Abstract
Background
Penile cancer (PeCa) is a rare, aggressive malignancy often associated with the human papillomavirus (HPV). The practice of a personalized risk-adapted approach is not yet established. This study is to assess the relationship between HPV tumor status and chemoradiotherapy (CRT) in PeCa locoregional control (LRC).
Methods
We retrospectively identified patients with HPV status who were diagnosed with squamous cell carcinoma of the penis and treated with surgical resection between 1999 and 2016. The relationship between tumor/treatment characteristics and LRC were analyzed with univariate and multivariate Cox proportional hazard regression analysis (UVA and MVA, respectively). Time-to-event outcomes were estimated with Kaplan-Meier curves and compared via log-rank tests.
Results
Fifty-one patients were identified. The median follow-up was 36.6 months. Patients were primarily HPV-negative (HPV−) (n = 28, 55%), and pathologic node positive (pN+) (55%). The 2 year LRC rate was 54%. pN+ patients had a significantly lower 2 year LRC (37 vs. 81%, p = 0.002). In the subgroup analysis of pN+ patients (n = 28), there was a LRC benefit associated with the addition of CRT (HR 0.19; 95% CI 0.05–0.70, p = 0.012) and HPV-positive (HPV+) disease (HR 0.18; 95% CI 0.039–0.80, p = 0.024) using MVA. HPV+ patients treated with CRT had improved 2 year LRC compared to HPV− patients (83 vs. 38%, p = 0.038).
Conclusions
Adjuvant CRT and HPV+ disease independently predicted for improved LRC in pN+ PeCa. In HPV+ PeCa, the LRC benefit was primarily observed in patients treated with adjuvant CRT. Prospective investigation of HPV+ and CRT is required to further delineate their roles in optimizing PeCa treatment.
Keywords: Chemoradiation therapy, HPV, Penile cancer, Locoregional recurrence, Surgery
Introduction
Penile cancer (PeCa) is relatively rare in Western countries, representing less than 1% of male malignancies in the United States [1]. However, the incidence is higher among men in Africa, South America, and some regions of Asia. PeCa is generally a squamous cell carcinoma, derived from the carcinogenesis of a penile intraepithelial neoplasia precursor [2]. Early diagnosis is very important in regard to both organ preservation and disease outcome, with 5 year survival rates estimated at approximately 50% [3]. Due to its rarity and consequent lack of randomized trials, PeCa poses a challenge for risk stratification and treatment decisions.
The extent of lymph node involvement is the major prognostic factor of survival in PeCa patients [4]. Five-year cancer-specific survivals for pathologic node negative (pN0), pN1, pN2 and pN3 are 85–100%, 79–89%, 17–60%, and 0–17%, respectively [5, 6]. Multimodal management including inguinal and/or pelvic lymphadenectomy is often required for patients with extensive lymph node metastasis [7]. Multiple retrospective studies have suggested that postoperative radiation therapy could improve LRC with an absolute benefit of 30–50% in patients with high nodal disease burden and extracapsular extension [8–10]. Addition of concomitant chemotherapy with better tumor response is well studied with level I evidence in head and neck, vulvar, and anal squamous cell carcinoma. Given the similar histology of PeCa, National Comprehensive Cancer Network (NCCN) treatment guideline currently recommends postoperative chemoradiotherapy for patients with bulky inguinal or enlarged pelvic lymphadenopathy, extracapsular extension, and positive margin in PeCa [11].
The primary risk factors for PeCa include chronic inflammation, lack of circumcision, tobacco smoking, poor hygiene, and human papillomavirus (HPV) infection [3]. HPV causes approximately 50% of PeCa worldwide [2, 12, 13]. PeCa can develop through malignant transformation of high-risk HPV infection, or independent of HPV with p53 mutation [2]. The differences in biological behavior, tumor aggressiveness, and response to chemoradiotherapy (CRT) between HPV-positive (HPV+) and HPV-negative (HPV−) carcinomas has been well characterized in head and neck squamous cell carcinoma (HNSCC) [14]. In HNSCC, a positive HPV/p16 predicts for improved locoregional control (LRC) in patients treated with CRT [15–17], a finding which has led to considerable changes in the management of this patient population [15]. Clinical trials for HPV/p16 positive HNSCC are currently investigating de-escalation of treatment to minimize toxicity without sacrificing oncologic outcomes. However, the impacts of etiologic risk stratifications, therapy, and prognosis are largely unexplored in the management of PeCa.
The main objective of this study is to determine the impact of HPV/p16 tumor status and adjuvant CRT on LRC in PeCa.
Patients and methods
This retrospective study was approved by the University of South Florida and Moffitt Cancer Center Institutional Review Board. We retrospectively reviewed a cohort of PeCa patients with histologically diagnosed squamous cell carcinoma treated at Moffitt Cancer Center between 1999 and 2016. HPV statuses were obtained using the frozen tissues, and p16 detection using formalin-fixed, paraffin-embedded tissue slides as described previously [18]. Briefly, the presence of HPV was detected with in situ hybridization (ISH; Family 16 probe, prediluted), which was used to assess for high-risk HPV subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66. Carcinomas were classified as HPV+ when a discrete signal (punctate and/or diffuse) was detected in the nuclei of tumor cells. Immunohistochemistry for p16 detection was performed using mouse monoclonal antibody E6H4 (Ventana). The expression for p16 was categorized as negative expression (complete absence of p16 staining or only focal positivity) and positive expression (strong and diffuse nuclear and cytoplasmic p16 positivity in most tumor cells) [19]. HPV+ tumors were defined as either positive HPV ISH or high expression of p16.
One hundred and nine patients were retrospectively identified. Patients who were lost to follow-up or referred to our institute with recurrent or distant metastatic disease were excluded if no complete medical records were retrievable. In total, 51 eligible PeCa patients were tested for HPV and p16 status. The staging was performed using the American Joint Committee on Cancer (AJCC) 7th edition staging system. Clinical parameters, in particular, demographic data, histopathology, surgery, chemotherapy and radiation history, and outcomes were abstracted from medical charts. Acute and late adverse events were abstracted from medical charts and graded using CTCAE version 4.0. Acute toxicity was defined as adverse events experienced during or up to 90 days after radiation therapy. Late adverse events were defined as side effects experienced 90 days after radiation therapy.
Statistical analysis
Descriptive statistics were used to summarize the cohort, including the median and range for continuous variables, or counts and percentages for categorical variables. The time-to-event outcomes were estimated via Kaplan-Meier curves and groups were compared via log-rank tests. LRC was defined as freedom from disease recurrence at the primary disease site or regional lymph nodes. Progression-free survival (PFS) was defined as the time from surgery to locoregional recurrence, distant metastasis, or death from any cause. Overall survival (OS) was defined as from the time of surgery to death from any cause. PFS and OS endpoints were censored for patients surviving at last follow-up or last known contact. The Cox proportional-hazards model was used for univariate and multivariate analysis (UVA and MVA, respectively) to assess the effect of patient, tumors, HPV status, and other significant predictive factors on the endpoints. Values considered significant or trending on UVA (p < 0.1) were included in MVA. The association between CRT and toxicity was compared via Pearson’s Chi-square (χ2) and Fisher’s exact tests as appropriate. A 2-tailed p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 24 (IBM, Chicago, IL).
Results
Clinicopathologic and treatment characteristics
The clinical characteristics of all 51 patients in our cohort are detailed in Table 1. Patients were primarily HPV− (n = 28, 55%), pathologic node positive (pN+, 55%), and AJCC stage II (43%). HPV+ patients (n = 23) included seventeen HPV ISH+/p16+, three HPV ISH+/p16−, and three HPV ISH−/p16+ tumors (Table S1). Most patients were treated with partial penectomy (76%), had negative surgical margins (94%), and underwent inguinal dissection (82%). Seventeen patients (33%) received adjuvant concurrent CRT, 13 of whom received weekly cisplatin-based chemotherapy and four of whom received 5-FU/Mitomycin C chemotherapy. Three patients received adjuvant platinum-based chemotherapy alone. Two patients received adjuvant radiation therapy alone. External beam RT was delivered in daily fractions of 180 or 200 cGy to the primary surgical stump in a median-prescribed dose of 4500 cGy (range 4250–5040 cGy). For patients who received pelvic radiation (n = 15, 29%), the positive pelvic lymph nodal region was given a median dose of 4500 cGy (range, 3960–5400 cGy). For patients who underwent inguinal radiation (n = 13, 25%), clinically or pathologically positive nodal areas were given a median dose of 4500 cGy (range 4250–6480 cGy).
Table 1.
Patient, tumor, and treatment characteristics of patients with penile squamous cell carcinoma
| Characteristics | All patients | pN+ | p value | ||
|---|---|---|---|---|---|
| Total (n = 51) | Total (n = 28) | CRT(+) (n = 14) | CRT(−) (n = 14) | ||
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Median age, Y (range) | 61 (37–91) | 58.5 (37–76) | 61 (47–71) | 54 (37–76) | 0.401 |
| ECOG | |||||
| 1 | 43 (84) | 22 (79) | 11 (79) | 11 (79) | 1 |
| 2 | 8 (16) | 6 (21) | 3 (21) | 3 (21) | |
| Race | |||||
| White | 42 (82) | 25 (89) | 12 (86) | 13 (93) | 0.219 |
| Black | 2 (4) | 2 (7) | 2 (14) | 0 (0) | |
| Hispanic | 5 (10) | 1 (4) | 0 (0) | 1 (7) | |
| Other | 2 (4) | 0 (0) | 0 (0) | 0 (0) | |
| Tumor stage | |||||
| T1 | 16 (31) | 8 (29) | 6 (43) | 2 (14) | 0.167 |
| T2 | 16 (31) | 9 (32) | 2 (14) | 7 (50) | |
| T3 | 17 (33) | 10 (36) | 6 (43) | 4 (29) | |
| T4 | 2 (4) | 1 (4) | 0 (0) | 1 (7) | |
| Nodal stage | |||||
| N0 | 23 (45) | 0 (0) | 0 (0) | 0 (0) | 0.632 |
| N1 | 6 (12) | 6 (21) | 2 (14) | 4 (29) | |
| N2 | 16 (31) | 16 (57) | 9 (64) | 7 (50) | |
| N3 | 6 (12) | 6 (21) | 3 (21) | 3 (21) | |
| Pathologic nodal status | |||||
| pN(−) | 23 (45) | 0 (0) | 0 (0) | 0 (0) | |
| pN(+) | 28 (55) | 28 (100) | 14 (100) | 14 (100) | |
| AJCC 7 STAGE | |||||
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.176 |
| II | 22 (43) | 0 (0) | 0 (0) | 0 (0) | |
| IIIA | 5 (10) | 5 (18) | 1 (7) | 4 (29) | |
| IIIB | 10 (20) | 10 (36) | 7 (50) | 3 (21) | |
| IV | 14 (27) | 13 (46) | 6 (43) | 7 (50) | |
| HPV/P16 status | |||||
| Negative | 28 (55) | 16 (57) | 7 (50) | 9 (64) | 0.7 |
| Positive | 23 (45) | 12 (43) | 7 (50) | 5 (36) | |
| Extracapsular extension | |||||
| ECE(−) | 45 (88) | 22 (79) | 11 (79) | 11 (79) | 1 |
| ECE(+) | 6 (12) | 6 (21) | 3 (21) | 3 (21) | |
| Lymphovascular invasion | |||||
| LVSI(−) | 35 (69) | 16 (57) | 7 (50) | 9 (64) | 0.7 |
| LVSI(+) | 16 (31) | 12 (43) | 7 (50) | 5 (36) | |
| Extent of surgery | |||||
| Partial penectomy | 39 (76) | 21 (75) | 12 (86) | 9 (64) | 0.39 |
| Total penectomy | 12 (24) | 7 (25) | 2 (14) | 5 (36) | |
| Pelvic dissection | |||||
| No | 46 (90) | 25 (89) | 13 (93) | 12 (86) | 1 |
| Yes | 5 (10) | 3 (11) | 1 (7) | 2 (14) | |
| Inguinal dissection | |||||
| No | 9 (18) | 0 (0) | 0 (0) | 0 (0) | |
| Yes | 42 (82) | 28 (100) | 14 (100) | 14 (100) | |
| Margin status | |||||
| SM(−) | 48 (94) | 26 (93) | 13 (93) | 13 (93) | 1 |
| SM(+) | 3 (6) | 2 (7) | 1 (7) | 1 (7) | |
| Chemoradiotherapy | |||||
| No | 34 (67) | 14 (50) | 0 (0) | 14 (100) | |
| Yes | 17 (33) | 14 (50) | 14 (100) | 0 (0) | |
| Chemotherapy | |||||
| No | 31 (61) | 13 (46) | 0 (0) | 13 (93) | |
| Yes | 20 (39) | 15 (54) | 14 (100) | 1 (7) | |
| Chemotherapy type | |||||
| None | 31 (61) | 13 (46) | 0 (0) | 13 (93) | |
| Platinum based | 17 (33) | 13 (46) | 12 (86) | 1 (7) | |
| Other | 3 (6) | 2 (7) | 2 (14) | 0 (0) | |
| Postoperative radiation | |||||
| No | 32 (63) | 13 (46) | 0 (0) | 13 (93) | |
| Yes | 19 (37) | 15 (54) | 14 (100) | 1 (7) | |
| Pelvic radiation | |||||
| No | 36 (71) | 16 (57) | 3 (21)a | 13 (93) | |
| Yes | 15 (29) | 12 (43) | 11 (79) | 1 (7) | |
| Inguinal radiation | |||||
| No | 38 (75) | 18 (64) | 4 (29)b | 14 (100) | |
| Yes | 13 (25) | 10 (36) | 10 (71) | 0 (0) | |
| RT dose to penis (Gy) | 0 (0–50.4) | 45 (0–50.4) | 45.5 (45–50.4) | 0 (0–50) | |
| RT dose to pelvis (Gy) | 0 (0–54) | 0 (0–54) | 45 (0–54) | 0 (0–50) | |
| RT dose to inguinal (Gy) | 0 (0–64.8) | 0 (0–64.8) | 45 (0–64.8) | 0 (0–0) |
CRT adjuvant concurrent chemoradiotherapy, ECOG Eastern Cooperative Oncology Group performance status
Received inguinal RT
Received pelvic RT
Predictors of locoregional control
The overall median follow-up was 36.6 months when estimated via the reverse Kaplan-Meier method. LRC at 2 years for this cohort was 54% (Fig. 1a). Using UVA in Cox proportional hazards analysis, predictors of LRC for the entire cohort (n = 51) included performance status, tumor stage, nodal stage, HPV status, and extent of primary surgery (all p < 0.1), but only pathologic nodal status continued to predict for LRC using MVA (p = 0.027) (Table 2). Patients with pathological node-positive disease (pN+) (n = 28, 55%), regardless of pN1, pN2, or pN3 metastatic burden, had a significantly worse LRC compared to pathological node-negative patients (pN0) (Fig. 1b, p = 0.003). Stratifying based on nodal involvement showed a significant 2 year LRC benefit in pN− patients (Fig. 1c, 81 vs. 37%, p = 0.002) when compared to pN+ patients by the log-rank test.
Fig. 1.
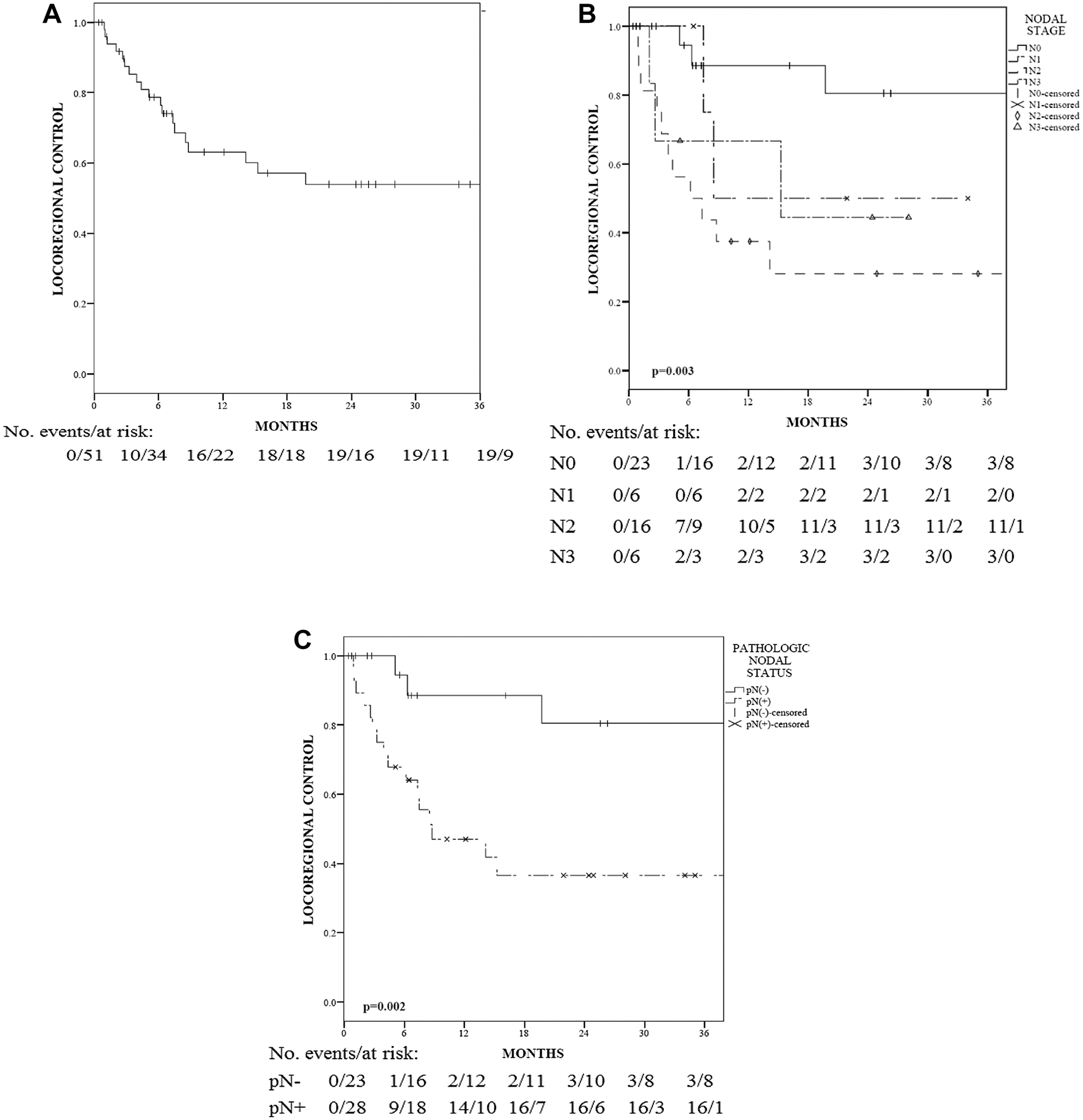
a Locoregional control (LRC) among all patients. b LRC among patients with different nodal stages. c LRC among patients with pathologic negative (pN−) or positive (pN+) nodal status. The p values are based on the log-rank test
Table 2.
Variables associated with locoregional control among all patients with PeCa (n = 51)
| Variables | UVA | MVA | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| ECOG | 3.88 (1.38–10.92) | 0.010 | 1.67 (0.49–5.76) | 0.415 |
| Tumor stage | ||||
| T1 | Reference | 0.047 | Reference | 0.551 |
| T2 | 1.42 (0.51–3.98) | 0.500 | 1.44 (0.36–5.72) | 0.605 |
| T3 | 1.00 (0.31–3.19) | 1.000 | 1.05 (0.28–3.98) | 0.943 |
| T4 | 54.27 (3.13–939.93) | 0.006 | 9.25 (0.39–220.24) | 0.169 |
| Nodal stage | ||||
| N0 | Reference | 0.014 | Reference | 0.027 |
| N1 | 2.64 (0.43–16.01) | 0.290 | 3.98 (0.57–27.64) | 0.163 |
| N2 | 7.67 (2.14–27.42) | 0.002 | 7.99 (2.01–31.75) | 0.003 |
| N3 | 4.62 (0.92–23.17) | 0.063 | 8.11 (1.30–50.48) | 0.025 |
| HPV/p16 status | 0.42 (0.16–1.1) | 0.076 | 0.41 (0.14–1.24) | 0.116 |
| Extent of surgery | 2.27 (0.91–5.64) | 0.079 | 2.10 (0.70–6.36) | 0.187 |
Bold indicates statistical significance p values (< 0.05)
ECOG Eastern Cooperative Oncology Group performance status, UVA cox univariate analysis, MVA cox multivariate analysis, HR hazard ratio
Predictors of locoregional control in node-positive disease
As our only independent predictor of LRC, pN+ subset (n = 28, 55%) was analyzed. There was a 2 year LRC benefit associated with adjuvant CRT (vs. without CRT: 54 vs. 13%, p = 0.006; Fig. 2). In Cox UVA, other variables associated with LRC in pN+ patients include tumor stage, HPV status, lymphovascular invasion (LVI), the extent of primary surgery, and adjuvant CRT (all p < 0.1; Table 3). In MVA, only HPV+ disease (HR 0.18, 95% CI 0.039–0.80, p = 0.024) and adjuvant CRT (HR 0.19, 95% CI 0.05–0.70, p = 0.012) predicted for LRC in pN+ patients (Table 3). In the group without adjuvant concurrent CRT, one patient received adjuvant RT only and one patient received adjuvant chemotherapy only; this treatment potentially may have improved LRC in these two patients. However, we continued to observe a significant improvement in LRC for CRT when these two patients were included in the group without CRT (−CRT). There was one T4 patient in the −CRT group, which potentially augmented outcome prediction for pN+ patients. A MVA was performed excluding the T4 patient, and adjuvant CRT (HR 0.188, 95% CI 0.05–0.702, p = 0.013) and HPV status (HR 0.177, 95% CI 0.039–0.803, p = 0.025) continued to predict for LRC.
Fig. 2.
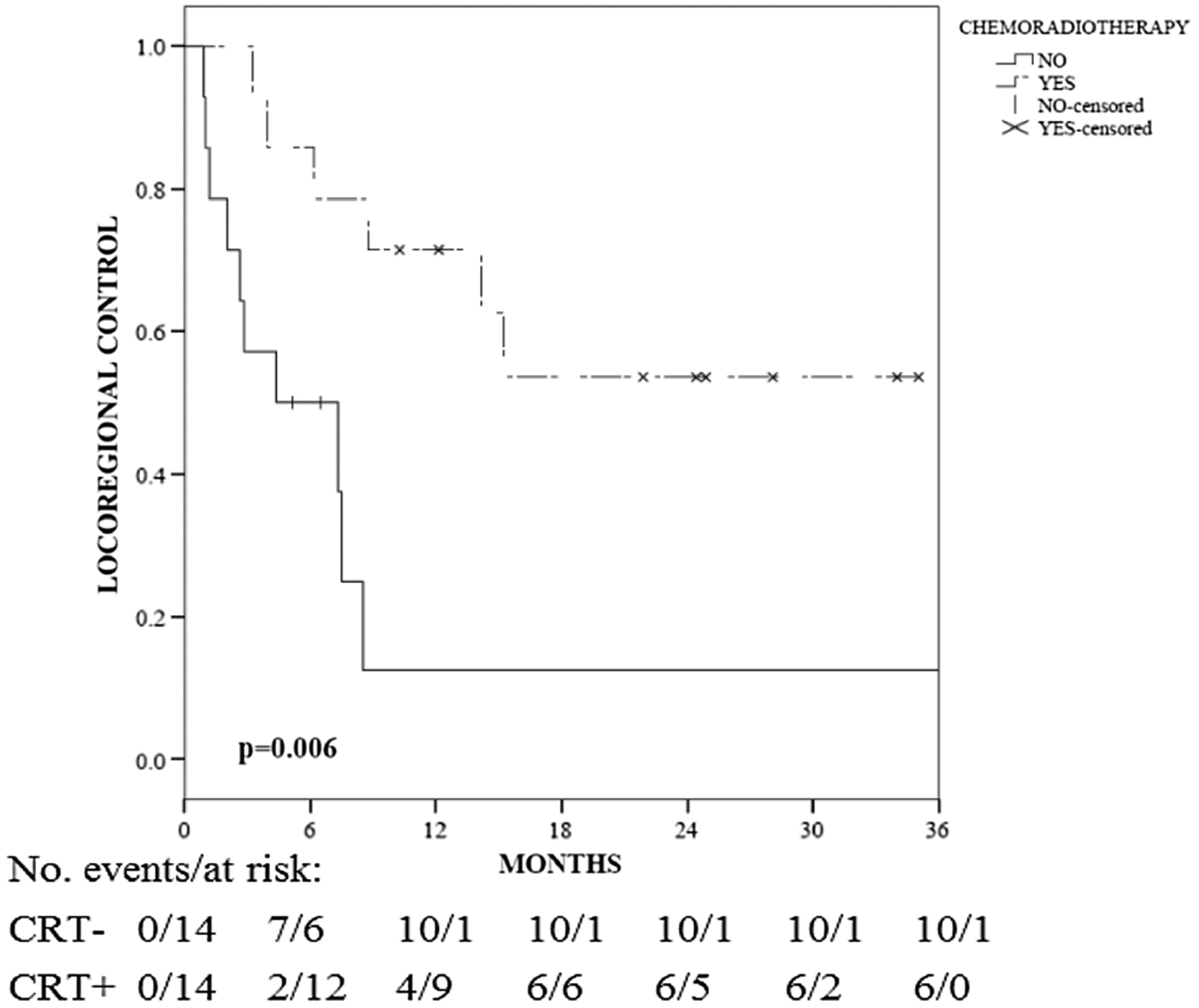
Locoregional control for patients with pathologic positive (pN+) nodal status treated with and without adjuvant CRT. The p values are based on the log-rank test
Table 3.
Variables associated with locoregional control among pN+ patients with PeCa (n = 28)
| Variables | UVA | MVA | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Tumor stage | ||||
| T1 | Reference | 0.022 | Reference | 0.648 |
| T2 | 3.72 (1.01–13.71) | 0.048 | 1.29 (0.29–5.63) | 0.739 |
| T3 | 1.23 (0.31–4.93) | 0.774 | 0.84 (0.16–4.37) | 0.839 |
| T4 | 53.15 (2.77–1020.21) | 0.008 | 5.44 (0.21–142.99) | 0.310 |
| HPV/p16 status | 0.24 (0.08–0.76) | 0.015 | 0.18 (0.039–0.80) | 0.024 |
| LVI | 0.36 (0.12–1.13) | 0.080 | 0.39 (0.10–1.60) | 0.192 |
| Extent of surgery | 2.55 (0.92–7.04) | 0.072 | 1.58 (0.46–5.41) | 0.463 |
| Adjuvant CRT | 0.24 (0.08–0.71) | 0.010 | 0.19 (0.05–0.70) | 0.012 |
Bold indicates statistical significance p values (< 0.05)
LVI Lymphovascular invasion, CRT Chemoradiotherapy, UVA Cox Univariate analysis, MVA Cox Multivariate analysis, HR Hazard ratio
HPV status stratified by treatment
When analyzing the entire cohort (n = 51), there was a non-significant 1 and 2 year LRC benefit associated with HPV+ patients (82 vs. 50% and 75 vs. 39%, p = 0.068), compared to HPV− patients (Fig. 3a).
Fig. 3.
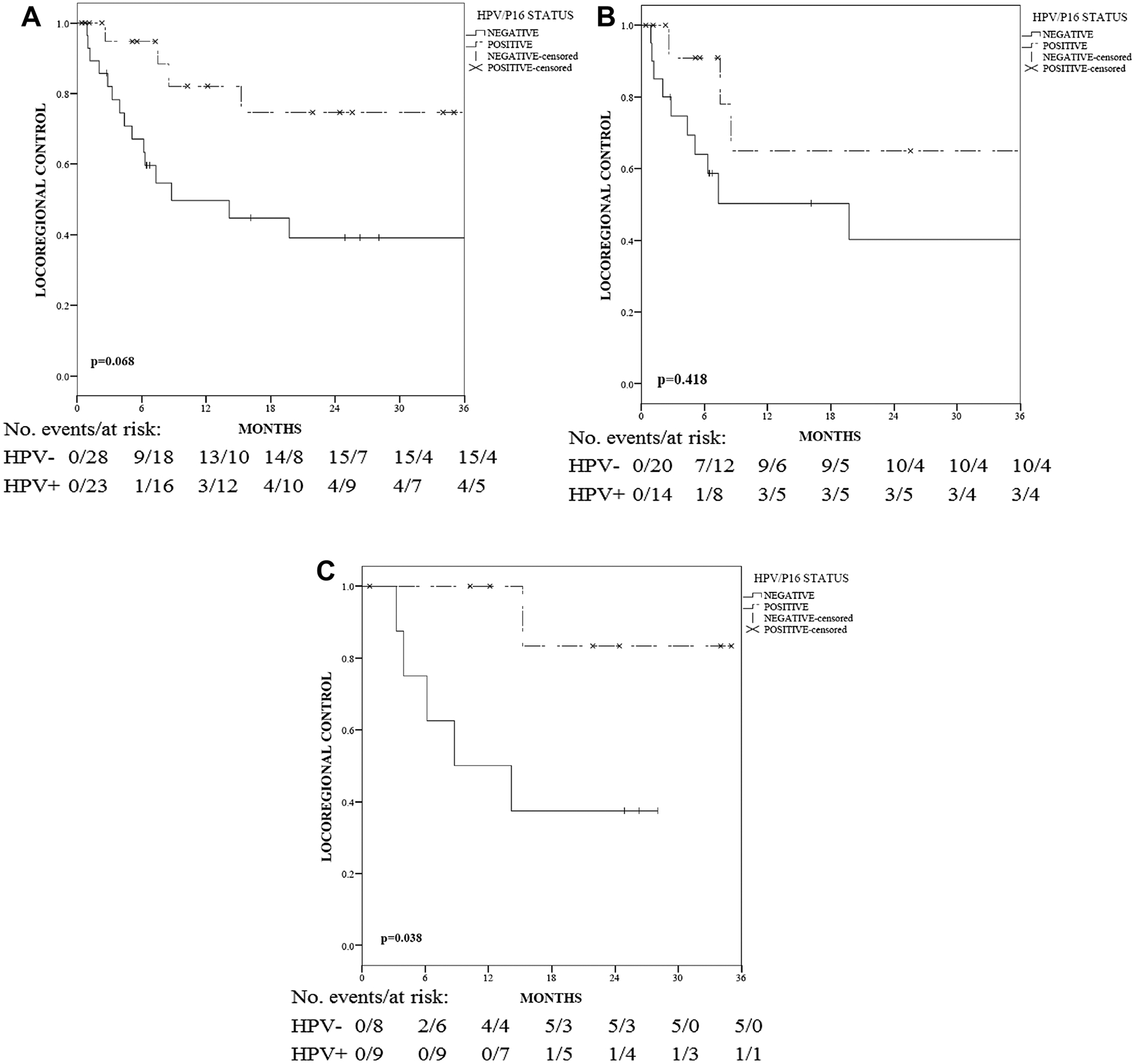
a Locoregional control (LRC) among all patients stratified by HPV status. b LRC among patients stratified by HPV status and treated with surgery without adjuvant CRT. c LRC among patients stratified by HPV status regardless of nodal stage and treated with surgery and adjuvant CRT. The p values are based on the log-rank test
When stratified by treatment, having HPV+ disease did not demonstrate a significant 1 and 2 year LRC benefit for patients treated with surgery without CRT (vs. HPV−: 65 vs. 51% and 65 vs. 40%, respectively, p = 0.418, Fig. 3b). However, in patients treated with adjuvant CRT, HPV+ PeCa predicted for 1 and 2 year absolute LRC benefits of 50% (100 vs. 50%, respectively) and 45% (83 vs. 38%, respectively), when compared to HPV− patients (p = 0.038) (Fig. 3c). The observed benefit was less likely influenced by the chemotherapy regimens of these two groups since they were well balanced (p = 0.39). In the HPV+/CRT+ group, eight patients received concurrent cisplatin chemotherapy and one patient received concurrent 5-FU/Mitomycin C. In the HPV−/CRT+ group, one patient received 5-FU/Mitomycin C, one received Cisplatin/5-FU, and others (n = 6) received concurrent Cisplatin.
Other clinical outcomes
Among all patients (Supplementary Table S2), HPV/p16 status and adjuvant CRT were not associated with PFS and OS (all p > 0.1). Patient nodal status was associated with PFS (p = 0.006). Patient performance status was associated with PFS (p = 0.005, 95% CI 1.46–8.19) and OS (p = 0.035, 95% CI 1.08–8.34).
Among pN+ patients (Supplementary Table S3), HPV/p16 status and adjuvant CRT were not associated with a benefit of PFS (p = 0.065). Patient performance status, HPV/p16 status, and adjuvant CRT were not associated with OS (all p > 0.1).
Acute and late toxicity
Acute and late toxicity was assessed for this cohort and compared to patients treated with adjuvant CRT (CRT+) versus without adjuvant CRT (CRT−) (Supplementary Table S4). There were no grade ≥ 3 toxicities noted in our study. The most common acute adverse events for patients treated with adjuvant CRT were grade 1 fatigue (88.2%), grade 2 skin toxicity (17.6%), grade 2 hematologic toxicity (11.8%), and grade 2 GI toxicity (11.8%). The most common late toxicities in the patients treated with adjuvant CRT were grade 1 skin toxicities (35.3%) and grade 1 lymphedema (17.6%). The addition of CRT did not significantly predict for an increase in acute or late grade 2 toxicity (all p > 0.1).
Discussion
In rare malignancies, such as PeCa, data from randomized controlled trials is limited, with a scarcity of data regarding treatment optimization. We analyzed the association between HPV status and CRT in the treatment of PeCa patients. To our knowledge, this is the first study to evaluate outcomes in PeCa in regard to HPV status and the addition of CRT. This study shows that patients with positive HPV/p16 status are associated with improved LRC when treated with adjuvant CRT. These findings suggest that HPV+ PeCa may be a distinct subset of PeCa with a different etiology and response to CRT [4, 12, 13], which may translate to the improved outcomes that are well understood in HNSCC [15–17, 20, 21].
The extent of nodal metastases is an important prognostic factor in patients with PeCa [22, 23], predicting for poor long-term survival [4, 24]. Retrospective studies have demonstrated that adjuvant chemotherapy might be correlated with improved outcome in pN+ patients [22, 25]. Sharma et al. reported that adjuvant chemotherapy was associated with improved overall survival in MVA (HR 0.40; 95% CI 0.19–0.87; p = 0.021) [22]. However, prospective data are lacking in the adjuvant setting to evaluate efficacy and causality. Recent multicenter retrospective analysis demonstrated that perioperative chemotherapy did not provide significant survival benefit in locally advanced PeCa [26]. In our study, chemotherapy was primarily cisplatin-based and acted both as a radiosensitizer and potentially improved regional control. The addition of CRT was well tolerated with no significant increase in grade 2 toxicity in this cohort.
The role of adjuvant radiation with or without concurrent chemotherapy is well established in the treatment of HNSCC and anogenital cancer, and data extrapolated from these disease sites have been used in the treatment of locally advanced PeCa. Postoperative CRT has been recommended by NCCN treatment guideline for patients with high-risk features (palpable bulky inguinal nodes, pelvic node involvement, extracapsular extension, and positive margin) [11]. Multiple retrospective studies demonstrated superior LRC in high risk, locally advanced PeCa treated with postoperative RT or CRT [8–10, 25, 27]. However, prospective studies are required to attest the impact of adjuvant RT or CRT in PeCa, especially with novel risk stratification strategies.
In our study, HPV-positive PeCa predicted for a 2 year LRC benefit that was limited to patients treated with adjuvant CRT (vs. HPV-negative: 83 vs. 38%, p = 0.038). To our knowledge, our study is the first to confirm the association between HPV status and radiosensitivity in PeCa. In addition, we note that PeCa patients with nodal involvement have improved outcomes when HPV-positive or treated with CRT, similar to findings of other HPV-related malignancies. Our findings suggest a prospective study is warranted, stratifying PeCa by HPV status, which would augment management in HPV-positive PeCa to a more chemoradiocentric approach.
An International Penile Advanced Cancer Trial (InPACT, ECOG-ACRIN EA8134) is ongoing to address the sequence of treatments including neoadjuvant CRT (concurrent cisplatin plus radiation therapy) versus neoadjuvant chemotherapy (Paclitaxel, Ifosfamide, and Cisplatin) versus standard surgery [28]. The InPACT study is also collecting HPV data for further analysis, which will provide important insights into the role of HPV tumor status in the management of PeCa in the neoadjuvant setting. Similar to findings in other disease sites [29, 30], it will be intriguing to further characterize the prognostic impact of HPV/p16, smoking, and p53 status in PeCa.
Limitations of this study include its retrospective nature and short follow-up period. Due to the rarity of PeCa, our study had a limited sample size and may have been under-powered for identifying all prognostic factors associated with PeCa, such as RT fields and the extent of nodal dissection. Given the retrospective nature, the documents for the long-term toxicities, p53 status, and smoking history were limited and the logistics of not receiving adjuvant CRT in a subset of pN+ patients was unknown. The conclusions on target coverage and dose were limited given that various radiation fields and doses were used in this small cohort study. Given these limitations, the impact of HPV+ tumor status and adjuvant CRT in PeCa should be considered useful for hypothesis-generating, rather than yielding definitive treatment options.
Conclusions
Our study demonstrated a significant correlation of HPV status with improved locoregional control of PeCa to adjuvant chemoradiation therapy. Our results warrant further investigation in prospective studies.
Supplementary Material
Footnotes
Conflict of interest Dr. Anna R. Giuliano is on a member of Merck’s Scientific and Global Advisory Boards and receives funding form Merck for investigator initiated studies related to HPV. The other authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00345-018-2280-0) contains supplementary material, which is available to authorized users.
References
- 1.Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300 [DOI] [PubMed] [Google Scholar]
- 2.Mannweiler S, Sygulla S, Winter E, Regauer S (2013) Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol 69:73–81 [DOI] [PubMed] [Google Scholar]
- 3.Pow-Sang MR, Ferreira U, Pow-Sang JM, Nardi AC, Destefano V (2010) Epidemiology and natural history of penile cancer. Urology 76:S2–S6 [DOI] [PubMed] [Google Scholar]
- 4.Srinivas V, Morse MJ, Herr HW, Sogani PC, Whitmore WF Jr (1987) Penile cancer: relation of extent of nodal metastasis to survival. J Urol 137:880–882 [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Akduman B, Bouchot O, Palou J, Tobias-Machado M (2010) Prognostic factors in penile cancer. Urology 76:S66–S73 [DOI] [PubMed] [Google Scholar]
- 6.Leone A, Diorio GJ, Pettaway C, Master V, Spiess PE (2017) Contemporary management of patients with penile cancer and lymph node metastasis. Nat Rev Urol 14:335. [DOI] [PubMed] [Google Scholar]
- 7.Ornellas AA, Kinchin EW, Nobrega BL, Wisnescky A, Koifman N, Quirino R (2008) Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J Surg Oncol 97:487–495 [DOI] [PubMed] [Google Scholar]
- 8.Chen MF, Chen WC, Wu CT, Chuang CK, Ng KF, Chang JT (2004) Contemporary management of penile cancer including surgery and adjuvant radiotherapy: an experience in Taiwan. World J Urol 22:60–66 [DOI] [PubMed] [Google Scholar]
- 9.Franks KN, Kancherla K, Sethugavalar B, Whelan P, Eardley I, Kiltie AE (2011) Radiotherapy for node positive penile cancer: experience of the Leeds teaching hospitals. J Urol 186:524–529 [DOI] [PubMed] [Google Scholar]
- 10.Tang DH, Djajadiningrat R, Diorio G, Chipollini J, Ma Z, Schaible BJ, Catanzaro M, Ye D, Zhu Y, Nicolai N et al. (2017) Adjuvant pelvic radiation is associated with improved survival and decreased disease recurrence in pelvic node-positive penile cancer after lymph node dissection: A multi-institutional study. Urol Oncol 35:605.e617–605.e623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penile cancer [https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf]
- 12.Diorio GJ, Giuliano AR (2016) The role of human papilloma virus in penile carcinogenesis and preneoplastic lesions: a potential target for vaccination and treatment strategies. Urol Clin North Am 43:419–425 [DOI] [PubMed] [Google Scholar]
- 13.Mannweiler S, Sygulla S, Beham-Schmid C, Razmara Y, Pummer K, Regauer S (2011) Penile carcinogenesis in a low-incidence area: a clinicopathologic and molecular analysis of 115 invasive carcinomas with special emphasis on chronic inflammatory skin diseases. Am J Surg Pathol 35:998–1006 [DOI] [PubMed] [Google Scholar]
- 14.Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, Leemans CR, Brakenhoff RH (2004) Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst 96:998–1006 [DOI] [PubMed] [Google Scholar]
- 15.Naghavi AO, Strom TJ, Ahmed KA, Echevarria MI, Abuodeh YA, Venkat PS, Frakes JM, Harrison LB, Trotti AM, Caudell JJ (2016) Management of oropharyngeal cancer in the HPV era. Cancer Control 23:197–207 [DOI] [PubMed] [Google Scholar]
- 16.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269 [DOI] [PubMed] [Google Scholar]
- 17.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A (2006) Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24:736–747 [DOI] [PubMed] [Google Scholar]
- 18.Zargar-Shoshtari K, Spiess PE, Berglund AE, Sharma P, Pow-sang JM, Giuliano A, Magliocco AM, Dhillon J (2016) Clinical significance of p53 and p16(ink4a) status in a contemporary North American penile carcinoma cohort. Clin Genitourin Cancer 14:346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezerra SM, Chaux A, Ball MW, Faraj SF, Munari E, Gonzalez-Roibon N, Sharma R, Bivalacqua TJ, Burnett AL, Netto GJ (2015) Human papillomavirus infection and immunohistochemical p16(INK4a) expression as predictors of outcome in penile squamous cell carcinomas. Hum Pathol 46:532–540 [DOI] [PubMed] [Google Scholar]
- 20.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J (2009) Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 27:1992–1998 [DOI] [PubMed] [Google Scholar]
- 21.Lewis JS Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK (2010) p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol 34:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma P, Djajadiningrat R, Zargar-Shoshtari K, Catanzaro M, Zhu Y, Nicolai N, Horenblas S, Spiess PE (2015) Adjuvant chemotherapy is associated with improved overall survival in pelvic node-positive penile cancer after lymph node dissection: a multi-institutional study. Urol Oncol 33(496):e417–e423 [DOI] [PubMed] [Google Scholar]
- 23.Sonpavde G, Pagliaro LC, Buonerba C, Dorff TB, Lee RJ, Di Lorenzo G (2013) Penile cancer: current therapy and future directions. Ann Oncol 24:1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu JY, Li YH, Zhang ZL, Yao K, Ye YL, Xie D, Han H, Liu ZW, Qin ZK, Zhou FJ (2013) The risk factors for the presence of pelvic lymph node metastasis in penile squamous cell carcinoma patients with inguinal lymph node dissection. World J Urol 31:1519–1524 [DOI] [PubMed] [Google Scholar]
- 25.Nicolai N, Sangalli LM, Necchi A, Giannatempo P, Paganoni AM, Colecchia M, Piva L, Catanzaro MA, Biasoni D, Stagni S et al. (2016) A combination of cisplatin and 5-fluorouracil with a taxane in patients who underwent lymph node dissection for nodal metastases from squamous cell carcinoma of the penis: treatment outcome and survival analyses in neoadjuvant and adjuvant settings. Clin Genitourin Cancer 14:323–330 [DOI] [PubMed] [Google Scholar]
- 26.Necchi A, Pond GR, Raggi D, Ottenhof SR, Djajadiningrat RS, Horenblas S, Khoo V, Hakenberg OW, Draeger D, Protzel C et al. (2017) Clinical outcomes of perioperative chemotherapy in patients with locally advanced penile squamous-cell carcinoma: results of a multicenter analysis. Clin Genitourin Cancer 15(548–555):e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapierre A, Riou O, Flechon A, Mottet N, Azria D (2017) Advanced penile cancer with iliac lymph node involvement treated with radiation and concurrent gemcitabine. Cancer Radiother 21:134–137 [DOI] [PubMed] [Google Scholar]
- 28.Nicholson S, Kayes O, Minhas S (2014) Clinical trial strategy for penis cancer. BJU Int 113:852–853 [DOI] [PubMed] [Google Scholar]
- 29.Lassen P, Lacas B, Pignon JP, Trotti A, Zackrisson B, Zhang Q, Overgaard J, Blanchard P (2018) Prognostic impact of HPV-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: the MARCH-HPV project. Radiother Oncol 126:107–115 [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Zhang W, Liu W, Mao Y, Fu Z, Liu J, Huang W, Zhang Z, An D, Li B (2017) Human papillomavirus infection increases the chemoradiation response of esophageal squamous cell carcinoma based on P53 mutation. Radiother Oncol 124:155–160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


