Abstract
Although the protective role of dietary fiber on cancer risk has been reported in several epidemiological studies, the association of fiber intake on head and neck cancer (HNC) risk is still unclear. We investigated the association between fiber intake and the risk of HNC using data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Among 101,700 participants with complete dietary information, 186 participants developed HNC during follow-up (January 1998 to May 2011). Dietary data were collected using a self-administered food-frequency questionnaire (1998–2005). We estimated hazard ratios (HRs) and the corresponding 95% confidence intervals (CI), using the Cox proportional hazards model. Higher intake of total fiber, insoluble fiber and soluble fiber was associated with decreased HNC risks, with a significant trend. The HRs of highest versus the lowest tertile of intake were 0.43 (95%CI: 0.25–0.76) for total fiber, 0.38 (95%CI: 0.22–0.65) for insoluble fiber, and 0.44 (95%CI: 0.25–0.79) for soluble fiber. These inverse association were consistent in oral cavity and pharyngeal cases, but the impact of fiber intake was weaker in laryngeal cases. We did not observe any significant interaction of potential confounders, including smoking and drinking, with total fiber intake on HNC risk. These findings support evidence of a protective role of dietary fiber on HNC risk.
Keywords: Dietary fiber, Insoluble fiber, Soluble fiber, Head and Neck Cancer, Prospective Cohort Study
Brief description
Although the protective role of dietary fiber on cancer risk has been reported in several epidemiological studies, the possible association of fiber intake on head and neck cancer (HNC) risk remains unclear. In this large scale prospective cohort study, we found a protective role of fiber intake on HNC risk after allowance for major potential confounders, including smoking and drinking. These findings support evidence of a protective role of dietary fiber on HNC risk.
Introduction
More than 500,000 incident head and neck cancer (HNC) cases are diagnosed annually, and this is the 6th most common cancer worldwide1. Anatomical sites of HNC include oral cavity, oropharynx, hypopharynx and larynx. Tobacco smoking and alcohol drinking are the predominant and established risk factors2. The association between human papilloma virus (HPV) infection and oropharyngeal cancer is also established3. However, the role of other environmental factors, including dietary factors, is less clear4.
Intake of non-starchy vegetables and fruit has been thought to decrease HNC risk4. Fruits and vegetables are rich in vitamins, minerals, fiber and antioxidants5–8. Among them, an inverse dose-risk association between fiber intake and HNC risk has been suggested in a few studies9–19. Thus, dietary fiber could decrease the risk of HNC. However, most investigations which evaluated this association were case-control in study design, with the inherent issues of recall and selection bias9–16, 18. Only two prospective cohort studies investigated this association, showing inverse associations17, 19. Therefore, the World Cancer Research Fund report concluded that the evidence linking dietary fiber with head and neck cancers was too limited to form a reliable conclusion4. We therefore investigated the association between fiber intake and the risk of HNC using data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial.
Materials and Methods
Study design
The PLCO cancer screening trial is a large-scale clinical trial designed to determine whether selected screening tests reduce deaths from prostate, lung, colorectal, and ovarian cancer20. Briefly, the trial started in 1992 and ended enrollment in 2001. Approximately 155,000 men and women between the ages of 55 and 74 were enrolled at 10 centers across the United States (Alabama, Michigan, Colorado, Hawaii, Wisconsin, Minnesota, Pennsylvania, Utah, Missouri, and Washington DC). At entry, participants were randomized to one of two study groups. The control group received routine health care from their health providers. The intervention group received a series of screening tests for prostate, lung, colorectal, and ovarian cancers, which included chest X-ray, flexible sigmoidoscopy, prostate-specific antigen screening, digital rectal examination, cancer antigen 125 screening, and transvaginal ultrasound. Screening of participants ended in late 2006. The data used for this study include the follow-up information up to May 2011. Written informed consent was obtained from all study participants. Ethical approval for human subject’s research was obtained at each of the centers.
Data collection
Subjects randomized to either study arm (control or intervention) were eligible if they had completed the baseline questionnaire and the diet history questionnaire, which was administered to participants in both groups starting in 1998. Before that time, diet history questionnaires were administered to only those in the intervention arm. A study update was mailed annually to the study participants to ascertain cancer diagnoses. Participants were asked if they were diagnosed with cancer, the type of cancer, date of diagnosis, hospital or clinic of diagnosis, and physician contact information. For every cancer reported, medical record abstraction included the cancer diagnosis date and International Classification of Disease for Oncology, second edition (ICD-O-2) code. Vital status was obtained by the administration of the Annual Study Update questionnaire, reports from relatives, friends, or physicians, and National Death Index searches. Study centers attempted to obtain a death certificate for each death. If the study participants were diagnosed with cancer after study entry, which ranged from 1992 to 2001, and before completion of the dietary questionnaire, they were not eligible.
Only malignant primary HNC cases were considered. Tumors were assigned to 1 of the 5 categories as follows: (1) oral cavity: ICD-O-2 codes C00.3 to C00.9, C02.0 to C02.3, C03.0, C03.1, C03.9, C04.0, C04.1, C04.8, C04.9, C05.0, C06.0 to C06.2, C06.8, and C06.9; (2) oropharynx: ICD-O-2 codes C01.9, C02.4, C05.1, C05.2, C09.0, C09.1, C09.8, C09.9, C10.0, C10.2-C10.4, C10.8, and C10.9; (3) hypopharynx: ICD-O-2 codes C12.9, C13.0 to C13.2, C13.8, and C13.9; (4) oral cavity or pharynx not otherwise specified (NOS): ICD-O-2 codes C02.8, C02.9, C05.8, C05.9, C14.0, C14.2, and C14.8; and (5) larynx: ICD-O-2codes C10.1, C32.0 to C32.3 and C32.8 to C32.9. Of the 154,897 participants recruited into the PLCO study, 111,511 participants completed both the baseline questionnaire and the diet history questionnaire. Of the 111,511 participants with valid questionnaires, participants were excluded because: (1) they had cancer before entry into the PLCO study (n= 9697); (2) they did not have follow-up time (n= 91); (3) they had the incidence of salivary gland cancer after baseline (n= 23). Thus, this study included 101,700 participants, and 186 cases with confirmed incident HNC. The number of HNC cases was equal between groups with 93 HNC cases in each group.
The baseline questionnaire included self-reported information on age, sex, race, education, tobacco smoking, alcohol drinking, family history of cancer, medical history, height, weight and other selected life style factors. Dietary data were collected using a self-administered food-frequency questionnaire, the Diet History Questionnaire, version 1.0 (National Cancer Institute, 2007). The diet history questionnaire included portion size and frequency of consumption of 124 food items and supplement use during the past year21.
The Nutrition Data System for Research (NDS-R) was used to estimate the amount of dietary fiber. The NDS-R combines nutrition information from the US Department of Agriculture Nutrient Database for Standard Reference, food manufacturers, scientific literature, and other published food tables22. Specific fiber groups were created based on the pyramid food groups by calculating the fiber content of each food item belonging to the group and multiplying it by the reported amount consumed23, 24. Main sources of dietary fiber were cereal/grain, vegetables, fruit, and legumes24.
Statistical analysis
We estimated hazard ratios (HRs), and the corresponding 95% confidence intervals (CIs), using the Cox proportional hazards model. Follow-up time was calculated from the date of entry until the occurrence of one of the following events: diagnosis of HNC, death, or the end of follow-up. Models included adjustment for age (categorical), sex (male vs female), race (White, Non-Hispanic vs Other), body mass index (BMI) at the time of enrollment (≤24.9 kg/m2 vs ≥25 kg/m2), education (≤high school vs ≥some college), pipe and cigar smoking (never vs former vs current), cigarette smoking status (never vs former vs current), pack-year cigarette smoking (never vs <20 vs ≥20), alcohol drinking status (never vs former vs current), alcohol drinking intensity [alcohol (g/day):never vs <5 vs ≥5 and <10 vs ≥10 and <20 vs ≥20 and <30 vs ≥30], non-alcohol total energy (continuous), total vegetable and fruit intake (continuous), and marital status (married or living as married vs widowed vs divorced vs separated vs never married). Missing values for covariates were treated as dummy variables in the models. Tests for linear trend were computed scoring the tertiles from 1 to 3. To test interactions, we performed likelihood-ratio tests, which compared models with and without the interaction term.
All statistical analyses were performed using the software STATA version 13 (Stata Corp, College Station, TX, USA). All tests were two-sided.
Results
The median follow-up was 12.5 years. Table 1 shows the characteristics of the PLCO cohort and the HNC cases. Higher proportions of male, smokers, and drinkers were observed in HNC cases. Other characteristics showed no remarkable differences between the overall cohort and HNC cases. HNC cases included 81 cases for oral cavity, 18 for oropharynx, 10 for hypopharynx, 1 for NOS, and 76 for larynx.
Table 1.
Characteristics of the PLCO cohort abd the head and neck cancer cases
| Characteristics | Cohort | Cases | ||
|---|---|---|---|---|
| No. of participants | % | No. of cases | % | |
| Total | 101,700 | 186 | ||
| Age | ||||
| ≤59 years | 34,950 | 35 | 63 | 34 |
| 60–64 years | 31,742 | 31 | 60 | 32 |
| 65–69 years | 22,526 | 22 | 44 | 24 |
| ≥ 70 years | 12,482 | 12 | 19 | 10 |
| Sex | ||||
| Male | 49,460 | 49 | 150 | 81 |
| Female | 52,240 | 51 | 36 | 19 |
| BMI | ||||
| ≤24.9 kg/m2 | 33,737 | 33 | 63 | 34 |
| ≥25.0 kg/m2 | 66,630 | 66 | 119 | 64 |
| Missing | 1,333 | 1 | 4 | 2 |
| Education | ||||
| ≤High school | 42,916 | 42 | 81 | 44 |
| ≥Some college | 58,587 | 58 | 105 | 56 |
| Missing | 197 | 0 | 0 | 0 |
| Race | ||||
| White, Non-Hispanic | 92,483 | 91 | 168 | 90 |
| Other | 9,217 | 9 | 18 | 10 |
| Cigarette smoking status | ||||
| Never | 48,544 | 48 | 36 | 20 |
| Former | 43,749 | 43 | 88 | 47 |
| Current | 9,394 | 9 | 62 | 33 |
| Missing | 13 | 0 | 0 | 0 |
| Pack-year cigarette smoking | ||||
| Never | 48,544 | 48 | 36 | 19 |
| <20 | 19,239 | 19 | 25 | 13 |
| ≥20 | 32,761 | 32 | 124 | 67 |
| Missing | 1,156 | 1 | 1 | 1 |
| Alcohol drinking status | ||||
| Never | 10,112 | 10 | 5 | 3 |
| Former | 14,752 | 14 | 32 | 17 |
| Current | 73,956 | 73 | 143 | 77 |
| Missing | 2,880 | 3 | 6 | 3 |
| Alcohol drinking intensity (g/day) | ||||
| Never | 27,744 | 27 | 43 | 23 |
| <5 | 39,748 | 39 | 47 | 25 |
| ≥5 and <10 | 9,912 | 10 | 11 | 6 |
| ≥10 and <20 | 9,713 | 10 | 23 | 13 |
| ≥20 and <30 | 7,464 | 7 | 13 | 7 |
| ≥30 | 7,119 | 7 | 49 | 26 |
| Pipe smoking | ||||
| Never | 86,543 | 85 | 146 | 78 |
| Former | 13,336 | 13 | 35 | 19 |
| Current | 937 | 1 | 5 | 3 |
| Missing | 884 | 1 | 0 | 0 |
| Cigar smoking | ||||
| Never | 88,217 | 87 | 147 | 79 |
| Former | 10,820 | 10 | 31 | 17 |
| Current | 1,678 | 2 | 7 | 4 |
| Missing | 985 | 1 | 1 | 0 |
| Non-alcohol total energy (kcal/day) | ||||
| Mean ± SD | 1670.91 ± 698.09 | 1767.20 ± 734.39 | ||
| Total vegetable intake (g/day) | ||||
| Mean ± SD | 284.03 ± 186.37 | 263.72 ± 162.46 | ||
| Total fruit intake (g/day) | ||||
| Mean ± SD | 273.91 ± 217.84 | 212.31 ± 281.97 | ||
| Marital status | ||||
| Married or living as married | 79,596 | 78 | 140 | 75 |
| Widowed | 8,201 | 8 | 13 | 7 |
| Divorced | 9,718 | 10 | 25 | 14 |
| Separated | 782 | 1 | 2 | 1 |
| Never married | 3,217 | 3 | 6 | 3 |
| Missing | 186 | 0 | 0 | 0 |
| Primary site | ||||
| Oral cavity | 81 | 44 | ||
| Oropharynx | 18 | 10 | ||
| Hypopharynx | 10 | 5 | ||
| NOS | 1 | 0 | ||
| Larynx | 76 | 41 | ||
Abbreviation: BMI, body mass index; SD, standard deviation; NOS, oral cavity or pharynx not otherwise specified.
Higher intake of total fiber, insoluble fiber and soluble fiber were associated with decreased HNC risks, with significant trends (Table 2). The multivariate HRs, adjusted by age, sex, BMI, education, race, pipe and cigar smoking, cigarette smoking status, pack-year cigarette smoking, alcohol drinking status, alcohol drinking intensity, non-alcohol total energy, total vegetable and fruit intake and marital status, of highest tertiles versus the lowest ones of intake were 0.43 (95%CI: 0.25–0.76) for total fiber, 0.38 (95%CI: 0.22–0.65) for insoluble fiber, and 0.44 (95%CI: 0.25–0.79) for soluble fiber. Although these trends were consistent across oral cavity and pharyngeal cases, the impact of fiber intake was weaker in laryngeal cases, especially for soluble fiber.
Table 2.
Fiber intake and the risk of head and neck cancer in the PLCO cohort.
| Head and Neck Cancer | Oral cavity and Pharynx | Larynx | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrients | Cohort | Cases | HR* | 95% CI | p -value | Cases | HR* | 95% CI | p -value | Cases | HR* | 95% CI | p -value |
| Total fiber (g/day) | |||||||||||||
| Q1 (<13.56) | 33,874 | 78 | 1.00 | - | - | 42 | 1.00 | - | - | 35 | 1.00 | - | - |
| Q2 (≥13.56 to <20.00) | 33,901 | 62 | 0.73 | 0.50–1.06 | 0.094 | 41 | 0.80 | 0.49–1.28 | 0.349 | 21 | 0.66 | 0.36–1.23 | 0.191 |
| Q3 (≥20.00) | 33,925 | 46 | 0.43 | 0.25–0.76 | 0.003 | 26 | 0.34 | 0.16–0.71 | 0.004 | 20 | 0.67 | 0.28–1.59 | 0.361 |
| ptrend | 0.004 | 0.007 | 0.294 | ||||||||||
| Insoluble fiber (g/day) | |||||||||||||
| Q1 (<8.85) | 33,853 | 86 | 1.00 | - | - | 48 | 1.00 | - | - | 37 | 1.00 | - | - |
| Q2 (≥8.85 to <13.18) | 33,929 | 54 | 0.57 | 0.39–0.83 | 0.003 | 34 | 0.57 | 0.36–0.93 | 0.023 | 20 | 0.59 | 0.32–1.08 | 0.089 |
| Q3 (≥13.18) | 33,918 | 46 | 0.38 | 0.22–0.65 | <0.001 | 27 | 0.31 | 0.15–0.62 | 0.001 | 19 | 0.57 | 0.24–1.32 | 0.189 |
| ptrend | <0.001 | 0.001 | 0.138 | ||||||||||
| Soluble fiber (g/day) | |||||||||||||
| Q1 (<4.53) | 33,785 | 75 | 1.00 | - | - | 42 | 1.00 | - | - | 32 | 1.00 | - | - |
| Q2 (≥4.53 to <6.67) | 33,996 | 63 | 0.75 | 0.51–1.09 | 0.132 | 41 | 0.75 | 0.47–1.22 | 0.247 | 22 | 0.78 | 0.42–1.46 | 0.443 |
| Q3 (≥6.67) | 33,919 | 48 | 0.44 | 0.25–0.79 | 0.006 | 26 | 0.29 | 0.14–0.62 | 0.001 | 22 | 0.87 | 0.36–2.13 | 0.761 |
| ptrend | 0.007 | 0.003 | 0.686 | ||||||||||
Abbreviations: HR, hazard ratio; CI, confidence interval.
Adjusted by age, sex, body mass index, education, race/ethnicity, pipe smoking status, cigar smoking status, cigarette smoking status, pack-year cigarette smoking, alcohol drinking status, alcohol drinking intensity, marital status, non-alcohol total energy, and total vegetable and fruit intake.
We also evaluated interactions of cigarette smoking and alcohol drinking with total fiber intake on HNC risk (Figure 1A and 1B). We did not observe any significant interaction of smoking and drinking with total fiber intake on HNC risk (pstrata for smoking= 0.151; pstrata for drinking= 0.451). Thus, under a multivariate model, the HR of high alcohol/low fiber intake versus low alcohol/high fiber intake was 2.77, and that for high tobacco/low fiber intake versus no tobacco/high fiber intake was 13.23. Increased HNC risks were observed for the lowest tertile of fiber intake among both never drinkers and never tobacco smokers.
Figure 1:
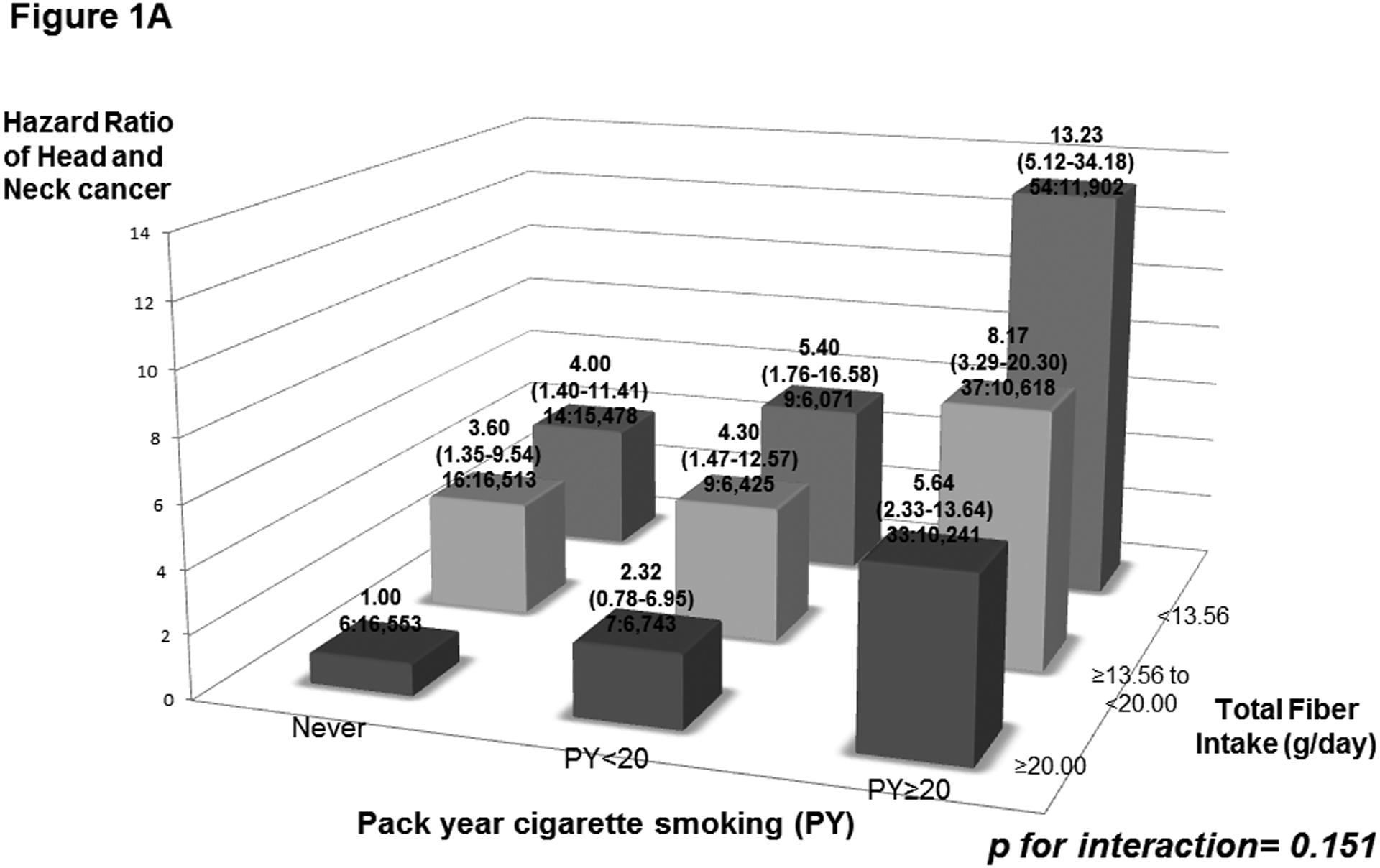
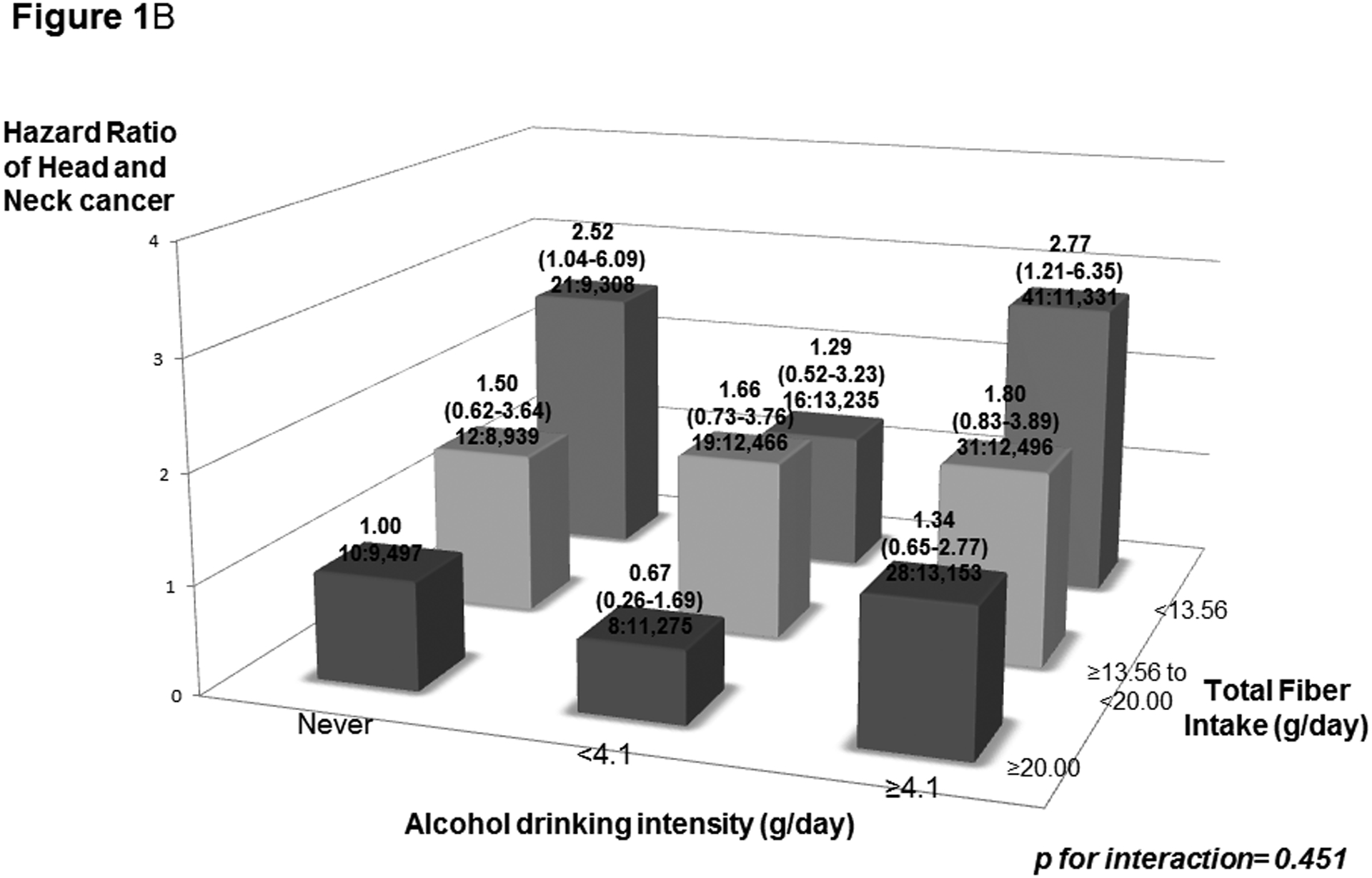
Hazard ratios (HRs) of head and neck cancer, and corresponding confidence intervals (95% CIs), according to pack year cigarette smoking (PY) (Figure 1A) or alcohol drinking intensity (g/day) (Figure 1B) and total fiber intake (g/day). The HRs were derived from Cox proportional hazard models adjusted for age, sex, body mass index, education, race/ethnicity, pipe smoking status, cigar smoking status, cigarette smoking status, pack-year cigarette smoking, alcohol drinking status, alcohol drinking intensity, non-alcohol total energy, total vegetable and fruit intake, and marital status. The number of cases and controls within each category was indicated below the corresponding HR as: “number of cases : number of controls.” We found no significant interaction of smoking and drinking with total fiber intake on HNC risk.
Additionally, we performed stratified analyses to evaluate the impact of total fiber intake on HNC risk across selected covariates (Figure 2). There was no interaction between total fiber intake and HNC risk when stratified by age, sex, BMI and education.
Figure 2:
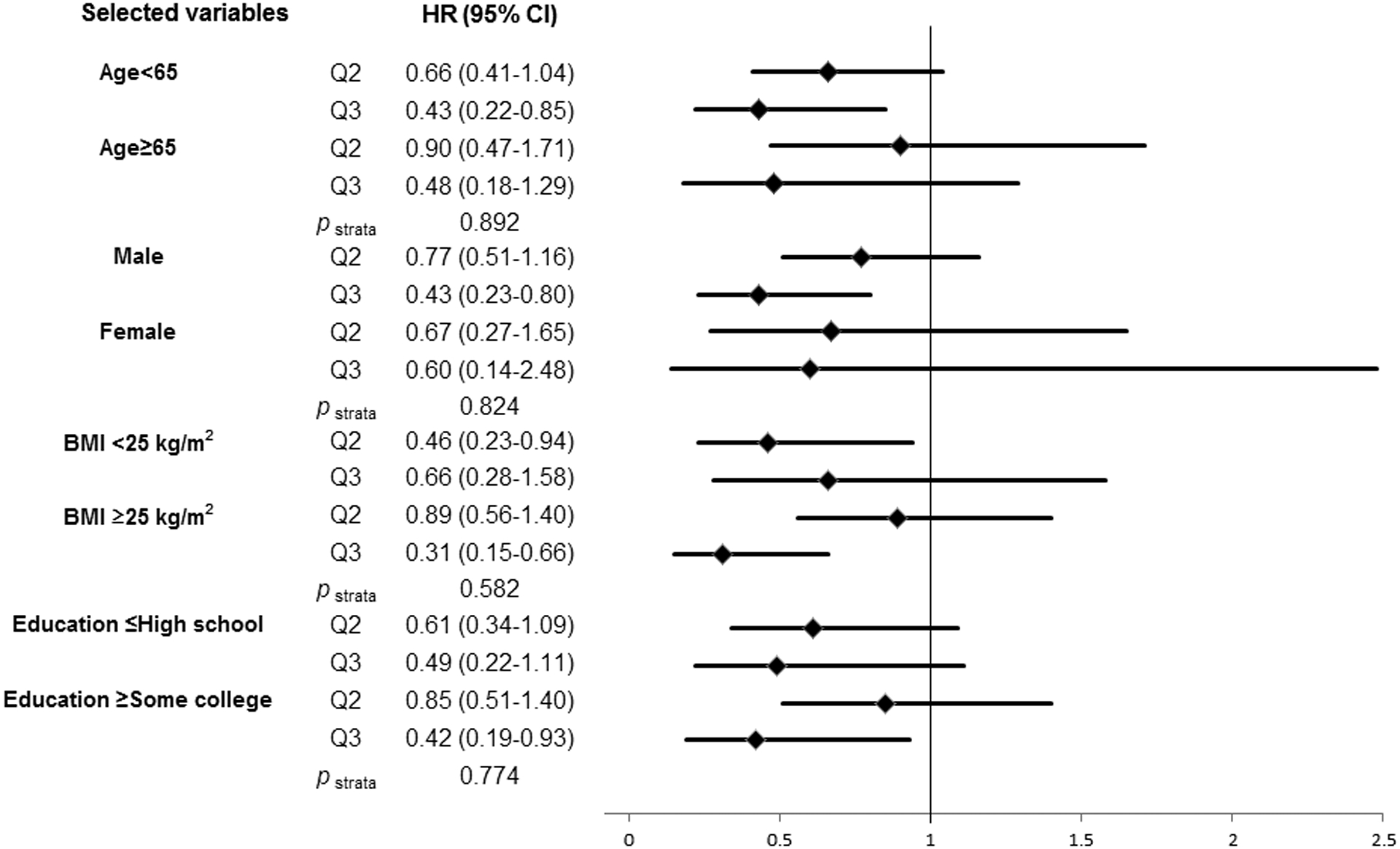
Impact of higher tertiles of total fiber intake (Q2, ≥13.56 to <20.00 g/day; Q3, ≥20.00 g/day) on head and neck cancer risk compaired with the lowest tertile of total fiber intake (Q1, <13.56 g/day) according to selected covariates. The hazard ratios (HRs) were derived from Cox proportional hazard models adjusted for age, sex, body mass index, education, race/ethnicity, pipe smoking status, cigar smoking status, cigarette smoking status, pack-year cigarette smoking, alcohol drinking status, alcohol drinking intensity, non-alcohol total energy, total vegetable and fruit intake, and marital status. We found no significant interaction of selected covariates with total fiber intake on HNC risk.
Discussion
In this prospective cohort study, we observed a protective role of fiber intake on HNC risk after adjustment for potential confounders. In this study, we were able to adjust for the intake of total fruits and vegetables, as well as socio-economic indicators. This association was consistent across subsites of HNC. The favorable role of fiber intake suggested a multiplicative interaction with smoking and drinking although the p-value was not statistically significant, and was not heterogeneous across strata of recognized confounders. The combination of high tobacco and low fibers led to an over 10-fold excess risk.
Several mechanisms have been proposed for the mechanism of fiber intake on HNC risk. Dietary fiber may bind carcinogens and thereby limit their contact with epithelia of the oral cavity, pharynx and larynx25, 26. Dietary fiber may reduce glycaemic load27, and improve insulin sensitivity, favourably influencing insulin-like growth factor I, which may promote carcinogenesis28. In addition, fiber-rich foods tend to have a high content of antioxidants26. However, a high fiber intake may simply be an indicator of a diet rich in fruit, vegetables, whole grains, and a better general lifestyle pattern29. In this study, however, we were able to adjust for the intake of total fruits and vegetables, as well as for socio-economic indicators.
To date, two prospective cohorts17, 19 and nine case-control studies9–16, 18 have considered the association between fiber intake and the risk of HNC or its subsites. The National Institutes of Health (NIH)-AARP Diet and Health Study, was the largest prospective cohort study, including 1,867 HNC cases during a 12-year follow-up, and reported on an inverse association of total fiber intake with HNC risk among women with a significant trend, and a weaker impact of total fiber intake among men (HR10g/day= 0.77, 95%CI: 0.64–0.93 for women; 0.93, 95%CI: 0.86–1.00 for men, p-interactions<0.001)19. In our study, we did not observe any meaningful differences by sex for total fiber intake and HNC risk, but this may be due to small sample sizes in the stratified analysis. The other prospective cohort study, the Iowa Women’s Health Study (IWHS), included 53 oral cavity and pharyngeal cancer cases and 21 laryngeal cancer cases from a cohort of 34,651 postmenopausal women with a 14-year follow-up17. The IWHS found a significant inverse association of total fiber intake with the risk of oral cavity and pharyngeal cancer (HR for highest vs lowest= 0.49), but no association with the risk of laryngeal cancer (HR for highest vs lowest= 1.82). We also observed that the impact of total fiber intake was weaker among laryngeal cancer cases, possible due to the fact that only the upper part of the larynx is in direct contact with foods.
Regarding case-control studies, a study in China reported on a significant inverse association of fiber from fruits and vegetables with the risk of oral cavity cancer, but not with fiber from other sources14. These favorable associations with fruit and vegetable fiber were also reported in Italian studies which also distinguished soluble and insoluble fibers16, 18. No association between fiber intake and the risk of HNC was observed in a case-control study conducted in Uruguay; however that study included only 33 oral cavity and pharyngeal cancer cases and 34 laryngeal cancer cases15.
Our study has several strengths. First, with its prospective study design, the questionnaire data were collected before cancer diagnosis. Thus, we can exclude a relevant role of recall bias. Second, we carefully adjusted for known confounders associated with fiber intake and HNC risk, including sex, BMI, education, tobacco smoking, alcohol drinking, the intake of total fruit and vegetable, and energy intake. Regarding residual confounding from cigarette smoking status, alcohol drinking status and marital status, the protective role of dietary fiber was consistent after adjustment for these factors. In addition, we did not have information on hypertension, hyperlipidemia, cirrhosis, type 2 diabetes mellitus, proton pump inhibitors, statins and metformin. Some of these factors (i.e., diabetes) have been moderately associated to the risk of HNC30, but are unlikely to have a material confounding effect on the association between fiber intake and HNC.
Statistical power was limited for subsite analysis. However, our cohort size is the second largest prospective cohort study. We did not have information about HPV infection. Since we would not expect HPV infection to be associated specifically with fiber intake, HPV infection status seems unlikely to meet the properties of a confounder. Furthermore, we divided HNC cases into two group of subsites, i.e. oral cavity and pharyngeal cases, and laryngeal cases. Although we considered the impact of fiber intake on oropharyngeal cases all (N=18) only, we found a similar decreased trend on oropharyngeal cases.
In summary, our findings support evidence of the protective role of dietary fiber on HNC risk. Future studies that elucidate which foods are the main source of dietary fiber which decrease the risk of HNC are warranted.
Acknowledgements
We thank the NIH PLCO study group for allowing us to use the data. DK, CLV, MH designed research and wrote the paper; DK performed statistical analyses; All authors read and approved the final manuscript. The authors declare no potential conflicts of interest. This work was supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS under Award Number N01-CN-25524 and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001067. In addition, this work was supported by JSPS Grant-in-Aid for Young Scientists (B) to D.Kawakita (No.15K21283 and No. 17K18006), and by Italian Foundation for Cancer Research (FIRC) to C La Vecchia.
Abbreviations
- HNC
head and neck cancer
- HPV
human papilloma virus
- PLCO
Prostate, Lung, Colorectal, and Ovarian
- ICD-O-2
International Classification of Disease for Oncology, second edition
- NDS-R
Nutrition Data System for Research
- NOS
not otherwise specified
- HRs
hazard ratios
- CIs
confidence intervals
Reference
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer 2010;127: 2893–917. [DOI] [PubMed] [Google Scholar]
- 2.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wunsch-Filho V, Franceschi S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Journal of the National Cancer Institute 2007;99: 777–89. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Seminars in oncology 2004;31: 744–54. [DOI] [PubMed] [Google Scholar]
- 4.Research. WCRFAIfC, Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective, 2007.
- 5.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. The American journal of clinical nutrition 2003;78: 559S–69S. [DOI] [PubMed] [Google Scholar]
- 6.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. The American journal of clinical nutrition 2006;83: 1126–34. [DOI] [PubMed] [Google Scholar]
- 7.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. International journal of cancer 2008;122: 2330–6. [DOI] [PubMed] [Google Scholar]
- 8.Lucenteforte E, Garavello W, Bosetti C, La Vecchia C. Dietary factors and oral and pharyngeal cancer risk. Oral oncology 2009;45: 461–7. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin JK, Gridley G, Block G, Winn DM, Preston-Martin S, Schoenberg JB, Greenberg RS, Stemhagen A, Austin DF, Ershow AG, et al. Dietary factors in oral and pharyngeal cancer. Journal of the National Cancer Institute 1988;80: 1237–43. [DOI] [PubMed] [Google Scholar]
- 10.Gridley G, McLaughlin JK, Block G, Blot WJ, Winn DM, Greenberg RS, Schoenberg JB, Preston-Martin S, Austin DF, Fraumeni JF Jr. Diet and oral and pharyngeal cancer among blacks. Nutrition and cancer 1990;14: 219–25. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JR, Graham S, Haughey BP, Shedd D, O’Shea R, Brasure J, Wilkinson GS, West D. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. European journal of cancer Part B, Oral oncology 1992;28B: 9–15. [DOI] [PubMed] [Google Scholar]
- 12.Freudenheim JL, Graham S, Byers TE, Marshall JR, Haughey BP, Swanson MK, Wilkinson G. Diet, smoking, and alcohol in cancer of the larynx: a case-control study. Nutrition and cancer 1992;17: 33–45. [DOI] [PubMed] [Google Scholar]
- 13.Kune GA, Kune S, Field B, Watson LF, Cleland H, Merenstein D, Vitetta L. Oral and pharyngeal cancer, diet, smoking, alcohol, and serum vitamin A and beta-carotene levels: a case-control study in men. Nutrition and cancer 1993;20: 61–70. [DOI] [PubMed] [Google Scholar]
- 14.Zheng T, Boyle P, Willett WC, Hu H, Dan J, Evstifeeva TV, Niu S, MacMahon B. A case-control study of oral cancer in Beijing, People’s Republic of China. Associations with nutrient intakes, foods and food groups. European journal of cancer Part B, Oral oncology 1993;29B: 45–55. [DOI] [PubMed] [Google Scholar]
- 15.De Stefani E, Ronco A, Mendilaharsu M, Deneo-Pellegrini H. Diet and risk of cancer of the upper aerodigestive tract--II. Nutrients. Oral oncology 1999;35: 22–6. [DOI] [PubMed] [Google Scholar]
- 16.Soler M, Bosetti C, Franceschi S, Negri E, Zambon P, Talamini R, Conti E, La Vecchia C. Fiber intake and the risk of oral, pharyngeal and esophageal cancer. International journal of cancer 2001;91: 283–7. [DOI] [PubMed] [Google Scholar]
- 17.Kasum CM, Jacobs DR Jr., Nicodemus K, Folsom AR. Dietary risk factors for upper aerodigestive tract cancers. International journal of cancer 2002;99: 267–72. [DOI] [PubMed] [Google Scholar]
- 18.Pelucchi C, Talamini R, Levi F, Bosetti C, La Vecchia C, Negri E, Parpinel M, Franceschi S. Fibre intake and laryngeal cancer risk. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2003;14: 162–7. [DOI] [PubMed] [Google Scholar]
- 19.Lam TK, Cross AJ, Freedman N, Park Y, Hollenbeck AR, Schatzkin A, Abnet C. Dietary fiber and grain consumption in relation to head and neck cancer in the NIH-AARP Diet and Health Study. Cancer causes & control : CCC 2011;22: 1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MA, Hayes RB, Johnson CC, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled clinical trials 2000;21: 273S–309S. [DOI] [PubMed] [Google Scholar]
- 21.Diet history questionnaire. Risk factor monitoring and methods. National Cancer Institute. Version current 30 Nov 2016. Internet: http://www.riskfactor.cancer.gov/DHQ (accessed 30 Nov 2016). [Google Scholar]
- 22.NDSR descriptive overview. University of Minnesota Nutrition Co-ordinating Center. Version current 30 Nov 2016. Internet: http://www.ncc.umn.edu/ (accessed 30 Nov 2016). [Google Scholar]
- 23.Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, Bresalier R, Weissfeld JL, Flood A, Schatzkin A, Hayes RB, Prostate LC, et al. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet 2003;361: 1491–5. [DOI] [PubMed] [Google Scholar]
- 24.Kunzmann AT, Coleman HG, Huang WY, Kitahara CM, Cantwell MM, Berndt SI. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. The American journal of clinical nutrition 2015;102: 881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism: clinical and experimental 2012;61: 1058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slavin J, Jacobs D, Marquart L. Whole-grain consumption and chronic disease: protective mechanisms. Nutrition and cancer 1997;27: 14–21. [DOI] [PubMed] [Google Scholar]
- 27.Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, Bjorck I, Brand-Miller JC, Brighenti F, Buyken AE, Ceriello A, La Vecchia C, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutrition, metabolism, and cardiovascular diseases : NMCD 2015;25: 795–815. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. Journal of the National Cancer Institute 2000;92: 1472–89. [DOI] [PubMed] [Google Scholar]
- 29.Bosetti C, Pelucchi C, La Vecchia C. Diet and cancer in Mediterranean countries: carbohydrates and fats. Public health nutrition 2009;12: 1595–600. [DOI] [PubMed] [Google Scholar]
- 30.Stott-Miller M, Chen C, Chuang SC, Lee YC, Boccia S, Brenner H, Cadoni G, Dal Maso L, La Vecchia C, Lazarus P, Levi F, Matsuo K, et al. History of diabetes and risk of head and neck cancer: a pooled analysis from the international head and neck cancer epidemiology consortium. Cancer epidemiology, biomarkers & prevention 2012;21: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]


