There are over 40 species within the genus Entamoeba, eight of which infect humans. Of these, four species (Entamoeba histolytica, E. dispar, E. moshkovskii, and E. bangladeshi) are morphologically indistinguishable from each other, and yet differentiation is important for appropriate treatment decisions. Here, we developed a hydrolysis probe-based tetraplex real-time PCR assay that can simultaneously detect and differentiate these four species in clinical samples.
KEYWORDS: Entamoeba histolytica, Entamoeba dispar, Entamoeba moshkovskii, Entamoeba bangladeshi, amebiasis, diagnosis, PCR, real-time PCR, pathogenicity
ABSTRACT
There are over 40 species within the genus Entamoeba, eight of which infect humans. Of these, four species (Entamoeba histolytica, E. dispar, E. moshkovskii, and E. bangladeshi) are morphologically indistinguishable from each other, and yet differentiation is important for appropriate treatment decisions. Here, we developed a hydrolysis probe-based tetraplex real-time PCR assay that can simultaneously detect and differentiate these four species in clinical samples. In this assay, multicopy small-subunit (SSU) ribosomal DNA (rDNA) sequences were used as targets. We determined that the tetraplex real-time PCR can detect amebic DNA corresponding to as little as a 0.1 trophozoite equivalent of any of these species. We also determined that this assay can detect E. histolytica DNA in the presence of 10-fold more DNA from another Entamoeba species in mixed-infection scenarios. With a panel of more than 100 well-characterized clinical samples diagnosed and confirmed using a previously published duplex real-time PCR (capable of detecting E. histolytica and E. dispar), our tetraplex real-time PCR assay demonstrated levels of sensitivity and specificity comparable with those demonstrated by the duplex real-time PCR assay. The advantage of our assay over the duplex assay is that it can specifically detect two additional Entamoeba species and can be used in conventional PCR format. This newly developed assay will allow further characterization of the epidemiology and pathogenicity of the four morphologically identical Entamoeba species, especially in low-resource settings.
INTRODUCTION
Amebiasis, a disease caused by the ameba Entamoeba histolytica, occurs in an estimated 500 million people worldwide and is responsible for 50,000 to 100,000 deaths annually, making it the third leading cause of death among parasitic diseases (1–3). It is transmitted through the fecal-oral route and is endemic in low-income regions that lack access to safe drinking water and sanitation, including Southeast Asia, Africa, Mexico, and South America (4–6). In developed countries, amebic infections are concentrated in certain high-risk groups, such as men who have sex with men, people who live in long-term-care facilities, and travelers returning from a country where the disease is endemic (6, 7). Nine of 10 E. histolytica infections remain asymptomatic; however, 10% of infections cause intestinal disease, such as amebic colitis (e.g., diarrhea or dysentery), or, less commonly, extraintestinal disease, such as amebic liver abscess (ALA), which can be fatal if not treated promptly (3, 8). In children, E. histolytica infections are linked to growth stunting (9) and delayed cognitive development (10). It remains unclear why only 10% of E. histolytica infections are symptomatic, but this is thought to be linked to parasite genotype (11, 12), host genetics (13, 14), and/or environmental factors (15, 16). The World Health Organization recommends treatment of all E. histolytica infections irrespective of associated symptoms (2). Treating asymptomatic carriers of E. histolytica not only eliminates future risk of disease but can prevent the spread of infectious ameba cysts excreted in stool.
While disease caused by E. histolytica is well documented, there are three additional species of Entamoeba also shown to infect humans: E. dispar, E. moshkovskii, and E. bangladeshi. Traditionally, E. dispar has been almost universally accepted as a nonpathogenic species. Recent data suggest E. moshkovskii might be an emerging pathogen in children, causing diarrhea and dysentery in Bangladesh (17). E. bangladeshi, originally identified in two individuals in Bangladesh in 2012 (18) and recently detected in South Africa, occurs in both asymptomatic and diarrheal individuals (19) and is commonly observed in stool of those infected with other Entamoeba species; however, limited epidemiologic data prevent a definitive conclusion on pathogenicity (19).
Accurate diagnosis of amebiasis is challenging, especially in countries where the disease is endemic that still rely on microscopic detection of ameba cysts and trophozoites in stool samples (20). Morphologic E. histolytica resemblance to other nonpathogenic or “undetermined-pathogenic” species makes the majority of earlier epidemiological data on amebiasis questionable (21–23). In addition to the fact that microscopy is a nonspecific diagnostic tool due to the existence of other morphologically identical species, its use is also confounded by false-positive results due to the presence of host macrophages and other cells resembling E. histolytica (24, 25). Additionally, microscopy is highly insensitive as it relies on intact ameba structures and because the trophozoite form of E. histolytica begins to disintegrate within 1 to 2 h of stool production unless properly preserved prior to microscopic examination (20). In most ALA cases, stool microscopy is not helpful as concurrent intestinal infection is usually not seen in a majority of cases (26–29).
Several enzyme-linked immunosorbent assays (ELISAs) that detect amebic antigen in stool or liver aspirate pus samples show variable success rates in the detection of E. histolytica (reviewed in references 20, 30, and 31). Among these, the E. histolytica II ELISA (TechLab, Blacksburg, VA) is approved by the U.S. Food and Drug Administration. It performed well in most countries where the disease is endemic (32–34) but poorly in some of the countries where the disease is not endemic (35, 36). The assay performs poorly with frozen and preserved samples and with samples that are collected from patients already treated with antiamebic drugs (28). Similar assays for species-specific detection of E. dispar, E. moshkovskii, or E. bangladeshi are unavailable.
Molecular diagnosis performed with conventional and real-time PCR is more sensitive and specific than the ELISAs and enables species-specific detection. Species-specific detection of E. histolytica, E. dispar, and E. moshkovskii is available in various formats, including conventional PCR (37, 38), nested PCR (39–41), and real-time PCR (42, 43). Several conventional and real-time PCR assays are also available for simultaneous detection and differentiation of E. histolytica and two other major diarrhea-causing parasites, Giardia and Cryptosporidium species (44–50). Real-time PCR assays have advantages over conventional PCR assays, as they are faster, can be monitored in real time, and are less prone to contamination since post-PCR processing steps to detect amplified products, such as running of gels, are not needed. However, real-time PCR assays require expensive equipment and supplies and technical expertise that are not readily available in resource-poor settings. Also, proper facilities (e.g., separate rooms for DNA purification and master mix preparation), knowledge (e.g., one-way direction of material and personnel, etc.), and participation in external quality assessment schemes may not be readily available in resource-poor settings. Recently, a hydrolysis probe-based real-time PCR for simultaneous detection and differentiation of four Entamoeba species (E. histolytica, E. dispar, E. moshkovskii, and E. bangladeshi) was reported (19). While the probes in that assay are species specific, it utilizes primers that are common to all four Entamoeba species. As a result, the assay does not provide species-specific information if used in the conventional PCR format in resource-poor settings, limiting its use to the real-time PCR format only.
We report a hydrolysis probe-based tetraplex real-time PCR assay developed to detect four Entamoeba species (E. histolytica, E. dispar, E. moshkovskii, and E. bangladeshi) in clinically relevant stool and liver aspirate pus samples. Because it utilizes four sets of species-specific primers, it offers an advantage over the existing tetraplex real-time PCR assay by allowing species-specific detection in a conventional PCR format in the absence of a real-time PCR instrument and the associated supplies and technical expertise. The performance characteristics of this tetraplex real-time PCR were compared with those of an established duplex real-time PCR assay for E. histolytica and E. dispar using DNA extracted from more than 100 intestinal and ALA samples from clinical patients.
MATERIALS AND METHODS
Cultures of Entamoeba.
E. histolytica HM-1:IMSS culture was maintained axenically in TYI-S-33 (Trypticase, yeast extract, and iron serum) medium at the U.S. Centers for Disease Control and Prevention (CDC) Free-Living and Intestinal Amebas (FLIA) laboratory as previously described (51). Extracted DNA from a known number of ameba trophozoites was used to determine the limit of detection (LOD) of the E. histolytica real-time PCR assay in singleplex and multiplexed real-time PCR assay formats. Axenic culture lysates with known numbers of trophozoites from E. dispar SAW760 and E. moshkovskii Laredo strains and xenic culture (52) lysate from known numbers of trophozoites of E. bangladeshi were kindly provided by Rashidul Haque in the Parasitology Laboratory of International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B). These were used to determine LODs for the individual and multiplexed real-time PCR assays and were also used to determine the linear range of individual singleplex real-time PCR assays.
DNA from culture isolates and clinical samples.
DNA from 27 xenic culture isolates (including 11 E. histolytica isolates, 9 E. dispar isolates, 3 E. moshkovskii isolates, 1 E. bangladeshi isolate, 2 Blastocystis hominis isolates, and 1 Entamoeba coli isolate), DNA from 46 clinical stool samples (including 12 samples positive for E. histolytica, 16 for E. dispar, and 1 for E. moshkovskii and 17 negative for Entamoeba spp.), and DNA from 41 aspirated liver abscess samples (including 38 samples positive for E. histolytica and 3 from patients with bacterial liver abscess) were utilized in this study. These DNAs were collected for other studies, but they were made available for Ph.D. research studies of I. K. M. Ali at the London School of Hygiene and Tropical Medicine, United Kingdom, during 2001 to 2005. The samples were deidentified prior to their use in this study. All samples had previously been characterized in ICDDR,B laboratories using one or more of the following methods (described elsewhere as indicated): isoenzyme analysis (24), nested PCR (24), antigen detection E. histolytica II ELISA (24) (TechLab, Blacksburg, VA), or a real-time PCR (53).
DNA purification.
DNA was purified from 50 μl of culture pellet dispersed in 250 μl of lysis buffer (0.25% sodium dodecyl sulfate–0.1 M EDTA, pH 8.0) containing100 μg/ml of proteinase K using the cetyltrimethylammonium bromide (CTAB) method, as described previously (54). DNA was purified from 0.1 g of stool samples using a QIAamp DNA stool minikit (catalog no. 51604, Qiagen, USA) as described previously (55). DNA was purified from 200-μl volumes of aspirated liver abscess samples using a DNeasy blood and tissue kit (catalog no. 69506, Qiagen, USA) according to the manufacturer’s instructions. A previously characterized Entamoeba-negative stool or liver abscess sample that had been spiked with an aliquot (∼100 μl) of a known Entamoeba culture lysate served as a positive extraction control. The same previously characterized Entamoeba-negative stool or liver abscess sample served as a negative extraction control, which was not spiked with an Entamoeba culture lysate.
Primer and probe design.
To design primers and probes, complete or partial 18S small-subunit rRNA gene sequences of E. histolytica, E. dispar, E. moshkovskii, and E. bangladeshi (only partial sequences were available for E. bangladeshi) were downloaded from PubMed (GenBank accession numbers: X75434.1 for E. histolytica, KP722600.1 for E. dispar, KP722602.1 for E. moshkovskii, and KR025411.1 for E. bangladeshi) and sequences were aligned using multiple-sequence-alignment software (http://multalin.toulouse.inra.fr/multalin/) (56). We chose the 18S small-subunit ribosomal rRNA genes as PCR targets because they occur in hundreds of copies per ameba genome (57). For each species, unique sequences were selected and sequence differences were chosen at the 3′ ends of primers to maximize the species specificity (Fig. 1). All probe sequences selected were species specific to increase the specificities of the individual and tetraplex real-time PCR assays (Table 1).
FIG 1.
Locations of primers and probes in the 18S small-subunit rRNA genes. Alignment of corresponding 18S small-subunit rRNA genes of four Entamoeba species was performed to show locations of primer and probe sequences. Sequences of forward primers are underlined with solid lines, sequences of reverse primers are underlined with double solid lines, and the sequences of the probes are underlined with dashed lines and italicized. Primer and probe sequences that differ from those of at least one other Entamoeba species are shown by bold fonts. (A) E. histolytica (Eh). (B) E. dispar (Ed). (C) E. moshkovskii (Em). (D) E. bangladeshi (Eb). The GenBank accession numbers are as follows: X75434.1 (E. histolytica), KP722600.1 (E. dispar), KP722602.1 (E. moshkovskii), and KR025411.1 (E. bangladeshi).
TABLE 1.
Primers and probesa
| Primer or probe name |
Sequence (5′ to 3′)b | Specificity | Product size (bp) |
|---|---|---|---|
| EhF3 | CAGTAATAGTTTCTTTGGTTAGTAAAA | E. histolytica | 133 |
| EhR3 | CTTAGAATGTCATTTCTCAATTCAT | ||
| EhP3 | HEX-GTTTGTATTAGTACAAAATGGC-BHQ1 | ||
| EdF3 | CAGTAATAGTTTCTTTGGTTAGTAAAG | E. dispar | 134 |
| EdR3 | CTTAGAATGTCATTTCTCAATTTAC | ||
| EdP3n1 | Cy5-GTATTAGTACAAAGTGGCCAA-BHQ3 | ||
| EmF4 | CAGATGGCTACCACTTCTAC | E. moshkovskii | 145 |
| EmR4 | GATTTCGTAAGAGTATTTACTTCT | ||
| EmP4 | FAM-CTCGAGGTGGTTAACTCCAC-BHQ1 | ||
| EbF2 | GTTTCTAGAGATGTGATAATGG | E. bangladeshi | 132 |
| EbR2 | CAATATTGTCCCATGCTTGAATATC | ||
| EbP2 | TAMRA-GGGTGTTTAAAGCAAAACATTAA-BHQ2 |
18S small-subunit rRNA gene sequences of Entamoeba species were used to design primers and probes. In the primer and probe names, “F” indicates forward primer, “R” indicates reverse primer, “P” indicates probe, “Eh” indicates E. histolytica, “Ed” indicates E. dispar, “Em” indicates E. moshkovskii, and “Eb” indicates E. bangladeshi.
5′ fluorescent dyes and the 3′ quencher molecules of the probes are shown at the respective ends. BHQ, black hole quencher; FAM, 6-carboxyfluorescein; HEX, 6-carboxy-2,4,4,5,7,7-hexachlorofluorescein; TAMRA, 6-carboxytetramethylrhodamine.
PCR assays.
Real-time PCR assays were performed in tetraplex or singleplex format in duplicate in a total volume of 20 μl using commercially available Platinum Quantitative PCR SuperMix-UDG with ROX (catalog no. 11743-500, Fisher Scientific, USA). Each DNA template was diluted 1:2 and 1:20 with sterile distilled water to further minimize any adverse effect of the presence of PCR inhibitors that could have still been copurified during DNA extraction steps despite the use of an extraction kit that should have removed inhibitors. Next, 5 μl of each dilution was added to each PCR mixture in two separate wells. Positive controls (either a mixture of 4 ameba DNAs for tetraplex real-time PCR or a single relevant ameba DNA for the singleplex real-time PCR) and negative controls (sterile water) were included in each PCR run. One microliter each of forward and reverse primers from a 20 μM stock and 1 μl of each probe from a 2 μM stock were added to the final reaction mixture. The PCR assay described above was also run in a conventional PCR format (i.e., without the use of probes) in singleplex PCR format. Results were considered valid when the results obtained with the replicates agreed. The duplex real-time PCR assay for E. histolytica and E. dispar was performed according to a method previously described by Qvarnstrom et al. (58). All PCR assays were carried out in an ABI7500 thermal cycler (Applied Biosystems, USA). The tetraplex or singleplex real-time PCR thermal cycles consisted of one cycle at 50°C for 2 min and 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 55°C for 1 min. Real-time PCR data were analyzed using Applied Biosystems 7500 software for 7500 and 7500 Fast real-time PCR systems (v2.3). The conventional singleplex PCR was performed exactly the same as the singleplex real-time PCR except that no probes were added to the reaction mixture.
Gel electrophoresis.
Amplified products (10 μl) from the conventional singleplex PCR assays were run on a 2% agarose gel (SeaKem LE Agarose, USA) containing a 1× final concentration of GelRed nucleic acid stain solution (Biotium, USA) for 2 h at a constant voltage (125 V) (59). A 100-bp DNA size marker (BioLabs, USA) was used to verify the sizes of amplicons.
Limit of detection (LOD) of singleplex and tetraplex real-time PCR assays.
In order to detect the minimum quantity of organism DNA needed to obtain a positive amplification signal (i.e., LOD), Entamoeba-negative (verified by microscopy and PCR) stool or liver aspirate samples were spiked with known numbers of trophozoites of E. histolytica, E. dispar, E. moshkovskii, or E. bangladeshi. DNA was extracted by the use of Qiagen stool kits or Qiagen blood and tissue kits. Eluted DNA was then serially diluted 10-fold to produce 1.0-μl volumes containing DNA from 10,000, 1,000, 100, 10, 1, 0.1, or 0.01 trophozoite equivalents. DNA was assayed using the singleplex or tetraplex real-time PCR format with species-specific primers and probes.
LOD of E. histolytica real-time PCR in mixed infections.
Mixed infections with more than one species of Entamoeba in the same individual are not uncommon. Detection of mixed infection of E. histolytica with another Entamoeba species is particularly important because only E. histolytica infection requires treatment. Here, we investigated the limit of the ability of E. histolytica real-time PCR and the tetraplex real-time PCR to detect E. histolytica DNA in the presence of various proportions of DNA from one or more of other Entamoeba species. We used E. histolytica DNA that had originated from 0.1, 1.0, 10, 100, 1,000, and 10,000 trophozoite equivalents in the presence of a base level of either 10 or 100 trophozoite DNA equivalents from other species in duplex or tetraplex PCR format.
Linear range of detection.
In order to determine the linear range of detection for each individual singleplex real-time PCR, standard curves were generated using DNA originating from 0.1 to 10,000 trophozoite equivalents per reaction mixture. Each assay was repeated in triplicate.
Specificity.
The specificity of each of the Entamoeba real-time PCRs was evaluated individually against the DNAs from other three Entamoeba species. For example, the E. histolytica primers and probe (Fig. 1) were allowed to amplify DNAs originating from 10,000 trophozoites of each of four Entamoeba species in separate wells. Additionally, the specificity of the primers and probes in this study was evaluated in singleplex and tetraplex formats against the DNAs from other intestinal organisms such as Entamoeba coli, Entamoeba hartmanni, Entamoeba polecki, Blastocystis hominis, Cryptosporidium, Giardia, Shigella, Campylobacter, and Escherichia coli.
Data analysis and calculation of comparative sensitivity and specificity.
In order to minimize the run-to-run variations, quantification cycle (Cq) values were manually adjusted with a known positive control in each run. If a clinical sample gave a Cq value of <37, it was considered positive. However, samples that gave late Cq values of between 37 and 40 were assayed at least twice, and if they still gave a similar or lower Cq value (i.e., <37), they were considered positive.
In comparison with the duplex real-time PCR for E. histolytica and E. dispar, the sensitivity and specificity of the tetraplex real-time PCR assay were calculated for the ability to detect E. histolytica and E. dispar with the following formulas: sensitivity = (no. of correct positive results)/(total no. of positive samples) × 100%; specificity = (no. of correct negative results)/(total no. of negative samples) × 100%.
RESULTS
Limit of detection (LOD).
Individually, E. histolytica, E. dispar, E. moshkovskii, and E. bangladeshi real-time PCR assays were sufficiently sensitive to detect the respective species DNAs originated from a 0.1 trophozoite equivalent (Table 2). The LODs remained the same when the real-time PCR was performed in the tetraplex PCR format (Table 2). Because the PCR target is multicopy small-subunit (SSU) ribosomal DNA (rDNA), which is maintained at about 200 copies per genome (57), a 0.1 trophozoite equivalent should contain about 20 copies of target DNA.
TABLE 2.
Limit of detectiona
| No. of trophozoite DNAs used |
Cq LOD |
|||||||
|---|---|---|---|---|---|---|---|---|
|
b
Singleplex real-time PCR |
c
Tetraplex real-time PCR |
|||||||
| E. histolytica | E. dispar | E. moshkovskii | E. bangladeshi | E. histolytica | E. dispar | E. moshkovskii | E. bangladeshi | |
| 10,000 | 16.4 | 19.8 | 18.3 | 16.9 | 16.5 | 18.8 | 14.9 | 16.4 |
| 1,000 | 19.7 | 22.7 | 21.7 | 20.3 | 21 | 22.8 | 17.9 | 19.9 |
| 100 | 23.1 | 26.3 | 25.2 | 22.6 | 24.4 | 26 | 21.5 | 22.4 |
| 10 | 27.4 | 29.7 | 29 | 26.9 | 27.7 | 30.2 | 25.5 | 26.3 |
| 1 | 31.7 | 35.6 | 33.1 | 27.9 | 30.6 | 34.3 | 27.8 | 26.6 |
| 0.1 | 35.1 | 38.1 | 36 | 34.6 | 38.2 | 38.3 | 33.1 | 34.3 |
| 0.01 | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq |
| None | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq |
Limit of detection (LOD) values were calculated in the singleplex and tetraplex real-time PCR formats.
For the singleplex PCRs, Cq values are shown with the respective primers and probe sets. All the signals were species specific. For example, E. histolytica primers and probe gave signals (i.e., Cq values) only with the E. histolytica DNA.
DNA from all 4 species was mixed together to perform the tetraplex real-time PCR.
Linear range of detection.
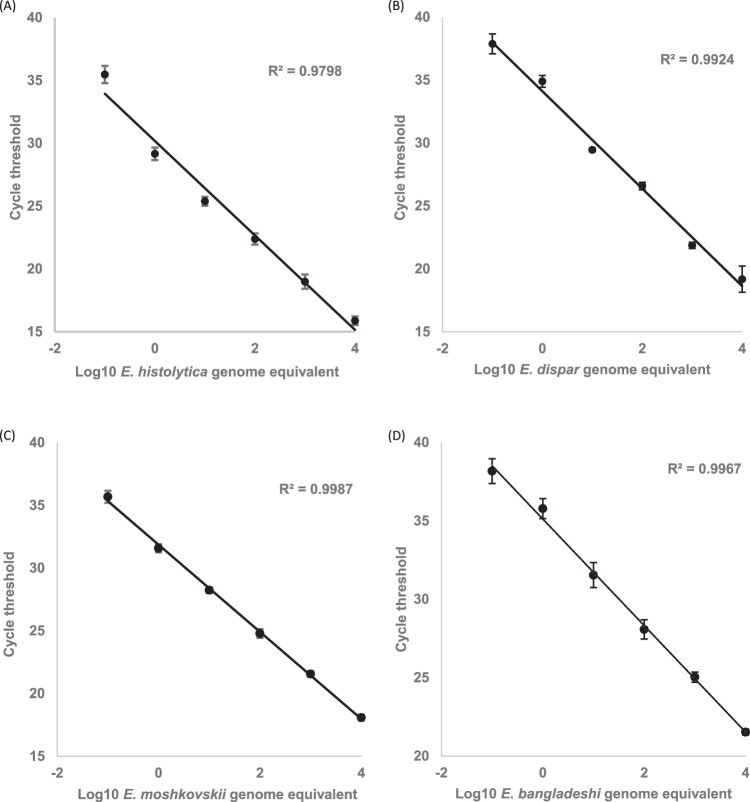
Standard curves for individual Entamoeba real-time PCR assays were calculated and found to be linear over a range from 0.1 to 10,000 trophozoite equivalents per reaction (Fig. 2) indicating that the individual real-time PCR assays could detect as little as 0.1 trophozoite equivalent per reaction, showing a high degree of correlation (r2 > 0.97).
FIG 2.
Linear dynamic range of the singleplex Entamoeba real-time PCR assays using the corresponding Entamoeba DNAs. (A) E. histolytica. (B) E. dispar. (C) E. moshkovskii. (D) E. bangladeshi.
Specificity.
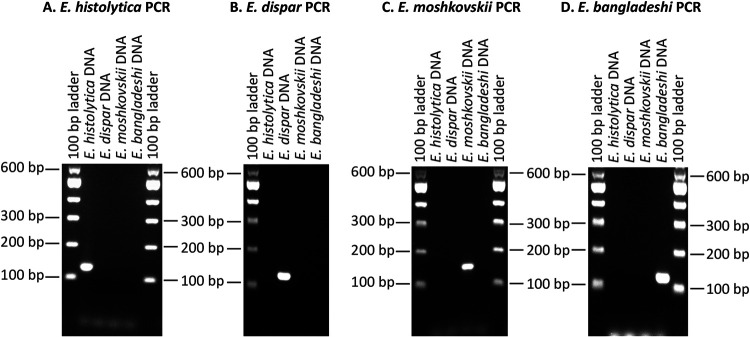
Each of the individual Entamoeba real-time PCR assays was evaluated individually against the DNAs from other three Entamoeba species. E. histolytica real-time PCR gave positive signal only with the E. histolytica DNA and did not show any cross-reaction with any other Entamoeba DNAs. Similarly, the other three Entamoeba real-time PCRs gave amplification signals only with the respective species DNAs and did not show cross-reaction with any other Entamoeba species DNA. Additionally, each Entamoeba primer set was checked for cross-reaction with DNA from the other three Entamoeba species in separate reaction wells in conventional PCRs. Each primer set gave expected amplification only with the respective Entamoeba DNA and did not cross-react with other Entamoeba DNAs (Fig. 3).
FIG 3.
Specificity of individual Entamoeba PCRs in the conventional PCR format. The final sets of primers from four Entamoeba species were evaluated for their species specificity. Each conventional PCR (A, E. histolytica PCR; B, E. dispar PCR; C, E. moshkovskii PCR; D, E. bangladeshi PCR) used a single set of primers (for example, E. histolytica PCR used only an E. histolytica-specific primer set as described for panel A) in the presence of DNAs from four Entamoeba species that originated from 10,000 trophozoites each. Amplified products were run on a 2% agarose gel. In each of the PCRs, we observed species-specific amplification and no cross-amplifications with DNA from other Entamoeba species. The DNA size marker used was a 100-bp ladder (BioLabs, USA).
The primers and probes in this study did not give nonspecific fluorescence signals in singleplex or tetraplex real-time PCRs against the DNAs from other intestinal organisms such as Entamoeba coli, Entamoeba hartmanni, Entamoeba polecki, Blastocystis hominis, Cryptosporidium, Giardia, Shigella, Campylobacter, and Escherichia coli. Similarly, nonspecific amplifications were not detected in conventional PCRs either in singleplex or tetraplex formats (data not shown). We conclude that the Entamoeba primers and probes in this study were unique to the species level.
LOD of E. histolytica real-time PCR in mixed infections.
In a mixed-infection scenario with more than one species of Entamoeba in the same individual, our results indicated that the E. histolytica real-time PCR assay was capable of detecting E. histolytica DNA in the presence of up to 100-fold more of another Entamoeba DNA in duplex real-time PCR or of up to 10-fold more of the remaining three Entamoeba DNAs in the tetraplex real-time PCR (Table 3). If the other Entamoeba DNAs were present at >100-fold compared to the E. histolytica DNA, then the E. histolytica real-time PCR failed to detect it. This is a limitation of this real-time PCR.
TABLE 3.
Detection of mixed infectionsa
| No. of E. histolytica trophozoite DNAs |
No. of other Entamoeba trophozoite DNAs |
Real-time PCR Cq value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duplex-1 |
Duplex-2 |
Duplex-3 |
Tetraplex |
||||||||
| E. histolytica | E. dispar | E. histolytica | E. moshkovskii | E. histolytica | E. bangladeshi | E. histolytica | E. dispar | E. moshkovskii | E. bangladeshi | ||
| 0.1 | 10 | 31.2 | 28.8 | 35.5 | 26.2 | 33.1 | 28 | No Cq | 28.7 | 26.9 | 29.6 |
| 1 | 10 | 27.9 | 28.4 | 28.3 | 26.4 | 28.4 | 29.4 | 28.7 | 27.8 | 26.8 | 29.2 |
| 10 | 10 | 24.9 | 27.7 | 24.9 | 26 | 25.3 | 26.5 | 25.2 | 29.3 | 26.2 | 27.4 |
| 100 | 10 | 21.7 | 32.8 | 21.7 | 27.3 | 22.1 | 23.7 | 22.1 | 34.7 | 28.9 | 23.3 |
| 1,000 | 10 | 18.4 | No Cq | 18.5 | No Cq | 28.6 | 20.7 | 18.7 | No Cq | No Cq | 19.9 |
| Blank | Blank | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq |
| 1 | 100 | 27.7 | 25.2 | 31.2 | 22.9 | 28.8 | 27.3 | No Cq | 25.1 | 23.4 | 27.8 |
| 10 | 100 | 24.7 | 24.8 | 25.1 | 22.7 | 25.6 | 25.9 | 25.2 | 24.9 | 23.3 | 26.2 |
| 100 | 100 | 21.7 | 24 | 21.6 | 22.6 | 22.1 | 23.6 | 21.8 | 24.7 | 23.2 | 23.7 |
| 1,000 | 100 | 18.5 | 28.6 | 18.5 | 23 | 18.7 | 20.3 | 18.7 | 29.4 | 24.9 | 20.5 |
| 10,000 | 100 | 15.4 | No Cq | 15.5 | No Cq | 15.6 | 17.5 | 15.8 | No Cq | No Cq | 17.6 |
| Blank | Blank | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq | No Cq |
Performance of E. histolytica real-time PCR in the presence of DNA of other Entamoeba species originating from 10 trophozoites or 100 trophozoites as indicated.
Comparison with the CDC diagnostic duplex real-time PCR for the detection of E. histolytica and E. dispar using clinical samples.
DNA from cultured organisms and stool and liver aspirate samples were available from 114 clinical patients from Bangladesh.
The duplex real-time PCR and tetraplex real-time PCR performed equally well with 11 E. histolytica-positive culture DNAs and 9 E. dispar-positive culture DNAs and detected all of these accurately (Table 4). Three Entamoeba-negative cultures (two of these were positive for Blastocystis hominis, and the other was positive for Entamoeba coli) were also accurately identified as negative for E. histolytica and E. dispar by both duplex and tetraplex real-time PCRs. Thus, the tetraplex real-time PCR showed 100% sensitivity and 100% specificity in detecting E. histolytica and E. dispar in culture DNAs compared to the duplex real-time PCR. Additionally, three E. moshkovskii culture DNAs and one E. bangladeshi culture DNA were also available. The tetraplex real-time PCR could positively identify the E. moshkovskii and the E. bangladeshi culture DNAs accurately; such identifications were outside the scope of the duplex real-time PCR, and it only identified these as negative for E. histolytica or E. dispar.
TABLE 4.
Performance of tetraplex real-time PCRa
| Source of DNA |
No. of samples |
Previous detectionb |
No. detected by: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Duplex real-time PCRc
|
Tetraplex real-time PCR |
|||||||||
| E. histolytica | E. dispar | Negative | E. histolytica | E. dispar | E. moshkovskii | E. bangladeshi | Negative | |||
| Culture (n = 27) | 11 | E. histolytica | 11 | 0 | 0 | 11 | 0 | 0 | 0 | 0 |
| 9 | E. dispar | 0 | 9 | 0 | 0 | 9 | 0 | 0 | 0 | |
| 3 | E. moshkovskii | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | |
| 1 | E. bangladeshi | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 3 | Negative | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 | |
| Stool (n = 46) | 12 | E. histolytica | 12 | 0 | 0 | 12 | 0 | 0 | 0 | 0 |
| 16 | E. dispar | 0 | 16 | 0 | 0 | 16 | 3d | 0 | 0 | |
| 1 | E. moshkovskii | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| 17 | Negative | 0 | 0 | 17 | 3 | 0 | 0 | 0 | 14 | |
| LA (n = 41) | 38 | E. histolytica | 38 | 0 | 0 | 38 | 0 | 0 | 0 | 0 |
| 3 | Negative | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 | |
The performance of the tetraplex real-time PCR was compared with that of an existing duplex real-time PCR for E. histolytica and E. dispar in clinical samples. LA, liver abscess (aspirate).
Previous diagnosis was based on a combination of different tests such as culture (and zymodeme), conventional and nested PCR, antigen detection E. histolytica II ELISA (TechLab), and duplex real-time PCR. “0” indicates a negative test result. Negative, previously negative for E. histolytica and E. dispar species.
Data were determined by the use of a previously described existing duplex real-time PCR (46).
Tetraplex real-time PCR detected these as mixed infections of E. dispar and E. moshkovskii.
The tetraplex Entamoeba real-time PCR performed slightly better than the duplex Entamoeba real-time PCR in clinical stool samples (n = 46). It detected E. histolytica in 3 additional samples among 17 negatives that the duplex real-time PCR failed to detect. A nested genotyping PCR (in locus STGA-D followed by Sanger sequencing [54]) confirmed the presence of E. histolytica DNAs in these 3 “negative” samples (data not shown). These three samples were likely negative by the duplex real-time PCR due to lower number of target DNAs being below its detection threshold. The target amplicon size of the duplex real-time PCR is 231 bp (i.e., 42.4% larger than that of the tetraplex real-time PCR, 133 bp). PCR with a larger target is less sensitive than PCR a smaller target, and this may explain why the tetraplex real-time PCR was more sensitive than the duplex real-time PCR. The tetraplex Entamoeba real-time PCR could also detect mixed infections of E. moshkovskii with E. dispar in 3 additional samples that the duplex PCR identified as representing E. dispar only. The presence of E. moshkovskii DNA in these samples was verified using a nested PCR specific for E. moshkovskii (60). Overall, the tetraplex real-time PCR showed 100% sensitivity and 82.4% specificity in detecting E. histolytica and E. dispar compared to the duplex real-time PCR. Both real-time PCRs accurately detected 38 positive E. histolytica DNAs purified from the ALA samples, while they detected three liver aspirate DNAs from nonamebic sources as negative for E. histolytica. With liver abscess samples, the tetraplex real-time PCR showed 100% sensitivity and 100% specificity in detecting E. histolytica compared to the duplex real-time PCR.
DISCUSSION
Approximately half a billion people worldwide are thought to acquire E. histolytica infections annually. Because these data are based mostly on the microscopic identification of E. histolytica, there is uncertainty about their validity. We speculate that an unknown portion of these “E. histolytica infections” are actually due to other morphologically indistinguishable Entamoeba species such as E. dispar, E. moshkovskii, and E. bangladeshi. In this study, our main objective was to develop a detection method that can specifically, and uniquely, identify the four Entamoeba species named above in clinical specimens and we successfully developed a highly sensitive and specific tetraplex real-time PCR assay. Not only can this PCR be performed in real-time PCR format, this novel PCR can be used as a conventional PCR if real-time PCR instrumentation, reagents, and expertise are unavailable, as is the case in most countries where amebiasis is endemic.
Performance characteristics of the tetraplex real-time PCR assay were compared with those of an existing duplex real-time PCR assay for E. histolytica and E. dispar (58) by the use of cultured ameba DNA and stool and liver abscess DNAs. Compared to the duplex real-time PCR assay, the tetraplex real-time PCR assay performed equally well with the cultured and liver abscess DNAs and showed an increase in sensitivity with stool DNAs by detecting three additional E. histolytica DNAs that the duplex real-time PCR failed to detect.
A real-time PCR capable of simultaneous detection of the four Entamoeba species named above has recently been reported (19). It uses a common primer set for all four Entamoeba species but uses a unique species-specific probe set for each Entamoeba species. Because it uses a common primer pair for the four Entamoeba species, a major limitation of this assay is that it cannot be used in conventional PCR format in the absence of real-time PCR instruments, reagents, and expertise. The tetraplex real-time PCR developed in our study has the added advantage of being useful in a conventional PCR format, if needed, because it uses four pairs of unique primers specific for each Entamoeba species. Moreover, the sensitivity of the published tetraplex real-time PCR was not evaluated or reported, and the authors admitted that some of the true Entamoeba-positive samples (verified by Sanger sequencing) were missed by their real-time PCR, likely because of low ameba burdens. Additionally, it is not known how versatile their real-time PCR system is for the detection of mixed infections of two or more Entamoeba species. This is particularly important for the detection of mixed infections of E. histolytica with another Entamoeba species in order to make correct treatment decisions.
The tetraplex real-time PCR that we developed was highly sensitive, and it could detect Entamoeba DNA that had originated from the equivalent of 0.1 trophozoite per reaction. Detection of DNA originating from just 0.1 trophozoite equivalent is not surprising given the hundreds of copies of target 18S small-subunit ribosomal rRNA gene molecules per ameba genome (57). It could also detect the target pathogen, E. histolytica DNA, in the presence of up to 10-fold more DNA from one or more other Entamoeba species. Another advantage of this tetraplex real-time PCR is its smaller (132-to-145-bp) amplicon sizes in comparison with those used by Ngobeni et al. (19), which are 250 bp each, that is, approximately 42% to 47% larger than those used this study. A large (≥250-bp) amplicon is generally harder to amplify, and a PCR amplifying a larger amplicon is hence less sensitive than a PCR amplifying a smaller amplicon (61). In this study, during the screening and optimization of primers and probes, we noticed that the E. dispar real-time PCR was 10-fold less sensitive when a larger amplicon was used as the target (194 bp) than when a smaller amplicon was used as the target (134 bp, which was the target ultimately chosen) (data not shown). A comparison of the two tetraplex real-time PCRs is provided in Table 5.
TABLE 5.
Comparison of tetraplex real-time PCRs developed previously by Ngobeni et al. and in this study
| Feature | Result for tetraplex real-time PCR developed by: |
|
|---|---|---|
| Ngobeni et al. (19) | Ali and Roy (this study) | |
| Simultaneous detection of E. histolytica, E. dispar, E. moshkovskii, and E. bangladeshi | Yes | Yes |
| Target DNA | 18S SSU rDNA | 18S SSU rDNA |
| Real-time PCR platform | Hydrolysis probes (TaqMan) | Hydrolysis probes (TaqMan) |
| Primers | One pair, common to all four Entamoeba species | Four pairs, each specific to an Entamoeba species |
| Probes | Four, each specific to an Entamoeba species | Four, each specific to an Entamoeba species |
| Sensitivity | Unknown | Well defined; capable of detecting ameba DNA originating from a single trophozoite |
| Amplicon size | Larger (250 bp) | Smaller (132–145 bp) |
| Use in conventional PCR format? | No | Yes |
| Detection limit for disproportionately infected mixed infections | Not tested | Can detect E. histolytica in the presence of up to 10-fold more DNA from one or more Entamoeba species |
| Compared with another established detection method? | No | Yes, compared with a diagnostic duplex real-time PCR for E. histolytica and E. dispar that is routinely used at U.S. CDC |
Several real-time PCR-based assays are available for the detection of E. histolytica (and sometimes of E. moshkovskii as well) along with other enteric pathogens of bacterial or viral origin (50, 62–65). However, although they are sensitive and specific and allow detection of multiple pathogens in the same assay, a major disadvantage that limits their use in countries where E. histolytica infection is endemic is that they require sophisticated and expensive instruments and trained experts to run these assays. Also, they do not include E. bangladeshi detection and cannot be used in Entamoeba species-specific epidemiological surveys.
Our Entamoeba tetraplex real-time PCR has several limitations, however, and should be used with the following cautions. (i) We evaluated a limited number of DNAs from samples positive for E. moshkovskii and E. bangladeshi. (ii) This method may fail to detect an Entamoeba species in mixed-infection cases when the DNA from another Entamoeba is present at >100-fold. In such a scenario, only the predominant Entamoeba species is detected. (iii) For the majority of the intestinal samples that we tested, the clinical status of ameba-infected individuals was not provided for this study. (iv) Assay performance may be compromised in formalin-fixed specimens since formalin-induced DNA degradation can interfere with detection, leading to false-negative results. In this study, 9 of the 46 stool samples were sodium acetate-acetic acid-formalin (SAF)-fixed samples, and the remaining samples were fresh. Those nine SAF samples performed equally well in both duplex and tetraplex real-time PCRs. (v) This method is not suitable for determining the absolute burden of amebas in patients since amebas may not be excreted uniformly in each stool specimen. (vi) Since the stool samples were collected for other studies at the ICDDR,B, we were unable to investigate the effect of sample transportation time on the performance of real-time PCR. (vii) Experiments to detect LOD of E. histolytica DNA in mixed infections in the tetraplex real-time PCR were performed with purified trophozoites. As a consequence, we do not know if the results obtained with clinical samples would be equally accurate if the predominant form of Entamoeba were cyst. (viii) The E. histolytica real-time PCR would give positive amplification results with DNA from Entamoeba nuttalli (a species that infects nonhuman primates, especially macaques, but may occasionally infect humans) since the 18S SSU rDNA sequences used for the design of the primers and probe are identical in the species.
Concluding remarks.
E. histolytica and E. dispar occupy opposite ends of the pathogenicity spectrum: E. histolytica is pathogenic, and E. dispar is nonpathogenic. However, the levels of pathogenicity of E. moshkovskii and E. bangladeshi likely lie somewhere between those extremes. We describe a sensitive and species-specific tetraplex real-time PCR for the simultaneous detection and differentiation of E. histolytica, E. dispar, E. moshkovskii, and E. bangladeshi by the use of DNAs from clinical samples such as stool samples or liver aspirates. This novel real-time PCR will help elucidate the true epidemiology and pathogenicity of these four morphologically indistinguishable Entamoeba species. In resource-poor settings, where real-time PCR instruments and expertise are unavailable, this tetraplex real-time PCR can be used in a conventional PCR format.
ACKNOWLEDGMENTS
We acknowledge Rashidul Haque, International Centre for Diarrheal Disease Research, Bangladesh, for providing ameba culture lysates and/or DNA samples used in the real-time PCR LOD assay. We thank Jennifer Murphy, U.S. Centers for Disease Control and Prevention (CDC), for critical reading of the manuscript.
The findings and conclusions in this report are ours and do not necessarily represent the views of the Centers for Disease Control and Prevention.
I.K.M.A. conceived the idea for the study, designed the experiments, analyzed the data, and wrote the manuscript. S.R. designed the experiments, performed the experiments, and analyzed the data.
The efforts of I.K.M.A. and S.R. were funded by HHS and the Centers for Disease Control and Prevention (CDC).
We declare that we have no competing interests to disclose.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1997. World Health Organization/Pan American Health Organization/UNESCO report of a consultation of experts on amebiasis. Wkly Epidemiol Rec 72:97–100. https://www.who.int/docstore/wer/pdf/1997/wer7214.pdf.9100475 [Google Scholar]
- 3.Stauffer W, Ravdin JI. 2003. Entamoeba histolytica: an update. Curr Opin Infect Dis 16:479–485. doi: 10.1097/00001432-200310000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr. 2003. Amebiasis. N Engl J Med 348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- 5.Shirley DT, Farr L, Watanabe K, Moonah S. 2018. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infect Dis 5:ofy161. doi: 10.1093/ofid/ofy161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley SL., Jr 2003. Amoebiasis. Lancet 361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 7.Petri WA., Jr 1996. Recent advances in amebiasis. Crit Rev Clin Lab Sci 33:1–37. doi: 10.3109/10408369609101485. [DOI] [PubMed] [Google Scholar]
- 8.Gathiram V, Jackson TF. 1987. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. S Afr Med J 72:669–672. [PubMed] [Google Scholar]
- 9.Petri WA Jr, Mondal D, Peterson KM, Duggal P, Haque R. 2009. Association of malnutrition with amebiasis. Nutr Rev 67(Suppl 2):S207–S215. doi: 10.1111/j.1753-4887.2009.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Tarleton JL, Haque R, Mondal D, Shu J, Farr BM, Petri WA Jr. 2006. Cognitive effects of diarrhea, malnutrition, and Entamoeba histolytica infection on school age children in Dhaka, Bangladesh. Am J Trop Med Hyg 74:475–481. doi: 10.4269/ajtmh.2006.74.475. [DOI] [PubMed] [Google Scholar]
- 11.Ali IK, Haque R, Alam F, Kabir M, Siddique A, Petri WA Jr. 2012. Evidence for a link between locus R-R sequence type and outcome of infection with Entamoeba histolytica. Clin Microbiol Infect 18:E235–E237. doi: 10.1111/j.1469-0691.2012.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali IK, Mondal U, Roy S, Haque R, Petri WA Jr, Clark CG. 2007. Evidence for a link between parasite genotype and outcome of infection with Entamoeba histolytica. J Clin Microbiol 45:285–289. doi: 10.1128/JCM.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC Jr, Myers MG Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA Jr. 2011. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest 121:1191–1198. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duggal P, Haque R, Roy S, Mondal D, Sack RB, Farr BM, Beaty TH, Petri WA Jr. 2004. Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J Infect Dis 189:520–526. doi: 10.1086/381272. [DOI] [PubMed] [Google Scholar]
- 15.MacFarlane RC, Singh U. 2006. Identification of differentially expressed genes in virulent and nonvirulent Entamoeba species: potential implications for amebic pathogenesis. Infect Immun 74:340–351. doi: 10.1128/IAI.74.1.340-351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rastew E, Vicente JB, Singh U. 2012. Oxidative stress resistance genes contribute to the pathogenic potential of the anaerobic protozoan parasite, Entamoeba histolytica. Int J Parasitol 42:1007–1015. doi: 10.1016/j.ijpara.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimokawa C, Kabir M, Taniuchi M, Mondal D, Kobayashi S, Ali IK, Sobuz SU, Senba M, Houpt E, Haque R, Petri WA Jr, Hamano S. 2012. Entamoeba moshkovskii is associated with diarrhea in infants and causes diarrhea and colitis in mice. J Infect Dis 206:744–751. doi: 10.1093/infdis/jis414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royer TL, Gilchrist C, Kabir M, Arju T, Ralston KS, Haque R, Clark CG, Petri WA Jr. 2012. Entamoeba bangladeshi nov. sp., Bangladesh. Emerg Infect Dis 18:1543–1545. doi: 10.3201/eid1809.120122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngobeni R, Samie A, Moonah S, Watanabe K, Petri WA Jr, Gilchrist C. 2017. Entamoeba species in South Africa: correlations with the host microbiome, parasite burdens, and first description of Entamoeba bangladeshi outside of asia. J Infect Dis 216:1592–1600. doi: 10.1093/infdis/jix535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali IK. 2015. Intestinal amebae. Clin Lab Med 35:393–422. doi: 10.1016/j.cll.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Ruiz A, Haque R, Aguirre A, Castanon G, Hall A, Guhl F, Ruiz-Palacios G, Miles MA, Warhurst DC. 1994. Value of microscopy in the diagnosis of dysentery associated with invasive Entamoeba histolytica. J Clin Pathol 47:236–239. doi: 10.1136/jcp.47.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque R, Faruque AS, Hahn P, Lyerly DM, Petri WA Jr. 1997. Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. J Infect Dis 175:734–736. doi: 10.1093/infdis/175.3.734. [DOI] [PubMed] [Google Scholar]
- 23.Strachan WD, Chiodini PL, Spice WM, Moody AH, Ackers JP. 1988. Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet 1:561–563. doi: 10.1016/s0140-6736(88)91355-4. [DOI] [PubMed] [Google Scholar]
- 24.Haque R, Ali IK, Akther S, Petri WA Jr. 1998. Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol 36:449–452. doi: 10.1128/JCM.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huston CD, Haque R, Petri WA Jr. 1999. Molecular-based diagnosis of Entamoeba histolytica infection. Expert Rev Mol Med 1999:1–11. doi: 10.1017/S1462399499000599. [DOI] [PubMed] [Google Scholar]
- 26.Alam F, Salam MA, Hassan P, Mahmood I, Kabir M, Haque R. 2014. Amebic liver abscess in northern region of Bangladesh: sociodemographic determinants and clinical outcomes. BMC Res Notes 7:625. doi: 10.1186/1756-0500-7-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Congly SE, Shaheen AA, Meddings L, Kaplan GG, Myers RP. 2011. Amoebic liver abscess in USA: a population-based study of incidence, temporal trends and mortality. Liver Int 31:1191–1198. doi: 10.1111/j.1478-3231.2011.02562.x. [DOI] [PubMed] [Google Scholar]
- 28.Haque R, Mollah NU, Ali IK, Alam K, Eubanks A, Lyerly D, Petri WA Jr. 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J Clin Microbiol 38:3235–3239. doi: 10.1128/JCM.38.9.3235-3239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzenstein D, Rickerson V, Braude A. 1982. New concepts of amebic liver abscess derived from hepatic imaging, serodiagnosis, and hepatic enzymes in 67 consecutive cases in San Diego. Medicine (Baltimore) 61:237–246. doi: 10.1097/00005792-198207000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2007. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev 20:511–532. doi: 10.1128/CMR.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verweij JJ, Stensvold CR. 2014. Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin Microbiol Rev 27:371–418. doi: 10.1128/CMR.00122-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haque R, Faruque AS, Petri WA Jr. 1997. Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. Arch Med Res 28:317–318. [PubMed] [Google Scholar]
- 33.Haque R, Neville LM, Hahn P, Petri WA Jr. 1995. Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J Clin Microbiol 33:2558–2561. doi: 10.1128/JCM.33.10.2558-2561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solaymani-Mohammadi S, Rezaian M, Babaei Z, Rajabpour A, Meamar AR, Pourbabai AA, Petri WA Jr. 2006. Comparison of a stool antigen detection kit and PCR for diagnosis of Entamoeba histolytica and Entamoeba dispar infections in asymptomatic cyst passers in Iran. J Clin Microbiol 44:2258–2261. doi: 10.1128/JCM.00530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gatti S, Swierczynski G, Robinson F, Anselmi M, Corrales J, Moreira J, Montalvo G, Bruno A, Maserati R, Bisoffi Z, Scaglia M. 2002. Amebic infections due to the Entamoeba histolytica-Entamoeba dispar complex: a study of the incidence in a remote rural area of Ecuador. Am J Trop Med Hyg 67:123–127. doi: 10.4269/ajtmh.2002.67.123. [DOI] [PubMed] [Google Scholar]
- 36.Gonin P, Trudel L. 2003. Detection and differentiation of Entamoeba histolytica and Entamoeba dispar isolates in clinical samples by PCR and enzyme-linked immunosorbent assay. J Clin Microbiol 41:237–241. doi: 10.1128/jcm.41.1.237-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamzah Z, Petmitr S, Mungthin M, Leelayoova S, Chavalitshewinkoon-Petmitr P. 2006. Differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii by a single-round PCR assay. J Clin Microbiol 44:3196–3200. doi: 10.1128/JCM.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zebardast N, Haghighi A, Yeganeh F, Seyyed Tabaei SJ, Gharavi MJ, Fallahi S, Lasjerdi Z, Salehi N, Taghipour N, Kohansal C, Naderi F. 2014. Application of multiplex PCR for detection and differentiation of Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii. Iran J Parasitol 9:466–473. [PMC free article] [PubMed] [Google Scholar]
- 39.ElBakri A, Samie A, Ezzedine S, Odeh RA. 2013. Differential detection of Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii in fecal samples by nested PCR in the United Arab Emirates (UAE). Acta Parasitol 58:185–190. doi: 10.2478/s11686-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 40.Khairnar K, Parija SC. 2007. A novel nested multiplex polymerase chain reaction (PCR) assay for differential detection of Entamoeba histolytica, E. moshkovskii and E. dispar DNA in stool samples. BMC Microbiol 7:47. doi: 10.1186/1471-2180-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngui R, Angal L, Fakhrurrazi SA, Lian YL, Ling LY, Ibrahim J, Mahmud R. 2012. Differentiating Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii using nested polymerase chain reaction (PCR) in rural communities in Malaysia. Parasit Vectors 5:187. doi: 10.1186/1756-3305-5-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamzah Z, Petmitr S, Mungthin M, Leelayoova S, Chavalitshewinkoon-Petmitr P. 2010. Development of multiplex real-time polymerase chain reaction for detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii in clinical specimens. Am J Trop Med Hyg 83:909–913. doi: 10.4269/ajtmh.2010.10-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau YL, Anthony C, Fakhrurrazi SA, Ibrahim J, Ithoi I, Mahmud R. 2013. Real-time PCR assay in differentiating Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii infections in Orang Asli settlements in Malaysia. Parasit Vectors 6:250. doi: 10.1186/1756-3305-6-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bairami A, Rezaei S, Rezaeian M. 2018. Synchronous identification of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. in stool samples using a multiplex PCR assay. Iran J Parasitol 13:24–30. [PMC free article] [PubMed] [Google Scholar]
- 45.Flecha MJ, Benavides CM, Tissiano G, Tesfamariam A, Cuadros J, de Lucio A, Bailo B, Cano L, Fuentes I, Carmena D. 2015. Detection and molecular characterisation of Giardia duodenalis, Cryptosporidium spp. and Entamoeba spp. among patients with gastrointestinal symptoms in Gambo Hospital, Oromia Region, southern Ethiopia. Trop Med Int Health 20:1213–1222. doi: 10.1111/tmi.12535. [DOI] [PubMed] [Google Scholar]
- 46.Haque R, Roy S, Siddique A, Mondal U, Rahman SM, Mondal D, Houpt E, Petri WA Jr. 2007. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am J Trop Med Hyg 76:713–717. doi: 10.4269/ajtmh.2007.76.713. [DOI] [PubMed] [Google Scholar]
- 47.Laude A, Valot S, Desoubeaux G, Argy N, Nourrisson C, Pomares C, Machouart M, Le Govic Y, Dalle F, Botterel F, Bourgeois N, Cateau E, Leterrier M, Le Pape P, Morio F. 2016. Is real-time PCR-based diagnosis similar in performance to routine parasitological examination for the identification of Giardia intestinalis, Cryptosporidium parvum/Cryptosporidium hominis and Entamoeba histolytica from stool samples? Evaluation of a new commercial multiplex PCR assay and literature review. Clin Microbiol Infect 22:190.e1–190.e8. doi: 10.1016/j.cmi.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Maas L, Dorigo-Zetsma JW, de Groot CJ, Bouter S, Plotz FB, van Ewijk BE. 2014. Detection of intestinal protozoa in paediatric patients with gastrointestinal symptoms by multiplex real-time PCR. Clin Microbiol Infect 20:545–550. doi: 10.1111/1469-0691.12386. [DOI] [PubMed] [Google Scholar]
- 49.Mero S, Kirveskari J, Antikainen J, Ursing J, Rombo L, Kofoed PE, Kantele A. 2017. Multiplex PCR detection of Cryptosporidium sp, Giardia lamblia and Entamoeba histolytica directly from dried stool samples from Guinea-Bissauan children with diarrhoea. Infect Dis (Lond) 49:655–663. doi: 10.1080/23744235.2017.1320728. [DOI] [PubMed] [Google Scholar]
- 50.Van den Bossche D, Cnops L, Verschueren J, Van Esbroeck M. 2015. Comparison of four rapid diagnostic tests, ELISA, microscopy and PCR for the detection of Giardia lamblia, Cryptosporidium spp. and Entamoeba histolytica in feces. J Microbiol Methods 110:78–84. doi: 10.1016/j.mimet.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Diamond LS, Harlow DR, Cunnick CC. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg 72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 52.Robinson GL. 1968. Laboratory cultivation of some human parasitic amoebae. J Gen Microbiol 53:69–79. doi: 10.1099/00221287-53-1-69. [DOI] [PubMed] [Google Scholar]
- 53.Roy S, Kabir M, Mondal D, Ali IK, Petri WA Jr, Haque R. 2005. Real-time-PCR assay for diagnosis of Entamoeba histolytica infection. J Clin Microbiol 43:2168–2172. doi: 10.1128/JCM.43.5.2168-2172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali IK, Zaki M, Clark CG. 2005. Use of PCR amplification of tRNA gene-linked short tandem repeats for genotyping Entamoeba histolytica. J Clin Microbiol 43:5842–5847. doi: 10.1128/JCM.43.12.5842-5847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blessmann J, Ali IK, Nu PA, Dinh BT, Viet TQ, Van AL, Clark CG, Tannich E. 2003. Longitudinal study of intestinal Entamoeba histolytica infections in asymptomatic adult carriers. J Clin Microbiol 41:4745–4750. doi: 10.1128/jcm.41.10.4745-4750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corpet F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharya S, Bhattacharya A, Diamond LS. 1988. Comparison of repeated DNA from strains of Entamoeba histolytica and other Entamoeba. Mol Biochem Parasitol 27:257–262. doi: 10.1016/0166-6851(88)90045-x. [DOI] [PubMed] [Google Scholar]
- 58.Qvarnstrom Y, James C, Xayavong M, Holloway BP, Visvesvara GS, Sriram R, da Silva AJ. 2005. Comparison of real-time PCR protocols for differential laboratory diagnosis of amebiasis. J Clin Microbiol 43:5491–5497. doi: 10.1128/JCM.43.11.5491-5497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 60.Ali IK, Hossain MB, Roy S, Ayeh-Kumi PF, Petri WA Jr, Haque R, Clark CG. 2003. Entamoeba moshkovskii infections in children, Bangladesh. Emerg Infect Dis 9:580–584. doi: 10.3201/eid0905.020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varga A, James D. 2006. Real-time RT-PCR and SYBR Green I melting curve analysis for the identification of Plum pox virus strains C, EA, and W: effect of amplicon size, melt rate, and dye translocation. J Virol Methods 132:146–153. doi: 10.1016/j.jviromet.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Buss SN, Leber A, Chapin K, Fey PD, Bankowski MJ, Jones MK, Rogatcheva M, Kanack KJ, Bourzac KM. 2015. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol 53:915–925. doi: 10.1128/JCM.02674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madison-Antenucci S, Relich RF, Doyle L, Espina N, Fuller D, Karchmer T, Lainesse A, Mortensen JE, Pancholi P, Veros W, Harrington SM. 2016. Multicenter valuation of BD Max enteric parasite real-time PCR assay for detection of Giardia duodenalis, Cryptosporidium hominis, Cryptosporidium parvum, and Entamoeba histolytica. J Clin Microbiol 54:2681–2688. doi: 10.1128/JCM.00765-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spina A, Kerr KG, Cormican M, Barbut F, Eigentler A, Zerva L, Tassios P, Popescu GA, Rafila A, Eerola E, Batista J, Maass M, Aschbacher R, Olsen KE, Allerberger F. 2015. Spectrum of enteropathogens detected by the FilmArray GI panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infect 21:719–728. doi: 10.1016/j.cmi.2015.04.007. [DOI] [PubMed] [Google Scholar]