Abstract
Introduction
Coronavirus disease of 2019 (COVID-19) is a lower respiratory tract infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This disease can impact the cardiovascular system and lead to abnormal electrocardiographic (ECG) findings. Emergency clinicians must be aware of the ECG manifestations of COVID-19.
Objective
This narrative review outlines the pathophysiology and electrocardiographic findings associated with COVID-19.
Discussion
COVID-19 is a potentially critical illness associated with a variety of ECG abnormalities, with up to 90% of critically ill patients demonstrating at least one abnormality. The ECG abnormalities in COVID-19 may be due to cytokine storm, hypoxic injury, electrolyte abnormalities, plaque rupture, coronary spasm, microthrombi, or direct endothelial or myocardial injury. While sinus tachycardia is the most common abnormality, others include supraventricular tachycardias such as atrial fibrillation or flutter, ventricular arrhythmias such as ventricular tachycardia or fibrillation, various bradycardias, interval and axis changes, and ST segment and T wave changes. Several ECG presentations are associated with poor outcome, including atrial fibrillation, QT interval prolongation, ST segment and T wave changes, and ventricular tachycardia/fibrillation.
Conclusions
This review summarizes the relevant ECG findings associated with COVID-19. Knowledge of these findings in COVID-19-related electrocardiographic presentations may assist emergency clinicians in the evaluation and management of potentially infected and infected patients.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Cardiac, ECG, EKG, Electrocardiogram, Emergency medicine, Arrhythmia, Dysrhythmia
1. Introduction
In December 2019, an outbreak of a lower respiratory tract disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China [1]. This was subsequently termed coronavirus disease of 2019 (COVID-19) by the World Health Organization and has been declared a global pandemic, infecting millions worldwide [2]. While much of the focus has been on the respiratory system, COVID-19 can also cause a variety of cardiac complications [[3], [4], [5], [6], [7], [8]] and a range of electrocardiographic abnormalities. Studies have described COVID-19 related myocarditis [[9], [10], [11], [12]], cardiac tamponade [13], Brugada-like pattern [14], transient ST segment elevations [15], as well as medication-induced cardiac dysrhythmias [[16], [17], [18]]. Myocardial involvement in COVID-19 has been associated with poor outcomes, especially in myocardial injury which occurs in 22–44% of critical or severe cases [[4], [5], [6], [7], [8]]. Therefore, it is crucial that the emergency clinician recognize the electrocardiographic (ECG) manifestations of COVID-19 [19,20]. This narrative review provides a discussion of the various electrocardiographic presentations encountered in the COVID-19-infected patient.
2. Methods
This narrative review outlines the underlying pathophysiology and ECG manifestations of COVID-19. A literature review of PubMed and Google Scholar databases was performed for articles up to October 23, 2020, using the keywords ‘COVID’ OR ‘SARS-CoV-2’ OR ‘coronavirus’ OR ‘SARS’ AND ‘ECG’ OR ‘EKG’ OR ‘electrocardiogram’ for this narrative review. The authors included case reports and series, retrospective and prospective studies, systematic reviews and meta-analyses, clinical guidelines, and other narrative reviews. Commentaries and letters were also included. The literature search was restricted to studies published or translated into English. The initial literature search revealed over 200 articles, with the majority of these articles consisting of case reports. Authors reviewed all relevant articles and decided which studies to include for the review by consensus, with focus on emergency medicine-relevant articles, including guidelines. A total of 80 resources were selected for inclusion in this review.
3. Discussion
3.1. Pathophysiology
SARS-CoV-2 is an RNA virus that utilizes the angiotensin converting enzyme 2 (ACE2) receptor to enter cells by receptor-mediated endocytosis [21,22]. Compared to SARS-CoV-1, SARS-CoV-2 displays tighter ligand binding to the ACE2 receptor, located in several tissues, including the lungs, heart, kidneys, gastrointestinal tract, vasculature, and skin [3,23]. Respiratory symptoms associated with COVID-19 primarily come from ACE2 expression in the type 2 lung alveolar cells; however, over 7.5% of myocardial cells also express the ACE2 receptor [[24], [25], [26], [27], [28]]. While the presence of the ACE2 receptor can account for cardiac injury, the etiology of the cardiovascular effects that may occur in COVID-19 is likely multifactorial. The virus can cause a hyperinflammatory state, leading to vascular inflammation, cardiac injury, plaque instability, hypercoagulability, and myocardial depression [[25], [26], [27], [28]]. Pathologic data reveal cardiac interstitial inflammatory infiltration and necrosis, with blood vessels demonstrating microthrombi and inflammation [26,[29], [30], [31]]. COVID-19 may also result in cytokine storm, sepsis, disseminated intravascular coagulation, and ultimately multiorgan dysfunction and death. Myocardial abnormalities with ECG changes in COVID-19 may be due to this cytokine storm, hypoxic injury, electrolyte abnormalities, plaque rupture, coronary spasm, and microthrombi, as well as direct endothelial or myocardial injury [[3], [4], [5], [6], [7], [8],11,32].
There are a variety of arrhythmias and ECG abnormalities that may occur in COVID-19 [3,4,19,20]. Interestingly, the interpretation of the ECG, with either dysrhythmia recognition or identification of a concerning morphologic issue, is unchanged from the non-COVID-19 patient; in contrast, the context of the patient presentation and thus the clinical impact of the ECG findings has significantly changed. ECG abnormalities are common, present in 93% of hospitalized critically ill patients in one study [32]. Palpitations, likely reflective of dysrhythmia, may be the initial presenting symptom in approximately 7% of patients, and one study suggests 17% of patients in the general cohort and 44% of patients in the intensive care unit (ICU) setting experience dysrhythmias [3,4,19,20,33]. Another study reported a 9.6% rate of dysrhythmias [34]. Arrhythmias and ECG abnormalities are more common in critically ill patients [33,35], which may occur in 33–93% of these cases [36,37], and they are also associated with increased risk of in-hospital mortality (odds ratio [OR]1.95) and need for mechanical ventilation [38]. A study of 1258 patients found atrial fibrillation/flutter (OR 2.5), right ventricular (RV) strain (OR 2.7), and ST segment abnormalities (OR 2.4) to be associated with increased risk of mechanical ventilation and death [19]. Emergency clinicians should note that RV strain electrocardiographically presents with ST segment depression and T wave inversion in the right to mid-precordial (leads V1-V4) and the inferior leads (leads II, III, and aVF). Of the inferior leads, lead III frequently demonstrates the most obvious findings due to it direct imaging of the right ventricular muscle mass. Over 8% of deaths may be due to dysrhythmias in the setting of COVID-19 [39].
3.2. Supraventricular tachycardias (SVT)
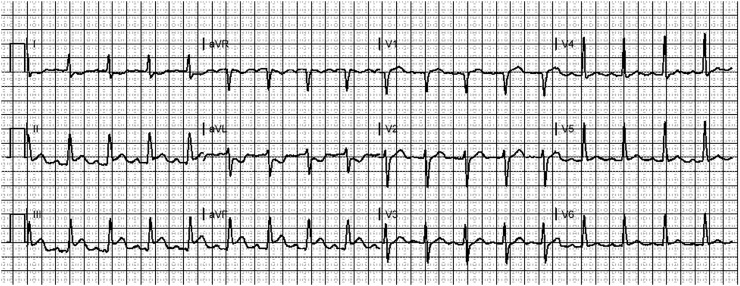
Sinus tachycardia is the most common supraventricular tachycardia encountered in the ill COVID-19 patient, resulting from the usual causes, including hypovolemia, hypoperfusion, hypoxia, elevated body temperature, pain, and anxiety. After sinus tachycardia, atrial fibrillation (Fig. 1 ) is the next most commonly seen SVT [[40], [41], [42]]; atrial fibrillation can present in a number of fashions, including new-onset, recurrence of a pre-existing dysrhythmia, and persistence of permanent atrial fibrillation with new rapid ventricular response. In the majority of patients with significant COVID-19 infection and atrial fibrillation, the rate will be quite rapid, thus the ECG will demonstrate atrial fibrillation with rapid ventricular response. Both sinus tachycardia and atrial fibrillation are independent predictors of illness severity, myocardial injury, and poor outcomes in COVID-19 [40]. One study conducted in New York hospitals found atrial fibrillation/flutter was present in 14.3% of patients at admission and occurred in 10.1% of patients during hospitalization [39]. Another study found atrial fibrillation/flutter to be present in 22% of critically ill patients requiring mechanical ventilation [32]. Atrial fibrillation is more common in patients following an inflammatory insult such as cardiomyopathy from COVID-19 and occurs in up to half of patients admitted to the ICU [[41], [42], [43], [44], [45], [46]]. Two case reports demonstrated the highly variable impact that COVID-19 has on the heart [47,48]; in one case [47], the patient experienced both atrial flutter and atrial fibrillation, eventually reverting to sinus rhythm 48 h later.
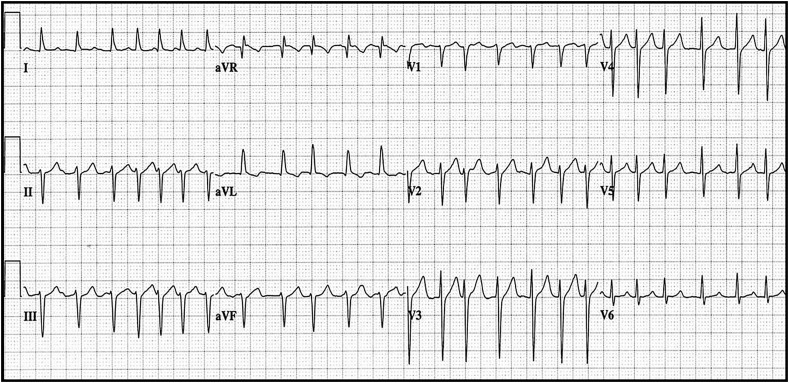
Fig. 1.
Atrial fibrillation with rapid ventricular response in a COVID-19-infected 76 year-old female with new-onset atrial fibrillation.
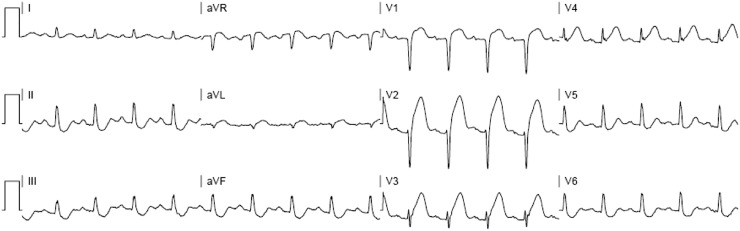
There is a paucity of literature evaluating the presence of other types of SVT, such as atrioventricular nodal reentry tachycardia (AVNRT), in COVID-19 patients. In most instances of AVNRT from the pre-COVID-19 era, patients with AVNRT are frequently younger and lack significant acute and chronic cardiorespiratory ailments; this typical AVNRT presentation likely explains the relative paucity of literature on this dysrhythmia in the COVID-19 patient. Refer to Fig. 2 for the ECG of a 19-year-old patient with palpitations and dyspnea who presented with AVNRT. The patient was appropriately treated with adenosine but experienced persistent dyspnea after uncomplicated conversion to sinus tachycardia. Further evaluation revealed that she was positive for COVID-19, and she was admitted to the hospital with persistently low oxygen saturations. She ultimately did well and was discharged from the hospital 5 days later, without AVNRT recurrence.
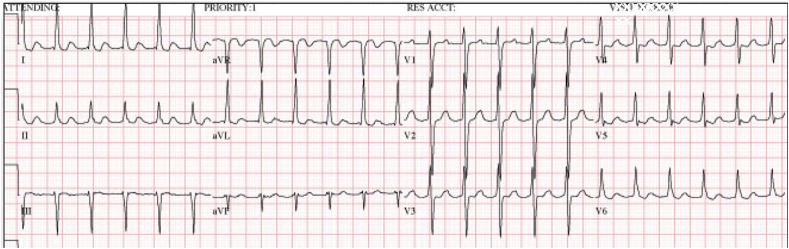
Fig. 2.
Atrioventricular nodal re-entrant tachycardia in a 19 year-old patient with palpitations and dyspnea who presented with AVNRT. The patient was appropriately treated with adenosine but experienced persistent dyspnea after uncomplicated conversion to sinus tachycardia; further evaluation revealed that she was positive for COVID-19.
3.3. Malignant ventricular dysrhythmias: ventricular tachycardia and ventricular fibrillation
Malignant ventricular arrhythmias are a known complication of viral myocarditis and cardiomyopathy, with ventricular tachycardia (VT) and/or ventricular fibrillation (VF) occurring in 1–6% of patients [19,35,36,[49], [50], [51], [52]]. In patients with COVID-19, these arrhythmias may be due to a combination of QT interval prolonging medications, metabolic abnormalities, and myocardial inflammation [19,20,46,[49], [50], [51], [52], [53]]. COVID-19 patients with elevated troponin have been shown to have a higher incidence of VT than those with normal troponins [5,44].
VT with a pulse is seen with both monomorphic and polymorphic presentations. Monomorphic ventricular tachycardia (MVT) is the most frequent form of VT seen in the COVID-19 patient, frequently resulting from structural heart disease such as acute coronary syndrome with STEMI, myocardial injury, or myocarditis; patients with pre-existing structural heart disease of many types can provide the substrate for MVT during periods of extreme physiologic stress due to the range of issues encountered in the COVID-19-infected patient. Polymorphic VT (PVT), including the PVT subtype torsade de pointes, results from functional (i.e., non-structural) heart disease and is likely less common; it most often occurs in situations involving medication toxicities, electrolyte abnormality, and various pro-arrhythmic states (e.g. Brugada syndrome, long QT syndrome).
Cardiac arrest dysrhythmias include the traditional 4 rhythm scenarios: the “shockable” (pulseless VT and VF) and “non-shockable” (pulseless electrical activity [PEA] and asystole) dysrhythmias. Specific dysrhythmia diagnosis in the cardiac arrest patient, whether out-of-hospital or hospital-based, is unchanged in the COVID-19 pandemic. During the pandemic, the occurrence of both out-of-hospital cardiac arrest (OHCA) and hospital-based cardiac arrest have increased based on recent data [[54], [55], [56], [57], [58]]. A study conducted in Italy found close to a 60% increase in OHCA in 2020 compared to 2019 [54]. A second study in France found a 52% increase in OHCA between February and April 2020, compared to 2019 [55]. This unfortunate increased rate of OHCA has also been seen in the United States. For example, in New York City there was a three-fold higher rate of patients undergoing resuscitation in the out-of-hospital setting when compared to a similar 2019 pre-pandemic period; the odds ratio (OR) of encountering “non-shockable” dysrhythmias increased significantly with an OR for PEA of 3.50 and an OR for asystole of 1.99 [56]. The mortality rate of patients in cardiac arrest increased in all three studies [[54], [55], [56]]. There are several factors that may contribute to this increased occurrence with more frequent poor outcome, including the COVID-19 infection itself as well as the delays in seeking care related to the pandemic, and lower rates of important bystander care in the pre-arrival period of cardiac arrest management [59].
Patients hospitalized with COVID-19 are at increased risk of cardiac arrest, but the rhythms associated with cardiac arrest vary in the inpatient population. A study focusing on hospitalized patients in New York City noted that asystole occurred most often, followed by PEA and then pulseless VT/VF [39]. Another study found 9 patients out of 700 experienced cardiac arrest, with only one having a shockable rhythm [57]. A study of 136 Chinese inpatients with COVID-19 complicated by cardiac arrest during hospitalization found most arrests were respiratory in origin with non-shockable rhythms (90% asystole and 4% PEA); only 3% survived to 30 days, with just 1% having intact neurologic function [58].
3.4. Bradycardia and atrioventricular block
Bradycardias and AVB are less commonly encountered as compared to tachydysrhythmias, though they may account for up to 11.8% of cardiac dysrhythmias [60,61]. A case report details a patient with COVID-19 presenting with first-degree atrioventricular block (AVB); during hospitalization, the rhythm transitioned to Mobitz type 1 second-degree AVB with further evolution ultimately to third-degree AVB [60]. Other cases describe older patients with multiple cardiac risk factors experiencing progression to high-grade AVB (second-degree type II and third-degree AVB) and/or intra-ventricular conduction block; many of these patients developing these more concerning conduction abnormalities progressed rapidly to cardiac arrest [[10], [56],[60], [61], [62], [63], [64]]. Three of these dysrhythmias (sinus bradycardia, junctional rhythm, idioventricular rhythm) occurred immediately prior to cardiac arrest onset – thus, in these cases, the development of significant bradycardia in the critically ill COVID-19 patient is a marker of risk of impending cardiovascular collapse [65].
3.5. Interval and axis abnormalities
QT interval prolongation may occur in over 13% of patients with COVID-19 infection, and several medications previously used for COVID-19 may prolong the QT interval, including chloroquine, hydroxychloroquine, and azithromycin [[66], [67], [68]]. QT interval prolongation is associated with more critical illness requiring ICU admission, as well as cardiac injury and mortality [61,69]. Left and right bundle branch blocks may occur in up to 12% of patients at the time of admission or during hospitalization [32,39]. Significant QT interval prolongation may lead to torsade des pointes. Fig. 3 is the ECG of a patient with COVID-19-related myocarditis; the ECG reveals atrial fibrillation with a mean rate of approximately 100 bpm, significant QRS complex widening, right axis deviation, poor R wave progression, and ST segment elevation in the anterolateral leads.
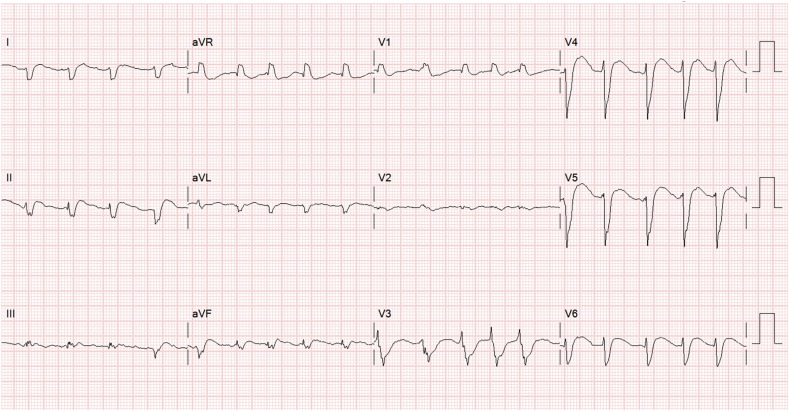
Fig. 3.
COVID-19-related myocarditis with atrial fibrillation, QRS complex widening, right axis deviation, poor R wave progression, and ST segment elevation.
QRS complex axis deviation has been reported, usually involving situations presenting with RV strain; such patients are frequently experiencing acute respiratory failure from multi-lobar pneumonia and/or pulmonary embolus with large clot burden. Electrocardiographically, these patients present with right axis deviation along with prominent R waves in leads V1 and V2 and ST segment depression/T wave inversion in leads II, III, aVF, and V1 to V4 [19]. Fig. 4 shows the inferior and right to mid precordial leads with T wave inversion, indicative of RV strain, in a patient with significant pulmonary embolus.
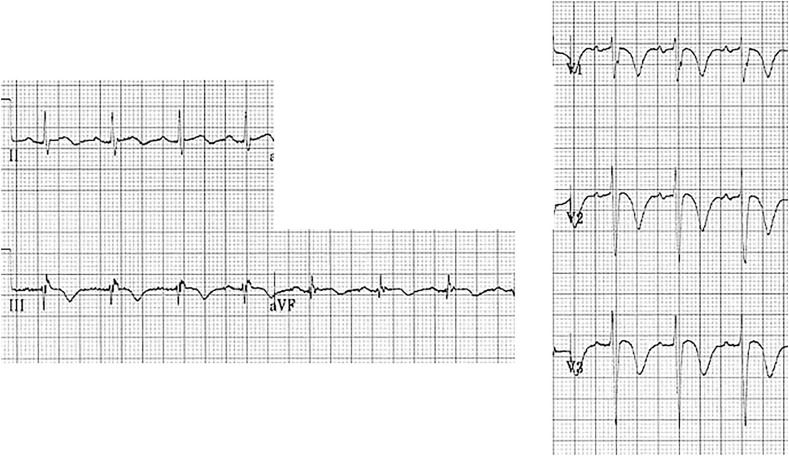
Fig. 4.
Right ventricular strain with T wave inversions in the inferior and right to mid precordial leads.
3.6. Morphologic presentations – ST segment, T wave, and QRS complex abnormalities
The interpretation of the ECG in the patient with chest pain or a similar presentation is more challenging in the COVID-19 pandemic. Emergency clinicians must consider STEMI, myocardial injury, and myocarditis, as all three clinical entities present with ST segment and T wave abnormalities. COVID-19 infection associated with myocardial injury may demonstrate ST segment deviations (elevation or depression), T wave inversion, and pathologic Q waves [[3], [4], [5],19,20,69]. One study found ST segment and T wave changes to be the most common abnormality in patients requiring ICU admission, occurring in 40% of patients [70]. Another investigation noted that nonspecific repolarization changes including ST segment and T wave abnormalities were encountered in 41% of patients; reportedly, these findings resulted from myocardial injury and are associated with poor outcomes, including increased need for ICU admission, more frequent mechanical ventilatory support, and increased mortality [32,40]. A case series included 18 patients with COVID-19 infection and ST segment elevation, 10 of which had ST segment elevation at the time of presentation and 8 developed ST segment elevation during hospitalization [71]. Ultimately, acute myocardial infarction (AMI) was diagnosed in 8 patients, while 10 demonstrated nonocclusive (i.e., not involving ACS) myocardial injury [71]. Myocarditis and/or myopericarditis associated with COVID-19 infection can demonstrate ECG findings resembling occlusive AMI, such as focal ST segment elevation with reciprocal change [11,72,73].
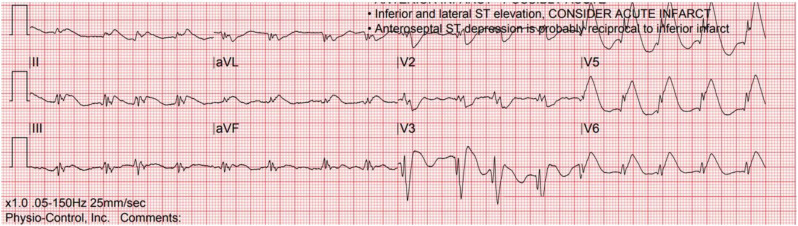
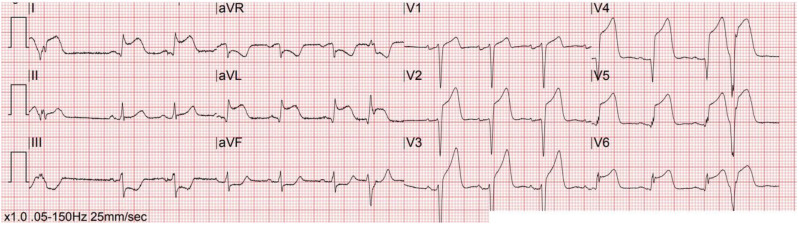
In the patient with ST segment elevation, the distinction between STEMI, myocardial injury, and myocarditis may not be possible strictly based upon ECG interpretation, which can make this particularly challenging. In the aforementioned case series of ST segment elevation in COVID-19-infected patients [71], STEMI was suggested with focal ST segment elevations, while myocardial injury demonstrated diffuse or widespread ST segment elevation. The clinical presentation can provide diagnostic clues and aid in the distinction of STEMI versus myocardial injury/myocarditis. Chest pain is a frequent presentation in the STEMI patient, while it is less common with myocardial injury/myocarditis which more often involves dyspnea and other symptoms consistent with acute viral infection [1]. Echocardiographic findings such as focal wall motion abnormalities with depressed left ventricular function suggest STEMI while myocardial injury more often demonstrates normal left ventricular function or diffuse dysfunction [2]. Fig. 3, Fig. 5, Fig. 6, Fig. 7, Fig. 8 depict ST segment elevation corresponding to STEMI (Fig. 5, Fig. 6) and myocardial injury (Fig. 7, Fig. 8) in COVID-19-infected patients.
Fig. 5.
Inferior STEMI with focal ST segment elevation in leads II, III, and aVF and reciprocal ST segment depression in lead aVL in a COVID-19-infected patient.
Fig. 6.
Anterior STEMI with focal ST segment elevation in leads V1 to V4 and reciprocal ST segment depression in the inferior leads in a COVID-19-infected patient.
Fig. 7.
Myocardial injury in a COVID-19-infected patient with diffuse ST segment elevation in the inferior, lateral, and anterior regions. Bedside echocardiography revealed no focal wall motion abnormalities with only mild diffuse hypokinesis.
Fig. 8.
Myocardial injury in a COVID-19-infected patient with diffuse ST segment elevation in the anterior and lateral regions. This patient was initially taken to the cardiac catheterization laboratory with no coronary occlusive lesions noted. The serum troponin values were extremely elevated but in a plateau pattern, rather than the typical rise and fall patient of acute myocardial infarction.
COVID-19 may also result in an unmasked Brugada pattern on ECG in patients with existing pathology. A pseudo-Brugada type 1 pattern is associated with fever and changes in myocardial sodium channels [[74], [75], [76]]. Type 1 is demonstrated by convex ST segment elevation > 2 mm in more than 1 lead of V1-V3, followed by a negative T wave.
3.7. Electrocardiographic presentations of COVID-19-related pulmonary embolism
PE has been recognized as a presenting issue and/or complication of COVID-19, particularly in patients with severe illness [[77], [78], [79]]. This COVID-19-related predisposition to venous thromboembolism likely occurs via several different mechanisms, including increased angiotensin II activity and related thrombogenic effects via enhanced coagulation system and platelet function, cytokine-mediated activation of the coagulation cascade, and a potential direct effect of the viral infection, causing localized inflammatory process and enhanced focal thrombosis [[77], [78], [79]].
Sinus tachycardia and/or atrial fibrillation with rapid ventricular response are commonly encountered in the setting of critical illness, including PE and COVID-19 [[77], [78], [79], [80]]. Electrocardiographic findings of right ventricular strain, as discussed previously, are also frequently encountered. In a recent review of PE in patients with COVID-19 infection, ECG findings most often involved non-specific abnormalities, including sinus tachycardia and minimal ST segment or T wave changes. More specific findings, suggestive of right ventricular strain, were encountered in only one-third of patients. The classic S1Q3T3 pattern was seen in less than 10% of COVID-19-related PE [77]. Other electrocardiographic presentations typical of PE include anterior T wave inversion and right bundle branch block [77,78]. In non-COVID-19 patients with PE, a normal ECG is encountered in approximately one-fifth of such individuals [80]. In patients with COVID-19 and critical illness, the ECG is rarely entirely normal when PE is diagnosed. While ECG findings may suggest PE, an ECG should not be used to rule in or rule out the disease.
4. Conclusions
COVID-19 can negatively impact the cardiovascular system and lead to abnormal ECG findings, which may be due to cytokine storm, hypoxic injury, electrolyte abnormalities, plaque rupture, coronary spasm, microthrombi, as well as direct endothelial or myocardial injury. The most common finding is sinus tachycardia, but others include SVTs such as atrial fibrillation or flutter, ventricular arrhythmias such as VT or VF, bradycardia, interval and axis changes (QT prolongation), and ST segment and T wave changes. Knowledge of these ECG abnormalities may assist emergency clinicians in evaluation and management of patients with COVID-19.
Declaration of Competing Interest
There are no conflicts of interest for any author.
Acknowledgements
BL, TM, MR, WB, MG, and MS conceived the idea for this manuscript and contributed substantially to the writing and editing of the review. This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, or SAUSHEC EM Residency Program.
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease (COVID-2019) Situation Report 124. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Last accessed: 5/24/20, 2020.
- 3.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.048. Epub 2020 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Published online March 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Chen C., Yan J., et al. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhong Hua Xin Xue Guan Bing Za Zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., He W., Yu X., et al. Prognostic value of myocardial injury in patients with COVID-19. Zhong Hua Xin Xue Guan Bing Za Zhi. 2020;56 doi: 10.3760/cma.j.cn112148-20200313-00202. [DOI] [PubMed] [Google Scholar]
- 8.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim I.C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. Epub 2020 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cizgici A.Y., Zencirkiran Agus H., Yildiz M. COVID-19 myopericarditis: it should be kept in mind in today’s conditions. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.080. Epub 2020 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabbagh M.F., Aurora L., D’Souza P., et al. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.009. Epub 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidovich M.I. Transient Brugada-like ECG pattern in a patient with coronavirus disease 2019 (COVID-19) JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.007. Epub 2020 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asif T., Ali Z. Transient ST segment elevation in two patients with COVID-19 and a normal transthoracic echocardiogram. Eur J Case Rep Intern Med. 2020;7(5) doi: 10.12890/2020_001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jankelson L., Karam G., Becker M.L., Chinitz L.A., Tsai M.C. QT prolongation, torsades de pointes and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.008. Epub 2020 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chorin E., Wadhwani L., Magnani S., et al. QT interval prolongation and torsade De pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.014. Epub 2020 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Broek M.P.H., Möhlmann J.E., Abeln B.G.S., et al. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth Heart J. 2020 doi: 10.1007/s12471-020-01429-7. Epub 2020 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias P., Poterucha T.J., Jain S.S., et al. The prognostic value of electrocardiogram at presentation to emergency department in patients with COVID-19. Mayo Clin Proc. 2020;95(10):2099–2109. doi: 10.1016/j.mayocp.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haseeb S., Gul E.E., Çinier G., et al. Value of electrocardiography in coronavirus disease 2019 (COVID-19) [published online ahead of print, 2020 Aug 6] J Electrocardiol. 2020;62:39–45. doi: 10.1016/j.jelectrocard.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [Epub ahead of print: 04 Mar 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Penninger J.M., Li Y., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 25.Zou X., Chen K., Zou J., et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020 doi: 10.1007/s11684-020-0754-0. [Epub ahead of print: 12 Mar 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao X.H., Li T.Y., He Z.C., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49 doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 27.Prabhu S.D. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 28.Levi M., van der Poll T., Büller H.R. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian S., Xiong Y., Liu H., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through post-mortem core biopsies. Preprints. 2020 doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox S.E., Akmatbekov A., Harbert J.L., et al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from new Orleans. medRxiv. 2020 doi: 10.1016/S2213-2600(20)30243-5. 2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertini M., Ferrari R., Guardigli G., et al. Electrocardiographic features of 431 consecutive, critically ill COVID-19 patients: an insight into the mechanisms of cardiac involvement. Europace. 2020 Sep 18:euaa258. doi: 10.1093/europace/euaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottlieb M., Sansom S., Frankenberger C., et al. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med. 2020 Aug 6 doi: 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Y., Tu L., Zhu P., et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clin Med. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehra M.R., Desai S.S., Kuy S.R., et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382 doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Abrams M.P., Wan E.Y., Waase M.P., et al. Clinical and cardiac characteristics of COVID-19 mortalities in a diverse New York City cohort. J Cardiovasc Electrophysiol. 2020 Oct 6 doi: 10.1111/jce.14772. 33022765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Chen L., Wang J., et al. Electrocardiogram analysis of patients with different types of COVID-19. Ann Noninvasive Electrocardiol. 2020 Sep;20 doi: 10.1111/anec.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradley A., Sheridan P. Atrial fibrillation. BMJ. 2013;346 doi: 10.1136/bmj.f3719. [DOI] [PubMed] [Google Scholar]
- 42.Brady W.J., Ferguson J.D., Ullman E.A., Perron A.D. Myocarditis: emergency department recognition and management. Emerg Med Clin North Am. 2004;22:865–885. doi: 10.1016/j.emc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Noubiap J.J., Bigna J.J., Agbor V.N., et al. Meta-analysis of atrial fibrillation in patients with various cardiomyopathies. Am J Cardiol. 2019;124:262–269. doi: 10.1016/j.amjcard.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 44.Kang Y., Chen T., Mui D., et al. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020 doi: 10.1136/heartjnl-2020-317056. Published online April 30, 2020:heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang E.Y., Hulme O.L., Khurshid S., et al. Initial precipitants and recurrence of atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047549. Published online April 15, 2020:CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 47.Seecheran R., Narayansingh R., Giddings S., et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620925571. 2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fried J.A., Ramasubbu K., Bhatt R., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047164. Published online April 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turagam M.K., Musikantow D., Goldman M.E., et al. Malignant arrhythmias in patients with COVID-19: incidence, mechanisms and outcomes. Circ Arrhythm Electrophysiol. 2020 Oct 7 doi: 10.1161/CIRCEP.120.008920. PMID: 33026892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ammirati E., Cipriani M., Moro C., et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: multicenter lombardy registry. Circulation. 2018;138:1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 51.Hufnagel G., Pankuweit S., Richter A., Schönian U., Maisch B. The European study of epidemiology and treatment of cardiac inflammatory diseases (ESETCID). First epidemiological results. Herz. 2000;25:279–285. doi: 10.1007/s000590050021. [DOI] [PubMed] [Google Scholar]
- 52.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047349. Published online April 16, 2020:CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.03.024. Published online April 2020:S002561962030313X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baldi E., Sechi G.M., Mare C., et al. For the Lombardia CARe researchers. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020;30(383):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldi E., Sechi G.M., Mare C., et al for the Lombardia CARe Researchers COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;41:3045–3054. doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai P.H., Lancet E.A., Weiden M.D., et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5:1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao F., Xu S., Ma X., et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramzy M., Montrief T., Gottlieb M., Brady W.J., Singh M., Long B. COVID-19 cardiac arrest management: a review for emergency clinicians. Am J Emerg Med. 2020;38(12):2693–2702. doi: 10.1016/j.ajem.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He J., Wu B., Chen Y., et al. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol. 2020 doi: 10.1016/j.cjca.2020.03.028. Published online March 2020:S0828282X20303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Liu T., Liu M., et al. Electrocardiogram abnormalities in patients with COVID-19. Zhong Hua Xin Lv Shi Chang Xue Za Zhi. 2020;24:12832. [Google Scholar]
- 62.Jean-Louis F., Adedayo A., Ajibawo T., et al. A rare case of resolution of high-degree atrioventricular block associated with COVID-19. J Med Cases North Am. 11, Jul 2020 doi: 10.14740/jmc3524. https://www.journalmc.org/index.php/JMC/article/view/3524/2837 Available from: Date accessed: 09 Oct. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azarkish M., Laleh Far V., Eslami M., Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020;41:2131. doi: 10.1093/eurheartj/ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho J.S., Sia C.H., Chan M.Y., Lin W., Wong R.C. Coronavirus-induced myocarditis: A meta-summary of cases. Heart Lung. 2020;49(6):681–685. doi: 10.1016/j.hrtlng.2020.08.013. PMID: 32861884; PMCID: PMC7440036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amaratunga E.A., Corwin D.S., Moran L., Snyder R. Bradycardia in patients with COVID-19: a calm before the storm? Cereus. 2020;12 doi: 10.7759/cureus.8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roden D.M., Harrington R.A., Poppas A., Russo A.M. Considerations for drug interactions on QTc in exploratory COVID-19 (coronavirus disease 2019) treatment. Circulation. 2020;141:e906–e907. doi: 10.1161/CIRCULATIONAHA.120.047521. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Mercuro N.J., Yen C.F., Shim D.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramireddy A., Chugh H., Reinier K., et al. Experience with hydroxychloroquine and azithromycin in the COVID-19 pandemic: implications for QT interval monitoring [published online ahead of print, 2020 May 28] J Am Heart Assoc. 2020 doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Feng Y., Tang J., et al. Surface electrocardiographic characteristics in coronavirus disease 2019: repolarization abnormalities associated with cardiac involvement. ESC Heart Fail. 2020 Sep 8 doi: 10.1002/ehf2.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y., Liu T., Tse G., Wu M., et al. Electrocardiograhic characteristics in patients with coronavirus infection: a single-center observational study. Ann Noninvasive Electrocardiol. 2020 Sep;20 doi: 10.1111/anec.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020:ehaa190. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sorgente A., Capulzini L., Brugada P. The known into the unknown: Brugada syndrome and COVID-19. JACC Case Rep. 2020;2:1250–1251. doi: 10.1016/j.jaccas.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang D., Saleh M., Garcia-Bengo Y., et al. COVID-19 infection unmasking Brugada syndrome. Heart Rhythm Case Rep. 2020;6:237–240. doi: 10.1016/j.hrcr.2020.03.012. Published 2020 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lugenbiel P., Roth L., Seiz M., et al. The arrhythmogenic face of COVID-19: Brugada ECG pattern during acute infection. Eur Heart J Case Rep. 2020;30(4):1–2. doi: 10.1093/ehjcr/ytaa230. PMID: 33089051; PMCID: PMC7454489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kho J., Ioannou A., Van den Abbeele K., Mandal A.K.J., Missouris C.G. Pulmonary embolism in COVID-19: clinical characteristics and cardiac implications. Am J Emerg Med. 2020;38:2142–2146. doi: 10.1016/j.ajem.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 May 5 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avila J., Long B., Holladay D., Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021;39:213–218. doi: 10.1016/j.ajem.2020.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ullman E., Brady W.J., Perron A.D., Chan T., Mattu A. Electrocardiographic manifestations of pulmonary embolism. Am J Emerg Med. 2001 Oct;19(6):514–519. doi: 10.1053/ajem.2001.27172. [DOI] [PubMed] [Google Scholar]