Abstract
Background:
Despite neoadjuvant chemoradiation (nCRT) followed by esophagectomy for locally advanced esophageal cancer, locoregional recurrence (LRR) is common and factors associated with LRR have not been clearly identified.
Methods:
Patients were identified from a single institution, prospectively maintained database (1996-2013). Patterns of recurrence were described and associated factors of LRR were analyzed using competing risks regression models.
Results:
Of the 456 patients treated with nCRT and surgery, 167 patients developed recurrence. Locoregional and distant recurrences were observed in 69 (15.1%) and 140 (30.9%) patients, respectively. Time to recurrence (13.6 vs 10.4 months, P = 0.20) and median overall survival (29.3 vs 19.1 months, P = 0.12) were no different among the 27 patients (6%) who developed a solitary LRR compared to patients who developed distant recurrence. Univariable analysis identified lymphovascular invasion (HR 1.46, P = 0.07), lymph node ratio >0.5 (HR 2.16, P = 0.02), positive margin (HR 1.95, P = 0.05), lack of response to neoadjuvant therapy (HR 1.99, P < 0.01), clinical T stage (HR 2.62, P < 0.01) and final T3/4 stage (HR 2.06, P < 0.01) as factors significantly associated with LRR. Clinical T stage and response to neoadjuvant treatment were independently associated with LRR on multivariable analysis.
Conclusions:
Although aggressive tumor biology plays a significant role in LRR, optimizing neoadjuvant treatments to obtain a complete pathologic response may lead to improved locoregional control.
Keywords: esophagectomy, outcomes, prognosis, risk factors
1 |. INTRODUCTION
Despite the expanded use of neoadjuvant chemoradiation (nCRT) in recent years, overall prognosis for esophageal cancer remains poor. In 2015, an estimated 16 980 patients were diagnosed with and 15 590 patients died of esophageal cancer in the United States.1 As these dismal statistics imply, many patients with esophageal cancer present with incurable disease2 and among the patients who do receive curative intent treatment, many suffer from recurrence with limited salvage treatment options.3,4
Recent randomized controlled trials5,6 and a meta-analysis7 have confirmed improved disease-free survival (DFS) and overall survival (OS) in patients with locally advanced esophageal cancer using nCRT followed by surgical resection. Based on these data, nCRT has become the standard of care for these patients. Most notably, the CROSS trial showed a prolonged median OS of 49.4 months with the addition of nCRT compared to 24.0 months with surgery alone (P = 0.003) and complete 5-year follow-up recently confirmed a doubling of the median OS with the use of nCRT.8
Despite radical esophageal resection, the rate of locoregional recurrence (LRR) with surgery alone has been reported up to 42%.9–12 Multimodality therapy is designed to optimize locoregional control of the disease and, indeed, leads to higher R0 resection rates,5 lower locoregional failure rates,13 and induces a pathologic complete response in up to 47% of patients.5,14,15 Yet, despite nCRT, approximately 20% of patients will develop LRR.9,13,16,17 Salvage treatment options for these patients are limited4,18,19 and the resultant progressive dysphagia can cause significant morbidity leading to malnutrition, refractory pain, and repeated palliative endoscopic dilations and/or stent placements. Unfortunately, the etiology of locoregional failure following nCRT in esophageal cancer patients has not been intently studied. It remains unclear if technical adjustments (such as higher radiation doses, expanded radiation fields, or more extensive lymphadenectomy) could be made to further improve LR control in these patients. The aims of this study are to (1) analyze the patterns of recurrence in patients with locally advanced esophageal cancer treated with nCRT and esophagectomy; (2) explore the survival implications of locoregional failure; and (3) identify potentially actionable factors associated with LRR.
2 |. METHODS
Patients were identified from a single institution IRB-approved database of patients undergoing esophagectomy for esophageal cancer. Data were retrospectively collected until 2006, after which data have been prospectively maintained. Patient demographics, clinical outcomes, perioperative variables, multimodality treatment regimens, and recurrence data were retrospectively analyzed. All patients who underwent nCRT followed by esophagectomy for curative intent were included. Patients who underwent palliative surgery, presented with synchronous hepatic or peritoneal metastases, or died within 90 days of surgery were excluded from the analysis.
Once the diagnosis of esophageal cancer was made, the patients’ clinical stage was established by computed tomography (CT) scans, endoscopic ultrasound (EUS), and positron emission tomography (PET) scans according to American Joint Committee on Cancer staging criteria.20 nCRT was recommended to patients with clinical ≥T2 or N1 disease. The preferred neoadjuvant regimen at our institution includes cisplatin (Days 1 and 29) and continuous 5-fluorouracil infusion (Monday through Friday) concurrent with intensity-modulated radiation therapy (IMRT) or 3D conformal radiation therapy (3DCRT) to a total dose ranging from 45 to 56 Gy over 25 to 28 fractions, as previously reported.14,21 Some patients were treated by referring centers and alternatively received weekly carboplatin and paclitaxel concurrent radiation using doses of 41.4 to 50 Gy over 23 to 25 fractions.5 The radiation fields were designed to encompass the gross tumor delineated by imaging or fiducial markers and an appropriate elective volume to encompass sites of potential microscopic disease. North American esophageal cancer consensus contouring guidelines were recently published22 and were not available for the time period of this study, thus there was inter-practitioner heterogeneity in contouring design amongst the radiation oncologists treating these cancers.
Patients were restaged with PET/CT scans approximately 6 weeks after completion of nCRT. Patients who did not have evidence of metastatic disease and were medically fit were offered surgical resection. Esophagectomy was generally performed 6 to 10 weeks following completion of nCRT. In general, patients were not treated with adjuvant therapy. PET response was noted when post-nCRT imaging revealed decreased or resolved PET avidity at the primary tumor site compared to pre-nCRT imaging. Complete pathologic response to nCRT was defined as no residual tumor cells seen at the tumor site or regional lymph nodes on pathologic evaluation. Patients who had residual microscopic cancer but were downstaged (improved T stage, N stage, or tumor size) from their pre-nCRT clinical stage were classified as having had a partial response. No response was noted when the pathologic stage was the same or more advanced than the pre-nCRT clinical stage.
When surveillance was performed at our institution, patients were followed every 3-6 months with a history and physical exam, basic metabolic panel, and complete blood count for 3 years, then every 6 months for 2 additional years. CT or PET scans of the chest, abdomen, and pelvis were performed annually for 5 years. During the initial years of the study period, a routine esophagogastroduodenoscopy (EGD) was performed 6-12 months after surgery; however, in our current practice, and for most of the study period, an EGD was only performed in patients with a history of Barrett’s esophagus or who developed symptoms. When recurrent esophageal cancer was suspected by EGD, imaging or symptoms, a biopsy for confirmation was obtained prior to further treatment. The type of recurrence was classified as follows: local recurrence when it involved the esophageal lumen, most often at the anastomotic site; regional recurrence when it involved adjacent mediastinal or celiac lymph nodes; LLR when having local and/or regional disease; or distant recurrence (DR) when it involved distant organs including non-regional lymph nodes. Many patients developed metastases at multiple sites. Recurrence-free survival (RFS) was calculated from the date of surgery to the date of recurrence. OS was calculated from the date of surgery to death or date of last follow-up. Time to recurrence and survival time after recurrence were calculated using the date when recurrence was determined by imaging or biopsy.
Descriptive statistics compared patients with any DR to those with only LRR using χ2 test for categorical predictor variables and Wilcoxon rank sum test for continuous predictor variables. For small sample sizes, Fisher’s exact test was used for comparison purposes. To estimate recurrence free survival, competing risk analysis was done. For RFS main event of interest was any DR and competing event were only LRR or Death. Cumulative incidence was estimated and a plot was generated using “cmprsk” R package. Multivariable competing risks regression models (sub distribution hazard models) were developed to determine the correlation of clinically important covariates to the RFS. Backward selection methods with SLS = 0.25 was used to choose covariates for the final model. To compare the post event survival for any DR and solitary LRR, Kaplan-Meier curve and estimates, with the log rank test, were used. SAS v9.4 was used to perform this analysis.
3 |. RESULTS
3.1 |. Demographics and patterns of recurrence
Out of 827 esophageal cancer patients from October 1996 to June 2013, 521 patients (63%) were treated with nCRT followed by esophagectomy. Based on the exclusion criteria outlined above, 65 patients were excluded (Figure 1). A summary of the demographics and clinicopathologic characteristics of the 456 patients who were included in our analysis is shown in Table 1. The nCRT regimens most often used were 5-fluorouracil/cisplatin (74%) or carboplatin/paclitaxel (14%) and the median dose of radiation was 50.4 Gy (range 16.2, 66.6 Gy). Planned doses of neoadjuvant chemotherapy and radiation were completed in 85% and 93% of patients, respectively. The median number of lymph nodes harvested was 10 (range 0-39). When the prognostic value of lymph node harvest became evident 8-10 years ago,23,24 our institution began to focus more intently on surgical harvest and, in particular, pathologic retrieval. As such, we found a significant difference in the median lymph node harvest before and after 2008 (7 vs 15, P < 0.001). A positive proximal, distal or radial margin was found in 19 patients. Our overall R0 resection rate was 96%. A complete pathologic response was observed in 181 patients (40%).
FIGURE 1.
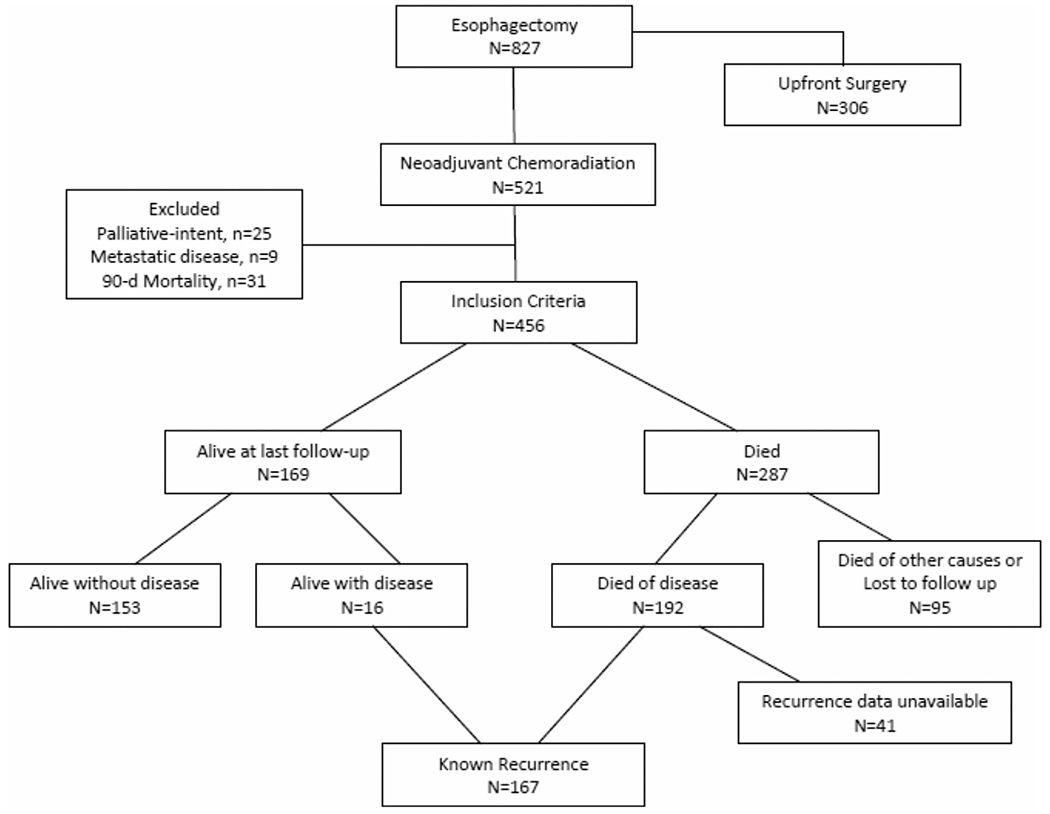
A flow chart describing the inclusion criteria and clinical outcomes of 827 patients treated with esophagectomy leading to our main study population of 167 patients with known disease recurrence following neadjuvant chemoradiation and esophagectomy
TABLE 1.
Demographic and clinicopathologic characteristics of 456 patients with locally advanced esophageal cancer treated with neoadjuvant chemoradiation and esophagectomy
| Patient variable | Total | LRR | DR | No known recurrence | P-value |
|---|---|---|---|---|---|
| N | 456 | 27 | 140 | 289 | |
| Age (mean ± SD) | 63 ± 11 | 64 ± 11 | 63 ± 11 | 63 ± 11 | 0.73 |
| Gender | |||||
| Male | 381 (84) | 21 (78) | 119 (85) | 241 (83) | 0.39 |
| Female | 75 (16) | 6 (22) | 21 (15) | 48 (17) | |
| Histology | |||||
| Adenocarcinoma | 389 (87) | 23 (85) | 124 (89) | 242 (86) | 0.70 |
| Squamous cell carcinoma | 55 (12) | 4 (15) | 13 (9) | 38 (13) | |
| Adenosquamous carcinoma | 4 (1) | 0 (0) | 3 (2) | 2 (1) | |
| Anatomic location | |||||
| Upper third (20-25 cm) | 6 (1) | 0 (0) | 1 (1) | 5 (2) | 0.75 |
| Middle third (26-30 cm) | 26 (6) | 1 (4) | 7 (5) | 18 (6) | |
| Lower third (31-40 cm) | 265 (58) | 18 (67) | 74 (53) | 173 (60) | |
| GE junction | 157 (35) | 8 (29) | 57 (41) | 92 (32) | |
| Clinical T stage (EUS) | |||||
| uT1 | 20 (5) | 2 (9) | 3 (2) | 15 (6) | 0.27 |
| uT2 | 45 (11) | 2 (9) | 6 (5) | 37 (15) | |
| uT3 | 289 (72) | 14 (61) | 95 (77) | 180 (70) | |
| uT4 | 48 (12) | 5 (21) | 19 (16) | 24 (9) | |
| Clinical N stage (EUS) | |||||
| uN0 | 101 (25) | 7 (30) | 26 (21) | 68 (27) | 0.51 |
| uN1 | 297 (75) | 16 (70) | 97 (79) | 184 (73) | |
| Location of neoadjuvant radiation | |||||
| Moffitt | 160 (36) | 8 (30) | 53 (39) | 99 (35) | 0.41 |
| Referring institution | 289 (64) | 19 (70) | 84 (61) | 186 (65) | |
| Radiation technique | |||||
| 2D | 4 (2) | 1 (10) | 1 (2) | 2 (1) | 0.10 |
| 3D | 65 (32) | 1 (10) | 20 (36) | 44 (32) | |
| IMRT | 135 (66) | 8 (80) | 34 (62) | 93 (67) | |
| Radiation dose | |||||
| <50.4 Gy | 111 (30) | 7 (35) | 35 (31) | 68 (28) | 0.80 |
| 50.4Gy | 172 (46) | 9 (45) | 57 (50) | 103 (43) | |
| >50.4 Gy | 92 (24) | 4 (20) | 22 (19) | 66 (28) | |
| PET response | |||||
| Yes | 124 (78) | 7 (78) | 31 (69) | 86 (83) | 0.71 |
| No | 34 (22) | 2 (22) | 14 (31) | 18 (17) | |
| Type of operation | |||||
| Ivor-Lewis | 284 (63) | 16 (62) | 86 (61) | 182 (63) | 0.61 |
| Laparoscopic Ivor-Lewis | 93 (20) | 7 (27) | 24 (17) | 62 (21) | |
| Transhiatal | 29 (6) | 0 (0) | 9 (7) | 20 (7) | |
| Laparoscopic transhiatal | 40 (9) | 3 (12) | 18 (13) | 19 (7) | |
| Three-field | 9 (2) | 0 (0) | 3 (2) | 6 (2) | |
| Grade | |||||
| Well/moderate differentiation | 163 (40) | 10 (40) | 49 (40) | 104 (40) | 0.85 |
| Poor differentiation | 119 (29) | 7 (28) | 47 (38) | 65 (25) | |
| No tumor present | 129 (31) | 8 (32) | 29 (22) | 92 (35) | |
| Lymphvascular invasion | |||||
| Yes | 79 (20) | 7 (29) | 33 (26) | 40 (16) | 0.83 |
| No | 316 (80) | 17 (71) | 95 (74) | 204 (74) | |
| Perineural invasion | |||||
| Yes | 67 (17) | 5 (22) | 26 (20) | 36 (15) | 1.0 |
| No | 327 (83) | 18 (78) | 104 (80) | 205 (85) | |
| Positive margin | |||||
| Yes | 19 (4) | 3 (11) | 9 (6) | 7 (2) | 0.09 |
| No | 435 (96) | 23 (89) | 131 (94) | 281 (98) | |
| Final tumor stage | |||||
| T0/Tis | 195 (43) | 9 (33) | 45 (32) | 141 (49) | 0.81 |
| T1 | 62 (14) | 5 (19) | 16 (11) | 41 (14) | |
| T2 | 80 (18) | 6 (22) | 29 (21) | 45 (16) | |
| T3 | 111 (24) | 6 (22) | 47 (34) | 58 (20) | |
| T4 | 7 (1) | 1 (4) | 3 (2) | 3 (1) | |
| Final nodal stage | |||||
| N0 | 311 (69) | 16 (59) | 74 (53) | 221 (77) | 0.86 |
| N1 | 118 (26) | 10 (37) | 54 (39) | 54 (19) | |
| N2 | 18 (4) | 1 (4) | 7 (5) | 10 (3) | |
| N3/4 | 6 (1) | 0 (0) | 4 (3) | 2 (1) | |
| Lymph node ratio | |||||
| ≤0.2 | 366 (81) | 19 (70) | 101 (72) | 246 (88) | 0.58 |
| 0.2 to 0.5 | 38 (8) | 4 (15) | 15 (11) | 19 (7) | |
| ≥0.5 | 38 (8) | 2 (7.5) | 20 (14) | 16 (5) | |
| Pathologic response | |||||
| Complete | 181 (40) | 8 (29) | 39 (28) | 134 (49) | 0.87 |
| Partial | 172 (37) | 11 (41) | 62 (44) | 99 (36) | |
| No response | 72 (16) | 4 (15) | 28 (20) | 40 (15) | |
| Any complication | |||||
| Yes | 272 (60) | 22 (82) | 77 (55) | 173 (60) | 0.02 |
| No | 181 (40) | 5 (18) | 63 (45) | 113 (40) | |
| Anastomotic leak | |||||
| Yes | 22 (5) | 3 (11) | 4 (3) | 15 (5) | 0.09 |
| No | 425 (95) | 24 (89) | 134 (97) | 267 (95) | |
| Adjuvant chemotherapy | |||||
| Yes | 64 (16) | 2 (8) | 30 (23) | 32 (12) | 0.17 |
| No | 347 (84) | 22 (92) | 99 (77) | 226 (88) |
Patients were stratified by pattern of disease recurrence: LRR includes patients with isolated locoregional recurrence and DR includes any patient with a distant recurrence.
Median follow-up in surviving patients was 50.1 months. At the time of last follow-up, 169 patients (37%) were still alive, 16 of who were alive with known recurrent disease (Figure 1). In contrast, 192 patients (42%) had died of disease and 95 patients (21%) had died of other or unknown causes. Details relating to known recurrence were available in 167 patients. The patterns and specific sites of recurrence are summarized in Figure 2. Among all patients who received nCRT, local recurrence, regional recurrence and DR occurred in 19 (4%), 56 (12%), and 140 (31%) patients, respectively. The most common sites of DR were lung, liver and bone, followed by non-regional lymph nodes, brain and peritoneum. Metastases at more than one site were common and occurred in 68 patients. Solitary LRR occurred in 27 patients (6%) while the remaining 42 patients with LRR also developed DR. As shown in Table 1, the only significant clinicopathologic feature differing between the LRR and DR groups was the rate of post-operative complications, which was more frequent in the solitary LRR group. The rate of anastomotic leak was also higher in the solitary LRR group (11%) compared to any patient who developed DR (3%) but the difference was not statistically significant (P = 0.09). Also noteworthy, patients who had a complete pathologic response to nCRT (n = 181) had fewer locoregional failures (n = 19, 11%) compared to patients with partial or no response (50 out of 244, 20%, P < 0.001).
FIGURE 2.
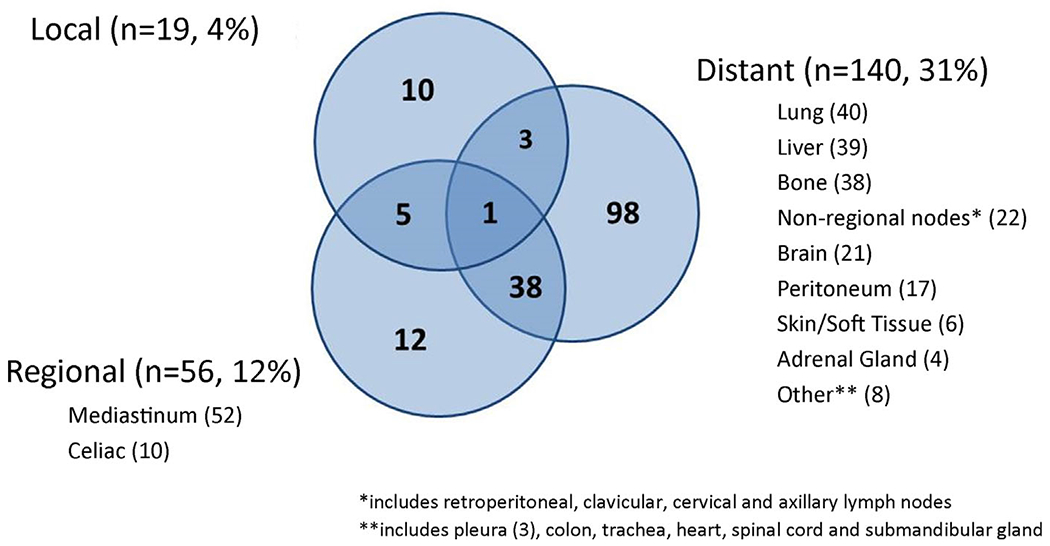
A summary of the patterns of disease recurrence (local, regional, and distant) and specific anatomic sites of recurrence in 167 out of 456 patients with esophageal cancer treated with neoadjuvant chemoradiation followed by esophagectomy
3.2 |. Timing of disease recurrence
The probability of recurrence over time in patients with solitary LRR or any DR is shown Table 2 and Figure 3. Ninety percent of all recurrences occurred within 36 months of surgery, 73% occurred within 18 months. The median time to recurrence in all patients with known recurrence was 10.6 months (range 1.2-101 months) and did not differ based on pattern of recurrence (LRR, 13.6 vs DR, 10.4 months; P = 0.27). The longest disease free interval to LRR and DR was 72.0 and 101 months, respectively. Treatment for recurrent disease was most often received at referring institutions, thus the specific therapies given were unknown in some patients. Among the patients with available follow-up data, only 13 patients underwent curative intent surgical resection for recurrent disease. Chemotherapy and additional radiation were given to 68 and 28 patients, respectively.
TABLE 2.
The probability of solitary locoregional recurrence (LRR) or distant recurrence (DR) at 1, 3, 5, and 10 years in patients with locally advanced esophageal cancer treated with neoadjuvant multimodality therapy
| LRR, N = 27 | DR, N = 140 | |
|---|---|---|
| Probability of recurrence | ||
| 1-year | 3% | 18% |
| 3-year | 6% | 27% |
| 5-year | 6% | 31% |
| 10-year | 7% | 32% |
| Median time to death after recurrence (months) | 8.4 | 6.1 |
FIGURE 3.
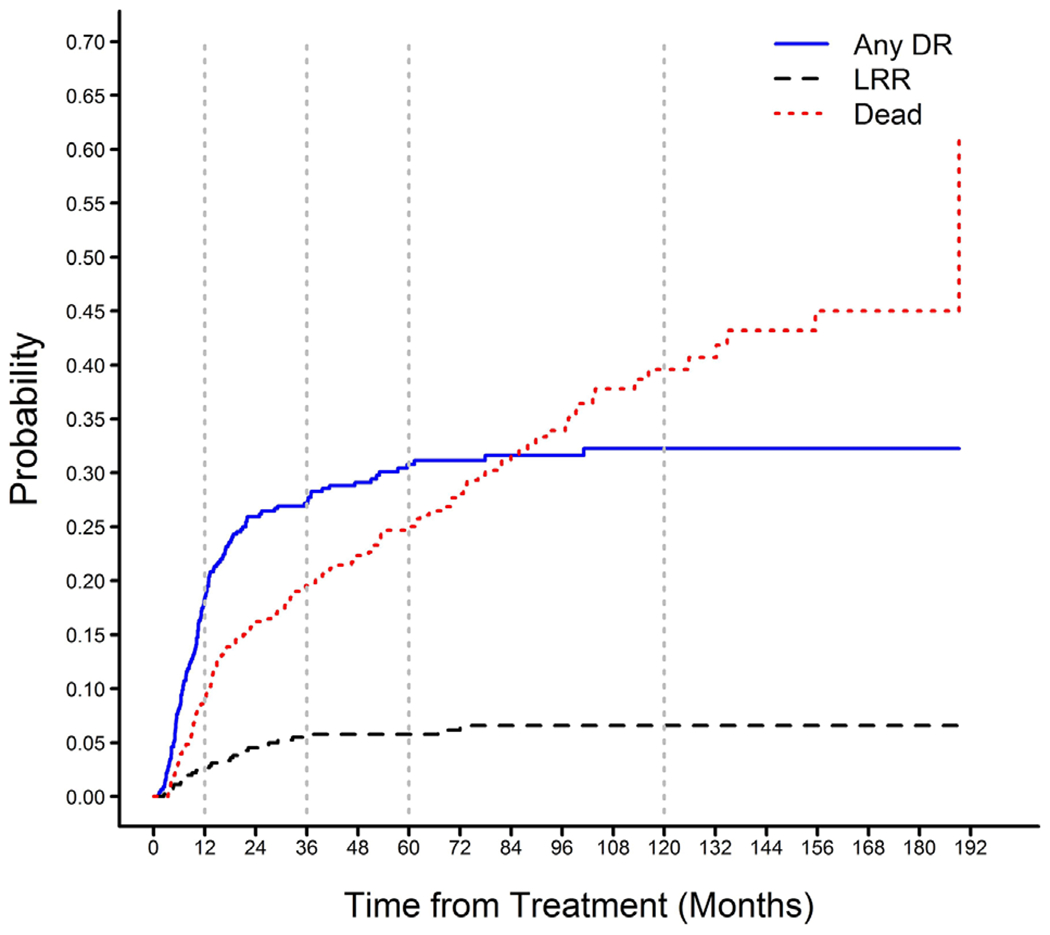
Competing risks curves for survival in patients with locally advanced esophageal cancer treated with neoadjuvant chemoradiation and esophagectomy stratified pattern of recurrence
Patients who died after solitary LRR survived a median of 8.4 months compared to 6.1 months for patients who died after any DR (P = 0.15). Median OS in patients with solitary LRR was not statistically different compared to patients with any DR (29.3 vs 19.1 months, P = 0.12).
3.3 |. Associated factors of locoregional recurrence
Factors associated with LRR on univariable analysis are shown in Table 3 and include: the presence of lymphovascular invasion, high lymph node ratio, positive margin, clinical and final T stage, and response to neoadjuvant treatment. Interestingly, many treatment-related factors were found not to be associated with locoregional failure including type of surgery (Ivor-Lewis vs transhiatal), lymph node harvest, radiation dose, the use of IMRT and the use of adjuvant chemotherapy. On multivariable analysis, only clinical T stage and response to neoadjuvant therapy were independently associated with LRR.
TABLE 3.
Univariate and multivariate analysis of factors associated with locoregional recurrence in patients with locally advanced esophageal cancer treated with neoadjuvant chemoradiation followed by esophagectomy
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio (95%CI) | P-value | Hazard ratio (95%CI) | P-value | |
| Lymphovascular invasion | 1.46 (0.97, 2.21) | 0.07 | ||
| Perineural invasion | 1.27 (0.82, 1.96) | 0.29 | ||
| Lymph node ratio (reference ≤0.2) | ||||
| 0.2 to 0.5 | 1.69 (0.97, 2.96) | 0.06 | ||
| >0.5 | 2.16 (1.32, 3.54) | 0.02 | ||
| Final T stage (reference T0) | ||||
| T1/2 | 1.50 (0.99, 2.28) | 0.06 | ||
| T3/4 | 2.06 (1.36, 3.11) | <0.01 | ||
| Positive margin | 1.95 (1.00,3.80) | 0.05 | 1.52 (0.75, 3.01) | 0.25 |
| Treatment response (reference complete response) | ||||
| Partial response | 1.80 (1.20, 2.70) | <0.01 | 1.69 (1.10, 2.59) | 0.02 |
| No response | 1.99 (1.21, 3.27) | <0.01 | 2.00 (1.19, 3.33) | <0.01 |
| Clinical uT stage (reference uT1/2) | ||||
| uT3/4 | 2.62 (1.33, 5.16) | <0.01 | 2.43 (1.24, 4.78) | <0.01 |
4 |. DISCUSSION
In the well-matched, randomized controlled CROSS trial,5 the addition of nCRT to surgical resection for locally advanced esophageal cancer was associated with an improved R0 resection rate (92% vs 69%, P < 0.001), a lower rate of metastatic lymph nodes found on final pathology (31% vs 75%, P < 0.001) and a complete pathologic response rate of 29%. This improved locoregional control seen with trimodality therapy led to a significantly longer median DFS and a doubling of the median OS (49.4 vs 24.0 months, P = 0.003). Despite the favorable locoregional efficacy of nCRT, 14.1% of patients experienced locoregional failures (compared to 33.5% of patients treated with surgery alone)13 which closely resembles the incidence of LRR (15%) in our series.
One of the most important findings highlighted in our analysis, and which is demonstrated by others as well,13,25 is that while 15% of patients developed LRR, most locoregional failures occurred in the setting of synchronous DR. In fact, relatively few patients in our series (6%) developed solitary LRR. OS in patients with combined LRR and DR was most similar to patients who develop DR without LRR suggesting that, as expected, survival in these patients is most dependent on distant organ involvement. At the same time, survival from the time of recurrence was not significantly different in patients with solitary LRR (8.4 months) compared to patients with any distant failure (6.1 months).
The observation that outcomes in patients with isolated LRR are no better than patients with DR was unexpected but could be explained by the significant morbidity of locoregional failure. These patients develop progressive dysphagia leading to weight loss, malnutrition, and declining performance status which can rapidly and significantly compromise the patient’s ability to receive further treatment. Furthermore, as our results imply, aggressive tumor biology is a significant contributing factor to LRR which may also explain the rapid deterioration and lack of effective treatments seen in patients with isolated locoregional disease.
Our analysis also offers important implications in terms of surveillance. First, isolated local failure is a rare event occurring in only 4% of our patients and between 2% and 3% in other series,4,13 providing further evidence that routine surveillance endoscopy is not necessary as outlined in the most recent NCCN guidelines.26 Second, almost 75% of patients who developed recurrence did so within 18 months and 90% of patients recurred within the first 3 years. This pattern of early disease recurrence has been demonstrated by others as well.4,27 However, the longest DFI in our cohort was 101 months before the patient developed lung metastases. These observations would suggest the importance of more frequent imaging surveillance during the first 3 years and the need to follow the patient well beyond 5 years. On the other hand, patient outcomes are dismal following recurrence from esophageal cancer because salvage resection for isolated disease is rarely possible and systemic strategies are largely ineffective.4,18,19 Therefore, until more effective salvage treatments are available, identifying recurrent disease earlier may not have a significant impact on long-term outcomes.
Despite these discouraging findings, efforts should continue to find ways to reduce the rate of LRR in patients treated with nCRT and surgery. Few authors have sought to identify clinicopathologic features associated with LRR. A small study28 reported no locoregional recurrences in 40 patients treated with nCRT and en bloc esophagectomy compared to 17% in 18 patients treated with nCRT and transhiatal esophagectomy, suggesting that more radical resection could reduce the rate of LRR. This finding was not confirmed in our study; however, only 2% of our patients were treated with a three-field or en bloc esophagectomy. In another study, Shaikh et al29 found that fewer lymph nodes assessed correlated to a higher rate of regional failure and reported that locoregional RFS was longer in patients who had >13 lymph nodes evaluated compared to patients with ≤13 nodes. In contrast, lymph node harvest was not found to be associated with LRR in our patients. As noted, lymph node harvest was higher in our patients treated after 2008. We believe this is primarily the results of more focused pathologic evaluation rather than a difference in surgical technique and, as such, would not be expected to independently influence LRR. However, we did find lower lymph node ratios were associated with fewer LRRs on univariable analysis and, in general, higher lymph node harvests will yield lower lymph node ratios. Taken together, these observations suggest that an appropriate lymphadenectomy could be essential, not only for accurate staging, but also in optimizing locoregional control.
We expected to find that positive margins would correlate to higher rates of LRR. A positive margin on final pathology was overall uncommon in our series (4%). Although, among the 69 patients who developed LRR, 8 (12%) had a positive proximal or radial margin and 4 patients out of 19 with LR (21%) had a positive margin. Despite these observations, a positive margin was not an independent risk factor for LRR on multivariable analysis. It is our standard practice to perform a wide lymphadenectomy to clear the radial margin and to obtain frozen sections of the proximal esophageal margin. If the frozen sections are positive for invasive disease or high grade dysplasia, an additional margin is obtained prior to performing the esophageal anastomosis. Despite these efforts, frozen sections can be falsely negative and, in rare cases, the tumor extent is submucosal and may not allow for proximal or radial clearance. Further analysis is required to evaluate the clinical significance of a positive margin in terms of LRR.
Another significant finding in our analysis is the lower LRR rate among patients who achieved a pathologic complete response compared to non-complete responders (11% vs 20%, P < 0.001). Other reports17,30 have also shown lower recurrence rates and improved survival in patients who achieve a complete pathologic response following nCRT and surgery. Meguid et al30 reported a median OS of 79 months in patients who achieved a complete pathologic response compared to 31 months and 19 months in partial and non-responders, respectively (P < 0.001) and found fewer overall recurrences in complete responders (22% vs 36% and 35%, P = 0.055). However, the rate of LRR was no different between these three groups (27%, 21%, and 22%, P = 0.829). The difference in LRR rates among complete responders in Meguid’s study (27%) compared to our cohort (11%) may be explained, in part, by the higher doses of radiation used in our patients, leading to a higher incidence of complete pathologic responses (31% vs 40%). While radiation dose, modality of radiation, and chemotherapy regimen were not associated with LRR in our series, such adjustments in neoadjuvant regimens to increase the incidence of complete pathologic responses may have an indirect, beneficial effect on the rate of locoregional failure.
The specific role of radiation in the incidence of LRR has received attention in several recent reports. Van Daele et al31 reported a higher rate of regional recurrence in 109 patients treated with 36 Gy compared to 38 patients treated with 41-50 Gy (28% vs 3%, P < 0.001). In our series, only nine patients received a radiation dose of <40 Gy and our median radiation dose was 50.4 Gy which could explain why we did not see a difference in LRR rates based on radiation dose. From the CROSS trial data, Oppendijk et al13 analyzed the relationship between LRR and radiation treatment fields and found that LRR developed at similar rates both in (5.2%) and outside (6.1%) the treated radiation fields. Likewise, Dorth et al25 analyzed LRR by comparing the specific location of recurrence on imaging with the radiation treatment fields and determined that 95% of nodal failures (including para-aortic) occurred outside or near the primary radiation fields. Yet, similar to our findings, most patients developed DR at the same time which led them to conclude that targeting additional nodal basins with nCRT would offer limited clinical benefit.
Many factors which could be optimized by oncology providers to potentially reduce the incidence of locoregional failures (radiation regimen, chemotherapy agent, type of surgery, and the use of adjuvant therapy) were not associated with LRR in our analysis. At the same time, factors which were associated with LRR in our analysis suggest that aggressive tumor biology (high lymph node ratios, the presence of lymphovascular invasion, poor treatment response, and advanced pathologic stage) plays a significant role in the incidence of LRR. Koshy et al also highlighted the role of tumor biology in these patients by reporting that longer tumor length, macroscopic residual disease, and adenocarcinoma histology correlated with worse LR control. These observations, taken together with the low incidence of solitary locoregional failure, suggest that further improvements in long-term patient survival will require new treatment strategies specifically targeting systemic control.
Our study is limited by its retrospective nature and its relatively long study period of 17 years. Admittedly, variation in practice habits evolve over time and standard treatment regimens were much different at the beginning compared to the end of the study period. However, it is precisely the relative heterogeneity in our series which permitted the identification of factors associated with locoregional failure. Additional studies evaluating LRR following current treatment protocols could provide additional understanding to recurrence patterns and associated risk factors. Another significant limitation is complete follow-up was not available in all patients. As a tertiary referral center, many of our patients returned to their local oncologist for surveillance. While great effort is made to monitor these patients’ progress, some were ultimately lost to follow-up or specific details regarding recurrence or subsequent treatments were not available.
5 |. CONCLUSION
In conclusion, despite aggressive multimodality neoadjuvant treatment and surgical resection in patients with locally advanced esophageal cancer, the most common site of relapse was regional lymph nodes. Although many factors associated with LRR suggest that locoregional failure is a function of aggressive tumor biology, administering more effective nCRT regimens to increase rates of complete pathologic responses may allow for more optimized locoregional control. However, given the high rate of multifocal LRR and DR, further improvements in locoregional control are not likely to alter the natural history of patients with recurrent esophageal cancer. Novel locoregional approaches and/or new systemic regimens are needed to further reduce recurrence rates and provide suitable salvage options.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies AR, Pillai A, Sinha P, et al. Factors associated with early recurrence and death after esophagectomy for cancer. J Surg Oncol. 2014;109:459–464. [DOI] [PubMed] [Google Scholar]
- 4.Sudo K, Taketa T, Correa AM, et al. Locoregional failure rate after preoperative chemoradiation of esophageal adenocarcinoma and the outcomes of salvage strategies. J Clin Oncol. 2013;31:4306–U4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof M, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 6.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro J, Van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 9.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. [DOI] [PubMed] [Google Scholar]
- 10.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. [DOI] [PubMed] [Google Scholar]
- 11.Abate E, DeMeester SR, Zehetner J, et al. Recurrence after esophagectomy for adenocarcinoma: defining optimal follow-up intervals and testing. J Am Coll Surg. 2010;210:428–435. [DOI] [PubMed] [Google Scholar]
- 12.Smit JK, Pultrum BB, van Dullemen HM, et al. Prognostic factors and patterns of recurrence in esophageal cancer assert arguments for extended two-field transthoracic esophagectomy. Am J Surg. 2010;200:446–453. [DOI] [PubMed] [Google Scholar]
- 13.Oppedijk V, van der Gaast A, van Lanschot JJB, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385–391. [DOI] [PubMed] [Google Scholar]
- 14.Almhanna K, Hoffe S, Strosberg J, Dinwoodie W, Meredith K, Shridhar R. Concurrent chemoradiotherapy with protracted infusion of 5-fluorouracil (5-FU) and cisplatin for locally advanced resectable esophageal cancer. J Gastrointest Oncol. 2015;6:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasini F, de Manzoni G, Zanoni A, et al. Neoadjuvant therapy with weekly docetaxel and cisplatin, 5-fluorouracil continuous infusion, and concurrent radiotherapy in patients with locally advanced esophageal cancer produced a high percentage of long-lasting pathological complete response A phase 2 study. Cancer. 2013;119:939–945. [DOI] [PubMed] [Google Scholar]
- 16.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. [DOI] [PubMed] [Google Scholar]
- 17.van Hagen P, Wijnhoven BPL, Nafteux P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg. 2013;100:267–273. [DOI] [PubMed] [Google Scholar]
- 18.Ichida H, Imamura H, Yoshimoto J, et al. Pattern of postoperative recurrence and hepatic and/or pulmonary resection for liver and/or lung metastases from esophageal carcinoma. World J Surg. 2013;37:398–407. [DOI] [PubMed] [Google Scholar]
- 19.Parry K, Visser E, van Rossum PSN, Mohammad NH, Ruurda JP, van Hillegersberg R. Prognosis and treatment after diagnosis of recurrent esophageal carcinoma following esophagectomy with curative intent. Ann Surg Oncol. 2015;22:S1292–S1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice TW, Blackstone EH, Rusch VW. 7th Edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. [DOI] [PubMed] [Google Scholar]
- 21.Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17:1159–1167. [DOI] [PubMed] [Google Scholar]
- 22.Wu AJ, Bosch WR, Chang DT, et al. Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer. Int J Radiat Oncol Biol Phys. 2015;92:911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg. 2007;11:1384–1393. [DOI] [PubMed] [Google Scholar]
- 24.Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–554. [DOI] [PubMed] [Google Scholar]
- 25.Dorth JA, Pura JA, Palta M, et al. Patterns of recurrence after trimodality therapy for esophageal cancer. Cancer. 2014;120:2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancer (Version 1.2017). Available online at: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- 27.Lou FR, Sima CS, Adusumilli PS, et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol. 2013;8:1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzetto C, DeMeester SR, Hagen JA, Peyre CG, Lipham JC, DeMeester TR. En bloc esophagectomy reduces local recurrence and improves survival compared with transhiatal resection after neoadjuvant therapy for esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2008;135:1228–1236. [DOI] [PubMed] [Google Scholar]
- 29.Shaikh T, Zaki MA, Dominello MM, et al. Patterns and predictors of failure following tri-modality therapy for locally advanced esophageal cancer. Acta Oncol. 2016;55:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meguid RA, Hooker CM, Taylor JT, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. 2009;138: 1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Daele E, Ceelen W, Boterberg T, et al. Effect of neoadjuvant radiation dose on surgical and oncological outcome in locally advanced esophageal cancer. Acta Chir Belg. 2015;115:8–14. [PubMed] [Google Scholar]


