Abstract
Background
Although chemotherapy saves lives, increasing evidence shows that chemotherapy accelerates aging. We previously demonstrated that mRNA expression of p16INK4a, a biomarker of senescence and molecular aging, increased early and dramatically after beginning adjuvant anthracycline-based regimens in early stage breast cancer patients. Here, we determined if changes in p16INK4a expression vary by chemotherapy regimen among early stage breast cancer patients.
Methods
We conducted a study of stage I-III breast cancer patients receiving adjuvant or neoadjuvant chemotherapy. p16INK4a expression was analyzed prechemotherapy and postchemotherapy (median 6.2 months after the last chemotherapy) in peripheral blood T lymphocytes. Chemotherapy-induced change in p16INK4a expression was compared among regimens. All statistical tests were 2-sided.
Results
In 146 women, chemotherapy was associated with a statistically significant increase in p16INK4a expression (accelerated aging of 17 years; P < .001). Anthracycline-based regimens were associated with the largest increases (accelerated aging of 23 to 26 years; P ≤ .008). Nonanthracycline-based regimens demonstrated a much smaller increase (accelerated aging of 9 to 11 years; P ≤ .15). In addition to the type of chemotherapy regimen, baseline p16INK4a levels, but not chronologic age or race, were also associated with the magnitude of increases in p16INK4a. Patients with lower p16INK4a levels at baseline were more likely to experience larger increases.
Conclusions
Our findings suggest that the aging effects of chemotherapy may be influenced by both chemotherapy type and the patient’s baseline p16INK4a level. Measurement of p16INK4a expression is not currently available in the clinic, but nonanthracycline regimens offering similar efficacy as anthracycline regimens might be favored.
There is increasing evidence that cancer treatment, especially chemotherapy, accelerates human aging (1-4). The most compelling evidence comes from childhood cancer survivorship studies that found that by age 50 years the cumulative incidence of at least 1 severe, disabling, or life-threatening condition was 53.6%. In addition, 30% of survivors who reached age 35 years without any severe or life-threatening condition developed a life-threatening condition within 10 years (5). Accumulation of chronic diseases due to accelerated aging led to an 8.4-fold increased risk of death compared with the general population.
Senescence is a cellular mechanism that is activated in response to stress and DNA damage and promotes permanent inhibition of the cell cycle of damaged cells. This targeted control of the cell cycle serves as a protective mechanism essential for minimizing the proliferation of damaged cells that can lead to tumor initiation and growth (6). Over time (or after repeated damage), an accumulation of senescent cells in tissues contributes to perturbations in the ability of tissues to undergo repair, in part because of the decline in the replicative function of self-renewing stem cells and an increase in local inflammation (7-10). Therefore, although senescent cells protect tissues from becoming cancerous, the accumulation of senescent cells promotes chronic inflammation, declines in organ function, and acceleration of age-related diseases (eg, atherosclerosis, idiopathic lung fibrosis, osteoarthritis, bone loss, hepatic steatosis, and cognitive decline) (11).
Finding biomarkers of aging that capture changes over the entire lifespan and reflect the clinical aspects of aging is a rich and expanding area of research (12,13). Potential biomarkers include inflammatory markers, telomere length (14-18), sarcopenia, DNA methylation (19,20), and maximal oxygen consumption (vO2max) (21). Expression of p16INK4a, a biomarker of senescence, is considered a fundamental and dynamic measurement of molecular aging with a 10-fold increase in gene expression between ages 20 and 80 years (22). p16INK4a is a tumor suppressor molecule that acts to arrest cell proliferation, resulting in multi-organ cell senescence in murine models (23). Maintenance of the p16INK4a transcript is required for maintenance of the senescent phenotype, and senescent cells remain in tissues indefinitely (24-27).
In humans, p16INK4a expression can be readily measured in T cells and is a robust marker of age in normal controls (28). Expression of p16INK4a in human T cells is influenced by a variety of age-promoting stimuli, including cigarette smoking, physical inactivity (28), cytotoxic chemotherapy administration (29), chronic HIV infection (30), and bone marrow transplantation (4). We previously reported rapid and persistent increases in p16INK4a mRNA expression in the peripheral blood T cells in a small cohort of women receiving anthracycline-based adjuvant chemotherapy for early stage breast cancer, whereas in a second cohort of early stage breast cancer patients who did not receive chemotherapy, p16INK4a expression levels were similar to healthy volunteers (29).
Our initial study of chemotherapy-induced aging was done in early stage breast cancer patients, almost all of whom received anthracycline-based regimens (29). In the current study, we postulated that currently used chemotherapy regimens containing agents with different mechanisms of action would differ in their propensity to induce senescence in T cells. To address this question, we evaluated if chemotherapy-induced changes in p16INK4a mRNA expression differed among 4 commonly used adjuvant chemotherapy regimens. We also examined the contribution of different patient and clinical characteristics, including chronologic age and baseline p16INK4a expression, in predicting the magnitude of increased p16INK4a expression in response to chemotherapy treatment.
Methods
Study Participants
Patients were recruited between April 2011 and August 2018 from university-affiliated hospitals. Women aged 21 years or older with histologically confirmed stage I-III breast cancer and scheduled for adjuvant or neoadjuvant chemotherapy were offered participation in 3 Lineberger Comprehensive Cancer Center (LCCC) studies: LCCC1027 (NCT01305954), LCCC1334 (NCT02167932), and LCCC1410 (NCT02328313). The protocols differed in age criteria for inclusion (LCCC1334 for women aged 21 to 64 years, LCCC1410 for women aged 65 years or older, LCCC1027 for women aged 18 years or older). All studies were approved by the University of North Carolina at Chapel Hill (UNC) LCCC protocol review committee and the institutional review boards of each study site. Written informed consent meeting all university and federal guidelines were obtained from each participant. A total of 19, 75, and 52 participants were enrolled in LCCC1027, LCCC1334, and LCCC1410, respectively.
Chemotherapy Regimens
Patients enrolled in these studies received chemotherapy as determined by their oncologist based on tumor stage and breast cancer phenotype as suggested by National Comprehensive Cancer Network guidelines (31). For statistical analysis, regimens were arranged into 4 groups: 1) doxorubicin, cyclophosphamide, and paclitaxel (AC-T); 2) docetaxel and cyclophosphamide ± anti-HER2 therapy (TC); 3) docetaxel and carboplatin + anti-HER2 therapy (TCH); and 4) doxorubicin and cyclophosphamide plus paclitaxel and carboplatin (AC-TC). Granulocyte colony-stimulating factors (pegfilgrastim) were routinely used in patients receiving docetaxel and cyclophosphamide, docetaxel and carboplatin, and doxorubicin and cyclophosphamide (when administrated every 2 weeks—“dose dense”). For patients on chemotherapy regimens that included anti-HER2 therapy for 12 months, postchemotherapy blood draws were done while patients were still receiving anti-HER2 therapy.
p16INK4a Assay
Blood samples were drawn prior to chemotherapy initiation and at least 60 days after the completion of chemotherapy. The median time from the last chemotherapy treatment to the postchemotherapy blood sample was 6.2 months (range = 2.1-14.3). Blood was drawn into lavender-top EDTA tubes, T cells were isolated, and expression of p16INK4a mRNA in peripheral blood T lymphocytes was determined using TaqMan real-time quantitative reverse transcription polymerase chain reaction. Expression analysis was performed by Sapere Bio (formerly HealthSpan Dx), using technology described previously (29). Every run included external and internal controls to monitor assay performance. Cycle threshold values over 37 were considered below detection and excluded from the analysis. The overall precision (reproducibility based on run-to-run and between operators’ analytical validation) of p16INK4a measurement was 0.8 Ct. The same assay and quality control procedures were used for all samples.
Statistical Analysis
Descriptive statistics are provided to summarize the study sample. Differences in age by regimen were compared using ANOVA, and paired t tests evaluated changes in p16INK4a expression overall and within each regimen. Linear regression was used to model the relationship between patient characteristics with the change in p16INK4a expression. Fisher exact test compared the rates of p16INK4a expression change by regimen. All analyses were conducted using SAS statistical software v9.4 (Cary, NC). Conversion of change in log2 p16 to years of chronologic aging was calculated using the formula: Δlog2p16/0.028 derived from a 633-patient cohort (32). All statistical tests were 2-sided, and a P value of less than .05 was considered statistically significant.
Results
Patient Characteristics
We enrolled 146 patients diagnosed with early stage breast cancer who received neoadjuvant or adjuvant chemotherapy. Their baseline characteristics are presented in Table 1. The mean age was 57 years (range = 28-81). Karnofsky Performance Status was greater than 80% in 92% of patients. African American patients comprised 15.8% of the cohort, 50.7% had hormone receptor-positive and HER2-negative tumors, and 62.3% were treated with adjuvant chemotherapy. Nearly half the patients (47.9%) were treated with anthracycline-based regimens (AC-T or AC-TC), 34.9% with docetaxel and cyclophosphamide (TC), and 17.1% with docetaxel and carboplatin + anti-HER2-directed therapy (TCH). Radiation therapy was administered to 73% of patients after the completion of chemotherapy. As shown in Table 2, patient characteristics were closely associated with the different chemotherapy regimens. For example and as expected, patients with higher stage cancers (stage II and III) were more likely to receive anthracycline (AC)–containing chemotherapy, whereas TC was more likely administered in women with estrogen receptor–positive tumors and lower stage tumors; the majority of women receiving AC-TC had triple-negative breast cancer.
Table 1.
Patient characteristics
| Variable | All patients |
|---|---|
| (n = 146) | |
| Overall age at consent, y | |
| Mean (SD) | 57 (12.2) |
| Range, y | 28-81 |
| Race, No. (%) | |
| White | 110 (75.3) |
| African American | 23 (15.8) |
| Other | 13 (8.9) |
| Breast cancer stage, No. (%)a | |
| I | 32 (21.9) |
| II | 81 (55.5) |
| III | 33 (22.6) |
| Phenotype, No. (%) | |
| Hormone receptor–positive and HER2-negative | 74 (50.7) |
| Hormone receptor–negative and HER2-negative (triple negative) | 42 (28.8) |
| HER2-positive and hormone receptor–negative | 14 (9.6) |
| HER2-positive and hormone receptor–positive | 16 (11.0) |
| Timing of chemotherapy, No. (%) | |
| Neoadjuvant | 54 (37.0) |
| Adjuvant | 91 (62.3) |
| Both | 1 (0.7) |
| Treatment regimens, No. (%) | |
| AC-T (doxorubicin and cyclophosphamide then paclitaxel and carboplatin)b | 53 (36.3) |
| AC-TC (doxorubicin and cyclophosphamide then paclitaxel and carboplatin)c | 17 (11.6) |
| TC (docetaxel and cyclophosphamide)d | 51 (34.9) |
| TCH (docetaxel and carboplatin + anti-HER2 therapy)e | 25 (17.1) |
American Joint Committee on Cancer stage version 7.
Nine patients had paclitaxel before doxorubicin and cyclophosphamide. Doxorubicin and cyclophosphamide given every 2 weeks with growth factors (5 patients got anthracycline every 3 weeks); 3 patients received anti-HER2 therapy with AC-T.
TC given first in 11 patients.
Given every 3 weeks with growth factors; 2 patients received anti-HER2 therapy with TC.
Given every 3 weeks with growth factors; all received trastuzumab, and 19 patients received pertuzumab in addition to trastuzumab.
Table 2.
Patient characteristics by chemotherapy regimen
| Variable | Regimen |
|||||
|---|---|---|---|---|---|---|
| AC-T | AC-TC | TC | TCH | P a | P | |
| (n = 53) | (n = 17) | (n = 51) | (n = 25) | AC vs non-ACa | ||
| Chronological age, y | ||||||
| Mean (SD) | 55.7 (11.5) | 48.9 (12.5) | 60.5 (11.3) | 58.0 (13.1) | .005 | .006 |
| Race, No. (%) | ||||||
| African American | 12 (22.6) | 2 (11.8) | 4 (7.8) | 5 (20.0) | .48 | .35 |
| White | 37 (69.8) | 14 (82.3) | 42(82.4) | 17 (68.0) | ||
| Other | 4 (7.5) | 1 (5.9) | 5 (9.8) | 3 (12.0) | ||
| Phenotype, No. (%) | ||||||
| Estrogen receptor–positive | 36 (67.9) | 1 (5.9) | 39 (76.5) | 14 (56.0) | <.001 | .04 |
| Estrogen receptor–negative | 17 (32.1) | 16 (94.1) | 12 (23.5) | 11 (44.0) | ||
| Stage, No. (%) | ||||||
| I | 7 (13.2) | 1 (5.9) | 22 (43.1) | 2 (8.0) | <.001 | <.001 |
| II | 27 (50.9) | 10 (58.8) | 29 (56.9) | 15 (15.0) | ||
| III | 19 (35.8) | 6 (35.3) | 0 (0) | 8 (32.0) | ||
| Timing of chemotherapy, No. (%) | ||||||
| Adjuvant | 29 (54.7) | 2 (11.8) | 49 (96.1) | 11 (44.0) | <.001 | <.001 |
| Neoadjuvant | 23 (43.4) | 15 (88.2) | 2 (3.9) | 14 (56.0) | ||
| Both | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) | ||
| Radiation postchemotherapy, No. (%) | ||||||
| Yes | 40 (75.5) | 13 (76.5) | 35 (68.6) | 18 (72.0) | .73 | .29 |
| No | 13 (24.5) | 4 (23.5) | 16 (31.4) | 7(28.0) | ||
ANOVA for age (continuous variable) and Fisher exact test for all categorical variables. All tests 2-sided. AC-T = doxorubicin and cyclophosphamide then paclitaxel and carboplatin; AC-TC = doxorubicin and cyclophosphamide then paclitaxel and carboplatin; TC = docetaxel and cyclophosphamide; TCH = docetaxel and carboplatin + anti-HER2 therapy.
Change in p16INK4a Expression From Baseline to Posttreatment
Mean differences in p16INK4a expression from baseline to the follow-up visit are presented in Table 3. Overall, chemotherapy regimens were associated with a 1.4-fold increase in p16INK4a expression (equivalent to a 17-year acceleration in aging; P < .001). Anthracycline-based regimens (AC-T or AC-TC) were associated with the largest mean change in p16INK4a expression (1.6- to 1.7-fold increase; equivalent to a 23- to 26-year acceleration in aging; P ≤ .008), whereas smaller mean changes in p16INK4a expression were observed in patients receiving nonanthracycline chemotherapy regimens: TCH (equivalent to a 9-year acceleration in aging; P = .03) or TC (equivalent to 11-year acceleration in aging; P = .15).
Table 3.
Chemotherapy-induced change from baseline in p16INK4a mRNA expression to the follow-up visit by chemotherapy regimen and overall
| Regimen | Time point | No. | Mean log2 p16INK4a (95% CI) | Mean log2 change in p16INK4a (95% CI) | P a | Absolute change in p16INK4 (95% CI) | Change in age (95% CI), y |
|---|---|---|---|---|---|---|---|
| Overall | Baseline | 146 | 9.62 (9.46 to 9.77) | 0.47 (0.33 to 0.61) | <.001 | 1.39 (1.25 to 1.54) | 17 (12 to 22) |
| Follow-up visit | 146 | 10.09 (9.93 to 10.24) | |||||
| AC-T (Dose-dense doxorubicin and cyclophosphamide followed by paclitaxel) | Baseline | 53 | 9.53 (9.28 to 9.78) | 0.65 (0.46 to 0.84) | <.001 | 1.57 (1.38 to 1.79) | 23 (16 to 30) |
| Follow-up visit | 53 | 10.18 (9.93 to 10.43) | |||||
| AC-TC (Doxorubicin and cyclophosphamide followed by paclitaxel and carboplatin) | Baseline | 17 | 9.34 (8.87 to 9.82 | 0.74 (0.22 to 1.25) | .008 | 1.67 (1.16 to 2.38) | 26 (8 to 45) |
| Follow-up visit | 17 | 10.08 (9.65 to 10.52) | |||||
| TC (Docetaxel and cyclophosphamide ± anti-HER2 therapy) | Baseline | 51 | 9.79 (9.52 to 10.05) | 0.30 (0.04 to 0.57) | .03 | 1.23 (1.03 to 1.48) | 11 (1 to 20) |
| Follow-up visit | 51 | 10.09 (9.78 to 10.40) | |||||
| TCH (Docetaxel and carboplatin + anti-HER2 therapy) | Baseline | 25 | 9.64 (9.25 to 10.02) | 0.26 (−0.10 to 0.63) | .15 | 1.20 (1.00 to 1.55) | 9 (−4 to 23) |
| Follow-up visit | 25 | 9.90 (9.57 to 10.23) |
Paired t test and all 2-sided. CI = confidence interval.
Baseline p16INK4a and Change in p16INK4a Expression
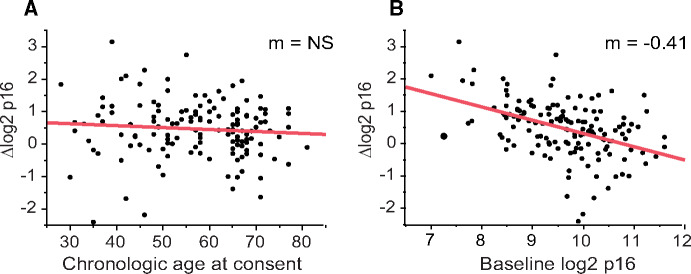
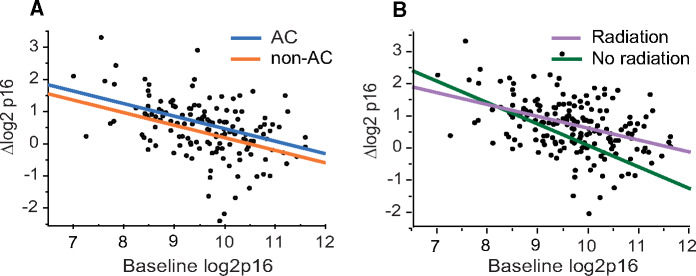
Having established that the increase in p16INK4a expression may be driven by the type of chemotherapy regimen, we tested if patient characteristics contributed to the magnitude of chemotherapy-induced increase in p16INK4a expression. Surprisingly, we found that baseline p16INK4a expression, and not chronologic age, correlated with the magnitude of chemotherapy-induced increase in p16INK4a expression (Figure 1). When we separated the cohort into patients receiving anthracycline- vs nonanthracycline-based chemotherapy, the regression slopes of association between baseline p16INK4a expression and chemotherapy-induced change in p16INK4a expression were parallel between treatment groups (Figure 2, A). These findings suggest that although anthracycline-containing chemotherapy regimens cause larger increases in p16INK4a expression levels than nonanthracycline regimens, the propensity to increase p16INK4a expression was dependent on the baseline p16INK4a expression and not an individual regimen.
Figure 1.
Chemotherapy-induced changes in p16INK4a mRNA expression correlate with baseline p16INK4a expression but not chronologic age. Correlation between chronologic age (A) or baseline p16INK4a expression (B) and chemotherapy-induced change in p16INK4a expression. AC = anthracycline; M = regression slope; non-AC = nonanthracycline-containing; NS = not statistically significant (P = .32).
Figure 2.
Patient’s expression levels of p16INK4a prior to chemotherapy are a major predictor of chemotherapy-induced change in p16INK4a mRNA expression. Correlation between baseline p16INK4a expression and chemotherapy-induced change in p16INK4a expression in patients receiving anthracycline (blue line) or nonanthracycline-containing regimens (orange line) (A) or patients who received (lavender line) or did not received (green line) postchemotherapy radiation (B). AC = anthracycline.
Breast or locoregional radiation in this cohort was given to 73% of patients. There was no statistically significant difference in the anthracycline chemotherapy–induced increase in p16INK4a expression compared with those who did not receive radiation therapy (P = .29). As shown in Figure 2, B, regardless of whether patients received radiation or not, a similar trend was observed for an inverse correlation between p16INK4a expression levels at baseline, and the magnitude of chemotherapy-induced change in p16INK4a expression. A more detailed quantitative analysis of the impact of radiation on the chemotherapy-induced increase in p16INK4a expression was not possible, because there was a statistical interaction between baseline p16INK4a expression and receipt of radiation (p16*radiation, P = .06). This finding is not surprising because the decision to administer radiation is based on the type or surgery (partial or total mastectomy) and stage.
To understand the effect of baseline p16INK4a expression, chemotherapy regimen, and race on the chemotherapy-induced increase in p16INK4a expression, we analyzed these variables using univariate and multivariate regression analyses. As shown in Table 4, in a multivariate analysis, both baseline p16INK4a expression and chemotherapy regimen, but not race, were statistically significant predictors of a chemotherapy-induced increase in p16INK4a expression. These findings suggest that patients with lower baseline levels of p16INK4a expression are more likely to experience larger chemotherapy-induced increases in p16INK4a expression.
Table 4.
Linear regression parameter estimates for the magnitude of change in p16INK4a mRNA expression
| Variable | Univariate |
Multivariablea |
||
|---|---|---|---|---|
| Estimate | P b | Estimate | P b | |
| Chronologic age at consent | −0.006 | .31 | 0.010 | .09 |
| Baseline p16INK4a mRNA expression | −0.410 | <.001 | −0.435 | <.001 |
| Regimen, AC vs non-AC | 0.380 | .008 | 0.325 | .01 |
| Race, African American vs all others | 0.224 | .26 | NS | NS |
Regimen-adjusted analysis was based on AC- vs non-AC–containing regimens. AC = anthracycline.
Linear regression and 2-sided.
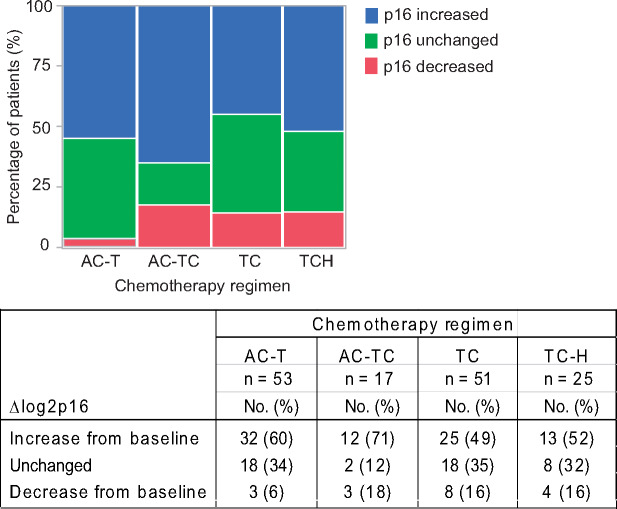
Finally, to better understand how these data might apply to a clinical setting, we evaluated whether specific chemotherapy regimens differed in their propensity to increase p16INK4a expression (Figure 3). Changes in p16INK4a expression were categorized as follows: increase in p16INK4a expression above the assay precision (an increase over 0.4); unchanged p16INK4a levels within the assay precision (between 0.4 and -0.4); and decrease in p16INK4a expression below the assay precision (below 0.4). Most patients had increases in p16INK4a expression, with percentages ranging from 49% for the TC group to 71% for the AC-TC group. The percentage of patients whose p16INK4a expression remained unchanged ranged from 12% in the AC-TC group to about 35% in both the AC-T and TC groups. Only a small percentage of patients (6%) in the AC-T had decreases in chemotherapy-induced p16INK4a expression, but this rose to 16%-18% in the other 3 chemotherapy groups. Therefore, although a choice of chemotherapy regimens may impact the magnitude of chemotherapy-induced increase in p16INK4a expression, an increase in p16INK4a expression is likely driven by the baseline p16INK4a expression and not chronological age or other patient and tumor characteristics.
Figure 3.
Patients whose p16INK4a expression levels are increased by chemotherapy are not restricted to any 1 treatment. Distribution of patients whose p16INK4a levels increased above assay precision is similar among chemotherapy regimens commonly used to treat early stage breast cancer patients.
Discussion
We have previously shown that anthracycline-based chemotherapy in women with early stage breast cancer induced expression of cell senescence biomarkers in vivo (29). In the current study, we determined if chemotherapy regimens commonly used in patients with early stage breast cancers have differential effects on p16INK4a expression. We found that anthracycline-based therapies were statistically significantly more pro-aging than nonanthracycline-based therapies. Adjuvant anthracycline-based chemotherapy regimens save the lives of many patients and lower their risk of breast cancer relapse and death (33). However, potential toxicities such as myelodysplasia, acute leukemia (34,35) and cardiac toxicity (36) detract from the overall benefit. For many breast cancer patients, nonanthracycline-containing adjuvant chemotherapy regimens are associated with similar improvements in disease-free and overall survival as anthracycline regimens (37). Therefore, choosing a chemotherapy regimen that has a smaller impact on the chemotherapy-induced acceleration of aging may prove to be important to minimize chemotherapy-induced acceleration of morbidity and mortality.
Interestingly, although chemotherapy regimen type may have been an important driver of the magnitude of change in p16INK4a expression, baseline p16INK4a expression levels also played a statistically significant role. Patients with lower p16INK4a values at baseline experienced a larger chemotherapy-induced increase in p16INK4a expression than patients with higher baseline p16INK4a values, and that association remained statistically significant even after adjusting for chemotherapy regimen. One potential limitation of our study is a smaller sample size (between 17 and 53 patients in each regimen group). Larger studies will improve the quantification of age acceleration induced by different chemotherapy regimens. In addition, similar effects of chemotherapy and irradiation on telomere length have been reported (14-18). Also, measurement of additional biomarkers of aging that capture various molecular mechanisms of aging, such as an increase in inflammation (the measurement of the senescence-associated secretory phenotype) (10), epigenetic changes (19,20) (DNA methylation panels), and changes in metabolism, may deepen our understanding of processes affected by chemotherapy. Taken together, our results suggest that the choice of chemotherapy regimen, as well as the baseline “molecular age” of the patient, are important factors that determine the pro-aging capacity of chemotherapy regimens.
Our observation that patients with lower baseline p16INK4a expression experience a larger increase in p16INK4a expression is unexpected. One explanation is that there may be a threshold to the number of senescent cells a tissue or organism can tolerate. Whereas senescence protects from cellular damage-induced tumorigenesis, the accumulation of senescent cells is detrimental to tissue function and regenerative capacity because of an increase in inflammation and depletion of stem cells. In naturally aged mice, the number of senescent cells has been reported as 1%-3% of total tissue (38,39), suggesting that the maximum number of senescent cells is capped and tightly controlled by the immune system. We have previously found in a cohort of human donors that p16INK4a expression levels in a population tend to plateau, suggesting that there is a saturation threshold for the accumulation of senescent cells in humans as well (40). Our data in this study demonstrate that patients with lower baseline p16INK4a expression experienced a larger increase in p16INK4a levels during chemotherapy treatments than patients with higher baseline p16INK4a expression, consistent with the observation that p16INK4a expression may plateau later in life.
Much research remains to be done to characterize the correlation between p16INK4a expression levels and human health. Although a link between p16INK4a expression and mortality has been demonstrated in animal models (41-43), a definitive link between senescence levels and the clinical manifestation of aging and mortality in humans has not been established. A few studies have begun to establish the correlation between p16INK4a expression and predisposition to chemotherapy-induced toxicities and the development of frailty. For example, we demonstrated that high levels of p16INK4a correlated with chemotherapy-induced adverse events, such as severe (grade 3 and 4) fatigue (44). In childhood survivors, chemotherapy-induced accelerated aging and increased morbidity and mortality have been well established, and new data suggest that chemotherapy increased p16INK4a expression in a dose-dependent manner and increased p16INK4a expression correlated with increased frailty in this cohort (45). Studies are underway to establish the correlation between p16INK4a expression and multimorbidities and mortality in middle-aged adult cancer patients as well as young adult childhood cancer survivors.
In addition to chemotherapy, radiation is also known to promote cellular senescence (46-49). In murine models, whole body radiation is a major inducer of senescence and p16INK4a expression (50). However, the effect of radiation on changes in p16INK4a expression in women with early stage breast cancer is less clear. In women with early stage breast cancers, radiation is limited to the breast (in earlier stage patients) or the chest wall and regional nodes (in women treated with mastectomy and with larger tumors and/or positive axillary lymph nodes). Because of the targeted tissue volume treated, radiation in breast cancer patients is not likely to have the same effect as in murine models. Our previous studies in early stage breast cancer patients who received anthracycline-containing chemotherapy did not find radiation to be a statistically significant contributor to the chemotherapy-induced increase in p16INK4a expression, and in a second larger cohort of patients not treated with chemotherapy, radiation had no effect on p16INK4a expression (29). In the current study, 73% of patients received breast or locoregional radiation. We are unable to clearly define the role, if any, of radiation and p16INK4a expression because of statistical interaction between variables, but we are able to demonstrate that the inverse correlation between baseline p16INK4a levels and the magnitude of chemotherapy-induced p16INK4a increases remain even in women who did not receive radiation treatment. Larger studies in cohorts of women who receive radiation only or chemotherapy with radiation would be able to tease out individual contributions of baseline p16INK4a expression, chemotherapy regimen, and radiation on the p16INK4a increases with treatment.
Altogether, our findings suggest that the pro-aging effects of chemotherapy regimens are mediated by the combined effects of the type of chemotherapy regimen and the patient’s baseline p16INK4a level. In addition, our data suggest that anthracycline regimens, especially when used in younger patients, accelerate biologic age. Adjuvant anthracycline-based chemotherapy regimens save the lives of many patients and lower their risk of breast cancer relapse and death (33,51). However, recent studies show that adjuvant nonanthracycline regimens may be as effective as anthracycline-containing regimens for large numbers of early stage patients (37). Our data provide additional support for selection on nonanthracycline regimens in women with early stage breast cancer unless the projected benefit for anthracycline regimens is clearly associated with improved outcomes (for example, women with triple-negative breast cancer or those with hormone receptor–positive breast cancer with 4 or more positive nodes). For those patients where anthracycline regimens are preferred, the protective effects of lifestyle interventions such as exercise or pharmacologic agents (52,53) (eg, senolytics) to retard accelerated aging effects in patients receiving chemotherapy are areas of intense interest requiring further research.
Funding
This work was supported by the National Cancer Institute (R01CA203023), Breast Cancer Research Foundation (New York, NY), Kay Yow Cancer Fund (Cary, NC), and University Cancer Research Fund (University of North Carolina, Chapel Hill, NC).
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to disclose.
Author contributions: SSS, NM, and HBM were involved in the conceptualization, methodology, data curation, data analysis, and writing. AMD was involved in the methodology, data curation, data analysis, and writing. KRH, CKA, LAC, ECD, TAJ, GGK, MSK, RER, JCS, and HBM contributed patient resources to this study. All authors contributed to the data analysis and writing.
Acknowledgments: We greatly appreciate the active support of the oncology clinicians and their research staff at multiple sites and, most importantly, the breast cancer patients participating in our studies. The study sites are Duke University Medical Center/Cancer Institute, Ohio State University Comprehensive Cancer Center, MD Anderson Cancer Center, UNC Rex Healthcare, and UNC Cancer Center. We also thank Tucker Brenizer, Erin O’Hare, Emily Bell, Chad Wagoner, Will Pulley, Nancy Burns, and Amy Garrett for their unwavering commitment to study implementation best practices. We are indebted to the pioneering research in biomarkers of aging by Dr Norman Sharpless and his initial involvement and encouragement of our investigations in women with breast cancer. We also appreciate the help of Lena Randhawa in the preparation of this manuscript.
Prior presentation: Preliminary results were presented at the American Society of Clinical Oncology 2017 Annual Meeting, June 3, 2017, Chicago, IL: Shachar SS, et al. (2017). Changes in p16INK4a (p16) expression, a biomarker of aging, in peripheral blood T cells (PBTC) in patients receiving anthracycline (A) vs non-anthracycline (NoA) chemotherapy (CRx) for early stage breast cancer (EBC). J Clin Oncol. 2017:35(15 suppl):10060-10060.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67(2):212–215. [DOI] [PubMed] [Google Scholar]
- 2. Buttiglieri S, Ruella M, Risso A, et al. The aging effect of chemotherapy on cultured human mesenchymal stem cells. Exp Hematol. 2011;39(12):1171–1181. [DOI] [PubMed] [Google Scholar]
- 3. Scuric Z, Carroll JE, Bower JE, et al. Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer. 2017;3(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood WA, Krishnamurthy J, Mitin N, et al. Chemotherapy and stem cell transplantation increase p16(INK4a) expression, a biomarker of T-cell aging. EBioMedicine. 2016;11:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A.. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99(2):1047–1078. [DOI] [PubMed] [Google Scholar]
- 7. He S, Sharpless NE.. Senescence in health and disease. Cell. 2017;169(6):1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharpless NE, DePinho RA.. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8(9):703–713. [DOI] [PubMed] [Google Scholar]
- 9. Coppe JP, Desprez PY, Krtolica A, Campisi J.. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol Mech Dis. 2010;5(1):99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campisi J, Andersen JK, Kapahi P, Melov S.. Cellular senescence: a link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21(6):354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ovadya Y, Krizhanovsky V.. Strategies targeting cellular senescence. J Clin Invest. 2018;128(4):1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hubbard JM, Cohen HJ, Muss HB.. Incorporating biomarkers into cancer and aging research. J Clin Oncol. 2014;32(24):2611–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franceschi C, Campisi J.. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Ser A Biol Sci Med Sci. 2014;69(suppl 1):S4–9. [DOI] [PubMed] [Google Scholar]
- 14. Unryn BM, Hao D, Gluck S, Riabowol KT.. Acceleration of telomere loss by chemotherapy is greater in older patients with locally advanced head and neck cancer. Clin Cancer Res. 2006;12(21):6345–6350. [DOI] [PubMed] [Google Scholar]
- 15. Pooley KA, Sandhu MS, Tyrer J, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010;70(8):3170–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diker-Cohen T, Uziel O, Szyper-Kravitz M, Shapira H, Natur A, Lahav M.. The effect of chemotherapy on telomere dynamics: clinical results and possible mechanisms. Leuk Lymphoma. 2013;54(9):2023–2029. [DOI] [PubMed] [Google Scholar]
- 17. Brouwers B, Hatse S, Dal Lago L, et al. The impact of adjuvant chemotherapy in older breast cancer patients on clinical and biological aging parameters. Oncotarget. 2016;7(21):29977–29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schroder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;84(10):1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11(2):303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sehl ME, Carroll JE, Horvath S, Bower JE.. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sorrentino JA, Sanoff HK, Sharpless NE.. Defining the toxicology of aging. Trends Mol Med. 2014;20(7):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song Z, von Figura G, Liu Y, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9(4):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brenner AJ, Stampfer MR, Aldaz CM.. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene. 1998;17(2):199–205. [DOI] [PubMed] [Google Scholar]
- 26. Te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP.. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62(6):1876–1883. [PubMed] [Google Scholar]
- 27. Kim WY, Sharpless NE.. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127(2):265–275. [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8(4):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson JA, Krishnamurthy J, Menezes P, et al. Expression of p16(INK4a) as a biomarker of T-cell aging in HIV-infected patients prior to and during antiretroviral therapy. Aging Cell. 2012;11(5):916–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(3):310–320. [DOI] [PubMed] [Google Scholar]
- 32. Muss HB Smitherman A Wood WA et al. p16 a biomarker of aging and tolerance for cancer therapy. Transl Cancer Res. 2020. doi: 10.21037/tcr.2020.03.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peto R, Davies C, et al. Early Breast Cancer Trialists’ Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25(24):3699–3704. [DOI] [PubMed] [Google Scholar]
- 35. Freedman RA, Seisler DK, Foster JC, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome among older women receiving anthracycline-based adjuvant chemotherapy for breast cancer on Modern Cooperative Group Trials (Alliance A151511). Breast Cancer Res Treat. 2017;161(2):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Du XL, Xia R, Liu CC, et al. Cardiac toxicity associated with anthracycline-containing chemotherapy in older women with breast cancer. Cancer. 2009;115(22):5296–5308. [DOI] [PubMed] [Google Scholar]
- 37. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: the ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017;35(23):2647–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hashimoto M, Asai A, Kawagishi H, et al. Elimination of p19(ARF)-expressing cells enhances pulmonary function in mice. JCI Insight. 2016;1(12):e87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsygankov D, Liu Y, Sanoff HK, Sharpless NE, Elston TC.. A quantitative model for age-dependent expression of the p16INK4a tumor suppressor. Proc Natl Acad Sci USA. 2009;106(39):16562–16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demaria M, O’Leary MN, Chang J, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smitherman AB Wood WA Mitin N et al. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16INK4a and frailty. Cancer. 2020. doi: 10.1002/cncr.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le ON, Rodier F, Fontaine F, et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010;9(3):398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Munoz-Espin D, Serrano M.. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–496. [DOI] [PubMed] [Google Scholar]
- 49. van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shao L, Feng W, Li H, et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood. 2014;123(20):3105–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clarke M, Coates AS, Darby SC, et al. Early Breast Cancer Trialists’ Collaborative Group. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371(9606):29–40. [DOI] [PubMed] [Google Scholar]
- 52. Wang YW, He SJ, Feng X, et al. Metformin: a review of its potential indications. Drug Des Devel Ther .2017;11:2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Munoz DP, Yannone SM, Daemen A, et al. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight. 2019;4(14):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.