Abstract
Background.
Isolated limb infusion (ILI) is a minimally invasive technique for delivering regional chemotherapy to an extremity for patients with locally advanced cutaneous malignancies and sarcoma.
Methods.
A single-institution, prospectively collected database was analyzed for intention-to-treat with ILI.
Results.
From 2007 to 2016, 163 patients underwent 205 procedures (201 were successfully completed), and four malignancies were treated: melanoma (72.1% of all ILIs), sarcoma (23.4%), squamous cell carcinoma (SCC; 2.0%) and Merkel cell carcinoma (MCC; 2.5%). A median grade II regional Wieberdink toxicity score was observed, with 88.1% of patients experiencing grade II or less. Median follow-up was 21.8 months, and overall response rate (ORR) was 59.0% for melanoma, 48.9% for sarcoma, 50.0% for SCC, and 60.0% for MCC. A significant difference (p = 0.04) between upper (76.9%) and lower extremity (55.1%) ORR was observed in patients with melanoma. When comparing responders with nonresponders, patients with melanoma had significantly longer in-field progression-free survival (IPFS; 14.1 vs. 3.2 months, p < 0.001), distant metastatic-free survival (DMFS; not reached vs. 25.8 months, p = 0.006), and overall survival (OS; 56.0 vs. 26.7 months, p = 0.0004). Sarcoma responders had a significantly longer IPFS (13.0 vs. 2.7 months, p < 0.0001), but no significant distant metastatic or OS advantage. Over a median follow-up of 19.3 months, sarcoma patients had an overall limb salvage rate of 68.4%.
Conclusion.
ILI is a well-tolerated procedure for patients with locally advanced melanoma, sarcoma, and other cutaneous malignancies. ILI responders had a significantly longer time to IPFS, while melanoma responders also had a DMFS and OS advantage.
The last decade has seen a surge in the adoption of new locoregional and systemic therapies for metastatic melanoma.1 As survival for these patients increases, so does the importance of locoregional control. For patients with locally advanced soft tissue sarcoma (STS), surgery ± noeadjuvant or adjuvant radiation is the mainstay of treatment, but if limb salvage is untenable, chemotherapy and external beam radiation therapy are adjuvants to attempt to convert to a limb-preserving resection.2 Isolated limb infusion (ILI) is a regionally directed intra-arterial perfusion-based therapy that has been developed to treat locoregionally advanced malignancies in an extremity.3–7
ILI has demonstrated response rates of between 54 and 84% for melanoma, with complete responses (CRs) between 24 and 38%.7–10 It has also been used to treat locally advanced STS and other nonmelanoma cutaneous malignancies, including Merkel cell carcinoma (MCC) and squamous cell carcinoma (SCC).9,11 In 2008, the Thompson group reported a 90% overall response rate (ORR) for patients with locally advanced STS,12 while Mullinax et al. recently reported a multi-institutional experience of 84 ILIs with locally advanced STS and reported an ORR of 58%, as well as limb salvage, in 77.9% of patients.2 While data are limited for ILI in patients with advanced, unresectable MCC and SCC of the extremity, response rates are encouraging, with a majority of patients experiencing an objective response and achieving limb salvage.9,11,13
The aim of this study was to review our experience using ILI for the treatment of locally advanced melanoma, sarcoma, MCC, and SCC. Our primary objective was to evaluate the response to ILI, and, secondarily, we wanted to determine if patients who respond to ILI have a survival advantage.
METHODS
Data were prospectively collected for all patients who underwent ILI at the Moffitt Cancer Center from May 2007 to December 2016, and analyzed for intention to treat. Patients who were offered ILI had unresectable extremity-threatening sarcomas otherwise facing amputation. All melanoma patients had stage IIIB or IIIC disease. Demographic data, indications for procedure, intraoperative parameters, and oncologic outcomes were reviewed. Response to therapy was measured at 3 months following each ILI, on computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET)/CT using Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 criteria for deep lesions or direct measurement for cutaneous and palpable lesions.14 Generally, cutaneous malignancies were followed by PET/CT and sarcomas were followed by MRI and CT for metastasis. Patients also had physical examinations to assess the response of in-transit metastases. Overall survival (OS), in-field progression, and out-of-field progression (distant metastasis) were analyzed. The study was approved by our Institutional Review Board.
Procedure
Under fluoroscopic guidance, arterial and venous catheters were placed percutaneously into the affected limb, with the catheter tips positioned distal to the tourniquet to ensure adequate extremity isolation. ILIs were performed in the operating room under general anesthesia. Patients were systemically heparinized at a dose of 300 units/kg to achieve an activated clotting time (ACT) > 400 s prior to inflating the tourniquet, and papaverine 60 mg was administered through the arterial catheter after tourniquet inflation. The tourniquet was inflated when the temperature of the extremity reached 37 °C. Isolation of the limb was checked, ensuring the arterial inflow was occluded by listening to cessation of flow in pedal or radial arteries using a Doppler probe. Limb volume measurements were used to determine the dose of chemotherapeutic agents, a combination of actinomycin-D (administered at 100 μg/L) and melphalan [administered at 7.5 mg/L for lower extremities (LEs) and 10 mg/L for upper extremities (UEs)]. The melphalan was corrected for ideal body weight. Four patients received temozolamide as part of a trial, but all others received actinomycin-D and melphalan. Chemotherapy was then manually infused with a syringe for 30 min (at a flow rate of approximately 80–100 cc/min) connected in-line to the closed circuit with a heating source. The limbs were also wrapped in Kimberly-Clark® Liquid warming blankets (Irving, TX, USA). Following infusion of chemotherapy, the limb was washed out with saline until the effluent was clear. Heparinization was reversed with protamine after the tourniquet had been released. Very few patients had regional nodal disease at evaluation for ILI, but, if performed, a completion lymphadenectomy was performed at the same operation and after reversal of heparin. The interval to a repeat ILI varied between 3 and 12 months depending on the response to initial therapy and/or recurrence after CR. If there was a response to the initial ILI, then we offered a repeat ILI to patients who progressed or recurred.
Postoperative Management
All patients were admitted to a monitored care unit. Extremity neurovascular checks were routinely performed and creatine phosphokinase (CPK) levels were checked every 12 h. If CPK levels exceeded 1000 U/L, normal saline intravenous fluids and corticosteroids were administered until CPK levels declined to < 1000 U/L. Patients were discharged after CPK levels returned towards baseline. Limb toxicity was measured using the Wieberdink toxicity scale throughout the hospital stay and at each postoperative visit,15 and the highest grade toxicity was recorded.
Statistics
Patient demographics, tumor characteristics, and perioperative variables were described with mean and standard deviation, proportion, or median and interquartile range, where applicable. Student’s t test, Kruskal–Wallis test, and Chi square association tests were performed. Time to local progression and death were calculated from the time of the procedure to last follow-up or corresponding survival events. Log-rank tests were used to compare the responders with nonresponders on distribution of time to event, and Kaplan–Meier curves were used to display the results of these tests. A two-sided p-value < 0.05 was considered significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
From 2007 to 2016, a total of 163 patients underwent an attempted 205 ILIs, with 201 successfully completed (Fig. 1). The median age was 74 years (range 27–94) and the majority of patients were women (60.1%) (Table 1). ILI procedures were performed for four malignancies: melanoma 72.1% (n = 148), sarcoma 23.4% (n = 48), SCC 2.0% (n = 4), and MCC 2.5% (n = 5). Repeat ILIs constituted 20.5% (n = 42) of our series, with two patients receiving three ILIs (Fig. 1).
FIG. 1.
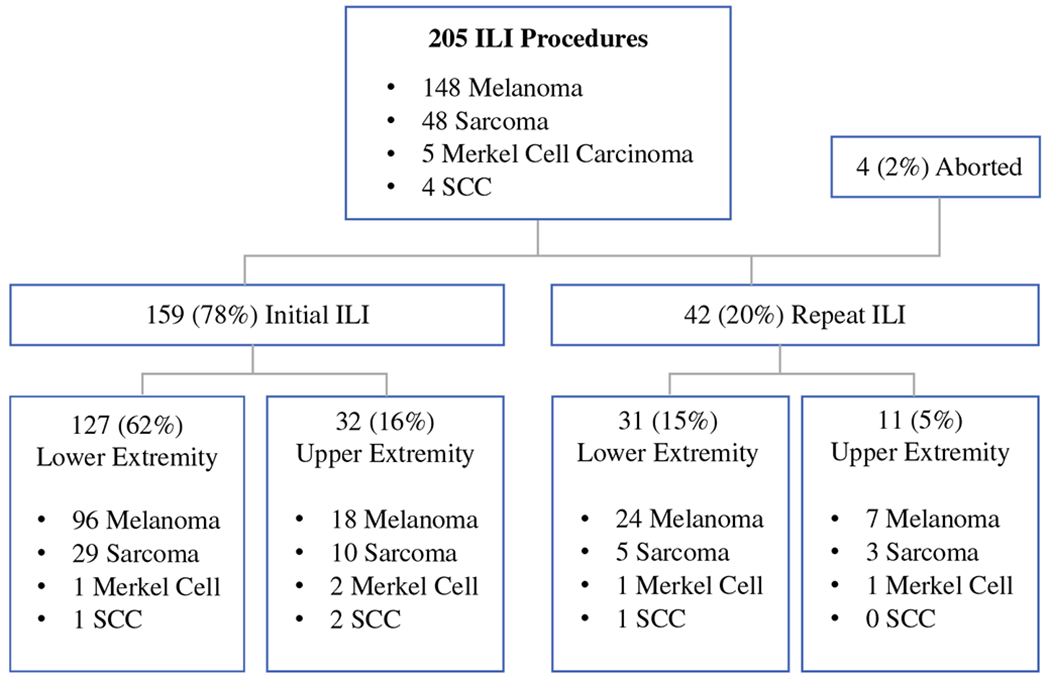
Isolated limb infusions performed, stratified by disease type, initial versus repeat procedure, and upper versus lower extremity. Final procedure numbers by extremity do not include the four aborted procedures. ILI isolated limb infusions, SCC squamous cell carcinoma
TABLE 1.
Patient and perioperative characteristics for isolated limb infusion
| Median | Range | |
|---|---|---|
| Median age (years) | 74 | 27–94 |
| Limb volume (L) | ||
| Upper extremity | 2.3 | 1.4–3.2 |
| Lower extremity | 7.7 | 3.2–22.7 |
| Melphalan dose (mg) | ||
| Upper extremity | 19.0 | 8.5–31.0 |
| Lower extremity | 41.0 | 20.0–90.7 |
| Actinomycin dose (μg) | ||
| Upper extremity | 240.0 | 320.0–900.0 |
| Lower extremity | 695.2 | 140.0–310.0 |
| Ischemia time (min) | ||
| Upper extremity | 47.0 | 42.0–86.0 |
| Lower extremity | 51.0 | 42.0–73.0 |
| Temperature at 30 min (°C) | ||
| Upper extremity | 39.3 | 36.8–40.6 |
| Lower extremity | 39.6 | 37.5–41.5 |
| pH at 30 min | ||
| Upper extremity | 7.09 | 6.89–7.27 |
| Lower extremity | 7.2 | 6.7–7.7 |
| Wieberdink toxicity score | ||
| Upper extremity | 2 | 1–3 |
| Lower extremity | 2 | 1–4 |
| CPK peak (IU/L) | ||
| Upper extremity | 184.0 | 21.0–2440.0 |
| Lower extremity | 590 | 27.0–12,808.0 |
| CPK peak POD (days) | ||
| Upper extremity | 2.0 | 1.0–5.0 |
| Lower extremity | 4.0 | 0.5–8.0 |
| Median LOS (days) | ||
| Upper extremity | 4.0 | 3.0–10.0 |
| Lower extremity | 6.0 | 3.0–13.0 |
CPK creatine phosphokinase, POD postoperative day, LOS length of stay
Intra- and Postoperative Outcomes
Four procedures were aborted before chemoperfusion for reasons that included failing to isolate the limb with the tourniquet on a patient with a LE limb volume of 22.7 L (body mass index 45), an arterial intimal flap noted during placement of catheters, and inability to place the catheters distal to the tourniquet. Perioperative characteristics and outcomes are listed in Table 1. The median ischemia time was 50 min, with a median temperature of 39.6 °C and pH of 7.2. The median CPK for LE ILIs was significantly higher at 590 IU/L compared with 184 IU/L for UEs (p = 0.001). This corresponded with a longer median length of stay (LOS) for LE ILIs where the peak CPK was observed at postoperative day (POD) 4 and median LOS was 6 days, versus peak CPK at POD 2 and median LOS of 4 days for UE ILIs. Initial and repeat ILIs did not statistically differ in median CPK elevation or LOS. The cohort had a median Wieberdink toxicity score of grade II (range I–IV), which did not differ between UE, LE, and initial or repeat procedures. Grade III toxicity was seen in 11.4% (23/201) of patients and one LE patient had a grade IV toxicity that resulted in a fasciotomy to treat compartment syndrome.
Disease Response to Isolated Limb Infusion
Response at 3 months was evaluable in 198 of the 201 completed procedures. The ORR at 3 months, defined as either a CR or partial response (PR), was 59.0% for melanoma, 48.9% for sarcoma, 50.0% for SCC, and 60.0% for MCC (Fig. 2). Responses for melanoma and sarcoma, by UE and LE, as well as initial and repeat procedures, can be seen in Table 2. One melanoma and two sarcoma patients did not have 3-month follow-up to assess their response. Responses did not differ significantly by disease type (p = 0.5) or initial versus repeat procedure (p = 0.5). A significant difference (p = 0.04) between UE (76.9%) and LE (55.1%) ORR was seen in melanoma but this was not observed in other disease types. Melanoma ORR for initial ILIs was 58.4% compared with 60.0% for repeat ILIs (p = 0.7). Sarcoma ORR for UE ILIs was 41.2% compared with 51.5% for LE ILIs (p = 0.9), and sarcoma ORR for initial ILIs was 41.2% compared with 66.6% for repeat ILIs (p = 0.2). CPK peak level did not correspond with response to treatment (p = 0.59). Smaller limb volumes were associated with an improved response; mean limb volumes in responders were 6.1 L compared with nonresponders, whose mean limb volumes were 6.9 L (p = 0.03).
FIG. 2.
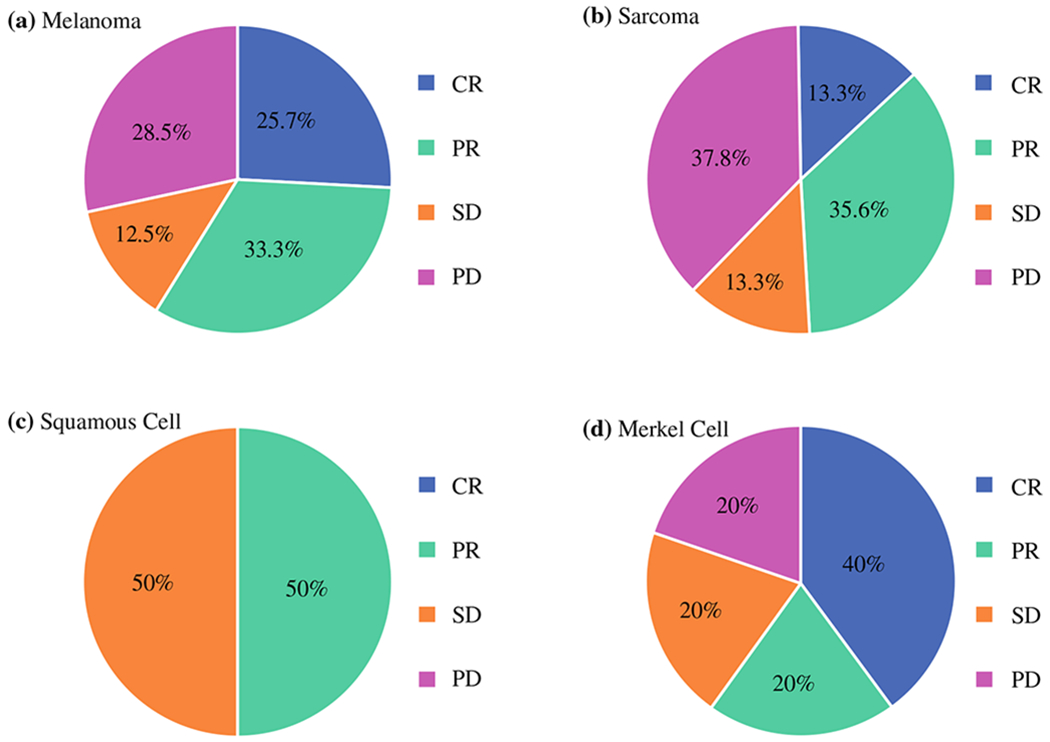
Response to ILI at 3 months by disease type. Response to therapy was measured at 3 months following each ILI on CT, MRI, or PET/CT using RECIST version 1.1 criteria for deep lesions or direct measurement for cutaneous and palpable lesions. CR was defined as the disappearance of all lesions; PR was defined as a ≥ 30% decrease in the diameter of the tumor (for sarcoma patients) or a ≥ 30% decrease in the number of in-field lesions (for cutaneous malignancies); PD was defined as a ≥ 20% increase in the tumor diameter or evidence of new nodules; and SD was defined as failure to meet the criteria for PR or PD. ILI isolated limb infusion, CT computed tomography, MRI magnetic resonance imaging, PET positron emission tomography, RECIST response evaluation criteria in solid tumors, CR complete response, PR partial response, PD progressive disease, SD stable disease
TABLE 2.
Disease response at 3 months by ILI procedure type
| Disease type | Overall response rate (%) | p value |
|---|---|---|
| Melanoma (n = 144) | ||
| UE (n = 26) | 76.9 | 0.04 |
| LE (n = 118) | 55.1 | |
| Initial (n = 113) | 58.4 | 0.8 |
| Repeat (n = 31) | 60.0 | |
| Sarcoma (n = 45) | ||
| UE (n = 12) | 41.2 | 0.5 |
| LE (n = 33) | 51.5 | |
| Initial (n = 38) | 41.2 | 0.2 |
| Repeat (n = 7) | 66.6 |
3-month follow-up on one melanoma patient and two sarcoma patients was unavailable, therefore their response data are not included in this table
ILI isolated limp perfusion, UE upper extremity, LE lower extremity
Patients who had a PR disease after ILI were offered resection if it would render them clinically disease-free (no evidence of disease (NED)). Patients with a PR who were then resected to NED included 12 of 47 melanoma (26%) and 1/16 sarcoma PRs (6%). Additionally, sarcoma patients had an overall limb salvage rate of 68.4% after a median follow-up of 19.3 months.
Survival Analysis
The overall median follow-up for the entire cohort was 21.8 months (22.1 months for melanoma and 19.3 months for sarcoma). For melanoma patients, responders had significantly longer in-field progression-free survival (IPFS; 14.1 vs. 3.2 months, p < 0.0001) and OS (56.0 months vs. 26.7 months, p = 0.0004) compared with nonresponders. For melanoma patients, distant metastatic-free survival (DMFS) was not reached for responders but was 25.8 months for nonresponders (p = 0.006) (Fig. 3a–c). Sarcoma responders had a longer IPFS (13.0 vs. 2.7 months, p < 0.0001), but no statistically significant DMFS as the median time to metastasis was not reached for responders or nonresponders. The median OS was not reached for sarcoma responders and was 52.8 months for nonresponders (p = 0.48) (Fig. 3d–f). There was no significant difference in IPFS or OS for patients who had a CR compared with patients who had a PR, for both melanoma and sarcoma patients. Melanoma IPFS did not differ significantly between initial versus repeat procedures (p = 0.69) or UE versus LE (p = 0.16) procedures. Kaplan–Meier plots for MCC and SCC were not generated due to the inclusion of only three patients in each group.
FIG. 3.
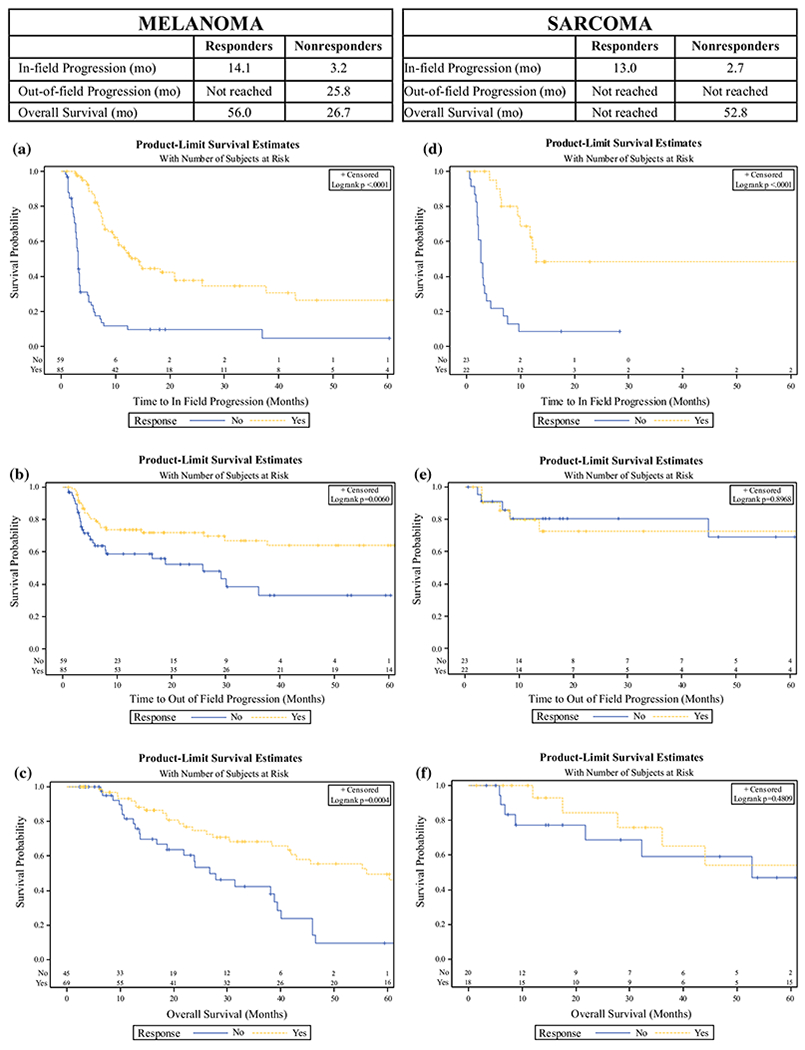
Progression and survival by response to treatment. Kaplan–Meier curves demonstrating the survival in years by response at 3 months after isolated limb infusion for responders (complete response and partial response) compared with nonresponders (stable disease and progressive disease) for patients with a melanoma in-field progression-free survival; b melanoma distant metastatic-free survival; c melanoma overall survival; d sarcoma in-field progression-free survival; e sarcoma distant metastatic-free survival; and f sarcoma overall survival
DISCUSSION
This study, the largest US single-center series, demonstrates that ILI is a well-tolerated procedure for advanced melanoma, sarcoma, and other cutaneous malignancies. Our experience of over 200 ILIs showed that most patients have little toxicity and were hospitalized for a median of 5 days. The majority of patients had a CR or PR, with an ORR of 59.0% for melanoma, 48.9% for sarcoma, 50% for SCC, and 60% for MCC. Melanoma and sarcoma responders had a significantly longer time to in-field progression and melanoma responders had longer time to distant metastatic events. Our updated experience demonstrated a survival advantage for melanoma responders, with a median OS of 56.0 months, i.e., 2.4 years longer than nonresponders.
This study updates our previous experience9 and our results are comparable with other published studies.8,10,16 A retrospective multi-institutional study of 162 ILIs performed for melanoma at eight institutions published a similar ORR of 64% (vs. 59.0% in the current series).8 A multi-institutional study in Australia of over 300 initial ILI procedures had a higher ORR of 75% (33% CR; 42% PR) and found a statistically significant difference in survival for patients achieving a CR (80 months) versus PR (30 months; p = 0.014).16 However, our series did not show this difference in survival between patients who experienced a CR and those who experienced a PR (p = 0.16). One explanation for this could be a difference in burden of disease (BOD) between these two studies. Several studies have shown that lower BOD (low defined as ≤ 10 lesions, none > 2 cm) is associated with increased ORR, with significantly improved progression-free survival and OS.17,18 In one such study, patients with a low BOD had an ORR of 73% with 50% CR, compared with those with a high BOD who had an ORR of 47% with 24% CR (p = 0.002). Another possible explanation for the lower responses rates in this study is that it spanned from 2006 to 2017 and captured patients who have not responded to contemporary treatments, including immunotherapy and targeted therapy, with ILI serving as a second- or third-line treatment, again with possible higher BOD. Our previous study published in 2013 likely reported patients treated with ILI earlier in the disease process and did not include many patients who did not respond to systemic treatments.9
For sarcoma, our response rates and limb salvage rates were slightly lower compared with a published multi-institutional study with an ORR of 58% and an overall limb salvage rate of 77.9%.2 Again, this might be due to the fact that other institutions treat patients with lower BOD, prior to failure of other systemic and local radiation therapies. We generally consider ILI as salvage therapy after patients with locally advanced and heavily pretreated STS have little to no other option than amputation. We also consider ILI for patients with intermediate- to high-grade tumors and exclude well-differentiated liposarcoma. We defined, as have others,2 limb salvage to be patients with locally advanced sarcoma who did not undergo an amputation after treatment with ILI, even if disease was left in situ.
Select patients can be made free of disease by resection after a PR following ILI, and previous studies have shown this improves disease-free survival, with OS similar to those with a CR after ILI alone.19 We were able to further resect approximately one-quarter of our melanoma patients who had a PR. Additionally, there is an option to offer repeat ILIs for those with persistent unresectable disease, even with a prior PR. Several studies have evaluated repeat ILIs for recurrent melanoma.20,21 Chai et al. showed that repeat ILI is well tolerated, as it was in this current series, with no significant difference between initial or repeat ILIs in Wieberdink toxicity score, median CPK value, or LOS.20 In contrast, Kroon et al. noted increased toxicity after repeat ILI, with an increase in the number of grade III (42%) and IV (10%) toxicities, but the majority tolerated the procedure well.21 Similarly, they did not see a significant difference in response rates between initial and repeat ILIs.
This study has several limitations. We did not evaluate the effect of other treatments such as immunotherapies or intralesional therapies prior to or after receiving ILI. Many of the nonresponders, or patients who eventually progressed, went on to receive therapies that included radiation, intralesional therapies including talimogene laherparepvec (TVEC) and rose bengal disodium 10% (PV-10), targeted therapies such as dabrafenib/trametinib, and immunotherapies including pembrolizumab and ipilimumab. The impact of such therapies on the response rate of ILI is unknown and may serve as an area for future investigation. Given the diversity of additional therapies and the time frame in which patients may have received therapies after ILI, we did not include this as part of our analysis. Patients with locoregional disease are discussed at our multidisciplinary tumor board and recommendations are discussed with the patient.
CONCLUSION
ILI is a safe and effective treatment for locally advanced melanoma, sarcoma, and other cutaneous malignancies. Future directions for research include studying response and survival to combination therapies such as ILI and systemic immunotherapies, and ILI and intralesional therapies, for which numerous trials are ongoing and others are currently in the planning phases.
Acknowledgments
DISCLOSURE Jonathan S. Zager—consultant for Amgen, Speakers Bureau for Amgen, Consultant for Castle Biosciences, Consultant and Medical Adivsory Board for Delcath Systems. Research support from Amgen, Provectus, Castle Biosciences, Novartis, Delcath Systems. Nothing to disclosure from all other authors.
REFERENCES
- 1.Specenier P Pembrolizumab use for the treatment of advanced melanoma. Expert Opin Biol Ther. 2017;17(6):765–80. [DOI] [PubMed] [Google Scholar]
- 2.Mullinax JE, Kroon HM, Thompson JF, et al. Isolated limb infusion as a limb salvage strategy for locally advanced extremity sarcoma. J Am Coll Surg. 2017;224(4):635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14(3):238–47. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg. 1997;132(8):903–7. [DOI] [PubMed] [Google Scholar]
- 5.Sanki A, Kam PC, Thompson JF. Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann Surg. 2007;245(4):591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunhagen DJ, de Wilt JH, van Geel AN, Verhoef C, Eggermont AM. Isolated limb perfusion with TNF-alpha and melphalan in locally advanced soft tissue sarcomas of the extremities. Recent Results Cancer Res. 2009;179:257–70. [DOI] [PubMed] [Google Scholar]
- 7.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15(11):3003–13. [DOI] [PubMed] [Google Scholar]
- 8.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208(5):706–15 (Discussion 715–707). [DOI] [PubMed] [Google Scholar]
- 9.Wong J, Chen YA, Fisher KJ, Zager JS. Isolated limb infusion in a series of over 100 infusions: a single-center experience. Ann Surg Oncol. 2013;20(4):1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbour AP, Thomas J, Suffolk J, Beller E, Smithers BM. Isolated limb infusion for malignant melanoma: predictors of response and outcome. Ann Surg Oncol. 2009;16(12):3463–72. [DOI] [PubMed] [Google Scholar]
- 11.Turaga KK, Beasley GM, Kane JM 3rd, et al. Limb preservation with isolated limb infusion for locally advanced nonmelanoma cutaneous and soft-tissue malignant neoplasms. Arch Surg. 2011;146(7):870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncrieff MD, Kroon HM, Kam PC, Stalley PD, Scolyer RA, Thompson JF. Isolated limb infusion for advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2008;15(10):2749–56. [DOI] [PubMed] [Google Scholar]
- 13.Zeitouni NC, Giordano CN, Kane JM 3rd. In-transit Merkel cell carcinoma treated with isolated limb perfusion or isolated limb infusion: a case series of 12 patients. Dermatol Surg. 2011;37(3):357–64. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 15.Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18(10):905–10. [DOI] [PubMed] [Google Scholar]
- 16.Kroon HM, Coventry BJ, Giles MH, et al. Australian Multicenter Study of Isolated Limb Infusion for Melanoma. Ann Surg Oncol. 2016;23(4):1096–103. [DOI] [PubMed] [Google Scholar]
- 17.Muilenburg DJ, Beasley GM, Thompson ZJ, Lee JH, Tyler DS, Zager JS. Burden of disease predicts response to isolated limb infusion with melphalan and actinomycin D in melanoma. Ann Surg Oncol. 2015;22(2):482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dossett LA, Ben-Shabat I, Olofsson Bagge R, Zager JS. Clinical response and regional toxicity following isolated limb infusion compared with isolated limb perfusion for in-transit melanoma. Ann Surg Oncol. 2016;23(7):2330–5. [DOI] [PubMed] [Google Scholar]
- 19.Wong J, Chen YA, Fisher KJ, Beasley GM, Tyler DS, Zager JS. Resection of residual disease after isolated limb infusion (ILI) is equivalent to a complete response after ILI-alone in advanced extremity melanoma. Ann Surg Oncol. 2014;21(2):650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai CY, Deneve JL, Beasley GM, et al. A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Ann Surg Oncol. 2012;19(5):1637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115(9):1932–40. [DOI] [PubMed] [Google Scholar]


