Abstract
Background:
Information about the frequency of cannabinoid use and the clinical characteristics of its users in oncology supportive care is limited. This study explored associations between cannabinoid use and cancer-related clinical characteristics in a cancer population.
Patients and Methods:
This retrospective review included 332 patients who had a urine drug test (UDT) for tetrahydrocannabinol (THC) together with completion of an Edmonton Symptom Assessment Scale (ESAS) and cannabinoid history questionnaire on the same day that urine was obtained during 1 year in the supportive care clinic.
Results:
The frequency of positive results for THC in a UDT was 22.9% (n=76). Significant statistical differences were seen between THC-positive and THC-negative patients for age (median of 52 [lower quartile, 44; upper quartile, 56] vs 58 [48; 67] years; P<.001), male sex (53.9% vs 39.5%; P=.034), and past or current cannabinoid use (65.8% vs 26.2%; P<.001). Statistical significance was observed in ESAS items between the THC-positive and THC-negative groups for pain (7 [lower quartile, 5; upper quartile; 8] vs 5 [3; 7]; P=.001), nausea (1 [0; 3] vs 0 [0; 3]; P=.049), appetite (4 [2; 7] vs 3 [0; 5.75]; P=.015), overall well-being (5.5 [4; 7] vs 5 [3; 6]; P=.002), spiritual well-being (5 [2; 6] vs 3 [1; 3]; P=.015), insomnia (7 [5; 9] vs 4 [2; 7]; P<.001), and total ESAS (52 [34; 66] vs 44 [29; 54]; P=.001). Among patients who reported current or past cannabinoid use, THC-positive patients had higher total scores and scores for pain, appetite, overall well-being, spiritual well-being, and insomnia than THC-negative patients.
Conclusions:
Patients with cancer receiving outpatient supportive care who had positive UDT results for THC had higher symptom severity scores for pain, nausea, appetite, overall and spiritual well-being, and insomnia compared with their THC-negative counterparts. These results highlight potential opportunities to improve palliative care.
Background
There has been an emerging interest in the use of cannabinoids for treatment of various medical conditions.1 Many states have approved the use of medical-grade cannabinoids (medical marijuana) for numerous symptoms and conditions, including chronic pain, spasticity, seizures, anorexia, nausea, and glaucoma.2-4 In addition, marijuana has been highlighted as a potential immune-modulating or anticancer agent.5 Given the symptom burden reported by patients in the oncology outpatient supportive care setting, it is not surprising that patient and family inquiries about purported cannabinoid benefits for common cancer-related symptoms, such as pain, nausea, and poor appetite, have become more frequent as medical marijuana programs have proliferated.6 Patients’ presumption of the positive effects of cannabinoid use are common during such discussions, and interest in exploring benefits are often expressed.7-9
In general, cannabinoids are classified into 3 major categories: endogenous, synthetic, and plant-derived. Anandamide and 2-arachidonylglycerol are naturally occurring and seem to be widely involved in regulatory functions through agonist or antagonist effects on not only cannabinoid receptors CB1 and CB2 but also certain ion channels10,11 or orphan receptors.12-17 Complex biologic actions and pathophysiologic mechanisms of cannabinoids have been proposed.18 Numerous synthetic chemical compounds have been synthesized,19 and >60 biologically active compounds derived from Cannabis sativa have been isolated.13 The pharmacokinetics and dynamics vary, depending on the source, concentration, delivery route, and ratios between the multiple plant-derived compounds and duration of exposure.20 Therefore, a variety of standardized, plant-derived, or synthetically produced cannabinoid products have been developed for medical use (medical-grade).20 In contrast, non-medical-grade products are nonstandardized and contain unknown amounts of cannabinoids.21
The Edmonton Symptom Assessment Scale (ESAS) has well-established reliability and validity when used in patients with cancer as a routine self-assessment tool.22 Patients use the ESAS to score their average symptom severity during the 24 hours before presenting for an outpatient clinic visit; the score is based on an 11-point numeric rating scale from 0 (absence of symptom) to 10 (worst intensity).23 We sought to determine the positive rate of cannabinoid metabolites through urine drug testing (UDT) and compare ESAS characteristics among patients with positive versus negative test results.
Patients and Methods
Study Population
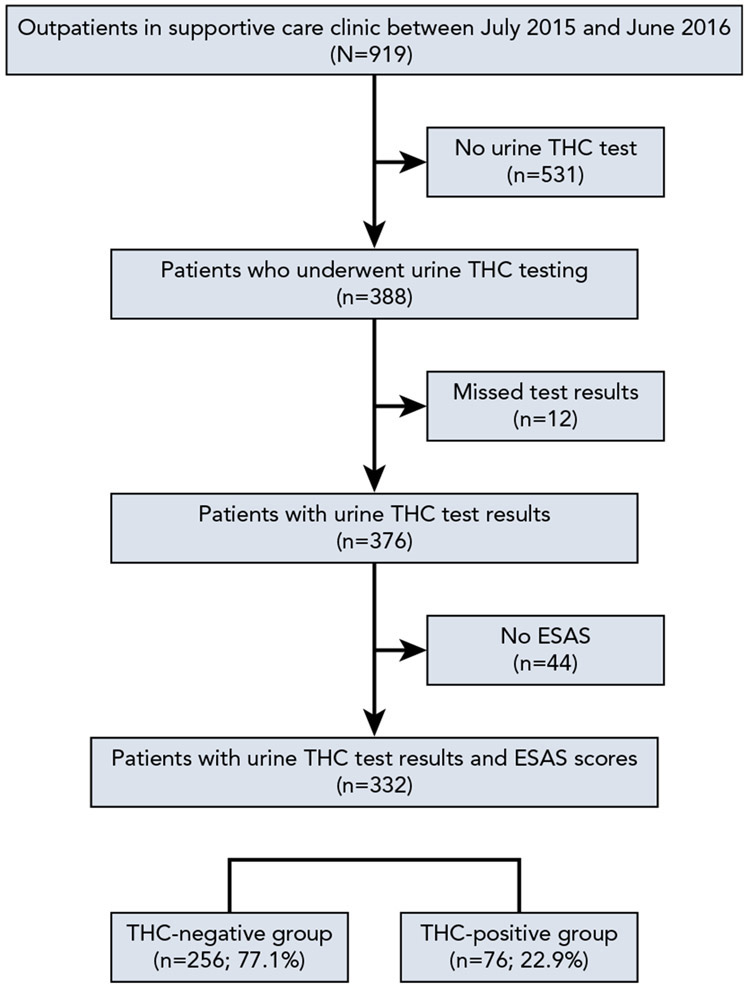
A retrospective medical records review was performed to identify reported differences in symptom characteristics of cannabinoid users and nonusers based on UDT results in the supportive care outpatient clinic at Moffitt Cancer Center from July 2015 through June 2016. Institutional Review Board approval was obtained. Medical records were reviewed for demographic data, presence of urine tetrahydrocannabinol (THC), ESAS-reported symptom severity, and personal report of cannabinoid use. Among 919 outpatients, 332 who underwent UDT and completed the self-reported questionnaire on the day of urine sampling were identified and included (Figure 1).
Figure 1.
Flow diagram of the study population.
Abbreviations: ESAS, Edmonton Symptom Assessment Scale; THC, tetrahydrocannabinol.
Statistical Analysis
All analyses were performed using SPSS Statistics, version 25.0 (IBM Corporation). The Kolmogorov-Smirnov method and Levene’s test were used to assess the normality and equality of variance assumptions, respectively. Continuous variables were presented as median (lower quartile; upper quartile) and categorical data as frequencies and percentages. The Mann-Whitney U test, chi-square test, or Fisher exact test was used to compare the endpoints between the THC-positive and THC-negative groups based on UDT. ESAS individual symptom severity scores and total scores were compared between the urinary THC-positive and THC-negative groups. Multiple logistic regression models were used to assess the differences in ESAS scores between the 2 groups, adjusted for age, sex, and cancer type. Statistical significance was declared for 2-sided Pvalues <.05.
Results
Of 332 patients, 76 (22.9%) had positive UDT results for cannabinoid metabolites (nor-9-carboxy-Δ9-tetrahydrocannabinol [THC-COOH]). Only 2 of these patients (2.6%) were prescribed synthetic cannabinoids, which also can be detected in urinary THC, and were enrolled in the THC-positive group.
There were significant statistical differences between the THC-positive and THC-negative groups for age (52 [lower quartile, 44; upper quartile, 56] vs 58 [48; 67] years; P<.001), male sex (53.9% vs 39.5%; P=.034), and personal report of cannabinoid use in the past or currently (65.8% vs 26.2%; P<.001). No statistical difference was seen for cancer types between the 2 groups (Table 1).
Table 1.
Clinical Characteristics According to Urinary THC Results
| Total n (%) |
THC-Positive Group n (%) |
THC-Negative Group n (%) |
P Valuea | |
|---|---|---|---|---|
| Total | 332 (100%) | 76 (22.9%) | 256 (77.1%) | |
| Male sex | 142 (42.8%) | 41 (53.9%) | 101 (39.5%) | .034 |
| Median age (LQ; UQ), y | 55 (46; 64) | 51 (44; 56) | 58 (48; 67) | <.001 |
| Age groups, y | ||||
| <30 | 18 (5.4%) | 3 (0.9%) | 15 (4.5%) | |
| 30–39 | 33 (10.0%) | 13 (4.0%) | 20 (6.0%) | |
| 40–49 | 62 (18.7%) | 19 (5.7%) | 43 (13.0%) | |
| 50–59 | 91 (27.4%) | 26 (7.8%) | 65 (19.6%) | |
| 60–69 | 83 (25.0%) | 15 (4.5%) | 68 (20.5%) | |
| 70–79 | 41 (12.3%) | 0 (0%) | 41 (12.3%) | |
| ≥80 | 4 (1.2%) | 0 (0%) | 4 (1.2%) | |
| Personal history of cannabinoid use (self-report) | <.001 | |||
| Never | 203 (61.1%) | 22 (28.9%) | 181 (70.7%) | |
| Yes | 117 (35.3%) | 50 (65.8%) | 67 (26.2%) | |
| Not answered | 12 (3.6%) | 4 (5.3%) | 8 (3.1%) | |
| Cancer type | .123 | |||
| Gynecologic | 70 (21.1%) | 18 (23.7%) | 52 (20.3%) | |
| Gastrointestinal | 49 (14.8%) | 16 (21.1%) | 33 (12.9%) | |
| Hematologic | 35 (10.5%) | 6 (7.9%) | 29 (11.3%) | |
| Lung | 34 (10.2%) | 5 (6.6%) | 29 (11.3%) | |
| Breast | 30 (9.0%) | 3 (3.9%) | 27 (10.5%) | |
| Sarcoma | 26 (7.8%) | 4 (5.3%) | 22 (8.6%) | |
| Melanoma | 20 (6.0%) | 6 (7.9%) | 14 (5.5%) | |
| Lymphoma | 19 (5.7%) | 3 (3.9%) | 16 (6.3%) | |
| Head and neck | 15 (4.5%) | 4 (5.3%) | 11 (4.3%) | |
| Urologic | 13 (3.9%) | 6 (7.9%) | 7 (2.7%) | |
| Neuroendocrine | 9 (2.7%) | 4 (5.3%) | 5 (2.0%) | |
| Unknown primary | 5 (1.5%) | 0 (0%) | 5 (2.0%) | |
| Prostate | 4 (1.2%) | 0 (0%) | 4 (1.6%) | |
| Brain | 3 (0.9%) | 1 (1.3%) | 2 (0.8%) | |
Abbreviations: LQ, lower quartile; THC, tetrahydrocannabinol; UQ, upper quartile.
Bold indicates statistically significant P values.
Although 203 patients (61.1%) denied any history of cannabinoid product use in the past or currently, 22 of these (10.8%) had positive UDT results for THC. Sensitivity and specificity of self-reported answers regarding personal history of cannabinoid use were 50 of 77 (65.8%) and 181 of 256 (70.7%), respectively (Table 1).
There were statistically significant differences between the THC-positive and THC-negative groups for some ESAS items, including pain (7 [lower quartile, 5; upper quartile, 8] vs 5 [3; 7]; P=.001), nausea (1 [0; 3] vs 0 [0; 3]; P=.049), appetite (4 [2; 7] vs 3 [0; 5.75]; P=.015), overall well-being (5.5 [4; 7] vs 5 [3; 6]; P=.002), spiritual well-being (5 [2; 6] vs 3 [1; 3]; P=.015), insomnia (7 [5; 9] vs 4 [2; 7]; P<.001), and total ESAS (52 [34; 66] vs 44 [29; 54]; P=.001) (Table 2). Multivariate logistic regression was performed to adjust for age, sex, and cancer type. The THC-positive group had a higher risk for total ESAS than the THC-negative group (odds ratio [OR], 1.027; 95% CI, 1.012–1.041). Similarly, individual items of pain (OR, 1.187; 95% CI, 1.066–1.323), appetite (OR, 1.130; 95% CI, 1.036–1.233), overall well-being (OR, 1.242; 95% CI, 1.096–1.407), spiritual well-being (OR, 1.125; 95% CI, 1.027–1.231), and insomnia (OR, 1.229; 95% CI, 1.118–1.350) were scored higher in the THC-positive group. We also performed a statistical comparison of symptom severity between the THC-positive and THC-negative groups for patients who reported actual cannabinoid use on their self-reported questionnaire (Table 3).
Table 2.
ESAS Scores According to Urinary THC Results
| Symptom | THC-Positive Group Median (LQ; UQ) |
THC-Negative Group Median (LQ; UQ) |
P Valuea | OR (95% CI) | P Valuea,b |
|---|---|---|---|---|---|
| Total, n (%) | 76 (22.9%) | 256 (77.1%) | |||
| Pain | 7 (5; 8) | 5 (3; 7) | .001 | 1.187 (1.066–1.323) | .002 |
| Fatigue | 4 (2; 6) | 6 (4; 8) | .290 | 1.076 (0.971–1.193) | .161 |
| Drowsiness | 1 (0; 3) | 3 (1; 6) | .152 | 1.067 (0.966–1.178) | .199 |
| Nausea | 1 (0; 3) | 0 (0; 3) | .049 | 1.105 (0.998–1.224) | .055 |
| Appetite | 4 (2; 7) | 3 (0; 5.75) | .015 | 1.130 (1.036–1.233) | .006 |
| Shortness of breath | 1 (0; 4) | 1 (0; 3) | .777 | 1.042 (0.942–1.151) | .425 |
| Depression | 3 (0; 6) | 2 (0; 5) | .544 | 1.071 (0.977–1.172) | .143 |
| Anxiety | 4 (1; 6) | 3 (1; 6) | .220 | 1.061 (0.969–1.160) | .199 |
| Overall well-being | 6 (4; 7) | 5 (3; 6) | .002 | 1.242 (1.096–1.407) | .001 |
| Spiritual well-being | 5 (2; 6) | 3 (1; 3) | .015 | 1.125 (1.027–1.231) | .011 |
| Constipation | 1 (0; 6) | 1 (0; 5) | .676 | 1.071 (0.993–1.166) | .117 |
| Insomnia | 7 (5; 9) | 4 (2; 7) | <.001 | 1.229 (1.118–1.350) | <.001 |
| Total ESAS | 52 (34; 66) | 44 (29; 54) | .001 | 1.027 (1.012–1.041) | <.001 |
Abbreviations: ESAS, Edmonton Symptom Assessment Scale; LQ, lower quartile; OR, odds ratio; THC, tetrahydrocannabinol; UQ, upper quartile.
Bold indicates statistically significant P values.
Multiple logistic regression for urinary THC results was adjusted for age, sex, and cancer type.
Table 3.
ESAS Scores According to Urinary THC Results Among Patients Reporting Cannabinoid Use
| THC-Positive Group Median (LQ; UQ) |
THC-Negative Group Median (LQ; UQ) |
P Valuea | OR (95% CI) | P Valuea,b | |
|---|---|---|---|---|---|
| Total, n (%) | 50 (42.7%) | 67 (57.3%) | |||
| Pain | 7 (5; 7) | 5 (3; 7) | .008 | 1.269 (1.070–1.505) | .006 |
| Fatigue | 6 (4; 8) | 6 (3; 7) | .236 | 1.124 (0.970–1.303) | .121 |
| Drowsiness | 4 (2; 6) | 4 (2; 5) | .421 | 1.075 (0.928–1.246) | .335 |
| Nausea | 1 (0; 3) | 1 (0; 2) | .365 | 1.082 (0.920–1.273) | .339 |
| Appetite | 5 (3; 7) | 2 (0; 5) | <.001 | 1.294 (1.121–1.492) | <.001 |
| Shortness of breath | 2 (0; 4.25) | 1 (0; 4) | .830 | 0.993 (0.862–1.145) | .928 |
| Depression | 3.5 (0.75; 7) | 3 (1; 5) | .353 | 1.117 (0.974–1.281) | .113 |
| Anxiety | 3 (1; 6.25) | 4 (1; 5) | .861 | 1.048 (0.914–1.201) | .503 |
| Overall well-being | 6 (4; 7.25) | 5 (3; 6) | .013 | 1.284 (1.059–1.557) | .011 |
| Spiritual well-being | 5 (2; 6) | 3 (1; 6) | .028 | 1.161 (1.010–1.335) | .036 |
| Constipation | 2.50 (0; 6) | 1 (0; 4) | .411 | 1.119 (0.973–1.286) | .115 |
| Insomnia | 7 (4.75; 8) | 4 (2; 7) | .001 | 1.203 (1.056–1.369) | .005 |
| Total ESAS | 52 (44.75; 64.50) | 40 (29; 54) | .003 | 1.032 (1.010–1.055) | .004 |
Abbreviations: ESAS, Edmonton Symptom Assessment Scale; LQ, lower quartile; OR, odds ratio; THC, tetrahydrocannabinol; UQ, upper quartile.
Bold indicates statistically significant P values.
Multiple logistic regression for urinary THC results was adjusted for age, sex, and cancer type.
Discussion
Use of medicinal cannabinoids seems to be more popular among patients with cancer compared with the general population.24,25 A recent survey suggested that patients want to learn more about cannabinoid use during cancer treatment from healthcare providers.26 However, a nationwide survey among oncologists revealed concerning gaps in data and education, with only 30% of oncologists reporting that they believe they are sufficiently knowledgeable to provide medical marijuana recommendations.27 Furthermore, there is a dearth of scientific evidence to support cannabinoid use in patients with cancer. Mücke et al28 conducted a systematic review and meta-analysis to evaluate the therapeutic use of cannabinoids in palliative/supportive care medicine and concluded that, because of the low quality of evidence, no recommendations can be made regarding the use of cannabinoids in this setting for patients with cancer. Another systematic review indicated statistically significant adverse event rates associated with cannabinoid use in patients with cancer.2 Furthermore, it is even more difficult to examine the efficacy and safety of unauthorized or sporadic use of cannabinoids in these patients. It is, of course, unknown how unauthorized cannabinoid use will affect the symptom burden or quality of life of patients living with cancer.6,29
Presence of the urine cannabinoid metabolite THC-COOH indicates that a patient was exposed to synthetic or plant-derived cannabinoids within approximately the past 10 days, but its concentration does not correlate with the amount, route, or frequency of use.30 Information about the prevalence of cannabinoid use in patients with cancer is limited. In one recent study at another comprehensive cancer center in a state that has approved the use of marijuana for medicinal and recreational purposes, the frequency of positive UDT results for THC was 14%.26 The state of Florida, where our study was conducted, was not actively dispensing state-authorized marijuana during our study period. In our data, the frequency of THC positivity was 22.9%, and the urinary THC-positive group was younger and predominantly male, and reported a history of cannabinoid use more frequently than the urinary THC-negative group. In our study, underreporting of cannabinoid use was evident, with UDT yielding THC-positive results for 10.8% of patients who denied use on clinic screening questionnaires.
Interestingly, the urinary THC-positive group reported higher total ESAS scores and higher individual scores for pain, nausea, appetite, insomnia, and both overall and spiritual well-being compared with the THC-negative group.
There are several potential explanations for these results. First, higher symptom burden with certain ESAS items may motivate patients to use cannabinoids. Second, using cannabinoids, especially unauthorized products or inappropriate use, could result in a higher symptom burden because of their own adverse effects. Third, cannabinoid users might have lower thresholds for certain symptoms than nonusers. Fourth, besides cannabinoid use, it is possible that suboptimal standard pharmacologic or nonpharmacologic symptom management resulted in higher ESAS scores among patients with THC-positive results. However, those symptoms might better respond to conventional treatment modalities when patients do not use cannabinoids.
This study has several limitations. First, significant potential selection bias exists. UDT and substance risk assessment have been performed sporadically, with no clear consensus or guideline in medical oncologic or supportive care clinics. Among our data, only 42% of patients underwent UDT during the study period. No patients were allowed to refuse UDT if a physician ordered it. However, we were unable to specify the reason that patients were chosen or not chosen for UDT on the day of their visit. Because of this selection bias, our results should be interpreted with caution. Second, in patients’ medical records, we were unable to distinguish between recreational and medicinal intent of cannabinoid use; determine delivery method or route; determine cannabinoid quality, dose, or frequency; or ascertain the last time of cannabinoid use. Without knowing these specific data, our cross-sectional ESAS scores could be mixed or contaminated, depending on the purpose or means of cannabinoid use. Lastly, although the ESAS has been shown to be a reliable tool to assess symptom burden in patients with cancer, it remains unclear whether this validation is also consistent in active cannabinoid users.
Conclusions
Patients with cancer receiving outpatient supportive care who had positive UDT results for THC had higher symptom severity scores for pain, nausea, appetite, and insomnia compared with their THC-negative counterparts. These results highlight potential opportunities to improve palliative care. Despite these findings, there are gaps in understanding of the symptoms that patients perceive as most likely to benefit from cannabinoid use and the actual impact of cannabinoid products on the symptom burden reported by patients, including the outpatient oncology supportive care population. With increasing state legalization of medical marijuana and ongoing unauthorized use likely to increase, it is important for researchers to address these critical gaps in the evidence base regarding marijuana use in patients with cancer. At a minimum, clinicians should consider collecting clinically relevant data on actual marijuana use in their patients, with the ultimate goal of optimizing safe prescribing and patient comfort. Further prospective research is necessary to achieve a better understanding of our findings.
Footnotes
Disclosures: The authors have not received any financial consideration from any person or organization to support the preparation, analysis, results, or discussion of this article.
References
- 1.Albright VA, Johnson EO. Emerging topics and innovative methodologies in cannabis research. Subst Abuse 2018;12:1178221818774264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician 2018;64:e78–e94. [PMC free article] [PubMed] [Google Scholar]
- 3.Carliner H, Brown QL, Sarvet AL, et al. Cannabis use, attitudes, and legal status in the U.S.: a review. Prev Med 2017;104:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacula RL, Smart R. Medical marijuana and marijuana legalization. Annu Rev Clin Psychol 2017;13:397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Śledziński P, Zeyland J, Słomski R, et al. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med 2018;7:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martell K, Fairchild A, LeGerrier B, et al. Rates of cannabis use in patients with cancer. Curr Oncol 2018;25:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bubis LD, Davis L, Mahar A, et al. Symptom burden in the first year after cancer diagnosis: an analysis of patient-reported outcomes. J Clin Oncol 2018;36:1103–1111. [DOI] [PubMed] [Google Scholar]
- 8.Kramer JL. Medical marijuana for cancer. CA Cancer J Clin 2015;65:109–122. [DOI] [PubMed] [Google Scholar]
- 9.Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm 2016;12:638–654. [DOI] [PubMed] [Google Scholar]
- 10.Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci 2019;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Petrocellis L, Orlando P, Moriello AS, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 2012;204:255–266. [DOI] [PubMed] [Google Scholar]
- 12.Lutz B The endocannabinoid system and extinction learning. Mol Neurobiol 2007;36:92–101. [DOI] [PubMed] [Google Scholar]
- 13.Backes M Cannabis Pharmacy: The Practical Guide to Medical Marijuana. New York, NY: Black Dog & Leventhal; 2014. [Google Scholar]
- 14.Davis MP. Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands. Expert Opin Investig Drugs 2014;23:1123–1140. [DOI] [PubMed] [Google Scholar]
- 15.Davis MP. Cannabinoids for symptom management and cancer therapy: the evidence. J Natl Compr Canc Netw 2016;14:915–922. [DOI] [PubMed] [Google Scholar]
- 16.Godlewski G, Offertáler L, Wagner JA, et al. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat 2009;89:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryberg E, Larsson N, Sjögren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 2007;152:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur R, Ambwani SR, Singh S. Endocannabinoid system: a multi-facet therapeutic target. Curr Clin Pharmacol 2016;11:110–117. [DOI] [PubMed] [Google Scholar]
- 19.Castaneto MS, Gorelick DA, Desrosiers NA, et al. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend 2014;144:12–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steele G, Arneson T, Zylla D. A comprehensive review of cannabis in patients with cancer: availability in the USA, general efficacy, and safety. Curr Oncol Rep 2019;21:10. [DOI] [PubMed] [Google Scholar]
- 21.Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 2018;84:2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage 2017;53:630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 24.Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington State. J Am Board Fam Med 2017;30:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johannigman S, Eschiti V. Medical use of marijuana in palliative care. Clin J Oncol Nurs 2013;17:360–362. [DOI] [PubMed] [Google Scholar]
- 26.Pergam SA, Woodfield MC, Lee CM, et al. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer 2017;123:4488–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun IM,Wright A, Peteet J, et al. Medical oncologists’ beliefs, practices, and knowledge regarding marijuana used therapeutically: a nationally representative survey study. J Clin Oncol 2018;36:1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mücke M, Weier M, Carter C, et al. Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopenia Muscle 2018;9:220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belle-Isle L, Walsh Z, Callaway R, et al. Barriers to access for Canadians who use cannabis for therapeutic purposes. Int J Drug Policy 2014;25:691–699. [DOI] [PubMed] [Google Scholar]
- 30.Brenneisen R, Meyer P, Chtioui H, et al. Plasma and urine profiles of Delta9-tetrahydrocannabinol and its metabolites 11-hydroxy-Delta9-tetrahydrocannabinol and 11-nor-9-carboxy-Delta9-tetrahydrocannabinol after cannabis smoking by male volunteers to estimate recent consumption by athletes. Anal Bioanal Chem 2010;396:2493–2502. [DOI] [PubMed] [Google Scholar]