOI] [PubMed] [Google Scholar]
- 6.Kroon HM, Moncrieff M, Kam PC, et al. Outcomes following isolated limb infusion for melanoma: a 14-year experience. Ann Surg Oncol. 2008;15:3003–13. [DOI] [PubMed] [Google Scholar]
- 7.O’Donoghue C, Perez MC, Mullinax JE, et al. Isolated limb infusion: a single-center experience with over 200 infusions. Ann Surg Oncol. 2017;24:3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–15; discussion 715–7. [DOI] [PubMed] [Google Scholar]
- 9.Kroon HM, Coventry BJ, Giles MH, et al. Australian multicenter study of isolated limb infusion for melanoma. Ann Surg Oncol. 2016;23:1096–103. [DOI] [PubMed] [Google Scholar]
- 10.Coventry BJ, Kroon HM, Giles MH, et al. Australian multicenter experience outside of the Sydney Melanoma Unit of isolated limb infusion chemotherapy for melanoma. J Surg Oncol. 2014;109:780–5. [DOI] [PubMed] [Google Scholar]
- 11.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 14.Weitman ES, Zager JS. Regional therapies for locoregionally advanced and unresectable melanoma. Clin Exp Metastasis. 2018;35:495–502. [DOI] [PubMed] [Google Scholar]
- 15.Miura JT, Zager JS. Intralesional therapy as a treatment for locoregionally metastatic melanoma. Expert Rev Anticancer Ther. 2018;18:399–408. [DOI] [PubMed] [Google Scholar]
- 16.Luu C, Khushalani NI, Zager JS. Intralesional and systemic immunotherapy for metastatic melanoma. Expert Opin Biol Ther. 2016;16:1491–9. [DOI] [PubMed] [Google Scholar]
- 17.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muilenburg DJ, Beasley GM, Thompson ZJ, et al. Burden of disease predicts response to isolated limb infusion with melphalan and actinomycin D in melanoma. Ann Surg Oncol. 2015;22:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroon HM, Huismans A, Waugh RC, et al. Isolated limb infusion: technical aspects. J Surg Oncol. 2014;109:352–6. [DOI] [PubMed] [Google Scholar]
- 20.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–205. [DOI] [PubMed] [Google Scholar]
- 21.Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–10. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 23.Hunter RD. World Health Organization (WHO) handbook for reporting results of cancer treatment. WHO, Geneva, Switzerland, 1979. [Google Scholar]
- 24.Chai CY, Deneve JL, Beasley GM, et al. A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Ann Surg Oncol. 2012;19:1637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NCCN clinical practice guidelines in oncology: melanoma. National Comprehensive Cancer Network, Montgomery: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–8. [DOI] [PubMed] [Google Scholar]
- 27.Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(Suppl 15):9568. [Google Scholar]
- 28.Ariyan CE, Brady MS, Siegelbaum RH, et al. Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res. 2018;6:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroon HM, Huismans AM, Kam PC, et al. Isolated limb infusion with melphalan and actinomycin D for melanoma: a systematic review. J Surg Oncol. 2014;109:348–51. [DOI] [PubMed] [Google Scholar]


