Abstract
Background:
Treatment of patients with Parkinson disease (PD) using autologous mesenchymal stem cells (MSCs) is a promising method to influence the pathogenesis of the disease. The aim of this study was to assess the immediate results of the introduction of MSCs on the effectiveness of motor and nonmotor symptoms in patients with PD.
Methods:
MSCs were transplanted to 12 patients with PD through intravenous and tandem (intranasal + intravenous) injections. Effectiveness of the therapy was evaluated 1 and 3 months posttransplantation. Neurological examination of the intensity of motor symptoms was carried out in the morning after a 12 or 24 h break in taking antiparkinsonian drugs, then 1 h after they were taken. The intensity of motor symptoms was assessed with the help of Section III of the Unified PD Rating Scale of the International Society for Movement Disorders (UPDRS). The intensity of nonmotor symptoms was assessed with the help of the following scales: Hamilton Depression Rating Scale, the Pittsburgh Sleep Quality Index, the Epworth Sleepiness Scale, Nonmotor Symptoms Scale, and the 39-item Parkinson’s Disease Questionnaire.
Results:
We found a statistically significant decrease in the severity of motor and nonmotor symptoms in the study group in the posttransplant period.
Conclusion:
Positive results allow us to consider MSCs transplantation as a disease-modifying therapeutic strategy in PD. However, this method of PD treatment is not a fully understood process, which requires additional studies and a longer follow-up period to monitor the patients’ condition posttransplantation.
Keywords: Mesenchymal stem cells, Motor symptoms, Nonmotor symptoms

INTRODUCTION
Parkinson disease (PD) is a chronic, steadily progressive neurodegenerative disease. It leads to severe motor impairment, social disadaptation, and a decrease in the quality of patient’s life.
One of the most promising ways of treatment PD is based on the cellular technologies.[32,38] Cell therapy for PD was performed for the 1st time in 1979.[23] Due to the progress in biotechnology, cell therapy is rapidly becoming a potential treatment option, changing the course of PD in patients.
Numerous experimental and clinical studies into PD therapy reference the application of cells of various origins: dopamine-secreting cells, fetal mesencephalon cells, embryonic stem cells, induced pluripotent stem cells, bone marrow stem cells (hematopoietic and mesenchymal cells), stem cells from other sources, and genetically modified cells.[28] Among them, the use of mesenchymal stem cells (MSCs) is one of the perspective directions of cellular therapy for many neurological disorders for the following reasons:
MSCs are easily obtained from various tissues[16]
MSCs are able to independently migrate to the damaged area when introduced into the human body by different routes of administration[12]
MSCs secrete various biological factors necessary for neuroprotection[36]
MSCs can differentiate into neuronal phenotypes under appropriate conditions[2]
Use of MSCs is not accompanied by ethical problems.
Recently, interest in cell therapy using MSCs in scientific and clinical research has increased exponentially.[33,37] Clinical trials have demonstrated the possibility of safe transplantation of autologous MSCs in patients with cerebral stroke,[19,27] multiple sclerosis,[7] cerebral palsy,[14] and amyotrophic lateral sclerosis.[21] In PD, the possibility of introducing MSCs by intracerebral, intra-arterial, intrathecal, and intravenous route has been described.[4,34,35] The efficacy of cell therapy using MSCs transplantation was evaluated on animal models with parkinsonism.[3,25] These studies demonstrated a positive therapeutic effect of both intact and differentiated MSCs on the symptoms of laboratory animal models with parkinsonism, such as the reduction of the motor symptoms, normalization the level of dopamine and other neurotransmitters, an increase in the number of neurons in the damaged area, and a delay in the disease progression.[6,11] These encouraging results present a convincing argument to perform similar studies in PD patients.
Two methods of cell implantations that improve the neuroregenerative potential of MSCs with a minimal risk of surgical complications were considered for this study. Local or intranasal transplantation is affordable and minimally invasive.[31] Systemic or intravenous method is advantageous, as it allows to easily perform repeated administrations and stimulate systemic immunomodulatory effects, relevant in conditions of neuroinflammation in PD.[9]
MATERIALS AND METHODS
The timeline of the study
By the end of 2018, we have successfully completed the preclinical stage of our research.[1] In November 2018, the clinical phase of our study was initiated. On January 17, 2019, the first autologous MSCs transplantation to a patient with PD was performed in the neurosurgical department of our clinical hospital. At present, the number of patients in the posttransplant period has increased to 12. Our objective was to make an assessment of the immediate results of the MSCs introduction effectiveness on motor and nonmotor symptoms in patients with PD.
The clinical examination of PD patients
Clinical examination at the selection stage included the clarification of complaints, the history of the disease, neurological examination, and neuroimaging (CT and MRI 1.5 T). In addition, the age of onset of PD, its duration and type of flow, family history of PD, the presence of nonmotor manifestations, chronic inflammatory diseases, significant stressful situations, and work with herbicides in history were specified. Furthermore, the duration of antiparkinsonian drugs usage, the regimen of their administration, the side effects, and another therapy were clarified. The diagnosis of PD was established in all participants of the current study in accordance with the United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria.[15] The clinical stage of the disease was indicated in the diagnosis in accordance with Hoehn and Yahr scale in the modification of Lindvall et al. (1998). The selection criteria and contraindications listed below are based on the opinions of foreign experts and our own experience.
The selection criteria:
A reliable diagnosis of Parkinson’s disease, according to, in accordance with, the United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria (UK Brain Bank Criteria, 1992)
Stage of the disease according to Hoehn and Yahr scale: 1.5–3.0 scores
Rapidly progressive type of the flow with changing the stages in no more than 4 years
A good response to levodopa treatment: positive dopamine test (difference between motor functions in the on- and off-period not less than 30% by a total score of three parts of the UPDRS scale)
The duration of the disease is not more than 8 years with the absence of motor fluctuations and dyskinesia
The age of patients is up to 65 years.
The contraindications:
Parkinsonism and parkinsonism-plus syndromes
Severe concomitant diseases (congestive heart failure, myocardial infarction; pneumonia, decompensated diabetes mellitus, cachexia, etc.)
Autoimmune diseases, bleeding, a history of sepsis
Oncological diseases
An acute stage or exacerbation of the chronic inflammatory process of the sinuses and oral cavity
A positive result for HIV, hepatitis B (HBV), hepatitis C (HCV), syphilis (RW)
Cognitive deficit (Montreal Cognitive Assessment (MOCA) <26)
Mental disorders – hallucinations, behavior disorders
Depression of a pronounced degree (not more than 19 points on the Hamilton scale)
Alcoholism, drug addiction, criminal liability in the patient’s history
Pregnancy, lactation.
Collection of the cellular material from patients with PD, who were subject to MSCs transplantation, was performed from the posterior upper crest of an iliac bone according to the standard method.
Preclinical work
Mononuclear cells from bone marrow punctate were isolated by the standard method of separation on the ficoll-verographin gradient (P = 1.077). The suspension was centrifuged for 10 min at 1500 rpm/min at room temperature. The pellet was resuspended in 10–15 ml of medium for culturing MSCs. The cell suspension was adjusted to the inoculum concentration with culture medium for MSCs and plated in culture vials. They were placed in a CO2 incubator (37°C, 5% CO2, 90% humidity). After 24–48 h of incubation, cells that had not been adhered to the surface of the vial were washed 3 times with a stream of sterile PBS, after which the vials were filled with specialized medium for culturing MSCs. Every 3–4 days, ½ of the volume of the cultivation medium was changed. At 70–80% confluency stage, the cells were removed from the surface of the culture vial using a trypsin/EDTA solution. Then, they were seeded into plastic sterile vials for adhesive cultures with gas permeable caps to obtain the first passage. When the first passage reached 75–90% confluency, the cells were reseeded to obtain subsequent passages until the amount of MSCs necessary for therapy was achieved.
Clinical study
The transplantation of autologous MSCs in patients with PD was performed using two methods, based on the literature data and our own experience with experimental animals in preparation for this study.[24,31,39]
Systemic (intravenous) administration method: the total dose of cells (0.5–2.0 million/kg of patient’s weight) is administered slowly intravenously in three stages with an interval of 7 days
Method of tandem (intranasal + intravenous) administration: a suspension of autologous MSCs is administered at a dose of 5.0–12.6 million cells in a volume of 2–5 ml of the prepared solution into the submucous layer of the olfactory epithelium zone from both sides. Seven days after intranasal administration, 10–50 million cells are injected slowly intravenously in two stages with an interval of a week.
The choice between systemic or tandem cell administrations was determined on the individual basis, considering the expected benefits and potential risks.
Effectiveness of the therapy was evaluated before the transplantation (day 0) and after the introduction of MSCs (month 1 and month 3) according to the effect on the motor and nonmotor symptoms. Nonmotor symptoms were assessed with help of the following scales: Hamilton Depression Rating Scale (HDRS), Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), Nonmotor Symptoms Scale (NMS), and the 39-item Parkinson’s Disease Questionnaire (PDQ-39).
The severity of motor symptoms of PD was evaluated on the basis of Section III of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) of the International Society for Movement Disorders (2008). The assessment of motor functions was performed in the morning after a 12–24 h break in taking antiparkinsonian drugs (off-period), then 1 h after they were taken (on-period).
Statistical data processing was performed using the Statistica 8.0 package. The data obtained are presented as the median with an interquartile interval (25–75th percentile). Comparison of the results of the two groups and determination of the statistical significance of the differences were carried out using nonparametric Wilcoxon criteria and the Mann–Whitney U-test. Differences were recognized as statistically significant at P <0.05.
RESULTS
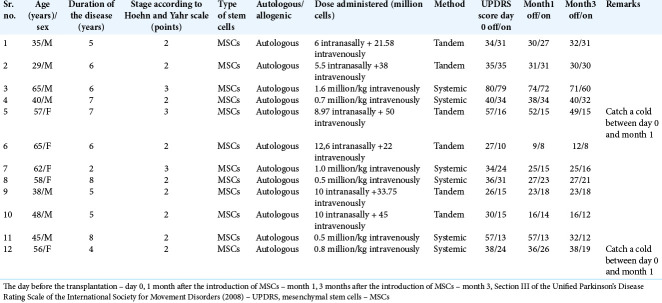
The study group consisted of 12 patients (m:f – 7:5) with a diagnosis of PD, randomized by sex and age, who underwent cell therapy of MSCs in various combinations: systemic or tandem administration. The average age of the patients was 52.0 (39.5; 59.0) years, the duration of the disease was 7.0 (5.0; 8.0) years, the severity of the disease according to the Hoehn and Yahr scale was 2.0 (2.0; 3.0) points. The details of these patients are mentioned in Table 1.
Table 1:
The details of the patients from the study group.
The comparison group included 11 patients (m:f – 6:5) with a diagnosis of PD, randomized by sex and age, received drug treatment with levodopa drugs, dopamine receptor agonists, and amantadine. The control subjects did not receive any injection, including placebo. The average age of the patients was 52.0 (47.5; 62.5) years, the duration of the disease was 6.0 (4.0; 7.0) years, and the severity of the disease on the Hoehn and Yahr scale was 2.0 (2.0; 2.0) points.
There were no statistically significant differences in gender, age, and stage of the disease distribution between the two groups (P > 0.05 according to Mann–Whitney U-test).
We revealed statistically significant differences (P = 0.02) between the MDSUPDRS score (Section III) before treatment (Me = 36.5 [30.0; 57.5]) and the score in month 1 (Me = 33.5 [26.0; 52.5]) in the off-period in the study group. The difference amounted to 9%. The achieved result was maintained for 3 months after transplantation (Me = 32.0 [23.0; 40.0]). In the on-period, statistically significant differences between the score before therapy (Me = 20.0 [15.5; 34.0]) and month 1 (Me = 22.0 [14.5; 31.0]), (P = 0.18), and in month 1 and month 3 (Me =19.0 [15.0; 31.0], [P = 0.2]) were not detected.
In the comparison group, statistically significant differences between the score in day 0 (Me = 20.0 [15.5; 34.0]) and in month 1 (Me = 22.0 [14.5; 31.0]), (P = 0.18), and in month 1 and month 3 (Me = 19.0 [15.0; 31.0], [P = 0.2]) were not detected both in the off- and on-periods.
An improvement of mood, significant decrease in daytime sleepiness, and the patients’ sleep quality were identified in the study group. By month 1, 36% decrease in score according to the Hamilton depression scale was observed compared to day 0 (P = 0.02). The improvement of mood and a decreased depressive mood were also detected after 3 months (P = 0.01) and amounted to 44%. By the month 3, sleep quality assessed by the PSQI scale, improved by 46% compared to the initial data, and daytime sleepiness, assessed by the Epworth scale, decreased by 42% [Table 2].
Table 2:
The intensity of nonmotor symptoms in the study group before the transplantation, 1 month, and 3 months after the introduction of MSCs (Me, Q25-Q75).
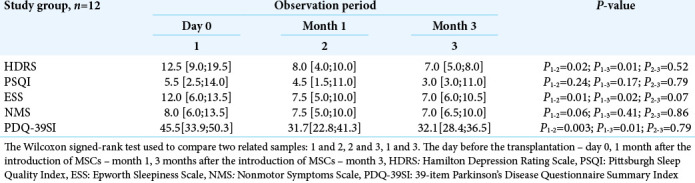
There was also a statistically significant increase (P = 0.003) in the overall quality of life by the PDQ-39 scale. The achieved result was maintained for 3 months after transplantation (P = 0.01).
There was no statistically significant difference when assessing the same symptoms in the dynamics in the comparison group (P ≥ 0.05) [Table 3].
Table 3:
The intensity of nonmotor symptoms in the comparison group in the baseline day, 1 month, and 3 months later (Me, Q25-Q75).
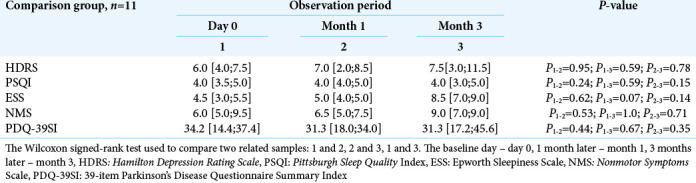
Effectiveness of the therapy was also assessed by the NMS scale. A tendency toward a decreased total number of nonmotor manifestations of PD between day 0 (Me = 8.0 [6.0; 13.5]) and month 3 (Me = 7.0 [6.0; 10.5]) [Table 1] has also been revealed. In contrast, the patients from the comparison group during the same observation period showed a tendency to the symptoms’ increase [Table 3].
DISCUSSION
At present, it is established that MSCs, similar to leukocytes, express a variety of receptors and cell adhesion molecules involved in homing and migration to the lesion sites both with local and systemic routes of administration.[8,13,22,29]
Experimental models of rodents have shown that if introduced through the intranasal route, MSCs are able to migrate to the brain as early as the 1st day after the administration and are present at the lesion site for 3 weeks or more.[26,31,39] In these studies, the implanted stem cells have shown a pronounced neurotrophic effect by producing growth factors, such as GDNF, BDNF, NGF, IGF-1, and VEGF. In addition, there is ample evidence that MSCs support the structural organization of both individual brain cells and the neuronal network as a whole, and contribute to the survival of neurons and oligodendrocytes in neurodestructive conditions.[18] Moreover, by exerting an immunoregulatory effect on both local and systemic levels,[5] MSCs decrease the inflammatory response from microglial cells, and, thus, restore the functional activity of neurons.[17,20]
At present, only preliminary data are available on the use of MSCs in human PD.[30] In an open-label study in 2010, Indian researchers administered 106 autologous bone marrow-derived MSCs per kilogram body weight unilaterally into the sublateral ventricular zone through stereotactic surgery in seven patients with PD.[34] The procedure was well tolerated. Three out of seven patients were reported to have lasting improvement in the unified Parkinson’s disease rating scale and other rating scales compared to baseline. In agreement with our findings, this study also demonstrates that intranasal and tandem (intranasal + intravenous) methods of autologous MSCs administration are safe and have beneficial neuroprotective and neurorestorative effects.
At this stage of the study, given a short period of the postoperative follow-up, we cannot fully exclude the influence of the possible placebo effect, which most often occurs in the early stages of open research.[10] Patient evaluation using standardized scales and a long period of posttransplant observation will help to answer this question definitively.
Undoubtedly, the data obtained are only preliminary, and there is a need for more detailed and lengthy study. In future, an additional set of evaluation studies is planned, along with an increased number of the transplanted MSCs.
Due to the fact that the lifetime of MSCs in the body is limited to several weeks, it is important to study the long-term effects of their transplantation in PD, the effectiveness of repeated MSCs reintroduction, and the time intervals over which they must be carried out.
CONCLUSION
The treatment of patients with PD with the use of MSCs has produced encouraging results that suggest influence on many symptoms of the PD pathogenesis, as well as their capability to modify the course of the disease and provide control over the manifestations of the motor symptoms of the disease. Our data demonstrate a decrease in the severity of motor and nonmotor symptoms of the disease in the posttransplant period. These encouraging results allow us to consider the application of MSCs in PD as a therapy modifying the course of the disease. However, this method of PD treatment is not a fully understood process, which requires additional studies and a longer follow-up period in the posttransplant period.
Footnotes
How to cite this article: Boika A, Aleinikava N, Chyzhyk V, Zafranskaya M, Nizheharodava D, Ponomarev V. Mesenchymal stem cells in Parkinson’s disease: Motor and nonmotor symptoms in the early posttransplant period. Surg Neurol Int 2020;11:380.
Contributor Information
Aliaksandr Boika, Email: aboika@tut.by.
Natallia Aleinikava, Email: aleina1974@mail.ru.
Veranika Chyzhyk, Email: chyzhykva@gmail.com.
Marina Zafranskaya, Email: -zafranskaya@gmail.com.
Darya Nizheharodava, Email: nzh@tut.by.
Vladimir Ponomarev, Email: professor.ponomarev@gmail.com.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Aleinikava NE, Boika AV, Nizheharodava DB, Ponomarev VV, Vanslau MI, Ustsiamchuk AM, et al. The obtaining of toxic chronic model of Parkinsonism syndrome in rats. Vestnik VSMU. 2018;17:92–9. [Google Scholar]
- 2.Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008;17:547–54. doi: 10.1089/scd.2007.0172. [DOI] [PubMed] [Google Scholar]
- 3.Blandini F, Cova L, Armentero MT, Zennaro E, Levandis G, Bossolasco P, et al. Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transplant. 2010;19:203–17. doi: 10.3727/096368909X479839. [DOI] [PubMed] [Google Scholar]
- 4.Brazzini A, Cantella R, De la Cruz A, Yupanqui J, León C, Jorquiera T, et al. Intraarterial autologous implantation of adult stem cells for patients with Parkinson disease. J Vasc Interv Radiol. 2010;21:443–51. doi: 10.1016/j.jvir.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen D, Fu W, Zhuang W, Lv C, Li F, Wang XJ. Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson’s disease. J Neurosci Res. 2017;95:907–17. doi: 10.1002/jnr.23879. [DOI] [PubMed] [Google Scholar]
- 7.Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–6. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao R, Zhang G, Chen X, Yang A, Smith G, Wong DF, et al. CSF biomarkers and its associations with 18F-AV133 cerebral VMAT2 binding in Parkinson’s disease-a preliminary report. PLoS One. 2016;11:e0164762. [Google Scholar]
- 10.Goetz CG, Wuu J, McDermott MP, Adler CH, Fahn S, Freed CR, et al. Placebo response in Parkinson’s disease: Comparisons among 11 trials covering medical and surgical interventions. Mov Disord. 2008;23:690–9. doi: 10.1002/mds.21894. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Wakao S, Kitada M, Ose T, Watabe H, Kuroda Y, et al. Autologous mesenchymal stem cell-derived dopaminergic neurons function in Parkinsonian macaques. J Clin Invest. 2013;123:272–84. doi: 10.1172/JCI62516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellmann MA, Panet H, Barhum Y, Melamed E, Offen D. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett. 2006;395:124–8. doi: 10.1016/j.neulet.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 13.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–41. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Zhang C, Gu J, Wu W, Shen Z, Zhou X, et al. A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transplant. 2018;27:325–34. doi: 10.1177/0963689717729379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–60. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 17.Jose S, Tan SW, Ooi YY, Ramasamy R, Vidyadaran S. Mesenchymal stem cells exert anti-proliferative effect on lipopolysaccharide-stimulated BV2 microglia by reducing tumour necrosis factor-α levels. J Neuroinflammation. 2014;11:149. doi: 10.1186/s12974-014-0149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koniusz S, Andrzejewska A, Muraca M, Srivastava AK, Janowski M, Lukomska B, et al. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci. 2016;10:109. doi: 10.3389/fncel.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Hong JM, Moon GJ, Lee P, Ahn YH, Bang OY, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhang R, Yan K, Chen F, Huang W, Lv B, et al. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation. 2014;11:135. doi: 10.1186/1742-2094-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: A long-term safety study. Cytotherapy. 2012;14:56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- 22.Nitzsche F, Müller C, Lukomska B. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35:1446–60. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]
- 23.Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Seiger A, Olson L, et al. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–7. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- 24.Ponomarev VV, Boyko AV, Zafranskaya MM. D, Aleynikova NE, Chyzhyk VA, et al. Minsk: Belarusian State Medical University; 2019. Assessment of Cell Therapy Effectiveness in Patients with Parkinson’s Disease using Different Cell Transplantation Routs; pp. 132–6. [Google Scholar]
- 25.Salama M, Sobh M, Emam M, Abdalla A, Sabry D, ElGamal M, et al. Effect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson’s disease. Exp Ther Med. 2017;13:976–82. doi: 10.3892/etm.2017.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanko Y, Navitskaya V, Zamaro A, Zafranskaya M, Krivenko S, Koulchitsky S, et al. Somatotopic principle of perineural implantation of stem cells in patients with brain injuries. J Neurol Stroke. 2018;8:259–61. [Google Scholar]
- 27.Shanko YG, Kulchitskiy VA, Novitskaya VV, Tokalchik YP, Zafranskaya MM, Krivenko SI, et al. Stem cells in the treatment of cerebral infarction: An analytical review of the literature. Med Novosti. 2019;1:9–11. [Google Scholar]
- 28.Shen Y, Huang J, Liu L, Xu X, Han C, Zhang G, et al. A compendium of preparation and application of stem cells in Parkinson’s disease: Current status and future prospects. Front Aging Neurosci. 2016;8:117. doi: 10.3389/fnagi.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staff NP, Jones DT, Singer W. Mesenchymal stromal cell therapies for neurodegenerative diseases. Mayo Clin Proc. 2019;94:892–905. doi: 10.1016/j.mayocp.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stukach YP, Shanko YG, Parkhach LP, Pashkevich SG, Kulchitckiy VA. In: Medical Electronics and New Medical Technologies: A Collection of Scientific Articles 9th International Scientific and Technical Conference. Minsk: Belorusskiy Gosudarstvennyy Universitet Informatiki I Radioelektroniki; 2016. The technology of delivery mesenchymal stem cells to different parts of the brain in the anterior or posterior cranial fossa; pp. 121–4. [Google Scholar]
- 32.Tozzi A, De Iure A, Bagetta V, Tantucci M, Durante V, QuirogaVarela A, et al. Alpha-synuclein produces early behavioral alterations via striatal cholinergic synaptic dysfunction by interacting with GluN2D N-Methyl-D-aspartate receptor subunit. Biol Psychiatry. 2016;79:402–14. doi: 10.1016/j.biopsych.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, et al. Open-labeled study of kunilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Venkataramana NK, Pal R, Rao SA, Naik AL, Jan M, Nair R, et al. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: A pilot clinical study. Stem Cells Int. 2012;2012:931902. doi: 10.1155/2012/931902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: Focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52. doi: 10.1186/1471-2202-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune-and inflammation-mediated diseases: Review of current clinical trials. J Biomed Sci. 2016;23:76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuhara T, Kameda M, Sasaki T, Tajiri N, Date I. Cell therapy for Parkinson’s disease. Cell Transplant. 2017;26:1551–9. doi: 10.1177/0963689717735411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zafranskaya MM, Nizhegorodova DB, Aleynikova NE, Kuznetsova TE, Vanslav MI, Ignatovich TV, et al. The migration of multipotent mesenchymal stromal cells after systemic and local administration in an experimental model of Parkinson’s disease. Ann Clin Exp Neurol. 2019;13:32–40. [Google Scholar]