Abstract
Purpose/Objectives:
Evidence from the management of oligometastases with stereotactic body radiation therapy (SBRT) reveals differences in outcomes based on primary histology. We have previously identified a multigene expression index for tumor radiosensitivity (RSI) with validation in multiple independent cohorts. In this study, we assessed RSI in liver metastases and assessed our clinical outcomes after SBRT based on primary histology.
Methods and Materials:
Patients were identified from our prospective, observational protocol. The previously tested RSI 10 gene assay was run on samples and calculated using the published algorithm. An independent cohort of 33 patients with 38 liver metastases treated with SBRT was used for clinical correlation.
Results:
A total of 372 unique metastatic liver lesions were identified for inclusion from our prospective, institutional metadata pool. The most common primary histologies for liver metastases were colorectal adenocarcinoma (n = 314, 84.4%), breast adenocarcinoma (n = 12, 3.2%), and pancreas neuroendocrine (n = 11, 3%). There were significant differences in RSI of liver metastases based on histology. The median RSIs for liver metastases in descending order of radioresistance were gastrointestinal stromal tumor (0.57), melanoma (0.53), colorectal neuroendocrine (0.46), pancreas neuroendocrine (0.44), colorectal adenocarcinoma (0.43), breast adenocarcinoma (0.35), lung adenocarcinoma (0.31), pancreas adenocarcinoma (0.27), anal squamous cell cancer (0.22), and small intestine neuroendocrine (0.21) (P<.0001). The 12-month and 24-month Kaplan-Meier rates of local control (LC) for colorectal lesions from the independent clinical cohort were 79% and 59%, compared with 100% for non-colorectal lesions (P = .019), respectively.
Conclusions:
In this analysis, we found significant differences based on primary histology. This study suggests that primary histology may be an important factor to consider in SBRT radiation dose selection.
Summary
We have previously validated a multigene expression index for tumor radiosensitivity (RSI). The current analysis reveals significant differences in RSI based on primary histology of liver metastases with colorectal adenocarcinoma found to be more radioresistant. Differences in RSI were validated in a cohort of 33 patients treated with stereotactic body radiation therapy (SBRT) to 38 liver metastases. This analysis reveals primary histology to be an important factor to consider in SBRT radiation dose selection.
Introduction
The management of liver metastases with stereotactic body radiation therapy (SBRT) has become increasingly common; several large prospective phase 1 and 2 trials have revealed excellent local control (LC) and minimal toxicity (1-5). Factors influencing clinical outcomes after SBRT for liver metastases are believed to include radiation dose (6, 7), tumor volume (1, 3, 5, 7), and previous lines of chemotherapy (2). Some reports have suggested a difference in outcomes based on primary histology. Reports have suggested poorer LC with colorectal histology (3, 8). Little research currently exists to help guide dose selection and predict outcomes in the management of liver metastases with SBRT based on primary histology.
We have previously developed a multigene expression model of tumor radiosensitivity (9). This model has been validated in multiple independent clinical cohorts including breast, rectal, esophageal, head and neck, glioblastoma, pancreas, and prostate malignancies (9-14). This model predicts a radiosensitivity index (RSI) that is directly proportional to tumor radioresistance (RSI: high index = radioresistance). A recent report from our group demonstrated significant differences in RSI based on the anatomic location of colon metastases (10). This was validated in an independent cohort of 23 metastatic colon cancer patients treated with SBRT to 29 lung and liver metastases. The purpose of this study was to assess differences in RSI between liver metastases based on histology and to verify these differences in RSI based on histology in an independent cohort of patients treated with liver SBRT.
Methods and Materials
Patients were identified from the institutional review board—approved Total Cancer Care (TCC) prospective, deidentified observational protocol at TCC (15). Data from a total of 380 unique, surgically resected metastatic liver lesions were obtained from the TCC metadata pool.
RNA preparation and gene expression profiling
These methods have been described previously (10). The expression values for the samples in this study and corresponding genes were extracted from the TCC database. These expression values were normalized against a median sample using iterative rank order normalization (16). An RNA quality-related batch effect was identified in the resulting normalized data, which was removed by training a partial least squares (PLS) model (17) to the RNA integrity number (RIN) for each sample and then subtracting the first partial least squares component.
Radiosensitivity signature
The previously tested 10-gene assay was run on tissue samples ranked according to gene expression. RSI was calculated using the previously published ranked based algorithm (9, 18).
Briefly, each of the 10 genes in the assay was ranked according to gene expression: from the highest expressed gene (10) to the lowest (1). RSI was determined using the previously published ranked based linear algorithm:
SBRT patient cohorts
An independent retrospective analysis was conducted on all consecutive patients treated with a dose of 50 or 60 Gy in 5 fractions given over 1 week. Each patient was immobilized with the Body-Fix whole-body or thoracic-T double vacuum immobilization system (Medical Intelligence, Schwabmünchen, Germany). Patients underwent placement of fiducial markers before simulation, guided by either computed tomographic (CT), endoscopic, or angiographic approaches. An individualized motion management strategy was chosen after conventional simulation with fluoroscopy to determine the amount of respiratory-associated fiducial marker motion with an intended technique of either incorporating an abdominal compression device or respiratory gating using an infrared reflector on the patient’s chest. The appropriate personalized motion management technique was then designed at simulation with axial CT images obtained on a Light Speed RT 16-slice CT simulator (GE HealthCare, Milwaukee, WI) with image acquisition set at 3-mm slice thickness. Four-dimensional CT imaging was performed using Varian RPM (Varian, Palo Alto, CA). For all patients, an initial cone beam computed tomography (CBCT) scan was acquired at the time of the first treatment for patients treated with an intensity modulated radiation therapy technique with subsequent daily CBCT films with appropriate shifts to confirm alignment. For patients treated with a 3-dimensional conformal radiation therapy (3DCRT) plan, a CBCT was performed on the preport day for verification, and then daily kilovoltage (KV) images were taken before each fraction with the patient in the maximum exhalation position to match the gated digitally reconstructed radiograph. For planning purposes, the planning target volume (PTV) equaled the gross tumor volume (GTV) +5 mm. The maximum doses to the bowel, cord, esophagus, and stomach were 30 Gy, 20 Gy, 20 Gy, and 30 Gy, respectively. In addition, the mean dose to the kidneys was <10 Gy, liver-GTV V15 <700 cc, lung V10 <1500 cc, and heart mean <10 Gy. The PTV was covered by 90% of the prescribed dose.
After treatment, patients were followed up by the treating radiation oncologist or medical oncologist with imaging at 2- to 3-month intervals. An independent review of imaging to assess LC was undertaken by 2 radiation oncologists (K.A.A. and J.J.C.) and a radiologist (G.E.H.). Local failure was defined by an increase in the size of the previously irradiated area according to the Response Evaluation Criteria in Solid Tumors criteria, version 1.1 (19).
Statistical analysis
Statistical analyses were performed with JMP 11 (SAS Institute Inc, Cary, NC). Descriptive statistics were used to summarize the cohort, including median and range for continuous variables or counts and percentages for categorical variables. When comparing RSI values between groups, the Kruskal-Wallis test was used. The Fisher exact and median test was used to test clinical differences between groups. The overall survival (OS) and LC rates were calculated from the date of treatment to the date of death or progression using the Kaplan-Meier method. A 2-tailed P<.05 was considered statistically significant. Cox proportional hazard model analyses were performed using univariate analysis and multivariate analysis. Box plots were generated using MATLAB (version R2013a, Math-Works Inc, 2010, Natick, MA).
Results
Differences in the RSI of liver metastases
In this analysis, primary histologies selected for inclusion were limited to sites with ≥3 tissue samples; this yielded a total of 372 metastatic samples for inclusion in the study. The most common primary histologies for liver metastases were colorectal adenocarcinoma (n = 314, 84.4%), breast adenocarcinoma (n = 12, 3.2%), pancreas neuroendocrine (n = 11, 3%), and melanoma (n = 9, 2.4%).
The median RSI for all liver lesions was 0.43 (quartile [Q]1, 0.28; Q3, 0.49). There were significant differences in RSIs of liver metastases based on primary histology. The median RSIs for liver metastases in descending order of radioresistance were gastrointestinal stromal tumor (GIST) (0.57), melanoma (0.53), colorectal neuroendocrine (0.46), pancreas neuroendocrine (0.44), colorectal adenocarcinoma (0.43), breast adenocarcinoma (0.35), lung adenocarcinoma (0.31), pancreas adenocarcinoma (0.27), anal squamous cell cancer (0.22), and small intestine neuroendocrine (0.21) (P<.0001). A box plot of RSI of liver metastases based on primary histology is displayed in Figure 1.
Fig. 1.
Box plot of radiosensitive index (RSI) values of liver metastases based on primary histology. Abbreviation: GIST = gastrointestinal stromal tumor. Unfilled diamonds represent outliers using the standard 1.5 interquartile range rule.
A total of 7 patients had multiple different liver tissue samples (n = 15) available for analysis. They included colorectal adenocarcinoma (n = 13, 86.7%) and breast adenocarcinoma (n = 2, 13.3%). The deviation from the mean from tumor samples from the same patient with the same histology was 0.026 (range, 0.004-0.09). The remaining 357 samples were from individual patients.
Clinical outcomes in liver metastases treated with SBRT
An independent cohort of 33 patients with 38 liver metastases treated with SBRT at our institution was assessed. This included a total of 27 colorectal metastases and 11 noncolorectal metastases (4 breast adenocarcinoma, 5 anal squamous cell cancer, and 2 lung adenocarcinoma). Of note, all histologies included in the noncolorectal group showed a more radiosensitive profile based on the RSI analysis. The characteristics of the lesions treated are shown in Table 1. The median follow-up time for all lesions was 21.2 months (range, 2.5-44.9 months). There were no differences between colorectal and noncolorectal metastases with respect to maximum diameter of the lesion (P = .29), SBRT dose (P = .69), or number of lines of previous chemotherapy (P = .59). There was a significant difference in LC of colorectal and noncolorectal liver metastases. Ten colorectal metastases showed local failure in the previously irradiated tumor bed on follow-up imaging, whereas no patients treated for noncolorectal metastases experienced local failure. The 12-month and 24-month Kaplan-Meier rates of LC for colorectal lesions were 79% and 59%, compared with 100% for non-colorectal lesions (P = .019) (Fig. 2). There was no significant difference in OS between patients treated for colorectal and noncolorectal metastases, with 12-month and 24-month rates of 100% and 73% and 82% and 73% (P = .75), respectively. There was a trend toward SBRT dose predicting response on univariate analysis: 50 Gy versus 60 Gy (hazard ratio 3.0; 95% confidence interval [CI]: 0.82-11.0; P = .10). Other factors including size ≥2 cm/<2 cm (P = .47) and number of lines of previous chemotherapy ≥2/1 (P = .42), age (P = .12), and gender (P = .47) were not found to be significant factors predicting LC. When factors that were trending on univariate analysis (age and dose) were taken into account, colorectal histology remained significant for local failure (P = .04) on multivariate analysis.
Table 1.
Clinical characteristics of colorectal and noncolorectal liver metastases
| Characteristic | Colorectal | Noncolorectal | P value |
|---|---|---|---|
| Patients (n) | 22 | 11 | |
| Age, y (range) | 67 (39-89) | 60 (46-74) | .11 |
| Male/female | 11/11 | 2/9 | .08 |
| Lesions (n) | 27 | 11 | |
| Diameter of lesion, cm (range) | 2 (0.6-6.7) | 2.7 (1.2-5.1) | .29 |
| Dose 50 Gy/60 Gy | 8/19 | 2/9 | .69 |
| Number of lines of previous chemotherapy (range) | 2 (0-5) | 2 (1-4) | .59 |
| Follow-up, mo (range) | 20.5 (3-44.9) | 28.4 (2.5-38.6) |
Fig. 2.
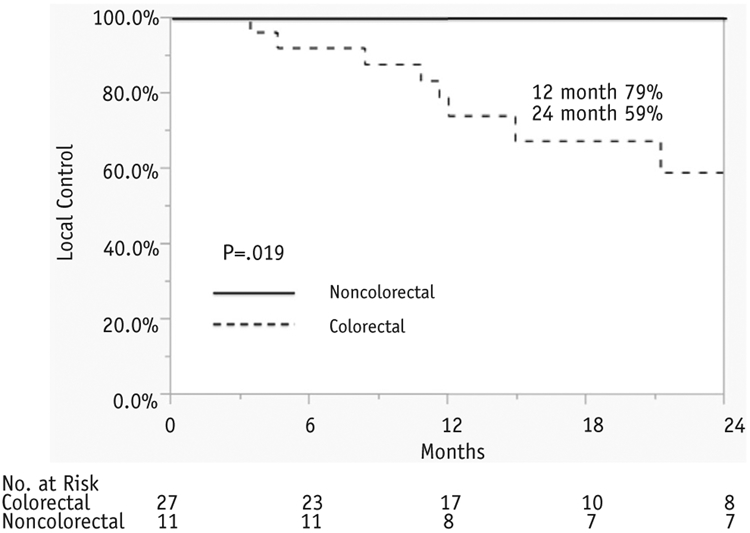
Kaplan-Meier local control rates for colorectal and noncolorectal liver metastases.
Discussion
In this analysis of liver metastases based on RSI, we noted several findings. First, significant differences were seen in liver metastases based on histology. Second, colorectal adenocarcinoma metastases were determined to be more radioresistant than were histologies such as anal squamous cell cancer, breast adenocarcinoma, and lung adenocarcinoma. Finally, these differences corresponded to clinical outcomes in an independent dataset of patients treated for liver metastases with SBRT at our institution with 50 Gy or 60 Gy in 5 fractions.
Several phase 1 and 2 studies have noted the safety and efficacy of SBRT as a treatment modality in the management of liver metastases (1-5). Hoyer et al (2) reported on the results of inoperable colorectal metastases treated with SBRT. There were 44 lesions treated in the liver, with a central dose of 45 Gy in 3 fractions. The 2-year rates of LC by tumor and patient were 86% and 63%, respectively. The treatment was well tolerated, but 1 case of liver failure was noted, with 2 severe late GI toxicities. The use of SBRT in the management of liver metastases is a well-accepted treatment modality, with LC rates in prospective and retrospective series ranging between 70% and 100% at 1 year and between 60% and 90% at 2 years after SBRT (20).
There is increasing evidence that primary histology may play a role in clinical outcomes after SBRT in the management of oligometastatic disease. Takeda et al (21) found differences in clinical outcomes between the LC of oligometastatic lung lesions compared with primary lung cancers. The study assessed 21 colorectal metastatic lung lesions, 23 lesions from other origins, and 188 primary lung cancers treated with 50 Gy in 5 fractions. The study found a 1-year LC of 80%, 94%, and 97% (P<.05) for colorectal primaries, oligometastases from other origins, and clinically diagnosed lung cancer, respectively. Milano et al (22) found improved outcomes in the management of oligometastatic disease of breast primaries versus nonbreast primaries, of which 38% were colorectal in origin. The 2-year, 4-year, and 6-year LC rates were 87% for breast primaries and 74%, 68%, and 65%, for nonbreast primaries, respectively. Lee et al (3) found a worse 1-year survival rate for colorectal than for breast patients treated for liver metastases, although the result was not statistically significant: 63% (95% CI: 44%-78%) versus 79% (95% CI: 36%-94%) (3). Although specific LC rates were not stated, the authors observed worse LC in patients treated for colorectal metastases (3, 20). Taken together, these studies indicate that histology is a variable which may affect outcomes and should potentially be taken into account in the development of SBRT dose and fractionation schedules.
Our previous analysis showed that in the management of colon metastases, RSI values differ based on anatomic location of the metastasis (10). Our own institutional experience suggests that clinical outcomes differ based on the treated anatomic location, with liver metastases found to be more radioresistant than lung metastases. In this study, we extended our work on liver metastases by assessing RSI values based on primary histology. Our study suggests that primary histology plays a significant role in determining radiosensitivity. Primary histologies, which we traditionally view as more radiosensitive, such as anal and breast, as predicted were found to have lower RSI values. Whereas when metastasized to the lung, colon cancer was more radiosensitive in our previous analysis (10), when it metastasized to the liver we found the histology to be more radioresistant in the present analysis. Clinical liver SBRT outcomes at our institution confirmed that when treated to a similar dose and fractionation, the results were worse than more radiosensitive histologies.
Previous studies have indicated that radiation dose also plays a significant role in LC outcomes after liver SBRT. A study from the University of Colorado by McCammon et al (7) reported on 246 lung and liver metastases treated with 3-fraction SBRT. Lesions treated to a dose of ≥54 Gy had a 3-year LC of 89.3% compared with 59.0% and 8.1% for those treated to 36 to 53.9 Gy and <36 Gy, respectively. Rusthoven et al (5) reported phase 1-2 results in 47 patients with 63 liver metastases with a maximal diameter <6 cm in a dose escalation trial of 36 to 60 Gy in 3 fractions. The LC rates at 1 and 2 years after SBRT were 95% and 92%, respectively, with a median survival of 20.5 months. This is higher than phase 1-2 reports from Lee et al (3) in 68 inoperable or medically unsuitable patients with lesions treated to a lower median SBRT dose of 41.8 Gy (range, 27.7-60 Gy) in 6 fractions over 2 weeks. A 1-year LC rate of 71% (95 CI, 58%-85%) was reported. Doses of 75 Gy in 3 fractions have been reported safe in phase 2 studies (4).
Although our series is smaller, there is an indication that dose may play a role in determining LC. Our study found a trend in lesions treated with 50 Gy faring worse than lesions treated with 60 Gy. The data from our study indicate that colorectal liver metastases and other more radioresistant metastases in the liver may fare better with higher effective doses of radiation and that when it is feasible, dose escalation should be used for radioresistant histologies. These data will need to be validated prospectively and will be the focus of a clinical trial at our institution.
This study of RSI values of liver metastases based on primary histology is not without limitations. First, genomic data were obtained from our institutional metadata pool; thus, not all clinical and baseline demographic characteristics of the 372 metastatic liver lesions are available. Second, RSI values for the 38 liver metastases are not available. This makes direct comparisons between datasets difficult. In addition, the majority of samples in this study, both in the genomic RSI analysis and in the separate clinical analysis, were of colorectal origin, which is not entirely unexpected, given the prevalence of management of oligometastatic colorectal liver metastases. However, these results are quite intriguing with regard to the management of oligometastatic liver metastases, and they may provide guidance toward novel dosing schedules with SBRT.
In this analysis assessing radiosensitivity of liver metastases based on primary histology, we noted significant differences based on primary histology. Primary histology played a significant role in determining radiosensitivity, with colorectal metastases found to be more radioresistant than histologies such as anal squamous cell cancer, breast adenocarcinoma, and lung adenocarcinoma. These findings correlated with clinical outcomes in an independent dataset of patients treated with liver SBRT. This study paves the way for a future clinical trial with genomically guided dose selection based on primary histology and RSI.
Acknowledgments
Supported by National Institutes of Health grants R21CA101355/R21CA135620, US Army Medical Research and Materiel Command (W81XWH-08-2-0101, subaward 12-15479-01-07), National Functional Genomics Center award 170220051, Bankhead-Coley Foundation award 09BB-22, and the Debartolo Family Personalized Medicine Institute; also supported in part under the Merck-Moffitt Cancer Center Research Collaboration.
Footnotes
Partially presented in oral form at the 57th Annual Meeting of the American Society for Radiation Oncology, San Antonio, Texas, Oct 21, 2015.
Conflict of interest: Steven A. Eschrich and Javier F. Torres-Roca report stock and leadership in Cvergenx, Inc and royalty and patents on RSI. The authors report no other conflict of interest.
References
- 1.Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: Results of a phase I/II trial. J Clin Oncol 2001; 19:164–170. [DOI] [PubMed] [Google Scholar]
- 2.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006; 45:823–830. [DOI] [PubMed] [Google Scholar]
- 3.Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009; 27:1585–1591. [DOI] [PubMed] [Google Scholar]
- 4.Scorsetti M, Arcangeli S, Tozzi A, et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radial Oncol Biol Phys 2013;86:336–342. [DOI] [PubMed] [Google Scholar]
- 5.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572–1578. [DOI] [PubMed] [Google Scholar]
- 6.Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006;45:838–847. [DOI] [PubMed] [Google Scholar]
- 7.McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radial Oncol Biol Phys 2009;73:112–118. [DOI] [PubMed] [Google Scholar]
- 8.Milano MT, Katz AW, Zhang H, et al. Oligometastases treated with stereotactic body radiotherapy: Long-term follow-up of prospective study. Int J Radial Oncol Biol Phys 2012;83:878–886. [DOI] [PubMed] [Google Scholar]
- 9.Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: Prediction of response and prognosis after chemoradiation. Int J Radial Oncol Biol Phys 2009;75:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed KA, Fulp WJ, Berglund AE, et al. Differences between colon cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential oligometastatic SBRT patient selection. Int J Radiat Oncol Biol Phys 2015;92:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed KA, Chinnaiyan P, Fulp WJ, et al. The radiosensitivity index predicts for overall survival in glioblastoma. Oncotarget 2015;6: 34414–34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eschrich SA, Fulp WJ, Pawitan Y, et al. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res 2012;18: 5134–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strom T, Hoffe SE, Fulp W, et al. Radiosensitivity index predicts for survival with adjuvant radiation in resectable pancreatic cancer. Radiother Oncol 2015;117:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres-Roca JF, Erho N, Vergara I, et al. A molecular signature of radiosensitivity (RSI) is an RT-specific biomarker in prostate cancer. Int J Radiat Oncol Biol Phys 2014;90:S157. [Google Scholar]
- 15.Fenstermacher DA, Wenham RM, Rollison DE, et al. Implementing personalized medicine in a cancer center. Cancer J 2011;17:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh EA, Eschrich SA, Berglund AE, et al. Iterative rank-order normalization of gene expression microarray data. BMC Bioinformatics 2013;14:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wold S, Ruhe A, Wold H, et al. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J Sci Stat Comput 1984;5:735–743. [Google Scholar]
- 18.Eschrich S, Zhang H, Zhao H, et al. Systems biology modeling of the radiation sensitivity network: A biomarker discovery platform. Int J Radiat Oncol Biol Phys 2009;75:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: A review of evidence. Int J Radiat Oncol Biol Phys 2012;82: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 21.Takeda A, Kunieda E, Ohashi T, et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011;101:255–259. [DOI] [PubMed] [Google Scholar]
- 22.Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008;112:650–658. [DOI] [PubMed] [Google Scholar]



