Abstract
Context:
There is a critical need for effective bladder-sparing therapies for bacillus Calmette-Guérin (BCG)-unresponsive non–muscle-invasive bladder cancer (NMIBC). Owing to the current lack of effective agents that can be used as a control, the US Food and Drug Administration began to accept single-arm trials for patients with carcinoma in situ (CIS), using complete response rate (CRR) and duration of response as the primary endpoints to support marketing applications. Despite the ensuing growth of clinical trials in this space, no consensus exists on a clinically relevant benchmark for CRR.
Objective:
To elucidate the CRR and recurrence-free rate (RFR) using bladder-sparing agents after BCG failure in order to provide a frame of reference for future clinical trial results.
Evidence acquisition:
We performed a systematic review of clinical trials utilizing bladder-sparing therapeutics for NMIBC recurring after intravesical BCG (PROSPERO CRD42019130553). The search was performed in MEDLINE, EMBASE, and Cochrane Library. Relevant studies identified from bibliography search and conference abstracts were searched to complement the systematic review. A total of 42 studies utilizing 24 treatment options and consisting of 2254 patients were included for final analysis.
Evidence synthesis:
Median CRRs in the treatment of CIS-containing tumors were 26% at 6 mo, 17% at 12 mo, and 8% at 24 mo after treatment. In comparison, median RFRs in the papillary-only studies were 67% at 6 mo, 44% at 12 mo, and 10% at 24 mo. Specifically in the BCG-unresponsive population, 6- and 12-mo CRRs in CIS-containing patients treated with Mycobacterium phlei cell wall-nucleic acid complex were 45% and 27%, respectively, and the median 6-, 12-, and 24-mo disease-free rates in the other studies were 43%, 35%, and 18%, respectively. The median progression-free rate was 91%: 95% in the CIS-containing studies and 89% in studies restricted to papillary-only recurrences. Toxicities of intravesical agents were generally mild, with very few dose limiting toxicities.
Conclusions:
We demonstrate that, to date, bladder-sparing therapies achieved modest efficacy in patients with NMIBC after BCG. Results from the current study will serve as a frame of reference for emerging trial results in the BCG-unresponsive space.
Patient summary:
In this study, we found that bladder-sparing therapies achieved modest efficacy in patients with non–muscle-invasive bladder cancer after bacillus Calmette-Guérin (BCG). These results will serve to inform future clinical trial results for salvage agents used to treat BCG-unresponsive bladder cancer.
Keywords: Bladder cancer, Bladder-sparing therapy, Non–muscle-invasive bladder cancer, Bacillus Calmette-Guérin–unresponsive non–muscle-invasive bladder cancer, Intravesical treatment
1. Introduction
There is a critical unmet need for effective bladder-sparing therapies to treat recurrent non–muscle-invasive bladder cancer (NMIBC) not responsive to intravesical (i/ves) bacillus Calmette-Guérin (BCG). Although radical cystectomy is the recommended standard-of-care treatment in this setting, a substantial proportion of patients are either unfit for or unwilling to undergo surgery, thus raising the need for bladder-sparing alternatives.
Development of new agents in this disease space has been hampered by the heterogeneity in patient population, poor definition of disease states, a lack of appropriate control arms, and consensus on trial endpoints. To address these concerns, workshops cosponsored by the US Food and Drug Administration (FDA) and American Urological Association (AUA), as well as expert consensus guidelines, have helped orchestrate the general framework for future studies [1–3]. Many of the recommendations were subsequently incorporated into the FDA’s Guidance Document on developing drugs and biologics for the treatment of BCG-unresponsive bladder cancer. In this document, the FDA indicated that open-label, single-arm clinical trials for carcinoma in situ (CIS)-containing BCG-unresponsive NMIBC with complete response rate (CRR) and duration of response (DoR) as the primary endpoints would be accepted to support a marketing application [4]. In contrast, efficacy of salvage agents for BCG-unresponsive patients with only resected papillary disease should be tested using a randomized controlled trial design with a time-to-event endpoint such as recurrence-free survival.
The FDA’s decision to accept single-arm clinical trials for novel agents tested in BCG-unresponsive NMIBC patients with CIS means that reference rates will be needed to guide discussions evaluating the effectiveness of such agents. Although recommendations for clinically relevant CRRs have been proposed [2], they were not data driven. Accordingly, we conducted a systematic review of clinical trials involving bladder-sparing therapies used in the post-BCG setting to identify evidence-based CRRs and recurrence-free rates (RFRs) and to provide a frame of reference to guide interpretation of future clinical trial results.
2. Evidence acquisition
2.1. Systematic literature review
We performed a systematic review of prospective clinical trials utilizing bladder-sparing therapeutics for NMIBC after prior i/ves BCG therapy following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PROSPERO CRD42019130553). Comprehensive search strategies were used to identify all relevant trials investigating the safety and/or efficacy of bladder-sparing therapeutics following persistent CIS and/or non–muscle-invasive papillary i/ves recurrence after BCG treatment. The search was performed in MEDLINE, EMBASE, and Cochrane Library using search terms defined by the population, interventions, comparisons, outcomes, and study design (PICOS) approach [5], using the terms “intravesical,” “non–muscle invasive bladder cancer” or “carcinoma” or “neoplasm” or “malignancy,” “refractory” or “recurring” or “failure” or “non-responsive” or “unresponsive,” and “BCG” or “bacillus Calmette-Guerin” (Supplementary material), with no limitations on publication year. Only English-language publications were considered. Observational studies, editorials, commentaries, review articles, and those not subject to peer review were excluded. Bibliographies of included studies were manually searched to ensure completeness. Conference abstracts of relevant medical societies up to and including the 2019 AUA Annual Meeting were searched to complement the systematic review. In cases where multiple reports were made on the same patient cohort, the most recent comprehensive publication was used for the analysis. To preserve fidelity of the analysis to outcomes in BCG-treated patients only, clinical trials including patients recurring after both BCG and non-BCG agents were excluded, unless a separate analysis was performed in the BCG recurrent subset. To accommodate for differences in study populations and disease descriptions before and after the definition of BCG-unresponsive NMIBC in 2015, all the trials accruing patients failing or recurring after induction or multiple courses of BCG, patients with both high-(HG) and low-grade (LG) recurrences, and patients with CIS only, papillary only, and any combinations thereof were captured. To assess specifically the efficacy of agents in the BCG-unresponsive setting, trials accruing patients satisfying the 2015 definition [2–4] were analyzed separately.
2.2. Study review methodology
Two authors (R.L. and D.S.) reviewed and selected studies independently; disagreements were resolved by discussion and consensus. Titles and abstracts were used to screen for initial study inclusion. Full texts of studies thought to meet or possibly meet the study inclusion were then reviewed. The same reviewers extracted relevant data independently using standardized data collection forms. Data retrieved from the reports include publication details (year of publication and authors), methodological components, and trial characteristics (sample size, therapeutic agent, and outcomes measures). The primary outcomes were stratified by tumor histology, using CRRs for studies limited to CIS-containing patients, RFRs for studies limited to patients with papillary disease, and disease-free rates (DFRs) for studies enrolling a combination of the two. CRR, RFR, and DFR were defined as the lack of a tumor or recurrence on cystoscopic evaluation with mandated/for-cause biopsies and negative urine cytology. Enhanced cystoscopy was not routinely used in any of the included studies. Secondary outcome measures included toxicity and progression-free rate (PFR) defined by progression to muscle-invasive or metastatic disease. In cases of comparative studies of more than one agent, outcomes in each treatment arm were separately extracted and analyzed.
2.3. Risk of bias assessment
A formal exclusion of studies due to risk of bias (RoB) assessment was not carried out as none of the existing RoB scales were felt to be appropriate for this systematic review. Many different factors could affect the outcome of the patients in the studies included in this systematic review. These and other limitations are presented in the Discussion. However, studies reported only in abstract form or not presenting complete outcome data were deemed to have a high RoB and excluded from the final analysis.
2.4. Data synthesis
Data synthesis was stratified by the histology of tumor after BCG, with CRRs reported for studies limited to CIS-containing patients, RFRs for studies limited to patients with papillary disease, and DFRs for studies enrolling a combination of the two. CRRs/RFRs/DFRs and PFRs reported at different time points in each study were collected and grouped by the post-treatment time intervals at which these were recorded. The median and range of the groups at 3, 6, 12, 18, and 24 mo following treatment were reported. Heterogeneity of study results was assessed by the inconsistency statistic (I2) and its connected chi-square test for heterogeneity. A meta-analysis was not feasible due to the extreme heterogeneity of the reported outcomes in each group. For illustrative purposes, the results were summarized using forest plots.
To further explore the efficacy of the bladder-sparing agents in different subgroups, separate analyses were carried out for studies accruing patients with BCG-unresponsive NMIBC [2–4]. Salvage agents were also broadly categorized as cytotoxic or immunogenic. CRRs/RFRs/DFRs of each drug type in the patient subsets were examined. No formal test was conducted for the purpose of subgroup comparisons and results were solely displayed in a descriptive manner.
3. Evidence synthesis
3.1. Literature search results
In total, 713 citations were retrieved from the databases and 12 additional abstracts were included from recent international conferences. After removing duplicates and screening 721 titles and abstracts, 619 citations were excluded from further analysis. The remaining 102 full texts were screened, of which 60 were excluded (Fig. 1). A total of 42 studies with 45 unique study arms (SAs) consisting of 2254 patients, with a median (interquartile range [IQR]) of 35 (18–47) patients were included for final analysis. Of these studies, three were randomized controlled trials, one was a nonrandomized comparative study, and 38 were single-arm studies. Ten of the 38 single-arm studies reported incomplete outcome data and/or were available in the abstract form only, and were deemed to have a high RoB. In total, 24 treatment options, including 19 i/ves, three oral, one intravenous, and one combination of oral and i/ves, were identified (Fig. 2). Therapeutic agents included i/ves BCG [6,7]; i/ves BCG + interferon alpha (IFNα) [8]; i/ves Mycobacterium phlei cell wall–nucleic acid complex (MCNA) [9]; i/ves oncolytic adenovirus CG0070 [10,11]; i/ves rAd-IFNα/Syn3 (Instiladrin) [12–14]; combination of i/ves ALT803 and BCG [15]; oral bropirimine [16]; intravenous pembrolizumab [17]; i/ves gemcitabine [6,7,18–25]; oral everolimus and i/ves gemcitabine [26]; combination of i/ves ALT-801 and gemcitabine [27]; i/ves mitomycin C (MMC) [19]; i/ves thermochemotherapy using MMC [28–30]; i/ves electromotive drug administration of MMC [31]; i/ves docetaxel [32]; combination of i/ves cabazitaxel, gemcitabine, and cisplatin [33]; i/ves nanoparticle albumin bound (nab-)paclitaxel [34,35]; i/ves paclitaxel-hyaluronic acid bioconjugate (Oncofid) [36,37]; i/ves valrubicin [38,39]; i/ves doxorubicin analog AD32 [40]; oral dovitinib [41]; i/ves oportuzumab monatox (Vicinium) [42–44]; oral sunitinib [45]; and i/ves photodynamic therapy (Fig. 2) [46,47].
Fig. 1 –
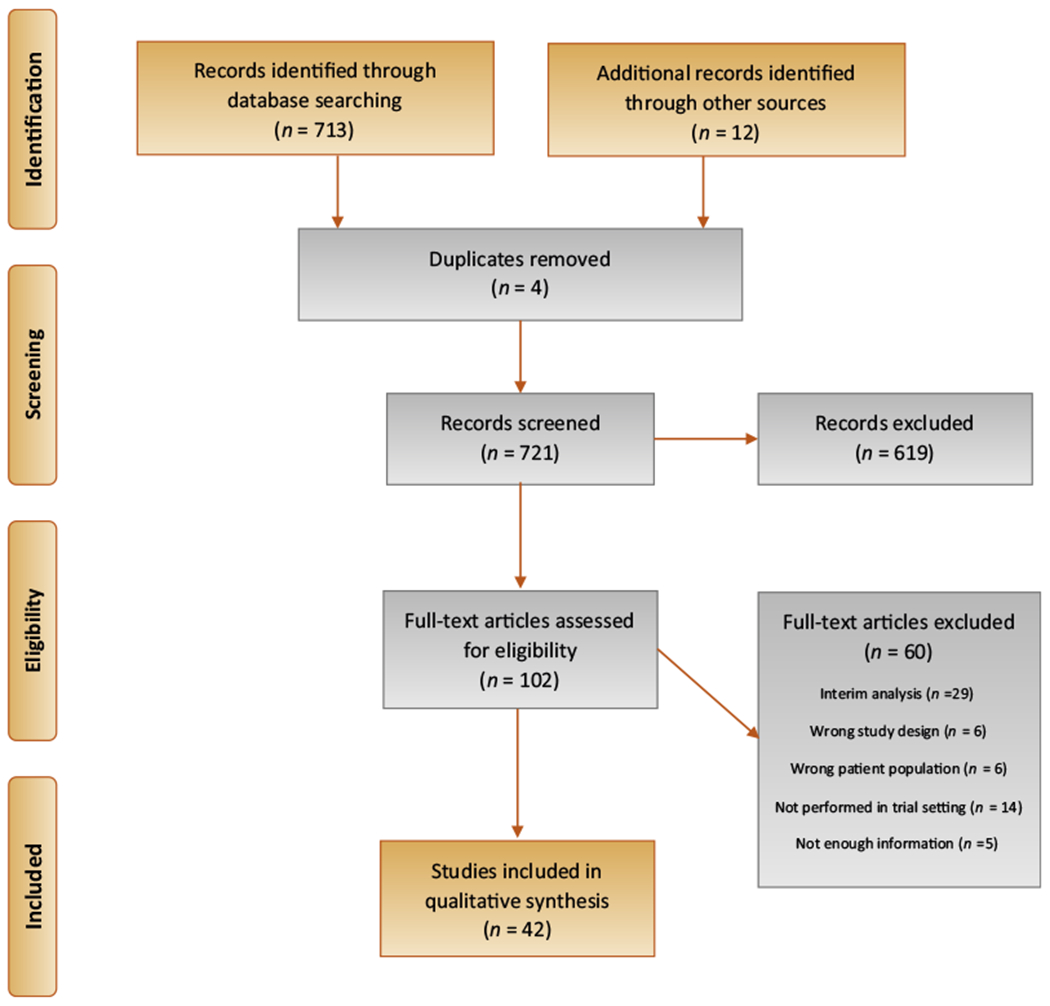
PRISMA flowchart of bladder-preserving treatments for recurrent non–muscle-invasive bladder cancer following intravesical BCG. BCG = bacillus Calmette-Guérin; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Fig. 2 –
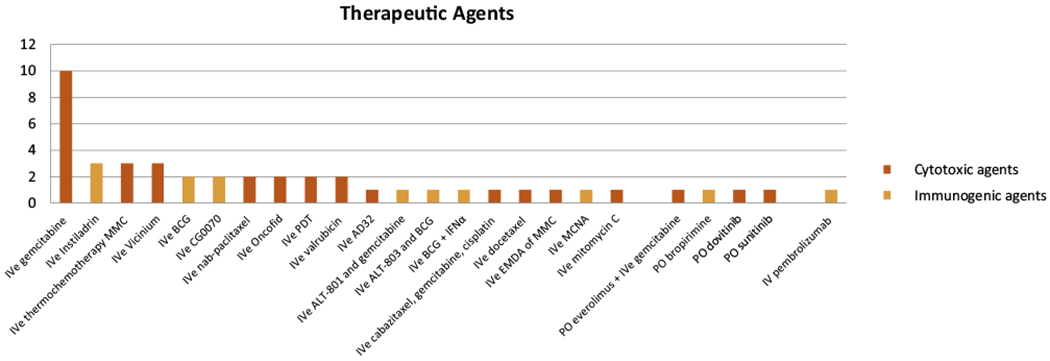
Breakdown of study arms by different therapeutic agents and route administered. Cytotoxic agents are shown as red bars; immunogenic agents are shown as blue bars. BCG = bacillus Calmette-Guérin; EMDA = electromotive drug administration; IFNα = interferon alpha; IVe = intravesical; MMC = mitomycin C; PDT = photodynamic therapy.
3.2. Data extraction
Supplementary Table 1 lists the pertinent clinicopathological characteristics of the studies. The median/mean age ranged from 54 to 77 yr. A majority of the SAs (28/45) enrolled patients with papillary disease and/or CIS following BCG therapy, nine enrolled patients with CIS with/without papillary disease, one enrolled patients with CIS only, six enrolled patients with papillary disease only, and another did not specify (Table 1). All but nine SAs comprised only patients who had recurred after at least one course of induction BCG. Of the remaining nine SAs, six included a minority of BCG-intolerant patients (3–18%) [8,19,21,26,38] and three studies did not specify [27,36,45]. Many of the studies published prior to the introduction of the definition for BCG-unresponsive NMIBC did not specify the number of patients who satisfied this term. Instead, the percentage of patients who recurred after two or more courses of BCG, ranging from 31% to 100%, was reported in 27 SAs. Two SAs and one post hoc analysis included only patients satisfying the BCG-unresponsive NMIBC definition (Table 1) [12–14,48]. Of these, only the SA by Li et al [48] separately presented CRRs in the BCG-unresponsive patients with CIS-containing tumors.
Table 1 –
Treatment efficacy and progression-free rates in all studies included.
| % Failed 1 prior BCG | % Failed 2 prior BCG | % BCG intolerant | CIS vs papillary | 3-mo CRR/RFR/DFR (%) | 6-mo CRR/RFR/DFR (%) | 12-mo CRR/RFR/DFR (%) | 18-mo CRR/RFR/DFR (%) | 24-mo CRR/RFR/DFR (%) | PFR (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Agent | n | ||||||||||
| Nseyo et al [47] | 1998 | PDT (C) | 36 | 100 | 50 | CIS ± papillary | 58.3 | 30.6 | 77.8 | ||||
| Sarosdy et al [16] | 1998 | Bropirimine (I) | 47 | 100 | 100 | 0 | CIS ± papillary | 14.9 | 89.5 | ||||
| Dalbagni et al [20] | 2002 | Gemcitabine (C) | 18 | 100 | 56 | 0 | Both | 38.9 | 94.4 | ||||
| Bassi et al [18] | 2005 | Gemcitabine (C) | 9 | 100 | 0 | CIS | 44.4 | 44.4 | 22.2 | 22.2 | 11.1 | 100 | |
| Dalbagni et al [21] | 2006 | Gemcitabine (C) | 30 | 90 | 67 | 10 | Both | 10.0 | 93.3 | ||||
| Joudi et al [8] | 2006 | BCG + IFNα (I) | 467 | 100 | 8 | Both | 45.0 | 93.1 | |||||
| Gacci et al [7] | 2006 | Gemcitabine (C) | 9 | 100 | 100 | 0 | Papillary | 100 | 66.7 | 44.4 | 11.1 | 0.0 | 80.0 |
| Gacci et al [7] | 2006 | BCG (I) | 10 | 100 | 100 | 0 | Papillary | 80.0 | 60.0 | 10.0 | 10.0 | 10.0 | 60.0 |
| McKiernan et al [32] | 2006 | Docetaxel (C) | 18 | 100 | Both | 55.6 | 100 | ||||||
| Gunelli et al [22] | 2007 | Gemcitabine (C) | 40 | 100 | 100 | Papillary | 95.0 | 77.5 | 70.0 | 70.0 | 100 | ||
| Mohanty et al [25] | 2008 | Gemcitabine (C) | 35 | 100 | 0 | Papillary | 60.0 | 91.4 | |||||
| Ignatoff et al [40] a | 2009 | AD32 (doxorubicin analog) (C) | 42 | 100 | Both | 19.0 | 16.7 | 78.6 | |||||
| Addeo et al [19] | 2009 | MMC (C) | 55 | 82 | 18 | Papillary | 89.1 | ||||||
| Addeo et al [19] | 2009 | Gemcitabine (C) | 54 | 85 | 15 | Papillary | 88.9 | ||||||
| Kowalski et al [42] | 2010 | Oportuzumab monatox (Vicinium; C) | 64 | 97 | 55 | Both | 39.3 | 100 | |||||
| Di Lorenzo et al [6] | 2010 | Gemcitabine (C) | 40 | 100 | Both | 19.0 | 82.5 | ||||||
| Di Lorenzo et al [6] | 2010 | BCG (I) | 40 | 100 | Both | 3.0 | 67.5 | ||||||
| Perdona et al [23] | 2010 | Gemcitabine (C) | 20 | 100 | 100 | Both | 75.0 | 65.0 | 50.0 | 40.0 | 35.0 | 75.0 | |
| McKiernan et al [34] | 2011 | Nab-paclitaxel (C) | 18 | 100 | Both | 27.8 | 94.4 | ||||||
| Bassi et al [36].a | 2011 | Paclitaxel-hyaluronic acid (Oncofid-P-B; C) | 15 | CIS ± papillary | 60.0 | 100 | |||||||
| Garcia et al [45].a | 2011 | Sunitinib (C) | 13 | 61.5 | 100 | ||||||||
| Burke et al [10] | 2012 | CG0070 (I) | 35 | 100 | ≥50 | 0 | Both | 48.6 | 100 | ||||
| Kowalski et al [43] | 2012 | Oportuzumab monatox (Vicinium; () | 45 | 100 | 0 | CIS ± papillary | 44.0 | 26.7 | 15.6 | 95.6 | |||
| Dinney et al [12] | 2013 | rAd-IFNα/Syn3 (I) | 17 | 94 | 88 | Both | 41.2 | 41.2 | 29.4 | 29.4 | 23.5 | 88.2 | |
| Dinney et al [38] (A9303) | 2013 | Valrubicin (C) | 78 | 96 | 39 | 4 | CIS ± papillary | 35.9 | 17.9 | 10.0 | 4.0 | 100 | |
| Dinney et al [38]/Steinberg et al [39] | 2013/2000 | Valrubicin (C) | 90 | 100 | 70 | CIS ± papillary | 17.8 | 13.0 | 97.8 | ||||
| Lee et al [46] | 2013 | PDT (C) | 34 | 100 | Both | 100 | 90.9 | 64.4 | 91.2 | ||||
| Skinner et al [24] | 2013 | Gemcitabine (C) | 47 | 100 | 100 | Both | 46.8 | 27.7 | 21.0 | 93.6 | |||
| Inman et al [28] | 2014 | MMC @ 42.2C (C) | 15 | 100 | Both | 73.3 | 66.7 | 66.7 | 40.0 | 26.7 | 100 | ||
| McKiernan et al [35] | 2014 | Nab-paclitaxel (C) | 28 | 100 | 75 | Both | 35.7 | 35.7 | 30.6 | 96.4 | |||
| Morales et al [9]/Li et al [48] | 2015/2017 | MCNA (I) | 129 | 100 | 75 | Both | 69.0 | 34.1 | 22.5 | 14.7 | 78.3 | ||
| Sonpavde et al [27].a | 2015 | IV ALT-801 + gemcitabine (C + I) | 9 | Both | 22.2 | 22.2 | 22.2 | 100 | |||||
| Navai et al [13] | 2016 | rAd-IFNα/Syn3 (I) | 7 | 100 | 100 | Both | 28.6 | 14.3 | 0.0 | 0.0 | 85.7 | ||
| Hahn et al [41] | 2016 | Dovitinib (C) | 13 | 100 | 100 | Both | 38.5 | 7.7 | 7.7 | 76.9 | |||
| Shore et al [14] | 2017 | rAd-IFNα/Syn3 (I) | 40 | 100 | 95 | Both | 57.5 | 42.5 | 35.0 | 95.0 | |||
| Dalbagni et al [26] | 2017 | PO everolimus, intravesical gemcitabine (C) | 23 | 100 | 68 | 11 | CIS ± papillary | 57.9 | 26.3 | 21.1 | 94.6 | ||
| Packiam et al [11] | 2018 | CG0070 (I) | 45 | 100 | Both | 46.7 | 29.5 | 95.6 | |||||
| Dickstein et al [44]a | 2018 | Oportuzumab monatox (Vicinium; C) | 111 | 100 | 100 | Both | 49.5 | 100 | |||||
| Racioppi et al [31] | 2018 | EMDA-MMC (C) | 26 | 100 | 31 | 0 | Both | 80.8 | 73.1 | 69.2 | 84.6 | ||
| Balar et al [17]a | 2019 | Pembrolizumab (I) | 103 | 100 | 100 | CIS ± papillary | 38.8 | 100 | |||||
| DeCastro et al [33]a | 2017 | Cabazitaxel, gemcitabine, cisplatin (C) | 18 | 100 | 100 | Both | 94.4 | 100 | |||||
| Tan et al [29] | 2019 | MMC @ 42.2C (C) | 48 | 100 | 63 | Both | 35.0 | 83.3 | |||||
| Tan et al [30]a | 2019 | MMC @ 42.2C (C) | 87 | 100 | 100 | Both | 55.2 | 48.3 | 95.4 | ||||
| Hurle et al [37]a | 2019 | Paclitaxel-hyaluronic acid (Oncofid-P-B; C) | 21 | 100 | CIS ± papillary | 85.7 | 76.2 | 100 | |||||
| Chamie et al [15].a | 2019 | BCG + ALT803 (I) | 24 | 100 | 100 | Both | 70.8 | 100 |
BCG = bacillus Calmette-Guérin; C = cytotoxic; CIS = carcinoma in situ; CRR = complete response rate; DFR = disease-free rate; EMDA = electromotive drug administration; I = immunogenic; IFNα = interferon α; IV = intravenous; MCNA = M. phiei cell wall–nucleic acid complex; MMC = mitomycin C; PDT = photodynamic therapy; PFR = progression-free rate; RFR = recurrence-free rate.
Studies having a high risk of bias.
CRR assessment and recurrence monitoring generally started 3 mo after the initiation of therapy, with a few studies expediting the first evaluation to immediately after the completion of induction therapy between 1 and 8 wk after initiation [3,8,18,20–22,27,36,46]. Random bladder biopsies (RBBs) were mandated as part of the surveillance protocol in 20 of the studies (Supplementary Table 1). While a majority of the studies defined recurrence as either a positive biopsy or a urine cytology, several published after the adoption of the BCG-unresponsive definition enumerated only HG recurrences on biopsy as an event.
3.3. Complete response/recurrence-free rates
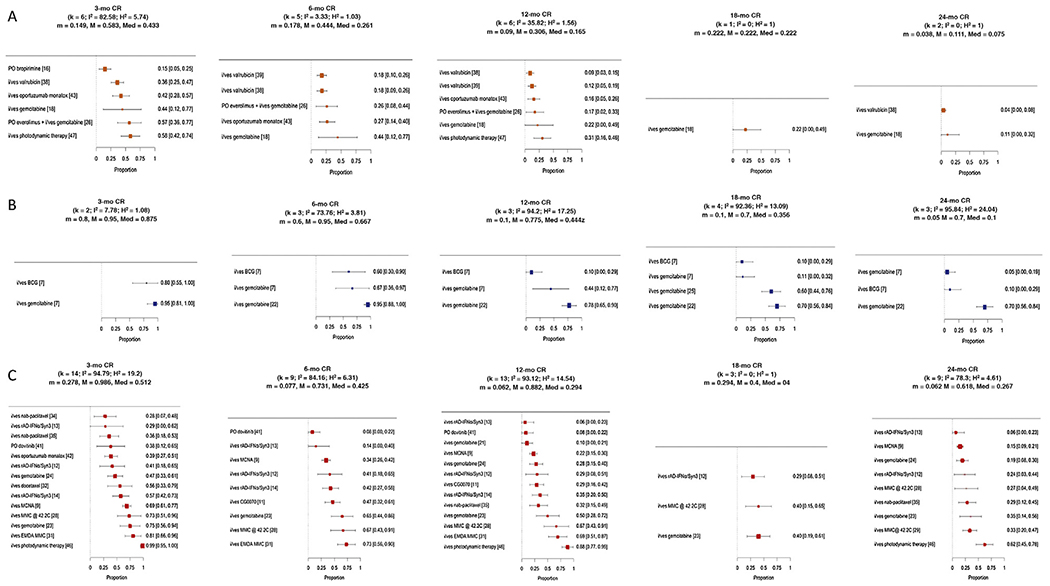
CRRs/RFRs/DFRs were variably reported at 3 mo (31 SAs), 6 mo (19 SAs), 12 mo (26 SAs), 18 mo (eight SAs), and 24 mo (19 SAs) after study initiation (Table 1). After exclusion of SAs with a high RoB, seven SAs accrued patients with CIS with/without papillary disease, six SAs accrued patients with papillary disease only, and 22 accrued patients SAs with both (Fig. 3). The median (range) CRRs in the seven SAs with CIS-containing patients were 43% (15–58%, n = 6) at 3 mo, 26% (18–44%, n = 5) at 6 mo, 17% (9–31%, n = 6) at 12 mo, 22% (22%, n = 1) at 18 mo, and 8% (4–11%, n = 2) at 24 mo (Fig. 4A and Supplementary Table 3). RFRs in the six SAs with papillary disease studies were 88% (80–95%, n = 2) at 3 mo, 67% (60–95%, n = 3) at 6 mo, 44% (10–78%, n = 3) at 12 mo, 36% (10–70%, n = 4) at 18 mo, and 10% (5–70%, n = 3) at 24 mo (Fig. 4B). DFRs in the 22 SAs enrolling both CIS and papillary patients were 51% (28–99%, n = 14) at 3 mo, 43% (8–73%, n = 9) at 6 mo, 29% (6–88%, n = 13) at 12 mo, 40% (29–40%, n = 3) at 18 mo, and 27% (6–62%, n = 9) at 24 mo (Fig. 4C).
Fig. 3 –
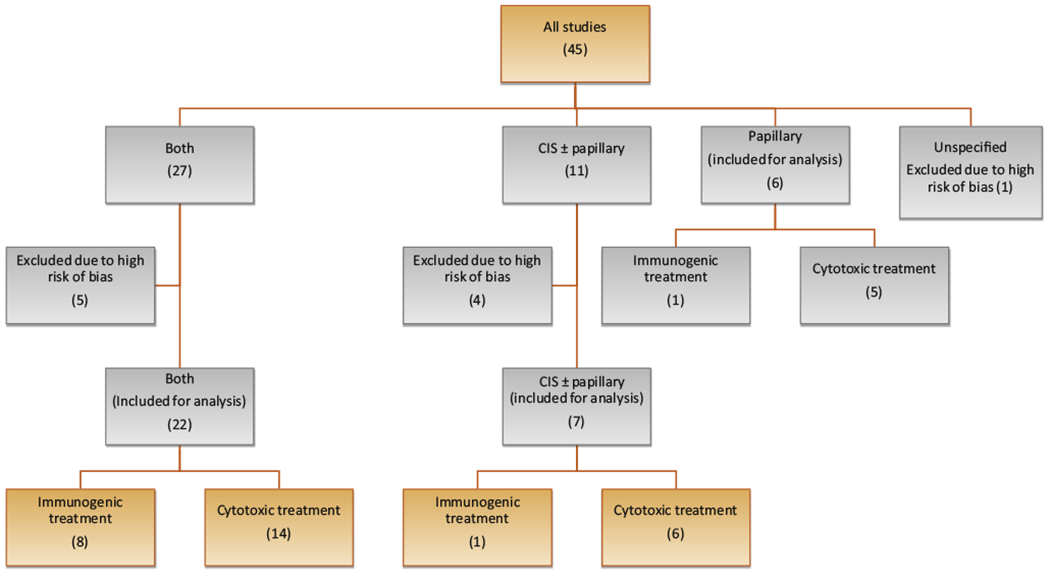
Diagram illustrating the stratification and inclusion of the analyzed studies. Studies were first stratified according to the tumor histology of patients accrued (CIS-containing tumors, papillary tumors, and both); those at a high risk of bias were then excluded. Studies were finally classified by the mechanism of the therapeutic agent. CIS = carcinoma in situ.
Fig. 4 –
Forest plots of complete response rates of CIS-containing studies, recurrence-free rates (RFRs) of papillary-only studies; and disease-free rates (DFR) of studies accruing both CIS and papillary disease patients at 3, 6,12,18, and 24 mo following treatment initiation excluding studies with a high risk of bias. BCG = bacillus Calmette-Guérin; CR = complete response; EMDA = electromotive drug administration; IFNα = interferon alpha; i/ves = intravesical; MCNA = M. phlei cell wall–nucleic acid complex; MMC = mitomycin C.
Of the studies analyzed, immunomodulatory agents were utilized in 10 SAs [6–14,16]. Stratified by tumor histology, Sarosdyetal [16] reported a 3-mo CRR of 15% after treatment using oral bropirimine in 47 patients with CIS-containing recurrences after failing two or more courses of BCG. In comparison, Gacci et al [7] reported a 3-mo RFR of 88%, but a 12-mo RFR of only 10% in 10 patients with recurrent papillary disease after failing two or more courses of BCG, who were then salvaged with reinduction BCG. In eight other SAs enrolling 780 patients with CIS and/or papillary recurrences (one SA i/ves BCG + IFNα, two SAs i/ves CG0070, one SA i/ves BCG, three SAs i/ves Instiladrin, and one SA i/ves MCNA), median (range) DFRs were 49% (29–69%, n = 4) at 3 mo, 41% (14–47%, n = 5) at 6 mo, and 29% (6–35%, n = 5) at 12 mo. Another 25 SAs evaluated the efficacy of cytotoxic salvage agents [6,7,18–26,28,29,31, 32,34,35,38,39,41–43,46,47]. Of these, six SAs consisting of 281 patients with CIS-containing recurrences (two SAs i/ves gemcitabine ± PO everolimus, two SAs i/ves valrubicin, one SA i/ves oportuzumab monatox, and one SA i/ves photodynamic therapy) demonstrated median (range) CRRs of 44% (36–58%, n = 5) at 3 mo, 26% (18–44%, n = 5) at 6 mo, and 17% (9–31%, n = 6) at 12 mo [18,26,38,39,43,47]. Another five SAs evaluated cytotoxic agents in 193 patients with papillary recurrences (one SA i/ves mitomycin and four SA i/ves gemcitabine), yielding median (range) RFRs of 100% (100%, n = 1) at 3 mo, 81% (67–95%, n = 2) at 6 mo, and 61% (44–78%, n = 2) at 12 mo [7,19,22,25]. Fourteen SAs evaluated cytotoxic agents in 419 patients with CIS and/or papillary recurrences (five SAs i/ves gemcitabine, three SA i/ves thermochemotherapy, one SA i/ves oportuzumab monatox, one SA i/ves photodynamic therapy, one SA i/ves docetaxel, two SAs i/ves paclitaxel, and one SA PO dovitinib), yielding median (range) DFRs of 51% (28–99%, n = 10) at 3 mo, 66% (8–73%, n = 4) at 6 mo, and 41% (8–88%, n = 8) at 12 mo.
Ten SAs enrolled patients having failed two or more courses of BCG [7,13,14,16,22–24,41,48]. Of those, three SAs were restricted to patients satisfying the BCG-unresponsive definition [13,14,48]. Another five SAs enrolled patients with LG recurrences after treatment with adequate BCG [7,22–24]. Baseline clinicopathological information and treatment efficacy results are summarized in Table 2. RFRs/DFRs at 12 mo ranged from 0% to 35% in the BCG-unresponsive trials. In a separate analysis of the CIS-containing cohort in the MCNA trial, CRRs were found to be 45% at 6 mo and 27% at 12 mo after treatment [48].
Table 2 –
Treatment efficacy in BCG-unresponsive trials (top) and all patients following adequate BCG (bottom).
| Authors | Year | Phase | Agent | n | Age | M (%)/F (%) | CIS vs papillary | Tumor stage |
Tumor grade |
Biopsy required | Recurrence definition | 3-mo CRR/RFR/DFR (%) | 6-mo CRR/RFR/DFR (%) | 12-mo CRR/RFR/DFR (%) | 18-mo CRR/RFR/DFR (%) | 24-mo CRR/RFR/DFR (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ta (%) | T1 (%) | CIS (%) | Low (%) | Int (%) | High (%) | |||||||||||||||
| Li et al [48] | 2017 | II | MCNA | 94 | 67.9 | 69/31 | Both | 42 | 4 | 72 | 0 | 0 | 100 | Yes | HG recurrences | 48.9 | 34.8 | 28.3 | ||
| Li et al [48] (CIS cohort) | 68 | 100 | HG recurrences | 44.8 | 26.5 | 16.6 | ||||||||||||||
| Navai et al [13] | 2016 | Ib | Instiladrin | 7 | 76.2 | 100/0 | Both | 57 | 14 | 71 | 0 | 0 | 100 | No | Positive biopsy or cytology | 28.6 | 14.3 | 0.0 | 0.0 | |
| Shore et al [14] | 2017 | II | Instiladrin | 40 | 70.5 | 83/17 | Both | 20 | 28 | 75 | 0 | 0 | 100 | Yes | HG recurrences | 57.5 | 42.5 | 35.0 | ||
| Gacci et al [7] | 2006 | II/III | Gemcitabine | 9 | 75 | 78/22 | Papillary | 44 | 56 | 0 | 11 | 33 | 56 | No | Positive biopsy or cytology | 100.0 | 66.7 | 44.4 | 11.1 | 0.0 |
| Gacci et al [7] | 2006 | II/III | BCG | 10 | 75.5 | 80/20 | Papillary | 70 | 30 | 0 | 10 | 60 | 30 | No | Positive biopsy or cytology | 80.0 | 60.0 | 10.0 | 10.0 | 10.0 |
| Gunelli et al [22] | 2007 | II | Gemcitabine | 40 | 66 | 95/5 | Papillary | 10 | 90 | 0 | 0 | 53 | 43 | No | Positive biopsy or cytology | 95.0 | 77.5 | 70.0 | 70.0 | |
| Perdona et al [23] | 2010 | II | Gemcitabine | 20 | 68.3 | 65/35 | Both | 20 | 80 | 35 | 25 | 0 | 75 | No | Positive biopsy | 75.0 | 65.0 | 50.0 | 40.0 | 35.0 |
| Skinner et al [24] | 2013 | II | Gemcitabine | 47 | 70 | 70/30 | Both | 36 | 4 | 60 | 11 | 0 | 89 | Yes | Positive biopsy or cytology | 46.8 | 27.7 | 21.0 | ||
| Dinney et al [12] | 2013 | I | Instiladrin | 17 | 72 | 94/6 | Both | 59 | 6 | 65 | 12 | 0 | 88 | Yes | Positive biopsy or cytology | 41.2 | 41.2 | 29.4 | 29.4 | 23.5 |
BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ; CRR = complete response rate; DFR = disease-free rate; HG = high grade; Int = intermediate; MCNA = M. phlei cell wall–nucleic acid complex; M/F = male/female; RFR = recurrence-free rate.
3.4. Progression-free rate
Overall, median follow-up in all studies excluding those with a high RoB was 17 mo (IQR 12–28.1 mo), with the median (range) PFR of 91% (60–99%; Fig. 5). Stratified by tumor histology, median PFRs were 96% (78–99%, n = 7) in SAs enrolling patients with recurrent CIS with/without papillary disease at a median follow-up of 12 mo (IQR 12 mo), 89% (60–99%, n = 6) in those enrolling patients with recurrent papillary disease only at a median follow-up of 19.9 mo (IQR 16.8–30 mo), and 94% (68–99%, n = 22) in studies enrolling both CIS and papillary patients at a median follow-up of 17 mo (IQR 9–29.6 mo).
Fig. 5 –
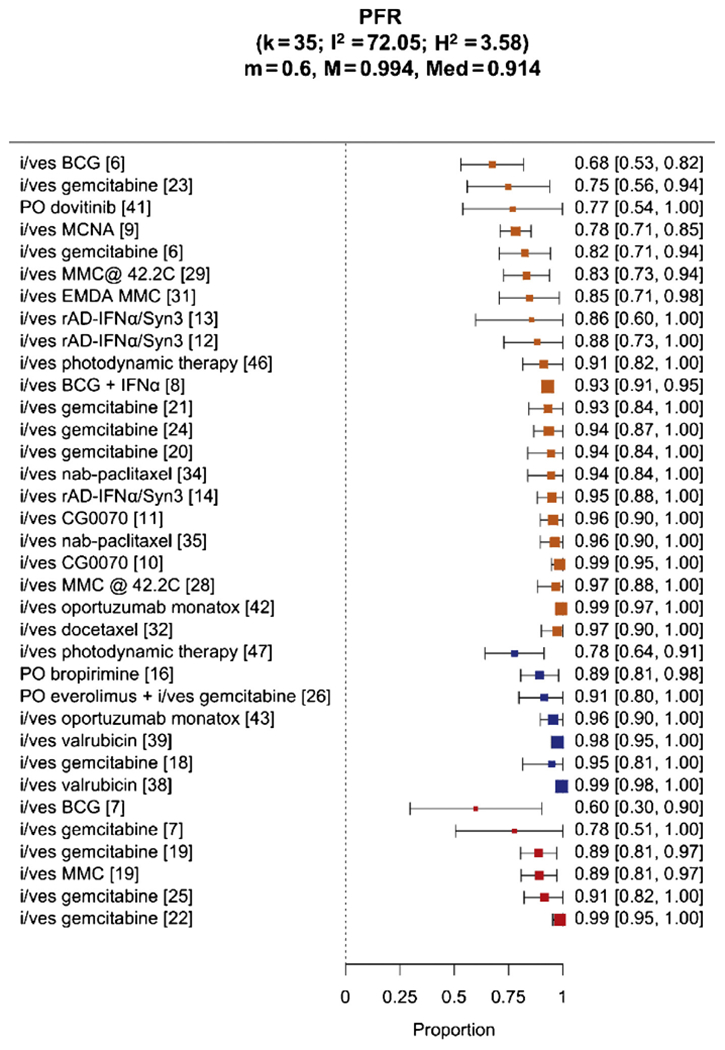
Comparison of 3-, 6-, 12-, 18-, and 24-mo progression-free rates (PFRs) in all study arms excluding those with a high risk of bias. BCG = bacillus Calmette-Guérin; IFNα = interferon alpha; i/ves = intravesical; MCNA = M. phlei cell wall–nucleic acid complex; MMC = mitomycin C.
3.5. Toxicity
Toxicity of i/ves treatments were relatively mild, with grade 3 or higher adverse events (AEs) ranging from 0% to 25%. In total, 23 dose limiting toxicities (DLTs) occurred in 2046 evaluable patients. Most of the AEs due to i/ves therapy were related to urinary bother, including dysuria, frequency, and hematuria. Notably, three episodes of lymphopenia were observed in patients treated with i/ves gemcitabine. Expectedly, severe toxicities were more frequently seen in trials using systemic therapies. A total of 18 DLTs occurred in 208 patients. Toxicities in these trials included hepatotoxicity, metabolic disorders, and death due to severe colitis.
3.6. Discussion
Despite the well-documented unmet need for bladder-sparing therapies in NMIBC patients recurring after i/ves BCG, innovation and progress have been hampered by the lack of consensus over trial design and primary study endpoints. Owing to the complex scheduling of BCG infusions, nuances in the timing, grade, and histology of recurrent tumors may reflect biological heterogeneity, leading to different prognosis and levels of response to salvage agents. To facilitate accurate interpretation of trial results, the term BCG-unresponsive NMIBC was developed to more precisely define the patient cohort refractory to i/ves BCG therapy in whom alternate treatment modalities should be sought [2,3]. Although subsequently incorporated into the FDA investigational guidelines for developing drugs and biologics for the treatment of BCG-unresponsive NMIBC [4], many questions still remain.
Foremost, there exists no data-driven efficacy benchmark for salvage bladder-sparing therapy. The only FDA-approved agent valrubicin was felt to provide inadequate cancer control, demonstrating a 12-mo CRR of only 13% [38]. Lacking standards, the expert panel at the public workshop cohosted by the AUA and FDA felt that “an initial response rate of 40–50% at 6 mo and a durable response rate of at least 30% for 18–24 mo with the lower bound of 95% confidence interval excluding 20% should be clinically meaningful” [1]. Our study demonstrated that, to date, bladder-sparing therapies achieved modest efficacy in NMIBC patients having recurred after BCG. Median CRRs in the treatment of CIS-containing tumors were 26% at 6 mo, 17% at 12 mo, and 8% at 24 mo after treatment. In comparison, median RFRs in the papillary-only studies were 67% at 6 mo, 44% at 12 mo, and 10% at 24 mo. Specifically in the BCG-unresponsive population, 6- and 12-mo CRRs in the CIS-containing patients from the MCNA trial were 45% and 27%, respectively. Median PFRs were 91% at a median follow-up of 17 mo, 95% in the CIS-containing studies (median 12-mo follow-up), and 89% in studies restricted to papillary-only recurrences (median 19.9-mo follow-up).
Although formal statistical comparison was not feasible due to clinical and study design heterogeneity, an interesting finding emerging from the current study was that patients with papillary-only recurrences were more effectively treated with adjuvant bladder-sparing therapy than patients with CIS-containing tumors. This pattern was observed across studies using both immunogenic and cytotoxic agents (Supplementary Table 3). The FDA specified that the primary endpoints of CRR and DoR in trials to support marketing applications should be assessed only in patients with active disease at the time of trial enrollment (ie, CIS-containing patients) [4]. Although pretreatment concomitant CIS has been demonstrated to predict poor progression-free survival and cancer-specific survival following induction BCG [49,50], its relevance in treating BCG recurrent NMIBC is relatively unknown. The differences seen in the salvage rates of recurrent papillary versus CIS-containing tumors point to possible intrinsic biological differences between these disease subtypes. On the contrary, differences seen in the early treatment response may merely reflect the ability to eradicate visible papillary tumor by transurethral resection. Either way, results herein support the FDA’s recommendation to stratify the analysis of trial results based on the type of disease at trial enrollment [4]. The relative inefficacy in treating CIS-containing tumors should also be considered in the discussions surrounding marketing applications of novel agents.
Another challenge in applying the BCG-unresponsive definition in clinical trials involves its restrictions on the timing of recurrence and the requirement for RBBs prior to enrollment. Despite the FDA’s efforts to relax the inclusion criteria, many patients fall out of the window for trial enrollment due to delays in referrals after being diagnosed with BCG-unresponsive disease. Kamat et al [51] described another clinical scenario where a BCG-unresponsive patient, having failed a course of salvage agent, will no longer qualify for trial enrollment due to the time elapsed since the receipt of the last BCG therapy. They suggested that these patients should qualify for clinical trials on the basis of previously being deemed BCG unresponsive. Moreover, mandatory bladder mapping biopsies prior to the initiation of salvage therapy in patients already diagnosed with CIS yield little additional prognostic information and can further delay trial enrollment. These inclusion criteria will likely need to be fine-tuned as additional BCG-unresponsive trials get underway.
A third factor that may introduce a bias into the interpretation of CRR pertains to the definition used for recurrence and the varying methods for its detection. Most commonly, studies have relied on a combination of cystoscopy and urine cytology to detect i/ves recurrences. However, as CIS and incipient papillary disease are notoriously elusive on cystoscopy and urine cytology [52,53], variations from trial to trial in the requirement of RBBs can lead to differences in CRRs/RFRs. In a recently completed trial using rAd-IFNα/Syn3, five patients with negative cystoscopy and cytology were found to have CIS on RBBs, underscoring the importance of incorporating RBBs into the surveillance monitoring protocol [54]. On the contrary, although specificity of urine cytology was as high as 86% [55], false positive results can occur and histological evidence of an HG tumor should be confirmed prior to enumerating cancer recurrence. Lastly, the use of enhanced cystoscopy has been demonstrated to improve detection rates of NMIBC [56]. The effects of its adoption in the assessment of treatment efficacy of bladder-sparing drugs in the BCG-unresponsive setting remain unclear.
Traditionally, patients with LG recurrences following BCG were included in bladder-sparing salvage trials. Compared with such trials encompassing patients with LG recurrences following adequate BCG, treatment efficacy was inferior in the bona fide BCG-unresponsive patients. This observation points to possible differences in the disease course following LG versus HG recurrences, corroborating with the FDA guidelines restricting BCG-unresponsive NMIBC to HG recurrences only. By contrast, there is emerging evidence that up to 14.4% of patients with LG recurrence will progress to muscle-invasive or metastatic disease [57]. As such, although LG recurrences do not constitute recurrences by the current guidelines, these patients still require close monitoring lest there is disease progression.
The study is not without limitations. First, in order to preserve the fidelity of our analysis to only patients having failed BCG, the scope of this systematic review was limited by excluding several clinical trials enrolling patients after failing both BCG and non-BCG agents. Second, due to the vast clinical and study design heterogeneity, a meta-analysis of the combined results was not feasible. As such, we used the narrative synthesis method recommended by the European Association of Urology to present the results of the systematic review [58]. Additionally, as some of the studies have been conducted over 2 decades ago, obtaining individual data for an individual patient data meta-analysis was not possible. Furthermore, as many of the studies were published prior to the formalization of the BCG-unresponsive NMIBC definition, patient enrollment and interpretation of results were affected as outlined in the Discussion. Relatively few studies were available that strictly conformed to the formal BCG-unresponsive definition, in which treatment efficacy was expectedly inferior to those in studies conducted with BCG recurrent patients overall. These results are in line with previous studies demonstrating the relative inefficacy of continued i/ves treatment in the BCG-unresponsive population [59]. Finally, response rates were reported at nonuniform time intervals after treatment initiation.
Nevertheless, results from the current study will serve as a frame of reference for emerging trial results in the BCG-unresponsive space. For instance, recently presented results from the phase 3 rAd-IFNα/Syn3 trial for BCG-unresponsive NMIBC demonstrated 3-, 6-, and 12-mo CRRs of 53.4%, 40.8%, and 24.3%, respectively, in 103 CIS-containing patients and RFRs of 72.9%, 58.3%, and 43.8%, respectively, in 48 patients with papillary disease [54], in line with results from previous trials conducted in the BCG-unresponsive population. In addition, 17% of 97 BCG-unresponsive CIS-containing patients treated with pembrolizumab had a durable complete response of ≥12 mo in the KEYNOTE-057 trial [60]. With the accumulation of efficacy data for bladder-sparing salvage agents tested in the precisely defined BCG-unresponsive population, more robust analytic methods can be applied to establish the efficacy benchmark to guide future trials.
4. Conclusions
We demonstrate that, to date, bladder-sparing therapies achieved modest efficacy in patients with NMIBC after BCG, with median 6-, 12-, and 24-mo CRRs of 26%, 17%, and 8%, respectively, in CIS-containing patients. Recurrent papillary disease was more effectively treated, with median 6-, 12-, and 24-mo RFRs of 67%, 44%, and 10%, respectively. The median PFR was 91%. Toxicities of i/ves agents were generally mild, with very few DLTs. Results from the current study will serve as a frame of reference for emerging trial results in the BCG-unresponsive space.
Supplementary Material
Acknowledgments:
The authors thank Clara Fowler and Susan Sharpe for their help in devising and executing the comprehensive search strategies.
Financial disclosures: Roger Li certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Roger Li: clinical trial protocol committee—Cold Genesys and BMS; scientific advisor/consultant—BMS and Ferring. Debasish Sundi: scientific advisor—Janssen and Cold Genesys. Jingsong Zhang: no relevant disclosures. Philippe E. Spiess: panel member—NCCN bladder and penile cancer. Michael A. Poch: no relevant disclosures. Wade J. Sexton: no relevant disclosures. Peter C. Black: clinical trial protocol committee—Genentech, Janssen, BMS, Astellas, Aitka, MDx Health, AstraZeneca, Theralase, and Pacific Edge; scientific advisor/consultant—AbbVie, Asieris, AstraZeneca, Astellas, Bayer, Biosyent, BMS, H3-Biomedicine, Janssen, Merck, Roche, Sanofi, Urogen, and Ferring; speaker bureau—AbbVie, Biosyent, Janssen, Ferring, TerSera, and Pfizer; grants/honorarium—Decipher Biosciences, iProgen, Sanofi, and Bayer; patent—Decipher. James M. McKiernan: no relevant disclosures. Gary D. Steinberg: clinical trial protocol committee—Merck, BMS, Janssen, and Cold Genesys; scientific advisor/consultant—Heat Biologics, Cold Genesys, PhotoCure, Merck, Roche/Genentech, Ciclomed, Taris Biomedical, MDxHealth, Fidia Farmaceuticals, Urogen, Ferring, Aduro, Boston Scientific, BMS, Astra Zeneca, Pfizer, Janssen, Epivax Oncology, Natera, FKD, Ferring, EnGene Bio, SesenBio, and BioCanCell; equity/stock options—Epivax Oncology and Urogen. Ashish M. Kamat: scientific advisor/consultant—Merck, BMS, Eisai, Arquer, MDx Health, Photocure, Astra Zeneca, IBCG, TMC Innovation, Theralase, BioClin Therapeutics, FKD Industries, Cepheid, Medac, Asieris, Pfizer, Abbott Molecular, US Biotest, Ferring, Imagin, Cold Genesys, Roviant, Sessen Bio, CEC Oncology, and Nucleix; intellectual property—CyPRIT (Cytokine Panel for Response to Intravesical Immunotherapy). Scott M. Gilbert: no relevant disclosures.
Funding/Support and role of the sponsor: This project has been partially supported by the Moffitt Foundation Grant.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2020.02.012.
References
- [1].Jarow JP, Lerner SP, Kluetz PG, et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: report of a Food and Drug Administration and American Urological Association public workshop. Urology 2014;83:262–5. [DOI] [PubMed] [Google Scholar]
- [2].Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol 2016;34:1935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lerner SP, Dinney C, Kamat A, et al. Clarification of bladder cancer disease states following treatment of patients with intravesical BCG. Bladder cancer (Amsterdam Netherlands) 2015;1:29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Administration UFD. In: UDoHaH rie, editor. BCG-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment guidance for industry. Silver Spring, MD: Office of Communications, Division of Drug Information; 2018. p. 1–10. [Google Scholar]
- [5].Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [6].Di Lorenzo G, Perdona S, Damiano R, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer 2010;116:1893–900. [DOI] [PubMed] [Google Scholar]
- [7].Gacci M, Bartoletti R, Cai T, et al. Intravesical gemcitabine in BCG-refractory T1G3 transitional cell carcinoma of the bladder: a pilot study. Urol Int 2006;76:106–11. [DOI] [PubMed] [Google Scholar]
- [8].Joudi FN, Smith BJ, O’Donnell MA, National BCGIPIG. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol 2006;24:344–8. [DOI] [PubMed] [Google Scholar]
- [9].Morales A, Herr H, Steinberg G, et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette-Guerin. J Urol 2015;193:1135–43. [DOI] [PubMed] [Google Scholar]
- [10].Burke JM, Lamm DL,Meng MV, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol 2012;188:2391–7. [DOI] [PubMed] [Google Scholar]
- [11].Packiam VT, Lamm DL, Barocas DA, et al. , editors.An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: interim results Urologic Oncology: Seminars and Original Investigations. Elsevier; 2018. [DOI] [PubMed] [Google Scholar]
- [12].Dinney CP, Fisher MB, Navai N, et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. J Urol 2013;190:850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Navai N, Benedict WF, Zhang G, et al. Phase 1b trial to evaluate tissue response to a second dose of intravesical recombinant adenoviral interferon alpha2b formulated in Syn3 for failures of bacillus Calmette-Guerin (BCG) therapy in nonmuscle invasive bladder cancer. Ann Surg Oncol 2016;23:4110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shore ND, Boorjian SA, Canter DJ, et al. Intravesical rAd-IFNalpha/ Syn3 for patients with high-grade, bacillus Calmette-Guerin-refractory or relapsed non-muscle-invasive bladder cancer: a phase II randomized study. J Clin Oncol 2017;35:3410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chamie K, Lee J, Rock A, Rhode P, Soon-Shiong P, Chang S. LBA-18 Preliminary phase 2 clinical results of IL-15RαFC superagonist N-803 with BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) patients demonstrates 82% CR of carcinoma in situ (CIS). J Urol 2019;201(Suppl 4):e999–1000. [Google Scholar]
- [16].Sarosdy MF, Manyak MJ, Sagalowsky AI, et al. Oral bropirimine immunotherapy of bladder carcinoma in situ after prior intravesical bacille Calmette-Guerin. Urology 1998;51:226–31. [DOI] [PubMed] [Google Scholar]
- [17].Balar AV, Kulkarni GS, Uchio EM, et al. Keynote 057: Phase II trial of pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to bacillus Calmette-Guérin (BCG). J Clin Oncol 2019;37(7_suppl):350.30557524 [Google Scholar]
- [18].Bassi P, De Marco V, Tavolini I, et al. Pharmacokinetic study of intravesical gemcitabine in carcinoma in situ of the bladder refractory to bacillus Calmette-Guerin therapy. Urol Int 2005;75:309–13. [DOI] [PubMed] [Google Scholar]
- [19].Addeo R, Caraglia M, Bellini S, et al. Randomized phase III trial on gemcitabine versus mitomycin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol 2009;28:543–8. [DOI] [PubMed] [Google Scholar]
- [20].Dalbagni G, Russo P, Sheinfeld J, et al. Phase I trial of intravesical gemcitabine in bacillus Calmette-Guerin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol 2002;20:3193–8. [DOI] [PubMed] [Google Scholar]
- [21].Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guerin-refractory transitional cell carcinoma of the bladder. J Clin Oncol 2006;24:2729–34. [DOI] [PubMed] [Google Scholar]
- [22].Gunelli R, Bercovich E, Nanni O, et al. Activity of endovesical gemcitabine in BCG-refractory bladder cancer patients: a translational study. Br J Cancer 2007;97:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perdona S, Di Lorenzo G, Cantiello F, et al. Is gemcitabine an option in BCG-refractory nonmuscle-invasive bladder cancer? A single-arm prospective trial. Anticancer Drugs 2010;21:101–6. [DOI] [PubMed] [Google Scholar]
- [24].Skinner EC, Goldman B,Sakr WA,et al. SWOGS0353: phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. J Urol 2013;190:1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mohanty NK, Nayak RL, Vasudeva P, Arora RP. Intravesical gemcitabine in management of BCG refractory superficial TCC of urinary bladder—our experience. Urol Oncol 2008;26:616–9. [DOI] [PubMed] [Google Scholar]
- [26].Dalbagni G, Benfante N, Sjoberg DD, et al. Single arm phase I/II study of everolimus and intravesical gemcitabine in patients with primary or secondary carcinoma in situ of the bladder who failed bacillus Calmette Guerin (NCT01259063). Bladder Cancer 2017;3:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sonpavde G, Rosser CJ, Pan C-X, et al. Phase I trial of ALT-801, a first-in-class T-cell receptor (TCR)-interleukin (IL)-2 fusion molecule, plus gemcitabine (G) for Bacillus Calmette Guerin (BCG)-resistant non-muscle-invasive bladder cancer (NMIBC). J Clin Oncol 2016;34 (2_suppl):451. [Google Scholar]
- [28].Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia 2014;30:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tan WS, Panchal A, Buckley L, et al. Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus Calmette-Guerin or institutional standard in patients with recurrence of non-muscle-invasive bladder cancer following induction or maintenance bacillus Calmette-Guerin therapy (HYMN): a phase III, open-label, randomised controlled trial. Eur Urol 2019;75:63–71. [DOI] [PubMed] [Google Scholar]
- [30].Tan Wei S, Hendricksen K, Wilby D, et al. PD13-10 Oncological outcomes of BCG unresponsive non-muscle invasive bladder cancer patients treated with postoperative chemohyperthermia: a multicentre European retrospective analysis. J Urol 2019;201(Suppl 4): e229–30. [Google Scholar]
- [31].Racioppi M, Di Gianfrancesco L, Ragonese M, Palermo G, Sacco E, Bassi PF. Electromotive drug administration (EMDA) of mitomycin C as first-line salvage therapy in high risk “BCG failure” non muscle invasive bladder cancer: 3 years follow-up outcomes. BMC Cancer 2018;18:1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McKiernan JM, Masson P, Murphy AM,et al. Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol 2006;24:3075–80. [DOI] [PubMed] [Google Scholar]
- [33].DeCastro GJ, Sui W, Pak JS, et al. A phase I trial for the use of intravesical cabazitaxel, gemcitabine, and cisplatin (CGC) in the treatment of BCG-refractory nonmuscle invasive urothelial carcinoma of the bladder. J Clin Oncol 2017;35(6_suppl):313. [Google Scholar]
- [34].McKiernan JM, Barlow LJ, Laudano MA, Mann MJ, Petrylak DP, Benson MC. A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guerin refractory nonmuscle invasive bladder cancer. J Urol 2011;186:448–51. [DOI] [PubMed] [Google Scholar]
- [35].McKiernan JM, Holder DD, Ghandour RA, et al. Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guerin treatment failure. J Urol 2014;192:1633–8. [DOI] [PubMed] [Google Scholar]
- [36].Bassi PF, Volpe A, D’Agostino D, et al. Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder: results of a phase I study. J Urol 2011; 185:445–9. [DOI] [PubMed] [Google Scholar]
- [37].Hurle R,Lazzeri M,Guazzoni G,et al. PD18-04 A phase 1 safety study of Oncofid-P-B administered for 12 consecutive weeks in BCG unresponsive/intolerant patients with bladder carcinoma in situ (CIS): preliminary results. J Urol 2019;201(Suppl 4):e312. [Google Scholar]
- [38].Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol 2013;31:1635–42. [DOI] [PubMed] [Google Scholar]
- [39].Steinberg G, Bahnson R, Brosman S, et al. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. J Urol 2000;163:761–7. [PubMed] [Google Scholar]
- [40].Ignatoff JM, Chen Y-H, Greenberg RE, Pow-Sang JM, Messing EM, Wilding G, editors. Phase II study of intravesical therapy with AD32 in patients with papillary urothelial carcinoma or carcinoma in situ (CIS) refractory to prior therapy with bacillus Calmette-Guerin (E3897): a trial of the Eastern Cooperative Oncology Group Urologic Oncology: Seminars and Original Investigations. Elsevier; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hahn NM, Bivalacqua TJ, Ross AE, et al. A phase II trial of dovitinib in BCG-unresponsive urothelial carcinoma with FGFR3 mutations or overexpression: Hoosier Cancer Research Network trial HCRN 12157. Clin Cancer Res 2017;23:3003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kowalski M, Entwistle J, Cizeau J, et al. A phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCG-refractory and BCG-intolerant patients. Drug Des Dev Ther 2010;4:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kowalski M, Guindon J, Brazas L, et al. A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with non-invasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guerin. J Urol 2012;188:1712–8. [DOI] [PubMed] [Google Scholar]
- [44].Dickstein R, Wu N, Cowan B, et al. LBA27 Phase 3 study of vicinium in BCG-unresponsive non-muscle invasive bladder cancer: initial results. J Urol 2018;199:e1167. [Google Scholar]
- [45].Garcia JA, Stephenson AJ, Ireland J, et al. Sunitinib in BCG-refractory non-muscle-invasive transitional cell carcinoma of the bladder. J Clin Oncol 2011;29(7_suppl):262. [Google Scholar]
- [46].Lee JY, Diaz RR, Cho KS, et al. Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guerin immunotherapy. J Urol 2013;190:1192–9. [DOI] [PubMed] [Google Scholar]
- [47].Nseyo UO, Shumaker B, Klein EA, Sutherland K. Photodynamic therapy using porfimer sodium as an alternative to cystectomy in patients with refractory transitional cell carcinoma in situ of the bladder. Bladder Photofrin Study Group J Urol 1998;160:39–44. [PubMed] [Google Scholar]
- [48].Li R, Amrhein J, Cohen Z, Champagne M, Kamat AM. Efficacy of Mycobacterium phlei cell wall-nucleic acid complex (MCNA) in BCG-unresponsive patients. Bladder Cancer 2017;3:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Davis JW, Sheth SI, Doviak MJ, Schellhammer PF. Superficial bladder carcinoma treated with bacillus Calmette-Guerin: progression-free and disease specific survival with minimum 10-year follow-up. J Urol 2002;167(2 Pt 1):494–500, discussion 501. [DOI] [PubMed] [Google Scholar]
- [50].Takashi M, Wakai K, Hattori T, et al. Multivariate evaluation of factors affecting recurrence, progression, and survival in patients with superficial bladder cancer treated with intravesical bacillus Calmette-Guerin (Tokyo 172 strain) therapy: significance of concomitant carcinoma in situ. Int Urol Nephrol 2002;33:41–7. [DOI] [PubMed] [Google Scholar]
- [51].Kamat AM, Lerner S,Black P, et al. Once BCG unresponsive, always BCG unresponsive: an open letter to the FDA to enhance recruitment into clinical trials in bladder cancer. Bladder Cancer 2017;3:145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schneeweiss S, Kriegmair M, Stepp H. Is everything all right if nothing seems wrong? A simple method of assessing the diagnostic value of endoscopic procedures when a gold standard is absent. J Urol 1999;161:1116–9. [PubMed] [Google Scholar]
- [53].Daneshmand S, Patel S, Lotan Y, et al. Efficacy and safety of blue light flexible cystoscopy with hexaminolevulinate in the surveillance of bladder cancer: a phase III, comparative, multicenter study. J Urol 2018;199:1158–65. [DOI] [PubMed] [Google Scholar]
- [54].Dinney C, editor. Phase 3 trial of rAd-IFNα/Syn3 for BCG unresponsive NMIBC. Society of Urologic Oncology Annual Meeting; 12/5/2019; Washington, DC. [Google Scholar]
- [55].Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, Kassouf W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol 2015;33:66, e25–31. [DOI] [PubMed] [Google Scholar]
- [56].Burger M, Grossman HB, Droller M, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol 2013;64:846–54. [DOI] [PubMed] [Google Scholar]
- [57].Li R, Metcalfe MJ, Tabayoyong WB, et al. Using grade of recurrent tumor to guide further therapy while on bacillus Calmette-Guerin: low-grade recurrences are not benign.Eur Urol Oncol 2019;2:286–93. [DOI] [PubMed] [Google Scholar]
- [58].Knoll T, Omar MI, Maclennan S, et al. Key steps in conducting systematic reviews for underpinning clinical practice guidelines: methodology of the European Association of Urology. Eur Urol 2018;73:290–300. [DOI] [PubMed] [Google Scholar]
- [59].Li R, Tabayoyong WB, Guo CC, et al. Prognostic implication of the United States Food and Drug Administration-defined BCG-unresponsive disease. Eur Urol 2019;75:8–10. [DOI] [PubMed] [Google Scholar]
- [60].US FDA. FDA / Merck, Sharpe & Dohme combined briefing information for the December 17, 2019 Meeting of the Oncologic Drugs Advisory Committee (PM session). www.fda.gov2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.