Abstract
Background
Evofosfamide is a hypoxia-activated prodrug of bromo-isophosphoramide mustard. We aimed to assess the benefit of adding evofosfamide to doxorubicin as first-line therapy for advanced soft-tissue sarcomas.
Methods
We did this international, open-label, randomised, phase 3, multicentre trial (TH CR-406/SARC021) at 81 academic or community investigational sites in 13 countries. Eligible patients were aged 15 years or older with a diagnosis of an advanced unresectable or metastatic soft-tissue sarcoma, of intermediate or high grade, for which no standard curative therapy was available, an Eastern Cooperative Oncology Group performance status of 0–1, and measurable disease by Response Evaluation Criteria in Solid Tumors version 1.1. Patients were randomly assigned (1:1) to receive doxorubicin alone (75 mg/m2 via bolus injection administered over 5–20 min or continuous intravenous infusion for 6–96 h on day 1 of every 21-day cycle for up to six cycles) or doxorubicin (given via the same dose procedure) plus evofosfamide (300 mg/m2 intravenously for 30–60 min on days 1 and 8 of every 21-day cycle for up to six cycles). After six cycles of treatment, patients in the single-drug doxorubicin group were followed up expectantly whereas patients with stable or responsive disease in the combination group were allowed to continue with evofosfamide monotherapy until documented disease progression. A web-based central randomisation with block sizes of two and four was stratified by extent of disease, doxorubicin administration method, and previous systemic therapy. Patients and investigators were not masked to treatment assignment. The primary endpoint was overall survival, analysed in the intention-to-treat population. Safety analyses were done in all patients who received any amount of study drug. This study was registered with ClinicalTrials.gov, number NCT01440088.
Findings
Between Sept 26, 2011, and Jan 22, 2014, 640 patients were enrolled and randomly assigned to a treatment group (317 to doxorubicin plus evofosfamide and 323 to doxorubicin alone), all of whom were included in the intention-to-treat analysis. The overall survival endpoint was not reached (hazard ratio 1·06, 95% CI 0·88–1·29; p=0·527), with a median overall survival of 18·4 months (95% CI 15·6–22·1) with doxorubicin plus evofosfamide versus 19·0 months (16·2–22·4) with doxorubicin alone. The most common grade 3 or worse adverse events in both groups were haematological, including anaemia (150 [48%] of 313 patients in the doxorubicin plus evofosfamide group vs 65 [21%] of 308 in the doxorubicin group), neutropenia (47 [15%] vs 92 [30%]), febrile neutropenia (57 [18%] vs 34 [11%]), leucopenia (22 [7%] vs 17 [6%]), decreased neutrophil count (31 [10%] vs 41 [13%]), and decreased white blood cell count (39 [13%] vs 33 [11%]). Grade 3–4 thrombocytopenia was more common in the combination group (45 [14%]) than in the doxorubicin alone group (four [1%]), as was grade 3–4 stomatitis (26 [8%] vs seven [2%]). Serious adverse events were reported in 145 (46%) of 313 patients in the combination group and 99 (32%) of 308 in the doxorubicin alone group. Five (2%) patients died from treatment-related causes in the combination group (sepsis [n=2], septic shock [n=1], congestive cardiac failure [n=1], and unknown cause [n=1]) versus one (<1%) patient in the doxorubicin alone group (lactic acidosis [n=1]).
Interpretation
The addition of evofosfamide to doxorubicin as first-line therapy did not improve overall survival compared with single-drug doxorubicin in patients with locally advanced, unresectable, or metastatic soft-tissue sarcomas and so this combination cannot be recommended in this setting.
Funding
Threshold Pharmaceuticals.
Introduction
Soft-tissue sarcomas comprise a diverse group of rare malignancies of mesenchymal or neural origin. 2-year overall survival for patients with newly diagnosed metastatic disease is around 20–30% when standard cytotoxic chemotherapy drugs such as doxorubicin or the combination of gemcitabine plus docetaxel are used.1–3 Doxorubicin remains a standard of treatment since its initial application in sarcoma in the 1970s.4 Median overall survival for patients with metastatic sarcoma who receive doxorubicin-based first-line therapy in randomised phase 3 studies ranges from 12 months to 17 months.1–3,5 More recent clinical trial data published in the past 3 years support incremental improvements in survival outcomes with the use of single-drug doxorubicin.1–3 This finding most likely reflects the complex nature of overall survival when used as an outcomes measure for first-line therapy, an improved biological understanding of the various sarcoma subtypes, additional options for the second and subsequent lines of therapy for individual sarcoma subtypes, and improvements in multidisciplinary and supportive care. Nonetheless, these outcomes are still suboptimum and highlight the need for new and innovative therapies for patients with advanced disease.
Evofosfamide is a nitroimidazole prodrug of the alkylating cytotoxin bromo-isophosphoramide mustard. Evofosfamide is reduced at the nitroimidazole site, and preferentially, under hypoxic conditions, releases bromo-isophosphoramide mustard, which can then function as a DNA cross-linking agent inducing intra-strand and inter-strand cross-links.6 Tumour hypoxia is a result of the disordered vasculature found in solid tumours and represents a compelling target for anticancer intervention. Few normal cells reach severe hypoxia. By contrast, tumours often consist of substantial areas of highly hypoxic cells that are known to be resistant to chemotherapy and radiotherapy.7–9 Evofos famide was designed to become activated in and selectively target hypoxic cells for presumed killing of this highly resistant cell population and to avoid standard ifosfamide-based haematological, renal, bladder, and CNS toxicities.
Encouraging results were reported with the combination of doxorubicin plus evofosfamide in a single-arm phase 2 study in 91 patients with advanced soft-tissue sarcomas in the first-line setting.10 In this study, evofosfamide (300 mg/m2) was given intravenously on days 1 and 8 in combination with doxorubicin (75 mg/m2) on day 1 of a 21-day cycle. After six cycles of treatment, patients with stable or responsive disease were allowed to continue with evofosfamide monotherapy until documented disease progression. Median overall survival was 21·5 months (95% CI 16·0–26·2), median progression-free survival was 6·5 months (5·8–7·7), and 32 (36%) of 89 patients had a radiographic response according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.0). Overall, the combination was well tolerated and without substantial renal, bladder, hepatic, or cardiac toxicity.
On the basis of these phase 2 trial result, we aimed to assess the efficacy of doxorubicin plus evofosfamide versus doxorubicin alone in a phase 3 trial in the first-line setting for patients with locally advanced unresectable or metastatic soft-tissue sarcomas.
Methods
Study design and participants
This international, open-label, randomised, phase 3 multicentre study was done at 81 academic or community investigational sites in the USA, Austria, Belgium, Canada, Denmark, France, Germany, Hungary, Israel, Italy, Poland, Russia, and Spain (appendix pp 38–39). Most patients were recruited through referral to major sarcoma centres. Eligible patients were 15 years or older with a diagnosis of an advanced unresectable or metastatic soft-tissue sarcoma, of intermediate or high grade, for which no standard curative therapy was available (appendix p 2). Patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, a life expectancy of at least 3 months, measurable disease according to RECIST version 1.1, and adequate end-organ and haemopoietic function. Patients were excluded if they had received previous systemic therapy for advanced or metastatic disease (neoadjuvant therapy followed by surgical resection and adjuvant therapy was permitted); had received previous therapy with ifosfamide, cyclophosphamide, another nitrogen mustard, or another hypoxic cytotoxin; had received previous systemic therapy with an anthracycline or anthracenedione, or previous mediastinal or cardiac radiotherapy; had a low-grade tumour according to standard grading systems (eg, American Joint Committee on Cancer grade 1 and 2 or Fédération Nationale des Centres de Lutte Contre le Cancer grade 1), significant cardiac dysfunction, severe chronic obstructive pulmonary disease, a known infection with HIV or active infection with hepatitis B or hepatitis C; had known brain metastases unless previously treated and well controlled for a period of 3 months or longer; or were pregnant or breastfeeding.
The protocol was approved by the institutional review boards or ethics committees, or both, at each participating site. Written informed consent was obtained for each patient. The study was done in accordance with the ethical principles that have their origins in the Declaration of Helsinki and with all appropriate national and local regulations and guidance, including the US Code of Federal Regulations on Good Clinical Practices and the International Conference on Harmonisation guideline E6: Good Clinical Practice.
Randomisation and masking
Patients were randomly assigned (1:1) to receive either single-drug doxorubicin or the combination of doxorubicin plus evofosfamide. Randomisation was stratified by extent of disease (locally advanced vs metastatic), doxorubicin administration schedule (bolus injection vs continuous infusion), and previous systemic therapy (previous adjuvant or neoadjuvant therapy vs no previous adjuvant or neoadjuvant therapy) to control for potential imbalance of factors that could have affected efficacy endpoints. The presence of liver metastases was not used as a stratification factor. We used a web-based central randomisation process with a randomisation schema generated by the clinical research organisation that allows sites to directly enrol patients into the study. The eight randomisation strata included block sizes of two and four. The blocks were filled on demand at time of randomisation. This was an open-label (non-placebo-controlled) trial; patients and investigators were not masked to the assigned treatment group.
Procedures
A schedule of study procedures and assessments is provided in the appendix (p 6). Doxorubicin was administered in both groups at 75 mg/m2 on day 1 of every 21-day cycle by either a bolus injection (no less than 5 min, but generally less than 20 min) or continuous intravenous infusion for 6–96 h for up to six cycles. The administration method for doxorubicin was decided on the basis of investigator discretion. Evofosfamide was administered intravenously at 300 mg/m2 for 30–60 min on day 1 and day 8 of every 21-day cycle. In the combination group, doxorubicin was administered 2–4 h after the completion of evofosfamide administration. After six cycles, patients in the single-drug doxorubicin group were followed up expectantly (ie, those patients who were not given any additional off-study anticancer therapy were monitored by serial scans for disease progression until initiation of anticancer treatment) whereas patients with stable or responsive disease in the combination group were allowed to continue with evofosfamide monotherapy until documented disease progression. The administration of a cardioprotectant (eg, dexrazoxane) was optional and left to the investigator’s discretion. Growth factor support (filgrastim or pegfilgrastim) was used prophylactically in the combination group and recommended in the doxorubicin group before initiation of dose reductions for neutropenia.
Dose modification rules were followed for all haematological and renal toxicities irrespective of causality, and for any other toxicity that was not clearly related to disease progression, intercurrent illness, concomitant drugs, or other non-drug intervention (appendix pp 4–5). Dose level reductions were allowed for both doxorubicin and evofosfamide, for both haematological and non-haematological toxicities (appendix pp 4–5). If a patient required more than two dose level reductions for toxicity, they were discontinued from the study. Any patient who missed more than one cycle of treatment (>3 weeks of day 1 of a cycle) for treatment-related toxicity was also discontinued from the study.
Tumour response was assessed using RECIST version 1.111 to determine objective tumour response, stable disease, duration of response, and progression-free survival. Tumour assessments were done at the end of cycles 2, 4, and 6; at the end of every third cycle for patients continuing on cycle 7 to cycle 12; and at the end of every third to fifth cycle thereafter. Tumour assessments were done at baseline until documentation of disease progression, initiation of other anticancer therapy, or death.
An electrocardiogram (ECG) along with an assessment of left ventricular ejection fraction, using either a multigated acquisition scan or echocardiogram was done at baseline, after completion of four cycles, and at the termination of doxorubicin treatment. Adverse events were collected from the start of the first dose of study drug until 30 days after discontinuation of the study drugs. Clinical laboratory toxicities and adverse events were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Pharmacokinetic samples for plasma evofosfamide and bromo-isophosphoramide mustard were collected on day 1 of cycle 1 for patients who received evofosfamide. Samples for plasma doxorubicin and doxorubicinol concentrations were collected on days 1 and 2 of cycle 1 at selected sites that administered doxorubicin bolus injection. Collection of serum and plasma samples and archival tissue for testing of hypoxia biomarkers (eg, carbonic anhydrase 9, neuron-specific enolase, osteopontin, and VEGF) was optional for all patients (appendix p 10).
Archival tissue specimen and associated pathology reports were required for a retrospective independent central pathology review done under a prespecified charter (appendix pp 35–37).
Patients were free to discontinue (withdraw) at any time during this clinical trial. If a patient withdrew from participation in the study during the treatment period, he or she was encouraged to return for an early termination visit for assessment of safety. The investigator also had the right to discontinue any patient from study drug administration or study participation. Criteria for a patient to be removed from the study included study completion, disease progression, clinically significant deterioration of the patient’s condition, and loss to followup, among other reasons (appendix p 7).
Outcomes
The primary efficacy outcome was overall survival, defined as the duration from date of randomisation to the date of death from any cause. Secondary efficacy outcome measures were progression-free survival and the proportion of patients achieving an overall response. Progression-free survival was defined as time from randomisation to the first occurrence of progression of disease or death from any cause within 63 days of last response assessment or randomisation. Overall response included complete and partial responses as defined by RECIST version 1.1. The window of 63 days was selected because it was halfway between the time between protocol-specified tumour assessment (42 days) and twice that amount (84 days). We theorised that deaths within that window would not overestimate the time to progressive disease. Patients who discontinued the study without progression were asked to be followed up until disease progression or initiation of additional cancer therapy. The RECIST measurements were investigator assessed, so all tumour response-related outcome including progression-free survival were investigator assessed. Any divergence from RECIST defined criteria were queried at the sites to conform to RECIST guidelines. Radiographs were collected for a potential central read, but after unblinding the results, the central read was deemed not necessary.
Tertiary efficacy outcomes were tumour resectability, progression-free survival at 3 and 6 months, duration of response, disease control (the proportion of patients achieving an overall response of stable disease or better), overall survival at 6 and 12 months (investigator-assessed), change in ECOG performance status, and quality of life and health status as measured by EuroQol five dimensions questionnaire with five-level scale (EQ-5D-5L). Safety endpoints were the incidence and severity of adverse events, serious adverse events, discontinuations due to adverse events, deaths from adverse events, left ventricular ejection fraction function, changes in ECG including the QTc, changes in clinical laboratory tests (haematology, serum chemistry, and urinalysis), changes in vital signs, changes in physical examination findings, and changes in use of concomitant drugs. Exploratory endpoints included the association of serum and plasma hypoxia biomarkers with efficacy and safety endpoints.
Statistical analysis
Originally, the study was designed to detect a 4-month improvement in median overall survival, assuming a median survival of 10 months in the doxorubicin control group and 14 months in the doxorubicin plus evofosfamide investigational group (eg, a hazard ratio [HR] of 0·714).5,12–15 However, several clinical trials1,2 were reported after the study initiation that indicated the median overall survival for patients with sarcoma receiving single-drug doxorubicin in the first-line setting was likely to be longer than 10 months. We therefore modified the study design assumptions to postulate a 12-month median overall survival in the doxorubicin control group and a 16-month median overall survival in the doxorubicin plus evofosfamide group. This modification was made under protocol amendment 3 (April 22, 2013), which was approved by regulatory agencies, including the US Food and Drug Administration (FDA), and by site institutional review boards and ethics committees. To maintain the same type I and type II error rates with the change in HR from 0·714 to 0·750, we increased the sample size from 450 patients to 620 patients. A sample size of 620 patients (1:1 randomisation) had 85% power to detect a 33% improvement in overall survival using a one-sided a level of 2·5%. This study was done to support regulatory approval and followed the convention to use hypothesis testing statistical boundaries that are one-sided to show superiority, but that the reported outcome is two-sided based on multiplying the one-sided p value by two. We designed the study as such, but we believe specifying the actual one-sided boundary that was used for the study to be appropriate. The protocol was conducted under a Special Protocol Agreement with the FDA. Under this agreement, the FDA reviewed and approved these statistical changes.
An Independent Data Monitoring Committee (IDMC) was appointed to monitor safety and efficacy during the study (appendix p 9). The IDMC met quarterly to assess the study safety and to do interim analyses. An interim futility analysis based on progression-free survival was to be done by the IDMC after 113 progression-free survival events. The timing was originally projected to occur after around half of the study participants were enrolled. However, the progression-free survival events occurred at a slower rate than projected and the interim futility analysis was cancelled under protocol amendment 3. Because this interim futility analysis was intended to stop the study for insufficient efficacy, the type I error was not affected.
An interim efficacy analysis based on overall survival was planned to occur after 235 survival events. After a survival sweep before the interim analysis, a total of 256 events were included in the analysis. We used the Lan-DeMets implementation of the O’Brien-Fleming group sequential method to determine the stopping boundaries and a spending. A one-sided value of 0·0035 based on 256 events was needed to reach statistical significance. On the basis of the interim survival analysis of 256 actual events, we adjusted the significance level to a planned one-sided value of 0·0239 for the final treatment comparison of the primary efficacy parameter that was scheduled to occur after 434 events. We included all patients randomly assigned to a treatment group (the intention-to-treat population) in the efficacy analyses and all patients who received any amount of study drug in the safety analyses.
We used the Kaplan-Meier method to estimate survival. We calculated estimates and CIs using the product limit method and Greenwood’s formula for the variance. We used a stratified log-rank test with the randomisation strata to test for statistical significance of progression-free and overall survival. Unstratified tests, strata defined by the clinical database, and pooling across strata subsets were done as part of the sensitivity analyses. We also did sensitivity analyses varying the window to include deaths as progression-free survival events. All statistical analyses were done with SAS version 9.1.
This study is registered with ClinicalTrials.gov, number NCT01440088.
Role of the funding source
The study was designed by the sponsor, Threshold Pharmaceuticals, with input from sarcoma experts and members of the Sarcoma Alliance for Research through Collaboration (SARC). Data were collected and entered into the study database by site personnel; the sponsor participated in the data review and data cleaning. The data were analysed and reported as specified in the statistical analysis plan by Threshold Pharmaceuticals with collaboration from the academic authors and SARC investigators. The academic authors and SARC had full access to all the data and vouch for the accuracy of the data and the analyses presented in this manuscript. The corresponding author wrote the initial draft of the manuscript and all authors contributed to subsequent drafts. The sponsor did not have a role in writing the initial draft. The corresponding author had final responsibility for the decision to submit for publication.
Results
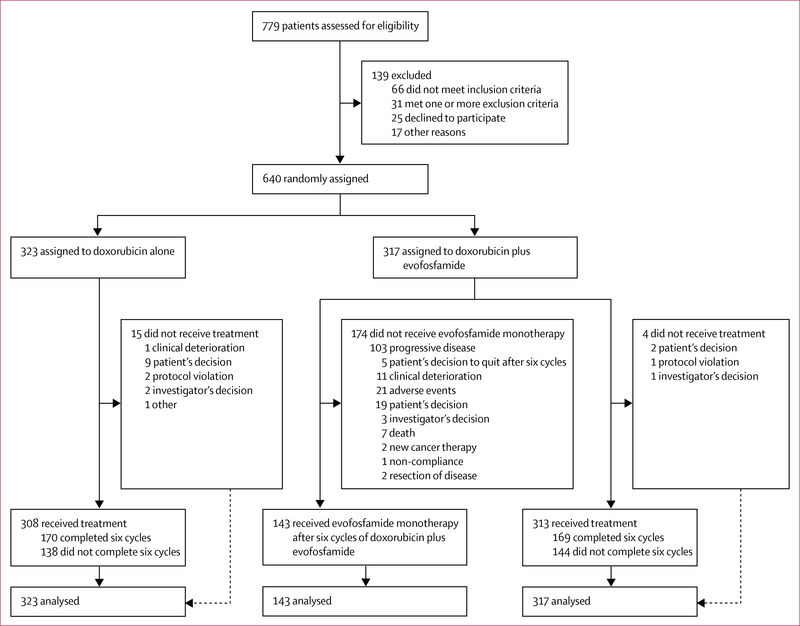
Between Sept 26, 2011, and Jan 22, 2014, we assessed 779 patients for eligibility and enrolled 640 eligible patients into the study (figure 1). 317 patients were randomly assigned to the doxorubicin plus evofosfamide group and 323 to the doxorubicin alone group. 19 patients who were randomly assigned did not receive treatment (four patients in the doxorubicin plus evofosfamide group and 15 in the doxorubicin alone group); however, these patients were still included in the intention-to-treat efficacy analyses. The safety analyses comprised the 621 patients who actually received study drug (313 in the doxorubicin plus evofosfamide group and 308 in the doxorubicin alone group). Overall, the two treatment groups were balanced with regard to demo graphics and disease characteristics (table 1). The median follow-up was 28·9 months (IQR 24·5–33·1). No patients were lost to follow-up in either treatment group.
Figure 1:
Trial profile
Table 1:
Baseline characteristics
| Doxorubicin alone (n=323) | Doxorubicin plus evofosfamide (n=317) | |
|---|---|---|
| Age (years) | 58 (49–66) | 60 (49–67) |
| <65 | 220 (68%) | 211 (67%) |
| ≥65 | 103 (32%) | 106 (33%) |
| Sex | ||
| Female | 172 (53%) | 173 (55%) |
| Male | 151 (47%) | 144 (45%) |
| Ethnicity | ||
| Hispanic or Latino | 15 (5%) | 18 (6%) |
| Not Hispanic or Latino | 308 (95%) | 299 (94%) |
| Race | ||
| White | 285 (88%) | 297 (94%) |
| Black | 16 (5%) | 11 (3%) |
| Asian | 12 (4%) | 5 (2%) |
| American Indian or Alaska Native | 3 (1%) | 0 |
| Other | 7 (2%) | 4 (1%) |
| ECOG performance status* | ||
| 0 | 184 (57%) | 181 (57%) |
| 1 | 137 (42%) | 133 (42%) |
| 2 | 1 (<1%) | 3 (1%) |
| Extent of disease | ||
| Locally advanced | 41 (13%) | 32 (10%) |
| Metastatic disease | 282 (87%) | 285 (90%) |
| Highest histological grade | ||
| Low grade | 1 (<1%) | 0 |
| Intermediate grade | 105 (33%) | 99 (31%) |
| Intermediate/high grade | 11 (3%) | 5 (2%) |
| High grade | 205 (63%) | 213 (67%) |
| Unknown grade | 1 (<1%) | 0 |
| Cellular classification site review | ||
| Leiomyosarcoma | 113 (35%) | 117 (37%) |
| Liposarcoma | 49 (15%) | 62 (20%) |
| Undifferentiated pleomorphic sarcoma | 43 (13%) | 36 (11%) |
| Other† | 118 (37%) | 102 (32%) |
| Previous radiotherapy | ||
| No | 203 (63%) | 200 (63%) |
| Yes | 120 (37%) | 117 (37%) |
| Previous adjuvant or neoadjuvant systemic therapy | ||
| No | 301(93%) | 297 (94%) |
| Yes | 22 (7%) | 20 (6%) |
Data are median (IQR) or n (%). Data are from the intention-to-treat population. ECOG=Eastern Cooperative Oncology Group.
ECOG data were only available for 322 patients in the doxorubicin alone group.
See appendix p 19.
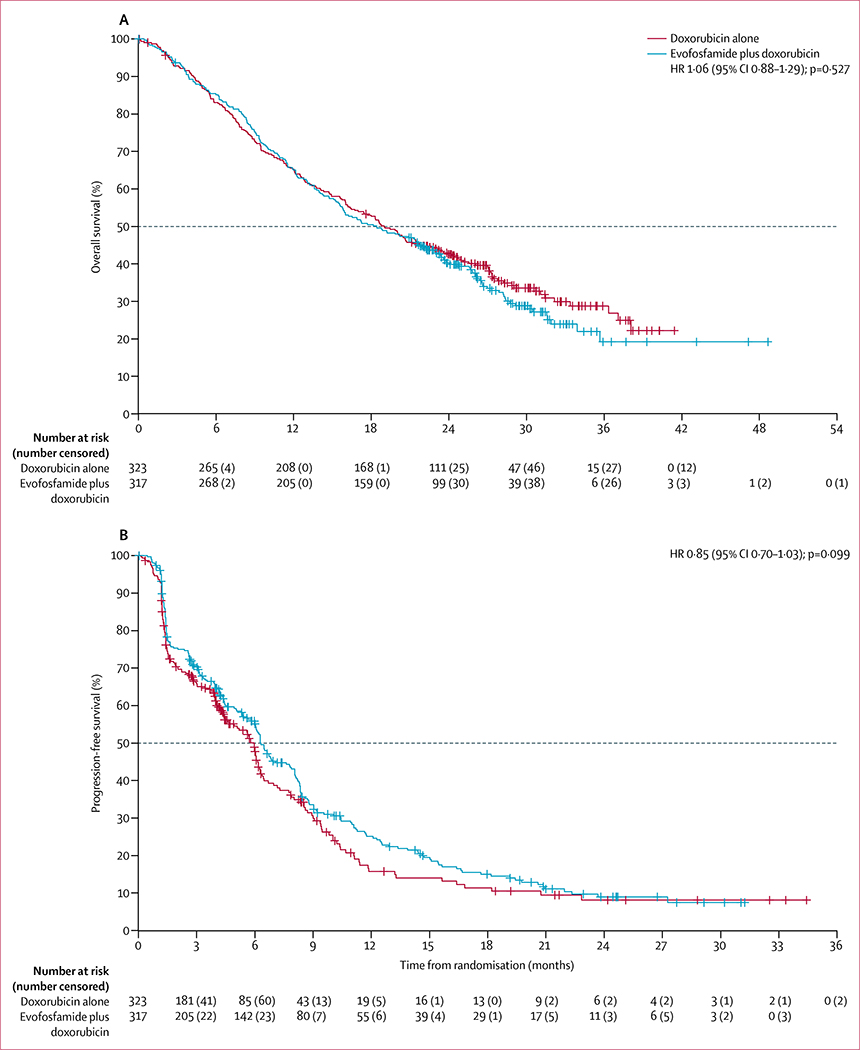
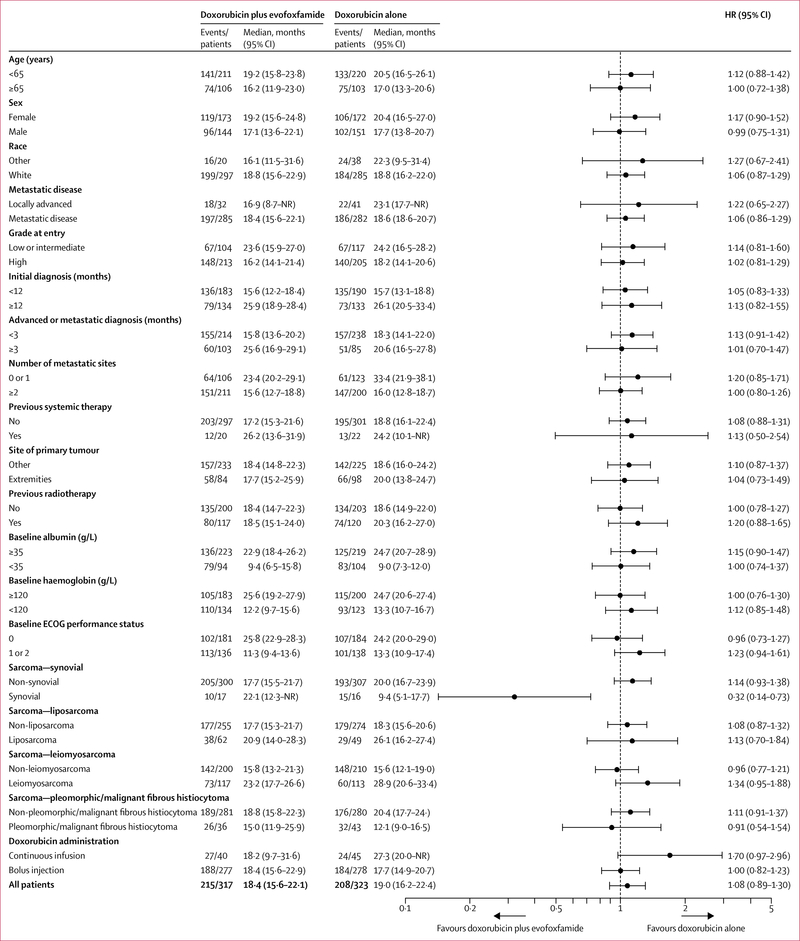
The date of database cutoff for the primary analysis was Oct 19, 2015. The final analysis for the overall survival primary endpoint was done after 423 events (deaths) had occurred (215 deaths in the combination group and 208 deaths in the doxorubicin alone group). The data cutoff date for the primary analysis was based on the projected date of the 434th death. After a survival sweep with a cutoff on Oct 19, 2015, 423 deaths were reported. The study retained more than 84% power. Discussions were held with the FDA and we agreed to do an additional sensitivity analysis on the basis of the 434 deaths. An additional survival sweep was not done because the study did not reach the primary efficacy endpoint of improving survival. The median overall survival follow-up was 28·3 months (IQR 24·6–34·0) in the doxorubicin alone group and 29·2 months (IQR 24·4–32·6) in the combination group. Median overall survival was similar in the two groups; median overall survival was 18·4 months (95% CI 15·6–22·1) in the combination group and 19·0 months (16·2–22·4) in the doxorubicin alone group (HR 1·06, 95% CI 0·88–1·29; p=0·527; figure 2A). The prespecified overall survival analyses by subgroups are summarised in figure 3 and showed no overall survival differences by treatment for all subgroups, except for the 33 patients with synovial sarcoma (HR 0·32 [95% CI 0·14–0·73]; p=0·0043). Additionally, no significant differences in median overall survival between the groups were reported in any stratification factor sensitivity analyses (appendix p 15).
Figure 2: Overall survival and progression-free survival.
(A) Overall survival. (B) Progression-free survival. HR=hazard ratio.
Figure 3: Overall survival by subgroup.
HR=hazard ratio. NR=not reached. ECOG=Eastern Cooperative Oncology Group
The final analysis for the progression-free survival secondary endpoint was done after 429 events occurred (236 in the combination group and 193 in the doxorubicin alone group). The median length of progression-free survival follow-up was 192 days (IQR 122–660) for the doxo rubicin alone group versus 639 days (275–752) for the combination group. Median progression-free survival was similar in the two groups (6·3 months [95% CI 6·0–7·8] in the combination group vs 6·0 months [4·6–6·2] in the doxorubicin alone group; HR 0·85 [95% CI 0·70–1·03], p=0·099; figure 2B). The sensitivity analyses with varying window sizes for including death were robust. With a 0-day window the HR was 0·84 (95% CI 0·69–1·02) and with a 126-day window the HR was 0·85 (0·70–1·03), which were both similar to the primary analysis HR of 0·85 (0·70–1·03) with the 63-day window. Progression-free survival analyses by subgroups are summarised in the appendix (p 35).
The proportion of patients who achieved an overall response (complete or partial response) was significantly higher in the combination treatment group than in the doxorubicin alone group (90 [28%] of 317 patients vs 59 [18%] of 323; p=0·0026; appendix p 16). A complete response was recorded in eight (1%) of 640 patients overall: five (2%) of 317 patients treated with doxorubicin plus evofosfamide and three (1%) of 323 patients treated with doxorubicin alone. A partial response was recorded in 141 (22%) of 640 patients overall (85 [27%] of 317 in the combination group vs 56 [17%] of 323 in the doxorubicin alone group). The median duration of response was similar in the combination treatment group and the doxorubicin alone group (6·7 months [IQR 3·9–13·8] vs 6·2 months [3·7–10·4]; HR 0·86 [95% CI 0·54–1·36], p=0·508). The proportion of patients achieving disease control (complete response, partial response, or stable disease) was 73% (232 of 317 patients) in the doxorubicin plus evofosfamide group and 66% (213 of 323) in the doxorubicin alone group (odds ratio 1·49 [95% CI 0·54–1·36], p=0·0473).
No differences in EQ-5D-5L outcome measures were reported between treatment groups on the basis of individual items scores, visual analogue scale, or health utility index (appendix pp 13–14).
A summary of exposure to evofosfamide and to doxorubicin is provided along with the doxorubicin administration schedules in the appendix (p 18). Patients received a median of six cycles (IQR 2–12) of evofosfamide. The median duration of exposure was 17·1 weeks (IQR 5·1–35·1) and the median number of evofosfamide doses ad ministered was 12 (IQR 4–23). Patients received a median of six cycles (IQR 2–6) of doxorubicin in both the doxorubicin alone group and doxorubicin plus evofosfamide group. The evofosfamide dose was modified in 141 (45%) of 313 patients; the doxorubicin dose was modified in 135 (43%) of 313 patients in the doxorubicin plus evofosfamide group and 106 (34%) of 308 patients in the doxorubicin alone group. The most common reason for treatment discontinuation was disease progression by tumour evaluation (311 [49%] of 640 patients overall; 197 [62%] of 317 patients in the doxorubicin plus evofosfamide group and 114 [35%] of 323 patients in the doxorubicin alone group; appendix p 18). Within each treatment group, the main other reasons for treatment discontinuation were for adverse events in the combination group (35 [11%] of 317 patients) and completion of six cycles of therapy in the doxorubicin alone group (151 [47%] of 323).
A higher proportion of patients in the doxorubicin alone group received subsequent therapy of any type than in the combination group (table 2), with more patients receiving subsequent chemotherapy (table 2) and initiating chemotherapy on average earlier from randomisation (6·6 months [SD 5·5] vs 8·6 months [6·6]). Regimens containing ifosfamide or dacarbazine were administered more commonly after treatment in the doxorubicin alone group than in the combination group (table 2). Response data to subsequent therapy were not collected in this trial.
Table 2:
Subsequent therapies
| Doxorubicin alone (n=323) | Doxorubicin plus evofosfamide (n=317) | |
|---|---|---|
| Total number of patients who had subsequent therapy | 260(80%) | 234 (74%) |
| Total number who had radiotherapy | 84 (26%) | 68 (21%) |
| Total number who had chemotherapy | 232 (72%) | 210 (66%) |
| Doxorubicin containing | 25 (8%) | 19 (6%) |
| Ifosfamide containing | 68 (21%) | 36 (11%) |
| Trabectedin containing | 59 (18%) | 62 (20%) |
| Gemcitabine plus taxane | 97 (30%) | 102 (32%) |
| Other gemcitabine containing | 38 (12%) | 38 (12%) |
| Pazopanib containing | 96 (30%) | 81 (26%) |
| Dacarbazine containing | 61 (19%) | 44 (14%) |
| Eribulin containing | 9 (3%) | 7 (2%) |
| Other or investigational | 64 (20%) | 43 (14%) |
Data are n (%).
In the combination group, 143 (46%) of 313 patients received evofosfamide monotherapy after completion of the combination treatment, as outlined in the protocol. The 143 patients who received evofosfamide monotherapy received a median of six evofosfamide monotherapy cycles (IQR 3–14; range 1–36); 55 (38%) patients had a response before starting maintenance therapy and 70 (49%) had a response at any point during the study. The proportion of patients achieving an overall response up to cycle 6 was 24% (75 of 317) in the combination group versus 16% (52 of 323) in the doxorubicin group. Seven of these patients in the doxorubicin group had subsequent assessments in response following additional post-cycle 6 assessments.
Tumour resectability, which was a prespecified tertiary endpoint, was followed up and measured in the trial. In total, only five (2%) of 323 patients in the doxorubicin group and seven (2%) of 317 patients in the combination group underwent resection of any lesion.
Baseline histology from the central pathology review is presented in the appendix (p 19). The findings from the central pathology report were similar to those from the investigator assessment. The central pathology had sufficient information to determine that 600 (94%) of 640 patients randomly assigned to a group had eligible histology for inclusion in the study on the basis of histological cellular classification and histological grade. Since the central pathology review was a retrospective analysis, it did not a?ect eligibility or randomisation, which was based on local site pathology.
In a prespecified pharmacokinetic analysis, in the combination group, mean maximum plasma concentrations were reached at the end of infusion for both evofosfamide and bromo-isophosphoramide mustard (appendix p 20). Once the intravenous infusion was terminated, plasma concentrations of evofosfamide decreased rapidly, with a geometric mean terminal half-life of 0·56 h. For the active metabolite, bromo-isophosphoramide mustard, plasma concentrations de creased rapidly, such that plasma concentrations were below quantifiable limits 2 h post-dose and terminal half-lives of bromo-isophosphoramide mustard could not be deter mined. Plasma concentrations for bromo-isophos-phoramide mustard were roughly 2% of that for evofosfamide. No interaction of doxorubicin with evofosfamide was apparent. Evofosfamide was administered at least 2 h before doxorubicin and has a terminal half-life of around 0·56 h; as such, most of the evofosfamide in the plasma was eliminated or distributed to tissues before the initiation of each doxorubicin infusion. The pharmacokinetics of doxorubicin was compared between the treatment groups and was found to be similar between the groups, indicating an absence of interaction between doxorubicin and evofosfamide. No statistically significant treatment–biomarker interactions were seen for median overall survival (appendix pp 33–34) or progression-free survival (data not shown).
Most patients in the doxorubicin plus evofosfamide group had adverse events, including 301 (96%) of 313 patients with adverse events judged to be related to evofosfamide and 304 (97%) with adverse events assessed as being related to doxorubicin. 292 (95%) of 308 patients in the doxorubicin alone group had adverse events assessed as related to doxorubicin. Adverse events of any grade that occurred in more than 10% of patients in either group are listed in the appendix (pp 21–27). Overall, incidences of adverse events were similar in the combination treatment group and the doxorubicin alone group. Nausea was the most common adverse event in both treatment groups, with an overall incidence of 63% (390 of 621 patients), including 180 (58%) of 308 patients in the doxorubicin alone group and 210 (67%) of 313 patients in the doxorubicin plus evofosfamide group. Around two-thirds of patients in the doxorubicin plus evofosfamide group also had study drug-related adverse events that were classified as grade 3 or worse (related to evofosfamide: 208 [66%] of 313 patients; related to doxorubicin: 209 [67%] of 313). 200 (65%) of 308 patients in the doxorubicin group had grade 3 or worse adverse events related to doxorubicin. Grade 1–2 events occurring in 10% or more of patients, grade 3–4 events occurring in 2% or more of patients, and all grade 5 events are listed in table 3.
Table 3:
Adverse events
| Doxorubicin alone group (n=308) |
Doxorubicin plus evofosfamide group (n=313) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
| Abdominal pain | 24 (8%) | 7 (2%) | 0 | 0 | 32 (10%) | 5 (2%) | 0 | 0 |
| Alopecia | 138 (45%) | 0 | 0 | 0 | 153 (49%) | 0 | 0 | 0 |
| Anaemia | 39 (13%) | 61 (20%) | 4 (1%) | 0 | 39 (12%) | 147 (47%) | 3 (1%) | 0 |
| Arthralgia | 17 (6%) | 1 (<1%) | 0 | 0 | 39 (12%) | 1 (<1%) | 0 | 0 |
| Asthenia | 33 (11%) | 1 (<1%) | 0 | 0 | 37 (12%) | 3 (1%) | 0 | 0 |
| Back pain | 26 (8%) | 0 | 0 | 0 | 34 (11%) | 5 (2%) | 0 | 0 |
| Cardiac failure congestive | 0 | 0 | 1 (<1%) | 0 | 0 | 1 (<1%) | 0 | 1 (<1%) |
| Cardiopulmonary failure | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Cellulitis | 2 (1%) | 2 (1%) | 0 | 0 | 11 (4%) | 7 (2%) | 0 | 0 |
| Completed suicide | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1%) |
| Constipation | 89 (29%) | 0 | 0 | 0 | 130 (42%) | 4 (1%) | 0 | 0 |
| Cough | 43 (14%) | 0 | 0 | 0 | 59 (19%) | 0 | 0 | 0 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1%) |
| Decreased appetite | 81 (26%) | 1 (<1%) | 0 | 0 | 109 (35%) | 4 (1%) | 0 | 0 |
| Dehydration | 10 (3%) | 3 (1%) | 0 | 0 | 21 (7%) | 7 (2%) | 0 | 0 |
| Diarrhoea | 67 (22%) | 1 (<1%) | 0 | 0 | 92 (29%) | 4 (1%) | 0 | 0 |
| Dizziness | 20 (6%) | 0 | 0 | 0 | 35 (11%) | 0 | 0 | 0 |
| Dry mouth | 12 (4%) | 0 | 0 | 0 | 33 (11%) | 0 | 0 | 0 |
| Dry skin | 16 (5%) | 1 (<1%) | 0 | 0 | 33 (11%) | 1 (<1%) | 0 | 0 |
| Dysgeusia | 41 (13%) | 0 | 0 | 0 | 70 (22%) | 0 | 0 | 0 |
| Dyspepsia | 38 (12%) | 1 (<1%) | 0 | 0 | 37 (12%) | 0 | 0 | 0 |
| Dyspnoea | 30 (10%) | 3 (1%) | 1 (<1%) | 0 | 60 (19%) | 6 (2%) | 0 | 0 |
| Ejection fraction decreased | 28 (9%) | 3 (1%) | 0 | 0 | 32 (10%) | 7 (2%) | 0 | 0 |
| Fatigue | 151 (49%) | 11 (4%) | 0 | 0 | 174 (56%) | 16 (5%) | 0 | 0 |
| Febrile neutropenia | 0 | 23 (7%) | 11 (4%) | 0 | 0 | 40 (13%) | 17 (5%) | 0 |
| Haemorrhoids | 11 (4%) | 0 | 0 | 0 | 42 (13%) | 1 (<1%) | 0 | 0 |
| Headache | 36 (12%) | 0 | 0 | 0 | 51 (16%) | 1 (<1%) | 0 | 0 |
| Hypokalaemia | 11 (4%) | 9 (3%) | 0 | 0 | 24 (8%) | 10 (3%) | 1 (<1%) | 0 |
| Hyponatraemia | 6 (2%) | 4 (1%) | 0 | 0 | 7 (2%) | 8 (3%) | 0 | 0 |
| Hypophosphataemia | 4 (1%) | 4 (1%) | 0 | 0 | 4 (1%) | 9 (3%) | 0 | 0 |
| Insomnia | 37 (12%) | 0 | 0 | 0 | 23 (7%) | 0 | 0 | 0 |
| Lactic acidosis | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Leucopenia | 11 (4%) | 13 (4%) | 4 (1%) | 0 | 8 (3%) | 8 (3%) | 14 (4%) | 0 |
| Lymphocyte count decreased | 5 (2%) | 6 (2%) | 2 (1%) | 0 | 4 (1%) | 12 (4%) | 3 (1%) | 0 |
| Meningitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1%) |
| Nausea | 178 (58%) | 2 (1%) | 0 | 0 | 205 (65%) | 5 (2%) | 0 | 0 |
| Neutropenia | 4 (1%) | 23 (7%) | 69 (22%) | 0 | 13 (4%) | 14 (4%) | 33 (11%) | 0 |
| Neutrophil count decreased | 3 (1%) | 9 (3%) | 32 (10%) | 0 | 3 (1%) | 12 (4%) | 19 (6%) | 0 |
| Oedema peripheral | 32 (10%) | 4 (1%) | 0 | 0 | 37 (12%) | 1 (<1%) | 0 | 0 |
| Pain in extremity | 20 (6%) | 2 (1%) | 0 | 0 | 32 (10%) | 0 | 0 | 0 |
| Palmar-plantar erythrodysaesthesia syndrome | 2 (1%) | 0 | 0 | 0 | 38 (12%) | 3 (1%) | 0 | 0 |
| Pancytopenia | 0 | 0 | 2 (1%) | 0 | 0 | 6 (2%) | 9 (3%) | 0 |
| Platelet count decreased | 15 (5%) | 4 (1%) | 1 (<1%) | 0 | 20 (6%) | 3 (1%) | 12 (4%) | 0 |
| Pneumonia | 2 (1%) | 3 (1%) | 0 | 1 (<1%) | 2 (1%) | 7 (2%) | 0 | 0 |
| Pulmonary embolism | 0 | 17 (6%) | 0 | 0 | 0 | 18 (6%) | 3 (1%) | 0 |
| Pyrexia | 39 (13%) | 2 (1%) | 0 | 0 | 69 (22%) | 2 (1%) | 0 | 0 |
| Sepsis | 0 | 0 | 3 (1%) | 0 | 0 | 3 (1%) | 1 (<1%) | 3 (1%) |
| Septic shock | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1%) |
| Skin hyperpigmentation | 1 (<1%) | 0 | 0 | 0 | 35 (11%) | 0 | 0 | 0 |
| Stomatitis | 99 (32%) | 7 (2%) | 0 | 0 | 136 (43%) | 26 (8%) | 0 | 0 |
| Thrombocytopenia | 15 (5%) | 1 (<1%) | 3 (1%) | 0 | 27 (9%) | 20 (6%) | 25 (8%) | 0 |
| Urinary tract infection | 24 (8%) | 2 (1%) | 0 | 0 | 41 (13%) | 6 (2%) | 0 | 0 |
| Vomiting | 61 (20%) | 1 (<1%) | 0 | 0 | 98 (31%) | 2 (1%) | 1 (<1%) | 0 |
| Weight decreased | 17 (6%) | 1 (<1%) | 0 | 0 | 41 (13%) | 3 (1%) | 0 | 0 |
| White blood cell count decreased | 6 (2%) | 12 (4%) | 21 (7%) | 0 | 6 (2%) | 14 (4%) | 25 (8%) | 0 |
Data are n (%) for all adverse events regardless of cause in the safety population. This table shows grade 1–2 events occurring in 10% or more patients, grade 3–4 events in 2% or more patients, and all grade 5 events. A full table of adverse events (showing grade 1–2 events in ≥10% patients and all grade 3–5 events is in the appendix pp 21–24).
A similar proportion of patients in the two treatment groups had an adverse event that resulted in discontinuation from treatment. In the doxorubicin plus evofosfamide group, 41 (13%) of 313 patients had an adverse event that resulted in the discontinuation of evofosfamide and 26 (8%) patients had an adverse event that resulted in the discontinuation of doxorubicin. In the doxorubicin alone group, 19 (6%) of 308 patients had an adverse event that resulted in the discontinuation of doxorubicin. In the doxorubicin plus evofosfamide group, 88 (28%) of 313 patients had a dose reduction of evofosfamide and 75 (24%) patients had a dose reduction of doxorubicin because of adverse events. In the doxorubicin alone group, 53 (17%) of 308 patients had a dose reduction of doxorubicin because of adverse events.
Haematological adverse events were more common in the doxorubicin plus evofosfamide group than in the doxorubicin alone group, with higher overall incidences of anaemia (189 [60%] of 313 vs 104 [34%] of 308), thrombocytopenia (72 [23%] vs 19 [6%]), and febrile neutropenia (57 [18%] vs 34 [11%]). Skin and mucosal toxicities were also reported, with a higher incidence in the combination treatment group (table 3). Stomatitis was reported in 106 (34%) of 308 patients in the doxorubicin alone group (including seven [2%] grade 3 or worse) and 162 (52%) of 313 patients in the doxorubicin plus evofosfamide group (including 26 [8%] grade 3 or worse). Adverse events including the term “rash” were reported in 17 (6%) of 308 patients in the doxorubicin alone group and 79 (25%) of 313 patients in the doxorubicin plus evofosfamide group. Only one rash adverse event was grade 3 in severity (exfoliative rash), which was in the doxorubicin plus evofosfamide group. Prophylactic and therapeutic measures such as topical applications and cryotherapy were implemented routinely to manage skin and mucosal toxicities.
Cardiac function, including left ventricular ejection fraction, was similar across treatment groups (appendix pp 29–30). At the termination visit, 15 (12%) of 130 patients from the doxorubicin plus evofosfamide group versus 13 (9%) of 144 patients from the doxorubicin alone group had a decrease in left ventricular ejection fraction of 10% or more, resulting in a left ventricular ejection fraction of less than 55%. The use of a cardiac protectant (eg, dexrazoxane) was generally site specific and when provided was generally given in both treatment groups.
No grade 3 increases in serum creatinine occurred and the overall grade 2 incidence was only 2% (ten of 621 patients). Glomerular filtration rates were calculated through all treatment cycles and no differences were recorded between treatment groups.
More patients in the doxorubicin plus evofosfamide group had at least one serious adverse event compared with the doxorubicin alone group (145 [46%] of 313 patients vs 99 [32%] of 308; appendix p 28). In the doxorubicin plus evofosfamide group, 96 (31%) of 313 patients had a serious adverse event that was assessed as related to evofosfamide and 100 (32%) of 313 patients had a serious adverse event that was assessed as related to doxorubicin. In the doxorubicin group, 58 (19%) of 308 patients had a serious adverse event that was assessed as related to doxorubicin. Most serious adverse events were haematological toxicities, with the most common in the doxorubicin group and the combination group being febrile neutropenia (27 [9%] of 308 patients vs 56 [18%] of 313) and anaemia (ten [3%] of 308 patients vs 19 [6%] of 313).
A total of 423 patients died during the study (either on treatment or during follow-up). There were 412 deaths in the safety population, of which 374 (91%) were due to progressive disease, ten (2%) adverse events, and 28 (7%) other causes (appendix p 31). 215 patients died in the doxorubicin plus evofosfamide group, including 212 in the safety population of which 191 (90%) were due to progressive disease, eight (4%) adverse events, and 13 (6%) other causes. 208 patients died in the doxorubicin alone group, including 200 in the safety population, of which 183 (92%) were due to progressive disease, two (1%) adverse events, and 15 (8%) from other causes.
Of the ten deaths due to adverse events, two were in the doxorubicin alone group and eight were in the doxorubicin plus evofosfamide group. Four deaths (two sepsis, one septic shock, and one unknown cause; all in the combination group) were deemed related to evofosfamide and doxorubicin. Two deaths (congestive cardiac failure in the combination group and lactic acidosis in the doxorubicin alone group) were deemed related to doxorubicin.
Discussion
To our knowledge, this study is the largest first-line trial to be done in advanced soft-tissue sarcomas and serves as a benchmark for future sarcoma trials. This international, randomised trial showed no meaningful differences in overall survival or progression-free survival between doxorubicin plus evofosfamide versus doxorubicin alone. The proportion of patients achieving an objective response was higher in the combination group than in the doxorubicin alone group; however, this finding did not translate into a difference in progression-free or overall survival. Although the proportion of patients achieving an overall response was improved with the addition of evofosfamide to doxorubicin, overall responses occurred in less than a third of patients and the degree and duration of responses were similar between treatment groups. The treatment of advanced inoperable, metastatic soft-tissue sarcomas continues to represent an area of great unmet medical need.
Our subgroup analysis of overall survival showed no significant difference in overall survival between the treatment groups in any of the histological subgroups analysed, with the exception of synovial sarcoma. The combination therapy seemed to benefit patients with synovial sarcoma, possibly due to the activity of the alkylator in this disease. The overall number of patients with synovial sarcoma in the study was low (33 patients overall; 17 in the combination group and 16 on single-drug doxorubicin). Therefore, the benefit noted needs to be taken with some consideration. We would like to see an additional study in synovial sarcoma and think the majority of the sarcoma community would be in favour and supportive of such a trial.
The decision to proceed with this phase 3 trial was based on strong, albeit non-randomised, phase 2 data. The interpretation of the data at the time was that the combination had substantial activity in soft-tissue sarcomas. Additionally, as outlined in the report of the phase 2 trial, additional responses were noted in patients receiving evofosfamide maintenance therapy after the combination, suggesting some continued activity of evofosfamide as a single drug.10 On initial design of the phase 3 study, the incorporation of continued evofosfamide therapy after six cycles of the combination was thought to be an important aspect of the study, especially in regard to facilitating an advantage in overall survival. In essence, this design compared the combination plus continued evofosfamide therapy to six cycles of doxorubicin alone. If evofosfamide had activity in soft-tissue sarcomas, patients randomly assigned to the combination would have been exposed to an active drug for much longer than just six cycles alone, possibly translating into a strong overall survival benefit. The phase 2 trial suggested continued exposure could have contributed to ongoing responses and a clinical benefit. However, the use of continued evofosfamide monotherapy after the combination was allowed rather than mandated in both the phase 2 and phase 3 studies.
A major concern of the investigators and the IDMC throughout the trial was that patients might withdraw consent after randomisation if they were not randomly assigned to the combination group. This withdrawal would have realised a significant bias of the open-label design. The study leaders notified all investigators of the importance of keeping patients on study, and patients were informed of study expectations as part of the consent process. The small number of patients randomly assigned to a group who did not initiate treatment (ie, withdrew consent; four in the doxorubicin plus evofosfamide group vs 15 in the doxorubicin alone group) was related to the commitment of the investigators and patients to the study and likely did not affect the overall statistical analyses.
Progression-free survival could have been confounded by inherent imbalances introduced by the design of this study, including an absence of placebo or study blind. Patients were censored from the progression-free survival analysis at the time they received off-study treatment. Patients in the doxorubicin alone group discontinued study treatment after six cycles irrespective of tumour status. At that point, they could receive other off-study treatments. Patients in the combination group who completed doxorubicin could continue on-study receiving evofosfamide as a single drug and, as such, were actively followed up for progression-free survival. This difference between treatment groups led to a larger number of patients being censored for progression-free survival (mainly for receiving additional sarcoma therapy) in the doxorubicin alone group. The median length of progression-free survival follow-up was 192 days for the doxorubicin alone group compared with 639 days for the combination group.
The imbalance in design could also have affected overall survival because patients in the doxorubicin group received subsequent therapy sooner and more frequently than those in the combination group. The study design compared overall survival of doxorubicin plus subsequent therapy versus doxorubicin plus evofosfamide followed by evofosfamide alone plus subsequent therapy. Additionally, once patients enrolled in the doxorubicin group were discontinued from treatment, there was a difference in assessment schedules between the two groups; tumour assessments were to be done every 9–15 weeks after week 18 for patients who were no longer receiving therapy. An enhanced activity of subsequent therapy relative to evofosfamide alone had the potential to affect overall survival, especially with the time on treatment of 4·5 months being much shorter than the time after treatment, with the median overall survival in the doxorubicin group being about 19 months. This time after treatment is not inconsequential because of recent advancements in the sarcoma treatment landscape (eg, trabectedin, eribulin, and votrient).16–18
The availability and effect of more efficacious second and subsequent lines of therapy is evident in the evolution over time of overall survival outcomes in the doxorubicin control group in first-line, randomised, phase 3 studies. The doxorubicin group of three studies done long-itudinally—notably the EORTC 62012 study (recruitment 2003–10),1 the PICASSO 3 study (recruitment 2010–12),2 and the present TH CR-406/SARC021 study (recruitment 2011–14)—show a progressive improvement in overall survival in patients given doxorubicin in the first-line setting. For these three studies, median overall survival for first-line doxorubicin was 12·8 months (95% CI 10·5–14·3), 16·9 months (0·79–1·39), and 19·0 months (16·2–22·4), respectively. Although these improvements could in part be due to patient selection and general improvements in multimodality patient care, the timing and effect of subsequent therapies cannot be discounted. Unfortunately, this study design specified that after a patient initiated any new off-study anti-tumour therapy, data for further tumour response assessments would not be collected. As such, the comparison of progression-free survival independent of subsequent treatment interventions is not possible.
Additionally, this trend over time of improving overall survival with doxorubicin in the first-line setting also allowed for possible over-interpretation of the phase 2 doxorubicin plus evofosfamide study, which showed an impressive median overall survival of 21·5 months.10 In retrospect, in view of the outcomes of this study and of sarcoma studies published in the past 2–3 years, the survival results of the phase 2 study could reflect the activity of doxorubicin alone when used as a first-line drug in patients with advanced or metastatic soft-tissue sarcoma. Exploratory imaging would have been helpful (eg, [F-18]fluoromisonidazole), but was beyond the financial and logistical scope of this large international study. Ideally, exploratory imaging could have been incorporated in the phase 2 trial.
A priori, with the initial study design, the true depth and degree of cytopenias that would occur with the combination therapy was uncertain. Although the phase 2 study suggested the combination would not be significantly more toxic than doxorubicin alone, the phase 2 study was not randomised. Therefore, prudence suggested the exclusion of patients with an ECOG performance status of 2 or higher to ensure that no undue harm would occur to patients if increased cytopenias were encountered. Adverse events were reported within 30 days after discontinuation of study drug. Because delayed adverse events can occur outside this window, the study stipulated that any late-occurring adverse events thought to be related to any of the study drugs be reported.
Overall, although the combination of doxorubicin plus evofosfamide in this phase 3 study was generally well tolerated, it was more toxic than doxorubicin monotherapy. Compared with the monotherapy, the combination group had a higher proportion of patients with febrile neutropenia, despite the use of growth factors, in addition to higher proportions of patients with anaemia or thrombocytopenia. Consistent with previous studies in patients with soft-tissue sarcomas, skin toxicity, mucosal toxicity, and enhanced myelosuppression were the most commonly reported adverse events associated with evofosfamide. These adverse events were manageable, as evidenced by the absence of an increase in treatment discontinuation in patients given evofosfamide compared with those given doxorubicin alone. The reported increase in skin and mucosal toxicities that was noted in the combination group was due to evofosfamide. A hypoxic region is believed to exist in the skin where evofosfamide is activated in minute amounts. Extensive precautions were taken to prevent skin toxicity on the trial, which might account for the lower proportion of patients with skin toxicity compared with previous studies with evofosfamide. No differences in EQ-5D-5L outcome measures were reported despite higher rates of toxicity in the combination group than in the doxorubicin alone group. An open-label study such as this one has the potential of confounding quality-of-life results, which are subjective endpoints with high amounts of variance.
Although our inclusion criteria stipulated that patients aged 15 years and older were eligible for this trial, no patients aged 15–18 years were enrolled. Single-drug doxorubicin might not be standard care for most children with sarcoma (at least in the USA). Rather, most paediatric patients with sarcoma would probably be treated with a doxorubicin-based combination. We suspect that children were not enrolled because their treating physicians did not want them to be randomly assigned to the doxorubicin only group. Paediatric patients might also have been preferentially enrolled on paediatric protocols with other drugs.
Tumour resectability was followed up and measured on this trial. Any form of surgical conversion would have been deemed a positive outcome or application of the study drug. We are uncertain as to why the proportion of patients achieving surgical conversion was so low despite the higher proportion of patients achieving an overall response reported in the combination group than in the doxorubicin alone group. Our entry criteria did select for patients with advanced and widespread disease. Therefore, the depth, extent, and duration of responses might not have been sufficient to justify removal of patients from the study to proceed with surgical resection.
This study highlights the challenges of designing trials to assess novel therapies in sarcomas, specifically calling into question optimum clinical trial design in this rare and heterogeneous group of malignancies. This study was limited by its initial design, including the absence of a placebo and study blind, the incorporation of a maintenance component with a potentially inactive drug, and the inclusion of several different sarcoma subtypes. For the sarcoma community, discussions should continue regarding the pitfalls of using the endpoint of overall survival in the first-line setting, the use of a placebo with proper trial blinding, and the validity of large trials inclusive of the numerous sarcoma subtypes.
In conclusion, this trial does not support the use of evofosfamide plus doxorubicin as a first-line treatment for patients with locally advanced or metastatic soft-tissue sarcoma. The study does provide a contemporary benchmark of the activity of doxorubicin for the design of future clinical trials in sarcomas and raises important questions regarding the management of patients follow ing maximum doses of an anthracycline (ie, expectant management vs the initiation of another therapy). This trial clearly shows the ability of the sarcoma community to do a large international study rapidly and collaboratively despite the rarity of these diseases, and also shows the feasibility of a new standardised protocol for central pathology review for future prospective trials in soft-tissue sarcomas.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for all randomised trials published in English between Jan 1, 1980, and Nov 30, 2016, that involved metastatic soft-tissue sarcoma and an anthracycline. We used the following search terms: “soft tissue”, “sarcoma”, “trial”, and “doxorubicin”. We identified 45 randomised clinical trials; 13 were reported as phase 3 clinical trials in patients with metastatic soft-tissue sarcoma, five of which were reported as being done in the first-line setting.
Added value of this study
To our knowledge, this trial is the largest randomised study done in soft-tissue sarcomas that compared a novel doxorubicin-based combination therapy to doxorubicin alone in the first-line setting for patients with locally advanced or metastatic disease. This study was a strongly collaborative effort done through the Sarcoma Alliance for Research through Collaboration (SARC) with contributions from international non-SARC study sites, highlighting the ability of the international sarcoma community to do large pivotal phase 3 clinical trials. Although the combination did not improve overall survival compared with doxorubicin alone, the study further defines outcomes for doxorubicin as a single drug when used in the first-line setting for patients with metastatic soft-tissue sarcoma and raises important issues regarding the design of large randomised clinical trials in this rare, heterogeneous, and difficult-to-treat group of malignancies.
Implications of all the available evidence
Doxorubicin is a standard treatment for metastatic soft-tissue sarcoma and remains a proper control group against which to compare new drugs and drug combinations. The survival of patients with metastatic soft-tissue sarcomas who receive doxorubicin in the first-line setting has improved over time. This improvement is most likely due to an improved biological understanding of the various sarcoma subtypes, additional options for the second and subsequent lines of therapy for individual sarcoma subtypes, and improvements in multidisciplinary and supportive care. For the sarcoma community, discussions should continue regarding the pitfalls of using the endpoint of overall survival to assess first-line treatments, the use of a placebo with a proper trial blind, and the conduct of large trials inclusive of numerous sarcoma subtypes.
Acknowledgments
We thank the patients, their family and caregivers, the investigators, and the members of the Sarcoma Alliance for Research through Collaboration and the international sarcoma community who participated in this study.
Declaration of interests
TA reports personal fees for activities outside the submitted work from Lilly, Novartis, and EMD Serono. GTB reports a grant from Threshold. SPC reports consultancy outside the submitted work for Amgen, CytRx, Novartis, Eisai, Janssen, and Threshold; and research funding from Amgen, CytRx, Threshold, Novartis, Eisai, and Bayer. LDC reports a grant from Threshold to support conduct of the clinical study; a grant and personal fees for activities outside the submitted work from Bristol-Myers Squibb; grants for activities outside the submitted work from Merck, Janssen, Eisai, and Amgen; and personal fees for activities the outside the submitted work from Altor Biosciences. GG reports a grant for activities outside the submitted work from PharmaMar; and personal fees for activities outside the submitted work from Lilly, Bayer, Pfizer, and Novartis. RLJ reports paid consultancy from Eisai, Pharmamar, Lilly, Merck, Immunedesign, Adaptimmune, Immodulon, and Blueprint. SK reports stock ownership with Threshold. TP is a fulltime employee and shareholder of Threshold, the sponsor of the study. DR reports consultancy with EMD Serrano. DKR reports a grant from Threshold. RFR reports personal fees for consultancy with EMD Serono; grants and personal fees for activities outside the submitted work from Lilly and Eisai; grants for clinical trial support outside the submitted work from Karyopharm, AADi, Plexxikon, Novartis, Tracon, Threshold, Astex, Ariad, and Ziopharm; and personal fees for consultancy outside the submitted work from Janssen. CWR reports a grant from Threshold to support this trial; personal fees for activities outside the submitted work from Eisai, EMD Serono, and Elexis; grants for activities outside the submitted work from CytRx, Eisai, and Tracon; and a grant and personal fees for activities outside the submitted work from Janssen. PS reports support for advisory functions from Merck and Threshold. SS reports grant support from SARC; non-financial support from Threshold; personal fees from EMD Serono; grant support, personal fees, and non-financial support from Janssen; and grants and non-financial support from Lilly, Amgen, AB Science, CytRx, and Plexxikon. WDT reports paid consultancy for activities outside the submitted work from Eisai, Janssen, Immune Design, Adaptimmune, Ariad, Daiichi Sanko, Plexxikon, Morphotek, Advaxis, and Tracon. HT reports grants and personal fees for activities outside the submitted work from GMD, Novartis, and Roche/Genentech; a grant outside the submitted work from Merck; and personal fees for consultancy outside the submitted work from EMD Serono. BAVT reports consultancy with EMD Serano and financial support from Threshold for participating in this trial. All other authors declare no competing interests.
Contributor Information
William D Tap, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Zsuzsanna Papai, Allami Egeszsegugyi Kozpont (State Health Center), Budapest, Hungary.
Brian A Van Tine, Washington University School of Medicine, St Louis, MO, USA.
Steven Attia, Mayo Clinic Florida, Jacksonville, FL, USA.
Kristen N Ganjoo, Stanford University Department of Medicine Division of Oncology, Stanford, CA, USA.
Robin L Jones, University of Washington Cancer Center/ Seattle Cancer Care Alliance, Seattle, WA, USA.
Scott Schuetze, University of Michigan Cancer Center, Ann Arbor, MI, USA.
Damon Reed, Moffitt Cancer Center, Tampa, FL, USA.
Sant P Chawla, Sarcoma Oncology Center, Santa Monica, CA, USA.
Richard F Riedel, Duke University Medical Center, Durham, NC, USA.
Anders Krarup-Hansen, Herlev Hospital, Herlev, Denmark.
Maud Toulmonde, Institut Bergonié, Bordeaux, France.
Isabelle Ray-Coquard, Centre Léon Bérard, Lyon, France.
Peter Hohenberger, Universitätsklinikum Mannheim, Mannheim, Germany.
Giovanni Grignani, Candiolo Cancer Institute, FPO, IRCCS, Turin, Italy.
Lee D Cranmer, University of Arizona Cancer Center, Seattle, WA, USA.
Scott Okuno, Mayo Clinic, Rochester, MN, USA.
Mark Agulnik, Robert H Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL, USA.
William Read, Winship Cancer Institute, Emory University, Atlanta, GA, USA.
Christopher W Ryan, Oregon Health and Science University, Portland, OR, USA.
Thierry Alcindor, Department of Oncology, McGill University, Montreal, QC, Canada.
Xavier F Garcia del Muro, ICO Hospital Duran i Reynals, Barcelona, Spain.
G Thomas Budd, Cleveland Clinic Foundation, Cleveland, OH, USA.
Hussein Tawbi, University of Pittsburgh Medical Center, Houston, TX, USA.
Tillman Pearce, Threshold Pharmaceuticals, South San Francisco, CA, USA.
Stew Kroll, Threshold Pharmaceuticals, South San Francisco, CA, USA.
Denise K Reinke, Sarcoma Alliance for Research through Collaboration, Ann Arbor, MI, USA.
Patrick Schöffski, UZ Leuven, Campus Gasthuisberg, Leuven, Belgium.
References
- 1.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014; 15: 415–23. [DOI] [PubMed] [Google Scholar]
- 2.Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: a phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol 2016; 34: 3898–905. [DOI] [PubMed] [Google Scholar]
- 3.Seddon BM, Whalen J, Strauss SJ, et al. GeDDiS: a prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas. Proc Am Soc Clin Oncol 2015; 33 (suppl): abstr 10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin RS, Wiernik PH, Bachur NR. Adriamycin chemotherapy—efficacy, safety, and pharmacologic basis of an intermittent single high-dosage schedule. Cancer 1974; 33: 19–27. [DOI] [PubMed] [Google Scholar]
- 5.Lorigan P, Verweij J, Papai Z, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 2007; 25: 3144–50. [DOI] [PubMed] [Google Scholar]
- 6.Duan JX, Jiao H, Kaizerman J, et al. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem 2008; 51: 2412–20. [DOI] [PubMed] [Google Scholar]
- 7.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11: 393–410. [DOI] [PubMed] [Google Scholar]
- 8.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004; 4: 437–47. [DOI] [PubMed] [Google Scholar]
- 9.Kaanders JH, Wijffels KI, Marres HA, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res 2002; 62: 7066–74. [PubMed] [Google Scholar]
- 10.Chawla SP, Cranmer LD, Van Tine BA, et al. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J Clin Oncol 2014; 32: 3299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 12.Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993; 11: 1269–75. [DOI] [PubMed] [Google Scholar]
- 13.Judson I, Radford JA, Harris M, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2001; 37: 870–77. [DOI] [PubMed] [Google Scholar]
- 14.Borden EC, Amato DA, Edmonson JH, Ritch PS, Shiraki M. Randomized comparison of doxorubicin and vindesine to doxorubicin for patients with metastatic soft-tissue sarcomas. Cancer 1990; 66: 862–67. [DOI] [PubMed] [Google Scholar]
- 15.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995; 13: 1537–45. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016; 34: 786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016; 387: 1629–37. [DOI] [PubMed] [Google Scholar]
- 18.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012; 379: 1879–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.