Abstract
We report clinical outcomes using multi-institutional data to evaluate oncologic efficacy of lymph node dissection (LND) at the time of cytoreductive nephrectomy. Number of positive lymph nodes was an independent predictor for cancer-specific survival. The performance of lymphadenectomy with standard templates in clinical trials of new systemic therapies could further ascertain prognostic value of LND.
Purpose:
To determine the therapeutic value of lymph node dissection (LND) during cytoreductive nephrectomy (CN) and assess predictors of cancer-specific survival (CSS) in metastatic renal-cell carcinoma.
Patients and Methods:
We identified 293 consecutive patients treated with CN at 4 academic institutions from March 2000 to May 2015. LND was performed in 187 patients (63.8%). CSS was estimated by the Kaplan-Meier method for the entire cohort and for a propensity score–matched cohort. Cox proportional hazards regression was used to evaluate CSS in a multivariate model and in an inverse probability weighting–adjusted model for patients who underwent dissection.
Results:
Median follow-up was 12.6 months (interquartile range, 4.47, 30.3), and median survival was 15.9 months. Of the 293 patients, 187 (63.8%) underwent LND. One hundred six patients had nodal involvement (pN+) with a median CSS of 11.3 months (95% confidence interval [CI], 6.6,15.9) versus 24.2 months (95% confidence interval, 14.1, 34.3) for pN− patients (log-rank P = .002). The hazard ratio for LND was 1.325 (95% CI, 1.002, 1.75) for the whole cohort and 1.024 (95% CI, 0.682, 1.537) in the propensity score–matched cohort. Multivariate analysis revealed that number of positive lymph nodes (P < .001) was a significant predictor of worse CSS.
Conclusion:
For patients with metastatic renal-cell carcinoma undergoing CN with lymphadenectomy, the number of nodes positive was predictive of survival at short-term follow-up. However, nonstandardized lymphadenectomy only provided prognostic information without therapeutic benefit. Prospective studies with standardized templates are required to further ascertain the therapeutic value of LND.
Keywords: Cytoreductive nephrectomy, Lymphadenectomy, Lymph node dissection, Metastatic renal cell carcinoma, Node density
Introduction
Approximately one third of patients diagnosed with renal-cell carcinoma (RCC) present with locally advanced or metastatic disease.1 The benefit of cytoreductive nephrectomy (CN) has been well established since before the arrival of targeted therapies.2,3 The role of lymph node dissection (LND) for RCC, however, has been controversial, with data questioning the advantage of node dissection as a result of its minimal impact on survival while adding time to the procedure and requiring manipulation of the great vessels.4–6
With the advent of new systemic therapies, the value of LND has been increasingly discussed in the metastatic RCC (mRCC) population.7 Although there is evidence that metastasectomy along with CN improves survival, the role of concomitant lymphadenectomy is not yet clear.8–10 Less information is available regarding histologic predictors of survival found at the time of LND.
Previous studies have evaluated the benefit of LND in the CN setting. One study found survival of patients with regional node involvement (pN+) was identical to that of patients with distant metastatic disease, while 2 other studies found those with node involvement had significantly shorter survival than those without regional disease.11,12 Furthermore, recent studies have not demonstrated improved outcomes for those undergoing LND during CN, yet pN+ disease is a predictor of more aggressive disease and shorter survival.4,13,14
The objectives of this study were to report clinical outcomes using multi-institutional data evaluating the therapeutic benefit of LND at the time of CN and to assess its impact on cancer-specific survival (CSS). In addition, we ascertain CSS on the basis of mRCC risk group classification as well as volume of regional disease based on number of positive lymph nodes (pN+) using propensity score–based analyses to minimize selection bias.
Patients and Methods
Data Source and Study Population
The study was performed after receipt of approval from the local institutional review board at each institution. We retrospectively reviewed medical records of 293 patients from 4 academic centers who sought care between March 2000 and May 2015 with mRCC. None of these patients received presurgical targeted therapies for neoadjuvant purposes before proceeding with CN. Chart review was performed to determine site and volume of metastatic disease at the time of nephrectomy. In general, LNDs were either hilar (with or without extension to pre- and para-aortic nodes for left sided tumors or pre- and paracaval nodes for right-sided tumors) or limited only to enlarged lymph nodes for which invasion was suspected (cN1) on cross-sectional imaging. Extent of dissection was not standardized across institutions and was unavailable for analysis.
Disease Classification and Disease-Specific Variables
Using previously defined prognostic factors as described by Motzer et al,15 patients were stratified on the basis of the 3-factor Memorial Sloan Kettering Cancer Center (MSKCC) criteria (favorable, intermediate, and poor). Other collected variables included age, Charlson comorbidity index, Karnofsky performance status, estimated blood loss, Fuhrman grade, RCC histology, margin status, presence of tumor necrosis, and sarcomatoid or rhabdoid features. All tissue was examined for the presence of metastases by genitourinary pathologists from each institution according to local institutional procedures. Pathologic stage was assigned according to the 2016 American Joint Committee on Cancer staging manual, 8th edition.
Statistical Analysis
Primary outcome was CSS and was calculated from the date of surgery until death from disease. Patient demographics and clinical characteristics were summarized using descriptive statistics. Univariate analyses were performed by chi-square test and Fisher’s exact test for categorical variables, and analysis of variance and Kruskal-Wallis test for numerical variables. Survival was estimated for those with complete follow-up by the Kaplan-Meier method and compared by the log-rank test.
Patients were compared on the basis of LND status, and a propensity score–matched model was developed using variables that were significantly different. The propensity score was calculated using the Logistic procedure in SAS 9.4 software (SAS Institute, Cary, NC) following the radius method described in Baser16 and further expounded in the proceedings of the SAS User Group.17,18 The variables included were grade, T stage, number of nodes removed, number of nodes positive, number of metastases, MSKCC category, and use of systemic therapy. We did not use imputation in the analysis and assumed missing data at random. For variables with substantial data missing, we checked to see if there were differences in missing and nonmissing values for variables for the analysis in question (eg, survival outcome).
Using Cox proportional hazards regression, the hazard ratio (HR) for LND was analyzed for the whole cohort and an inverse probability weighting–adjusted cohort to minimize selection bias.19 Within the sample for LND (n = 187), we performed univariate Cox proportional hazards regression followed by a backward selection multivariate model with a significance level of .10 with CSS as the primary outcome of interest. Statistical analyses were performed by SAS 9.4 software.
Results
Study Cohort Characteristics
Demographic and tumor characteristics for the entire cohort are provided in Table 1. Median age of patients was 61 (interquartile range [IQR], 54.7, 70.3) years with a median follow-up of 12.6 (IQR, 4.47, 30.3) months. Median survival of the entire cohort was 15.9 months. Of the 293 patients, 187 (63.8%) underwent LND. Patients who received LND had tumors with significantly higher Fuhrman grades, more sarcomatoid features, more papillary tumor architecture, and a nonsignificant trend to higher stage (Supplemental Table 1 in the online version). One hundred six patients with pN+ disease were found with a median CSS of 11.3 (95% confidence interval, 6.6, 15.9) versus 24.2 (95% confidence interval, 14.1, 34.3) months for patients with pN− disease (log-rank P = .002).
Table 1.
Demographic and Clinical Characteristics of 293 Patients
| Characteristic | Variable | Value |
|---|---|---|
| KPS | <80 | 33 (11.3%) |
| ≥80 | 260 (88.7%) | |
| Fuhrman grade | 1 | 3 (1.0%) |
| 2 | 27 (9.3%) | |
| 3 | 116 (39.9%) | |
| 4 | 145 (49.8%) | |
| Unknown | 2 | |
| RCC histology | Clear | 261 (90.9%) |
| Papillary | 26 (9.1%) | |
| Unknown | 6 | |
| AJCC tumor stage | T3a | 84 (28.7%) |
| T3b | 137 (46.8%) | |
| T3c | 34 (11.6%) | |
| T4 | 38 (13.0%) | |
| AJCC node stage | pN negative | 68 (52.8%) |
| pN positive | 119 (47.2%) | |
| Unknown | 106 | |
| Sarcomatoid features | No | 236 (80.8%) |
| Yes | 56 (19.2%) | |
| Unknown | 1 | |
| Rhabdoid features | No | 278 (94.9%) |
| Yes | 15 (5.1%) | |
| Tumor Necrosis | No | 77 (29.2%) |
| Yes | 187 (70.9%) | |
| Unknown | 29 | |
| Soft tissue margin positive | No | 265 (90.4%) |
| Yes | 28 (9.6%) | |
| No. of metastases | 1 | 171 (62.0%) |
| ≥2 | 105 (38.0%) | |
| Unknown | 17 | |
| Lung metastases | No | 107 (36.5%) |
| Yes | 186 (63.5%) | |
| Bone metastases | No | 238 (81.5%) |
| Yes | 54 (18.5%) | |
| Brain metastases | No | 283 (96.6%) |
| Yes | 10 (3.4%) | |
| Liver metastases | No | 258 (88.1%) |
| Yes | 35 (11.9%) | |
| MSKCC prognostic model | Favorable | 62 (21.2%) |
| Intermediate | 148 (50.7%) | |
| Poor | 82 (28.1%) | |
| Unknown | 1 | |
| Age (years) | Mean | 61.64 |
| Median | 61 | |
| Minimum | 24.10 | |
| Maximum | 86.30 | |
| SD | 10.62 | |
| Unknown | 4 | |
| Charlson comorbidity index | Mean | 7.39 |
| Median | 8 | |
| Minimum | 1 | |
| Maximum | 14 | |
| SD | 2.21 | |
| Unknown | 74 |
Abbreviations: AJCC = American Joint Committee on Cancer; KPS = Karnofsky performance status; MSKCC = Memorial Sloan Kettering Cancer Center; pN = pathologic node stage; RCC = renal-cell carcinoma; SD = standard deviation.
There was no significant difference in age, performance status, intraoperative blood loss, or proportion of bone, brain, liver, or polymetastatic disease. Large intraoperative blood loss was explained by numerous level 3 and 4 thrombus patients requiring complex vascular reconstruction. One hundred ninety-four patients (66.2%) received postoperative systemic therapies, with 42.7% receiving tyrosine kinase inhibitors and the rest receiving chemotherapy, immunotherapy, or combined therapy. Propensity score matching produced 65 pairs with adequate balance between LND and no LND for clinical and pathologic covariates (Supplemental Table 1 in the online version).
Oncologic Outcomes of LND
Overall median survival was 24.3 (IQR, 16.6, 33.7) months for no LND versus 15.8 (12.4, 20.5) months for LND (log-rank P = .047) with a HR for LND of 1.325 (1.002, 1.75; P = .048) (Figure 1A). In the propensity score–matched cohort, median survival was 24.6 (IQR, 16.1, 33.7) months for no LND versus 23.2 (16.2, 29.6) months for LND (log-rank P = .909) with a HR of 1.024 (0.682, 1.537; P = .909) (Figure 1B). The favorable MSKCC risk group had a median survival of 43.9 (IQR, 27.3, 65.5) months for no LND versus 20.5 (11.7, 24.9) months for LND (log-rank P = .038). In the intermediate-risk group, median survival was 18.2 (IQR, 12.3, 24.7) months for no LND versus 16.6 (11.5, 24.3) months for LND (log-rank P = .973). In the poor-risk group, median survival was 16.1 (IQR, 6.8, 47.6) months for no LND versus 12.4 (6.8, 18.1) months for LND (log-rank P = .101) (Supplemental Figure 1 in the online version).
Figure 1.
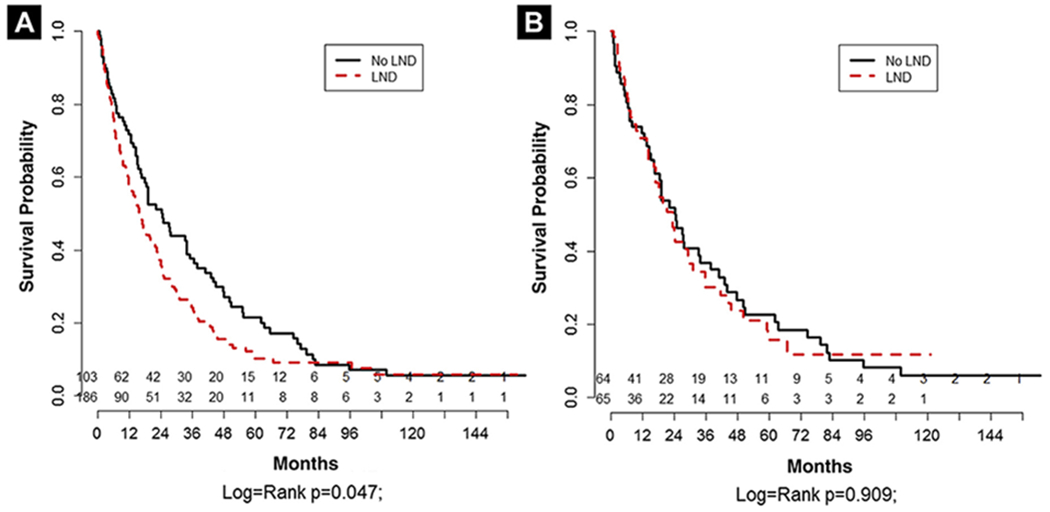
Kaplan-Meier Estimate for Cancer-Specific Survival for Entire Cohort (A) and Propensity Score–Matched Cohort (B) Based on Lymph Node Dissection (LND)
Subgroup Analysis for LND Patients
Cox proportional hazards analysis revealed number of positive nodes to be a significant predictor of worse CSS for those undergoing LND (HR 1.08; 95% confidence interval, 1.03, 1.13; P = .001) (Table 2). After inverse probability weighting adjustment, number of positive nodes remained significantly associated with poorer CSS (HR 1.08; 95% CI, 1.03, 1.14; P = .0009) (Table 3).
Table 2.
Cox Proportional Hazards Regression of CSS for 187 Patients Who Dissection
| Characteristic | Variable | HR (95% CI) | P | Backward Selection HR (95% CI)a | Backward Selection Pa |
|---|---|---|---|---|---|
| Fuhrman grade | 4 | 1.44 (1.02, 2.03) | .038b | ||
| ≤3 | — | — | |||
| AJCC tumor stage | T3b | 0.79 (0.52, 1.22) | .3 | 0.80 (0.51, 1.26) | .34 |
| T3c | 1.31 (0.72, 2.37) | .37 | 1.41 (0.75, 2.63) | .283 | |
| T4 | 1.68 (1.00, 2.84) | .05 | 1.41 (0.80, 2.47) | .23 | |
| T3a | — | — | |||
| No. of nodes removed | 1.01 (0.99, 1.04) | .28 | |||
| No. of positive nodes | 1.09 (1.05, 1.14) | <.001b | 1.08 (1.03, 1.13) | .001b | |
| Sarcomatoid features | Yes | 1.48 (1.00, 2.21) | .05 | — | |
| No | — | — | |||
| Rhabdoid features | Yes | 1.74 (0.81, 3.73) | .16 | — | |
| No | — | — | |||
| Tumor necrosis | 1 | 1.57 (1.07, 2.30) | .021b | — | |
| 2 | 0 | .98 | |||
| 0 | — | — | |||
| Soft tissue margin positive | 1 | 1.08 (0.63, 1.86) | .77 | — | |
| 0 | — | — | |||
| Systemic therapy | 1 | 1.16 (0.79, 1.69) | .45 | — | |
| 0 | — | — | |||
| No. of metastases | >1 | 1.25 (0.88, 1.78) | .21 | ||
| 1 | |||||
| MSKCC | Favorable | 1.00 (0.64, 1.57) | .99 | ||
| Intermediate | 1.44 (0.89, 2.33) | .14 | |||
| Poor | — | ||||
| Lymph node density | 2.29 (1.51, 3.49) | <.001b |
Abbreviations: AJCC = American Joint Committee on Cancer; CI = confidence interval; HR = hazard ratio; MSKCC = Memorial Sloan Kettering Cancer Center.
Results from backward-selection multivariate Cox proportional hazards regression.
Statistically significant.
Table 3.
Inverse Probability Weighting Cox Proportional Hazards Regression of CSS for 187 Patients Who Received Lymph Node Dissection
| Characteristic | Variable | HR (95% CI) | P | Backward Selection HR (95% CI)a | Backward Selection Pa |
|---|---|---|---|---|---|
| Furhman grade | 4 | 1.34 (0.96, 1.87) | .08 | ||
| ≤3 | — | — | |||
| AJCC tumor stage | T3b | 0.79 (0.53, 1.17) | .24 | 0.76 (0.5, 1.16) | .21 |
| T3c | 1.35 (0.73, 2.50) | .34 | 1.47 (0.78, 2.78) | .23 | |
| T4 | 1.72 (1.03, 2.88) | .038b | 1.39 (0.80, 2.42) | .25 | |
| T3a | — | — | |||
| No. of nodes removed | 1.01 (0.99, 1.04) | .25 | |||
| No. of positive nodes | 1.10 (1.05, 1.14) | <.001b | 1.08 (1.03, 1.14) | .0009b | |
| Sarcomatoid features | Yes | 1.46 (0.98, 2.18) | .06 | — | |
| No | — | — | |||
| Rhabdoid features | Yes | 1.88 (0.90, 3.90) | .09 | — | |
| No | — | — | |||
| Tumor necrosis | 1 | 1.78 (1.21, 2.61) | .003b | — | |
| 2 | 0 | .99 | |||
| 0 | — | — | |||
| Soft tissue margin positive | 1 | 1.16 (0.80, 1.69) | .87 | — | |
| 0 | — | — | |||
| Systemic therapy | 1 | 1.16 (0.79, 1.69) | .44 | — | |
| 0 | — | — | |||
| No. of metastases | >1 | 1.21 (0.85, 1.71) | .29 | ||
| 1 | |||||
| MSKCC | Favorable | 1.15 (0.65, 1.57) | .52 | ||
| Intermediate | 1.58 (0.89, 2.33) | .06 | |||
| Poor | — | ||||
| Lymph node density | 2.29 (1.50, 3.48) | <.001b |
Variables included in model were grade, T stage, number of nodes removed, number of nodes positive, number of metastases, MSKCC category, and use of systemic therapy.
Abbreviations: AJCC = American Joint Committee on Cancer; CI = confidence interval; CSS = cancer-specific survival; HR = hazard ratio; MSKCC = Memorial Sloan Kettering Cancer Center.
Results from backward-selection multivariate Cox proportional hazards regression.
Statistically significant.
Discussion
Approximately 25% to 30% of RCC cases present with metastatic disease at the time of diagnosis.20 Pending the results of randomized controlled trials, CN with integration of systemic therapies appears to be the treatment of choice in mRCC patients.21,22 While metastasectomy has demonstrated a therapeutic benefit with multimodal approaches, the value of LND has not proven beneficial in these patients.7,8,10–14,20,23,24 With the new arrival of systemic therapies, it will become valuable to continue to stratify those who will benefit from aggressive surgical interventions while adding additional prognostic information in regards to disease survival.
Cancer survival for RCC patients is known to be greatly affected by the presence of lymph node metastasis.7,25 Although the value of lymphadenectomy has not been proven to offer a survival advantage in the nonmetastatic setting, prospective trials have suggested a staging role for lymphadenectomy in disease prognosis, especially in higher stages of the disease.4,6 In the metastatic setting, reports on lymphadenectomy have been conflicting. Although it has not been shown to have a survival benefit in pN− patients, it has shown benefit in pN+ patients undergoing CN while improving response to postoperative therapy.11,13
Population studies using Surveillance, Epidemiology, and End Results (SEER) registry data have shown patients undergoing CN with concomitant LND carry worse disease-specific survival than those with CN alone.22 Finding a positive node decreased median cancer survival from 22 months to 9 months, with more positive nodes decreasing survival in these patients. Another SEER study found nodal disease worsened CSS and overall survival by 68% and 69%, respectively, with similar detrimental effect per additional positive node found (5.1% and 5.6%, respectively).26 A recent well-designed study from the Mayo Clinic demonstrated that patients with pN+ disease harbor more aggressive primary tumor features, which agrees with our results.14
We extrapolate that these aggressive phenotypes allow for early lymphatic spread in addition to hematogenous spread. Although LND did not show a therapeutic benefit, it highlights the staging significance of the procedure, as shown by the difference in median cancer survival (11.3 vs. 24.2 months) between pN+ and pN− patients, which is congruent with previous mRCC series.11–13 Furthermore, the proportion of positive nodes remained an independent risk factor and adds additional prognostic information in this select high-risk group of patients. Given that nodal involvement conveys an aggressive biology with potential for poor response to systemic therapies, complete removal of regional node disease would seem to be beneficial. Nonetheless, LND continues to not demonstrate any improvement in outcomes for RCC patients. Future studies with novel targeted therapies may expand the role of node dissection in this population.11,13,22,26
In this study, we report outcomes of node dissection during CN using a contemporary, multicenter cohort with a wide range of geographic locations and heterogeneous populations, which allows for generalization of our results. However, we acknowledge some important limitations that cannot be overcome. Our retrospective design carries inherent limitations associated with a retrospective chart review and a significant selection bias that may confound our results. For example, the nonstandardization of LND templates can cause significant variability in lymph node yields across centers, with surgeon discretion driving the extent of LND. Radiographic nodal size was missing from medical records, and follow-up for the cohort was relatively short. Likewise, lymph node yields can vary depending on laboratory processing of the lymph node packets. With robust prospective data likely not forthcoming, our study adds a multicenter view on the poor survival outcomes for those found to have regional lymph node involvement in the mRCC setting. With the recent availability of novel therapeutic systemic agents, the role of LND will need to be revisited for this select group of patients.
Conclusion
Lymphadenectomy was found to play a prognostic role with no therapeutic advantage in patients with mRCC at short follow-up. Pathologic features such as degree of lymph node involvement demonstrated prognostic significance. Performance of lymphadenectomy with standard templates in clinical trials of new systemic therapies continues to be needed.
Supplementary Material
Clinical Practice Points.
We evaluated CN and LND in a large multicenter cohort of patients.
LND offered prognostic but not therapeutic value.
The number of metastatic lymph node involvement was predictive of CSS after CN.
Footnotes
Disclosure
The authors have stated that they have no conflict of interest.
Supplemental Data
A supplemental table and figure accompanying this article can be found in the online version at https://doi.org/10.1016/j.clgc.2017.10.004.
References
- 1.Lam JS, Belldegrun AS, Pantuck AJ. Long-term outcomes of the surgical management of renal cell carcinoma. World J Urol 2006; 24:255–66. [DOI] [PubMed] [Google Scholar]
- 2.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 2001; 345:1655–9. [DOI] [PubMed] [Google Scholar]
- 3.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa–based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001; 358:966–70. [DOI] [PubMed] [Google Scholar]
- 4.Blom JH, van Poppel H, Marechal JM, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol 2009; 55:28–34. [DOI] [PubMed] [Google Scholar]
- 5.Leibovich BC, Blute ML. Lymph node dissection in the management of renal cell carcinoma. Urol Clin North Am 2008; 35:673–8. [DOI] [PubMed] [Google Scholar]
- 6.Capitanio U, Jeldres C, Patard JJ, et al. Stage-specific effect of nodal metastases on survival in patients with non-metastatic renal cell carcinoma. BJU Int 2009; 103:33–7. [DOI] [PubMed] [Google Scholar]
- 7.Godoy G, O’Malley RL, Taneja SS. Lymph node dissection during the surgical treatment of renal cancer in the modern era. Int Braz J Urol 2008; 34:132–42. [DOI] [PubMed] [Google Scholar]
- 8.Russo P, Synder M, Vickers A, et al. Cytoreductive nephrectomy and nephrectomy/complete metastasectomy for metastatic renal cancer. ScientificWorldJournal 2007; 7:768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell SC, Flanigan RC, Clark JI. Nephrectomy in metastatic renal cell carcinoma. Curr Treat Options Oncol 2003; 4:363–72. [DOI] [PubMed] [Google Scholar]
- 10.Zaid HB, Parker WP, Safdar NS, et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: a systematic review and meta-analysis. J Urol 2017; 197:44–9. [DOI] [PubMed] [Google Scholar]
- 11.Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol 2003; 169:2076–83. [DOI] [PubMed] [Google Scholar]
- 12.Vasselli JR, Yang JC, Linehan WM, et al. Lack of retroperitoneal lymphadenopathy predicts survival of patients withmetastatic renal cell carcinoma.J Urol 2001; 166:68–72. [PubMed] [Google Scholar]
- 13.Feuerstein MA, Kent M, Bernstein M, et al. Lymph node dissection during cytoreductive nephrectomy: a retrospective analysis. Int J Urol 2014; 21:874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershman B, Thompson RH, Moreira DM, et al. Lymph node dissection is not associated with improved survival among patients undergoing cytoreductive nephrectomy for metastatic renal cell carcinoma: a propensity score based analysis. J Urol 2017; 197:574–9. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004; 22:454–63. [DOI] [PubMed] [Google Scholar]
- 16.Baser O Too much ado about propensity score models? Comparing methods of propensity score matching. Value Health 2006; 9:377–85. [DOI] [PubMed] [Google Scholar]
- 17.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques [paper 214-26]. Paper presented at: SAS User Group (SUGI) 26, Long Beach, CA, April 22-24, 2001, Available at: http://www2.sas.com/proceedings/sugi26/p214-26.pdf. Accessed: October 22, 2017. [Google Scholar]
- 18.Parsons LS. Performing a 1:N case–control match on propensity score [paper 165-29], Paper presented at: SAS User Group (SUGI) 29, Montreal, Quebec, Canada, May 9-12, 2004 Available at: http://www2.sas.com/proceedings/sugi29/165-29.pdf. Accessed: October 22, 2017. [Google Scholar]
- 19.Narduzzi S, Golini MN, Porta D, et al. Inverse probability weighting (IPW) for evaluating and “correcting” selection bias. Epidemiol Prev 2014; 38:335–41. [PubMed] [Google Scholar]
- 20.Gupta K, Miller JD, Li JZ, et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 2008; 34:193–205. [DOI] [PubMed] [Google Scholar]
- 21.Kenney PA, Wood CG. Integration of surgery and systemic therapy for renal cell carcinoma. Urol Clin North Am 2012; 39:211–31. [DOI] [PubMed] [Google Scholar]
- 22.Patel HD, Gorin MA, Gupta N, et al. Mortality trends and the impact of lymphadenectomy on survival for renal cell carcinoma patients with distant metastasis. Can Urol Assoc J 2016; 10:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joslyn SA, Sirintrapun SJ, Konety BR. Impact of lymphadenectomy and nodal burden in renal cell carcinoma: retrospective analysis of the National Surveillance, Epidemiology, and End Results database. Urology 2005; 65:675–80. [DOI] [PubMed] [Google Scholar]
- 24.Capitanio U, Leibovich BC. The rationale and the role of lymph node dissection in renal cell carcinoma. World J Urol 2017; 35:497–506. [DOI] [PubMed] [Google Scholar]
- 25.Lughezzani G, Capitanio U, Jeldres C, et al. Prognostic significance of lymph node invasion in patients with metastatic renal cell carcinoma: a population-based perspective. Cancer 2009; 115:5680–7. [DOI] [PubMed] [Google Scholar]
- 26.Trinh QD, Sukumar S, Schmitges J, et al. Effect of nodal metastases on cancer-specific mortality after cytoreductive nephrectomy. Ann Surg Oncol 2013; 20: 2096–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


