Summary
Background
Immune checkpoint inhibitors are a new standard of care for patients with advanced non-small-cell lung cancer (NSCLC) without EGFR tyrosine kinase or anaplastic lymphoma kinase (ALK) genetic aberrations (EGFR−/ALK−), but clinical benefit in patients with EGFR mutations or ALK rearrangements (EGFR+/ALK+) has not been shown. We assessed the effect of durvalumab (anti-PD-L1) treatment in three cohorts of patients with NSCLC defined by EGFR/ALK status and tumour expression of PD-L1.
Methods
ATLANTIC is a phase 2, open-label, single-arm trial at 139 study centres in Asia, Europe, and North America. Eligible patients had advanced NSCLC with disease progression following at least two previous systemic regimens, including platinum-based chemotherapy (and tyrosine kinase inhibitor therapy if indicated); were aged 18 years or older; had a WHO performance status score of 0 or 1; and measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Key exclusion criteria included mixed small-cell lung cancer and NSCLC histology; previous exposure to any anti-PD-1 or anti-PD-L1 antibody; and any previous grade 3 or worse immune-related adverse event while receiving any immunotherapy agent. Patients in cohort 1 had EGFR+/ALK+ NSCLC with at least 25%, or less than 25%, of tumour cells with PD-L1 expression. Patients in cohorts 2 and 3 had EGFR−/ALK− NSCLC; cohort 2 included patients with at least 25%, or less than 25%, of tumour cells with PD-L1 expression, and cohort 3 included patients with at least 90% of tumour cells with PD-L1 expression. Patients received durvalumab (10 mg/kg) every 2 weeks, via intravenous infusion, for up to 12 months. Retreatment was allowed for patients who benefited but then progressed after completing 12 months. The primary endpoint was the proportion of patients with increased tumour expression of PD-L1 (defined as ≥25% of tumour cells in cohorts 1 and 2, and ≥90% of tumour cells in cohort 3) who achieved an objective response, assessed in patients who were evaluable for response per independent central review according to RECIST version 1.1. Safety was assessed in all patients who received at least one dose of durvalumab and for whom any post-dose data were available. The trial is ongoing, but is no longer open to accrual, and is registered with ClinicalTrials.gov, number NCT02087423.
Findings
Between Feb 25, 2014, and Dec 28, 2015, 444 patients were enrolled and received durvalumab: 111 in cohort 1, 265 in cohort 2, and 68 in cohort 3. Among patients with at least 25% of tumour cells expressing PD-L1 who were evaluable for objective response per independent central review, an objective response was achieved in 9 (12·2%, 95% CI 5·7–21·8) of 74 patients in cohort 1 and 24 (16·4%, 10·8–23·5) of 146 patients in cohort 2. In cohort 3, 21 (30·9%, 20·2–43·3) of 68 patients achieved an objective response. Grade 3 or 4 treatment-related adverse events occurred in 40 (9%) of 444 patients overall: six (5%) of 111 patients in cohort 1, 22 (8%) of 265 in cohort 2, and 12 (18%) of 68 in cohort 3. The most common treatment-related grade 3 or 4 adverse events were pneumonitis (four patients [1%]), elevated gamma-glutamyltransferase (four [1%]), diarrhoea (three [1%]), infusion-related reaction (three [1%]), elevated aspartate aminotransferase (two [<1%]), elevated transaminases (two [<1%]), vomiting (two [<1%]), and fatigue (two [<1%]). Treatment-related serious adverse events occurred in 27 (6%) of 444 patients overall: five (5%) of 111 patients in cohort 1, 14 (5%) of 265 in cohort 2, and eight (12%) of 68 in cohort 3. The most common serious adverse events overall were pneumonitis (five patients [1%]), fatigue (three [1%]), and infusion-related reaction (three [1%]). Immune-mediated events were manageable with standard treatment guidelines.
Interpretation
In patients with advanced and heavily pretreated NSCLC, the clinical activity and safety profile of durvalumab was consistent with that of other anti-PD-1 and anti-PD-L1 agents. Responses were recorded in all cohorts; the proportion of patients with EGFR−/ALK− NSCLC (cohorts 2 and 3) achieving a response was higher than the proportion with EGFR+/ALK+ NSCLC (cohort 1) achieving a response. The clinical activity of durvalumab in patients with EGFR+ NSCLC with ≥25% of tumour cells expressing PD-L1 was encouraging, and further investigation of durvalumab in patients with EGFR+/ALK+ NSCLC is warranted.
Funding
AstraZeneca.
Introduction
Treatment options for patients with advanced non-small-cell lung cancer (NSCLC) have expanded over the past 15 years. Targeted therapies have radically changed the treatment paradigm for patients with EGFR tyrosine kinase mutations and anaplastic lymphoma kinase (ALK) rearrangements (EGFR+/ALK+). For patients without targetable EGFR or ALK genetic aberrations (EGFR−/ALK−), chemotherapy has long been the cornerstone of treatment, with moderate outcomes.1 However, over the past 3 years, immune checkpoint inhibitors have become another important treatment modality for advanced NSCLC.2,3 Despite these developments, standard options are not available for patients with NSCLC who have disease progression after two lines of therapy. Only erlotinib is indicated in third-line treatment and beyond, with minor and debatable activity, particularly in the EGFR− population.4 Gemcitabine and vinorelbine are often administered in advanced lines in clinical practice because of their acceptable safety profile; however, the benefit of this treatment is unclear.
The inhibitory programmed cell death-1 (PD-1)–programmed cell death ligand-1 (PD-L1) pathway plays a major role in controlling T-cell activation and is used by tumour cells to evade antitumour responses.5,6 The anti-PD-1 monoclonal antibodies nivolumab and pembrolizumab, and the anti-PD-L1 monoclonal antibody atezolizumab have received approval from the European Commission in the EU and by the US Food and Drug Administration for the treatment of metastatic NSCLC.7-9 Meta-analyses in advanced NSCLC suggest that patients with higher tumour PD-L1 expression achieve improved responses to treatment with anti-PD-1 and anti-PD-L1 agents, compared with patients with lower PD-L1 expression.10,11 Retrospective analyses suggest that EGFR+/ALK+ tumours respond less well to these treatments than EGFR−/ALK− tumours.12,13
Durvalumab is a selective, high-affinity human immunoglobulin G1 κ monoclonal antibody that has been engineered to reduce antibody-dependent cell-mediated cytotoxicity.14 It blocks PD-L1 binding to PD-1 (half maximum inhibitory concentration [IC50] 0·1 nmol/L) and CD80 (B7.1; IC50 0·04 nmol/L), allowing T cells to recognise and kill tumour cells.15 Durvalumab is approved in the USA for the treatment of post-platinum locally advanced or metastatic urothelial carcinoma and unresectable, stage III NSCLC that has not progressed following concurrent platinum-based chemotherapy and radiation therapy, and has shown encouraging antitumour activity in a phase 1–2 clinical study across multiple advanced solid tumours,16 including NSCLC.17 Compared with patients who had tumours with less than 25% of tumour cells expressing PD-L1, a larger proportion of patients with advanced NSCLC with at least 25% of tumour cells expressing PD-L1 (assessed using the Ventana PD-L1 [SP263] Assay [Ventana Medical Systems, Tucson, AZ, USA]18) achieved an objective response, and their overall survival was longer.17
We report findings from the phase 2 ATLANTIC study, which evaluated the clinical activity and safety of third-line and later treatment with durvalumab in advanced NSCLC. The study included three independent patient cohorts defined by EGFR/ALK status and tumour PD-L1 expression.
Methods
Study design and participants
ATLANTIC was a phase 2, open-label, single-arm study done at 139 study centres across Asia, Europe, and North America. Patients were aged 18 years and older, had either histologically or cytologically documented NSCLC (stage IIIB or IV) or recurrent or progressive disease following multimodal therapy, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,19 a life expectancy of at least 12 weeks at day 1, and a WHO performance status score of 0 or 1. Patients had disease progression (investigator-determined, using RECIST version 1.1) or recurrence after at least two previous systemic treatment regimens for NSCLC, including one platinum-based chemotherapy regimen. Patients with EGFR+ NSCLC must have received an EGFR tyrosine kinase inhibitor, and patients with ALK+ NSCLC must have received an ALK tyrosine kinase inhibitor, before or after the platinum-based chemotherapy regimen. Eligible patients had to have adequate bone marrow and organ function, defined as platelet count of greater than 100 000 platelets per μL, an absolute neutrophil count of greater than 1500 cells per μL, haemoglobin of at least 9 g/dL, serum bilirubin up to 1·5 times the upper limit of normal (ULN; except for patients with Gilbert’s syndrome who were allowed in consultation with their physician); aspartate aminotransferase and alanine aminotransferase up to 2·5 times ULN (≤5 times ULN for patients with liver metastasis); and serum creatinine clearance greater than 40 mL/min as calculated with the Cockcroft-Gault equation or by 24-h urine collection for determination of creatinine clearance.
Key exclusion criteria included mixed small-cell lung cancer and NSCLC histology; previous exposure to any anti-PD-1 or anti-PD-L1 antibody; active or previously documented autoimmune disease; any previous grade 3 or worse immune-related adverse event while receiving any immunotherapy agent; brain metastasis or spinal cord compression unless asymptomatic, treated, and stable off steroids and anticonvulsants for at least 1 month before study entry; and current or previous use of immunosuppressive medication at least 28 days before durvalumab dosing. Full eligibility criteria are described in the appendix (pp 8–9).
Tumours were classified as EGFR+/ALK+ or EGFR−/ALK− on the basis of local testing and information provided by the trial sites; central testing was not done. Eligible patients with unknown EGFR/ALK status were enrolled into the EGFR−/ALK− cohort.
Patients were originally included in the study irrespective of tumour PD-L1 expression status because they were enrolled prior to the availability of a PD-L1 diagnostic. However, after an immunohistochemical assay was validated to assess PD-L1 expression in tumour tissue samples (the Ventana PD-L1 [SP263] Assay), a protocol amendment (amendment 1) was implemented to include only patients with at least 25% of tumour cells with membranous staining, at any intensity, for PD-L1 expression per central assessment of recent or archival samples. This cutoff of 25% was chosen on the basis of initial observations from a phase 1–2 study (NCT01693562) showing that the proportion of patients with NSCLC with at least 25% of tumour cells expressing PD-L1 who achieved an objective response was higher than the proportion of patients with less than 25% of tumour cells expressing PD-L1.20 To assess the potential benefit of further enriching the patient population on the basis of increased PD-L1 expression, a further protocol amendment (amendment 2) was incorporated to add a third cohort of patients with EGFR−/ALK− NSCLC, whose tumours had at least 90% of cells expressing PD-L1. The cutoff of 90% was chosen on the basis of data on file from the previous phase 1–2 study (NCT01693562) indicating that a subpopulation of patients with at least 90% of tumour cells expressing PD-L1 seemed to have a high likelihood of response, with seven responses in 18 patients (ie, 39% of patients achieved an objective response). The diagnostic assay and the decision to use the cutoff of 25% for PD-L1 expression in cohorts 1 and 2 (amendment 1) and 90% in cohort 3 (amendment 2) were developed using data external to this study (NCT01693562). The results that had been generated within this study at the time of the amendments did not influence selection of the cutoffs. After the second protocol amendment, the study included three independent patient cohorts (appendix p 3): cohort 1, including patients with EGFR+/ALK+ NSCLC with at least 25%, or less than 25%, of tumour cells with PD-L1 expression; cohort 2, including patients with EGFR−/ALK− NSCLC with at least 25%, or less than 25%, of tumour cells with PD-L1 expression; and cohort 3, including patients with EGFR−/ALK− NSCLC, with at least 90% of tumour cells with PD-L1 expression. Patients were enrolled sequentially into cohorts 2 and 3, whereas enrolment into cohort 1 continued throughout the study.
The study was undertaken in accordance with the ethical principles of the Declaration of Helsinki and the International Council on Harmonisation guidelines on Good Clinical Practice. The study protocol was reviewed and approved by the Institutional Review Board or Independent Ethics Committee at all participating centres. The full trial protocol is available. All patients provided written informed consent.
Procedures
All patients received durvalumab (10 mg/kg every 2 weeks) via intravenous infusion until disease progression was confirmed (unless the investigator considered that the patient would continue to receive benefit from treatment) or unacceptable toxicity, or for up to a maximum of 12 months. Following discontinuation or completion of durvalumab treatment, patients were followed up for survival. Patients who achieved and maintained disease control (ie, complete response, partial response, or stable disease) through to the end of the 12-month treatment period entered follow-up and were offered retreatment on evidence of disease progression.
Permanent discontinuation criteria included patient decision, loss to follow-up, severe non-compliance to the study protocol, one or more of the exclusion criteria being met at study entry, adverse event contraindicating further dosing, any adverse event meeting criteria for discontinuation (grade 4 non-immune-mediated and immune-mediated events [except endocrinopathy; for grade 4 laboratory abnormalities the decision to discontinue was made on the basis of accompanying clinical signs and symptoms and the investigator’s clinical judgment]; some grade 3 immune-mediated events [pneumonitis or interstitial lung disease; diarrhoea; elevations in transaminases >8 times ULN or bilirubin >5 times ULN or any case meeting Hy’s law criteria; elevations in serum creatinine]; grade 3 or 4 infusion reaction), initiation of alternative anticancer therapy, confirmed disease progression, and pregnancy or intent to become pregnant. Dose reductions were not permitted. For grade 2 and 3 non-immune-mediated events, grade 2 immune-mediated events, grade 3 immune-mediated events that did not meet criteria for discontinuation, and grade 4 endocrinopathy, durvalumab treatment was interrupted until resolution of the event to grade 1 or less, or baseline. Isolated grade 2, 3, or 4 hypothyroidism could be treated with replacement therapy without treatment interruption.
Tumour assessments by CT (preferred) or MRI were done during screening and every 8 weeks thereafter (for the first 48 weeks, after which assessments were done every 12 weeks), until confirmed objective disease progression.
Routine clinical and laboratory assessments were done at screening or day 1, and throughout the study. Serum chemistry, haematology, and vital signs were measured every 2 weeks; physical examinations were done every 2 weeks until week 8, then every 4 weeks thereafter; urinalysis, amylase, lipase, and thyroid function were measured every 4 weeks; electrocardiograms were done every 8 weeks. Adverse events and serious adverse events were recorded on or after the date of first dose, up to and including 90 days after the date of the last dose of study medication, and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Blood sampling for pharmacokinetic and immunogenicity analyses was done on days 1 and 29, and every 12 weeks thereafter. A validated electrochemiluminescence assay was used to quantify durvalumab concentrations in serum (lower limit of quantitation of 50 ng/mL) for pharmacokinetic analyses. A validated electrochemiluminescence assay using a Meso Scale Discovery platform (Meso Scale Diagnostics, Rockville, MD, USA) was used to detect anti-drug antibodies.
Outcomes
The primary endpoint was the proportion of patients who achieved an objective response (a confirmed complete response or partial response) in patients whose tumours expressed PD-L1 (≥25% of tumour cells expressing PD-L1 in cohorts 1 and 2; ≥90% of tumour cells expressing PD-L1 in cohort 3), using independent central review according to RECIST version 1.1.19 Secondary activity endpoints (summarised in the appendix, p 10) were overall survival (time from first dose until death due to any cause), progression-free survival (time from first dose until objective disease progression or death), duration of response (time from first confirmed response until disease progression or death), disease control (proportion of patients who had a best objective response of complete response or partial response in the first 6 months or stable disease for 6 months or more), and time to response (time from first dose until the first documented response that was subsequently confirmed). In cohort 2, these activity endpoints were analysed in a number of prespecified patient subsets according to PD-L1 expression and histology, including patients with at least 25% of tumour cells expressing PD-L1 and non-squamous histology, patients with at least 90% of tumour cells expressing PD-L1, and patients with at least 90% of tumour cells expressing PD-L1 and non-squamous histology (a comprehensive list of these prespecified subsets is provided in the appendix, p 10). The activity endpoints were also analysed in patients with at least 90% of tumour cells expressing PD-L1 (both all patients and those with non-squamous histology only) from cohorts 2 and 3 combined. Other secondary endpoints were: safety and tolerability assessed via analyses of adverse events, physical examinations, laboratory findings (including clinical chemistry, haematology, and urinalysis), and vital signs (including blood pressure, pulse, and electrocardiograms); pharmacokinetics; and immunogenicity.
Statistical analysis
The study was designed to enrol and treat approximately 94 patients with prospectively determined PD-L1-expressing tumours in each cohort (≥25% tumour cells expressing PD-L1 in cohorts 1 and 2; ≥90% tumour cells expressing PD-L1 in cohort 3); over-recruitment in cohorts was permitted to accommodate protocol amendment 1. The sample size was determined by estimation precision instead of the power for a hypothesis test, because ATLANTIC was a single-arm trial and our aim was to estimate the treatment effect instead of comparing it to a control group. The length (or half width) of the CI provides a measure for the precision of the treatment estimates. Since the width of the CI is dependent on the point estimate for the proportion of patients who achieved an objective response, two probable scenarios were examined. Under the assumption that 80 patients would have measurable disease per independent central review, two-sided 95% exact CIs for the proportion of patients who achieved an objective response would be 16·0–35·9 or 29·2–51·6, provided the observed proportion of patients who achieved an objective response was 25·0% (based on 20/80 patients responding) or 40·0% (based on 32/80 patients responding). For each of these scenarios, the sample size was considered to provide sufficient precision for the treatment effect estimation, given the width of the CIs.
Final analysis of the primary endpoint was planned for approximately 6 months after the enrolment of the last patient within each individual cohort. The proportion of patients who achieved an objective response and the proportion with disease control (best objective response of complete response or partial response in the first 6 months or stable disease for 6 months or more) were summarised with two-sided 95% exact CIs by the Clopper-Pearson method; duration of response, progression-free survival, and overall survival were assessed via the Kaplan-Meier product-limit method. Descriptive statistics were used to summarise the time to response. The primary analysis was based on the programmatically derived proportion of patients who achieved an objective response using independent central review assessments, and through the use of all scans irrespective of whether they were scheduled or not. An analysis of the proportion of patients who achieved an objective response using the site investigator tumour data according to RECIST version 1.1 was done as a sensitivity analysis to confirm the results of the primary analysis. In cohort 2, sensitivity analyses were also done for the proportion of patients who achieved an objective response using independent central review data according to RECIST version 1.1 in all treated patients who had a baseline tumour assessment and measurable disease at baseline according to the investigator (ie, the full analysis set), and according to RECIST modified for confirmation of progression in the patients evaluable for response per independent central review subset. In cohort 2, a prespecified subgroup analysis of the proportion of patients who achieved an objective response was done (prespecified analyses were by sex, geographical region, race, age, smoking history, WHO performance status, disease stage, site of disease, histology, and line of treatment).
The full analysis set (or intention-to-treat population) was defined as all treated patients who had a baseline tumour assessment and measurable disease at baseline according to the investigator site assessment. The primary endpoint (ie, the proportion of patients who achieved an objective response) and associated response endpoints (ie, duration of response, disease control, and time to response) were analysed in all treated patients who had a baseline tumour assessment and measurable disease at baseline according to the independent central review (patients evaluable for response per independent central review; a subset of the full analysis set). Progression-free survival and overall survival were assessed in the full analysis set. Patients who did not have measurable disease at baseline according to the investigator were excluded from the full analysis set. Patients who did not have measurable disease at baseline according to the independent central review were excluded from the patients evaluable for response per independent central review subset.
Safety data (including adverse events of special interest on the basis of their likely immune cause, and immune-mediated adverse events—ie, an adverse event of special interest requiring treatment with systemic steroids, other immunosuppressants, or endocrine therapy for specific endocrine events, and with no clear alternative cause) and immunogenicity data were summarised in the safety analysis set (all patients who received at least one dose of durvalumab and for whom any post-dose data were available). All patients who received at least one dose of durvalumab per the protocol and for whom any post-dose pharmacokinetic data were available were included in the pharmacokinetic analysis set.
SAS (version 9.1.3 or higher) was used for all analyses. The study is registered with ClinicalTrials.gov, number NCT02087423, and EudraCT, number 2013-005427-16.
Role of the funding source
The funder contributed to the design and implementation of the study, data collection, data management, data analysis, data interpretation, and writing of the report. All authors had full access to the data used to write the report, and the corresponding author had final responsibility for the decision to submit for publication.
Results
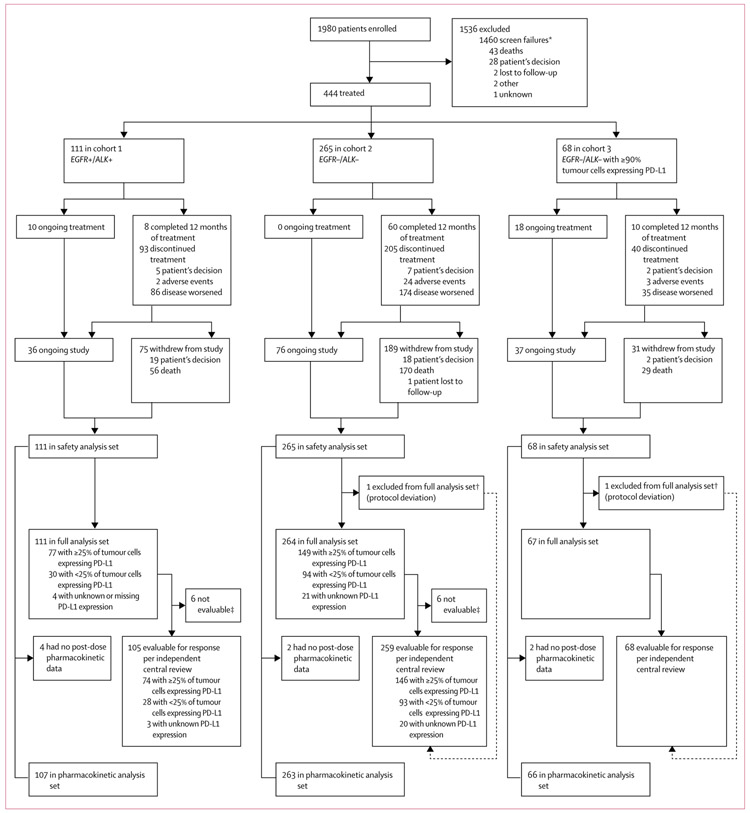
Patients were enrolled between Feb 25, 2014, and Dec 28, 2015. The first protocol amendment to include only patients with at least 25% of tumour cells expressing PD-L1 was implemented on May 13, 2014, and the second protocol amendment to add cohort 3 was implemented on Nov 28, 2014. In cohorts 1 and 2, 38 and 167 patients were enrolled under the original protocol and a further 35 and 92 were enrolled under amendment 1. 1980 patients were enrolled and screened; after exclusions, (mainly for screen failures) 444 patients received durvalumab (safety analysis set): 111 in cohort 1 (EGFR+/ALK+), 265 in cohort 2 (EGFR−/ALK−), and 68 in cohort 3 (EGFR−/ALK−, ≥90% of tumour cells with PD-L1 expression; figure 1). Baseline characteristics are shown in table 1. Patients had received at least two, and as many as 11, previous anticancer regimens. A greater proportion of patients had received at least four previous therapies in cohorts 1 and 2, compared with cohort 3. Previous anticancer treatments are listed in the appendix (p 11). More Asian patients were enrolled in cohort 1 than in cohorts 2 and 3, as expected in a predominantly EGFR+ population.21 Cohort 1 also had a greater proportion of women, patients who had never smoked, and patients with a performance status score of 0, and fewer patients with baseline squamous histology (only one patient), than cohorts 2 and 3. The prevalence of patients with at least 90%, at least 25%, and less than 25% of tumour cells expressing PD-L1 among those screened for participation in the three study cohorts is shown in the appendix (p 12).
Figure 1: Trial profile.
Of the 1980 enrolled patients, 85 were counted twice because they were rescreened because of programmed cell death ligand-1 (PD-L1) expression results not being obtained within the screening window. EGFR+=EGFR tyrosine kinase mutations. ALK+=anaplastic lymphoma kinase (ALK) rearrangements. EGFR−=EGFR wild type. ALK−=ALK rearrangement negative. *Most screen failures occurred because of the protocol amendment to include only patients with at least 25% of tumour cells with PD-L1 expression (patients with <25% of tumour cells with PD-L1 expression enrolled before the amendment who had not started treatment did not go on to receive treatment). †Patients met the independent central review conditions, but did not have measurable disease at baseline according to the investigator; a protocol deviation was reported for each patient. ‡Patients were not evaluable for response per independent central review because they did not have measurable disease at baseline according to the independent central review.
Table 1:
Patient demographics and baseline characteristics (safety analysis set)
| Cohort 1, EGFR+/ALK+ (n=111) |
Cohort 2, EGFR−/ALK−* (n=265) |
Cohort 3, EGFR−/ALK−* (≥90%†; n=68) |
|
|---|---|---|---|
| Median age, years | 61·0 (51·0–67·0) | 62·0 (55·0–68·0) | 61·0 (55·0–67·0) |
| Sex | |||
| Men | 41 (37%) | 162 (61%) | 39 (57%) |
| Women | 70 (63%) | 103 (39%) | 29 (43%) |
| Race or ethnicity | |||
| White | 44 (40%) | 212 (80%) | 42 (62%) |
| Asian | 66 (59%) | 51 (19%) | 24 (35%) |
| Black or African American | 1 (1%) | 2 (1%) | 2 (3%) |
| WHO performance status | |||
| 0 | 45 (41%) | 86 (32%) | 19 (28%) |
| 1 | 65 (59%) | 178 (67%) | 49 (72%) |
| Histology | |||
| Squamous | 1 (1%) | 55 (21%) | 20 (29%) |
| Non-squamous | 110 (99%) | 210 (79%) | 48 (71%) |
| Overall disease classification | |||
| Metastatic | 102 (92%) | 245 (92%) | 61 (90%) |
| Locally advanced | 9 (8%) | 20 (8%) | 7 (10%) |
| Smoking history | |||
| Never smoked | 65 (59%) | 39 (15%) | 9 (13%) |
| Ex-smoker | 42 (38%) | 203 (77%) | 51 (75%) |
| Current smoker | 4 (4%) | 22 (8%) | 8 (12%) |
| CNS metastases‡ | 25 (23%) | 38 (14%) | 9 (13%) |
| Mutation status | |||
| EGER+ | 97 (87%) | 0 | 0 |
| ALK+ | 15 (14%) | 0 | 0 |
| EGER+ and ALK+ | 1 (1%)§ | 0 | 0 |
| Number of previous anticancer regimens | |||
| 2 | 32 (29%) | 106 (40%) | 41 (60%) |
| 3 | 33 (30%) | 70 (26%) | 18 (26%) |
| ≥24 | 46 (41%) | 89 (34%) | 9 (13%) |
| Mean | 3·8 (2·0) | 3·2 (1·4) | 2·6 (0·8) |
| Median | 3·0 (2·0–5·0) | 3·0 (2·0–4·0) | 2·0 (2·0–3·0) |
| Programmed cell death ligand-1 (PD-L1) expression status | |||
| <25% of tumour cells† | 30 (27%)¶ | 95 (36%)¶ | 0 |
| ≥25% of tumour cells† | 77 (69%) | 149 (56%) | 68 (100%) |
| ≥90% of tumour cells† | 47 (42%) | 72 (27%) | 67 (99%)∥ |
| Unknown | 3 (3%)¶ | 21 (8%)¶ | 0 |
| Missing | 1 (1%)¶ | 0 | 0 |
Data are median (IQR), n (%), or mean (SD). EGER+=EGER mutated. ALK+=anaplastic lymphoma kinase (ALK) rearrangements. EGER−=EGER wild type. ALK−=ALK rearrangement negative.
Includes patients with unknown EGER/ALK status.
Tumour cells with membrane staining for PD-L1.
Brain metastases were a study exclusion criterion unless patients were asymptomatic, treated, and stable off steroids and anticonvulsants for at least 1 month before entry into the study.
This patient was also counted in the EGER+ and ALK+ subgroups.
Recruited before the protocol amendment to include only patients with at least 25% of tumour cells with PD-L1 expression.
One patient who had less than 90% (70%) of tumour cells with PD-L1 expression was initially enrolled and treated in cohort 1 but was subsequently discovered to be EGER−/ALK− and, because cohort 2 was no longer enrolling, this patient was enrolled in cohort 3; a protocol deviation was reported, but the patient was still included in analyses.
At data cutoff (June 3, 2016), the median follow-up in all patients and in censored patients only was 6·7 months (IQR 2·5–11·3) and 10·3 months (5·9–13·9), respectively, in cohort 1, 9·2 (3·8–15·7) and 16·9 (15·5–19·0) in cohort 2, and 7·0 (3·4–9·7) and 9·2 (7·1–11·6) in cohort 3. A median of 6·0 (3–15) infusions of durvalumab had been received at data cutoff in cohort 1, 8·0 (4–21) in cohort 2, and 12·5 (4–20) in cohort 3. Cohort 3 had a longer median actual duration of exposure to durvalumab (24·1 weeks [8·0–38·4] excluding dose delays [36 (53%) of 68 patients on treatment for ≥24 weeks]), than cohorts 1 and 2 (12·0 weeks [6·0–30·0; 35 (32%) of 111 patients on treatment for ≥24 weeks] in cohort 1 and 16·1 weeks [8·0–42·3; 107 (40%) of 265 patients on treatment for ≥24 weeks] in cohort 2). The primary reason for ending treatment before completion of the maximum 12-month treatment period was disease progression (figure 1); most study withdrawals were because of death, predominantly due to disease progression. After discontinuation of durvalumab, 100 (23%) of 444 patients received subsequent systemic anticancer therapy—most commonly erlotinib (22 patients; 5%; appendix p 13). Three patients (1%) received subsequent immune checkpoint inhibitors (nivolumab in all cases). After durvalumab, 61 (14%) of 444 patients received subsequent radiotherapy (appendix p 13).
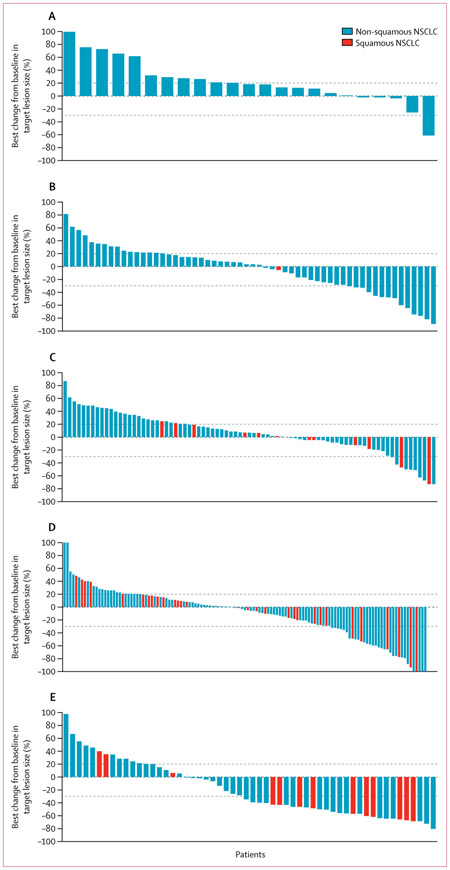
In cohort 1, 77 patients had at least 25% of tumour cells expressing PD-L1, 74 of whom were evaluable for response per independent central review, and 30 patients had less than 25% of tumour cells expressing PD-L1, 28 of whom were evaluable for response per independent central review; four patients (three of whom were evaluable for response per independent central review) had unknown or missing PD-L1 expression status. In cohort 2, 149 patients had at least 25% of tumour cells expressing PD-L1, 146 of whom were evaluable for response per independent central review, and 95 patients had less than 25% of tumour cells expressing PD-L1, 93 of whom were evaluable for response per independent central review; 21 patients (20 of whom were evaluable for response per independent central review) had unknown status. All 68 patients in cohort 3 were evaluable for response per independent central review. One patient in cohort 3 had less than 90% (70%) of tumour cells with PD-L1 expression. In the patients with tumours expressing PD-L1, the proportion of patients who achieved an objective response was 9 (12·2%, 95% CI 5·7–21·8) of 74 patients in cohort 1 (EGFR+/ALK+, ≥25% of tumour cells expressing PD-L1), 24 (16·4%, 10·8–23·5) of 146 patients in cohort 2 (EGFR−/ALK−, ≥25% of tumour cells expressing PD-L1), and 21 (30·9%, 20·2–43·3) of 68 patients in cohort 3 (EGFR−/ALK−, ≥90% of tumour cells expressing PD-L1; table 2). More patients with higher tumour expression of PD-L1 achieved an objective response than patients with lower or no PD-L1 expression, irrespective of EGFR/ALK status (table 2; appendix pp 14–15). In cohort 2, of the 146 patients with at least 25% of tumour cells with PD-L1 expression, 70 had at least 90% of cells with PD-L1 expression; the proportion of this group who had an objective response was 11 (15·7%, 95% CI 8·1–26·4) of 70 patients. In cohorts 2 and 3 combined, 138 patients with at least 90% of cells expressing PD-L1 were included, with an objective response of 32 (23·2%, 16·4–31·1) of 138 patients (appendix p 14). Results in subsets of patients with non-squamous histology from cohorts 2 and 3 were consistent with those of the overall population (appendix p 15). The sensitivity analyses were generally consistent with the primary analysis (data not shown). Our prespecified analysis of the proportion of patients who achieved an objective response according to subgroups by demographics and disease characteristics in cohort 2 showed consistent results across all subgroups of patients with at least 25% of tumour cells expressing PD-L1 (appendix p 4). Greater antitumour activity in patients with higher tumour PD-L1 expression levels (ie, ≥25% or ≥90% of tumour cells expressing PD-L1) was also apparent for best change in tumour size compared with baseline (figure 2). The median time to response from the first dose ranged from 1·8 (IQR 1·8–1·8) to 2·1 (1·8–3·7) months across the cohorts (table 2). Findings for duration of response showed that responses were durable in all cohorts irrespective of PD-L1 expression status (table 2). In cohort 1, in patients with at least 25% of tumour cells expressing PD-L1, five (56%) of nine responders were progression free at data cutoff (June 3, 2016); the responder with less than 25% of tumour cells expressing PD-L1 had progressed after their partial response. In cohort 2, in patients with less than 25% of tumour cells expressing PD-L1, four (57%) of seven were progression free at data cutoff, compared with 12 (50%) of 24 responders with 25% or more tumour cells expressing PD-L1. In cohort 3, 18 (86%) of 21 responders were progression free at data cutoff (appendix p 5). Disease control rates at 6 months were higher in patients with EGFR−/ALK− versus EGFR+/ALK+ NSCLC and in patients with higher PD-L1 expression versus those with lower or no PD-L1 expression (table 2).
Table 2:
Summary of clinical activity
| Cohort 1, EGFR+/ALK+ |
Cohort 2, EGFR−/ALK−* |
Cohort 3, EGFR−/ALK−* (≥90%†) |
|||
|---|---|---|---|---|---|
| <25%† | ≥25%†‡ | <25%† | ≥25%†‡ | ||
| Patients evaluable for response per independent central review§ | |||||
| Total | 28 | 74 | 93 | 146 | 68 |
| Confirmed objective response | 1 (3·6%, 0·1–18·3) | 9 (12·2%, 5·7–21·8) | 7 (7·5%, 3·1–14·9) | 24 (16·4%, 10·8–23·5) | 21 (30·9%, 20·2–43·3) |
| Confirmed disease control at 6 months¶ | 2 (7·1%, 0·9–23·5) | 15 (20·3%, 11·8–31·2) | 19 (20·4%, 12·8–30·1) | 42 (28·8%, 21·6–36·8) | 26 (38·2%, 26·7–50·8) |
| Best overall response | |||||
| Complete response | 0 | 0 | 0 | 1 (1%) | 0 |
| Partial response | 1 (4%) | 9 (12%) | 7 (8%) | 23 (16%) | 21 (31%) |
| Stable disease∥ | 5 (18%) | 23 (31%) | 27 (29%) | 51 (35%) | 12 (18%) |
| Progressive disease | 22 (79%) | 40 (54%) | 59 (63%) | 70 (48%) | 35 (51%) |
| Not evaluable | 0 | 2 (3%) | 0 | 1 (1%) | 0 |
| TTR, months | 1·8 (1·8–1·8) | 1·8 (1·8–1·8) | 2·1 (1·8–3·7) | 1·9 (1·8–3·7) | 1·9 (1·8–3·5) |
| DoR, months | 7·9 (7·9–7·9) | 7·4 (5·6–9·2) | NR (7·2–NR) | 12·3 (7·5–NR) | NR (NR–NR) |
| Full analysis set** | |||||
| Total | 30 | 77 | 94 | 149 | 67 |
| PFS, months | 1·9 (1·8–1·9) | 1·9 (1·8–3·6) | 1·9 (1·8–1·9) | 3·3 (1·9–3·7) | 2·4 (1·8–5·5) |
| OS, months | 9·9 (4·2–13·0) | 13·3 (8·1–NC) | 9·3 (5·9–10·8) | 10·9 (8·6–13·6) | NR (5·9–NC) |
| OS at 1 year | 40·0% (22·1–57·4) | 54·8% (41·5–66·3) | 34·5% (25·0–44·1) | 47·7% (39·3–55·5) | 50·8% (36·9–63·2) |
| OS follow-up, months | 8·2 (3·0–13·3) | 6·5 (2·5–10·9) | 9·3 (3·8–15·3) | 9·4 (4·2–15·9) | 7·0 (3·4–9·7) |
Data are n, n (%, 95% CI), n (%), median (IQR), or % (95% CI). EGER+=EGER mutated. ALK+=anaplastic lymphoma kinase (ALK) rearrangements. EGER−=EGER wild type. ALK−=ALK rearrangement negative. TTR=time to response. DoR=duration of response. NR=not reached. PFS=progression-free survival. OS=overall survival. NC=not calculated.
Includes patients with unknown EGER/ALK status.
Tumour cells with membrane staining for programmed cell death ligand-1 (PD-L1). Four patients in cohort 1 and 21 patients in cohort 2 had an unknown or missing PD-L1 expression status.
Patients with ≥90% of tumour cells expressing PD-L1 are included within the ≥25% group.
All treated patients who had a baseline tumour assessment and measurable disease at baseline according to the independent central review.
Confirmed complete or partial response, or stable disease for 6 months or more.
Unconfirmed complete or partial response, or stable disease for at least 8 weeks.
All treated patients who had a baseline tumour assessment and had measurable disease at baseline according to the investigator site assessment.
Figure 2: Best change from baseline in tumour size over time.
(A) Cohort 1 (EGFR+/ALK+ non-small-cell lung cancer [NSCLC]) patients with less than 25% of tumour cells expressing programmed cell death ligand-1 (PD-L1). (B) Cohort 1 patients with at least 25% of tumour cells expressing PD-L1. (C) Cohort 2 (EGFR−/ALK− NSCLC) patients with less than 25% of tumour cells expressing PD-L1. (D) Cohort 2 patients with at least 25% of tumour cells expressing PD-L1. (E) Cohort 3 (EGFR−/ALK− NSCLC) patients with at least 90% of tumour cells expressing PD-L1. Dashed reference lines at −30% and +20% indicate thresholds for partial response and disease progression. Values greater than 100% or less than −100% are displayed as 100% and −100%. The charts show patients evaluable for response per independent central review (all treated patients who had a baseline tumour assessment and had measurable disease at baseline according to the independent central review; patients also had to have at least one post-baseline tumour assessment to be included in the analysis). EGFR+=EGFR tyrosine kinase mutations.
ALK+=anaplastic lymphoma kinase (ALK) rearrangements. EGFR−=EGFR wild type. ALK−=ALK rearrangement negative.
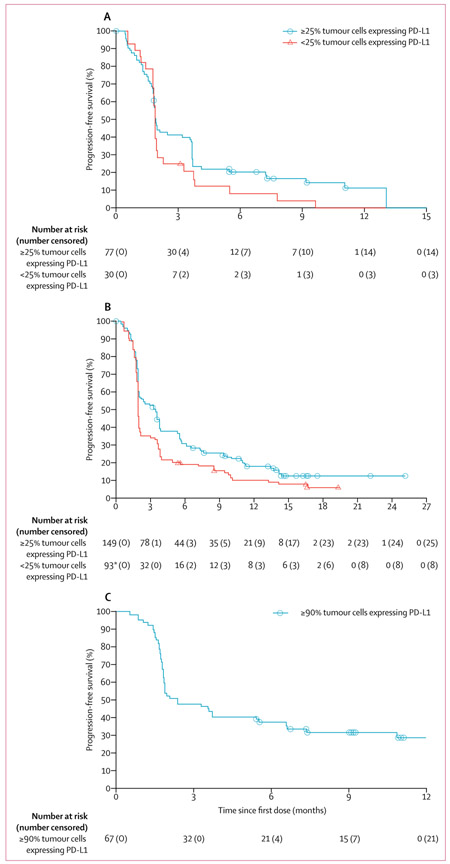
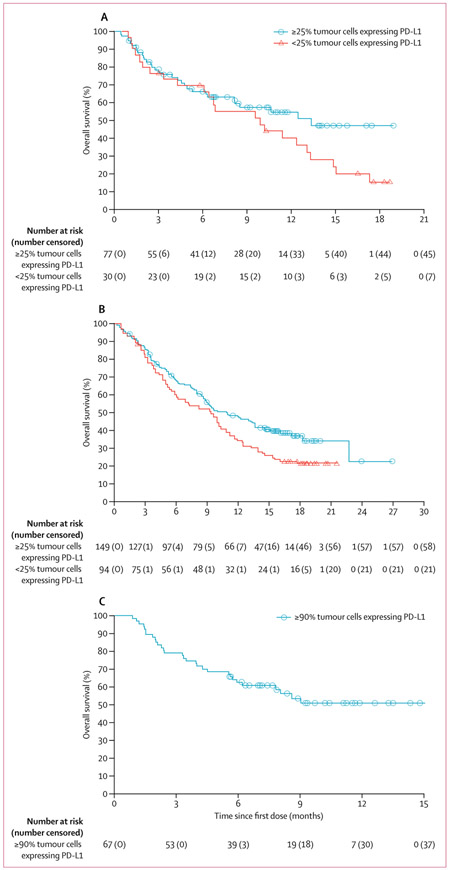
At data cutoff, 63 (82%) of 77 patients in cohort 1 (≥25% of tumour cells expressing PD-L1), 124 (83%) of 149 in cohort 2 (≥25% of tumour cells expressing PD-L1), and 46 (69%) of 67 in cohort 3 had disease progression or died in the full analysis set. Median progression-free survival was longer for patients with higher PD-L1 expression (ie, ≥25% or ≥90% of tumour cells expressing PD-L1) versus those with lower or no expression (ie, <25% of tumour cells expressing PD-L1) in patients with EGFR−/ALK− NSCLC (ie, patients in cohorts 2 and 3), but not in those with EGFR+/ALK+ NSCLC (ie, patients in cohort 1; table 2, figure 3). At data cutoff, 32 (42%) of 77 patients in cohort 1 (≥25% of tumour cells expressing PD-L1), 91 (61%) of 149 in cohort 2 (≥25% of tumour cells expressing PD-L1), and 30 (45%) of 67 in cohort 3 had died. Median overall survival was longer for patients with at least 25% of tumour cells with PD-L1 expression versus those with less than 25%, regardless of EGFR/ALK status (table 2, figure 4). At data cutoff, median overall survival had not been reached in cohort 3 (table 2, figure 4).
Figure 3: Progression-free survival.
(A) Cohort 1 (EGFR+/ALK+ non-small-cell lung cancer [NSCLC]; full analysis set).
(B) Cohort 2 (EGFR−/ALK− NSCLC; full analysis set). (C) Cohort 3 (EGFR−/ALK− NSCLC with ≥90% tumour cells with programmed cell death ligand-1 [PD-L1] expression; full analysis set). EGFR+=EGFR tyrosine kinase mutations. ALK+=anaplastic lymphoma kinase (ALK) rearrangements.
EGFR−=EGFR wild type. ALK−=ALK rearrangement negative. *In patients with less than 25% of tumour cells expressing PD-L1, the number of patients at risk is per independent central review and therefore smaller than the total number of patients in the full analysis set.
Figure 4: Overall survival.
(A) Cohort 1 (EGFR+/ALK+ non-small-cell lung cancer [NSCLC]; full analysis set). (B) Cohort 2 (EGFR−/ALK− NSCLC; full analysis set). (C) Cohort 3 (EGFR−/ALK− NSCLC with ≥90% tumour cells with programmed cell death ligand-1 [PD-L1] expression; full analysis set). EGFR+=EGFR tyrosine kinase mutations.
ALK+=anaplastic lymphoma kinase (ALK) rearrangements. EGFR−=EGFR wild type. ALK−=ALK rearrangement negative.
In an exploratory post-hoc analysis (appendix pp 6–7, p 16), clinical activity in patients with at least 25% of tumour cells with PD-L1 expression from cohort 1 was analysed according to whether they had EGFR+ or ALK+ NSCLC (one patient had both EGFR mutation and ALK rearrangement). All objective responses in cohort 1 occurred in EGFR+ patients; the proportion of patients with at least 25% of tumour cells with PD-L1 expression who achieved an objective response was 9 (14·1%, 95% CI 6·6–25·0) of 64 patients. Baseline characteristics of the ten responders in cohort 1 (including the responder with <25% of tumour cells expressing PD-L1) are provided in the appendix (p 17).
256 (58%) of 444 patients had treatment-related adverse events (table 3), the most common of which were fatigue (50 [11%]), hypothyroidism (36 [8%]), asthaenia (31 [7%]), nausea (28 [6%]), pruritus (28 [6%]), diarrhoea (27 [6%]), pyrexia (26 [6%]; data not shown), hyperthyroidism (24 [5%]; data not shown), and decreased appetite (24 [5%]; data not shown). Treatment-related grade 3 or 4 adverse events occurred in 40 (9%) of 444 patients—six (5%) of 111 in cohort 1 (EGFR+/ALK+), 22 (8%) of 265 in cohort 2 (EGFR−/ALK−), and 12 (18%) of 68 in cohort 3 (EGFR−/ALK−, ≥90% of tumour cells expressing PD-L1). Grade 3 or 4 events reported by more than one patient were pneumonitis (four [1%]), elevated gamma-glutamyltransferase (four [1%]), diarrhoea (three [1%]), infusion-related reaction (three [1%]), elevated aspartate aminotransferase (two [<1%]), elevated transaminases (two [<1%]), vomiting (two [<1%]), and fatigue (two [<1%]; table 3). All patients with treatment-related grade 3 or 4 pneumonitis, diarrhoea, infusion-related reaction, elevated transaminases, and vomiting recovered from these adverse events. Of the four patients with at least grade 3 elevated gamma-glutamyltransferase, one recovered, for two the event was ongoing at the time of death (20 and 119 days after the onset of the event), and for one the event was ongoing at the time they withdrew consent (approximately 3 months after the onset of the event). Of the two patients with grade 3 elevated aspartate aminotransferase, one was treated with steroids and recovered, and for the other patient the event was ongoing at the time of death (event reported on day 166, patient discontinued durvalumab because of disease progression on day 167, and died on day 169 because of NSCLC). Of the two patients with grade 3 fatigue, for one patient the event was ongoing at the time they withdrew consent, for the other patient the event was ongoing 49 days after onset (7 days after becoming grade 3) when the patient discontinued durvalumab because of disease progression.
Table 3:
Treatment-related adverse events (safety analysis set)
| Cohort 1, EGFR+/ALK+ (n=111) | Cohort 2, EGFR−/ALK−* (n=265) | Cohort 3, EGFR−/ALK−* (≥90%†; (n=68) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Any events‡ | 47 (42%) | 4 (4%) | 2 (2%) | 135 (51%) | 20 (8%) | 2 (1%) | 34 (50%) | 12 (18%) | 0 |
| Fatigue | 7 (6%) | 0 | 0 | 28 (11%) | 1 (<1%) | 0 | 13 (19%) | 1 (1%) | 0 |
| Hypothyroidism | 10 (9%) | 0 | 0 | 17 (6%) | 0 | 0 | 9 (13%) | 0 | 0 |
| Asthenia | 4 (4%) | 0 | 0 | 26 (10%) | 1 (<1%) | 0 | 0 | 0 | 0 |
| Nausea | 6 (5%) | 0 | 0 | 15 (6%) | 1 (<1%) | 0 | 6 (9%) | 0 | 0 |
| Pruritus | 2 (2%) | 0 | 0 | 18 (7%) | 0 | 0 | 8 (12%) | 0 | 0 |
| Diarrhoea | 4 (4%) | 0 | 0 | 17 (6%) | 1 (<1%) | 0 | 3 (4%) | 2 (3%) | 0 |
| Vomiting | 6 (5%) | 0 | 0 | 4 (2%) | 2 (1%) | 0 | 2 (3%) | 0 | 0 |
| Anaemia | 1 (1%) | 0 | 0 | 7 (3%) | 0 | 0 | 0 | 1 (1%) | 0 |
| Pneumonitis | 1 (1%) | 1 (1%) | 0 | 3 (1%) | 3 (1%) | 0 | 1 (1%) | 0 | 0 |
| Aspartate aminotransferase increased | 1 (1%) | 0 | 0 | 1 (<1%) | 0 | 0 | 2 (3%) | 2 (3%) | 0 |
| Gamma-glutamyltransferase increased |
0 | 0 | 0 | 2 (1%) | 3 (1%) | 1 (<1%) | 0 | 0 | 0 |
| Infusion-related reaction | 0 | 1 (1%) | 0 | 3 (1%) | 1 (<1%) | 0 | 0 | 1 (1%) | 0 |
| Malaise | 2 (2%) | 0 | 0 | 2 (1%) | 1 (<1%) | 0 | 1 (1%) | 0 | 0 |
| Rash maculo-papular | 2 (2%) | 0 | 0 | 2 (1%) | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
| Hypotension | 0 | 0 | 0 | 2 (1%) | 0 | 0 | 0 | 1 (1%) | 0 |
| Tumour flare | 0 | 0 | 0 | 2 (1%) | 1 (<1%) | 0 | 0 | 0 | 0 |
| Adrenal insufficiency | 0 | 0 | 0 | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 | 0 |
| Bradycardia | 0 | 0 | 0 | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 | 0 |
| Dermatitis | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 1 (1%) | 0 | 0 |
| Lymphocyte count decreased | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
| Lymphopenia | 0 | 0 | 0 | 1 (<1%) | 0 | 1 (<1%) | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 1 (1%) | 0 | 0 |
| Transaminases increased | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 1 (1%) | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Agitation | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Bronchitis | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diabetes insipidus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Diverticulitis | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Gastrointestinal disorder | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Hepatic atrophy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Hepatic enzyme increased | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Hypokalaemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Hypopituitarism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Hypovolaemic shock | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 |
| Meningitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Neutrophil count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Pericardial effusion | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spinal cord compression | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tumour haemorrhage | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
Data are n (%). Grade 1–2 events occurring in at least 10% of patients in any cohort, and all events of grade 3 or worse. Adverse events are reported in accordance with the Medical Dictionary for Regulatory Activities (version 19.0)-preferred term. Events are listed in order of descending frequency in the total population. Events causally related to treatment, as assessed by the investigator; missing responses are counted as related. There were no treatment-related deaths at the time of analysis. Includes adverse events with an onset date on or after the date of first dose or pretreatment adverse events that increase in severity on or after the date of first durvalumab dose up to and including 90 days following the date of last dose of study medication or up to and including the date of initiation of the first subsequent therapy (whichever occurred first). EGER+=EGER tyrosine kinase mutations. ALK+= anaplastic lymphoma kinase (ALK) rearrangements. EGER−=EGER wild type. ALK−=ALK rearrangement negative.
Includes patients with unknown EGER/ALK status.
Tumour cells with membrane staining for programmed cell death ligand-1.
Each patient has been represented once with the maximum reported Common Terminology Criteria for Adverse Events (version 4.03) grade for each adverse event. If a patient has multiple events within an adverse event, then the maximum reported Common Terminology Criteria for Adverse Events grade across those events is counted for that preferred term.
Treatment-related serious adverse events occurred in 27 (6%) of 444 patients (cohort 1, five [5%] of 111; cohort 2, 14 [5%] of 265; cohort 3, eight [12%] of 68; appendix p 18). The most common overall were pneumonitis (five [1%]), fatigue (three [1%]), and infusion-related reaction (three [1%]). Treatment-related adverse events leading to treatment discontinuation occurred in ten (2%) of 444 patients (cohort 1, one [1%] of 111; cohort 2, eight [3%] of 265; cohort 3, one [1%] of 68); these comprised pneumonitis (n=3), elevated hepatic enzymes (n=2), anaemia (n=1), hypovolaemic shock (n=1), nephritis (n=1), infusion-related reaction (n=1), and diarrhoea (n=1). All events were grade 2 or 3.
159 (36%) of 444 patients died while on treatment or within 90 days of the last dose of durvalumab; most (n=146) were considered to have died solely as a result of their underlying NSCLC. Of the remaining 13 patients who died in this timeframe, four were considered to have died both as a result of their underlying NSCLC and a fatal adverse event (considered not to be related to durvalumab); the fatal adverse events were pneumonia, pulmonary sepsis, pulmonary embolism, and acute myocardial infarction (appendix p 19). Two patients were lost to follow-up or withdrew consent; thus, cause of death is unknown. Seven patients died solely due to a fatal adverse event; for six patients, the fatal adverse event was considered not to be related to durvalumab, whereas for one patient, the relation of the event (pneumonitis) to durvalumab could not be completely excluded. The fatal treatment-related pneumonitis event occurred after the start of subsequent therapy. The patient developed pneumonitis 65 days after discontinuing durvalumab because of disease progression and 2 days after starting subsequent therapy with erlotinib. There were no treatment-related deaths before the start of subsequent therapy. Causes of death are summarised in the appendix (p 19).
140 (32%) of 444 patients had a treatment-related adverse event of special interest (cohort 1, 28 [25%] of 111; cohort 2, 80 [30%] of 265; cohort 3, 32 [47%] of 68; appendix p 20). 12 (3%) of 444 patients had a treatment-related adverse event of special interest of pneumonitis (cohort 1, two [2%] of 111; cohort 2, seven [3%] of 265; cohort 3, three [4%] of 68). The two patients in cohort 1 who had pneumonitis had both received three previous EGFR tyrosine kinase inhibitors (appendix p 2). Grade 3 or 4 treatment-related adverse events of special interest were reported in 22 (5%) of 444 patients (cohort 1, two [2%] of 111; cohort 2, 12 [5%] of 265; cohort 3, eight [12%] of 68). Overall, 55 (12%) of 444 patients had an immune-mediated adverse event; these events are summarised in table 4.
Table 4:
Immune-mediated adverse events* by grouped preferred term (safety analysis set)
| Cohort 1, EGFR+/ALK+ (n=111) |
Cohort 2, EGFR−/ALK−† (n=265) |
Cohort 3, EGFR−/ALK−† (≥90%‡; n=68) |
||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | |
| Any event§ | 13 (12%) | 1 (1%) | 22 (8%) | 5 (2%) | 9 (13%) | 5 (7%) |
| Adrenal insufficiency | 0 | 0 | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Colitis | 0 | 0 | 1 (<1%) | 0 | 1 (1%) | 0 |
| Dermatitis | 1 (1%) | 0 | 2 (1%) | 1 (<1%) | 0 | 0 |
| Diarrhoea | 1 (1%) | 0 | 1 (<1%) | 0 | 0 | 1 (1%) |
| Hyperthyroidism | 3 (3%) | 0 | 4 (2%) | 0 | 3 (4%) | 0 |
| Hypophysitis | 0 | 0 | 0 | 0 | 0 | 1 (1%) |
| Hypothyroidism | 11 (10%) | 0 | 13 (5%) | 0 | 8 (12%) | 0 |
| Pneumonitis | 1 (1%) | 1 (1%) | 3 (1%) | 2 (1%) | 2 (3%) | 0 |
| Rash | 0 | 0 | 1 (<1%) | 0 | 1 (1%) | 1 (1%) |
| Select hepatic events | 0 | 0 | 0 | 1 (<1%) | 0 | 2 (3%) |
Data are n (%). Adverse events are reported in accordance with the Medical Dictionary for Regulatory Activities (version 19.0)-preferred term. There were no grade 4 or 5 immune-mediated adverse events at the time of analysis. Includes adverse events with an onset date on or after the date of first dose or pretreatment adverse events that increase in severity on or after the date of first durvalumab dose up to and including 90 days following the date of last dose of study medication or up to and including the date of initiation of the first subsequent therapy (whichever occurred first). ALK+= anaplastic lymphoma kinase (ALK) rearranged. ALK−=ALK rearrangement negative. EGFR+=EGFR mutated. EGER−=EGER wild type.
Adverse events of special interest that required the use of systemic steroids, other immunosuppressants, or endocrine therapy, and with no clear other cause.
Includes patients with unknown EGFR/ALK status.
Tumour cells with membrane staining for programmed cell death ligand-1.
Each patient has been represented once with the maximum reported Common Terminology Criteria for Adverse Events (version 4.03) grade for each adverse event, sorted alphabetically by grouped term. If a patient has multiple events within an adverse event, then the maximum Common Terminology Criteria for Adverse Events grade across those events is counted for that preferred term.
After treatment with durvalumab (10 mg/kg) every 2 weeks, the durvalumab trough concentrations were similar in the three cohorts at all the timepoints (appendix pp 2 and 21). A low incidence (16 [4%] of 442 patients) of immunogenicity was observed (appendix pp 2 and 22). No association between the development of anti-drug antibodies and pharmacokinetic exposure was observed, nor was any effect of anti-drug antibodies on efficacy or safety observed (data not shown).
Discussion
The results of the ATLANTIC study show that the anti-PD-L1 monoclonal antibody durvalumab has clinical activity and an acceptable tolerability profile in heavily pretreated patients with advanced NSCLC. Durable responses and encouraging overall survival data were observed across cohorts of patients with NSCLC defined by EGFR and ALK status and tumour PD-L1 expression. Our findings confirm preliminary results from a previous phase 1–2 study of durvalumab in patients with advanced NSCLC,17 and show that the clinical activity and safety profile of durvalumab is consistent with other anti-PD-1 and anti-PD-L1 agents.
We report the final analysis of the primary efficacy endpoint, the proportion of patients who achieved an objective response. Responses were recorded across the three cohorts, and the proportions of patients who achieved a response were generally lower in patients with EGFR+/ALK+ NSCLC than in those with EGFR−/ALK− NSCLC. Higher PD-L1 expression enriched for response both in patients with EGFR+/ALK+ NSCLC (cohort 1) and those with EGFR−/ALK− NSCLC (cohort 2). The highest proportion of patients achieving an objective response (31%) was in cohort 3 (EGFR−/ALK−, ≥90% of tumour cells expressing PD-L1), although the proportion with an objective response in a subset of patients in cohort 2 with at least 90% of tumour cells expressing PD-L1 was lower (16%). Thus, the higher proportion of patients who achieved an objective response in cohort 3 might be due to a factor other than the increased PD-L1 expression; for example, patients in cohort 3 were less heavily pretreated than those in cohort 2. Good durability of response was seen across responders in all cohorts, irrespective of PD-L1 expression; however, duration of follow-up was not consistent between the cohorts. In the cohort 2 subpopulation with at least 25% of tumour cells expressing PD-L1, the proportions of patients achieving an objective response were similar in all subgroups by baseline characteristics, including squamous and non-squamous histologies, current or former smokers and those who had never smoked, and CNS metastases (present and absent).
Irrespective of EGFR or ALK status, median overall survival was higher in patients with at least 25% of tumour cells expressing PD-L1 (approximately 11–13 months), although the values in patients with less than 25% of tumour cells expressing PD-L1 (approximately 9–10 months) still remain encouraging. At data cutoff, median overall survival had not been reached in cohort 3, although the overall survival at 1 year was favourable at 51%.
The ATLANTIC data reflect the durvalumab clinical activity observed in a phase 1–2 study17 in patients with advanced NSCLC: the proportion of patients who achieved an objective response was 17·5% in the overall population (n=285), and 13·0% in the third-line and later treatment setting (n=146). Similar to ATLANTIC, the proportion of patients with at least 25% of tumour cells expressing PD-L1 who achieved an objective response was higher, and their overall survival was longer, than patients with less than 25% of tumour cells expressing PD-L1 (in patients treated with third-line and later durvalumab, 22·0% vs 6·1% achieved an objective response, and median overall survival was 13·0 vs 7·6 months).17
Although several immune checkpoint inhibitor trials have included patients with advanced NSCLC who have received at least two previous lines of therapy, few have focused only on the third-line and later setting, and none have prospectively analysed EGFR-driven or ALK-driven tumours. In CheckMate 063,22 a phase 2, single-arm trial of third-line and later nivolumab in advanced squamous NSCLC, the proportion of patients who achieved an objective response was 14·5% in the overall population and 24% in patients with at least 5% of tumour cells with PD-L1 expression; median progression-free survival was 1·9 months (95% CI 1·8–3·2) and median overall survival was 8·2 months (6·1–10·9) in the overall population. In patients with previously treated advanced NSCLC who received pembrolizumab in the uncontrolled KEYNOTE 001 trial, the proportion of patients who achieved an objective response was 18%, median progression-free survival was 3·0 months (2·2–4·0), and median overall survival was 9·3 months (8·4–12·4).23 These activities seem generally comparable with that of durvalumab in the treatment of patients with EGFR−/ALK− NSCLC in ATLANTIC.
The inclusion of an independent cohort of patients with EGFR+/ALK+ NSCLC in ATLANTIC permitted prospective assessment of an immune checkpoint inhibitor in a population who have a distinct clinical course and prognosis compared with EGFR−/ALK− patients.24 Retrospective analyses in patients with advanced NSCLC have shown no survival benefit of anti-PD-1 or anti-PD-L1 over docetaxel in EGFR+ subgroups (overall survival hazard ratios vs docetaxel: nivolumab 1·18, 95% CI 0·69–2·00;25 pembrolizumab 0·88, 0·45–1·70;26 atezolizumab 1·24, 0·71–2·1827 and 0·99, 0·29–3·40).28 A meta-analysis29 of three of these studies25,26,28 showed that the immune checkpoint inhibitors significantly prolonged overall survival compared with docetaxel in the overall population and EGFR− subgroup, but not in the EGFR+ subgroup. The preliminary antitumour activity and overall survival data observed in the present study with durvalumab in heavily pretreated patients with EGFR+ NSCLC (including previous EGFR tyrosine kinase inhibitor therapy) with at least 25% of tumour cells with PD-L1 expression seem encouraging on the basis of historical comparisons with studies in patients with pretreated, predominantly EGFR− NSCLC.22,23,25-28 The proportion of patients with at least 25% of tumour cells expressing PD-L1 who achieved an objective response in cohort 1 (EGFR+/ALK+) was not substantially lower than that in cohort 2 (EGFR−/ALK−; 12·2% vs 16·4%) and the difference in the proportion of patients achieving a response was even smaller when focusing on the EGFR+ subpopulation (proportion of EGFR+ patients in cohort 1 who achieved an objective response 14·1%; appendix p 16). However, patients with EGFR+ NSCLC with PD-L1 expression in at least 25% of cells are a small subset (approximately 26% of patients with EGFR+/ALK+ NSCLC and 24% of patients with EGFR+ NSCLC in this study; appendix p 12). The small number of patients with ALK+ NSCLC leaves the role of immune checkpoint inhibitors unresolved in this subpopulation.
Both ALK+ and EGFR+ lung cancer, which mostly occur in patients who have never smoked, have been shown to have low mutational burden.30 This low burden might explain the reduced activity of immune checkpoint inhibitors in patients with EGFR+ NSCLC, compared with patients with EGFR− NSCLC, observed consistently across studies.29 So far, little is known about the responsiveness of ALK+ NSCLC to immune checkpoint inhibitors. With no comparator arm, the uncontrolled nature of data from the ATLANTIC study is subject to patient selection; thus, further study in both EGFR+ and ALK+ subpopulations is warranted. Furthermore, because the duration of follow-up varied across the cohorts, subsequent analyses of the data from this study will be needed to determine whether the duration of responses is similar between patients with EGFR+/ALK+ NSCLC and those with EGFR−/ALK− NSCLC. Future analyses could also include evaluation of whether durvalumab activity in the EGFR+/ALK+ population is related to increased tumour mutational burden after multiple disease progressions, and whether patients with EGFR+ NSCLC progressed because of the onset of EGFR Thr790Met mutation.
Durvalumab monotherapy had an acceptable tolerability profile in the ATLANTIC study: most adverse events were low grade, and immune-mediated adverse events were manageable with standard treatment guidelines. The percentage of patients who discontinued durvalumab because of treatment-related adverse events was low across all three cohorts. Cohort 3 had higher incidences of treatment-related grade 3 or 4 adverse events, serious adverse events, adverse events of special interest, and immune-mediated adverse events than cohorts 1 and 2, which might be explained by the longer duration of treatment exposure in cohort 3. In cohort 1, previous treatment with at least one tyrosine kinase inhibitor was a study inclusion criterion; however, the proportion of patients who had a treatment-related adverse event of special interest of pneumonitis was lower in cohort 1 than in cohorts 2 and 3. In cohort 1, 98% of patients did not experience pneumonitis, which suggests there is no increased risk of pneumonitis in patients treated with durvalumab following previous tyrosine kinase inhibitors at some point in their treatment history. The safety profile of durvalumab in ATLANTIC was consistent with other anti-PD-1 and anti-PD-L1 monoclonal antibodies in previously treated patients with advanced NSCLC.25-27
Limitations of our study include the absence of a comparator arm and the short duration of follow-up, particularly in cohorts 1 and 3. Comparison between the cohorts was not an objective of the study; thus, no formal statistical comparison was done. The three cohorts were independent and enrolled in different timeframes. Between-cohort differences in treatment exposure (cohort 1 had the shortest duration and cohort 3 had the longest duration) and demographic and disease characteristics (in general, the characteristics of cohorts 2 and 3 were similar and representative of an EGFR−/ALK− population, whereas cohort 1 showed differences consistent with their EGFR+/ALK+ status) further preclude any informal comparison of efficacy or safety results. The inclusion criterion of WHO performance status score of 0 or 1 is a good performance status for such heavily pretreated patients; therefore, the trial population might not be truly representative of real-world patients. However, at the time that ATLANTIC was initiated, the safety profile of durvalumab in patients with NSCLC after at least two previous therapies was unknown. The performance status restriction was a means to ensure patients were fit enough to tolerate unanticipated toxicities, and is in keeping with trials that included patients in the third-line and later treatment setting.22,23,27,28
The ATLANTIC study makes an important contribution to the body of evidence on the efficacy of immune checkpoint inhibitors in NSCLC. Our results show that durvalumab has clinical activity in patients with NSCLC who are heavily pretreated; the proportion of patients achieving a response were higher in patients whose tumours expressed higher levels of PD-L1, but responses were durable irrespective of PD-L1 expression status. Although clinical applications of these data might be few, one potentially interesting question is whether durvalumab has a role in the treatment of EGFR+ tumours with high PD-L1 expression, and could be the subject of further clinical investigation.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for reports published in English from Jan 1, 2010, to July 31, 2017, using the terms “advanced non-small-cell lung cancer” AND (“anti-PD-1” OR “anti-PD-LL”) AND (“recurrent” OR “relapsed” OR “previously treated” OR “third-line”), and limited our results to clinical trials. We did similar searches in PubMed, adding the terms “EGFR mutation” and “ALK rearrangement”. Although several trials of immune checkpoint inhibitors in advanced non-small-cell lung cancer (NSCLC) have included some patients who received at least two previous lines of therapy, few have focused on a heavily pretreated population. CheckMate 063, a phase 2, single-arm, nivolumab trial, exclusively enrolled patients who had previously received two or more treatments. We found no studies that prospectively analysed immune checkpoint inhibitors in patients with EGFR-driven or ALK-driven tumours, although retrospective analyses suggest that tumours with EGFR mutations or ALK rearrangements (EGFR+/ALK+) respond less well to immunotherapy than tumours without EGFR or ALK genetic aberrations (EGFR−/ALK−). Most studies suggest that patients with advanced NSCLC with higher tumour expression of PD-L1 achieve improved responses to anti-PD-1 and anti-PD-L1 treatment (eg, nivolumab, pembrolizumab, and atezolizumab) compared with patients with lower PD-L1 expression. A phase 1–2 study of durvalumab showed that patients with advanced NSCLC with at least 25% of tumour cells expressing PD-L1 had a higher objective response rate and longer overall survival than patients with less than 25% of tumour cells expressing PD-L1.
Added value of this study
In our heavily pretreated patient population, durvalumab had clinical activity and a tolerability profile consistent with other anti-PD-1 and anti-PD-L1 agents. Durable responses and encouraging overall survival data were observed across all cohorts, with a higher proportion of patients with EGFR−/ALK− tumours achieving a response versus patients with EGFR+/ALK+ tumours. Clinical activity was generally enhanced in patients with increased tumour expression of PD-L1, irrespective of EGFR/ALK status.
Implications of all the available evidence
Our results add to the information about the clinical efficacy of immune checkpoint inhibitors in NSCLC and the role of tumour expression of PD-L1. ATLANTIC contributes to the body of evidence in advanced lines of treatment and in patients with EGFR+/ALK+ NSCLC. The results suggest durvalumab might have a role in the treatment of EGFR+ tumours with high PD-L1 expression. Additional prospectively designed controlled studies in patients with EGFR+/ALK+ NSCLC are warranted to further understand the activity of immune checkpoint inhibitors in this population.
Acknowledgments
We thank the patients, their families, and their caregivers for participating in the ATLANTIC study, and all investigators and site personnel. We thank Marc Ballas, formerly of AstraZeneca, for his contribution to the study. This study was sponsored by AstraZeneca. Medical writing and editorial assistance were provided by Samantha Holmes, of Cirrus Communications, an Ashfield Company (Macclesfield, UK), and was funded by AstraZeneca.
Footnotes
Declaration of interests
MCG received personal fees for advisory board participation from AstraZeneca, Roche, Bristol-Myers Squibb, and Merck Sharp and Dohme during this study. JV received an institutional research grant from AstraZeneca and served AstraZeneca in an advisory capacity during this study. HL received personal fees from AstraZeneca, Roche, Merck Sharp and Dohme, Bristol-Myers Squibb, Pfizer, Novartis, Lilly, and Amgen, and non-financial support from AstraZeneca, Roche, Merck Sharp and Dohme, Bristol-Myers Squibb, Pfizer, Lilly, and Amgen, all outside the submitted work. JEG received a research fund grant from AstraZeneca and personal fees for advisory services, outside the submitted work. JP received clinical trial funding from AstraZeneca during this study; received clinical trial funding from Genentech, Bristol-Myers Squibb, Curis, Corvus, EMD Serono, and Macrogenics outside the submitted work; reports DSMC and speakers’ bureau participation with Bristol-Myers Squibb outside the submitted work; reports speakers’ bureau participation with Genentech and Merck outside the submitted work; has a patent T-cell immunotherapy development pending; is a founder and owner of BioCytics, which is a clinical research laboratory developing T-cell immunotherapy; and has previously bought stock in the T-cell companies LionBiotech, Juno, Blue Bird, Kite Pharma, and ZioPharm. CC has received fees during the past 5 years for attending scientific meetings, speaking, organising research, and providing consulting services from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche, Sanofi-Aventis, Lilly, Novartis, Merck Sharp and Dohme, Bristol-Myers Squibb, and Amgen, outside the submitted work. PB participated in advisory boards for Eli Lilly, Bristol-Myers Squibb, and Boehringer Ingelheim, outside the submitted work. PW-P received personal fees for advisory board participation from AstraZeneca, Merck, Bristol-Myers Squibb, and Lilly, outside the submitted work. RAS received a research grant from AstraZeneca and personal fees from AstraZeneca, Boehringer Ingelheim, Merck, Novartis, Lilly, Pfizer, Roche, Taiho, and Bristol-Myers Squibb, outside the submitted work. YH, CW, and PAD are employees of AstraZeneca and hold shares in AstraZeneca. NAR received personal fees from Merck, Bristol-Myers Squibb, Roche, Novartis, Pfizer, and Lilly, outside the submitted work. All other authors declare no competing interests.
Contributor Information
Marina Chiara Garassino, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Byoung-Chul Cho, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, South Korea.
Joo-Hang Kim, CHA Bundang Medical Center, CHA University, Gyeonggi-do, South Korea.
Julien Mazières, Toulouse University Hospital, Université Paul Sabatier, Toulouse, France.
Johan Vansteenkiste, University Hospitals KU Leuven, Leuven, Belgium.
Hervé Lena, CHU Rennes–Hôpital Pontchaillou, Rennes University, Rennes, France.
Jesus Corral Jaime, Hospital Universitario Virgen del Rocio, Seville, Spain.
Jhanelle E Gray, H Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
John Powderly, Carolina BioOncology Institute, Huntersville, NC, USA.
Christos Chouaid, Centre Hospitalier Intercommunal de Créteil, Créteil, France.
Paolo Bidoli, Azienda Ospedaliera San Gerardo, Monza, Italy.
Paul Wheatley-Price, The Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON, Canada.
Keunchil Park, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
Ross A Soo, National University Hospital and National University Cancer Institute, Singapore.
Yifan Huang, AstraZeneca, Gaithersburg, MD, USA.
Catherine Wadsworth, AstraZeneca, Alderley Park, UK.
Phillip A Dennis, AstraZeneca, Gaithersburg, MD, USA.
Naiyer A Rizvi, Columbia University Medical Center, New York, NY, USA.
References
- 1.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27 (suppl 5): v1–27 [DOI] [PubMed] [Google Scholar]
- 2.Califano R, Kerr K, Morgan RD, et al. Immune checkpoint blockade: a new era for non-small cell lung cancer. Curr Oncol Rep 2016; 18: 59. [DOI] [PubMed] [Google Scholar]
- 3.Marrone KA, Brahmer JR. Immune checkpoint therapy in non-small cell lung cancer. Cancer J 2016; 22: 81–91. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014; 32: 1902–08. [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33: 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genentech. Tecentriq (atezolizumab) prescribing information. 2017. http://www.gene.com/download/pdf/tecentriq_prescribing.pdf (accessed Aug 29, 2017).
- 8.Merck Sharp & Dohme. Keytruda (pembrolizumab) summary of product characteristics. 2017. https://www.medicines.org.uk/emc/medicine/30602 (accessed Aug 29, 2017).
- 9.Bristol-Myers Squibb. Opdivo (nivolumab) prescribing information. 2017. http://packageinserts.bms.com/pi/pi_opdivo.pdf (accessed Aug 29, 2017).
- 10.Abdel-Rahman O Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Oncol Hematol 2016; 101: 75–85. [DOI] [PubMed] [Google Scholar]
- 11.Zhou GW, Xiong Y, Chen S, Xia F, Li Q, Hu J. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: a meta-analysis of randomized clinical trials. Medicine (Baltimore) 2016; 95: e4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bylicki O, Paleiron N, Margery J, et al. Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol 2017; 12: 563–69. [DOI] [PubMed] [Google Scholar]
- 13.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22: 4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oganesyan V, Gao C, Shirinian L, Wu H, Dall’Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008; 64: 700–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015; 3: 1052–62. [DOI] [PubMed] [Google Scholar]
- 16.Segal NH, Hamid O, Hwu W, et al. A phase I multi-arm dose-expansion study of the anti-programmed cell death-ligand-1 (PD-L1) antibody MEDI4736: preliminary data. Ann Oncol 2014; 25 (suppl 4): 1058PD. [Google Scholar]
- 17.Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Ann Oncol 2016; 27 (suppl 6): 1216PD. [Google Scholar]
- 18.Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol 2016; 11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47 [DOI] [PubMed] [Google Scholar]
- 20.Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014; 32 (suppl 15): 8021. [Google Scholar]
- 21.Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2016; 7: 78985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015; 16: 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 24.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol 2012; 19 (suppl 1): S52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 27.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–46. [DOI] [PubMed] [Google Scholar]
- 29.Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—a meta-analysis. J Thorac Oncol 2017; 12: 403–07 [DOI] [PubMed] [Google Scholar]
- 30.Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. Proc Am Soc Clin Oncol 2016; 34 (suppl 15): 9017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.