Abstract
Background:
The impact of oncogene panel results on the surgical management of indeterminate thyroid nodules (ITNs) is currently unknown.
Methods:
Surgical management of 649 patients consecutively evaluated from October 2008 to April 2016 with a single nodule biopsied and indeterminate cytology (193 evaluated with and 456 without oncogene panels) was assessed and compared. Histological features of 629 consecutively resected ITNs (164 evaluated with and 465 without oncogene panels) were also characterized and compared.
Results:
Oncogene panel evaluation was associated with higher rates of total thyroidectomy (45% vs 28%; P = .006), and central lymph node dissection (19% vs 12%; P = .03) without increasing the yield of malignancy or decreasing the rate of completion thyroidectomy. Most malignancies (64%), including 83% of those with driver mutation identified, were low-risk cancers for which a lobectomy could have been sufficient initial treatment.
Conclusion:
Current oncogene panel results seem insufficient to guide the surgical extent of solitary ITNs.
Keywords: molecular marker tests, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), thyroid cancer, thyroid nodule, thyroid surgery
1 |. INTRODUCTION
Several molecular marker tests are commercially available to characterize thyroid nodules with indeterminate cytology. These tests can be classified according to their ability to identify nodules that are most likely benign (“rule-out” tests) or malignant (“rule-in” tests).1 Whereas the potential benefit of rule-out tests is avoiding unnecessary diagnostic surgery; the potential benefit of the rule-in tests is avoiding the need for a completion thyroidectomy by identifying cancer before a diagnostic procedure.1,2 Oncogene panels are generally considered rule-in tests, although more recent panels like ThyroSeq version 2 (University of Pittsburgh Medical Center, Pittsburgh, PA) also claim rule-out ability.3,4 In our experience, this panel seemed reliable as rule-out test in follicular neoplasms but not in atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS).5 Besides the need to bring the performance of oncogene panels into sharper focus, their role in planning the extent of surgery is controversial and needs to be defined.2,6–8 The 2015 American Thyroid Association (ATA) recommends thyroid lobectomy for solitary, cytologically indeterminate thyroid nodules (ITNs) but suggest that total thyroidectomy may be preferred if a mutation specific for carcinoma is identified, given the higher risk for cancer (recommendation #20).9 This was our institutional approach until recently, when total thyroidectomy was the preferred treatment for all thyroid cancers >1 cm.10 However, current data support that thyroid lobectomy may be sufficient initial treatment for low-risk differentiated thyroid cancer.9
Recent publications suggest that most malignant ITNs are follicular variant of papillary thyroid carcinomas (FVPTCs) or minimally invasive follicular thyroid carcinomas.11–15 These tumors have very low invasive and metastatic potential and may not need completion thyroidectomy, as suggested in the 2015 ATA guidelines.9,16,17 This is particularly true for the encapsulated noninvasive FVPTCs for which a nomenclature change has been recently proposed to noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) that removes the term carcinoma recognizing their indolent behavior.18 The NIFTPs seem to be very prevalent among RAS-mutant tumors8,19; and RAS mutations are the most frequently identified mutations by oncogene panels in ITNs.3,4,20,21 If thyroid cancers among ITNs are mostly low-risk malignancies, a completion thyroidectomy could be unnecessary after most diagnostic lobectomies; and a total thyroidectomy may put patients at higher surgical risks to gain no clinical benefit.22
The purposes of this study were to: (1) determine the impact and appropriateness of oncogene panel evaluation on the surgical management of ITNs; and (2) characterize the histological features of ITNs looking for differences between nodules with positive and negative oncogene panel results.
2 |. PATIENTS AND METHODS
2.1 |. Study cohort
We retrospectively reviewed the medical records of 1053 consecutive ITNs (AUS/FLUS or follicular neoplasm) evaluated at our institution between October 2008 and April 2016 under an institutional review board-approved study. The ITNs were evaluated with oncogene panels sporadically until February 2014 (<5% of the ITNs); however, they have been consistently offered since then (65% of the ITNs; 85% of those biopsied at H. Lee Moffitt Cancer Center); except for a 3-month period in 2015 due to licensing issues of ThyroSeq version 2 in Florida. We excluded 24 nodules (2%) with presurgical evidence of thyroid malignancy despite the indeterminate cytology (see Figure 1). The other 1029 ITNs were classified into 2 groups: nodules evaluated with oncogene panels (OP group) or without oncogene panels (control group). The OP group was further subclassified into nodules with positive (OP_positive) or negative (OP_negative) oncogene panel results.
FIGURE 1.
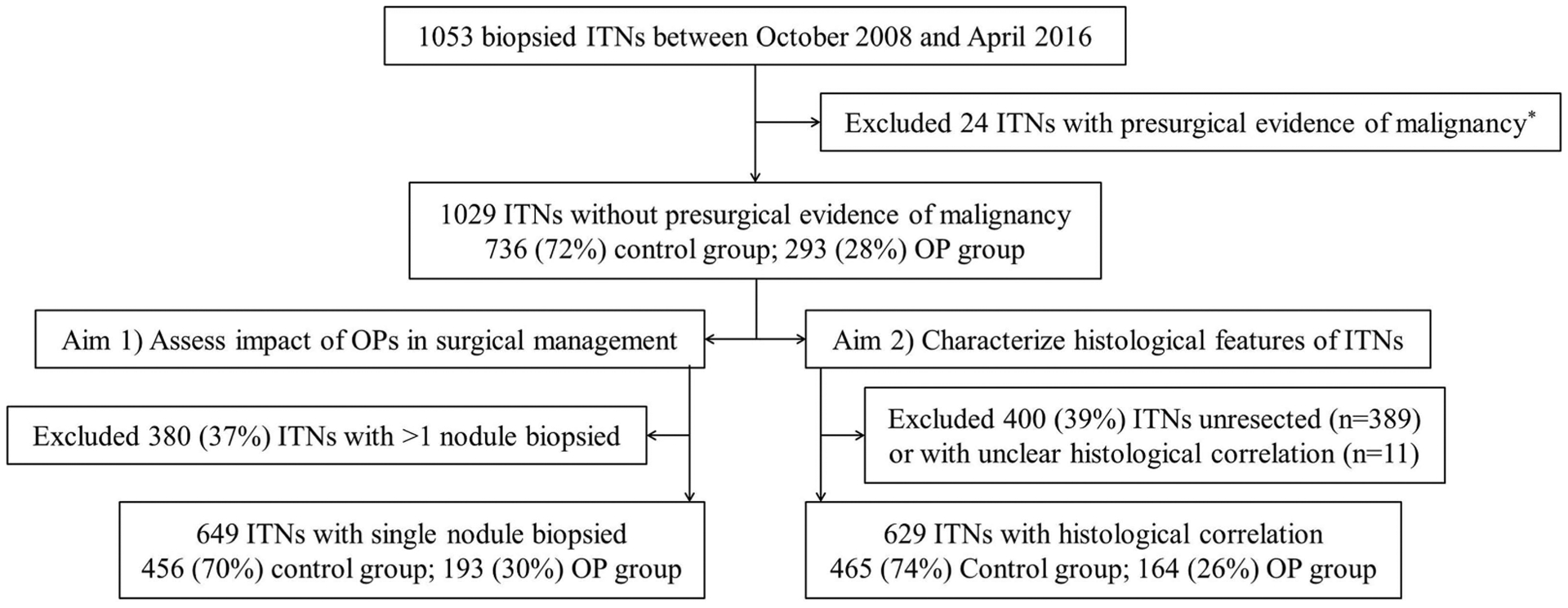
Patient cohort selection process. *Due to vocal cord paralysis (n = 5); proven cervical lymph node metastasis (n = 14); vocal cord paralysis and proven lymph node metastasis (n = 1); proven distant metastasis (n = 1); elevated basal serum calcitonin (>100 pg/mL; n = 2); and explosive growth (n = 1). Control group, nodules evaluated without oncogene panels; ITNs, Cytologically indeterminate thyroid nodules; OP, oncogene panel; OP group, nodules evaluated with oncogene panels
2.2 |. Aim 1: Impact of oncogene panels on surgical management of indeterminate thyroid nodules
To avoid confounding factors in this analysis we excluded patients with multiple nodules biopsied (see Figure 1). Rate of resection (any extent), prevalence of malignancy, extent of initial surgery, and rate of completion thyroidectomy were calculated for all the groups. Differences in surgical extent were adjusted for other factors that could have influenced the surgical management: family history of thyroid cancer in first-degree relatives; personal medical history of radiation exposure to the neck; known hereditary syndrome associated with thyroid cancer; other cancer diagnosis; presence of multinodular goiter (defined as ≥2 nodules >5 mm); significant contralateral nodules (defined as at least one nodule >1 cm on the contralateral lobe to the nodule biopsied); and presurgical thyroid dysfunction. We also evaluated the appropriateness of the initial surgical extent considering that a lobectomy alone would be sufficient initial treatment for low-risk malignancies but not for intermediate or high-risk cancers (see section 2.4 “Pathology”).
2.3 |. Aim 2: Histological characterization of indeterminate thyroid nodules
Histopathological diagnoses and features were characterized in all ITNs with available histological correlation (see Figure 1). Tumor staging was based on the seventh edition of the American Joint Committee on Cancer classification for differentiated thyroid carcinoma.23 Recurrence and response to therapy at last follow-up visit were assessed for all malignancies without concurrent thyroid cancer (defined as any foci of non-papillary thyroid carcinoma (PTC) or foci of PTC >1 cm) and at least 6 months of follow-up, in accord with the 2015 ATA recommendation for malignancies treated with total thyroidectomy and radioactive iodine; and as suggested in a recent publication in the rest.9,24
2.4 |. Pathology
Cytology was classified according to the Bethesda system for reporting thyroid cytopathology by board certified cytopathologists from the Department of Anatomic Pathology at Moffitt Cancer Center.25 Cytological diagnoses were extracted from pathology reports. In nodules biopsied multiple times, only the most recent report was used for the analysis. Histological correlation was done matching nodule size and location in the ultrasound and in the pathology report. Only the histological diagnosis of the biopsied nodule was used for this correlation. All cases in which this matching was unclear were excluded. Histopathological information was extracted from reports issued by our pathology department in 611 resected nodules (97%), and from external reports in the other 18 (3%). Given the retrospective design of this study, we acknowledge that pathologists were aware of both the cytological and molecular results at the time of histological diagnosis. However, all available specimens with malignant diagnosis were re-reviewed blinded to those results (129/166 malignancies; 78%) by 3 pathologists with focused experience in head and neck pathology at our pathology department. The FVPTCs were reclassified as NIFTPs if they met the recommended criteria; and as PTC in the presence of >1% “true” papillary formation, psammoma bodies, necrosis, high mitotic rate, and/or morphologic characteristics of other variants of PTC.18
We defined “low-risk” malignancies as differentiated thyroid carcinomas (DTCs) <4 cm with all the following features: no extrathyroidal extension; negative resection margins; no lymphovascular invasion (<4 foci for minimally invasive follicular thyroid carcinomas); clinical N0 or ≤5 lymph node metastases all ≤2 mm; and no distant metastasis. “High-risk” cancers were defined as DTC with any of the following features: gross extrathyroidal extension (T4); N1b or any lymph node metastasis >1 cm; and/or distant metastasis. All other malignancies were “intermediate-risk.”
2.5 |. Molecular markers
A 7-gene panel that looked for 14 point mutations in 4 genes (BRAF, HRAS, KRAS, and NRAS) and 3 rearrangements (PAX8/PPARG, RET/PTC1, and RET/PTC3), was used in 102 nodules (miRInform; Asuragen, Austin, TX, in 101 of them) until September 2014.21 ThyroSeq version 2 that searches for >1000 hotspots in 14 genes (AKT1, BRAF, CTNNB1, GNAS, HRAS, KRAS, NRAS, PIK3CA, PTEN, RET, TP53, TSHR, TERT, and EIF1AX) and 42 gene fusions (ALK, BRAF, IGF2BP3, NTRK1, NTRK3, PPARG, and RET) was used thereafter in 190 nodules.5 The change of the panel was based on hypothetical improved performance for our institutional pretest risk of malignancy.21 Sample collection for molecular analysis was done prospectively in all cases in accord with the manufacturer’s instructions at the time of the biopsy, and sent for analysis if cytology was indeterminate (AUS/FLUS and follicular neoplasm). The OP-group largely overlaps with the cohorts previously used to evaluate miRInform and ThyroSeq version 2 performance in our institution, and results were considered positive or negative, as previously described.5,21 One nodule evaluated with a 3-gene panel (BRAF, KRAS, and NRAS) was considered negative because no mutations were detected. Because differences in test performance between miRInform and ThyroSeq version 2 were not significantly different (Supporting Information Table S1, and Supporting Information Figure S1), all tests were combined for the analyses.
2.6 |. Institutional approach to the surgical management of indeterminate thyroid nodules
In February 2014, an institutional thyroid nodule management pathway, developed by our Department of Head and Neck-Endocrine Oncology, was implemented. According to this pathway, patients with indeterminate cytology undergo neck ultrasound evaluation. In the absence of pathological lymph nodes in the ultrasound, a total thyroidectomy is the preferred approach for ITNs under the following circumstances: bilateral nodularity; strong family history of thyroid cancer; thyroid dysfunction; patient preference; or oncogene panel result suggesting >50% risk of malignancy. In the latter scenario, it was also recommended to inspect the central compartment and to prophylactically remove nodes and node bearing tissue, due to the increased cancer risk. A lobectomy was recommended in the remaining patients. Before 2014, surgical management was not standardized in a protocol. The criteria followed to decide the extent of surgery, however, were basically the same, except that molecular markers were seldom available, and, therefore, were infrequently weighed in the decision. Dissection of the central compartment was usually decided on intraoperative findings, as it was in ITNs resected after 2014 that were not evaluated with oncogene panels. Completion thyroidectomy and radioactive iodine treatment were indicated, as suggested in the 2009 ATA guidelines and in agreement with patient preferences.10
2.7 |. Statistical analysis
Descriptive statistics and percentages are presented in the tables. Comparisons were done between the OP and control groups, and between the OP_positive and OP_negative groups using chi-square tests, and Fisher exact tests for categorical variables. Univariate logistic regression and backward selection multivariable logistic regression models were used to generate odds ratios, 95% confidence intervals, and P values. The significance level for entering effects was 0.30 and the significance level for removing effects was 0.10. Model diagnostics included Pearson and deviance residual plots. The Hosmer and Lemeshow goodness-of-fit test was used to assess the fit of the models. All analyses were done in SAS version 9.4 (SAS Institute, Cary, NC).
3 |. RESULTS
3.1 |. Study cohort
A total of 1029 ITNs (47% AUS/FLUS and 53% follicular neoplasm; mean size 2.5 cm) from 938 patients (75% women, mean age 54.6 years) were included in the study. Of these, 293 nodules (28%) were evaluated with oncogene panels (OP group). The test result was positive in 61 nodules (21%; Table 1), negative in 230 nodules (78%), and not adequate for evaluation in 2 nodules (1%). Patients in the OP group were significantly older at the time of biopsy (56.3 vs 53.8 years; P = .01) and were more likely euthyroid (79% vs 74%; P = .04) than the control group; however, other baseline characteristics were not significantly different (Table 2). Most nodules in the control group were evaluated before February 2014 (81%), whereas most nodules in the OP group were evaluated afterward (90%; P < .0001).
TABLE 1.
Molecular alterations detected by oncogene panels and associated risk of malignancy
| Molecular alteration | No. | % malignancya (no. malignant/no. resected) | Histological diagnosis |
|---|---|---|---|
| Point mutations | |||
| BRAF V600E | 2 | 100 (2/2) | 2 CVPTC |
| BRAF K601E | 2 | 50 (1/2) | AN; FVPTC |
| EIF1AX | 5 | 0 (0/3) | HP; 2 FA |
| EIF1AX + NRAS | 1 | N/A (0/0) | N/A |
| EIF1AX + NRAS + TERT | 1 | 100 (1/1) | FVPTC |
| EIF1AX + TSHR | 1 | 0 (0/1) | AN |
| HRAS | 6 | 75 (3/4) | AN; 2 NIFTP; FTC-MI |
| KRAS | 4 | 0 (0/4) | AN; FA; 2 HCA |
| NRAS | 23 | 40 (8/20) | 4 AN; 7 FA; 1 HCA; 4 NIFTP; 2 FVPTC; CVPTC; FTC-MI |
| NRAS + RET/PTC1 | 1 | 100 (1/1) | NIFTP |
| TERT | 1 | N/A (0/0) | N/A |
| TSHR | 4 | 0 (0/1) | HCA |
| Gene fusions | |||
| THADA/IGF2BP3 | 3 | 100 (3/3) | 2 NIFTP; FTC-MI |
| PAX8/PPARG | 4 | 100 (3/3) | 2 FVPTC; HCC-MI |
| Gene expression alterations | |||
| MET overexpression | 2 | 50 (1/2) | FA; OVPTC |
| ALK TK domain overexpressionb | 1 | 0 (0/1) | FA |
Abbreviations: AN, adenomatous nodule; CVPTC, conventional variant papillary thyroid carcinoma; FA, follicular adenoma; FTC-MI, follicular thyroid carcinoma, minimally invasive; FVPTC, follicular variant papillary thyroid carcinoma; HCA, Hürthle cell adenoma; HCC-MI, Hürthle cell carcinoma minimally invasive; HP, hyperplastic nodule; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; N/A, not available; OVPTC, oncocytic variant papillary thyroid carcinoma; PTC, papillary thyroid carcinoma; TK, tyrosine kinase.
NIFTP considered malignant.
Disproportional expression of the ALK tyrosine kinase domain as compared to the extracellular domain.
TABLE 2.
Baseline characteristics of the study cohort
| Patient level | All nodules (n = 938) No. (%) | Control group (n = 666) No. (%) | OP group (n = 272) No. (%) | P values (control vs OP) |
|---|---|---|---|---|
| Age, mean years | 54.6 | 53.8 | 56.3 | .01 |
| Male sex | 231 (25) | 173 (26) | 58 (21) | .13 |
| Thyroid functiona | 852 (91) | 585 (88) | 267 (98) | |
| Euthyroidism | 643 (75) | 433 (74) | 210 (79) | |
| Hypothyroidism | 175 (21) | 122 (21) | 53 (20) | |
| Hyperthyroidism | 34 (4) | 30 (5) | 4 (1) | .04 |
| Risk factorsb | ||||
| Contralat >1 cm | 261/760 (34) | 178/507 (35) | 83/253 (33) | .39 |
| Family history | 37/727 (5) | 30/493 (6) | 7/234 (3) | .07 |
| RT history | 18/727 (2) | 11/493 (2) | 7/234 (3) | .56 |
| Nodule level | (n = 1029) | 72 (736) | 28 (293) | |
| Cytology | ||||
| AUS/FLUS | 483 (47) | 334 (45) | 149 (51) | .13 |
| Follicular neoplasm/HCN | 546 (53) | 402 (55) | 144 (49) | |
| Size (cm)c | 2.5 | 2.4 | 2.6 | .07 |
| <2 | 439 (43) | 325 (45) | 114 (39) | |
| 2–3.9 | 454 (44) | 308 (43) | 146 (50) | .12 |
| ≥4 | 117 (11) | 84 (12) | 33 (11) | |
| Ultrasound pattern | 557 (89) | 397/465 (85) | 160/164 (98) | |
| Very-low | 50 (9) | 36 (9) | 14 (9) | |
| Low | 207 (37) | 158 (40) | 49 (31) | |
| Intermediate | 67 (12) | 45(11) | 22 (14) | |
| High | 66 (12) | 46 (12) | 20 (13) | |
| Non-ATAd | 168 (30) | 112 (28) | 56 (35) | .35 |
| Before 2014 | 627 (61) | 597 (81) | 30 (10) | < .0001 |
| After 2014e | 402 (39) | 139 (19) | 263 (90) |
Abbreviations: ATA, American Thyroid Association; AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; Contralat >1 cm, presence of contralateral nodules >1 cm; Control group, nodules evaluated without oncogene panels; Family history, family history of thyroid cancer; HCN, Hürthle cell neoplasm; OP group, nodules evaluated with oncogene panels (includes 2 nodules with complete test failure due to scant cellularity); RT history, medical history of radiation exposure to the neck; Ultrasound patterns, sonographic pattern as defined in the classification proposed in the 2015 ATA guidelines (evaluated in resected nodules only with ultrasound images available for review by a single observer and blinded to histological and molecular results).
Thyroid function was not available in 86 patients (9%), 81 in the control group (12%) and 5 in the OP group (2%).
Number with the risk factor/total number with information available.
Largest dimension determined by ultrasound. Not available in 19 nodules (all in the control group).
Non-ATA pattern includes heteroechoic nodules without other suspicious features, and isoechoic/hyperechoic/heteroechoic nodules with ≥1 of the suspicious features of the high-suspicion sonographic pattern.
Biopsy was performed outside Moffitt Cancer Center in 92 (66%) of the 139 nodules not evaluated with oncogene panels after 2014.
3.2 |. Impact of oncogene panels on surgical management of indeterminate thyroid nodules
Of the 938 patients in the study, 649 (69%) had just 1 thyroid nodule biopsied and were included for this analysis. The rate of resection and the prevalence of cancer were not significantly different between the control and OP groups (Table 3). However, nodules in the OP group were treated with higher rates of total thyroidectomy (45% vs 28%; P = .006), and central lymph node dissection (19% vs 12%; P = .03); and lower rates of lobectomy (50% vs 69%; P < .001) compared with nodules in the control group; due to more aggressive management of OP_positive nodules. After multivariable logistic regression, multinodular goiter and thyroid dysfunction were the only variables significantly associated with thyroid surgical extent (odds ratios 2.5 [1.6–4.2; P < .001] and 3.0 [1.8–5.2; P < .001] in the entire ITN cohort, respectively; and 2.7 [1.6–4.6; P = .02] and 3.2 [1.8–5.5; P = .03] in the OP group, respectively). Holding these variables at fixed values, the odds of having a total thyroidectomy for the OP group over the control group is 2.6 (1.6–4.4; P < .001); and 12.1 (4.8–30.1; P < .001) for the OP_positive group over the OP_negative group. Oncogene panel evaluation increased the odds of being treated with total thyroidectomy by 160%. For both multivariable logistic regression models (entire ITN cohort and OP group) the P values for the Hosmer and Lemeshow goodness-of-fit tests were 0.65 and 0.87. The odds ratio for central lymph node dissection was 1.7 (0.9–3.1; P = .11) in the OP group over the control group; and 5.7 (2.7–11.9; P < .001) in the OP_positive group over the OP_negative group.
TABLE 3.
Impact of oncogene panels on surgical management of cytologically indeterminate thyroid nodules
| Surgical management | All nodules (n = 649)a No. (%) | Control group (n = 456)a No. (%) | OP group (n = 193) No. (%) | P value control vs OP No. (%) | OP_pos (n = 44) No. (%) | OP_nege (n = 149) No. (%) | P value OP_pos vs OP_neg |
|---|---|---|---|---|---|---|---|
| % of resectionb | 395 (61) | 289 (63) | 106 (55) | .09 | 34 (77) | 72 (48) | .001 |
| % of malignancy | 117 (30) | 91 (32) | 26 (25) | .17 | 17 (50) | 9 (13) | < .001 |
| Total thyroidectomy | 127 (32) | 79 (28) | 48 (45) | .006s | 25 (74) | 23 (32) | < .001 |
| PoM among TT | 55 (43) | 37 (47) | 18 (38) | .36 | 15 (60) | 3 (13) | .001 |
| Intermediate/high-risk | 23 (18) | 18 (23) | 5 (10) | .16 | 4 (16) | 1 (4) | > .99 |
| CLND | 55 (14) | 35 (12) | 20 (19) | .03 | 15 (44) | 5 (7) | < .001 |
| PoM among CLND | 27 (49) | 20 (57) | 7 (35) | .27 | 7(47) | 0 | .04 |
| Presence of LNMc | 10 (18) | 8 (23) | 2 (10) | .45 | 1 (7) | 1 (20) | > .99 |
| Lobectomy | 251 (64) | 198 (69) | 53 (50) | < .001 | 6 (18) | 47 (65) | < .001 |
| % completion | 38 (15) | 32 (16) | 6 (11) | .52 | 2 (33) | 4 (9) | .14 |
| For index noduled | 32 (13) | 27 (14) | 5 (9) | > .99 | 1 (17) | 4 (9) | .33 |
| Intermediate/high-risk | 19 (8) | 17 (9) | 2 (4) | > .99 | 0 | 2 (4) | > .99 |
Abbreviations: CLND, patients managed with central lymph node dissection; Control group, nodules evaluated without oncogene panels; LNM, lymph node metastasis; OP group, nodules evaluated with oncogene panels; OP_neg, nodules with negative oncogene panel results; OP_pos, nodules with positive oncogene panel results; PoM, prevalence of malignancy, TT, nodules managed with total thyroidectomy.
Three patients had unclear cytology/histology correlation and were only used to calculate the percentage of resection and excluded for all other calculations.
Note that the sum of patients with total thyroidectomy and those with lobectomy do not equal the total number of resected nodules in each group because 17 nodules were resected with a different procedure (completion thyroidectomy after a previous remote lobectomy in most of them).
Patients with LNM among those treated with CLND. Includes LNM from incidental thyroid cancer in 2 patients with benign histology in the biopsied nodule, 1 in the control group and 1 in the OP_negative cohort.
Completion thyroidectomy done due to malignancy of the index (biopsied) nodule.
Includes two nodules with complete test failure due to scant cellularity. Only one was resected with a lobectomy and was benign.
The prevalence of malignancy was higher in the OP_positive than in the OP_negative nodules treated with total thyroidectomy or central lymph node dissection. However, only 16% (4/25) of the patients with OP_positive disease treated with total thyroidectomy had intermediate or high-risk malignancies, and a lobectomy could have been sufficient for the remainder. Similarly, only 7% of patients (1/15) with OP_positive disease with a formal central lymph node dissection had nodal metastases. Finally, the proportion of patients who required a completion thyroidectomy after an initial lobectomy was not significantly different between the OP_positive and OP_negative groups; nor was the proportion of intermediate or high-risk malignancies treated with a lobectomy. Similar findings were noted for both oncogene panels (Supporting Information Table S2).
3.3 |. Histological characterization of indeterminate thyroid nodules
Histological correlation was available in 629 nodules (61%; Table 4) and the overall prevalence of malignancy was 26% (n = 166). The most common malignant diagnosis was FVPTC (57%), two thirds of which would meet current criteria for NIFTP. Additionally, 12% of the malignancies were minimally invasive follicular thyroid carcinomas. The distribution of histological diagnosis was significantly different between resected nodules with and without a mutation identified (P < .001); but not between the control and OP groups. The prevalence of malignancy was significantly higher in the OP_positive group compared to the OP_negative group (48% vs 10%; P < .001); but differences were nonsignificant between the control and OP groups.
TABLE 4.
Histology of resected cytologically indeterminate thyroid nodules
| Histological diagnosis | All nodules (n = 629) No. (%) | Control group (n = 465) No. (%) | OP group (n = 164) No. (%) | P value control vs OP | OP_pos (n = 48) No. (%) | OP_nega (n = 116) No. (%) | P value OP_pos vs OP_neg |
|---|---|---|---|---|---|---|---|
| HP/AN/CLT | 169 (27) | 123 (26) | 46 (28) | .53 | 9 (19) | 37 (32) | < .001 |
| Adenomab [FA/HCA] | 294 [186/106] (47) | 211 [131/79] (45) | 83 [55/27] (51) | 16 [12/4] (33) | 67 [43/23] (58) | ||
| NIFTP | 56 (9) | 44 (9) | 12 (7) | 9 (19) | 3 (1) | ||
| FVPTC | 38 (6) | 30 (6) | 8 (5) | 6 (13) | 2 (4) | ||
| Other PTC [CVPTC] | 39 [34] (6) | 32 [29] (7) | 7 [5] (5) | 4 [3] (8) | 3 [2] (3) | ||
| FTC/HCC [MI] | 27 [21] (4) | 20 [14] (4) | 7 [7] (4) | 4 [4] (8) | 3 [3] (3) | ||
| Otherc | 6 (1) | 5 (1) | 1 (1) | 0 | 1 (1) | ||
| PoM | 166 (26) | 131 (28) | 35 (21) | .10 | 23 (48) | 12 (10) | < .001 |
Abbreviations: AN, adenomatous nodule; CLT, chronic lymphocytic thyroiditis; Control group, nodules evaluated without oncogene panels; CVPTC, conventional variant papillary thyroid carcinoma; FA, Follicular adenoma; FTC, follicular thyroid carcinoma; HCA, Hürthle cell adenoma; HCC, Hürthle cell carcinoma; HP, hyperplastic nodule; FVPTC, follicular variant papillary thyroid carcinoma; MI, minimally invasive; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; OP group, nodules evaluated with oncogene panels; OP_neg, nodules with negative oncogene panel results; OP_pos, nodules with positive oncogene panel results; PoM, prevalence of malignancy, PTC, papillary thyroid carcinoma.
Includes 1 hyperplastic nodule with complete test failure due to scant cellularity.
Includes 2 intrathyroidal parathyroid adenomas.
Includes 2 B-cell lymphoma, 2 intrathyroidal parathyroid carcinomas, and 2 medullary thyroid carcinomas.
Histological features of the 160 DTCs are summarized in Table 5. Most malignancies (64%) were low-risk malignancies; 33% were intermediate-risk malignancies; and 3% were high-risk malignancies. The proportion of low-risk, intermediate-risk, and high-risk malignancies was significantly different between the control and OP groups (P = .04), with a higher proportion of low-risk malignancies in the latter; but no differences were identified between the OP_positive and OP_negative groups, perhaps due to sample size. Oncogene panels detected mainly low-risk malignancies (83%). There were nonsignificant differences for any of the histological features evaluated between the control and OP groups; or the OP_positive and OP_negative groups, although we acknowledge the potential for a type 2 error given the small number of malignancies in these 2 groups. Similar histological findings were observed regardless of the oncogene panel (Supporting Information Table S3). If all ITNs in this series had a diagnostic lobectomy, <10% would have required a completion thyroidectomy due to invasive histological features (intermediate or high-risk cancers).
TABLE 5.
Histological features of cytologically indeterminate malignancies
| Histological features | All cancers (n = 160)a No. (%) | Control group (n = 126) No. (%) | OP group (n = 34) No. (%) | P value control vs OP | OP_pos (n = 23) No. (%) | OP_neg (n = 11) No. (%) | P value OP_pos vs OP_neg |
|---|---|---|---|---|---|---|---|
| Size ≥4 cm | 22 (14) | 17 (13) | 5(15) | .79 | 2 (9) | 3 (27) | .30 |
| ETE (n = 159) | 21 (13) | 19 (15) | 2 (6) | .25 | 1 (4) | 1 (9) | > .99 |
| T3 | 11 (7) | 11 (9) | 0 | 0 | 0 | ||
| T4a | 1 (1) | 0 | 1 (3) | 0 | 1 (9) | ||
| N/A (positive margins) | 9 (6) | 8 (6) | 1 (3) | 1 (4) | 0 | ||
| Positive margins (n = 157) | 16 (10) | 14 (11) | 2(6) | .53 | 0 | 1 (9) | > .99 |
| Rl (microscopic) | 15 (10) | 14 (11) | 1 (3) | 0 | 0 | ||
| R2 (macroscopic) | 1 (1) | 0 | 1 (3) | 0 | 1 (9) | ||
| Capsule (n = 147) | 132 (90) | 103 (90) | 29 (91) | .80 | 20 (91) | 9 (90) | > .99 |
| Total | 118 (80) | 93 (81) | 25 (78) | 18 (82) | 7 (70) | ||
| Partial | 14 (10) | 10 (9) | 4 (13) | 2(9) | 2 (20) | ||
| Capsule invasionb (n = 130) | 52 (40) | 41 (40) | 11 (38) | .83 | 8 (40) | 3 (33) | > .99 |
| Lymphovascular invasion (n = 154) | 24 (16) | 18 (15) | 6 (18) | .60 | 4 (18) | 2 (18) | > .99 |
| LNMc | 16 (10) | 14 (12) | 2 (6) | .53 | 1 (4) | 1 (9) | > .99 |
| LNM >0.2 cm | 11 (7) | 11 (9) | 0 | 0 | 0 | ||
| ENEd | 1 (6) | 1 (7) | 0 | 0 | 0 | ||
| N1a | 13 (8) | 11 (9) | 2 (6) | 1 (4) | 1 (9) | ||
| N1b | 3 (2) | 3 (2) | 0 | 0 | 0 | ||
| Distant metastasis | 1 (1) | 0 | 1 (3) | .15 | 1 (4) | 0 | > .99 |
| Stage | |||||||
| I/II | 134 (84) | 104 (83) | 30 (88) | .60 | 21 (92) | 9 (82) | .58 |
| III/IV | 26 (16) | 22 (17) | 4 (12) | 2 (8) | 2 (18) | ||
| Low-riske | 102 (64; 16) | 76 (60; 16) | 26 (76; 16) | .04 | 19 (83; 40) | 7 (64; 6) | .35 |
| Intermediate-riske | 53 (33; 8) | 47 (37; 10) | 6 (18; 4) | 3 (13; 6) | 3 (27; 3) | ||
| High-riske | 5 (3; 1) | 3 (2; 1) | 2 (6; 1) | 1 (4; 2) | 1 (9; 1) |
Abbreviations: Control group, nodules evaluated without oncogene panels; ENE, extranodal extension; ETE, extrathyroidal extension; LNMs, lymph node metastases; N/A, not applicable; OP group, nodules evaluated with oncogene panels; OP_neg, nodules with negative oncogene panel results; OP_pos, nodules with positive oncogene panel results.
Excluded 6 nodules with histology of B-cell lymphoma (n = 2), intrathyroidal parathyroid carcinoma (n = 2), and medullary thyroid carcinoma (n = 2).
Percentage of nodules with capsular invasion calculated for encapsulated nodules only.
Percentage calculated at the patient level (n = 155 all nodules, n = 121 in the control group). Of the 3 patients with N1b disease, 1 had a single LNM <0.1 cm (1/40 resected) identified during prophylactic lateral neck dissection done at the time of thyroidectomy for concomitant neck melanoma and no LNM in the central neck, and was considered cN0 for the risk categorization; and the other 2 were diagnosed during postsurgical whole-body iodine scan.
Percentage of nodules with at least 1 LNM.
See Methods section for characteristics of low, intermediate, and high-risk malignancies. In parenthesis: percentage of follicular-cell derived malignancies in the group; percentage of all nodules resected in the group.
Response to therapy was analyzed in 116 (73%) of the follicular derived thyroid cancers without concurrent thyroid cancer and follow-up ≥6 months (Table 6). The mean follow-up time was 34 months (range 6–137 months). No recurrences occurred during that time. At last visit, 88 (76%) had excellent response, 24 (21%) had indeterminate response, 3 (3%) had biochemically incomplete response; and 1 (1%) had structural incomplete response. There were no differences in treatment extent, cancer-risk, or response to therapy between the control and OP groups but median follow-up time was significantly longer in the control group (38 months vs 27 months; P < .0001).
TABLE 6.
Follow-up of differentiated thyroid cancers with indeterminate cytology
| Treatment and outcome details | All cancers (n = 116) No. (%) | Control group (n = 93) No. (%) | OP group (n = 23) No. (%) | P value (Control group vs OP group) |
|---|---|---|---|---|
| Treatment details | ||||
| Total thyroidectomya | 87 (75) | 66 (71) | 21 (91) | .06 |
| % treated with RAI | 59 (51) | 49 (53) | 10 (43) | .49 |
| Mean RAI dose (range) | 47 (0–250) | 49 (0–250) | 39 (0–200) | .43 |
| Cancer riskb | ||||
| Low | 76 (66) | 59 (63) | 17 (74) | .56 |
| Intermediate | 36 (31) | 31 (33) | 5(22) | |
| High | 4 (3) | 3 (3) | 1 (4) | |
| Months of follow-up (range) | 37 (6–137) | 38 (6–137) | 27 (7–35) | < .0001 |
| Response to therapy | ||||
| Excellent response | 89 (77) | 74 (80) | 15 (65) | .44 |
| Indeterminate responsec | 23 (20) | 16 (17) | 7 (30) | |
| Biochemically incompleted | 3 (3) | 2 (2) | 1 (4) | |
| Structurally incompletee | 1 (1) | 1 (1) | 0 (0) |
Abbreviations: Control group, nodules evaluated without oncogene panels; OP group, nodules evaluated with oncogene panels; RAI, radioactive iodine.
Performed in 1 or 2 steps.
See Methods section for characteristics of low, intermediate, and high-risk malignancies.
Thyroglobulin antibodies positive (stable or declining) in 11; detectable thyroglobulin in the established ranges in 10 (thyroid-stimulating hormone >1 in 4 of them); detectable thyroglobulin in the established ranges and positive thyroglobulin antibodies in 1; detectable thyroglobulin in the established ranges and indeterminate <1 cm nodule in the thyroid bed in 1.
Elevated thyroglobulin in all 3. One of them has developed small lung nodules no RAI avid that are indeterminate.
Persistent LNM after lateral neck dissection that is being followed.
4 |. DISCUSSION
In this study, we found a significant increase in the extent of surgery with which ITNs evaluated with oncogene panels were managed. However, despite a higher rate of resection in OP_positive than in OP_negative nodules, we did not observe an increase in the yield of malignancy or a decrease in the rates of completion thyroidectomy among nodules evaluated with oncogene panels compared to the control group. Finally, we found that most malignant ITNs were low-risk malignancies for which a lobectomy could be sufficient initial treatment, regardless of oncogene panel results.
4.1 |. Limitations and strengths
This is a single center retrospective study. Therefore, results may be limited by this design and may not be applicable to other centers. The cytology of all ITNs included in this study was consecutively evaluated at our institution and most histological specimens (97%) were issued at our pathology department by experienced pathologists, although this cannot obviate the limitations of light microcopy.26 Furthermore, the histology of most (78%) malignancies was reviewed blinded to the oncogene panel results to assure compliance with current diagnostic criteria and to avoid bias in histologic interpretation. Most patients in the control group were evaluated before 2014, whereas most patients in the OP group were evaluated afterward, when our institutional clinical pathway was implemented. This pathway, however, was an encapsulation of our standard of care at the time, and the only significant change was that oncogene panel evaluation was routinely recommended for the evaluation of ITNs. We believe that this is a strength rather than a limitation as it allows us to truly assess the impact of oncogene panel testing on the management of ITNs. Our results could also be limited by having analyzed the impact of different oncogene panels together. However, we believe that this is not a significant limitation because, in our experience, their clinical performance was not meaningfully different, and we found no differences in management or histological outcomes with either test. Nonetheless, we acknowledge that these results are not entirely conclusive due to wide 95% confidence intervals.
4.2 |. Interpretation of the results
Few studies to date have shown the impact of oncogene panels on the surgical management of ITNs and were conducted when a total thyroidectomy was the treatment of choice for all thyroid cancers >1 cm.2,7 One could argue that our results are biased because we were recommending total thyroidectomy in OP_positive nodules if the estimated prevalence of malignancy was >50%. However, our recommendation is similar to that in the 2015 ATA guidelines, which suggest: “total thyroidectomy might be preferred for ITNs if a cancer-specific mutation is identified.”9 For that reason, we believe that our findings are particularly relevant. Our study suggests that driver mutations are often identified in ITNs that do not have other clinical indications for total thyroidectomy, as reflected in the dramatic increase in the rate of total thyroidectomies despite the low rate of cancer-specific mutations identified by oncogene panels (17% of all nodules tested, excluding EIF1AX and TSHR mutations). Furthermore, our histological findings suggest that this ATA recommendation might result in overtreatment in a significant number of patients according to the new thyroid cancer treatment standards set by those same guidelines in which a lobectomy is recognized as sufficient initial treatment for low-risk malignancies.9
In our experience, the positive predictive value of oncogene panels was lower than in previous publications (48%).3,4,20 In particular, the prevalence of malignancy among our RAS-mutant tumors was 47% (14/30 nodules), which is significantly lower than the >80% usually quoted but similar to that found more recently by other groups.27,28 Nonetheless, the rate of invasive RAS-mutant tumors in our series was 20% (6/30 nodules), which is more consistent among all series. This suggests that the well-recognized low interobserver agreement for the classification of noninvasive encapsulated lesions with follicular pattern that have, if at all, very-low malignant potential, is behind the differences in the prevalence of malignancy of RAS and perhaps other mutations.18,29–31 A recent publication on 94 RAS-mutant tumors has also suggested that a lobectomy seems appropriate for these tumors.8 However, the authors of that study concluded that finding a RAS mutation preoperatively could be used to guide the extent of thyroidectomy.8 We would argue that because lobectomy is the default surgical management for ITNs in the absence of other indications for total thyroidectomy, identifying an RAS-mutation only adds cost to the evaluation of the nodule.
The BRAF-V600E mutation in tumors >1 cm is considered in the 2015 ATA guidelines as an intermediate-risk for recurrence criterion.9 However, the ATA does not recommend routinely checking the BRAF status given its limited prognostic utility taken in isolation.9 We found this mutation in <1% (n = 2) of all ITNs evaluated with oncogene panels, which is consistent with the literature (<5% in a recent meta-analysis).32 Both tumors with BRAF-V600E mutation were encapsulated, noninvasive, conventional variant of PTCs without nodal or distant metastasis, and had no evidence of disease at last follow-up visit. Other studies have also found limited impact of BRAF testing in ITNs for surgical planning.6,7,33 Therefore, screening for this mutation on ITNs may have limited clinical value as well.33
Mutations in TERT or TP53 have been associated with more aggressive cancers, particularly when combined with EIF1AX and RAS or BRAF-V600E mutations.34 In agreement with that, the only malignancy with distant metastasis in our cohort was a 6.8-cm FVPTC with mutations in RAS, EIF1AX, and TERT. The identification of multiple cancer-specific mutations might be the only oncogene panel result in which a total thyroidectomy would be preferred up-front. However, the extremely low frequency of this finding in ITNs (<1%) limits its clinical impact. In 3 of the other 4 high-risk malignancies in this series, the initial surgery was performed at an outside institution, and the preoperative evaluation may have differed from our protocols. Two had evident lateral lymph node metastases in the whole body iodine scan 3 months after surgery; and another one had gross extrathyroidal extension (T4a) and was incompletely resected per the surgical report.
4.3 |. Implications and future directions
To attenuate the impact of thyroid nodule and thyroid cancer overdiagnosis on healthcare costs and limit the complications derived from its treatment, it is necessary to identify nodules that can be managed less aggressively.35 In this study, we show that most malignant ITNs are low-risk cancers, which is in agreement with other recent publications.11–14 Our study shows that these histological findings are also the rule for malignancies identified by oncogene panels. Therefore, if surgery is indicated, lobectomy seems the preferred initial treatment for most solitary ITNs for 3 reasons: (1) oncogene panels detect mainly low-risk malignancies and may have a lower positive predictive value than anticipated; (2) total thyroidectomy may be appropriate for <10% of ITNs; and (3) the complication rate of total thyroidectomy is similar to that of 2-stage thyroidectomy but higher than that of lobectomy.22,36–38
This study also highlights the uncertain mid-term and long-term impact of molecular marker tests on health care costs and outcomes. The negative predictive value of molecular marker tests has been poorly validated on independent studies as very few nodules with a “benign”/negative result have been resected.39 Nonetheless, these results seem to be significantly decreasing the rate of resection of ITNs.40,41 Furthermore, several recent studies suggest that molecular marker tests are being overused7,42–44; and their performance might be different for nodules with different sonographic pattern and/or cytological diagnosis as they have demonstrated to impact on the pretest risk of malignancy.45–47 Future research should focus on identifying scenarios in which they may provide informative results.
5 |. CONCLUSION
In conclusion, current oncogene panel results seem insufficient to guide the surgical extent of solitary ITNs unless multiple cancer-specific mutations are identified in the same nodule, which is extremely unusual. For most solitary ITNs without other indications for total thyroidectomy, a lobectomy is likely the most appropriate treatment. The benefit of pure “rule-in” molecular marker tests in the evaluation of ITNs may be clinically limited. Implementation of recommendation #20 of the ATA guidelines may lead to overtreatment of indolent tumors and should be revisited.
Supplementary Material
ACKNOWLEDGMENTS
This work has been supported in part by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute (NCI) designated comprehensive cancer center (P30-CA076292).
DISCLOSURE STATEMENT
McIver and Centeno receive grant support from GeneproDx., Leon received a sponsored research grant from Rosetta Genomics, and holds equity in Rosetta Genomics. The rest of the authors have nothing to disclose.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- [1].Ferris RL, Baloch Z, Bernet V, et al. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015;25(7):760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yip L, Wharry LI, Armstrong MJ, et al. A clinical algorithm for fine-needle aspiration molecular testing effectively guides the appropriate extent of initial thyroidectomy. Ann Surg. 2014;260 (1):163–168. [DOI] [PubMed] [Google Scholar]
- [3].Nikiforov YE, Carty SE, Chiosea SI, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120(23):3627–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nikiforov YE, Carty SE, Chiosea SI, et al. Impact of the multigene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid. 2015;25(11):1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valderrabano P, Khazai L, Leon ME, et al. Evaluation of ThyroSeq v2 performance in thyroid nodules with indeterminate cytology. Endocr Relat Cancer. 2017;24(3):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aragon Han P, Olson MT, Fazeli R, et al. The impact of molecular testing on the surgical management of patients with thyroid nodules. Ann Surg Oncol. 2014;21(6):1862–1869. [DOI] [PubMed] [Google Scholar]
- [7].Noureldine SI, Najafian A, Aragon Han P, et al. Evaluation of the effect of diagnostic molecular testing on the surgical decision-making process for patients with thyroid nodules. JAMA Otolaryngol Head Neck Surg. 2016;142(7):676–682. [DOI] [PubMed] [Google Scholar]
- [8].Patel SG, Carty SE, McCoy KL, et al. Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery. 2017;161(1):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- [11].Trimboli P, Bongiovanni M, Rossi F, et al. Differentiated thyroid cancer patients with a previous indeterminate (Thy 3) cytology have a better prognosis than those with suspicious or malignant FNAC reports. Endocrine. 2015;49(1):191–195. [DOI] [PubMed] [Google Scholar]
- [12].Rago T, Scutari M, Latrofa F, et al. The large majority of 1520 patients with indeterminate thyroid nodule at cytology have a favorable outcome, and a clinical risk score has a high negative predictive value for a more cumbersome cancer disease. J Clin Endocrinol Metab. 2014;99(10):3700–3707. [DOI] [PubMed] [Google Scholar]
- [13].Kleiman DA, Beninato T, Soni A, Shou Y, Zarnegar R, Fahey TJ III. Does Bethesda category predict aggressive features in malignant thyroid nodules? Ann Surg Oncol. 2013;20(11):3484–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].VanderLaan PA, Marqusee E, Krane JF. Features associated with locoregional spread of papillary carcinoma correlate with diagnostic category in the Bethesda System for reporting thyroid cytopathology. Cancer Cytopathol. 2012;120(4):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu X, Medici M, Kwong N, et al. Bethesda categorization of thyroid nodule cytology and prediction of thyroid cancer type and prognosis. Thyroid. 2016;26(2):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ganly I, Wang L, Tuttle RM, et al. Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Hum Pathol. 2015;46(5):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goffredo P, Cheung K, Roman SA, Sosa JA. Can minimally invasive follicular thyroid cancer be approached as a benign lesion? A population-level analysis of survival among 1,200 patients. Ann Surg Oncol. 2013;20(3):767–772. [DOI] [PubMed] [Google Scholar]
- [18].Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gupta N, Dasyam AK, Carty SE, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98 (5):E914–E922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96(11): 3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Valderrabano P, Leon ME, Centeno BA, et al. Institutional prevalence of malignancy of indeterminate thyroid cytology is necessary but insufficient to accurately interpret molecular marker tests. Eur J Endocrinol. 2016;174(5):621–629. [DOI] [PubMed] [Google Scholar]
- [22].Hauch A, Al-Qurayshi Z, Randolph G, Kandil E. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. 2014;21(12): 3844–3852. [DOI] [PubMed] [Google Scholar]
- [23].Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A. Thyroid AJCC Cancer Staging Manual. 7th edition New York, New York, Springer, 2010:87–96. [Google Scholar]
- [24].Momesso DP, Vaisman F, Yang SP, et al. Dynamic risk stratification in patients with differentiated thyroid cancer treated without radioactive iodine. J Clin Endocrinol Metab. 2016;101(7): 2692–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19(11):1159–1165. [DOI] [PubMed] [Google Scholar]
- [26].Cibas ES, Baloch ZW, Fellegara G, et al. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med. 2013;159(5):325–332. [DOI] [PubMed] [Google Scholar]
- [27].Eszlinger M, Piana S, Moll A, et al. Molecular testing of thyroid fine-needle aspirations improves presurgical diagnosis and supports the histologic identification of minimally invasive follicular thyroid carcinomas. Thyroid. 2015;25(4):401–409. [DOI] [PubMed] [Google Scholar]
- [28].Krane JF, Cibas ES, Alexander EK, Paschke R, Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. 2015; 123(6):356–361. [DOI] [PubMed] [Google Scholar]
- [29].Elsheikh TM, Asa SL, Chan JK, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008;130(5):736–744. [DOI] [PubMed] [Google Scholar]
- [30].Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28(10):1336–1340. [DOI] [PubMed] [Google Scholar]
- [31].Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002;26(11):1508–1514. [DOI] [PubMed] [Google Scholar]
- [32].Trimboli P, Treglia G, Condorelli E, et al. BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2016;84(3):315–320. [DOI] [PubMed] [Google Scholar]
- [33].Kleiman DA, Sporn MJ, Beninato T, et al. Preoperative BRAF (V600E) mutation screening is unlikely to alter initial surgical treatment of patients with indeterminate thyroid nodules: a prospective case series of 960 patients. Cancer. 2013;119(8):1495–1502. [DOI] [PubMed] [Google Scholar]
- [34].Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. [DOI] [PubMed] [Google Scholar]
- [36].Erdem E, Gülçelik MA, Kuru B, Alagöl H. Comparison of completion thyroidectomy and primary surgery for differentiated thyroid carcinoma. Eur J Surg Oncol. 2003;29(9):747–749. [DOI] [PubMed] [Google Scholar]
- [37].Tan MP, Agarwal G, Reeve TS, Barraclough BH, Delbridge LW. Impact of timing on completion thyroidectomy for thyroid cancer. Br J Surg. 2002;89(6):802–804. [DOI] [PubMed] [Google Scholar]
- [38].Untch BR, Palmer FL, Ganly I, et al. Oncologic outcomes after completion thyroidectomy for patients with well-differentiated thyroid carcinoma. Ann Surg Oncol. 2014;21(4):1374–1378. [DOI] [PubMed] [Google Scholar]
- [39].Nishino M Molecular cytopathology for thyroid nodules: a review of methodology and test performance. Cancer Cytopathol. 2016;124(1):14–27. [DOI] [PubMed] [Google Scholar]
- [40].Sipos JA, Blevins TC, Shea HC, et al. Long-term nonoperative rate of thyroid nodules with benign results on the Afirma gene expression classifier. Endocr Pract. 2016;22(6):666–672. [DOI] [PubMed] [Google Scholar]
- [41].Duick DS, Klopper JP, Diggans JC, et al. The impact of benign gene expression classifier test results on the endocrinologist-patient decision to operate on patients with thyroid nodules with indeterminate fine-needle aspiration cytopathology. Thyroid. 2012;22(10):996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dedhia PH, Rubio GA, Cohen MS, Miller BS, Gauger PG, Hughes DT. Potential effects of molecular testing of indeterminate thyroid nodule fine needle aspiration biopsy on thyroidectomy volume. World J Surg. 2014;38(3):634–638. [DOI] [PubMed] [Google Scholar]
- [43].Noureldine SI, Olson MT, Agrawal N, Prescott JD, Zeiger MA, Tufano RP. Effect of gene expression classifier molecular testing on the surgical decision-making process for patients with thyroid nodules. JAMA Otolaryngol Head Neck Surg. 2015;141(12): 1082–1088. [DOI] [PubMed] [Google Scholar]
- [44].Sacks WL, Bose S, Zumsteg ZS, et al. Impact of Afirma gene expression classifier on cytopathology diagnosis and rate of thyroidectomy. Cancer Cytopathol. 2016;124(10):722–728. [DOI] [PubMed] [Google Scholar]
- [45].Valderrabano P, Khazai L, Thompson ZJ, et al. Cancer risk stratification of indeterminate thyroid nodules: a cytological approach. Thyroid. 2017;27(10):1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tang AL, Falciglia M, Yang H, Mark JR, Steward DL. Validation of American Thyroid Association ultrasound risk assessment of thyroid nodules selected for ultrasound fine-needle aspiration. Thyroid. 2017;27(8):1077–1082. [DOI] [PubMed] [Google Scholar]
- [47].Baca SC, Wong KS, Strickland KC, et al. Qualifiers of atypia in the cytologic diagnosis of thyroid nodules are associated with different Afirma gene expression classifier results and clinical outcomes. Cancer Cytopathol. 2017;125(5):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


